The aim of this review was to focus on CS in IR by
evaluating radiosensitivity, IR-induced side effects, tumour cell
biological behavioural changes after IRIS and underlying
mechanisms. It was hypothesized that a comprehensive understanding
may provide new insights into novel therapeutic modalities in RT to
improve the outcomes of cancer patients.
IR kills tumour cells by causing lethal DNA damage,
which can ignite the DNA damage response (DDR), and non-homologous
end joining (NHEJ) and homologous recombination (HR) are the two
main pathways for repairing double-strand breaks (DSBs) induced by
DNA damage. The accuracy of DNA damage repair by related downstream
signalling pathways determines cell fate, including senescence and
apoptosis (12). Generally, DNA DSBs
are an especially potent stimulus for inducing CS (13). IRIS, is also a form of stress-induced
premature senescence (SIPS) (14) and
can occur in many types of cells, including cancer cells,
fibroblasts, epithelial cells, endothelial cells (ECs), immune
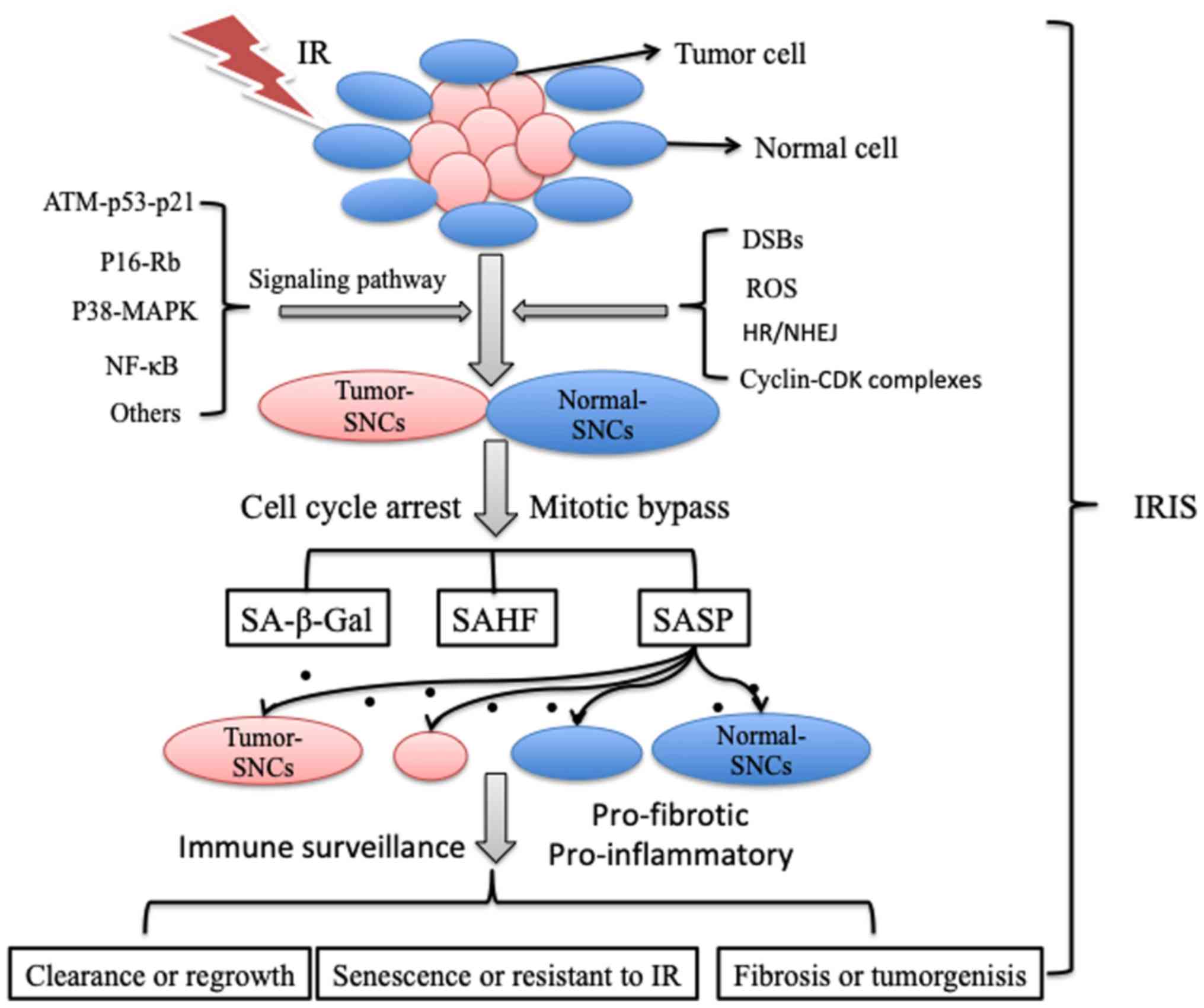
cells, and stem cells. Senescent cells (SCNs) always exhibit
apoptosis resistance, metabolic activity, proinflammatory and
profibrotic molecule secretion and neighbouring microenvironment
alteration despite that they have no cell division capacity and
permanently arrested proliferation (15) (Fig.
1).
In the senescence process induced by IR, the cell
cycle is interrupted by G2 arrest after inevitable DNA damage,
accompanied by mitotic bypass into the G1 phase (16). Ataxia telangiectasia-mutated protein
(ATM), p53, p21, p16-Rb, p38-mitogen-activated protein kinase
(p38-MAPK), NF-κB signalling pathway factors, reactive oxygen
species (ROS), senescence-associated secretory phenotype (SASP)
factors and cyclin-CDK complexes are involved in this process
(9,16,17).
Different doses of IR and DNA damage can lead to various types of
cells with mitotic cell cycle delays, including arrests in the G1,
G2 or S phase. G2 arrest and G2 slippage has been linked to IRIS in
most previous studies and reviews (18–21). SNCs
can be identified by prominent β-galactosidase activity, increased
p53, p21 and p16 expression, and decreased levels of Cdc2 and
survivin. Notably, some features of IRIS in normal cells and cancer
cells are summarized in Table I.
The function of the tumour suppressor protein p53 is
related to cell cycle control, DNA repair and apoptosis (40). p53 and phosphorylated retinoblastoma
protein (pRB) are the main proteins involved in establishing and
maintaining the state of irreversible growth arrest in replicative
senescence in normal human cells, and p53 inactivation could
reverse CS in BJ cells with a low level of p16 (41). Many studies (42–45) have
been carried out to explore the influence of p53 on IR-induced
effects. For example, HCT116 p53+/+ cells were found to
be much more susceptible to IRIS than p53–/– cells
(43). IR-induced mitotic skipping
during senescence-like growth arrest is associated with p53
function (24). Therefore, the
mechanisms of p53, the guardian of the genome, and its related
signalling pathways are well characterized in IRIS.
Increasing evidence supports that insulin-like
growth factor-binding protein 5 (IGFBP-5) plays a crucial role in
CS via a p53-dependent pathway and especially functions in the
coagulation factor Xa- or interleukin-6 (IL-6)-induced premature
senescence of ECs, smooth muscle cells (SMCs), and fibroblasts
(46–48). Exogenous IGFBP-5 or IGFBP-5
overexpression induces premature senescence in human umbilical vein
endothelial cells (HUVECs) in vitro, and knocking down
IGFBP-5 can partially alter the senescence process in vitro
(48). Notably, IGFBP-5 is
upregulated in the IRIS of HUVECs after chronic low-dose IR
(49) and may therefore be a
significant target to reduce IRIS in normal cells. In addition, the
BRE gene (BRCC45) is also associated with the DNA damage-induced
premature senescence of fibroblasts resulting from γ-IR (50). Downregulation of the lamin-B receptor
(LBR) and LB1 is a primary response of cells to various stresses
leading to senescence, and the loss of LB1 can even serve as a
biomarker of senescence (51,52). Naturally, other factors involved in
IRIS are independent of p53. For instance, oestrogen E2 suppressed
IRIS by inhibiting the binding of cyclin E with p21 and the
functional inactivation of p21, followed by permanent Rb
hyperphosphorylation, but it did not affect p53 activation in MCF-7
breast cancer cells (53).
Other factors also affect the process of IRIS. For
example, the IR dose plays a crucial role in inducing senescence or
apoptosis upon cell exposure; a low dose (0.5–10 Gy) of IR induces
senescence, while a very high dose (>10 Gy) induces apoptosis
(30), and this phenomenon is related
to the level of DNA damage and function of the DDR network.
Recently, Velegzhaninov et al (56) reported that a single low dose (30–50
mGy) of gamma irradiation could suppress CS in normal human
fibroblasts. Similarly, a single low-dose X-ray could promote the
proliferation of normal cells but not of cancer cells (29). However, low-dose fractionated IR (5×1
Gy) induced temporal patterns of p53/p21 expression in MRC5
fibroblasts, resulting in more significant CS than that generated
by a single 5 Gy pulse of IR, as indicated by an integrated
stochastic model of DNA damage repair (57). Therefore, the fraction regimen also
appears to affect IRIS and may respond differently in different
cells. For example, lymphocytic leukaemia cells with exponential
growth similar to that of rapidly proliferating tumour cells are
not very sensitive to fraction size, while slow-growing fibroblasts
and most late-responding cells show high sensitivity (31). Therefore, haematological toxicity
occurs early during the RT process, and monitoring and preventing
the development of leukopenia is of great importance. Other side
effects of IRIS are discussed more specifically in section
four.
Surviving non-tumourigenic cells were revealed to be
more prone to CS, while breast cancer initiating cells (CICs) could
be mobilized from the quiescent/G0 phase of the cell cycle to
actively cycling cells after sublethal doses of radiation (33). CICs, also called cancer stem cells
(CSCs), derived from many types of human cancers and cancer cell
lines demonstrate increased therapeutic resistance, partly because
they can evade differentiation and senescence induced by the
immune-suppression cytokine interferon (IFN) signalling pathway
(58–60).
The mechanisms underlying IRIS are becoming
increasingly abundant and clear, ranging from the classical cell
cycle regulation, DDR and DNA damage repair processes to related
miRNAs, lncRNAs, IR factors and cell heterogeneity. Moreover,
numerous unelucidated and unsolved problems related to the
induction of CS by IR remain, and IRIS appear to be more complex in
cancer cells than in normal cells partly because of the intricate
biological features of tumour cells.
IRIS is the result of the inaccurate repair of
damaged DNA after IR. Targeting accelerated and increased IRIS has
been an important method for increasing the effectiveness of
RT.
Poly (ADP-ribose) polymerase (PARP) is known to
function in various DNA repair mechanisms, such as base excision
repair, HR and NHEJ. PARP inhibitor (PARPi) has been used to treat
tumours with BRCA1 or BRCA2 mutations (62) and can be used in combination with
other treatment measures. Many studies have indicated that PARPis
can sensitize most cancer cells to IR by prolonging growth arrest
and CS (63–65). Concurrent therapy with blockade of
DNA-dependent protein kinase (DNA-PK) and PARP-1 can accelerate the
senescence of irradiated non-small cell lung cancer (NSCLC) cells
and irradiated H460 ×enografts further than that achieved with IR
alone (66) (Table II).
Other evidence has also demonstrated that CS and
irradiation have a synergistic effect when applied in combination
with irradiation. Phosphorothioate-modified antisense
oligonucleotide (PS62ASODN), which inhibits human telomerase
reverse transcriptase (hTERT) to stimulate senescence, enhanced the
inhibition of tumour characteristics in liver cancer cells
(67). Telomeric repeat-binding
factor 2 (TRF2), a member of the shelterin complex that plays a key
role in protecting and stabilizing chromosomal ends, markedly
increased the radiosensitivity of human mesenchymal stem cells
(hMSCs) compared to that of controls in both proliferation and
senescence assays (68). Similarly,
inhibition of the mammalian target of rapamycin (MTOR) pathway can
augment the radiosensitivity of cancer cells by promoting CS
(69). In glioblastoma (GBM) cells,
silencing both histone deacetylase 4 (HDAC4) and erythropoietin
receptor (EPOR) promoted IR-induced senescence and reversed
radioresistance (70–71). Moreover, GBM cells treated with
verapamil in combination with carmustine and irradiation were more
vulnerable to IRIS than those subjected to individual or
dual-combination treatment (72).
Irradiated non-small cell lung cancer (NSCLC) cells
can be rendered more radiosensitive by inhibiting epidermal growth
factor receptor (EGFR) in a p53-dependent senescence pathway
(73). However, other evidence has
revealed that senescence is a prominent mechanism of
radiosensitization in 45% of NSCLC cell lines and occurs
independent of the p53 status but is linked to p16 induction.
Senescence and radiosensitization have also been linked to an
increase in residual radiation-induced DNA damage, especially DSBs,
regardless of the p53/p16 status (73). Notably, irrespective of the cell-based
assay employed, caution should be paid to avoid misinterpreting
radiosensitivity data in terms of reduced viability (74). Furthermore, similar to receptor
tyrosine kinase (RTK) targeting strategies in cancer, IRIS could
represent a potential alternative treatment outcome, both allowing
tumour growth control and enabling patients to have a better
quality of life (75). However, as
the SASP incidence increases, IRIS appears to be a candidate
mechanism contributing to Fanconi anaemia complementation group A
(FancA)-mediated radioresistance in head and neck squamous cell
carcinoma (11).
Collectively, these findings indicate that many
radiosensitizers function based on CS. Limited benefits suggest
that more complicated mechanisms should be considered and explored
because CS may facilitate radioresistance in tumour cells and
increase the radiosensitivity of surrounding normal cells.
CS induced by IR in normal cells leads to tissue
fibrosis and organ dysfunction and increases the risk of secondary
neoplasms in almost all bodily systems (42,64). As a
result, decreasing these side effects induced by IRIS has been a
direction for improving the therapeutic radiation ratio with the
exception of radiosensitizers. An increasing number of researchers
are exploring the deeper mechanisms underlying this process, and
some interference targets have exhibited potential to suppress CS
in normal cells (Table III).
The length of telomeres in somatic cells shortens
over time due to increasing age or pathogenic factors, resulting in
CS. Both chemotherapy and RT significantly impair telomere
maintenance and function in normal human cells, which may lead to
CS and ultimately result in tissue/organ damage and secondary
malignancies in long-term survivors of cancer (78). However, the telomere length and the
telomere length distribution in peripheral leukocytes was revealed
to remain unchanged after RT (79).
Residual NP-2 cells (human glioma-derived cells) exhibited CS
without changes in telomere length after 6 Gy of C-ion irradiation
(80).
IR-induced pulmonary fibrosis (PF) is a severe late
side effect of thoracic RT. Irradiated mice administered with an
inhibitor of B-cell lymphoma-2 (Bcl-2)/B-cell lymphoma-extra large
(BCL-xL) via gavage after persistent PF developed reduced type II
pneumocyte senescence, and PF was reversed (81). Both recombinant truncated plasminogen
activator inhibitor-1 (PAI-1) protein (rPAI-1) and rapamycin, were
revealed to prevent radiation-induced fibrosis in the lungs of mice
(82,83). In terms of CS, these data indicate
that PF is less challenging to treat and more preventable than
ever.
Total body irradiation (TBI) induces long-term bone
marrow (BM) suppression via the induction of premature senescence
in haematopoietic stem cells (HSCs) in a p16-independent manner
(84). The selective clearance of
SCNs, including senescent BM-derived HSCs and senescent muscle stem
cells, by a pharmacological agent or small-molecule inhibitor of
p38 MAPK was beneficial in part through its rejuvenation of aged
tissue stem cells and rescue of long-term myelosuppression
(85,86).
Childhood cancer survivors are at an increased risk
of frailty, which is partly a result of RT (87); however, IR-reduced CS in children has
more profound influences. The leukocyte telomere length (LTL) was
shorter in childhood acute lymphocytic leukaemia (ALL) survivors
who underwent treatment with cranial IR than in survivors in the
control group, which may lead to the premature development of
age-related chronic conditions in survivors (88). Notably, a regeneration defect in
ageing germline stem cells after IR could be treated by the loss of
FOXO in an adult model of stem cell injury induced by low-dose IR
(89).
These researchers also justified that MSCs in which
members of the RB gene family were silenced did not exhibit
increased apoptosis, necrosis or senescence compared with untreated
cells after exposure to X-rays at 40 and 2,000 mGy. These surviving
MSCs exhibited accumulated DNA damage and may have undergone
neoplastic transformation (90).
Therefore, attention should be paid to cancer patients with RB gene
mutations in terms of evaluating the onset of secondary neoplasms
following RT. Another research group used weighted gene
co-expression network analysis (WGCNA) to screen for differentially
expressed genes between the senescence and non-senescence groups
following RT and identified six hub genes: BANK1, Tomm70a, AFAP1,
Cd84, Nuf2 and NFE2 (91). The
authors provided an alternate method to search for key genes linked
to IRIS and built a foundation for exploring these genes (91).
Different radiation sources used in IR have
different effects on normal cells. Alessio et al (92) revealed that IR with α particles
created less apoptosis and senescence in BM-MSCs; that is, α
particles may spare healthy stem cells more efficaciously than
X-rays. Low-level laser therapy (LLLT) enhanced viability and
proliferation and reduced senescence of fibroblasts following γ-IR
exposure, while LLLT resulted in decreased proliferation and
increased senescence in breast cancer cells (MDA-MB-231 cells)
(93). It is worth mentioning that
the greater biological efficacy of C ions compared to that of low
linear energy transfer (LET) radiation (X-rays) may be misevaluated
in 2D culture experiments (94).
Relevant models and beams are necessary to promote the use of
charged particles with increased patient safety.
The application of senolytic agents that selectively
kill senescent cells may improve organ function, including SNCs
induced by IR (81,95). Other therapeutic methods, including
antioxidants, free radical scavengers, mTOR inhibitors,
anti-inflammatory agents, stem cell therapy and senomorphics, also
have the potential to reduce side effects induced by IRIS (9). MnTnBuOE-2-PyP could inhibit
radiation-induced collagen contraction and CS in fibroblasts but
could not protect colorectal cancer cells from IR damage (93,96),
potentially providing new options for reducing IR-induced damage.
However, further investigations need to be performed in humans to
evaluate their safety and efficacy.
In fact, the SLGA response to IR may reflect a key
mechanism of residual-cell survival, ultimately resulting in
radioresistance, tumour regrowth and dormant tumour recurrence
(102). Recently, the phenomenon
that SCNs can regrow after exposure to IR has attracted increasing
attention, which reflects that CS plays ‘opposing roles’ in RT and
other genotoxic therapies (23,103,104).
SNCs appearing in the context of neoadjuvant chemoradiotherapy for
rectal cancer can promote epithelial-mesenchymal transition (EMT)
and further affect the residual tumour microenvironment (105).
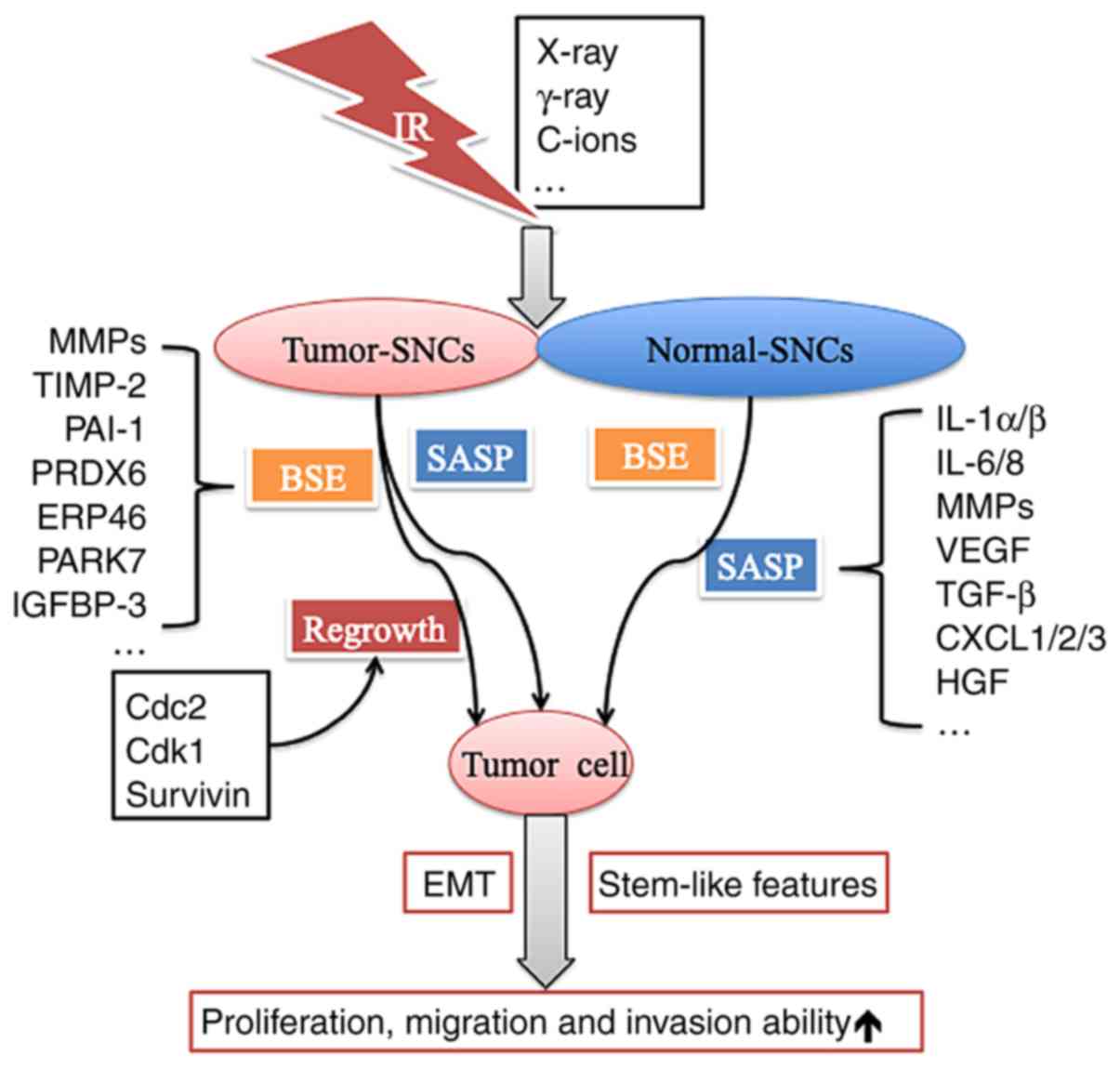
Some DNA damage foci induced by IR may persist for a
long time. However, the repair of DSBs in SCNs may ultimately
result in recovery and regrowth after combination IR/PARPi
treatment (106,107). Furthermore, the cells regrown after
IRIS may exhibit more aggressive biological behaviours, such as
enhanced proliferative ability and increased invasion and migration
capacities, than those existing before IR. SCNs also acquired the
ability to secrete many types of factors to facilitate growth and
invasion in vitro and in vivo (5) (Fig.
2).
Normal cells are more sensitive to the IR dose
regarding the changes in proliferative ability induced by IRIS,
while tumour cells seem to dull to the IR dose and segmentation
mode. Fractionated radiation and single IR (e.g., 6 or 3×2, 12 or
6×2 Gy) exposures have equivalent abilities to inhibit tumour
growth via IRIS in vitro and in vivo (32). Ablative doses (18 Gy) of radiation
exhibit more inhibitory effects on the proliferative, migratory and
invasive capacities of lung cancer-associated fibroblasts (CAFs)
because CAFs play significant roles in cancer cell invasion and
metastasis (108). A low dose of 30
mGy γ-IR was revealed to increase the overall proliferative
potential of normal human fibroblasts (HELF-104) (56), while γ-IR could inhibit the growth of
primary prostate epithelial cells by inducing senescence, not
apoptosis (109).
Apart from proliferative arrest, the SASP is another
prominent feature of senescent cells (110). The SASP includes cytokines,
chemokines, growth factors and proteases and can trigger the
activation of a complex signalling network (111). Irradiated ECs may adversely affect
non-irradiated surrounding cells via the SASP, which has been
linked to radiation-induced cardiovascular disease (98). The cytokine IL-6, an SASP component,
is highly upregulated in many cancers and is considered one of the
most important cytokines involved in pro- and anti-tumourigenic
effects (112).
Senescence-associated IL-6 and IL-8 cytokines can be triggered by
paracrines, autocrines, and endocrines, which reinforce the
senescent milieu and inflammatory microenvironment in breast cancer
cells (36).
Furthermore, the IR-induced bystander effect (BSE)
may have important implications in RT (113). The IR-induced BSE describes how
cells not exposed to IR show biological changes under the influence
of molecular signals secreted by irradiated neighbouring cells
(113,114). Several pathways are involved in the
paracrine circuit that induces senescence in neighbouring cells,
such as the matrix metalloproteinase-2 (MMP-2)/tissue inhibitor of
metalloproteinase-2 (TIMP-2), IGFBP3/PAI-1, and peroxiredoxin
6/endoplasmic reticulum protein 46 (ERP46)/Parkinsonism-associated
deglycase (PARK7)/cathepsin D/major vault protein pathways
(115). Moreover, lung fibroblasts
with premature senescence resulting from IR may strongly enhance
the growth of malignant human lung cancer cells (A549 and H1299)
in vitro and in immunocompromised mice through increasing
the expression of matrix MMPs (38).
In other words, it is common that various tumour
cells can undergo SLGA after different types of IR. However, these
tumour SNCs may recover their proliferative ability and exhibit
more aggressive biological behaviour when the environment is
suitable. While the SASP exhibited by tumour SCNs and normal SCNs
is mostly responsible for this process, the BSE induced by IR also
plays a crucial role via various pathways. However, the complexity
of the SASP and various mechanisms of action still restrict our
understanding of IRIS (35). The
mechanism underlying IR-induced BSE and tumour cell escape from
IRIS remains unknown, and further research is urgently required to
solve this problem.
Although the p16-pRB and p53-p21 tumour suppressor
pathways are widely recognized as the main mechanisms underlying
SLGA, it is still unclear what makes this arrest stable and what
makes CS act as a double-edged sword in cancer treatment (116), especially in terms of improving the
efficacy of RT. There may be other related mechanisms contributing
to IR-induced senescence.
Mitochondria play an important role in
radiation-induced cellular damage, and different qualities of
radiation affect the changes in mitochondrial dynamics (117). Cells exposed to low-dose X-rays and
replicative senescent cells exhibit a residual capacity to use
fatty acids and glutamine as alternative fuels, respectively
(118). Several mitochondrial
signalling pathways have been revealed to induce CS (119). DNA cleavage occurring in senescent
HDFs after γ-irradiation was triggered by a modest decrease in the
mitochondrial membrane potential, which was strong enough to
release mitochondrial endonuclease G (EndoG). Then, EndoG
translocated into the nucleus to induce the nonlethal cleavage of
damaged DNA (27).
IR-induced senescence in quiescent ECs is mediated
by at least 2 different pathways dependent on the mitochondrial
oxidative stress response and p53 activation (120). hTERT suppression caused by either C
ion irradiation or MST-312 impairs mitochondrial function, and
telomere-mitochondrion links play a role in the induction of
senescence in MCF-7 cells after C ion irradiation (76).
Ferroptosis is a form of regulated necrotic cell
death controlled by glutathione peroxidase 4 (GPX4). Ferritinophagy
is a lysosomal process that promotes ferritin degradation and
ferroptosis. Iron accumulation in SNCs is driven by impaired
ferritinophagy. The autophagy activator rapamycin could prevent
both the iron accumulation phenotype of SNCs and the increase in
TfR1, ferritin and intracellular iron, however, rapamycin failed to
re-sensitize these cells to ferroptosis (121).
Acyl-CoA synthetase long-chain family member 4
(Acsl4) is preferentially expressed in a panel of basal-like breast
cancer cell lines and predicts their sensitivity to ferroptosis.
Acsl4 inhibition is a viable therapeutic approach for preventing
ferroptosis-related diseases (122).
cGAS is a DNA sensor in the DDR process. Genomic DNA
damage leads to cGAS activation, stimulation of inflammatory
responses, CS and cancer via the cGAMP/stimulator of interferon
genes (STING) pathway (123). cGAS
deletion also abrogated SASPs induced by IR. cGAS mediated CS and
inhibited immortalization, and cGAS activated antitumour immunity
(124). cGAS recognized cytosolic
chromatin fragments in SNCs. The activation of cGAS, in turn,
triggered the production of SASP factors via STING, thereby
promoting paracrine senescence (125).
Although our understanding of CS in IR is still
initial, similar to RT in the treatment of cancer, IRIS functions
as a ‘double-edged sword’ and crucially influences the
comprehensive results of RT. First, because the SASPs created by
different types of SCNs are highly different, SCNs play a
complicated role in the response of cancer to RT via SASPs.
Developing effective pharmacological methods, such as senolytic
agents, to remove accumulated SNCs or weaken SASP intensity may be
a promising method (126). In
addition, combining prosenescence therapy with checkpoint
immunotherapy may contribute to eradicating cancer cells from the
viewpoint of CS (127). Finally,
more well-designed preclinical and clinical trials have the
potential to facilitate the development of targeted SNC therapy,
which will ultimately improve the clinical outcomes of cancer
patients subjected to RT.
Not applicable.
The present study was supported by the National
Natural Science Foundation of China (nos. 81560488, 81660504 and
81860536), the Yunnan Provincial Training Special Funds for
High-level Health Technical Personnel (no. H-201624), the Yunnan
Health Science Foundation (nos. 2017NS191, 2018NS0065 and
2018NS0064), the Doctoral Scholar Newcomer Award of Yunnan Province
and Graduate Student Innovation Fund of Kunming Medical University
(2019D015).
Not applicable.
ZC, LC, YX and WL conceived and designed the study.
KC, YL, LW, LL and YH researched the literature. ZC, LC and WL
wrote the manuscript. KC, YX and YL performed data analysis and
designed the figures. ZC, KC, YX, YL, YH, LW, LL, LC and WL revised
and edited the article. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hayflick L and Moorhead PS: The serial
cultivation of human diploid cell strains. Exp Cell Res.
25:585–621. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharpless NE and Sherr CJ: Forging a
signature of in vivo senescence. Nat Rev Cancer. 15:397–408. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sulli G, Rommel A, Wang X, Kolar MJ, Puca
F, Saghatelian A, Plikus MV, Verma IM and Panda S: Pharmacological
activation of REV-ERBs is lethal in cancer and oncogene-induced
senescence. Nature. 553:351–355. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McRobb LS, McKay MJ, Gamble JR, Grace M,
Moutrie V, Santos ED, Lee VS, Zhao Z, Molloy MP and Stoodley MA:
Ionizing radiation reduces ADAM10 expression in brain microvascular
endothelial cells undergoing stress-induced senescence. Aging
(Albany NY). 9:1248–1262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davalos AR, Coppe JP, Campisi J and
Desprez PY: Senescent cells as a source of inflammatory factors for
tumor progression. Cancer Metastasis Rev. 29:273–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Childs BG, Gluscevic M, Baker DJ, Laberge
RM, Marquess D, Dananberg J and van Deursen JM: Senescent cells: An
emerging target for diseases of ageing. Nat Rev Drug Discov.
16:718–735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eriksson D and Stigbrand T:
Radiation-induced cell death mechanisms. Tumour Biol. 31:363–372.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Wang Y, Liu S, Liu Y, Xu H, Liang
J, Zhu J, Zhang G, Su W, Dong W and Guo Q: Upregulation of EID3
sensitizes breast cancer cells to ionizing radiation-induced
cellular senescence. Biomed Pharmacother. 107:606–614. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen HQ, To NH, Zadigue P, Kerbrat S, De
La Taille A, Le Gouvello S and Belkacemi Y: Ionizing
radiation-induced cellular senescence promotes tissue fibrosis
after radiotherapy. A review. Crit Rev Oncol Hematol. 129:13–26.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Y, Campisi J, Higano C, Beer TM,
Porter P, Coleman I, True L and Nelson PS: Treatment-induced damage
to the tumor microenvironment promotes prostate cancer therapy
resistance through WNT16B. Nat Med. 18:1359–1368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hess J, Unger K, Orth M, Schötz U,
Schüttrumpf L, Zangen V, Gimenez-Aznar I, Michna A, Schneider L,
Stamp R, et al: Genomic amplification of Fanconi anemia
complementation group A (FancA) in head and neck squamous cell
carcinoma (HNSCC): Cellular mechanisms of radioresistance and
clinical relevance. Cancer Lett. 386:87–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Noda A, Hirai Y, Hamasaki K, Mitani H,
Nakamura N and Kodama Y: Unrepairable DNA double-strand breaks that
are generated by ionising radiation determine the fate of normal
human cells. J Cell Sci. 125:5280–5287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rossiello F, Herbig U, Longhese MP,
Fumagalli M and d'Adda di Fagagna F: Irreparable telomeric DNA
damage and persistent DDR signalling as a shared causative
mechanism of cellular senescence and ageing. Curr Opin Genet Dev.
26:89–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki M and Boothman DA: Stress-induced
premature senescence (SIPS)-influence of SIPS on radiotherapy. J
Radiat Res. 49:105–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He S and Sharpless NE: Senescence in
health and disease. Cell. 169:1000–1011. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li M, You L, Xue J and Lu Y: Ionizing
radiation-induced cellular senescence in normal, non-transformed
cells and the involved DNA damage response: A mini review. Front
Pharmacol. 9:5222018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagane M, Kuppusamy ML, An J, Mast JM,
Gogna R, Yasui H, Yamamori T, Inanami O and Kuppusamy P:
Ataxia-telangiectasia mutated (ATM) kinase regulates eNOS
expression and modulates radiosensitivity in endothelial cells
exposed to ionizing radiation. Radiat Res. 189:519–528. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gire V and Dulic V: Senescence from G2
arrest, revisited. Cell Cycle. 14:297–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krenning L, Feringa FM, Shaltiel IA, Van
Den Berg J and Medema RH: Transient activation of p53 in G2 phase
is sufficient to induce senescence. Mol Cell. 55:59–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Müllers E, Silva Cascales H, Jaiswal H,
Saurin AT and Lindqvist A: Nuclear translocation of Cyclin B1 marks
the restriction point for terminal cell cycle exit in G2 phase.
Cell Cycle. 13:2733–2743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye C, Zhang X, Wan J, Chang L, Hu W, Bing
Z, Zhang S, Li J, He J, Wang J and Zhou G: Radiation-induced
cellular senescence results from a slippage of long-term G2
arrested cells into G1 phase. Cell Cycle. 12:1424–1432. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuilman T, Michaloglou C, Mooi WJ and
Peeper DS: The essence of senescence. Genes Dev. 24:2463–2479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Scheiber MN, Neumann C, Calin GA
and Zhou D: MicroRNA regulation of ionizing radiation-induced
premature senescence. Int J Radiat Oncol Biol Phys. 81:839–848.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki M, Yamauchi M, Oka Y, Suzuki K and
Yamashita S: Live-cell imaging visualizes frequent mitotic skipping
during senescence-like growth arrest in mammary carcinoma cells
exposed to ionizing radiation. Int J Radiat Oncol Biol Phys.
83:e241–e250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hudson D, Kovalchuk I, Koturbash I, Kolb
B, Martin OA and Kovalchuk O: Induction and persistence of
radiation-induced DNA damage is more pronounced in young animals
than in old animals. Aging (Albany NY). 3:609–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim BC, Han NK, Byun HO, Kim SS, Ahn EK,
Chu IS, Leem SH, Lee CK and Lee JS: Time-dependently expressed
markers and the characterization for premature senescence induced
by ionizing radiation in MCF7. Oncol Rep. 24:395–403.
2010.PubMed/NCBI
|
|
27
|
Studencka M and Schaber J: Senoptosis:
Non-lethal DNA cleavage as a route to deep senescence. Oncotarget.
8:30656–30671. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki K, Mori I, Nakayama Y, Miyakoda M,
Kodama S and Watanabe M: Radiation-induced senescence-like growth
arrest requires TP53 function but not telomere shortening. Radiat
Res. 155:248–253. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang X, Gu J, Yu D, Wang G, Zhou L, Zhang
X, Zhao Y, Chen X, Zheng S, Liu Q, et al: Low-dose radiation
induces cell proliferation in human embryonic lung fibroblasts but
not in lung cancer cells: Importance of ERK1/2 and AKT signaling
pathways. Dose-Response. 14:15593258156221742016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Boerma M and Zhou D: Ionizing
radiation-induced endothelial cell senescence and cardiovascular
diseases. Radiat Res. 186:153–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rezáčová M, Rudolfová G, Tichý A, Bačíková
A, Mutná D, Havelek R, Vávrová J, Odrážka K, Lukášová E and Kozubek
S: Accumulation of DNA damage and cell death after fractionated
irradiation. Radiat Res. 175:708–718. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim BC, Yoo HJ, Lee HC, Kang KA, Jung SH,
Lee HJ, Lee M, Park S, Ji YH, Lee YS, et al: Evaluation of
premature senescence and senescence biomarkers in carcinoma cells
and xenograft mice exposed to single or fractionated irradiation.
Oncol Rep. 31:2229–2235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lagadec C, Vlashi E, Della Donna L, Meng
Y, Dekmezian C, Kim K and Pajonk F: Survival and self-renewing
capacity of breast cancer initiating cells during fractionated
radiation treatment. Breast Cancer Res. 12:R132010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liakou E, Mavrogonatou E, Pratsinis H,
Rizou S, Evangelou K, Panagiotou PN, Karamanos NK, Gorgoulis VG and
Kletsas D: Ionizing radiation-mediated premature senescence and
paracrine interactions with cancer cells enhance the expression of
syndecan 1 in human breast stromal fibroblasts: The role of TGF-β.
Aging (Albany NY). 8:1650–1668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hernandez-Segura A, de Jong TV, Melov S,
Guryev V, Campisi J and Demaria M: Unmasking transcriptional
heterogeneity in senescent cells. Curr Biol. 27:2652–2660.e4. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ortiz-Montero P, Londoño-Vallejo A and
Vernot JP: Senescence-associated IL-6 and IL-8 cytokines induce a
self- and cross-reinforced senescence/inflammatory milieu
strengthening tumorigenic capabilities in the MCF-7 breast cancer
cell line. Cell Commun Signal. 15:172017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Johnston CJ, Hernady E, Reed C, Thurston
SW, Finkelstein JN and Williams JP: Early alterations in cytokine
expression in adult compared to developing lung in mice after
radiation exposure. Radiat Res. 173:522–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Papadopoulou A and Kletsas D: Human lung
fibroblasts prematurely senescent after exposure to ionizing
radiation enhance the growth of malignant lung epithelial cells in
vitro and in vivo. Int J Oncol. 39:989–999. 2011.PubMed/NCBI
|
|
39
|
Liao EC, Hsu YT, Chuah QY, Lee YJ, Hu JY,
Huang TC, Yang PM and Chiu SJ: Radiation induces senescence and a
bystander effect through metabolic alterations. Cell Death Dis.
5:e12552014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sugrue MM, Shin DY, Lee SW and Aaronson
SA: Wild-type p53 triggers a rapid senescence program in human
tumor cells lacking functional p53. Proc Natl Acad Sci USA.
94:9648–9653. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Beauséjour CM, Krtolica AF, Galimi F,
Narita M, Lowe SW, Yaswen P and Campisi J: Reversal of human
cellular senescence: Roles of the p53 and p16 pathways. EMBO J.
22:4212–4222. 2014. View Article : Google Scholar
|
|
42
|
Cheng Z, Zheng YZ, Li YQ and Wong CS:
Cellular senescence in mouse hippocampus after irradiation and the
role of p53 and p21. J Neuropathol Exp Neurol. 76:260–269. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Widel M, Lalik A, Krzywon A, Poleszczuk J,
Fujarewicz K and Rzeszowska-Wolny J: The different radiation
response and radiation-induced bystander effects in colorectal
carcinoma cells differing in p53 status. Mutat Res. 778:61–70.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lindgren T, Stigbrand T, Råberg A, Riklund
K, Johansson L and Eriksson D: Genome wide expression analysis of
radiation-induced DNA damage responses in isogenic HCT116 p53+/+
and HCT116 p53-/- colorectal carcinoma cell lines. Int J Radiat
Biol. 91:99–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gong L, Gong H, Pan X, Chang C, Ou Z, Ye
S, Yin L, Yang L, Tao T, Zhang Z, et al: p53 isoform
Δ113p53/Δ133p53 promotes DNA double-strand break repair to protect
cell from death and senescence in response to DNA damage. Cell Res.
25:351–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sanada F, Taniyama Y, Muratsu J, Otsu R,
Iwabayashi M, Carracedo M, Rakugi H and Morishita R: Activated
factor X induces endothelial cell senescence through IGFBP-5. Sci
Rep. 6:355802016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sanada F, Taniyama Y, Muratsu J, Otsu R,
Shimizu H, Rakugi H and Morishita R: IGF binding protein-5 induces
cell senescence. Front Endocrinol (Lausanne). 9:532018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim K, Seu Y, Baek S, Kim MJ, Kim KJ, Kim
JH and Kim JR: Induction of cellular senescence by insulin-like
growth factor binding protein-5 through a p53-dependent mechanism.
Mol Biol Cell. 18:4543–4552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rombouts C, Aerts A, Quintens R, Baselet
B, El-Saghire H, Harms-Ringdahl M, Haghdoost S, Janssen A, Michaux
A, Yentrapalli R, et al: Transcriptomic profiling suggests a role
for IGFBP5 in premature senescence of endothelial cells after
chronic low dose rate irradiation. Int J Radiat Biol. 90:560–574.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shi W, Tang MK, Yao Y, Tang C, Chui YL and
Lee KK: BRE plays an essential role in preventing replicative and
DNA damage-induced premature senescence. Sci Rep. 6:235062016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lukášová E, Kovarˇík A, Bacˇíková A, Falk
M and Kozubek S: Loss of lamin B receptor is necessary to induce
cellular senescence. Biochem J. 474:281–300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Freund A, Laberge RM, Demaria M and
Campisi J: Lamin B1 loss is a senescence-associated biomarker. Mol
Biol Cell. 23:2066–2075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Toillon RA, Magné N, Laïos I, Castadot P,
Kinnaert E, Van Houtte P, Desmedt C, Leclercq G and Lacroix M:
Estrogens decrease gamma-ray-induced senescence and maintain cell
cycle progression in breast cancer cells independently of p53. Int
J Radiat Oncol Biol Phys. 67:1187–1200. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Abdelmohsen K, Panda A, Kang MJ, Xu J,
Selimyan R, Yoon JH, Martindale JL, De S, Wood WH III, Becker KG
and Gorospe M: Senescence-associated lncRNAs: Senescence-associated
long noncoding RNAs. Aging Cell. 12:890–900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sohn D, Peters D, Piekorz RP, Budach W and
Jänicke RU: miR-30e controls DNA damage-induced stress responses by
modulating expression of the CDK inhibitor p21WAF1/CIP1 and
caspase-3. Oncotarget. 7:15915–15929. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Velegzhaninov IO, Ermakova AV and Klokov
DY: Low dose ionizing irradiation suppresses cellular senescence in
normal human fibroblasts. Int J Radiat Biol. 94:825–828. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dolan DW, Zupanic A, Nelson G, Hall P,
Miwa S, Kirkwood TB and Shanley DP: Integrated stochastic model of
DNA damage repair by non-homologous end joining and
p53/p21-mediated early senescence signalling. PLoS Comput Biol.
11:e10042462015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Celià-Terrassa T, Liu DD, Choudhury A,
Hang X, Wei Y, Zamalloa J, Alfaro-Aco R, Chakrabarti R, Jiang YZ,
Koh BI, et al: Normal and cancerous mammary stem cells evade
interferon-induced constraint through the miR-199a-LCOR axis. Nat
Cell Biol. 19:711–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bai X, Fisher DE and Flaherty KT:
Cell-state dynamics and therapeutic resistance in melanoma from the
perspective of MITF and IFNγ pathways. Nat Rev Clin Oncol. Apr
9–2019.(Epub ahead of print) Doi: 10.1038/s41571-019-0204-6.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Braumüller H, Wieder T, Brenner E, Aßmann
S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M,
Griessinger C, et al: T-helper-1-cell cytokines drive cancer into
senescence. Nature. 494:361–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sato K, Kato A, Sekai M, Hamazaki Y and
Minato N: Physiologic thymic involution underlies age-dependent
accumulation of senescence-associated CD4(+) T cells. J Immunol.
199:138–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lord CJ and Ashworth A: PARP inhibitors:
Synthetic lethality in the clinic. Science. 355:1152–1158. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Efimova EV, Mauceri HJ, Golden DW, Labay
E, Bindokas VP, Darga TE, Chakraborty C, Barreto-Andrade JC,
Crawley C, Sutton HG, et al: Poly(ADP-ribose) polymerase inhibitor
induces accelerated senescence in irradiated breast cancer cells
and tumors. Cancer Res. 70:6277–6282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Barreto-Andrade JC, Efimova EV, Mauceri
HJ, Beckett MA, Sutton HG, Darga TE, Vokes EE, Posner MC, Kron SJ
and Weichselbaum RR: Response of human prostate cancer cells and
tumors to combining PARP inhibition with ionizing radiati on. Mol
Cancer Ther. 10:1185–1193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chatterjee P, Choudhary GS, Sharma A,
Singh K, Heston WD, Ciezki J, Klein EA and Almasan A: PARP
inhibition sensitizes to low dose-rate radiation TMPRSS2-ERG fusion
gene-expressing and PTEN-def icient prostate cancer cells. PLoS
One. 8:e604082013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Azad A, Bukczynska P, Jackson S, Haupt Y,
Cullinane C, McArthur GA and Solomon B: Co-targeting
deoxyribonucleic acid-dependent protein kinase and poly(adenosine
diphosphate-ribose) polymerase-1 promotes accelerated senescence of
irradiated cancer cells. Int J Radiat Oncol Biol Phys. 88:385–394.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cao F, Ju X, Chen D, Jiang L, Zhu X, Qing
S, Fang F, Shen Y, Jia Z and Zhang H: Phosphorothioatemodified
antisense oligonucleotides against human telomerase reverse
transcriptase sensitize cancer cells to radiotherapy. Mol Med Rep.
16:2089–2094. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Orun O, Tiber PM and Serakinci N: Partial
knockdown of TRF2 increase radiosensitivity of human mesenchymal
stem cells. Int J Biol Macromol. 90:53–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nam HY, Han MW, Chang HW, Kim SY and Kim
SW: Prolonged autophagy by MTOR inhibitor leads radioresistant
cancer cells into senescence. Autophagy. 9:1631–1632. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Marampon F, Megiorni F, Camero S,
Crescioli C, McDowell HP, Sferra R, Vetuschi A, Pompili S, Ventura
L, De Felice F, et al: HDAC4 and HDAC6 sustain DNA double strand
break repair and stem-like phenotype by promoting radioresistance
in glioblastoma cells. Cancer Lett. 397:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pérès EA, Gérault AN, Samuel V, Roussel S,
Toutain J, Divoux D, Guillamo JS, Sanson M, Bernaudin M and Petit
E: Silencing erythropoietin receptor on glioma cells reinforces
efficacy of temozolomide and X-rays through senescence and mitotic
catastrophe. Oncotarget. 6:2101–2119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ham SW, Jeon HY and Kim H: Verapamil
augments carmustine- and irradiation-induced senescence in glioma
cells by reducing intracellular reactive oxygen species and calcium
ion levels. Tumour Biol. 39:10104283176922442017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang M, Morsbach F, Sander D, Gheorghiu L,
Nanda A, Benes C, Kriegs M, Krause M, Dikomey E, Baumann M, et al:
EGF receptor inhibition radiosensitizes NSCLC cells by inducing
senescence in cells sustaining DNA double-strand breaks. Cancer
Res. 71:6261–6269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mirzayans R, Andrais B and Murray D:
Impact of premature senescence on radiosensitivity measured by high
throughput cell-based assays. Int J Mol Sci. 18:E14602017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Francica P, Aebersold DM and Medova M:
Senescence as biologic endpoint following pharmacological targeting
of receptor tyrosine kinases in cancer. Biochem Pharmacol.
126:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Miao GY, Zhou X, Zhang X, Xie Y, Sun C,
Liu Y, Gan L and Zhang H: Telomere-mitochondrion links contribute
to induction of senescence in MCF-7 cells after carbon-ion
irradiation. Asian Pac J Cancer Prev. 17:1993–1998. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ernst A, Anders H, Kapfhammer H, Orth M,
Hennel R, Seidl K, Winssinger N, Belka C, Unkel S and Lauber K:
HSP90 inhibition as a means of radiosensitizing resistant,
aggressive soft tissue sarcomas. Cancer Lett. 365:211–222. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li P, Hou M, Lou F, Björkholm M and Xu D:
Telomere dysfunction induced by chemotherapeutic agents and
radiation in normal human cells. Int J Biochem Cell Biol.
44:1531–1540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Maeda T, Nakamura K, Atsumi K, Hirakawa M,
Ueda Y and Makino N: Radiation-associated changes in the length of
telomeres in peripheral leukocytes from inpatients with cancer. Int
J Radiat Biol. 89:106–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jinno-Oue A, Shimizu N, Hamada N, Wada S,
Tanaka A, Shinagawa M, Ohtsuki T, Mori T, Saha MN, Hoque AS, et al:
Irradiation with carbon ion beams induces apoptosis, autophagy, and
cellular senescence in a human glioma-derived cell line. Int J
Radiat Oncol Biol Phys. 76:229–241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Pan J, Li D, Xu Y, Zhang J, Wang Y, Chen
M, Lin S, Huang L, Chung EJ, Citrin DE, et al: Inhibition of
Bcl-2/xl with ABT-263 selectively kills senescent type II
pneumocytes and reverses persistent pulmonary fibrosis induced by
ionizing radiation in mice. Int J Radiat Oncol Biol Phys.
99:353–361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chung EJ, McKay-Corkum G, Chung S, White
A, Scroggins BT, Mitchell JB, Mulligan-Kehoe MJ and Citrin D:
Truncated plasminogen activator inhibitor-1 protein protects from
pulmonary fibrosis mediated by irradiation in a murine model. Int J
Radiat Oncol Biol Phys. 94:1163–1172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chung EJ, Sowers A, Thetford A,
McKay-Corkum G, Chung SI, Mitchell JB and Citrin DE: Mammalian
target of rapamycin inhibition with rapamycin mitigates
radiation-induced pulmonary fibrosis in a murine model. Int J
Radiat Oncol Biol Phys. 96:857–866. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shao L, Feng W, Li H, Gardner D, Luo Y,
Wang Y, Liu L, Meng A, Sharpless NE and Zhou D: Total body
irradiation causes long-term mouse BM injury via induction of HSC
premature senescence in an Ink4a- and Arf-independent manner.
Blood. 123:3105–3115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chang J, Wang Y, Shao L, Laberge RM,
Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W,
et al: Clearance of senescent cells by ABT263 rejuvenates aged
hematopoietic stem cells in mice. Nat Med. 22:78–83. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lu L, Wang YY, Zhang JL, Li DG and Meng
AM: p38 MAPK inhibitor insufficiently attenuates HSC senescence
administered long-term after 6 Gy total body irradiation in mice.
Int J Mol Sci. 17:E9052016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ness KK, Armstrong GT, Kundu M, Wilson CL,
Tchkonia T and Kirkland JL: Frailty in childhood cancer survivors.
Cancer. 121:1540–1547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ariffin H, Azanan MS, Abd Ghafar SS, Oh L,
Lau KH, Thirunavakarasu T, Sedan A, Ibrahim K, Chan A, Chin TF, et
al: Young adult survivors of childhood acute lymphoblastic leukemia
show evidence of chronic inflammation and cellular aging. Cancer.
123:4207–4214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Artoni F, Kreipke RE, Palmeira O, Dixon C,
Goldberg Z and Ruohola-Baker H: Loss of foxo rescues stem cell
aging in Drosophila germ line. eLife. 6:e278422017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Alessio N, Capasso S, Di Bernardo G,
Cappabianca S, Casale F, Calarco A, Cipollaro M, Peluso G and
Galderisi U: Mesenchymal stromal cells having inactivated RB1
survive following low irradiation and accumulate damaged DNA: Hints
for side effects following radiotherapy. Cell Cycle. 16:251–258.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xing Y, Zhang J, Lu L, Li D, Wang Y, Huang
S, Li C, Zhang Z, Li J and Meng A: Identification of hub genes of
pneumocyte senescence induced by thoracic irradiation using
weighted gene coexpression network analysis. Mol Med Rep.
13:107–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Alessio N, Esposito G, Galano G, De Rosa
R, Anello P, Peluso G, Tabocchini MA and Galderisi U: Irradiation
of mesenchymal stromal cells with low and high doses of alpha
particles induces senescence and/or apoptosis. J Cell Biochem.
118:2993–3002. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ramos Silva C, Cabral FV, de Camargo CF,
Núñez SC, Mateus Yoshimura T, de Lima Luna AC, Maria DA and Ribeiro
MS: Exploring the effects of low-level laser therapy on fibroblasts
and tumor cells following gamma radiation exposure. J Biophotonics.
9:1157–1166. 2016. View Article : Google Scholar
|
|
94
|
Hamdi DH, Chevalier F, Groetz JE, Durantel
F, Thuret JY, Mann C and Saintigny Y: Comparable senescence
induction in three-dimensional human cartilage model by exposure to
therapeutic doses of x-rays or C-ions. Int J Radiat Oncol Biol
Phys. 95:139–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Baker DJ, Wijshake T, Tchkonia T,
LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL and van
Deursen JM: Clearance of p16Ink4a-positive senescent cells delays
ageing-associated disorders. Nature. 479:232–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kosmacek EA, Chatterjee A, Tong Q, Lin C
and Oberley-Deegan RE: MnTnBuOE-2-PyP protects normal colorectal
fibroblasts from radiation damage and simultaneously enhances
radio/chemotherapeutic killing of colorectal cancer cells.
Oncotarget. 7:34532–34545. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Beach TA, Johnston CJ, Groves AM, Williams
JP and Finkelstein JN: Radiation induced pulmonary fibrosis as a
model of progressive fibrosis: Contributions of DNA damage,
inflammatory response and cellular senescence genes. Exp Lung Res.
43:134–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Philipp J, Azimzadeh O, Subramanian V,
Merl-Pham J, Lowe D, Hladik D, Erbeldinger N, Ktitareva S, Fournier
C, Atkinson MJ, et al: Radiation-induced endothelial inflammation
is transferred via the secretome to recipient cells in a
STAT-mediated process. J Proteome Res. 16:3903–3916. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chatterjee A, Kosmacek EA and
Oberley-Deegan RE: MnTE-2-PyP treatment, or NOX4 inhibition,
protects against radiation-induced damage in mouse primary prostate
fibroblasts by inhibiting the TGF-Beta 1 signaling pathway. Radiat
Res. 187:367–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Li H, Wang Y, Pazhanisamy SK, Shao L,
Batinic-Haberle I, Meng A and Zhou D: Mn(III)
meso-tetrakis-(N-ethylpyridinium-2-yl) porphyrin mitigates total
body irradiation-induced long-term bone marrow suppression. Free
Radic Biol Med. 51:30–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Xu G, Wu H, Zhang J, Li D, Wang Y, Wang Y,
Zhang H, Lu L, Li C, Huang S, et al: Metformin ameliorates ionizing
irradiation-induced long-term hematopoietic stem cell injury in
mice. Free Radic Biol Med. 87:15–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kaur E, Rajendra J, Jadhav S, Shridhar E,
Goda JS, Moiyadi A and Dutt S: Radiation-induced homotypic cell
fusions of innately resistant glioblastoma cells mediate their
sustained survival and recurrence. Carcinogenesis. 36:685–695.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Roberson RS, Kussick SJ, Vallieres E, Chen
SY and Wu DY: Escape from therapy-induced accelerated cellular
senescence in p53-null lung cancer cells and in huma n lung
cancers. Cancer Res. 65:2795–2803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Chakradeo S, Elmore LW and Gewirtz DA: Is
senescence reversible? Curr Drug Targets. 17:460–466. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Tato-Costa J, Casimiro S, Pacheco T, Pires
R, Fernandes A, Alho I, Pereira P, Costa P, Castelo HB, Ferreira J
and Costa L: Therapy-induced cellular senescence induces
epithelial-to-mesenchymal transition and increases invasiveness in
rectal cancer. Clin Colorectal Cancer. 15:170–178.e3. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gewirtz DA, Alotaibi M, Yakovlev VA and
Povirk LF: Tumor cell recovery from senescence induced by radiation
with PARP inhibition. Radiat Res. 186:327–332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Alotaibi M, Sharma K, Saleh T, Povirk LF,
Hendrickson EA and Gewirtz DA: Radiosensitization by PARP
inhibition in DNA repair proficient and deficient tumor cells:
Proliferative recovery in senescent cells. Radiat Res. 185:229–245.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hellevik T, Pettersen I, Berg V, Winberg
JO, Moe BT, Bartnes K, Paulssen RH, Busund LT, Bremnes R, Chalmers
A and Martinez-Zubiaurre I: Cancer-associated fibroblasts from
human NSCLC survive ablative doses of radiation but their invasive
capacity is reduced. Radiat Oncol. 7:592012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Frame FM, Savoie H, Bryden F, Giuntini F,
Mann VM, Simms MS, Boyle RW and Maitland NJ: Mechanisms of growth
inhibition of primary prostate epithelial cells following gamma
irradiation or photodynamic therapy include senescence, necrosis,
and autophagy, but not apoptosis. Cancer Med. 5:61–73. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Malaquin N, Martinez A and Rodier F:
Keeping the senescence secretome under control: Molecular reins on
the senescence-associated secretory phenotype. Exp Gerontol.
82:39–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wiley CD and Campisi J: From ancient
pathways to aging cells-connecting metabolism and cellular
senescence. Cell Metab. 23:1013–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Rodier F and Campisi J: Four faces of
cellular senescence. J Cell Biol. 192:547–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Rzeszowska-Wolnyab J and Widel M: Ionizing
radiation-induced bystander effects, potential targets for
modulation of radiotherapy. Eur J Pharmacol. 625:156–164. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Jalal N, Haq S, Anwar N, Nazeer S and
Saeed U: Radiation induced bystander effect and DNA damage. J
Cancer Res Ther. 10:819–833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Özcan S, Alessio N, Acar MB, Mert E,
Omerli F, Peluso G and Galderisi U: Unbiased analysis of senescence
associated secretory phenotype (SASP) to identify common components
following different genotoxic stresses. Aging (Albany NY).
8:1316–1329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Mowla SN, Lam EW and Jat PS: Cellular
senescence and aging: The role of B-MYB. Aging Cell. 13:773–779.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Jin X, Li F, Liu B, Zheng X, Li H, Ye F,
Chen W and Li Q: Different mitochondrial fragmentation after
irradiation with X-rays and carbon ions in HeLa cells and its
influence on cellular apoptosis. Biochem Biophys Res Commun.
500:958–965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Capasso S, Alessio N, Squillaro T, Di
Bernardo G, Melone MA, Cipollaro M, Peluso G and Galderisi U:
Changes in autophagy, proteasome activity and metabolism to
determine a specific signature for acute and chronic senescent
mesenchymal stromal cells. Oncotarget. 6:39457–39468. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ziegler DV, Wiley CD and Velarde MC:
Mitochondrial effectors of cellular senescence: Beyond the free
radical theory of aging. Aging Cell. 14:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lafargue A, Degorre C, Corre I,
Alves-Guerra MC, Gaugler MH, Vallette F, Pecqueur C and Paris F:
Ionizing radiation induces long-term senescence in endothelial
cells through mitochondrial respiratory complex II dysfunction and
superoxide generation. Free Radic Biol Med. 108:750–759. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Masaldan S, Clatworthy SAS, Gamell C,
Meggyesy PM, Rigopoulos AT, Haupt S, Haupt Y, Denoyer D, Adlard PA,
Bush AI and Cater MA: Iron accumulation in senescent cells is
coupled with impaired ferritinophagy and inhibition of ferroptosis.
Redox Biol. 14:100–115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Li T and Chen ZJ: The cGAS-cGAMP-STING
pathway connects DNA damage to inflammation, senescence, and
cancer. J Exp Med. 215:1287–1299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Yang H, Wang H, Ren J, Chen Q and Chen ZJ:
cGAS is essential for cellular senescence. Proc Natl Acad Sci USA.
114:E4612–E4620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Glück S, Guey B, Gulen MF, Wolter K, Kang
TW, Schmacke NA, Bridgeman A, Rehwinkel J, Zender L and Ablasser A:
Innate immune sensing of cytosolic chromatin fragments through cGAS
promotes senescence. Nat Cell Biol. 19:1061–1070. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sun Y, Coppé JP and Lam EW: Cellular
senescence: The sought or the unwanted? Trends Mol Med. 24:871–885.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Leite de Oliveira R and Bernards R:
Anti-cancer therapy: Senescence is the new black. EMBO J.
37:e993862018. View Article : Google Scholar : PubMed/NCBI
|