Introduction
Osteosarcoma (OS) is one of the most common primary
malignant bone tumors. It most commonly occurs in the metaphysis of
long bones in children and adolescents. Approximately 10–20% of
patients have metastasized disease at initial diagnosis (1,2). OS is
highly invasive and leads to progressive bone destruction.
Currently, the main clinical treatment of patients with OS is still
surgery-based, supplemented by chemotherapy (1) or radiotherapy (2). Although the treatment for OS has
improved over the past few decades, the 5-year overall survival
rate remains extremely low, at ~60% (3). Intraoperative contamination of radical
surgery, microlesions in the peritumoral tissue that are unable to
be completely removed, and microresidual tumors are the primary
causes of local recurrence in patients with OS, and the prognosis
of these patients is often poor (4,5).
Therefore, new treatment methods are urgently required in order to
control the intraoperative tumor contamination and residuals,
decrease the recurrence rate and improve the survival rate of
patients.
Cold atmospheric plasma (CAP) is the fourth basic
material state after solid, liquid and gas. Plasma is a partially
ionized gas generated by a focused discharge (6). It contains a variety of physical and
chemical components, including electric field, ions, photons, free
radicals and other unknown active substances (7). Conventional plasma can only be applied
to industry under high temperatures of over 10,000°C. The
temperature range of CAP is 20–50°C, and then, the molecular
structure and cellular integrity can be maintained (8). Reports have demonstrated that CAP can
be used in wound healing (9),
sterilization (10) and food
preservation (11). The antitumor
capabilities of CAP have become a popular topic for research,
including restoring the sensitivity of chemotherapy-resistant
cancer cells (12). Although the
direct application of CAP has proven to be sensitive for a number
of different types of cancer, the poor penetration of CAP limits
its applications in cancer treatments in the clinical practice,
particularly for cancer metastasis. Recently, CAP-activated media
were demonstrated to have sufficient antitumor effects toward a
number of cell types (13),
including HeLa (14), glioblastoma
(15), breast cancer (16), ovarian cancer (17) and pancreatic cancer cells (18). There are also reports on the
selective antitumor effects of buffered saline (19) and Ringer's solution (20). These findings suggest that CAP
treatment is more than a drug therapy. According to previous
studies, ROS production may be the main mechanism of CAP-induced
apoptosis (21–23). However, the specific description of
this mechanism remains unclear. Furthermore, there are reports that
different cell lines reveal different responses to CAP
treatment.
The present study used Ringer's solution as the
mediator of CAP treatment as its composition is simple and easy to
obtain. CAP-activated Ringer's solution was prepared and used to
treat human OS cells. MTT assay, apoptosis detection
[Annexin-V/propidium iodide (PI)], ROS level determination, JC-1
assay and western blot analysis were performed to determine the
effects of CAP-activated Ringer's solution on OS cells. The aim of
the present study was to analyze the effects of CAP-activated
Ringer's solution on human OS cell lines MG63 and U2OS, and to
further characterize its cellular effects and potential molecular
mechanisms. These results represent an important advancement in the
clinical applications of plasma-activated solutions.
Materials and methods
Cell culture
The OS cell lines MG63 and U2OS and the human
osteoblast hFOB1.19 cell line were obtained from the Shanghai
Institute of Life Sciences, Chinese Academy of Sciences. OS cells
were cultured in high glucose Dulbecco's modified Eagle medium
(DMEM; HyClone; pH 7.0–7.4) with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin, and
100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.). All
cells were incubated in a humidified cell incubator (Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2. The adherent OS
cells were trypsinized, collected by centrifugation, resuspended in
fresh medium and passaged at a ratio of 1:4.
Preparation of plasma-activated
solution
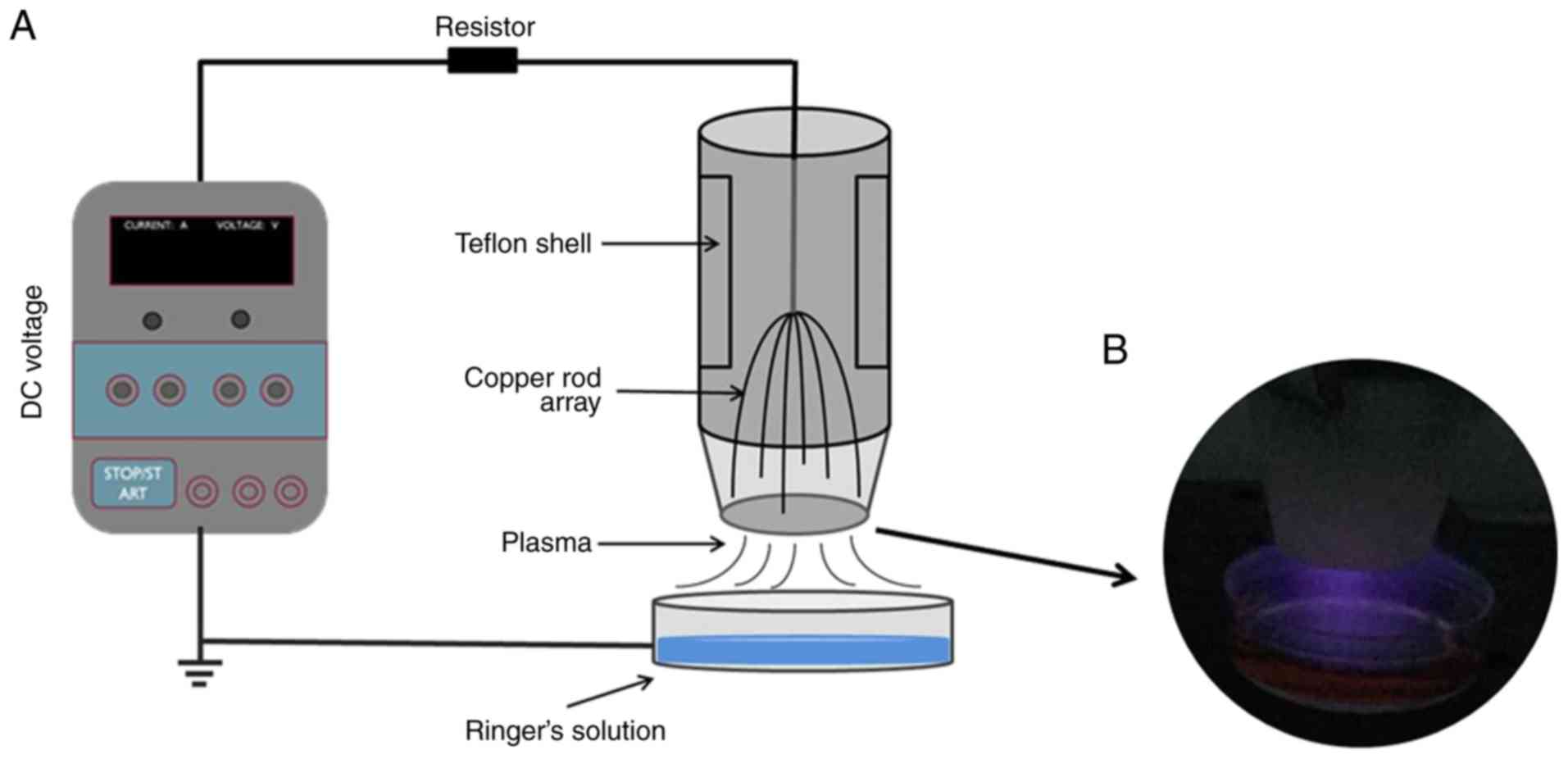
The plasma device used in the present study was an
air-dependent instrument designed and developed by the Institute of
Plasma Physics, Chinese Academy of Sciences. The schematic graph
and image are presented in Fig. 1.
The air plasma discharge electrode consists of six copper rods with
a diameter of 1 mm. Other articles have reported similar types of
configuration (24,25). In the present study, the six
electrodes were driven by high voltage direct current (DC) without
the requirement for any external gas supply. Ringer's solution
acted as a grounded electrode. The input voltage was 10 kV, the
discharge current was 5 mA, and the ballast resistor R that limited
the discharge current was 30 mΩ. Therefore, the gas temperature of
the air plasma generated was controlled at 20–30°C. In the present
study, 3 ml Ringer's solution (lactated Ringer's solution) was
placed in a 35-mm Petri dish with a distance from the electrode of
1.0 cm, and was treated with a CAP device for 60, 120, 180, 240 or
300 sec.
Treatment of cells with
plasma-activated solution
MG63, U2OS and hFOB1.19 cells were incubated with
CAP-activated Ringer's solution for 30 min, washed twice with
phosphate-buffered saline (PBS), and cultured for 24 h with fresh
medium. For some cells, ROS and JC-1 detection was immediately
performed following treatment with CAP-activated Ringer's
solution.
Cell viability assay
The effect of CAP-activated Ringer's solution on
cell viability was determined using an MTT assay (Sigma Aldrich;
Merck KGaA) according to the manufacturer's protocol. Cells were
trypsinized with trypsin/ethylenediaminetetraacetic acid
(trypsin/EDTA) (HyClone), seeded into 96-well plates at a density
of 10,000 cells/well in triplicate, and cultured for 24 h.
CAP-activated Ringer's solution was used in the experimental
groups, and untreated Ringer's solution functioned as the control
group. Cells were incubated with either CAP-activated Ringer's
solution or untreated Ringer's solution for 30 min, washed with
PBS, and cultured with fresh medium for 24 h Then, 10 µl MTT
solution (5 mg/ml) was added to each well and incubated for 4 h.
Next, 100 µl DMSO was added to each well and thoroughly mixed on a
shaker to dissolve the purple-violet crystal. Finally, the optical
density (OD) value of the purple-violet crystal at 570 nm was
measured using the EnSpire Multimode Plate Reader R (PerkinElmer).
In addition, cell viability was calculated as Experimental group OD
value/Control group OD value ×100%.
Apoptosis determination
Apoptosis induced by CAP-activated Ringer's solution
was measured using Annexin V-FITC (BB-4101-50T) according to the
manufacturer's protocol. The MG63 cells were treated with
CAP-activated Ringer's solution for 30 min and cultured for 24 h.
OS cells were digested with trypsin without EDTA and washed twice
with PBS. A total of 1×105 cells were resuspended in the
binding buffer, and 5 µl Annexin V-FITC was added to the cell
suspension and incubated for 15 min at 4°C in the dark. The cells
were then mixed with 10 µl PI and incubated for another 5 min at
4°C in the dark. Finally, cells were detected by flow cytometry (BD
Biosciences) and analyzed for apoptosis using FlowJo7.6.5 software
(Tree Star Inc.).
Determination of mitochondrial
membrane potential (ΔΨm)
The ΔΨm was determined using JC-1
(5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocya-nineiodide;
Beyotime Biotechnology; C2006). According to the manufacturer's
protocol, the cells were incubated with CAP-activated Ringer's
solution for 30 min, washed twice with PBS, and resuspended in 0.5
ml cell culture medium. Then, 0.5 ml JC-1 staining solution was
added and inverted several times to mix well. Next, the cells were
incubated at 37°C for 20 min and centrifuged at 600 × g for 3–4 min
at 4°C to form a pellet. The cells were washed twice with JC-1
staining buffer (1X) and resuspended in an appropriate amount of
JC-1 staining buffer (1X). The fluorescence intensities of the
fluorescent dye excited at 490 and 590 nm were measured by flow
cytometry (BD Biosciences), and the ΔΨm was reflected by the JC-1
polymer/monomer ratio or the absorbance of the JC-1 polymer.
Western blot analysis
The expression of apoptotic proteins at 0, 2, 4 and
8 h after MG63 cells were treated with CAP-activated Ringer's
solution was analyzed via western blotting. Cells were incubated
with CAP-activated Ringer's solution for 30 min, and adherent and
floating cells were collected. Mitochondria and cytoplasmic
proteins were extracted using a mitochondrial extraction kit
(Solarbio) according to the manufacturer's protocol. Protein
quantification was performed using a BCA assay, and 40 µg of
protein samples were separated via SDS-PAGE (10% gel) and
transferred to a polyvinylidene difluoride (PVDF) membrane. The
membrane was blocked with Tris-buffered saline (TBST) buffer and 5%
(M/V) skimmed milk powder, and incubated with caspase-3 (dilution
1:500; cat. no. WL03339), cleaved caspase-3 (dilution 1:1,000; cat.
no. WL01857), PARP (dilution 1:500; cat. no. WL01578), cytochrome
c (Cyt c; dilution 1:500; cat. no. WL01571) and β-actin
(dilution 1:1,000; cat. no. WL01845) primary antibody at 4°C
overnight (all antibodies were purchased from Wanleibio Co., Ltd.).
After washing, the blot was incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(dilution 1:5,000; cat. no. WLA023; Wanleibio Co., Ltd.) for 45 min
at room temperature. After washing with TBST, the blot was exposed
in a dark room using an ECL+ chemiluminescence kit. The film was
scanned, and the OD of the target bands was analyzed using a gel
image processing system (Gel-Pro Analyzer software; Tanon
Science & Technology Co., Ltd.).
Intracellular reactive oxygen species
(ROS) detection
The ROS level changes in OS cells treated with
CAP-activated Ringer's solution were detected using
2′,7′-dichlorofluorescein diacetate (DCFH-DA). OS cells were seeded
at a density of 4×105 cells/well in 6-well plates in
triplicate and cultured for 24 h. Immediately after treatment of
cells with CAP-activated Ringer's solution, 10 µM DCFH-DA was added
and incubated for 30 min at 37°C in the dark with 5%
CO2. The intracellular ROS level was measured using an
EnSpire multimode plate reader (PerkinElmer) with 488 nm excitation
wavelength and 525 nm emission filter.
Statistical analysis
The data are presented as the mean ± standard
deviation of at least three independent experiments. Statistical
analysis was performed using GraphPad Prism 7.0 software (GraphPad
Software, Inc.). Differences were assessed by two-sample t-test or
one-way ANOVA. LSD post hoc and Dunnett's post hoc tests were used
where appropriate. P≤0.05 and P≤0.01 were considered to indicate a
statistically significant difference.
Results
CAP-activated Ringer's solution
affects cell morphology
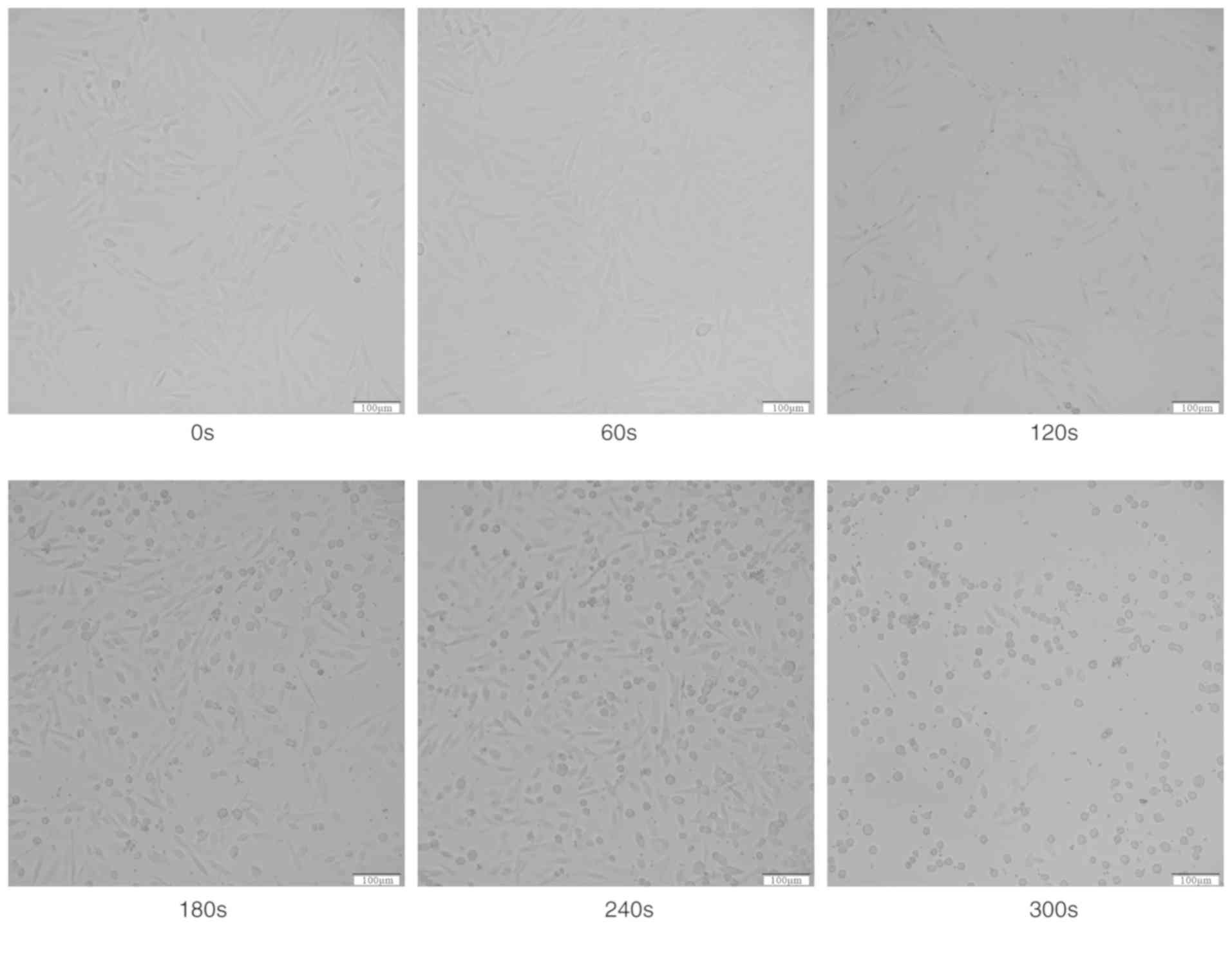
The present study observed that treatment with
CAP-activated Ringer's solution altered the morphology of OS cells.
Compared with the control cells, the cells demonstrated shrinkage
following treatment with Ringer's solution that was exposed to CAP
for 180 sec, and a small amount of cells became suspended in the
cell culture medium. When the exposure time reached 300 sec, the
spindle cells rounded and shriveled, and more cells were suspended
in the medium (Fig. 2).
CAP-activated Ringer's solution
decreases cell viability
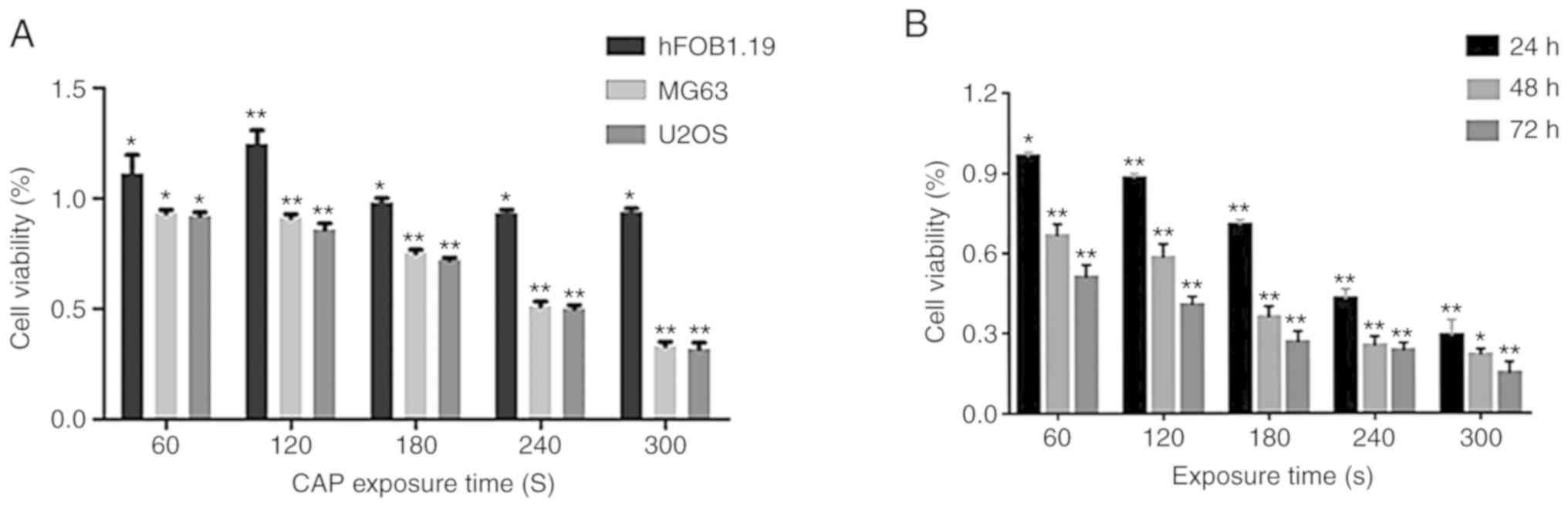
OS cells treated with CAP-activated Ringer's
solution were assayed using the MTT method after 24 h.
CAP-activated Ringer's solution treatment inhibited the
proliferation of MG63 and U2OS OS cells in a dose-dependent manner
(Fig. 3A). When the exposure time
was extended to 240 sec, the viability of MG63 and U2OS cells
treated with CAP-activated Ringer's solution was significantly
decreased to 40.04 and 35.00%, respectively. When the exposure time
was extended to 300 sec, the inhibition rates were >70%.
However, the human osteoblast hFOB1.19 cells treated with
CAP-activated Ringer's solution did not exhibit significant
inhibition of growth, but instead displayed promotion of growth
under the exposure time of <120 sec. The inhibition effect was
still detectable 48 and 72 h after treatment with CAP-activated
Ringer's solution (Fig. 3B).
CAP-activated Ringer's solution
induces apoptosis
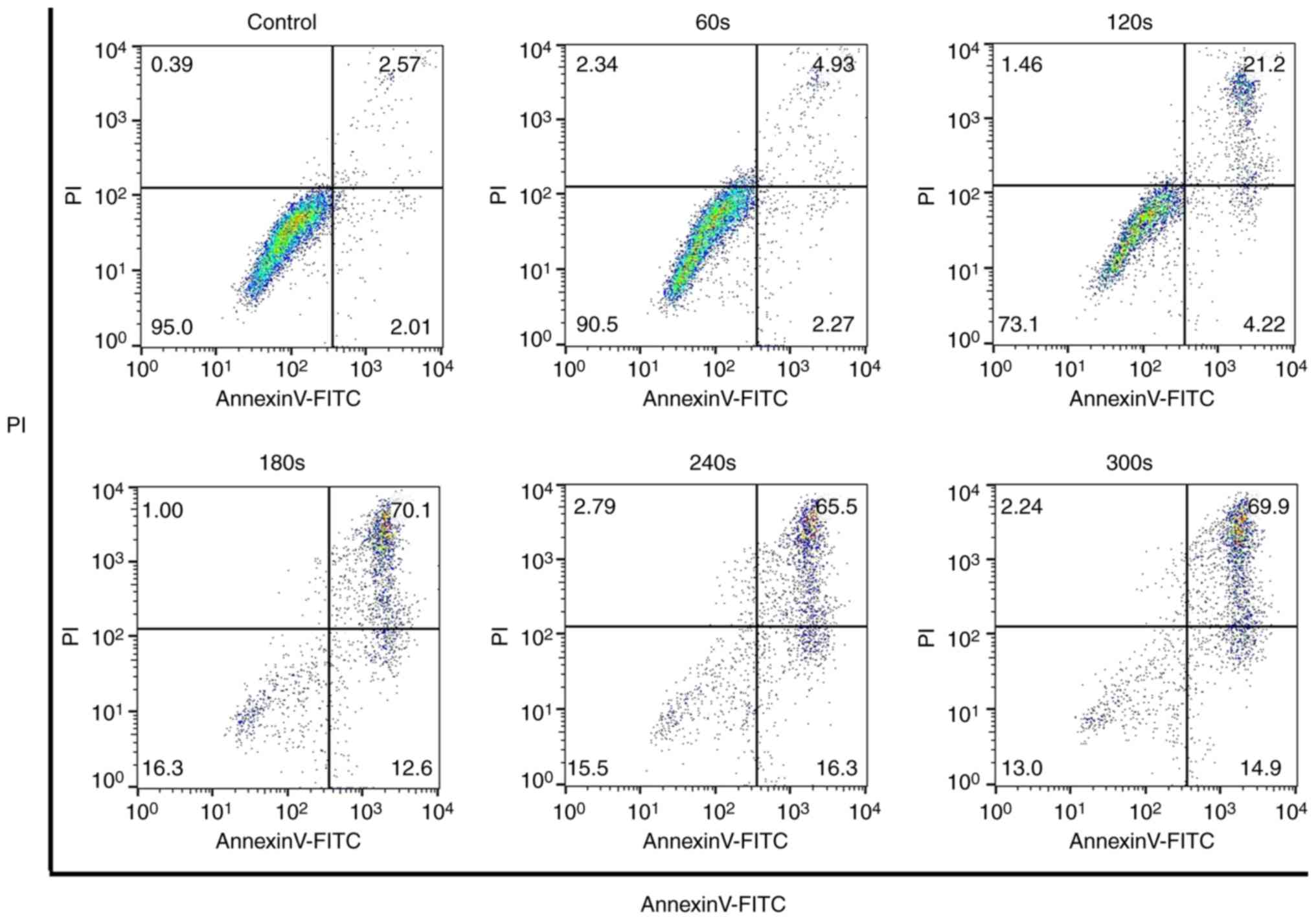
The Annexin V-FITC and PI staining confirmed that
when the exposure time to CAP reached 180 sec, the apoptotic rate
of OS cells was significantly increased (Fig. 4). When the exposure time reached 300
sec, ~84% of the cells were in an apoptotic state. The expression
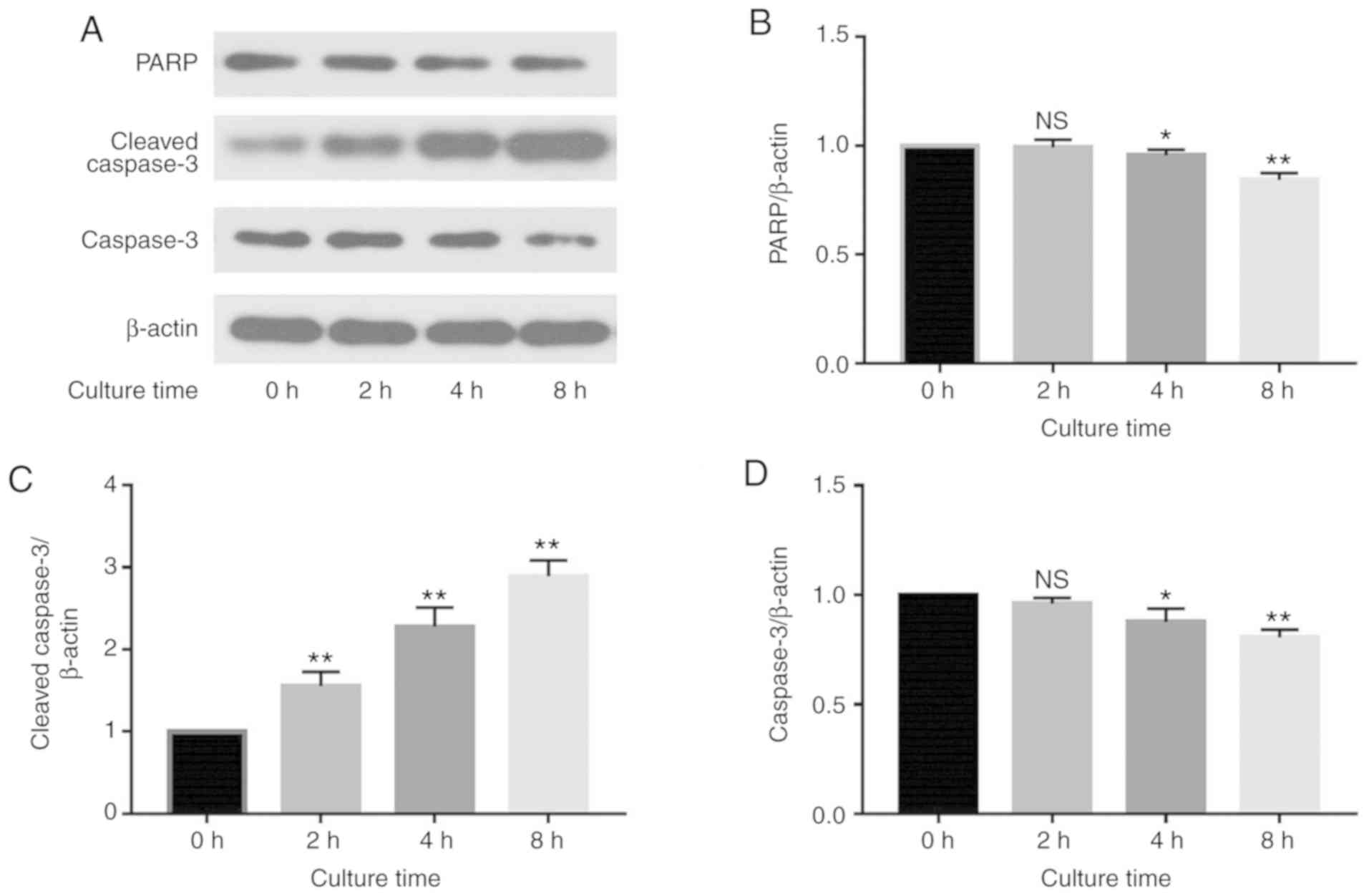
of caspase-3 and PARP in MG63 cells was assessed via western
blotting (Fig. 5). The results
revealed that at 0, 2, 4 and 8 h after the 30-min incubation with
CAP-activated Ringer's solution (CAP exposure time of 180 sec), the
expression levels of caspase-3 and PARP were decreased in a
time-dependent manner (Fig. 5B and
D), and the level of cleaved caspase-3 was significantly
increased in a time-dependent manner (Fig. 5C) (P<0.05). These findings
indicate that CAP-activated Ringer's solution induces apoptosis in
MG63 cells via the caspase-3-dependent pathway.
CAP-activated Ringer's solution
reduces cell mitochondrial membrane potential
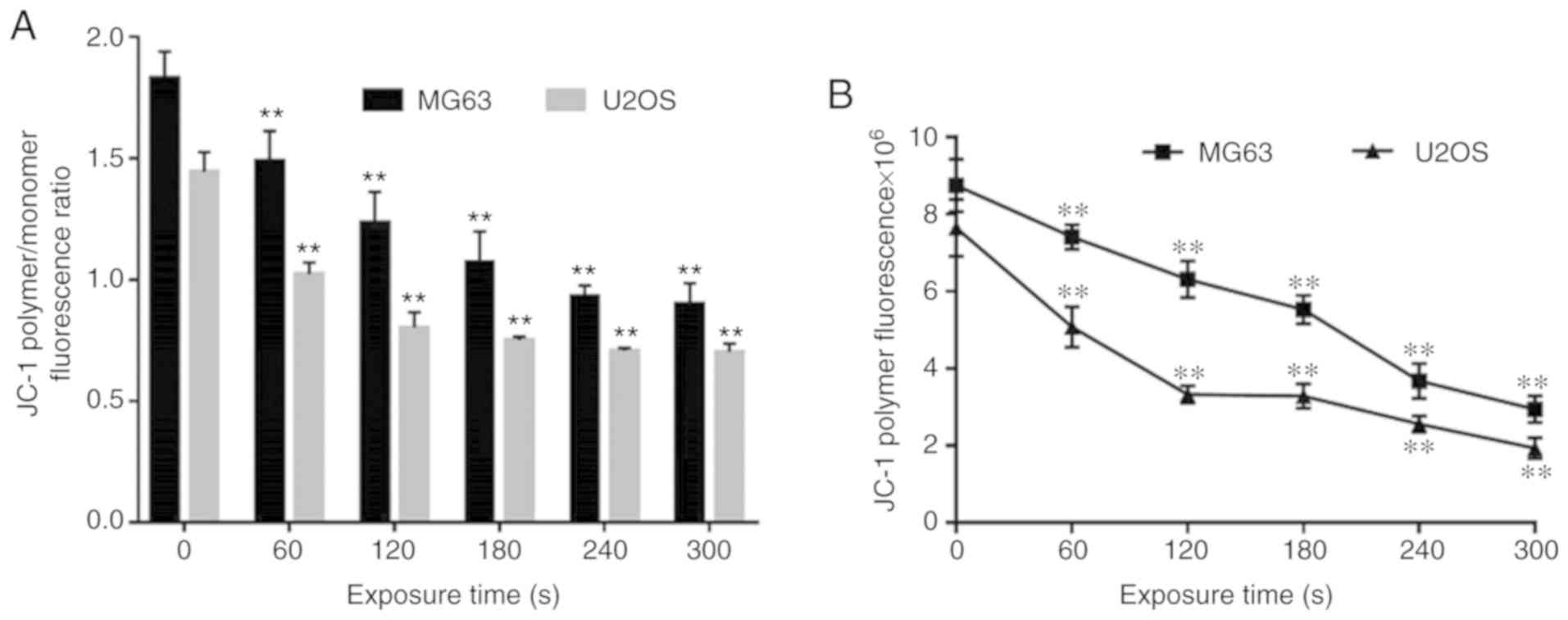
With the elongation of exposure time of Ringer's
solution to CAP, the content of JC-1 polymers in the mitochondria
of cells of the experimental groups was significantly decreased
compared with that of the control group (Fig. 6B), and the JC-1 polymer/monomer
ratio was also significantly decreased (Fig. 6A), which indicates a decrease in
mitochondrial membrane potential (P<0.01).
CAP-activated Ringer's solution
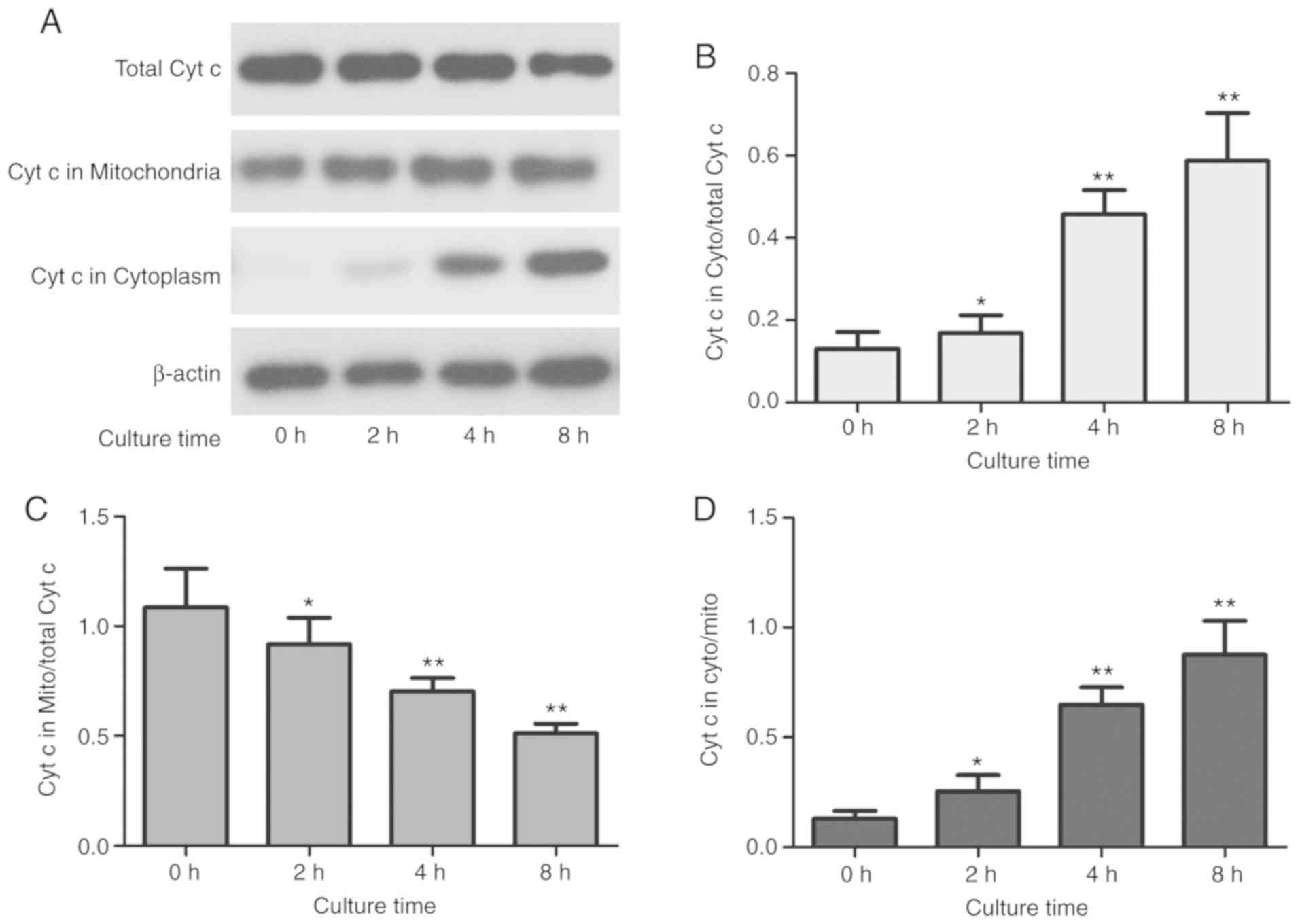
induces cytochrome c (Cyt c) release
OS cells treated with CAP-activated Ringer's
solution with an exposure time of 180 sec showed decreased Cyt c
expression in the mitochondria, but significantly increased Cyt c
levels in the cytoplasm (P<0.01) (Fig. 7B and C), The total cytochrome
c content did not change significantly, suggesting that Cyt
c is released from mitochondria into the cytosol in MG63 cells
treated with CAP-activated Ringer's solution (Fig. 7A). Furthermore, the level of Cyt c
in each component was altered in a time-dependent manner (Fig. 7B-D).
CAP-activated Ringer's solution causes
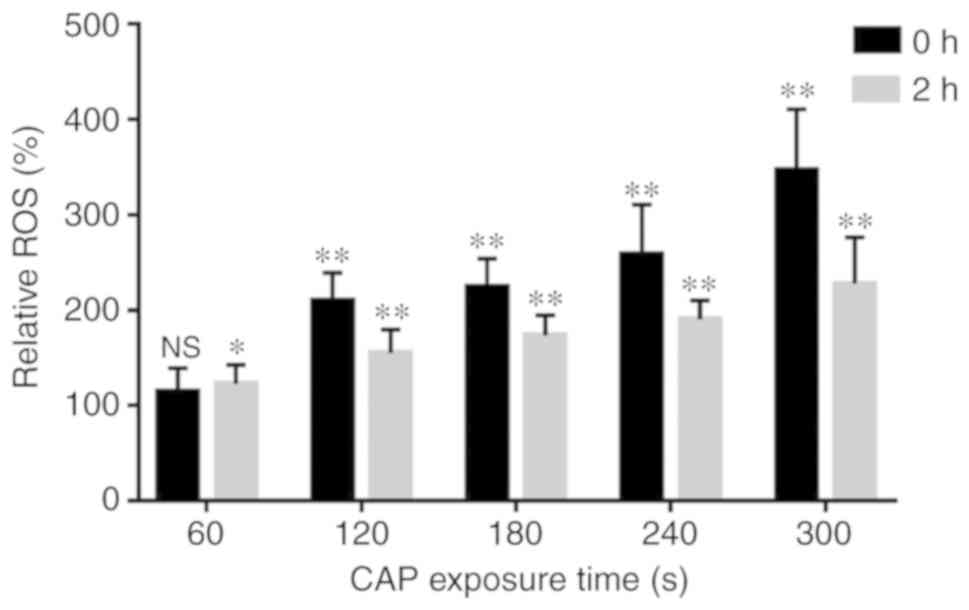
production of intracellular ROS
The present study detected a significant increase in
intracellular ROS upon the exposure of Ringer's solution to CAP
(P<0.01). The ROS level reached its maximum when the exposure
time was 300 sec, which was >3 times that without exposure. At 2
h after treatment with CAP-activated Ringer's solution, the
intracellular ROS content was significantly decreased compared with
the level detected immediately after treatment (Fig. 8).
Discussion
In recent years, techniques for limb salvage surgery
have greatly advanced with the improvement of osteosarcoma (OS)
treatments (26). However, the
accompanying tumor contamination of the surgical area and the
microresidual lesions in the peritumoral tissue have become a
problem for the orthopedist. Negative microscopic observation
results of the margin of the tumor do not guarantee maximum tumor
resection (27). The same situation
is also present in benign tumors that are prone to recurrence, such
as giant cell tumor of bone and osteoblastoma. Akihiko et al
performed a long-term follow-up of 461 patients with OS, and the
results revealed that the 5-year survival rate of 45 patients with
recurrence was 30% and the 10-year survival rate was only 13%
(28). Therefore, the residual
tumor cells in surgeries that are invisible to the naked eye are
expected to be destroyed through plasma-activated solution,
achieving controlled tumor recurrence. In the meantime, studies
have confirmed that cold atmospheric plasma (CAP)-activated
solution has little effect on normal cells (19,29).
Schuster et al found that CAP can increase the frequency of
tumor surface response and the rate of apoptosis in clinical
studies of CAP in the treatment of human head and neck squamous
cell carcinoma (30). Both in
vivo and in vitro experiments have demonstrated that CAP
inhibition of a variety of tumor cells is exerted through apoptosis
(15,31–34).
The determination of apoptosis is usually conducted by a
combination of multiple methods. In the present study, the
inhibition of proliferation by CAP-activated Ringer's solution was
first tested on OS cells using an MTT assay, and the results
revealed that the cell proliferation was significantly inhibited
when OS cells were treated with activated Ringer's solution, with
the inhibition rate reaching 70%. It was also revealed that plasma
inhibition of cell proliferation was significantly time- and
dose-dependent. Activated Ringer's solution has no obvious
inhibitory effect on the growth of human osteoblasts, and so it was
also observed that plasma had a selective inhibitory effect on
tumor cells. The present study demonstrated that CAP-activated
Ringer's solution exhibited a significant inhibitory effect on OS
cell viability. In order to prove that this inhibitory effect leads
to OS cell death induced by CAP-activated Ringer's solution through
programmed apoptosis, the present study examined the double
staining of Annexin V/PI on cells via flow cytometry, and revealed
that the cell numbers in the early and late stages of apoptosis
caused by plasma treatment were higher than those in the
no-treatment control group, indicating that plasma treatment can
induce apoptosis of OS cells. This apoptosis also demonstrated a
significant time- and dose-dependence.
Mitochondria are considered the most important sites
of apoptosis during the apoptotic process in a number of different
types of cells (35). Decreased
mitochondrial membrane potential (ΔΨm) is an early marker of
apoptosis (36,37). When the mitochondrial membrane of
normal live cells is relatively intact, the membrane potential is
high, and JC-1 exists in the form of polymers in the mitochondria.
When cells undergo apoptosis, the mitochondrial membrane is
damaged, the mitochondrial membrane potential is decreased, and
JC-1 is present as a monomer (38,39).
Therefore, the present study used JC-1 to analyze ΔΨm. The results
of the present study also revealed that as the plasma exposure time
increased, the mitochondrial membrane potential gradually
decreased.
There is a large number of apoptosis-inducing
factors in mitochondria and on the mitochondrial membrane, such as
cytochrome c (Cyt c) and caspase precursors. Changes in
mitochondrial membrane permeability and mitochondrial membrane
potential result in the release of Cyt c from the mitochondria into
the cytoplasm, which activates caspase-3 and thereby activates
downstream polyADP ribose polymerase (PARP) to amplify apoptotic
signals. PARP is an important apoptotic executive protein that
directly leads to apoptosis (35,40).
Further confirming that the CAP-activated Ringer's solution induces
the apoptosis signaling pathway, the western blotting results of
the present study revealed a decrease in mitochondrial Cyt c
expression, an increase in cytosolic Cyt c expression, and
time-dependent expression of caspase-3 and PARP.
Reactive oxygen species (ROS) are a class of
oxygen-containing substances produced by aerobic cells during
aerobic metabolism that have extremely high biological activities.
ROS play an important role in the proliferation and apoptosis of
cells. A certain amount of ROS in the cells is necessary for the
maintenance of normal physiological condition of the cells
(41). However, high concentrations
of ROS can directly induce changes in mitochondrial membrane
permeability and even mitochondrial damage, cause damage to
intracellular proteins, lipids and DNA molecules, and lead to cell
death (42). Studies have
demonstrated that ROS are an important factor in CAP-induced
apoptosis; however, the stability of ROS is poor (43). The present study used DCFH-DA as a
fluorescent probe to measure intracellular ROS levels. The results
revealed that as the plasma exposure time increased, the
intracellular ROS level gradually increased, indicating that ROS
are involved in plasma-induced apoptosis. When the exposure time
reached 300 sec, the ROS level reached the maximum. As the
post-treatment time was prolonged, the level of ROS was decreased,
which may be due to the instability of intracellular ROS or the
antioxidant capacity of the cells per se that prevents
further decrease of ROS. However, 48 and 72 h after CAP-activated
Ringer's solution treatment, OS cells still demonstrated certain
inhibitory effects, which were stronger than those at 24 h,
indicating that ROS may not be the only factor inhibiting the
growth of MG63 cells. The activated Ringer's solution may contain
substances that are more stable and can continuously inhibit the
proliferation of OS cells.
Tanaka et al detected little ROS in
glioblastoma cells treated with plasma-activated Ringer's solution,
while detecting a large amount of H2O2 in the
plasma-activated Ringer's solution, and suggested that
H2O2 is the primary factor that induces
apoptosis in plasma-activated Ringer's solution (20). However, in that study, cells were
treated with diluted PAL (plasma-activated lactated Ringer's
solution) solution, which may result in the active ingredients in
diluted plasma-activated Ringer's solution not reaching the
threshold to induce massive ROS production. Alternatively, this
different result may be due to the different plasma devices and
different cell types. The composition of Ringer's solution is
simple (NaCl, 6.0 g/l; KCl, 0.3 g/l; CaCl2, 0.2 g/l; and
L-sodium lactate, 3.1 g/l). Tanaka et al used CAP to
separately activate a double concentrated solution of each
component and demonstrated that L-lactate exhibited an antitumor
effect on U251SP cells (20).
However, that study failed to analyze the association between more
than two components. In the future, it will be necessary to conduct
an in-depth study on the interaction between the components of
activated Ringer's solution, to confirm the feasibility of
activated Ringer's solution in clinical applications, and to
determine the storage methods and conditions.
The present study verified that CAP-activated
Ringer's solution has a significant selective inhibition of OS
cells, and intracellular ROS was an important factor during this
process. The potential mechanism may be that the increase in
intracellular ROS content leads to changes in mitochondrial
membrane permeability and mitochondrial membrane potential, which
lead to the release of Cyt c into the cytoplasm. The release of Cyt
c further activates a series of downstream apoptotic responses to
induce apoptosis, indicating that activated Ringer's solution
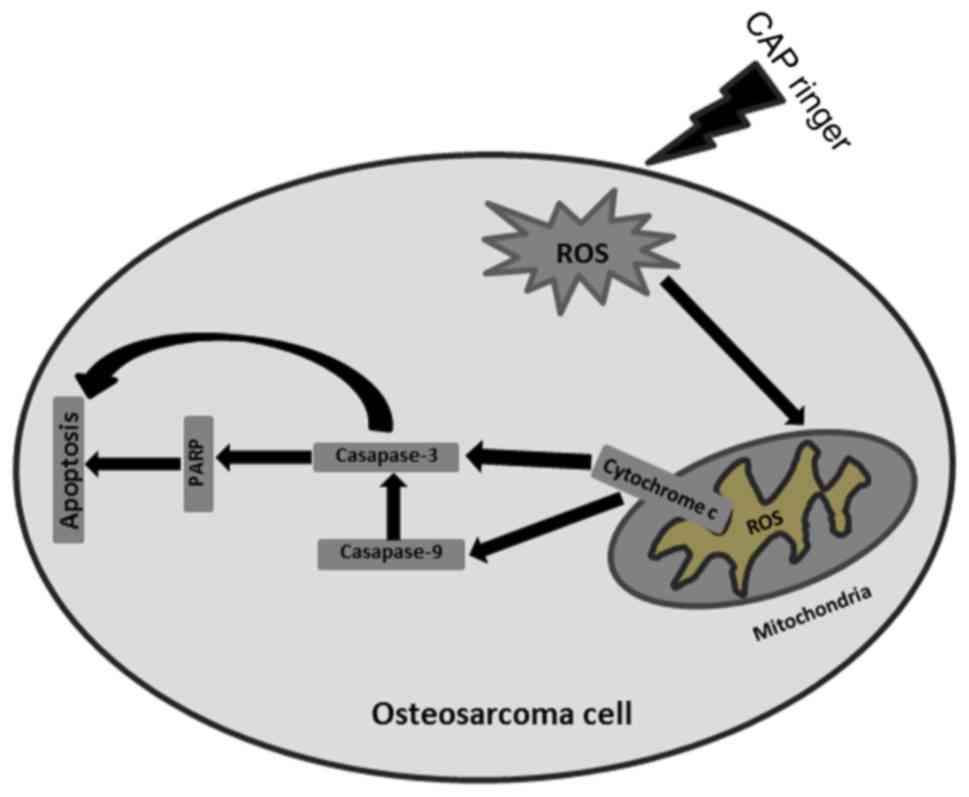
activates the mitochondrial pathway of apoptosis (Fig. 9).
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Natural Science Foundation Project of Anhui Province (grant no.
1708085MH215) and the School Fund of Anhui Medical University
(grant no. 2015×kj088).
Availability of data and materials
The data used to support the findings of the present
study are available from the corresponding author upon request.
Authors' contributions
YW and SX conceived and designed the experiments. YW
performed the experiments. CM, YQ, SC, HW and XY collected the data
and performed the statistical analysis. YW with the help of GZ and
YH wrote the manuscript and revised it critically for important
intellectual content. CY and CC provided the plasma equipment and
guided the experiments. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experiments conducted in the present study were
approved by the Ethics Committee of The First Affiliated Hospital,
Anhui Medical University (Anhui, China).
Patient consent for publication
Not applicable.
Competing interests
The authors have declared that they have no
competing interests.
Glossary
Abbreviations
Abbreviations:
|
CAP
|
cold atmospheric plasma
|
|
OS
|
osteosarcoma
|
|
ROS
|
reactive oxygen species
|
|
Ringer
|
lactated Ringer's solution
|
References
|
1
|
Geller DS and Gorlick R: Osteosarcoma: A
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.PubMed/NCBI
|
|
2
|
Blattmann C, Oertel S, Schulz-Ertner D,
Rieken S, Haufe S, Ewerbeck V, Unterberg A, Karapanagiotou-Schenkel
I, Combs SE, Nikoghosyan A, et al: Non-randomized therapy trial to
determine the safety and efficacy of heavy ion radiotherapy in
patients with non-resectable osteosarcoma. BMC Cancer. 10:962010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bacci G, Longhi A, Versari M, Mercuri M,
Briccoli A and Picci P: Prognostic factors for osteosarcoma of the
extremity treated with neoadjuvant chemotherapy: 15-year experience
in 789 patients treated at a single institution. Cancer.
106:1154–1161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang W, Yang J, Wang Y, Wang D, Han G, Jia
J, Xu M and Bi W: Survival and prognostic factors in Chinese
patients with osteosarcoma: 13-year experience in 365 patients
treated at a single institution. Pathol Res Pract. 213:119–125.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan D, Sherman JH and Keidar M: Cold
atmospheric plasma, a novel promising anti-cancer treatment
modality. Oncotarget. 8:15977–15995. 2017.PubMed/NCBI
|
|
7
|
Keidar M, Yan D, Beilis II, Trink B and
Sherman JH: Plasmas for treating cancer: Opportunities for adaptive
and self-adaptive approaches. Trends Biotechnol. 36:586–593. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee HJ, Shon CH, Kim YS, Kim S, Kim GC and
Kong MG: Degradation of adhesion molecules of G361 melanoma cells
by a non-thermal atmospheric pressure microplasma. New J Phys.
11:1150262009. View Article : Google Scholar
|
|
9
|
Schmidt A and Bekeschus S: Redox for
repair: Cold physical plasmas and Nrf2 signaling promoting wound
healing. Antioxidants (Basel). 7(pii): E1462018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iseki S, Ohta T, Aomatsu A, Ito M, Kano H,
Higashijima Y and Hori M: Rapid inactivation of penicillium
digitatum spores using high-density nonequilibrium atmospheric
pressure plasma. Appl Phys Lett. 96:1537042010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calvo T, Alvarez-Ordóñez A, Prieto M,
Bernardo A and López M: Stress adaptation has a minor impact on the
effectivity of non-thermal atmospheric plasma (NTAP) against
salmonella spp. Food Res Int. 102:519–525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Köritzer J, Boxhammer V, Schäfer A,
Shimizu T, Klämpfl TG, Li YF, Welz C, Schwenk-Zieger S, Morfill GE,
Zimmermann JL and Schlegel J: Restoration of sensitivity in
chemo-resistant glioma cells by cold atmospheric plasma. PLoS One.
8:e644982013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chauvin J, Gibot L, Griseti E, Golzio M,
Rols MP, Merbahi N and Vicendo P: Elucidation of in vitro cellular
steps induced by antitumor treatment with plasma-activated medium.
Sci Rep. 9:48662019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boehm D, Curtin J, Cullen PJ and Bourke P:
Hydrogen peroxide and beyond-the potential of high-voltage
plasma-activated liquids against cancerous cells. Anticancer Agents
Med Chem. 18:815–823. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan D, Cui H, Zhu W, Nourmohammadi N,
Milberg J, Zhang LG, Sherman JH and Keidar M: The specific
vulnerabilities of cancer cells to the cold atmospheric
plasma-stimulated solutions. Sci Rep. 7:44792017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiang L, Xu X, Zhang S, Cai D and Dai X:
Cold atmospheric plasma conveys selectivity on triple negative
breast cancer cells both in vitro and in vivo. Free Radic Biol Med.
124:205–213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Utsumi F, Kajiyama H, Nakamura K, Tanaka
H, Mizuno M, Ishikawa K, Kondo H, Kano H, Hori M and Kikkawa F:
Effect of indirect nonequilibrium atmospheric pressure plasma on
anti-proliferative activity against chronic chemo-resistant ovarian
cancer cells in vitro and in vivo. PLoS One. 8:e815762013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bekeschus S, Käding A, Schröder T, Wende
K, Hackbarth C, Liedtke KR, van der Linde J, von Woedtke T,
Heidecke CD and Partecke LI: Cold physical plasma-treated buffered
saline solution as effective agent against pancreatic cancer cells.
Anticancer Agents Med Chem. 18:824–831. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Yang X, Yang C, Gao J, Zhao Y,
Cheng C, Zhao G and Liu S: The inhibition effect of cold
atmospheric plasma-activated media in cutaneous squamous carcinoma
cells. Future Oncol. 15:495–505. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka H, Nakamura K, Mizuno M, Ishikawa
K, Takeda K, Kajiyama H, Utsumi F, Kikkawa F and Hori M:
Non-thermal atmospheric pressure plasma activates lactate in
Ringer's solution for anti-tumor effects. Sci Rep. 6:362822016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Judée F, Fongia C, Ducommun B, Yousfi M,
Lobjois V and Merbahi N: Short and long time effects of low
temperature plasma activated media on 3D multicellular tumor
spheroids. Sci Rep. 6:214212016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cooper M, Fridman G, Staack D, Gutsol AF,
Vasilets VN, Anandan S, Cho YI, Fridman A and Tsapin A:
Decontamination of surfaces from extremophile organisms using
nonthermal atmospheric-pressure plasmas. IEEE Trans Plasma Sci.
37:866–871. 2009. View Article : Google Scholar
|
|
23
|
Chauvin J, Judée F, Yousfi M, Vicendo P
and Merbahi N: Analysis of reactive oxygen and nitrogen species
generated in three liquid media by low temperature helium plasma
jet. Sci Rep. 7:45622017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ruan Z, Guo Y, Gao J, Yang C, Lan Y, Shen
J, Xu Z, Cheng C, Liu X, Zhang S, et al: Control of
multidrug-resistant planktonic Acinetobacter baumannii: Biocidal
efficacy study by atmospheric-pressure air plasma. Plasma Sci
Technol. 20:0655132018. View Article : Google Scholar
|
|
25
|
Liu Y, Tan S, Zhang H, Kong X, Ding L,
Shen J, Lan Y, Cheng C, Zhu T and Xia W: Selective effects of
non-thermal atmospheric plasma on triple-negative breast normal and
carcinoma cells through different cell signaling pathways. Sci Rep.
7:79802017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ottaviani G, Robert RS, Huh WW, Palla S
and Jaffe N: Sociooccupational and physical outcomes more than 20
years after the diagnosis of osteosarcoma in children and
adolescents: Limb salvage versus amputation. Cancer. 119:3727–3736.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mavrogenis AF, Abati CN, Romagnoli C and
Ruggieri P: Similar survival but better function for patients after
limb salvage versus amputation for distal tibia osteosarcoma. Clin
Orthop Relat Res. 470:1735–1748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takeuchi A, Lewis VO, Satcher RL, Moon BS
and Lin PP: What are the factors that affect survival and relapse
after local recurrence of osteosarcoma? Clin Orthop Relat Res.
472:3188–3195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guerrero-Preston R, Ogawa T, Uemura M,
Shumulinsky G, Valle BL, Pirini F, Ravi R, Sidransky D, Keidar M
and Trink B: Cold atmospheric plasma treatment selectively targets
head and neck squamous cell carcinoma cells. Int J Mol Med.
34:941–946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schuster M, Seebauer C, Rutkowski R,
Hauschild A, Podmelle F, Metelmann C, Metelmann B, von Woedtke T,
Hasse S, Weltmann KD and Metelmann HR: Visible tumor surface
response to physical plasma and apoptotic cell kill in head and
neck cancer. J Craniomaxillofac Surg. 44:1445–1452. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koensgen D, Besic I, Gümbel D, Kaul A,
Weiss M, Diesing K, Kramer A, Bekeschus S, Mustea A and Stope MB:
Cold atmospheric plasma (CAP) and CAP-stimulated cell culture media
suppress ovarian cancer cell growth-A putative treatment option in
ovarian cancer therapy. Anticancer Res. 37:6739–6744.
2017.PubMed/NCBI
|
|
32
|
Yan D, Talbot A, Nourmohammadi N, Cheng X,
Canady J, Sherman J and Keidar M: Principles of using cold
atmospheric plasma stimulated media for cancer treatment. Sci Rep.
5:183392015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Z, Li L, Cheng X, Gjika E and Keidar
M: Effects of cold atmospheric plasma generated in deionized water
in cell cancer therapy. Plasma Process Polym. 13:1151–1156. 2016.
View Article : Google Scholar
|
|
34
|
Li XY, Feng Z, Pu SC, Yun Y, Shi XM and Xu
Z: Cold atmospheric plasma jet-generated oxidized derivatives of
tryptophan and their selective effects on murine melanoma and
fibroblast cells. Plasma Chem Plasma Process. 38:919–936. 2018.
View Article : Google Scholar
|
|
35
|
Qiao J, Wu Y, Liu Y, Li X, Wu X, Liu N,
Zhu F, Qi K, Cheng H, Li D, et al: Busulfan triggers intrinsic
mitochondrial-dependent platelet apoptosis independent of platelet
activation. Biol Blood Marrow Transplant. 22:1565–1572. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lemeshko VV: VDAC electronics: 3.
VDAC-Creatine kinase-dependent generation of the outer membrane
potential in respiring mitochondria. Biochim Biophys Acta.
1858:1411–1418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lemeshko VV: VDAC electronics: 5 Mechanism
and computational model of hexokinase-dependent generation of the
outer membrane potential in brain mitochondria. Biochim Biophys
Acta Biomembr. 1860:2599–2607. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elefantova K, Lakatos B, Kubickova J,
Sulova Z and Breier A: Detection of the mitochondrial membrane
potential by the cationic dye JC-1 in L1210 cells with massive
overexpression of the plasma membrane ABCB1 drug transporter. Int J
Mol Sci. 19(pii): E19852018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brooks MM, Neelam S, Fudala R, Gryczynski
I and Cammarata PR: Lenticular mitoprotection. Part A: Monitoring
mitochondrial depolarization with JC-1 and artifactual fluorescence
by the glycogen synthase kinase-3β inhibitor, SB216763. Mol Vis.
19:1406–1412. 2013.PubMed/NCBI
|
|
40
|
Haeberlein SL: Mitochondrial function in
apoptotic neuronal cell death. Neurochem Res. 29:521–530. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee HS, Hwang CY, Shin SY, Kwon KS and Cho
KH: MLK3 is part of a feedback mechanism that regulates different
cellular responses to reactive oxygen species. Sci Signal.
7:ra522014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song HJ, Lee EK, Lee JA, Kim HL and Jang
KW: The addition of mifamurtide to chemotherapy improves lifetime
effectiveness in children with osteosarcoma: A Markov model
analysis. Tumour Biol. 35:8771–8779. 2014. View Article : Google Scholar : PubMed/NCBI
|