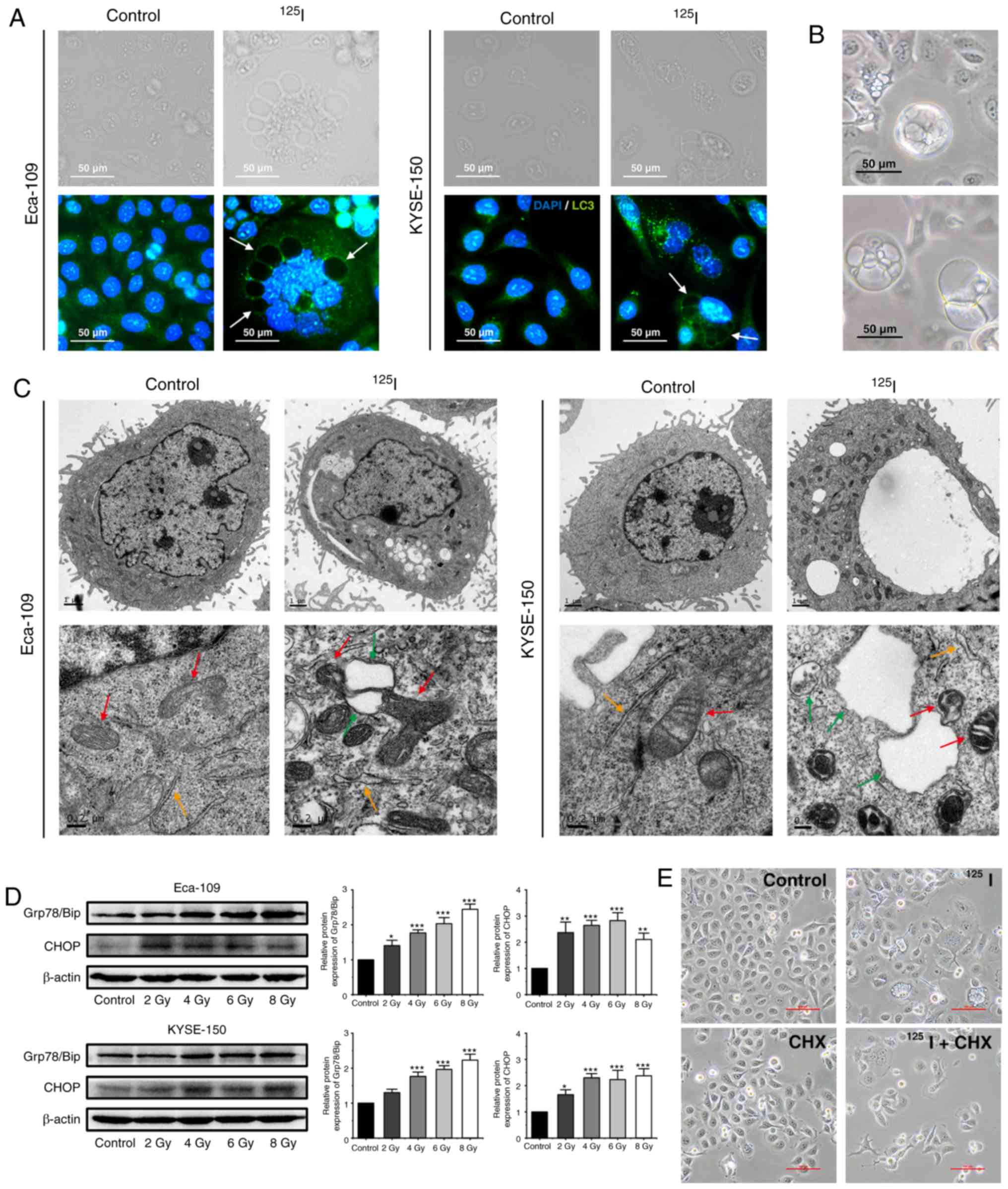
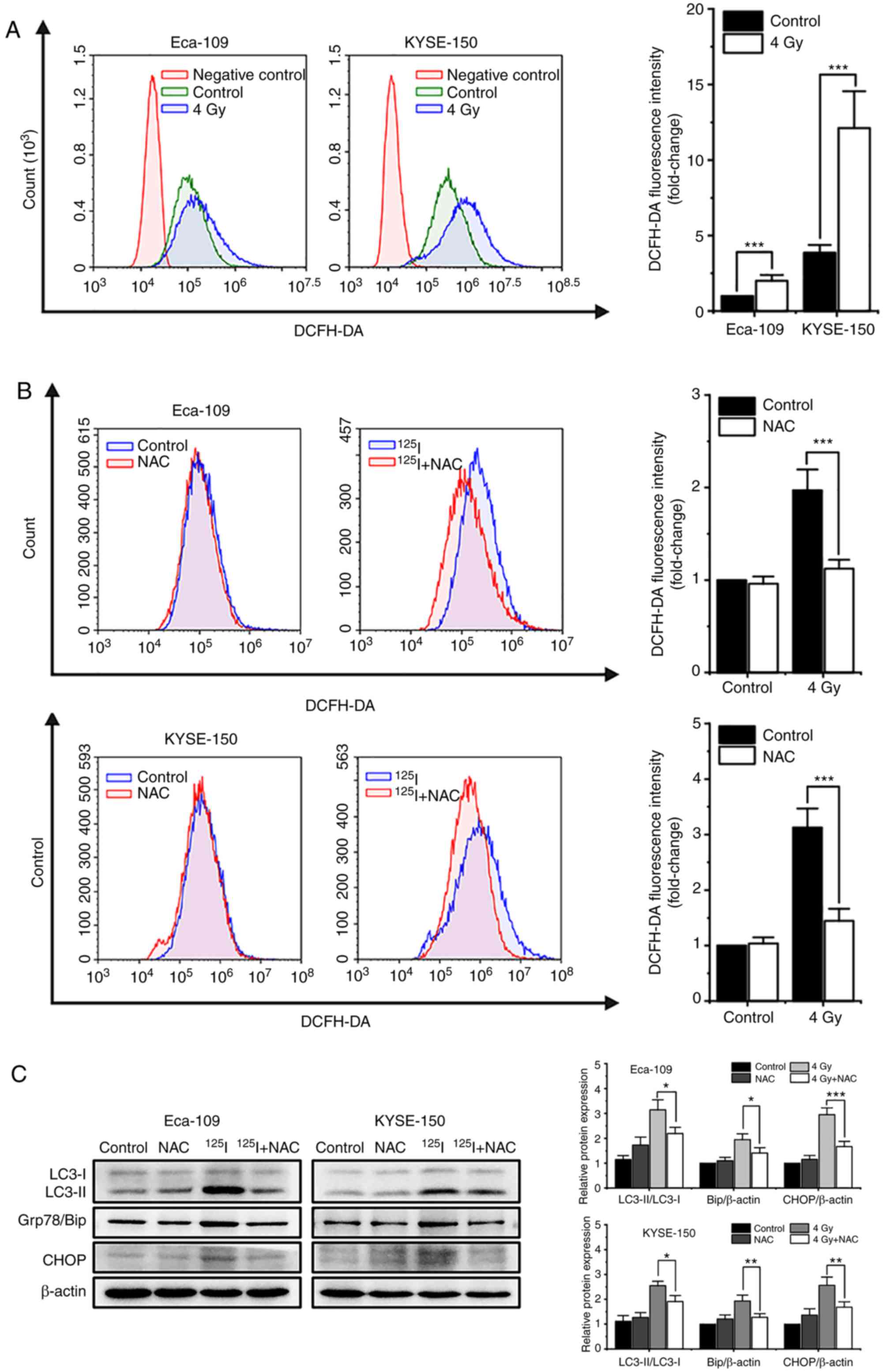
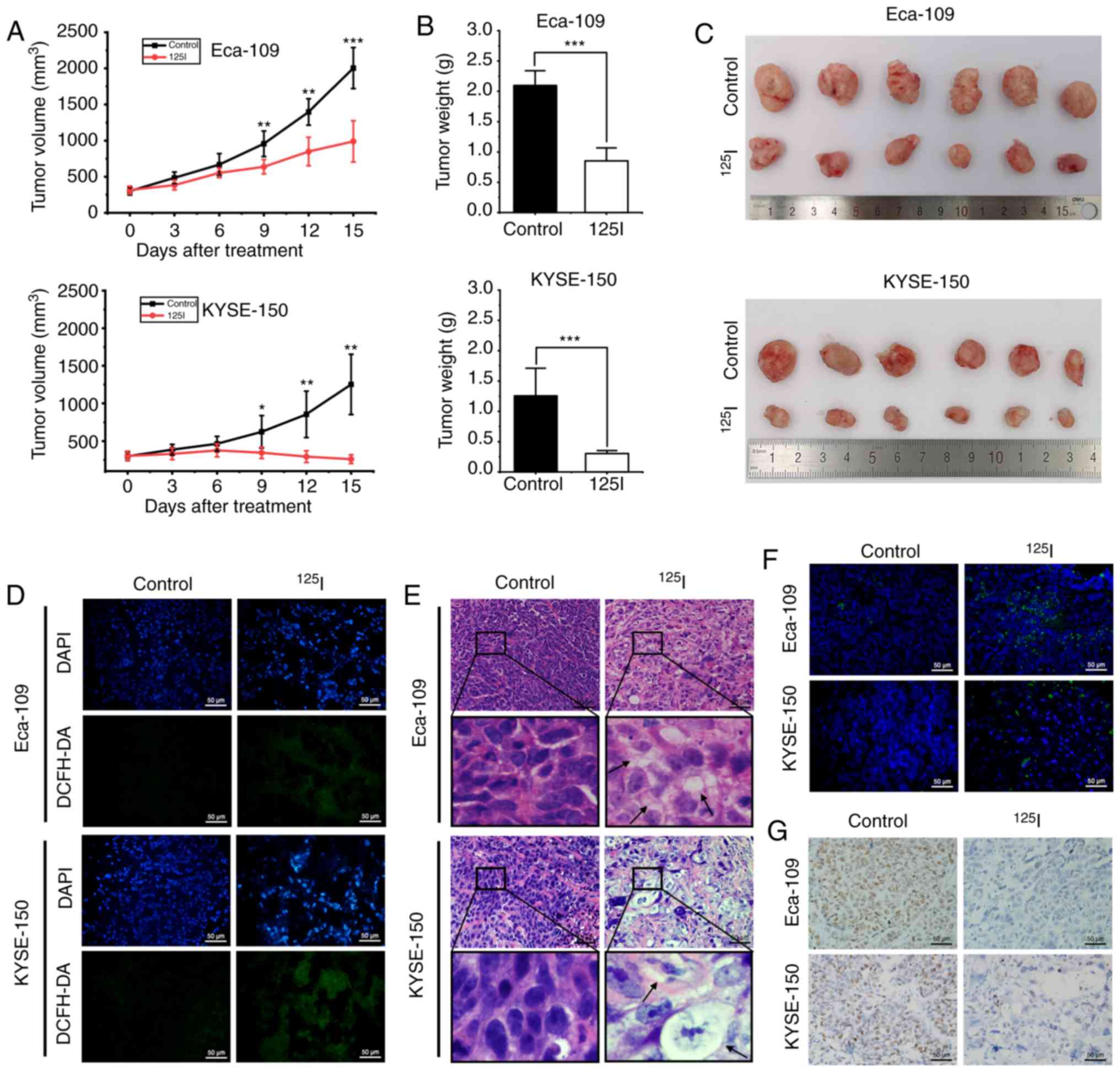
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar
|
|
2
|
Lagergren J, Smyth E, Cunningham D and
Lagergren P: Oesophageal cancer. Lancet. 390:2383–2396. 2017.
View Article : Google Scholar
|
|
3
|
Jatoi A, Martenson JA, Foster NR, McLeod
HL, Lair BS, Nichols F, Tschetter LK, Moore DF Jr, Fitch TR and
Alberts SR; North Central Cancer Treatment Group (N0044), :
Paclitaxel, carboplatin, 5-fluorouracil, and radiation for locally
advanced esophageal cancer: Phase II results of preliminary
pharmacologic and molecular efforts to mitigate toxicity and
predict outcomes: North central cancer treatment group (N0044). Am
J Clin Oncol. 30:507–513. 2007. View Article : Google Scholar
|
|
4
|
Tsushima T, Mizusawa J, Sudo K, Honma Y,
Kato K, Igaki H, Tsubosa Y, Shinoda M, Nakamura K, Fukuda H, et al:
Risk factors for esophageal fistula associated with
chemoradiotherapy for locally advanced unresectable esophageal
cancer: A supplementary analysis of JCOG0303. Medicine (Baltimore).
95:e36992016. View Article : Google Scholar :
|
|
5
|
Schnell O, Scholler K, Ruge M, Siefert A,
Tonn JC and Kreth FW: Surgical resection plus stereotactic 125I
brachytherapy in adult patients with eloquently located
supratentorial WHO grade II glioma-feasibility and outcome of a
combined local treatment concept. J Neurol. 255:1495–1502. 2008.
View Article : Google Scholar
|
|
6
|
Zhang F, Wang J, Guo J, Li Y, Huang X,
Guan Z, Lei G, Wang J, Ye X, Zhao X, et al: Chinese expert
consensus workshop report: Guideline for permanent iodine-125 seed
implantation of primary and metastatic lung tumors. Thorac Cancer.
10:388–394. 2019. View Article : Google Scholar
|
|
7
|
Mo Z, Zhang T, Zhang Y, Xiang Z, Yan H,
Zhong Z, Gao F and Zhang F: Feasibility and clinical value of
CT-guided (125)I brachytherapy for metastatic soft tissue sarcoma
after first-line chemotherapy failure. Eur Radiol. 28:1194–1203.
2018. View Article : Google Scholar
|
|
8
|
Zhuang HQ, Wang JJ, Liao AY, Wang JD and
Zhao Y: The biological effect of 125I seed continuous low dose rate
irradiation in CL187 cells. J Exp Clin Cancer Res. 28:122009.
View Article : Google Scholar :
|
|
9
|
Wang H, Li J, Qu A, Liu J, Zhao Y and Wang
J: The different biological effects of single, fractionated and
continuous low dose rate irradiation on CL187 colorectal cancer
cells. Radiat Oncol. 8:1962013. View Article : Google Scholar :
|
|
10
|
Guo JH, Teng GJ, Zhu GY, He SC, Fang W,
Deng G and Li GZ: Self-expandable esophageal stent loaded with 125I
seeds: Initial experience in patients with advanced esophageal
cancer. Radiology. 247:574–581. 2008. View Article : Google Scholar
|
|
11
|
Zhu HD, Guo JH, Mao AW, Lv WF, Ji JS, Wang
WH, Lv B, Yang RM, Wu W, Ni CF, et al: Conventional stents versus
stents loaded with (125)iodine seeds for the treatment of
unresectable oesophageal cancer: A multicentre, randomised phase 3
trial. Lancet Oncol. 15:612–619. 2014. View Article : Google Scholar
|
|
12
|
Chen HL, Shen WQ and Liu K: Radioactive
self-expanding stents for palliative management of unresectable
esophageal cancer: A systematic review and meta-analysis. Dis
Esophagus. 30:1–16. 2017. View Article : Google Scholar
|
|
13
|
Qu A, Wang H, Li J, Wang J, Liu J, Hou Y,
Huang L and Zhao Y: Biological effects of (125)i seeds radiation on
A549 lung cancer cells: G2/M arrest and enhanced cell death. Cancer
Invest. 32:209–217. 2014. View Article : Google Scholar
|
|
14
|
Wang ZM, Lu J, Zhang LY, Lin XZ, Chen KM,
Chen ZJ, Liu FJ, Yan FH, Teng GJ and Mao AW: Biological effects of
low-dose-rate irradiation of pancreatic carcinoma cells in vitro
using 125I seeds. World J Gastroenterol. 21:2336–2342. 2015.
View Article : Google Scholar :
|
|
15
|
Kim BM and Hong Y, Lee S, Liu P, Lim JH,
Lee YH, Lee TH, Chang KT and Hong Y: Therapeutic implications for
overcoming radiation resistance in cancer therapy. Int J Mol Sci.
16:26880–26913. 2015. View Article : Google Scholar :
|
|
16
|
Hu L, Wang H, Zhao Y and Wang J: (125)I
seeds radiation induces paraptosis-like cell death via PI3K/AKT
signaling pathway in HCT116 cells. Biomed Res Int.
2016:81454952016. View Article : Google Scholar :
|
|
17
|
Chaurasia M, Bhatt AN, Das A, Dwarakanath
BS and Sharma K: Radiation-induced autophagy: Mechanisms and
consequences. Free Radic Res. 50:273–290. 2016. View Article : Google Scholar
|
|
18
|
Leidal AM, Levine B and Debnath J:
Autophagy and the cell biology of age-related disease. Nat Cell
Biol. 20:1338–1348. 2018. View Article : Google Scholar
|
|
19
|
Lu C and Xie C: Radiation-induced
autophagy promotes esophageal squamous cell carcinoma cell survival
via the LKB1 pathway. Oncol Rep. 35:3559–3565. 2016. View Article : Google Scholar
|
|
20
|
Tao H, Qian P, Lu J, Guo Y, Zhu H and Wang
F: Autophagy inhibition enhances radiosensitivity of Eca109 cells
via the mitochondrial apoptosis pathway. Int J Oncol. 52:1853–1862.
2018.
|
|
21
|
Lee D, Kim IY, Saha S and Choi KS:
Paraptosis in the anti-cancer arsenal of natural products.
Pharmacol Ther. 162:120–133. 2016. View Article : Google Scholar
|
|
22
|
Prokhorova EA, Egorshina AY, Zhivotovsky B
and Kopeina GS: The DNA-damage response and nuclear events as
regulators of nonapoptotic forms of cell death. Oncogene. 39:1–16.
2020. View Article : Google Scholar
|
|
23
|
Chen X, Chen X, Zhang X, Wang L, Cao P,
Rajamanickam V, Wu C, Zhou H, Cai Y, Liang G and Wang Y:
Curcuminoid B63 induces ROS-mediated paraptosis-like cell death by
targeting TrxR1 in gastric cells. Redox Biol. 21:1010612019.
View Article : Google Scholar
|
|
24
|
Fontana F, Moretti RM, Raimondi M,
Marzagalli M, Beretta G, Procacci P, Sartori P, Montagnani Marelli
M and Limonta P: delta-Tocotrienol induces apoptosis, involving
endoplasmic reticulum stress and autophagy, and paraptosis in
prostate cancer cells. Cell Prolif. 52:e125762019. View Article : Google Scholar :
|
|
25
|
Seo MJ, Lee DM, Kim IY, Lee D, Choi MK,
Lee JY, Park SS, Jeong SY, Choi EK and Choi KS: Gambogic acid
triggers vacuolization-associated cell death in cancer cells via
disruption of thiol proteostasis. Cell Death Dis. 10:1872019.
View Article : Google Scholar :
|
|
26
|
Zhao H, Xu X, Lei S, Shao D, Jiang C, Shi
J, Zhang Y, Liu L, Lei S, Sun H and Huang Q: Iturin A-like
lipopeptides from Bacillus subtilis trigger apoptosis, paraptosis,
and autophagy in Caco-2 cells. J Cell Physiol. 234:6414–6427. 2019.
View Article : Google Scholar
|
|
27
|
Kessel D: Apoptosis, paraptosis and
autophagy: Death and survival pathways associated with photodynamic
therapy. Photochem Photobiol. 95:119–125. 2019. View Article : Google Scholar
|
|
28
|
Weinberg F and Chandel NS: Reactive oxygen
species-dependent signaling regulates cancer. Cell Mol Life Sci.
66:3663–3673. 2009. View Article : Google Scholar
|
|
29
|
Aird EG, Folkard M, Mayes CR, Bownes PJ,
Lawson JM and Joiner MC: A purpose-built iodine-125 irradiation
plaque for low dose rate low energy irradiation of cell lines in
vitro. Br J Radiol. 74:56–61. 2001. View Article : Google Scholar
|
|
30
|
Gan Z, Jing J, Zhu G, Qin Y, Teng G and
Guo J: Preventive effects of (1)(2)(5)I seeds on benign restenosis
following esophageal stent implantation in a dog model. Mol Med
Rep. 11:3382–3390. 2015. View Article : Google Scholar
|
|
31
|
Fernandez-Capetillo O, Chen HT, Celeste A,
Ward I, Romanienko PJ, Morales JC, Naka K, Xia Z, Camerini-Otero
RD, Motoyama N, et al: DNA damage-induced G2-M checkpoint
activation by histone H2AX and 53BP1. Nat Cell Biol. 4:993–997.
2002. View
Article : Google Scholar
|
|
32
|
Chen YS, Song HX, Lu Y, Li X, Chen T,
Zhang Y, Xue JX, Liu H, Kan B, Yang G and Fu T: Autophagy
inhibition contributes to radiation sensitization of esophageal
squamous carcinoma cells. Dis Esophagus. 24:437–443. 2011.
View Article : Google Scholar
|
|
33
|
Shubin AV, Demidyuk IV, Komissarov AA,
Rafieva LM and Kostrov SV: Cytoplasmic vacuolization in cell death
and survival. Oncotarget. 7:55863–55889. 2016. View Article : Google Scholar :
|
|
34
|
Zaorsky NG, Davis BJ, Nguyen PL, Showalter
TN, Hoskin PJ, Yoshioka Y, Morton GC and Horwitz EM: The evolution
of brachytherapy for prostate cancer. Nat Rev Urol. 14:415–439.
2017. View Article : Google Scholar
|
|
35
|
Wu C, Li B, Sun G, Peng C and Xiang D:
Efficacy and safety of iodine-125 brachytherapy combined with
chemotherapy in the treatment of advanced NSCLC in the elderly.
Onco Targets Ther. 11:6617–6624. 2018. View Article : Google Scholar :
|
|
36
|
Lehnert S, Reniers B and Verhaegen F:
Relative biologic effectiveness in terms of tumor response of 125I
implants compared with 60Co gamma rays. Int J Radiat Oncol Biol
Phys. 63:224–229. 2005. View Article : Google Scholar
|
|
37
|
Liu C, Wang L, Qiu H, Dong Q, Feng Y, Li
D, Li C and Fan C: Combined strategy of radioactive (125)I seeds
and salinomycin for enhanced glioma chemo-radiotherapy: Evidences
for ROS-mediated apoptosis and signaling crosstalk. Neurochem Res.
43:1317–1327. 2018. View Article : Google Scholar
|
|
38
|
Zhang WF, Jin WD, Li B, Wang MC, Li XG,
Mao WY and Luo KY: Effect of brachytherapy on NF-kB and VEGF in
gastric carcinoma xenografts. Oncol Rep. 32:635–640. 2014.
View Article : Google Scholar
|
|
39
|
Lee DM, Kim IY, Seo MJ, Kwon MR and Choi
KS: Nutlin-3 enhances the bortezomib sensitivity of p53-defective
cancer cells by inducing paraptosis. Exp Mol Med. 49:e3652017.
View Article : Google Scholar :
|
|
40
|
Zhou Y, Huang F, Yang Y, Wang P, Zhang Z,
Tang Y, Shen Y and Wang K: Paraptosis-inducing nanomedicine
overcomes cancer drug resistance for a potent cancer therapy.
Small. 14:2018.
|
|
41
|
Kim JY, Lee DM, Woo HG, Kim KD, Lee HJ,
Kwon YJ and Choi KS: RNAi screening-based identification of USP10
as a novel regulator of paraptosis. Sci Rep. 9:49092019. View Article : Google Scholar :
|
|
42
|
Sperandio S, de Belle I and Bredesen DE:
An alternative, nonapoptotic form of programmed cell death. Proc
Natl Acad Sci USA. 97:14376–14381. 2000. View Article : Google Scholar
|
|
43
|
Wang G, Liu L, Sharma S, Liu H, Yang W,
Sun X and Dong Q: Bmi-1 confers adaptive radioresistance to
KYSE-150R esophageal carcinoma cells. Biochem Biophys Res Commun.
425:309–314. 2012. View Article : Google Scholar
|
|
44
|
Zhang H, Luo H, Jiang Z, Yue J, Hou Q, Xie
R and Wu S: Fractionated irradiation-induced EMT-like phenotype
conferred radioresistance in esophageal squamous cell carcinoma. J
Radiat Res. 57:370–380. 2016. View Article : Google Scholar :
|
|
45
|
Mirzayans R, Andrais B, Scott A, Wang YW,
Kumar P and Murray D: Multinucleated giant cancer cells produced in
response to ionizing radiation retain viability and replicate their
genome. Int J Mol Sci. 18:E3602017. View Article : Google Scholar
|
|
46
|
Denisenko TV, Sorokina IV, Gogvadze V and
Zhivotovsky B: Mitotic catastrophe and cancer drug resistance: A
link that must to be broken. Drug Resist Updat. 24:1–12. 2016.
View Article : Google Scholar
|
|
47
|
Weihua Z, Lin Q, Ramoth AJ, Fan D and
Fidler IJ: Formation of solid tumors by a single multinucleated
cancer cell. Cancer. 117:4092–4099. 2011. View Article : Google Scholar :
|
|
48
|
Srinivas US, Tan BWQ, Vellayappan BA and
Jeyasekharan AD: ROS and the DNA damage response in cancer. Redox
Biol. 25:1010842019. View Article : Google Scholar
|
|
49
|
Zou Z, Chang H, Li H and Wang S: Induction
of reactive oxygen species: An emerging approach for cancer
therapy. Apoptosis. 22:1321–1335. 2017. View Article : Google Scholar
|
|
50
|
Tang JY, Ou-Yang F, Hou MF, Huang HW, Wang
HR, Li KT, Fayyaz S, Shu CW and Chang HW: Oxidative
stress-modulating drugs have preferential anticancer
effects-involving the regulation of apoptosis, DNA damage,
endoplasmic reticulum stress, autophagy, metabolism, and migration.
Semin Cancer Biol. 58:109–117. 2019. View Article : Google Scholar
|