Introduction
Tranilast [N-(3′,4′-dimethoxycinnamoyl)-anthranilic
acid] was developed as an antiallergic drug for the treatment of
inflammatory diseases, including bronchial asthma, atypical
dermatitis, allergic conjunctivitis, keloids and hypertrophic scars
(1). It has also been shown that
tranilast is effective in a wide range of conditions such as
vascular injury (2), osteoporosis
(3), diabetes, autoimmune disease,
ocular disease and renal fibrosis (1). The largest clinical trial to explore
new applications of tranilast was the Prevention of REStenosis with
Tranilast and its Outcomes (PRESTO) trial (4), which examined whether tranilast
decreased the frequency of angiographic restenosis after
percutaneous coronary intervention. Although the PRESTO trial
failed to show improvement in the incidence of restenosis,
researchers have been investigating the efficacy of tranilast for
several other diseases (3,5,6).
Tranilast has been shown to have an inhibitory effect on the growth
of various types of cancer cells, including breast (7,8),
prostate (9), pancreas (10), lung (11) and stomach (12). However, tranilast has not been
approved for cancer treatment.
Osteosarcoma is the most common primary malignant
bone tumor and is generally treated with preoperative chemotherapy,
surgery and postoperative chemotherapy. Although prognosis has
improved for patients with localized disease, patients with
metastatic disease still have a poor prognosis. The most popular
chemotherapeutic drugs, including high-dose methotrexate,
doxorubicin, cisplatin and ifosfamide, are used to eradicate
micrometastases; however, 20–30 % of patients cannot be cured of
metastatic disease, especially in the lungs (13). Although new anticancer agents for
soft tissue sarcoma (pazopanib, eribulin and trabectedin) have been
approved over the past 10 years, only one drug (mifamurtide) has
been approved for osteosarcoma in Europe but not in the USA) in the
past 20 years. Therefore, we investigated whether tranilast, which
has been widely used clinically without severe adverse effects,
enhances the anticancer effect of existing chemotherapeutic drugs.
Furthermore, we analyzed the mechanism of action of tranilast when
it acts synergistically with anticancer drugs.
Materials and methods
Cell culture
Osteosarcoma cell lines 143B, U2OS and MG-63 were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). HOS and normal dermal fibroblast WI-38 cell lines were
obtained from RIKEN BioResource Center (Tsukuba, Japan). These cell
lines were cultured in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich, Merck KGaA; Darmstadt, Germany) supplemented with
10% fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 100 U/ml penicillin (Thermo Fisher Scientific, Inc.) and
100 µg/ml streptomycin (Thermo Fisher Scientific, Inc.). Cell lines
were maintained for up to 20 passages at 37°C in 5% CO2.
Regarding p53 status, HOS, 143B and MG-63 express mutant p53
(14,15), whereas U2OS expresses wild-type p53
(16).
Analysis of cell viability
Cells were treated with tranilast (Tokyo Chemical
Industry, Co., Ltd., Tokyo, Japan), doxorubicin (Sigma-Aldrich,
Merck KGaA) and cisplatin (Tokyo Chemical Industry). After 48 h of
treatment, cell viability was analyzed using a colorimetric assay
for mitochondrial dehydrogenase activity (WST-1; Roche Diagnostics,
Basel, Switzerland), as previously described (17).
Cell cycle analysis
Human osteosarcoma cells were cultured with 2 µM
cisplatin or 100 nM doxorubicin with or without 200 µM tranilast.
An equivalent volume of vehicle was used as the control. Cell cycle
analysis was performed 48 h after treatment, as previously reported
(17). Cells were collected, fixed
with 70% ethanol at −20°C, washed with phosphate-buffered saline
(PBS), and treated with Guava Cell Cycle reagent (Merck Millipore,
Burlington, MA, USA). DNA content was examined using flow cytometry
(CytoFLEX; Beckman Coulter, Brea, CA, USA).
Cell apoptosis analysis
Human osteosarcoma cells were cultured for 48 h with
or without 200 µM tranilast or 2 µM cisplatin, and an equivalent
volume of vehicle was used as the control. Cells were treated with
the Annexin V-FITC/7-ADD kit (Beckman Coulter), and
fluorescence-activated cell sorting was performed on a CytoFLEX
flow cytometer (Beckman Coulter).
Western blotting
Western blotting was performed as previously
described (18). Cells were lysed
using NP40 buffer, which contained 0.5% NP-40, 10 mM Tris-HCl (pH
7.4), 150 mM NaCl, 3 mM phenylmethylsulfonyl fluoride (Wako Pure
Chemical Industries Co., Ltd., Kanagawa, Japan), 5 mg/ml aprotinin
(Sigma-Aldrich; Merck KGaA), 2 mM sodium orthovanadate (Wako Pure
Chemical Industries) and 5 mM EDTA. Lysates were boiled in SDS
sample buffer (10 µg of protein loaded per lane), separated using
SDS-PAGE (4–15% gradient gel; cat no. 456-1086; Bio-Rad
Laboratories, Hercules, CA, USA) and transferred to polyvinylidene
fluoride (PVDF) membranes (Caliper Life Sciences, Inc., Mountain
View, CA, USA). Membranes were blocked in 5% non-fat dry milk in
Tris-buffered saline Tween-20 (TBST) and incubated with primary
antibodies against cleaved caspase-3 (dilution 1:500; cat. no.
9661; Cell Signaling Technology, Danvers, MA, USA), cleaved
poly(ADP-ribose) polymerase (cleaved PARP; dilution 1:500; cat. no.
5625; Cell Signaling Technology), H2A histone family member X
(H2AX; dilution 1:1,000; cat. no. 7631; Cell Signaling Technology)
phospho-H2AX (p-H2AX; dilution 1:1,000; cat. no. 2577; Cell
Signaling Technology), p21 (dilution 1:1,000; cat. no. 2947; Cell
Signaling Technology), Bim (dilution 1:1,000; cat. no. 2933; Cell
Signaling Technology), ataxia telangiectasia and Rad3-related
kinase (ATR; dilution 1:500; cat. no. 13934; Cell Signaling
Technology), phospho-ATR (p-ATR; dilution 1:500; cat. no. 58014;
Cell Signaling Technology), checkpoint kinase 1 (CHK1; dilution
1:500; cat. no. 2360; Cell Signaling Technology), phospho-CHK 1
(p-CHK1; dilution 1:500; cat. no. 12302; Cell Signaling
Technology), cyclin-dependent kinase 1 (CDK1; dilution 1:500; cat.
no. 28439; Cell Signaling Technology), phospho-CDK1 at Y15 (p-CDK1
Y15; dilution 1:500; cat. no. 9111; Cell Signaling Technology),
p-CDK1 T161 (dilution 1:500; cat. no. 9114; Cell Signaling
Technology), Wee1 (dilution 1:500; cat. no. 13084; Cell Signaling
Technology), poshpo-Wee1 (p-Wee1; dilution 1:500; cat. no. 4910;
Cell Signaling Technology) and tubulin (dilution 1:1,000; cat. no.
66031-1-IG; Proteintech Group, Inc., Rosemont, IL, USA) diluted in
TBST overnight at 4°C. Blots were washed using TBST and incubated
with horseradish-peroxidase-conjugated secondary antibodies
(anti-rabbit; dilution 1:5,000; cat. no. 7074; Cell Signaling
Technology, or anti-mouse; dilution 1:5,000; cat. no. 7076; Cell
Signaling Technology) in TBST for 1 or 2 h at room temperature.
Immunocomplexes were visualized using an enhanced chemiluminescence
kit (ECL; GE Healthcare, Tokyo, Japan).
Drug combination studies
Synergism after treatment with tranilast and
cisplatin was evaluated using CalcuSyn software version 2 (Biosoft,
Ferguson, MO, USA), which is based on the median-effect principle
originally established by Chou and Talalay (19). From the fraction affected by the
dose obtained from cell proliferation assays and the dose of drug,
the software draws a dose-effect curve and calculates the
median-effect dose (ED50). For each combined dose
effect, a combination index (CI) was generated. The effects of the
combinations were then transformed into and displayed as
fraction-affected CI plots. If the data of single-agent and
combination use were inputted, the software calculated the CI,
which represented the pharmacological interaction of two drugs. A
CI value of 1 indicates an additive effect between the two agents,
whereas CI<1 or CI>1 indicates synergism or antagonism,
respectively.
Animal studies
Experiments with a xenograft mouse model were
performed as follows: a total of 24 mice (5-week-old; nude mice;
weight, 18–24 g, Nihon SLC, Hamamatsu, Japan) were housed in the
animal facility under a 12-h day/night cycle, temperature of
23±1°C, relative humidity of 50±10% with ad libitum access
to food and water. Suspensions of 1×106 143B cells in
100 µl Matrigel (Corning Inc., Corning, NY, USA) were
subcutaneously inoculated into the flank of mice. Xenograft models
were randomly divided into four groups of either treatment with
tranilast 400 mg/kg/day, cisplatin 4 mg/kg twice weekly, a
combination of tranilast and cisplatin, or an equal volume of
vehicle as a control. Tumor volume and body weight was measured
twice a week. Tumor volume (V) was calculated using the following
formula: V = LW2/2, where L and W indicate the length
and width of the tumors, respectively. All animal humanely
sacrificed by CO2 inhalation when they met the following
humane endpoint criteria: Severe tumor burden (the maximal diameter
of tumor exceeded 20 mm), weight loss exceeded 10% of the total
weight, prostration and difficulty of breathing. All animal
experiments were performed in compliance with the guidelines of the
Institute of Laboratory Animal Sciences, Graduate School of Medical
and Dental Sciences, Kagoshima University. Every effort was made to
minimize both the number of animals used and animal pain.
Statistical analysis
For the in vitro experiments, data are
expressed as mean ± standard deviation (SD) values shown from three
independent experiments. Statistical analysis was performed by
one-way analysis of variance (ANOVA) followed by Tukey's post hoc
test. To analyze the difference between the dose-response curves
for tranilast in osteosarcoma and fibroblast, ANCOVA (analysis of
covariance) was used. For the in vivo experiment,
statistical analysis was performed with a non-parametric multiple
comparison test using the Steel-Dwass method. P<0.05 was
considered to indicate a statistically significant difference.
Results
Tranilast inhibits the proliferation
of osteosarcoma cells
Osteosarcoma cell lines (HOS, 143B, U2OS and MG-63)
and normal fibroblasts (WI-38) were treated with tranilast (50,
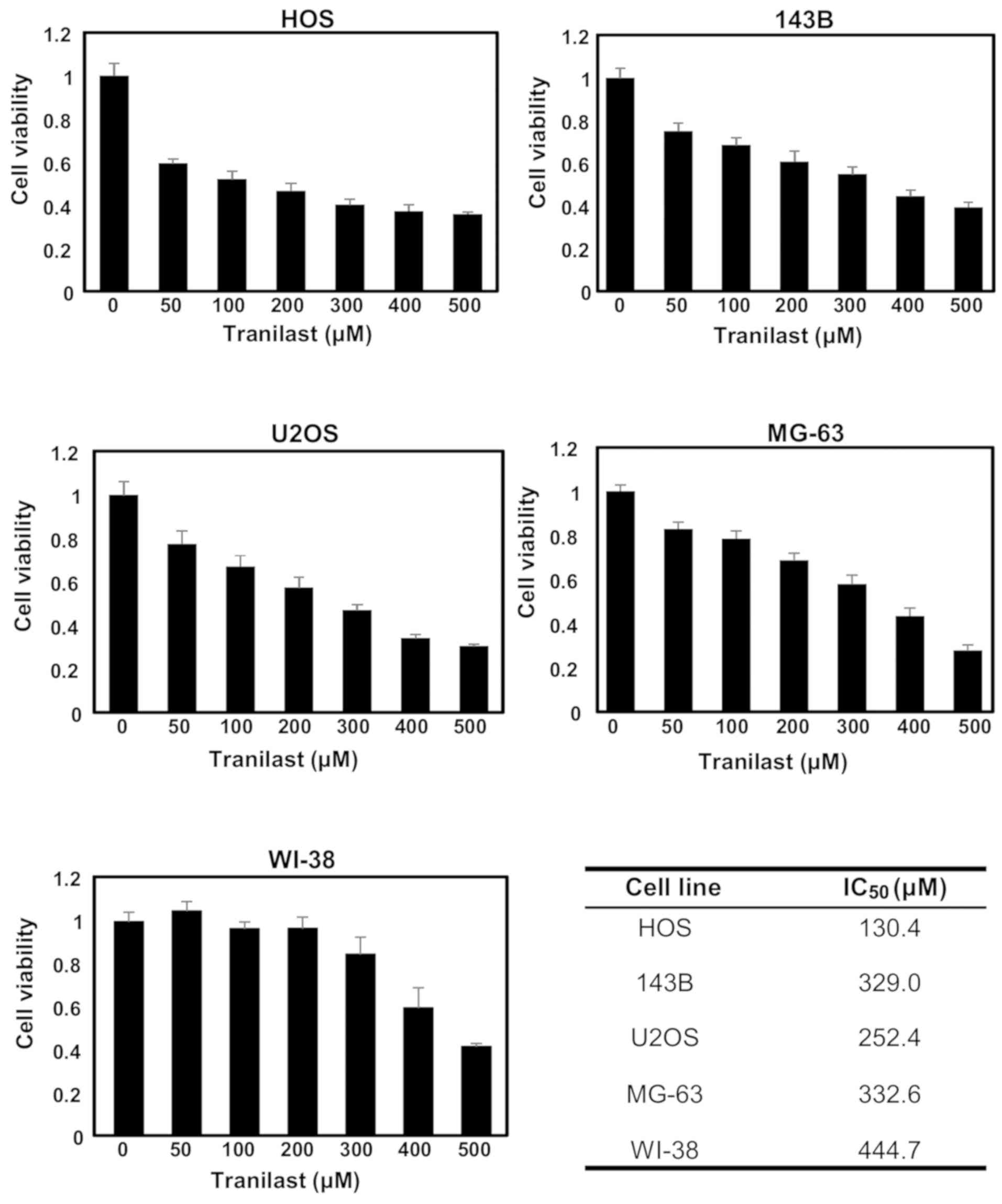
100, 200, 300, 400 and 500 µM) for 48 h and cell viability was
determined. The effect of tranilast was small, although
proliferation was inhibited in all four cell lines in a
dose-dependent manner (Fig. 1).
IC50 values for HOS, 143B, U2OS and MG-63 cells were
130.4, 329.0, 252.4 and 332.6 µM, respectively. In contrast, the
IC50 value for WI-38 was 444.7 µM. At 200 µM of
tranilast, the viability of all four osteosarcoma cell lines were
significantly reduced compared with that of WI-38 fibroblasts
(ANOVA with Tukey's test, vs. HOS, P=0.00001; vs. 143B, P=0.0008;
vs. U2OS, P=0.001; vs. MG-63, P=0.02). Therefore, we performed
experiments using the combination treatment of tranilast and
anticancer drugs at 200 µM of tranilast. Analysis of covariance
(ANCOVA) of cell viability at 0–500 µM demonstrated significant
statistical difference between the four osteosarcoma cell lines and
normal fibroblast WI-38 (vs. HOS, P=0.0007; vs. 143B, P=0.0005; vs.
U2OS, P=0.0001; vs. MG-63, P=0.0003).
Combined treatment with tranilast and
anticancer agents, cisplatin and doxorubicin enhances the cytotoxic
effect on osteosarcoma cells
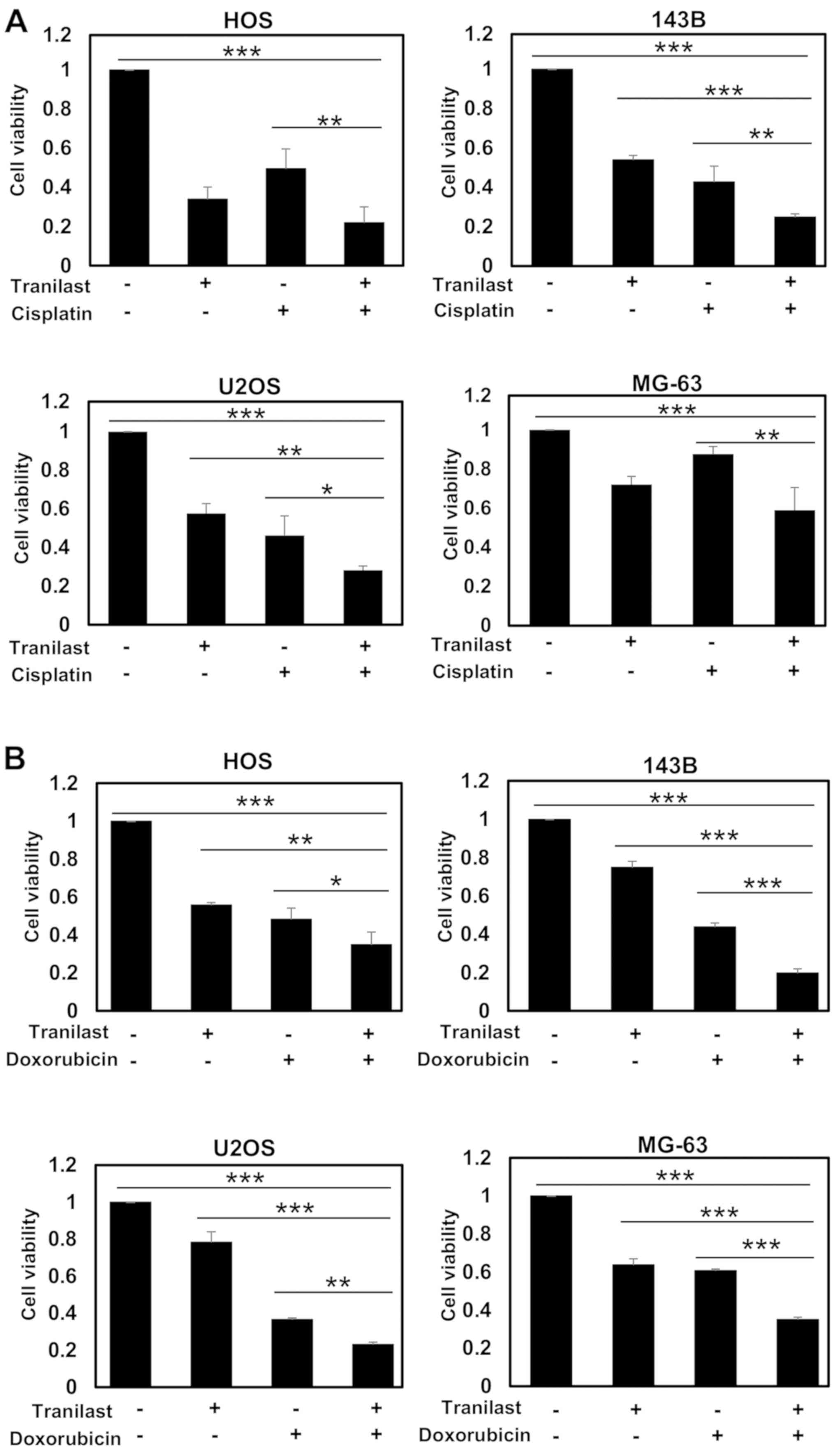
To determine whether tranilast enhances the effect
of anticancer agents, osteosarcoma cells were treated with combined
tranilast and anticancer drugs. Tranilast significantly enhanced
the effect of cisplatin on osteosarcoma cell lines in regards to
reduced cell viability (Fig. 2A).
Tranilast also significantly enhanced the cytotoxic effect of
doxorubicin in regards to reduced cell viability (Fig. 2B). However, the enhancement was less
than that for cisplatin because the cell lines were relatively
sensitive to doxorubicin. Next, we examined whether tranilast and
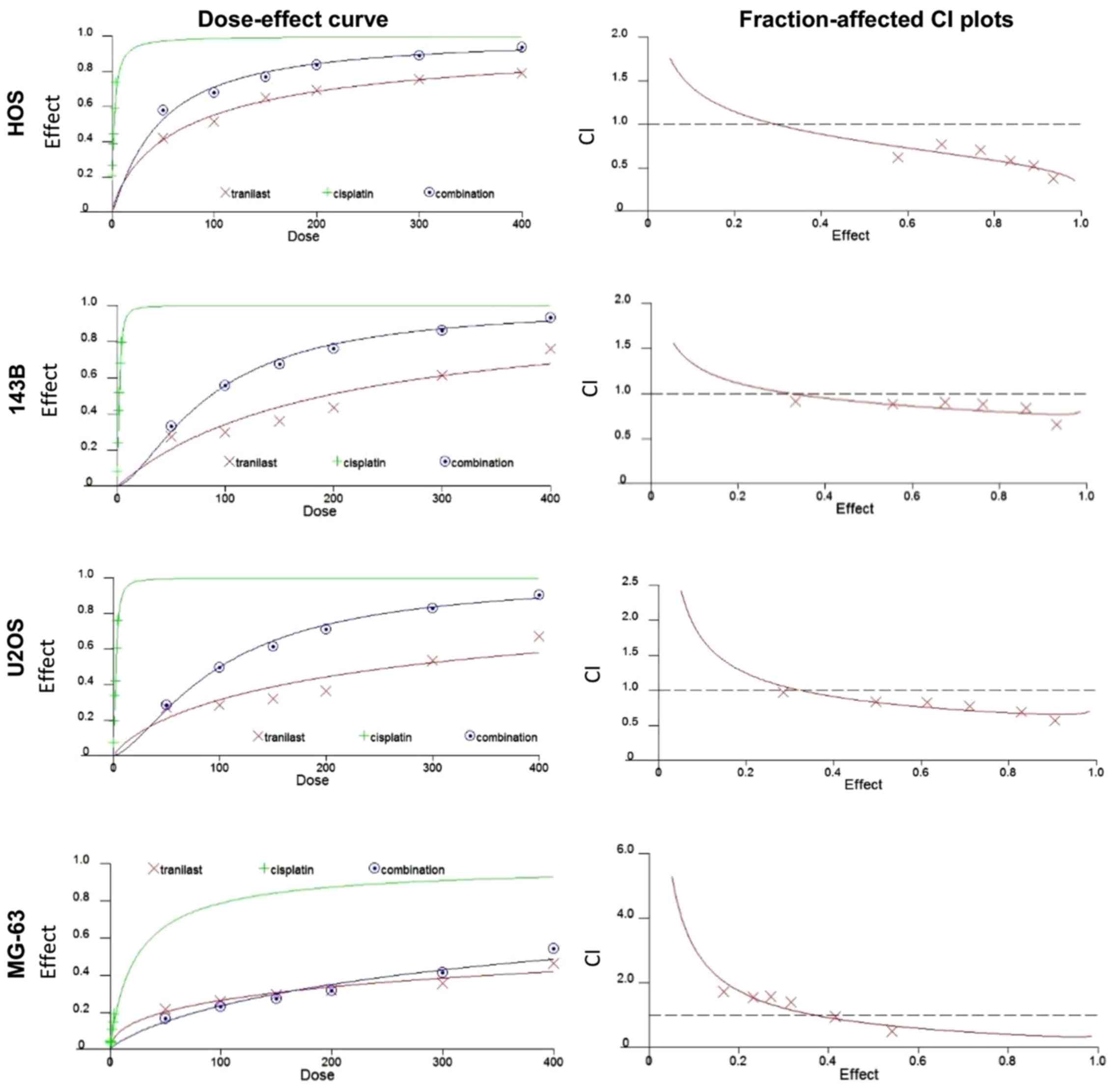
cisplatin inhibited cell proliferation in a synergistic manner. We
examined CIs using synergistic characteristics after treatment with
tranilast and cisplatin. Dose-effect and fraction-affected CI plots
in HOS, 143B, U2OS and MG-63 cells are shown in Fig. 3. Average CI values at
ED50, ED75 and ED90
(ED50-ED90 CI) for tranilast in combination
with cisplatin were 0.57 in HOS, 0.4 in 143B, 0.39 in U2OS and 0.51
in MG-63 cells. The results demonstrated that tranilast and
cisplatin synergistically inhibited the viability of the
osteosarcoma cell lines.
Tranilast enhances cisplatin-mediated
apoptotic cell death in osteosarcoma cells
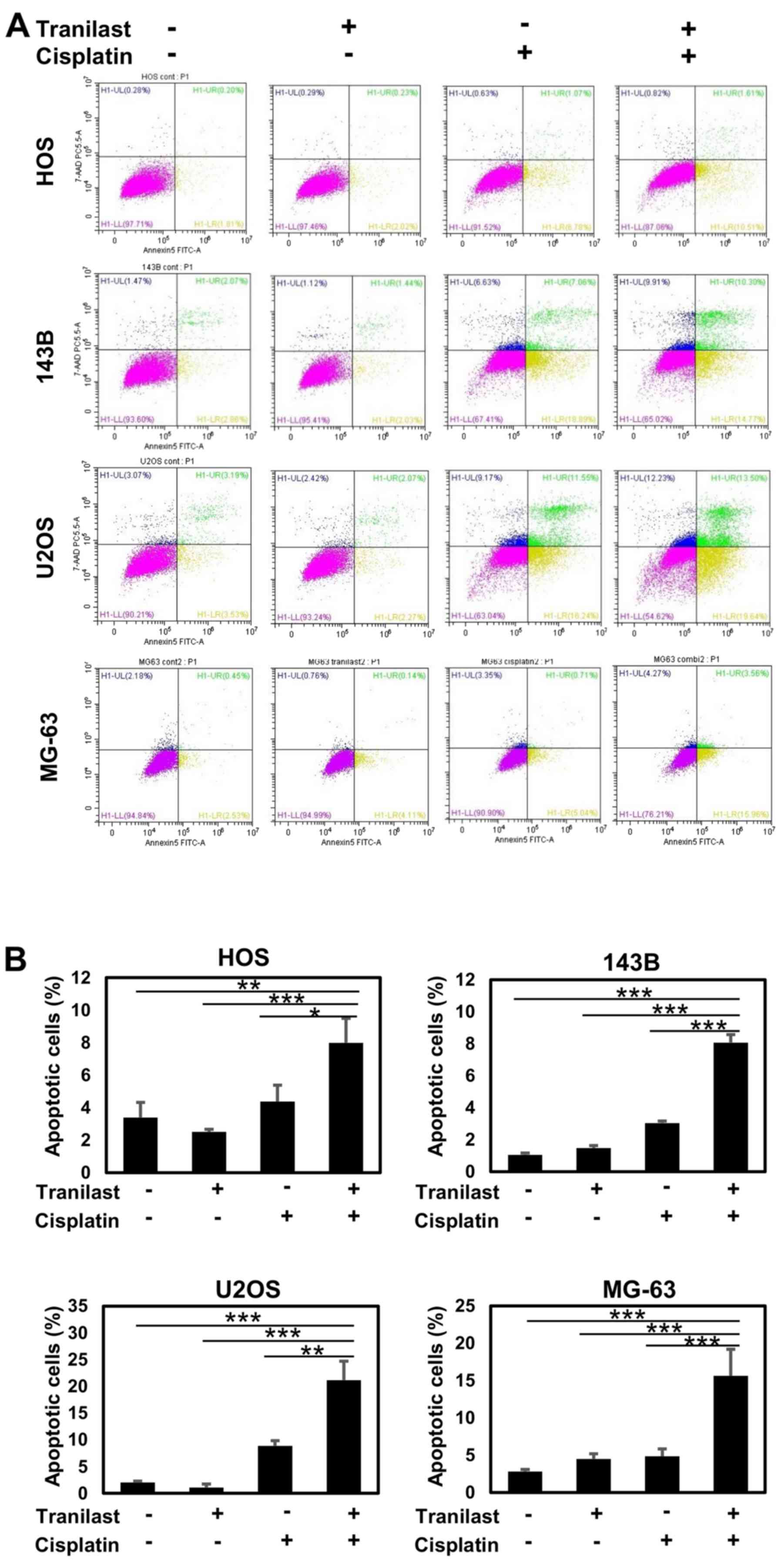
To analyze the mode of cell death induced by the
combined treatment with tranilast and cisplatin in HOS, 143B, U2OS
and MG-63 osteosarcoma cells, flow cytometry was performed.
Tranilast alone did not induce significant apoptotic death in all
cell lines (Fig. 4). Cisplatin
induced apparent accumulation of the cells in early and late
apoptosis. The combination of tranilast and cisplatin enhanced both
early and late apoptotic cell death (Fig. 4A). The increase in apoptotic cell
fraction by combined tranilast and cisplatin was statistically
significant in all four osteosarcoma cell lines compared with
single treatment with tranilast or cisplatin (Fig. 4B). Especially, combined treatment
induced a significantly higher percentage of apoptotic cells in HOS
(P=0.03), 143B (P=0.0001), U2OS (P=0.02) and MG-63 (P=0.007) than
that by cisplatin alone.
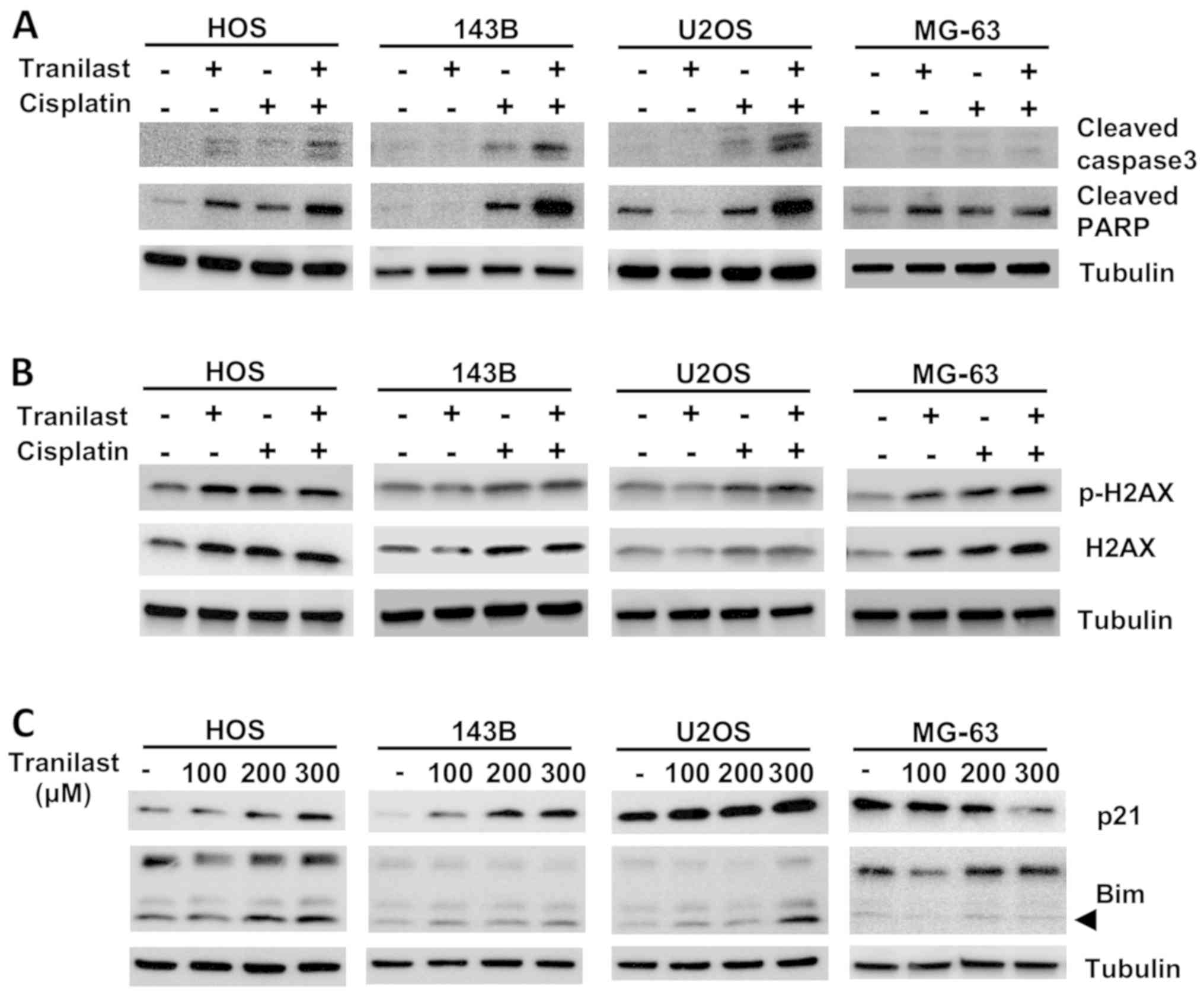
Western blotting demonstrated increased cleaved
PARP, cleaved caspase-3 and p-H2AX, which are hallmarks of
cisplatin-induced apoptotic cell death (Fig. 5A). Expression of cleaved caspase-3,
cleaved PARP and p-H2AX was enhanced by combined tranilast and
cisplatin (Fig. 5A and B).
Expression of p21 was increased in a dose-dependent manner by
tranilast (Fig. 5C). Expression of
Bim, a proapoptotic protein, was also increased by tranilast
treatment (Fig. 5C). Apoptotic
protein induction by tranilast and cisplatin, and upregulation of
p21 and Bim protein levels by tranilast were less in the MG-63
osteosarcoma cell line.
Tranilast enhances cisplatin-induced
G2/M arrest in osteosarcoma cells
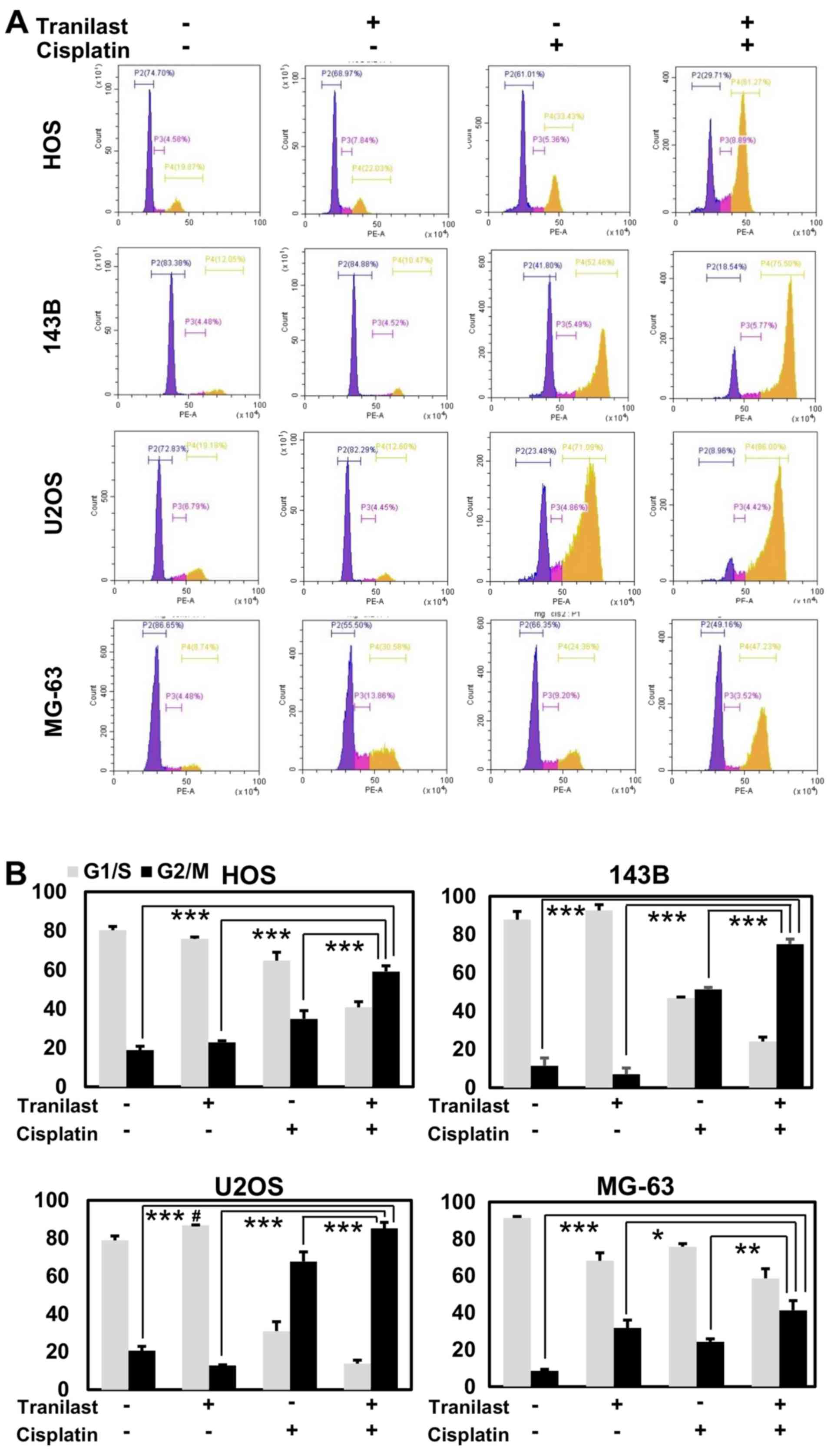
We examined the cell cycle population after
tranilast and/or cisplatin treatment in osteosarcoma cells. HOS,
143B, U2OS and MG-63 cells were treated with cisplatin (2 µM),
tranilast (200 µM), and the combination of both drugs, and were
analyzed using flow cytometry at 48 h after treatment. Tranilast
induced a small increase in the G1 population of U2OS cells (p53
wild-type) but not of HOS, 143B, or MG-63 cells (p53 mutant).
Cisplatin drastically increased the G2/M population in all cell
lines. Combined tranilast and cisplatin further increased the
proportion of cells in the G2/M phase from 34.8 to 59.1% in HOS
cells (P=0.001), from 51.4 to 75.0% in 143B cells (P=0.0001), from
67.7 to 85.1% in U2OS cells (P=0.007), and from 24.2 to 41.3% in
MG-63 cells (P=0.006) compared with cisplatin alone (Fig. 6).
Tranilast and cisplatin enhance the
ATR/CHK1 pathway in osteosarcoma cells
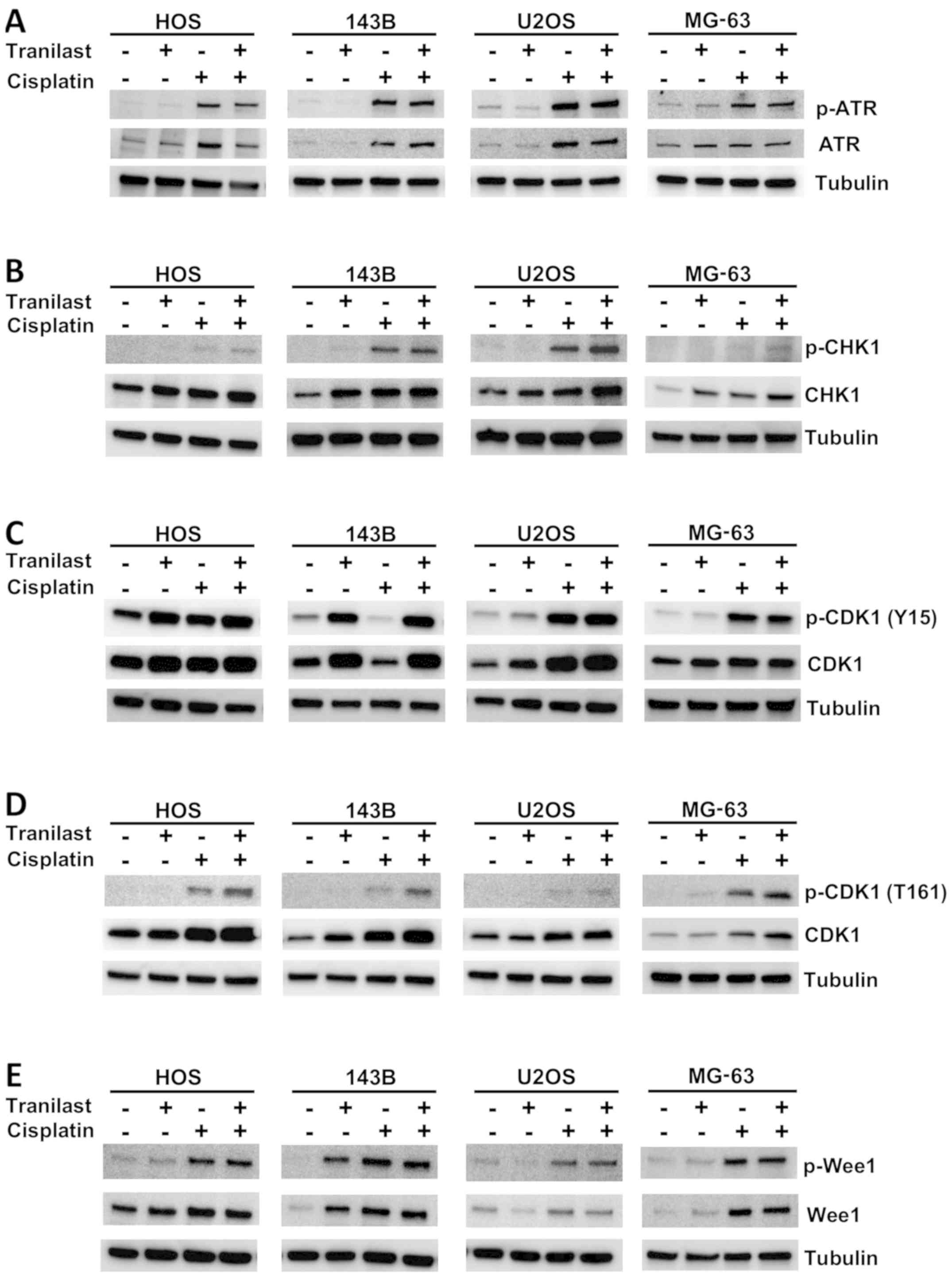
To explore the mechanism of enhancement of the G2/M
phase after tranilast/cisplatin treatment, HOS, 143B and U2OS
osteosarcoma cells were collected 48 h after treatment for western
blotting with antibodies against cell-cycle regulators (Fig. 7). Tranilast enhanced expression of
CHK1 and p-CDK1 (Y15), which is an inactivated form. Cisplatin
enhanced expression of p-ATR, p-CHK1, p-CDK1 (Y15) and p-Wee1
(Fig. 7). Combined treatment
increased p-CDK1 (Y15) more than cisplatin alone in all cell lines.
These results suggest that G2/M arrest was enhanced by increased
expression of CHK1 under the cytotoxic ATR pathway and CDK1
inactivation was induced.
Tranilast enhances the effect of
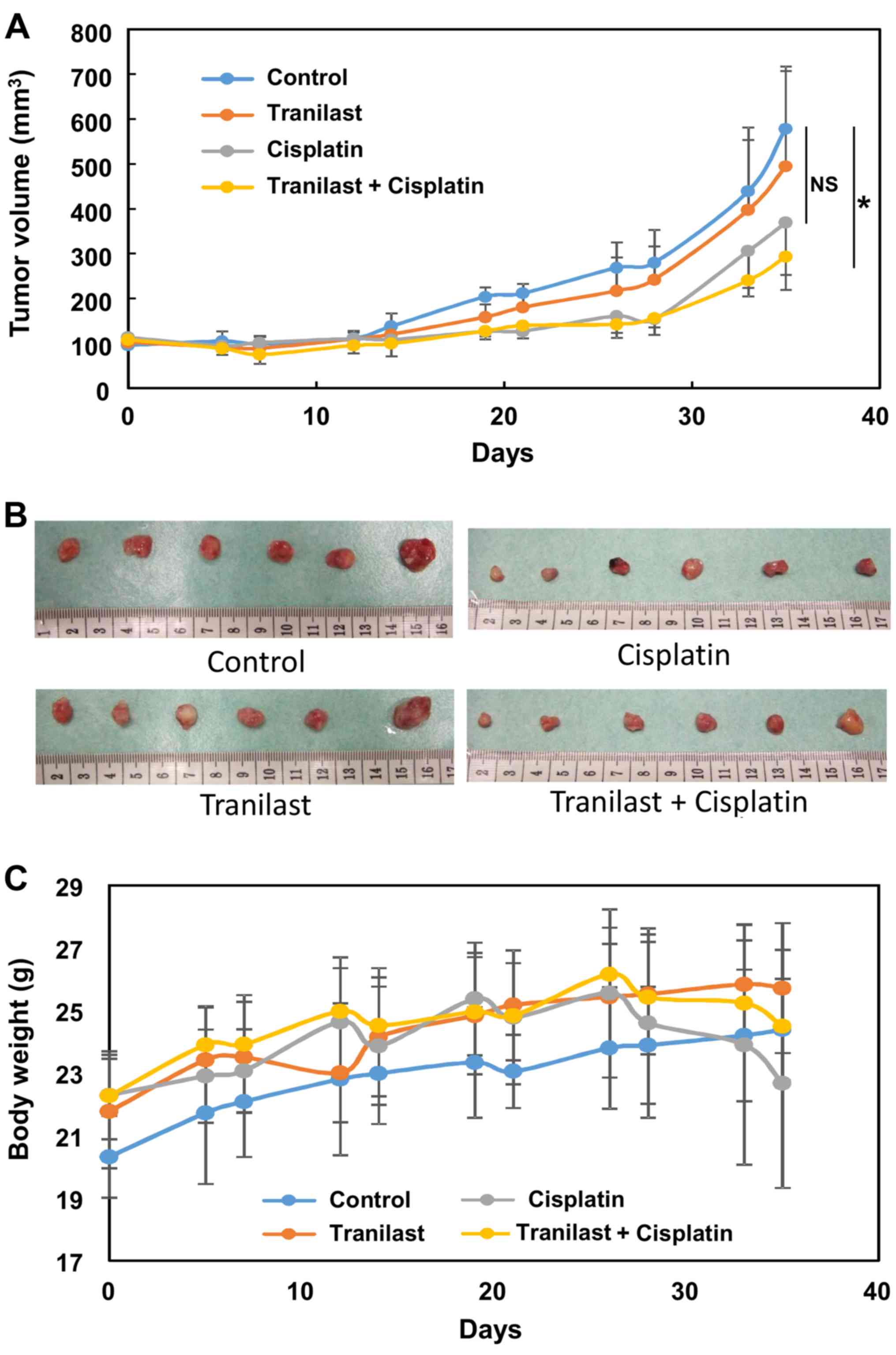
cisplatin on growth of osteosarcoma xenografts in mice
The osteosarcoma cell line 143B was implanted into
the flank of nude mice to explore the effect of tranilast in
combination with cisplatin. Tumor growth was significantly
inhibited in the combination group compared with the control group
(P=0.02), whereas cisplatin failed to reduce tumor volume
significantly (P=0.11; Fig. 8A and
B). Cisplatin-treated mice showed significant loss of body
weight at 4 weeks after initiation of the treatment (Fig. 8C). Combined treatment did not
enhance this adverse effect, and the reduction in body weight was
less than with cisplatin alone, although it was not statistically
significant (Fig. 8C).
Discussion
Tranilast [N-(3′,4′-dimethoxycinnamoyl)-anthranilic
acid] was originally thought to exert its antiallergic effect via
inhibition of chemical mediator release from mast cells (20). Additional effects of inhibition of
keloids and hypertrophic scar are mediated by inhibited production
of collagen by skin fibroblasts (21). It is also suggested that tranilast
inhibits the release of transforming growth factor (TGF)-β1 from
fibroblasts (21). The mechanism of
the anticancer effect of tranilast has not been completely
clarified. Subramaniam et al showed that tranilast induced
G1/S arrest and reduced migration in a murine breast cancer cell
line, which seemed to be mediated through TGF-β modulation
(22).
Cisplatin mainly induces apoptosis in osteosarcoma
cells via G2/M arrest; thus, we explored cell-cycle regulators
after treatment. DNA-damaging treatment, including cisplatin,
activates cell-cycle checkpoints, which induce G1/S arrest followed
by G2/M arrest (23). Cancer cells
defective in the p53 pathway lack the G1 checkpoint and depend on
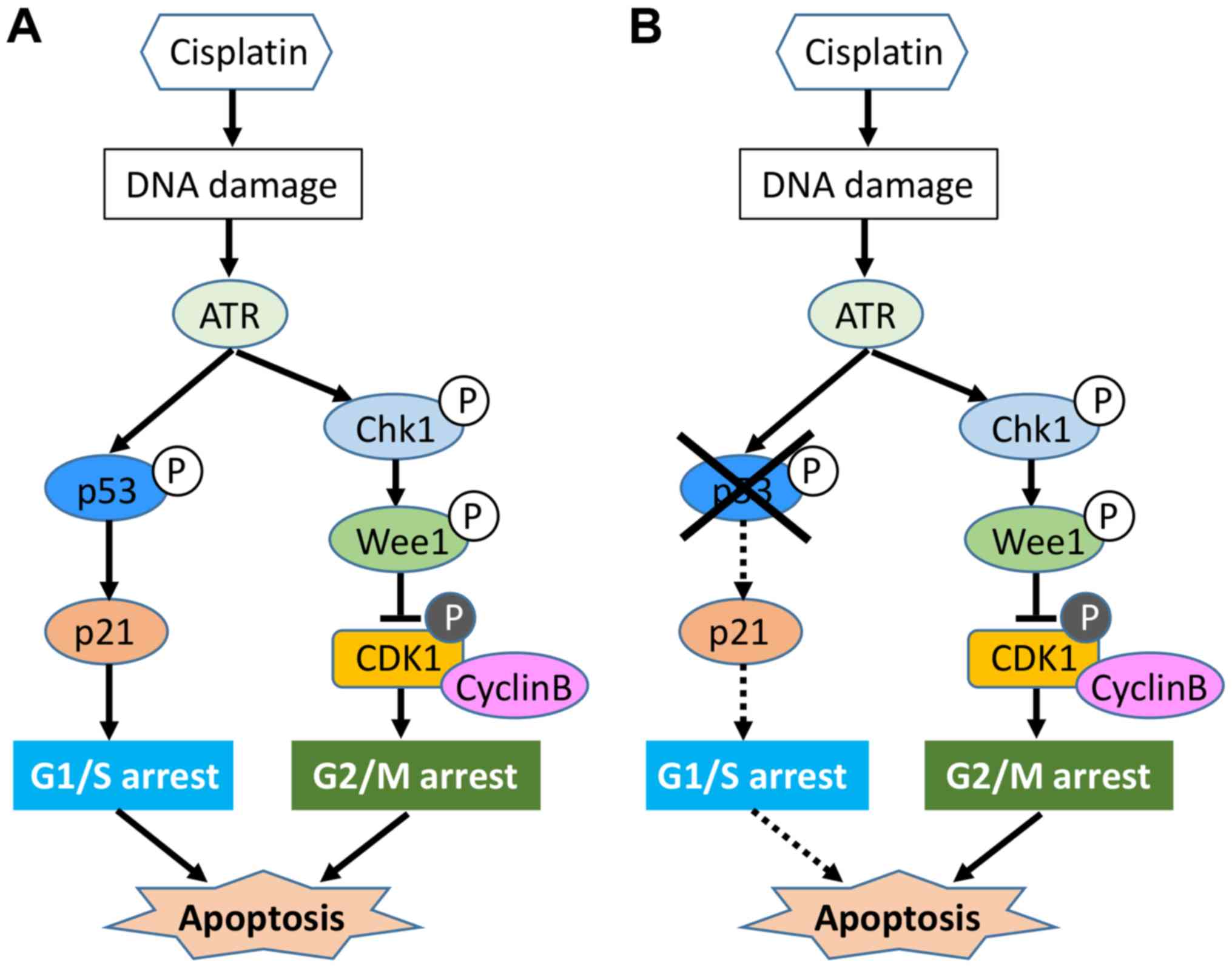
the G2/M checkpoint. Our results showed that cisplatin induced G2/M
arrest in all four osteosarcoma cell lines, although U2OS cells
harbored wild-type p53. This could be explained by previous
evidence that cisplatin does not frequently induce significant G1
phase accumulation, largely because cells remain trapped in the
G2/M phase (24). Cisplatin is
known to induce ATR kinase (24),
thereby activating downstream CHK1.
In the present study, this pathway was activated to
induce G2/M arrest, regardless of p53 status in osteosarcoma cells.
This suggests that in osteosarcoma cells, at least in part,
ATR/CHK1 may work independently of p53 function. Regarding
induction of the cyclin-dependent kinase inhibitor p21 after
treatment, p53-expressing U2OS cells showed higher p21 levels after
cisplatin and/or tranilast treatment than those observed in HOS and
143B, p53 mutant cells. Therefore, in p53-expressing osteosarcoma
cells, p21 and p53 cooperatively work to induce apoptosis via
several factors including pro-apoptotic protein Bim. Nevertheless,
our results demonstrated that tranilast could enhance sensitivity
to cisplatin, irrespective of the status of the p53 pathway
(Fig. 9), which is frequently
impaired in osteosarcoma patients (25).
Advancement of multiagent chemotherapy regimens for
osteosarcoma has led to a dramatic improvement in the prognosis for
patients with localized disease. The first chemotherapeutic agents
were doxorubicin and high-dose methotrexate with leucovorin in the
1970s. Other drugs such as cisplatin, ifosfamide and
cyclophosphamide have proven efficacy in the treatment of
osteosarcoma; however, they do not completely eradicate metastatic
lesions (26). Although a
combination of cytotoxic agents produces enhanced anticancer
efficacy, adverse effects sometimes compromise the condition of the
patient and treatment may need to be terminated. Non-cytotoxic
agents that potentiate cancer cells to chemotherapeutic drugs have
been discovered. Caffeine has been shown to enhance the effect of
anticancer drugs by inhibiting DNA repair and cell-cycle
checkpoints (27). The natural
antioxidant resveratrol is reported to overcome multidrug
resistance by modulating ABC transporter proteins (28).
Tranilast, which has been used in many patients
since the 1980s, has demonstrated its activity as an enhancer or
sensitizer of several anticancer drugs in different types of cancer
(29). Murahashi et al have
shown that the combined treatment of cisplatin and tranilast
decreased fibrosis and mitosis and increased apoptosis in scirrhous
gastric cancer cells (30).
Tranilast also sensitizes pancreatic cancer cells to gemcitabine
through suppression of DNA synthesis enzymes (31). In breast cancer, tranilast has been
shown to synergistically act with tamoxifen, which was mediated by
vascular endothelial growth factor and matrix metalloproteinase-9
(32). In the present study, we
first showed that tranilast alone inhibited proliferation in
osteosarcoma cell lines and synergistically acted in combination
with cisplatin.
It was reported that serum concentrations of
tranilast reach 30–300 µM in vivo after oral administration
of 600 mg/day tranilast (33).
Since the IC50 values of tranilast in osteosarcoma cells
were 130–330 µM (Fig. 1), tranilast
monotherapy may not be sufficient to exert strong antitumor effects
in vivo. However, the concentration of tranilast when used
in combination with cisplatin was 200 µM, which can be achieved by
the oral administration of tranilast at the currently approved dose
(600 mg/day). In addition, since normal fibroblasts were not
significantly damaged at 200 µM of tranilast (Fig. 1), tranilast can be used safely in
patients.
High-dose cisplatin therapy for osteosarcoma
patients may cause severe toxicity including permanent hearing loss
and kidney damage. The prevalence of hearing loss in children
treated with platinum analogs ranges from 2 to 90%. Recently, a
clinical trial with pantoprazole, an inhibitor of the organic
cation transporter 2, has been performed; however, it did not
ameliorate ototoxicity and nephrotoxicity induced by cisplatin
(34). If tranilast sufficiently
improves the therapeutic outcome of osteosarcoma patients,
decreasing the dose of cisplatin or other agents may reduce the
risk of severe adverse effects caused by anticancer drugs.
In conclusion, tranilast was originally established
as an antiallergic agent. It has a cytostatic effect in
osteosarcoma cells and enhances the effect of anticancer drugs,
especially cisplatin in vitro and in vivo. The
enhanced sensitivity to cisplatin is mediated by enhanced apoptosis
induced by G2/M arrest. Tranilast has been clinically approved and
has few adverse effects; therefore, clinical trials that evaluate
tranilast in combination with chemotherapy in osteosarcoma should
be undertaken.
Acknowledgements
We are grateful to Hui Gao (Technical assistant at
the Department of Orthopaedic Surgery, Graduate School of Medical
and Dental Sciences, Kagoshima University) for her excellent
technical assistance. We wish to thank the Joint Research
Laboratory of Kagoshima University Graduate School of Medical and
Dental Sciences.
Funding
The present study was supported by Grants-in-Aid for
Scientific Research (KAKENHI) (C) 15K10452 and (C) 17K10973.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TN, SN, HS, SM, SK and NT were involved in the
conception and design of the study; TN and YS performed the
experiments; SM and HS were involved in planning and supervised the
experiments of western blotting and cell viability assays; TS and
SK were involved in planning and supervision of the animal
experiments: TN, HS, YS and SN analyzed the data; SN, TS and SM
performed the interpretation of the results and drafted the
manuscript; SK and NT provided critical revisions of the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The animal experiment was approved by the
Association for the Accreditation and Assessment of Laboratory
Animal Care (Kagoshima, Japan). All animal experiments were
performed in compliance with the guidelines of the Institute of
Laboratory Animal Sciences, Graduate School of Medical and Dental
Sciences, Kagoshima University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Darakhshan S and Pour AB: Tranilast: A
review of its therapeutic applications. Pharmacol Res. 91:15–28.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ward MR, Sasahara T, Agrotis A, Dilley RJ,
Jennings GL and Bobik A: Inhibitory effects of tranilast on
expression of transforming growth factor-beta isoforms and
receptors in injured arteries. Atherosclerosis. 137:267–275. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Phan TV, Ke K, Sul OJ, Park YK, Kim KK,
Cho YS, Chung HT and Choi HS: Protection against
ovariectomy-induced bone loss by tranilast. PLoS One. 9:e955852014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holmes DR Jr, Savage M, LaBlanche JM, Grip
L, Serruys PW, Fitzgerald P, Fischman D, Goldberg S, Brinker JA,
Zeiher AM, et al: Results of prevention of REStenosis with
tranilast and its outcomes (PRESTO) trial. Circulation.
106:1243–1250. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang Y, Jiang H, Chen Y, Wang X, Yang Y,
Tao J, Deng X, Liang G, Zhang H, Jiang W and Zhou R: Tranilast
directly targets NLRP3 to treat inflammasome-driven diseases. EMBO
Mol Med. 10:e86892018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tokuyama H, Kelly DJ, Cox A, Zhang Y, Thai
K, Nikolic-Paterson DJ and Gilbert RE: Tranilast ameliorates
experimental mesangial proliferative glomerulonephritis. Nephron
Exp Nephrol. 109:e1–e7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Darakhshan S, Bidmeshkipour A, Mansouri K,
Saeid HM and Ghanbari A: The effects of tamoxifen in combination
with tranilast on CXCL12-CXCR4 axis and invasion in breast cancer
cell lines. Iran J Pharm Res. 13:683–693. 2014.PubMed/NCBI
|
|
8
|
Subramaniam V, Ace O, Prud'homme GJ and
Jothy S: Tranilast treatment decreases cell growth, migration and
inhibits colony formation of human breast cancer cells. Exp Mol
Pathol. 90:116–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Izumi K, Mizokami A, Li YQ, Narimoto K,
Sugimoto K, Kadono Y, Kitagawa Y, Konaka H, Koh E, Keller ET and
Namiki M: Tranilast inhibits hormone refractory prostate cancer
cell proliferation and suppresses transforming growth factor
beta1-associated osteoblastic changes. Prostate. 69:1222–1234.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaneda M, Obara H, Suzuki K, Takeuchi O,
Takizawa A, Osaku M, Matsubara H and Kitagawa Y: Evaluation of
suppressive effects of tranilast on the invasion/metastasis
mechanism in a murine pancreatic cancer cell line. Pancreas.
46:567–574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yatsunami J, Aoki S, Fukuno Y, Kikuchi Y,
Kawashima M and Hayashi SI: Antiangiogenic and antitumor effects of
tranilast on mouse lung carcinoma cells. Int J Oncol. 17:1151–1156.
2000.PubMed/NCBI
|
|
12
|
Yashiro M, Murahashi K, Matsuoka T,
Nakazawa K, Tanaka H, Osaka H, Koyama T, Ohira M and Chung KH:
Tranilast (N-3,4-dimethoxycinamoyl anthranilic acid): A novel
inhibitor of invasion-stimulating interaction between gastric
cancer cells and orthotopic fibroblasts. Anticancer Res.
23:3899–3904. 2003.PubMed/NCBI
|
|
13
|
Meazza C and Scanagatta P: Metastatic
osteosarcoma: A challenging multidisciplinary treatment. Expert Rev
Anticancer Ther. 16:543–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chandar N, Billig B, McMaster J and Novak
J: Inactivation of p53 gene in human and murine osteosarcoma cells.
Br J Cancer. 65:208–214. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ganjavi H, Gee M, Narendran A, Parkinson
N, Krishnamoorthy M, Freedman MH and Malkin D: Adenovirus-mediated
p53 gene therapy in osteosarcoma cell lines: Sensitization to
cisplatin and doxorubicin. Cancer Gene Ther. 13:415–419. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Allan LA and Fried M: p53-dependent
apoptosis or growth arrest induced by different forms of radiation
in U2OS cells: p21WAF1/CIP1 repression in UV induced apoptosis.
Oncogene. 18:5403–5412. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakamura S, Nagano S, Nagao H, Ishidou Y,
Yokouchi M, Abematsu M, Yamamoto T, Komiya S and Setoguchi T:
Arsenic trioxide prevents osteosarcoma growth by inhibition of GLI
transcription via DNA damage accumulation. PLoS One. 8:e694662013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi K, Setoguchi T, Tsuru A, Saitoh
Y, Nagano S, Ishidou Y, Maeda S, Furukawa T and Komiya S:
Inhibition of casein kinase 2 prevents growth of human
osteosarcoma. Oncol Rep. 37:1141–1147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Isaji M, Miyata H, Ajisawa Y, Takehana Y
and Yoshimura N: Tranilast inhibits the proliferation, chemotaxis
and tube formation of human microvascular endothelial cells in
vitro and angiogenesis in vivo. Br J Pharmacol. 122:1061–1066.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzawa H, Kikuchi S, Arai N and Koda A:
The mechanism involved in the inhibitory action of tranilast on
collagen biosynthesis of keloid fibroblasts. Jpn J Pharmacol.
60:91–96. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Subramaniam V, Chakrabarti R, Prud'homme
GJ and Jothy S: Tranilast inhibits cell proliferation and migration
and promotes apoptosis in murine breast cancer. Anticancer Drugs.
21:351–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shapiro GI and Harper JW: Anticancer drug
targets: Cell cycle and checkpoint control. J Clin Invest.
104:1645–1653. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goto A, Kanda H, Ishikawa Y, Matsumoto S,
Kawaguchi N, Machinami R, Kato Y and Kitagawa T: Association of
loss of heterozygosity at the p53 locus with chemoresistance in
osteosarcomas. Jpn J Cancer Res. 89:539–547. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jaffe N: Historical perspective on the
introduction and use of chemotherapy for the treatment of
osteosarcoma. Adv Exp Med Biol. 804:1–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sabisz M and Skladanowski A: Modulation of
cellular response to anticancer treatment by caffeine: Inhibition
of cell cycle checkpoints, DNA repair and more. Curr Pharm
Biotechnol. 9:325–336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu Y, Li C, Li H, Li M and Shu X:
Resveratrol-mediated reversal of tumor multi-drug resistance. Curr
Drug Metab. 15:703–710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rogosnitzky M, Danks R and Kardash E:
Therapeutic potential of tranilast, an anti-allergy drug, in
proliferative disorders. Anticancer Res. 32:2471–2478.
2012.PubMed/NCBI
|
|
30
|
Murahashi K, Yashiro M, Inoue T, Nishimura
S, Matsuoka T, Sawada T, Sowa M and Hirakawa-Ys Chung K: Tranilast
and cisplatin as an experimental combination therapy for scirrhous
gastric cancer. Int J Oncol. 13:1235–1240. 1998.PubMed/NCBI
|
|
31
|
Mitsuno M, Kitajima Y, Ohtaka K, Kai K,
Hashiguchi K, Nakamura J, Hiraki M, Noshiro H and Miyazaki K:
Tranilast strongly sensitizes pancreatic cancer cells to
gemcitabine via decreasing protein expression of ribonucleotide
reductase 1. Int J Oncol. 36:341–349. 2010.PubMed/NCBI
|
|
32
|
Darakhshan S, Bidmeshkipour A, Khazaei M,
Rabzia A and Ghanbari A: Synergistic effects of tamoxifen and
tranilast on VEGF and MMP-9 regulation in cultured human breast
cancer cells. Asian Pac J Cancer Prev. 14:6869–6874. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kusama H, Kikuchi S, Tazawa S, Katsuno K,
Baba Y, Zhai YL, Nikaido T and Fujii S: Tranilast inhibits the
proliferation of human coronary smooth muscle cell through the
activation of p21waf1. Atherosclerosis. 143:307–313. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fox E, Levin K, Zhu Y, Segers B, Balamuth
N, Womer R, Bagatell R and Balis F: Pantoprazole, an inhibitor of
the organic cation transporter 2, does not ameliorate
cisplatin-related ototoxicity or nephrotoxicity in children and
adolescents with newly diagnosed osteosarcoma treated with
methotrexate, doxorubicin, and cisplatin. Oncologist. 23:762–e779.
2018. View Article : Google Scholar : PubMed/NCBI
|