Introduction
Colon cancer is ranked as the third most common
cancer among men and the second most common cancer among women in
the world, with the highest incidence between 40 and 50 years of
age (1). Among adults less than 50
years of age, the incidence of colon cancer has increased by 22%
from 2000 to 2013, and the rate of mortality has increased by 13%
from 2000 to 2014 (2). Metastasis is
the leading cause of death in colon cancer patients, and ~50% of
these patients develop metastatic disease (3,4).
Therefore, it is necessary to understand the mechanism of cancer
metastasis in order to identify new targets to suppress cancer
metastasis.
NF-κB activating protein (NKAP) is a conserved
protein and is named based on its ability to enhance NF-κB activity
(5). Previous research has revealed
that NKAP is a transcriptional repressor of the Notch signaling
pathway and plays an essential role in the development of T cells
and invariant NKT (iNKT) cells, as well as T cell maturation and
the acquisition of functional competency (6–8). NKAP is
also crucial for maintenance and survival of hematopoietic stem
cells (HSCs), and its deficiency could result in decreased
proliferation and increased apoptosis in HSCs (9). Thapa et al found that NKAP is
also required for proliferation burst and differentiation of NKT17
cells (10). Additionally, a new role
for NKAP as a nuclear speckle protein in RNA splicing and
processing in HeLa cells has been reported (11). NKAP is also reported to play a role in
the regulation of mitosis, and its dysregulation can lead to
chromosomal instability, which may result in tumorigenesis
(12).
In this study, we aimed to determine whether NKAP
was upregulated in colon cancer. To further investigate whether
NKAP was involved in the progression and metastasis of colon
cancer, we knocked down the expression of NKAP in HCT116 and HT-29
cells and upregulated its expression in HCT-15 cells. Our data
showed that NKAP knockdown resulted in a significant decrease in
the proliferation and invasion of colon cancer cells, as well as
induction of apoptosis and autophagy, whereas NKAP overexpression
promoted cell proliferation and invasion, and inhibited apoptosis
and autophagy.
Materials and methods
Tissue specimens
The Research Ethics Committee of Shandong Provincial
Hospital Affiliated to Shandong University (no. 2018-220) approved
the present study. A total of 75 cases of colon cancer tissues and
corresponding paracancerous tissues were obtained from patients who
underwent colon cancer resection at Shandong Provincial Hospital
from June 1, 2013 to September 30, 2016 for immunohistochemical
staining. The patient ages ranged from 26–83 years (median, 63.5
years), and included 43 males and 32 females. The
clinicopathological features of colon cancer patients are shown in
Table I. An additional 25 pairs of
colon cancer tissues and corresponding paracancerous tissues were
collected for western blot assay. Written informed consent was
obtained from all the patients. All the specimens remained
anonymous throughout the study and were handled according to the
ethical and legal standards of the hospital.
 | Table I.Associations between the NKAP
expression levels and the clinicopathological characteristics of
colon cancer patients. |
Table I.
Associations between the NKAP
expression levels and the clinicopathological characteristics of
colon cancer patients.
|
|
| NKAP
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Cases n=75 | High (n=41) | Low (n=34) | P-value |
|---|
| Age (years) |
|
≤60 | 26 | 12 | 14 | 0.2807 |
|
>60 | 49 | 29 | 20 |
|
| Sex |
|
Male | 43 | 27 | 16 | 0.1013 |
|
Female | 32 | 14 | 18 |
|
| Tumor size
(cm) |
| ≤3 | 14 | 4 | 10 | 0.0296 |
|
>3 | 61 | 37 | 24 |
|
| Distant
metastasis |
|
Yes | 9 | 5 | 4 | 0.9545 |
| No | 66 | 36 | 30 |
|
| Lymph node
metastasis |
|
Yes | 36 | 20 | 16 | 0.8819 |
| No | 39 | 21 | 18 |
|
| Stage |
|
I+II | 13 | 3 | 10 | 0.0119 |
|
III+IV | 62 | 38 | 24 |
|
Cell culture and infection
Human colon cancer cell lines HCT116, LoVo, SW480
and HCT-15 and colorectal adenocarcinoma cell line HT-29 (STR
profiling was used for authentication), were obtained from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China). Cells
were cultured in RPMI-1640 (HyClone, Thermo Fisher Scientific)
medium containing 10% FBS (Gibco, Thermo Fisher Scien-tific) at
37°C with 5% CO2. The sequence of shRNA targeting NKAP
was obtained from Ribobio (Guangzhou). Virapower system
(Invitrogen, Thermo Fisher Scien-tific) was used for lentivirus
packaging to construct stably expressing shRNA-NKAP or shRNA
control (negative control, NC) (12).
NKAP cDNA sequences were cloned in pcDNA3.1 plasmid then
transfected into HCT-15 cells, and the empty plasmid was used as
negative control. Cells at 40–50% confluency were infected with
lentivirus expressing shRNA-NKAP or pcDNA3.1-NKAP, and shRNA
control or pcDNA3.1 was used as the negative control. Infected
cells were harvested for drug selection with puromycin, and cells
stably expressing shRNA or NKAP were used for subsequent
experiments.
Immunohistochemical staining
Tissues were stained using the EliVision™plus kit
(Maixin, Fuzhou, China) according to the manufacturer's
instructions (13). Anti-NKAP was
obtained from Abcam. The percentage of positive stained cells was
graded as follows: 1, 6–25%; 2, 26–50%; 3, 51–75%; 4, >75% and
0, ≤5%. The staining intensity was graded as follows: No staining
(0), weak staining (faintly yellow; 1), moderate staining (brownish
yellow; 2), and strong staining (brown; 3). The percentage of
NKAP-positive cells multiplied with the staining intensity was the
final score of samples. Colon cancer patients were divided into two
groups based on this score, in which scores ≤6 were regarded as
NKAP low-expression group, and scores >6 were regarded as NKAP
high-expression group.
Reverse transcription-PCR
(RT-PCR)
Total RNA was extracted using TRIzol (PuFei) and
reverse transcribed to cDNA using SuperRT cDNA Synthesis kit
(CWBIO). The reaction system was as follows: dNTP Mix, 4 µl; primer
Mix, 2 µl; mRNA, 2 µl; RT buffer, 4 µl; SuperRT, 1 µl; RNase-Free
water, 7 µl; 42°C for 30 min; 85°C for 5 min. The NKAP mRNA
expression was detected using SYBR Master Mixture (Takara Bio
Inc.). The reaction conditions were as follows: 95°C for 30 sec;
and 95°C for 5 sec, 55°C for 30 sec and 72°C for 30 sec for 40
cycles. Primers used were: NKAP: upstream,
5′-CGGCAGAAGAGATTAAGTGAG-3′, downstream,
5′-CTCCACTGGTGTATGTTCATC-3′; GAPDH: upstream,
5′-TGACTTCAACAGCGACACCCA-3′, downstream,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The comparative Cq (ΔΔcq) method was
used to analyze the obtained data (14).
Western blot assay
RIPA lysis buffer (CWBIO) was used for cell lysis in
order to perform protein extraction, and protein concentration was
measured with a bicinchoninic acid protein assay kit (Beyotime,
Beijing, China). Protein (20 µg) was separated by 10% SDS-PAGE gel
and then transferred to polyvinylidene fluoride mem-branes (PVDF).
The primary antibodies were added for incubation with the membrane
in 5% non-fat milk in TBST (150 mM NaCl, 20 mM Tris, 0.05%
Tween-20) at 4°C overnight. Hereafter, the membrane was incubated
with secondary antibodies for 1 h and treated with an enhanced
chemiluminescence kit for signal development. ImageJ software was
performed to quantify the density of each band. The primary
antibodies, which were obtained from Abcam, were as follows:
Anti-NKAP (rabbit polyclonal; dilution 1:1,000; cat. no. ab229096),
anti-Bcl-2 (rabbit polyclonal; dilution 1:1,000; cat. no. ab59348),
anti-Bax (rabbit polyclonal; dilution 1:1,000; cat. no. ab53154),
anti-p-Akt (rabbit monoclonal; dilution 1:1,000; cat. no. ab81283),
anti-Akt (rabbit monoclonal; dilution 1:1,000; cat. no. ab32505),
anti-mTOR (rabbit polyclonal; dilution 1:1,000; cat. no. ab2732),
anti-p-mTOR (rabbit polyclonal; dilution 1:1,000; cat. no.
ab131538), anti-P70 (Rabbit monoclonal; dilution 1:1,000; cat. no.
ab32529), anti-Cyclin D1 (rabbit monoclonal; dilution 1:1,000; cat.
no. ab134175), anti-β-catenin (rabbit monoclonal; dilution 1:1,000;
cat. no. ab32572), anti-E-cad (rabbit polyclonal; dilution 1:1,000;
cat. no. ab15148), anti-P62 (rabbit polyclonal; dilution 1:1,000;
cat. no. ab91526), anti-Beclin1 (rabbit polyclonal; dilution
1:1,000; cat. no. ab62557), anti-LC3B (rabbit polyclonal; dilution
1:1,000; cat. no. ab48394), and anti-GAPDH (rabbit polyclonal;
dilution 1:1,000; cat. no. ab9485). The anti-active Caspase-3
p17-specific (rabbit polyclonal; dilution 1:1,000; cat. no.
19677-1-AP) and all secondary antibodies (anti-rabbit IgG; dilution
1:2,000; cat. no. SA00001-2) were obtained from Proteintech Group
(Rosemont).
CCK-8 assay
After infection, the cells were seeded in 96-well
plates at a density of 1×103/well and cultured for 72 h.
Then, 10 µl of CCK-8 reagent (Solarbio Science & Technology)
was added into each well and incubated for 90 min at 37°C. The OD
value of excitation light was detected using enzyme standard
instrument at a wavelength of 450 nm.
Colony formation assay
After infection for 24 h, 5×102 cells
were seeded into a 10 cm dish containing 5 ml of medium and
cultured at 37°C with 5% CO2 until cells had formed
sufficiently large clones. The medium was removed, and colonies
were stained with 0.1% crystal violet for 30 min. Images were
captured and the number of clones was counted.
Invasion assay
Cell invasion experiments were performed using
Transwell chambers coated with Matrigel. Infected cells were
cultured in serum-free medium for 24 h and digested. Cells
(1×104) were transferred to the top chamber, and the
complete medium was added into the lower chamber. After 24 h of
incubation, the invasive cells were fixed with 4% paraformaldehyde
at room temperature for 30 min. The filters were then stained with
0.1% crystal violet at room temperature for another 20 min. The
invasive cells were captured and counted under the dissecting
microscope (magnification, ×100).
Apoptosis detection assay
Apoptosis of infected cells was determined using
Annexin V PE/7-AAD kit (KeyGen BioTECH). Infected cells were
harvested and resuspended in 1X binding buffer at a density of
1–5×106 cells/ml. Next, 100 µl cell suspensions were
incubated with Annexin V PE/7-AAD apoptosis kit for staining. The
samples were analyzed using the FACS caliber instrument. The rate
of apoptosis was analyzed by BD FACSDiva software.
Statistical analysis
Analyses were performed using SPSS 18.0 software.
Data were obtained from three independent experiments and expressed
as mean ± standard deviation. The Chi-square test was used to
evaluate associations. Comparisons between the two groups were
analyzed using Student's t-test. P<0.05 was considered
statistically significant.
Results
NKAP is upregulated in colon cancer
and its expression is associated with clinicopathological
parameters of colon cancer
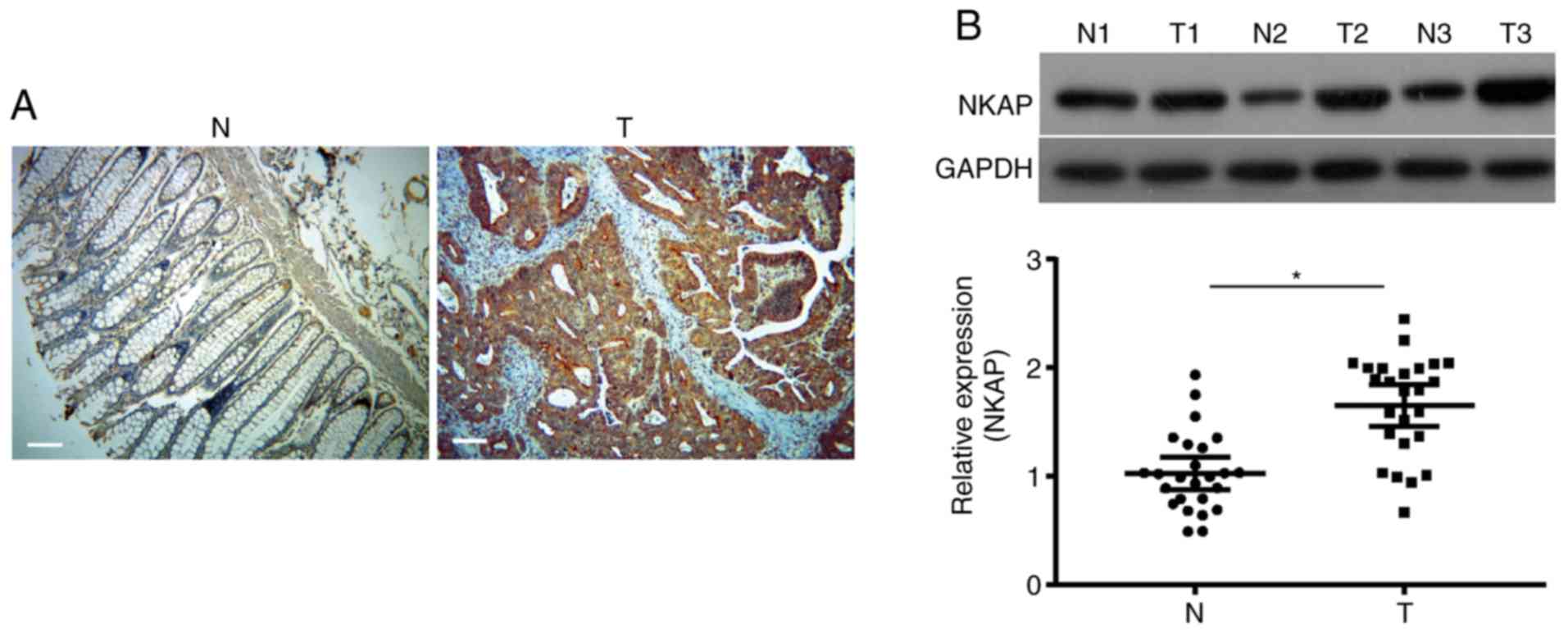
Immunohistochemistry assay was performed to
investigate the expression of NKAP in colon cancer. As shown in
Fig. 1A, the NKAP-positive signal was
located in the nucleus and cytoplasm of tumor cells. NKAP
expression in colon cancer tissues (54.67%, 41/75) was much higher
than NKAP expression in paracancerous tissues (17.33%, 13/75).
Moreover, the expression of NKAP was positively associated with
tumor size and stages (both P<0.05, Table I). Western blot analysis revealed that
the protein expression of NKAP was significantly upregulated in
colon cancer tissues compared to paracancerous tissues (P<0.05,
Fig. 1B, n=25).
NKAP regulates the proliferation and
viability of colon cancer cells
We observed high expression of NKAP mRNA in HT-29
and HCT116 cells, and low expression of NKAP in HCT-15 cells
Fig. S1A). In order to investigate
the role of NKAP in the progression of colon cancer, NKAP
expression was knocked down by shRNA interference in HCT116 and
HT-29 cells at the mRNA (Fig. 2A) and
protein (Fig. 2B) levels. HCT-15
cells were transfected with pcDNA3.1-NKAP plasmid to upregulate its
expression (Fig. 2A and B).
Furthermore, CCK-8 assay was performed to examine the effect of
NKAP on proliferation in colon cancer cells. Our data showed that
after 72 h of incubation, the proliferation of HCT116 cells with
shNKAP interference was clearly inhibited compared to the NC
(P<0.05, Fig. 2C). Similarly, NKAP
knockdown also reduced the proliferation of colorectal
adenocarcinoma HT-29 cells (P<0.05, Fig. 2D). As shown in Fig. 2E, upregulation of NKAP significantly
increased the proliferation of HCT-15 cells (P<0.05). The colony
formation assay demonstrated a significant decrease in colony
forming ability. NKAP knocked down HCT116 and HT-29 cells compared
to the NC, while an increase in colony forming ability was observed
in HCT-15 cells with NKAP overexpression (both P<0.05, Fig. 2F and G). Taken together, these results
indicated that NKAP played an oncogenic role in the proliferation
and viability of colon cancer cells in vitro.
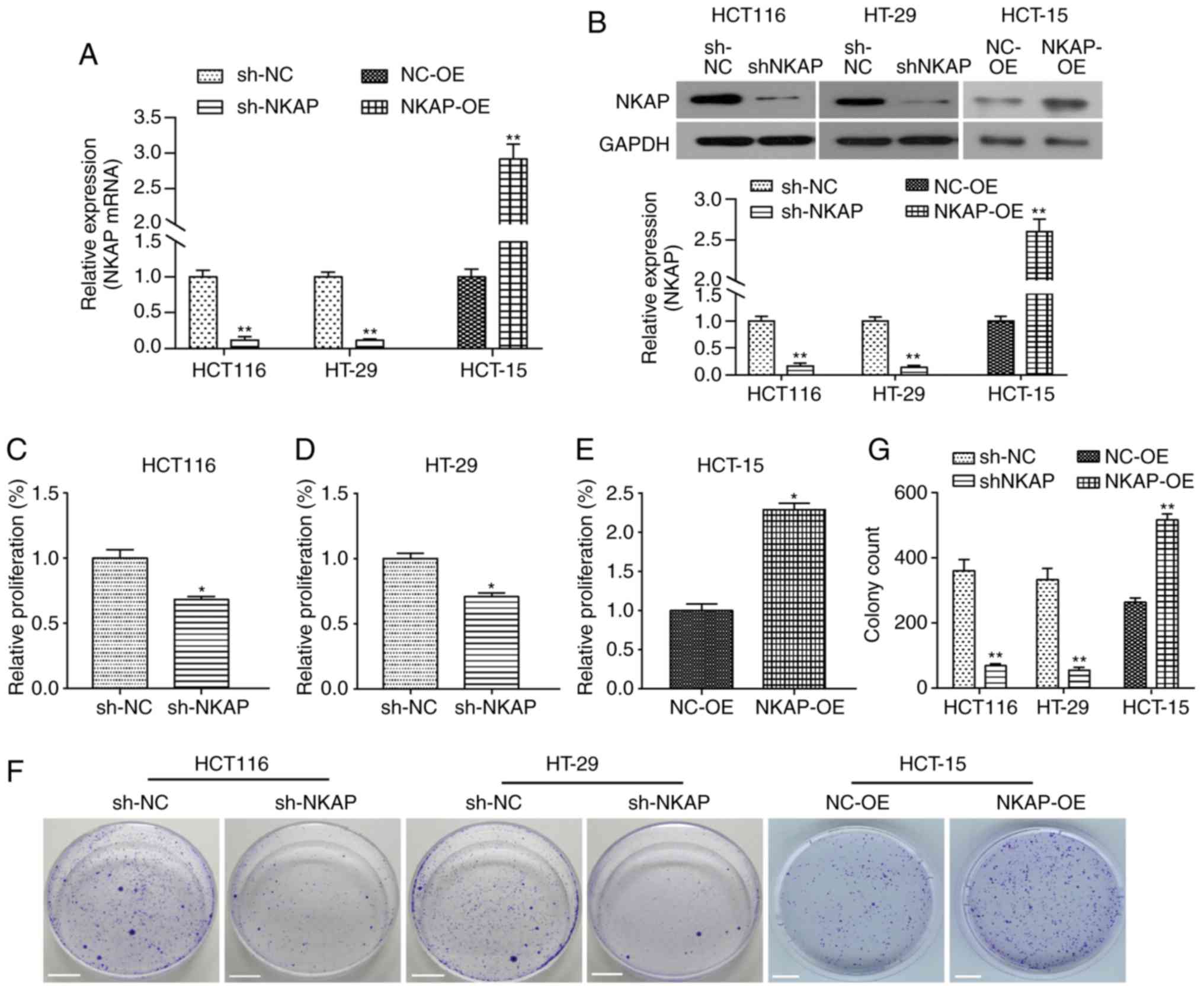 | Figure 2.NKAP regulates the proliferation and
viability of HCT116, HT-29 and HCT-15 cells. HCT116 and HT-29 cells
were transfected with shRNA-NKAP (sh-NKAP), shRNA control was used
as negative control (sh-NC); HCT-15 cells were transfected with
pcDNA3.1-NKAP (NKAP overexpression, NKAP-OE), and the empty plasmid
was used as negative control (NC-OE). (A) After 24 h of
transfection, the expression of NKAP mRNA was detected using RT-PCR
in HCT116, HT-29 and HCT-15 cells. (B) Following transfection for
48 h, the expression of NKAP protein in HCT116, HT-29 and HCT-15
cells was examined using western blot analysis. (C-E) Following
transfection for 72 h, cell viability was assessed using CCK-8
assay in HCT116 (C), HT-29 (D) and HCT-15 (E) cells. (F) Cell
proliferation ability was detected using colony formation assay in
HCT116 (left), HT-29 (middle) and HCT-15 (right) cells. Scale bar:
1 cm. *P<0.05, **P<0.01 compared with negative control
(NC). |
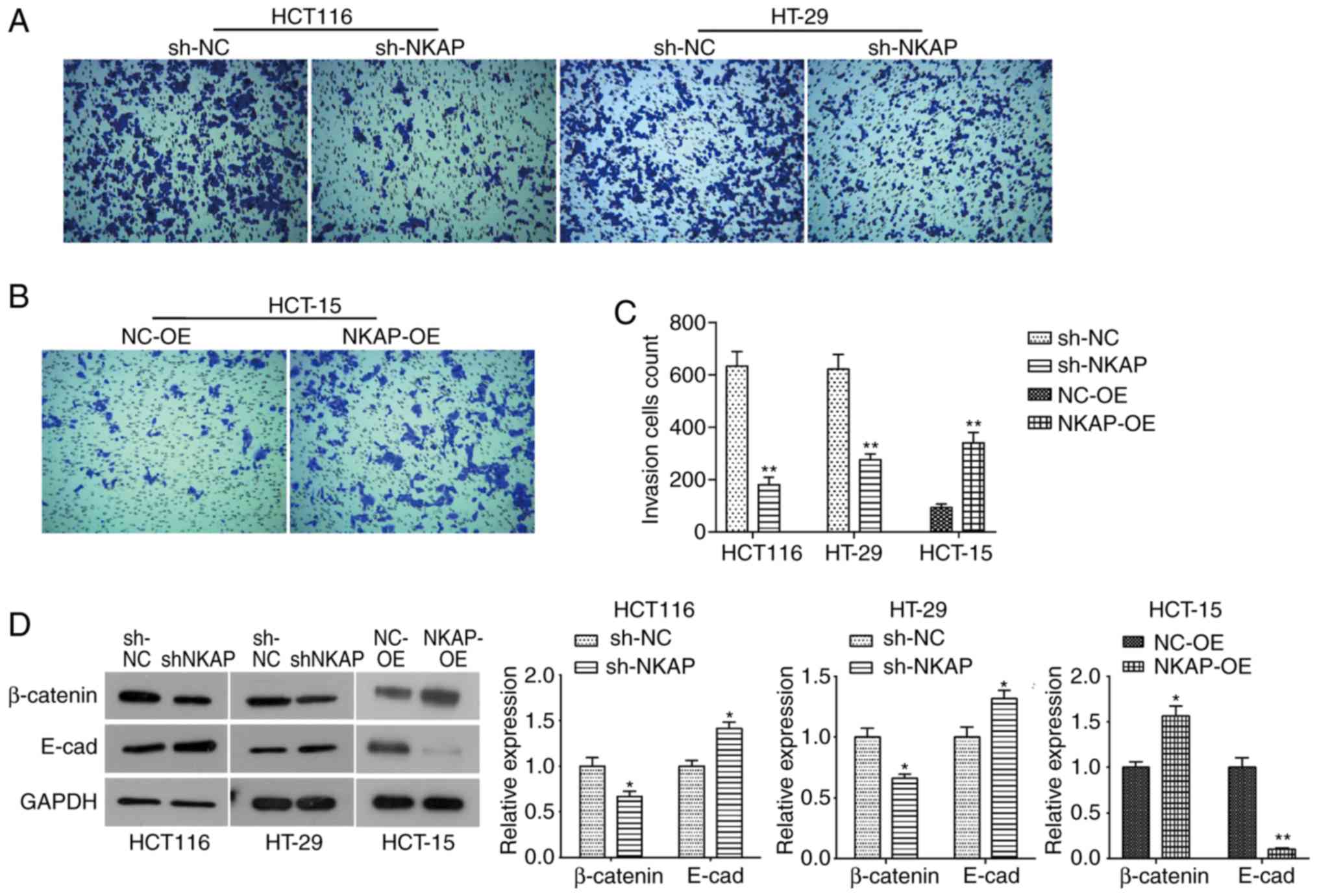
NKAP regulated the invasion and
epithelial-mesenchymal transition (EMT) of colon cancer cells
Metastatic potential is one of the most important
features of all tumors. Therefore, we examined the effect of NKAP
on colon cancer invasion using Transwell assay. As shown in
Fig. 3A and C, silencing of NKAP
significantly reduced the invasion of HCT116 and HT-29 cells (both
P<0.05) compared to NC cells. Consistently, NKAP overexpression
markedly resulted in an increase in invasion in HCT-15 cells
(Fig. 3B and C). Furthermore, we
observed significant upregulation of adhesion factor β-catenin in
colon cancer tissues (Fig. S1C and
D), and it was significantly downregulated in NKAP-deficient
cells but upregulated in NKAP overexpression cells (Fig. 3D). The epithelial marker protein E-cad
was upregulated in NKAP-silenced cells, while it was markedly
downregulated in NKAP overexpressed HCT-15 cells (Fig. 3D). This was an indication that NKAP
regulated the EMT process in colon cancer cells in
vitro.
NKAP regulates apoptosis and autophagy
of colon cancer cells
One hallmark of tumors is uncontrolled apoptosis
(15). Therefore, flow cytometric
assay was performed in order to examine the effect of NKAP on cell
apoptosis. As shown in Fig. 4A and C,
the rate of apoptosis was significantly elevated following
downregulation of NKAP compared to the NC cells, both in HCT116 and
HT-29 cells (both P<0.05). Consistently, NKAP overexpression
inhibited apoptosis in HCT-15 cells (P<0.05, Fig. 4B and C). In order to determine the
related mechanism of apoptosis induction by NKAP, changes in
expression of apoptosis-related proteins were assessed. Western
blot results showed a significant decrease in the expression of
anti-apoptotic protein Bcl-2 in NKAP-silenced cells, and a notable
increase in the expression of pro-apoptotic proteins Bax and Active
Caspase-3 was observed in both HCT116 and HT-29 cells interfered
with shRNA-NKAP (both P<0.05, Fig.
4D). An increase in the expression of Bcl-2 was observed in
NKAP overexpression cells, as well as a decrease in the expression
of Bax and Active Caspase-3 (Fig.
4D). In addition, we determined that the expression of
autophagy-related proteins was modulated by NKAP. As indicated by
western blotting, there was an increase in Beclin1 and LC3-II
expression and a decrease in LC3-I expression in NKAP silenced
cells, while a decrease in Beclin1 and LC3-II expression and
increase in P62 and LC3-I expression (both P<0.05, Fig. 4E). These data suggested that NKAP
inhibited apoptosis and autophagy in colon cancer cells, while
silencing its expression can promote apoptosis and autophagy.
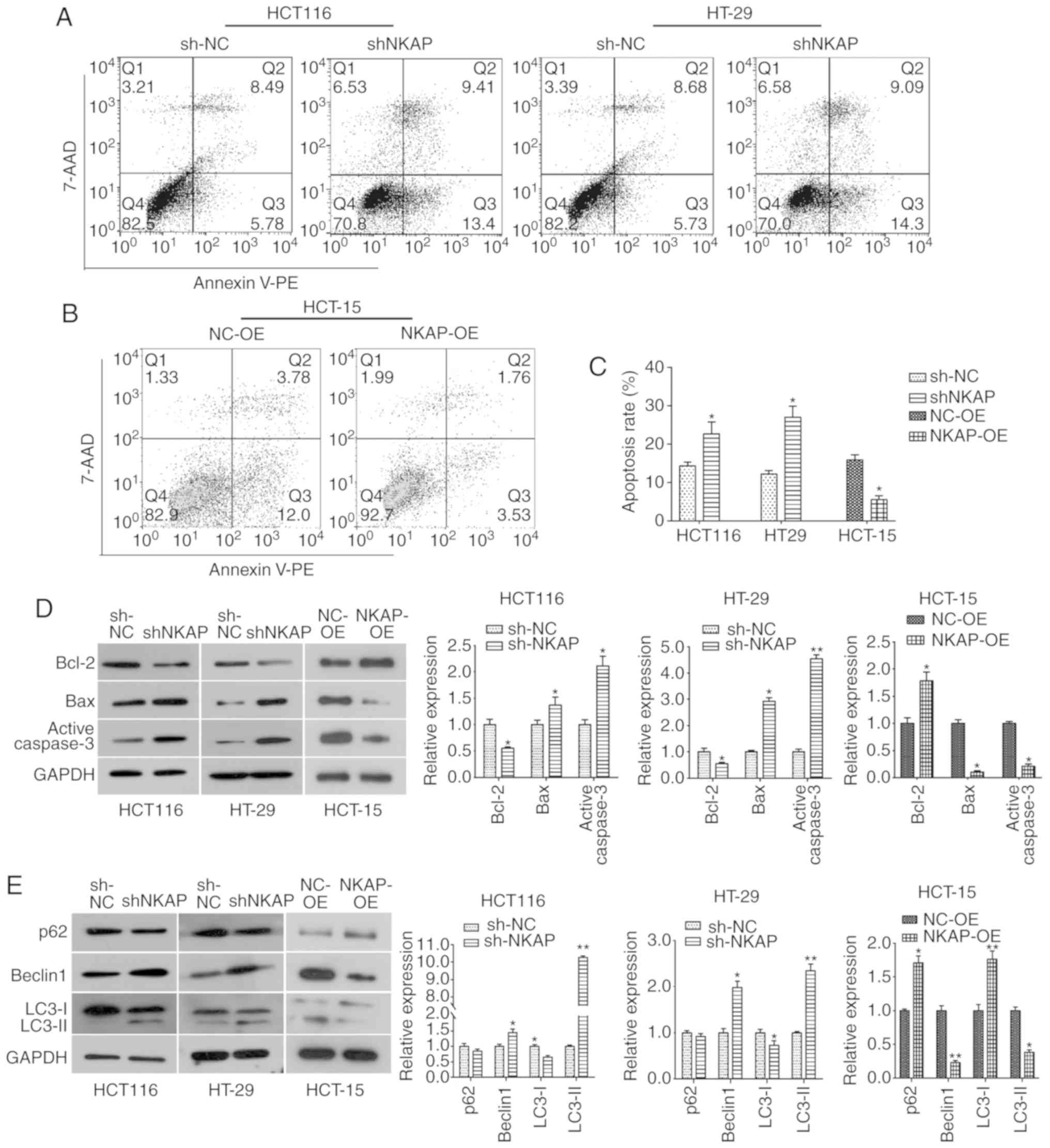 | Figure 4.NKAP modulates apoptosis and
autophagy in colon cancer cells. (A) Cell apoptosis of HCT116 and
HT-29 cells was detected by flow cytometry. (B) Effect of NKAP
overexpression on apoptosis in HCT-15 cells. (C) Quantitative
analysis of apoptosis results. (D) Western blot analysis was
performed to detect the expression of apoptosis-related proteins,
Bcl-2, Bax and Active Caspase-3, in HCT116, HT-29 and HCT-15 cells.
(E) Expression of autophagy-related proteins was assessed using
western blotting. sh-NKAP, cells transfected with shRNA-NKAP;
sh-NC, cells transfected with shRNA control; NKAP-OE, NKAP
overexpression, cells transfected with pcDNA3.1-NKAP; NC-OE, cells
transfected with the empty plasmid. *P<0.05, **P<0.01
compared with negative control (NC). |
NKAP regulates activation of the
Akt/mTOR and NF-κB signaling pathways in colon cancer cells
Considering the pivotal role of the Akt/mTOR
signaling pathway in cell growth and survival, as well as tumor
progression and metastasis, the expression of its key components
was examined in NKAP-silenced cells. As is shown in Fig. S1B and D, the Akt/mTOR pathway was
overactivated in colon cancer tissues as a result of the
upregulation of p-Akt and p-mTOR expression. Our data showed that
NKAP knockdown did not affect the total amount of Akt and mTOR, but
it significantly reduced Akt and mTOR phosphorylation in HCT116 and
HT-29 cells (Fig. 5). Accordingly,
the expression of its downstream proteins, Cyclin D1 and P70, was
decreased in NKAP-deficient cells compared to NC cells (Fig. 5). By contrast, upregulation of NKAP
promoted Akt and mTOR phosphorylation and expression of Cyclin D1
and P70 in HCT-15 cells (Fig. 5).
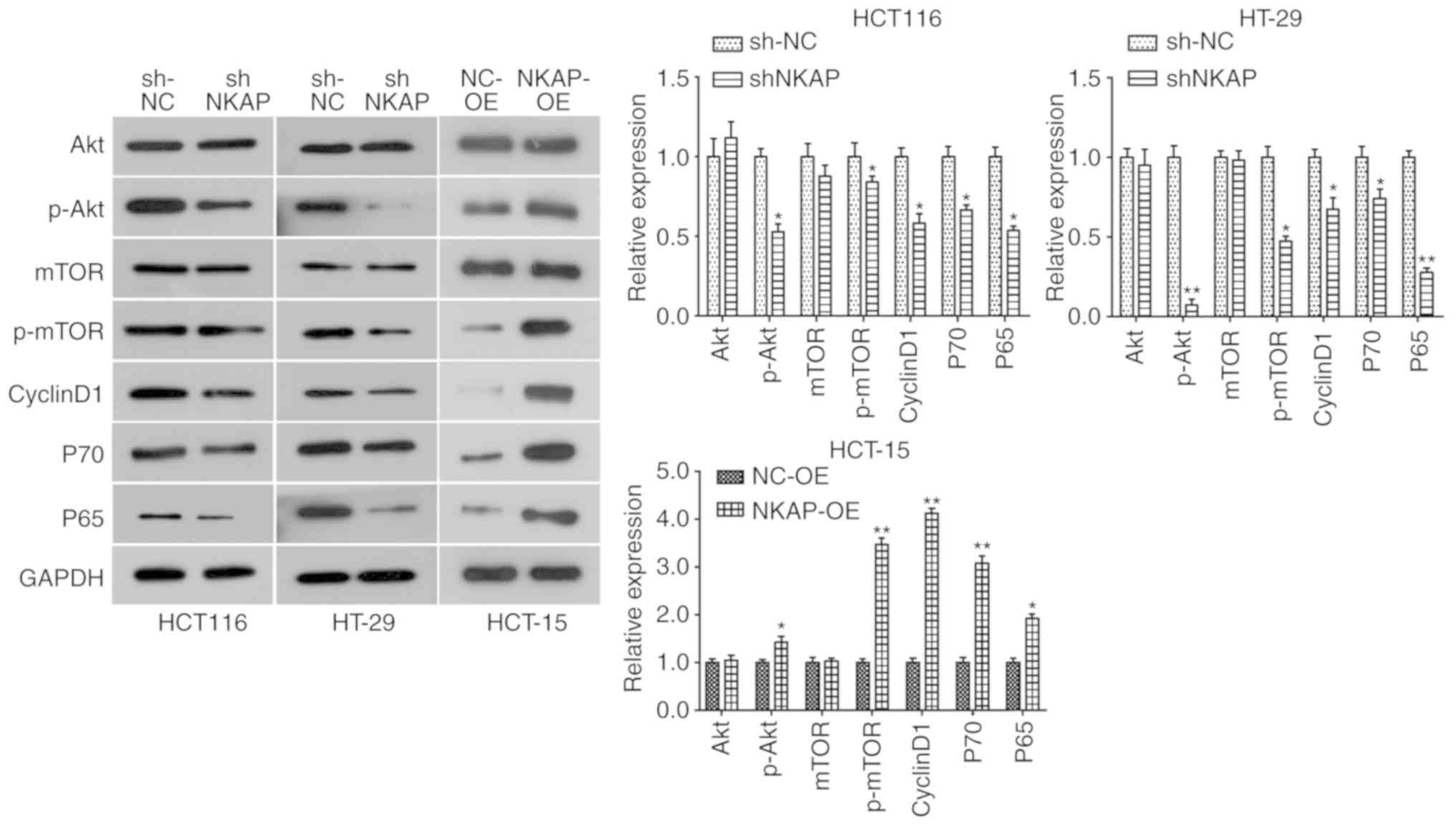 | Figure 5.NKAP regulates the activation of the
Akt/mTOR and NF-κB signaling pathways in colon cancer cells.
Following transfection for 48 h, the expression of Akt/mTOR
pathway-related proteins (Akt, p-Akt, mTOR, p-mTOR, Cyclin D1, and
P70) was detected using western blotting in HCT116, HT-29 and
HCT-15 cells. Expression of the key component of NF-κB signaling
pathway P65 was also assessed using western blotting. sh-NKAP,
cells transfected with shRNA-NKAP; sh-NC, cells transfected with
shRNA control; NKAP-OE, NKAP overexpression, cells transfected with
pcDNA3.1-NKAP; NC-OE, cells transfected with the empty plasmid.
*P<0.05, **P<0.01 compared with negative control (NC). |
Since NKAP could enhance NF-κB activity, we examined
the effect of NKAP expression on the NF-κB signaling pathway in
colon cancer cells. P65 is a subunit of NF-κB, which is a crucial
nuclear transcription factor involved in cellular functions. The
level of P65 was increased in colon cancer tissues (Fig. S1D) and decreased in NKAP-silenced
cells, while P65 expression was significantly upregulated by NKAP
overexpression (Fig. 5). This
indicated that the NF-κB signaling pathway was suppressed by NKAP
knockdown and promoted by NKAP overexpression in colon cancer
cells. Taken together, we can conclude that the Akt/mTOR and NF-κB
signaling pathways may be involved in the oncogenic role of NKAP in
the progression of colon cancer.
Discussion
It has been reported that NKAP is involved in cell
development and proliferation of T cell and iNKT cells (6–8). A recent
study has revealed that the dysregulation of NKAP results in
chromosomal instability which is associated with tumorigenesis
(12). However, there is still a
knowledge gap regarding the role of NKAP in colon cancer
progression. Herein, we observed that the expression of NKAP was
upregulated in colon cancer tissues. Its expression demonstrated a
positive association with tumor size and stages (Table I), suggesting potential involvement of
NKAP in the progression of colon cancer. Interestingly, our data
showed that NKAP knockdown significantly reduced cell proliferation
and viability in HCT116 and HT-29 cells, while NKAP overexpression
promoted the proliferation and viability of HCT-15 cells. As is
commonly known, invasion is a common hallmark of malignant tumors,
resulting in tumor metastasis, which is the leading cause of death
in colon cancer patients (16). The
present study showed that the invasion of colon cancer cells was
inhibited by NKAP knockdown and promoted by NKAP overexpression
in vitro, which was consistent with the effect of NKAP on
breast cancer cells (17). This
finding suggests that NKAP functions as an oncogene in the
proliferation and invasion of colon cancer cells. In addition, NKAP
was found to regulate the EMT by modulating the expression of
β-catenin and E-cad in colon cancer cells. β-catenin has been
reported to accumulate in the invasive front of colon cancer
(18,19). E-cad, a key epithelial marker protein,
is involved in metastasis, drug resistance and prognosis of colon
cancer (20–22). Therefore, the regulation of NKAP on
EMT may be involved in its effect on cell invasion.
Apoptosis is a major regulatory mechanism for the
control of cell growth, while dysregulation of apoptosis and
resistance to cell death are common hallmarks of cancer (23). Promotion of apoptosis is an important
aspect of cancer therapy (24,25),
Additionally, loss of NKAP is reported to promote apoptosis in HSCs
cells (9). Herein, our results showed
that NKAP knockdown significantly increased the rate of apoptosis
in colon cancer cells in vitro, while NKAP overexpression
inhibited apoptosis. The intrinsic mitochondrial pathway is a
pivotal mechanism for the regulation of apoptosis (26). However, it is reported that tumor
cells generally evade regulation of apoptosis by impairing the
expression of anti-apoptotic or pro-apoptotic proteins (27). It is widely held that the Caspase and
Bcl-2 families are key apoptosis-related proteins involved in
initiating the mitochondrial apoptosis pathway (28–30). As
described above, we found that loss of NKAP decreased the
expression of anti-apoptotic protein Bcl-2 and increased the
expression of pro-apoptotic proteins Bax and Active Caspase-3,
while NKAP overexpression caused the opposite effect (Fig. 4). These findings suggest that NKAP
knockdown could trigger cell apoptosis in a mitochondrial pathway
in colon cancer in vitro. In addition, we detected the
effect of NKAP on autophagy in colon cancer cells. Autophagy is a
complex cellular process regulated by multiple signaling pathways
(31). Autophagy has been reported to
play different roles in different tumor model systems, suppressing
or promoting cancer cell proliferation or tumorigenesis, indicating
that the effect of autophagy on cancer progression is
context-dependent (32,33). During autophagy, the cytosolic form of
LC3, LC3-I, is translocated to the autophagic membrane-bound form,
LC3-II. Therefore, the relative expressions of LC3-I and II are
considered to be an index of autophagy (34,35). In
the present study, loss of NKAP resulted in decreased LC3-I level
and increased LC3-II level in colon cancer cells, and NKAP
overexpression causes the opposite effect, indicating that NKAP
could regulate cell autophagy in colon cancer cells, and silencing
NKAP promotes autophagy.
NKAP has been shown to function as an RNA-binding
protein in RNA splicing (11). Li
et al have confirmed that NKAP functions as a mitotic
regulator in chromosome alignment, and loss of NKAP results in
misalignment of chromosomes which leads to mitotic arrest in human
soft tissue sarcomas (12). However,
the specific mechanism of NKAP in colon cancer remains unclear. The
PI3K/Akt signaling pathway has been implicated in various cellular
processes and is reported to play an important role in the
initiation and progression of colon cancer by regulating a series
of oncogenes or anti-oncogenes (36,37). Our
data revealed that the Akt/mTOR pathway was overactivated in colon
cancer through upregulation of p-Akt and p-mTOR (Fig. S1B and D). Herein, we found that NKAP
knockdown significantly inhibited activation of the Akt/mTOR
pathway, which was further confirmed by decreased expression of
downstream proteins, Cyclin D1 and P70 (Fig. 4A). Cyclin D1 is a pivotal
proto-oncogene involved in cancer progression by cell cycle
regulation (38). P70 is a
serine/threonine protein kinase that has been confirmed to be
positively associated with tumor growth and angiogenesis (39). Additionally, we found that the NF-κB
pathway was also suppressed as NKAP was knocked down and
upregulated in NKAP overexpression cells. Based on the above
results, we are able to conclude that the Akt/mTOR and NF-κB
pathways may be involved in the oncogenic role of NKAP in colon
cancer.
In summary, to the best of our knowledge, results of
the present study reveal for the first time that NKAP is
upregulated in colon cancer and plays an oncogenic role in the
progression of colon cancer, whereby NKAP knockdown suppresses the
proliferation and invasion of colon cancer cells, as well as
promote apoptosis and autophagy. Therefore, our study provides new
insight regarding the role of NKAP in tumor progression, which may
provide a potential target for the treatment of colon cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by National Natural Science
Foundation of China (nos. 81502508 and 81600121) and Natural
Science Foundations of Shandong Province (no. ZR2016HQ46).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS, GL, JH and XL participated in the design of the
study and drafted the manuscript. YD, AF and ZC participated in
data analyses and helped draft the manuscript. All authors gave
final approval for publication.
Ethics approval and consent to
participate
Primary human tumor specimen collection was granted
prior approval from the Ethics Committee of Shandong Provincial
Hospital Affiliated to Shandong University (no. 2018-220), Jinan,
China. Tissue analysis was approved by the Ethics Committee of
Shandong Provincial Hospital Affiliated to Shandong University. All
of the patients provided informed consent to participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vatandoust S, Price TJ and Karapetis CS:
Colorectal cancer: Metastases to a single organ. World J.
Gastroenterol. 21:11767–11776. 2015.
|
|
4
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen D, Li Z, Yang Q, Zhang J, Zhai Z and
Shu HB: Identification of a nuclear protein that promotes NF-kappaB
activation. Biochem Biophys Res Commun. 310:720–724. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pajerowski AG, Nguyen C, Aghajanian H,
Shapiro MJ and Shapiro VS: NKAP is a transcriptional repressor of
notch signaling and is required for T cell development. Immunity.
30:696–707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thapa P, Das J, Mcwilliams D, Shapiro M,
Sundsbak R, Nelson-Holte M, Tangen S, Anderson J, Desiderio S,
Hiebert S, et al: The transcriptional repressor NKAP is required
for the development of iNKT cells. Nat Commun. 4:15822013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu FC, Pajerowski AG, Nelson-Holte M,
Sundsbak R and Shapiro VS: NKAP is required for T cell maturation
and acquisition of functional competency. J Exp Med. 208:1291–1304.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pajerowski AG, Shapiro MJ, Gwin K,
Sundsbak R, Nelson-Holte M, Medina K and Shapiro VS: Adult
hematopoietic stem cells require NKAP for maintenance and survival.
Blood. 116:2684–2693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thapa P, Chen MW, Mcwilliams DC, Belmonte
P, Constans M, Sant'Angelo DB and Shapiro VS: NKAP regulates
invariant NKT cell proliferation and differentiation into
ROR-γt-expressing NKT17 cells. J Immunol. 196:4987–4998. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burgute BD, Peche VS, Steckelberg AL,
Glöckner G, Gaßen B, Gehring NH and Noegel AA: NKAP is a novel
RS-related protein that interacts with RNA and RNA binding
proteins. Nucleic Acids Res. 42:3177–3193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li T, Chen L, Cheng J, Dai J, Huang Y,
Zhang J, Liu Z, Li A, Li N, Wang H, et al: SUMOylated NKAP is
essential for chromosome alignment by anchoring CENP-E to
kinetochores. Nat Commun. 7:129692016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu QB and Sun GP: Expression of COX-2 and
HER-2 in colorectal cancer and their correlation. World J
Gastroenterol. 21:6206–6214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cotter TG: Apoptosis and cancer: The
genesis of a research field. Nat Rev Cancer. 9:501–507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang X, Zha L, Li H, Liao G, Huang Z, Peng
X and Wang Z: Upregulation of GNL3 expression promotes colon cancer
cell proliferation, migration, invasion and epithelial-mesenchymal
transition via the Wnt/β-catenin signaling pathway. Oncol Rep.
38:2023–2032. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Wang H, Yin Y, Li Q and Zhang M:
NKAP functions as an oncogene and its expression is induced by
CoCl2 treatment in breast cancer via AKT/mTOR signaling
pathway. Cancer Manag Res. 10:5091–5100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oguma K, Oshima H, Aoki M, Uchio R, Naka
K, Nakamura S, Hirao A, Saya H, Taketo MM and Oshima M: Activated
macrophages promote Wnt signalling through tumour necrosis
factor-alpha in gastric tumour cells. EMBO J. 27:1671–1681. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amado NG, Predes D, Moreno MM, Carvalho
IO, Mendes FA and Abreu JG: Flavonoids and Wnt/β-catenin signaling:
Potential role in colorectal cancer therapies. Int J Mol Sci.
15:12094–12106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saito T, Masuda N, Miyazaki T, Kanoh K,
Suzuki H, Shimura T, Asao T and Kuwano H: Expression of EphA2 and
E-cadherin in colorectal cancer: Correlation with cancer
metastasis. Oncol Rep. 11:605–611. 2004.PubMed/NCBI
|
|
21
|
Murray L: The role of E-cadherin in colon
cancer drug resistanceUniv Glasgow; 2010
|
|
22
|
Kwak JM, Min BW, Lee JH, Choi JS, Lee SI,
Park SS, Kim J, Um JW, Kim SH and Moon HY: The prognostic
significance of E-cadherin and liver intestine-cadherin expression
in colorectal cancer. Dis Colon Rectum. 50:1873–1880. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Croce CM and Reed JC: Finally, an
apoptosis-targeting therapeutic for cancer. Cancer Res.
76:5914–5920. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang Q, Li S, Cheng P, Deng M, He X, Wang
Z, Yang CH, Zhao XY and Huang J: High expression of anti-apoptotic
protein Bcl-2 is a good prognostic factor in colorectal cancer:
Result of a meta-analysis. World J Gastroenterol. 23:5018–5033.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parrish AB, Freel CD and Kornbluth S:
Cellular mechanisms controlling caspase activation and function.
Cold Spring Harb Perspect Biol. 5:2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Igney FH and Krammer PH: Death and
anti-death: Tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gui D, Guo Y, Wang F, Liu W, Chen J, Chen
Y, Huang J and Wang N: Astragaloside IV, a novel antioxidant,
prevents glucose-induced podocyte apoptosis in vitro and in vivo.
PLoS One. 7:e398242012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu R, Tang S, Wang M, Xu X, Yao C and Wang
S: MicroRNA-497 induces apoptosis and suppresses proliferation via
the Bcl-2/Bax-caspase9-caspase3 pathway and cyclin D2 protein in
HUVECs. PLoS One. 11:e01670522016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
White E: The role for autophagy in cancer.
J Clin Invest. 125:42–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burman C and Ktistakis NT: Autophagosome
formation in mammalian cells. Semin Immunopathol. 32:397–413. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tan S, Shi H, Ba M, Lin S, Tang H, Zeng X
and Zhang X: miR-409-3p sensitizes colon cancer cells to
oxaliplatin by inhibiting Beclin-1-mediated autophagy. Int J Mol
Med. 37:1030–1038. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang QG, Li TY, Liu DN and Zhang HT:
PI3K/Akt pathway involving into apoptosis and invasion in human
colon cancer cells LoVo. Mol Biol Rep. 41:3359–3367. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Malinowsky K, Nitsche U, Janssen KP, Bader
FG, Späth C, Drecoll E, Keller G, Höfler H, Slotta-Huspenina J and
Becker KF: Activation of the PI3K/AKT pathway correlates with
prognosis in stage II colon cancer. Br J Cancer. 110:2081–2089.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alao JP: The regulation of Cyclin D1
degradation: Roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang BH: PI3K/AKT and mTOR/p70S6K1
signaling pathways in human cancer. Curr Cancer Drug Targets.
13:2332013. View Article : Google Scholar : PubMed/NCBI
|