Introduction
Prostate cancer (PCa) is a type of cancer
specifically diagnosed in males, with higher incidence in European
and American countries (1).
Androgen deprivation therapy (ADT) is the standard of treatment for
patients with advanced/metastatic PCa (2). However, patients may inevitably
develop castration-resistant prostate cancer (CRPC), which has a
poor prognosis (3). Therefore, it
is necessary to identify novel molecular mechanisms that regulate
cancer progression and develop targeted therapies to improve PCa
patient outcomes.
Gene therapy with conditionally replicative
adenovirus (CRAds) is an emerging experimental therapeutic strategy
for treating refractory cancer resistant to treatment. CRAds appear
to be an effective option for targeting androgen-independent
prostate cancer. CRAds exert intrinsic anticancer activity through
selective replication, resulting in target tumor cell death
(4).
Enhancer of zeste homolog 2 (EZH2) is a member of
the polycomb group (PcG) protein family, whose main role is to
remodel the structure of chromatin. Polycomb repressor complex
(PRC)1 and PRC2 are classified according to their functions. EZH2
is one of the core enzymatic subunits of PRC2, that methylates
histone H3 at lysine 27 to interfere with the transcription of
several genes (5–7). EZH2 is overexpressed in many cancers.
It has been reported that multiple gain or loss-of-function EZH2
mutations occur in distinct cancer types (8), including myelodysplastic syndromes
(MDS), breast cancer, and PCa. EZH2 is not only a key epigenetic
inhibitor of histone methylation, but also a gene expression
activator through different pathways (9). Many studies have highlighted the
association between EZH2 expression and PCa development. Varambally
et al (10) demonstrated a
positive association between EZH2 protein expression and PCa
invasiveness (7,11). Following radical prostatectomy, EZH2
overexpression is associated with both metastasis and higher risk
of PCa recurrence (12).
Furthermore, it has been reported that EZH2 overexpression is a
common phenomenon in PCa that is associated with a poor clinical
outcome in PCa patients (13,14).
Therefore, EZH2 was proposed to be an oncogene, and its increased
expression may be used as a marker of prostate tumors with
aggressive and metastatic potential (8). These studies demonstrate the role of
EZH2 in PCa invasiveness, and suggest that EZH2 may be an effective
therapeutic target in PCa therapy. Therefore, the elucidating the
mechanisms that regulate EZH2 function may provide therapeutic
insights into the treatment of this cancer.
The catalytic component of human telomerase reverse
transcriptase (hTERT) is not expressed in the majority of primary
somatic human cells, whereas most cancer cells reactivate
telomerase by transcriptional upregulation of Htert (15). It has been demonstrated that the
hTERT promoter can be used to restrict gene expression of
E1-deleted replication defective adenoviral vectors to
telomerase-positive cancer cells (16).
In the present study, the generation of a new
adenovirus Ad-hTERT-EZH2 small hairpin (sh)RNA was reported, in
which EZH2 shRNA expression cassettes containing the hTERT promoter
were inserted. It was found that the Ad-hTERT-EZH2shRNA showed
excellent antitumor efficacy on PCa in vitro. The results
suggested that Ad-hTERT-EZH2shRNA may be a promising agent for the
treatment of PCa.
Materials and methods
Tissue specimens
CRPC and benign prostate hyperplasia (BPH) specimens
were obtained from transurethral resection of the prostate
procedures, following the occurrence of lower urinary tract
symptoms. Androgen-dependent prostate cancer (ADPC) samples were
obtained from radical prostatectomies. No other treatments,
including ADT, radiotherapy and chemotherapy, were performed.
Clinical pathological data were obtained from the clinical medical
college of Yangzhou University from January 1, 2014 to December 31,
2018. In this prospective study, 120 patients aged 62–77 were
enrolled, including 10 CRPC cases, 60 ADPC cases and 50 BPH cases.
All of the tissue specimens were confirmed by two pathologists. The
present study was approved by the Institutional Review Board of
Subei People's Hospital of Jiangsu Province and patient consent was
obtained prior to tissue collection.
CRAd construction
The hTERT promoter sequence (Table I) was obtained from the relevant
reference (17). The conventional
cytomegalovirus (CMV) promoter was replaced with a hTERT promoter
in the adenovirus plasmid with green fluorescence.
Ad-hTERT-EZH2shRNA was constructed by encoding EZH2-shRNA, which
had the following sequences: shRNA1, 5′-GCTAGGTTAATTGGGACCAAA-3′;
shRNA2, 5′-GGATGGTACTTTCATTGAAGA-3′; negative control,
5′-TTCTCCGAACGTGTCACGT-3′ (17).
The construction of CRAds was as described previously (18). The other two complexes were also
constructed to act as controls (Ad-CMV and Ad-CMV-EZH2-shRNA). All
plasmid constructs were confirmed by detecting the DNA sequences
(19).
 | Table I.Sequences of hTERT promoter. |
Table I.
Sequences of hTERT promoter.
| Name | Sequences |
|---|
| h-TERT |
GGCCCCTCCCTCGGGTTACCCCACAGCCTAGGCCGATTCGACCTCTCTCCGCTGGGGCCCTCGCTGGCGTCCCTGCACCCTGGGAGCGCGAGCGGCGCGCGGGCGGGGAAGCGCGGCCCAGACCCCCGGGTCCGCCCGGAGCAGCTGCGCTGTCGGGGCCAGGCCGGGCTCCCAGTGGATTCGCGGGCACAGACGCCCAGGACCGCGCTCCCCACGTGGCGGAGGGACTGGGGACCCGGGCACCCGTCCTGCCCCTTCACCTTCCAGCTCCGCCTCCTCCGCGCGGACCCCGCCCCGTCCCGACCCCTCCCGGGTCCCCGGCCCAGCCCCCTCCGGGCCCTCCCAGCCCCTCCCCTTCCTTTCCGCGGCCCCGCCCTCTCCTCGCGGCGCGAGTTTCAGGCAGCGCTGCGTCCTGCTGCGCACGTGGGAAGCCCTGGCCCCGGCCACCCCCGCG |
Immunohistochemistry assay
All specimens were fixed in 4% formaldehyde at room
temperature for 6 h following excision. Paraffin sections were
dewaxed by xylene and hydrated in a gradient ethanol series. All
immunohistochemical tissue sections were evaluated by two
independent pathologists. Immunohistochemistry was performed with a
primary antibody against human EZH2 (cat. no. 3147S; 1:200; Cell
Signaling Technology, Inc.) at 4°C overnight. The secondary
antibody (anti mouse IgG; cat. no. 7076P2; 1:2,000; Cell Signaling
Technology, Inc.) was applied at 37°Cfor 15 min. For EZH2
expression scoring, the intensity of positive signal was evaluated
by eye, as follows: 0, no staining; 1, low staining; 2, medium
staining; and 3, strong staining (20). The samples were then categorized
into two groups according to the scores assessed: <2, low
expression; 2–3, high expression.
Cell culture
Human PCa cell lines (PC3 and DU145) were provided
by the Stem Cell Bank, Chinese Academy of Science (Shanghai,
China). Cells were cultured at 37°C with 5% CO2 in
RPMI-1640 (HyClone Laboratories; GE Healthcare Life Sciences), 10%
fetal bovine serum (FBS; HyClone Laboratories; GE Healthcare Life
Sciences), 50 IU/ml penicillin and 50 mg/ml streptomycin (Beyotime
Institute of Biotechnology).
Transient transfection
Cells were cultured at 37°C overnight in 6-well
plates for 24 h, until 50–70 % confluence was reached. The frozen
virus was thawed in an ice bath, prior to transfection on the 2nd
day. The virus was diluted to the desired density according to the
preliminary experiment (1×1010 pfu/ml). Cells and virus
were mixed and incubated at 37°C for 2 h. Next, the medium was
replaced with fresh medium and cells were incubated for 24–36 h
before infection was observed by fluorescence microscopy.
Transfection efficiency was confirmed by western blotting. Cell
proliferation and invasion were assayed following transfection.
Western blotting
Western blot analysis was performed as described
previously (21). Cells were
collected and lysed in radioimmunoprecipitation assay (RIPA) buffer
and the protein samples (50 µg) were separated by 10% SDS-PAGE and
electrophoretically transferred to a polyvinylidene difluoride
membrane. The membrane was incubated with the following primary
antibodies at 4°C overnight: EZH2 (dilution 1:1,000; cat. no.
3147S; Cell Signaling Technology, Inc.), E-cadherin (dilution
1:1,000; cat. no. 4A2C7; Thermo Fisher Scientific, Inc.), Cyclin D1
(CCND1; dilution 1:1,000; cat. no. A0310; Shenzhen Yinji
Technology, Co., Ltd.) and proliferation marker protein Ki-67
(dilution 1:1,000; cat. no. 14-5698-82; Cell Signaling Technology,
Inc.), followed by secondary antibody (dilution 1:2,000; cat. no.
7076P2; Cell Signaling Technology, Inc.) incubation at room
temperature for 2 h. Finally, signal detection was performed by
chemiluminescence using an ECL kit (Thermo Fisher Scientific,
Inc.). GAPDH (dilution 1:10,000; Santa Cruz Biotechnology, Inc.)
was used as a protein loading control. Signals were visualized by
using a Molecular Image ChemiDocÔ XRS+ system with Image Lab
software version 5.2.1 (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR assays were used to detect EZH2 transcript
expression. According to the manufacturer's instructions, total RNA
was extracted from cell samples using TRIzol reagent (Thermo Fisher
Scientific, Inc.), and 1 µg total RNA was reverse transcribed into
cDNA using SuperScript II reverse transcriptase (cat. no. 18090050;
Thermo Fisher Scientific, Inc.). EZH2 and internal control GAPDH
were then amplified by qPCR using the following primers: EZH2
forward, 5′-CCAAGAGAGCCATCCAGACT-3′ and reverse,
5′-CGATGCCGACATACTTCAGG-3′; GAPDH forward, 5′-GCATCAAGGGAGACACCA-3′
and reverse, 5′-TGACCTAACTAAAGCACCAGA-3′ (22). qPCR was conducted by using the Step
One PlusÔ Real-Time PCR system (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). All samples were amplified in triplicate. The
PCR thermocycling conditions were as follows: 95°C for 30 sec, then
40 cycles of 10 sec at 95°C, 60°C for 20 sec and 70°C for 10 sec.
The 2−ΔΔCq method was used to calculate relative gene
expression (23).
Transwell Matrigel invasion assay
Cell invasion ability was detected in 24-well
plates. Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) was
diluted to 8-fold with serum-free medium, which was then injected
into the upper chambers with polycarbonate membranes (8 µm pore
size) the night before the experiment. Next, transfected PC3 and
DU145 cells were diluted to 5×104/ml and added into the
upper chamber with serum-free medium (200 µl), and the lower
chambers were filled with 200 µl complete medium (RPMI-1640 with
10% FBS). Cells were cultured for 12 h at 37°C at an atmosphere of
5% CO2. Non-invading cells were removed from the top of
the filter and invaded cells were fixed with 100% methanol for 30
min at room temperature. Crystal violet (0.1%) was used to stain
the cells at room temperature for 10 min and the invaded cell
number was counted in 10 random fields of view using an inverted
light microscope.
Cell Counting Kit (CKK)-8 assay
Cell growth was analyzed by using a WST-8 Cell
Counting Kit-8 (Dojindo Molecular Technologies, Inc., Shanghai,
China). Cells (2.5×103) suspended in RPMI-1640 medium
containing 10% FBS were seeded in 96-well plates and incubated for
24, 48 and 72 h, respectively. CCK-8 solution (10 µl) was added to
each well for 30 min at 37°C. Absorbance was subsequently measured
at 450 nm with a microplate reader.
Statistical analysis
Statistical analyses were performed with SPSS
version 25.0 (IBM Corp., Armonk, NY, USA). Values are presented as
the mean ± standard deviation from at least three independent
experiments. Differences were assessed by Student's t-test or
one-way analysis of variance followed by Tukey's post hoc test.
Recurrence survival was analyzed using the Kaplan-Meier method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical relevance of EZH2 expression
in human PCa specimens
First, the expression of EZH2 in BPH, ADPC and CRPC
patients was examined by immunohistochemistry. Compared with the
BPH specimens, the expression of EZH2 was markedly upregulated in
ADPC and CRPC specimens (Fig. 1A).
The expression of EZH2 was associated with the category of
specimens (P<0.01; Fig. 1B).
After 18–30 months on ADT, large amounts of patients of ADPC
progress to CRPC, particularly in patients whose EZH2 intensity
scores were equal to 3 (P<0.05; Fig.
1C). The time of progression-free survival in ADPC patients
with EZH2 scores <2 was significantly increased compared to
patients with EZH2 scores ≥2 (P<0.05; Fig. 1D).
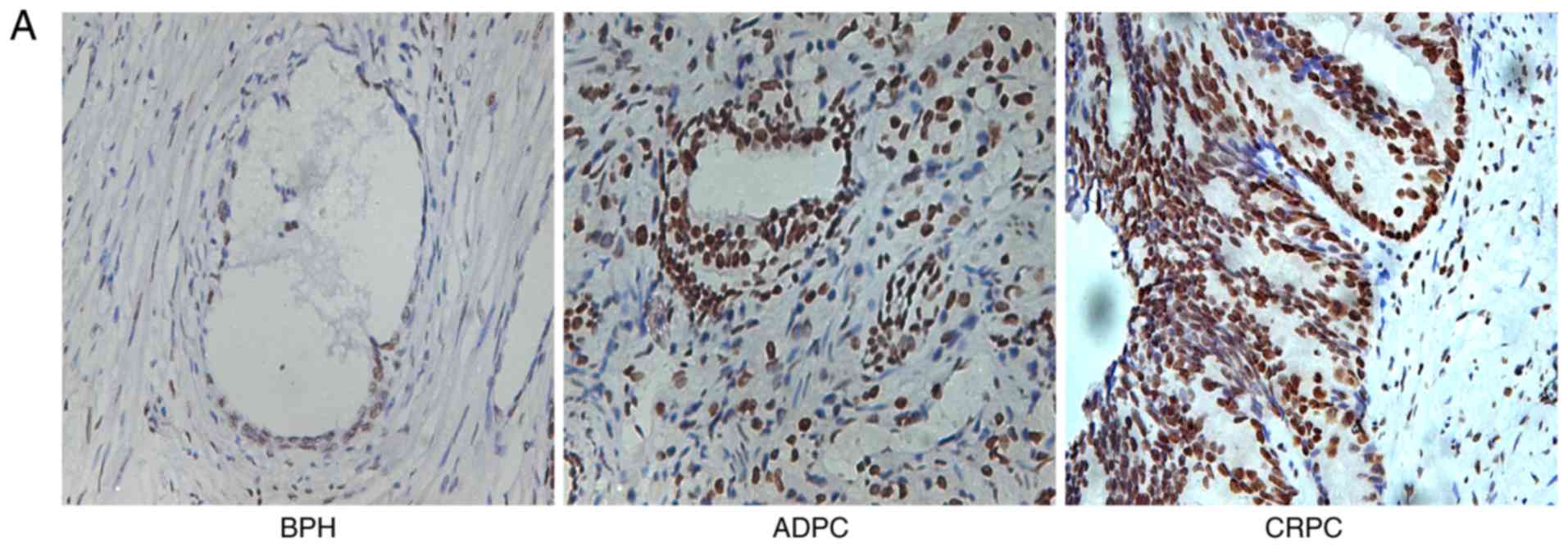 | Figure 1.EZH2 expression in BPH, ADPC and CRPC
specimens. (A) Immunohistochemical analysis of EZH2 in BPH, ADPC
and CRPC specimens (original magnification, ×400). (B) Scoring of
the staining intensities indicated that EZH2 protein expression was
significantly increased in ADPC and CRPC specimens, compared with
the BPH tissues. (C) After 18–30 months of ADT, the majority of
patients progressed from CRPC to ADPC. (D) Kaplan-Meier analyses of
cumulative progression-free survival compared with the scores of
the staining intensities (P<0.05). EZH2, enhancer of zeste
homolog 2; BPH, benign prostate hyperplasia; ADPC,
androgen-dependent prostate cancer; CRPC, castration-resistant
prostate cancer; ADT, androgen deprivation therapy. |
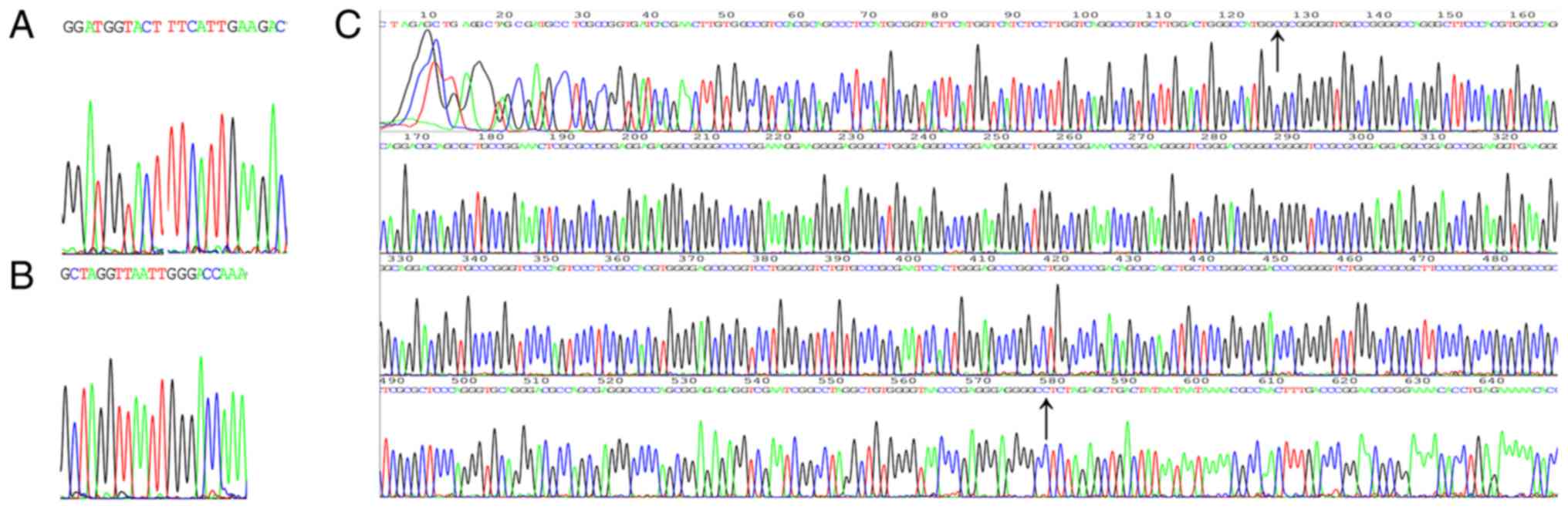
Successful construction of
conditionally replicative adenovirus
Following CRAd construction, shRNA and hTERT
promoter expression was identified in the monoclonal colonies by
sequencing (Fig. 2A-C). For the
hTERT promoter, the arrow symbol indicated the reverse
complementary sequence from the beginning to the end. The correct
clone was the successfully constructed target gene of adenovirus
vector. CCK-8 assays were used to detect the transfection
efficiency of the two shRNAs in the PC3 cell line. shRNA2 exhibited
better inhibition of PC3 proliferation (Fig. 2D). As EZH2 and its protein are
overexpressed in human lung adenocarcinoma A549 cells (24), adenovirus was transfected into these
cells, which were then observed under a microscope 24 h, 48 h and
72 h after transfection. The fluorescence indicated that the
transfected cells increased gradually with the extension of time
which indirectly represented that adenovirus vector was
successfully constructed (Fig.
2E).
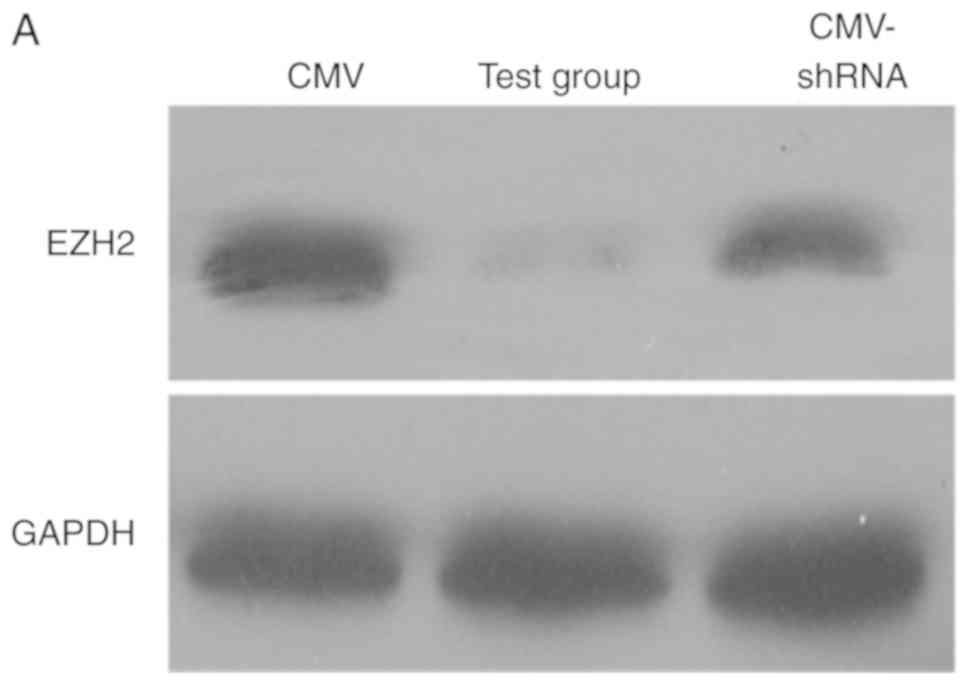
EZH2 expression is reduced by
Ad-hTERT-EZH2shRNA
PCa cells (PC3 and DU145) were transfected with
three types of adenoviruses, respectively. After 72 h of
transfection, the expression of EZH2 was detected by western
blotting (Fig. 3A). The results
suggested that Ad-hTERT-EZH2shRNA (Test group) transfection most
significantly inhibited the expression of EZH2 gene in PCa cells,
followed by Ad-CMV-EZH2shRNA (CMV-shRNA). RT-qPCR also showed that
Ad-hTERT-EZH2shRNA significantly reduced the gene expression of
EZH2 (Fig. 3B).
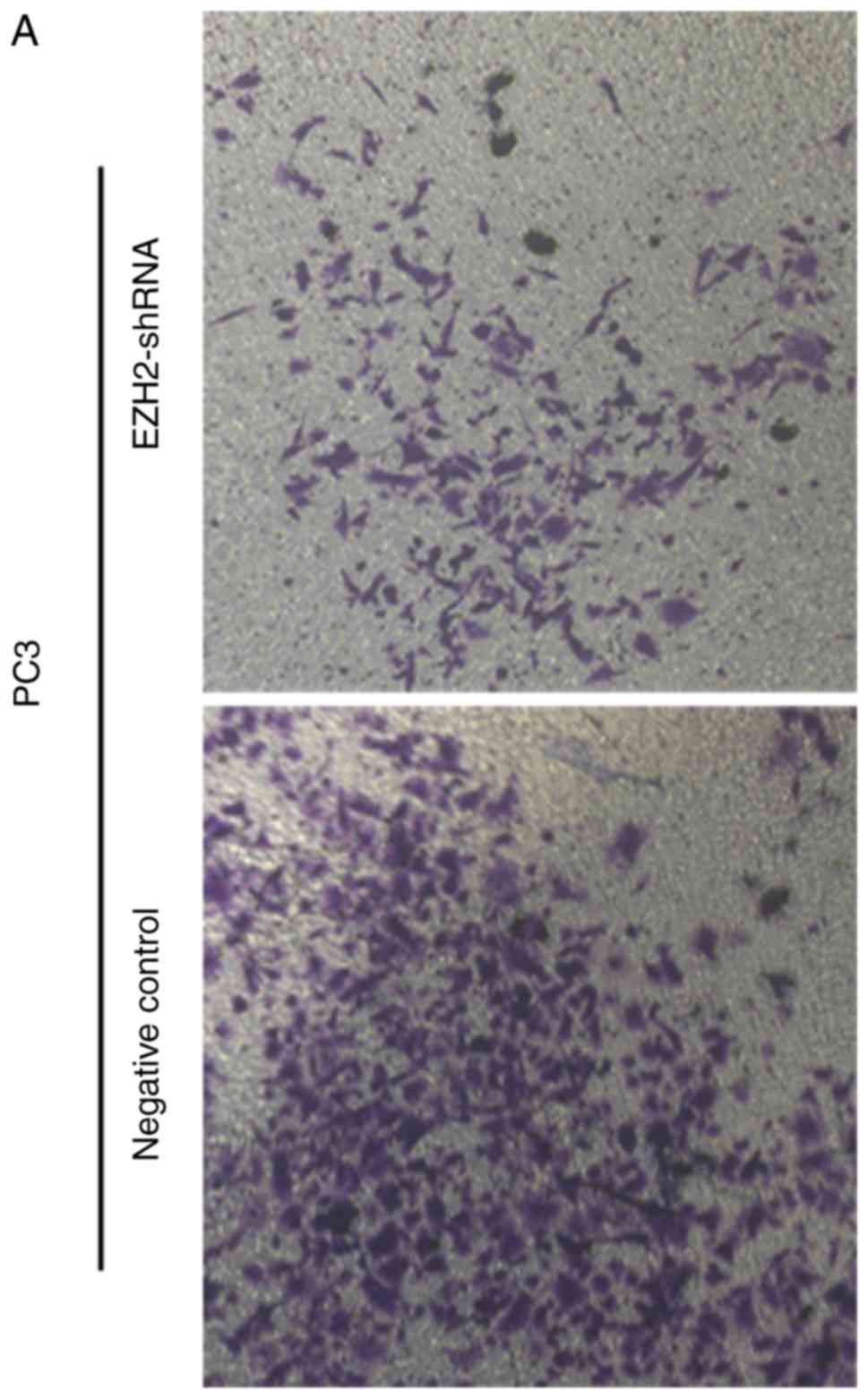
Cell invasion assay
To examine the invasion ability of the PCa cells,
Transwell assays were performed following cell transfection and
compared with the negative control group. Ad-hTERT-EZH2shRNA
transfected cells exhibited a decrease in cell invasion after
culture for 48 h, respectively (Fig.
4).
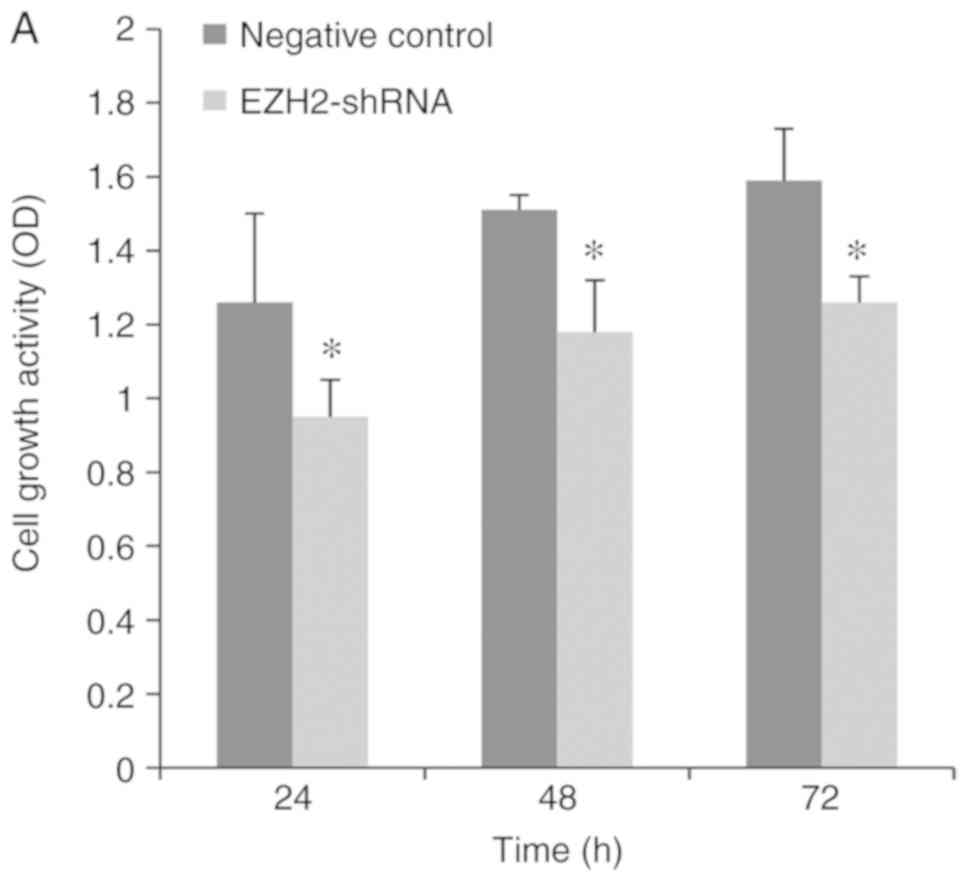
Cell proliferation assay
To examine the tumor-suppressive function of EZH2,
the effect of EZH2 knockdown on cell proliferation was measured
using CCK-8 assays. Following transfection, shRNA was stably
expressed in PCa cell lines, compared with the negative control.
Transfection with EZH2-shRNA resulted in a significant decrease of
cell growth in the PCa cell lines (P<0.05; Fig. 5A and B).
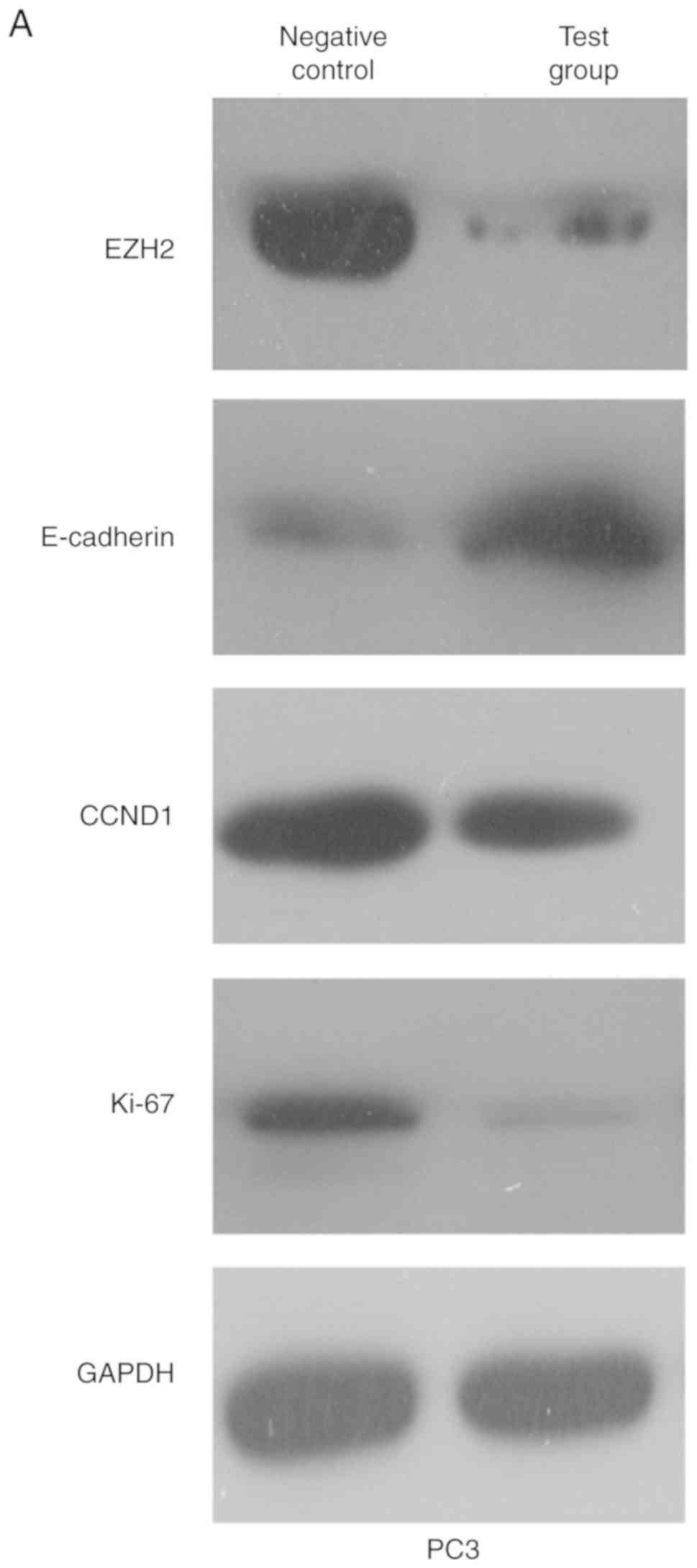
EZH2 knockdown upregulates E-cadherin
and represses Ki67 and CCND1 expression in PCa cell lines
In order to explore the mechanism of EZH2 in PCa,
the relationship between EZH2, E-cadherin, Ki67 and CCND1 was
examined. The expression of EZH2, E-cadherin, Ki67 and CCND1 was
measured by western blotting following transfection with
Ad-hTERT-EZH2shRNA. Untreated cells were used as a negative
control. The expression level of EZH2, Ki67 and CCND1 was decreased
by shRNA, whereas E-cadherin expression was repressed (Fig. 6A and B).
Discussion
Despite advances in technology that have allowed
earlier diagnoses, PCa morbidity remains high, particularly in CRPC
cases. CRPC occurs after 18 to 30 months of ADT (3). Limited therapeutic options are
available once androgen resistance develops. Therefore, increasing
amount of research has focused on gene therapy for patients with
CRPC (25).
Oncolytic viral therapy is a novel method of tumor
treatment, where tumor cells are selectively infected with viruses,
resulting in tumor cell death and stimulation of a specific
anti-tumor immune response (26–28).
Oncolytic viruses are modified with attenuated or genetically
engineered viruses, which are highly specific to tumor cells,
meaning they have limited effects on normal tissues (29). The main side effects are
influenza-like symptoms (<grade 3). In nearly 100 clinical
studies of oncolytic viruses, no serious side effects caused by
viruses have been found. No dose limiting toxicity was observed,
and the safety was reliable (30).
Due to their stability and well-established
methodology, traditional transgenic and gene targeting technologies
currently remain the main strategy used for model construction,
rather than the CRISPR/Cas system (31). CRAds selectively replicate and
spread within tumor cells, meaning this tool not only has a strong
tumor scavenging ability, but can also be used in reduced doses.
This consequently reduces any toxic liver effects of the liver, and
thus CRAds have gradually become a widespread concern in gene
therapy (31).
EZH2 serves a pivotal role in regulating chromosomal
structure (32). High EZH2
expression has been detected in a variety of tumors and is
associated with the degree of tumor malignancy, invasion and
metastasis (33,34). Varambally (10) et al showed that EZH2 siRNA
transfection markedly inhibited benign prostate cell growth. A
previous study also reported that EZH2 may be a potential target in
the treatment of PCa (8). In the
present study, it was demonstrated that EZH2 staining was localized
in the nucleus of tumor cells, and EZH2 was overexpressed in PCa
tissues compared to BPH. EZH2 shRNA transfection reduced the RNA
and protein expression of EZH2, as determined by RT-qPCR and
western blot analysis. In addition, EZH2 gene expression is low in
early stage PCa, but significantly increased in CRPC, compared with
ADPC (35,36). EZH2 knockdown inhibits cancer cell
growth and metastasis (37,38). Studies have confirmed that EZH2 is
positively correlated with tumor angiogenesis, as EZH2 is involved
in the formation of VEGF (39,40).
Researchers also found that in ADPC, EZH2 binds to androgen
receptors as an androgen receptor transcription factor, in place of
androgen, thereby contributing to the progression of PCa (9). In the present study, the effects of
EXH2 on PCa cell growth and invasiveness was demonstrated in two
CRPC cell lines. This indicated that EZH2 shRNA had potential
anti-CRPC tumor activity.
Studies have shown that the aberrant expression of
EZH2 in PCa at an early stage may promote the progression of PCa
into a more aggressive form (36).
In the present study, decreased EZH2 expression reduced PCa cell
proliferation and invasion. Taken together, these findings indicate
that EZH2 is involved in the proliferation, invasion, progression,
metastasis of PCa cells (41,42).
Telomerase is positively expressed in >90% of
human tumor cells but absent in almost all of the normal cells
(43). hTERT is a determinant
factor in telomerase activity among human telomerase components.
hTERT is highly expressed in ~90% of malignant tumors, but inactive
in normal cells (44,45). Research has shown that hTERT
activity levels exhibit a prevalence range of 63–94 % for PCa
(46). Vectors carrying the hTERT
promoter have a targeted effect on malignant tumors (47,48).
Based on the information above, hTERT was selected as a core
promoter in the present study, in order to drive a specific target
sequence in CRAds so that it could specifically be expressed in PCa
tissues, but not in normal tissues. Western blotting showed that
the experimental group (Ad-hTERT-EZH2shRNA) had obvious advantages
in inhibiting the expression of EZH2 compared to the other two
controls, and that the hTERT promoter was more effective in
delivering the EZH2-shRNA.
Patients with the same PCa stage or grade often
exhibit different clinical features, making it difficult to
accurately predict prognosis with clinical stage and pathology data
alone. In the present study, the expression of Ki67, E-cadherin and
CCND1 may have also been associated with both PCa development and
recurrence (5,49,7).
E-Cadherin is a classical member of the cadherin superfamily. Loss
of function is thought to contribute to progression in cancer by
increasing proliferation, invasion and/or metastasis (50,51).
Antigen Ki67 is a nuclear protein that is associated with and may
be necessary for cellular proliferation. Furthermore, it is
associated with ribosomal RNA transcription (52). Ki67 is an excellent marker to
determine the growth fraction of a given cell population. The
fraction of Ki67-positive tumor cells is often correlated with the
clinical outcome in cancer. Finally, the main function of CCND1 is
to promote cell proliferation. A previous study has demonstrated
that CCND1 significantly decreased in EZH2-deficient cells
(53). In the present study, the
results indicated that silencing EZH2 led to decreased expression
of CCND1 and Ki67, and increased the expression of E-cadherin. When
combined with other clinicopathological information, such as
pathological grade, CCND1, Ki67 and E-cadherin could be used as
prognostic biomarkers. However, the signaling pathways between
EZH2, CCND1, Ki67 and E-cadherin remain unclear. The underlying
mechanism should be investigated in our future studies.
The current study provided evidence that EZH2 was
correlated with CRPC cell invasion and proliferation, but there are
several limitations. First, the number of CRPC specimens obtained
was small, and more samples should be used in further studies.
Second, an animal model should be established in the future to
better demonstrate the efficacy of the Ad-hTERT-EZH2-shRNA. In
addition, gene over-expression experiments was absent in the
present study, as a large number of studies have proved that the
EZH2 gene is abundantly expressed in cancer cells (8,13).
Collectively, the present study showed that CRAds
armed with EZH2 shRNA exhibited significant antitumor effects in
human PCa cells. EZH2 knockdown suppressed PCa cell proliferation
and invasion. Additionally, silencing EZH2 led to the decreased
expression of CCND1 and Ki67, and increased E-cadherin expression.
To the best of our knowledge, the present study revealed a novel
mechanism by which CRAds with EZH2 shRNA inhibited PCa cell growth,
and highlighted the potential clinical importance of CRAds in CRPC
therapies.
Acknowledgements
The authors thank the laboratory staff at the
Clinical Medical College of Yangzhou University (Subei People's
Hospital of Jiangsu Province) for technical support.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81402101).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XG, YXW and GCZ designed the study. SGX and JJY
wrote the manuscript, collected clinical information and performed
statistical analyses; QS, QN, ZG and BYG assisted with
immunohistochemical analysis, cell culture, PCR, western blotting
and in vitro experiments. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by institutional
Review Board of Clinical Medical College of Yangzhou University
(Subei People's Hospital of Jiangsu Province) and patient consent
was obtained prior to tissue collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klil-Drori AJ, Tascilar K, Yin H, Aprikian
A, Bitton A and Azoulay L: Androgen deprivation therapy and the
incidence of inflammatory bowel disease in patients with prostate
cancer. Am J Epidemiol. 184:15–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Massard C and Fizazi K: Targeting
continued androgen receptor signaling in prostate cancer. Clin
Cancer Res. 17:3876–3883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Irving J, Wang Z, Powell S, O'Sullivan C,
Mok M, Murphy B, Cardoza L, Lebkowski JS and Majumdar AS:
Conditionally replicative adenovirus driven by the human telomerase
promoter provides broad-spectrum antitumor activity without liver
toxicity. Cancer Gene Ther. 11:174–185. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani
RS, Tomlins SA, Mehra R, Laxman B, Cao X, Yu J, et al: Repression
of E-cadherin by the polycomb group protein EZH2 in cancer.
Oncogene. 27:7274–7284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu L, Xu Z, Zhong L, Wang H, Jiang S,
Long Q, Xu J and Guo J: Enhancer of zeste homolog 2 (EZH2) promotes
tumour cell migration and invasion via epigenetic repression of
E-cadherin in renal cell carcinoma. BJU Int. 117:351–362. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lobo J, Rodrigues Â, Antunes L, Graça I,
Ramalho-Carvalho J, Vieira FQ, Martins AT, Oliveira J, Jerónimo C
and Henrique R: High immunoexpression of Ki67, EZH2, and SMYD3 in
diagnostic prostate biopsies independently predicts outcome in
patients with prostate cancer. Urol Oncol. 36:161.e167–161.e117.
2018. View Article : Google Scholar
|
|
8
|
Kim KH and Roberts CW: Targeting EZH2 in
cancer. Nat Med. 22:128–134. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis
RT, Wu X, Stack EC, Loda M, Liu T, et al: EZH2 oncogenic activity
in castration-resistant prostate cancer cells is
Polycomb-independent. Science. 338:1465–1469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RG, Otte AP, et al: The polycomb group protein EZH2 is involved in
progression of prostate cancer. Nature. 419:624–629. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiang S, Zou P, Tang Q, Zheng F, Wu J,
Chen Z and Hann SS: HOTAIR-mediated reciprocal regulation of EZH2
and DNMT1 contribute to polyphyllin I-inhibited growth of
castration-resistant prostate cancer cells in vitro and in vivo.
Biochim Biophys Acta Gen Subj. 1862:589–599. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laitinen S, Martikainen PM, Tolonen T,
Isola J, Tammela TL and Visakorpi T: EZH2, Ki-67 and MCM7 are
prognostic markers in prostatectomy treated patients. Int J Cancer.
122:595–602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu X, Gao XS, Bai Y, et al: EZH2
overexpression as a biomarker of poor prognosis in prostate cancer.
2016.
|
|
14
|
Cao P, Deng Z, Wan M, Huang W, Cramer SD,
Xu J, Lei M and Sui G: MicroRNA-101 negatively regulates Ezh2 and
its expression is modulated by androgen receptor and
HIF-1alpha/HIF-1beta. Mol Cancer. 9:1082010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wirth T, Zender L, Schulte B, Mundt B,
Plentz R, Rudolph KL, Manns M, Kubicka S and Kühnel F: A
Telomerase-dependent conditionally replicating adenovirus for
selective treatment of cancer. Cancer Res. 63:3181–3188.
2003.PubMed/NCBI
|
|
16
|
Kirch HC, Ruschen S, Brockmann D, Esche H,
Horikawa I, Barrett JC, Opalka B and Hengge UR: Tumor-specific
activation of hTERT-derived promoters by tumor suppressive
E1A-mutants involves recruitment of p300/CBP/HAT and suppression of
HDAC-1 and defines a combined tumor targeting and suppression
system. Oncogene. 21:7991–8000. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu L, Liu Y, Zhang T, Wu H, Lin M, Wang
C, Zhan Y, Zhou Q, Qiao B, Sun X, et al: Synthetic Bax-Anti Bcl2
combination module actuated by super artificial hTERT promoter
selectively inhibits malignant phenotypes of bladder cancer. J Exp
Clinl Cancer Res. 35:32016. View Article : Google Scholar
|
|
18
|
Cebrià F, Kobayashi C, Umesono Y, Nakazawa
M, Mineta K, Ikeo K, Gojobori T, Itoh M, Taira M, Sánchez Alvarado
A and Agata K: FGFR-related gene nou-darake restricts brain tissues
to the head region of planarians. Nature. 419:620–624. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanger F, Nicklen S and Coulson AR: DNA
sequencing with chain-terminating inhibitors. Proc Natl Acad Sci
USA. 74:5463–5467. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruan J, Wei B, Xu Z, Yang S, Zhou Y, Yu M,
Liang J, Jin K, Huang X, Lu P and Cheng H: Predictive value of Sox2
expression in transurethral resection specimens in patients with T1
bladder cancer. Med Oncol. 30:4452013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu J, Lu Y, Cui D, Li E, Zhu Y, Zhao Y,
Zhao F and Xia S: miR-200b suppresses cell proliferation, migration
and enhances chemosensitivity in prostate cancer by regulating
Bmi-1. Oncol Rep. 31:910–918. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li K, Liu C, Zhou B, Bi L, Huang H, Lin T
and Xu K: Role of EZH2 in the growth of prostate cancer stem cells
isolated from LNCaP cells. Int J Mol Sci. 14:11981–11993. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou W, Wang J, Man WY, Zhang QW and Xu
WG: siRNA silencing EZH2 reverses cisplatin-resistance of human
non-small cell lung and gastric cancer cells. Asian Pac J Cancer
Prev. 16:2425–2430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jalava SE, Urbanucci A, Latonen L,
Waltering KK, Sahu B, Jänne OA, Seppälä J, Lähdesmäki H, Tammela TL
and Visakorpi T: Androgen-regulated miR-32 targets BTG2 and is
overexpressed in castration-resistant prostate cancer. Oncogene.
31:4460–4471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Small EJ, Carducci MA, Burke JM, Rodriguez
R, Fong L, van Ummersen L, Yu DC, Aimi J, Ando D, Working P, et al:
A phase I trial of intravenous CG7870, a replication-selective,
prostate-specific antigen–targeted oncolytic adenovirus, for the
treatment of hormone-refractory, metastatic prostate cancer. Mol
Ther. 14:107–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pol J, Buqué A, Aranda F, Bloy N, Cremer
I, Eggermont A, Erbs P, Fucikova J, Galon J, Limacher JM, et al:
Trial watch-oncolytic viruses and cancer therapy. Oncoimmunology.
5:e11177402016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Gruijl TD, Janssen AB and van Beusechem
VW: Arming oncolytic viruses to leverage antitumor immunity. Expert
Opin Biol Ther. 15:959–971. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Twumasi-Boateng K, Pettigrew JL, Kwok YYE,
Bell JC and Nelson BH: Oncolytic viruses as engineering platforms
for combination immunotherapy. Nat Rev Cancer. 18:419–432. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Delwar Z, Zhang K, Rennie PS and Jia W:
Oncolytic virotherapy for urological cancers. Nat Rev Urol.
13:334–352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fang W, Ping WH, Fang CX, Yuan LC and Qian
H: Construction and characterization of oncolytic adenovirus
controlled under heat shock protein70 gene promoter. Prog Biochem
Biophys. 2009:1536–1543. 2009.
|
|
32
|
Li J, You Y, Yue W, Yu H, Lu T, Wu Z, Jia
M, Ruan Y, Liu J, Zhang D and Wang L: Chromatin remodeling gene
EZH2 involved in the genetic etiology of autism in Chinese Han
population. Neurosci Lett. 610:182–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simon JA and Lange CA: Roles of the EZH2
histone methyltransferase in cancer epigenetics. Mutat Res.
647:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu X, Wu Q and Li L: Functional and
therapeutic significance of EZH2 in urological cancers. Oncotarget.
8:38044–38055. 2017.PubMed/NCBI
|
|
35
|
Saramaki OR, Tammela TL, Martikainen PM,
Vessella RL and Visakorpi T: The gene for polycomb group protein
enhancer of zeste homolog 2 (EZH2) is amplified in late-stage
prostate cancer. Genes Chromosomes Cancer. 45:639–645. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van Leenders GJ, Dukers D, Hessels D, van
den Kieboom SW, Hulsbergen CA, Witjes JA, Otte AP, Meijer CJ and
Raaphorst FM: Polycomb-group oncogenes EZH2, BMI1, and RING1 are
overexpressed in prostate cancer with adverse pathologic and
clinical features. Eur Urol. 52:455–463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bryant RJ, Cross NA, Eaton CL, Hamdy FC
and Cunliffe VT: EZH2 promotes proliferation and invasiveness of
prostate cancer cells. Prostate. 67:547–556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cai H, Memarzadeh S, Stoyanova T, Beharry
Z, Kraft AS and Witte ON: Collaboration of Kras and Androgen
receptor signaling stimulates EZH2 expression and tumor propagating
cells in prostate cancer. Cancer Res. 72:4672–4681. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu C, Han HD, Mangala LS, Ali-Fehmi R,
Newton CS, Ozbun L, Armaiz-Pena GN, Hu W, Stone RL, Munkarah A, et
al: Regulation of tumor angiogenesis by EZH2. Cancer Cell.
18:185–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu ZQ, Zhang L, Gao BS, Wan YG, Zhang XH,
Chen B, Wang YT, Sun N and Fu YW: EZH2 promotes tumor progression
by increasing VEGF expression in clear cell renal cell carcinoma.
Clin Transl Oncol. 17:41–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu C, Jin X, Yang J, Yang Y, He Y, Ding L,
Pan Y, Chen S, Jiang J and Huang H: Inhibition of EZH2 by chemo-
and radiotherapy agents and small molecule inhibitors induces cell
death in castration-resistant prostate cancer. Oncotarget.
7:3440–3452. 2016.PubMed/NCBI
|
|
42
|
Debeb BG, Gong Y, Atkinson RL, Sneige N,
Huo L, Gonzalez-Angulo AM, Hung MC, Valero V, Ueno NT and Woodward
WA: EZH2 expression correlates with locoregional recurrence after
radiation in inflammatory breast cancer. J Exp Clin Cancer Res.
33:582014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Trujillo KA, Hines WC, Vargas KM, Jones
AC, Joste NE, Bisoffi M and Griffith JK: Breast field
cancerization: Isolation and comparison of telomerase-expressing
cells in tumor and tumor adjacent, histologically normal breast
tissue. Mol Cancer Res. 9:1209–1221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Komata T, Kanzawa T, Kondo Y and Kondo S:
Telomerase as a therapeutic target for malignant gliomas. Oncogene.
21:656–663. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Köchling M, Ewelt C, Fürtjes G,
Peetz-Dienhart S, Koos B, Hasselblatt M, Paulus W, Stummer W and
Brokinkel B: hTERT promoter methylation in pituitary adenomas.
Brain Tumor Pathol. 33:27–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang J, Wang Z, Li X, Li J and Shi H:
Human telomerase reverse transcriptase expression correlates with
vascular endothelial growth factor-promoted tumor cell
proliferation in prostate cancer. Artif Cells Blood Substit Immobil
Biotechnol. 36:83–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
March-Villalba JA, Martínez-Jabaloyas JM,
Herrero MJ, Santamaria J, Aliño SF and Dasí F: Cell-free
circulating plasma hTERT mRNA is a useful marker for prostate
cancer diagnosis and is associated with poor prognosis tumor
characteristics. PLoS One. 7:e434702012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou J, Wang H, Cannon V, Wolcott KM, Song
H and Yates C: Side population rather than CD133+ cells
distinguishes enriched tumorigenicity in hTERT-immortalized primary
prostate cancer cells. Mol Cancer. 10:1122011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Abdelrahman AE, Arafa SA and Ahmed RA:
Prognostic value of Twist-1, E-cadherin and EZH2 in prostate
cancer: An immunohistochemical study. Turk Patoloji Derg.
1:198–210. 2017.PubMed/NCBI
|
|
50
|
Beavon IR: The E-cadherin–catenin complex
in tumour metastasis: Structure, function and regulation. Eur J
Cancer. 36:1607–1620. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Harrington KJ: The biology of cancer.
Medicine. 44:1–5. 2016. View Article : Google Scholar
|
|
52
|
Bullwinkel J, Baron-Lühr B, Lüdemann A,
Wohlenberg C, Gerdes J and Scholzen T: Ki-67 protein is associated
with ribosomal RNA transcription in quiescent and proliferating
cells. J Cell Physiol. 206:624–635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yoo KH and Hennighausen L: EZH2
methyltransferase and H3K27 methylation in breast cancer. Int J
Biol Sci. 8:59–65. 2012. View Article : Google Scholar : PubMed/NCBI
|