Introduction
Head and neck squamous cell carcinoma (HNSCC) is a
type of cancer that develops from the mucosal lining of the upper
aerodigestive tract, and is associated with a variety of
pathophysiological risk factors, such as smoking, betel nut
chewing, alcohol consumption and human papillomavirus infection.
Approximately 600,000 new cases of HNSCC are currently diagnosed
worldwide annually, making HNSCC the sixth most common type of
cancer among human malignancies (1–3).
Although clinical treatments for patients with HNSCC, such as
surgery, radiation therapy, chemotherapy, targeted therapy and
combination treatments, have advanced over the last two decades,
the 5 year survival rate of patients diagnosed with HNSCC is
estimated to be 65% (3). The
clinical symptoms of HNSCC generally include a white or red patch
on the gums or tongue and swelling of the jaw, accompanied by
discomfort, abnormal bleeding and pain in the oral cavity, pharynx,
larynx, salivary glands, paranasal sinuses and nasal cavity. The
clinical treatments for HNSCC may cause side effects associated
with functional changes in the ability to chew, swallow or talk,
and may lead to multiple long-lasting physical and mental
comorbidities (4). Recently,
natural products used in traditional folk medicine have attracted
attention as chemotherapeutic compounds in order to reduce the side
effects of clinical HNSCC treatments (5).
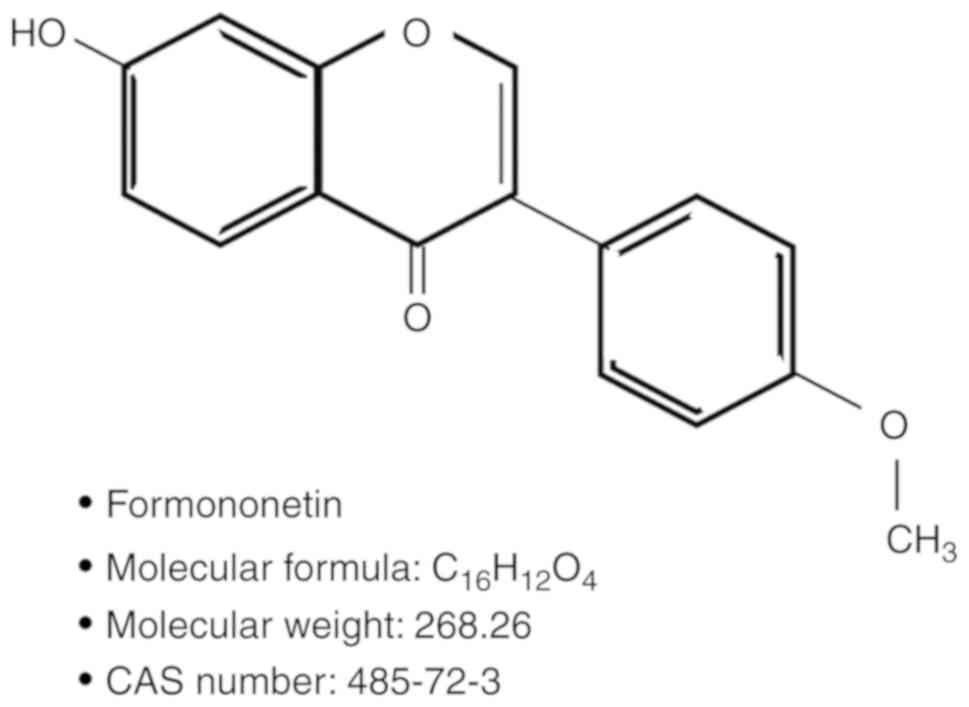
As shown in Fig. 1,
formononetin [C16H12O4, CAS no:
485-72-3, synonym:
7-Hydroxy-3-(4′-methoxyphenyl)-4H-benzopyran-4-one] is a
phytoestrogen and natural isoflavone isolated from Pongamia
pinnata (6), Astragalus
membranaceus (7), Ononis
angustissima (8) and
Trifolium pratense (T. pratense) (9). Recent studies investigating the
physiological roles of formononetin have reported a variety of
biological effects, including antioxidant activity (10), estrogenic effects (10), anti-inflammatory properties
(11,12), neuronal protection against
hypoxia-induced cytotoxicity (13)
and anti-neovascularization (14).
Moreover, the anticancer properties of formononetin have been
observed in several types of cancer cells, such as the human
urinary bladder cancer cell line T24 (15), the human non-small-cell lung cancer
cell lines A549 and NCI-H23 (16),
the human prostate cancer cell lines PC-3 and DU145 (17), the human breast cancer cell line
MDA-MB-231 (18), the human
cervical cancer cell line HeLa (19), and the human colorectal carcinoma
cell line HCT116 (20), and are
mediated through anti-angiogenic effects, cell cycle arrest,
inhibition of proliferation and induction of apoptosis.
Hence, the aim of the present study was to determine
the chemotherapeutic effects of formononetin and its potential
signaling pathways on HNSCC cells.
Materials and methods
Cell culture
L929 normal mouse fibroblasts and FaDu cells were
obtained from the American Type Culture Collection (ATCC).
According to the ATCC instructions, L929 cells were grown in
Eagle's minimum essential medium (Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.), and FaDu cells were grown in minimum essential
medium (Thermo Fisher Scientific, Inc.) supplemented with 10% FBS;
both were cultured in a humidified incubator at 37°C with 5%
CO2.
Cytotoxicity assessment
To determine the biological safety and cytotoxicity
of formononetin (SC-202614; Santa Cruz Biotechnology Inc.), L929
and FaDu cells (1×105 cells/ml) were cultured in 96-well
plates. Thereafter, the cells were treated with 0, 5, 10, 25 or 50
µM formononetin for 24 h at 37°C. Following incubation, the cells
were incubated for a further 4 h in 20 µl of 5 mg/ml MTT solution
(Thermo Fisher Scientific, Inc.). The supernatant was subsequently
removed, and 200 µl/well dimethyl sulfoxide (Sigma-Aldrich; Merck
KGaA) was added to dissolve the MTT crystals. The optical density
(OD) of each well was then measured at 570 nm, using an Epoch
microplate spectrophotometer (BioTek Instruments). The experiments
were independently repeated three times. The mean OD ± standard
deviation for each group of replicates was calculated, and the
entire procedure was then repeated three times.
Live/dead cell assay
The Live/Dead cell viability assay kit was obtained
(Thermo Fisher Scientific, Inc.), which uses green calcein AM to
stain the live cells (green fluorescence) and ethidium homodimer-1
to stain the dead cells (red fluorescence). L929 and FaDu cells
(1×105 cells/ml) were cultured in an 8-well chamber
slide (Nunc® Lab-Tek® Chamber Slide™ system;
Sigma-Aldrich; Merck KGaA), and allowed to attach to the bottom of
the chamber slide overnight. Thereafter, cultured cells were
treated with 0, 10 or 25 µM formononetin for 24 h at 37°C and
stained using the Live/Dead cell viability assay kit, according to
the manufacturer's instructions. Cells were imaged using a
fluorescence microscope (Eclipse TE2000; Nikon Instruments).
Hematoxylin and eosin (H&E)
staining
To investigate the morphological changes in FaDu
cells induced by formononetin treatment, H&E staining (3 min
for hematoxylin and 30 sec for eosin) was performed at room
temperature. Briefly, FaDu cells (1×105 cells/ml) were
cultured in an 8-well chamber slide (Nunc®
Lab-Tek® Chamber Slide™ system; Sigma-Aldrich; Merck
KGaA), and were allowed to adhere to the well overnight. Cultured
cells were then treated with 0, 10 or 25 µM formononetin for 24 h
at 37°C. Thereafter, the cells were rinsed three times with
phosphate-buffered saline (PBS) at 4°C and fixed with 4%
paraformaldehyde for 30 min at 4°C. H&E staining was performed
in order to observe morphological changes in the cells. The cells
were observed and imaged using a Leica DM750 light microscope
(Leica Microsystems).
Nuclear staining
To investigate nuclear condensation in FaDu cells
treated with formononetin, DAPI staining was performed. Briefly,
FaDu cells (1×105 cells/ml) were cultured in an 8-well
chamber slide (Nunc® Lab-Tek® Chamber Slide™
system; Sigma-Aldrich; Merck KGaA), and allowed to adhere to the
well overnight. Cultured cells were treated with 0, 10 or 25 µM
formononetin for 24 h at 37°C. Thereafter, the cells were rinsed
three times with PBS at 4°C and stained with 1 mg/ml DAPI
(Sigma-Aldrich; Merck KGaA) for 20 min. Nuclear condensation was
observed and imaged using a fluorescence microscope (Eclipse
TE2000; Nikon Instruments).
Chromosomal DNA fragmentation
assay
FaDu cells (1×105 cells/ml) were cultured
in 6-well plates and allowed to adhere overnight. Cultured FaDu
cells were treated with 0, 10 or 25 µM formononetin for 24 h at
37°C, and rinsed three times with PBS at 4°C. The cells were
treated with 100 µl cell lysis buffer [1% NP-40, 20 mM EDTA, 50 mM
Tris-HCl (pH 7.5 (all from Sigma-Aldrich; Merck KGaA)] and
incubated at 4°C for 10 min, then centrifuged at 12,000 × g at 4°C
for 30 min. RNase A (Bioneer) was added to the supernatant and
incubated at 37°C for 1 h. Subsequently, proteinase K (Bioneer) was
added to the supernatant and incubated at 37°C for 8 h. An equal
volume of isopropanol (Sigma-Aldrich; Merck KGaA) was then added,
and the lysates were incubated at −80°C for 24 h to precipitate
genomic DNA. Following centrifugation at 12,000 × g for 15 min at
4°C, the supernatant was removed, allowed to dry naturally, and
dissolved in Tris-EDTA buffer. Thereafter, the genomic DNA isolated
from each sample was electrophoresed on a 1.5% agarose gel
(Bioneer). A gel imaging system (Transilluminator, Bioneer) was
used for observation, and images were captured.
Caspase-3 and −7 activity assay
The activity of caspase-3 and −7 was assessed using
the cell-permeable fluorogenic substrate
PhiPhiLux-G1D2 (OncoImmunin Inc.) according
to the manufacturer's instructions, and was imaged using
fluorescence microscopy (Eclipse TE200; Nikon Instruments).
Fluorescence-activated cell sorting
(FACS)
FACS (BD Cell Quest® version 3.3) was
performed using Annexin V-FITC Early Apoptosis Detection Kit (Cell
Signaling Technology, Inc.) composed of Annexin V-FITC and
propidium iodide (PI) to investigate changes in the apoptotic
populations of FaDu cells treated with formononetin according to
manufacturer's instructions. FaDu cells (5×105 cells/ml)
were cultured on a 6-well plate for 24 h, and then treated with 0,
10 or 25 µM formononetin for a further 24 h. The cells were
collected, washed with ice-cold PBS and resuspended in 1X binding
buffer (BD Biosciences). Thereafter, 1 µl Annexin V-FITC and 12.5
µl PI were added to 96 µl cell suspension and incubated for 15 min
at 37°C. Changes in the apoptotic populations were analyzed using
BD Cell Quest® version 3.3 (Becton-Dickinson and
Company).
Western blot analysis
FaDu cells (5×105 cells/ml) were cultured
on a 6-well plate and then treated with 0, 10 or 25 µM formononetin
for a further 24 h. Thereafter, cell lysates were prepared using a
cell lysis buffer (Cell Signaling Technology, Inc.), according to
the manufacturer's instructions. The protein concentration of the
cell lysates was determined using a bicinchoninic acid protein
assay (Thermo Fisher Scientific, Inc.). An equal amount (30 µg) of
each cell lysate was denatured at 100°C for 10 min. Each cell
lysate was electrophoresed on 10% sodium dodecyl sulfate
polyacrylamide gel using mini-PROTEAN® Tetra Cell
(Bio-Rad Laboratories, Inc.) attached with PowerPac™ HC Power
Supply (Bio-Rad Laboratories, Inc.) and subsequently transferred to
polyvinylidene fluoride (PVDF) membrane (Immobilon-P PVDF membrane,
EMD Millipore) using mini Trans-Blot® Cell (Bio-Rad
Laboratories, Inc.) attached with PowerPac™ HC Power Supply
(Bio-Rad Laboratories, Inc.) at 4°C. Thereafter, the PVDF membranes
were blocked using 5% (v/v) bovine serum albumin (BSA;
Sigma-Aldrich; Merck KGaA) prepared in Tris-buffered saline with
0.05% Tween-20 (TBS-T; Santa Cruz Biotechnology Inc.) and then
incubated with the primary antibodies at 4°C overnight. The
following primary antibodies were purchased from Santa Cruz
Biotechnology, Inc. and were diluted at 1:1,000 in TBS-T containing
5% (v/v) BSA to perform western blotting: Antibodies against Fas
Ligand (FasL; sc-33716), B-cell lymphoma-2 (Bcl-2; sc-56015),
B-cell lymphoma-extra large (Bcl-xL; sc-136207) and Bax-like BH3
protein (Bid; sc-514622). In addition, the following primary
antibodies were purchased from Cell Signaling Technology, Inc. and
were diluted at 1:1,000 in TBS-T containing 5% (v/v) BSA to perform
western blotting: Antibodies against caspase-8 (cat. no. 9746),
caspase-3 (cat. no. 9662), poly(ADP-ribose) polymerase (PARP; cat.
no. 9532), Bcl-2-interacting killer (Bik; cat. no. 4592),
Bcl-2-like protein 11 (Bim, cat. no. 2933), Bcl-2-antagonist of
cell death (Bad; cat. no. 9292), Bcl-2-associated X protein (Bax;
cat. no. 2772), caspase-9 (cat. no. 9508), β-actin (cat. no. 4970),
phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2;
cat. no. 9101), total ERK1/2 (cat. no. 9102), p-p38 (cat. no.
4092), total p38 (cat. no. 9212), p-nuclear factor (NF)-κB (cat.
no. 3033), and total NF-κB (cat. no. 6956). Subsequently, they were
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibodies (1:2,000; Santa Cruz Biotechnology, Inc.) for 2 h at
room temperature. The immunoreactive bands were visualized using an
ECL system (GE Healthcare), exposed on radiographic film or
MicorChemi 4.2 (Dong-il SHIMADZU Corp.). Thereafter, the
densitometric analysis of western blots was performed using ImageJ
software 1.51j8 (W. Rasband, National Institutes of Health;
available at http://rsb.info.nih.gov/ij/), an open source image
processing program designed for scientific multidimensional images.
The densitometric analysis of each image was repeated 3 times for
statistical analysis.
In vivo study using an animal tumor
model generated by a FaDu cell xenograft
All animal studies were performed under the protocol
CIACUC2017-A0054 approved by the Institutional Animal Care and Use
Committee of Chosun University (Gwangju, Korea). A total of 10
BALB/c male nude mice, aged 5 weeks and weighing 25.15±1.3 g, were
purchased from Damul Science. All animals were housed and were
allowed to recover form shipping-associated stress for 1 week in a
specific pathogen-free experimental animal housing center
(temperature 25±10°C, relative humidity 60±10%, 12 h light/dark
cycle) with free access to autoclaved food and water. Following
adaptation, the body weight of the animals was measured using a lab
balance (Sartorius). Thereafter, FaDu cells (1×107
cells/100 µl PBS) were injected subcutaneously to generate a
xenograft tumor model (21,22). At 14 days after the injection, tumor
formation was detected under the skin of mice that had received
FaDu cell xenografts. Thereafter, the animals were divided into a
xenograft positive control group (n=5) and an experimental group
(n=5), in which the anticarcinogenic effect of formononetin was
tested. A total of 10 mg/kg formononetin dissolved in 5% ethanol
and 5% ethanol without formononetin were administered orally to the
experimental and control groups, respectively, every other day for
21 days (3 weeks). The volume of the xenograft tumors was measured
twice per week using the following formula (23): Volume (mm3) of xenograft
tumor = 1/2 × length (mm) × width (mm)2
Body weight was also recorded twice per week. At the
end of the study, all animals were anesthetized with 1.5%
isoflurane (Piramal Critical Care, Inc.), perfused with saline and
fixed in 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA). Finally,
the tumors were dissected surgically and embedded in paraffin for
immunohistochemical examination.
Immunohistochemistry
Dissected tumor tissues were fixed in 4%
paraformaldehyde (Sigma-Aldrich; Merck KGaA) at 4°C for 72 h, and
were then processed with 70, 95 and 100% ethanol (Sigma-Aldrich;
Merck KGaA), xylene (Sigma-Aldrich; Merck KGaA), and paraffin
(Paraplast plus®; Sigma-Aldrich; Merck KGaA).
Thereafter, tumor tissues were embedded in paraffin, prepared and
cut into 8 µm sections using a Leica® RM2235 manual
rotary microtome (Leica Biosystems Inc.), and mounted on glass
slides. The sections were deparaffinized using xylene, rehydrated
with two washes each of 100 and 95% ethanol and then rinsed with
tap water. The sections were incubated at 4°C with caspase-3
antibody overnight [cat. no. 9662, Cell Signaling Technology, Inc.;
dilution, 1:1,000 in 5 ml PBS-T (PBS with 0.05% Tween-20)
containing 250 µl normal goat serum (cat. no. 5425; Cell Signaling
Technology, Inc.)]. Thereafter, immunohistochemistry was performed
using the Vectastain® ABC kit (Vector Laboratories,
Inc.) according to the manufacturer's instructions. The sections
were subsequently transferred to mounting reagent, and examined
under a fluorescence microscope (Eclipse TE200; Nikon
Instruments).
Statistical analysis
The experimental data are presented as the mean ±
standard deviation and were compared using analysis of variance,
followed by post-hoc multiple comparison (Tukey's test) using SPSS
software version 25 (IBM Corp.) The Student's t-test was performed
to analyze the significance of differences between animal groups.
P<0.05 was considered to indicate statistically significant
differences. All the data, except the animal study, were obtained
from three independent experiments.
Results
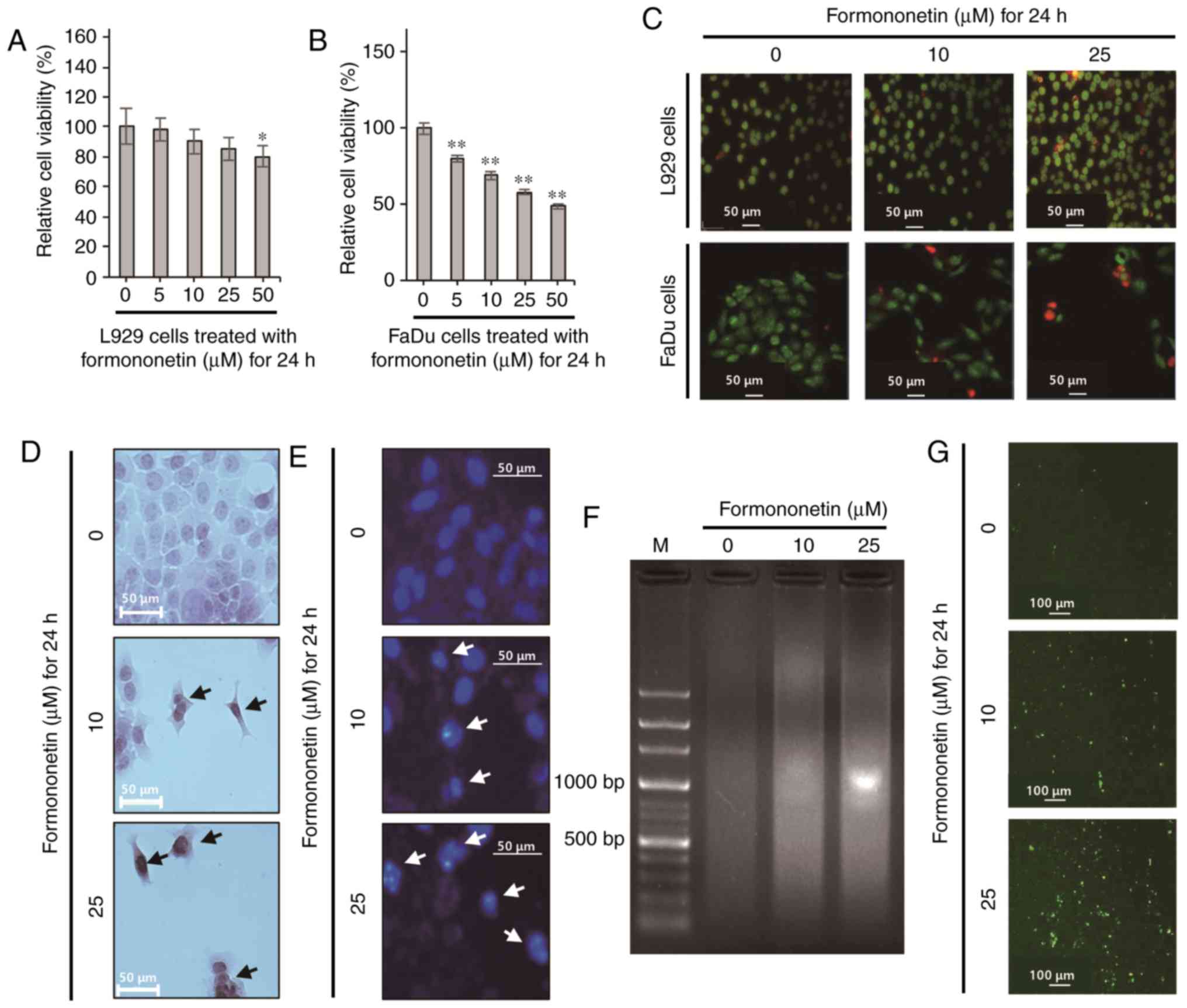
Formononetin decreases the viability
of FaDu cells without affecting the viability of L929 mouse
fibroblasts
L929 mouse fibroblasts, which were used as normal
cells, were cultured in 5–50 µM formononetin for 24 h. The MTT
assay was then performed to evaluate the cytotoxicity of
formononetin. As shown in Fig. 2A,
treatment with 5–25 µM formononetin did not significantly decrease
the viability of L929 mouse fibroblasts; however, treatment with 50
µM formononetin decreased the viability of L929 mouse fibroblasts
to 82±3% compared with the control (P<0.05). The relative cell
viability was found to be 79±2, 65±3, 58±3 and 46±2% in FaDu cells
treated with 5, 10, 25 and 50 µM formononetin, respectively (all
P<0.01; Fig. 2B). These data
demonstrate that formononetin induces FaDu cell death in a
dose-dependent manner, and the IC50 value of
formononetin was ~39.7 µM in FaDu cells. To confirm the viability
of L929 mouse fibroblasts and FaDu cells treated with 10 or 25 µM
formononetin for 24 h, a Live/Dead cell assay was performed, using
green calcein AM and ethidium homodimer-1 to stain live and dead
cells, respectively. As shown in Fig.
2C, almost all L929 cells treated with formononetin emitted
green fluorescence following staining with green calcein AM, which
stains live cells. However, FaDu cells exposed to formononetin
emitted red fluorescence in a dose-dependent manner following
staining with ethidium homodimer-1, which stains dead cells. Taken
together, these findings indicate that formononetin at 10 and 25 µM
induces FaDu cell death without affecting the viability of normal
fibroblasts (Fig. 2A-C). Hence, 10
and 25 µM formononetin was used to treat FaDu cells in the present
study.
Formononetin induces apoptotic death
in FaDu cells
To elucidate the cellular mechanism through which
formononetin induces FaDu cell death, the formation of apoptotic
bodies and morphological changes in FaDu cells treated with
formononetin were observed following H&E staining. As shown in
Fig. 2D, the number of FaDu cells
was progressively reduced with increasing concentrations of
formononetin. Furthermore, morphological changes similar to
apoptotic body formation, including outward membrane bulges and
shrinkage, were observed in FaDu cells treated with formononetin.
Therefore, to determine whether formononetin-induced FaDu cell
death is due to apoptosis, DAPI staining was performed to detect
nuclear condensation, a typical feature of apoptosis. As shown in
Fig. 2E, the number of FaDu cells
with condensed nuclei increased upon exposure to formononetin in a
dose-dependent manner. Moreover, genomic DNA fragmentation, another
characteristic of apoptosis, was increased in FaDu cells treated
with formononetin, as shown in Fig.
2F (arrows). As shown in Fig.
2G, the number of FaDu cells emitting green fluorescence due to
the cleavage of the cell permeable fluorogenic substrate
PhiPhiLux-G1D2 by cleaved caspase-3 increased
significantly in a dose dependent manner in the presence of
formononetin.
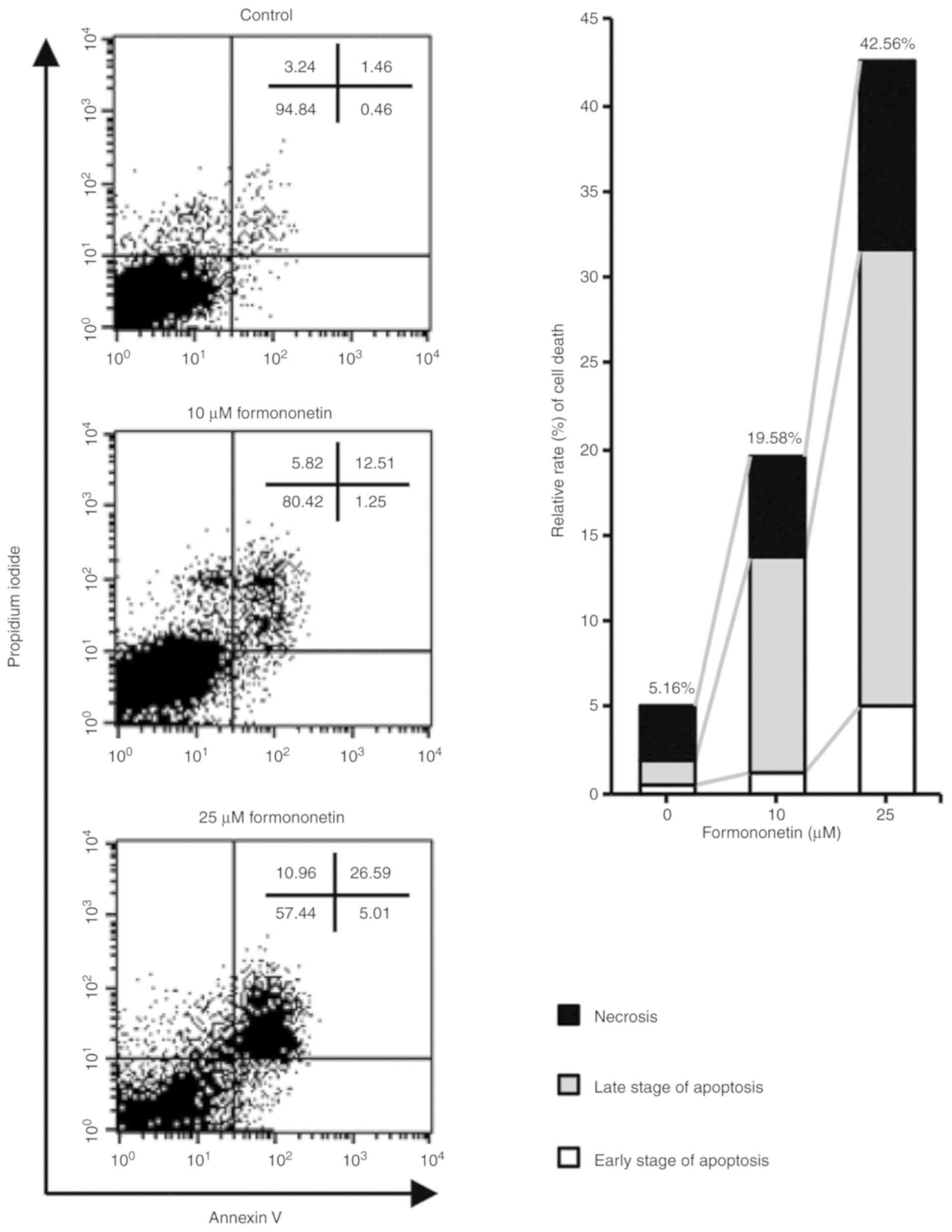
FACS analysis using Annexin V and PI staining was
subsequently performed to confirm formononetin-induced apoptosis of
FaDu cells. As shown in Fig. 3, the
relative rates of cell death were 19.58 and 42.56% in FaDu cells
treated with 10 and 25 µM formononetin for 24 h, respectively.
Furthermore, the early- and late-stage apoptotic cell populations
were 13.76 and 31.6% in FaDu cells treated with 10 and 25 µM
formononetin for 24 h, respectively. Therefore, the FACS analysis
indicated that formononetin induces cell death by increasing the
population of apoptotic FaDu cells. These data strongly suggest
that formononetin induces apoptotic cell death in FaDu cells in a
dose-dependent manner.
Formononetin-induced FaDu cell death
involves death receptor-mediated extrinsic and
mitochondria-dependent intrinsic apoptosis
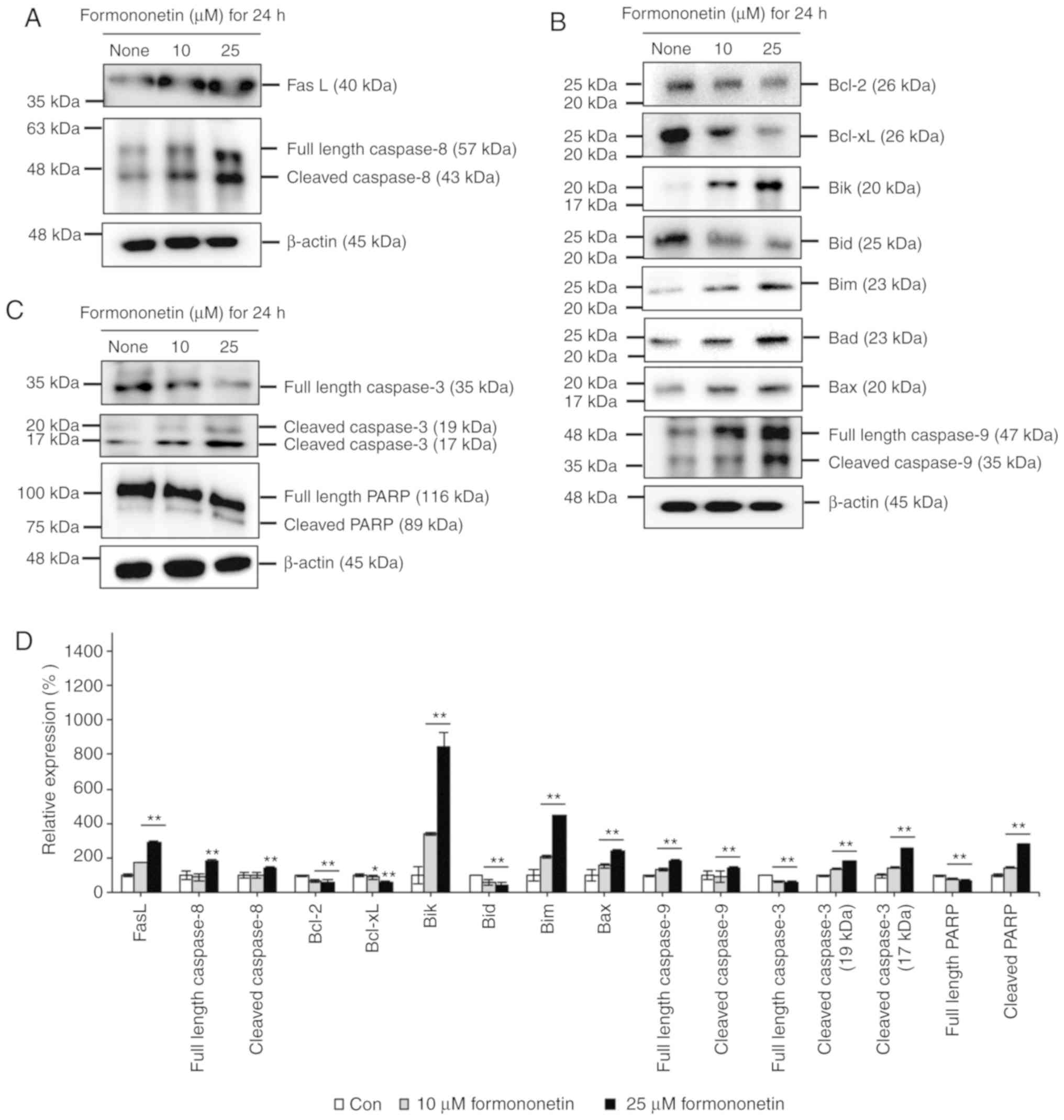
To investigate formononetin-induced FaDu cell death,
western blot analyses were performed using antibodies associated
with death receptor-mediated extrinsic and mitochondria
dependent-intrinsic apoptosis, as shown in Fig. 4. The level of FasL significantly
increased in FaDu cells treated with formononetin, as shown in
Fig. 4A. Additionally, cleaved
caspase-8 levels increased in a dose-dependent manner in FaDu cells
treated with 10 and 25 µM formononetin. These data indicate that
formononetin-induced apoptosis is coordinated by an extrinsic death
receptor-mediated apoptotic pathway through FasL and caspase-8 in
FaDu cells.
Furthermore, the expression of anti-apoptotic
factors, such as Bcl-2 and Bcl-xL, was found to be decreased in
FaDu cells upon treatment with 10 and 25 µM formononetin in a
dose-dependent manner. In addition, the expression of Bid, which is
a precursor of truncated Bid (tBid) acting as a pro-apoptotic
factor, decreased dose-dependently by formononetin in FaDu cells.
Conversely, the expression of Bik, Bim, Bax and cleaved caspase-9,
which are pro-apoptotic factors associated with the
mitochondria-dependent intrinsic apoptosis pathway, increased in
FaDu cells treated with formononetin, as shown in Fig. 4B. These data indicate that
formononetin-induced FaDu cell apoptosis is mediated by the
mitochondria-dependent intrinsic apoptosis pathway, involving
downregulation of anti-apoptotic factor expression, upregulation of
pro-apoptotic factor expression, and the activation of caspase-9.
As shown in Fig. 4C, the levels of
cleaved caspase-3 increased in FaDu cells treated with 10 and 25 µM
formononetin due to the cleavage of pro-caspase-3, a downstream
substrate of cleaved caspase-8 and cleaved caspase-9. Additionally,
the levels of cleaved PARP increased due to the cleavage of
pro-PARP, a downstream substrate of cleaved caspase-3. These
findings indicate that formononetin-induced FaDu cell death is
coordinated by death receptor-mediated extrinsic and
mitochondria-dependent intrinsic apoptosis through activation of
the caspase cascade in FaDu cells.
The chemotherapeutic effects of
formononetin are mediated by the suppression of mitogen-activated
protein kinases (MAPKs) in FaDu cells
To investigate the signal transduction pathways
associated with formononetin-induced apoptosis in FaDu cells,
western blotting was performed, using antibodies specific to MAPKs,
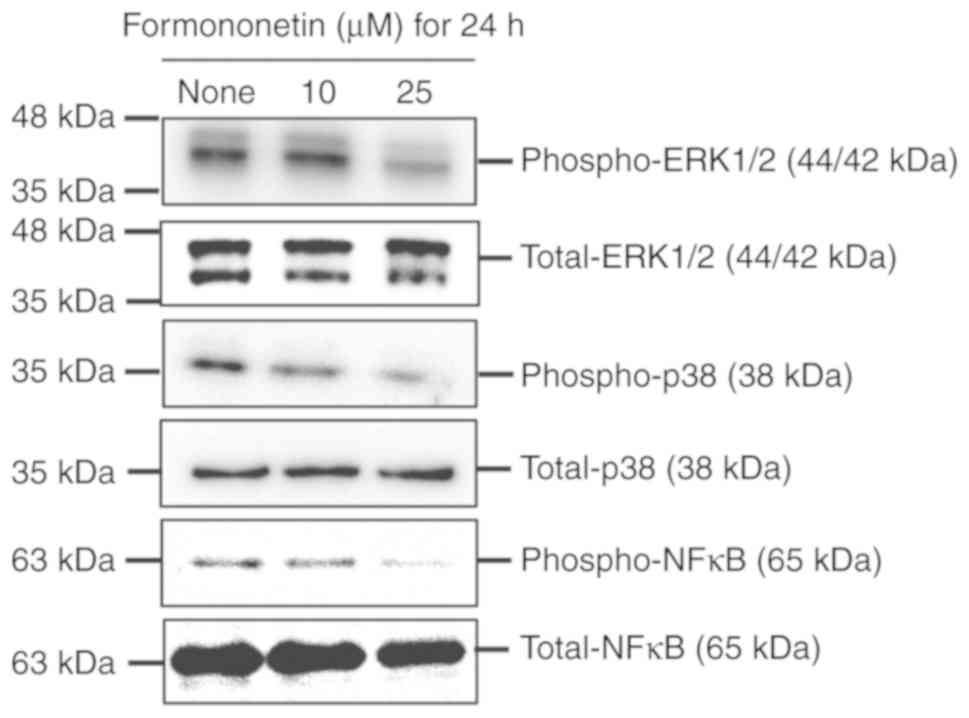
such as ERK1/2 and p38, and NF-κB, as shown in Fig. 5. Formononetin was found to suppress
the phosphorylation of ERK1/2, p38 and NF-κB in FaDu cells in a
dose-dependent manner. These findings indicate that
formononetin-induced FaDu cell apoptosis is mediated by the
suppression of the ERK1/2, p38 and NF-κB phosphorylation.
Formononetin suppresses tumor
formation in a FaDu cell xenograft animal model
To assess the effects of formononetin on FaDu tumor
growth in vivo, FaDu cells were xenografted into
experimental mice and the resulting tumor sizes and body weights
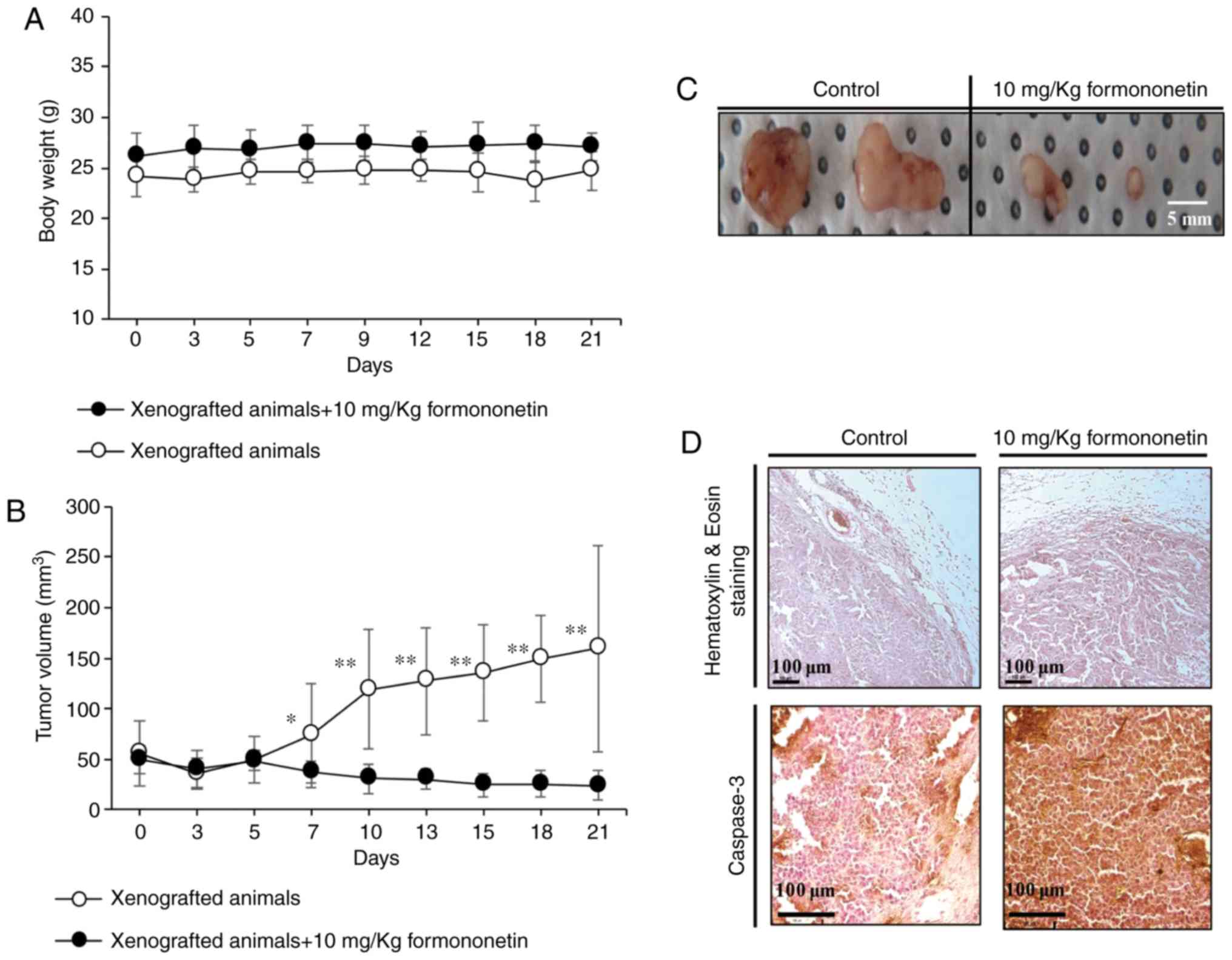
were measured weekly for up to 3 weeks. As shown in Fig. 6A, the body weight of the xenograft
group administered 10 mg/kg formononetin was 27.05±1.35 g, which
was ~9.5% higher compared with that of the control group (24.7±1.98
g) at 21 days. There was no obvious loss of body weight in
xenograft animals over the 3 weeks, indicating that formononetin
was well-tolerated. Tumor volume and weight were significantly
lower in mice receiving formononetin compared with those in the
control group, as shown in Fig. 6B and
C. Furthermore, immunohistochemical analysis of the tumors
demonstrated that the expression of cleaved caspase-3 was markedly
increased in formononetin-treated tumors compared with the controls
(Fig. 6D). Taken together, these
data indicate that formononetin suppressed tumor formation through
apoptotic cell death in a FaDu xenograft animal model.
Discussion
HNSCC forms on the mucosal surfaces of the upper
aerodigestive tract, including the oral cavity, pharynx, larynx and
paranasal sinuses, and is generally treated by surgery, alone or in
combination with radiotherapy and chemotherapy, depending on tumor
stage and location (21,22). However, these HNSCC treatments
frequently compromise the quality of life of the patients due to
their side effects, such as masticatory dysfunction and
psychological problems caused by altered orofacial appearance
(22). Therefore, the development
of biologically safe chemotherapeutic agents, which display
outstanding anticancer efficacy by focusing on cancer cell-specific
apoptosis and reducing side effects, is of great importance. Recent
studies associated with the development of chemotherapeutic agents
have focused on the anticancer properties of natural compounds
isolated from herbal plants used in folk medicine or biologically
proven natural materials.
Phytoestrogens, which are natural compounds similar
to 17β-estradiol, are present in various edible fruits, vegetables,
and some herbal plants (23,24).
Phytoestrogens have been found to mediate physiological and
pathological responses associated with reproduction, bone
remodeling, cardiovascular function, immune system activity, and
several other metabolic diseases, through interaction with the
estrogen receptor (23,24). In addition, it was recently reported
that phytoestrogens play a role in the prevention and treatment of
various cancers, including liver, lung, colon, breast, prostate and
oral cancers (25). In our previous
study, we reported that biochanin-A, a phytoestrogen derived from
T. pretense (known as red clover in traditional medicine)
induced the apoptosis of FaDu cells via the death
receptor-dependent extrinsic and mitochondria dependent-intrinsic
apoptosis pathways (26). We herein
demonstrated that formononetin, a phytoestrogen isolated from
herbal plants, induced FaDu cell death via death receptor-mediated
extrinsic and mitochondria-dependent intrinsic apoptosis.
In the present study, formononetin did not affect
the viability of L929 fibroblasts used as normal cells. Huh et
al reported that the proliferation of human umbilical vein
endothelial cells was accelerated when treated with 1–100 µM
formononetin (27); however, this
did not affect the viability of normal subchondral osteoblasts
(28). Hence, these results
indicate that the 5–25 µM formononetin dose range used in the
present study may be biologically safe for normal cells, whereas
the viability of FaDu cells treated with 5–50 µM formononetin
decreased in a dose-dependent manner. In agreement with our
results, formononetin was also found to decrease the viability of
human osteosarcoma U2OS cells (29), human non-small-cell lung cancer A549
and NCL-H23 cells (16), human
prostate cancer DU-145 cells (30),
human cervical cancer HeLa cells (19), and human breast cancer MCF-7 cells
(31), in a time- and
dose-dependent manner. Although these studies have consistently
demonstrated that formononetin is toxic to various cancer cells, to
the best of our knowledge, the present study was the first to
identify that formononetin induces cell death in human HNSCC.
Furthermore, the present study determined the concentration of
formononetin that increased FaDu cell death without affecting
normal fibroblasts.
Apoptosis, also referred to as programmed cell
death, is mediated by biochemical alterations that include cell
shrinkage (32), nuclear
condensation (33), chromosomal DNA
fragmentation (34), and activation
of the caspase cascade (35). The
results of the present study indicated that formononetin-induced
FaDu cell death was mediated by apoptosis. Moreover, FACS analysis
with Annexin V and PI staining revealed that cell populations in
both the early and late stages of apoptosis gradually increased.
These data strongly indicate that formononetin induces apoptosis in
FaDu cells.
Targeting the regulation of cellular mechanisms to
accelerate cell death is a highly effective strategy for cancer
therapy. Apoptosis is a particularly important mechanism associated
with the elimination of unwanted cells during development to
maintain homeostasis in long-lived mammals (36). Therefore, a number of recent studies
associated with the development of chemotherapeutic agents have
focused on inducing cancer cell-specific apoptosis by modulating
apoptotic signaling pathways.
Apoptosis is generally activated by either the death
receptor-mediated extrinsic or mitochondria dependent-intrinsic
pathways, and is highly regulated by the activation (cleavage) of
caspases (cysteine aspartyl-specific proteases) to induce cell
death (36). The death
receptor-mediated extrinsic apoptosis pathway is triggered by the
recruitment of adaptor proteins, such as Fas-associated death
domain (FADD) and tumor necrosis factor (TNF) receptor-associated
death domain (TRADD), through the binding of death ligands [e.g.
FasL, TNF-related apoptosis-inducing ligand (TRAIL) and TNF] to TNF
family death receptors (37–39).
Subsequently, adaptor proteins induce the activation of caspase-8
through the cleavage of pro-caspase-8, then activated caspase-8
induces the activation of executioner caspase-3, which leads to
cell death (38). In the present
study, formononetin increased the expression of FasL and the
activation of caspase-8 in FaDu cells. Consequently, the activation
of caspase-3, a downstream target molecule of caspase-8 and
caspase-9, and its downstream pro-apoptotic substrate PARP, was
also increased in FaDu cells treated with formononetin. These
results indicate that formononetin-induced apoptosis is coordinated
by the death receptor-mediated extrinsic apoptosis pathway, through
the upregulated expression of the death ligand FasL in FaDu
cells.
Generally, the mitochondria-dependent intrinsic
apoptosis pathway is initiated by the cleavage of Bid to tBid in
response to apoptotic stress, such as DNA injury, upregulation of
oncogenes, growth factor deprivation, increased Ca2+
levels, DNA-damaging molecules, oxidants and microtubule-targeting
drugs (40). In addition, the
activation of caspase-8, which is involved in the death
receptor-mediated extrinsic apoptosis pathway, can initiate the
mitochondria-dependent intrinsic apoptosis pathway by cleaving Bid
to tBid (41). The oligomerization
of activated Bax and Bcl-2-antagonist/killer (Bak) lead to the
release of intermembrane cytochrome c by inducing
mitochondrial outer membrane permeabilization (42). Although anti-apoptotic factors, such
as Bcl-2 and Bcl-xL, regulate the oligomerization of activated Bax
and Bak, the anti-apoptotic activity of these factors is regulated
by pro-apoptotic factors, such as Bik, Bim and Bad (43,44).
In the present study, the activation of caspase-8 decreased Bid
levels by cleaving Bid to tBid in FaDu cells treated with
formononetin. In addition, formononetin reduced anti-apoptotic
activity by downregulating the expression of anti-apoptotic
factors, such as Bcl-2 and Bcl-xL, in FaDu cells. Moreover,
formononetin increased the expression of mitochondria-dependent
pro-apoptotic factors, such as Bik and Bim. Alterations in the
levels of these anti- and pro-apoptotic factors associated with the
mitochondria-dependent apoptosis pathway subsequently induced the
activation cascade of caspase-9, caspase-3 and PARP in FaDu cells
treated with formononetin. Taken together, these findings indicate
that formononetin-induced apoptosis is mediated by
mitochondria-dependent intrinsic apoptotic pathways through the
activation of caspase-8, which is triggered by upregulated FasL
expression in FaDu cells. In agreement with the findings of the
present study, formononetin has also been found to induce
mitochondria-dependent apoptotic pathways by downregulating Bcl-2
and upregulating Bax in U2OS human osteosarcoma cells, A549 human
non-small-cell lung cancer cells, and DU-145 and PC-3 human
prostate cancer cells (16,29,30,45).
Generally, MAPKs are closely associated with tumor
cell proliferation, differentiation, apoptosis, angiogenesis,
invasion and metastasis (46). In
the present study, the phosphorylation of ERK1/2 and p38 was
suppressed in a dose-dependent manner in FaDu cells treated with
formononetin. Recent studies have reported that the MAPK signaling
pathway may directly or indirectly suppress the activation of
caspase-3 and caspase-9 by inhibiting the release of cytochrome
c from mitochondria (46–49).
NF-κB is a cell signaling molecule that regulates the expression of
several genes associated with immune responses, cell adhesion, cell
differentiation, cell proliferation, angiogenesis and apoptosis
(50). However, NF-κB activation is
suppressed by the binding of the NF-κB inhibitor, IκB, which is
activated by Akt phosphorylation (51). Recently, Alam et al reported
that the expression of the anti-apoptotic factor Bcl-2 was
positively correlated with NF-κB activity and closely associated
with the progression and resistance to treatment of oral squamous
carcinoma (52). Therefore, as MAPK
and NF-κB signaling is frequently activated in cancer cells, the
inactivation of this signaling pathway may contribute to the
anticarcinogenic effects.
In the present study, orally administered
formononetin effectively suppressed tumor growth compared with the
control group. Consistently with our results, formononetin-induced
antitumor effects, including apoptosis and the suppression of tumor
growth, have been reported in several types of cancer cells, such
as human breast cancer (53),
osteosarcoma (29), cervical cancer
(19) and colon cancer (20) cell lines. Moreover, Tyagi et
al reported that the oral administration of resveratrol, a
representative phytoestrogen derived from red wine, induced the
apoptosis of FaDu cells through the upregulation of cleaved
caspase-3 in xenograft animal models (54). Similarly, the histological results
of the present study demonstrated that the expression of cleaved
caspase-3 was significantly upregulated in the tumor tissues
dissected from xenograft animals receiving formononetin compared
with those in the control group. These data indicate that
formononetin induces the expression of cleaved caspase-3 through
both the death receptor-mediated extrinsic and
mitochondria-dependent intrinsic apoptotic pathways.
Recent studies reported that formononetin exerts
additional chemotherapeutic effects, such as inhibition of cancer
cell proliferation (15) and
angiogenesis (53), and suppression
of invasion and migration in various types of tumors (18). However, the present study only
focused on formononetin-induced apoptotic cell death and its
cellular signaling mechanism. Other formononetin-induced antitumor
effects, including the inhibition of proliferation, angiogenesis,
invasion and migration, will be investigated in further in
vitro and in vivo studies.
In conclusion, formononetin, a phytoestrogen derived
from T. pratense, effectively promotes cell death via death
receptor-mediated extrinsic and mitochondria-dependent intrinsic
apoptosis in FaDu cells. These findings suggest that formononetin
may be a promising chemotherapeutic candidate for the treatment of
HNSCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by a research fund
from Chosun University Dental Hospital, 2019.
Availability of data and materials
The analyzed datasets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JSO, THK, JHP, HL, IAC and JSK substantially
contributed to the conception and design of the manuscript, drafted
the article and revised it for important intellectual content. JSO,
JSY, GJL, YSS, DKK, CSK, SKY, HJK, SGK and JSK acquired, analyzed
and interpreted the data. All authors have read and approved the
final version of the manuscript and agree to be accountable for all
aspects of the research, ensuring that the accuracy or integrity of
any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
All animal studies were performed according to the
protocol (CIACUC2017-A0054) approved by the Institutional Animal
Care and Use Committee of Chosun University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
T. pratense
|
Trifolium pratense
|
|
ATCC
|
American Type Culture Collection
|
|
FBS
|
fetal bovine serum
|
|
OD
|
optical density
|
|
H&E
|
hematoxylin and eosin
|
|
PBS
|
phosphate-buffered saline
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
dihydrochloride
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
PI
|
propidium iodide
|
|
SDS-PAGE
|
sodium dodecyl sulfate polyacrylamide
gel electrophoresis
|
|
FasL
|
Fas ligand
|
|
Bid
|
Bax-like BH3 protein
|
|
tBid
|
truncated Bid
|
|
Bcl-2
|
B-cell lymphoma-2
|
|
Bcl-xL
|
B-cell lympoma-extra large
|
|
Bik
|
Bcl-2 interacting killer
|
|
Bim
|
Bcl-2-like protein 11
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
NF-κB
|
nuclear factor-κB
|
|
MAPK
|
mitogen-activated protein kinase
|
|
FADD
|
Fas-associated death domain
|
|
TNF
|
tumor necrosis factor
|
|
TRADD
|
TNF-receptor-associated death
domain
|
|
TRAIL
|
TNF-related apoptosis-inducing
ligand
|
|
Bax
|
Bcl-2-associated X protein
|
|
Bak
|
Bcl-2-antagonist/killer
|
References
|
1
|
Qi X, Jia B, Zhao X and Yu D: Advances in
T-cell checkpoint immunotherapy for head and neck squamous cell
carcinoma. Onco Targets Ther. 10:5745–5754. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bonomo P, Loi M, Desideri I, Olmetto E,
Delli Paoli C, Terziani F, Greto D, Mangoni M, Scoccianti S,
Simontacchi G, et al: Incidence of skin toxicity in squamous cell
carcinoma of the head and neck treated with radiotherapy and
cetuximab: A systematic review. Crit Rev Oncol Hematol. 120:98–110.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Belcher R, Hayes K, Fedewa S and Chen AY:
Current treatment of head and neck squamous cell cancer. J Surg
Oncol. 110:551–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kundu SK and Nestor M: Targeted therapy in
head and neck cancer. Tumour Biol. 33:707–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shrotriya S, Agarwal R and Sclafani RA: A
perspective on chemoprevention by resveratrol in head and neck
squamous cell carcinoma. Adv Exp Med Biol. 815:333–348. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Jiang Z, Li X, Hou Y, Liu F, Li N,
Liu X and Yang L: Natural therapeutic agents for neurodegenerative
diseases from a traditional herbal medicine Pongamia pinnata
(L.) Pierre. Bioorg Med Chem Lett. 25:53–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li W, Sun YN, Yan XT, Yang SY, Kim S, Lee
YM, Koh YS and Kim YH: Flavonoids from Astragalus
membranaceus and their inhibitory effects on LPS-stimulated
pro-inflammatory cytokine production in bone marrow-derived
dendritic cells. Arch Pharm Res. 37:186–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghribi L, Waffo-Teguo P, Cluzet S, Marchal
A, Marques J, Mérillon JM and Ben Jannet H: Isolation and structure
elucidation of bioactive compounds from the roots of the Tunisian
Ononis angustissima L. Bioorg Med Chem Lett. 25:3825–3830.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tava A, Pecio L, Stochmal A and Pecetti L:
Clovamide and flavonoids from leaves of Trifolium pratense
and T. pratense subsp. Nivale grown in Italy. Nat Prod
Commun. 10:933–936. 2015.PubMed/NCBI
|
|
10
|
Mu H, Bai YH, Wang ST, Zhu ZM and Zhang
YW: Research on antioxidant effects and estrogenic effect of
formononetin from Trifolium pratense (red clover).
Phytomedicine. 16:314–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu N and An J: Formononetin ameliorates
mast cell-mediated allergic inflammation via inhibition of
histamine release and production of pro-inflammatory cytokines. Exp
Ther Med. 14:6201–6206. 2017.PubMed/NCBI
|
|
12
|
Ma Z, Ji W, Fu Q and Ma S: Formononetin
inhibited the inflammation of LPS-induced acute lung injury in mice
associated with induction of PPAR gamma expression. Inflammation.
36:1560–1566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun M, Zhou T, Zhou L, Chen Q, Yu Y, Yang
H, Zhong K, Zhang X, Xu F, Cai S, et al: Formononetin protects
neurons against hypoxia-induced cytotoxicity through upregulation
of ADAM10 and sAβPPα. J Alzheimers Dis. 28:795–808. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu J, Ke X, Ma N, Wang W, Fu W, Zhang H,
Zhao M, Gao X, Hao X and Zhang Z: Formononetin, an active compound
of Astragalus membranaceus (Fisch) Bunge, inhibits
hypoxia-induced retinal neovascularization via the HIF-1α/VEGF
signaling pathway. Drug Des Devel Ther. 10:3071–3081. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu Y, Zhang X, Li Z, Yan H, Qin J and Li
T: Formononetin inhibits human bladder cancer cell proliferation
and invasiveness via regulation of MiR-21 and PTEN. Food Funct.
8:1061–1066. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y, Zhao Y, Ai X, Cheng B and Lu S:
Formononetin suppresses the proliferation of human non-small cell
lung cancer through induction of cell cycle arrest and apoptosis.
Int J Clin Exp Pathol. 7:8453–8461. 2014.PubMed/NCBI
|
|
17
|
Li T, Zhao X, Mo Z, Huang W, Yan H, Ling Z
and Ye Y: Formononetin promotes cell cycle arrest via
downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer
cells. Cell Physiol Biochem. 34:1351–1358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou R, Xu L, Ye M, Liao M, Du H and Chen
H: Formononetin inhibits migration and invasion of MDA-MB-231 and
4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through
PI3K/AKT signaling pathways. Horm Metab Res. 46:753–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin YM, Xu TM, Zhao YH, Wang YC and Cui
MH: In vitro and in vivo anti-cancer activity of formononetin on
human cervical cancer cell line HeLa. Tumour Biol. 35:2279–2284.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Auyeung KK, Law PC and Ko JK: Novel
anti-angiogenic effects of formononetin in human colon cancer cells
and tumor xenograft. Oncol Rep. 28:2188–2194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lydiatt WM, Patel SG, O'Sullivan B,
Brandwein MS, Ridge JA, Migliacci JC, Loomis AM and Shah JP: Head
and Neck cancers-major changes in the American Joint Committee on
cancer eighth edition cancer staging manual. CA Cancer J Clin.
67:122–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martino R and Ringash J: Evaluation of
quality of life and organ function in head and neck squamous cell
carcinoma. Hematol Oncol Clin North Am. 22:1239–1256. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soni M, Rahardjo TB, Soekardi R,
Sulistyowati Y, Lestariningsih, Yesufu-Udechuku A, Irsan A and
Hogervorst E: Phytoestrogens and cognitive function: A review.
Maturitas. 77:209–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sirotkin AV and Harrath AH: Phytoestrogens
and their effects. Eur J Pharmacol. 741:230–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qadir MI and Cheema BN: Phytoestrogens and
related food components in the prevention of cancer. Crit Rev
Eukaryot Gene Expr. 27:99–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cho IA, You SJ, Kang KR, Kim SG, Oh JS,
You JS, Lee GJ, Seo YS, Kim DK, Kim CS, et al: Biochanin-A induces
apoptosis and suppresses migration in FaDu human pharynx squamous
carcinoma cells. Oncol Rep. 38:2985–2992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huh JE, Nam DW, Baek YH, Kang JW, Park DS,
Choi DY and Lee JD: Formononetin accelerates wound repair by the
regulation of early growth response factor-1 transcription factor
through the phosphorylation of the ERK and p38 MAPK pathways. Int
Immunopharmacol. 11:46–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huh JE, Seo DM, Baek YH, Choi DY, Park DS
and Lee JD: Biphasic positive effect of formononetin on metabolic
activity of human normal and osteoarthritic subchondral
osteoblasts. Int Immunopharmacol. 10:500–507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu W and Xiao Z: Formononetin induces
apoptosis of human osteosarcoma cell line U2OS by regulating the
expression of Bcl-2, Bax and MiR-375 in vitro and in vivo. Cell
Physiol Biochem. 37:933–939. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu XJ, Li YQ, Chen QY, Xiao SJ and Zeng
SE: Up-regulating of RASD1 and apoptosis of DU-145 human prostate
cancer cells induced by formononetin in vitro. Asian Pac J Cancer
Prev. 15:2835–2839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen J, Zhao X, Ye Y, Wang Y and Tian J:
Estrogen receptor beta-mediated proliferative inhibition and
apoptosis in human breast cancer by calycosin and formononetin.
Cell Physiol Biochem. 32:1790–1797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bortner CD and Cidlowski JA: Cell
shrinkage and monovalent cation fluxes: Role in apoptosis. Arch
Biochem Biophys. 462:176–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kass GE, Eriksson JE, Weis M, Orrenius S
and Chow SC: Chromatin condensation during apoptosis requires ATP.
Biochem J. 318:749–752. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Higuchi Y: Chromosomal DNA fragmentation
in apoptosis and necrosis induced by oxidative stress. Biochem
Pharmacol. 66:1527–1535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chai F, Truong-Tran AQ, Ho LH and Zalewski
PD: Regulation of caspase activation and apoptosis by cellular zinc
fluxes and zinc deprivation: A review. Immunol Cell Biol.
77:272–278. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pfeffer CM and Singh ATK: Apoptosis: A
target for anticancer therapy. Int J Mol Sci. 19:4482018.
View Article : Google Scholar :
|
|
37
|
Zaman S, Wang R and Gandhi V: Targeting
the apoptosis pathway in hematologic malignancies. Leuk Lymphoma.
55:1980–1992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu H, Su D, Zhang J, Ge S, Li Y, Wang F,
Gravel M, Roulston A, Song Q, Xu W, et al: Improvement of
pharmacokinetic profile of TRAIL via trimer-tag enhances its
antitumor activity in vivo. Sci Rep. 7:89532017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Green DR and Llambi F: Cell death
signaling. Cold Spring Harb Perspect Biol. 1:a0060802015.
View Article : Google Scholar
|
|
42
|
Suhaili SH, Karimian H, Stellato M, Lee TH
and Aguilar MI: Mitochondrial outer membrane permeabilization: A
focus on the role of mitochondrial membrane structural
organization. Biophys Rev. 9:443–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Czabotar PE, Colman PM and Huang DC: Bax
activation by Bim? Cell Death Differ. 16:1187–1191. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gogada R, Yadav N, Liu J, Tang S, Zhang D,
Schneider A, Seshadri A, Sun L, Aldaz CM, Tang DG and Chandra D:
Bim, a proapoptotic protein, up-regulated via transcription factor
E2F1-dependent mechanism, functions as a prosurvival molecule in
cancer. J Biol Chem. 288:368–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang X, Bi L, Ye Y and Chen J:
Formononetin induces apoptosis in PC-3 prostate cancer cells
through enhancing the Bax/Bcl-2 ratios and regulating the p38/Akt
pathway. Nutr Cancer. 66:656–661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peng Q, Deng Z, Pan H, Gu L, Liu O and
Tang Z: Mitogen-activated protein kinase signaling pathway in oral
cancer. Oncol Lett. 15:1379–1388. 2018.PubMed/NCBI
|
|
47
|
Huang RH, Quan YJ, Chen JH, Wang TF, Xu M,
Ye M, Yuan H, Zhang CJ, Liu XJ and Min ZJ: Osteopontin promotes
cell migration and invasion, and inhibits apoptosis and autophagy
in colorectal cancer by activating the p38 MAPK signaling pathway.
Cell Physiol Biochem. 41:1851–1864. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lv D, Wu H, Xing R, Shu F, Lei B, Lei C,
Zhou X, Wan B, Yang Y, Zhong L, et al: HnRNP-L mediates bladder
cancer progression by inhibiting apoptotic signaling and enhancing
MAPK signaling pathways. Oncotarget. 8:13586–13599. 2017.PubMed/NCBI
|
|
49
|
Chen H, Jin ZL and Xu H: MEK/ERK signaling
pathway in apoptosis of SW620 cell line and inhibition effect of
resveratrol. Asian Pac J Trop Med. 9:49–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sun XF and Zhang H: NFKB and NFKBI
polymorphisms in relation to susceptibility of tumour and other
diseases. Histol Histopathol. 22:1387–1398. 2007.PubMed/NCBI
|
|
51
|
Yap TA, Garrett MD, Walton MI, Raynaud F,
de Bono JS and Workman P: Targeting the PI3K-AKT-mTOR pathway:
Progress, pitfalls, and promises. Curr Opin Pharmacol. 8:393–412.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Alam M, Kashyap T, Pramanik KK, Singh AK,
Nagini S and Mishra R: The elevated activation of NFkB and AP-1 is
correlated with differential regulation of Bcl-2 and associated
with oral squamous cell carcinoma progression and resistance. Clin
Oral Investig. 21:2721–2731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu XY, Xu H, Wu ZF, Chen C, Liu JY, Wu GN,
Yao XQ, Liu FK, Li G and Shen L: Formononetin, a novel FGFR2
inhibitor, potently inhibits angiogenesis and tumor growth in
preclinical models. Oncotarget. 6:44563–44578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tyagi A, Gu M, Takahata T, Frederick B,
Agarwal C, Siriwardana S, Agarwal R and Sclafani RA: Resveratrol
selectively induces DNA damage, independent of Smad4 expression, in
its efficacy against human head and neck squamous cell carcinoma.
Clin Cancer Res. 17:5402–5411. 2011. View Article : Google Scholar : PubMed/NCBI
|