Introduction
Cholangiocarcinoma is an aggressive malignancy, and
the difficulties associated with its diagnosis at the operative
stage and high recurrence rate after surgery result in high
mortality (1,2). Cholangiocarcinoma may be resistant to
conventional chemotherapeutic agents, and non-surgical therapeutic
regimens result in minimally improved patient survival (3). Therefore, the discovery of new
therapeutic targets is required to increase favorable outcomes
among cholangiocarcinoma patients.
Cyclin-dependent kinases (CDKs) belong to a family
of protein kinases that play critical roles in the regulation of
the cell cycle machinery, and consist of 21 hypotypes (4). CDK activation is required for cell cycle
progression, and is positively regulated by cyclins and negatively
regulated by CDK inhibitors. CDK1-cyclin B was revealed to be
important for the progression of G2/M, and CDK2-cyclin E promoted
cell cycle transition from the G1 to S phase. Furthermore, CDK5 has
been revealed to be associated with the regulation of cell
migration and invasion (4).
The cell cycle is dysregulated during the
carcinogenic process, which is accompanied by the overexpression of
positive cell cycle regulators (CDKs and cyclins) and the loss of
function of CDK inhibitors (5–7).
Dysregulated CDKs have been linked to cancer initiation and
progression, and the up-regulated expression of CDKs is closely
associated with the poor prognosis of patients with various types
of cancers (8–12). Thus, the targeting of CDK pathways
represents an effective therapeutic strategy against cancer, and
there are several ongoing clinical trials using CDK inhibitors
(13).
In pancreatic ductal adenocarcinoma (PDAC), the
pharmacological inhibition of CDKs was revealed to be effective in
experimental studies, and several CDK inhibitors are currently
being examined in clinical trials (13–15). A
recent study reported that CDK1 is a synthetic lethal target for
KRAS mutant tumors including PDAC (16). Since activating KRAS mutations have
been reported in cholangiocarcinoma (17–19), and
PDAC shares many biological properties with cholangiocarcinoma, the
inhibition of CDKs may be beneficial for the treatment of
cholangiocarcinoma.
Several experimental studies on cholangiocarcinoma
have examined alterations in the expression of CDKs and cell cycle
progression following treatments with anticancer agents other than
CDK inhibitors (20–23). However, evidence to directly support
the involvement and biological significance of the CDK pathways in
cholangiocarcinoma is limited. Therefore, we herein examined CDK
pathways in cholangiocarcinoma, with the aim of developing a novel
therapeutic approach for the disease.
Materials and methods
Ethical statement
Experiments using human materials were performed
with the approval of the Ethics Committee of Kanazawa University
Graduate School of Medicine (Permit no. 1985-3). Protocols for
animal studies were approved by the Committee of the Institute for
Experimental Animals, Kanazawa University Advanced Science Research
Center (Permit no. AP-173905).
Cell culture
The human cholangiocarcinoma cell lines, CCKS-1,
TFK-1 and HUCCT-1, were used. CCKS-1 was established in our
laboratory from abdominal metastasis of cholangiocarcinoma
(moderately differentiated adenocarcinoma) (24). TFK1 and HUCCT-1 were provided by Cell
Resource Center for Biochemical Research, Tohoku University,
Sendai, Japan. CCKS-1 was grown in Dulbecco's modified Eagle's
medium/F-12 (Gibco; Thermo Fisher Scientific, Inc.), and TFK-1 and
HUCCT-1 were grown in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum and 1%
antibiotic-antimycotic solution (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in an atmosphere of 5% CO2. To block CDK
signaling, the multi-CDK inhibitor roscovitine (Cell Signaling
Technology, Inc.) was used.
Cell proliferation assay
Cell proliferative activity was examined using the
WST-1 assay (Roche Diagnostics GmbH) according to the
manufacturer's instructions. Cells were seeded on a 96-well plate
at a density of ~2×103−1×104 cells/well.
Cells were treated with roscovitine at the concentrations of 2, 10
and 20 µM. After 24, 48 and 72 h following the treatment, the WST-1
reagent was added and incubated for 1 h before reading the plate.
Absorbance at 450 nm was measured using a microplate reader. Each
assay was conducted in eight sets.
Flow cytometry
Cells were treated with 20 µM roscovitine for 24 h
and processed for cell cycle analysis using the BrdU Flow Kit
(Nippon Becton Dickinson Company, Ltd.) according to the
manufacturer's instructions. Samples were analyzed using BD
FACSCanto™ II Flow Cytometry (BD Biosciences). The percentages of
cells in the G1, S, and G2/M phases of the cell cycle were assessed
using BD FACSDiva™ Software (BD Biosciences).
Western blotting
Cells were treated with 20 µM roscovitine for 72 h,
and proteins were extracted using the T-PER Protein Extraction
Reagent (Gibco; Thermo Fisher Scientific, Inc.). Protein
concentrations were determined using the Bradfold method. Equal
amounts of protein (50 µg) were loaded in each lane of an
SDS-polyacrylamide gel (5–20%). Proteins were separated by
electrophoresis and transferred to a nitrocellulose membrane. After
blocking, the membrane was incubated at 4°C overnight with primary
antibodies against p-CDK1 (Thr161; 1:500 dilution; cat. no.
bs-3481R; rabbit polyclonal; Bioss Antibodies, Inc.), CDK1 (1:1,000
dilution; mouse monoclonal; cat. no. ab18; Abcam), and β-actin
(1:1,000 dilution; cat. no. 4967). rabbit monoclonal; Cell
Signaling Technology, Inc.). Following an incubation with the
secondary antibody conjugated with peroxidase using Histofine
Simple Stain MAX PO (Nichirei Corp.; cat. no. 424142) at room
temperature for 1 h, the protein was visualized using
3,3′-diaminobenzidine tetrahydrochloride (DAB). Image analysis was
performed using the NIH ImageJ software (National Institutes of
Health). Each assay was conducted in five sets.
ELISA assay
The protein levels of p-CDK1 (Thr161) and total CDK1
in cholangiocarcinoma cell lines were determined using an
enzyme-linked immunosorbent assay (ELISA). The same samples used
for the western blotting were analyzed, and the protein levels were
determined using a RayBio® Human phospho-CDK1 (Thr161)
and Total CDK1 ELISA kit (RayBiotech) according to the
manufacturer's instructions. Optical density was measured using a
microplate reader at 450 nm, and the ratio of p-CDK1/CDK1 was
calculated for each sample. Each assay was conducted in six
sets.
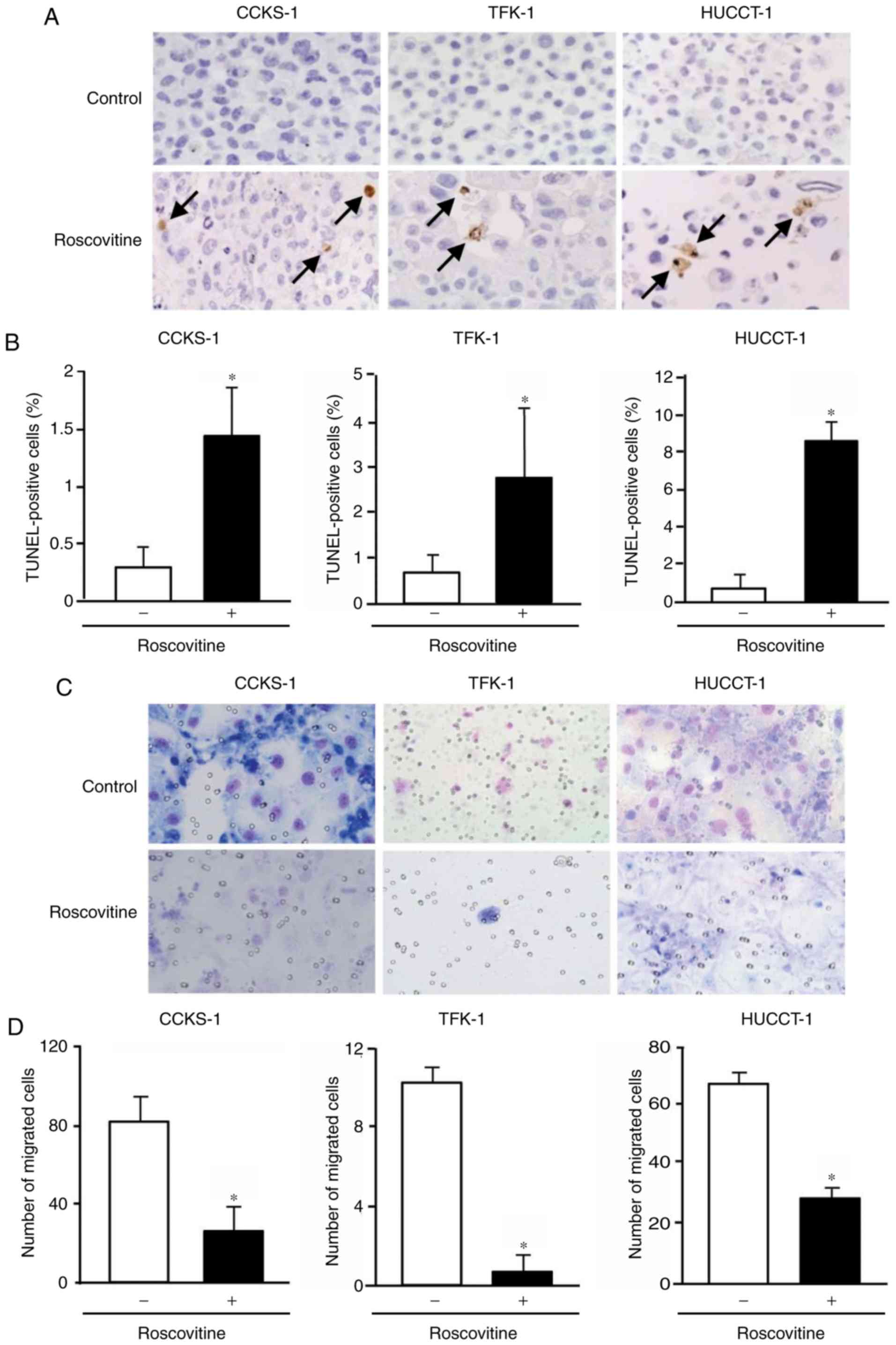
Apoptosis assay
DNA fragmentation attributable to apoptosis was
assessed by the terminal deoxynucleotide transferase-mediated dUTP
nick end-labeling (TUNEL) method. Cells were treated with 20 µM
roscovitine for 72 h and processed in a paraffin-embedded cell
block. After deparaffinization, sections were stained using a
commercial kit (in situ Apoptosis Detection kit; Takara Bio,
Inc.) according to the manufacturer's instructions. Color
development was performed using DAB, and sections were
counterstained with hematoxylin. Five high-power fields were
randomly selected under a light microscope, and the percentage of
apoptotic cells was calculated.
Invasion assay
Carcinoma cell invasion was examined using the
Corning® BioCoat™ Matrigel®
Invasion Chamber (Corning, Inc.) according to the manufacturer's
instructions. Cells (~2-15×104 cells/well) in a 0.5-ml
suspension with or without 20 µM roscovitine were seeded in each
well. Cells were incubated at 37°C for 72 h and then fixed at room
temperature for 12 h with 10% neutral formalin. Cells were stained
with Diff-Quick (Sysmex Corp.) at room temperature for 15 sec.
Migrated cells were visualized under a light microscope, and the
number of cells was counted in five randomly selected high-power
fields.
Murine xenograft model
Five-week-old BALB/cAnNCrj-nu/nu nude mice (male)
were obtained from Charles River Laboratories, Inc. CCKS-1 was
implanted subcutaneously into the right flanks of mice. On day 20,
tumor-bearing mice were randomly divided into two groups (control
and treatment groups), and five mice were included in each group.
Roscovitine (ChemScene) was administered to mice by an
intraperitoneal injection at a dose of 50 mg/kg for two 5-day
series with a 2-day break in between. Control mice were treated
with carrier solution alone. Tumor sizes were measured every day,
and the tumor volumes were calculated using the following formula:
(1/2) LxW2, where L and W represented the longest tumor
axis (mm) and the shortest tumor axis (mm), respectively. The fold
difference from the initial tumor volume (measured at day 20) was
defined as the tumor growth rate (25).
On day 33 (13 days after the first injection of
roscovitine), the mice were sacrificed by inhalation of
CO2 with a 20% volume/min gas displacement flow rate
until euthanasia was confirmed by the arrest of heartbeat and
breathing. Formalin-fixed paraffin-embedded sections of dissected
tumors were used for the apoptosis assay and the immunostaining of
Ki-67.
Immunohistochemistry
Fifty-four cases of extrahepatic cholangiocarcinoma
collected from Kanazawa University Hospital and its affiliated
hospitals were examined. Sample collection was conducted during the
periods from August 1994 to July 2016. All cases surgically
resected were diagnosed as conventional cholangiocarcinoma. The
median age of the patients was 69 years ranging from 35 to 84
years, and the male:female ratio was 33:21. The
tumor-node-metastasis (TNM) classification was used according to
the guidelines of the International Union Against Cancer (26). Cholangiocarcinoma cell lines (CCKS-1,
TFK-1, and HUCCT-1) and tumor tissues obtained from a murine
xenograft model were also used for the immunohistochemical
analysis. Samples were fixed at room temperature for 48 h in 10%
neutral formalin, embedded in paraffin, and 4-µm-thick
paraffin-embedded sections were prepared.
After deparaffinization, antigen retrieval was
performed by microwaving in 10 mmol/l citrate buffer (pH 6.0) for
the immunostaining of p-CDK1, cyclin B1, and cyclin E1. Regarding
the immunostaining of Ki-67, antigen retrieval was performed by
heating in Tris-ethylenediaminetetraacetic acid buffer (pH 9.0)
with a pressure cooker. Sections were immersed in 0.3% hydrogen
peroxidase in methanol for 20 min to block endogenous peroxidase
activity and then incubated in protein block solution (Dako;
Agilent Technologies, Inc.). Sections were incubated overnight at
4°C with primary antibodies against p-CDK1 (Thr161; 1:100 dilution;
cat. no. bs-3481R), rabbit polyclonal; Bioss Antibodies, Inc.),
cyclin B1 (1:100 dilution; cat. no. ab32053; rabbit; monoclonal;
Abcam), cyclin E1 (1:100 dilution; cat. no. ab33911; rabbit
monoclonal; Abcam), or Ki-67 (prediluted; cat. no. 418071; rabbit
monoclonal; Nichirei Corp.). Sections were then incubated with the
secondary antibody conjugated with peroxidase using Histofine
Simple Stain MAX PO [Nichirei Corp. (cat. no. 424142)] at room
temperature for 1 h. Color development was performed using DAB, and
sections were counterstained with hematoxylin.
The immunostaining of p-CDK2 was performed using the
Novolink™ Polymer Detection System (Leica Biosystems)
according to the manufacturer's protocol. Briefly, after blocking
endogenous peroxidase using Peroxidase Block for 5 min and a
pretreatment with Protein Block for 5 min, sections were incubated
at 4°C overnight with the primary antibody against p-CDK2 (Thr160;
1:100 dilution; rabbit polyclonal; Abcam cat. no. ab194868).
Sections were then treated with Post Primary Block followed by
Novolink Polymer at room temperature for 30 min. Immunoreactivity
was visualized using DAB Working Solution, and sections were
counterstained with hematoxylin.
Histological evaluation
A semiquantitative analysis of the
immunohistochemical staining of p-CDK1, p-CDK2, cyclin B1, and
cyclin E1 was performed for surgically resected specimens. Staining
in the nuclei or cytoplasm of cholangiocarcinoma cells was
individually evaluated, and staining in each section was graded as
follows: (−), negative or <5% of carcinoma cells were positive;
(+), >5% of carcinoma cells were positive.
The expression of Ki-67 was assessed by counting at
least 1,000 cholangiocarcinoma cells in an area with the greatest
staining density that was selected in an ×200 magnification field
under a light microscope. The percentage of cells that were
positive for Ki-67 was expressed as the Ki-67-labeling index.
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD). The significance of differences was analyzed using the
Student's t-test and chi-squared test. In the univariate analysis,
postoperative survival probability was calculated by the
Kaplan-Meier method, and survival curves were compared by the
Log-rank test using BellCurve® for Excel software
(Social Survey Research Information Co., Ltd.). A P-value <0.05
was considered to indicate a statistically significant
difference.
Results
Upregulation of CDK and cyclin
expression in cholangiocarcinoma tissues
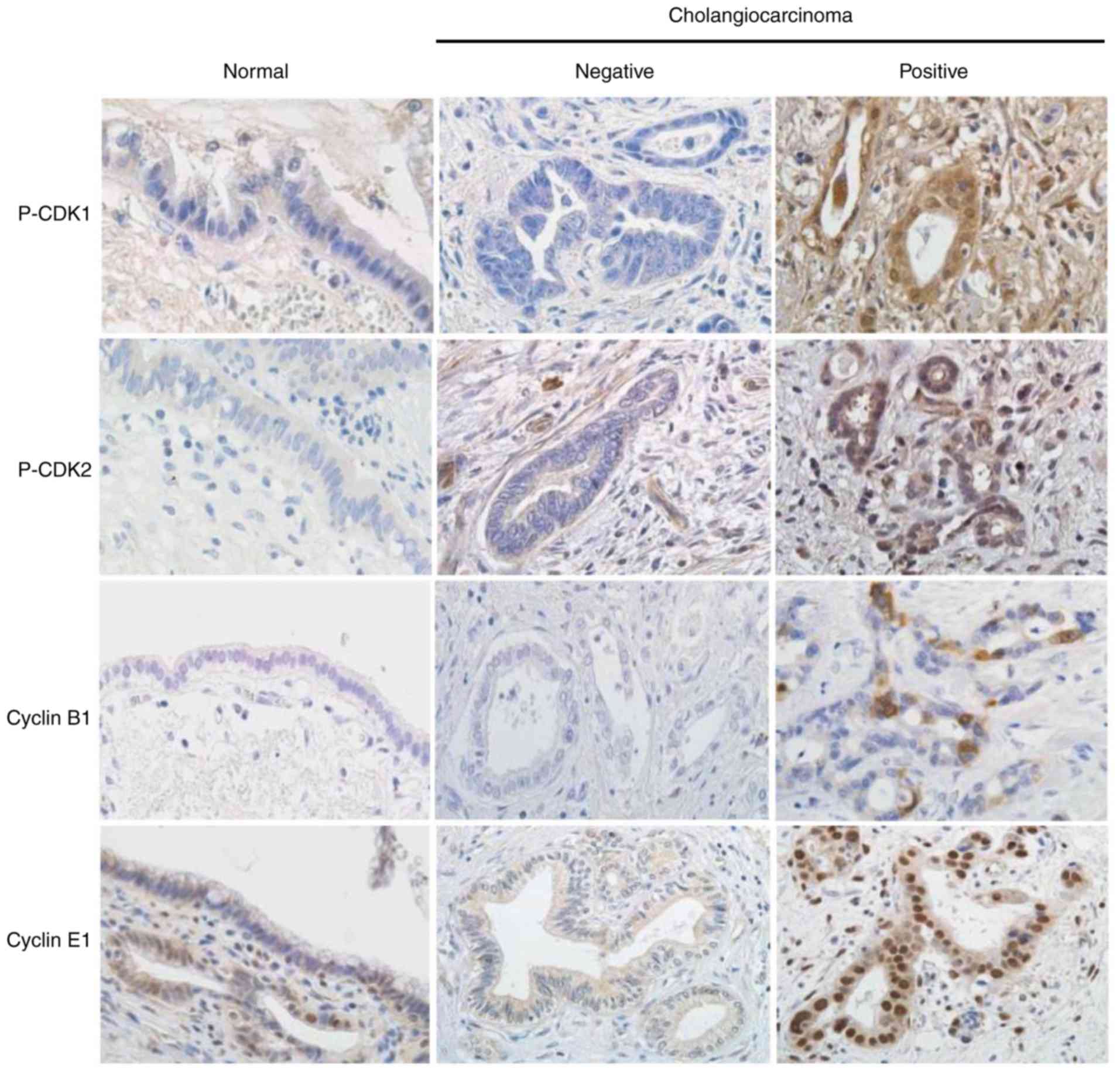
The immunohistochemical expression of p-CDK1,
p-CDK2, cyclin B1, and cyclin E1 was absent or weak in
non-neoplastic biliary epithelial cells. In cholangiocarcinoma, the
upregulation of protein expression was observed in a number of
cases (Fig. 1). Positive
immunohistochemical signals for CDKs and cyclins were observed in
both the nuclei and cytoplasm of carcinoma cells, and the extent of
positive staining varied from patchy to diffuse. Among the 54
cholangiocarcinoma cases examined, positive nuclear expression for
p-CDK1, p-CDK2, cyclin B1, and cyclin E1 was observed in 29 cases
(54%), 18 cases (33%), 32 cases (59%), and 34 cases (63%),
respectively (Table I). No
correlations were noted between the positivity of p-CDK1 and cyclin
B1 or the positivity of p-CDK2 and cyclin E1 (data not shown).
 | Table I.Relationship between the
immunohistochemical expression of CDKs/cyclins and
clinicopathological factors in cholangiocarcinoma. |
Table I.
Relationship between the
immunohistochemical expression of CDKs/cyclins and
clinicopathological factors in cholangiocarcinoma.
|
|
| p-CDK1a |
| p-CDK2a |
| Cyclin
B1a |
| Cyclin
E1a |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Factor | N (%) | - (%) | + (%) | P-value | - (%) | + (%) | P-value | - (%) | + (%) | P-value | - (%) | + (%) | P-value |
|---|
| Sex |
|
|
| 0.686 |
|
| 0.554 |
|
| 0.752 |
|
| 0.480 |
|
Male | 33 (61) | 16 (48) | 17 (52) |
| 21 (64) | 12 (36) |
| 14 (42) | 19 (58) |
| 11 (33) | 22 (67) |
|
|
Female | 21 (39) | 9 (43) | 12 (57) |
| 15 (71) | 6 (29) |
| 8 (38) | 13 (62) |
| 9 (43) | 12 (57) |
|
| Histology |
|
|
| 0.599 |
|
| 0.441 |
|
| 0.182 |
|
| 0.440 |
|
Well | 26 (48) | 13 (50) | 13 (50) |
| 16 (62) | 10 (38) |
| 13 (50) | 13 (50) |
| 11 (42) | 15 (58) |
|
|
Mod-Poor | 28 (52) | 12 (43) | 16 (57) |
| 20 (71) | 8 (29) |
| 9 (32) | 19 (68) |
| 9 (32) | 19 (68) |
|
| Depth of
invasion |
|
|
| 0.984 |
|
| 0.070 |
|
| 0.151 |
|
| 0.358 |
| T1,
2 | 28 (52) | 13 (46) | 15 (54) |
| 18 (64) | 10 (36) |
| 14 (50) | 14 (50) |
| 12 (43) | 16 (57) |
|
| T3,
4 | 26 (48) | 12 (46) | 14 (54) |
| 18 (69) | 8 (31) |
| 8 (31) | 18 (69) |
| 8 (31) | 18 (69) |
|
| Lymph node
metastasis |
|
|
| 0.032 |
|
| 0.245 |
|
| 0.004 |
|
| 0.777 |
|
Negative | 24 (44) | 15 (63) | 9 (38) |
| 18 (75) | 6 (25) |
| 15 (63) | 9 (38) |
| 12 (50) | 12 (50) |
|
|
Positive | 30 (56) | 10 (33) | 20 (67) |
| 18 (60) | 12 (40) |
| 7 (23) | 23 (77) |
| 8(27) | 22 (73) |
|
| Stage |
|
|
| 0.034 |
|
| 0.433 |
|
| 0.001 |
|
| 0.288 |
|
I–IIa | 22 (41) | 14 (64) | 8 (36) |
| 16 (73) | 6 (27) |
| 15 (68) | 7 (32) |
| 10 (45) | 12 (55) |
|
|
>IIb | 32 (59) | 11 (34) | 21 (66) |
| 20 (63) | 12 (37) |
| 7 (22) | 25 (78) |
| 10 (31) | 22 (69) |
|
Clinicopathological significance of
CDK and cyclin expression in cholangiocarcinoma
The relationship between clinicopathological factors
and the immunohistochemical expression of CDKs and cyclins was
examined. The nuclear expression of p-CDK1 and cyclin B1 was
positively associated with the presence of lymph node metastasis
and the clinical stage (Table I). The
nuclear expression of p-CDK2 and cyclin E1 was not associated with
these clinicopathological factors. The expression of p-CDK1,
p-CDK2, cyclin B1, and cyclin E1 in the cytoplasm of carcinoma
cells was not associated with any clinicopathological factors (data
not shown).
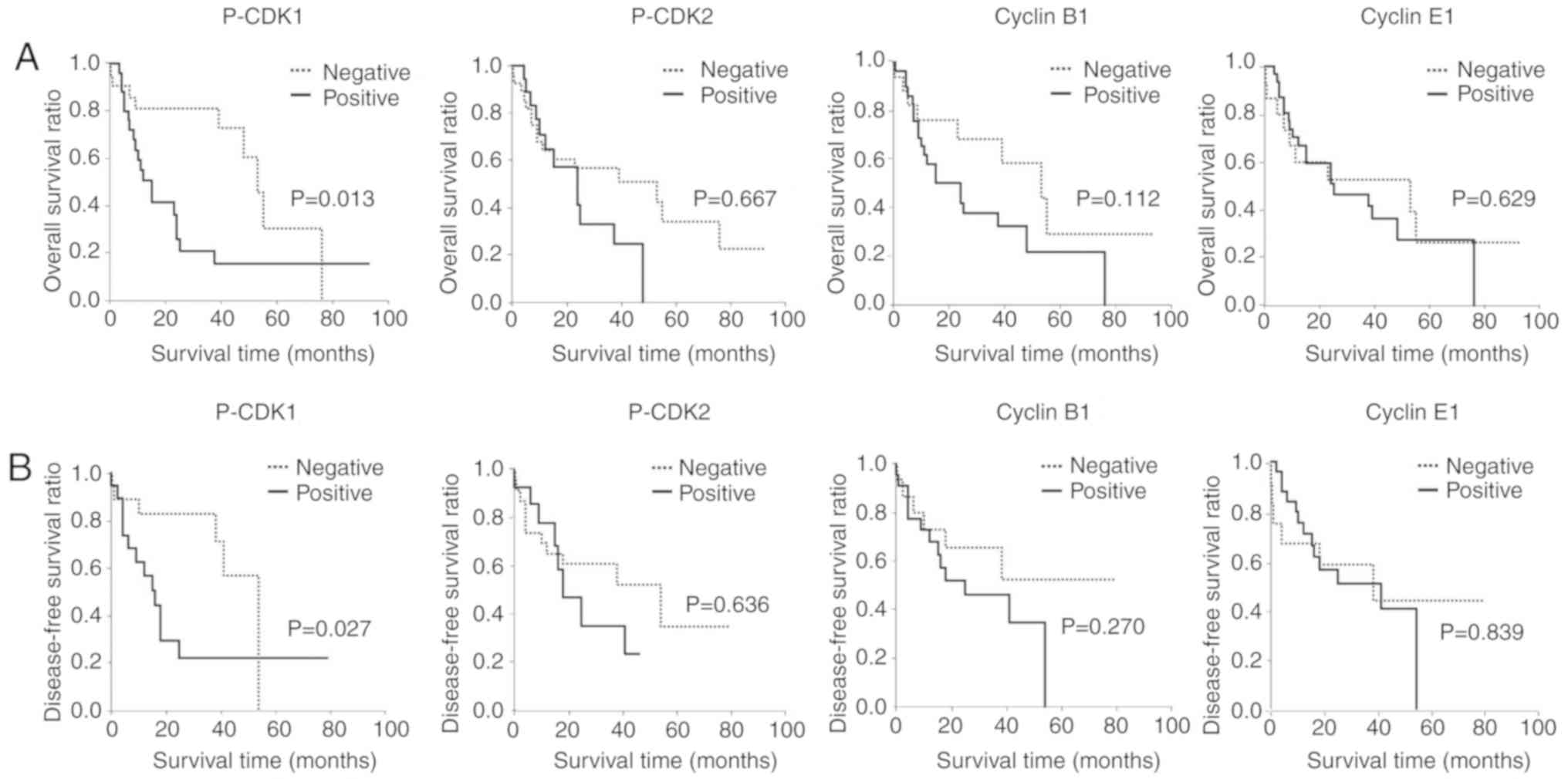
Postoperative follow-up data were analyzed with a
median follow-up period of 21.8 months for the overall survival
ratio and 18.0 months for the disease-free survival ratio. Patients
with the positive nuclear expression of p-CDK1 had a significantly
poor prognosis (Fig. 2). In contrast,
no correlation was observed between patient prognosis and the
nuclear expression of p-CDK2, cyclin B1, and cyclin E1 (Fig. 2). The cytoplasmic expression of
p-CDK1, p-CDK2, cyclin B1, and cyclin E1 was not associated with
patient prognosis (data not shown).
A group of disease-free surviving individuals was
observed within the p-CDK1-positive cohort of patients.
Disease-free surviving patients were also present in the p-CDK2-,
cyclin B1-, and cyclin E1-negative groups. These surviving patients
appeared to have cholangiocarcinoma with a well-differentiated
histology.
The present results indicated that the activation of
the CDK pathway involving CDK1 rather than CDK2 defined the tumor
aggressiveness of cholangiocarcinoma. To further clarify their
involvement in cholangiocarcinoma, in vitro experiments were
performed.
Effects of CDK inhibition on
cholangiocarcinoma biology in vitro
The human cholangiocarcinoma cell lines, CCKS-1,
TFK-1, and HUCCT-1 were used. Immunostaining revealed that p-CDK1
was expressed in the nuclei of all three cell lines, and its
expression was stronger in CCKS-1 and HUCCT-1 than in TFK-1
(Fig. 3A). When cells were treated
with the multi-CDK inhibitor roscovitine, cell proliferative
activity was inhibited in the three cell lines in time- and
dose-dependent manners, with 20 µM roscovitine exerting the
strongest effects (Fig. 3B). In
subsequent in vitro experiments, roscovitine was used at a
dose of 20 µM.
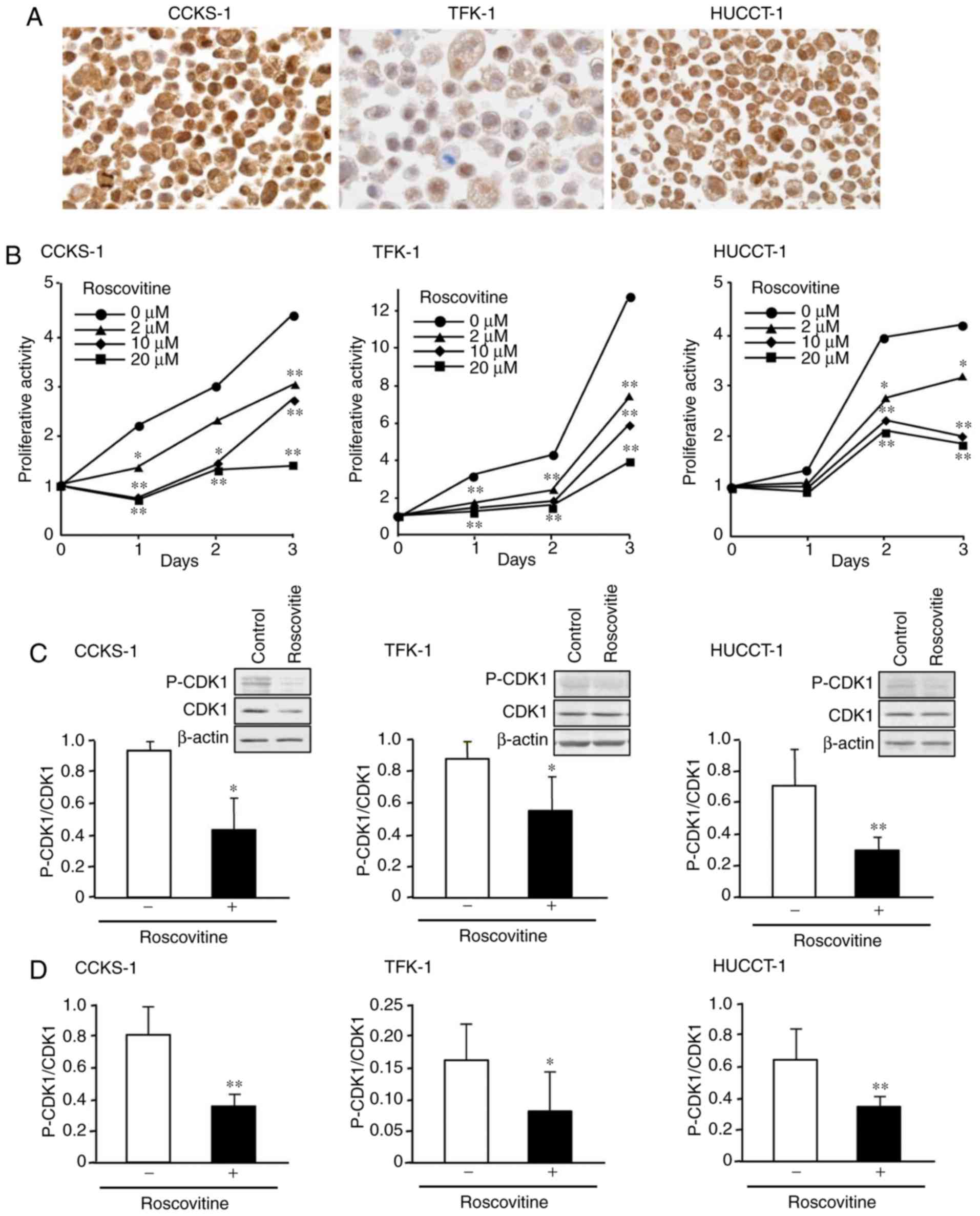 | Figure 3.Effects of roscovitine on the cell
proliferative activity of cholangiocarcinoma in vitro. (A)
Immunohistochemical expression of p-CDK1 in the human
cholangiocarcinoma cell lines, CCKS-1, TFK-1, and HUCCT-1. (B) The
inhibition of the cell proliferative activity of these cell lines
following the treatment with roscovitine that was assessed using
the WST-1 assay. (C) A western blot analysis indicating the reduced
expression of p-CDK1 following roscovitine treatment. The ratio of
p-CDK1/CDK1 was determined for each sample. Representative western
blots are presented in the upper right corner of each figure, in
which the image in each square was captured from the same blotting
membrane. (D) An ELISA analysis revealing that roscovitine
treatment reduced the ratio of p-CDK1/CDK1 in the cells. Data were
expressed as the mean ± SD of (B) eight, (C) five, and (D) six
sets. The significance of differences was assessed using the
Student's t-test. *P<0.05, **P<0.01. Original magnification,
×1,000 (A). p-CDK1, phosphorylated cyclin-dependent kinase 1. |
The western blot analysis indicated that the
treatment with roscovitine reduced the expression of p-CDK1 in the
cells (Fig. 3C), and the analysis
using ELISA further confirmed that the ratio of p-CDK1/CDK1 was
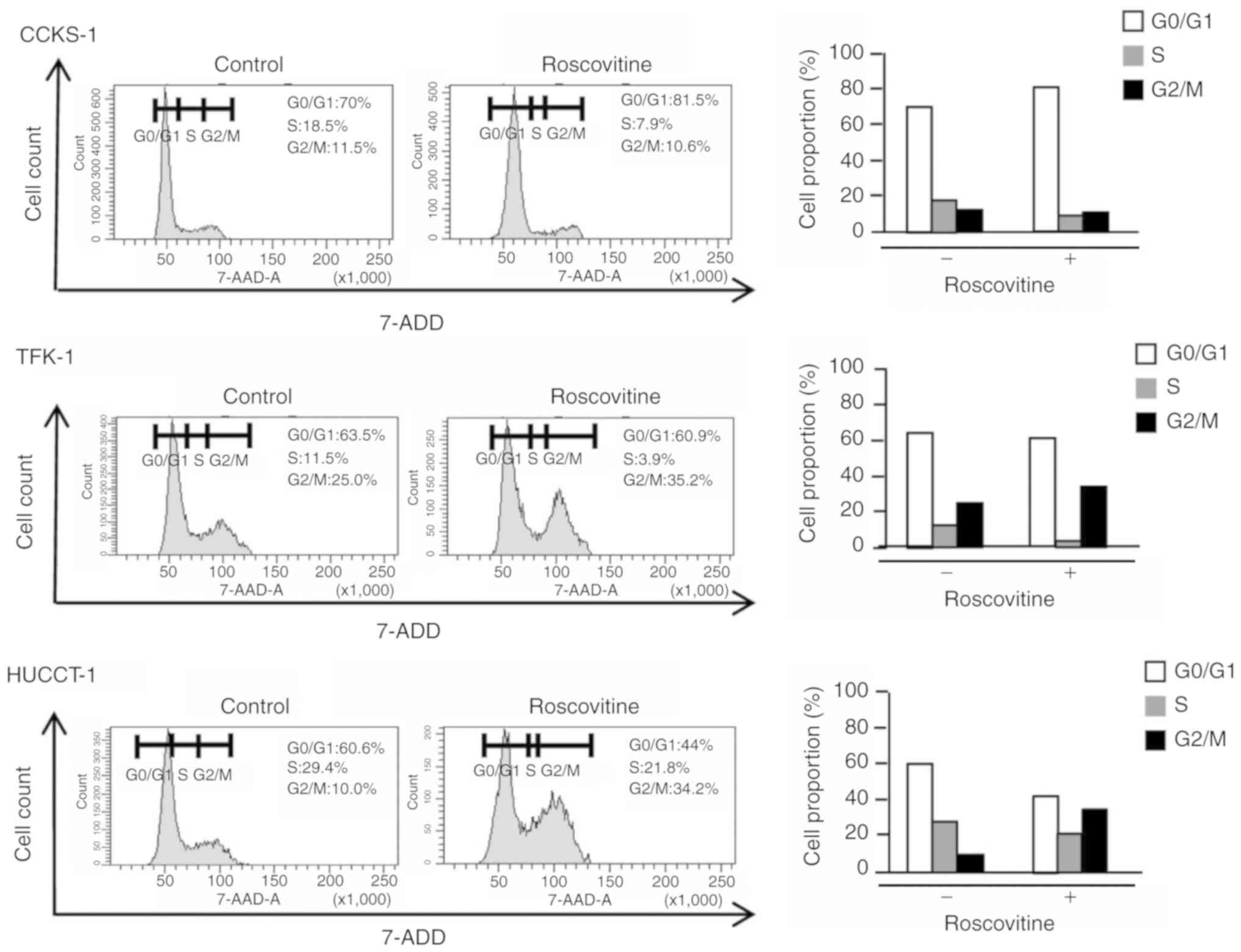
significantly reduced by the treatment (Fig. 3D). Following treatment with
roscovitine, the cell cycle was arrested at the G1 or G2/M phase in
CCKS-1, TFK-1, and HUCCT-1 (Fig. 4),
and this was accompanied by an increase in the percentage of
apoptotic cells (Fig. 5A and B).
Furthermore, the invasion of cholangiocarcinoma cells was
significantly inhibited by the treatment with roscovitine (Fig. 5C and D).
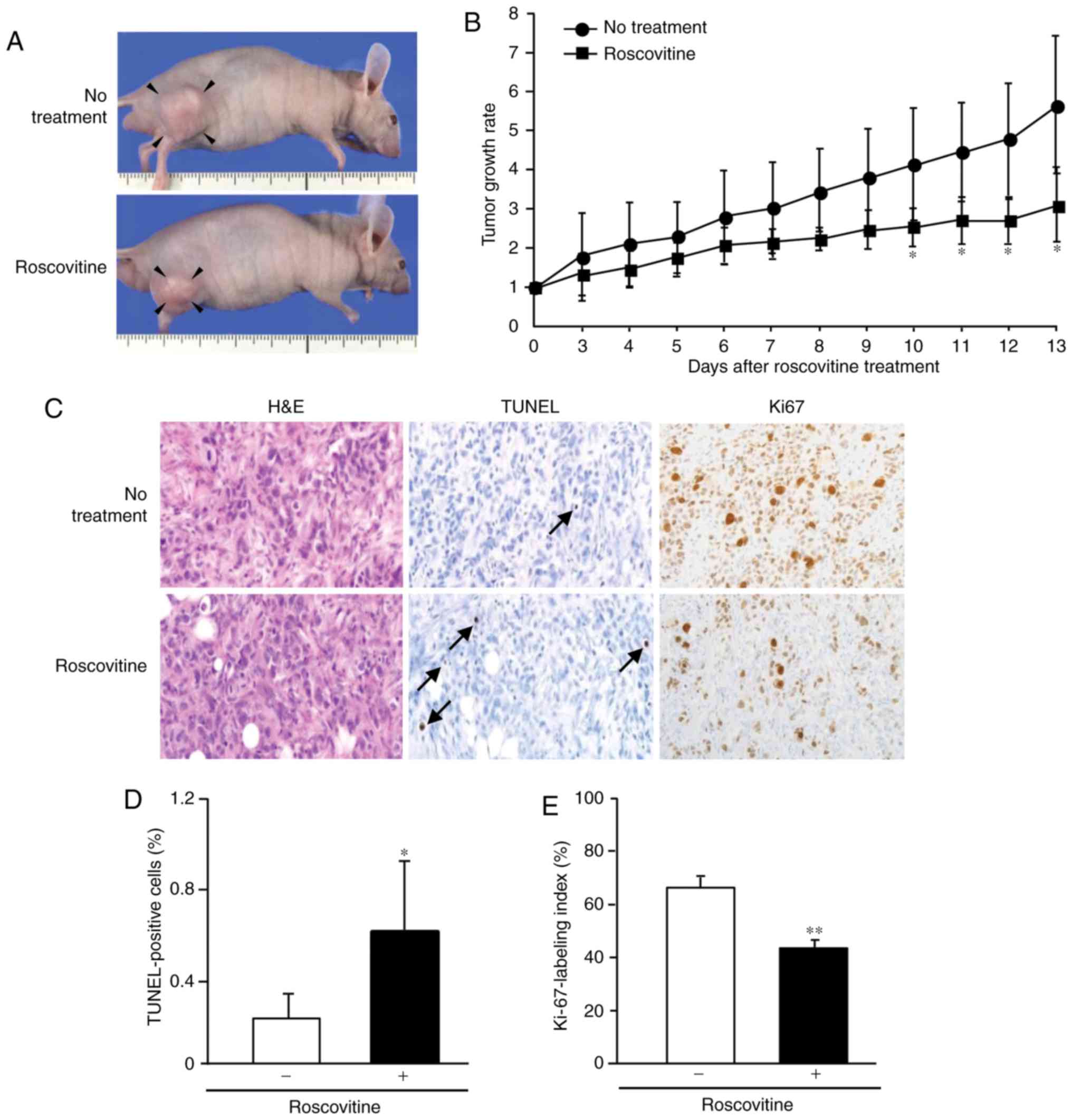
Effects of CDK inhibition on
cholangiocarcinoma in a murine xenograft model
The subcutaneous implantation of CCKS-1 was used in
the analysis of the murine xenograft model. An intraperitoneal
injection of roscovitine inhibited tumor growth in this model, and
a significant difference was noted in the tumor growth rate between
the untreated and treated groups between 10 and 13 days after
roscovitine treatment (Fig. 6A and
B). Tumors in the untreated group grew up to a volume of 847
mm3 (6.7-fold growth against the initial size), whereas
tumors in the treated group had a maximum volume of 600
mm3 (3.0-fold growth against the initial size).
Similar to the results of the in vitro
experiments, the apoptotic response of cholangiocarcinoma cells was
significantly induced by the treatment (Fig. 6C and D). The Ki-67-labeling index of
cholangiocarcinoma cells was significantly reduced by the treatment
(Fig. 6C and E). Thus, roscovitine
was an effective agent to treat cholangiocarcinoma in
vivo.
Discussion
The present study revealed the significant
involvement of the CDK pathway and CDK1 in cholangiocarcinoma. CDK1
and related molecules play key roles in the cell cycle, and the
upregulated expression of CDK1 occurs in a number of human
malignant tumors (9–12). CDK1 forms a complex with cyclin B1,
and the CDK1-cyclin B1 complex (M-phase promoting factor) is
indispensable for cell cycle transition into the G2/M phase
(27,28). The CDK1 protein is activated by
phosphorylation at the Thr161 amino acid activation site and by
dephosphorylation at the Thr14/Tyr15 amino acid inhibition sites.
CDK1 and cyclin B1 are located in both the nuclei and cytoplasm of
cells, and the activation of CDK1-cyclin B1 triggers its rapid
accumulation in nuclei (28,29).
The immunohistochemical analysis revealed that the
nuclear expression of p-CDK1 (Thr161) and cyclin B1, rather than
their cytoplasmic expression, was correlated with
clinicopathological factors and/or patient prognosis. These results
indicated that the activation of CDK1-cyclin B1 contributes to the
aggressive behavior of cholangiocarcinoma. Since the
immunohistochemical expression of p-CDK1 and cyclin B1 was absent
or weak in normal biliary epithelial cells and hepatocytes, the
therapeutic inhibition of the CDK pathway involving CDK1 may have
less hepatotoxicity for the normal liver physiology.
Positive correlations have been suggested between
CDK phosphorylation and their downstream targets, including
cyclins. However, the immunohistochemical analysis revealed no
correlations between p-CDK1/cyclin B1 and p-CDK2/cyclin E1
positivity. After the mitotic phase, p-CDK1/cyclin B1 are
inactivated by dephosphorylation and the destruction of cyclin B1
(28). The activation of CDK2
requires the assembly of cyclin E and phosphorylation at the Thr160
amino acid activation site by CDK activation enzymes. p-CDK2/cyclin
E1 are inactivated by dephosphorylation at the Thr14/Tyr15 amino
acid inhibition site by cdc25A, and the destruction of cyclin E
occurs via ubiquitination and proteasome processing during the S
phase (30,31). Although the exact reason for this
currently remains unclear, the complex interactions between CDKs,
cyclins, and other related molecules may account for the lack of
correlations between p-CDK1/cyclin B1 and p-CDK2/cyclin E1
positivity.
CDK inhibitors exert antitumor effects in various
types of cancers, and CDK1 is a target molecule (13–15,32–34).
Roscovitine is a broad-range purine inhibitor that inhibits CDK1,
CDK2, CDK5, and CDK 7 through direct competition at the ATP-binding
site. Roscovitine inhibits cell proliferation in various cancers,
and the anti-proliferative effect is mediated by cell cycle arrest,
which is accompanied by the induction of apoptosis (35–38).
Regarding the application of roscovitine to biliary diseases, it
was revealed to inhibit cholangiocyte growth (liver cystogenesis)
in a mouse model of autosomal dominant polycystic kidney disease
(39). Consistent with previously
reported findings, it was revealed that roscovitine inhibited the
cell proliferation of cholangiocarcinoma, decreased the expression
of p-CDK1, arrested the cell cycle at the G1 or G2/M phase, and
induced apoptosis. Furthermore, roscovitine inhibited the invasion
of cholangiocarcinoma cells in vitro. The activation of CDK5
was revealed to be involved in the invasion of PDAC, and the
inhibition of cholangiocarcinoma cell invasion may be associated
with the inhibitory effects of roscovitine on CDK5 activation
(40).
In vivo studies further confirmed that
roscovitine significantly inhibited cell proliferative activity,
and induced apoptosis in a murine xenograft model of
cholangiocarcinoma. These results were consistent with the data of
previous studies that demonstrated the antitumor effects of
roscovitine in vivo (25,37,41). It
has been reported that roscovitine induced apoptosis in a xenograft
model of Ewing's sarcoma family tumor by a caspase-dependent
mechanism (25). Another study
revealed that roscovitine enhanced the antitumor effects of
doxorubicin by inducing G2/M arrest rather than apoptosis
accompanied by the increase of p27 expression in a breast cancer
xenograft model (41). Although the
detailed mechanism requires study, it is plausible that the
xenograft growth inhibition by roscovitine relates to the complex
interaction of molecules included in different signaling pathways,
and the inhibition of cell cycle progression and the induction of
apoptosis may be important factors attributable to the antitumor
effects of roscovitine in vivo. The inhibition of tumor
invasion may also contribute to the antitumor effects.
KRAS mutant tumor cells have elevated CDK1 activity,
and CDK1 may be a synthetic lethal target for KRAS mutant tumors
including PDAC (16). Activating KRAS
mutations are frequently observed in cholangiocarcinoma
predominantly of the perihilar and distal types and are reported to
be present in up to 40% of cases (17–19).
CCKS-1 and HUCCT-1 were revealed to harbor a KRAS mutation (G12D),
whereas TFK-1 had no mutation at codon 12 of KRAS (42,43). The
stronger nuclear expression of p-CDK1 in CCKS-1 and HUCCT-1 than in
TFK-1 may reflect the presence of KRAS mutations in cells, and
suggests that CDK1 is a preferable therapeutic target for
cholangiocarcinoma with KRAS mutations.
In the present study, the nuclear expression of
p-CDK1 was also observed in TFK-1. Although p-CDK1 expression in
TFK-1 was weak on immunostaining, the presence of p-CDK1 indicates
its potential as a therapeutic target. The multi-CDK inhibitor
roscovitine inhibited cell proliferative activity and arrested the
cell cycle in TFK-1 as well as in CCKS-1 and HUCCT-1; however, the
extent of the involvement of CDK1 inhibition in these inhibitory
effects was unclear, and the effects of roscovitine on other CDKs
may be involved. Therefore, studies using specific inhibitors of
CDK1 are required to further clarify the biological significance of
CDK1 in cholangiocarcinoma. Furthermore, the relationship between
the KRAS mutation status and the extent of p-CDK1 expression needs
to be analyzed in patients with cholangiocarcinoma.
In summary, the present study demonstrated that the
pharmacological inhibition of CDK pathways using roscovitine
reduced cell proliferation and the invasion of cholangiocarcinoma.
These inhibitory effects were accompanied by cell cycle arrest at
the G1 or G2/M phase and the induction of apoptosis. The
immunohistochemical expression of p-CDK1 and cyclin B1 predicted
the aggressive behavior of cholangiocarcinoma, and p-CDK1 may be a
useful prognostic marker of cholangiocarcinoma. These results
indicated that the CDK pathway involving CDK1 has potential as a
therapeutic target for cholangiocarcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MY performed the experiments and wrote the
manuscript with technical support from KT. YS and KH conceived and
designed the study. MS analyzed the data and provided comments. All
authors participated in data interpretation and critically reviewed
and approved the manuscript and agree to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Experiments using human materials were performed
with the approval of the Ethics Committee of Kanazawa University
Graduate School of Medicine (Permit no. 1985-3). Protocols for
animal studies were approved by the Committee of the Institute for
Experimental Animals, Kanazawa University Advanced Science Research
Center (Permit no. AP-173905).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CDK
|
cyclin-dependent kinase
|
|
DAB
|
3,3′-diaminobenzidine
tetrahydrochloride
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
p-CDK
|
phosphorylated CDK
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
SD
|
standard deviation
|
|
TUNEL
|
terminal dUTP nick-end labeling
|
References
|
1
|
Razumilava N and Gores GJ:
Cholangiocarcinoma. Lancet. 383:2168–2179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rizvi S, Khan SA, Hallemeier CL, Kelley RK
and Gores GJ: Cholangiocarcinoma-evolving concepts and therapeutic
strategies. Nat Rev Clin Oncol. 15:95–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cereda S, Passoni P, Reni M, Viganò MG,
Aldrighetti L, Nicoletti R and Villa E: The cisplatin, epirubicin,
5-fluorouracil, gemcitabine (PEFG) regimen in advanced biliary
tract adenocarcinoma. Cancer. 116:2208–2214. 2010.PubMed/NCBI
|
|
4
|
Cheng W, Yang Z, Wang S, Li Y, Wei H, Tian
X and Kan Q: Recent development of CDK inhibitors: An overview of
CDK/inhibitor co-crystal structures. Eur J Med Chem. 64:615–639.
2019. View Article : Google Scholar
|
|
5
|
Schwartz GK and Shah MA: Targeting the
cell cycle: A new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deep G and Agarwal R: New combination
therapies with cell cycle agents. Curr Opin Investig Drugs.
9:591–604. 2008.PubMed/NCBI
|
|
7
|
Coxon CR, Anscombe E, Harnor SJ, Martin
MP, Carbain B, Golding BT, Hardcastle IR, Harlow LK, Korolchuk S,
Matheson CJ, et al: Cyclin-dependent kinase (CDK) inhibitors:
Structure-activity relationships and insights into the CDK-2
selectivity of 6-substituted 2-arylaminopurines. J Med Chem.
60:1746–1767. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai J, Li B, Zhu Y, Fang X, Zhu M, Wang M,
Liu S, Jiang X, Zheng J, Zhang X, et al: Prognostic biomarker
identification through integrating the gene signatures of
hepatocellular carcinoma properties. EbioMedicine. 19:18–30. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sung WW, Lin YM, Wu PR, Yen HH, Lai HW, Su
TC, Huang RH, Wen CK, Chen CY, Chen CJ, et al: High
nuclear/cytoplasmic ratio of Cdk1 expression predicts poor
prognosis in colorectal cancer patients. BMC Cancer. 14:9512014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hongo F, Takaha N, Oishi M, Ueda T,
Nakamura T, Naitoh Y, Naya Y, Kamoi K, Okihara K, Matsushima T, et
al: CDK1 and CDK2 activity is a strong predictor of renal cell
carcinoma recurrence. Urol Oncol. 32:1240–1246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi YX, Zhu T, Zou T, Zhuo W, Chen YX,
Huang MS, Zheng W, Wang CJ, Li X, Mao XY, et al: Prognostic and
predictive values of CDK1 and MAD2L1 in lung adenocarcinoma.
Oncotarget. 7:85235–85243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang C, Elkahloun AG, Robertson M, Gills
JJ, Tsurutani J, Shih JH, Fukuoka J, Hollander MC, Harris CC,
Travis WD, et al: Loss of cytoplasmic CDK1 predicts poor survival
in human lung cancer and confers chemotherapeutic resistance. PLoS
One. 6:e238492011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
García-Reyes B, Kretz AL Ruff JP, von
Karstedt S, Hillenbrand A, Knippschild U, Henne-Bruns D and Lemke
J: The emerging role of cyclin-dependent kinases (CDKs) in
pancreatic ductal adenocarcinoma. Int J Mol Sci. 19(pii):
E32192018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feldmann G, Mishra A, Bisht S, Karikari C,
Garrido-Laguna I, Rasheed Z, Ottenhof NA, Dadon T, Alvarez H,
Fendrich V, et al: Cyclin-dependent kinase inhibitor Dinaciclib
(SCH727965) inhibits pancreatic cancer growth and progression in
murine xenograft models. Cancer Biol Ther. 12:598–609. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Whittaker SR, Mallinger A, Workman P and
Clarke PA: Inhibitors of cyclin-dependent kinases as cancer
therapeutics. Pharmacol Ther. 173:83–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Costa-Cabral S, Brough R, Konde A, Aarts
M, Campbell J, Marinari E, Riffell J, Bardelli A, Torrance C, Lord
CJ, et al: CDK1 is a synthetic lethal target for KRAS mutant
tumours. PLoS One. 11:e01490992016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Churi CR, Shroff R, Wang Y, Rashid A, Kang
HC, Weatherly J, Zuo M, Zinner R, Hong D, Meric-Bernstam F, et al:
Mutation profiling in cholangiocarcinoma: Prognostic and
therapeutic implications. PLoS One. 9:e1153832014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura H, Arai Y, Totoki Y, Shirota T,
Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S, et
al: Genomic spectra of biliary tract cancer. Nat Genet.
47:1003–1010. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mertens JC, Rizvi S and Gores GJ:
Targeting cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis.
1864:1454–1460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He W, Wang B, Zhuang Y, Shao D, Sun K and
Chen J: Berberine inhibits growth and induces G1 arrest and
apoptosis in human cholangiocarcinoma QBC939 cells. J Pharmacol
Sci. 119:341–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng J, Li Q, Wang W, Wang Y, Fu X, Wang
W, Fan L and Yan W: Apoptosis-related protein-1 acts as a tumor
suppressor in cholangiocarcinoma cells by inducing cell cycle
arrest via downregulation of cyclin-dependent kinase subunits.
Oncol Rep. 35:809–816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Samukawa E, Fujihara S, Oura K, Iwama H,
Yamana Y, Tadokoro T, Chiyo T, Kobayashi K, Morishita A, Nakahara
M, et al: Angiotensin receptor blocker telmisartan inhibits cell
proliferation and tumor growth of cholangiocarcinoma through cell
cycle arrest. Int J Oncol. 51:1674–1684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boueroy P, Aukkanimart R, Boonmars T,
Sriraj P, Ratanasuwan P, Juasook A, Wonkchalee N, Vaeteewoottacharn
K and Wongkham S: Inhibitory effect of aspirin on
cholangiocarcinoma cells. Asian Pac J Cancer Prev. 18:3091–3096.
2017.PubMed/NCBI
|
|
24
|
Sugawara H, Yasoshima M, Katayanagi K,
Kono N, Watanabe Y, Harada K and Nakanuma Y: Relationship between
interleukin-6 and proliferation and differentiation in
cholangiocarcinoma. Histopathology. 33:145–153. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tirado OM, Mateo-Lozano S and Notario V:
Roscovitine is an effective inducer of apoptosis of Ewing's sarcoma
family tumor cells in vitro and in vivo. Cancer Res. 65:9320–9327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brierley JD, Gospodarowics MK and
Wittekind C: International union against cancer (UICC)TNM
Classification of Malignant Tumors. 8th. New York: John Wiley and
Sons, LTD.; 2017
|
|
27
|
Salaun P, Rannou Y and Prigent C: Cdk1,
Plks, Auroras, and Neks: The mitotic bodyguards. Adv Exp Med Biol.
617:41–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gavet O and Pines J: Progressive
activation of cyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell.
18:533–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gavet O and Pines J: Activation of cyclin
B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at
mitosis. J Cell Biol. 189:247–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Keenan SM, Bellone C and Baldassare JJ:
Cyclin-dependent kinase 2 nucleocytoplasmic translocation is
regulated by extracellular regulated kinase. J Biol Chem.
276:22404–22409. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Won KA and Reed SI: Activation of cyclin
E/CDK2 is coupled to site-specific autophosphorylation and
ubiquitin-dependent degradation of cyclin E. EMBO J. 15:4182–4193.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parry D, Guzi T, Shanahan F, Davis N,
Prabhavalkar D, Wiswell D, Seghezzi W, Paruch K, Dwyer MP, Doll R,
et al: Dinaciclib (SCH 727965), a novel and potent cyclin-dependent
kinase inhibitor. Mol Cancer Ther. 9:2344–2353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei D, Parsels LA, Karnak D, Davis MA,
Parsels JD, Marsh AC, Zhao L, Maybaum J, Lawrence TS, Sun Y, et al:
Inhibition of protein phosphatase 2A radiosensitizes pancreatic
cancers by modulating CDC25C/CDK1 and homologous recombination
repair. Clin Cancer Res. 19:4422–4432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nilubol N, Boufraqech M, Zhang L, Gaskins
K, Shen M, Zhang YQ, Gara SK, Austin CP and Kebebew E: Synergistic
combination of flavopiridol and carfilzomib targets commonly
dysregulated pathways in adrenocortical carcinoma and has
biomarkers of response. Oncotarget. 9:33030–33042. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coley HM, Shotton CF and Thomas H:
Seliciclib (CYC202; r-roscovitine) in combination with cytotoxic
agents in human uterine sarcoma cell lines. Anticancer Res.
27:273–278. 2007.PubMed/NCBI
|
|
36
|
Wesierska-Gadek J, Borza A, Komina O and
Maurer M: Impact of roscovitine, a selective CDK inhibitor, on
cancer cells: Bi-functionality increases its therapeutic potential.
Acta Biochim Pol. 56:495–501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nair BC, Vallabhaneni S, Tekmal RR and
Vadlamudi RK: Roscovitine confers tumor suppressive effect on
therapy-resistant breast tumor cells. Breast Cancer Res.
13:R802011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cicenas J, Kalyan K, Sorokinas A,
Stankunas E, Levy J, Meskinyte I, Stankevicius V, Kaupinis A and
Valius M: Roscovitine in cancer and other diseases. Ann Transl Med.
3:1352015.PubMed/NCBI
|
|
39
|
Bukanov NO, Moreno SE, Natoli TA, Rogers
KA, Smith LA, Ledbetter SR, Oumata N, Galons H, Meijer L and
Ibraghimov-Beskrovnaya O: CDK inhibitors R-roscovitine and S-CR8
effectively block renal and hepatic cystogenesis in an orthologous
model of ADPKD. Cell Cycle. 11:4040–4046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jin X, Yang C, Fan P, Xiao J, Zhang W,
Zhan S, Liu T, Wang D and Wu H: CDK5/FBW7-dependent ubiquitination
and degradation of EZH2 inhibits pancreatic cancer cell migration
and invasion. J Biol Chem. 292:6269–6280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Appleyard MV, O'Neill MA, Murray KE,
Paulin FE, Bray SE, Kernohan NM, Levison DA, Lane DP and Thompson
AM: Seliciclib (CYC202, R-roscovitine) enhances the antitumor
effect of doxorubicin in vivo in a breast cancer xenograft model.
Int J Cancer. 124:465–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saijyo S, Kudo T, Suzuki M, Katayose Y,
Shinoda M, Muto T, Fukuhara K, Suzuki T and Matsuno S:
Establishment of a new extrahepatic bile duct carcinoma cell line,
TFK-1. Tohoku J Exp Med. 177:61–71. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoshikawa D, Ojima H, Kokubu A, Ochiya T,
Kasai S, Hirohashi S and Shibata T: Vandetanib (ZD6474), an
inhibitor of VEGFR and EGFR signalling, as a novel
molecular-targeted therapy against cholangiocarcinoma. Br J Cancer.
100:1257–1266. 2009. View Article : Google Scholar : PubMed/NCBI
|