Introduction
Glioma cancer is also referred to as neuroectodermal
tumor or neuroepithelial tumor since the tumor develops in the
neuroderm. According to the World Health Organization, ~75%
malignant primary brain tumors are glioma cancer (1). Meanwhile, the annual morbidity rate of
5/10,000 and the high mortality rate of glioma have attracted
extensive attention (2). It has
been reported in the literature that the annual morbidity rate of
glioma in China is 3–6/100,000, with a higher incidence among male
patients than female patients. In addition, ~30,000 patients
succumb to this disease annually (3). Glioma can occur at all age groups,
which is dominated by adults, with 30–40 years being the peak age
of morbidity (2). Glioma in adults
is commonly observed in the supratentorial region, while that in
children is mostly in the cerebellum (4). The majority of gliomas are tumors with
invasive growth, which are associated with the possibility of
recurrence following resection (4).
Common clinical symptoms of glioma are associated with the tumor
site and pathological type of glioma (5).
As one of the most common malignant primary
intracranial tumors, glioma cancer is the central nervous system
tumor with the poorest therapeutic effects at present (6). Medical professionals have performed a
large number of studies on the molecular biology of glioma,
including its association with microRNA (miRNA) (7). It has been demonstrated that microRNA
is associated with glioma stem cells (7). This is of crucial importance to study
microRNA and it has attracted extensive attention (8). miRNA acts on downstream target genes
or proteins through multiple signaling pathways and transcription
factors (8). Therefore, miRNAs can
regulate the expression of oncogenes or tumor suppressor genes, and
result in cell cycle changes in glioma stem cells. In addition,
this will lead to changes in cell apoptosis, proliferation and
differentiation, and affect the differentiation of glioma and its
self-renewal ability (9). miRNA
expression has been demonstrated to be upregulated or downregulated
in glioma stem cells. This renders corresponding changes in
signaling pathways, or all types of transcription or growth factors
(9). Furthermore, certain tumor
stem cell-related characteristics can also be affected, including
proliferation, differentiation, invasion and migration. Therefore,
microRNA can inhibit the growth and proliferation of tumor stem
cells (9).
The phosphatase and tensin homolog (PTEN) gene is a
new gene isolated from the homologous deletion region of primary
breast cancer (10). It is the
first tumor suppressor gene possessing dual phosphatase activity
discovered thus far, and is located on chromosome 10q23 (10). The PTEN gene inhibits tumor cell
proliferation, induces apoptosis, and suppresses tumor cell
migration and local adhesion, thereby exerting effects on the tumor
(10). Deletion or mutation of the
PTEN gene can result in abnormal cell proliferation (11). Loss of homozygosity and
heterozygosity, as well as abnormal promoter methylation of the
gene, can result in its inactivation (11). A previous study demonstrated that
the PTEN gene is associated with the genesis and development of
multiple solid tumors (12). A
previous study suggested that abnormal methylation of the PTEN gene
promoter may be observed in different types of tumors (11), including breast cancer, esophageal
cancer, thyroid carcinoma, renal carcinoma, oral squamous cell
carcinoma and ovarian clear cell carcinoma (13).
The phosphoinositide 3-kinase (PI3K)/protein kinase
B (Akt) signaling pathway is extensively distributed in cells. It
exerts important physiological functions through multiple pathways,
including regulating cell cycle and cellular energy metabolism
(14). It has been demonstrated
that the PI3K/Akt signaling pathway is excessively activated in
glioma cancer, suggesting an association between this pathway, and
the genesis and development of glioma (15). Meanwhile, PI3K and Akt may be
effective targets for the clinical treatment of glioma cancer
(15). Zhang et al (16) reported that miRNA-494 promotes
colorectal (16), epithelial
ovarian (17) and pancreatic cancer
(18). The aim of the present study
was to analyze the possible association between microRNA-494 and
cell proliferation in glioma.
Materials and methods
Glioma samples
The peripheral blood of patients with glioma (n=58;
age range, 44–70 years; mean age, 57±12.5 years; 28 males and 30
females) and healthy volunteers (n=28; age range, 51–69 years; mean
age, 59.5±9 years; 14 males and 14 females) were collected and
centrifuged at 10,000 × g at 4°C for 10 min. Serum was collected
and stored at −70°C, prior to being used for quantitative
polymerase chain reaction (qPCR).
RNA isolation and qPCR
Total RNA was extracted from serum samples using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), cDNA was amplified from 10 ng RNA using Taqman
MicroRNA assays (Applied Biosystems; Thermo Fisher Scientific,
Inc.). cDNA was prepared using SYBR Premix Ex Taq™ II (Takara
Biotechnology Co., Ltd., Dalian, China). MicroRNA-494 forward,
5′-CATAGCCCGTGAAACATACACG-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′;
and U6 forward, 5′-CGCTTCGGCAGCACATATACTA-3′ and reverse,
5′-GCGAGCACAGAATTAATACGAC-3′. The thermocycling conditions for this
stage were as follows: 95°C for 5 min; 40 cycles of denaturation at
95°C for 25 sec; and annealing/extension at 60°C for 30 sec. The
miRNA expression was determined using the comparative Cq
(2−ΔΔCq) method method (19). Low miRNA-494 expression was
categorized as 1–2 times that of the control group (U6), and high
miRNA-494 expression was categorized as >2 times that of the
control group (U6).
Cell lines and transfection
Human glioma U251 cells were maintained in RPMI-1640
medium (HyClone™; GE Healthcare Life Sciences, Logan, UT, USA),
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and cultured at 37°C in a 5% CO2
incubator. A total of 100 ng anti-miR-494, miR-494, si-PTEN, PTEN
plasmid and negative control were synthesized by Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). Anti-miR-494
(5′-UUCUCCGAACGUGUCACGUUU-3′ and 5′-ACGUACACGUUCGGAGAAUU-3′),
miR-494 (5′UGAAACAUACACGGGAAACCUC3′ and
5′-GGUUUCCCGUGUAUGUUUCAUU-3′) and negative control were transfected
into cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Next, anti-miR-494 and si-PTEN (cat. no.
sc-44272; Santa Cruz Biotechnology) or miR-494 and PTEN plasmid
(5′-ACTCTTGCCTGGTTGCAAGTGTCAA-3′ and
5′-TCTGAATTTTTTTTTATCAAGAGGGGATAAA-3′) were transfected into cells
using Lipofectamine 2000. After 4 h of transfection, fresh
RPMI-1640 medium was added to cells.
Luciferase reporter assay
The PTEN 3′-UTR containing the microRNA-494 target
site was cloned into the pGL3 luciferase reporter vector (Sangon
Biotech Co., Ltd. Shanghai, China). The PTEN 3′-UTR containing the
microRNA-494 plasmid was transfected into cells using Lipofectamine
2000. A Dual-Luciferase Reporter assay system (Promega Corporation,
Madison, WI, USA) was used to measure luciferase activity levels at
48 h after transfection through comparison with Renilla
luciferase activity using a Renilla luciferase activity kit
(Promega Corporation).
MTT assay
Cell proliferation was analyzed at 24, 48 and 72 h
after transfection by MTT assay. A total of 20 µl MTT was added to
cells, followed by incubation for 4 h at 37°C. Medium was removed
and 150 µl dimethyl sulfoxide (DMSO) was added to cells, followed
by agitation for 20 min at 37°C. Optical absorbance was read by
EnSpire Multimode Plate Reader (PerkinElmer, Inc., Waltham, MA,
USA) at 490 nm.
Transwell assay for cell invasion
Cells (1×105) were seeded into upper
chambers (8-µm pore size; Merck KGaA, Darmstadt, Germany) in
RPMI-1640 medium, and RPMI-1640 medium supplemented with 10% fetal
bovine serum was plated into the lower chambers. Meanwhile, cells
were placed into the upper chambers and pre-coated with Matrigel
(BD Biosciences, Franklin Lakes, NJ, USA). Following incubation for
48 h, the chambers were fixed with 4% paraformaldehyde at room
temperature for 30 min and then stained with 0.1% crystal violet
(BD Biosciences) at room temperature for 15 min. Cell was observed
using a Zeiss Axioplan 2 confocal microscope (magnification, ×100;
Carl Zeiss MicroImaging).
Flow cytometric analysis and
caspase-3/-9 activity
Cell proliferation was analyzed at 48 h after
transfection, and cells were resuspended in PBS and stained with
Annexin V-fluorescein isothiocyanate/propidium iodide (5 µl; BD
Biosciences) for 15 min at room temperature. The stained cells were
quantified using a flow cytometer (BD Influx™ Instrument; BD
Biosciences) and analyzed using FlowJo 7.6.1 software (FlowJo LLC,
Ashland, OR, USA) Protein was extracted from cells using
radioimmunoprecipitation assay (RIPA) buffer and the liquid was
placed in an ice bath for 30 min. Total protein concentration was
determined using a bicinchoninic acid (BCA) protein assay kit.
Proteins were used to measure caspase-3/-9 activity, and optical
absorbance was read by an EnSpire Multimode Plate Reader
(PerkinElmer, Inc.) at 405 nm.
Western blot analysis. Protein was extracted from
cells using RIPA buffer and the liquid was placed in an ice bath
for 30 min. Total protein concentration was determined using BCA
protein assay kit. Proteins were resolved through 8–10% SDS-PAGE,
prior to transfer onto PVDF membranes (EMD Millipore, Billerica,
MA, USA). The membranes were blocked with 5% dry skimmed milk for 1
h at 37°C, and probed with Bax (cat. no. ab32503; 1:2,000; Abcam,
Cambridge, MA, USA), LC3 (cat. no. ab48394; 1:2,000; Abcam), PTEN
(cat. no. ab228466, 1:2,000; Abcam), p-Akt (cat. no. 4060; 1:2,000;
Cell Signaling Technology, Inc.), p-mTOR (cat. no. 5536; 1:2,000;
Cell Signaling Technology, Inc.) and GAPDH (cat. no. ab8245,
1:5,000; Abcam) primary antibodies at 4°C for 6–8 h. Membranes were
washed with TBST, incubated with horseradish peroxidase-conjugated
goat anti-rabbit IgG antibodies (cat. no. 7074; 1:5,000; Cell
Signaling Technology, Inc.) at room temperature for 2 h, visualized
using chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.)
and analyzed using Image_Lab_3.0 software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
The results are expressed as the mean ± standard
error of the mean. Data were analyzed using Student's t-test or
one-way analysis of variance, followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
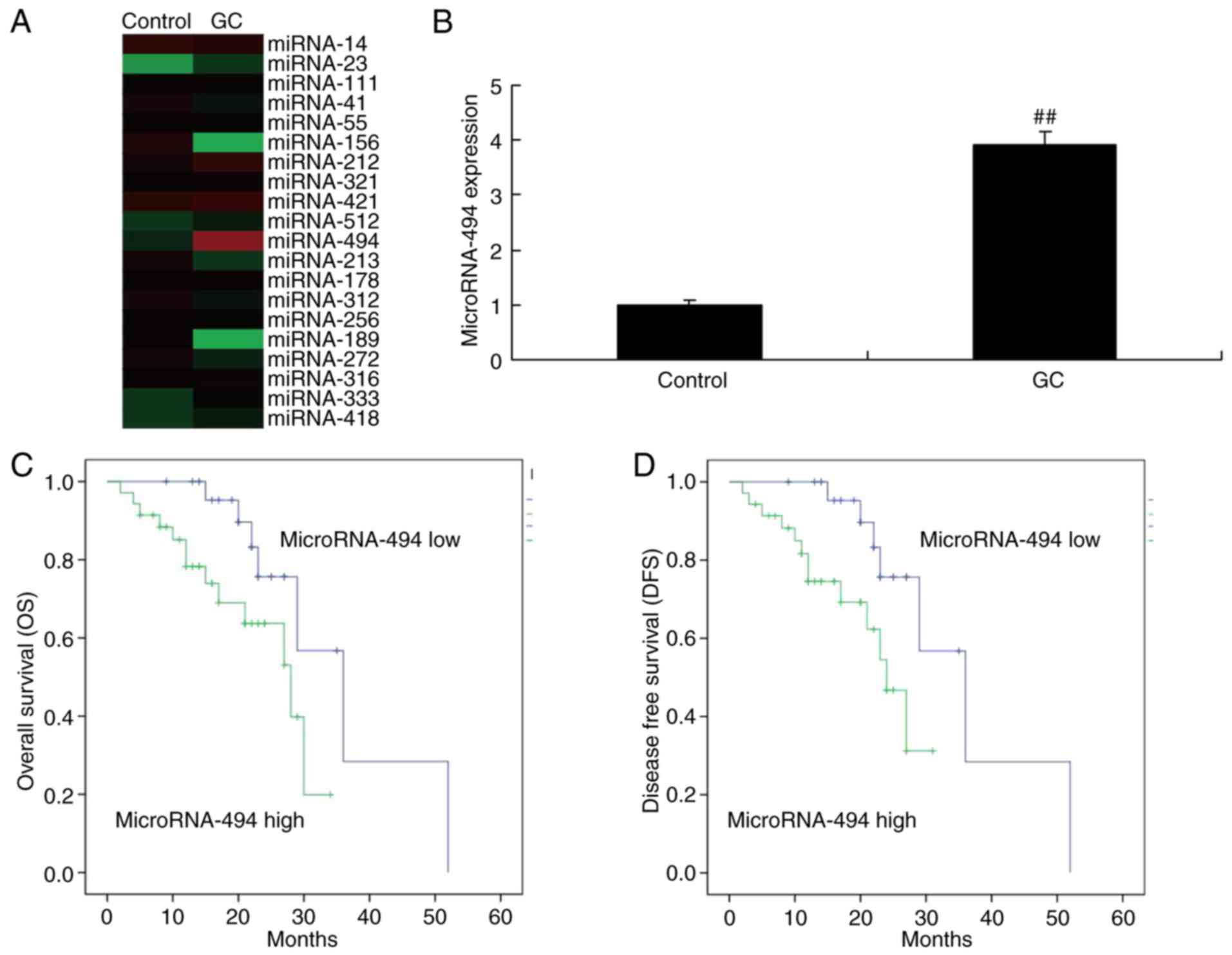
The expression of miR-494
To determine whether miR-494 from the serum of
glioma samples, the miR-494 expression was examined. As
demonstrated in Fig. 1A and B,
miR-494 expression was upregulated in the serum of patients with
glioma, compared with the normal group. Subsequently, the
association between miR-494 expression and survival rate was
analyzed in patients with glioma. As a result, the overall survival
(OS) and disease-free survival (DFS) rates in patients with glioma
with a lower expression of miR-494 were higher than those in
patients with a higher expression of miR-494 (Fig. 1C and D).
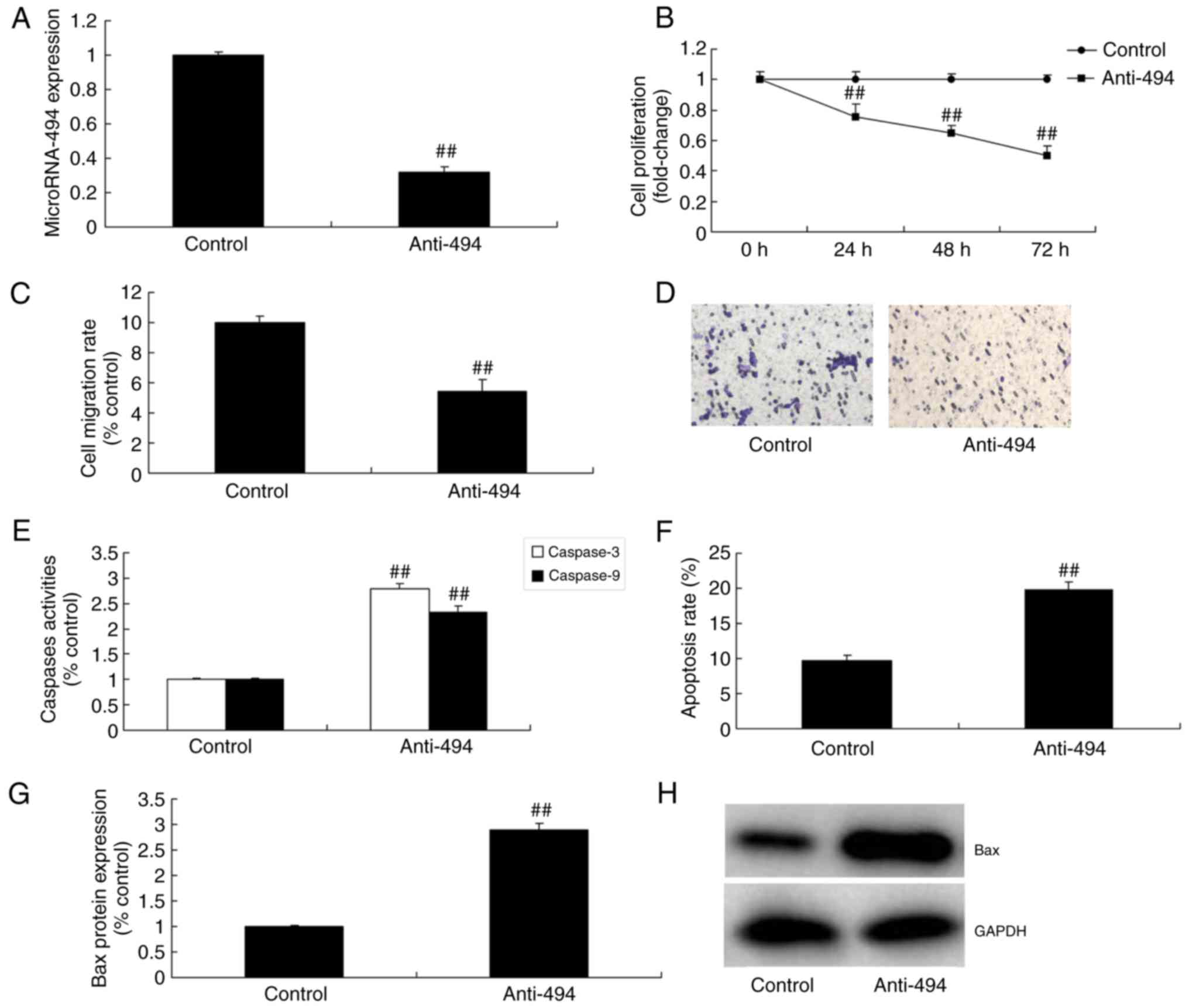
Anti-miR-494 inhibits the growth and
migration of glioma cells
Furthermore, the growth and migration of glioma
cells was assessed following downregulation of miR-494. As
demonstrated in Fig. 2A-D,
anti-miR-494 mimics decreased miR-494 expression, and inhibited the
growth and migration of glioma cells, compared with the control
group. Furthermore, caspase-3 and caspase-9 activity levels,
apoptosis rate and Bax protein expression were induced by
anti-miR-494 in glioma, compared with the control group (Fig. 2E-H).
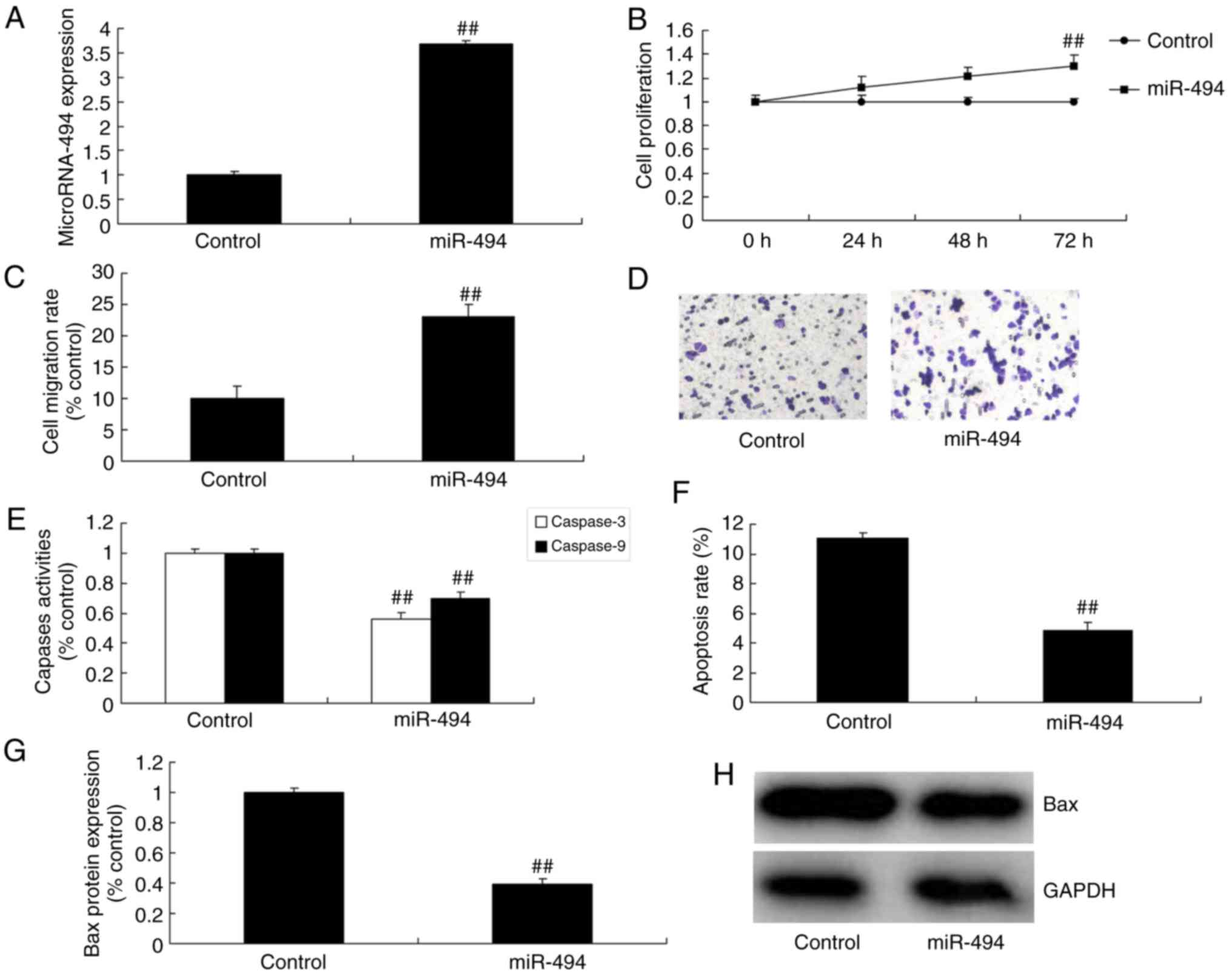
miR-494 promotes the growth and
migration of glioma cells
The expression of miR-494 was upregulated by miR-494
mimics, followed by analysis of the function of miR-494 on the
growth and migration of glioma cells. miR-494 expression of glioma
in miR-494 group was increased, compared with the control group
(Fig. 3A). Growth and migration of
glioma cells were promoted by overexpression of miR-494, compared
with the control group (Fig. 3B-D).
However, caspase-3 and caspase-9 activity levels, apoptosis rate
and Bax protein expression in glioma were suppressed by miR-494
upregulation, compared with the control group (Fig. 3E-H).
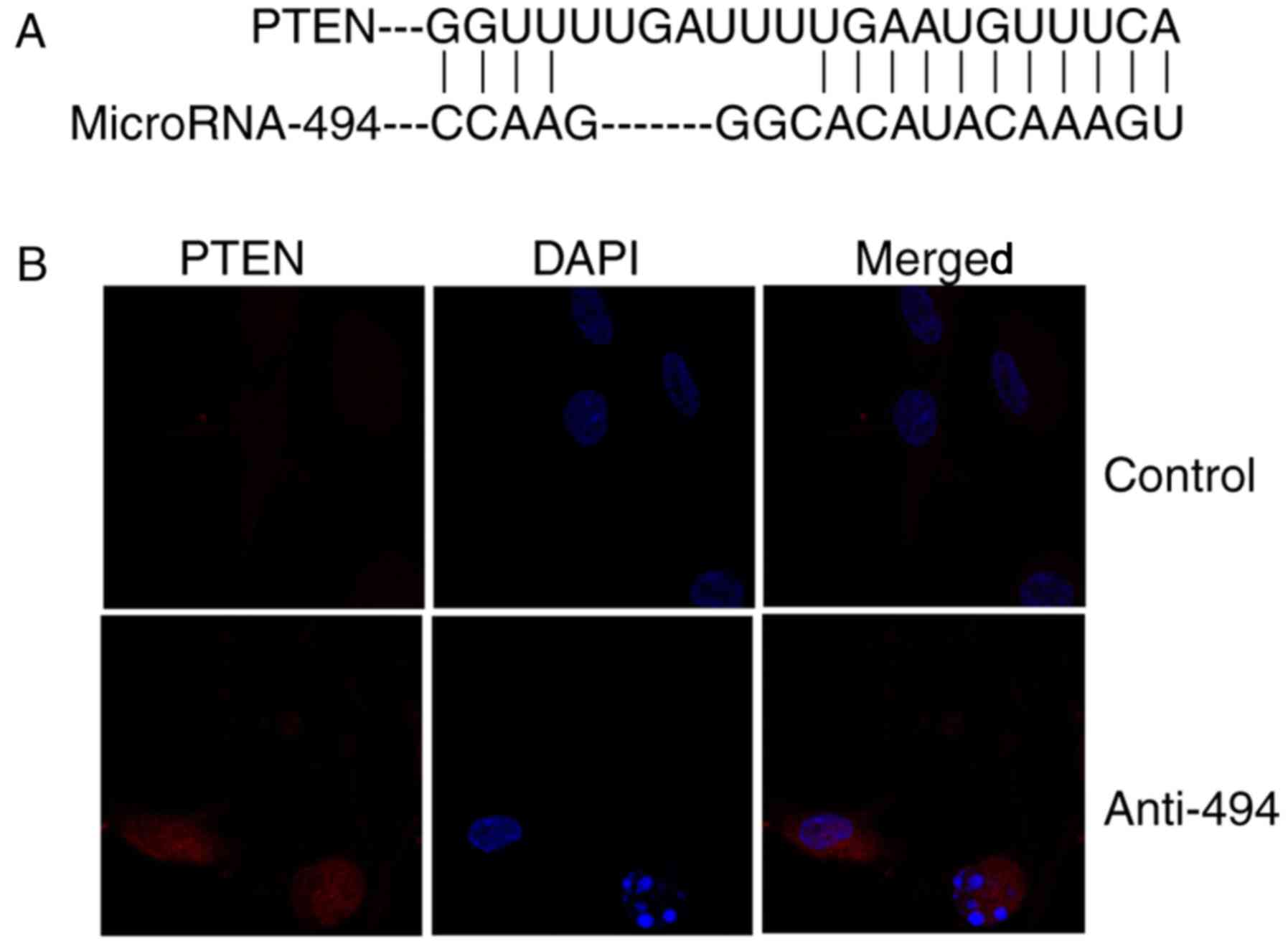
The effects of miR-494 regulates the
PTEN/Akt/mTOR pathway in glioma
As demonstrated in Fig.
4A, PTEN is a direct target of miR-494. Immunofluorescence
revealed that anti-miR-494 induced the protein expression of PTEN
in glioma, compared with the control group (Fig. 4B). Next, western blot analysis was
used to measure changes in the PTEN/Akt/mTOR pathway in glioma
using anti-miR-494 or miR-494 mimics. As a result, PTEN protein
expression was induced, and p-Akt and p-mTOR protein expression
were suppressed by anti-miR-494 in glioma, compared with the
control group (Fig. 5A-D).
Overexpression of miR-494 suppressed PTEN protein expression, and
induced p-Akt and p-mTOR protein expression in glioma, compared
with the control group (Fig.
5E-H).
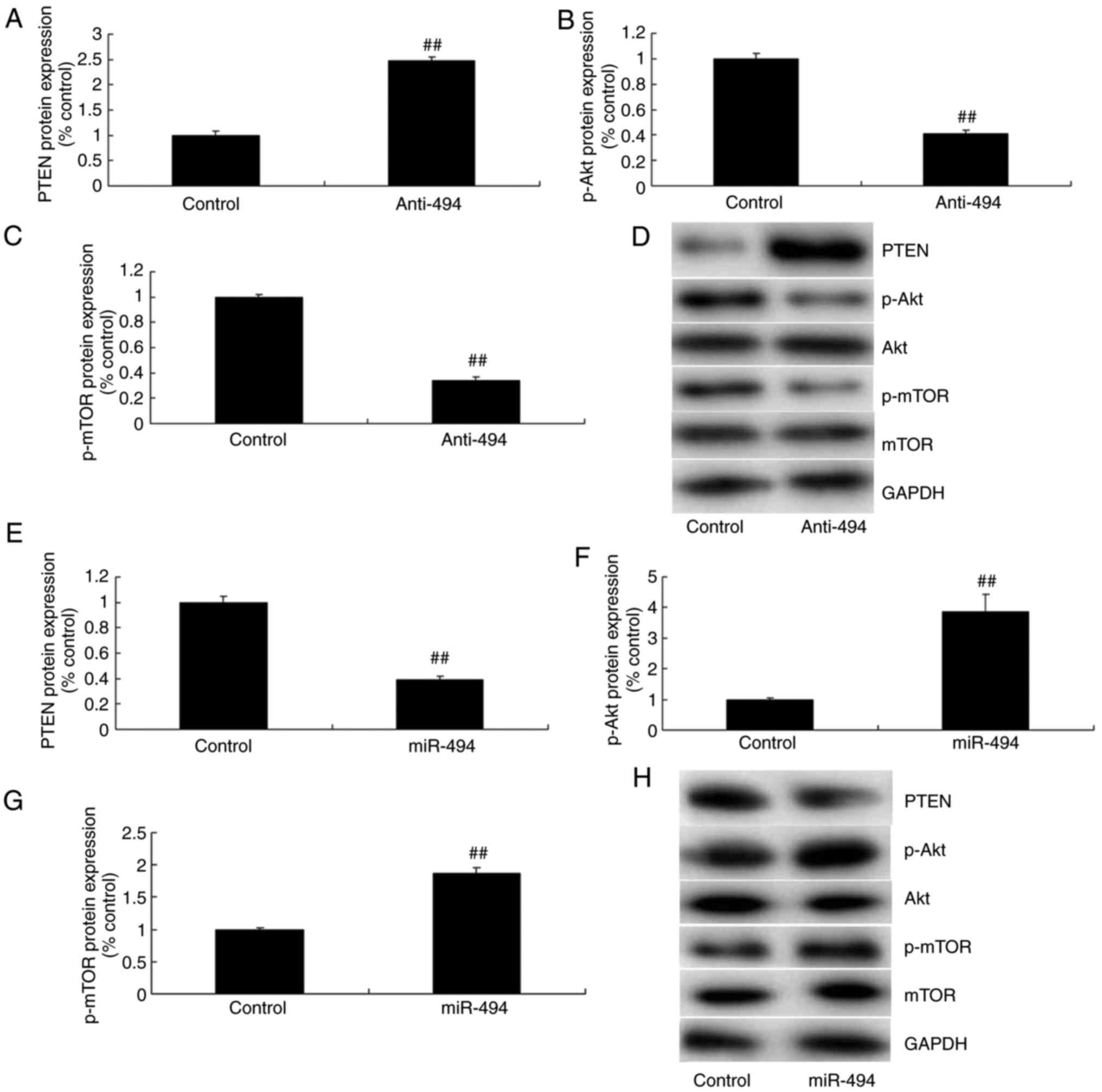 | Figure 5.Effects of the microRNA-494-regulated
PTEN/Akt/mTOR pathway in glioma. (A) PTEN, (B) p-Akt and (C) p-mTOR
protein expression by statistical analysis, and (D) western blot
analysis following downregulation of miRNA-494. (E) PTEN, (F) p-Akt
and (G) p-mTOR protein expression by statistical analysis, and (H)
western blot analysis following overexpression of miRNA-494.
Control, negative control group (n=3); anti-miRNA-494,
downregulation of miRNA-494 (n=3); miRNA-494, overexpression of
miRNA-494 (n=3). ##P<0.01, compared with the negative
control group. PTEN, phosphatase and tensin homolog; Akt, protein
kinase B; mTOR, mechanistic target of rapamycin; p-,
phosphorylated; miR, microRNA. |
The inhibition of PTEN reduces the
function of miR-494 in glioma cell growth through the Akt/mTOR
pathway
To determine the role of PTEN in the function of the
miR-494/Akt/mTOR pathway in glioma, si-PTEN was used to suppress
PTEN protein expression in glioma following anti-miR-494
transfection, compared with the anti-miR-494 alone group (Fig. 6A and B). Consequently, the protein
expression of p-Akt and p-mTOR in glioma was induced by the
inhibition of PTEN, compared with anti-miR-494 (Fig. 6A, C and D). The function of miR-494
in the inhibition of cell growth and migration in glioma was
reversed following PTEN interference, compared with anti-miR-494
alone (Fig. 7A-C). The inhibition
of PTEN reduced the effect of miR-494 on glioma cell apoptosis and
Bax protein expression, compared with anti-miR-494 (Fig. 7D-G).
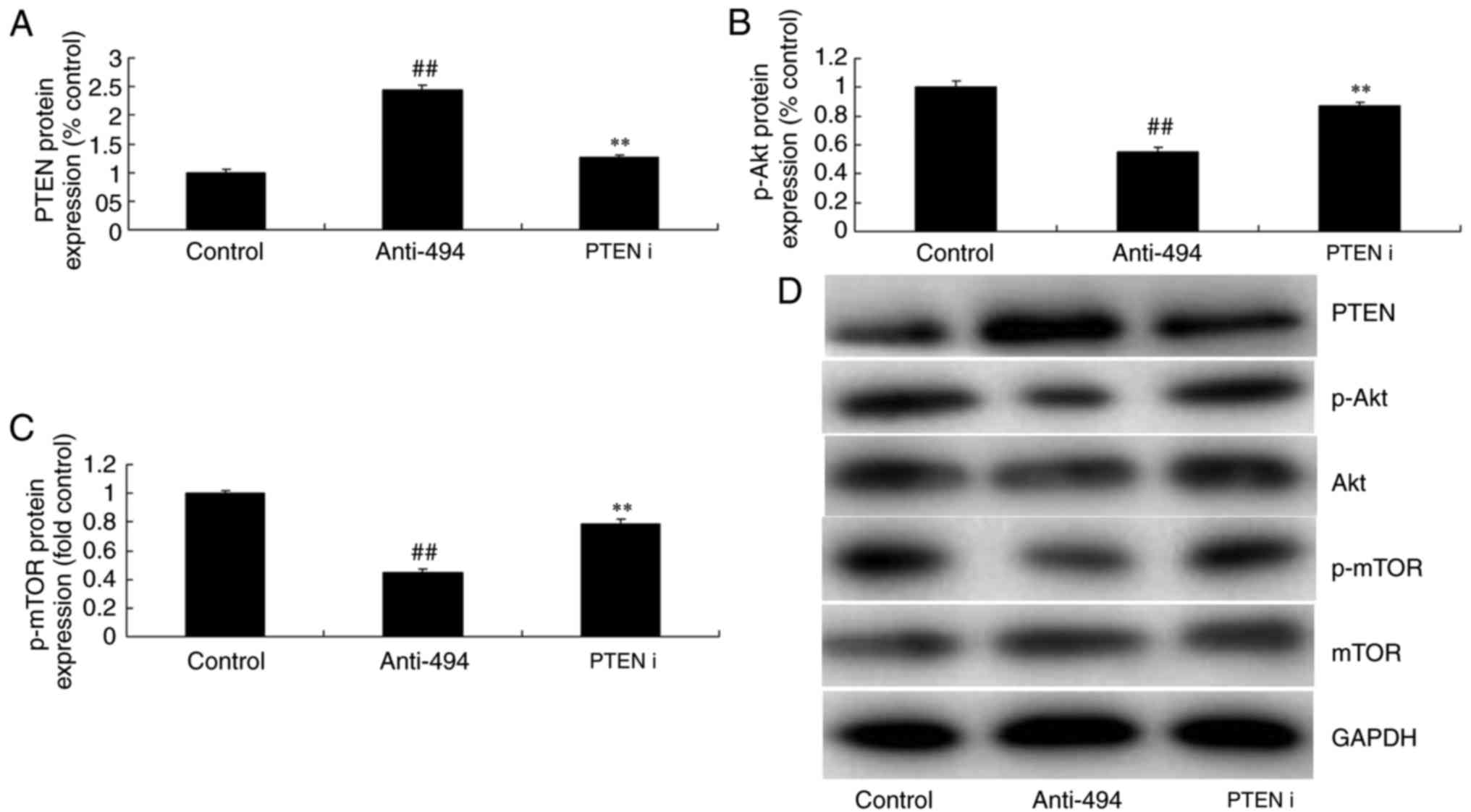 | Figure 6.Inhibition of PTEN reduces the effect
of microRNA-494 on the Akt/mTOR pathway. (A) PTEN, (B) p-Akt and
(C) p-mTOR protein expression by statistical analysis, and (D)
western blot analysis. Control, negative control group (n=3);
anti-miRNA-494, downregulation of microRNA-494 (n=3); PTEN i,
si-PTEN and downregulation of microRNA-494 (n=3).
##P<0.01, compared with the negative control group;
**P<0.01, compared with the downregulation of microRNA-494
group. PTEN, phosphatase and tensin homolog; Akt, protein kinase B;
mTOR, mechanistic target of rapamycin; p-, phosphorylated; miR,
microRNA; I, interference. |
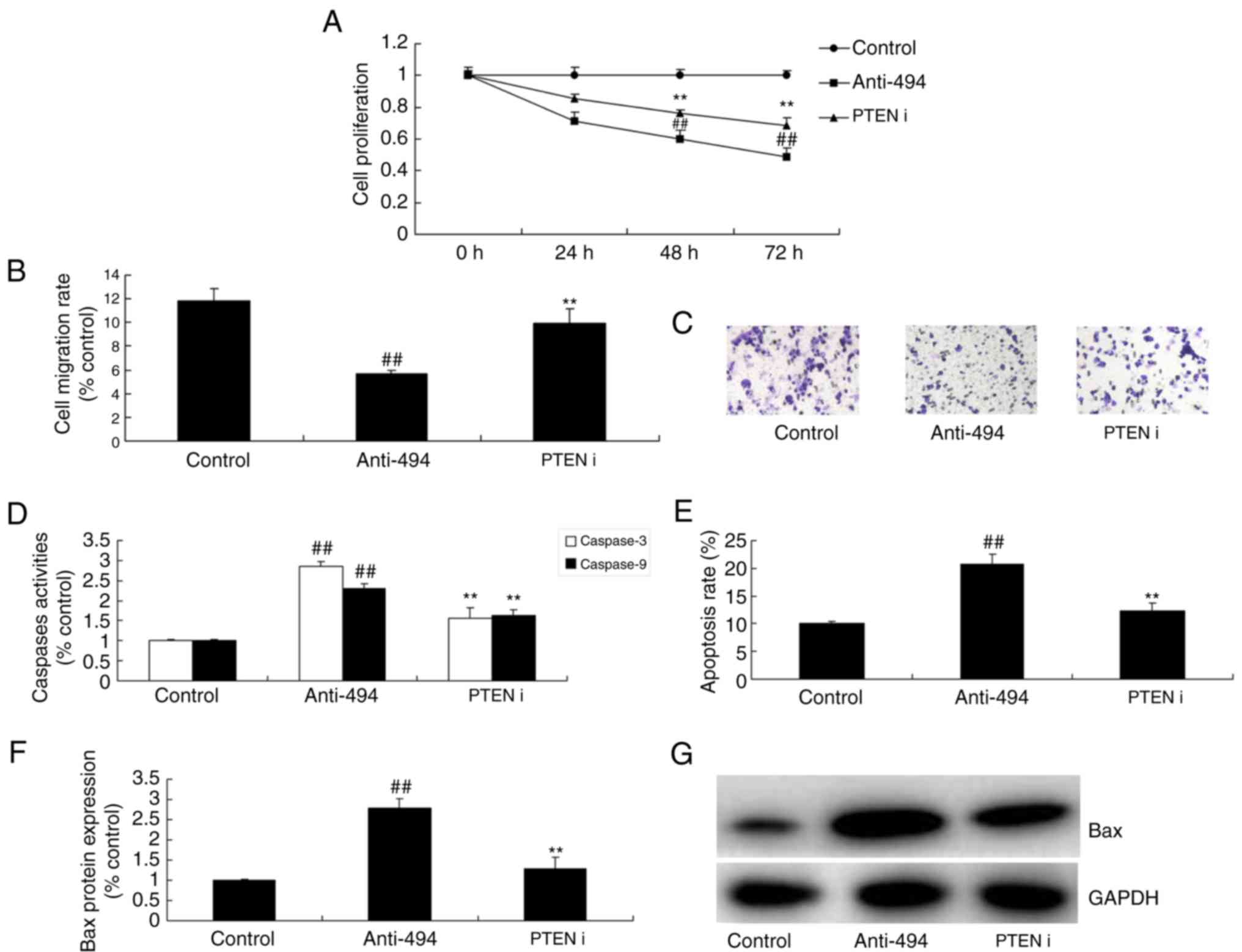 | Figure 7.Inhibition of PTEN reduces the effect
of microRNA-494 on glioma cancer cell growth through the Akt/mTOR
pathway. (A) Cell proliferation, (B) migration rate and (C)
migration rate by statistical analysis, (D) Caspase-3/-9 activity,
(E) apoptosis rate and Bax protein expression by (F) statistical
analysis and (G) western blot analysis in glioma. Control, negative
control group (n=3); anti-miRNA-494, downregulation of microRNA-494
(n=3); PTEN i, si-PTEN and downregulation of microRNA-494 (n=3).
##P<0.01, compared with the negative control group;
**P<0.01, compared with the downregulation of microRNA-494
group. PTEN, phosphatase and tensin homolog; Akt, protein kinase B;
mTOR, mechanistic target of rapamycin; i, interference. |
The promotion of PTEN promotes the
function of miR-494 in glioma cancer cell proliferation through the
Akt/mTOR pathway
Finally, to further determine the role of PTEN in
the anticancer effect of miR-494 on glioma, PTEN plasmid induced
the protein expression in glioma following anti-miR-494, compared
with anti-microRNA-494 alone (Fig. 8A
and B). The promotion of PTEN suppressed the protein expression
of p-Akt and p-mTOR in glioma following anti-miR-494 transfection,
compared with anti-miR-494 alone (Fig.
8A, C and D). The promotion of PTEN reduced cell growth and
migration, and induced apoptosis and Bax protein expression in
glioma following anti-miR-494, compared with anti-miR-494 alone
(Fig. 9).
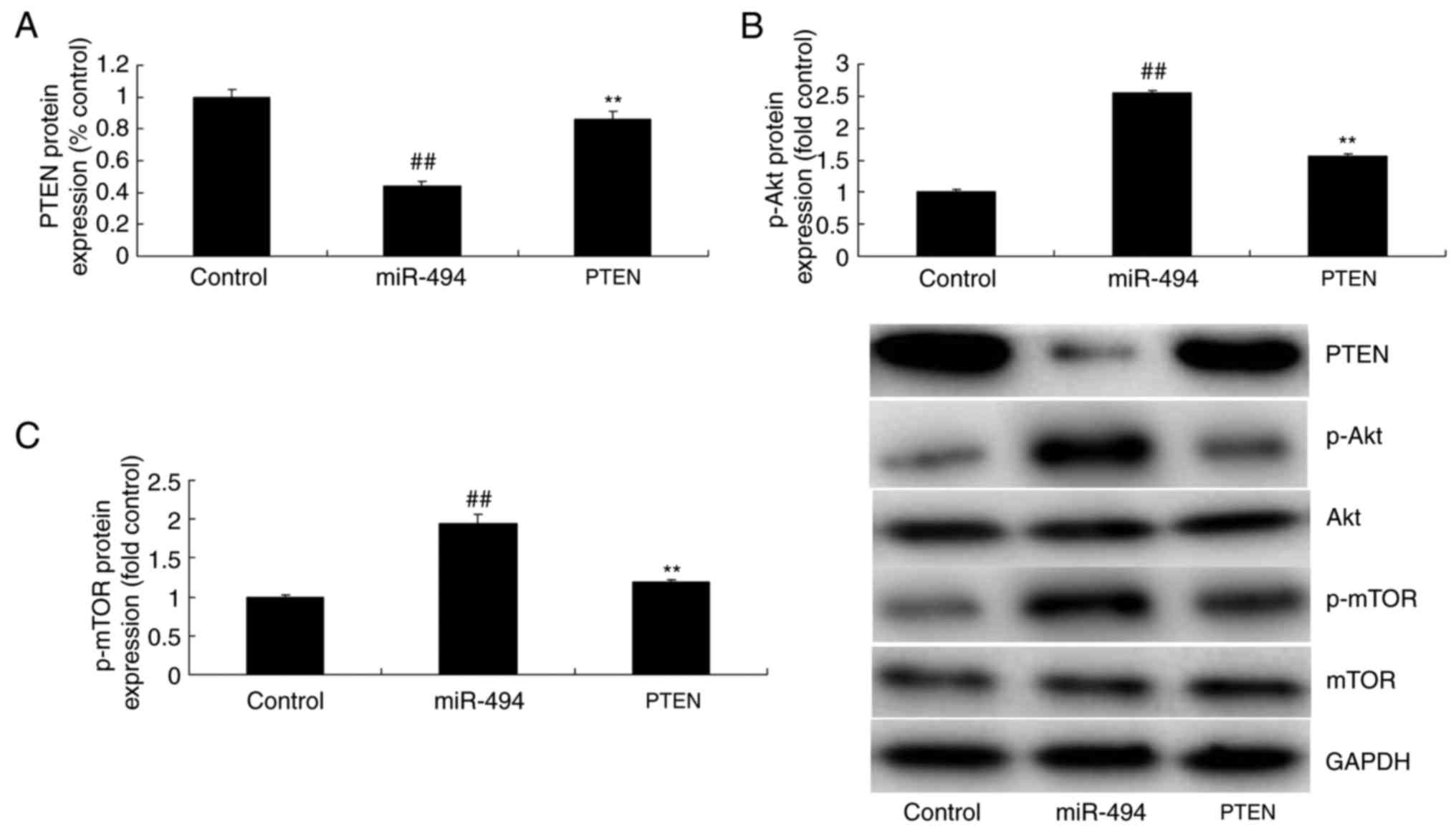 | Figure 8.Promotion of PTEN promotes the effect
of microRNA-494 on the Akt/mTOR pathway. (A) PTEN, (B) p-Akt and
(C) p-mTOR protein expression by statistical analysis, and (D)
western blot analysis. Control, negative control group (n=3);
miR-494, overexpression of miR-494 (n=3); miR-494 + PTEN, PTEN
plasmid overexpression of miR-494 (n=3). ##P<0.01,
compared with the negative control group, **P<0.01, compared
with the overexpression of microRNA-494 group. PTEN, phosphatase
and tensin homolog; Akt, protein kinase B; mTOR, mechanistic target
of rapamycin; p-, phosphorylated; i, interference; miR,
microRNA. |
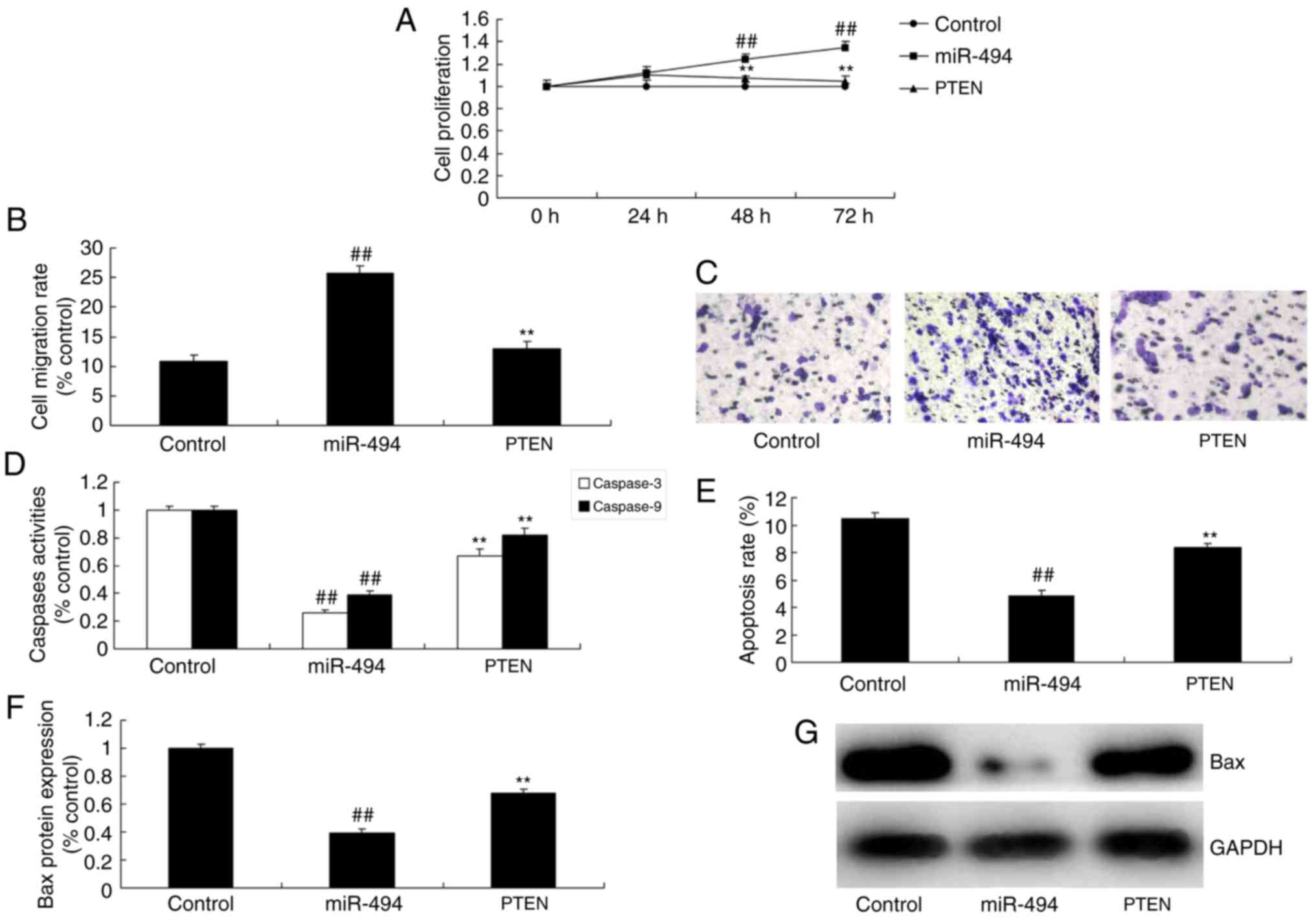 | Figure 9.Promotion of PTEN promotes the effect
of microRNA-494 on glioma cancer cell growth through the Akt/mTOR
pathway. (A) Cell proliferation, (B) migration rate and (C)
migration rate by statistical analysis, (D) Caspase-3/-9 activity,
(E) apoptosis rate, and Bax protein expression by (F) statistical
analysis and (G) Bax protein expression of glioma. Control, control
negative group (n=3); miRNA-494, overexpression of miR-494 (n=3);
miR-494 + PTEN, PTEN plasmid overexpression of miR-494 (n=3).
##P<0.01, compared with the negative control group;
**P<0.01, compared with overexpression of miR-494 group. PTEN,
phosphatase and tensin homolog; Akt, protein kinase B; mTOR,
mechanistic target of rapamycin; miR, microRNA. |
Discussion
Glioma is an intracranial tumor with a high
morbidity rate and it cannot be cured (4). WHO has classified it as the most
severe astrocytoma with the highest degree of invasion (20). Previous studies have demonstrated
that miRNA expression profiles in glioma and multiple malignant
tumor tissues are different from those in normal tissues (4,21).
These differentially expressed microRNAs exert irreplaceable
functions in the genesis and development of tumors through
regulating the expression of oncogenes and tumor suppressor genes
(20). Therefore, studying microRNA
is required in order to elucidate the pathogenesis, diagnosis and
treatment of cancer (22).
Furthermore, the present study demonstrated that microRNA-494
expression is upregulated in the serum of patients with glioma,
compared with the normal group. Taken together, the results of the
present study suggested that miR494 acts as an oncogene in glioma.
Zhang et al (16) reported
that microRNA-494 promotes colorectal, epithelial ovarian (17) and pancreatic cancer (18). These results are in line with those
of the present study and demonstrated that miR-494 participates in
the development and progression of glioma.
In the pathogenesis of autophagy, the most dominant
morphological feature of autophagy is the presence of a large
amount of foam-like structures in cells (13). They are the double membrane
phagocytic vacuoles that contain cytoplasm and organelles. The
genesis of autophagy can be classified into the following 4 steps
based on this (23): Autophagy
induction, formation of autophagy vacuoles, fusion of autophagy
vacuoles with lysosome, and degradation and recycling of materials.
There are numerous cellular transduction signals regulating
autophagy. Of them, PI3K and mTOR are relatively well known
(24). PI3K is of crucial
importance in the formation of early phagocytic vacuoles. For
instance, PIK3C3-BECN1 in mammals serves a key role in regulating
phosphatidylinositol in autophagy. In addition, it serves an
important role in the formation of double molecular membrane
structure of autophagy (25). The
results of the present study demonstrated that anti-microRNA-494
induced PTEN expression and suppressed the Akt/mTOR pathway in
glioma. Su et al (25)
demonstrated that miR-494 upregulates the PI3K/Akt pathway through
targeting PTEN in ischemia/reperfusion injury (26). Li et al (26) demonstrated that miR-494-3p regulates
cellular proliferation and apoptosis by PTEN/Akt signaling in human
glioblastoma cells (27). These
results demonstrated that miR-494 participated in the growth and
apoptosis of human glioblastoma cells, and it was established that
PTEN is a direct target of miR-494 and that miR-494 regulates the
Akt/mTOR pathway through PTEN expression.
The PTEN gene can promote autophagy. As a type of
lipid phosphatase, PTEN can transform its substrate
3,4,5-phosphatidylinostiol triphosphate into 4,5-diphosphoinositide
(28). Therefore, it serves a
negative regulatory role in the PI3K-Akt signal transduction
pathway. This pathway involves multiple downstream events,
including apoptosis inhibition and stimulation of protein synthesis
through the sirolimus target protein (mTOR) pathway (11). In the present study, the inhibition
of PTEN reduced the microRNA-494-promoted apoptosis of glioma
cells. Zhu et al (28)
demonstrated that microRNA-494 inhibits apoptosis by modulating the
PTEN/Akt/mTOR pathway in rats following spinal cord injury
(29). However, only si-PTEN was
used, and PTEN affected the function of miR-494, which demonstrated
that PTEN only participated in the function of miR-494 in glioma.
Further experimental methods are required in order to verify these
results.
The present study demonstrated that microRNA-494 was
involved in the growth and migration of glioma cells, and promoting
the apoptosis of U251 cells. These results suggested that a high
microRNA-494 expression level is a potential risk factor for
glioma, and that the therapeutic potential of anti-miRNA-494
promoted the apoptosis of glioma cells through the Akt/mTOR pathway
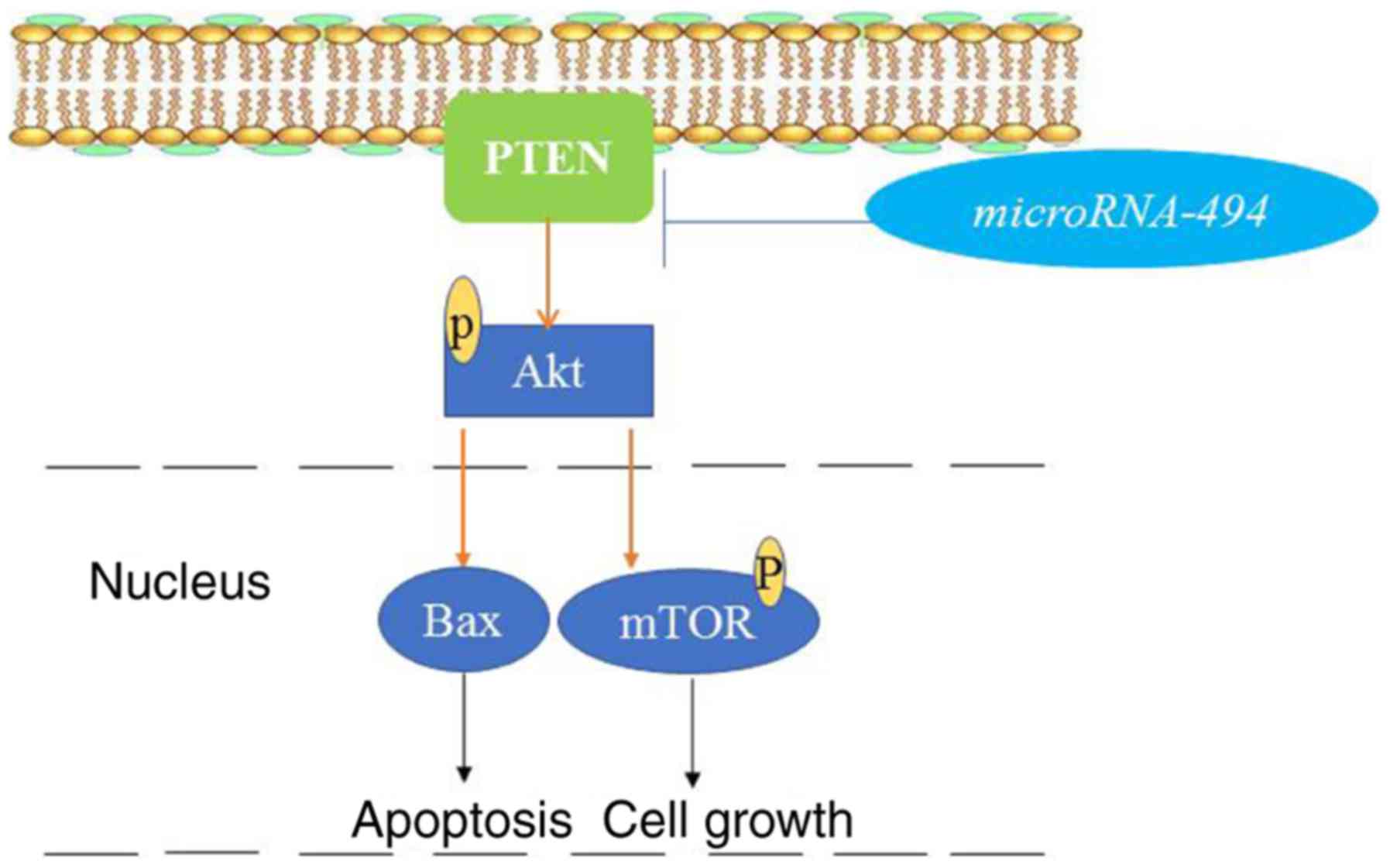
via PTEN expression (Fig. 10).
Therefore, miRNA-494 may be considered as a potential therapeutic
target for the treatment of glioma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
study are available from the corresponding author on reasonable
request.
Authors' contributions
PS designed the experiment, analyzed the data and
wrote the manuscript; KH and ZJL performed the experiment. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First Affiliated Hospital of Qiingdao University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Buckner JC, Shaw EG, Pugh SL, Chakravarti
A, Gilbert MR, Barger GR, Coons S, Ricci P, Bullard D, Brown PD, et
al: Radiation plus procarbazine, CCNU, and vincristine in low-grade
glioma. N Engl J Med. 374:1344–1355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crane CA, Han SJ, Ahn B, Oehlke J, Kivett
V, Fedoroff A, Butowski N, Chang SM, Clarke J, Berger MS, et al:
Individual patient-specific immunity against high-grade glioma
after vaccination with autologous tumor derived peptides bound to
the 96 KD chaperone protein. Clin Cancer Res. 19:205–214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pan E, Yu D, Yue B, Potthast L, Chowdhary
S, Smith P and Chamberlain M: A prospective phase II
single-institution trial of sunitinib for recurrent malignant
glioma. J Neurooncol. 110:111–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li HL, Cui XL, Zhang JN and Lin S:
Chemotherapy alleviates subacute recurrent glioma-associated
refractory cerebral edema by downregulating vascular endothelial
growth factor. Med Oncol. 31:132014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo X, Zheng X and Huang H: Protective
effects of dexmedetomidine on brain function of glioma patients
undergoing craniotomy resection and its underlying mechanism. Clin
Neurol Neurosurg. 146:105–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li P, Wang X, Shan Q, Wu Y and Wang Z:
MicroRNA-130b promotes cell migration and invasion by inhibiting
peroxisome proliferator-activated receptor-gamma in human glioma.
Oncol Lett. 13:2615–2622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Chen J and Zhang J:
AdipoR1-mediated miR-3908 inhibits glioblastoma tumorigenicity
through downregulation of STAT2 associated with the AMPK/SIRT1
pathway. Oncol Rep. 37:3387–3396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pal R and Greene S: microRNA-10b is
overexpressed and critical for cell survival and proliferation in
medulloblastoma. PLoS One. 10:e01378452015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mazurowski MA, Clark K, Czarnek NM,
Shamsesfandabadi P, Peters KB and Saha A: Radiogenomics of
lower-grade glioma: Algorithmically-assessed tumor shape is
associated with tumor genomic subtypes and patient outcomes in a
multi-institutional study with The Cancer Genome Atlas data. J
Neurooncol. 133:27–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang MH, Lin CL, Zhang JJ, Weng ZP, Hu T,
Xie Q and Zhong XY: Role of PTEN in cholera toxin-induced SWO38
glioma cell differentiation. Mol Med Rep. 7:1912–1918. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elhag R, Mazzio EA and Soliman KF: The
effect of silibinin in enhancing toxicity of temozolomide and
etoposide in p53 and PTEN-mutated resistant glioma cell lines.
Anticancer Res. 35:1263–1269. 2015.PubMed/NCBI
|
|
12
|
Lester A, Rapkins R, Nixdorf S, Khasraw M
and McDonald K: Combining PARP inhibitors with radiation therapy
for the treatment of glioblastoma: Is PTEN predictive of response?
Clin Transl Oncol. 19:273–278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koul D, Wang S, Wu S, Saito N, Zheng S,
Gao F, Kaul I, Setoguchi M, Nakayama K, Koyama K, et al:
Preclinical therapeutic efficacy of a novel blood-brain
barrier-penetrant dual PI3K/mTOR inhibitor with preferential
response in PI3K/PTEN mutant glioma. Oncotarget. 8:21741–21753.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koul D, Shen R, Kim YW, Kondo Y, Lu Y,
Bankson J, Ronen SM, Kirkpatrick DL, Powis G and Yung WK: Cellular
and in vivo activity of a novel PI3K inhibitor, PX-866, against
human glioblastoma. Neuro Oncol. 12:559–569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paul-Samojedny M, Pudelko A, Kowalczyk M,
Fila-Daniłow A, Suchanek-Raif R, Borkowska P and Kowalski J:
Combination therapy with AKT3 and PI3KCA siRNA enhances the
antitumor effect of temozolomide and carmustine in T98G
glioblastoma multiforme cells. BioDrugs. 30:129–144. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Guo L, Li Y, Feng GH, Teng F, Li
W and Zhou Q: MicroRNA-494 promotes cancer progression and targets
adenomatous polyposis coli in colorectal cancer. Mol Cancer.
17:12018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang A, Wang X, Yu C, Jin Z, Wei L, Cao J,
Wang Q, Zhang M, Zhang L, Zhang L, et al: microRNA-494 is a
potential prognostic marker and inhibits cellular proliferation,
migration and invasion by targeting SIRT1 in epithelial ovarian
cancer. Oncol Lett. 14:3177–3184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma YB, Li GX, Hu JX, Liu X and Shi BM:
Correlation of miR-494 expression with tumor progression and
patient survival in pancreatic cancer. Genet Mol Res.
14:18153–18159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ducassou A, Uro-Coste E, Verrelle P,
Filleron T, Benouaich-Amiel A, Lubrano V, Sol JC, Delisle MB, Favre
G, Ken S, et al: Alphavbeta3 integrin and fibroblast growth factor
receptor 1 (FGFR1): Prognostic factors in a phase I–II clinical
trial associating continuous administration of Tipifarnib with
radiotherapy for patients with newly diagnosed glioblastoma. Eur J
Cancer. 49:2161–2169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bartels U, Wolff J, Gore L, Dunkel I,
Gilheeney S, Allen J, Goldman S, Yalon M, Packer RJ, Korones DN, et
al: Phase 2 study of safety and efficacy of nimotuzumab in
pediatric patients with progressive diffuse intrinsic pontine
glioma. Neuro Oncol. 16:1554–1559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karremann M, Hoffmann M, Benesch M,
Kwiecien R, von Bueren AO and Kramm CM: Secondary solid
malignancies after high-grade glioma treatment in pediatric
patients. Pediatr Hematol Oncol. 32:467–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang C, Liu S, Yuan X, Hu Z, Li H, Wu M,
Yuan J, Zhao Z, Su J, Wang X, et al: Valproic acid promotes human
glioma U87 cells apoptosis and inhibits glycogen synthase
kinase-3beta through ERK/Akt signaling. Cell Physiol Biochem.
39:2173–2185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Y, Dong L, Bao S, Wang M, Yun Y and Zhu
R: FK228 augmented temozolomide sensitivity in human glioma cells
by blocking PI3K/AKT/mTOR signal pathways. Biomed Pharmacother.
84:462–469. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin CJ, Chen TL, Tseng YY, Wu GJ, Hsieh
MH, Lin YW and Chen RM: Honokiol induces autophagic cell death in
malignant glioma through reactive oxygen species-mediated
regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol Appl
Pharmacol. 304:59–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su S, Luo, Liu X, Liu J, Peng F, Fang C
and Li B: miR-494 up-regulates the PI3K/Akt pathway via targetting
PTEN and attenuates hepatic ischemia/reperfusion injury in a rat
model. Biosci Rep. 37:2017. View Article : Google Scholar
|
|
27
|
Li XT, Wang HZ, Wu ZW, Yang TQ, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Huang YL, et al: miR-494-3p
regulates cellular proliferation, invasion, migration, and
apoptosis by PTEN/AKT signaling in human glioblastoma cells. Cell
Mol Neurobiol. 35:679–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen JH, Zhang P, Chen WD, Li DD, Wu XQ,
Deng R, Jiao L, Li X, Ji J, Feng GK, et al: ATM-mediated PTEN
phosphorylation promotes PTEN nuclear translocation and autophagy
in response to DNA-damaging agents in cancer cells. Autophagy.
11:239–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu H, Xie R, Liu X, Shou J, Gu W, Gu S
and Che X: MicroRNA-494 improves functional recovery and inhibits
apoptosis by modulating PTEN/AKT/mTOR pathway in rats after spinal
cord injury. Biomed Pharmacother. 92:879–887. 2017. View Article : Google Scholar : PubMed/NCBI
|