Introduction
Hepatoblastoma, the most commonly diagnosed
malignant pediatric liver tumor, is frequently diagnosed in the
first 3 years of life. In recent years, the combination of surgery
and chemotherapy has improved the prognosis of patients with
hepatoblastoma (1). Furthermore,
chemotherapeutic agents, including cisplatin, have been applied in
therapeutic strategies for hepatoblastoma (2,3).
However, conventional chemotherapy agents frequently have limited
clinical applications due to the adverse side effects and drug
resistance acquired following long-term use. Consequently, it is
vital to develop safe and affordable alternative therapeutic agents
for the treatment of hepatoblastoma.
Recently, naturally occurring compounds have been
valued as potential anticancer therapies due to their safety and
efficacy. Additionally, the majority of clinical chemotherapeutic
drugs have an alkaloid structure, suggesting that alkaloids are
important antitumor agent candidates. Lycorine, a crude alkaloid
extracted from Amaryllidaceae genera, is reported to have
antimalarial, antiviral and anti-inflammatory properties (4–7).
Notably, lycorine is at least 15-fold more effective against cancer
cells compared with normal cells, suggesting that lycorine is a
selective anti-tumor compound (8).
Multiple molecular mechanisms have been reported to be involved in
the anticancer effects of lycorine. Inducing apoptosis of cancer
cells has a pivotal role among these mechanisms. For example,
lycorine induces apoptosis of A549 cells via the adenosine
monophosphate-activated protein kinase/serine/threonine-protein
kinase mTOR/S6 kinase signaling pathway (9), and lycorine induces apoptosis of
bladder cancer T24 cells by inhibiting protein kinase B
phosphorylation and activating the intrinsic apoptotic cascade
(10). However, increasing evidence
has emphasized that lycorine also inhibits cancer cell
proliferation and migration (11).
Thus, it is important to examine the molecular mechanisms
underlying the anti-proliferative and anti-migration effects.
In the present study, the HepG2 hepatoblastoma cell
line was used to investigate the inhibitory effects of lycorine on
cell proliferation and migration. Lycorine inhibited the
proliferation of HepG2 cells and induced cell cycle arrest at the
G2/M phase. Additionally, lycorine inhibited the migration of HepG2
cells. Furthermore, mechanistic analyses revealed that lycorine
inhibited HepG2 cell proliferation and migration through
suppression of Rho associated coiled-coil containing protein kinase
1 (ROCK1)/cofilin-induced actin dynamics. Furthermore,
pre-incubation of cells with Y-27632, a specific ROCK1 inhibitor,
significantly attenuated the anti-proliferative and anti-migration
effects of lycorine. Additionally, Y-27632 attenuated
lycorine-induced cofilin downregulation and G2/M phase cell cycle
arrest. These results suggested that lycorine may be useful as a
promising agent for treating hepatoblastoma.
Materials and methods
Cells and antibodies
Lycorine (cat. no. A0415) was purchased from Chengdu
Must Biotechnology Co., Ltd. (Chengdu, China) and dissolved in PBS
as a stock solution. Y-27632 (cat. no. S1049) was obtained from
Selleck Chemicals (Houston, TX, USA). Antibodies against cyclin A
(cat. no. sc-751; 1:500), cyclin B1 (cat. no. sc-752; 1:500),
cyclin dependent kinase 1 (cdc2; cat. no. sc-8395; 1:1,000) and
GAPDH (cat. no. sc-51905; 1:10,000) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA); antibodies against cofilin
(cat. no. ab42824; 1:2,000) and ROCK1 (cat. no. ab45171; 1:1,000)
were from Abcam (Cambridge, MA, USA); and antibodies against matrix
metalloproteinase (MMP)-9 (cat. no. 13667; 1:1,000) and MMP-2 (cat.
no. 40994; 1:1,000) were from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
Cell culture
The human HepG2 hepatoblastoma cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA). Cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; cat. no. PM150212; Procell Life Science & Technology
Co., Ltd., Wuhan, China) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
cultured in a 37°C incubator with a humidified atmosphere of 5%
CO2. Once cells were adhering to the flask, the medium
was changed every 2 days and the cells were digested using 0.25%
trypsin (Gibco; Thermo Fisher Scientific, Inc.).
MTT assay
Cells were seeded in a 96-well culture plate at a
density of 1×104 cells/well overnight, and treated with
various concentrations of lycorine (0.2, 0.5, 1, 2, 10, 20 and 100
µM) the following day. Following incubation in a 5% CO2
incubator at 37°C for 24 h or 48 h, the medium was removed and 20
µl MTT solution (5 mg/ml) was added to each well. Following
incubation at 37°C for an additional 4 h, 150 µl dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to
dissolve the dark blue crystals. The optical density value was
determined at 570 nm and measured on a microplate reader (Varioskan
Flash; Thermo Fisher Scientific, Inc.). All these results are
expressed as a percentage of the control, which was set at 100%.
Each experiment was repeated three times individually.
Clone formation assay
Cells (200 cells/well) were seeded in a 6-well
plate. Cells were allowed to attach overnight and exposed to
different concentrations of lycorine (10 and 20 µM) for 48 h,
following which the culture medium was replaced with fresh DMEM and
cultured for 2 weeks; cells were subsequently fixed with 4%
paraformaldehyde for 15 min and stained with 0.1% crystal violet
for 10 min at room temperature. The number of colonies was counted
using Photoshop CS6 software (Adobe Systems, Inc., San Jose, CA,
USA). Each group had three repeat wells and this experiment was
repeated three times.
Flow cytometry
The cell cycle distribution was determined by flow
cytometry. Cells were seeded in a 6-well culture plate at a density
of 1×106 cells/well. Lycorine (10 and 20 µM) were added
the subsequent day. Following incubation for 48 h, cells were
harvested and washed twice with PBS. Cells were fixed in cold 75%
ethanol overnight in 4°C. Cells were washed twice with cold PBS and
suspended in PBS with 200 µg/ml RNase and 50 µg/ml propidium iodide
(cat. no. 556547; BD Biosciences, San Jose, CA, USA) in the dark
for 30 min. The results were measured by flow cytometry (FACScan;
BD Biosciences) and analyzed using ModFit LT 3.2 software (Verity
Software House, Inc., Topsham, ME, USA).
Wound healing assay
A wound healing assay was used to assess the
migration ability of HepG2 cells. Briefly, cells were seeded in a
6-well culture plate. When cells reached 90% confluence, a wound
was scratched with a 200 µl pipette tip. Cells were washed with PBS
three times to remove the scratched cells, and lycorine (10 and 20
µM) was added and incubated for 24 or 48 h. The cells were imaged
following replacement of the medium. The wound width was measured
using ImageJ software (version 1.48; National Institutes of Health,
Bethesda, MD, USA): Wound healing rate (%) = 100 × (0 h width -
24/48 h width)/2/0 h width.
Transwell assay
HepG2 cells were adjusted to a density of
2×104 cells/well, resuspended and seeded in the upper
chamber of Transwell chambers with 200 µl serum-free medium, and
600 µl complete medium containing 30% FBS was added in the lower
chamber. Lycorine (10 and 20 µM) was added to the two chambers.
Following incubation for 48 h, non-migrated cells on the top
surface of the upper chamber were gently scraped away with a cotton
swab. The lower membrane containing migrated cells was fixed in 4%
paraformaldehyde for 10 min and stained with 0.1% crystal violet
for 15 min at room temperature. A microscope (×4 magnification;
Olympus IX51; Olympus Corporation, Tokyo, Japan) was used to image
the migrated cells. The total cell numbers were calculated from
three different fields and three independent experiments.
Western blot analysis
Cells were harvested and lysed in lysis buffer
containing 1 mM phenylmethylsulfonyl fluoride (Beyotime Institute
of Biotechnology, Haimen, China). Bicinchoninic acid protein
quantification kits (Beyotime Institute of Biotechnology) were used
to measure protein concentrations. A total of 15 µg protein was
separated via 12% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Following
blocking with 5% skim milk in Tris-buffered saline (TBS) containing
0.1% Tween-20 for 2 h at room temperature, specific primary
antibodies were added to the membranes and incubated at 4°C
overnight on a shaker. Membranes were washed in TBS with Tween-20
three times and incubated with anti-rabbit or anti-mouse
horseradish peroxidase secondary antibodies (cat. nos. 074-1516 and
074-1802, respectively; 1:100,000; Kirkegaard & Perry
Laboratories Inc., Gaithersburg, MD, USA) for a further 2 h at room
temperature. Enhanced chemiluminescence reagent (EMD Millipore) was
used to visualize the bands. Densitometric analysis was performed
using Quantity One software version 4.6.2 (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). GAPDH was used as an internal
control.
Immunofluorescence assay
Following treatment with lycorine (10 and 20 µM) for
48 h, cells were washed twice with PBS and fixed with ice-cold 75%
ethanol for 15 min at room temperature. Cells were permeabilized
with 0.1% Triton X-100 for 5 min, fluorescent staining of
filamentous and globular actin was performed by staining with
fluorescent deoxyribonuclease I conjugates and fluorescent
phallotoxins (Molecular Probes; Thermo Fisher Scientific, Inc.) for
30 min in the dark, and slides were washed and stained with DAPI
for 5 min at room temperature (cat. no. C1002; Beyotime Institute
of Biotechnology). Images were captured using a Leica scanning
confocal microscope (×40 magnification; TCS SP2 AOB; Leica
Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
All data were analyzed using GraphPad Prism 6.0
software (GraphPad Software, Inc., San Diego, CA, USA). Data
presented are expressed as the mean ± standard deviation at least
three independent experiments. Differences between groups were
analyzed by one-way analysis of variance (ANOVA) with Dunnett's or
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Lycorine inhibits the proliferation of
HepG2 cells
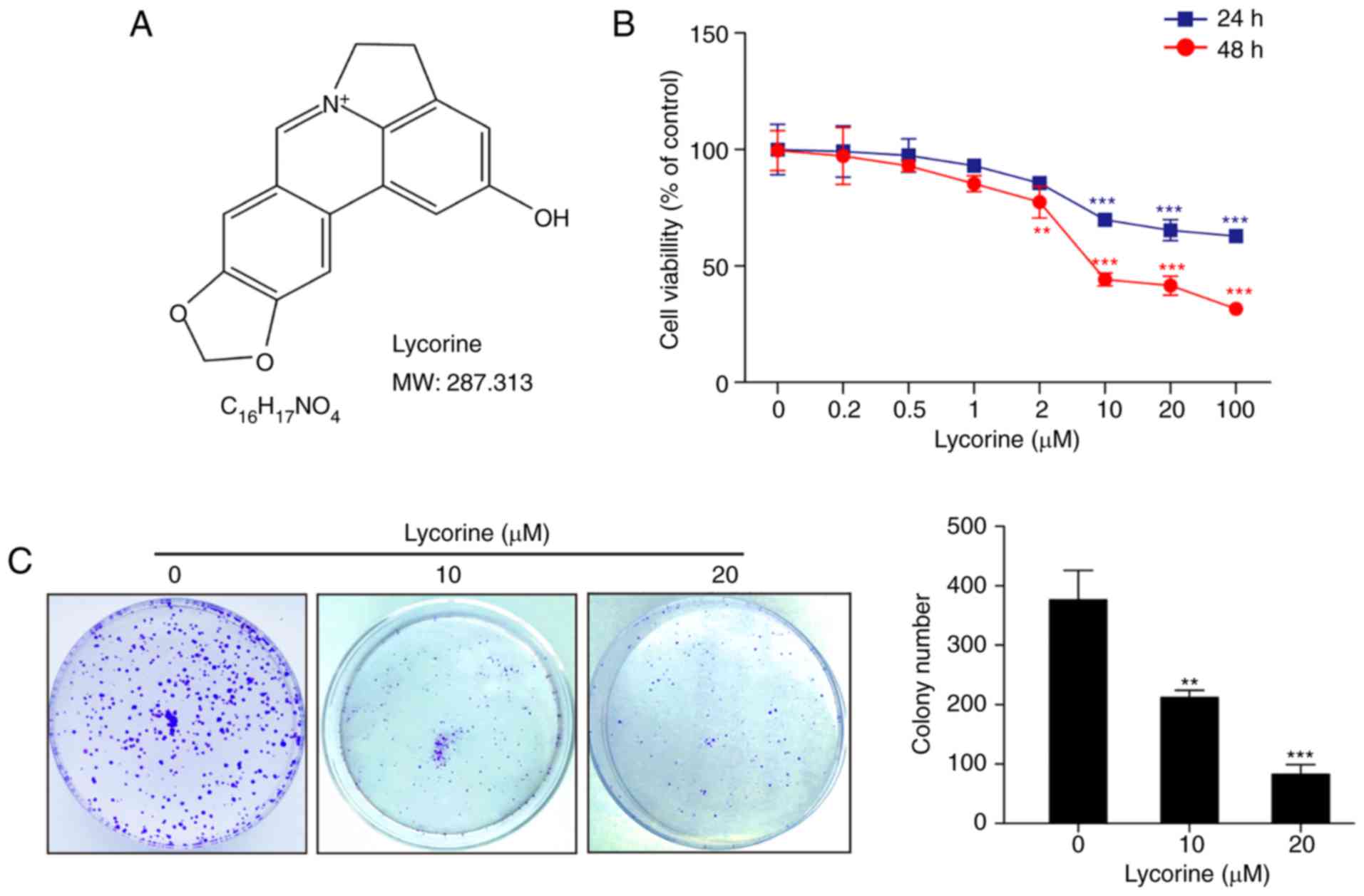
The chemical structure and molecular weight of
lycorine is presented in Fig. 1A.
The cytotoxicity of lycorine in HepG2 cells was determined by MTT
assay. Cells were treated with various concentrations of lycorine
(0.2, 0.5, 1, 2, 10, 20 and 100 µM) for 24 or 48 h, which resulted
in significant decreases in cell viability in a dose-dependent
manner (P<0.01, P<0.001; Fig.
1B). Exposure of cells to 2 µM lycorine resulted in a modest
decrease in cell viability, and these events became significant
following exposure of cells to ≥10 µM lycorine (Fig. 1B). To further confirm the
anti-proliferative effect of lycorine, a clone formation assay was
performed. In accordance with the results of the MTT assay,
lycorine significantly inhibited the clone formation of HepG2 cells
compared with the control cells (P<0.01, 10 µM; P<0.001, 20
µM; Fig. 1C). Taken together, these
results demonstrated that lycorine inhibited the proliferation of
HepG2 cells.
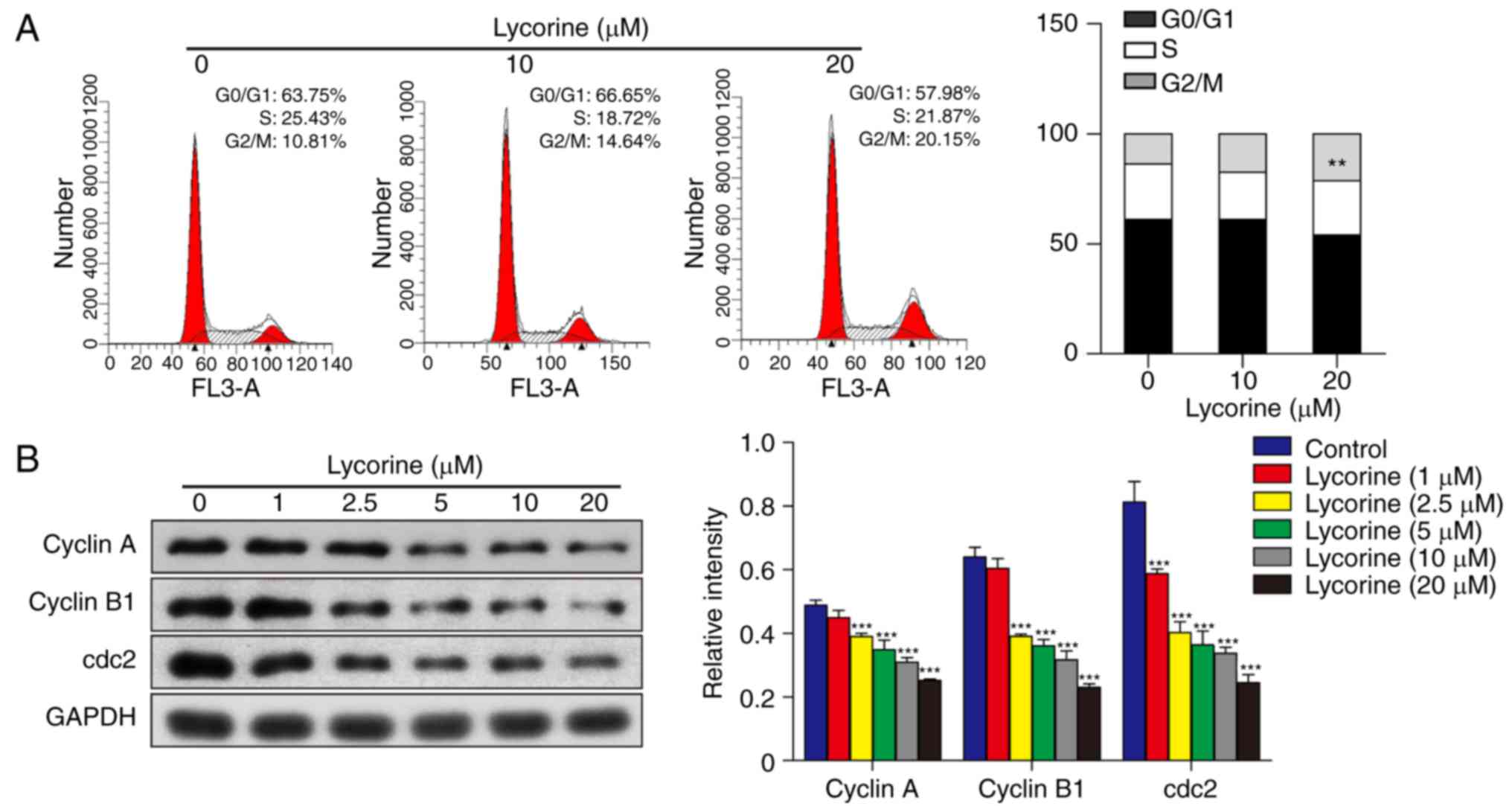
Lycorine induces HepG2 cell cycle
arrest at the G2/M phase via downregulation of cyclin A, cyclin B1
and cdc2
Cell cycle arrest is an important mechanism involved
in the inhibition of cell growth (12). To examine the effect of lycorine on
the cell cycle of HepG2 cells, cell cycle dynamics were assessed by
flow cytometry. Following exposure of cells to lycorine (10 and 20
µM) for 48 h, the proportion of cells in the G2/M phase increased
from 10.81% (without lycorine) to 14.64% (in the presence of 10 µM
lycorine) and 20.15% (in the presence of 20 µM lycorine; P<0.01
vs. control; Fig. 2A). These
results indicated that lycorine induced HepG2 cell cycle arrest at
the G2/M phase. To further examine the molecular mechanisms under
lycorine-induced G2/M phase arrest, western blot analysis was
performed to assess the expression of cyclin A, cyclin B1 and cdc2.
The results revealed that exposure of cells to lycorine resulted in
a marked decrease in the expression of cyclin A, cyclin B1 and cdc2
in HepG2 cells (P<0.001; Fig.
2B). These data suggested that lycorine has an inhibitory
effect on HepG2 cell cycle progression, which may cause the
inhibition of HepG2 cell proliferation.
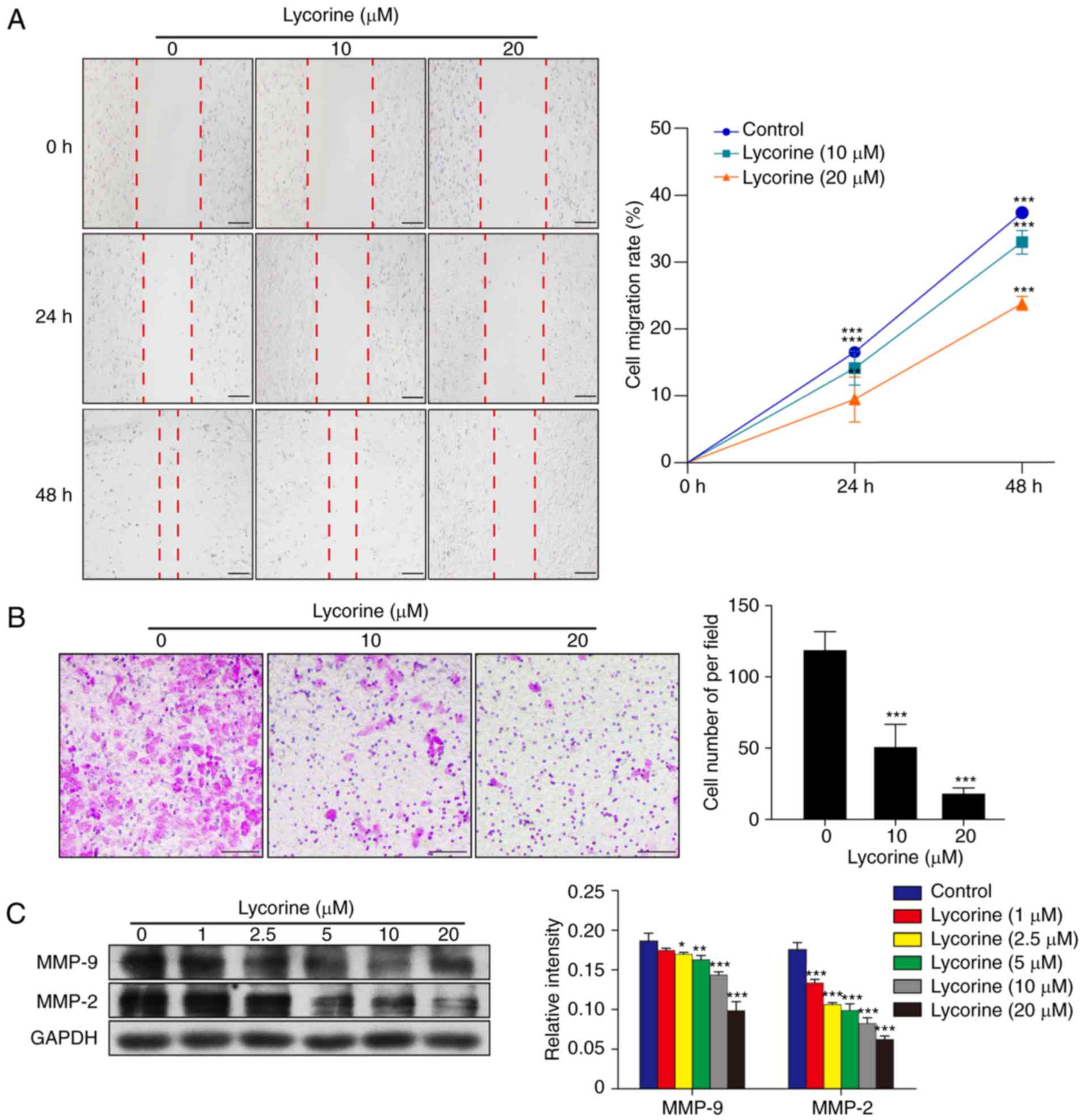
Lycorine inhibits the migration of
HepG2 cells
The wound healing assay indicated that lycorine
induced a marked decrease in cell migration in HepG2 cells in a
dose and time-dependent manner (P<0.01 and P<0.001; Fig. 3A). Furthermore, the Transwell assay
revealed that lycorine significantly inhibited the migratory
ability of HepG2 cells (P<0.001; Fig. 3B). Evidence has confirmed that MMPs,
particularly MMP-9 and MMP-2, are important regulators in the
process of cancer migration (13,14).
To investigate whether lycorine alters the expression of MMP-9 and
MMP-2, western blot analysis was performed. The results
demonstrated that compared with the control group, lycorine
decreased the expression levels of MMP-9 and MMP-2 in a
concentration-dependent manner (P<0.05, P<0.01 and
P<0.001; Fig. 3C). These data
indicated that lycorine inhibits HepG2 cell migration.
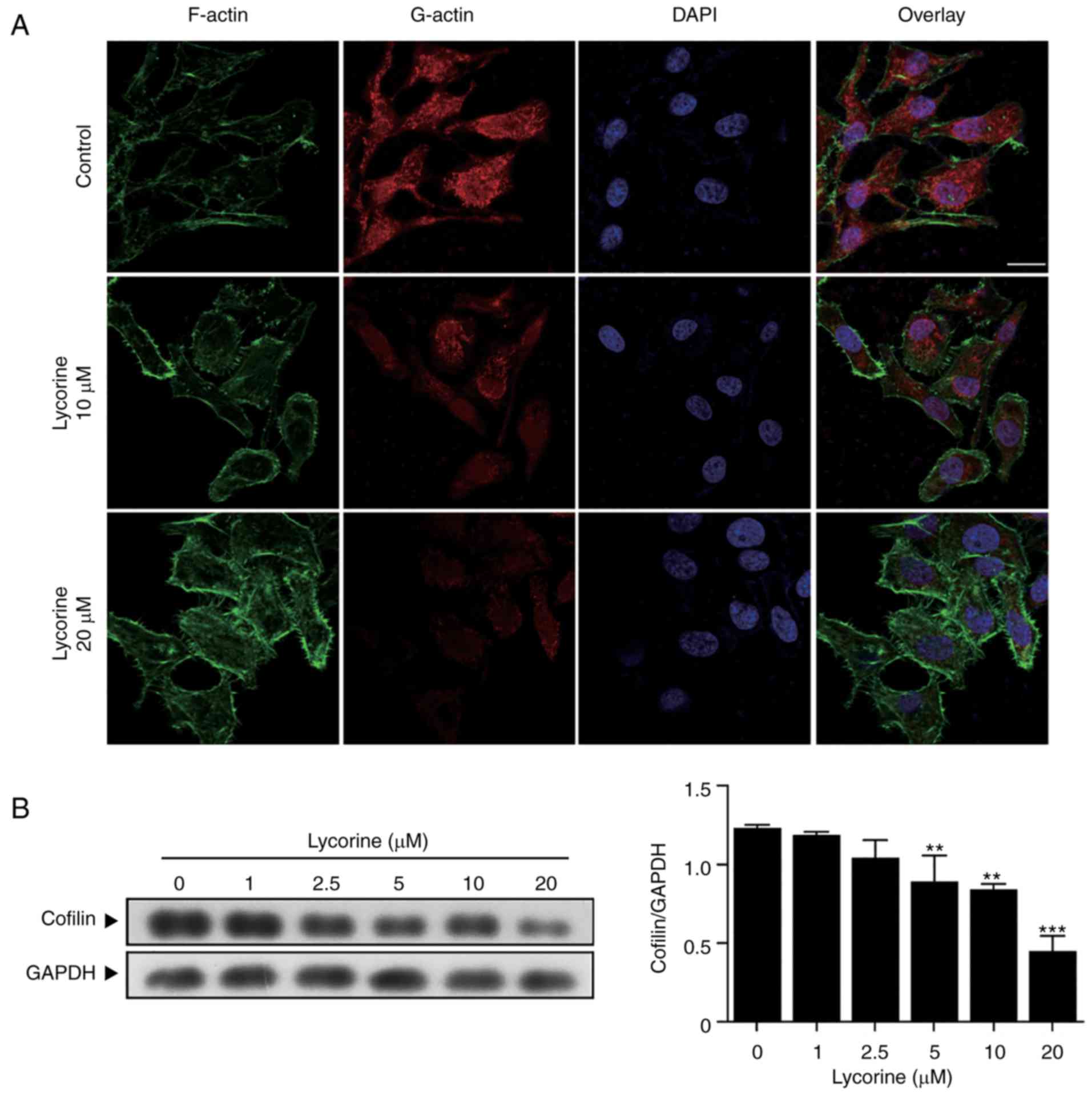
Lycorine alters actin cytoskeletal
dynamics by suppressing cofilin expression
Actin, an essential component of the cytoskeleton,
has a critical role in a wide range of cellular processes,
including cell migration and cell division (15). In the current study, exposure of
cells to lycorine (10 and 20 µM) resulted in an increase in
polymerized filamentous actin (F-actin) and a decrease in
depolymerized globular actin (G-actin; Fig. 4A). It has been reported that cofilin
regulates actin dynamics by severing actin filaments (16). Therefore, western blot analysis was
performed to determine whether cofilin is involved in the
lycorine-induced altering of actin cytoskeletal dynamics. As
presented in Fig. 4B, HepG2 cells
were treated with lycorine (1, 2.5, 5, 10 and 20 µM) for 48 h,
which resulted in a decrease in the expression of cofilin
(P<0.01 and P<0.001). Collectively, these results suggested
that lycorine suppresses the expression of cofilin and alters actin
cytoskeletal dynamics.
ROCK1 activation has an important role
in lycorine-induced anti-proliferative and anti-migration
effects
ROCK1 has been confirmed to have an important role
in regulating cell polarity and migration (17,18).
In the present study, it was investigated whether ROCK1 activation
is involved in lycorine-induced anticancer effects. Treatment of
HepG2 cells with lycorine (1, 2.5, 5, 10 and 20 µM) for 48 h
induced cleavage/activation of ROCK1 in a dose-dependent manner
(Fig. 5A). To further confirm the
role of ROCK1 in lycorine-induced cell proliferation and migration
inhibition, Y-27632, a ROCK1 specific inhibitor, was used. Western
blot analysis indicated that pre-incubation of cells with Y-27632
inhibited lycorine-induced ROCK1 cleavage/activation (Fig. 5B). Pre-incubation of cells with
Y-27632 attenuated the lycorine-induced cofilin decrease (Fig. 5C). Furthermore, pre-incubation with
Y-27632 also attenuated the decreases in cyclin A, cyclin B1, cdc2,
MMP-9 and MMP-2, which indicated that Y-27632 attenuated
lycorine-induced G2/M cell cycle arrest and migration ability
(Fig. 5D and E). A clone formation
assay demonstrated that combined treatment with Y-27632 and
lycorine significantly increased the colony number compared with
treatment with lycorine alone (P<0.01; Fig. 5F). The wound healing and Transwell
assays revealed that co-administration of Y-27632 and lycorine
markedly attenuated the lycorine-induced inhibitory effect on cell
migration (P<0.01, P<0.001; Fig.
5G and H). Collectively, these results demonstrated that ROCK1
activation has a critical role in the lycorine-induced effects on
actin cytoskeletal dynamics, and anti-proliferative and
anti-migration activity in HepG2 cells.
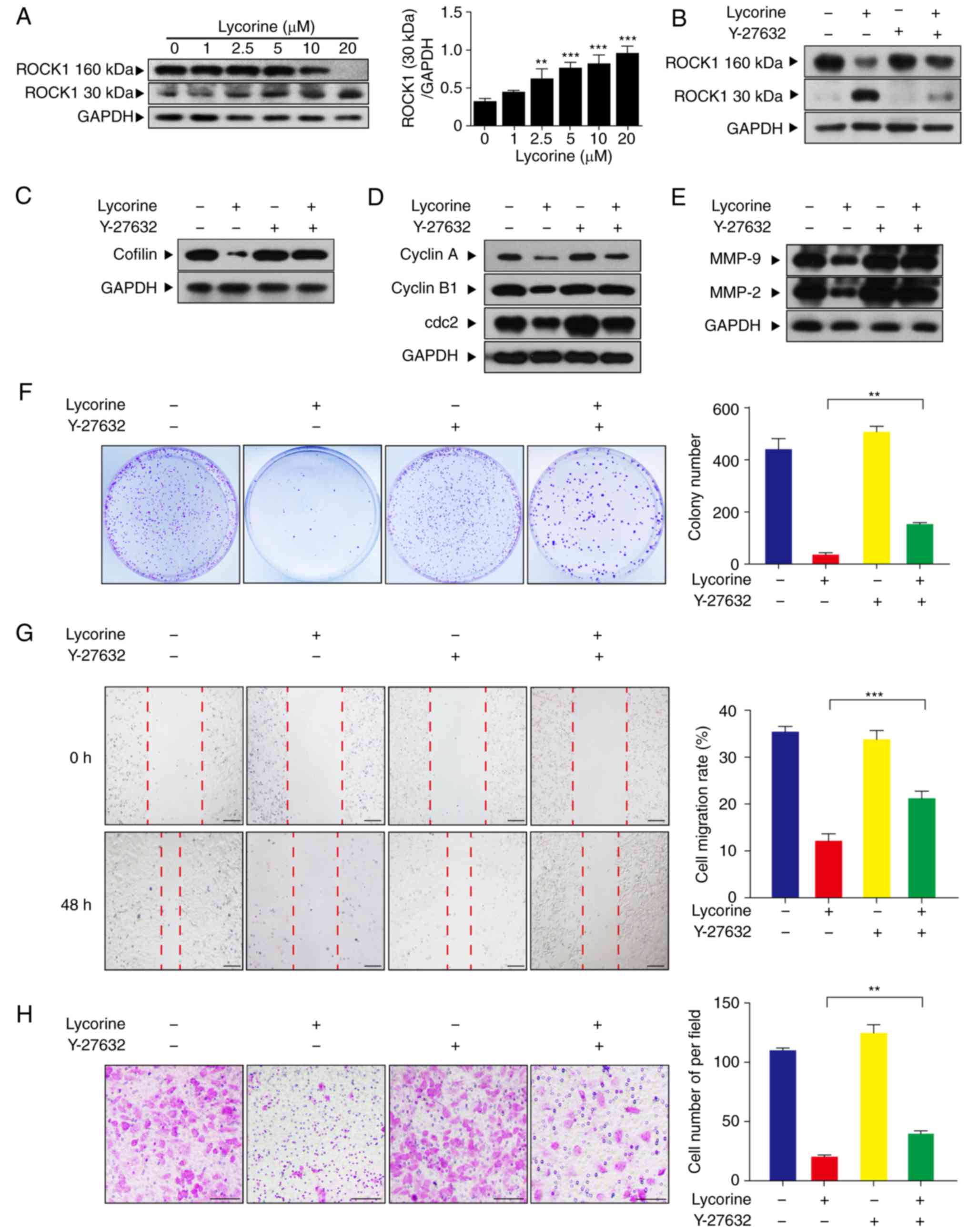 | Figure 5.ROCK1 activation has an important
role in lycorine-induced anti-proliferative and anti-migration
effects. (A) Cells were treated with lycorine (1, 2.5, 5, 10 and 20
µM) for 48 h and western blot analysis was used to assess the
expression levels of ROCK1 (160 kDa) and cleaved ROCK1 (30 kDa).
The relative quantification of proteins was analyzed using Quantity
One software. Data are presented the mean ± SD (n=3). **P<0.01
and ***P<0.001 vs. control. (B) Cells were pretreated with
Y-27632 (20 µM), a specific ROCK1 inhibitor, for 2 h, followed by
treating with lycorine (20 µM) for 48 h. Western blotting was used
to assess the expression of ROCK1 (160 kDa) and cleaved ROCK1 (30
kDa). (C) Following preincubation of cells with Y-27632, western
blotting was used to assess the expression of cofilin. (D)
Following preincubation of cells with Y-27632, western blotting was
used to assess the expression of cyclin A, cyclin B1 and cdc2. (E)
Following preincubation of cells with Y-27632, western blotting was
used to assess the expression of MMP-9 and MMP-2. (F) Cells were
fixed and stained with crystal violet, the number of cell colonies
was counted. (G) Cell migration was measured by wound healing assay
and results are presented graphically. Scale bar, 200 µm. (H) Cell
migration was measured in a Transwell assay. Scale bar, 200 µm.
Data are presented the mean ± SD (n=3). **P<0.01 and
***P<0.001 vs. the lyorine-only treatment group. ROCK1, Rho
associated coiled-coil containing protein kinase 1; SD, standard
deviation; MMP, matrix metalloproteinase; cdc2, cyclin dependent
kinase 1. |
Discussion
In recent years, naturally occurring compound have
received increasing attention in cancer research. Lycorine, a
natural compound obtained from the Amaryllidaceae plant family,
possesses anti-cancer activity in breast cancer, bladder cancer and
multiple myeloma (10,19,20).
However, the function and associated mechanisms of lycorine have
not been examined in hepatoblastoma. In the current study, the
inhibitory effects of lycorine on cell proliferation and migration
were investigated in HepG2 cells. The HepG2 cell line was
originally established in 1979 by Aden et al (21) and mistakenly reported as
hepatocellular carcinoma. In recent years, the HepG2 cell line has
frequently been used for hepatoblastoma research (3). The results of the present study
indicated that lycorine inhibited HepG2 cell proliferation by
inducing cell cycle arrest at the G2/M phase, and decreasing the
expression of cyclin A, cyclin B1 and cdc2. In addition, lycorine
decreased the migration ability of HepG2 cells. Furthermore,
lycorine altered actin cytoskeletal dynamics by suppressing the
expression of cofilin, and ROCK1 activation was demonstrated to
have an important role in the anti-proliferative and anti-migration
effects of lycorine in HepG2 hepatoblastoma cells.
Agents that possess the ability to inhibit the
proliferation and migration of tumor cells may be used to inhibit
cancer progression and increase survival rates (22). In the present study, treatment with
lycorine effectively inhibited the cell proliferation and colony
formation of HepG2 cells. It has been previously reported that
lycorine induces cell cycle arrest at the G0/G1 phase in K562 cells
(23) and KM3 cells (24). However, in the current study,
lycorine induced HepG2 cell cycle arrest at the G2/M phase in a
dose-dependent manner. The cell cycle is a physiological process,
including the G0/G1, S and G2/M phases. A series of proteins,
including cyclins and cyclin-dependent kinases, regulate the cell
cycle (25). In the present study,
cyclin A, cyclin B1 and cdc2 were significantly downregulated in
HepG2 cells following treatment with lycorine. These findings
suggested that lycorine induced cell cycle arrest at the G2/M phase
via inhibition of cyclin A, cyclin B1 and cdc2 expression in HepG2
cells.
Metastasis is considered to be the primary cause of
mortality in the majority of patients with cancer. The migration
and invasion ability of tumor cells are key factors in tumor
metastasis. Previous studies have demonstrated that lycorine
inhibits the growth and metastasis of breast cancer through
inhibition of signal transducer and activator of transcription 3
signaling (19). Similarly, in the
current study, lycorine inhibited the migration of HepG2 cells. The
MMP family, generally considered to be biomarkers for cancers, are
reportedly upregulated in the majority of types of cancer (26). Furthermore, MMPs have also been
considered to be potential therapeutic targets in cancer (27–29).
In particular, MMP-9 and MMP-2, exhibiting enzymatic collagenase
activity, are typical members among them (30). ROCK1, which serves an important role
in cell polarity and migration, regulates the expression of MMP-9
and MMP-2 (31,32). Jeong et al (33) demonstrated that lysophosphatidic
acid increases ovarian cancer cell invasion via a Ras/Rho/ROCK
signaling pathway and subsequent production of the proteolytic
enzyme MMP-9. Cofilin is an important downstream mediator of ROCK1,
and the present study revealed that inhibition of the ROCK1/cofilin
pathway decreased the expression of MMP-9 and MMP-2. These results
indicated that the anti-migration effects of lycorine on HepG2
cells may be associated with the lycorine-induced downregulation of
MMP-9 and MMP-2.
Previous studies have indicated that the
reorganization of the actin cytoskeleton is the basis of cancer
cell migration, adhesion and invasion (34–36).
The current findings demonstrated that lycorine blocked the normal
dynamic turnover of the actin cytoskeleton, with an increase in
polymerized F-actin and a loss of depolymerized G-actin. A number
of actin-binding proteins have been reported to be involved in the
regulation of actin dynamics. For example, cofilin, a member of the
actin depolymerizing factor/cofilin family, exerts its effects on
actin filament dynamics by binding to F-actin and severing actin
filaments. Cofilin has been reported to be overexpressed in
pancreatic cancer cells, A549 lung cancer cells and the rat C6
glioblastoma cell line (14,37,38).
Furthermore, Yap et al (39)
reported that overexpression of cofilin enhanced cell motility in
U373 astrocytoma cells. In the present study, treatment with
lycorine decreased the expression of cofilin in a dose-dependent
manner. ROCK1, an upstream regulator of cofilin, has an important
role in regulating cell polarity and migration (34,40–42).
Furthermore, Y-27632, a ROCK1 specific inhibitor, stimulates
proliferation in various cell lines (43–45),
indicating that the activation of ROCK1 inhibits cell
proliferation. In the present study, ROCK1 activation was
associated with lycorine-induced anti-proliferative and
anti-migration effects in HepG2 cells. Treatment with lycorine
resulted in the cleavage/activation of ROCK1, and pre-incubation of
cells with Y-27632 blocked lycorine-induced decreases in cofilin,
cyclin A, cyclin B1, cdc2, MMP-9 and MMP-2. Furthermore, combined
treatment with Y-27632 and lycorine markedly attenuated
lycorine-induced clone formation inhibition and its inhibitory
effects on migratory ability. Taken together, these results
demonstrated that lycorine alters actin cytoskeletal dynamics by
suppressing the expression of cofilin and activating ROCK1.
In conclusion, the data demonstrated that lycorine
inhibited HepG2 hepatoblastoma cell proliferation and migration
through inhibition of ROCK1/cofilin-induced actin dynamics. All of
the findings provide support for the development of lycorine as a
potential drug candidate for anti-hepatoblastoma therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 31600806),
Clinical Research Projects of Xinqiao Hospital, Army Medical
University (grant no. 2016YLC12), and the Foundation of Chongqing
Science and Technology Commission (grant no.
CSTC2015SHMSZX120078).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL, RZ and GL conceived and designed the study. WL,
QZ, QT and CH performed the experiments. JH, YLi, YLu and QW
analyzed the data. WL and RZ wrote the manuscript. GL and QZ
reviewed and edited the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the report work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zsiros J, Maibach R, Shafford E, Brugieres
L, Brock P, Czauderna P, Roebuck D, Childs M, Zimmermann A,
Laithier V, et al: Successful treatment of childhood high-risk
hepatoblastoma with dose-intensive multiagent chemotherapy and
surgery: Final results of the SIOPEL-3HR study. J Clin Oncol.
28:2584–2590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang YT, Feng LH, Zhong XD, Wang LZ and
Chang J: Single-agent cisplatin treatment of children with
high-risk hepatoblastoma. J Pediatr Hematol Oncol. 36:271–275.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu Y, Zhao X, Zhang Y, Kang Y, Wang J and
Liu Y: Antitumor activity of YM155, a selective survivin
suppressant, in combination with cisplatin in hepatoblastoma. Oncol
Rep. 34:407–414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jimenez A, Santos A, Alonso G and Vazquez
D: Inhibitors of protein synthesis in eukarytic cells. Comparative
effects of some amaryllidaceae alkaloids. Biochim Biophys Acta.
425:342–348. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toriizuka Y, Kinoshita E, Kogure N,
Kitajima M, Ishiyama A, Otoguro K, Yamada H, Omura S and Takayama
H: New lycorine-type alkaloid from Lycoris traubii and
evaluation of antitrypanosomal and antimalarial activities of
lycorine derivatives. Bioorg Med Chem. 16:10182–10189. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen JW, Ruan Y, Ren W, Ma BJ, Wang XL and
Zheng CF: Lycorine: A potential broad-spectrum agent against crop
pathogenic fungi. J Microbiol Biotechnol. 24:354–358. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo Y, Wang Y, Cao L, Wang P, Qing J,
Zheng Q, Shang L, Yin Z and Sun Y: A conserved inhibitory mechanism
of a lycorine derivative against enterovirus and hepatitis C virus.
Antimicrob Agents Chemother. 60:913–924. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamoral-Theys D, Andolfi A, Van
Goietsenoven G, Cimmino A, Le Calvé B, Wauthoz N, Mégalizzi V, Gras
T, Bruyère C, Dubois J, et al: Lycorine, the main phenanthridine
Amaryllidaceae alkaloid, exhibits significant antitumor activity in
cancer cells that display resistance to proapoptotic stimuli: An
investigation of structure-activity relationship and mechanistic
insight. J Med Chem. 52:6244–6256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng H, Fu R, Yan L and Huang J: Lycorine
induces apoptosis of A549 cells via AMPK-mammalian target of
rapamycin (mTOR)-S6K signaling pathway. Med Sci Monit.
23:2035–2041. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang C, Wang Q, Li X, Jin Z, Xu P, Xu N,
Xu A, Xu Y, Zheng S, Zheng J, et al: Lycorine induces apoptosis of
bladder cancer T24 cells by inhibiting phospho-Akt and activating
the intrinsic apoptotic cascade. Biochem Biophys Res Commun.
483:197–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao Z, Yu D, Fu S, Zhang G, Pan Y, Bao M,
Tu J, Shang B, Guo P, Yang P, et al: Lycorine hydrochloride
selectively inhibits human ovarian cancer cell proliferation and
tumor neovascularization with very low toxicity. Toxicol Lett.
218:174–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
King KL and Cidlowski JA: Cell cycle
regulation and apoptosis. Annu Rev Physiol. 60:601–617. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. Febs J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sinha P, Hütter G, Köttgen E, Dietel M,
Schadendorf D and Lage H: Increased expression of epidermal fatty
acid binding protein, cofilin, and 14-3-3-sigma (stratifin)
detected by two-dimensional gel electrophoresis, mass spectrometry
and microsequencing of drug-resistant human adenocarcinoma of the
pancreas. Electrophoresis. 20:2952–2960. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gourlay CW and Ayscough KR: The actin
cytoskeleton: A key regulator of apoptosis and ageing? Nat Rev Mol
Cell Biol. 6:583–589. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gross SR: Actin binding proteins: Their
ups and downs in metastatic life. Cell Adh Migr. 7:199–213. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding W, Tan H, Zhao C, Li X, Li Z, Jiang
C, Zhang Y and Wang L: MiR-145 suppresses cell proliferation and
motility by inhibiting ROCK1 in hepatocellular carcinoma. Tumour
Biol. 37:6255–6260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Genda T, Sakamoto M, Ichida T, Asakura H,
Kojiro M, Narumiya S and Hirohashi S: Cell motility mediated by rho
and Rho-associated protein kinase plays a critical role in
intrahepatic metastasis of human hepatocellular carcinoma.
Hepatology. 30:1027–1036. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Xu J and Xing G: Lycorine inhibits
the growth and metastasis of breast cancer through the blockage of
STAT3 signaling pathway. Acta Biochim Biophys Sin. 49:771–779.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roy M, Liang L, Xiao X, Peng Y, Luo Y,
Zhou W, Zhang J, Qiu L, Zhang S, Liu F, et al: Lycorine
downregulates HMGB1 to inhibit autophagy and enhances bortezomib
activity in multiple myeloma. Theranostics. 6:2209–2224. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aden DP, Fogel A, Plotkin S, Damjanov I
and Knowles BB: Controlled synthesis of HBsAg in a differentiated
human liver carcinoma-derived cell line. Nature. 282:615–616. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Bi T, Shen G, Li Z, Wu G, Wang Z,
Qian L and Gao Q: Lupeol induces apoptosis and inhibits invasion in
gallbladder carcinoma GBC-SD cells by suppression of EGFR/MMP-9
signaling pathway. Cytotechnology. 68:123–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Dai HJ, Ye M, Wang SL, Xiao XJ,
Zheng J, Chen HY, Luo YH and Liu J: Lycorine induces cell-cycle
arrest in the G0/G1 phase in K562 cells via HDAC inhibition. Cancer
Cell Int. 12:492012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Liu J, Tang LJ, Shi YW, Ren W and Hu
WX: Apoptosis induced by lycorine in KM3 cells is associated with
the G0/G1 cell cycle arrest. Oncol Rep. 17:377–384. 2007.PubMed/NCBI
|
|
25
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Benson CS, Babu SD, Radhakrishna S,
Selvamurugan N and Sankar Ravi B: Expression of matrix
metalloproteinases in human breast cancer tissues. Dis Markers.
34:395–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Komiya Y, Kurabe N, Katagiri K, Ogawa M,
Sugiyama A, Kawasaki Y and Tashiro F: A novel binding factor of
14-3-3beta functions as a transcriptional repressor and promotes
anchorage-independent growth, tumorigenicity, and metastasis. J
Biol Chem. 283:18753–18764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang Y, Lv P, Sun Z, Han L and Zhou W:
14-3-3β promotes migration and invasion of human hepatocellular
carcinoma cells by modulating expression of MMP2 and MMP9 through
PI3K/Akt/NF-kappaB pathway. PLoS One. 11:e01460702016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mendes O, Kim HT and Stoica G: Expression
of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat
model. Clin Exp Metastasis. 22:237–246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schram K, Ganguly R, No EK, Fang X, Thong
FS and Sweeney G: Regulation of MT1-MMP and MMP-2 by leptin in
cardiac fibroblasts involves Rho/ROCK-dependent actin cytoskeletal
reorganization and leads to enhanced cell migration. Endocrinology.
152:2037–2047. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu X, Chen D and Liu G: Overexpression of
RhoA promotes the proliferation and migration of cervical cancer
cells. Biosci Biotechnol Biochem. 78:1895–1901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jeong KJ, Park SY, Cho KH, Sohn JS, Lee J,
Kim YK, Kang J, Park CG, Han JW and Lee HY: The Rho/ROCK pathway
for lysophosphatidic acid-induced proteolytic enzyme expression and
ovarian cancer cell invasion. Oncogene. 31:4279–4289. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ishaq M, Lin BR, Bosche M, Zheng X, Yang
J, Huang D, Lempicki RA, Aguilera-Gutierrez A and Natarajan V: LIM
kinase 1-dependent cofilin 1 pathway and actin dynamics mediate
nuclear retinoid receptor function in T lymphocytes. BMC Mol Biol.
12:412011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Flamini MI, Fu XD, Sanchez AM, Giretti MS,
Garibaldi S, Goglia L, Pisaneschi S, Tosi V, Genazzani AR and
Simoncini T: Effects of raloxifene on breast cancer cell migration
and invasion through the actin cytoskeleton. J Cell Mol Med.
13:2396–2407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Freitas VM, Rangel M, Bisson LF, Jaeger RG
and Machado-Santelli GM: The geodiamolide H, derived from Brazilian
sponge Geodia corticostylifera, regulates actin
cytoskeleton, migration and invasion of breast cancer cells
cultured in three-dimensional environment. J Cell Physiol.
216:583–594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Keshamouni VG, Michailidis G, Grasso CS,
Anthwal S, Strahler JR, Walker A, Arenberg DA, Reddy RC, Akulapalli
S, Thannickal VJ, et al: Differential protein expression profiling
by iTRAQ-2DLC-MS/MS of lung cancer cells undergoing
epithelial-mesenchymal transition reveals a migratory/invasive
phenotype. J Proteome Res. 5:1143–1154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang W, Goswami S, Lapidus K, Wells AL,
Wyckoff JB, Sahai E, Singer RH, Segall JE and Condeelis JS:
Identification and testing of a gene expression signature of
invasive carcinoma cells within primary mammary tumors. Cancer Res.
64:8585–8594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yap CT, Simpson TI, Pratt T, Price DJ and
Maciver SK: The motility of glioblastoma tumour cells is modulated
by intracellular cofilin expression in a concentration-dependent
manner. Cell Motil Cytoskeleton. 60:153–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Salvarezza SB, Deborde S, Schreiner R,
Campagne F, Kessels MM, Qualmann B, Caceres A, Kreitzer G and
Rodriguez-Boulan E: LIM kinase 1 and cofilin regulate actin
filament population required for dynamin-dependent apical carrier
fission from the trans-Golgi network. Mol Biol Cell.
20:438–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kaji N, Muramoto A and Mizuno K: LIM
kinase-mediated cofilin phosphorylation during mitosis is required
for precise spindle positioning. J Biol Chem. 283:4983–4992. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Martin San A, Lee MY, Williams HC, Mizuno
K, Lassègue B and Griendling KK: Dual regulation of cofilin
activity by LIM kinase and Slingshot-1L phosphatase controls
platelet-derived growth factor-induced migration of human aortic
smooth muscle cells. Circ Res. 102:432–438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Okumura N, Ueno M, Koizumi N, Sakamoto Y,
Hirata K, Hamuro J and Kinoshita S: Enhancement on primate corneal
endothelial cell survival in vitro by a ROCK inhibitor. Invest
Ophthalmol Vis Sci. 50:3680–3687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakamura K, Yoshimura A, Kaneko T, Sato K
and Hara Y: ROCK inhibitor Y-27632 maintains the proliferation of
confluent human mesenchymal stem cells. J Periodontal Res.
49:363–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Del Debbio CB, Santos MF, Yan CY, Ahmad I
and Hamassaki DE: Rho GTPases control ciliary epithelium cells
proliferation and progenitor profile induction in vivo. Invest
Ophthalmol Vis Sci. 55:2631–2641. 2014. View Article : Google Scholar : PubMed/NCBI
|