Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer and the second leading cause of cancer-related
deaths worldwide (1). CRC has
become a critical health problem with an increase in its incidence
and mortality over the past decade (2). Despite advances in surgical resection
combined with chemotherapy and radiotherapy, to the best of our
knowledge, progress in treatment of CRC remains sluggish in recent
years (3). Therefore, numerous
studies have attempted to illuminate the underlying mechanisms on
the development of CRC and develop novel methods to improve
therapeutic effects.
Catalpol, a bioactive component extracted from
traditional Chinese medicine Rehmannia glutinosa (Di
Huang), has various pharmacological activities (4). Several studies indicated that catalpol
has inhibitory effects on proliferation and induced apoptosis in
several cancer types including human CRC (3–5). Our
previous study also focused on the therapeutic effect of catalpol
against acute liver injury via alleviation of inflammatory response
(6). However, the underlying
mechanisms of catalpol in CRC treatment are still unclear, which
limits the application of catalpol in CRC treatment.
microRNAs (miRs) are small noncoding RNAs which are
involved in numerous biological processes. Accumulating evidence
has demonstrated that miRs are aberrantly expressed in multiple
human cancers and stimulate inappropriate cellular programs
(7). The antitumor and
antiproliferative activities of catalpol are closely associated
with aberrant versions of miRs (8–10),
which provides insights on the interaction between catalpol and the
expression of miRs. Our previous studies indicated that miR-34a has
a tumor-suppressive role in human CRC and cholangiocarcinoma
(11,12); thus, we hypothesized that there may
be a correlation between catalpol and miR-34a in the progression of
CRC.
Recently, the roles of autophagy regulated by miRs
in the progression of human cancers have been of concern (13,14).
Our previous studies demonstrated that the resistance of
oxaliplatin in CRC cells was connected with miR-34a-mediated
autophagy (11). In addition, it
was reported that inhibition of autophagy attenuated the
anti-inflammatory effect regulated by catalpol on liver fibrosis
(15). Moreover, catalpol was
revealed to suppress autophagy to prevent denervated muscular
atrophy (16). Considering the
association between autophagy, miRNAs and catalpol, it was surmised
that the autophagy regulated by miRNAs may be involved in mediating
the functional role of catalpol. However, the exact role of miR-34a
mediated by catalpol in CRC cell autophagy and apoptosis awaits to
be elucidated.
Materials and methods
Patients and tissue samples
CRC samples and adjacent normal colon tissues were
obtained from 60 CRC patients (median age, 62 years; range, 47–76
years; 26 mmale and 34 mfemale patients) between January 2018 and
December 2018 at the Second Affiliated Hospital of Harbin Medical
University. All patients provided written informed consent
according to our institutional guidelines, and the study protocol
was approved by the Institutional Review Board of Harbin Medical
University (KY2018-208). The samples were trimmed and snap frozen
in liquid nitrogen immediately after surgical resection. The tumor
stage was classified according to the 7th tumor-node-metastasis
(TNM) classification of the International Union against Cancer
(UICC) (17). Data of clinical
characteristics were collected from medical records.
Animal model of azoxymethane
(AOM)-induced CRC
Three-week-old male Wistar rats (40–60 g) obtained
from the Animal Center of the Second Affiliated Hospital of Harbin
Medical University were housed with free access to sterile food and
water under a standard 12-h light/dark cycle and a controlled
temperature (22±2°C) and humidity (55±5%). Animal experiments were
carried out in strict accordance with the Harbin Medical University
Institutional Animal Care guidelines and was approved by the Animal
Research Ethics Use Committee of Harbin Medical University
(KY2018-208). Thirty-six rats were randomly divided into 3 groups
of 12 rats each. Animals in the experimental groups (AOM and
catalpol) received AOM (15 mg/kg i.p.) and the control group
received an equal volume of normal saline, once weekly for 2 weeks.
Catalpol dissolved in phosphate-buffered saline (PBS; 10 mg/kg) was
administered intragastrically daily from week 5 to 25. The AOM
group was administered the same volume of PBS orally. Rats health
and behavior were monitored every 2 days. At the end of the
experiment, euthanasia by anesthesia overdose with intraperitoneal
injection of sodium pentobarbital (Nembutal; 200 mg/kg body weight)
was perfomed. Then colon tissue samples were obtained from each rat
after decapitation was performed to confirm euthanasia (6,18).
Cell culture and treatment
The human colon epithelial cell line FHC obtained
from the American Type Culture Collection (ATCC) was cultured in
DMEM/F12 medium (GIBCO; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS) at 37°C in a
humidified 5% CO2 atmosphere. The human CRC cell lines
HCT116, HT29, SW620 and SW480 were purchased from the Cell Bank of
the Chinese Academy of Sciences and were routinely cultured using
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing
10% FBS. The miR-34a mimics or miR-34a inhibitor purchased from
Shanghai GenePharma Co., Ltd., were transfected into the CRC cells
for 48 h using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.)
in indicated concentrations according to the supplier's
instructions. Rapamycin (10 nM; Cell Signaling Technology, Inc.)
and 3-methyladenine (3-MA; 5 mM; Sigma-Aldrich; Merck KGaA) were
added to further activate or inhibit the flux of autophagy in CRC
cells as previously described (12).
Bioinformatics analysis and luciferase
assay
Since miRNAs function by base-pairing with sequences
in the 3′-UTR of their target mRNA, we used TargetScan (http://www.targetscan.org/) to search for potential
targets of miR-34a. For luciferase reporter assay, the CRC cells
were co-transfected with 80 ng mutant reporter plasmids, 10 ng
pRL-TK-Renilla-luciferase plasmid, and sirtuin 1 (SIRT1)
RNAs (20 nmol/l). Luciferase activity was assessed by quantifying
the ratio of firefly luciferase activity to Renilla
luciferase activity using the Dual-Luciferase Reporter Assay system
(Promega Corporation) as previously described (12) at 24 h after transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA or miRs were isolated from cultured cells
and fresh surgical tissues using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) or mirVana miRNA Isolation kit (Ambion;
Thermo Fisher Scientific, Inc.). They were reverse-transcribed to
cDNA by High Capacity cDNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.). RT-qPCR was performed in triplicate with the
Power SYBR Green (Thermo Fisher Scientific, Inc.) using the ABI
StepOne system. The thermocycling parameters were as follows: 10
min at 95°C for polymerase activation followed by 40 cycles
consisting of 95°C for 15 sec and 60°C for 60 sec. The expression
levels of SIRT1 were normalized to β-actin, and U6 small nuclear
RNA (U6) was used as the internal control for miR-34a. The
2−∆∆Cq method was used to normalize the relative levels
of the target genes. The details of all the primers for RT-qPCR
used in this study are presented in Table I (19).
 | Table I.Primers used for RT-qPCR. |
Table I.
Primers used for RT-qPCR.
| Primer name | Primer sequence:
5′-3′ |
|---|
| SIRT1 | F:
CCCAGAACATAGACACGCTGGA |
|
| R:
ATCAGCTGGGCACCTAGGACA |
| β-actin | F:
ATGTTGAGACCTTCAACACC |
|
| R:
AGGTAGTCAGTCAGGTCCCGGCC |
| miR-34a | F:
TGGTGTCGTGGAGTCG |
|
| R:
GGCATCTCTCGCTTCATCTT |
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
Western blotting
Cell extracts were collected and proteins were
extracted using RIPA buffer (Beyotime Institute of Biotechnology).
The concentration of total protein was measured using a BCA Protein
Assay Kit (Beyotime Institute of Biotechnology). Thirty µg protein
lysates was electrophoresed by 12% SDS-PAGE and then transferred to
nitrocellulose membranes (Bio-Rad Laboratories, Inc.). After
blocking with 5% fat-free milk for 2 h, the membranes were probed
with the relevant primary antibodies (SIRT1, LC3, Beclin 1, Bcl-2,
Bax, cytochrome c and β-actin; Table II) at 4°C overnight. Finally an
enhanced chemiluminescence detection reagents Alexa Fluor 680
donkey anti-mouse IgG or Alexa Fluor 680 donkey anti-rabbit IgG
(Table II) secondary antibody was
incubated with the membranes for 12 h at 4°C and visualized with an
Odyssey™ Infrared Imaging system (LI-COR Biosciences).
Densitometry was performed using Alpha Imager 2200 (Alpha Innotech
Corp.). β-actin was used as a control for whole-cell lysates.
 | Table II.Antibodies used for western
blotting. |
Table II.
Antibodies used for western
blotting.
| Antibody | Manufacturer | Species | Dilution |
|---|
| SIRT1 | cat. no. sc-135792
(Santa Cruz Biotechnology, Inc.) | Mouse | 1:500 |
| LC3-II
(MAPLC3β) | cat. no. sc-28266
(Santa Cruz Biotechnology, Inc.) | Rabbit | 1:500 |
| Beclin 1 | cat. no. sc-11427
(Santa Cruz Biotechnology, Inc.) | Rabbit | 1:500 |
| Bcl-2 | cat. no. sc-7382
(Santa Cruz Biotechnology, Inc.) | Mouse | 1:200 |
| Bax | cat. no. sc-526
(Santa Cruz Biotechnology, Inc.) | Mouse | 1:200 |
| Cytochrome
c | cat. no. sc-13156
(Santa Cruz Biotechnology, Inc.) | Mouse | 1:200 |
| β-actin | cat. no. sc-69879
(Santa Cruz Biotechnology, Inc.) | Mouse | 1:500 |
| Alexa Fluor 680
donkey anti-mouse IgG anti-mouse IgG | A10038 (Thermo
Fisher Scientific, Inc.) | Donkey | 1:5,000 |
| Alexa Fluor 680
donkey anti-rabbit IgG | A10043 (Thermo
Fisher Scientific, Inc.) | Donkey | 1:5,000 |
Cell viability and cytotoxicity
assay
Cell Counting Kit-8 (CCK-8; Boster Biological
Technology) was used to assess cell viability as previously
described (11). The CRC cells were
seeded at a density of 3×103/well in 96-well plates and
maintained at 37°C overnight. CCK-8 solution (10 µl) was added into
each well and incubated for another 1 h. The absorbance at 450 nm
was measured using a Microplate Reader (Bio-Rad Laboratories,
Inc.). For the cytotoxicity assay, lactate dehydrogenase (LDH)
activity in culture medium was determined using an LDH release
assay kit (Boster Biological Technology).
Evaluation of autophagy and apoptosis
by flow cytometry
Cell autophagy was assessed by detecting the
mono-dansylcadaverine (MDC; Sigma-Aldrich; Merck KGaA) positively
stained cells using flow cytometry. Cells (104) were
washed with PBS and then incubated with 0.05 mmol/l MDC in PBS at
37°C for 45 min. After washing three times with PBS, the cell
suspension was analyzed by flow cytometry immediately. For cell
apoptosis detection, 104 cells were collected in 200 ml
RPMI-1640 medium without FBS. Following resuspension, approximately
10 ml Annexin V solution (BD Biosciences) was added. Fifteen min
later, 300 ml medium buffer and 5 ml propidium iodide (PI; BD
Biosciences) were added, and the cell suspension was analyzed by
flow cytometry (BD Biosciences) immediately (11).
Electron microscopy
Cells from each group were harvested and fixed with
2.5% glutaraldehyde at 4°C for 2 h. The samples were suspended in
PBS with 1% osmic acid. After dehydration and embedding, the
ultrathin sections were prepared on uncoated copper grids with an
Ultrotome (Reichert/Leica Ultracut S) and stained with 3% uranyl
acetate and lead citrate for 5 min at room temperature. Images were
acquired using a JEM 1230 transmission electron microscope (JEOL,
Ltd.).
Statistical analysis
Statistical analysis was carried out by SPSS version
21 (IBM Corp.) or GraphPad Prism version 5.0 (GraphPad Software,
Inc.). All the data from at least three independent experiments
were expressed as the mean ± standard deviation. Comparisons of
quantitative data between two groups were performed using paired
two-tailed Student's t-tests. Comparisons among three or more
groups were performed by one-way ANOVA test followed by Tukey's
test. Correlation analysis between miR-34a and SIRT1 mRNA
expression levels was performed by Pearson's rank correlation
coefficient analysis. The impact of clinical parameters was
estimated by multivariate logistic regression analysis and Cox
regression analysis (Table III).
P<0.05 was considered to indicate a statistically significant
difference.
 | Table III.Relationship between miR-34a
expression and clinicopathological features in CRC patients. |
Table III.
Relationship between miR-34a
expression and clinicopathological features in CRC patients.
|
|
| miR-34a |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | n | Low | High | P-value |
|---|
| Age (years) |
|
|
| 0.603 |
|
<60 | 27 | 14 | 13 |
|
|
≥60 | 33 | 16 | 17 |
|
| Sex |
|
|
| 0.397 |
|
Male | 26 | 14 | 12 |
|
|
Female | 34 | 16 | 18 |
|
| Tumor size
(cm) |
|
|
| 0.094 |
|
<5 | 36 | 19 | 17 |
|
| ≥5 | 24 | 11 | 13 |
|
|
Differentiation |
|
|
| <0.001 |
|
Well/moderate | 20 | 2 | 18 |
|
|
Poor | 40 | 28 | 12 |
|
| Lymphatic node
metastasis |
|
|
| <0.001 |
|
Present | 43 | 28 | 15 |
|
|
Absent | 17 | 2 | 15 |
|
| Clinical
stages |
|
|
| <0.001 |
|
I/II | 17 | 2 | 15 |
|
|
III/IV | 43 | 28 | 15 |
|
| CA199 level
(U/ml) |
|
|
| 0.500 |
|
<37 | 31 | 15 | 16 |
|
|
≥37 | 29 | 15 | 14 |
|
| CEA level
(ng/ml) |
|
|
| <0.001 |
|
<5 | 16 | 2 | 14 |
|
| ≥5 | 44 | 28 | 16 |
|
Results
miR-34a is downregulated and SIRT1 is
upregulated in CRC tissues and cell lines
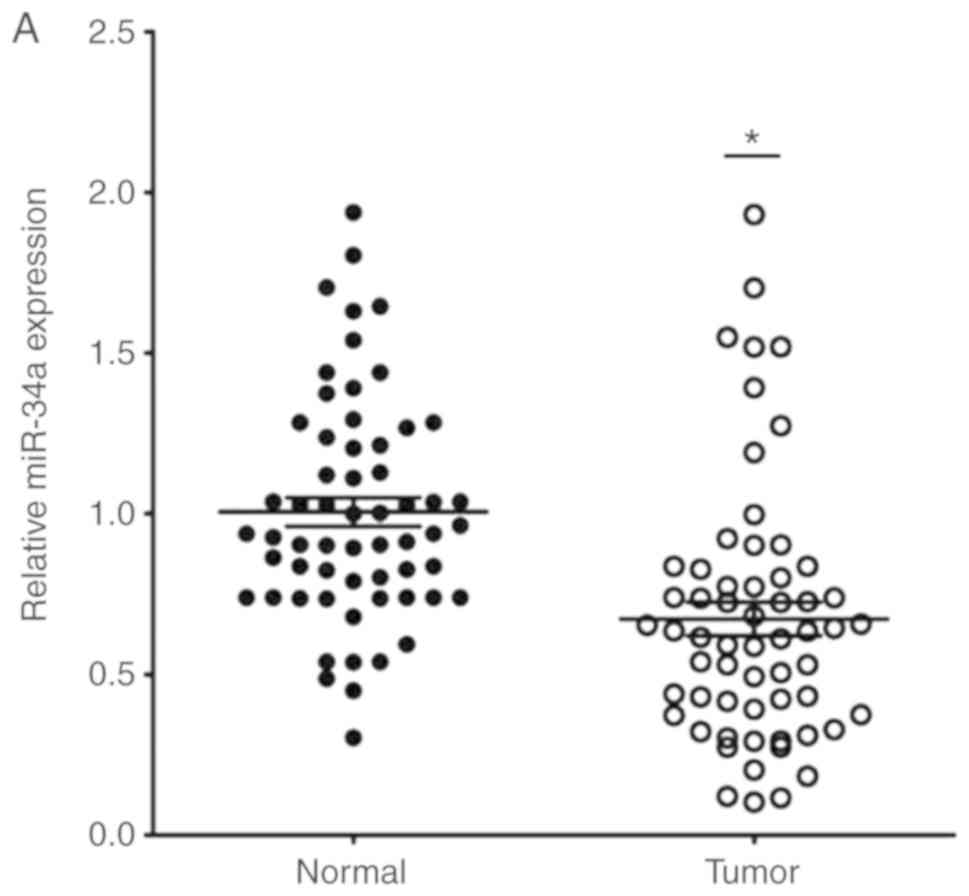
The expression of miR-34a in CRC tissues was
significantly decreased compared with the adjacent non-tumor
tissues (Fig. 1A). SIRT1 mRNA
expression was upregulated in CRC tissues compared to the adjacent
normal colon tissues (Fig. 1B).
Notably, the expression levels of miR-34a and SIRT1 had a
significant inverse correlation (Fig.
1C) as revealed by Spearman correlation analysis. The 60 CRC
patients were divided into two groups according to the median value
(0.6725) of the miR-34a expression level (0.6725) in CRC tissues to
further evaluate the association between the expression levels of
miR-34a and the clinicopathological characteristics of CRC
(Table III). The data indicated
that miR-34a expression was decreased in samples with poor cell
differentiation, lymphatic metastasis, high expression of
carcinoembryonic antigen (CEA) and advanced clinical stages.
However, age, sex, tumor size, expression of carbohydrate antigen
(CA)19-9 had no association with miR-34a expression (P>0.05).
miR-34a expression was further detected in FHC and HT29, HCT116,
SW480 and SW620 CRC cell lines. As revealed in Fig. 1D, the expression levels of miR-34a
were significantly decreased in all the CRC cell lines compared to
FHC cells. The expression levels of SIRT1 in CRC cell lines were
significantly higher than that in FHC cells (Fig. 1E). HT29 and HCT116 exhibited
moderate expression levels of miR-34a and SIRT1, thus these two
cell lines were selected to further explore the role of the
miR-34a/SIRT1 axis in the progression of CRC.
miR-34a directly targets SIRT1 in CRC
cells
Data from TargetScan (http://www.targetscan.org/) revealed that the 3′-UTR
of SIRT1 contained the complementary site which may play a role in
translational repression for the seed region of miR-34a (Fig. 2A). A dual luciferase assay was
conducted in HT29 and HCT116 cells. As revealed in Fig. 2B, miR-34a mimic transfection
(PGL3′-UTR WT) decreased luciferase activity compared to the
control group. Additionally, transfection with the mutated 3′-UTR
(PGL3′-UTR MUT) of the SIRT1 gene, revealed no significant
difference in luciferase activities between the miR-34a mimic
transfection and the control groups. Moreover, compared to the
normal control group, miR-34a mimic transfection and miR-34a
inhibitor successfully increased and decreased, respectively, the
endogenous miR-34a expression levels in CRC cells (Fig. 2C). Results of western blotting
revealed that the transfection with the miR-34a mimics decreased
SIRT1 expression while transfection with the miR-34a inhibitor
promoted SIRT1 expression at the protein level in CRC cells
(Fig. 2D). These data indicated
that SIRT1 was one of the direct targets of miR-34a in CRC
cells.
Catalpol suppresses autophagy and
induces apoptosis through the miR-34a/SIRT1 axis in CRC cell
lines
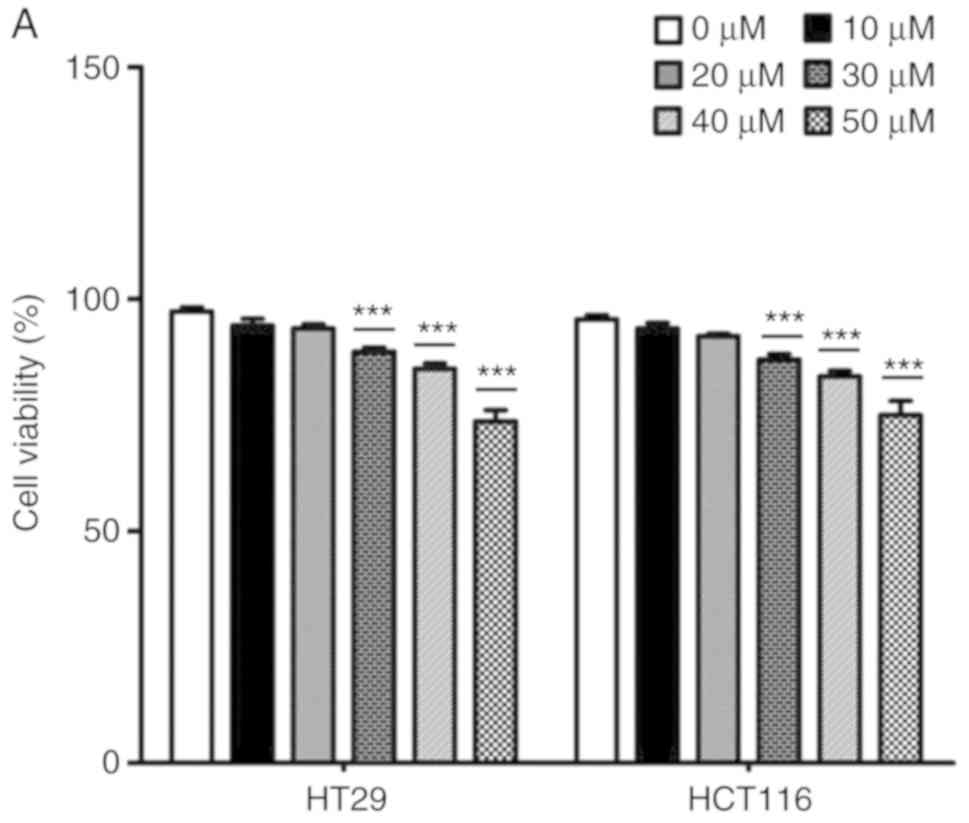
The effects of catalpol on CRC cell viability and
cytotoxicity were initially evaluated in vitro. CRC cell
viability was significantly decreased by 30–50 µM catalpol
treatment compared to the control group (Fig. 3A). Cytotoxicity was revealed at a
concentration of 40 µM (Fig. 3B).
Thus, 30 µM was used in the following experiments. Whether catalpol
significantly inhibited autophagy in CRC cells was next
investigated. MDC-positively stained CRC cells were significantly
decreased by catalpol treatment (Fig.
3C). Consistent with the flow cytometric results, Fig. 3D reveals the decreased
autophagosomes associated with catalpol treatment in CRC cells.
Compared with the normal controls, expression of autophagy-related
protein Beclin 1 and LC3-II (also referred as MAPLC3β) was
significantly downregulated by catalpol (Fig. 3G). Induction of apoptosis of CRC
cells by catalpol was demonstrated by flow cytometry (Fig. 3E) and Bax, cytochrome c
expression levels were increased, while Bcl-2 protein expression
levels were reduced (Fig. 3G).
Consistent with previous studies (8–10),
expression of miR-34a in CRC cells was markedly upregulated by
catalpol treatment compared to control cells (Fig. 3F). As anticipated, expression of
SIRT1 in both HT29 and HCT116 cells was downregulated by catalpol
(Fig. 3G). These results indicated
that suppression of autophagy and induction of apoptosis of CRC
cells by catalpol may be partly achieved through the miR-34a/SIRT1
axis.
Catalpol-regulated autophagy is
involved in mediating apoptosis in CRC cells
To address whether catalpol regulated
autophagy-modulated apoptosis of CRC cells, the CRC cells were
subjected to catalpol with or without 3-MA and rapamycin treatment.
The results revealed that catalpol inhibited autophagy and induced
apoptosis of CRC cells, as indicated by decreased MDC-positive
cells (Fig. 4A) and autophagosomes
(Fig. 4B); decreased expression
levels of LC3-II, Beclin 1 and Bcl-2; and higher expression levels
of Bax and cytochrome c (Fig.
4D) as well as the apoptotic rate (Fig. 4C). 3-MA significantly suppressed
autophagy and induced apoptosis of CRC cells while rapamycin
activated autophagy but had no effect on apoptosis in CRC cells, as
revealed by increased MDC-positive cells (Fig. 4A) and autophagosomes (Fig. 4B) and higher expression of
autophagy-related proteins (Fig.
4D). In addition, 3-MA combined with catalpol did not affect
either autophagy activity or apoptosis of CRC cells as compared
with treatment with catalpol alone. However, compared with
treatment with catalpol alone, rapamycin in combination with
catalpol attenuated the inhibitory effect on autophagy and induced
apoptosis of CRC cells (Fig. 4).
These results strongly indicated that inhibition of autophagy by
catalpol may exert an accelerated effect on apoptosis of CRC
cells.
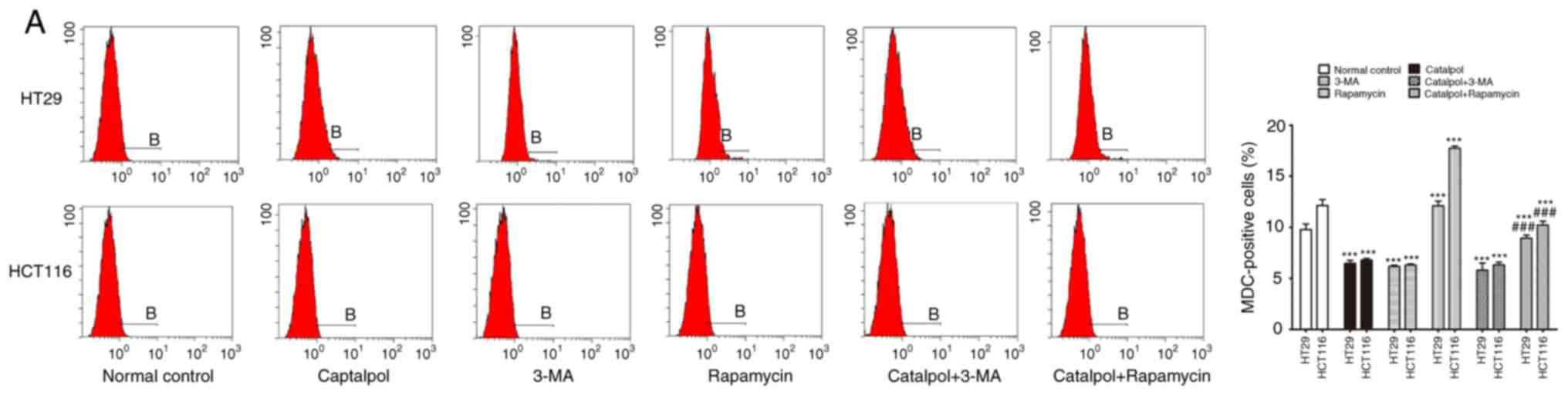 | Figure 4.Inhibition of autophagy regulated by
catalpol contributes to apoptosis in CRC cells. (A) CRC cells were
exposed to 30 µM catalpol for 24 h and treated with or without 3-MA
and rapamycin, and then flow cytometry was used to detect the
autophagic levels (***P<0.001 and ###P<0.001). (B)
Autophagic vacuole formation was revealed by representative
electron micrographs in CRC cell lines. The arrows indicate the
autophagosomes. (C) Apoptosis was analyzed by flow cytometry in CRC
cells after various treatments (***P<0.001 and
###P<0.001). (D) Western blotting revealed the
expression of SIRT1, LC3II, Beclin 1, Bcl-2, Bax, and cytochrome c
in HT29 and HCT116 cells. β-Actin was used as a loading control.
Quantitation of the results was performed (***P<0.001 indicates
a significant difference vs. the normal control group;
#P<0.05, ##P<0.01 and
###P<0.001 indicates a significant difference vs. the
group treated with catalpol). CRC, colorectal cancer; SIRT1,
sirtuin 1; 3-MA, 3-methyladenine. |
The miR-34a/SIRT1 pathway participates
in regulating catalpol-suppressed CRC cell autophagy and induces
apoptosis
miR-34a and catalpol have been demonstrated as key
regulators of autophagy (11,15);
thus, it was investigated whether miR-34a was involved in mediating
catalpol-regulated autophagy and apoptosis of CRC cells. Both the
cell lines were treated by catalpol and transfected with miR-34a
mimics or inhibitors. The number of MDC-positive cells detected by
flow cytometry (Fig. 5A) and
autophagosomes detected by electron microscopy (Fig. 5B) were significantly decreased
compared to the controls. Western blotting revealed decreased
expression of SIRT1, LC3-II, Beclin 1 and Bcl-2, and higher
expression of Bax and cytochrome c (Fig. 5D) in CRC cells in the catalpol
treatment and miR-34a mimics transfection groups compared to the
control group. Similarly, the apoptotic rate was significantly
increased in both cell lines by treatment with catalpol or miR-34a
mimics compared to the control group (Fig. 5C). The level of autophagy was
enhanced but apoptosis was not weakened in both HT29 and HCT116
cell lines by the miR-34a inhibitor, since the results revealed
increased MDC-positive cells (Fig.
5A) and autophagosomes (Fig.
5B), along with increased SIRT1, LC3-II and Beclin 1 protein
expression levels (Fig. 5D). The
effect on CRC cell apoptosis was not significant compared to the
control group (Fig. 5C and D).
Treatment with catalpol in combination with miR-34a mimic had no
significant effect on autophagy flux or apoptosis compared with
catalpol alone in HT29 and HCT116 cells (Fig. 5). Compared with catalpol alone, the
inhibitory effect of autophagy and induction of apoptosis were
impaired by catalpol in combination with miR-34a inhibitor in CRC
cells, as indicated by increased MDC-positive cells (Fig. 5A) and autophagosomes (Fig. 5B), increased expression of SIRT1,
LC3-II, Beclin 1 and Bcl-2, and decreased expression of Bax and
cytochrome c (Fig. 5D) in
CRC cells. Moreover, the inhibitory effect on apoptosis was
significantly reduced in both HT29 and HCT116 cell lines compared
to catalpol treatment alone (Fig.
5C). These data indicated that the miR-34a/SIRT1 axis at least
partly participated in mediating catalpol-suppressed autophagy and
catalpol-induced apoptosis of CRC cells.
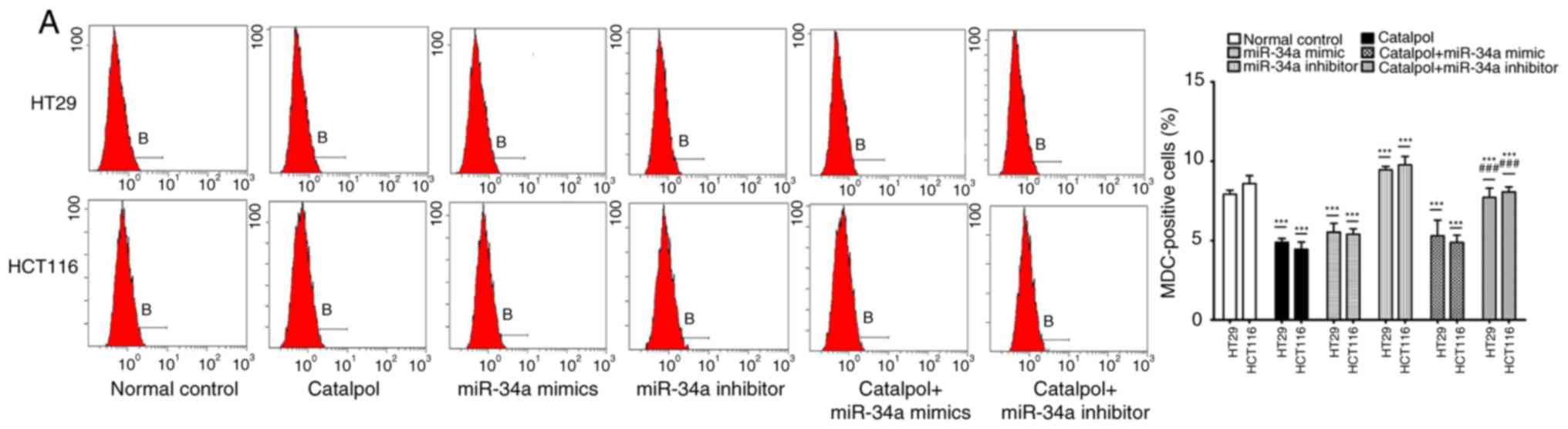 | Figure 5.The miR-34a/SIRT1 axis participates
in regulating catalpol-suppressed CRC cell autophagy and induces
apoptosis. (A) HT29 and HCT116 cells were treated with 30 µM
catalpol for 24 h and in combination with miR-34a mimic or
inhibitor, and then flow cytometry was used to examine autophagic
levels (***P<0.001 and ###P<0.001). (B) Autophagic
vacuole formation was revealed by representative electron
micrographs in CRC cell lines. The arrows indicate the
autophagosomes. (C) The CRC cell apoptotic rate was analyzed by
flow cytometry (***P<0.001 and ###P<0.001). (D)
Western blotting revealed the expression of SIRT1, LC3, Beclin 1,
Bcl-2, Bax, and cytochrome c in HT29 and HCT116 cells
(*P<0.05 and ***P<0.001 indicates a significant difference
vs. the normal control group; #P<0.05,
##P<0.01 and ###P<0.001 indicates a
significant difference vs. the group treated with catalpol). miR,
microRNA; SIRT1, sirtuin 1; CRC, colorectal cancer. |
Catalpol inhibits tumorigenesis
through regulation of the miR-34a/SIRT1 pathway in a rat model
The AOM-induced CRC rat model was used to evaluate
the chemopreventive effect and toxicity of catalpol in vivo.
First, percentage weight gain among each group was observed,
however the differences were not significant (data not shown). As
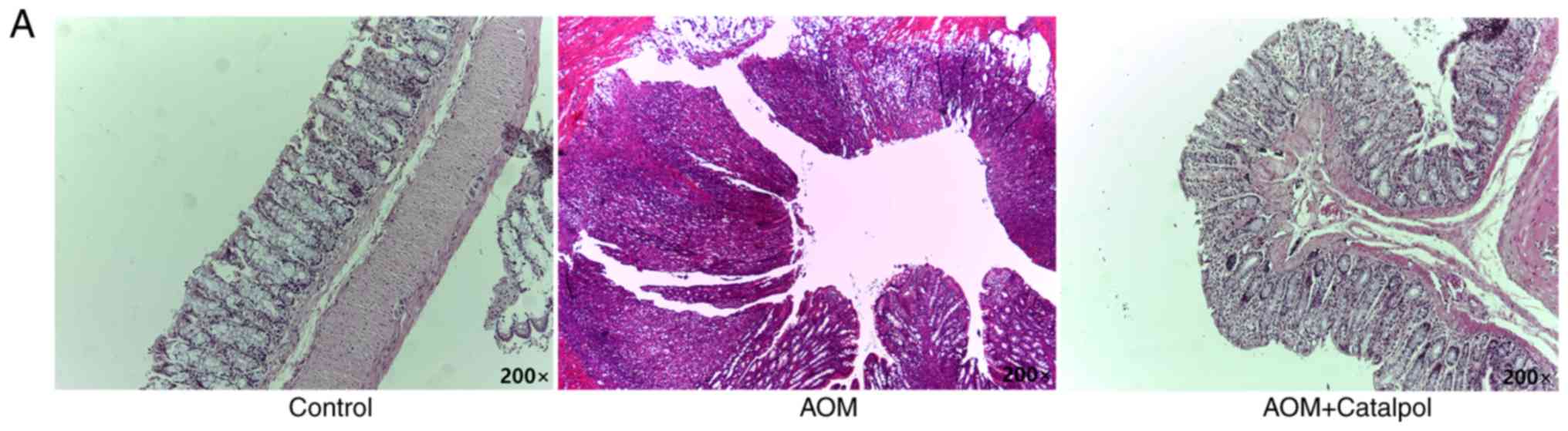
revealed in Fig. 6A, catalpol
intervention markedly prevented the formation of colon tumors. All
rats treated with AOM developed preneoplastic aberrant crypt foci
(ACF) (Fig. 6B). The catalpol group
had a significantly decreased number of ACFs with ≤4 crypts as well
as total ACF compared to the AOM group (Fig. 6B). No significant difference was
revealed in the number of ACFs containing >5 crypts among the
groups (Fig. 6B). To determine the
potential mechanism of catalpol in vivo, miR-34a and SIRT1
mRNA expression levels in colon tissues from each group were
examined by RT-qPCR. Consistent with the results in vitro,
expression of miR-34a was significantly decreased in the
AOM-treated group compared to the control group, and catalpol
treatment promoted the expression of miR-34a compared to AOM
treatment (Fig. 6C). The expression
of SIRT1 mRNA was significantly upregulated by treatment with AOM
compared to the control while treatment with catalpol in
combination with AOM inhibited the promoting effect on the
expression of SIRT1 compared to AOM treatment alone (Fig. 6D). These results provided evidence
that the catalpol-mediated miR-34a/SIRT1 pathway was involved in
inhibition of malignant behavior in vivo.
Discussion
Catalpol is one of the main active ingredients of
R. glutinosa (Di Huang) which has been revealed to
have antitumor effects on various of human cancers (5,8–10).
Recently, the inhibited effect on inflammation and tumor
angiogenesis of catalpol in CRC cells was revealed and catalpol was
considered as a potential candidate compound for treating CRC
(3,10,20).
Significant changes in the miRNA expression profiles were observed
in cancer cells after catalpol treatment (8–10,21).
Moreover, the present findings indicated that miR-34a played a
tumor-suppressive role during progression of human CRC and
cholangiocarcinoma (11,12). Thus, miR-34a was selected as a
candidate gene for catalpol in the present study. The present
research revealed that miR-34a was aberrantly downregulated in most
of human CRC tissues and all the CRC cell lines. Since miRNAs exert
physiological functions by directly modulating their target mRNA
expression, bioinformatics analysis was performed to predict if
SIRT1 may be one of the potential targets of miR-34a. SIRT1 is a
class III nuclear deacetylase that can mediate genetic programs by
modifying histones and transcription factors (22). SIRT1 is reported to inactivate p53
by deacetylating a critical lysine residue, and blocking SIRT1 may
lead to the activation of the p53 pathway and suppress the
progression of human cancers including CRC (23). Moreover, miR-34a could bind the site
within the 3′-UTR of SIRT1 to silence the mRNA as revealed in a
previous study (24). Consistent
with previous research, ectopic expression of miR-34a interposed
SIRT1 protein expression in CRC cell lines, and miR-34a may be a
tumor-suppressor and function by directly inhibiting SIRT1
expression in the progression of CRC.
Mounting evidence has revealed that catalpol has
inhibitory effects on proliferation and inducing effects on
apoptosis in several types of human malignancies (8–10,21).
Moreover, catalpol was revealed to inhibit autophagy and decrease
apoptosis via the mammalian target of rapamycin pathway in
denervated muscular atrophy (16).
In addition, it was reported that resistance of autophagy
attenuated the catalpol-induced anti-inflammatory effect on liver
fibrosis (15). In the present
study, it was revealed that 30–50 µM catalpol suppressed cell
viability and 40 µM catalpol resulted in toxicity in CRC cells.
Thus, 30 µM was used in the subsequent experiments. As documented
previously, modest autophagy may protect cells from apoptosis under
different conditions (11,25). The present data indicated that CRC
cell autophagy was significantly inhibited and apoptosis was
induced by catalpol treatment. Moreover, it was revealed that
catalpol upregulated miR-34a expression and suppressed SIRT1
expression in CRC cells. Catalpol has been revealed to target
multiple signaling pathways, including Bcl-2/Bax, JNK, NF-κB to
exert its pharmacological effects (26,27).
Thus, the antitumor effects and the potential mechanisms underlying
the catalpol-mediatied miR-34a/SIRT1 axis may be regulated by
complex signaling pathways. The present study indicated that the
miR-34a/SIRT1 axis was likely involved in regulating catalpol
suppression of autophagy and induction of apoptosis in CRC cells,
however, more studies are required in the future.
To date, there is wide recognition that autophagy
plays a dual role in regulating the fate of cancer cells (28). Although increasing evidence has
focused on the association between autophagy and progression of
malignant tumors, it is far from being clarified. The present study
indicated that catalpol and 3-MA attenuated activation of autophagy
and induced apoptosis of CRC cells. In addition, 3-MA in
combination with catalpol did not show any effect on autophagy or
apoptosis of CRC cells. Notably, rapamycin impaired the promoting
effect of catalpol on CRC cell apoptosis. Therefore, it was
suggested that catalpol may induce CRC cell apoptosis by
suppressing autophagy.
To determine whether miR-34a participated in
mediating catalpol-regulated autophagy and apoptosis, CRC cells
were treated by catalpol and concurrent promotion or silencing of
miR-34a expression. It was confirmed that both catalpol and miR-34a
overexpression antagonized cell autophagy and promoted apoptosis of
CRC cells. The autophagy was aggravated but apoptosis was not
restrained when miR-34a was exogenously inhibited. Combination
treatment with catalpol and miR-34a mimic revealed no effect on
autophagy or apoptosis in CRC cells. Moreover, the antitumor
effects of catalpol were neutralized by concurrent silencing of
miR-34a expression. Previous studies indicated that catalpol
administration significantly alleviated symptoms in a rat colitis
model (21,29). The present results indicated that
there was no significant weight gain in any of the experimental
groups, which may be because the experiment period was not long
enough. However, all rats treated with AOM developed preneoplastic
ACF in the colon. Catalpol treatment impaired the carcinogenic
effects of AOM. Moreover, as was anticipated, catalpol promoted the
expression of miR-34a and inhibited the expression of SIRT1 in the
colon of rats. Conversely, Xiong et al reported that
activation of SIRT1 by catalpol attenuated infiltration of
inflammatory cells, cytokine profiles, oxidative responses, and
epithelial cell apoptosis in colitis rats (21). The appearance of this discrepancy in
SIRT1 expression by catalpol treatment may be due to the totally
different diseases and AOM-induced stages as well as the different
cell lines used in in vitro investigations. Thus, it was
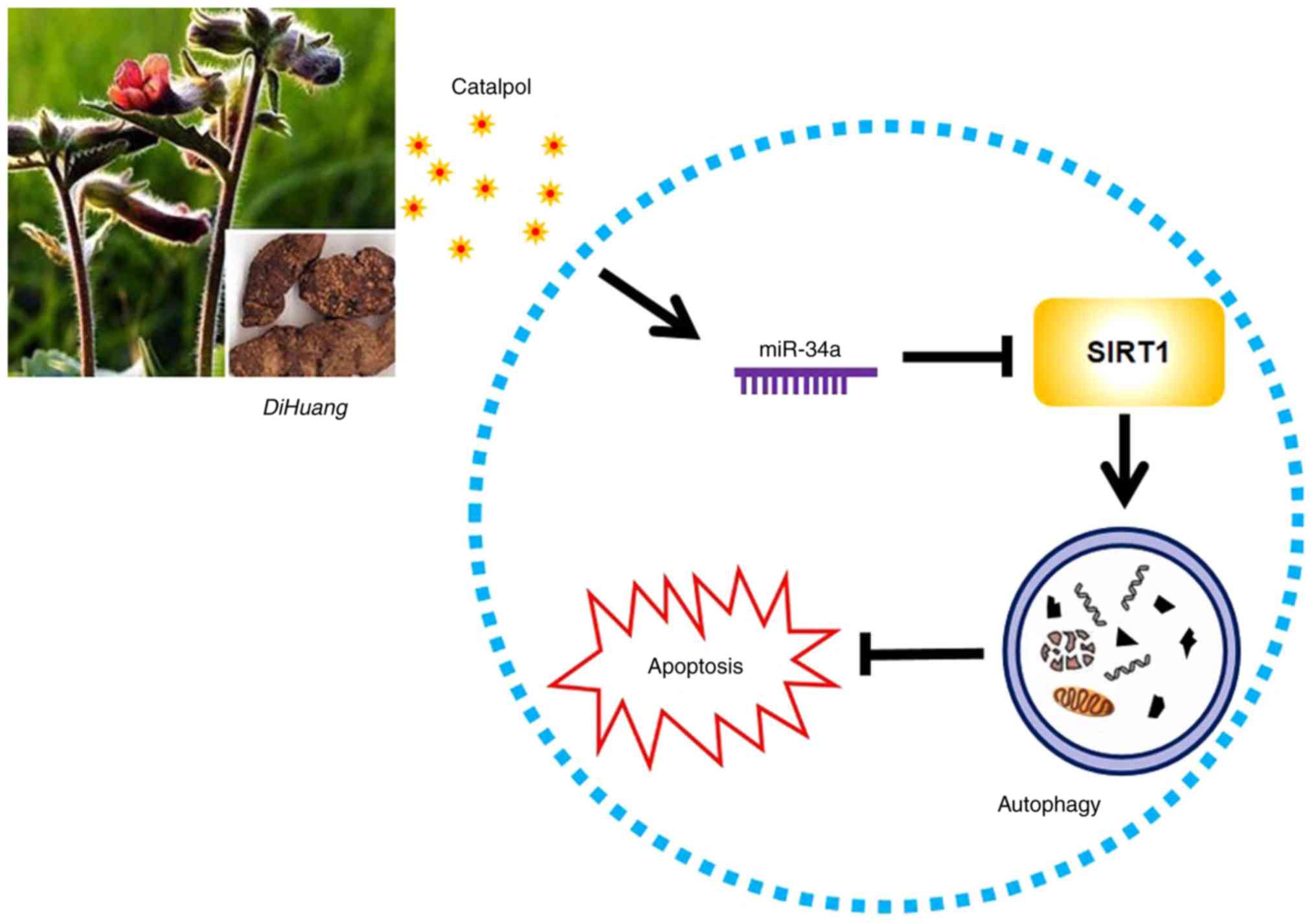
speculated that catalpol may induce apoptosis and inhibit autophagy
at least partly by upregulating miR-34a, along with suppression of
SIRT1 expression (Fig. 7).
In summary, it was demonstrated that miR-34a
functioned as an antioncogene in human CRC, which was at least in
part through the suppression of SIRT1 expression. Aberrantly
downregulated expression of miR-34a in CRC patients was correlated
with adverse clinical characteristics. In addition, cell autophagy
was suppressed while apoptosis was induced by catalpol treatment in
human CRC cells. Catalpol-regulated autophagy was demonstrated to
participate in mediating apoptosis in CRC cells. Furthermore,
miR-34a was upregulated by catalpol treatment and catalpol may
function by modulating the miR-34a/SIRT1 axis, which at least
partly provides an insight into the mechanisms underlying catalpol
treatment and may be considered as a novel therapeutic target for
CRC.
Acknowledgements
Not applicable.
Funding
The present study was supported by Heilongjiang
Postdoctoral Funds for Scientific Research Initiation (LBH-Q17129)
and the Science Foundation of the Health Commission of Heilongjiang
Province (2018346).
Availability of data and materials
The data used in the present study are available
from the corresponding author upon reasonable request.
Authors' contributions
PFQ designed the experiments. PFQ and ZLZ performed
the experiments. LY analyzed the data and wrote the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All patients provided written informed consent
according to our institutional guidelines and the study protocol
was approved by the Institutional Review Board of Harbin Medical
University. The animal experiments were carried out in strict
accordance with the Harbin Medical University Institutional Animal
Care and Use Committee (KY2018-208).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
SIRT1
|
sirtuin 1
|
|
miRs
|
miRNAs/microRNAs
|
|
UTR
|
untranslated region
|
|
AOM
|
azoxymethane
|
|
PBS
|
phosphate-buffered saline
|
|
MDC
|
mono-dansylcadaverine
|
|
NC
|
negative control
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
CA
|
carbohydrate antigen
|
|
CEA
|
carcinoembryonic antigen
|
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang Y, Weng W, Peng J, Hong L, Yang L,
Toiyama Y, Gao R, Liu M, Yin M, Pan C, et al: Fusobacterium
nucleatum increases proliferation of colorectal cancer cells and
tumor development in mice by activating Toll-like receptor 4
signaling to nuclear factor-κB, up-regulating expression of
microRNA-21. Gastroenterology. 152:851–866.e24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu P, Wu Y, Yang A, Fu X, Mao M and Liu
Z: Catalpol suppressed proliferation, growth and invasion of CT26
colon cancer by inhibiting inflammation and tumor angiogenesis.
Biomed Pharmacother. 95:68–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiu LZ, Bo J, Zhi BL, Hao S and An LJ:
Catalpol ameliorates cognition deficits and attenuates oxidative
damage in the brain of senescent mice induced by D-galactose.
Pharmacol Biochem Behav. 88:64–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang ZH and Zhansheng H: Catalpol inhibits
migration and induces apoptosis in gastric cancer cells and in
athymic nude mice. Biomed Pharmacother. 103:1708–1719. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Jia R, Wang F, Qiu G, Qiao P, Xu
X and Wu D: Catalpol protects mice against
Lipopolysaccharide/D-galactosamine-induced acute liver injury
through inhibiting inflammatory and oxidative response. Oncotarget.
9:3887–3894. 2018.PubMed/NCBI
|
|
7
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian MicroRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao N, Tian JX, Shang YH, Zhao DY and Wu
T: Catalpol suppresses proliferation and facilitates apoptosis of
OVCAR-3 ovarian cancer cells through upregulating microRNA-200 and
downregulating MMP-2 expression. Int J Mol Sci. 15:19394–19405.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu C, Wu F, Liu Y and Meng C: Catalpol
suppresses proliferation and facilitates apoptosis of MCF-7 breast
cancer cells through upregulating microRNA-146a and downregulating
matrix metalloproteinase-16 expression. Mol Med Rep. 12:7609–7614.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu L, Gao H, Wang H, Zhang Y, Xu W, Lin
S, Wang H, Wu Q and Guo J: Catalpol promotes cellular apoptosis in
human HCT116 colorectal cancer cells via microRNA-200 and the
downregulation of PI3K-Akt signaling pathway. Oncol Lett.
14:3741–3747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun C, Wang FJ, Zhang HG, Xu XZ, Jia RC,
Yao L and Qiao PF: miR-34a mediates oxaliplatin resistance of
colorectal cancer cells by inhibiting macroautophagy via
transforming growth factor-β/Smad4 pathway. World J Gastroenterol.
23:1816–1827. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiao P, Li G, Bi W, Yang L, Yao L and Wu
D: microRNA-34a inhibits epithelial mesenchymal transition in human
cholangiocarcinoma by targeting Smad4 through transforming growth
factor-beta/Smad pathway. BMC Cancer. 15:4692015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun L, Hu L, Cogdell D, Lu L, Gao C, Tian
W, Zhang Z, Kang Y, Fleming JB and Zhang W: MIR506 induces
autophagy-related cell death in pancreatic cancer cells by
targeting the STAT3 pathway. Autophagy. 13:703–714. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Z, Zhu P, Zhang L, Xiong B, Tao J,
Guan W, Li C, Chen C, Gu J, Duanmu J and Zhang W: Autophagy
inhibition attenuates the induction of anti-inflammatory effect of
catalpol in liver fibrosis. Biomed Pharmacother. 103:1262–1271.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Shao Y, Gao Y, Wan G, Wan D, Zhu
H, Qiu Y and Ye X: Catalpol prevents denervated muscular atrophy
related to the inhibition of autophagy and reduces BAX/BCL2 ratio
via mTOR pathway. Drug Des Devel Ther. 13:243–253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sobin LH, Gospodarowicz KM and Wittekind
CH: TNM classification of malignant tumors. UICC International
Union Against Cancer. 7th. Wiley-Blackwell; 2009
|
|
18
|
Xu Y, Yang X, Mei S, Sun Y and Li J:
Acquisition of temozolomide resistance by the rat C6 glioma cell
line increases cell migration and side population phenotype. Oncol
Rep. 42:2355–2362. 2019.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong J, Liang W, Wang T, Sui J, Wang J,
Deng Z and Chen D: Saponins regulate intestinal inflammation in
colon cancer and IBD. Pharmacol Res. 144:66–72. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiong Y, Shi L, Wang L, Zhou Z, Wang C,
Lin Y, Luo D, Qiu J and Chen D: Activation of sirtuin 1 by
catalpol-induced down-regulation of microRNA-132 attenuates
endoplasmic reticulum stress in colitis. Pharmacol Res. 123:73–82.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad
Sci. 105:13421–13426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang C, Qiu S, Sun F, Li W, Wang Z, Yue B,
Wu X and Yan D: Long non-coding RNA HNF1A-AS1 mediated repression
of miR-34a/SIRT1/p53 feedback loop promotes the metastatic
progression of colon cancer by functioning as a competing
endogenous RNA. Cancer Lett. 410:50–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai M, Du G, Shi R, Yao J, Yang G, Wei Y,
Zhang D, Xu Z, Zhang R, Li Y, et al: miR-34a inhibits migration and
invasion by regulating the SIRT1/p53 pathway in human SW480 cells.
Mol Med Rep. 11:3301–3307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiao PF, Yao L, Zhang XC, Li GD and Wu DQ:
Heat shock pretreatment improves stem cell repair following
ischemia-reperfusion injury via autophagy. World J Gastroenterol.
21:12822–12834. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou J, Xu G, Ma S, Li F, Yuan M, Xu H and
Huang K: Catalpol ameliorates high-fat diet-induced insulin
resistance and adipose tissue inflammation by suppressing the JNK
and NF-κB pathways. Biochem Biophys Res Commun. 467:853–858. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li DQ, Bao YM, Li Y, Wang CF, Liu Y and An
LJ: Catalpol modulates the expressions of Bcl-2 and Bax and
attenuates apoptosis in gerbils after ischemic injury. Brain Res.
1115:179–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin S and White E: Role of autophagy in
cancer: Management of metabolic stress. Autophagy. 3:28–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Crespo I, San-Miguel B, Prause C, Marroni
N, Cuevas MJ, González-Gallego J and Tuñón MJ: Glutamine treatment
attenuates endoplasmic reticulum stress and apoptosis in
TNBS-induced colitis. PLoS One. 7:e504072012. View Article : Google Scholar : PubMed/NCBI
|