Introduction
The incidence of RCC is increasing worldwide. RCC
accounts for approximately 2–3% of tumor diagnoses and tumor
mortality cases worldwide; most RCC cases are resistant to
traditional radiotherapy and chemotherapy and follow an
unpredictable disease course (1,2). In recent
years, great progress has been made in the diagnosis and treatment
of RCC, although the prognosis remains unsatisfactory. To achieve a
better therapeutic effect, early anticipation or identification of
patients with aggressive disease or poor prognosis is urgently
needed. More importantly, a more efficient and safer therapeutic
strategy for eliminating RCC is required to prevent relapse.
One general characteristic of tumor cells is the
secretion of membrane microvesicles, which are called exosomes.
Exosomes, especially tumor-derived exosomes (TEXs), which are
membrane-bound extracellular vesicles 30–100 nm in size secreted by
all tumor cells (3), have attracted
considerable attention as potential tools for diagnosing cancer and
delivering therapeutic anti-cancer drugs (4,5). However,
some studies have shown that TEXs alone are less efficient in
promoting T cell activation, and may even induce T cell apoptosis;
moreover, tumor progression is enhanced and immune escape is
promoted due to the expression of immunosuppressive mediators, such
as FasL, TNF-associated apoptosis inducing ligand (TRAIL) (6), programmed death-ligand 1 (PD-L1)
(7), transforming growth factor-β
(TGF-β) (8–11) and HSP72 (12). TEXs are highly enriched in
tumor-associated antigens (TAAs) and a series of immune-associated
proteins involved in antigen presentation, such as major
histocompatibility complex (MHC) molecules (13), inducible co-stimulatory molecules
(ICOS) (14) and heat shock proteins
(HSP) (15). Functional analyses have
demonstrated that TEXs can trigger potent CD8+ T
cell-dependent antitumor responses and induce antitumor immunity
via the transfer of exosomal molecules to dendritic cells (DCs)
(5,16–18).
Therefore, TEXs are an attractive potential vehicle for
immunotherapeutics due to their positive role in intercellular
communication.
Cytotoxic T cells (CTLs) can mediate their lytic
effects through the Fas ligand (L)-Fas pathway, in which the
interaction between FasL expressed on CTLs and Fas expressed on
target cells triggers apoptosis of the target cells (19). Notably, not all tumor cells, including
RenCa cells, express high levels of the Fas protein on the cell
surface; this expression is necessary for stimulated CTL-mediated
killing (20). CTLs initially
recognize target cells through the T cell receptor (TCR) and then
strongly adhere to these cells via accessory molecules. After the
TCR engages antigenic peptides presented by MHC molecules on target
cells, CTLs ultimately induce apoptosis via the interaction between
FasL and Fas proteins (21). However,
it remains unknown whether RenCa cell-derived TAA-stimulated CTLs
induce the FasL/Fas pathway on the surface of other types of tumor
cells. Therefore, whether this process of recognition and apoptosis
is antigen-specific requires further investigation.
Previous reports showed that TEX-stimulated
CD8+ T cells exhibit stronger cytotoxicity and more
efficient antitumor immunity against autologous tumor cells than
against other types of tumor cells due to the presentation of
immune-associated proteins (16–18). Our
pre-experimental study found that RCC-associated antigen G250,
which is highly expressed in renal cancer-derived exosomes and has
limited expression in normal tissue, may play an important role in
antigen-specific antitumor immunity (22). However, in general, few previous
studies have investigated the cytotoxicity of RDE-stimulated
CD8+ T cells. Therefore, in this study, for further
verification, exosomes derived from RenCa cell supernatants after
48 h of culture without fetal bovine serum (FBS) were isolated and
characterized, and the activating effect of RDEs combined with
GM-CSF and IL-12 on CD8+ T cells and the subsequent
cytotoxicity of RDE-stimulated CD8+ T cells was also
determined. Finally, the potent antigen-specific immunity mediated
by RDE-stimulated CD8+ T cells against RenCa cells in
comparison with other types of tumor cells were investigated using
a variety of methods both in vivo and in vitro.
Materials and methods
Mice
A total of 200 six-to-eight-week-old (17.40–20.25 g)
BALB/c mice were supplied by the Animal Experimental Center of
Chongqing Medical University (Chongqing, China) and housed under
standard laboratory conditions in the Laboratory Animal Care
Facility of Chongqing Medical University (Chongqing, China). Animal
care was provided in accordance with institutional guidelines, and
all experimental protocols involving animals were performed using a
protocol approved by the Ethics Committee of Chongqing Medical
University.
Cell lines and culture
The mouse renal cortical adenocarcinoma RenCa cell
line, mouse breast cancer 4T1 cell line, and mouse colon cancer
CT26 cell line were purchased from Shanghai Cell Bank (Shanghai,
China). The cells were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc. Waltham, MA, USA) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin and
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in an
incubator with a humidified atmosphere of 5% CO2 at
37°C. When the RenCa cells had grown to 80–90% confluency in the
plates, the supernatants were harvested for the isolation of RDEs
after 48 h of culture without FBS.
Isolation of RDEs
RDEs were extracted and purified as described
previously (22). Briefly, culture
supernatants were collected and successively centrifuged at 300 × g
for 10 min, 800 × g for 30 min, and 10,000 × g for 30 min, after
which cells and debris were removed (Ultracentrifuge CP100WX;
Hitachi Koki Himac, NuAire, Tokyo, Japan). After concentration by
ultrafiltration using a 100 kDa molecular weight cutoff (MWCO)
Centriplus centrifugal ultrafiltration tube (EMD Millipore,
Billerica, MA, USA) at 1,000 × g for 30 min, the exosome-enriched
ultrafiltrate was added to a 30% sucrose/D2O density cushion
(Tenglong Weibo Technology, Qingdao, China), followed by
ultracentrifugation at 100,000 × g for 60 min at 4°C. The bottom of
the cushion was collected and diluted with phosphate-buffered
saline (PBS). The exosomes were further concentrated by
centrifugation for 30 min at 1,000 × g in 100 kDa MWCO Amicon
Ultra-15 ultrafiltration tubes (EMD Millipore). The collected fluid
passed through a membrane filter (0.22 µm) for sterilization, and
the exosome sample was stored at −80°C.
Electron microscopy
The BCA method (Beyotime Institute of Biotechnology,
Shanghai, China) was used to quantify the total protein in the
RDEs. After quantification, a 20 µl drop of the suspension was
loaded onto an electron microscopy grid. Heavy metal staining was
performed with 2% phosphotungstic acid (pH 6.8) for 1 min and the
morphological characteristics of the RDEs were visualized and
observed under a transmission electron microscope (TEM) (JEM-2010,
Jeol Ltd., Tokyo, Japan).
Flow cytometry
RDEs and PBS were injected into BALB/c mice via the
caudal vein at 10 µg/mouse and 200 µl/mouse per injection,
respectively, a total of three times per week for 4 weeks. For
tumor inoculation, 200 µl of PBS containing 2×106 RenCa
cells was injected subcutaneously into the right flanks of the
mice. The mice were sacrificed under deep inhalation anesthesia
(2–3% isoflurane; Zhenzhun Institute of Biotechnology, Shanghai,
China) and local analgesia (oxybuprocaine hydrochloride; Zhenzhun
Institute of Biotechnology) for isolation of splenocytes and tumor
single cell suspensions one day after the last injection.
Splenocytes and tumor single cell suspensions were generated from
mice subjected to three different treatments on days 10, 20 and 30,
as described previously (23,24). In brief, erythrocyte-free single cell
suspensions were acquired by grinding the spleens several times in
RPMI-1640 and incubating with red blood cell lysis buffer (Beyotime
Institute of Biotechnology) on ice for 5 min. Tumor biopsies were
cut into several small fragments (2–3 mm in length) and placed in
an enzyme digest mix consisting of 3,000 U/ml DNAse, 10 mg/ml
collagenase and 10 mg/ml hyaluronidase (all from Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) at 37°C for 2 h. Splenocytes
and tumor single cell suspensions were collected, After several
washes, membrane markers of T cell subsets were stained with
anti-CD3-APC (Miltenyi Biotec, Bergisch Gladbach, Germany),
anti-CD4-FITC (Miltenyi Biotec) and anti-CD8-PE (Miltenyi Biotec)
antibodies. Then, the proportions of CD3+,
CD4+ and CD8+ T cells in splenocytes and
tumor single cell suspensions were analyzed using flow cytometry
(Beckman Coulter, Pasadena, CA, USA).
IFN-γ measurements
Next, the mice were divided into three groups and
vaccinated intravenously (i.v.) three times per week for 2 weeks.
For each injection, three groups of mice received 5 µg RDEs/mouse,
10 µg RDEs/mouse and 200 µl PBS/mouse, respectively. Two weeks
after the last immunization, splenic CD8+ T cells from
PBS- or RDE-immunized mice were sorted by magnetic beads (Miltenyi
Biotec) according to the manufacturer's protocol and as previously
described (24), seeded at a
concentration of 2.0×106 cells per well in 24-well
plates and incubated for 48 h at 37°C. After incubation, the
culture supernatants were collected for cytokine detection. IFN-γ
levels in the culture supernatants were measured by enzyme-linked
immunosorbent assays (ELISAs) using a commercial kit (R&D
Systems, Minneapolis, MN, USA) based on the manufacturer's
instructions.
Proliferation assay for
CD8+ T cells
Splenocytes from the mice were harvested and
depleted of red blood cells. Then, CD8+ T cells were
sorted using magnetic beads according to the manufacturer's
protocol as previously described (24). The isolated CD8+ T cells
were resuspended in PBS at a concentration of 1×107
cells/ml and labeled with 2.5 µM CFSE (Macklin Biochemical Co.,
Ltd., Shanghai, China) for 10 min at room temperature in the dark.
After quenching CFSE with five volumes of RPMI-1640 medium
containing 10% FBS and washing several times with PBS containing 2%
FBS, CD8+ T cells at a concentration of 1×106
cells/ml were co-cultured with 100 µl of PBS, 5 µg/ml
phytohemagglutinin (PHA) (Sigma-Aldrich; Merck Millipore), 5 µg/ml
PHA+20 µg/ml RDEs, 20 ng/ml GM-CSF (PeproTech, Shanghai, China), 20
ng/ml IL-12 (PeproTech), 5 µg/ml PHA+20 µg/ml RDEs+20 ng/ml GM-CSF,
5 µg/ml PHA+20 µg/ml RDEs+20 ng/ml IL-12 and 5 µg/ml PHA+20 µg/ml
RDEs+20 ng/ml GM-CSF+20 ng/ml IL-12 for a period of 48 h. The
cultured cells were harvested, and the proliferation of
CD8+ T cells was evaluated by flow cytometry. The
supernatant was harvested and analyzed for the release of
IFN-γ.
Tumor growth assays
The RenCa cell line, 4T1 cell line, and CT26 cell
line were prepared for injection from cultures grown to nearly 95%
confluence. The mice were randomly divided into groups, and the
three types of tumor cells were enumerated, adjusted to a
concentration of ~2×106 in 200 µl of PBS as described,
and injected subcutaneously into the right flanks of the mice to
generate three different kinds of tumor model. Then, the mice from
the experimental group and the control group were vaccinated three
times per week for 4 weeks. For each injection, each mouse in the
experimental group received 10 µg of RDEs via the caudal vein and
200 µl of PBS alone as a control. Tumor volume was measured by
caliper every three days and calculated according to the formula
V=π/6×LxW2 (L, length; W, width). The mice were
sacrificed under deep inhalation anesthesia (2–3% isoflurane) and
local analgesia (oxybuprocaine hydrochloride) for the isolation of
tumor tissues.
Cytotoxicity assays
BALB/c mice were i.v. immunized with RDEs and PBS.
Seven days later, the spleens were ground, and an erythrocyte
dissolving agent was used to acquire splenocyte single cell
suspensions after the final stimulation. To perform in vitro
CD8+ T cell assessments, erythrocyte-free single cell
suspensions were sorted by magnetic beads to obtain CD8+
T cells according to the manufacturer's protocol. Next, the
isolated CD8+ T cells were re-stimulated with irradiated
RenCa cells (4,000 rads; 40 Gy) in the presence of 20 ng/ml IL-2
(PeproTech, Shanghai, China) in vitro for three days. Then,
the re-stimulated CD8+ T cells were treated with 20
ng/ml GM-CSF, 20 ng/ml IL-12 or 20 ng/ml GM-CSF in combination with
20 ng/ml IL-12 for 48 h. PBS-treated CD8+ T cells were
treated with 20 ng/ml GM-CSF or 20 ng/ml IL-12.
Following five days of treatment, the
CD8+ T cells derived from different treatments were
harvested as effector cells and RenCa cells were used as target
cells to establish a co-culture system with different E/T
(effector/target) ratios for the LDH release assay via a CytoTox96
Non-Radioactive Cytotoxicity Assay Kit (Promega Biological
Products, Ltd., Shanghai, China) and E/T=3 for the apoptosis assay
via flow cytometry. Subsequently, RDE-stimulated CD8+ T
cells were used as new effector cells, whereas 4T1 cells and CT26
cells served as control target cells for the LDH release assay,
apoptosis assay and cell cycle analysis. Briefly, co-culture
systems were established with different E/T ratios of
RDE-stimulated CD8+ T cells and three types of tumor
cells at 37°C for 4 h. For the inhibition of FasL-dependent
cytotoxicity, co-cultures (RenCa cells and CD8+ T cells)
were treated or not with 10 µg/ml anti-FasL antibody (MFL-3,
555291; BD Bioscience, San Jose, CA) or isotype control
(eBioscience, San Diego, CA, USA) and maintained at 37°C. Specific
lysis (%) was calculated as follows: (Experimental LDH
release-effector cells-target spontaneous LDH release)/(target
maximum LDH release) ×100 (10).
Then, when E/T=10, the three types of tumor cells were digested by
trypsinization (Beyotime Biotechnology, Shanghai, China) and washed
several times for apoptosis assays and cell cycle analysis via flow
cytometry. Subsequently, CytoFLEX software (Beckman Coulter, CA,
USA) was used to analyze the flow cytometry data. The percentage of
apoptosis was calculated from early apoptosis (Annexin
V+/PI−) plus late apoptosis (Annexin
V+/PI+), and the cell cycle analysis was
compared with the S-phase ratio. Each experiment was performed in
triplicate.
Western blot analysis
Western blotting was performed as described
previously (25). A total of 20 µg of
RDEs or crude protein extracted from RenCa cell lysates was
fractionated by 10–15% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to polyvinylidene
difluoride (PVDF) membranes (Millipore). The membranes were blocked
in Tris-buffered saline with Tween (TBST) and 5% nonfat milk for 1
h at room temperature. The rabbit anti-mouse HSP70 monoclonal
antibody (ab181606; Abcam, Cambridge, MA, USA), rabbit anti-mouse
CD63 monoclonal antibody (ab217345; Abcam) and rabbit anti-mouse
CD81 monoclonal antibody (ab109201; Abcam) diluted 1:1,000 were
added and incubated at 4°C overnight. The next day, after
incubation with the appropriate horseradish peroxidase
(HRP)-conjugated goat anti-rabbit secondary antibodies (ab6721;
Abcam) (1:5,000 dilution), the bots were then detected using an
Odyssey Infrared System (LI-COR Bioscience, Lincoln, NE, USA).
The cell fractions of three types of tumor cells
from different treatments and CD8+ T cells from
different treatments were prepared, and protein quantification was
performed using the BCA method. Equal amounts of protein were
separated via 10–15% SDS-PAGE and transferred to PVDF membranes.
The membranes were incubated with TBST and 5% non-fat milk to block
nonspecific binding at room temperature, and then they were
incubated overnight at 4°C with rabbit anti-mouse FasL polyclonal
antibody (ab15285; Abcam) (for CD8+ T cells), rabbit
anti-mouse Fas polyclonal antibody (ab82419; Abcam) (for tumor
cells) and rabbit anti-mouse β-actin polyclonal antibody (ab8227;
Abcam) (1:1,000 dilution). The next day, after incubation with the
HRP-conjugated goat anti-rabbit secondary antibodies (1:5,000
dilution), the immunoassociated protein bands on the membrane were
visualized using a chemiluminescence kit (Beyotime Biotechnology,
Shanghai, China) and detected using an Odyssey Infrared System. The
density of each band was normalized to its loading control
(β-actin).
Statistical analysis
All experiments were performed at least three times
in triplicate, and all data represent the mean ± SEM. One-way ANOVA
or two-way ANOVA followed by Tukey's test was used to analyze the
data. Statistical analysis was performed using GraphPad Prism
software 7.0 for Mac (GraphPad Software, Inc., San Diego, CA,
USA).
Results
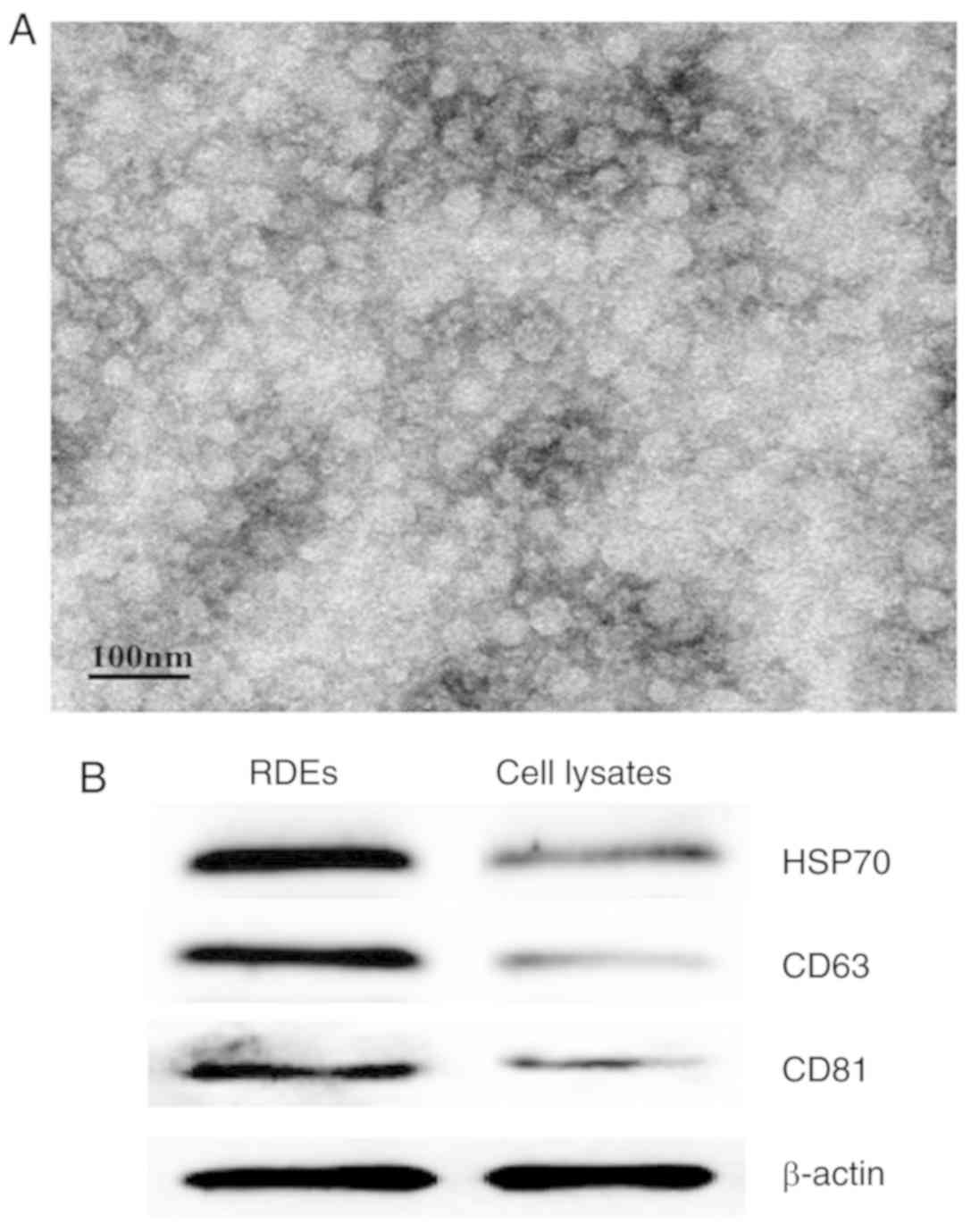
Isolation and characterization of
RDEs
Whether RenCa cells produce and secrete exosomes was
determined by TEM (Fig. 1A). We first
isolated and purified exosomes from the culture supernatants of
RenCa cells by ultrafiltration and sucrose density gradient
centrifugation. Subsequently, the RDEs exhibited the expected
morphology and were visualized as spherical membrane-bound vesicles
with a diameter of 30–100 nm as determined by TEM. In addition, the
western blot analysis results showed that the molecular markers
HSP70, CD63 and CD81 were highly expressed in RDEs but relatively
rare in the lysates of RenCa cells (Fig.
1B).
RDEs alter T cell subset ratios and
stimulate CD8+ T cells
Exosomes from tumor cells have been shown to either
suppress or stimulate the immune response (26). Because exosomes have complex immune
functions due to their composition, we initiated the study by
detecting T cell subset ratios (CD3+ CD4+ and
CD8+) in splenocytes from RDE-immunized mice and tumor
single cell suspensions from PBS-treated mice via flow cytometry at
different time points. An examination of the T cell subset ratios
in splenocytes (Fig. 2C and D) from
RDE-immunized mice indicated a significant increase in the ratio of
CD8+/CD4+ T cells compared with that of the
PBS treatment group, although no difference was observed in the
number of CD3+ T cells in the splenocytes (Fig. 2A and B) on days 10, 20 and 30.
However, the CD3+ T cells in splenocytes (Fig. 2A and B) from the tumor-bearing mice
were significantly decreased. We also found that the proportion of
CD8+/CD4+ T cells in RenCa tumor tissues
gradually increased with tumor progression (Fig. 2E and F). To further investigate the
immune response induced by RDEs, the levels of IFN-γ in culture
supernatants of splenic CD8+ T cells obtained from
RDE-immunized mice were measured via an ELISA kit, according to the
manufacturer's instructions. As shown in Fig. 2G, compared with CD8+ T
cells from PBS-immunized mice, RDEs induced splenic CD8+
T cells obtained from RDE-immunized mice to produce a large amount
of IFN-γ.
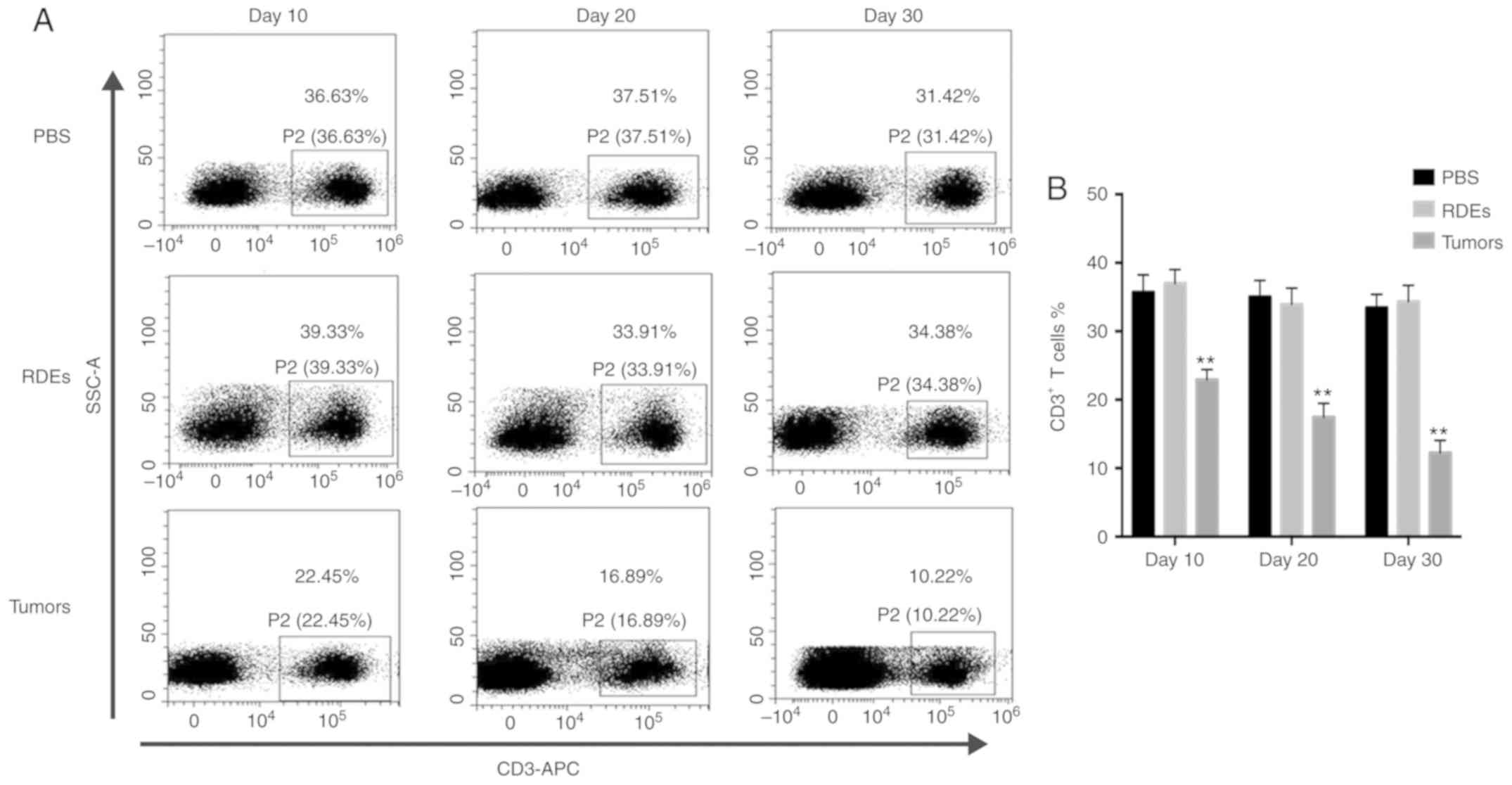 | Figure 2.Evaluation of the immune properties
of RDEs. Seven-week-old BLAB/c mice were immunized intravenously
with PBS and RDEs three times per week for 4 weeks, and RenCa cells
were simultaneously injected subcutaneously to generate a
tumor-bearing mouse model. One day after a 10-, 20- or 30-day
immunization with RDEs, splenocytes without erythrocytes and tumor
single-cell suspensions were isolated from mice in the different
treatment groups to determine the percentages of T cell subsets [(A
and B) CD3+ T cells in splenocytes, (C and D)
CD8+/CD4+ T cells in splenocytes in tumor
single cell suspensions] via flow cytometry. Similar data were
obtained from three independent experiments. Asterisks indicate
significant differences (**P<0.01). RDEs, RenCa cell-derived
exosomes; CD, cluster of differentiation; IFN-γ, interferon-γ;
ELISA, enzyme-linked immunosorbent assay; APC, allophycocyanin;
FITC, fluorescein isothiocyanate; PE, phycoerythrin; SSC-A, side
scatter area. Evaluation of the immune properties of RDEs.
Seven-week-old BLAB/c mice were immunized intravenously with PBS
and RDEs three times per week for 4 weeks, and RenCa cells were
simultaneously injected subcutaneously to generate a tumor-bearing
mouse model. One day after a 10-, 20- or 30-day immunization with
RDEs, splenocytes without erythrocytes and tumor single-cell
suspensions were isolated from mice in the different treatment
groups to determine the percentages of T cell subsets [(E and F)
CD8+/CD4+ T cells in tumor single cell
suspensions] via flow cytometry. (G) Splenic CD8+ T
cells obtained from mice immunized with PBS and RDEs were incubated
under a humidified atmosphere of 5% CO2 at 37°C in
vitro. After 48 h, the supernatants were cleared of dead cells
and cell debris, collected and utilized for ELISA to measure the
quantity of IFN-γ secreted from RDE-stimulated splenic
CD8+ T cells. Similar data were obtained from three
independent experiments. Asterisks indicate significant differences
(**P<0.01). RDEs, RenCa cell-derived exosomes; CD, cluster of
differentiation; IFN-γ, interferon-γ; ELISA, enzyme-linked
immunosorbent assay; APC, allophycocyanin; FITC, fluorescein
isothiocyanate; PE, phycoerythrin; SSC-A, side scatter area. |
RDEs combined with GM-CSF and IL-12
promoted the proliferation of CD8+ T cells more
effectively than RDEs alone with GM-CSF or IL-12 in vitro
To investigate whether RDE immunization could induce
the proliferation of CD8+ T cells effectively in
vitro, we initiated the study by determining the effect of RDEs
on the proliferation rate of CD8+ T cells. Splenic
CD8+ T cells obtained from the mice were labeled with
CFSE and stimulated with PBS, PHA, PHA+RDEs, GM-CSF, IL-12,
PHA+RDEs+GM-CSF, PHA+RDEs+IL-12 and PHA+RDEs+GM-CSF+IL-12. As shown
in Fig. 3A and B, the proliferation
index of CD8+ T cells was significantly enhanced by
stimulation with PHA compared with PBS; enhanced by stimulation
with PHA+RDEs compared with PHA; enhanced by stimulation with
GM-CSF or IL-12 compared with PBS; and enhanced by stimulation with
PHA+RDEs+GM-CSF or PHA+RDEs+IL-12 compared with PHA+RDEs. The
maximum proliferative index was observed in CD8+ T cells
stimulated with PHA+RDEs+GM-CSF+IL-12.
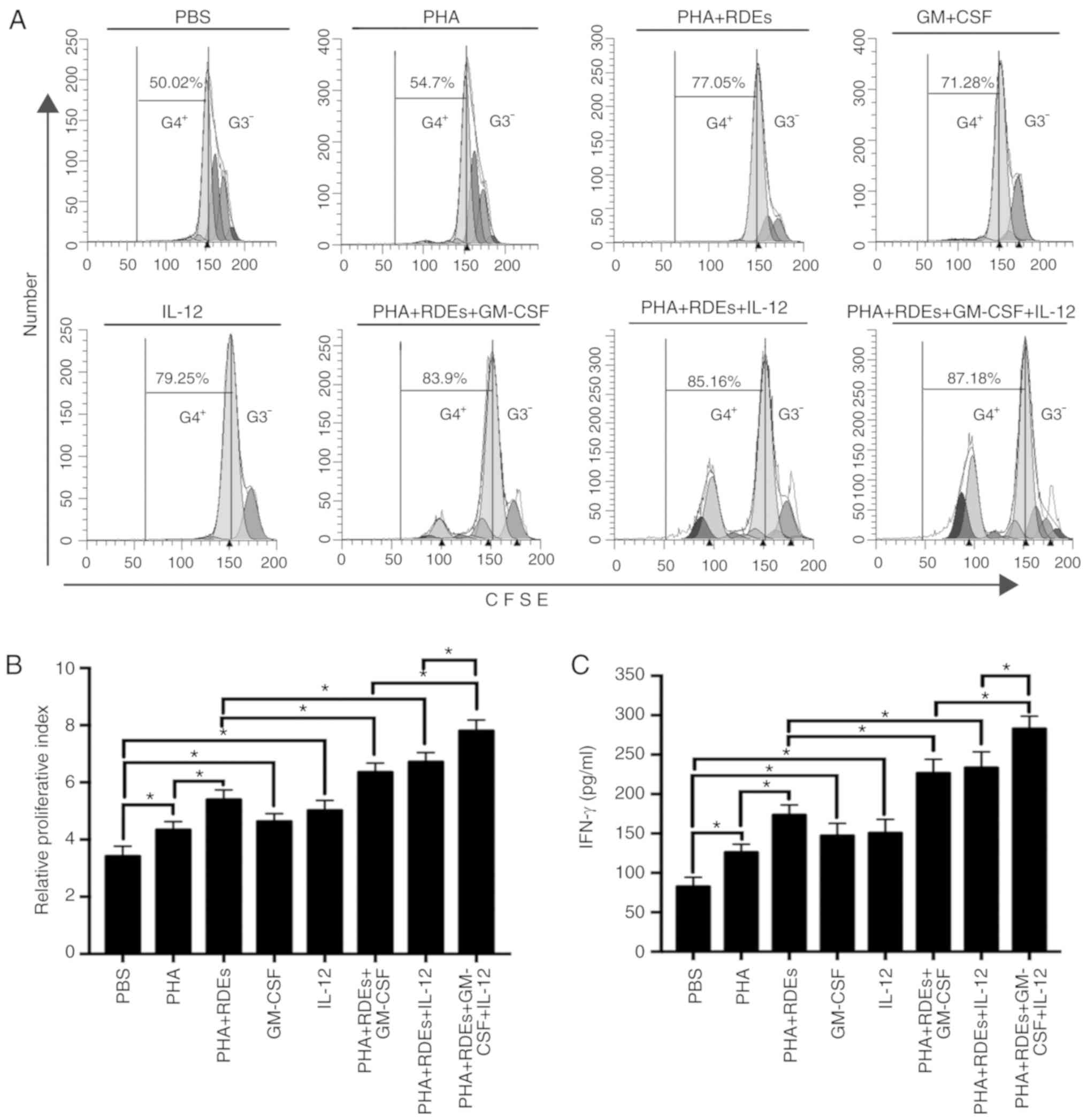 | Figure 3.RDEs combined with GM-CSF and IL-12
induce the proliferation and stimulation of CD8+ T cells
more effectively than RDEs alone with GM-CSF or IL-12 in
vitro. Splenic CD8+ T cells were labeled with CFSE
and treated with PBS, PHA, PHA+RDEs, GM-CSF, IL-12,
PHA+RDEs+GM-CSF, PHA+RDEs+IL-12 and PHA+RDEs+GM-CSF+IL-12 for 48 h.
(A) Number of cell divisions was assessed by successive halving of
the fluorescence intensity of CFSE by flow cytometry. RDE-treated
CD8+ T cells exhibited a smaller number of cells with
lower (G1, G2 and G3) cell divisions and a greater number of cells
with higher (G4+) cell divisions than non-RDE-treated
CD8+ T cells. The application of GM-CSF and IL-12
enhanced this effect. (B) Relative proliferation index of each
treatment group was also assessed by flow cytometry. (C)
Supernatants of different treatment groups were harvested, and the
release of IFN-γ was determined by ELISA. Each group was tested in
triplicate wells, and the values were statistically analyzed by
one-way ANOVA (*P<0.05). RDEs, RenCa cell-derived exosomes;
ELISA, enzyme-linked immunosorbent assay; IFN-γ, interferon-γ;
GM-CSF, granulocyte-macrophage colony stimulating factor; G1-4,
generation 1–4; IL-12, interleukin-12; PHA, phytohemagglutinin;
CFSE, carboxyfluorescein diacetate succinimidyl ester; ANOVA,
analysis of variance; CD, cluster of differentiation. |
To further investigate the ability of RDEs to induce
the release of IFN-γ by CD8+ T cells, the cell
supernatants of the different treatments described above were
harvested and subjected to an IFN-γ release assay by ELISA. The
results demonstrated that RDEs induced the production of IFN-γ by
CD8+ T cells in vitro, and similar results can be
observed above (Fig. 3C). This
finding indicates that RDEs can effectively promote the
proliferation and activation of CD8+ T cells.
Furthermore, the combination of RDEs with GM-CSF and IL-12 may also
facilitate a stronger positive effect than RDEs alone with GM-CSF
or IL-12 on the stimulation and proliferation of CD8+ T
cells.
More effective induction of cytotoxic
effects in RDE-stimulated CD8+ T cells treated with
GM-CSF and IL-12
To explore whether RDE-stimulated CD8+ T
cells combined with GM-CSF and IL-12 could induce more potent
cytotoxic effects, we harvested RDE-stimulated CD8+ T
cells treated with GM-CSF, IL-12 or GM-CSF in combination with
IL-12 to establish a co-culture system with different E/T ratios.
GM-CSF, IL-12-treated CD8+ T cells served as the
control. The results (Fig. 4A) showed
that RDE-stimulated CD8+ T cells treated with GM-CSF or
IL-12 could kill RenCa cells more effectively than RDE-stimulated
CD8+ T cells alone, and GM-CSF in combination with IL-12
could achieve the maximum cytotoxic effect. However, GM-CSF or
IL-12 alone failed to increase the cytotoxic effect of control
CD8+ T cells. Similar results were observed when flow
cytometry was used to measure the proportion of apoptotic RenCa
cells when E/T=3 (Fig. 4B and C). The
results demonstrated the antitumor effects of RDE-stimulated
CD8+ T cells combined with GM-CSF and IL-12.
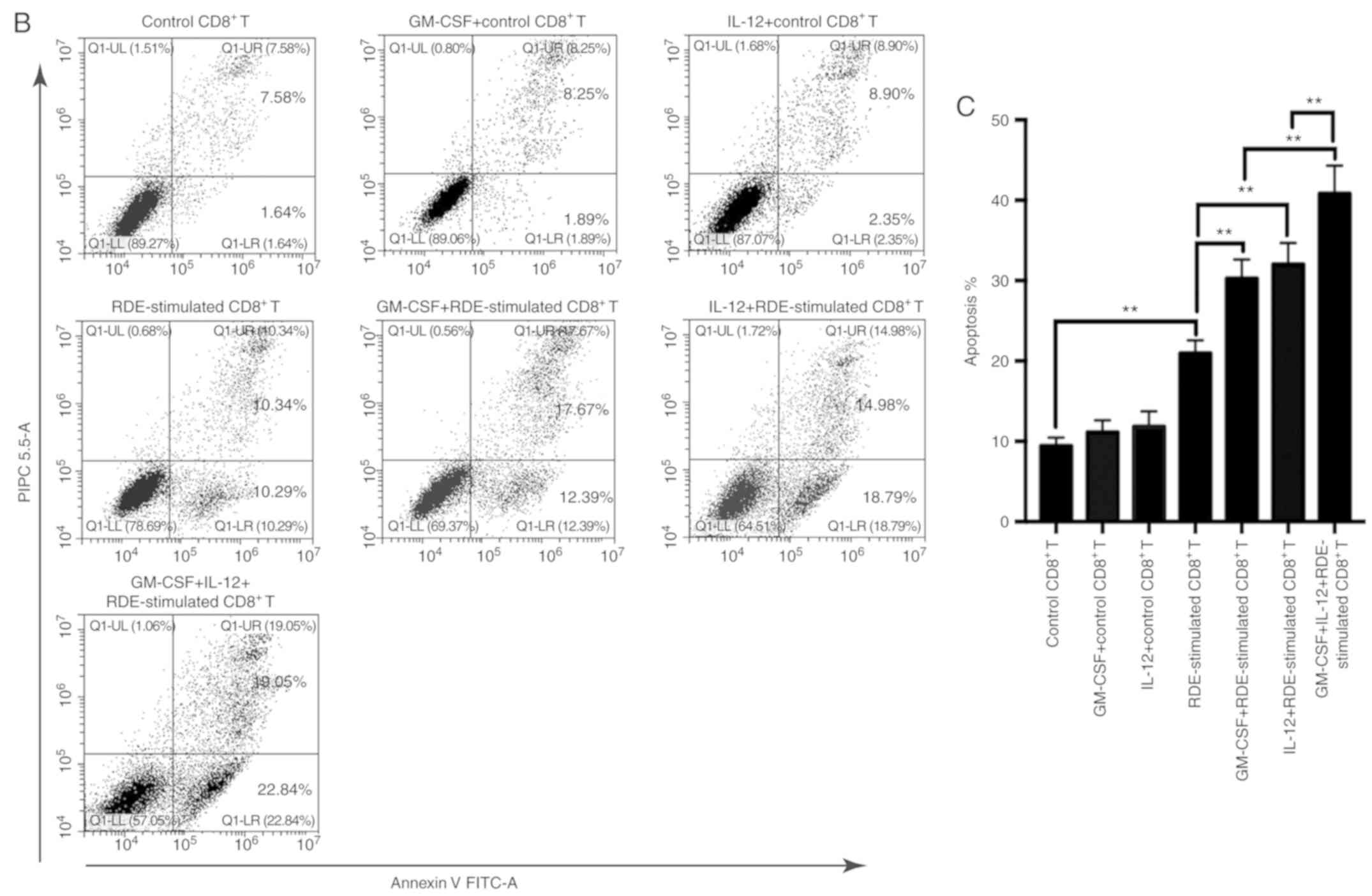 | Figure 4.Increased cytotoxicity of
RDE-stimulated CD8+ T cells in combination with GM-CSF
and IL-12. Splenic CD8+ T cells from immunized mice were
re-stimulated with irradiated RenCa cells in the presence of IL-2
in vitro. Three days later, re-stimulated CD8+ T
cells were treated with GM-CSF, IL-12 and GM-CSF in combination
with IL-12 for 48 h. Control CD8+ T cells were treated
with GM-CSF or IL-12. CD8+ T cells derived from the
different treatments were used as effectors, and the target cells
were RenCa cells. (A) Cytotoxic assay of CD8+ T cells
was performed using the LDH release assay with the following
formula: Lysis (%)=(experimental LDH release-effector cells-target
spontaneous LDH release)/(target maximum LDH release) ×100. (B and
C) Apoptosis analysis was performed when E/T=3, and the proportion
of apoptotic RenCa cells was detected by flow cytometry
(**P<0.01). IL, interleukin; GM-CSF, granulocyte-macrophage
colony stimulating factor; CD, cluster of differentiation; RDEs,
RenCa cell-derived exosomes; LDH, lactate dehydrogenase; E/T,
effector/target; FITC-A, fluorescein isothiocyanate-area; PI PC
5.5-A, propidium iodide PC 5.5-area. |
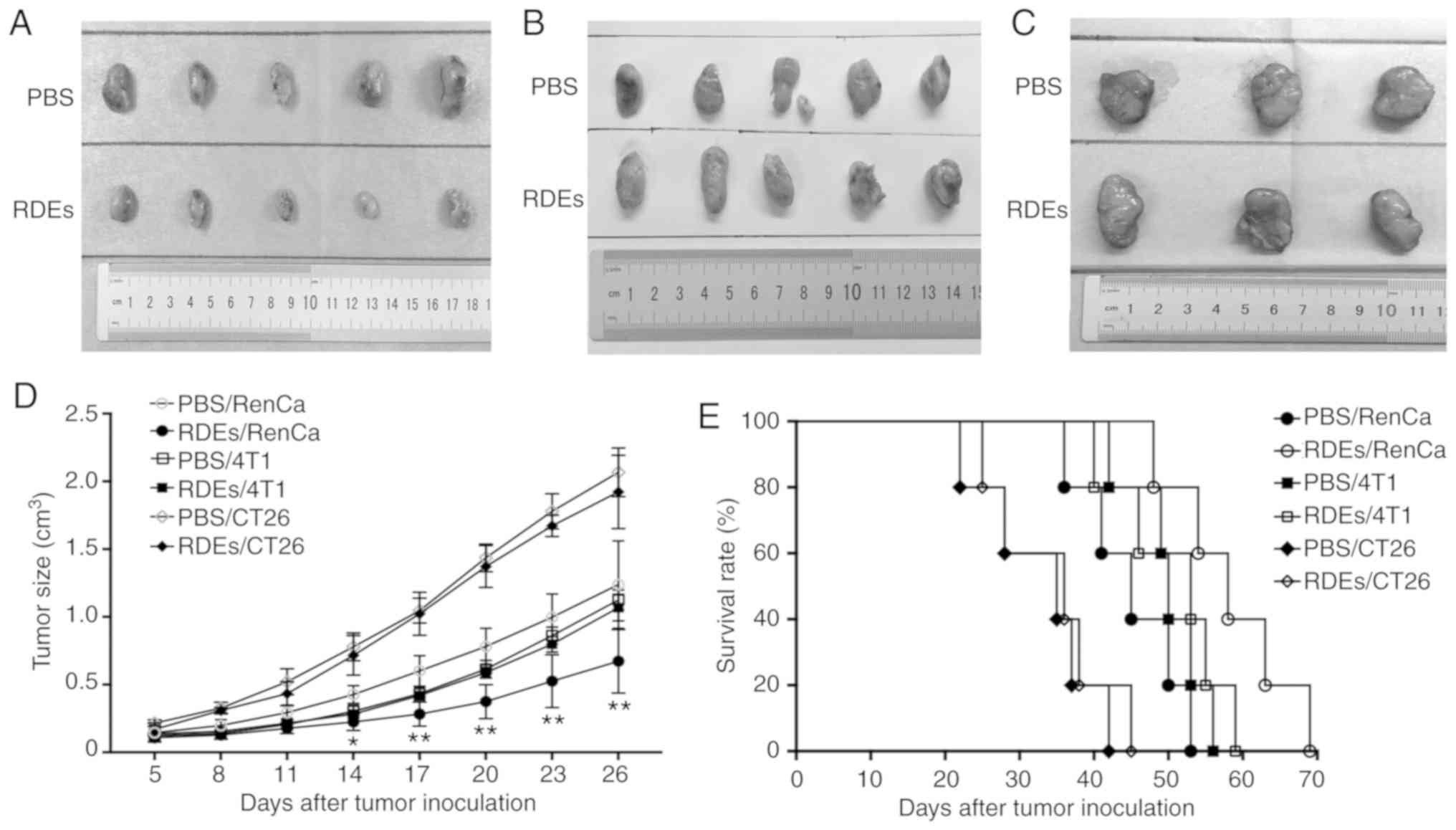
RDEs specifically inhibit the growth
of RenCa tumors
To determine whether RenCa tumors were more affected
by RDEs than other types of tumors, we used RDEs and PBS to treat
BALB/c mice bearing established subcutaneous RenCa, 4T1 and CT26
tumors and followed the tumor growth. Tumor size was measured using
calipers, and overall survival was monitored and recorded. As shown
in Fig. 5A and D, compared with PBS,
RDE vaccination promoted a marked inhibition of RenCa tumor growth.
In addition, a phenomenon occurred in which the difference in tumor
growth between the RDE groups and the PBS groups was not observed
in mice with 4T1 (Fig. 5B and D) and
CT26 (Fig. 5C and D) tumors.
Moreover, the survival rate of RenCa tumor-challenged mice was also
significantly increased by RDE immunization, although mice with 4T1
and CT26 tumors did not exhibit corresponding differences between
treatment groups (Fig. 5E). Taken
together, the data indicate that RDEs induced more effective
therapeutic immunity against renal cortical adenocarcinoma than
against other cancer types.
RDE-stimulated CD8+ T cells
induce stronger immunity against renal cancer than against other
cancer types in vitro
Next, we investigated the specific cytotoxic effects
of RDE-stimulated splenic CD8+ T cells against three
types of tumor cells using the LDH release method. Seven days after
intravenous immunization with RDEs, splenic CD8+ T cells
re-stimulated with irradiated RenCa cells were sorted and
co-cultured with different target cells at various E/T ratios. As
shown in Fig. 6A, RDE-stimulated
CD8+ T cells displayed potent cytotoxic ability against
RenCa cells at all E/T ratios, while no detectable CD8+
T response was induced by PBS. A less dramatic increase in the
killing rate occurred when 4T1 and CT26 cells were treated with
RDE-stimulated CD8+ T cells at E/T ratios over 12:1.
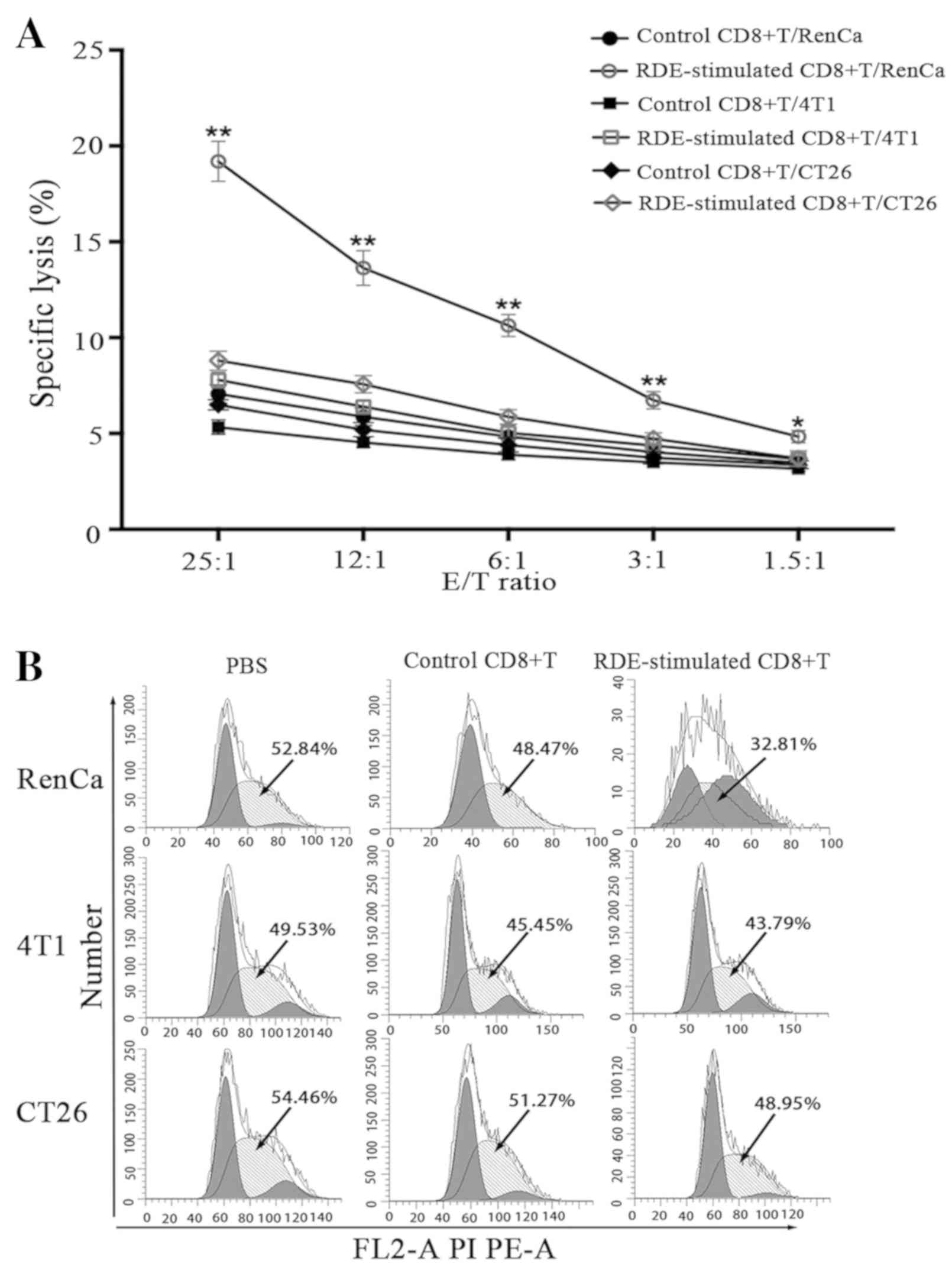 | Figure 6.Development of antigen-specific
CD8+ T cell cytotoxic activity in vitro. (A)
Splenic CD8+ T cells from immunized mice were
re-stimulated with irradiated RenCa cells in the presence of IL-2
in vitro. Three days later, stimulated CD8+ T
cells were harvested and used as effector (E) cells in the LDH
release assay, whereas RenCa cells or control 4T1 and CT26 cells
were used as the target (T) cells and mixed with effector cells at
different ratios. Specific lysis (%) was calculated with the
following formula on the basis of the amount of LDH release:
(Experimental LDH release-effector cells-target spontaneous LDH
release)/(target maximum LDH release) ×100. RDE-stimulated
CD8+ T cells and control CD8+ T cells were
co-cultured with three types of tumor cells at a 10:1 ratio. (B)
Cell cycle distribution of the three types of tumor cells were
detected by flow cytometry. The data are representative of three
independent experiments and expressed as the mean ± SD (*P<0.05,
**P<0.01). IL, interleukin; CD, cluster of differentiation;
RDEs, RenCa cell-derived exosomes; FITC-A, fluorescein
isothiocyanate-area; FL2-A PI PE-A, FL2-A propidium iodide PE
5.5-area; PI PC 5.5-A, propidium iodide PC 5.5-area; SD, standard
deviation; LDH, lactate dehydrogenase; FasL, Fas ligand.
Development of antigen-specific CD8+ T cell cytotoxic
activity in vitro. RDE-stimulated CD8+ T cells
and control CD8+ T cells were co-cultured with three
types of tumor cells at a 10:1 ratio. (C and D) Percentage of
apoptosis of the three types of tumor cells was detected by flow
cytometry. (E) FasL protein levels on the surface of
CD8+ T cells after different treatments and Fas levels
on the surface of RenCa, 4T1 and CT26 cells were assessed by
western blot analysis. The upper panel and lower panel show
histogram analyses of the expression levels of the FasL and Fas
proteins, respectively. β-actin was used as an internal control.
(F) Cytotoxic assay performed in the presence of anti-FasL antibody
or isotype control (10 µg/ml). The data are representative of three
independent experiments and expressed as the mean ± SD (*P<0.05,
**P<0.01). IL, interleukin; CD, cluster of differentiation;
RDEs, RenCa cell-derived exosomes; FITC-A, fluorescein
isothiocyanate-area; FL2-A PI PE-A, FL2-A propidium iodide PE
5.5-area; PI PC 5.5-A, propidium iodide PC 5.5-area; SD, standard
deviation; LDH, lactate dehydrogenase; FasL, Fas ligand. |
Subsequently, to determine the effect of effector
cells on apoptosis and the cell cycle in three types of target
cells, the percentage of cells undergoing apoptosis and the cell
cycle distribution of target cells were determined and quantified
using an apoptosis assay and a cell cycle assay, respectively, via
flow cytometry. As shown in Fig. 6C and
D, Annexin V-based apoptosis analysis revealed significant
increases in the percentages of apoptotic cells in the lower right
and upper right quadrants of the histograms, called early and late
apoptotic cells, respectively, in RenCa cells treated with
RDE-stimulated CD8+ T cells, compared with controls at
an E/T ratio of 10:1. By contrast, compared with the controls,
there was a less marked increase in the apoptosis rate when 4T1 and
CT26 cells were tested with RDE-stimulated CD8+ T cells.
Furthermore, in cell cycle assays (Fig.
6B), RenCa cells treated with RDE-stimulated CD8+ T
cells showed a significantly decreased S-phase ratio compared with
the controls, but the 4T1 and CT26 cells did not, indicating that
the killing activity of these RDE-stimulated CD8+ T
cells was exosome antigen-specific.
A previous study demonstrated that cytotoxic T cells
can mediate their lytic effects through a distinct pathway called
the Fas-based pathway, in which the interaction between FasL
expressed on CTLs and Fas presented on target cells triggers
apoptosis and cell death (20).
Hence, in the present study, the expression of FasL on
CD8+ T cells and Fas on tumor cells was detected by
western blotting. As shown in Fig.
6E, the co-culture of RDE-stimulated CD8+ T cells
and RenCa cells resulted in an increase in FasL expression on the
CD8+ T cell surface and an increase in Fas expression on
RenCa cells, compared with the control levels. However, in other
types of tumor cells, similar results were not observed. The
perforin inhibitor blocked the majority of RenCa cell CTL lysis,
suggesting a major effector role for granule-mediated lysis in
vitro (21). However, some
inhibition of lysis was also observed when the anti-FasL antibody
was present (Fig. 6F). These findings
suggest that the FasL/Fas-mediated mechanism also plays a
significant role in the cytotoxicity of RDE-stimulated
CD8+ T cells.
Discussion
Research on exosomes has attracted substantial
attention since their initial discovery. TEXs are not only involved
in establishing a favorable microenvironment surrounding the tumor
but also induce a comprehensive alteration of the immune system
(27,28).
A full understanding of TEX immunization includes
the observation that TEXs are a source of shared TAA and
stimulatory molecules for the innate immune response, which can
induce T cell-dependent immunity in mice or human tumor models
under some conditions (8–11,16–18). This
property makes TEXs novel potential vaccines for the treatment of
tumors. However, according to previous studies, TEXs can be
equipped with either immune-stimulatory ability or
immunosuppressive ability due to the complexity of their components
(26). The immunosuppressive
abilities of TEXs have been characterized in numerous cancer types
and in the context of several different mediators, such as the
amplification of myeloid-derived suppressor cell (MDSC) (23), the induction of effector T cell
apoptosis (29,30), the suppression of natural killer (NK)
cell capability (31) and the
disruption of dendritic cell (DC) differentiation and maturation
(32). However, this effect has no
negative impact on their availability to transmit TAA to mature DCs
and induce an immune-stimulatory response that is superior to their
immunosuppressive effect in tumor-bearing hosts. Therefore, we have
demonstrated by means of T cell subset ratio analyses, tumor growth
assays, IFN-γ secretion assays, proliferation assays of
CD8+ T cells and cytotoxicity assays of RDE-stimulated
CD8+ T cells that RDEs express immunologically relevant
molecules to induce in vivo and in vitro immune
activation and elicit a potent immune response. Additionally, when
RDE-stimulated CD8+ T cells are combined with GM-CSF and
IL-12, the immune response is further optimized. Furthermore, we
can conclude from the tumor growth assay that in the case of RDEs,
their immune-stimulatory ability is greater than their
immunosuppressive ability.
Previous studies have reported that the injection of
an increasing number of TEXs could provide a source of
tumor-rejection antigens relevant for immunization (33) and the crucial components of the immune
response are DCs that participate in antigen presentation and
mediate antigen specificity of the immune response (34). Therefore, based on the positive
effects of RDEs on immune cells, we investigated the specific
cytotoxic function of RDE-stimulated splenic CD8+ T
cells. As expected, RDE antigen-specific splenic CD8+ T
cells displayed stronger cytotoxicity for the autologous tumor
cells than the other types of tumor cells at any E/T ratio. In
vitro experiments have shown that TEXs may need to rely on host
DCs for the induction of an immune response (8–11,12–14);
however, TEXs can be taken up by CD8+ T cells, although
a lack of DC participation was reported in some experiments, and
TEXs can directly induce specific killing activity of
CD8+ T cells via exosomal MHC molecules (35). Notably, we observed a dramatic
phenomenon in which RDE-stimulated CD8+ T cells
displayed a slightly weaker lethality for CT26 cells and 4T1 cells.
This effect could be caused by nonspecific cytotoxicity or a
mixture of RDE-stimulated NK cells that lack specific cytotoxicity
for tumor cells among the CD8+ T cells derived from the
splenocytes of immunized mice (15).
Two major molecular pathways of CD8+ T
cell-regulated cytotoxicity have been proven: i) the exocytosis of
granules containing perforin and granzyme molecules and ii) the
interaction of FasL on T cells with the apoptosis-inducing Fas
molecule on target cells (19). CTLs
initially recognize target cells using the TCR and strongly adhere
to these cells via accessory molecules; finally, FasL partially
transmits death signals to Fas-positive target cells and induces
cell apoptosis. These anti-RenCa tumor CD8+ T cells can
lyse RenCa cells in vitro via the perforin/granzyme and
FasL/Fas pathways. Moreover, proinflammatory cytokines, such as
IFN-γ and TNF-α could play a key role in the effector phase of the
immune response because these cytokines sensitize tumor cells to
lysis by both of the above pathways (21). This observation may explain why
RDE-stimulated CD8+ T cell-treated RenCa cells highly
expressed the Fas protein compared with RenCa cells treated with
control CD8+ T cells or PBS. T-cell-mediated
cytotoxicity in vitro was enhanced via triggering through
the crossing-linking of TCR, which is known to be necessary for the
up-regulation of cell surface FasL (36). However, similar results were not
observed in other types of tumor cells, suggesting that the effect
of pro-inflammatory cytokines is based on the specific recognition
and combination of FasL and Fas and that apoptosis mediated by the
FasL-Fas pathway is characterized by antigenic specificity.
However, controlling tumor growth and metastasis using CTLs alone
is difficult due to the complex mechanisms of tumor cell
proliferation, progression and metastasis. CTLs expressing FasL can
induce apoptosis in tumor cells, whereas tumor cells expressing
FasL can also induce the elimination of CTLs according to the tumor
anti-killing theory (37), which
necessitates the optimization of RDE-based vaccines, such as the
use of RDE-stimulated CD8+ T cells in combination with
GM-CSF and IL-12.
Taken together, these data demonstrate that
investigating TEX-associated mechanisms in the context of the
immune response is vital for the development of novel therapies.
Additional mechanisms that modify TEXs to suppress and evade the
immune system have attracted attention. Immunosuppressive mediators
that are present in TEXs, such as FasL, TRAIL (6), TGF-β (8–11), PD-L1
(7), and HSP72 (12), can exert adverse effects on the
antitumor response. Furthermore, a negative effect of the
immunogenicity of TEX-based vaccines arises when TEXs lack the
expression of MHC complexes (13),
adhesion molecule signals (38) and
co-stimulatory molecules (14) that
can enhance antitumor immunity. Therefore, extensive research has
already demonstrated that downregulating these mediators of
immunosuppression and avoiding the absence of these
immune-stimulatory factors can lead to a more efficient induction
of systemic antitumor immunity and CTL responses. Furthermore, the
combination of TEXs with GM-CSF (39)
and IL-12 (22) may also facilitate a
stronger positive effect on the antitumor response. The activation
of NK cells induced by TEXs from one of these tumors may have a
relatively weak nonspecific killing effect on other tumors in
patients who have two or more tumors simultaneously. In summary,
exosome therapy offers a promising immune-based strategy for the
development of cancer vaccines; however, we must still optimize the
TEX-based treatment program in clinical trials due to the complex
role of TEX components in immune regulation.
In conclusion, our study demonstrated that RDEs
could be used as vaccines to effectively retard renal cancer growth
and prolong survival in mice. RDEs can promote the proliferation
and activation of CD8+ T cells, and the combination of
RDE-stimulated CD8+ T cells with GM-CSF or IL-2 may
achieve a more satisfactory antitumor immune response. Furthermore,
RDE-stimulated CD8+ T cells can induce antigen-specific
anti-renal cancer effects through the FasL/Fas signaling pathway.
Therefore, our study provides a promising therapy for renal cancer
using exosome-based antigen-specific vaccines.
Acknowledgements
Not applicable.
Funding
The National Natural Science Foundation of China
(grant no. 81272572) provided financial support for this study.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article, and are available on reasonable
request.
Authors contributions
HYX conducted experiments, data acquisition and
analysis and was a major contributor in writing the manuscript; YZ
made a significant contribution to the conception and design of the
experiments; NL and NY were involved in cell culture and exosome
extraction; XFX, HXW and XYL collected and analyzed the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Chongqing Medical University
approved this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests, and all authors have confirmed the accuracy of this
statement.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ljungberg B, Cowan NC, Hanbury DC, Hora M,
Kuczyk MA, Merseburger AS, Patard JJ, Mulders PF and Sinescu IC;
European association of urology guideline group, : EAU guidelines
on renal cell carcinoma: The 2010 update. Eur Urol. 58:398–406.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sundararajan V, Sarkar FH and Ramasamy TS:
Correction to: The versatile role of exosomes in cancer
progression: Diagnostic and therapeutic implications. Cell Oncol
(Dordr). 41:223–252. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gu X, Erb U, Büchler MW and Zöller M:
Improved vaccine efficacy of tumor exosome compared to tumor lysate
loaded dendritic cells in mice. Int J Cancer. 136:E74–E84. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stenqvist AC, Nagaeva O, Baranov V and
Mincheva-Nilsson L: Exosomes secreted by human placenta carry
functional Fas ligand and TRAIL molecules and convey apoptosis in
activated immune cells, suggesting exosome-mediated immune
privilege of the fetus. J Immunol. 191:5515–5523. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M,
Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1
contributes to immunosuppression and is associated with anti-PD-1
response. Nature. 560:382–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wieckowski EU, Visus C, Szajnik M,
Szczepanski MJ, Storkus WJ and Whiteside TL: Tumor-derived
microvesicles promote regulatory T cell expansion and induce
apoptosis in tumor-reactive activated CD8+ T
lymphocytes. J Immunol. 183:3720–3730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valenti R, Huber V, Filipazzi P, Pilla L,
Sovena G, Villa A, Corbelli A, Fais S, Parmiani G and Rivoltini L:
Human tumor-released microvesicles promote the differentiation of
myeloid cells with transforming growth factor-beta-mediated
suppressive activity on T lymphocytes. Cancer Res. 66:9290–9298.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang F, Wan J, Hao S, Deng X, Chen L and
Ma L: TGF-β1-silenced leukemia cell-derived exosomes target
dendritic cells to induce potent anti-leukemic immunity in a mouse
model. Cancer Immunol Immunother. 66:1321–1331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang F, Wan J, Hu W and Hao S:
Enhancement of anti-leukemia immunity by leukemia-derived exosomes
via downregulation of TGF-β1 expression. Cell Physiol Biochem.
44:240–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chalmin F, Ladoire S, Mignot G, Vincent J,
Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau
D, et al: Membrane-associated Hsp72 from tumor-derived exosomes
mediates STAT3-dependent immunosuppressive function of mouse and
human myeloid-derived suppressor cells. J Clin Invest. 120:457–471.
2010.PubMed/NCBI
|
|
13
|
Yang MQ, Du Q, Varley PR, Goswami J, Liang
Z, Wang R, Li H, Stolz DB and Geller DA: Interferon regulatory
factor 1 priming of tumour-derived exosomes enhances the antitumour
immune response. Br J Cancer. 118:62–71. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Escola JM, Kleijmeer MJ, Stoorvogel W,
Griffith JM, Yoshie O and Geuze HJ: Selective enrichment of
tetraspan proteins on the internal vesicles of multivesicular
endosomes and on exosomes secreted by human B-lymphocytes. J Biol
Chem. 273:20121–20127. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie Y, Bai O, Zhang H, Yuan J, Zong S,
Chibbar R, Slattery K, Qureshi M, Wei Y, Deng Y and Xiang J:
Membrane-bound HSP70-engineered myeloma cell-derived exosomes
stimulate more efficient CD8(+) CTL- and NK-mediated antitumour
immunity than exosomes released from heat-shocked tumour cells
expressing cytoplasmic HSP70. J Cell Mol Med. 14:2655–2666. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bu N, Wu H, Sun B, Zhang G, Zhan S, Zhang
R and Zhou L: Exosome-loaded dendritic cells elicit tumor-specific
CD8+ cytotoxic T cells in patients with glioma. J
Neurooncol. 104:659–667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hao S, Bai O, Yuan J, Qureshi M and Xiang
J: Dendritic cell-derived exosomes stimulate stronger
CD8+ CTL responses and antitumor immunity than tumor
cell-derived exosomes. Cell Mol Immunol. 3:205–211. 2006.PubMed/NCBI
|
|
18
|
Yao Y, Chen L, Wei W, Deng X, Ma L and Hao
S: Tumor cell-derived exosome-targeted dendritic cells stimulate
stronger CD8+ CTL responses and antitumor immunities.
Biochem Biophys Res Commun. 436:60–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kreuwel HT, Morgan DJ, Krahl T, Ko A,
Sarvetnick N and Sherman LA: Comparing the relative role of
perforin/granzyme versus Fas/Fas ligand cytotoxic pathways in
CD8+ T cell-mediated insulin-dependent diabetes
mellitus. J Immunol. 163:4335–4341. 1999.PubMed/NCBI
|
|
20
|
Sayers TJ, Brooks AD, Lee JK, Fenton RG,
Komschlies KL, Wigginton JM, Winkler-Pickett R and Wiltrout RH:
Molecular mechanisms of immune-mediated lysis of murine renal
cancer: Differential contributions of perforin-dependent versus
Fas-mediated pathways in lysis by NK and T cells. J Immunol.
161:3957–3965. 1998.PubMed/NCBI
|
|
21
|
Seki N, Brooks AD, Carter CR, Back TC,
Parsoneault EM, Smyth MJ, Wiltrout RH and Sayers TJ: Tumor-specific
CTL kill murine renal cancer cells using both perforin and Fas
ligand-mediated lysis in vitro, but cause tumor regression in vivo
in the absence of perforin. J Immunol. 168:3484–3492. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Luo CL, He BC, Zhang JM, Cheng G
and Wu XH: Exosomes derived from IL-12-anchored renal cancer cells
increase induction of specific antitumor response in vitro: A novel
vaccine for renal cell carcinoma. Int J Oncol. 36:133–140.
2010.PubMed/NCBI
|
|
23
|
Wang J, De Veirman K, Faict S, Frassanito
MA, Ribatti D, Vacca A and Menu E: Multiple myeloma exosomes
establish a favourable bone marrow microenvironment with enhanced
angiogenesis and immunosuppression. J Pathol. 239:162–173. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baldan V, Griffiths R, Hawkins RE and
Gilham DE: Efficient and reproducible generation of
tumour-infiltrating lymphocytes for renal cell carcinoma. Br J
Cancer. 112:1510–1518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan Y, Zhang Y, Zhao S, Chen J, Yang J,
Wang T, Zou H, Wang Y, Gu J, Liu X, et al: Cadmium-induced
apoptosis in neuronal cells is mediated by Fas/FasL-mediated
mitochondrial apoptotic signaling pathway. Sci Rep. 8:88372018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Junker K, Heinzelmann J, Beckham C, Ochiya
T and Jenster G: Extracellular vesicles and their role in urologic
malignancies. Eur Urol. 70:323–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Graner MW, Schnell S and Olin MR:
Tumor-derived exosomes, microRNAs, and cancer immune suppression.
Semin Immunopathol. 40:505–515. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barros FM, Carneiro F, Machado JC and Melo
SA: Exosomes and immune response in cancer: Friends or foes? Front
Immunol. 9:7302018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ludwig S, Floros T, Theodoraki MN, Hong
CS, Jackson EK, Lang S and Whiteside TL: Suppression of lymphocyte
functions by plasma exosomes correlates with disease activity in
patients with head and neck cancer. Clin Cancer Res. 23:4843–4854.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Troyer RM, Ruby CE, Goodall CP, Yang L,
Maier CS, Albarqi HA, Brady JV, Bathke K, Taratula O, Mourich D and
Bracha S: Exosomes from osteosarcoma and normal osteoblast differ
in proteomic cargo and immunomodulatory effects on T cells. Exp
Cell Res. 358:369–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szczepanski MJ, Szajnik M, Welsh A,
Whiteside TL and Boyiadzis M: Blast-derived microvesicles in sera
from patients with acute myeloid leukemia suppress natural killer
cell function via membrane-associated transforming growth
factor-beta1. Haematologica. 96:1302–1309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kunigelis KE and Graner MW: The dichotomy
of tumor exosomes (TEX) in cancer immunity: Is it all in the
ConTEXt? Vaccines (Basel). 3:1019–1051. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mignot G, Roux S, Thery C, Ségura E and
Zitvogel L: Prospects for exosomes in immunotherapy of cancer. J
Cell Mol Med. 10:376–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C, Du
Z and Yin H: Tumor-derived exosomes elicit tumor suppression in
murine hepatocellular carcinoma models and humans in vitro.
Hepatology. 64:456–472. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ichim TE, Zhong Z, Kaushal S, Zheng X, Ren
X, Hao X, Joyce JA, Hanley HH, Riordan NH, Koropatnick J, et al:
Exosomes as a tumor immune escape mechanism: Possible therapeutic
implications. J Transl Med. 6:372008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vignaux F, Vivier E, Malissen B,
Depraetere V, Nagata S and Golstein P: TCR/CD3 coupling to
Fas-based cytotoxicity. J Exp Med. 181:781–786. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lenardo M, Chan KM, Hornung F, McFarland
H, Siegel R, Wang J and Zheng L: Mature T lymphocyte
apoptosis-immune regulation in a dynamic and unpredictable
antigenic environment. Annu Rev Immunol. 17:221–253. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang YT, Kim YJ, Bu J, Cho YH, Han SW and
Moon BI: High-purity capture and release of circulating exosomes
using an exosome-specific dual-patterned immunofiltration (ExoDIF)
device. Nanoscale. 9:13495–13505. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dai S, Wei D, Wu Z, Zhou X, Wei X and
Huang H: Phase I clinical trial of autologous ascites-derived
exosomes combined with GM-CSF for colorectal cancer. Mol Ther.
16:782–790. 2008. View Article : Google Scholar : PubMed/NCBI
|