Introduction
Redd1 (also known as RTP801, DDIT4 or Dig2) is a
highly conserved stress response gene that is induced by various
stresses, such as hypoxia, ionizing radiation (IR), DNA damage and
energy depletion (1–4). Redd1 acts primarily as an inhibitor of
mTORC1, a central regulator of protein translation, resulting in
the inhibition of cell growth and proliferation (1,5,6).
However, paradoxically, high Redd1 expression favors cancer
progression and generates resistance to cancer therapy. It was
reported that Redd1 overexpression could predict poor prognosis of
ovarian cancer (7) and promote the
development of drug resistance in myeloma cells and prostate cancer
cells (8,9). In silico analysis revealed that
higher expression levels of Redd1 protein were associated with
worse outcomes in acute myeloid leukemia, glioblastoma multiforme,
as well as in breast, colon, skin and lung cancer (10). In our previous study, it was
demonstrated that constitutive overexpression of Redd1 led to
mTORC1 inhibition and to consequent AKT activation which was
involved in lung cancer cell survival and resistance to
chemotherapeutic drugs (11).
Heat shock proteins (HSPs) are highly conserved
molecular chaperones that play essential roles in protein
homeostasis, transport processes and signal transduction (12). HSPs protect cells from environmental
stress damage by stabilizing the native folding of proteins, and
help to sequester severely damaged proteins for degradation. HSPs
are classified according to their size, and include HSP90, HSP70,
HSP60, HSP40 and HSP27 (13). When
proteotoxic damage is present in the cells, the demand on HSPs is
increased. In particular, HSP27 and HSP70 are the most strongly
induced by anticancer drugs, oxidative stress or IR (14). Overexpressed HSP27 and HSP70 have
been revealed to be associated with tumor metastasis, poor
prognosis and resistance to chemotherapy (15). Therefore, inhibition of HSP27 and
HSP70 has emerged as a novel therapeutic strategy for cancer
therapy.
In the present study, we found that constitutive
overexpression of Redd1 led to HSP27 and HSP70 induction in lung
cancer cells. Inhibition of HSP27 or HSP70 suppressed AKT
phosphorylation, which was induced by constitutive overexpression
of Redd1 and enhanced the inhibitory effects on the viability of
Redd1-overexpressing cells. Inhibition of AKT phosphorylation
resulted in a decrease of HSP27 and HSP70 expression in
Redd1-overexpressing cells. These data indicated that HSPs and AKT
in Redd1-overexpressing cells positively and mutually regulated
their function and expression, and were involved in lung cancer
cell survival. Knockdown of HSP27, HSP70 or AKT enhanced IR
sensitivity, particularly in lung cancer cells in which Redd1 was
stably overexpressed. Collectively, constitutive overexpression of
Redd1 led to AKT activation and HSP27 and HSP70 induction, all of
which were involved in lung cancer cell survival and resistance to
IR, suggesting that Redd1 may be used as a therapeutic target for
lung cancer.
Materials and methods
Cell culture, reagents and γ-ionizing
radiation
H1299 lung cancer cells that stably overexpressed
vector or Redd1 (11) were
maintained in RPMI-1640 medium (Welgene, Inc., Gyeongsangbuk-do,
Korea) supplemented with 10% fetal bovine serum (FBS) and 1 µg/ml
puromycin (Sigma- Aldrich; Merck KGaA, Darmstadt, Germany).
Cisplatin (cis-diammineplatinum (II) dichloride) and
thiazolyl blue tetrazolium bromide (MTT) were purchased from
Sigma-Aldrich (Merck KGaA). 137Cesium (137Cs)
was used as a source of γ-radiation (Atomic Energy of Canada
Limited, Chalk River, ON, Canada).
Measurement of cell viability
Cell viability was assessed by measuring the
mitochondrial conversion of MTT. The proportion of converted MTT
was calculated by measuring the absorbance at 570 nm. The results
are expressed as the percentage reduction in MTT, assuming that the
absorbance of the control cells was 100%. The MTT experiments were
repeated 3 times.
Isolation of RNA and reverse
transcription PCR analysis
Total RNA was isolated from cells using TRIzol
reagent, according to the manufacturer's instructions (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). cDNA primed with
oligo dT was prepared from 2 µg total RNA using M-MLV Reverse
Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.). The
following specific primers were used for PCR: Redd1
(5′-GAACTCCCACCCCAGATCGG-3′ and 5′-CGAGGGTCAGCTGGAAGGTG-3′; 468-bp
product) (16), HSP27
5′-AAGGATGGCGTGGTGGAGATC-3′ and 5′-TCGTTGGACTGCGTGGCTAG-3′; 194-bp
product) (17), HSP70
5′-ATGAAGCACTGGCCTTTCCA-3′ and 5′-TTGTTCTGGCTGATGTCCTT-3′; 512-bp
product) (18), HSP90
(5′-TCTGGAAGATCCCCAGACAC-3′ and 5′-AGTCATCCCTCAGCCAGAGA-3′; 189-bp
product) (19) and β-actin
(5′-GGATTCCTATGTGGGCGACAG-3′ and 5′-CGCTCGGTGAGGATCTTCATG-3′;
438-bp product) (16).
Real-time PCR
Real-time PCR was conducted using TaqMan gene
expression assays (Applied Biosystems; Thermo Fisher Scientific,
Inc.) on an ABI 7500 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The following primers/probes were
used: Redd1 (assay ID: Hs01111681_g1), HSP27 (assay
ID: Hs00356629_g1) and HSP70 (assay ID: Hs00271229_s1). The
expression of each genes were normalised to β-actin (assay
ID: Hs01060665_g1). The thermocycling included an initial step at
50°C for 2 min, followed by 10 min at 95°C and 40 cycles of 15 sec
at 95°C and 1 min at 60°C. The fold-change of gene expression was
determined using the comparative Cq (2−ΔΔCq) method
(20).
Redd1, HSP27 and HSP70 mRNA levels in lung cancer
were analyzed using a commercially available TissueScan Lung Cancer
Tissue qPCR panel (Lung Cancer cDNA array II #HLRT502; Origene
Technologies, Inc., Rockville, MD, USA). This contained cDNA from 5
normal lungs, 25 stage I, 6 stage II, 10 stage III, and 2 stage IV
lung cancer samples.
siRNAs and transfections
Redd1 (cat. no. sc-45806), AKT1/2 (cat. no.
sc-43609), Rictor (cat. no. sc-61478) and control (cat. no.
sc-37007) siRNAs were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). HSP70 siRNAs (20) were synthesized as follows (Bioneer,
Co., Daejeon, Korea): siRNA-HSP70 sense, CGGUUUCUACAUGCAGAGA-dT dT
and siRNA-HSP70 antisense, UCUCUGCAUGUAGAAACCG-dT dT. The
transfection experiments were performed using Lipofectamine 2000,
according to the manufacturer's instructions (Invitrogen; Thermo
Fisher Scientific, Inc.).
Western blot analysis
Cells were lysed in RIPA buffer [50 mM Tris (pH
7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1% sodium
deoxycholate, 5 mM EDTA, 100 mM NaF and 1 mM
Na3VO4] containing protease inhibitor
cocktail (Roche Diagnostics GmbH, Penzberg, Germany) for 30 min at
4°C. Cell lysates were cleared by centrifugation at 12,000 × g for
20 min at 4°C, and the protein concentrations were measured by
Bradford reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Protein samples (15–30 µg) were separated using 8–12% SDS-PAGE gels
and transferred onto nitrocellulose membranes. The membranes were
blocked in Tris-buffered saline (TBS) containing 0.1% Tween (TBST)
and 5% non-fat dry milk for 1 h at room temperature. The primary
antibodies were incubated overnight at 4°C. The following
antibodies were used: Redd1 (dilution 1:1,000; cat. no. 10638-1-AP)
was obtained from ProteinTech Group, Inc. (Rosemont, IL, USA); AKT
(dilution 1:1,000; cat. no. 9272), p-AKT at Ser473 (dilution
1:1,000; cat. no. 9271), HSP27 (dilution 1:2,000; cat. no. 2402),
Rictor (dilution 1:1,000; cat. no. 2114), S6 (dilution 1:2,000;
cat. no. 2217) and p-S6 at Ser240/244 (dilution 1:2,000; cat. no.
4838) were all obtained from Cell Signaling Technology, Inc.
(Beverly, MA, USA); HSP70 (dilution 1:2,000; cat. no. ADI-SPA-812)
was purchased from Enzo Life Sciences, Inc. (Farmingdale, NY, USA);
HSP90 (dilution 1:2,000; cat. no. sc-69703) was obtained from Santa
Cruz Biotechnology, Inc.; and β-actin (dilution 1:3,000; cat. no.
A5316) was purchased from Sigma-Aldrich (Merck KGaA). After 3
washes in TBST, the membranes were incubated for 1 h at room
temperature with horseradish peroxidase-conjugated anti-rabbit IgG
(dilution 1:3,000; cat. no. sc-2030; Santa Cruz Biotechnology,
Inc.) and anti-mouse IgG (dilution 1:3,000; cat. no. sc-2005; Santa
Cruz Biotechnology, Inc.) secondary antibodies. The immunoreactive
bands were visualized using SuperSignal West Pico Chemiluminescent
substrates (Pierce; Thermo Fisher Scientific Inc.). Where
indicated, western blot images were quantified using ImageJ
software (version 1.52a; NIH; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
The results are expressed as the mean ± standard
deviation (SD) of 3 independent experiments. Statistical analysis
was performed using one-way analysis of variance (ANOVA), followed
by Tukey's post hoc test of GraphPad Prism software (version 5.0;
GraphPad Software Inc., San Diego, CA, USA). P<0.05, P<0.01
and P<0.001 were considered to indicate statistically
significant results.
Results
Expression of HSP27 and HSP70 is
increased in Redd1-overexpressing cells
We first analyzed proteins with expression levels
that had changed in Redd1-overexpressing cells using
two-dimensional gel electrophoresis-based proteomic analysis, and
we observed increased levels of HSP70 protein (data not shown).
Next, we used western blot analysis to further investigate the
expression of HSPs in H1299 lung cancer cells that stably
overexpressed Redd1. Increased levels of HSP27 and HSP70 protein
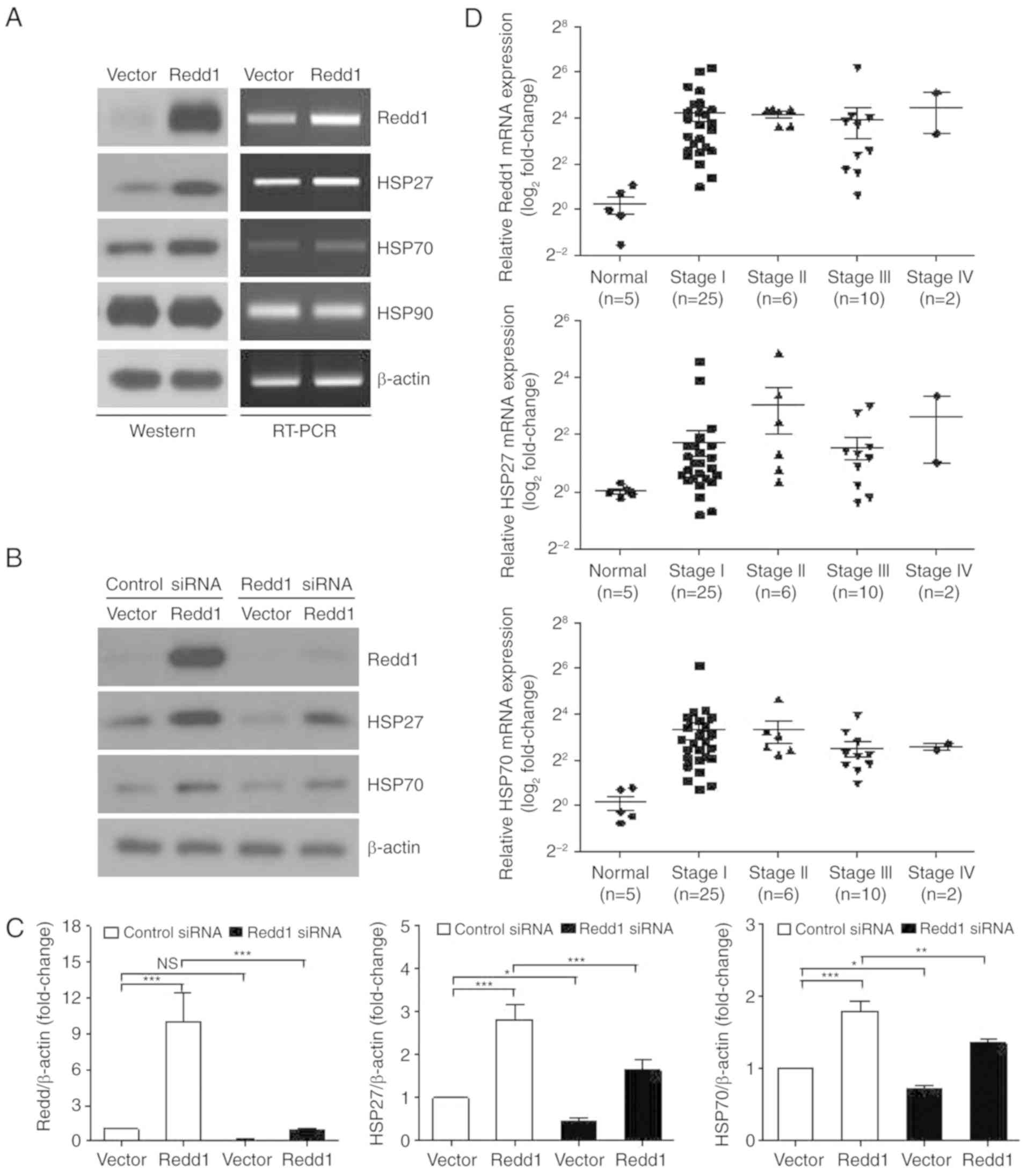
were observed in Redd1-overexpressing cells (Fig. 1A, left panel). However, constitutive
overexpression of Redd1 did not change the levels of HSP90 protein
(Fig. 1A, left panel). Expression
of HSP27 and HSP70 mRNA was also increased in Redd1-overexpressing
cells (Fig. 1A, right panel). To
further ascertain whether constitutive overexpression of Redd1
induced HSP27 and HSP70 expression, Redd1 siRNAs were transfected
in Redd1-overexpressing cells. As revealed in Fig. 1B and C, treatment with Redd1 siRNA
reduced Redd1-mediated HSP27 and HSP70 expression. Based on these
results, it was indicated that constitutive overexpression of Redd1
induces HSP27 and HSP70 expression.
Clinically, increased expression of HSP27 and HSP70
has been shown in high-grade malignant tumors (21–23).
Redd1 expression was significantly increased in non-small lung
cancer tissues compared with that in normal lung tissues (24). To evaluate HSP27, HSP70 and Redd1
mRNA expression in the different stages of lung cancer, we used a
commercially available Lung Cancer Tissue qPCR panel. This included
cDNAs obtained from 48 patients with histopathologically confirmed
lung cancer, representing all stages (stage I, n=25; stage II, n=6;
stage III, n=10; stage IV, n=2), and normal lung samples (n=5).
Redd1, HSP27 and HSP70 mRNA levels were higher in lung cancer
tissues than in normal lung tissues (Fig. 1D).
Knockdown of HSP27 or HSP70 inhibits
AKT phosphorylation and enhances the inhibitory effects on the
viability of Redd1-overexpressing cells
HSP27 has been reported to control apoptosis by
regulating the AKT signaling pathway (25,26).
We previously reported that constitutive overexpression of Redd1
resulted in AKT activation, which was involved in lung cancer cell
survival (11). Thus, we examined
AKT phosphorylation levels and viability in Redd1-overexpressing
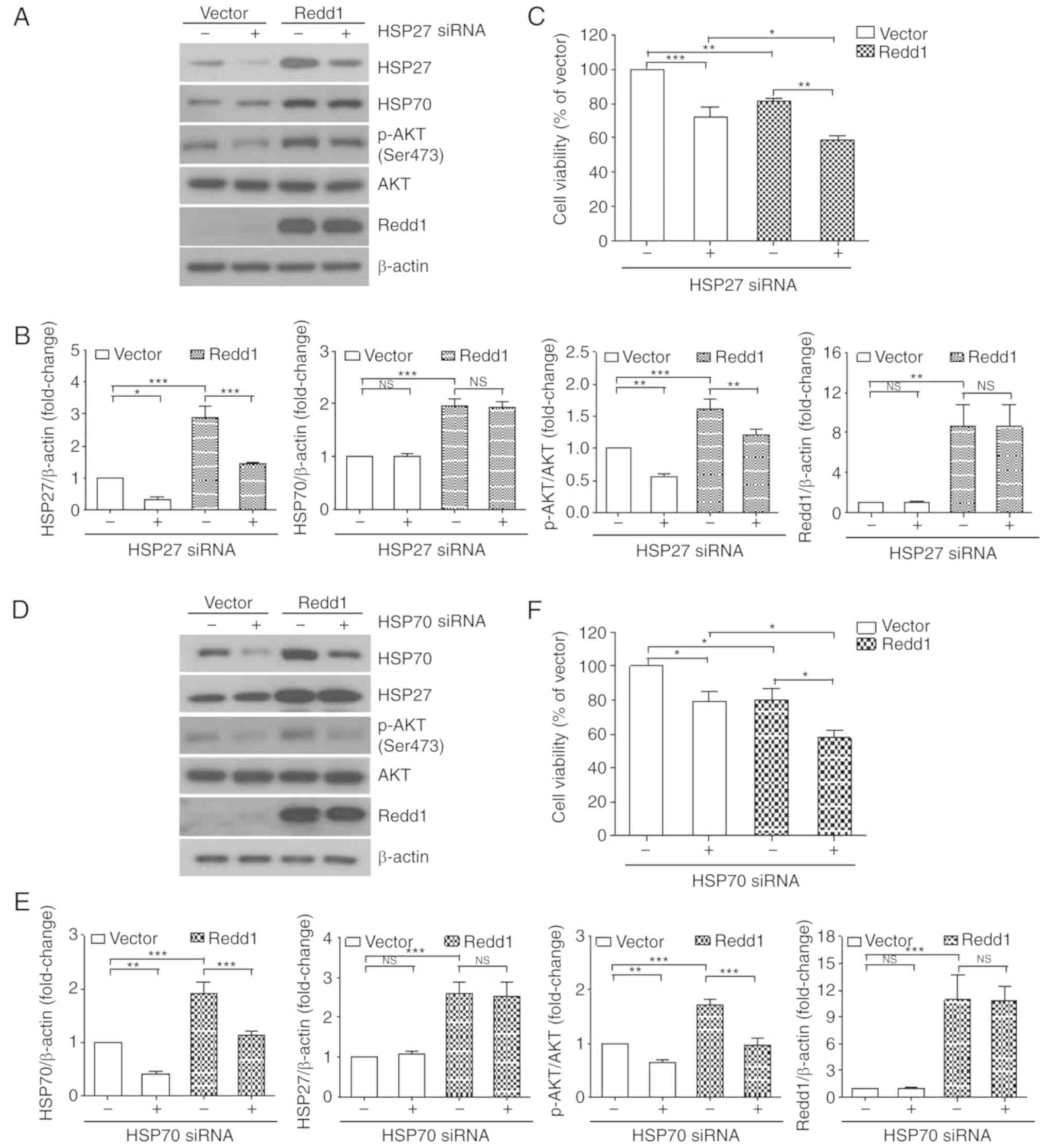
cells following treatment with a siRNA against HSP27. As
anticipated, treatment with HSP27 siRNA resulted in decreased
levels of AKT phosphorylation, which was induced by constitutive
overexpression of Redd1 (Fig. 2A and
B). Moreover, cell viability was significantly reduced in the
HSP27 siRNA-treated, Redd1-overexpressing cells when compared with
the control siRNA-treated, Redd1-overexpressing cells (Fig. 2C). However, knockdown of HSP27 did
not affect the levels of HSP70 in either the vector or the
Redd1-overexpressing cells (Fig. 2A and
B).
Next, we examined AKT phosphorylation levels and
viability in Redd1-overexpressing cells following treatment with a
siRNA against HSP70. HSP70 siRNA inhibited AKT phosphorylation
which was induced by constitutive overexpression of Redd1 (Fig. 2D and E), and enhanced the inhibitory
effects on the viability of Redd1-overexpressing cells (Fig. 2F). However, knockdown of HSP70 did
not affect the levels of HSP27 in either the vector or the
Redd1-overexpressing cells (Fig. 2D and
E). These data indicated that HSP27 and HSP70 induction by
constitutive overexpression of Redd1 was required for AKT
phosphorylation, and was involved in lung cancer cell survival.
Inhibition of AKT phosphorylation
reduces HSP27 and HSP70 expression induced by constitutive
overexpression of Redd1
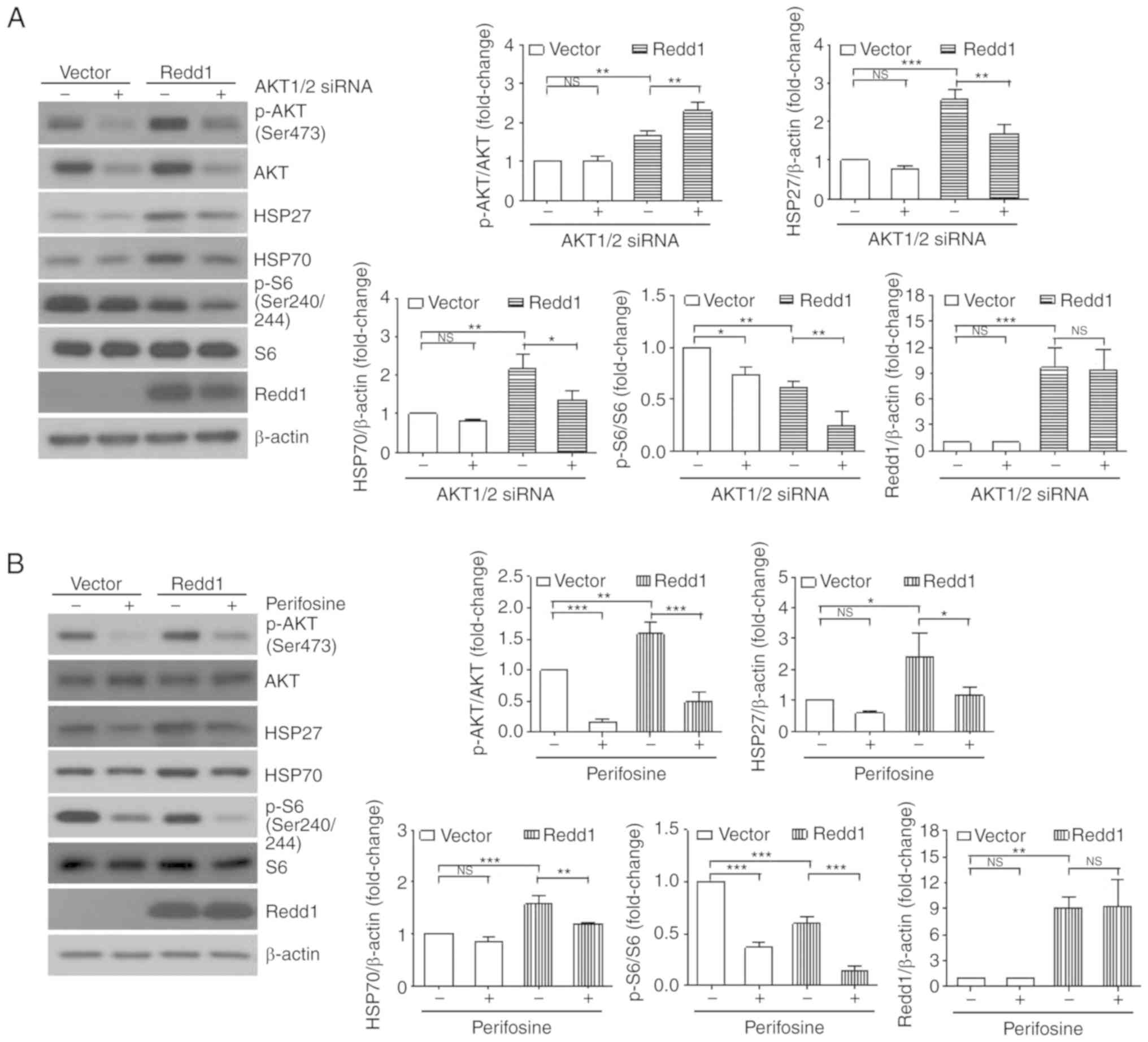
To examine the effects of AKT on HSP27 and HSP70
expression induced by constitutive overexpression of Redd1, we
investigated the expression of HSP27 and HSP70 following treatment
with AKT1/2 siRNA or perifosine, a selective AKT inhibitor. As
revealed in Fig. 3A and B,
depletion of Akt by siRNAs or inhibition of AKT by perifosine
suppressed Redd1-induced HSP27 and HSP70 expression, indicating
that AKT activity was required for HSP27 and HSP70 expression in
Redd1-overexpressing cells. Treatment of AKT1/2 siRNA or perifosine
also further enhanced the inhibition of mTORC1 activity by Redd1,
as judged by the decrease in S6 phosphorylation. We previously
reported that Redd1-induced AKT activation was mediated by mTORC2
(11). Thus, we investigated the
effects of mTORC2 on Redd1-induced HSP27 and HSP70 expression. The
expression of Rictor, a key component of mTORC2, was suppressed by
treatment with Rictor siRNA. As revealed in Fig. 3C, Rictor siRNA not only inhibited
AKT phosphorylation as previously reported (11), but also decreased HSP27 and HSP70
expression in Redd1-overexpressing cells. Knockdown of Rictor led
to further increases in the cisplatin sensitivity of
Redd1-overexpressing cells when compared with that of
vector-overexpressing cells (Fig.
3D). These data indicated that mTORC2 activity was required for
HSP27 and HSP70 expression in Redd1-overexpressing cells.
Inhibition of HSP27, HSP70 or AKT
increases the sensitivity of Redd1-overexpressing cells to IR
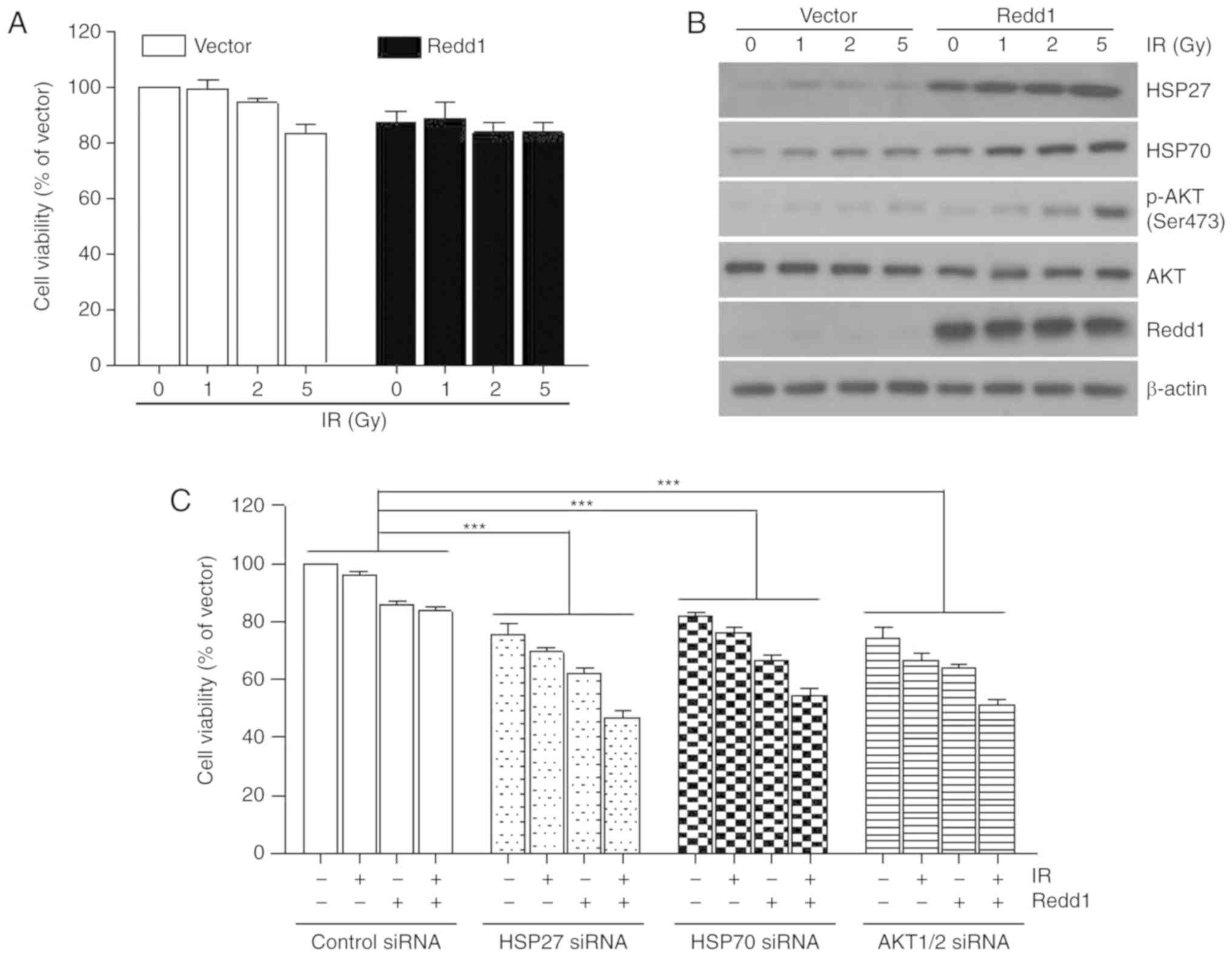
Radiation therapy for lung cancer is effective for
destroying cancer cells and shrinking tumors. Thus, H1299 cells
that stably overexpressed vector or Redd1 were exposed to
increasing doses of IR for 24 h. The cell viability rate was
>80%, despite the exposure of both cells to 5 Gy of IR (Fig. 4A). IR increased the levels of HSP27
and HSP70 expression and AKT phosphorylation in a dose-dependent
manner in H1299 cells that stably overexpressed vector or Redd1
(Fig. 4B). Transfection of HSP27,
HSP70 or AKT1/2 siRNAs in Redd1-overexpressing cells led to further
increases in IR sensitivity when compared with the control
siRNA-treated, Redd1-overexpressing cells (Fig. 4C). These data indicated that
inhibition of HSP27, HSP70, or AKT enhanced IR sensitivity,
particularly in cells in which Redd1 was stably overexpressed.
Discussion
Redd1 (also known as RTP801, DDIT4 or Dig2) acts as
a negative regulator of mechanistic target of rapamycin complex I
(mTORC1), which integrates diverse signals to regulate cell growth
and metabolism (1,5,6). In
the present study, we revealed that constitutive overexpression of
Redd1 induced HSP27 and HSP70 expression. Clinically, increased
expression of HSP27 and HSP70 has been monitored in high-grade
malignant tumors, such as osteosarcoma, leukemia, breast, ovarian
and endometrial cancer, and renal cell carcinoma (21–23).
Redd1 was significantly increased in non-small cell lung cancer
(NSCLC) tissue compared with normal lung tissue (24). In the present study, qRT-PCR human
lung cancer tissue cDNA arrays demonstrated that Redd1, HSP27 and
HSP70 mRNA expression levels were increased in lung cancer tissue
compared with those in normal lung tissue.
Several studies have reported that the resistance to
apoptosis and antiproliferative signals observed in cancer cells is
actually correlated with HSP27 and HSP70 induction (14). Pre-clinical and patient studies
demonstrated that overexpression of HSP27 and HSP70 corresponded
with an increased proliferation of malignant cells, and that the
inhibition of HSP27 and HSP70 expression and function reduced the
proliferation and increased the susceptibility of tumor cells to
chemotherapy and radiotherapy (27–29).
Disruption of HSP27 or HSP70 enhanced the viability of
Redd1-overexpressing H1299 lung cancer cells (Fig. 2). Ionizing radiation (IR) increased
HSP27 and HSP70 expression levels in a dose-dependent manner in
cells that stably overexpressed vector or Redd1 (Fig. 4B). The inhibition of HSP27 and HSP70
with siRNA in Redd1-overexpressing cells led to further increases
in IR sensitivity when compared with the vector-overexpressing
cells (Fig. 4C). These data
indicated that HSP27 and HSP70 induction, which occured as a
consequence of the constitutive overexpression of Redd1, played a
role in lung cancer cell survival and resistance to IR.
Several studies have shown that HSP27 and HSP70
activate an adaptive mechanism to preserve cell survival through
AKT signalling (30,31). The AKT signaling pathway was also
revealed to regulate the expression of HSP27 and HSP70, which
contributed to tumor cell survival (32,33).
We also observed that inhibition of HSP27 or HSP70 function
suppressed AKT phosphorylation induced by the constitutive
overexpression of Redd1, and the inhibition of AKT function
suppressed Redd1-mediated HSP27 or HSP70 expression. These data
indicated that heat shock proteins (HSPs) and AKT positively
regulated each other in Redd1-overexpressing cells, and that they
were involved in lung cancer cell survival.
We previously reported that Redd1-induced AKT
activation was mediated by mTORC2 (11). Thus, we investigated the effects of
mTORC2 on Redd1-induced HSP27 and HSP70 expression. Inhibition of
mTORC2 using Rictor siRNA led to decreases in HSP27 and HSP70
expression and further increases in cisplatin sensitivity in
Redd1-overexpressing cells (Fig. 3C and
D), suggesting that mTORC2 activity was partially required for
HSP27 and HSP70 expression in Redd1-overexpressing cells.
Collectively, constitutive overexpression of Redd1
led to HSP27 and HSP70 induction and AKT activation, all of which
were involved in lung cancer cell survival, suggesting that Redd1
may be used as a therapeutic target for lung cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education
(NRF-2017R1D1A1B03029209/50604-2019), the Mid-Career Researchers
Program through the NRF funded by the Ministry of Science and ICT
(MIST) (NRF-2017R1A2B2008398/50649-2019) and the Korea Institute of
Radiological and Medical Sciences (KIRAMS) funded by the MIST
(50531–2019), Republic of Korea.
Availability of data and materials
The authors declare that the materials included in
the manuscript, including all relevant raw data, will be freely
available to any researchers who wish to use them for
non-commercial purposes, while preserving any necessary
confidentiality and anonymity.
Authors' contributions
HOJ and ICP developed the concept and designed the
study. HOJ, SEH, JYK and MRK performed the experiments. YHC, YJH
and JKL provided technical support and conceptual advice. HOJ and
ICP wrote the manuscript. HOJ, YHC, YJH, JKL and ICP reviewed and
edited the manuscript. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brugarolas J, Lei K, Hurley RL, Manning
BD, Reiling JH, Hafen E, Witters LA, Ellisen LW and Kaelin WG Jr:
Regulation of mTOR function in response to hypoxia by REDD1 and the
TSC1/TSC2 tumor suppressor complex. Genes Dev. 18:2893–2904. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reiling JH and Hafen E: The
hypoxia-induced paralogs Scylla and Charybdis inhibit growth by
down-regulating S6K activity upstream of TSC in Drosophila.
Genes Dev. 18:2879–2892. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ellisen LW, Ramsayer KD, Johannessen CM,
Yang A, Beppu H, Minda K, Oliner JD, McKeon F and Haber DA:
REDD1, a developmentally regulated transcriptional target of
p63 and p53, links p63 to regulation of reactive oxygen species.
Mol Cell. 10:995–1005. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sofer A, Lei K, Johannessen CM and Ellisen
LW: Regulation of mTOR and cell growth in response to energy stress
by REDD1. Mol Cell Biol. 25:5834–5845. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeYoung MP, Horak P, Sofer A, Sgroi D and
Ellisen LW: Hypoxia regulates TSC1/2-mTOR signaling and tumor
suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev.
22:239–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Corradetti MN, Inoki K and Guan KL: The
stress-inducted proteins RTP801 and RTP801L are negative regulators
of the mammalian target of rapamycin pathway. J Biol Chem.
280:9769–9772. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia W, Chang B, Sun L, Zhu H, Pang L, Tao
L, Zou H, Du J, Dong Y, Qi Y, et al: REDD1 and p-AKT
over-expression may predict poor prognosis in ovarian cancer. Int J
Clin Exp Pathol. 7:5940–5949. 2014.PubMed/NCBI
|
|
8
|
Decaux O, Clément M, Magrangeas F, Gouraud
W, Charbonnel C, Campion L, Loiseau HA and Minvielle S: Inhibition
of mTORC1 activity by REDD1 induction in myeloma cells resistant to
bortezomib cytotoxicity. Cancer Sci. 101:889–897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barakat DJ, Mendonca J, Barberi T, Zhang
J, Kachhap SK, Paz-Priel I and Friedman AD: C/EBPβ regulates
sensitivity to bortezomib in prostate cancer cells by inducing
REDD1 and autophagosome-lysosome fusion. Cancer Lett.
375:152–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pinto JA, Rolfo C, Raez LE, Prado A,
Araujo JM, Bravo L, Fajardo W, Morante ZD, Aguilar A, Neciosup SP,
et al: In silico evaluation of DNA Damage Inducible Transcript 4
gene (DDIT4) as prognostic biomarker in several
malignancies. Sci Rep. 7:15262017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin HO, Hong SE, Kim JH, Choi HN, Kim K,
An S, Choe TB, Hwang CS, Lee JH, Kim JI, et al: Sustained
overexpression of Redd1 leads to Akt activation involved in cell
survival. Cancer Lett. 336:319–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vahid S, Thaper D, Gibson KF, Bishop JL
and Zoubeidi A: Molecular chaperone Hsp27 regulates the Hippo tumor
suppressor pathway in cancer. Sci Rep. 6:318422016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seigneuric R, Mjahed H, Gobbo J, Joly AL,
Berthenet K, Shirley S and Garrido C: Heat shock proteins as danger
signals for cancer detection. Front Oncol. 1:372011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garrido C, Brunet M, Didelot C, Zermati Y,
Schmitt E and Kroemer G: Heat shock proteins 27 and 70:
Anti-apoptotic proteins with tumorigenic properties. Cell Cycle.
5:2592–2601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lianos GD, Alexiou GA, Mangano A, Mangano
A, Rausei S, Boni L, Dionigi G and Roukos DH: The role of heat
shock proteins in cancer. Cancer Lett. 360:114–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin HO, Seo SK, Woo SH, Kim ES, Lee HC,
Yoo DH, Choe TB, Hong SI, Kim JI and Park IC: SP600125 negatively
regulates the mammalian target of rapamycin via ATF4-induced Redd1
expression. FEBS Lett. 583:123–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sheng B, Qi C, Liu B, Lin Y, Fu T and Zeng
Q: Increased HSP27 correlates with malignant biological behavior of
non-small cell lung cancer and predicts patient's survival. Sci
Rep. 7:138072017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Endo H, Yano M, Okumura Y and Kido H:
Ibuprofen enhances the anticancer activity of cisplatin in lung
cancer cells by inhibiting the heat shock protein 70. Cell Death
Dis. 5:e10272014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mehta A, Shervington L, Munje C and
Shervington A: A novel therapeutic strategy for the treatment of
glioma, combining chemical and molecular targeting of hsp90a.
Cancers. 3:4228–4244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Parcellier A, Schmitt E, Brunet M, Hammann
A, Solary E and Garrido C: Small heat shock proteins HSP27 and
alphaB- crystallin: Cytoprotective and oncogenic functions.
Antioxid Redox Signal. 7:404–413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santarosa M, Favaro D, Quaia M and
Galligioni E: Expression of heat shock protein 72 in renal cell
carcinoma: Possible role and prognostic implications in cancer
patients. Eur J Cancer. 33:873–877. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nanbu K, Konishi I, Mandai M, Kuroda H,
Hamid AA, Komatsu T and Mori T: Prognostic significance of heat
shock proteins HSP70 and HSP90 in endometrial carcinomas. Cancer
Detect Prev. 22:549–555. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su J, Huang H, Ju S and Shi J: Elevated
RTP801 promotes cell proliferation in non-small cell lung cancer.
IUBMB Life. 70:310–319. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rane MJ, Pan Y, Singh S, Powell DW, Wu R,
Cummins T, Chen Q, McLeish KR and Klein JB: Heat shock protein 27
controls apoptosis by regulating Akt activation. J Biol Chem.
278:27828–27835. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y and Shen X: Heat shock protein 27
protects L929 cells from cisplatin-induced apoptosis by enhancing
Akt activation and abating suppression of thioredoxin reductase
activity. Clin Cancer Res. 13:2855–2864. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aloy MT, Hadchity E, Bionda C, Diaz-Latoud
C, Claude L, Rousson R, Arrigo AP and Rodriguez-Lafrasse C:
Protective role of Hsp27 protein against gamma radiation-induced
apoptosis and radiosensitization effects of Hsp27 gene silencing in
different human tumor cells. Int J Radiat Oncol Biol Phys.
70:543–553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heinrich JC, Tuukkanen A, Schroeder M,
Fahrig T and Fahrig R: RP101 (brivudine) binds to heat shock
protein HSP27 (HSPB1) and enhances survival in animals and
pancreatic cancer patients. J Cancer Res Clin Oncol. 137:1349–1361.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schultz CR, Golembieski WA, King DA, Brown
SL, Brodie C and Rempel SA: Inhibition of HSP27 alone or in
combination with pAKT inhibition as therapeutic approaches to
target SPARC-induced glioma cell survival. Mol Cancer. 11:202012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu R, Kausar H, Johnson P, Montoya-Durango
DE, Merchant M and Rane MJ: Hsp27 regulates Akt activation and
polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt
signal complex. J Biol Chem. 282:21598–21608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Powers MV, Clarke PA and Workman P: Dual
targeting of HSC70 and HSP72 inhibits HSP90 function and induces
tumor-specific apoptosis. Cancer Cell. 14:250–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chatterjee M, Andrulis M, Stühmer T,
Müller E, Hofmann C, Steinbrunn T, Heimberger T, Schraud H,
Kressmann S, Einsele H and Bargou RC: The PI3K/Akt signaling
pathway regulates the expression of Hsp70, which critically
contributes to Hsp90-chaperone function and tumor cell survival in
multiple myeloma. Haematologica. 98:1132–1141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghosh A, Lai C, McDonald S, Suraweera N,
Sengupta N, Propper D, Dorudi S and Silver A: HSP27 expression in
primary colorectal cancers is dependent on mutation of KRAS
and PI3K/AKT activation status and is independent of TP53.
Exp Mol Pathol. 94:103–108. 2013. View Article : Google Scholar : PubMed/NCBI
|