Introduction
Glioblastoma (GBM) is one of the most aggressive
types of brain tumor. Despite great progress in the therapeutic
strategies for GBM over the past few decades, the prognosis remains
poor (1–4). The median overall survival is ~14.6
months from the time of diagnosis and the 5-year survival rates are
<9.8% following standard care (5,6). With
the continuing advances in molecular biology, gene therapy has
become a focus for cancer treatment (7). Thus, deeper research and better
understanding of the molecular mechanisms of glioma formation and
progression is necessary to establish the diagnostic and
therapeutic targets.
E3 ubiquitin-protein is a peptide ligase that have
been identified as a bio-marker and therapeutic target of
glioblastoma (8). Ring finger
protein 138 (RNF138) is a member of the E3 ligase family, which
contains an N-terminal Cys(3)-His-Cys(4) ring finger protein
domain, three zinc-finger-like domains and a C-terminal
ubiquitin-interacting motif (UIM) type (9). Our previous study confirmed that
RNF138 expression was significantly increased in glioma tissues and
in the glioma cell lines U87 and U251 compared with non-cancerous
brain tissues by reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) (10).
However, the mechanism of RNF138 in the progression of glioma has
not been fully elucidated.
Epithelial-mesenchymal-transition (EMT) is a
developmental process, where polarized epithelial cells undergo
multiple biochemical changes and assume a mesenchymal phenotype
(11). This subsequently increases
the migratory capacity, invasiveness, resistance to apoptosis and
expression of extracellular matrix components in cells (12–14).
Tumor recurrence in glioma is partly attributed to the increased
EMT, enhanced aggressive behavior and treatment resistance of the
tumor cells (15,16). The extracellular signal-regulated
protein kinases (Erk) signaling pathways have been reported to have
an important role in modulating cell invasion and progression of
EMT. Thus, it is necessary to understand the role of Erk signaling
pathways and RNF138 in the EMT process in human glioblastoma.
Therefore, the present study explored whether RNF138
has a role in the proliferation, migration and invasion of glioma.
A lentiviral-mediated RNA interference (RNAi) system was used to
explore the effect of RNF138 on the migration, proliferation of U87
and U251 cells in vitro. Furthermore, the possible
mechanisms involved in this process were also investigated. The
study suggested that RNF138 was important for the proliferation,
migration and invasion of glioma cells. Downregulation of RNF138
inhibited the process of EMT by suppressing Erk signaling pathway
in glioma.
Materials and methods
Cell lines
The cell lines U87 ATCC (glioblastoma cells of
unknown origin) and U251 (astrocytoma) were purchased from the Cell
Bank of Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). All cell lines were maintained in Dulbecco's
modified Eagle's medium (DMEM; HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 100 U/ml of penicillin,
100 µg/ml of streptomycin and 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a
humidified atmosphere of 5% CO2 in air.
RNF138 small interfering RNA (siRNA)
lentiviral vectors
siRNAs sequences targeting human RNF138 gene were
designed by Shanghai GenePharm Co., Ltd. (Shanghai, China). The
selected template was 5′-CTGTAACAGTAATCACCTA-3′. Following
confirmation by sequencing, the sequences were cloned into
pGCSIL-green fluorescent protein (GFP; Shanghai GenePharm Co.,
Ltd., Shanghai, China) to generate the lentiviral vectors. The
following sequences were used: RNF138, sense
5′-GATCCGGATCACTGTAACAGTAATCATTCAAGAGATGATTACTGTTACAGTGATCCTTTTTTG-3′,
antisense
5′-AATTCAAAAAAGGATCACTGTAACAGTAATCATCTCTTGAATGATTACTGTTACAGTGATCCG-3′),
NC, sense 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. Lentiviral vectors construction and
production were completed by Shanghai GenePharm Co., Ltd. The
average titre of the lentiviral vectors was ≥5×108
transducing units/ml.
Cell cultures and lentivirus siRNA
gene transduction
U87 and U251 cells in the exponential phase were
resuspended in 0.25% trypsin. Cells were plated in 6-well plates
(5×104 cells/well) until cell density reached 30%
confluence. Then, according to the multiplicity of infection value
(number of lentiviruses:number of cells), appropriate amount of
lentiviruses were added to the cells. After 24 h, the medium was
replaced with fresh DMEM medium and incubated further for 48 h.
Then, cells were observed under a fluorescence inverted microscope
and flow cytometry analysis was performed to detect for GFP
expression.
RNA extraction RT-qPCR
Total RNA was extracted from U87 and U251 cell
cultures after 5 days of transfection using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Concentration and purity of RNA were
determined spectrophotometrically by measuring its optical density
(absorbance 260/280 >2.0; absorbance 260/230 >1.8) using a
NanoDrop ND-1000 (Thermo Fisher Scientific, Inc.). Total RNA (2 µg)
was used for RT in a 20 µl reaction containing 10 U M-MLV Reverse
Transcriptase and 0.5 µg oligo (dT) primer. Samples were incubated
for 5 min at 25°C followed by 60 min at 42°C and the reaction was
terminated by heating at 70°C for 5 min. cDNA (2 µl) was used for
qPCR. The specific primer pairs used are as follows: RNF138, sense
5′-ATGTCCTATTTGTGTGTCTCTTCC-3′, antisense
5′-GCAGTTTGGTATTGGGTTTCTTC-3′; product size was 144 bp. GAPDH was
used as the internal control gene and the primer pairs used are as
follows: sense 5′-CAAGGTCATCCATGACAACTTTG-3′, antisense
5′-GTCCACCACCCTGTTGCTGTAG-3′, product size was 496 bp. The number
of cycles of PCR was optimized to ensure the product intensity to
be within the linear phase of amplification. The PCR protocol
consisted of an initial denaturation step at 95°C for 5 min,
followed by 30 cycles of three-step program at 95°C for 30 sec,
60°C for 30 sec, 72°C for 30 sec, and a final extension step of
72°C for 10 min to amplify RNF138. The RNF138 and GAPDH genes were
amplified with SYBR Master Mixture (Takara, Japan). Results are
presented as quantitation cycle (Cq) values, and were defined as
the PCR cycle quantification number at which an amplified product
was first detected. The average Cq was calculated for RNF138 and
GAPDH, and 2−ΔΔCq was determined as the mean of the
triplicate Cq values for RNF138 minus the mean of the triplicate Cq
values for GAPDH. The 2−ΔΔCq method (17) was used to analyze the relative
changes in the gene expression.
Colony suppression assay
For colony formation assay, 200 cells/well were
seeded into 6-well plates for 48 h after transduction. Then the
cells were cultured at 37°C in a 5% CO2 incubator with
the medium changed every 2 days. On day 14, the culture medium was
removed, and the cells were washed twice with PBS. Subsequently,
the colonies were fixed in 10% methanol at room temperature for 15
min, stained with 1% crystal violet (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at room temperature for 30 min, and then
washed. Colonies were counted and photographed.
Analysis of cell proliferation
Cells (at a density of 2×105/100 µl) were
seeded into 96-well plates following transduction with siRNA or the
negative control lentivirus for 12 h. Then, the Cell Counting kit-8
(CCK-8; Beyotime Institute of Biotechnology, Haimen, China) reagent
(10 µl) was added to each well at 24, 48 and 72 h
post-transduction. The plates were then incubated for 4 h at 37°C.
Absorbance values were measured at a wavelength of 450 nm in an
ELISA reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and
the proliferation rate of each group was analyzed.
Wound healing assay
Cells were seeded into 6-well plates and cultured
till the density reaches 80–90% confluence. A wound was made using
a 1 ml plastic pipette tip then cells were washed twice with PBS.
Subsequently, the medium was replaced by DMEM containing 2% FBS.
The size of the wound was measured under a microscope (Olympus
Corporation, Tokyo, Japan) at 0 and 24 h after wounding. For each
wound, the width of the wound at 0 h was designated as 100%, and
after 24 h the relative width of the open wound was calculated
using ImageJ software (National Institutes of Health, Bethesda, MD,
USA) (18).
Transwell invasion assay
Cell invasion assay was carried out using a 24-well
Transwell chamber (Corning Incorporated, Corning, NY, USA).
Matrigel (BD Biosciences, San Jose, CA, USA) was diluted to 1:8
with cold DMEM without FBS and used to coat the upper compartment
chamber. Different groups of cells (2×104) were then
plated into the upper wells with 100 µl serum-free DMEM and the
bottom chamber was filled with DMEM containing 10% FBS. After
incubation at 37°C for 24 h, noninvasive cells on the top chambers
were gently wiped with cotton wool. Invasive cells on the bottom
surface were fixed with 4% paraformaldehyde for 30 min at 37°C and
stained with 0.1% crystal violet for 2 h at room temperature, then
counted under a light microscope. The experiment for each group was
performed in triplicate and the results were averaged.
Flow cytometry analysis of
apoptosis
Apoptosis was measured with Annexin V-phycoerythrin
(PE) Apoptosis Detection kit (BD Biosciences). The cells
(2×105 cells/well) were plated in 6-well plates for 24
h. Then, the cells were washed with cold PBS and resuspended in 100
µl 1X binding buffer, followed by the addition of 5 µl Annexin V-PE
and 5 µl 7-aminoactinomycin D (7-AAD). The cells were incubated for
15 min at room temperature in the dark. Finally, 380 µl 1X binding
buffer was added to the cells. Cell apoptosis was analyzed using a
flow cytometer and CXP analysis software 2.2 (Beckman Coulter,
Inc., Brea, CA, USA).
Western blot analysis
Cells were harvested, washed twice with PBS, lysed
in ice-cold radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology) with a freshly added 0.01% protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA) and incubated on ice
for 30 min. Protein concentration was measured by bicinchoninic
acid protein assay kit (Beyotime Institute of Biotechnology). Equal
amounts of protein (30 µg) were subjected to 10% SDS-PAGE and then
were transferred onto the polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with
Western Blocking Buffer (Beyotime Institute of Biotechnology) for 2
h at room temperature and incubated with primary antibodies at 4°C
overnight. The membranes were washed with PBS with 0.1% Tween-20
before incubating them with horseradish peroxidase (HRP)-conjugated
anti-rabbit secondary antibodies (1:5,000; cat. no. 7074; Cell
Signaling Technology, Inc., Danvers, MA, USA) or HRP-conjugated
anti-mouse secondary antibodies (1:3,000; cat. no. XR-9720; ProSci
Incorporated, Poway, CA, USA) for 2 h at room temperature. After
washing again, protein levels were analyzed by Pierce™ Fast Western
Blot Kit Enhanced Chemiluminescence Substrate (ECL; cat. no. 3050;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and western
blotting detection system (Odyssey CLx; Gene Company, Ltd., Hong
Kong, China). The bands were quantified by densitometry using
ImageJ 1.46r software (National Institutes of Health, Bethesda, MD,
USA). The following antibodies were used in this study: GAPDH
(1:1,000; cat. no. sc-69778) and β-actin (1:1,000; cat. no.
sc-58673; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) were
used as internal controls. Bcl2 associated X apoptosis regulator
(BAX; 1:1,000; cat. no. sc-4239 WB; Santa Cruz Biotechnology,
Inc.), apoptosis regulator Bcl2 (Bcl2; 1:1,000; cat. no. sc-23960;
Santa Cruz Biotechnology, Inc.); vascular endothelial growth factor
(VEGF; 1:1,000; cat. no. sc-365578) and hypoxia-inducible factor-1α
(HIF-1α; 1:1,000; cat. no. sc-13515); Santa Cruz Biotechnology,
Inc.), cleaved caspase3 (cat. no. 9664), caspase3 (cat. no. 9662),
Erk1/2 (cat. no. 4695), phospho (p)-Erk1/2 (cat. no. 4370), matrix
metalloproteinase 2 (MMP2; cat. no. 40994), E-cadherin (cat. no.
14472) and vimentin (cat. no. 5741) were acquired from Cell
Signaling Technology, Inc. and RNF138 (cat. no. DF4025; Affinity
Biosciences, Cincinnati, OH, USA) at a dilution of 1:1,500.
Nude mouse model of intracranial
glioma
All experimental protocols were approved by the
Institutional Review Board of the Department of Laboratory Animal
Science of the First Affiliated Hospital of Soochow University
(Suzhou, China). All animal manipulations were performed under
sterile conditions. BALB/c-A nude female mice at 4 weeks of age
were purchased from the Shanghai Experimental Animal Center of the
Chinese Academy of Sciences (Shanghai, China) were randomly
assigned into two groups (n=11 per group). U87 cells
(3×105 cell transduced with lentivirus containing
siRNA-RNF138 or negative control sequences) in 4 µl DMEM/F12 were
injected 3 mm deep into the frontal lobe, 1.8–2.0 mm to the right
side of the sagittal suture and 1.8–2 mm anterior to the coronal
suture using an astereotactic frame. Mice were observed daily,
weighed two times a week until severe neurological deficit and/or
20% weight-loss and the following day was recorded as the survival
date (19,20). For mice found dead, the previous day
was recorded as the survival date.
Histology and immunohistochemical
staining
Tissues were fixed in 4% buffered formaldehyde for
24 h at 4°C. Paraffin-embedded tissue sections (5 µm thickness)
were placed on positively charged slides and air-dried. Following
drying, slides were incubated in a 60°C oven for 30 min. Sections
were deparaffinized with two xylene washes for 10 min each at room
temperature, then rehydrated using two incubations in absolute
alcohol of 5 min each at room temperature, then incubated in 95%
alcohol for 2 min and 70% alcohol for 2 min at room temperature.
Sections were washed briefly in distilled water and stain in Harris
hematoxylin solution for 10 min at room temperature prior to
washing in running tap water for 5 min at room temperature.
Differentiation was peformed in 1% acid alcohol for 30 sec at room
temperature then sections were washed in running tap water for 1
min. Bluing was performed in 0.2% ammonia water for 30 sec at room
temperature. Sections were washed in running tap water for 5 min at
room temperature and rinsed in 95% alcohol, 10 dips at room
temperature. Counterstaining was performed using eosin-phloxine
solution for 30 sec at room temperature; then sections were
dehydrated through 95% alcohol, two changes of absolute alcohol for
5 min each at room temperature. Sections were cleared in two
incubations with xylene for 5 min each at room temperature. Digital
images were acquired using a light microscope (Olympus BX40;
Olympus Corporation).
For immunoreactivity staining, 5 µm deparaffinized
polysine-coated sections were treated with 3% hydrogen peroxide for
5 min at room temperature to remove endogenous oxides. The cells
were treated using citrate buffer (BioGenex Laboratories, Fremont,
CA, USA) for 5 min at 100°C, and permeabilized with 0.3% Triton
X-100. The slides were incubated with primary antibodies, including
Ki-67 (1:50; cat. no. sc-56319; Santa Cruz Biotechnology, Inc.) and
MMP2 (1:150; cat. no. 40994), E-cadherin (1:100; cat. no. 14472),
VEGF (1:100; cat. no. sc-365578) and vimentin (1:100; cat. no.
5741; Cell Signaling Technology, Inc.) at 4°C overnight, followed
by incubation with a biotin-labeled secondary antibody (1:100
dilution; cat. no. ab64264; Abcam, Cambridge, MA, USA) for 1 h at
room temperature. 5% diaminobenzidine (Abcam) solution for 10 min
at room temperature was used for brown color development.
Immunohistochemical signals were observed under a microscope
(Olympus Corporation).
Statistical analysis
All results are expressed as the mean ± standard
deviation from at least three replicates. One-way analysis of
variance and Tukey test, or Student's unpaired t-test was used to
analyze significance using SPSS 16.0 software (SPSS, Inc., Chicago,
IL, USA). The Kaplan-Meier method was used to plot the survival
curves followed by a log-rank test. Survival analysis was performed
using GraphPad Software version 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Successful establishment of stable
RNF138 expression downregulation in U87 and U251 cell lines
According to our previous research (10), U87 and U251 cell lines exhibited
relatively higher RNF138 expression compared with noncancerous
brain samples, and hence, these two cell lines were chose for
RNF138 silencing experiments. To determine the effect of RNF138,
lentiviral siRNA was used to stably and specifically reduce the
expression of RNF138 in U87 and U251 cells established from
high-grade tumors.
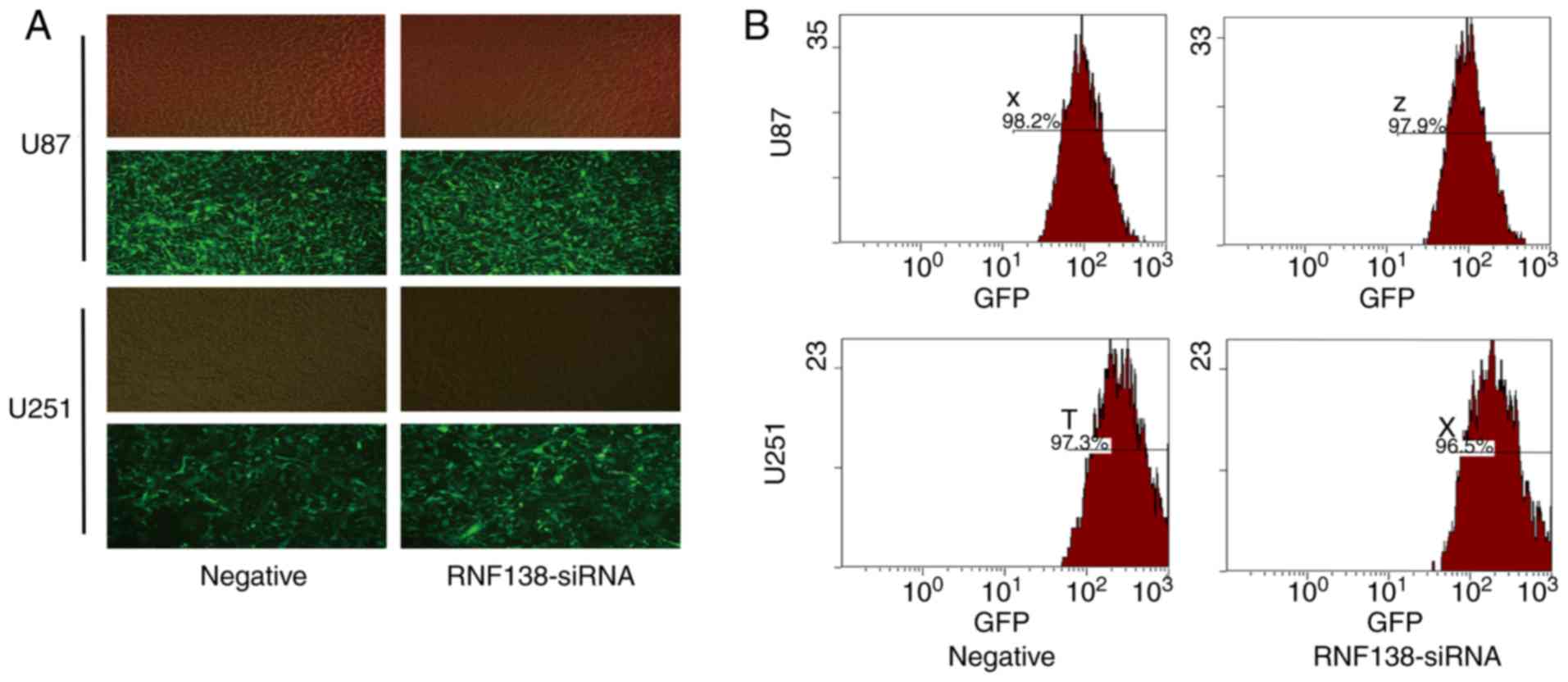
The transfection efficiency of U87 and U251 cells
transfected with RNF138-siRNA and the negative control lentivirus
was determined by fluorescence inverted microscope and flow
cytometry. The phase contrast fluorescence inverted microscopic
evaluation revealed >95% transfected cells (Fig. 1A) and flow cytometry analysis
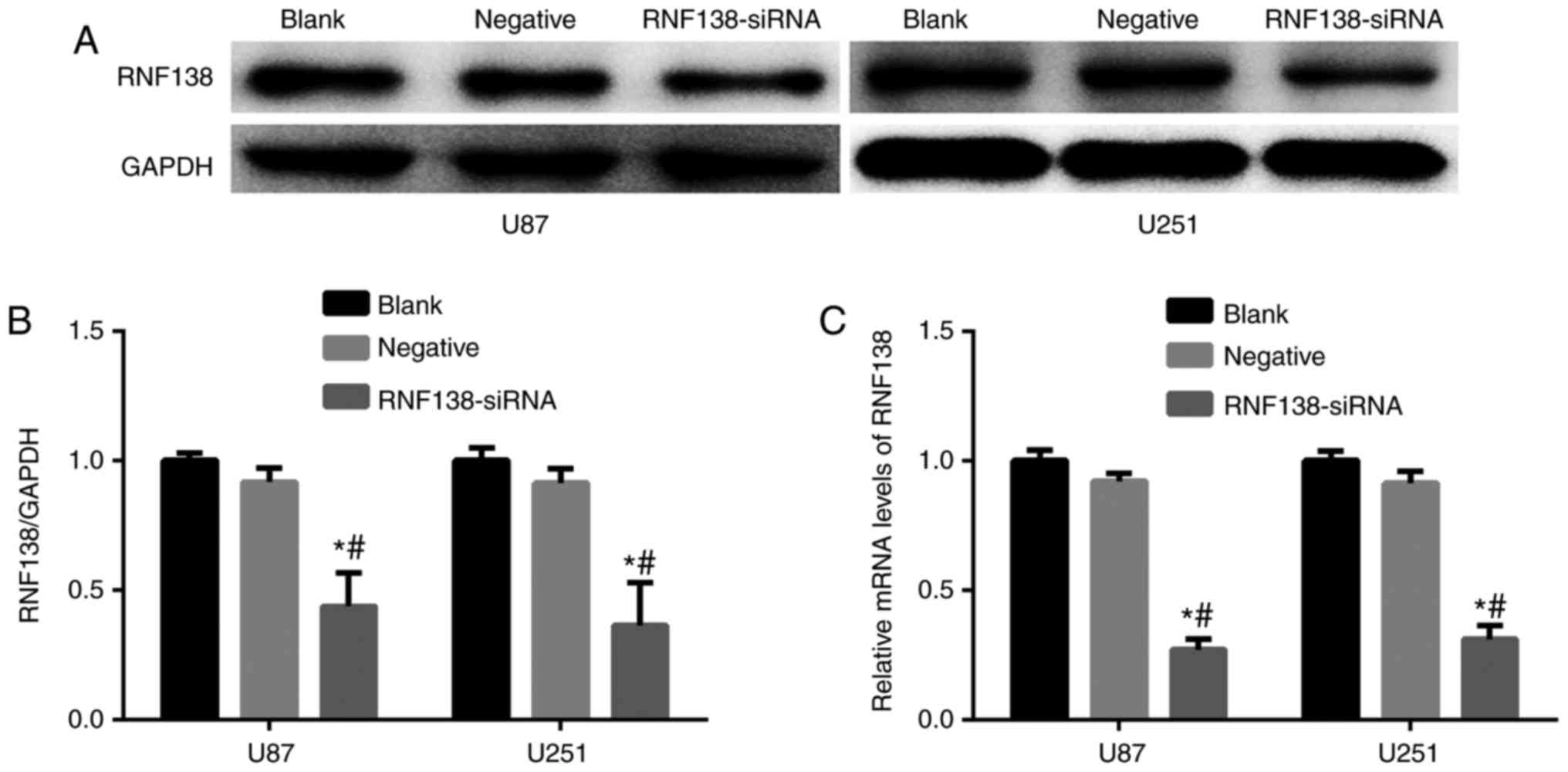
revealed >95% GFP-positive cells (Fig. 1B). RNF138 mRNA and protein
expression levels were measured by RT-qPCR and western blotting
analyses, respectively. Results revealed that RNF138 protein
(Fig. 2A and B) and mRNA expression
levels (Fig. 2C) were significantly
attenuated compared with the negative control lentiviral groups
(P<0.01).
Stable downregulation of RNF138
expression inhibits malignant glioma cell proliferation, migration
and invasion in vitro
The biological function of RNF138 in malignant
glioma was analyzed. U87 and U251 cell lines were selected to
investigate the effects of RNF138 on cell proliferation, migration
and invasion following transduction with RNF138-siRNA and negative
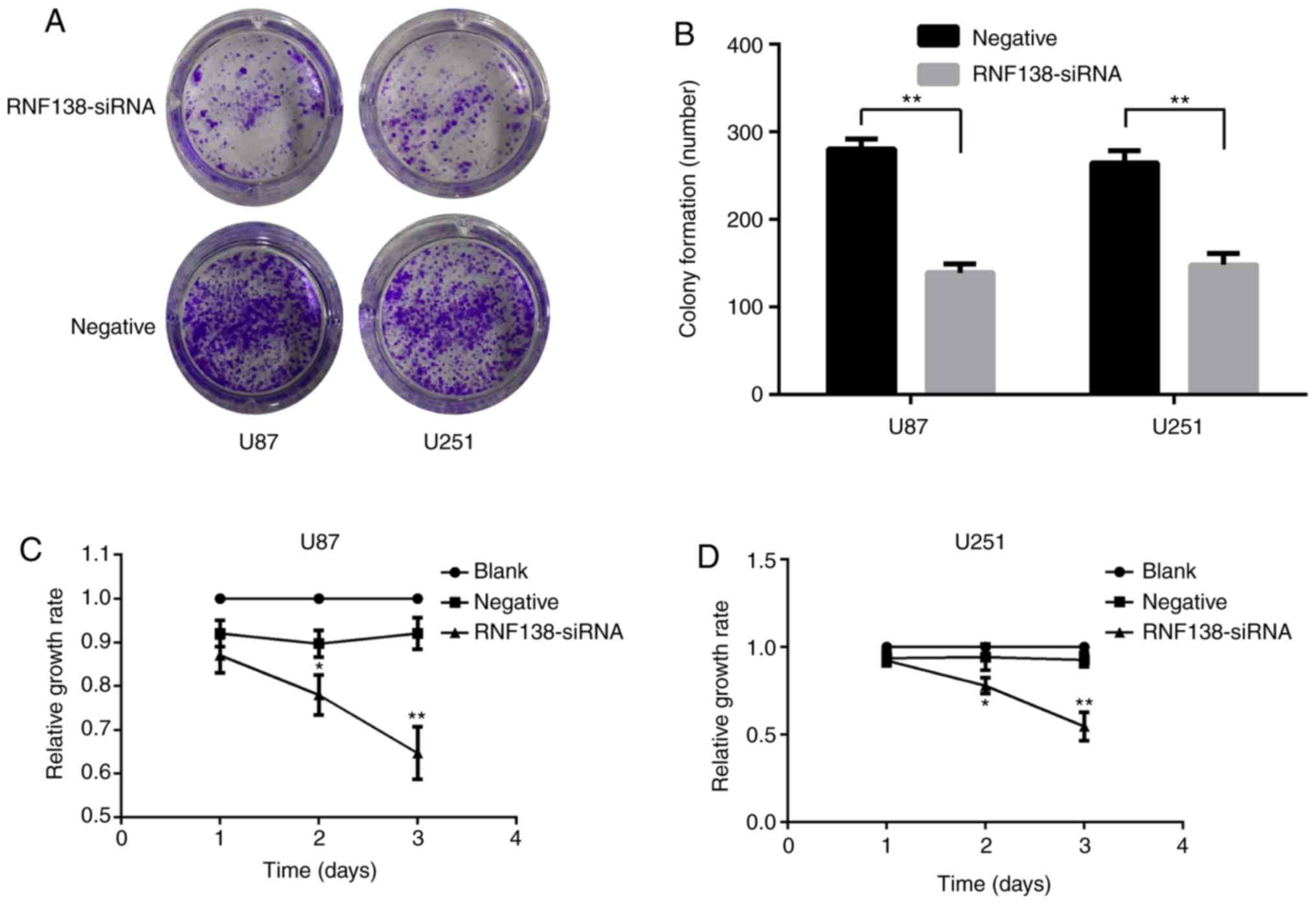
control lentiviruses. Colony formation and CCK-8 assays were
performed after transfection of the cells. Colony formation assay
(Fig. 3A) demonstrated that U87 and
U251 cells infected with RNF138-siRNA decreased the colony
formation compared with the negative control lentiviral groups
(P<0.01; Fig. 3B). CCK-8 assay
revealed that the downregulation of RNF138 led to a significant
reduction of cell proliferation in U251 and U87 cells compared with
negative control lentiviral groups (P<0.01; Fig. 3C and D). These results indicated
that silencing of RNF138 suppresses glioma cell proliferation.
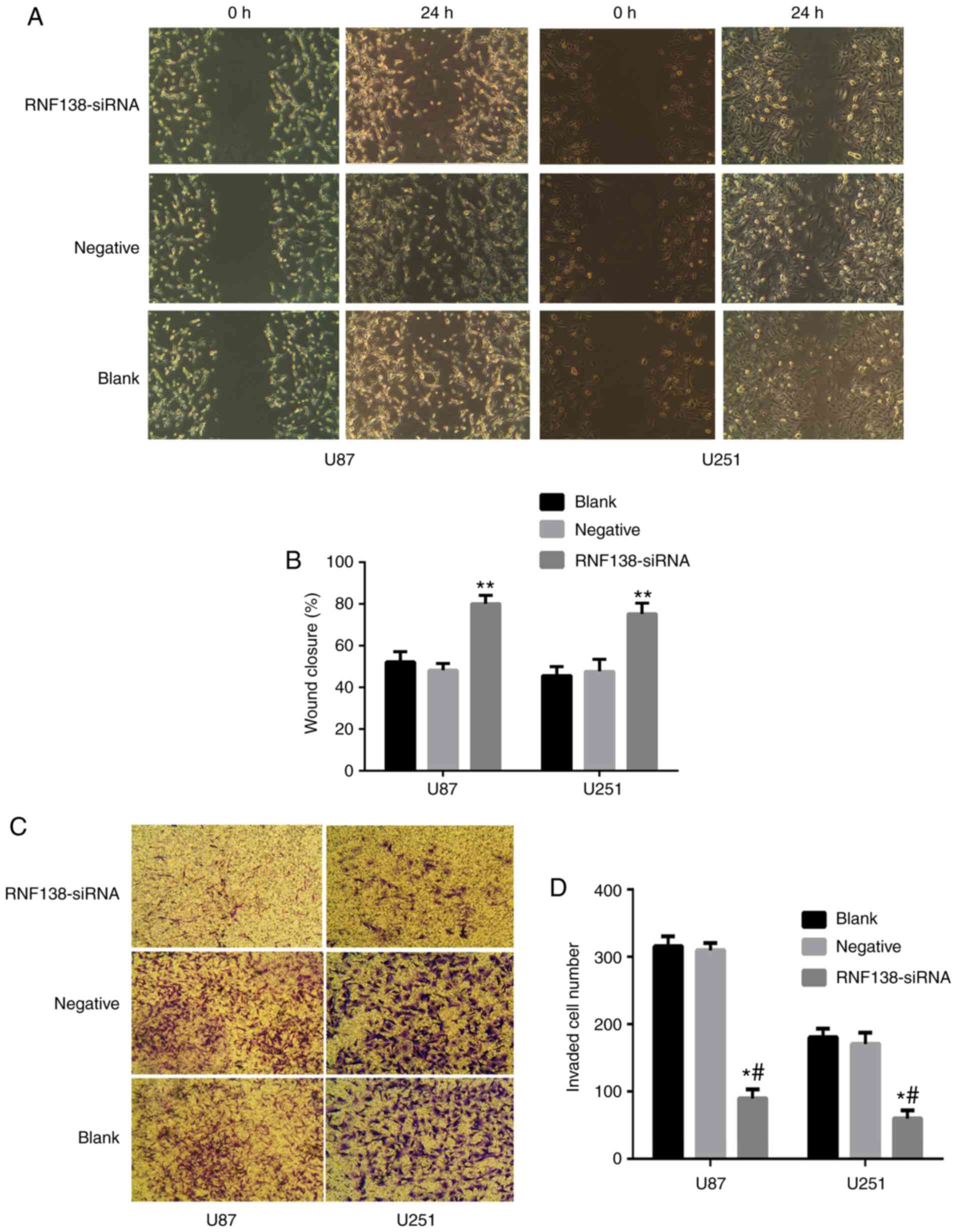
To investigate whether the downregulation of RNF138
in U87 and U251 cells affected their migratory and invasive
abilities in vitro, wound healing and Transwell assays were
performed. Results of wound healing assay (Fig. 4A) demonstrated that downregulation
of RNF138 resulted in a decrease in the migration compared with
negative control lentivirus groups (P<0.01; Fig. 4B), suggesting the reduced
migration-promoting abilities of RNF138 in the knockdown samples.
Additionally, the experimental results of the Transwell assay
demonstrated that the number of cells invading through the chamber
was decreased significantly by RNF138-siRNA compared to negative
control lentiviral groups (P<0.01; Fig. 4C and D), indicating that RNF138 has
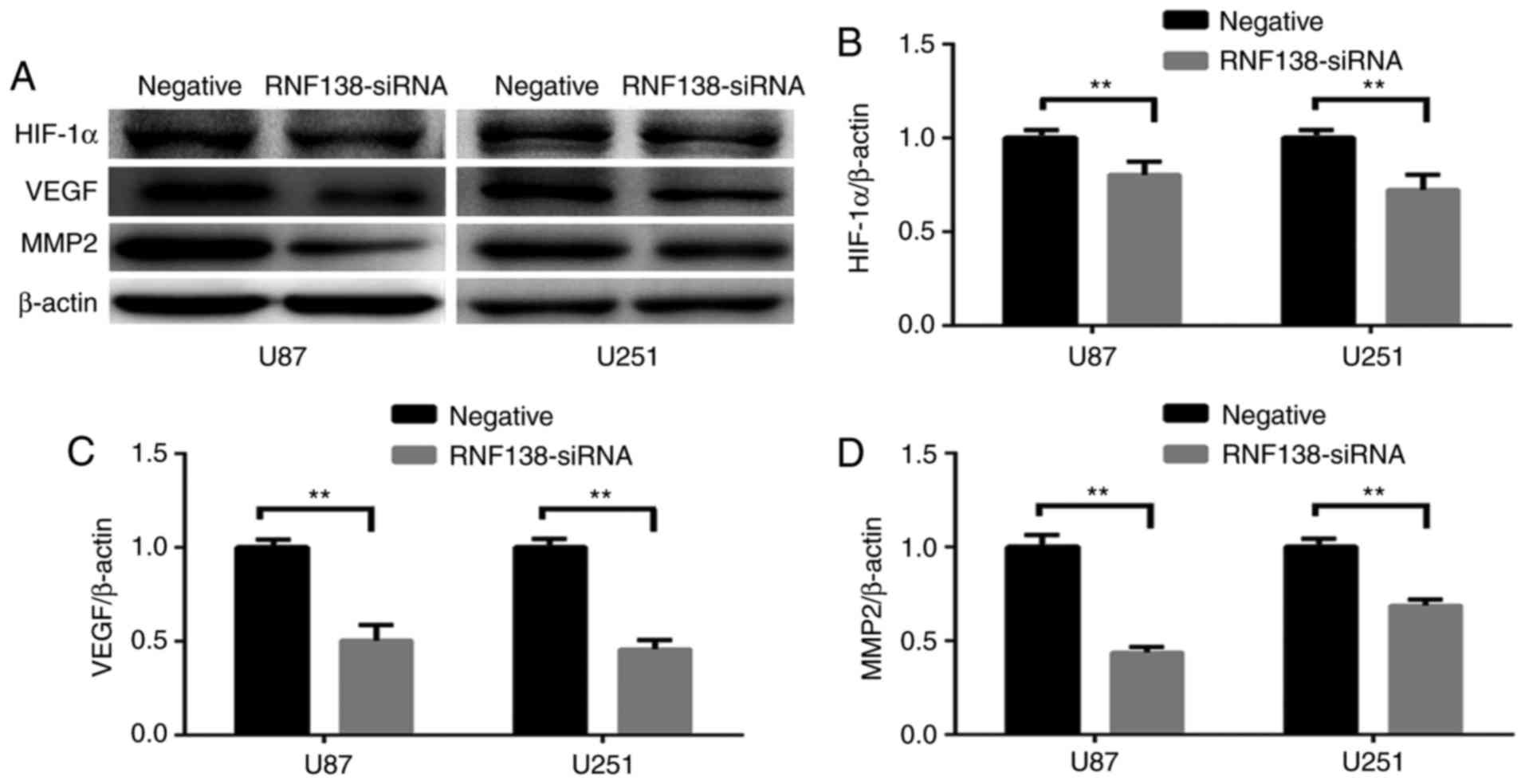
an important role in glioma cell invasion. To further elucidate the
detailed mechanism, the protein level changes of HIF-1α, VEGF and
MMP2 were determined by western blotting (Fig. 5A). Western blot analysis revealed
significant decrease of HIF-1α, VEGF and MMP2 protein levels by
downregulating RNF138 compared with the negative control
(P<0.01; Fig. 5B-D). These data
demonstrated that knockdown of RNF138 inhibited glioma cell
migration and invasion, potentially via downregulation of HIF-1α,
VEGF and MMP2 expression levels. The data implied that RNF138 has a
critical role in glioma cell proliferation, migration and
invasion.
Knockdown of RNF138 increased
apoptosis of glioma cells through caspase pathway
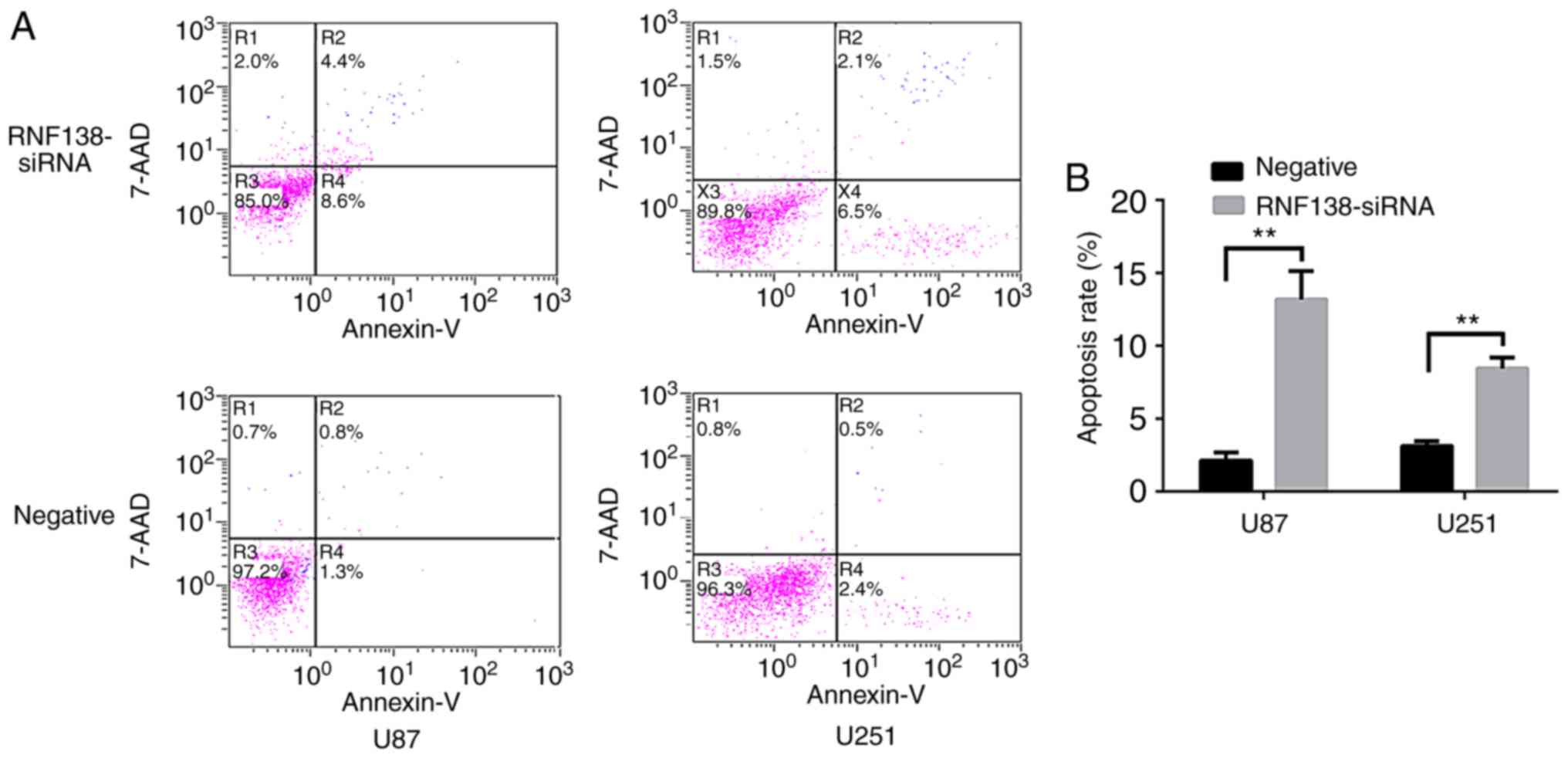
Annexin V-PE apoptosis detection and flow cytometry
analysis were used to examine whether knockdown of RNF138 inhibited
glioma cell proliferation by inducing cell apoptosis. Annexin V-PE
and 7-AAD to evaluate the apoptosis of GFP-positive U251 and U87
cell lines. The results revealed that knockdown of RNF138 may
induce cell apoptosis (Fig. 6A).
The proportion of apoptotic cells following RNF138 siRNA was
significantly increased in U87 and U251 cells compared with
negative control lentiviral groups (P<0.01; Fig. 6B). Subsequently, the protein levels
of apoptotic-associated factors (BAX, Bcl2, cleaved caspase3 and
caspase3) were determined using western blotting (Fig. 7A). Notably, the protein of caspase3
was unchanged in U87 and U251 cells with RNF138 knockdown compared
with the negative control lentivirus group (P<0.01; Fig. 7B). Additionally, the protein
expression of BAX and cleaved caspase3 (Fig. 7C and D) were upregulated, while Bcl2
(Fig. 7E) was downregulated in the
RNF138-siRNA group compared with the negative lentivirus group.
Taken together, these data suggested that downregulation of RNF138
inhibited glioma cell proliferation, increased apoptosis of glioma
cells through caspase signaling pathway.
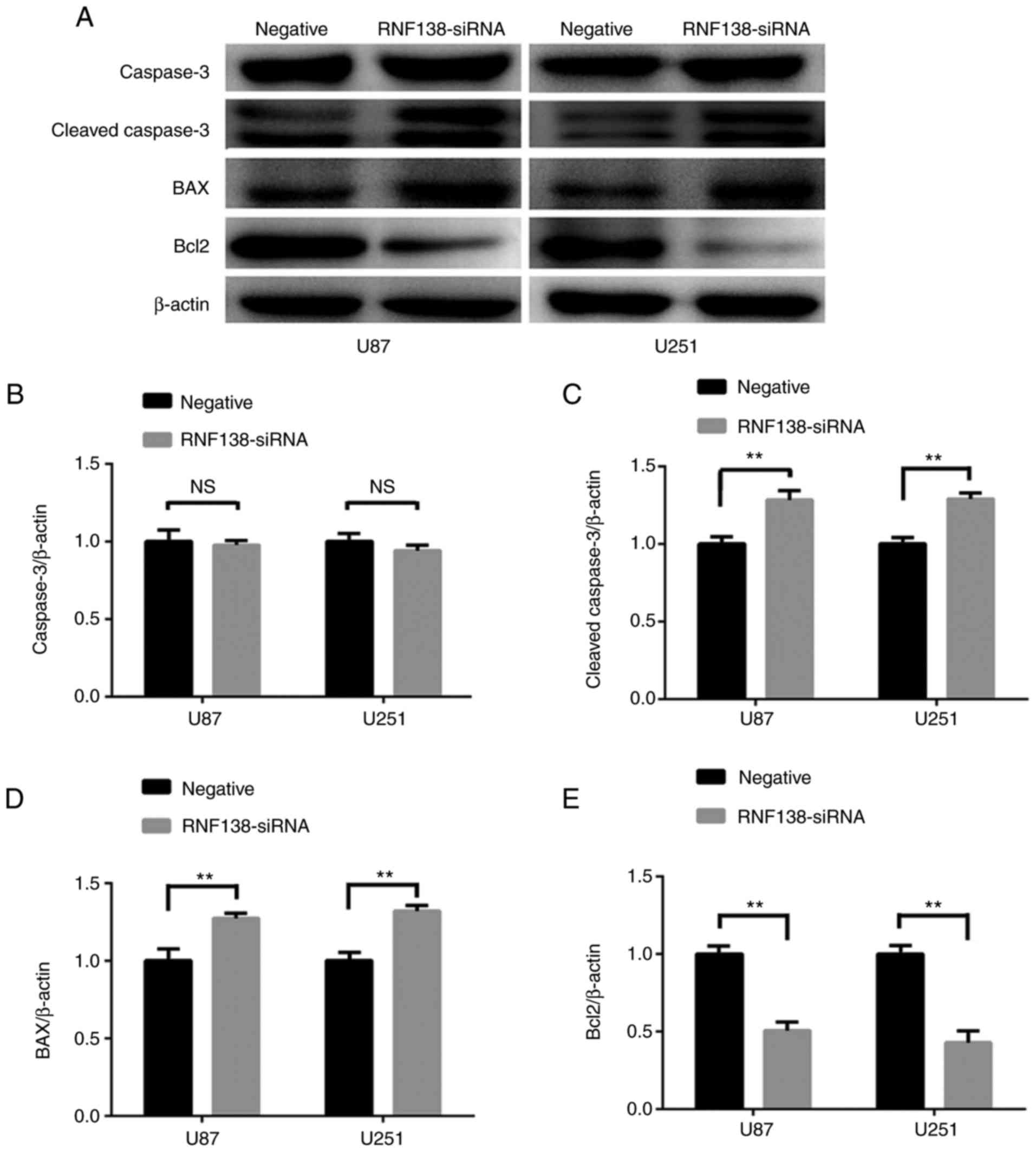 | Figure 7.Protein levels of
apoptotic-associated factors in U87 and U251 cells transduced with
RNF138-siRNA and negative control lentivirus. (A) Expression of
caspase3, cleaved caspase3, BAX and Bcl2 were detected by western
blot analysis. β-actin served as the loading control. Densitometry
analysis of (B) caspase3, (C) cleaved caspase3, (D) BAX and (E)
Bcl2, relative to β-actin expression in U87 and U251 cells with
ImageJ analysis. Data are presented as the mean ± standard
deviation for three independent experiments. **P<0.01. RNF138,
ring finger protein 138; siRNA, small interfering RNA; BAX, Bcl2
associated X apoptosis regulator; Bcl2, Bcl2 apoptosis regulator;
NS, not significant. |
Downregulation of RNF138 inhibited EMT
in U87 and U251 cells and suppressed the Erk signaling pathway
EMT is a reversible biological process that occurs
in cells during normal development and aberrantly activated in
various cancer types (21).
Previous studies have reported that EMT is closely associated with
malignant tumor cell invasion capacity. To further explore whether
downregulation of RNF138 expression level could influence EMT in
glioma cell lines, the EMT markers E-cadherin and vimentin were
measured using western blot analysis (Fig. 8A). Compared with the negative
control lentiviral cells, the expression level of E-cadherin was
significantly increased (P<0.01; Fig. 8B), while the vimentin levels were
decreased in RNF138-siRNA cells (P<0.01; Fig. 8C).
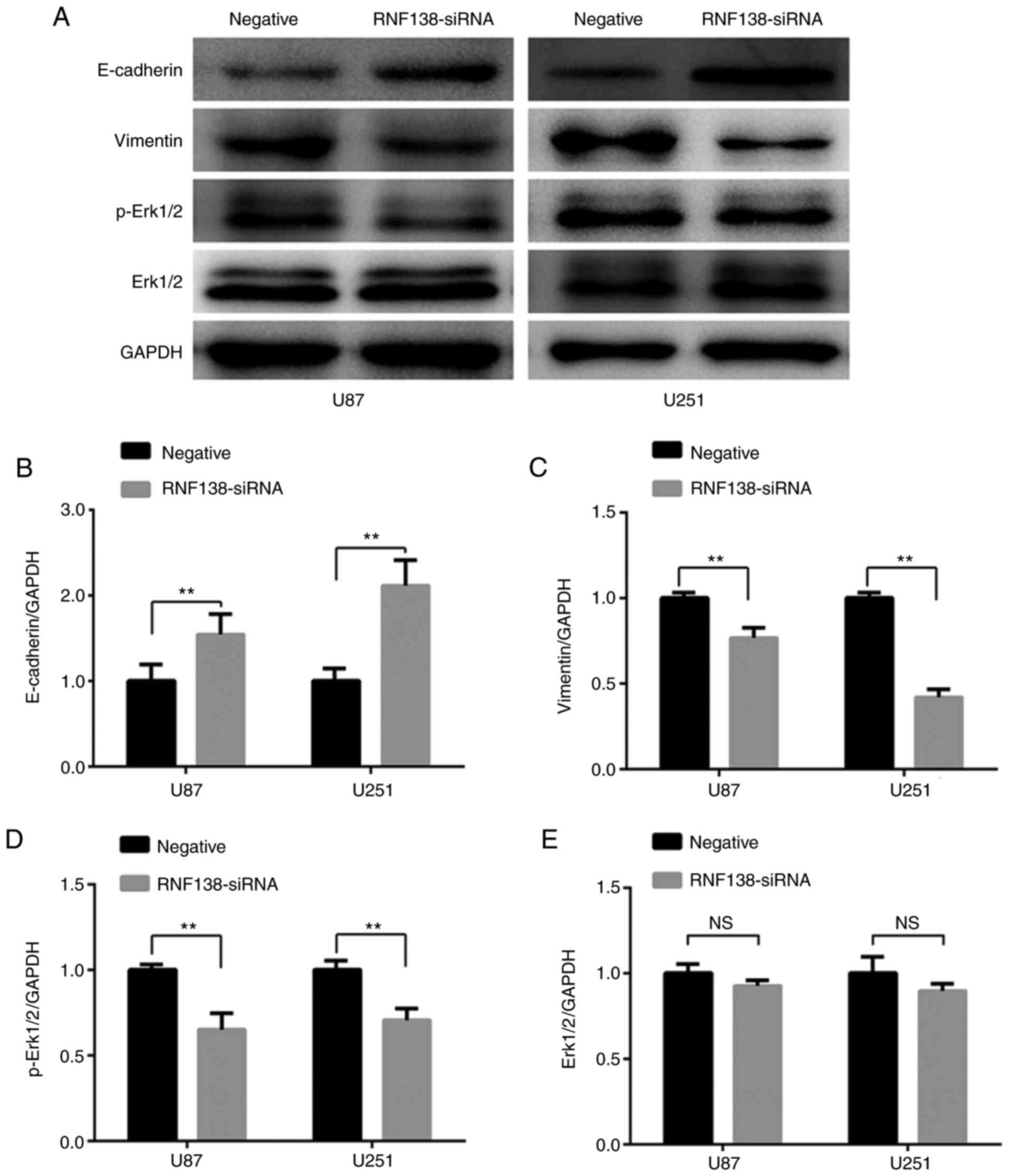 | Figure 8.Downregulation of RNF138 inhibits
epithelial-mesenchymal transition of U87 and U251 cells by
suppressing the Erk signaling pathway. (A) Expression of
E-cadherin, vimentin, p-Erk1/2, Erk1/2 and GAPDH in different
groups were detected by western blot analysis. Relative gray values
of (B) E-cadherin, (C) vimentin, (D) p-Erk1/2 and (E) Erk1/2
relative to GAPDH expression levels in U87 and U251 cells were
detected using ImageJ analysis. Data are presented as the mean ±
standard deviation for three independent experiments. **P<0.01.
p-, phospho-; Erk, extracellular signal-regulated kinase; RNF138,
ring finger protein 138; siRNA, small interfering RNA; NS, not
significant. |
There are several cell signaling pathways implicated
in the process of EMT. Erk signaling pathways has been reported to
have a role in modulating cell invasion and progression of EMT
(22,23). Western blot analysis was performed
to further investigate whether inhibition of RNF138 expression
could influence EMT via the Erk signaling pathway. These results
revealed that downregulation of RNF138 significantly inhibited the
activation of Erk1/2 (P<0.01; Fig.
8D), however, total Erk1/2 levels were unchanged (P>0.01;
Fig. 8E). These data indicate that
the downregulation of RNF138 may reverse the EMT process via the
Erk signaling pathway in glioblastoma.
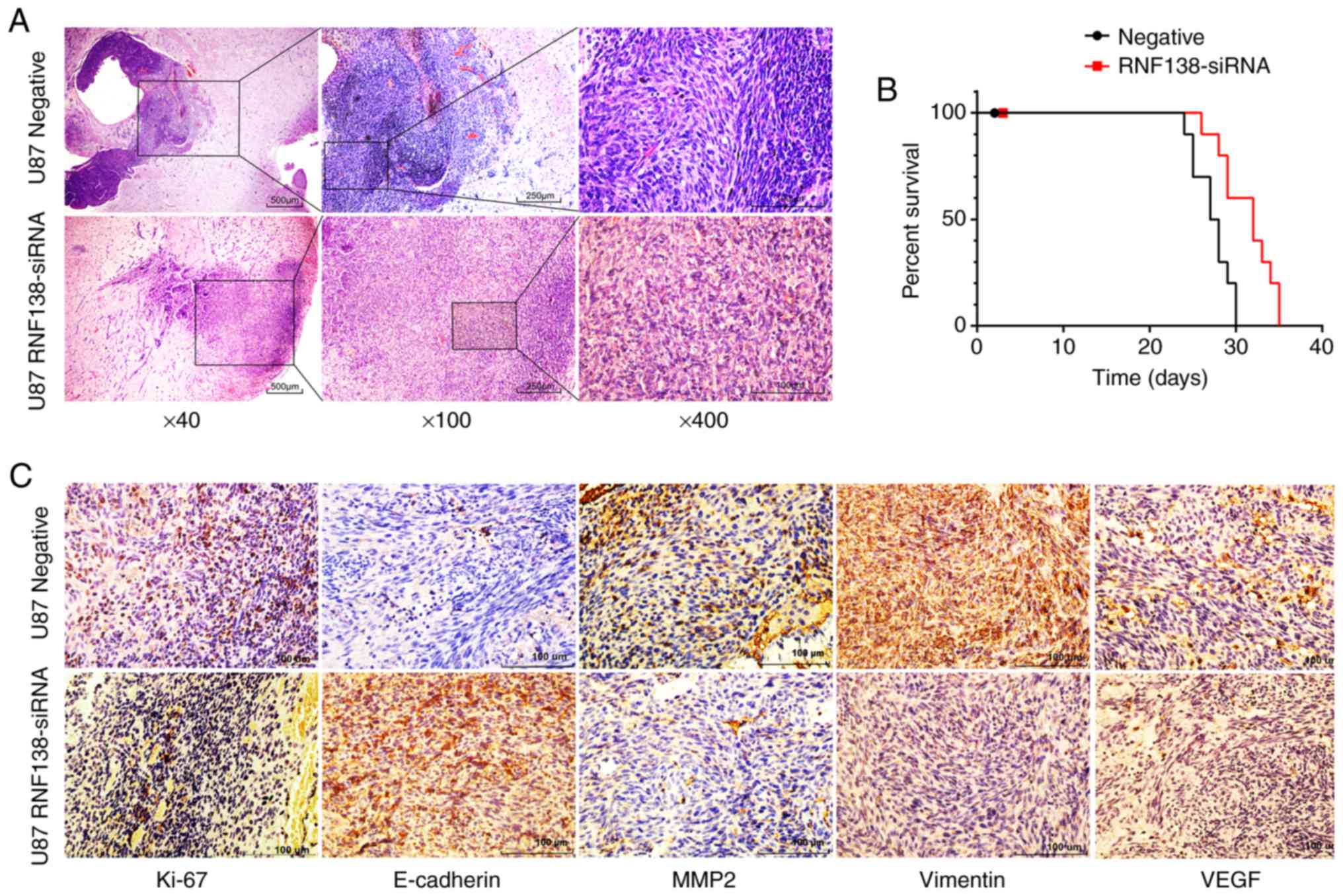
Knockdown of RNF138 suppresses tumor
growth in an orthotopic nude mice model
As described above, downregulation of RNF138
suppressed the tumorigenicity of glioma cells in vitro and
further analysis is required in vivo. Tumorigenicity was
determined in an orthotopic nude mouse model to confirm the
function of RNF138 knockdown in vivo. U87 cells with stable
expression of RNF138-siRNA or negative control lentivirus were
intracranially injected into nude mice. Eleven mice were collected
for RNF138-siRNA cells treated group (experiment group), and
another eleven mice were treated with Sci-siRNA as control group.
Hematoxylin and eosin (H&E) staining of the tumors resulting
from injection with U87 cells revealed decreased cell motion in the
RNF138-siRNA group compared with the scramble controls (Fig. 9A), Kaplan-Meier analysis showed
survival was prolonged in the RNF138-siRNA group compared with the
negative control group (P<0.01; Fig.
9B). Immunohistochemistry revealed that the expression of
Ki-67, vimentin, VEGF and MMP2 were lower in the RNF138-siRNA group
compared with the negative group, while E-cadherin expression was
notably higher than the negative control group (Fig. 9C). These results were consistent
with the in vitro results, indicating that knockdown of
RNF138 suppressed tumorigenesis of malignant glioma in
vivo.
Discussion
Ubiquitylation is a dynamic and reversible
post-translational modification that regulates a wide range of
biological processes, including cell cycle progression,
transcription, apoptosis and inflammation (24). RNF138 is characterized as an E3
ubiquitin ligase that contains a RING, zinc finger and UIM domains.
RNF138 was originally reported to act as a negative regulator of
Wnt signaling through Nemo-like kinase (25). Although the upregulation of RNF138
in human glioma tissues has been previously identified (10), the biological functions of RNF138 in
glioma cell lines remain largely unknown.
Therefore, CCK-8 proliferation assay, colony
formation assay and flow cytometry were used to explore the effects
of RNF138 on migration, proliferation and apoptosis of U87 and U251
cells in vitro. The current study demonstrated decreased
migration and invasion, and increased the apoptosis of U87 and U251
glioma cells with RNF138 knockdown. However, the exact molecular
mechanisms on how tumor cell proliferation, migration, apoptosis
and invasive potential are affected remained unclear.
Apoptosis is a well-orchestrated cellular mechanism
of programmed cell death that is mediated through a caspase cascade
(26,27). Therefore, the protein levels of
apoptosis-associated factors were determined in
RNF138-siRNA-infected glioblastoma cells. The levels of both
cleaved caspase3 and BAX were upregulated in U87 and U251 cells
with RNF138 silencing. Conversely, the expression levels of Bcl2
were downregulated by RNF138 knockdown. The current study
demonstrated the critical role of regulatory role of RNF138 in
apoptosis via the caspase3 pathway in U87 and U251 cells. Apoptotic
assay further confirmed the apoptosis inducing function of
downregulated RNF138. These findings are supported by the fact that
RNF138 silencing induced an increase in the abundance of cleaved
caspase3 protein.
Hou et al (28) suggested that cancer cells obtain
invasion ability via EMT, whereby epithelial cells lose their
cell-cell adhesion and attain mesenchymal characteristics. This
process has a critical role in the development and progression,
invasion and migration of diverse human tumors (28–31).
Therefore, to elucidate the precise mechanisms involved in cell
migration and invasion, the effects of RNF138 on EMT-associated
proteins were examined. The suppression of RNF138 expression
resulted in elevated expression of E-cadherin and reduced
expression of vimentin, which acts as a crucial step for cancer
cell migration and invasion in various cancer types (32–34).
These findings indicate that the knockdown of RNF138 potentially
reduced EMT in the glioma cell lines.
Erk signaling is associated with the process of EMT,
and an essential component of the mitogen-activated protein kinase
signal cascades. Erk is associated with the regulation of glioma
proliferation, differentiation, migration and apoptosis (35–38).
The effect of RNF138 on Erk signaling pathway was also
investigated. The level of p-Erk1/2 was notably decreased in
RNF138-siRNA glioma cells compared with negative control glioma
cells, and cell migration was suppressed following RNF138
knockdown. Thus, the data confirmed that lower expression of RNF138
in glioma cells reversed the EMT process, potentially via the Erk
pathway.
In addition, RNF138 knockdown notably decreased
MMP2, HIF-1α and VEGF protein expression levels. MMP2, HIF-1α and
VEGF have been reported to participate in EMT progression in
different types of cancer, which was regulated via Erk signaling
(39–41). HIF-1α is stabilized by
hypoxia-induced reactive oxygen species, which results in the
enhanced expression of several of hypoxia-associated genes,
including the VEGF, which is an angiogenic activator (42). Furthermore, immunohistochemistry
staining revealed that vimentin, VEGF and MMP2-positive cells were
reduced, while E-cadherin was higher in tumors produced from RNF138
knockdown cells than the negative control group. Taken together,
these results suggest that suppression of RNF138 reduced the
invasion and migration of glioma cells, and regulated the protein
levels of HIF-1α, VEGF and MMP2 potentially by reversing EMT via
Erk signaling.
Acknowledgements
The authors thank Shanghai GenePharm Co., Ltd.
(Shanghai, China) for providing the interference sequence and
technical assistance, the central lab for providing technical
instruction and assistance and the Cell Bank Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China) for
offering glioma cell lines.
Funding
This study was partially supported by the National
Science Foundation of China (grant no. 81572475) and the
Anti-Cancer Association Foundation of China (grant no.
CSNO-2016-MSD04).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HW and XL conducted the majority of experiments,
analyzed the results and wrote the majority of the paper. YZ and MF
designed the study, coordinated the study and wrote the paper. LY,
GZ and ZDe conducted the experiments on cell cultures and
lentivirus siRNA gene transfection. SC offered technical
instruction and assistance. ZDu performed analysis and
interpretation of data. All the authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Institutional Review Board of the Department of Laboratory Animal
Science of the First Affiliated Hospital of Soochow University
(Suzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Malzkorn B and Reifenberger G: Practical
implications of integrated glioma classification according to the
World Health Organization classification of tumors of the central
nervous system 2016. Curr Opin Oncol. 28:494–501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Winter SF, Loebel F and Dietrich J: Role
of ketogenic metabolic therapy in malignant glioma: A systematic
review. Crit Rev Oncol Hematol. 112:41–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ostrom QT, Gittleman H, Liao P, Rouse C,
Chen Y, Dowling J, Wolinsky Y, Kruchko C and Barnholtz-Sloan J:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 16
Suppl 4:iv1–iv63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanaka S, Louis DN, Curry WT, Batchelor TT
and Dietrich J: Diagnostic and therapeutic avenues for
glioblastoma: No longer a dead end? Nat Rev Clin Oncol. 10:14–26.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carlsson SK, Brothers SP and Wahlestedt C:
Emerging treatment strategies for glioblastoma multiforme. EMBO Mol
Med. 6:1359–1370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang J, Wang WF, Xie S, Zhang XL, Qi WF,
Zhou XP, Hu JX, Shi Q and Yu RT: Expression of β-transducin
repeat-containing E3 ubiquitin protein ligase in human glioma and
its correlation with prognosis. Oncol Lett. 9:2651–2656. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Wang F, Liu Y, Yao Y, Lv X, Dong B,
Li J, Ren S, Yao Y and Xu Y: RNF135, RING finger protein, promotes
the proliferation of human glioblastoma cells in vivo and in vitro
via the ERK pathway. Sci Rep. 6:206422016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu L, Lu Y, Han D, Yao R, Wang H, Zhong S,
Luo Y, Han R, Li K, Fu J, et al: Rnf138 deficiency promotes
apoptosis of spermatogonia in juvenile male mice. Cell Death Dis.
8:e27952017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou YX, Chen SS, Wu TF, Ding DD, Chen XH,
Chen JM, Su ZP, Li B, Chen GL, Xie XS, et al: A novel gene RNF138
expressed in human gliomas and its function in the glioma cell line
U251. Anal Cell Pathol (Amst). 35:167–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han MZ, Huang B, Chen AJ, Zhang X, Xu R,
Wang J and Li XG: High expression of RAB43 predicts poor prognosis
and is associated with epithelial-mesenchymal transition in
gliomas. Oncol Rep. 37:903–912. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Zhang W, Li Y, Alvarez A, Li Z,
Wang Y, Song L, Lv D, Nakano I, Hu B, et al: SHP-2-upregulated ZEB1
is important for PDGFRalpha-driven glioma epithelial-mesenchymal
transition and invasion in mice and humans. Oncogene. 35:5641–5652.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bustin SA, Benes V, Garson JA, Hellemans
J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL,
et al: The MIQE guidelines: Minimum information for publication of
quantitative real-time PCR experiments. Clin Chem. 55:611–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Zhang G, Wang Y, Elgehama A, Sun Y,
Li L, Gu Y, Guo W and Xu Q: Loss of periplakin expression is
associated with the tumorigenesis of colorectal carcinoma. Biomed
Pharmacother. 87:366–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Hu Y, Liu C, Wang Q, Han X, Han Y,
Xie XS, Chen XH, Li X, Siegel ER, et al: Human fibulin-3 protein
variant expresses anti-cancer effects in the malignant glioma
extracellular compartment in intracranial xenograft models.
Oncotarget. 8:106311–106323. 2017.PubMed/NCBI
|
|
20
|
Clingerman KJ and Summers L: Validation of
a body condition scoring system in rhesus macaques (Macaca
mulatta): inter- and intrarater variability. J Am Assoc Lab Anim
Sci. 51:31–36. 2012.PubMed/NCBI
|
|
21
|
Deng J, Wang L, Chen H, Hao J, Ni J, Chang
L, Duan W, Graham P and Li Y: Targeting epithelial-mesenchymal
transition and cancer stem cells for chemoresistant ovarian cancer.
Oncotarget. 7:55771–55788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buonato JM and Lazzara MJ: ERK1/2 blockade
prevents epithelial-mesenchymal transition in lung cancer cells and
promotes their sensitivity to EGFR inhibition. Cancer Res.
74:309–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding C, Tang W, Fan X, Wang X, Wu H, Xu H,
Xu W, Gao W and Wu G: Overexpression of PEAK1 contributes to
epithelial-mesenchymal transition and tumor metastasis in lung
cancer through modulating ERK1/2 and JAK2 signaling. Cell Death
Dis. 9:8022018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown JS and Jackson SP: Ubiquitylation,
neddylation and the DNA damage response. Open Biol. 5:1500182015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han D, Liang J, Lu Y, Xu L, Miao S, Lu LY,
Song W and Wang L: Ubiquitylation of Rad51d mediated by E3 ligase
Rnf138 promotes the homologous recombination repair pathway. PLoS
One. 11:e01554762016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang B, Qin J, Nie Y, Li Y and Chen Q:
Brain-derived neurotrophic factor propeptide inhibits proliferation
and induces apoptosis in C6 glioma cells. Neuroreport. 28:726–730.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang XQ, Bai HM, Li ST, Sun H, Min LZ, Tao
BB, Zhong J and Li B: Knockdown of HDAC1 expression suppresses
invasion and induces apoptosis in glioma cells. Oncotarget.
8:48027–48040. 2017.PubMed/NCBI
|
|
28
|
Hou M, Cheng Z, Shen H, He S, Li Y, Pan Y,
Feng C, Chen X, Zhang Y, Lin M, et al: High expression of CTHRC1
promotes EMT of epithelial ovarian cancer (EOC) and is associated
with poor prognosis. Oncotarget. 6:35813–35829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jayachandran A, Dhungel B and Steel JC:
Epithelial-to-me-senchymal plasticity of cancer stem cells:
Therapeutic targets in hepatocellular carcinoma. J Hematol Oncol.
9:742016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Krebs AM, Mitschke J, Losada Lasierra M,
Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D,
Reichardt W, Bronsert P, et al: The EMT-activator Zeb1 is a key
factor for cell plasticity and promotes metastasis in pancreatic
cancer. Nat Cell Biol. 19:518–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pietilä M, Ivaska J and Mani SA: Whom to
blame for metastasis, the epithelial-mesenchymal transition or the
tumor microenvironment? Cancer Lett. 380:359–368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-me-senchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zeng Z, Leng T, Feng X, Sun H, Inoue K,
Zhu L and Xiong ZG: Silencing TRPM7 in mouse cortical astrocytes
impairs cell proliferation and migration via ERK and JNK signaling
pathways. PLoS One. 10:e01199122015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Heigener DF, Gandara DR and Reck M:
Targeting of MEK in lung cancer therapeutics. Lancet Respir Med.
3:319–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Z, Dai H, Jia G, Li Y, Liu X and Ren
W: Insufficient radiofrequency ablation promotes human hepatoma
SMMC7721 cell proliferation by stimulating vascular endothelial
growth factor overexpression. Oncol Lett. 9:1893–1896. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong F, Tian H, Yan S, Li L, Dong X, Wang
F, Li J, Li C, Cao Z, Liu X and Liu J: Dihydroartemisinin inhibits
endothelial cell proliferation through the suppression of the ERK
signaling pathway. Int J Mol Med. 35:1381–1387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang D, Lu C and Ai H: Rab5a is
overexpressed in oral cancer and promotes invasion through ERK/MMP
signaling. Mol Med Rep. 16:4569–4576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morishita Y, Ookawara S, Hirahara I, Muto
S and Nagata D: HIF-1α mediates Hypoxia-induced
epithelial-mesenchymal transition in peritoneal mesothelial cells.
Ren Fail. 38:282–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mori H, Yao Y, Learman BS, Kurozumi K,
Ishida J, Ramakrishnan SK, Overmyer KA, Xue X, Cawthorn WP, Reid
MA, et al: Induction of WNT11 by hypoxia and hypoxia-inducible
factor-1α regulates cell proliferation, migration and invasion. Sci
Rep. 6:215202016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Park SY, Jang WJ, Yi EY, Jang JY, Jung Y,
Jeong JW and Kim YJ: Melatonin suppresses tumor angiogenesis by
inhibiting HIF-1alpha stabilization under hypoxia. J Pineal Res.
48:178–184. 2010. View Article : Google Scholar : PubMed/NCBI
|