Introduction
Vascular endothelial growth factor-A (VEGF-A) is a
key mediator of angiogenesis via class IV tyrosine kinase receptor
family of VEGF receptors (VEGFRs) (1). Three types of membrane VEGFRs have
been described, VEGFR-1-3, which are encoded by different genes.
The various VEGF-A isoforms facilitate angiogenesis via the
activation of VEGFR-1 and VEGFR-2, whereas VEGF-C and VEGF-D bind
to VEGFR-3, which promotes the formation of lymph vessels (2). It is generally agreed that VEGFR-2 is
the major receptor mediating mitogenic, angiogenic and permeability
effects under physiological conditions (3). In contrast, VEGFR-1 does not play a
relevant role in physiological angiogenesis in the adult but does
serve an important role in tumour angiogenesis. Furthermore,
VEGFR-1 ligands directly stimulate signalling pathways crucial for
tumour growth, progression and metastasis in cancer cells (4), and VEGFR-1 activation in a variety of
tumour types, including melanoma, inhibits apoptosis, induces
chemoresistance and is associated with a less favourable prognosis
and recurrence (5–8). VEGFR-1 is also activated by the
placenta growth factor (PlGF), a member of the VEGF family, which
is an exclusive ligand of this receptor and does not interact with
the other VEGFRs (9). VEGFR-1
exists as a full-length membrane protein or as several soluble
forms (sVEGFR-1) deriving from alternative splicing of the
corresponding pre-mRNA (10). The
most abundant sVEGFR-1 is comprised of the extracellular region
with a carboxyl-terminal end of 31 amino acids from intron 13. This
soluble form is released into the extracellular matrix and exerts
anti-angiogenic effects by sequestering VEGF-A or PlGF, thus
reducing their availability for membrane receptor activation
(11).
Several antitumour agents target VEGF-A signalling,
including the monoclonal antibodies (mAbs) bevacizumab and
ramucirumab, which block VEGF-A and VEGFR-2, respectively, the
fusion protein aflibercept, which prevents VEGF-A and PlGF
interaction with the membrane receptors, and a number of
multi-targeted small-molecule kinase inhibitors. Despite the
evidence of efficacy in the acute treatment of a number of
different types of solid tumours, the approved therapeutics
targeting VEGF-A/VEGFR-2 signalling lack long-term efficacy
(1). In addition, long-lasting
treatments are associated with unwanted side effects due to the
inhibition of physiological angiogenesis, resulting in bleeding,
hypertension and delayed wound healing (3,12).
Therefore, it may be possible that molecules selectively inhibiting
VEGFR-1 may exhibit improved safety profiles compared with agents
targeting VEGF-A and/or VEGFR-2, while concurrently maintaining
significant efficacy in antagonizing tumour vascularization and
metastasis (4,13).
In our previous studies, it was shown that the
anti-VEGFR-1 mAb D16F7, which blocks receptor signal transduction
without interfering with ligand binding (14), inhibited the chemotaxis and
invasiveness of glioblastoma (GB) cells and patient-derived GB stem
cells in response to VEGF-A and PlGF (8). This property is particularly important
as this mAb did not interfere with the decoy function of sVEGFR-1,
preserving its physiological anti-angiogenic activity. In addition,
in an in vivo orthotopic model, D16F7 reduced glioma growth,
tumour-associated vessel formation and increased median survival
time of mice, with a high percentage of long-term survivors
(13). These data suggest that
VEGFR-1 represents a therapeutic target for the treatment of GB,
which is the most aggressive primary brain tumour, characterised by
a high rate of therapeutic failure and less favourable prognosis.
Resistance to chemotherapy is frequently observed and recurrence
following initial therapy is common (15,16).
The anti-VEGF-A mAb bevacizumab has been approved for the treatment
of recurrent GB by the US-Food and Drug Administration (FDA) but
not by the European Medicines Agency (EMA). However, in this
clinical setting bevacizumab does not improve overall survival
(17–19). Intrinsic or acquired resistance
mechanisms toward anti–VEGF-A treatments may include: i) Increased
expression/activation of VEGF-A tyrosine kinase transmembrane
receptors in the tumour and tumour microenvironment; ii)
upregulation of different angiogenic factors including PlGF; iii)
phenotypic changes of tumour cells and/or iv) upregulation of
alternative angiogenic pathways (20). In addition, rescue mechanisms to
metabolic changes and hypoxia, mesenchymal cell transition, M2
microglia/macrophage polarization and myeloid cell infiltration may
contribute to the resistance towards anti-VEGF-A therapies
(18). Therefore, both GB cells and
the tumour microenvironment play a role in the failure of
treatments targeting the VEGF-A/VEGFRs axis.
Regarding the GB microenvironment in particular,
GB-associated microglia/macrophages (GAMs) represent the largest
proportion of tumour-infiltrating cells, contributing 30–70% of the
glioma mass (21). VEGFR-1 has been
demonstrated to be expressed in macrophages and its activation
favours the production of VEGF-A and PlGF or other angiogenic
factors and matrix metalloproteases (MMPs) which enhance cancer
invasiveness (22–26). However, to date, no data are
available on the expression of VEGFR-1 in human GAMs.
In recent years, our studies have demonstrated the
role of GAMs in different murine and human models of the GB
microenvironment, examining GAM polarization, the involvement of
the mTOR pathway in GAM activation, as well as the role of the
chemokine receptor CCR5 in GAM migration and activation (27–31).
In the present study, VEGFR-1 expression in GAMs of surgical
specimens collected from 42 patients with GB was assessed and it
was shown that the percentage of VEGFR-1-positive GAMs was higher
in the tumour tissue than in the surrounding parenchyma.
Materials and methods
Patients and tissue specimens
A total of 42 adults (mean age 60, range 34–79; 27
males/15 females), who underwent surgery for primary GB at the
Neurosurgery Department, Foundation ‘Agostino Gemelli’ University
Hospital (Rome, Italy), between March 2005 and September 2011 were
recruited for the present study. Diagnosis of GB was established by
histological examination according to the WHO classification (grade
IV) of central nervous system (CNS) tumours. In all cases total
removal of the tumour was achieved, and tissue samples from both
the tumour and the surrounding macroscopic normal brain tissue were
obtained (1–2 cm away from the tumour border; larger resections
were performed in tumours that grew far from eloquent areas). The
characteristics of patients are presented in Table I. All patients provided written
consent for use their specimens for research and the research
proposal was approved by the Ethics Committee of Foundation
‘Agostino Gemelli’ University Hospital (Rome, Italy) (8,30,31).
 | Table I.Demographic characteristics of the GB
patients. |
Table I.
Demographic characteristics of the GB
patients.
|
|
|
|
|
| VEGFR-1-positive
cells (%) |
|---|
|
|
|
|
|
|
|
|---|
| Patient | Age (years) | Sex | Tumour
location | Primary (P) vs.
Recurrent (R) | Tumour | Parenchyma |
|---|
| 1 | 67 | M | NAa | P | 79 | 44 |
| 2 | 70 | M | Temporal | P | 48 | 56 |
| 3 | 34 | M | Temporal | P | 57 | 0 |
| 4 | 72 | F | Frontal | R | 52 | 20 |
| 5 | 62 | F | Frontal | P | 100 | 0 |
| 6 | 66 | M | NA | P | 100 | 20 |
| 7 | 76 | M | Frontal | P | 100 | 64 |
| 8 | 79 | M | Frontal | P | 100 | 8 |
| 9 | 37 | M | Temporal | P | 100 | 84 |
| 10 | 71 | M | Occipital | P | 38 | 42 |
| 11 | 70 | M | Temporal | P | 0 | 0 |
| 12 | 52 | F | NA | P | 40 | 44 |
| 13 | 48 | M | NA | R | 43 | 62 |
| 14 | 46 | F | Temporal | P | 46 | 34 |
| 15 | 44 | F | Parietal | P | 38 | 5 |
| 16 | 71 | F | NA | P | 100 | 35 |
| 17 | 67 | F | Frontal | P | 36 | 38 |
| 18 | 49 | M | Frontal | P | 100 | 42 |
| 19 | 47 | F | Tempo-Parietal | R | 81 | 75 |
| 20 | 50 | M | Temporal | P | 100 | 72 |
| 21 | 62 | M | NA | P | 61 | 65 |
| 22 | 71 | F | Temporal | P | 32 | 20 |
| 23 | 75 | M | Parietal | P | 100 | 32 |
| 24 | 73 | F | NA | P | 71 | 20 |
| 25 | 42 | M | Temporal | P | 100 | 54 |
| 26 | 64 | M | NA | R | 50 | 22 |
| 27 | 66 | F | Frontal | R | 29 | 55 |
| 28 | 69 | M | Occipital | P | 100 | 100 |
| 29 | 66 | F | NA | P | 100 | 86 |
| 30 | 38 | M | Temporal | P | 100 | 8 |
| 31 | 74 | M | NA | P | 66 | 44 |
| 32 | 76 | M | Temporal | P | 100 | 88 |
| 33 | 73 | M | Temporal | P | 39 | 26 |
| 34 | 51 | F | NA | P | 100 | 44 |
| 35 | 58 | M | Frontal | P | 34 | 44 |
| 36 | 70 | M |
Fronto-temporal | P | 66 | 62 |
| 37 | 50 | F | Occipital | P | 100 | 80 |
| 38 | 62 | F | Frontal | P | 16 | 40 |
| 39 | 51 | M | Temporal | P | 100 | 40 |
| 40 | 51 | M | NA | P | 16 | 56 |
| 41 | NA | M | NA | R | 100 | 72 |
| 42 | 61 | M | Frontal | P | 100 | 40 |
| Total |
|
| Mean ± SEM |
| 69.9±4.8 |
43.9±4.0a,b |
Tissue preparation and
immunohistochemistry
Human tumour tissues were fixed in 4%
paraformaldehyde in 0.1 M phosphate buffer pH 7.6 overnight at 4°C.
Dehydration of tissue was performed using an alcohol series of 80
and 95% ethanol for 1 h each followed by 100% ethanol overnight.
Two 100% xylene washes were performed for 1 h each and then 1 h at
60°C in Paraplast Plus (Tyco/Healthcare). After a change of
Paraplast Plus, tissue was incubated in a 60°C vacuum oven for 2 h
prior to placing in molds to cool and solidify. Sections, 3- to
4-µm thick, were cut and collected on Superfrost Plus slides
(Thermo Fisher Scientific, Inc.). PT Link (Dako; Agilent
Technologies, Inc.) was used to deparaffinise and rehydrate the
sections and for antigen retrieval. Slides were immersed in 10 mM
citrate buffer, pH 6.0, for 10 min at 97°C and then cooled and
washed in PBS or TBS. Endogenous peroxidase activity was inhibited
by incubating the slides with Peroxide Block (ScyTek Laboratories)
for 7 min, after which, slides were washed with PBS and underwent
single staining procedure, whereas slides washed with TBS underwent
a double staining procedure.
For single staining, the slides were incubated with
Avidin/Biotin Blocking System (Spring Bioscience Corp.) and washed
3 times in PBS. Non-specific binding was blocked by incubating
tissues with Super Block Solution (ScyTek Laboratories) for 5 min.
Sections were incubated for 10 min at room temperature with rabbit
anti-human Flt-1/VEGFR-1 polyclonal antibody (dilution 1:50; cat.
no. E2800; Spring Bioscience Corp.), or overnight at 4°C with goat
anti-human ionised calcium binding adaptor molecule 1 (Iba1)
polyclonal antibody (dilution 1:250; cat. no. NB100-1028; Novus
Biologicals). Sections were washed extensively with PBS and
subsequently treated with Ultra Tek horseradish peroxidase
anti-polyvalent kit (ScyTek Laboratories). Finally, after 3 washes
with PBS, the sections were treated with the chromogen
3,3′-diaminobenzidine (ScyTek Laboratories), counterstained with
haematoxylin and mounted.
For double staining, non-specific binding was
blocked incubating tissues with Background Punisher
(Biocare-Medical Pacheco) for 10 min. Sections were incubated for
10 min at room temperature with rabbit anti-human Flt-1/VEGFR-1
polyclonal antibody (dilution 1:50; Spring Bioscience Corp.) and
overnight at 4°C with goat anti-human Iba1 polyclonal antibody
(dilution 1:250; Novus Biologicals). Thereafter, sections were
washed extensively in TBS and subsequently incubated with the MACH
2 rabbit horseradish peroxidase-polymer (Biocare-Medical) for
Flt-1/VEGFR-1 and with Ultratek horseradish peroxidase kit (ScyTek
Laboratories) for Iba1. Finally, after 3 washes in TBS, sections
were treated with 3,3′-diaminobenzidine (Biocare-Medical) as the
chromogen for Iba1 and with Vina Green (Biocare-Medical) as the
chromogen for Flt-1/VEGFR-1 and then counterstained with
haematoxylin and mounted.
Immunostaining analysis
Quantitative analyses were performed by counting
under the microscope (Optech Optical Technology) the number of
VEGFR-1+, Iba1+, or VEGFR-1+ and
Iba1+ double-positive cells in 50 cells. Two blinded
examiners evaluated three different areas of the same slides and
counted 50 cells that included the number of positive cells for
each antibody, the number of positive cells for both antibodies and
the number of negative cells. The average of 6 counts was reported
as percentage of positive cells.
Immortalised human microglia cell
line
The immortalised human microglia-SV40 (IMhu) cell
line was purchased from Applied Biological Materials Inc. The IMhu
cells were grown in Prigrow III media containing 10% foetal calf
serum and antibiotics in PriCoat T25 flasks (all from Applied
Biological Materials Inc.) and seeded at a density of
4×104 cells per cm2. Cells were split when
they reached ~80% confluence. For an extensive characterization of
the IMhu cell line and its culture conditions refer to Chiavari
et al (32).
Reverse transcription-quantitative
(RT-q)PCR
Total cellular RNA was prepared using an RNeasy Midi
kit from Qiagen, Inc., according to the manufacturer's protocol.
Total RNA (3 µg per sample) was subjected to reverse transcription
using SuperScript III enzyme (Invitrogen; Thermo Fisher Scientific,
Inc.) at 50°C for 60 min. Quantification of membrane VEGFR-1 levels
was performed by RT-qPCR using a dual-labelled fluorogenic probe
method and an ABI Prism 7000 sequence detector (PerkinElmer, Inc.),
as previously described (33). The
2−ΔΔCq relative quantification method was utilised to
calculate relative mRNA expression levels. The sequences of the
primers were as follows: VEGFR-1, forward 5′-ACCGAATGCCACCTCCATG-3′
and reverse 5′-AGGCCTTGGGTTTGCTGTC-3′. The level of VEGFR-1
transcript was normalised to that of 18S RNA (TaqMan®
Gene Expression Assay, Applied Biosystems, Inc.) and referred to
the values obtained for the VEGFR-1 negative human melanoma M14
cell line, to which an arbitrary value of 1 was assigned.
Immunofluorescence
Millicell EZ slide 8-well glass (EMD Millipore) were
used to seed the T98G and IMhu cells (5×104 cells/well).
Cells were fixed with 4% paraformaldehyde in PBS with calcium and
magnesium for 20 min at room temperature. After three washes in
PBS, cells were blocked with BSA and incubated overnight in the
presence of the primary anti-VEGFR-1 antibody (Spring Bioscience
Corp.; dilution 1:50). After three washes in PBS with gentle
agitation, the cells were incubated with a secondary donkey
anti-rabbit antibody (dilution 1:1,000; cat. no. A16028;
Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h and mounted
with Vectashield with DAPI (Vector Laboratories, Inc.).
Statistical analysis
Statistical comparison of the differences between
pairs of groups was performed using a Student's t-test or a
Wilcoxon signed-rank test. Statistical significance was determined
at α=0.05 level. P<0.05 was considered to indicate a
statistically significant difference. The non-parametric log-rank
test was performed to compare the survival distributions of the
Kaplan-Meier curve.
Results
Relevance of VEGFR-1 ligands
expression on the survival of patients with GB
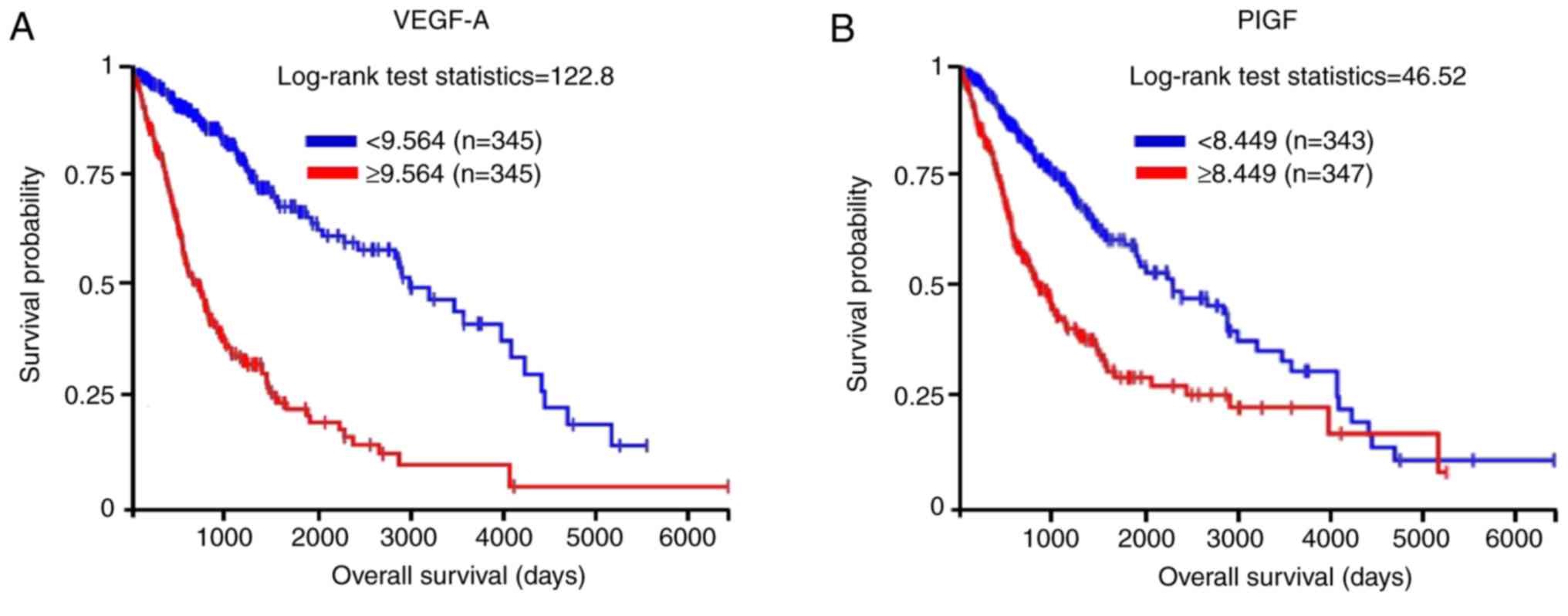
The role of the VEGFR-1 ligands VEGF-A and PlGF on
survival of patients with GB was assessed using data generated by
the TCGA Research Network (https://www.cancer.gov/tcga). The results showed that
345 of 690 patients (dataset, TCGA lower grade glioma and
glioblastoma) exhibited upregulated expression of VEGF-A, and this
was significantly associated with a ~66% reduction in overall
survival (Fig. 1A). High levels of
the VEGFR-1 specific ligand PlGF were detected in 347 of 690
patients and were significantly associated with reduced survival
(~60%; Fig. 1B). These data support
the involvement of VEGFR-1 expression in the aggressiveness of
GB.
Differences in VEGFR-1 expression
between tumour and brain parenchyma in human GB specimens
To analyse the distribution of VEGFR-1 expression in
GB, tissue specimens of the tumour and matching surrounding
parenchyma of patients with GB were collected following surgical
removal of the tumours from 42 patients. In agreement with our
previous study (8), the majority of
GB tissue samples (>90%) showed >25% of total cells positive
for VEGFR-1 staining (Table I).
There was no significant association of VEGFR-1 expression with age
or primary and recurrent GB.
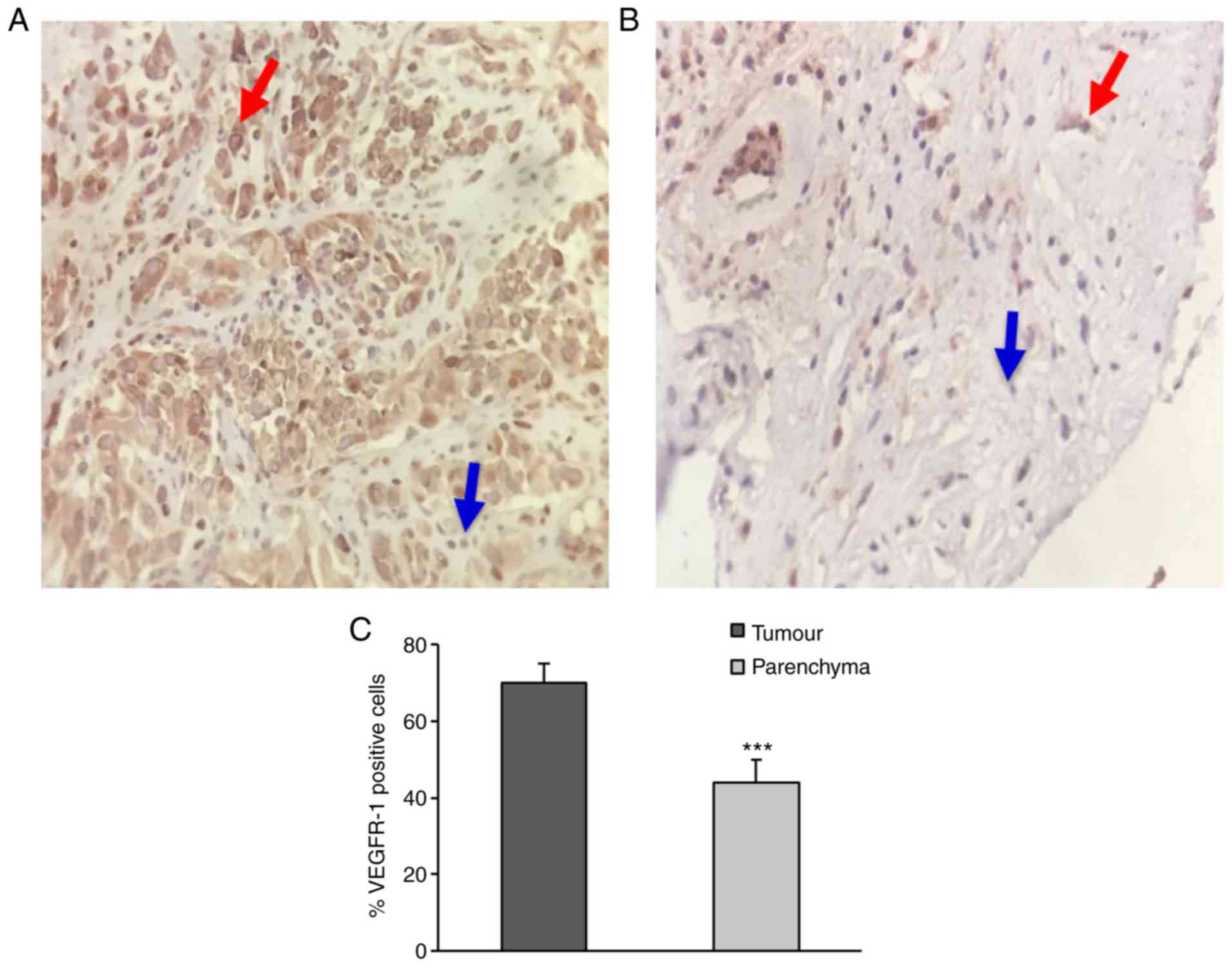
The tumour tissue presented a significantly higher
number of VEGFR-1-stained cells compared with the surrounding
parenchyma (Fig. 2). In fact, in
the parenchyma ~40% of the cells were positive for VEGFR-1
expression; whereas in the tumour, the percentage of cells
expressing VEGFR-1 was ~70% (Figs.
2 and S1). These data suggest
that VEGFR-1 is expressed in both GB cells and cells of the
microenvironment.
VEGFR-1 expression is increased in
GB-associated microglia
As VEGFR-1 staining was observed also in cells of
the tumour-associated microenvironment, and microglia are the
principal resident immune cells in the CNS, the expression of
VEGFR-1 was analysed in GAMs. To determine whether VEGFR-1 was
differentially expressed in the microglia infiltrating the tumours
compared with those present in the peripheral parenchyma, 23
samples were selected with similar number of cells positive for
Iba1, a microglia-macrophage biomarker, in the parenchyma and the
tumour (36.9 vs. 35.6%, respectively), but with different levels of
VEGFR-1 expression (Fig. 3A-D).
Using double staining for VEGFR-1 and Iba1 in these samples, it was
shown that the percentage of microglia-macrophages expressing
VEGFR-1 present in the tumour (Fig.
3E) was significantly higher compared with the parenchyma
(Fig. 3F). Taking into account only
the microglia-macrophage cell population in the tumour, 24% of the
Iba1-positive cells also expressed VEGFR-1, whereas in the
parenchyma the percentage of double-positive cells was 11%
(Table SI). When considering all
the cells, ~9 and 4% of the cells were Iba1/VEGFR-1 double-positive
in the tumour and the surrounding tissue, respectively (data not
shown).
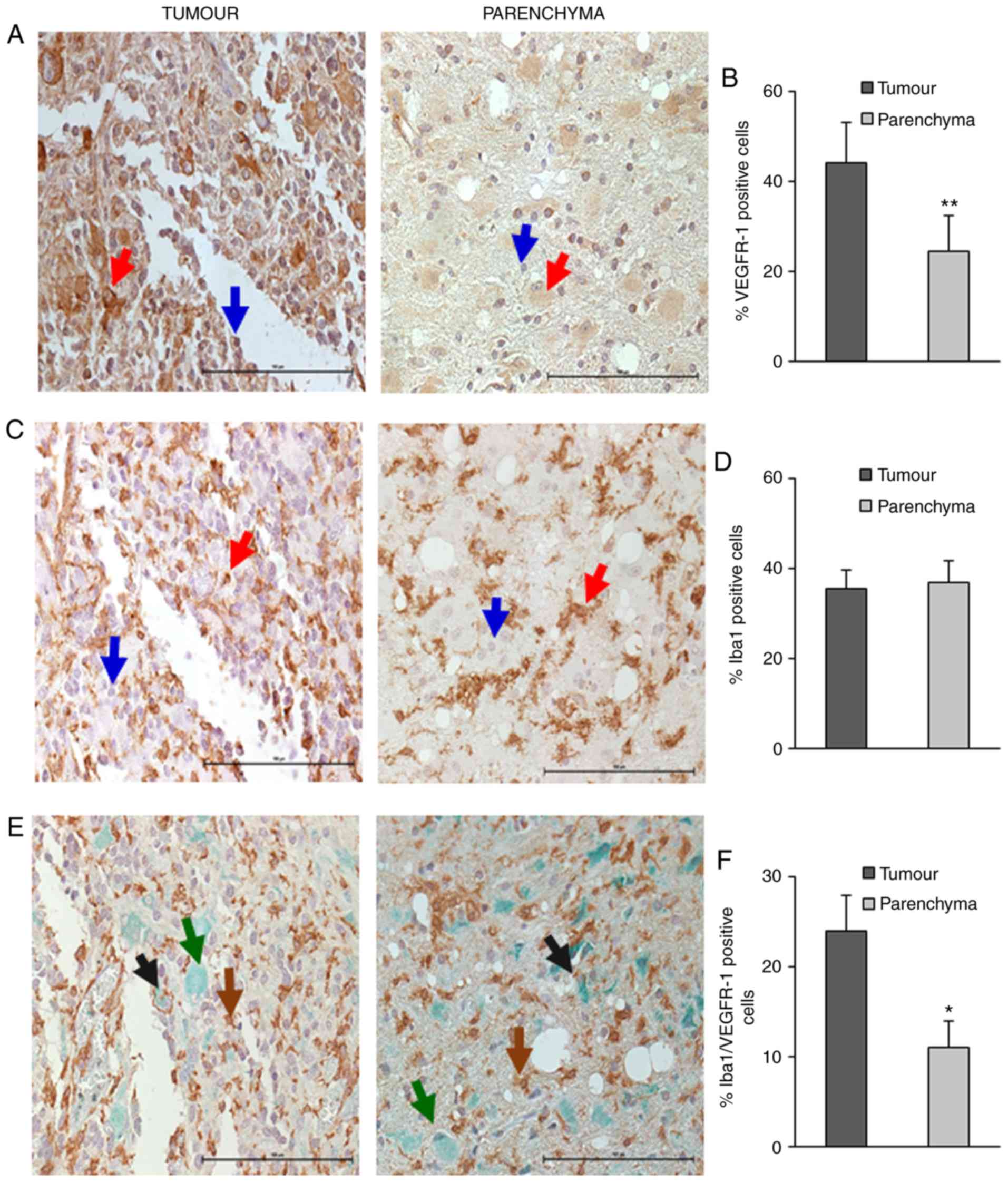 | Figure 3.Iba1 and VEGFR-1 staining of
microglia present in the parenchyma or tumour regions in GB
specimens. Representative images of tumour regions stained for
VEGFR-1 (A), Iba1 (C) and both proteins (E), and of parenchyma
regions stained for VEGFR-1 (B), Iba1 (D) and both proteins (F).
Magnification, ×40. Red and blue arrows point to positive and
negative cells, respectively. Brown, green and black arrows point
to VEGFR-1, Iba1 and double-positive cells, respectively.
Histograms indicate the percentages of VEGFR-1, Iba1 and
Iba1/VEGFR-1 positive cells in the tumour and parenchyma regions.
Data are expressed as mean ± SEM of 23 samples. Scale bar, 100 µm.
**P<0.01; *P<0.05. |
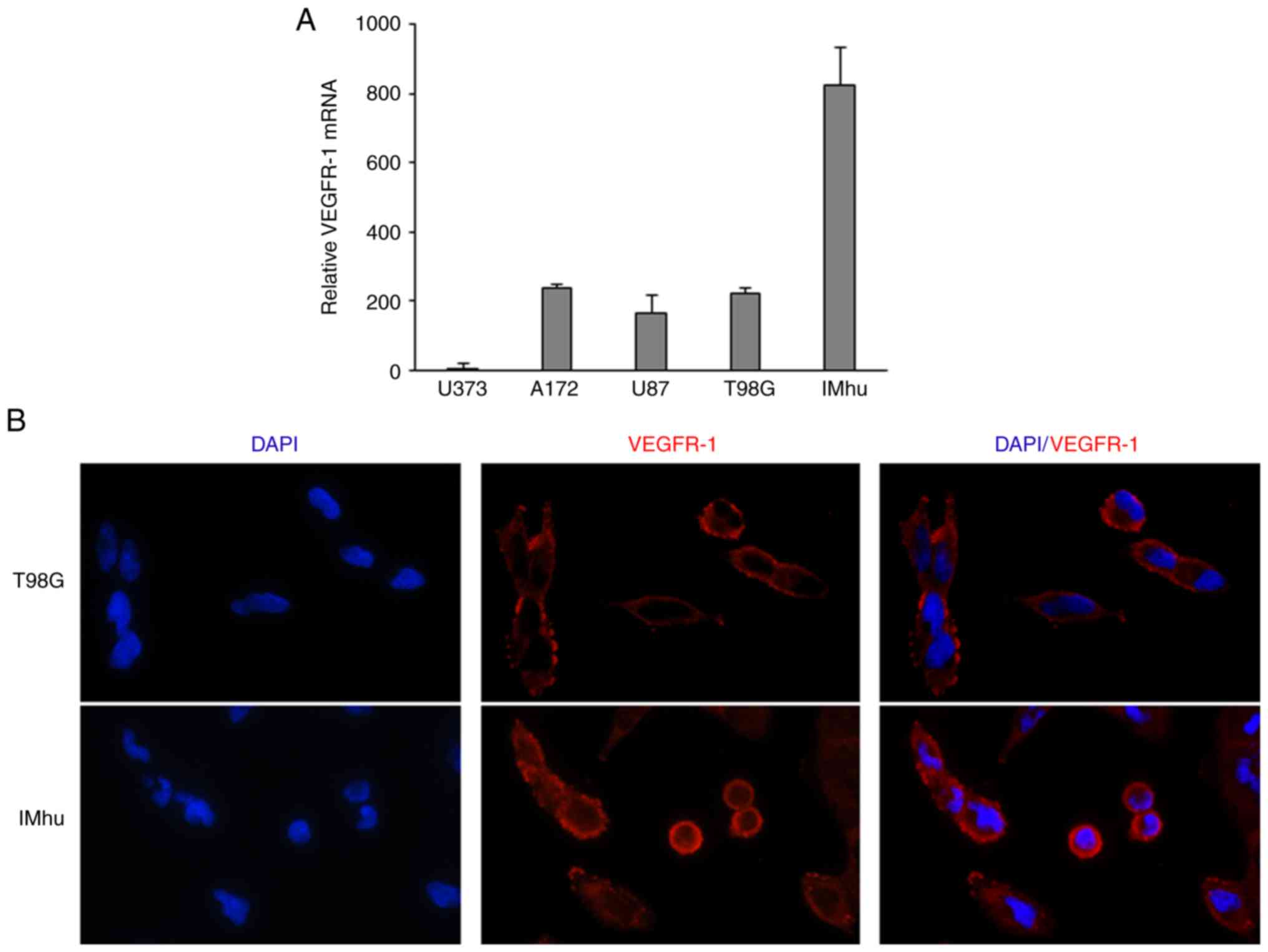
To confirm VEGFR-1 expression in human microglia,
the expression of this receptor was assessed in an immortalised
human microglial cell line (IMhu). This cell line was shown to
express considerable levels of the receptor compared with other GB
cell lines. RT-qPCR analysis showed that VEGFR-1 expression was 4
times higher in IMhu cells compared with that found in the T98G,
U87MG and A123 GB cell lines. The U373 line expressed very low
levels of VEGFR-1 (Fig. 4A). To
study the localisation of VEGFR-1, immunofluorescence experiments
were performed using T98G and IMhu cells. As shown in Fig. 4B, both cell lines expressed VEGFR-1
on the cell surface, and a minor amount of expression was also
observed at the cytoplasmic level. In addition, qualitative
analysis performed by a researcher in a blinded manner confirmed
that the expression of VEGFR-1 was higher in IMhu compared with
that in the T98G cell line.
Discussion
In our previous studies, it was demonstrated that in
GB tissues a high percentage of cells express VEGFR-1 (8). In the present study, by analysing
brain tissue specimens collected from patients with GB, it was
demonstrated for the first time that: i) The number of
VEGFR-1-positive cells was significantly higher in the tumour
tissue compared with that noted in the surrounding parenchyma; ii)
VEGFR-1-positive cells included a considerable quantity of GAMs;
and iii) the number of VEGFR-1-positive microglia-macrophages was
significantly higher in the tumour area compared with the
parenchymal region.
The data in the present study support the notion
that VEGFR-1 and its ligands are involved in the pathology of GB.
High expression levels of VEGF-A and of the selective VEGFR-1
ligand PlGF are inversely associated with overall survival.
Furthermore, in GB tissue sections a high percentage of cells,
including the tumour and microenvironment cells, were found to
express VEGFR-1. A reliable analysis of the effect of
membrane-bound VEGFR-1 expression on survival could not be
performed, as the probe utilised in the consulted database did not
discriminate between the membrane and soluble forms of the
receptor, which possess opposite effects on angiogenesis and tumour
progression.
VEGF-A has been extensively studied in regard to its
role in a number of different types of cancer, including GB, with
particular attention to VEGF-A/VEGFR-2-mediated pathways. In 2009
bevacizumab as a single-agent therapeutic was granted provisional
approval under the FDA's accelerated approval program for the
treatment of recurrent GB, based on the observation of durable
objective responses in two phase II clinical trials (34,35).
In 2017, bevacizumab received full approval based on the results of
a phase III clinical trial on the combination of bevacizumab with
lomustine. An increase of 2.7 months in progression-free survival
(PFS) was observed in the cohort treated with the drug combination
compared with chemotherapy alone (36). However, EMA considered these data
insufficient and refused the marketing authorization of bevacizumab
to treat GB. In line with the EMA decision, in 2014, a phase III
trial in newly diagnosed GB, testing bevacizumab in addition to the
standard therapy (radiotherapy-temozolomide followed by maintenance
temozolomide), did not show significantly improved survival
(37). Another phase III trial in
the same clinical setting reported a higher PFS in the bevacizumab
cohort compared with the control cohort, but with no difference in
overall survival. Furthermore, in contrast to other phase III
clinical trials, a decline in health-related quality of life and a
greater deterioration in neurocognitive function were more
frequently observed in patients receiving bevacizumab compared with
those receiving the placebo (38).
The causes of bevacizumab failure/resistance are not
yet completely understood but are often associated with changes in
the tumour microenvironment. In preclinical models and in clinical
specimens from patients with GB whose tumours progressed during
bevacizumab treatment, an increase in the presence of GAMs was
reported, which was also correlated with a less favourable survival
(39–41). In addition, resistance to
bevacizumab was associated with decreased expression of macrophage
migration inhibitory factor, which drives polarization of
antitumour M1 macrophages, and with an increase of pro-tumoural M2
macrophages (42). These data
provide a rationale for combining anti-angiogenic therapies with
strategies which target M2 macrophages or promote polarization of
macrophage-microglia toward the M1 phenotype.
GAMs are known to stimulate angiogenesis and
invasion in response to various cytokines or growth factors,
including basic fibroblast growth factor, MMP9 and VEGF-A and this
has previously been reviewed elsewhere (21). Under pathological conditions, GAMs
are a mixture of antitumour (M1) and pro-tumoural (M2) phenotypes.
Our laboratory previously demonstrated a characterization of the GB
microenvironment showing that a significant proportion of cells
expressing the M2 and M1 markers, CD163 or arginase 1 and the
inducible nitric oxide synthase, respectively, are present in the
GB tissue (30). In addition, bone
marrow-derived cells, including CD163+ M2 GAMs, have
been associated with tumour progression, angiogenesis and treatment
failure (43,44).
In a murine model, VEGFR-1 was found to be
preferentially expressed in M2 tumour-associated macrophages (TAMs)
(45). Moreover, VEGFR-1 activation
by PlGF was able to stimulate angiogenesis initiated by TAM
polarised toward the pro-tumoural M2 phenotype (46). It should be noted that the
expression of VEGFR-1 and VEGF-A might not necessarily correlate.
In fact, in the tumour microenvironment other cell types different
from cancer cells can produce the VEGFR-1 ligands VEGF-A and PlGF.
Therefore, we focused our attention on VEGFR-1 expression in
tumours as well as in microglial cells. The present study
demonstrated that VEGFR-1 is expressed in a quarter of GAMs and the
percentage of positive cells was significantly higher in the tumour
compared with the parenchyma. These results reinforce the rationale
for targeting VEGFR-1 in GB, as blockade of this receptor may exert
antitumour activity via various direct and indirect mechanisms
involving tumour, endothelial cells and GAMs. Thus, VEGFR-1
inhibition may impair: i) GB cell invasiveness and vasculogenic
mimicry (formation of vascular channels similar to those produced
by endothelial cells); ii) tumour-associated angiogenesis, and iii)
activation of GAMs with the pro-tumoural M2 phenotype which further
stimulates formation of new vessels and brain parenchyma
infiltration by GB cells. A critical issue associated with VEGFR-1
targeting is that molecules directed toward this receptor should
inhibit signal transduction through the membrane protein whilst
maintaining the antitumour/antiangiogenic activity of the soluble
form, which is able to sequester VEGF-A and PlGF released in the
extracellular matrix. In fact, a low sVEGFR-1/VEGF-A ratio in GB
has been associated with higher aggressiveness compared with
astrocytoma (47). These properties
are recapitulated in the anti-VEGFR-1 mAb D16F7 developed by our
laboratory previously, since this mAb inhibits the membrane
receptor activation without affecting ligand binding, and it does
not interfere with the decoy function of the soluble receptor.
Therefore, the manipulation of GAMs-glioblastoma crosstalk through
the VEGFR-1 axis may represent a suitable and promising therapeutic
strategy for the treatment of GB.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Paola Lanza
(Division of Anatomic Pathology and Histology, Catholic University
of Sacred Heart, Foundation ‘Agostino Gemelli’ University Hospital,
Rome, Italy) for her valuable suggestions on the
immunohistochemistry protocol. The results shown here are in part
based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga.
Funding
This study was supported by ‘Fondi di Ateneo 2016’
(to PN) and in part by the Italian Association for Cancer Research
(AIRC under IG 2017-ID. 20353 project-Principal Investigator GG)
and by the Italian Ministry of Health (grant no. RC18-2638151 to
PML).
Availability of data and materials
The analysed datasets generated during the study are
available from TCGA database in Xena browser (https://xenabrowser.net).
Authors' contributions
LL, PML, GG and PN designed the experiments and
drafted the manuscript. All authors critically reviewed and revised
the article. LL, GMPC, MC, FR and PML performed the experiments and
analysed the results. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All patients signed an informed consent form, and
the experimental protocol was approved by the Ethics Committee of
Foundation ‘Agostino Gemelli’ University Hospital (Rome,
Italy).
Patient consent for publication
Not applicable.
Competing interests
The authors have declared no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GAMs
|
glioblastoma-associated
microglia/macrophages
|
|
GB
|
glioblastoma
|
|
Iba1
|
ionised calcium binding adaptor
molecule 1
|
|
mAb
|
monoclonal antibody
|
|
MMP
|
matrix metalloprotease
|
|
PlGF
|
placenta growth factor
|
|
sVEGFR-1
|
soluble vascular endothelial growth
factor receptor-1
|
|
TCGA
|
The Cancer Genome Atlas
|
|
VEGF
|
vascular endothelial growth factor
|
|
VEGFR
|
VEGF receptor
|
References
|
1
|
Peach CJ, Mignone VW, Arruda MA, Alcobia
DC, Hill SJ, Kilpatrick LE and Woolard J: Molecular pharmacology of
VEGF-A isoforms: Binding and Signalling at VEGFR2. Int J Mol Sci.
19(pii): E12642018. View Article : Google Scholar
|
|
2
|
Bowler E and Oltean S: Alternative
splicing in angiogenesis. Int J Mol Sci. 20:20672019. View Article : Google Scholar :
|
|
3
|
Higa GM and Abraham J: Biological
mechanisms of bevacizumab-associated adverse events. Expert Rev
Anticancer Ther. 9:999–1007. 2009. View Article : Google Scholar
|
|
4
|
Lacal PM and Graziani G: Therapeutic
implication of vascular endothelial growth factor receptor-1
(VEGFR-1) targeting in cancer cells and tumour microenvironment by
competitive and non-competitive inhibitors. Pharmacol Res.
136:97–107. 2018. View Article : Google Scholar
|
|
5
|
Lacal PM, Ruffini F, Pagani E and D'Atri
S: An autocrine loop directed by the vascular endothelial growth
factor promotes invasiveness of human melanoma cells. Int J Oncol.
27:1625–1632. 2005.
|
|
6
|
Levati L, Ruffini F, Muzi A, Umezawa K,
Graziani G, D'Atri S and Lacal PM: Placenta growth factor induces
melanoma resistance to temozolomide through a mechanism that
involves the activation of the transcription factor NF-κB. Int J
Oncol. 38:241–247. 2011.
|
|
7
|
Li C, Liu B, Dai Z and Tao Y: Knockdown of
VEGF receptor-1 (VEGFR-1) impairs macrophage infiltration,
angiogenesis and growth of clear cell renal cell carcinoma (CRCC).
Cancer Biol Ther. 12:872–880. 2011. View Article : Google Scholar :
|
|
8
|
Atzori MG, Tentori L, Ruffini F, Ceci C,
Lisi L, Bonanno E, Scimeca M, Eskilsson E, Daubon T, Miletic H, et
al: The anti-vascular endothelial growth factor receptor-1
monoclonal antibody D16F7 inhibits invasiveness of human
glioblastoma and glioblastoma stem cells. J Exp Clin Cancer Res.
36:1062017. View Article : Google Scholar :
|
|
9
|
Cao Y: Positive and negative modulation of
angiogenesis by VEGFR1 ligands. Sci Signal. 24(2): re12009.
|
|
10
|
Abou-Fayçal C, Hatat AS, Gazzeri S and
Eymin B: Splice variants of the RTK family: Their Role in tumour
progression and response to targeted therapy. Int J Mol Sci.
18(pii): 3832017. View Article : Google Scholar :
|
|
11
|
Stevens M and Oltean S: Modulation of
receptor tyrosine kinase activity through alternative splicing of
ligands and receptors in the VEGF-A/VEGFR Axis. Cells. 8(pii):
E2882019. View Article : Google Scholar
|
|
12
|
Sia D, Alsinet C, Newell P and Villanueva
A: VEGF signaling in cancer treatment. Curr Pharm Des.
20:2834–2842. 2014. View Article : Google Scholar
|
|
13
|
Atzori MG, Tentori L, Ruffini F, Ceci C,
Bonanno E, Scimeca M, Lacal PM and Graziani G: The anti-vascular
endothelial growth factor receptor-1 monoclonal antibody D16F7
inhibits glioma growth and angiogenesis in vivo. J Pharmacol Exp
Ther. 364:77–86. 2018. View Article : Google Scholar
|
|
14
|
Graziani G, Ruffini F, Tentori L, Scimeca
M, Dorio AS, Atzori MG, Failla CM, Morea V, Bonanno E, D'Atri S and
Lacal PM: Antitumour activity of a novel anti-vascular endothelial
growth factor receptor-1 monoclonal antibody that does not
interfere with ligand binding. Oncotarget. 7:72868–72885. 2016.
View Article : Google Scholar :
|
|
15
|
Weller M: Next generation neuro-oncology.
Eur J Cancer. 96:1–5. 2018. View Article : Google Scholar
|
|
16
|
Lu VM, Jue TR, McDonald KL and Rovin RA:
The survival effect of repeat surgery at glioblastoma recurrence
and its trend: A systematic review and meta-analysis. World
Neurosurg. 115:453–459.e3. 2018. View Article : Google Scholar
|
|
17
|
Diaz RJ, Ali S, Qadir MG, De La Fuente MI,
Ivan ME and Komotar RJ: The role of bevacizumab in the treatment of
glioblastoma. J Neurooncol. 133:455–467. 2017. View Article : Google Scholar
|
|
18
|
Hundsberger T, Reardon DA and Wen PY:
Angiogenesis inhibitors in tackling recurrent glioblastoma. Expert
Rev Anticancer Ther. 17:507–515. 2017. View Article : Google Scholar
|
|
19
|
Tipping M, Eickhoff J and Ian Robins H:
Clinical outcomes in recurrent glioblastoma with bevacizumab
therapy: An analysis of the literature. J Clin Neurosci.
44:101–106. 2017. View Article : Google Scholar :
|
|
20
|
Itatani Y, Kawada K, Yamamoto T and Sakai
Y: Resistance to anti-angiogenic therapy in cancer-alterations to
anti-VEGF pathway. Int J Mol Sci. 19(pii): E12322018. View Article : Google Scholar
|
|
21
|
Dello Russo C, Lisi L, Tentori L, Navarra
P, Graziani G and Combs CK: Exploiting microglial functions for the
treatment of glioblastoma. Curr Cancer Drug Targets. 17:267–281.
2017. View Article : Google Scholar
|
|
22
|
Hiratsuka S, Nakamura K, Iwai S, Murakami
M, Itoh T, Kijima H, Shipley JM, Senior RM and Shibuya M: MMP9
induction by vascular endothelial growth factor receptor-1 is
involved in lung-specific metastasis. Cancer Cell. 2:289–300. 2002.
View Article : Google Scholar
|
|
23
|
Rolny C, Mazzone M, Tugues S, Laoui D,
Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, et
al: HRG inhibits tumour growth and metastasis by inducing
macrophage polarization and vessel normalization through
downregulation of PlGF. Cancer Cell. 19:31–44. 2011. View Article : Google Scholar
|
|
24
|
Zhou X and Qi Y: Larynx carcinoma
regulates tumour-associated macrophages through PLGF signaling. Sci
Rep. 5:100712015. View Article : Google Scholar :
|
|
25
|
Li N, Qin J, Lan L, Zhang H, Liu F, Wu Z,
Ni H and Wang Y: PTEN inhibits macrophage polarization from M1 to
M2 through CCL2 and VEGF-A reduction and NHERF-1 synergism. Cancer
Biol Ther. 16:297–306. 2015. View Article : Google Scholar :
|
|
26
|
Incio J, Tam J, Rahbari NN, Suboj P,
McManus DT, Chin SM, Vardam TD, Batista A, Babykutty S, Jung K, et
al: PlGF/VEGFR-1 signaling promotes macrophage polarization and
accelerated tumour progression in obesity. Clin Cancer Res.
22:2993–3004. 2016. View Article : Google Scholar :
|
|
27
|
Lisi L, Stigliano E, Lauriola L, Navarra P
and Dello Russo C: Proinflammatory-activated glioma cells induce a
switch in microglial polarization and activation status, from a
predominant M2b phenotype to a mixture of M1 and M2a/B polarized
cells. ASN Neuro. 6:171–183. 2014. View Article : Google Scholar
|
|
28
|
Lisi L, Laudati E, Navarra P and Dello
Russo C: The mTOR kinase inhibitors polarize glioma-activated
microglia to express a M1 phenotype. J Neuroinflammation.
11:1252014. View Article : Google Scholar :
|
|
29
|
Laudati E, Currò D, Navarra P and Lisi L:
Blockade of CCR5 receptor prevents M2 microglia phenotype in a
microglia-glioma paradigm. Neurochem Int. 108:100–108. 2017.
View Article : Google Scholar
|
|
30
|
Lisi L, Ciotti GM, Braun D, Kalinin S,
Currò D, Dello Russo C, Coli A, Mangiola A, Anile C, Feinstein DL
and Navarra P: Expression of iNOS, CD163 and ARG-1 taken as M1 and
M2 markers of microglial polarization in human glioblastoma and the
surrounding normal parenchyma. Neurosci Lett. 645:106–112. 2017.
View Article : Google Scholar
|
|
31
|
Lisi L, Ciotti GMP, Chiavari M,
Pizzoferrato M, Mangiola A, Kalinin S, Feinstein DL and Navarra P:
Phospho-mTOR expression in human glioblastoma microglia-macrophage
cells. Neurochem Int. 129:1044852019. View Article : Google Scholar
|
|
32
|
Chiavari M, Ciotti GMP, Navarra P and Lisi
L: Pro-inflammatory activation of a new immortalized human
microglia cell line. Brain Sci. 9(pii): E1112019. View Article : Google Scholar
|
|
33
|
Ruffini F, Failla CM, Orecchia A, Bani MR,
Dorio AS, Fortes C, Zambruno G, Graziani G, Giavazzi R, D'Atri S
and Lacal PM: Expression of the soluble vascular endothelial growth
factor receptor-1 in cutaneous melanoma: Role in tumour
progression. Br J Dermatol. 164:1061–1070. 2011. View Article : Google Scholar
|
|
34
|
Friedman HS, Prados MD, Wen PY, Mikkelsen
T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen
R, et al: Bevacizumab alone and in combination with irinotecan in
recurrent glioblastoma. J Clin Oncol. 27:4733–4740. 2009.
View Article : Google Scholar
|
|
35
|
Kreisl TN, Kim L, Moore K, Duic P, Royce
C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, et al:
Phase II trial of single-agent bevacizumab followed by bevacizumab
plus irinotecan at tumour progression in recurrent glioblastoma. J
Clin Oncol. 27:740–745. 2009. View Article : Google Scholar
|
|
36
|
Wick W, Gorlia T, Bendszus M, Taphoorn M,
Sahm F, Harting I, Brandes AA, Taal W, Domont J, Idbaih A, et al:
Lomustine and bevacizumab in progressive glioblastoma. N Engl J
Med. 377:1954–1963. 2017. View Article : Google Scholar
|
|
37
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar
|
|
38
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014. View Article : Google Scholar :
|
|
39
|
Piao Y, Liang J, Holmes L, Zurita AJ,
Henry V, Heymach JV and de Groot JF: Glioblastoma resistance to
anti-VEGF therapy is associated with myeloid cell infiltration,
stem cell accumulation, and a mesenchymal phenotype. Neuro Oncol.
14:1379–1392. 2012. View Article : Google Scholar :
|
|
40
|
Gabrusiewicz K, Liu D, Cortes-Santiago N,
Hossain MB, Conrad CA, Aldape KD, Fuller GN, Marini FC, Alonso MM,
Idoate MA, et al: Anti-vascular endothelial growth factor
therapy-induced glioma invasion is associated with accumulation of
Tie2-expressing monocytes. Oncotarget. 5:2208–2220. 2014.
View Article : Google Scholar :
|
|
41
|
Lu-Emerson C, Snuderl M, Kirkpatrick ND,
Goveia J, Davidson C, Huang Y, Riedemann L, Taylor J, Ivy P, Duda
DG, et al: Increase in tumour-associated macrophages after
antiangiogenic therapy is associated with poor survival among
patients with recurrent glioblastoma. Neuro Oncol. 15:1079–1087.
2013. View Article : Google Scholar :
|
|
42
|
Castro BA, Flanigan P, Jahangiri A,
Hoffman D, Chen W, Kuang R, De Lay M, Yagnik G, Wagner JR,
Mascharak S, et al: Macrophage migration inhibitory factor
downregulation: A novel mechanism of resistance to anti-angiogenic
therapy. Oncogene. 36:3749–3759. 2017. View Article : Google Scholar :
|
|
43
|
Kitamura T, Qian BZ and Pollard JW: Immune
cell promotion of metastasis. Nat Rev Immunol. 15:73–86. 2015.
View Article : Google Scholar :
|
|
44
|
Pollard JW: Trophic macrophages in
development and disease. Nat Rev Immunol. 9:259–270. 2009.
View Article : Google Scholar :
|
|
45
|
Melton DW, McManus LM, Gelfond JA and
Shireman PK: Temporal phenotypic features distinguish polarized
macrophages in vitro. Autoimmunity. 48:161–176. 2015. View Article : Google Scholar :
|
|
46
|
Jetten N, Verbruggen S, Gijbels MJ, Post
MJ, De Winther MP and Donners MM: Anti-inflammatory M2, but not
pro-inflammatory M1 macrophages promote angiogenesis in vivo.
Angiogenesis. 17:109–118. 2014. View Article : Google Scholar
|
|
47
|
Lamszus K, Ulbricht U, Matschke J,
Brockmann MA, Fillbrandt R and Westphal M: Levels of soluble
vascular endothelial growth factor (VEGF) receptor 1 in astrocytic
tumours and its relation to malignancy, vascularity, and VEGF-A.
Clin Cancer Res. 9:1399–1405. 2003.
|