Introduction
Lung cancer remains the leading cause of
cancer-associated mortality worldwide, and adenocarcinoma is the
most common histological type, accounting for almost half of these
mortalities (1). Environmental and
genetic factors favoring lung cancer initiation and progression
through clinical, pathological and molecular mechanisms interact in
a complex and poorly understood fashion (2,3). Owing
to tumor heterogeneity, molecularly targeted therapies have
effectively improved treatment for patients exhibiting mutated
epidermal growth factor receptor or translocated anaplastic
lymphoma kinase genes (4). However,
patients without identifiable target genes are treated with
conventional chemotherapy (5).
Therefore, it is necessary to investigate the underlying molecular
mechanisms of lung cancer to develop more efficient targeted
therapies.
Tumor necrosis factor α (TNF-α) is a pleiotropic
proinflammatory cytokine involved in the regulation of various
physiological and pathological signaling pathways, including
inflammation, differentiation, proliferation and apoptosis
induction (6,7). In the tumor microenvironment, TNF-α
accelerates multistep cancer progression by promoting
proliferation, migration and adhesion of cancer cells, and genesis
of neo-tumor vessels (8,9). However, TNF-α has also been
demonstrated to be an antitumor cytokine exhibiting cancer cell
toxicity in vitro and in vivo, by directly inducing
apoptosis and necrosis of cancer cells, increasing antitumor
immunity and impairing tumor blood vessel formation (7,10–12).
The dual and opposing functions of TNF-α in cancer progression and
regression are likely to occur in response to various routes and
dosages of administration of TNF-α, which may activate different
signal transduction pathways, a mechanism which requires further
elucidation (13–15).
Hypoxia-inducible factor 1α (HIF-1α) is an
oxygen-sensitive subunit of the heterodimeric transcription factor
HIF-1, associated with the constitutively expressed subunit HIF-1β
via targeting the hypoxia-responsive element, causing downstream
gene promoters to activate transcription in the nucleus (16,17).
It has been identified that HIF-1α, characterized by high
expression during hypoxia and rapid degradation in normoxia, is
regulated by the tumor suppressor von Hippel-Lindau protein
(18). HIF-1α functions as a tumor
promoter at the crossroads of inflammation and cancer, and is
widely activated in various types of human tumor tissue (19,20).
Evidence indicates that high nuclear expression levels of HIF-1α in
cancer tissues are directly correlated with decrease survival times
in lymph node-positive patients (21). In contrast, HIF-1α has been
demonstrated to regulate tumor metabolism as a tumor suppressor in
breast cancer cells, but not in cancer-associated fibroblasts
(22). Further studies revealed
that HIF-1α expression is significantly negatively correlated with
the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2), and
significantly positively correlated with the pro-apoptotic factor
Bcl-2-associated X protein in lung cancer (23). Currently, the function of HIF-1α
remains controversial and it has been reported to differ with
various tumor and stromal cell types (24).
The vasodilator-stimulated phosphoprotein
(VASP) gene is located on human chromosome 19q13.2–13.3
(25). As a member of the Ena/VASP
protein family, VASP is an actin-associated protein, which promotes
actin filament elongation by assembling actin monomers to the minus
end, and contributes to cytoskeleton rearrangement-based cellular
protrusion formation, cell adhesion and movement (26–28).
Galler et al (29)
identified that VASP regulates cytoskeletal stability as well as
focal adhesion in VASP-knockout mice. VASP has been
investigated with respect to its ability to alter the biology of
tumor cells regarding the processes of proliferation, migration and
invasion (30). Knockdown of VASP
has been revealed to inhibit the neoplastic transformation of
NIH3T3 fibroblasts in nude mice (31). Likewise, we demonstrated previously
that VASP is overexpressed in human primary breast cancer and is
involved in the antitumor activity of TNF-α, depending on HIF-1α,
in the luminal breast cancer cell line MCF-7 (32,33).
The aim of the present study was to understand the function of
TNF-α/HIF-1α/VASP signaling in vitro and in vivo, and
also to investigate a potential therapeutic mechanism for the
treatment of lung cancer.
Materials and methods
Plasmid constructs
Human VASP cDNA (pEGFP-C1-VASP) or
HIF1A cDNA (pEGFP-C1-HIF-1α) was cloned into the pEGFP-C1
vector (Clontech Laboratories, Inc., Mountainview, CA, USA). The
5′-flanking region of the putative VASP promoter was
amplified from human genomic DNA using the polymerase chain
reaction and cloned into the pGL3-Basic vector (Promega
Corporation, Madison, WI, USA) to construct the VASP
reporter plasmids. pGL3-2.1K reporter plasmids contained the
HIF-1α-binding site (HBS) which spanned the VASP promoter
from −1921 to +230 bp. pGL3-362 lacked the HBS which spanned the
sequence from −132 to +230 bp. All constructs were confirmed by
sequencing.
Cell culture and transfection
The human lung adenocarcinoma cell line A549 was
obtained from the China Center for Type Culture Collection, Wuhan
University (Wuhan, China). The human squamous lung carcinoma cell
line H226 was obtained from the Cell Bank of the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
Cells were cultured in RPMI-1640 medium (HyClone; GE Healthcare,
Logan, UT, USA) supplemented with 10% fetal bovine serum (HyClone;
GE Healthcare), 1% penicillin G and 1% streptomycin. The cells were
maintained at 37°C in a humidified atmosphere containing 5%
CO2. Transient transfection was performed using
TurboFect Transfection Reagent (Roche Diagnostics, Basel,
Switzerland).
Cell viability and adhesion assay
An MTT assay (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was used to determine cell viability. Cells were seeded in
96-well plates at a density of 1×105 cells/ml (100
µl/well). Cells in each experimental group were treated with TNF-α
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
concentrations of 0, 7.5, 30, 120 and 240 ng/ml, and treated with
the equivalent volume of nutrient medium in the control group.
Laminin (7,200 µg/ml; BD Biosciences, Franklin Lakes, NJ, USA) had
been previously coated onto 96-well plates for the determination of
the adhesive ability of cancer cells. The plates were incubated at
37°C for 2 h, and then washed with PBS to remove all non-adherent
cells. MTT was added to each well (20 µl; 5 mg/ml in PBS), and the
plates were incubated at 37°C for 4 h. A total of 200 µl dimethyl
sulfoxide was added to dissolve the formazan crystals that formed.
Spectrophotometric absorbance was measured at a wavelength of 570
nm using a microplate reader to determine the optical density (OD).
The viability of cells was calculated by comparison with the
vehicle control-treated cells, which were arbitrarily assigned a
viability of 100%. The percentage of adhesion was calculated
according to the following formula: Percentage of adhesion =
(OD570 of treated cells/OD570 of untreated
cells) × 100%. The experiments were performed in triplicate,
independently.
RNA interference
Short hairpin RNA (shRNA) duplexes were designed
against VASP (GenBank accession no. BC038224) with the
sequence
5′-TGCTGTAAAGCATCACAGTGGCCCGGGTTTTGGCCACTGACTGACCCGGGCCAGTGATGCTTTA-3′
and were inserted into the pcDNA™6.2-GW/EmGFP vector (Invitrogen;
Thermo Fisher Scientific, Inc.) to generate
pcDNA™6.2-GW/EmGFP-miR-VASP. HIF-1α short interfering RNA (siRNA)
(31) duplexes were designed
against HIF1A (GenBank accession no. NM_001530) with the
sequence 5′-CUGAUGACCAGCAACUUGAdTdT-3′, and synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China). A scrambled siRNA with the
sequence 5′-AGUUCAACGACCAGUAGUCdTdT-3′ and a scrambled shRNA
(Invitrogen; Thermo Fisher Scientific, Inc.) were used as negative
controls.
Luciferase assay
Cells were seeded in 24-well plates at a density of
5×105 cells/ml (300 µl/well) and co-transfected with 100
ng luciferase reporter construct, 20 ng Renilla luciferase
pRL-TK reporter and 400 ng pEGFP-C1-HIF-1α the next day. After 24
h, the cells were harvested and the luciferase activity was
determined using a Dual-Luciferase Reporter assay system (Promega
Corporation) with a GloMax 2020 Luminometer (Promega Corporation),
according to the manufacturer's protocol. Finally, the results were
normalized to Renilla luciferase activity. Each experiment
was performed in triplicate wells at least 3 times.
Isolation of RNA and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was isolated from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The cDNA was synthesized with a RevertAid FirstStrand cDNA
Synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.). The
resulting cDNA was analyzed using qPCR and a 7500 Fast Real-Time
PCR system. The PCR primers used were as follows: VASP, forward,
5′-AAAGTCAGCAAGCAGGAGGA-3′, and reverse,
5′-ATTCATCCTTGGGGGTTTTC-3′; HIF-1α, forward,
5′-GAAAGCGCAAGTCCTCAAAG-3′ and reverse, 5′-TGGGTAGGAGATGGAGATGC-3′;
and GAPDH, forward, 5′-CCTTCCTGACAGCCAGTGTG-3′ and reverse,
5′-CAGAATGGAAATACTGGAGCAAG-3′. All PCRs were performed 3 times each
with 3 or more replicates. Relative mRNA expression was calculated
using the 2−ΔΔCq method (34). HIF-1α thermocycling conditions
consisted of 40 cycles of 94°C for 10 sec, 54°C for 10 sec and 72°C
for 10 sec. VASP thermocycling conditions consisted of 40 cycles of
94°C for 40 sec, 58°C for 10 sec and 72°C for 10 sec.
Western blotting
Cells were harvested and then lysed in a modified
radioimmunoprecipitation assay buffer. The lysates were centrifuged
at 13,400 × g, for 15 min at 4°C. Bicinchoninic Acid Protein assay
kit (Pierce; Thermo Fisher Scientific, Inc.) was used to detect
protein concentration. Equal amounts of total protein (10 µg) were
separated by SDS-PAGE (10% gel) and transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Following
blocking in 5% non-fat milk in PBS for 2 h at room temperature, the
membranes were incubated overnight at 4°C with primary antibodies
against the following: Rabbit VASP (1:1,000; cat. no. 13472-1-AP;
ProteinTech Group Inc., Chicago, IL, USA), rabbit HIF-1α (1:1,000;
cat. no. ab82832; Abcam, Cambridge, UK) and mouse GAPDH (1:2,000;
cat. no. sc-69778; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). The membranes were washed and then incubated with horseradish
peroxidase-labeled secondary antibody, either goat anti-rabbit IgG
(1:5,000; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) or goat
anti-mouse IgG (1:5,000; cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. The blots were
visualized with Enhanced Chemiluminescence reagents (Pierce; Thermo
Fisher Scientific, Inc.). All experiments were performed 3
times.
Tumor xenograft model
Animal experiments were carried out according to the
guidelines of the Laboratory Animal Center of Wuhan University.
Male BALB/c athymic nude mice, 6 weeks in age, were purchased from
the Model Animal Research Center of Wuhan University. A549 cells
were injected subcutaneously (1×107 tumor cells in 0.1
ml PBS) into the right-lower flank of the carrier mice. Mice were
divided randomly into two groups (6 animals/group) when the tumor
volume reached 50–60 mm3. TNF-α was delivered at 54
µg/kg, intraperitoneally, once every 3 days for 15 days (5 times in
total), and the control group was injected with normal saline. The
weight of the mice and their tumor volumes were measured every 3
days. The tumor volumes were measured with Vernier calipers and
calculated using the following formula: L × W2/2, where
L is the larger and W is the smaller of the 2 dimensions. The
relative tumor volume (RTV) was calculated as follows: (mean tumor
volume during treatment)/(mean tumor volume prior to treatment).
The animal experiments were approved by the Experimental Ethics
Committee in Basic Medical Sciences, Wuhan University (Wuhan,
China). At 24 h after the final injection, mice were sacrificed by
anesthesia followed by cervical dislocation. Mice were also
sacrificed if any single tumor reached a diameter of 10 mm or if
any animal was adjudged to be in poor health and did not eat. No
multiple tumors were observed in any individual mouse.
Hematoxylin and eosin (H&E)
staining, immunohistochemistry and immunohistochemistry
The transplanted tumors were separated from the
surrounding muscles and dermis, excised, weighed and then fixed in
4% paraformaldehyde for H&E staining and immunohistochemistry
(HIF-1α, VASP and caspase-3) or prefixed in 2.5% glutaraldehyde for
transmission electron microscopy (Hitachi, Ltd., Tokyo, Japan) for
examination.
The tumor sections were collected and fixed in 4%
paraformaldehyde. Paraffin-embedded tissue sections (5 mm) were
stained with H&E, according to standard techniques (35). Images were captured using a Nikon
Eclipse Ci light microscope (Nikon Instruments, Inc., Tokyo,
Japan).
The lung tumor was cut into three 1-mm sections, and
fixed by immersion in 4% prechilled glutaraldehyde. Subsequently,
it was followed by post-fixation with 1% osmium tetroxide. Samples
were dehydrated in a graded ethanol series, embedded in Epon 812
(SPI Supplies/Structure Probe, Inc., West Chester, PA, USA). The
sections were examined under by transmission electron microscopy
(35).
Slides were deparaffinized, dehydrated through a
graded alcohol series and rinsed with distilled water. Antigen
retrieval was carried out by boiling slides in 1 mM sodium citrate
buffer (pH 6.0) followed by 20 min at a sub-boiling temperature and
incubating with 3% H2O2 for 12 min at room
temperature to block endogenous peroxidase. Next, the sections were
washed with PBS (pH 7.5), and incubated in protein blocking
solution [0.5% normal goat serum (Thermo Fisher Scientific, Inc.)
in PBS] for 30 min. The sections were then incubated with anti-VASP
(1:200), anti-HIF-1α (1:200) and anti-rabbit cleaved caspase-3
(1:200; cat. no. 9661; Cell Signaling Technology, Inc., Danvers,
MA, USA) primary antibody in a humidified chamber at 37°C for 2 h,
rinsed with PBS 3 times, and incubated with rhodamine red
X-conjugated anti-immunoglobulin G secondary antibody (1:200; cat.
no. 109-297-008; Jackson ImmunoResearch Laboratories, Inc., West
Grove, PA, USA) at room temperature for 1 h. 3,3′-Diaminobenzidine
(OriGene Technologies, Inc., Beijing, China) was used to stain
slides for 2 min at room temperature and the coloring reaction was
stopped using distilled water, counterstained with Gill's
hematoxylin for 1 min at room temperature (Sigma; Merck KGaA) and
observed under a BX53 light microscope (Olympus Corporation, Tokyo,
Japan). The mean densities of the sections were analyzed using
Image-Pro Plus 4.5 software (National Institutes of Health,
Bethesda, MD, USA).
Online databases
Data from the Kaplan the patients with Lung cancer
were divided into a low expression level group and a high
expression level group, according to the median VASP or HIF-1α mRNA
expression levels, using KM-Plotter (kmplot.com/analysis/index.php?p=service&cancer=lung).
VASP Affy ID, 202205_at; HIF-1α Affy ID, 200989_at. The restriction
of histology was adenocarcinoma and squamous cell carcinoma.
Statistical analysis
Results are presented as the mean ± standard error
of the mean. Statistical significance between groups was tested
using one-way analysis of variance followed by Bonferroni's post
hoc test. Multivariate Cox regression analysis was used to analyze
overall survival in association with clinicopathological features
and VASP/HIF-1α expression in lung adenocarcinoma. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of VASP in regulation of
proliferation and adhesion of A549 cells
To identify the effects of TNF-α on the
proliferative and adhesive ability of lung carcinoma, dose-response
and time-course experiments were performed using A549 and H226
cells. Cell viability was analyzed using an MTT assay and the
extracellular matrix component laminin was used to analyze adhesive
ability. The results suggested that TNF-α did not cause cell death
at low concentrations, which is consistent with our previous study
(33). TNF-α inhibited
proliferation and adhesion of A549 and H226 cells in a
dose-dependent manner (Fig. 1A and
B). Inhibition of cell proliferation was evident when cells
were treated with 120 ng/ml TNF-α for 24 h, and cell viability
rates decreased significantly (Fig.
1C). Therefore, TNF-α was used at a concentration of 120 ng/ml
for 24 h in subsequent experiments.
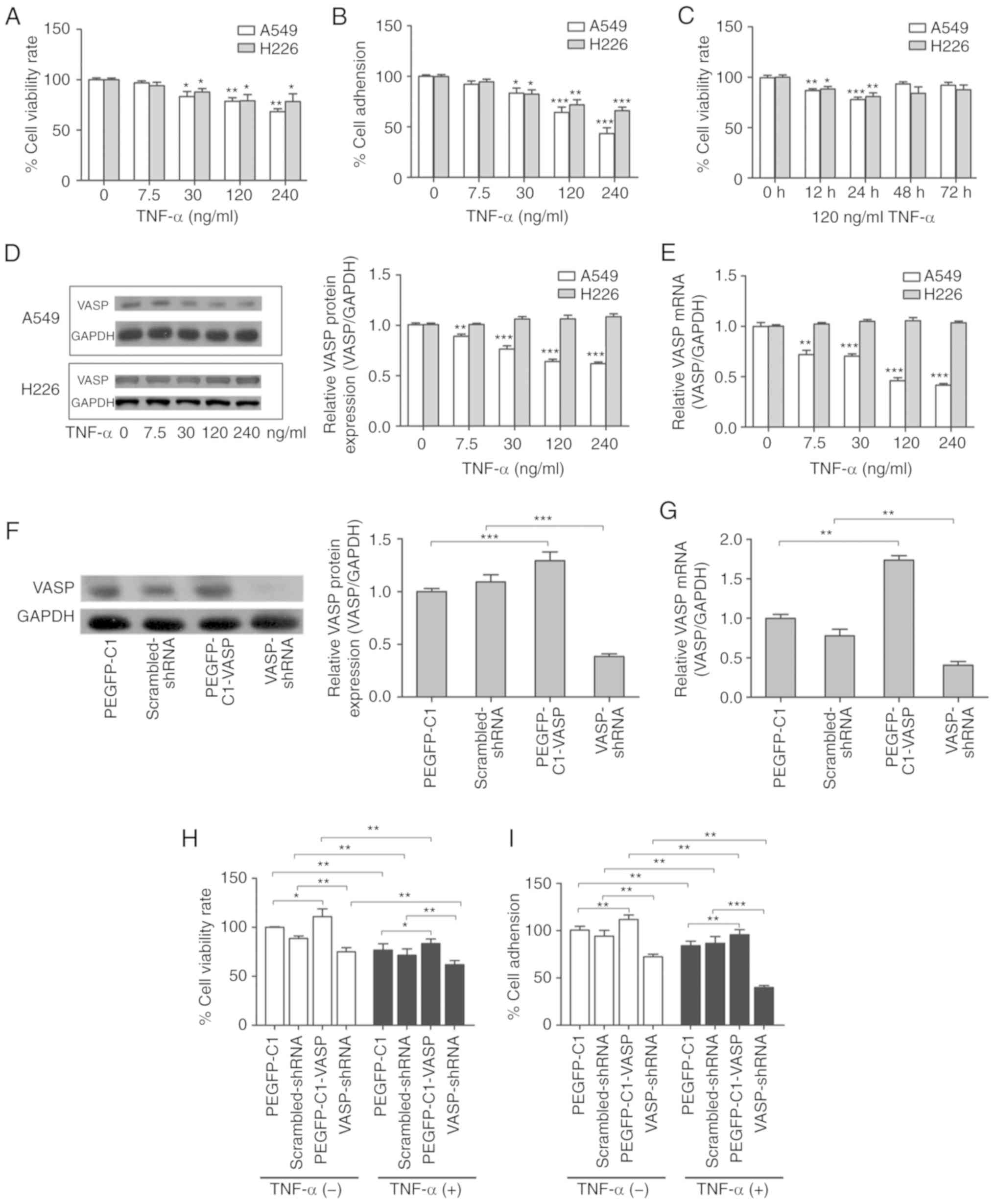 | Figure 1.Expression of VASP and the regulation
of proliferation and adhesion of A549 cells. (A) TNF-α
dose-dependently decreases the viability of A549 and H226 lung
cancer cells. *P<0.05, **P<0.01 vs. untreated control. (B)
TNF-α dose-dependently decreases the adhesion of A549 and H226 lung
cancer cells. *P<0.05, **P<0.01, ***P<0.001 vs. untreated
control. (C) Time-course analysis of the viability of A549 and H226
cancer cells treated with 120 ng/ml TNF-α. *P<0.05, **P<0.01,
***P<0.001 vs. untreated control. TNF-α inhibited the protein
and mRNA expression levels of VASP in A549, but not H226, cells.
A549 and H226 cells were treated with TNF-α for 24 h prior to (D)
western blot and (E) RT-qPCR analyses. GAPDH was used as an
internal control. **P<0.01, ***P<0.001 vs. untreated control.
(F) Western blotting and (G) RT-qPCR validation of VASP
overexpression or knockdown by transient transfection with
pEGFP-C1-VASP or VASP shRNA for 24 h. **P<0.01, ***P<0.001.
(H) Cell viability and (I) cell adhesion analysis following VASP
overexpression or knockdown, with or without 120 ng/ml TNF-α
treatment. The pEGFP-C1 vector or scrambled shRNA was used as
negative control. *P<0.05, **P<0.01, ***P<0.001. Results
are presented as the mean ± standard deviation of 3 independent
experiments (n=6 replicates). VASP, vasodilator-stimulated
phosphoprotein; TNF-α, tumor necrosis factor α; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; shRNA, short
hairpin RNA. |
VASP serves a critical function in regulating cell
physiology and has been identified to be associated with various
human diseases (26,30). However, it remains unclear whether
VASP is involved in the mechanism by which TNF-α inhibits lung
carcinoma cell proliferation and adhesion. To investigate whether
the inhibitory effects on cell proliferation and adhesion result
from the regulation of VASP expression by TNF-α, the protein and
mRNA expression levels of VASP were analyzed in A549 and H226
cells. Treatment with a high concentration of TNF-α (120 or 240
ng/ml) for 24 h effectively decreased VASP expression at the
protein and mRNA levels in A549 cells (Fig. 1D and E). However, there was no
significant difference in VASP expression at the protein or mRNA
levels with TNF-α treatment in H226 cells (Fig. 1D and E). These results indicated
that TNF-α treatment resulted in a significant downregulation of
VASP expression in A549 cells, but not in H226 cells. In brief,
these results suggest that VASP may be a contributor to antitumor
activities of TNF-α in A549 cells.
To investigate the biological consequences of VASP
overexpression or knockdown, mRNA and protein expression analysis
were used to confirm the expression or suppression of VASP by
transient transfection with pEGFP-C1-VASP or VASP shRNA (Fig. 1F and G). A cell viability assay
indicated that overexpression of VASP caused a significant increase
in the cell viability rate, whereas knockdown of VASP caused a
decrease (Fig. 1H). Consistent with
the cell viability results, a cell adhesion assay demonstrated that
overexpression of VASP led to a significant increase in cell
adhesive ability, whereas knockdown of VASP led to a decrease
(Fig. 1I). As VASP promoted A549
cell proliferation and adhesion in the absence of TNF-α, it was
subsequently investigated whether VASP was required for the effects
of TNF-α. A549 cell viability was assessed following transient
transfection with pEGFP-C1-VASP or VASP shRNA in the presence of
TNF-α (120 ng/ml). As expected, cell viability rates significantly
decreased with TNF-α treatment (Fig.
1H). Exogenous overexpression of VASP increased cell viability
rates compared with the control (pEGFP-C1) when cells underwent
TNF-α treatment. Loss of VASP expression significantly decreased
the cell viability rate compared with the control (Fig. 1H). These results indicated that
TNF-α-induced inhibition of cell proliferation was significantly
enhanced by knockdown of VASP expression and that exogenous VASP
expression partially rescued cells from TNF-α treatment-induced
cell death. We hypothesized that VASP may be required for
TNF-α-induced inhibition of A549-cell proliferation. Additional
experiments revealed that TNF-α-induced inhibition of cell adhesion
was similarly promoted by pretreatment with the knockdown plasmid,
and attenuated by exogenous VASP expression, which is consistent
with the cell viability results (Fig.
1I). Overexpression of VASP increased cell adhesion rates in
the presence of TNF-α, whereas loss of VASP decreased the cell
adhesion rates (Fig. 1I). These
results suggest that VASP may be required for TNF-α-induced
inhibition of A549 cell adhesion. Together, these results suggest
that VASP contributes to TNF-α-induced inhibition of proliferation
and adhesion of A549 cells in vitro.
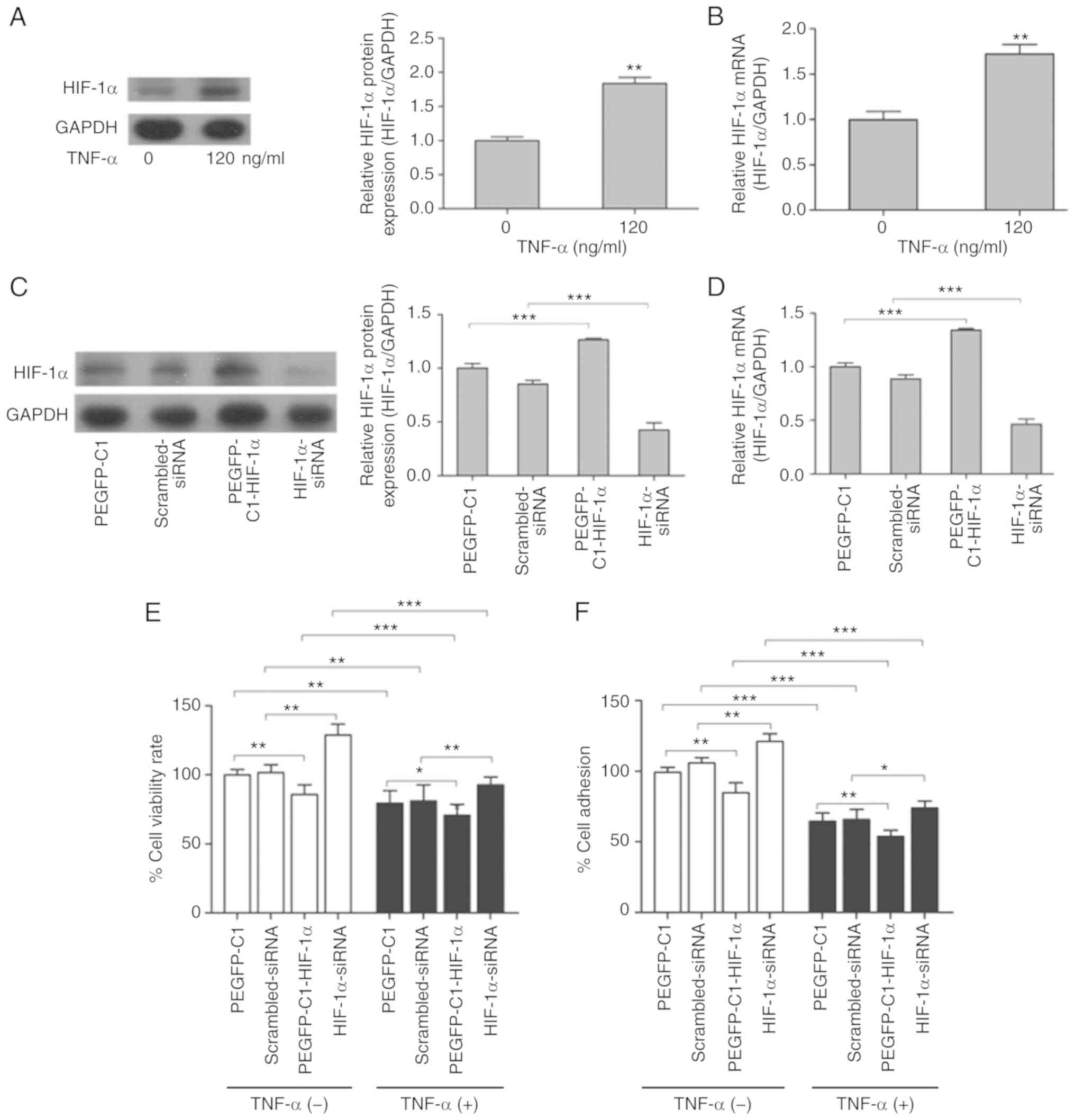
HIF-1α is required for TNF-α-induced
inhibition of proliferation and adhesion of A549 cells
Our previous study indicated that TNF-α
significantly increases the activity of HIF-1α in MCF-7 cells
(33). HIF-1α is a critical
transcription factor that regulates a variety of biological
processes, including cell survival, proliferation, apoptosis and
differentiation through downstream signal transduction cascades
(19). To investigate the function
of HIF-1α in TNF-α-induced inhibition of cell proliferation and
adhesion in lung cancer, protein expression and mRNA levels of
HIF-1α in TNF-α-treated A549 cells were determined. Western blot
results indicated that TNF-α effectively activated HIF-1α protein
expression in A549 cells treated with 120 ng/ml TNF-α for 24 h
(Fig. 2A). The results of RT-qPCR
indicated that HIF1A mRNA levels were also significantly
increased in A549 cells treated similarly (Fig. 2B). Thus, these results suggest that
HIF-1α is upregulated by TNF-α in A549 cells.
To investigate the effect of HIF-1α on the cell
proliferative and adhesive ability, the protein and mRNA expression
levels of HIF-1α were analyzed by overexpression (pEGFP-C1-HIF-1α)
and RNA interference (HIF-1α-siRNA) experiments in A549 cells
(Fig. 2C and D). Cell viability
assays identified that ectopic expression of HIF-1α decreased cell
viability in the absence of TNF-α, whereas siRNA-mediated knockdown
of HIF-1α increased viability of A549 cells (Fig. 2E). However, TNF-α treatment
significantly decreased cell viability rates (Fig. 2E). Exogenous overexpression of
HIF-1α decreased cell viability rates when A549 cells were treated
with TNF-α compared with the control (pEGFP-C1), similar to the
results obtained in the absence of TNF-α (Fig. 2E). HIF-1α deficiency significantly
blocked cell death induced by TNF-α treatment in A549 cells
(Fig. 2E). These results suggest
that the viability response induced by TNF-α was at least partially
HIF-1α-dependent. The cell adhesion assay demonstrated that
TNF-α-induced inhibition of cell adhesion was also attenuated by
knockdown of HIF-1α, and enhanced by HIF-1α overexpression
(Fig. 2F), which was consistent
with the cell viability results. The results of these complementary
experiments suggest that HIF-1α may be a negative regulator of
proliferative and adhesive ability in A549 cells. Therefore, we
hypothesize that TNF-α upregulates HIF-1α, and that HIF-1α affects
downstream proteins and regulates the proliferative and adhesive
ability of A549 cells.
HIF-1α inhibits VASP expression at the
transcriptional level
To determine whether the suppression of VASP by
TNF-α in A549 lung adenocarcinoma cells was dependent on HIF-1α,
the VASP genomic sequence was analyzed, and 2 canonical HBSs
located in the VASP promoter region were identified. Next,
the HBS sequences were cloned into a luciferase reporter, and a
luciferase assay was performed to assess the regulatory function of
HIF-1α (Fig. 3A). HIF-1α
significantly suppressed the VASP promoter activity, and
lack of the HBSs abrogated this suppression in A549 cells (Fig. 3B). The results indicated that HIF-1α
bound to the HBS compared with other regions.
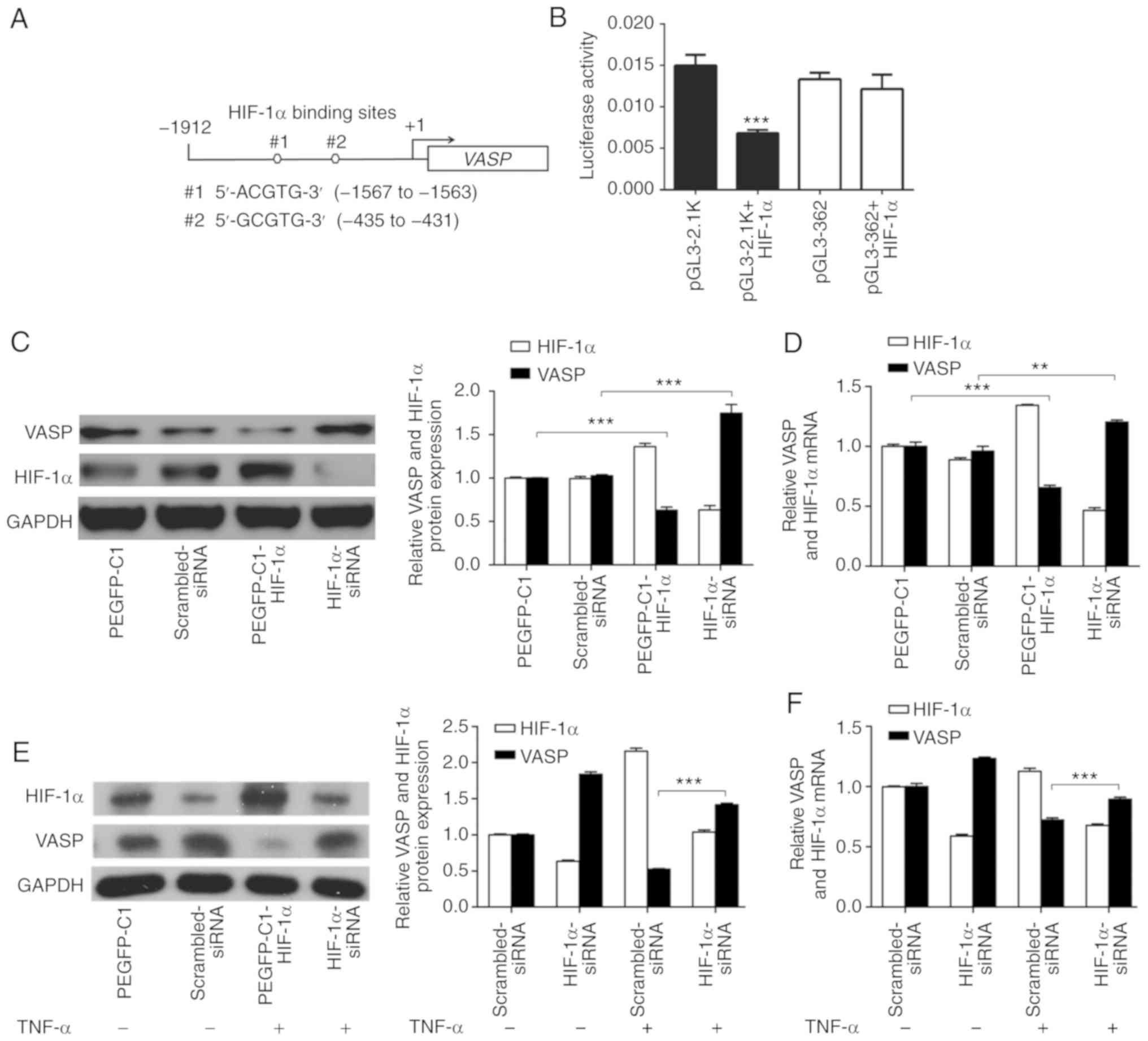 | Figure 3.HIF-1α inhibits VASP
expression at the transcriptional level. (A) HIF-1α regulates
VASP transcription. The VASP promoter region of the
HBS. (B) The HBS region and the VASP promoter sequence with
the HBS region deleted were cloned into pGL3 luciferase reporter
plasmids. Reporter plasmid, pRL Renilla luciferase vector
and pEGFP-C1-HIF-α plasmid were transfected into A549 cells.
***P<0.001 vs. pGL3-2.1K. (C) Western blot and (D) RT-qPCR
analyses in HIF-1α overexpression or knockdown. pEGFP-C1 vector or
scrambled siRNA was used as negative control. TNF-α regulates
VASP, mediated by HIF-1α. **P<0.01, ***P<0.001. A549
cells transfected with HIF-1α-siRNA, in the presence or absence of
TNF-α (120 ng/ml) for 24 h, were subjected to (E) western blot and
(F) RT-qPCR analyses. GAPDH was used as an internal control.
Scrambled siRNA was used as negative control. ***P<0.001.
Results are presented as the mean ± standard deviation of 3
independent experiments. HIF-1α, hypoxia-inducible factor 1α; VASP,
vasodilator-stimulated phosphoprotein; HBS, HIF-1α-binding site;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; TNF-α, tumor necrosis factor α; siRNA, short interfering
RNA. |
To further verify that HIF-1α signaling directly
regulates VASP expression, overexpression and knockdown experiments
were performed in A549 cells. Consistent with Fig. 2C, western blot analysis suggested
that VASP expression was significantly increased by siRNA-mediated
knockdown of HIF-1α (Fig. 3C). By
contrast, ectopically upregulated expression of HIF-1α caused a
decrease in VASP expression in A549 cells (Fig. 3C). In addition, RT-qPCR results
suggested further that the VASP mRNA expression level was
significantly decreased by overexpression of HIF-1α and increased
by knockdown of HIF-1α (Fig. 3D).
These results indicated that VASP is a direct target of
HIF-1α, and suggested a negative association between the expression
of the 2 proteins in A549 cells.
TNF-α treatment resulted in downregulation of VASP
expression in A549 cells and caused upregulation of HIF-1α
expression, suggesting that TNF-α may regulate VASP in a
HIF-1α-dependent manner (Figs. 1D and
E, 2A and B). To determine the
association between VASP and HIF-1α following TNF-α treatment,
western blot and RT-qPCR analyses were performed in A549 cells
(Fig. 3E and F). The results
indicated that knockdown of HIF-1α increased VASP expression in the
absence of TNF-α, and that VASP expression was partially suppressed
at the protein and mRNA levels when cells were treated with TNF-α
(Fig. 3C-F). Additionally,
HIF-1α-knockdown significantly restored VASP protein expression and
mRNA levels when A549 cells were treated with TNF-α compared with
control (scrambled siRNA) (Fig. 3E and
F). These results suggest that HIF-1α acts upstream of the VASP
signaling pathway following TNF-α treatment in A549 cells.
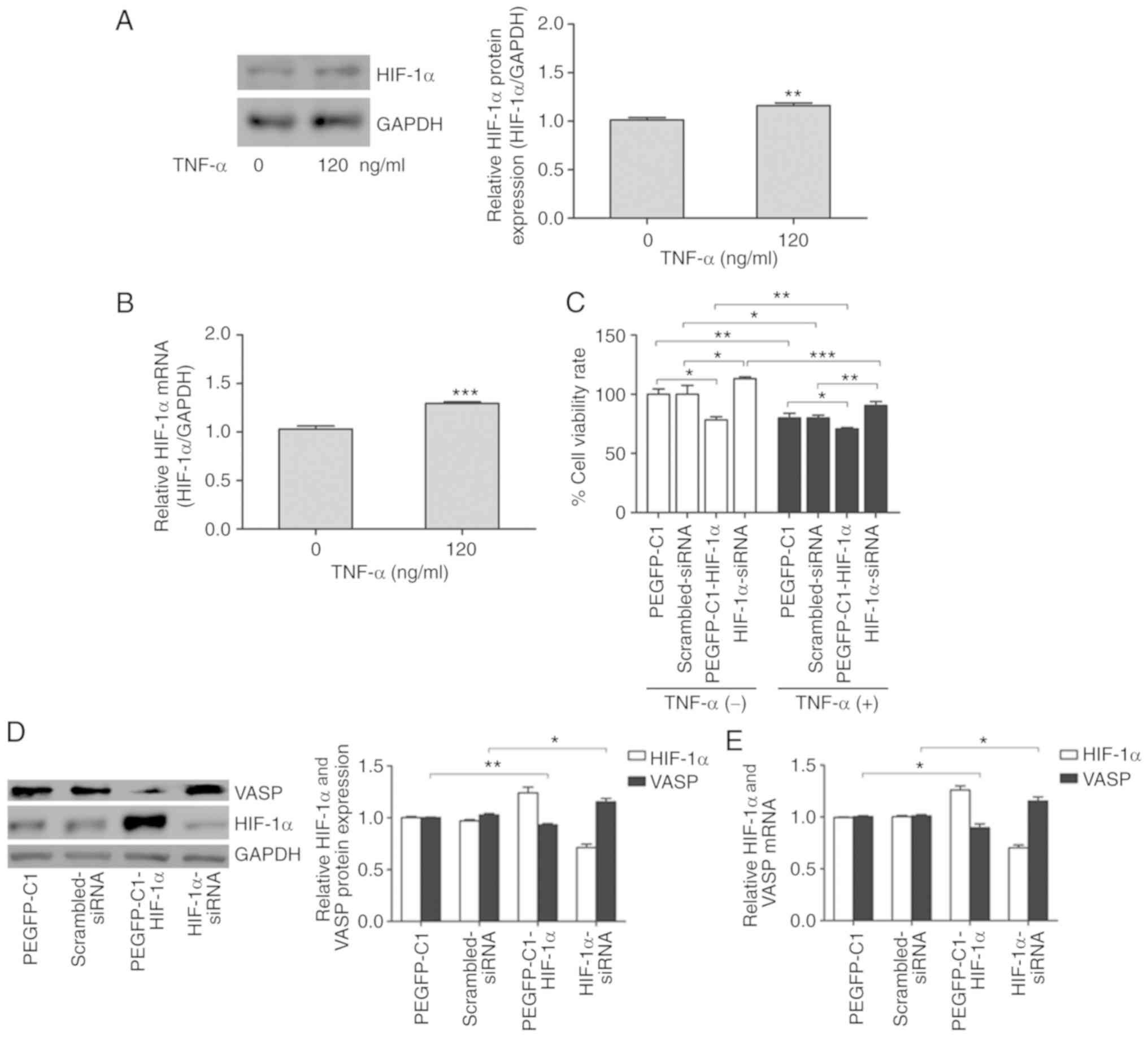
To further investigate the biological consequences
of the HIF-1α/VASP signaling pathway in response to TNF-α, H226
cells were used. mRNA and protein analysis revealed that TNF-α
promoted HIF-1α expression in H226 cells (Fig. 4A and B). Additionally, the function
of HIF-1α on H226 cell proliferation was investigated. Exogenous
overexpression of HIF-1α significantly decreased cell viability,
and HIF-1α deficiency partially rescued H226 cells from
TNF-α-induced cell death (Fig. 4C).
However, HIF-1α inhibited VASP expression in H226 cells (Fig. 4D and E). In this context, HIF-1α
acted as a transcriptional activator, downregulating the expression
of a large number of genes, including VASP, although the
direct targets were not identified. VASP may be regulated by
a subset of transcriptional activators in gene expression
regulatory networks; however, this remains to be elucidated.
TNF-α inhibits transplanted tumor
growth in vivo in the xenograft model
To investigate the antitumor effects of TNF-α in
vivo, nude mice bearing established A549 tumor xenografts were
treated with vehicle (saline) or TNF-α (54 µg/kg body weight) by
intraperitoneal injection. The body weight of each mouse was
measured every 3 days. Decreased body weights were observed in
TNF-α-treated mice (Fig. 5A),
although these were typically not significantly different from body
weights of control mice.
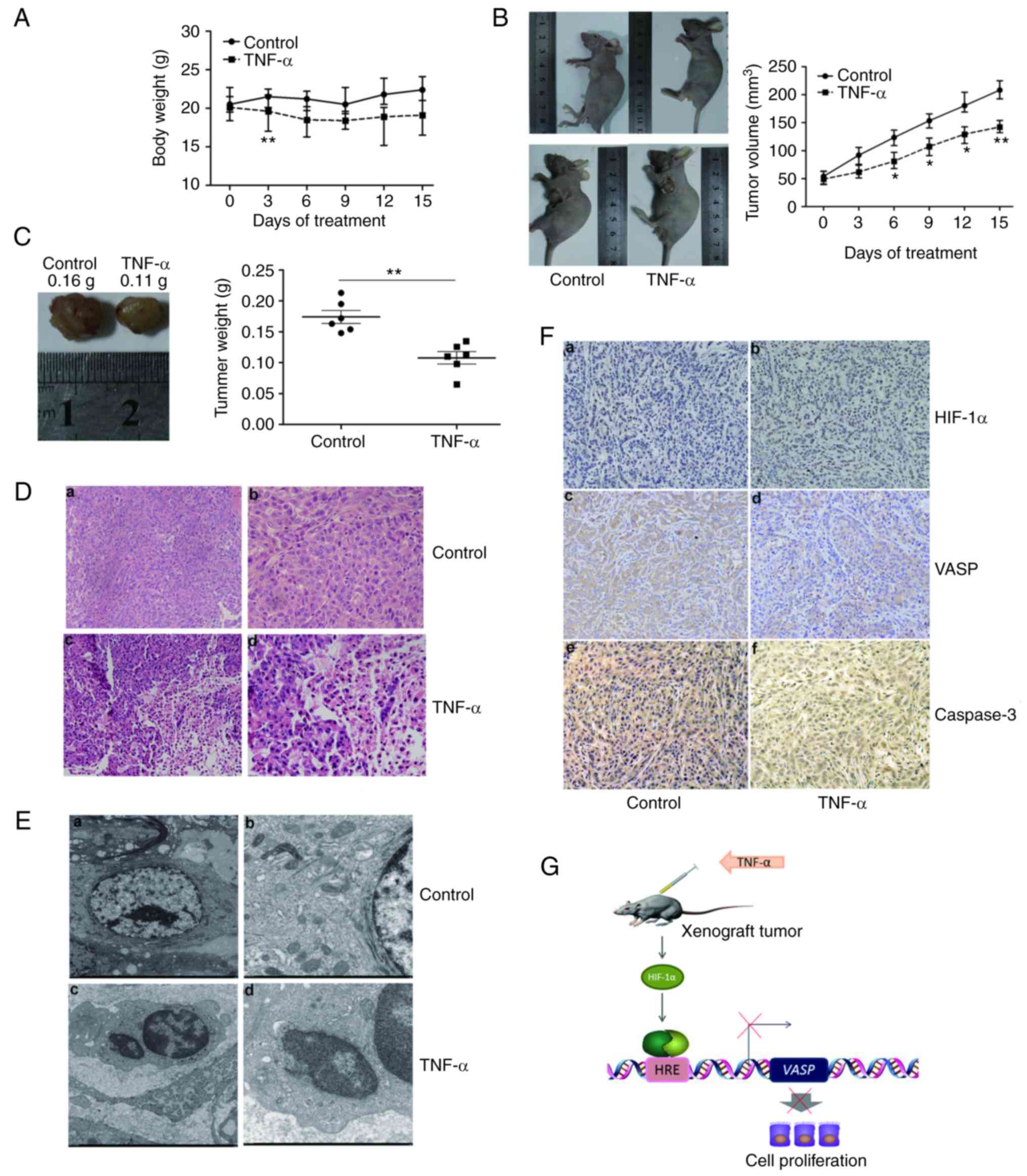 | Figure 5.TNF-α inhibits transplanted tumor
growth in vivo. (A) Effect of TNF-α on the body weight of
xenograft tumors in nude mice. The body weights of mice were
determined every 3 days. (B) TNF-α decreased the volume of
xenograft tumors in nude mice. Average tumor volumes were
determined every 3 days. (C) TNF-α decreased the weight of
xenograft tumors in nude mice. Average tumor weights at the end of
the indicated treatment are presented. (D) TNF-α affects the
tissues of xenograft lung tumors. Histopathological changes of the
xenograft in (a and b) control mice, or (c and d) following TNF-α
treatment, observed by hematoxylin and eosin staining (a and c,
×200 magnification; b and d, ×400 magnification). (E) TNF-α affects
the ultrastructure of xenograft lung tumor tissues. Morphological
changes of xenograft tumor tissues in (a and b) control mice, or (c
and d) mice treated with TNF-α, visualized by transmission electron
microscopy. (a) Tumor tissues in the control group: Large tumor
cells, normal morphology, approximately circular or oval in shape,
large and irregular nuclei, rich in euchromatin, homogeneous in
shape; (c) cytoplasm abundant, mitochondria and other organelles
were visible. (b) TNF-α treatment group tumor tissues: Nucleus
heterochromatin increased with cohesion, common tumor cell
apoptosis; (d) formation of apoptotic bodies. (a, ×2,000
magnification; b, ×2,500, magnification; c and d, ×7,000
magnification). (F) TNF-α affected HIF-1α, VASP and caspase-3
expression in xenograft lung tumor tissues. Immunohistochemistry
for (a and b) HIF-1α, (c and d) VASP and (e and f) caspase-3 in
xenograft tumor tissues of (a, c and e) control mice or (b, d and
f) treated mice. (a-f, ×200 magnification). (G) Schematic model of
the decrease in VASP gene expression following TNF-α
treatment. The extracellular signal molecule causes HIF-1α
activation, binding to the promoter region and resulting in the
downregulation of VASP. TNF-α, tumor necrosis factor α;
HIF-1α, hypoxia-inducible factor 1α; VASP, vasodilator-stimulated
phosphoprotein; HRE, hypoxia-response element. *P<0.05,
**P<0.01. |
Mice were treated as aforementioned, and the volume
of the xenograft tumor was determined every 3 days. The relative
tumor volume (RTV) of tumor-bearing mice treated with vehicle
increased linearly, and TNF-α significantly inhibited tumor growth
compared with vehicle (P<0.05; Fig.
5B).
Consistent with the tumor volume analysis, the
average tumor weights associated with vehicle and TNF-α treatment
were 0.16 and 0.11 g, respectively (P<0.05; Fig. 5C). Therefore, these results
suggested that TNF-α was effective against tumors and caused
minimal damage to normal cells (Fig.
5A-C).
The tumor tissues of nude mice treated with the
vehicle were nodular, encapsulated, exhibited medium hardness and
no distant infiltration, with a gray cut surface and festered (data
not shown). In addition, tumor cells were arranged densely,
exhibiting significant atypia, large nuclei, abundant cytoplasm,
and minimal intercellular substance were observed by H&E
staining (Fig. 5D-a and -b).
When mice were treated with TNF-α, the tissues of
xenograft lung tumors exhibited significant pathological changes,
including irregular necrosis, hemorrhage regions and flaky
eosinophilic structures (Fig. 5D-c and
-d).
Transmission electron microscopy was used to observe
the ultra-pathological changes of xenograft lung tumors. Tumor
cells in the control group were large, and approximately circular
or oval, with large and irregular nuclei (Fig. 5E-a and -b). In contrast, tumor cells
in the TNF-α-treated group were distributed sparsely, and cell size
was generally lower compared with that of control, with evident
cell debris. Parts of the cell envelope and nuclear membrane were
significantly swollen, and cytoplasmic mitochondria were swollen
with a rounded shape, ridge-reduced and demonstrating malalignment
(Fig. 5E-c and -d).
Positive HIF-1α protein expression in the nucleus,
and VASP and caspase-3 protein expression in the cytoplasm
exhibited brown granular or clustered distribution (Fig. 5F). Following TNF-α treatment for 15
days, HIF-1α was overexpressed in a number of xenograft lung tumors
compared with the control group (Fig.
5F-a and -b). Marked VASP staining intensity was easily
observed in the control group, but not in the therapeutic groups
(Fig. 5F-c and -d). Furthermore,
marked expression of caspase-3 was present in TNF-α-treated
xenograft lung tumor tissue (Fig. 5F-e
and -f). Quantification analysis for HIF-1α, VASP and caspase-3
protein expression was performed (Table
I).
 | Table I.Immunohistochemistry analysis of
HIF-1α, VASP and caspase-3 expression of xenograft lung tumor
tissues. |
Table I.
Immunohistochemistry analysis of
HIF-1α, VASP and caspase-3 expression of xenograft lung tumor
tissues.
|
|
| HIF-1α | VASP | Caspase-3 |
|---|
|
|
|
|
|
|
|---|
|
| Cases (n) | + | ++ | +++ | + | ++ | +++ | + | ++ | +++ |
|---|
| Control | 6 | 2 | 3 | 1 | 1 | 1 | 4 | 2 | 2 | 2 |
| TNF-α | 6 | 0 | 3 | 3 | 2 | 3 | 1 | 1 | 2 | 3 |
Given these results in the A549 cell line and tumor
xenograft model, we propose a schematic model for the decrease in
VASP expression mediated by TNF-α treatment (Fig. 5G).
Long non-coding RNAs (lncRNAs) have been identified
to be dysregulated in various diseases. lncRNA-LET has been
identified to serve a key function in the metastasis of certain
solid tumors by regulating HIF-1α (32). RT-qPCR was used to detect the mRNA
expression levels of lncRNA-LET in xenograft lung tumor
tissues in nude mice. The mRNA expression levels of
lncRNA-LET were decreased in the therapeutic group compared
with in the control (data not shown). These results suggest that
the lncRNA-LET-associated molecular regulation pathway may
be involved in the TNF-α-mediated decrease in the HIF-1α expression
level; however, the specific regulatory mechanism requires further
investigation.
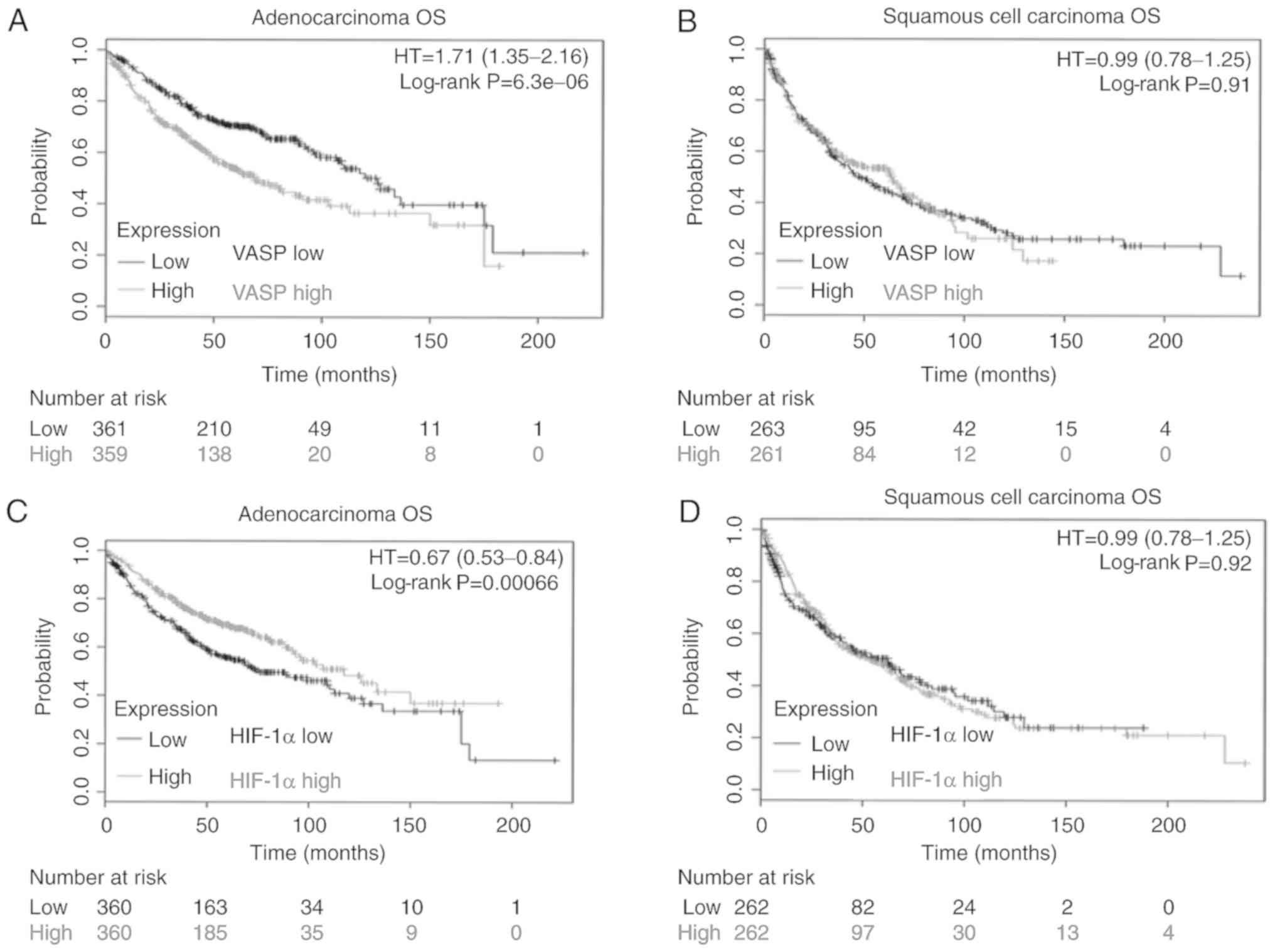
High VASP or low HIF-1α expression is
associated with poor prognosis in lung adenocarcinoma
Since VASP expression in paraffin-embedded tissue
specimens in lung cancer was significantly higher compared with
that of the control, the association between VASP expression and
outcome was investigated using the online KM-Plotter website for
lung cancer using public data (36,37).
Patients with lung adenocarcinoma were categorized using the median
value of VASP expression, and high expression of VASP mRNA
was associated with a significant decrease in overall survival
rates (Fig. 6A). No such
association was observed among patients with lung squamous cell
carcinoma subtypes (Fig. 6B). These
data suggest that VASP expression may serve a critical function in
lung adenocarcinoma. It was then analyzed whether the HIF1A
mRNA expression was associated with outcome in lung cancer.
Coincidently, lower HIF-1α expression in lung adenocarcinoma was
associated with poor prognosis, whereas no association was evident
among patients with squamous cell carcinoma subtypes (Fig. 6C and D). In addition, multivariate
analysis indicated that VASP expression was associated with overall
survival, independent of sex, smoking history and tumor stage
(Table II). However, HIF-1α
expression was not an independent predictor of overall survival
(Table III). These results
strongly support the hypothesis that VASP expression may be a
valuable prognostic biomarker and have a significant effect on the
clinical management of patients with lung adenocarcinoma.
 | Table II.Multivariate Cox regression analysis
of overall survival in association with clinicopathological
features and VASP expression in lung adenocarcinoma. |
Table II.
Multivariate Cox regression analysis
of overall survival in association with clinicopathological
features and VASP expression in lung adenocarcinoma.
| Characteristic | P-value | Hazard ratio (95%
CI) |
|---|
| Stage | 0.1367 | 3.64
(0.66–19.99) |
| AJCC stage T | 0.0096a | 2.52
(1.25–5.06) |
| AJCC stage N | 0.9009 | 0.9
(0.16–4.98) |
| Sex | 0.1698 | 1.5
(0.84–2.68) |
| Smoking
history | 0.4203 | 0.72
(0.32–1.6) |
| VASP | 0.0256a | 1.93
(1.08–3.44) |
 | Table III.Multivariate Cox regression analysis
of overall survival in association with clinicopathological
features and HIF-1α expression in lung adenocarcinoma. |
Table III.
Multivariate Cox regression analysis
of overall survival in association with clinicopathological
features and HIF-1α expression in lung adenocarcinoma.
| Characteristic | P-value | Hazard ratio (95%
CI) |
|---|
| Stage | 0.2392 | 2.78
(0.51–15.31) |
| AJCC stage T | 0.0085a |
2.57(1.27–5.18) |
| AJCC stage N | 0.8344 | 1.2
(0.21–6.79) |
| Sex | 0.1616 | 1.53
(0.84–2.75) |
| Smoking
history | 0.844 | 0.92
(0.42–2.03) |
| HIF-1α | 0.8016 | 1.08
(0.61–1.89) |
Discussion
The treatment of lung adenocarcinoma has been
advanced by the development of multiple therapies, including
surgery, chemotherapy, radiotherapy and molecular targeted therapy
(38). However, the high
heterogeneity and disorder of tumor cells present challenges in
cancer research. The results of the present study indicated a
function of VASP protein in TNF-α-induced lung cancer cell death.
The ability of TNF-α to activate HIF-1α, which binds to the
VASP promoter and suppresses its transcription, was also
demonstrated, suggesting that VASP acts downstream of the
TNF-α/HIF-1α signaling pathway.
As a cytokine, TNF-α may lead to either promotion or
prevention of tumor growth, depending on the dose or route of
injection, and the type of tumor model (13–15).
In 3LL-A9 Lewis lung carcinoma cells transplanted into C57BL/6
mice, TNF-α together with interferon α and perforin participated in
T and natural killer cell-mediated elimination of carcinoma cells,
demonstrating robust antitumor activity (39,40).
Targeted tumor endothelium delivering a hybrid adeno-associated
virus phage vector expressing TNF-α resulted in apoptosis in tumor
vessels, and significant inhibition of human melanoma xenograft
tumor growth (41). In agreement,
it was demonstrated in the present study that TNF-α directly
inhibited A549 cell proliferation and adhesion, and xenograft tumor
growth in nude mice. However, the underlying molecular mechanism
for TNF-α inhibition of tumor growth remains unknown and requires
further investigation.
VASP is an actin-associated protein, associated with
tumor progression and metastasis via regulation of cell adhesion,
migration and invasion. Previous studies demonstrated that a
decrease in VASP expression significantly inhibited the
proliferation and migration of MCF-7 luminal breast cancer cells
and BGC-823 gastric cancer cells (28,32,42).
Dertsiz et al (36)
determined that VASP expression levels in 26 cases of lung
adenocarcinoma were significantly higher than those of 14 cases of
adjacent normal lung tissues using immunohistochemistry, suggesting
that VASP may serve an important function in lung adenocarcinoma
progression. Henes et al (43) hypothesized that TNF-α directly
regulated the biological effects of VASP by activating the
transcription factor nuclear factor κB. As another nuclear
transcription factor, HIF-1α is involved in a complex molecular
network and is a mediator of cell proliferation and cell apoptosis
(44,45). Haddad and Harb (46) identified that TNF-α promoted HIF-1α
translocation into the nucleus and activated HIF-1α to bind
downstream target genes through increasing the production of ROS in
normoxic conditions in primary fetal rat alveolar epithelial type 2
cells. In addition, HIF-1-dependent expression of L1 cell adhesion
molecule inhibits vascular metastasis of hypoxic breast cancer
cells to the lungs (47). Our
previous study identified that HIF-1α mediated the repression of
VASP expression in MCF-7 cells (33). HIF-1α has the potential to regulate
a subset of downstream genes to inhibit cell proliferation in A549
and H226 lung cancer cells.
In the present study, the expression of VASP was
decreased at the mRNA and protein levels following TNF-α treatment
in 2 lung cancer cell lines. It was identified that VASP
overexpression promoted the proliferation and adhesion of A549 lung
adenocarcinoma cells in vitro. However, in the A549 cells,
the expression of HIF-1α was increased at the mRNA and protein
levels following TNF-α treatment, and HIF-1α overexpression
inhibited the proliferation and adhesion of A549 cells in
vitro. Furthermore, TNF-α inhibited cell proliferation and
adhesion following inhibition of VASP expression, whereas it
promoted cell proliferation and adhesion following inhibition of
HIF-1α expression in A549 cells. The results of the present study
provide a novel perspective for understanding the molecular
mechanism underlying the antitumor effect of TNF-α.
Furthermore, results from a luciferase assay
identified HBSs in the promoter region of VASP. In addition,
interference of HIF-1α in the cells led to a significant increase
in VASP expression at the mRNA and protein levels, and TNF-α is
able to inhibit VASP expression at the mRNA and protein levels
induced by interference of HIF-1α. The results of the present study
revealed that decreased HIF-1α expression, mediated by
HIF-1α-siRNA, rescued the proliferation ability inhibited by TNF-α
in 2 lung cancer cell lines. Indeed, VASP was not revealed
to be the effective target gene of HIF-1α, undergoing no
significant increase/decrease with TNF-α treatment in H226 cells
(data not shown). Subsequently, it was identified that VASP acts
downstream of HIF-1α, with the existence of HBSs in the VASP
promoter region using luciferase analysis. It was confirmed that
downregulated VASP promoter activity occurred via HIF-1α in
A549 cells. Furthermore, the results of the present study revealed
that HIF-1α mediated the antitumor effect of TNF-α in A549
cells.
Public databases containing clinical data indicate
that lung adenocarcinoma patients exhibiting different VASP and
HIF-1α expression levels may have different survival outcomes
(37). In the present study, an
association between TNF-α/HIF-1α and the VASP signaling pathway was
identified in A549 cells; however, the underlying molecular
mechanism remains unclear, and the potential use of VASP as a novel
prognostic marker or a therapeutic target for lung carcinoma
requires further investigation.
In summary, the results of the present study
indicate that TNF-α inhibits the proliferation and adhesion of A549
cells, and growth of transplanted tumors in nude mice, by
suppressing the expression of VASP by activating the
transcriptional activity of HIF-1α. These data suggest that the
HIF-1α/VASP signaling pathway serves an important function in the
regulation of TNF-α-induced suppression of xenograft tumor growth,
suggesting that our understanding of the antitumor effect of TNF-α
requires further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472765), the Hubei
Science Foundation (grant no. 2014CKB508), and the Laboratory and
Equipment Administration of Wuhan University under the ‘Open
Experimentation Program of Experimental Teaching Center of Wuhan
University’ project.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL and XC conceived and designed the experiments.
YT, LW and JZ performed the experiments. PH, KS and ZL analyzed the
data. YH, LX and YM contributed reagents, materials and analysis
tools. LX was responsible for cell culture, and YM was responsible
for plasmid construction. YH and XC wrote the paper. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The animal experiments were approved by the
Experimental Ethics Committee in Basic Medical Sciences, Wuhan
University (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TNF-α
|
tumor necrosis factor α
|
|
HIF-1α
|
hypoxia-inducible factor 1α
|
|
VASP
|
vasodilator-stimulated
phosphoprotein
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zagryazhskaya A, Gyuraszova K and
Zhivotovsky B: Cell death in cancer therapy of lung adenocarcinoma.
Int J Dev Biol. 59:119–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yatabe Y, Borczuk AC and Powell CA: Do all
lung adenocarcinomas follow a stepwise progression? Lung Cancer.
74:7–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen ZY, Zhong WZ, Zhang XC, Su J, Yang
XN, Chen ZH, Yang JJ, Zhou Q, Yan HH, An SJ, et al: EGFR
mutation heterogeneity and the mixed response to EGFR tyrosine
kinase inhibitors of lung adenocarcinomas. Oncologist. 17:978–985.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Devarakonda S, Morgensztern D and Govindan
R: Genomic alterations in lung adenocarcinoma. Lancet Oncol.
16:e342–e351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ben-Baruch A: The tumor-promoting flow of
cells into, within and out of the tumor site: Regulation by the
inflammatory axis of TNFα and chemokines. Cancer
Microenviron. 5:151–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wajant H: The role of TNF in cancer.
Results Probl Cell Differ. 49:1–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zelová H and Hošek J: TNF-α
signalling and inflammation: Interactions between old
acquaintances. Inflamm Res. 62:641–651. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Y and Zhou BP:
TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and
invasion. Br J Cancer. 102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carswell EA, Old LJ, Kassel RL, Green S,
Fiore N and Williamson B: An endotoxin-induced serum factor that
causes necrosis of tumors. Proc Natl Acad Sci USA. 72:3666–3670.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haranaka K, Satomi N and Sakurai A:
Antitumor activity of tumor necrosis factor (TNF) in vitro and in
vivo. Gan To Kagaku Ryoho. 11:1387–1393. 1984.(In Japanese).
PubMed/NCBI
|
|
12
|
Calzascia T, Pellegrini M, Hall H, Sabbagh
L, Ono N, Elford AR, Mak TW and Ohashi PS: TNF-alpha is critical
for antitumor but not antiviral T cell immunity in mice. J Clin
Invest. 117:3833–3845. 2007.PubMed/NCBI
|
|
13
|
Fajardo LF, Kwan HH, Kowalski J, Prionas
SD and Allison AC: Dual role of tumor necrosis factor-alpha in
angiogenesis. Am J Pathol. 140:539–544. 1992.PubMed/NCBI
|
|
14
|
Weishaupt A, Gold R, Hartung T, Gaupp S,
Wendel A, Brück W and Toyka KV: Role of TNF-alpha in high-dose
antigen therapy in experimental autoimmune neuritis: Inhibition of
TNF-alpha by neutralizing antibodies reduces T-cell apoptosis and
prevents liver necrosis. J Neuropathol Exp Neurol. 59:368–376.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bertazza L and Mocellin S: The dual role
of tumor necrosis factor (TNF) in cancer biology. Curr Med Chem.
17:3337–3352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weidemann A and Johnson RS: Biology of
HIF-1alpha. Cell Death Differ. 15:621–627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schindl M, Schoppmann SF, Samonigg H,
Hausmaninger H, Kwasny W, Gnant M, Jakesz R, Kubista E, Birner P
and Oberhuber G; Austrian Breast and Colorectal Cancer Study Group,
: Overexpression of hypoxia-inducible factor 1alpha is associated
with an unfavorable prognosis in lymph node-positive breast cancer.
Clin Cancer Res. 8:1831–1837. 2002.PubMed/NCBI
|
|
22
|
Chiavarina B, Whitaker-Menezes D, Migneco
G, Martinez-Outschoorn UE, Pavlides S, Howell A, Tanowitz HB,
Casimiro MC, Wang C, Pestell RG, et al: HIF1-alpha functions as a
tumor promoter in cancer associated fibroblasts, and as a tumor
suppressor in breast cancer cells: Autophagy drives
compartment-specific oncogenesis. Cell Cycle. 9:3534–3551. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan LF, Diao LM, Chen DJ, Liu MQ, Zhu LQ,
Li HG, Tang ZJ, Xia D, Liu X and Chen HL: Expression of HIF-1 alpha
and its relationship to apoptosis and proliferation in lung cancer.
Ai Zheng. 21:254–258. 2002.(In Chinese). PubMed/NCBI
|
|
24
|
Balamurugan K: HIF-1 at the crossroads of
hypoxia, inflammation, and cancer. Int J Cancer. 138:1058–1066.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haffner C, Jarchau T, Reinhard M, Hoppe J,
Lohmann SM and Walter U: Molecular cloning, structural analysis and
functional expression of the proline-rich focal adhesion and
microfilament-associated protein VASP. EMBO J. 14:19–27. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pula G and Krause M: Role of Ena/VASP
proteins in homeostasis and disease. Handb Exp Pharmacol. 39–65.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen XJ, Squarr AJ, Stephan R, Chen B,
Higgins TE, Barry DJ, Martin MC, Rosen MK, Bogdan S and Way M:
Ena/VASP proteins cooperate with the WAVE complex to regulate the
actin cytoskeleton. Dev Cell. 30:569–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Han G, Fan B, Zhou Y, Zhou X, Wei
L and Zhang J: Green tea (−)-epigallocatechin-3-gallate
down-regulates VASP expression and inhibits breast cancer cell
migration and invasion by attenuating Rac1 activity. Eur J
Pharmacol. 606:172–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Galler AB, García Arguinzonis MI,
Baumgartner W, Kuhn M, Smolenski A, Simm A and Reinhard M:
VASP-dependent regulation of actin cytoskeleton rigidity, cell
adhesion, and detachment. Histochem Cell Biol. 125:457–474. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knauer O, Binai NA, Carra G, Beckhaus T,
Hanschmann KM, Renné T, Backert S, Karas M and Wessler S:
Differential phosphoproteome profiling reveals a functional role
for VASP in Helicobacter pylori-induced cytoskeleton
turnover in gastric epithelial cells. Cell Microbiol. 10:2285–2296.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu K, Li L, Nisson PE, Gruber C, Jessee J
and Cohen SN: Reversible tumorigenesis induced by deficiency of
vasodilator-stimulated phosphoprotein. Mol Cell Biol. 19:3696–3703.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su K, Hu P, Wang X, Kuang C, Xiang Q, Yang
F, Xiang J, Zhu S, Wei L and Zhang J: Tumor suppressor berberine
binds VASP to inhibit cell migration in basal-like breast cancer.
Oncotarget. 7:45849–45862. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Su K, Tian Y, Wang J, Shi W, Luo D, Liu J,
Tong Z, Wu J, Zhang J and Wei L: HIF-1α acts downstream
of TNF-α to inhibit vasodilator-stimulated
phosphoprotein expression and modulates the adhesion and
proliferation of breast cancer cells. DNA Cell Biol. 31:1078–1087.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Hu PC, Gao FF, Lv JW, Xu S, Kuang
CC, Wei L and Zhang JW: The protective effect of curcumin on
hepatotoxicity and ultrastructural damage induced by cisplatin.
Ultrastruct Pathol. 38:358–362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dertsiz L, Ozbilim G, Kayisli Y, Gokhan
GA, Demircan A and Kayisli UA: Differential expression of VASP in
normal lung tissue and lung adenocarcinomas. Thorax. 60:576–581.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gyorffy B, Surowiak P, Budczies J and
Lanczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang X and Adjei AA: Lung cancer and
metastasis: New opportunities and challenges. Cancer Metastasis
Rev. 34:169–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baxevanis CN, Voutsas IF, Tsitsilonis OE,
Tsiatas ML, Gritzapis AD and Papamichail M: Compromised anti-tumor
responses in tumor necrosis factor-alpha knockout mice. Eur J
Immunol. 30:1957–1966. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Prevost-Blondel A, Roth E, Rosenthal FM
and Pircher H: Crucial role of TNF-alpha in CD8 T cell-mediated
elimination of 3LL-A9 Lewis lung carcinoma cells in vivo. J
Immunol. 164:3645–3651. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tandle A, Hanna E, Lorang D, Hajitou A,
Moya CA, Pasqualini R, Arap W, Adem A, Starker E, Hewitt S and
Libutti SK: Tumor vasculature-targeted delivery of tumor necrosis
factor-alpha. Cancer. 115:128–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J, Zhang J, Wu J, Luo D, Su K, Shi W,
Liu J, Tian Y and Wei L: MicroRNA-610 inhibits the migration and
invasion of gastric cancer cells by suppressing the expression of
vasodilator-stimulated phosphoprotein. Eur J Cancer. 48:1904–1913.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Henes J, Schmit MA, Morote-Garcia JC,
Mirakaj V, Köhler D, Glover L, Eldh T, Walter U, Karhausen J,
Colgan SP and Rosenberger P: Inflammation-associated repression of
vasodilator-stimulated phosphoprotein (VASP) reduces
alveolar-capillary barrier function during acute lung injury. FASEB
J. 23:4244–4255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Azoitei N, Becher A, Steinestel K, Rouhi
A, Diepold K, Genze F, Simmet T and Seufferlein T: PKM2 promotes
tumor angiogenesis by regulating HIF-1α through NF-κB
activation. Mol Cancer. 15:32016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Carmeliet P, Dor Y, Herbert JM, Fukumura
D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R,
Maxwell P, et al: Role of HIF-1alpha in hypoxia-mediated apoptosis,
cell proliferation and tumour angiogenesis. Nature. 394:485–490.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Haddad JJ and Harb HL: Cytokines and the
regulation of hypoxia-inducible factor (HIF)-1alpha. Int
Immunopharmacol. 5:461–483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang H, Wong CC, Wei H, Gilkes DM,
Korangath P, Chaturvedi P, Schito L, Chen J, Krishnamachary B,
Winnard PT Jr, et al: HIF-1-dependent expression of
angiopoietin-like 4 and L1CAM mediates vascular metastasis of
hypoxic breast cancer cells to the lungs. Oncogene. 31:1757–1770.
2012. View Article : Google Scholar : PubMed/NCBI
|