Introduction
Lung cancer is the leading cause of cancer-related
deaths worldwide and over 80% of patients with lung cancer are
diagnosed with non-small cell lung cancer (NSCLC) (1,2).
Platinum-based doublets, established in a series of clinical trials
as the standard treatment for NSCLC, have increased the response
rates and median survival of patients with NSCLC compared with the
same platinum alone (3). However,
the median survival of these patients remains less than 14
months.
Tumour angiogenesis, the formation of new
vasculature to supply nutrition and oxygen in a direction from
pre-existing blood vessels toward tumours, is prerequisite for
tumour progression (4). Most
tumours, including NSCLC, associated with tumour angiogenesis
overexpress vascular endothelial growth factors (VEGFs), and
VEGF/VEGF receptor (VEGFR) axes, particularly the VEGF-A/VEGFR2
axis, play a pivotal role in angiogenesis (5). Binding of VEGF-A to VEGFR2 results in
tyrosine phosphorylation in VEGFR2 and subsequent activation of
cellular substrates including extracellular-regulated kinase 1/2
(ERK1/2), members of the mitogen-activated protein kinase
superfamily, which regulate cell proliferation, differentiation and
survival (5).
Anti-angiogenic therapy targeting the VEGF-A/VEGFR2
axis is a promising strategy aimed at preventing tumour growth,
invasion and metastasis (6). The
addition of bevacizumab, a monoclonal antibody to VEGF-A, to the
platinum-based doublets is currently used for first-line therapy
for advanced and unresectable NSCLC except in the case of squamous
cell carcinoma (3). Recently, the
combination of docetaxel and ramucirumab, a monoclonal antibody to
VEGFR2, was approved for second-line therapy for advanced and
unresectable NSCLC including squamous cell carcinoma (3,7). Both
anti-angiogenic therapies significantly improve the response rates,
progression-free survival and overall survival of patients with
NSCLC, yet the median survival of the patients was shorter than 18
months. These studies suggest that NSCLC cells secrete
VEGF-independent angiogenic factors and that more effective
antiangiogenic-antibody therapies are expected to be developed.
In the present study, we explored VEGF-independent
angiogenic factors in NSCLC and demonstrated that hepatoma-derived
growth factor (HDGF) enhances VEGF-dependent angiogenesis and that
fibroblast growth factor-2 (FGF-2) is a VEGF-independent angiogenic
factor in human NSCLC cells.
Materials and methods
Cell culture, morphological
observation and reagents
Human NSCLC cell lines, A549, Lu99 and EBC-1, (Riken
BioResearch Center, Tsukuba, Japan) were cultured in 100-mm dishes
(Becton Dickinson Labware) in RPMI-1640 medium supplemented with
10% fetal bovine serum (FBS) (Moregate Biotech), penicillin (5
µg/ml), streptomycin (5 µg/ml) and neomycin (10 µg/ml). Human
umbilical vein endothelial cells (HUVECs) isolated from human
umbilical cord were purchased from Lonza Walkersville, Inc. and
cultured as described previously (8). Incubation was carried out at 37°C in
95% air and 5% CO2. Phase contrast imaging with a light
microscope (Nikon, Tokyo, Japan) was performed at the indicated
time points. Representative images of phase contrast were obtained.
Type I collagen solution (Atelocollagen Bovine Dermis, IPC-30) was
purchased from Koken, Co., Ltd. Other chemicals were purchased from
Sigma-Aldrich; Merck KGaA unless otherwise stated. All cells used
in this study were authenticated by short tandem repeat analysis
and confirmed to be mycoplasma-negative.
Serum-free culture
Serum-free culture was performed as described
previously (8) with slight
modifications. The NSCLC cell lines were trypsinised, spun down at
4°C and washed twice with cold serum-free MCDB-104GK medium (Nihon
Pharmaceutical Co., Ltd.). The cell lines were then serum-deprived,
seeded at a density of 0.2 or 2×105 cells/cm2
in 35-mm dishes (Asahi Techno Glass), 60-mm dishes (Asahi Techno
Glass) or 100-mm dishes (Becton Dickinson Labware) in serum-free
MCDB-104GK medium supplemented with penicillin (5 µg/ml),
streptomycin (5 µg/ml) and neomycin (10 µg/ml) and incubated for 24
h. The serum-free culture supernatant derived from EBC-1 cells
(EBC-1 supernatant) and the cells were spun down, and each
supernatant was filtrated through a 0.45-µm polyvinylidene
difluoride membrane filter (Merck Millipore, Italy) and
concentrated by ultrafiltration (Amicon Ultra 3K, Merck Millipore).
Protein concentrations in EBC-1 supernatants were determined using
the Bradford method.
Flow cytometric analysis of cell
death
Cell death analyses in EBC-1 cells were performed
using the Muse™ Cell Analyzer (Merck Millipore) according to the
manufacturer's instructions as described previously (8) with slight modifications. Briefly,
EBC-1 cells were harvested after 24-h cultures with or without 10%
FBS and suspended at 3×105 cells/ml in
phosphate-buffered saline (PBS) containing 1% FBS. Each 100-µl cell
suspension was then labelled for 20 min in the dark with the same
volume of Annexin-V/7-amino-actinomycin D (7-AAD) reagent (Muse™
Annexin-V & Dead Cell kit, Merck Millipore). Quantitative
detection of Annexin V/7-AAD-positive cells was performed using the
Muse™ Cell Analyzer.
Formation of capillary-like tube
structures (tube formation) in sandwich culture and quantitative
analysis
Tube formation in sandwich culture was performed as
described previously (8) with
slight modifications. Briefly, HUVECs (1.1×105
cells/cm2) were sandwiched between two layers of
collagen gel (0.258% type I collagen) with tube-induction medium
composed of MCDB-104GK medium and 199 medium at a 13:7 ratio,
supplemented with 2% FBS, l-ascorbic acid (25 µg/ml), penicillin (5
µg/ml), streptomycin (5 µg/ml) and neomycin (10 µg/ml) in 24-well
culture plates (Becton Dickinson Labware) for 24 h. Tube formation
was induced in the presence of tube-induction medium containing or
stratifying culture supernatants derived from NSCLC cell lines,
recombinant human VEGF-A (rhVEGF-A; HumanZyme), rhHDGF (Abnova) or
rhFGF-2 (Fuji Film Wako Pure Chemical Corp.). Inhibitory analysis
of tube formation was performed using tube-induction medium
containing or stratifying the supernatants or the rhGFs together
with neutralising antibodies listed in Table SI. Tube formation was quantified as
described previously (8). Tube
areas were quantified as the ratio of the area of the formed tubes
to that of the imaged field using the Scion Image 4.0.3 program
(Scion Corp.), and the ratio of tube areas of vehicle treatment or
control was regarded as 1.0.
Cell viability analysis of
three-dimensional (3D) culture of HUVECs
Tube formation of HUVECs in 3D culture was performed
as described previously (8) with
slight modifications. Briefly, HUVECs were trypsinised and spun
down. The culture supernatants were aspirated, and the cell pellets
were mixed with 0.258% type I collagen gels as described above.
HUVECs mixed with collagen gel were added to each well
(2.86×106 cells/ml) of 96-well culture plates (Becton
Dickinson Labware) for cell viability analysis as described below.
The plates were incubated at 37°C for 1 h to allow the collagen gel
to solidify. HUVECs were then incubated for 24 h to induce tube
formation in the abovementioned tube-induction medium containing
EBC-1 supernatants at 2–50 µg/ml. Cell viabilities were determined
using the Cell Counting Kit-8 (Dojindo) as described previously
(8). Cell viability assays in 3D
culture were performed in triplicate.
Cell proliferation analysis of HUVECs
in monolayer culture
Cell proliferation was analysed as described
previously (8) with slight
modifications. Briefly, HUVECs (5.0×103
cells/cm2) in collagen-coated 24-well culture plates
were treated with the abovementioned tube-induction medium
containing EBC-1 supernatants (2–50 µg/ml) at the indicated time
points. The cells were harvested by trypsinisation, and the cell
counts were determined with the Coulter Counter Z1 (Coulter Japan).
Cell proliferation assays were performed in duplicate.
Western blotting and antibodies
Western blotting was performed as described
previously (8) with slight
modifications. Briefly, HUVECs (1×105
cells/cm2) were seeded in collagen-coated 60-mm culture
dishes and incubated in the culture medium. The culture medium was
aspirated after 24 h and the dishes were washed with PBS. HUVECs
were then incubated in tube-induction medium without FBS for 3 h
before stimulation. Stimulation was achieved by supplementing the
serum-free tube-induction medium containing EBC-1 supernatant (50
µg/ml), 3D culture supernatant derived from Lu99 cells described
below or rhVEGF-A (30 ng/ml) with or without a mouse monoclonal
anti-IgG2B antibody (10 µg/ml) or an anti-VEGF-A neutralising
antibody (10 µg/ml) at the indicated time points. After the
stimulations, proteins were extracted from HUVECs. Culture
supernatants were collected from serum-free cultures of EBC-1 cells
and 3D culture of Lu99 cells treated with small interfering RNA
(siRNA) described below. Protein concentration of each supernatant
was determined using the Bradford method. Proteins extracted from
HUVECs, siRNA-treated EBC-1 supernatants (50 µg) and siRNA-treated
Lu99 cells were used for western blotting as described previously
(8). The antibodies used for
western blotting are described in Table SI.
RNA interference (RNAi) in NSCLC cell
lines
Stealth RNAi Negative Control Duplexes (#12935-113)
were purchased from Thermo Fisher Scientific, Inc. and used as a
control. Each Stealth siRNA duplex oligoribonucleotide against
VEGF-A, midkine (MK), HDGF, granulin (GRN) and FGF-2 (GenBank™
accession nos. NM_003376, NM_00101233, NM_004494, NM_002087 and
NM_002006, respectively) were synthesised by Thermo Fisher
Scientific, Inc. (sequences shown in Table SII). The duplex
oligoribonucleotides were dissolved in diethyl
pyrocarbonate-treated water to 20 µM. EBC-1 and Lu99 cells were
transfected with siRNAs using Lipofectamine RNAiMAX (Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's
instructions. Stealth RNAi compounds were transfected at a final
concentration of 5 nM in culture medium as described previously
(8) with slight modifications.
Briefly, 1×106 cells were incubated overnight in 100-mm
dishes containing 10 ml of RPMI-1640 medium supplemented with 10%
FBS without penicillin, streptomycin and neomycin. Lipofectamine
RNAiMAX and siRNA were each diluted in 1 ml of RPMI-1640 medium for
5 min at room temperature, and then they were combined and
incubated for 15 min at room temperature to form complexes. Two
millilitre of the mixture was added to each dish and the cells were
further incubated. The old RPMI-1640 medium containing the mixture
was aspirated after a 48-h incubation, the dishes were washed with
PBS, and 10 ml of fresh RPMI-1640 medium supplemented with 10% FBS
was added and incubated for another 24 h (a total of 72-h
incubations after starting the siRNA transfections). The cells were
harvested by trypsinisation. EBC-1 cells were then seeded at a
density of 2×105 cells/cm2 in 35-mm, 60-mm or
100-mm dishes in serum-free MCDB-104GK medium supplemented with
penicillin, streptomycin and neomycin and incubated further for 24
h as described above. The serum-free culture supernatants from
siRNA-treated EBC-1 cells were collected after the 24-h incubations
(a total of 96-h incubations after starting the siRNA
transfection). Following trypsinisation, Lu99 cells were incubated
in 3D cultures as described below.
Enzyme-linked immunosorbent assay
(ELISA) for VEGF-A
The levels of VEGF-A protein in serum-free culture
supernatants (50 µg/ml) from EBC-1 cells treated with or without
siRNAs were measured using the Human VEGF-A ELISA Kit (RayBiotech,
Inc.) according to the manufacturer's instructions as described
previously (8).
Identification of proteins by mass
spectrometry (MS)
After EBC-1 supernatant was collected as described
above, 20 µl (≥1 mg/ml) of each supernatant was precipitated using
trichloroacetic acid. The precipitates derived from each
supernatant were dissolved in Tris buffer (2 mM EDTA/250 mM
Tris-HCl) at pH 8.5, reduced with 0.67 M dithiothreitol, alkylated
with 1.4 M iodoacetamide and digested with trypsin. The recovered
peptides were analysed using a Q Exactive Plus MS instrument
(Thermo Fisher Scientific, Inc.) coupled with a capillary
high-performance liquid chromatography system (EASY-nLC 1200,
Thermo Fisher Scientific, Inc.) to acquire MS/MS spectra. A
0.075×150 mm-EASY-Spray column (3-µm particle diameter, 100-Å pore
size, Thermo Fisher Scientific, Inc.) was used with mobile phases
of 0.1% formic acid and 0.1% formic acid/80% acetonitrile. Data
derived from the MS/MS spectra were searched in the SWISS-Prot
database using the MASCOT Server (http://www. matrixscience. com) and proteins were
identified using the Scaffold viewer program (http://www.proteomesoftware.
com/products/scaffold). Protein identification by MS was
performed in two independent experiments, and proteins identified
in both experiments are shown in Table
SIII.
ELISA for HDGF
The levels of HDGF protein in EBC-1 supernatants (50
µg/ml) and those in Lu99 supernatants were measured using the Human
HDGF ELISA Kit (Arigo biolaboratories Corp., Hsinchu, Taiwan)
according to the manufacturer's instructions. Briefly, each
microtitre plate was pre-coated with a polyclonal anti-human HDGF
antibody (Arigo Biolaboratories Corp.). After collecting the
supernatants, EBC-1 supernatants (50 µg/ml), Lu99 supernatants or
rhHDGF (Arigo biolaboratories Corp.) were added to each well for 2
h at 37°C. After 3 washes with wash buffer, 100 µl of
peroxidase-linked anti-HDGF antibody (ARG81356, Arigo
biolaboratories Corp.) was added to each well for 1 h at 37°C.
Detection was performed with tetramethyl-benzidine,
dihydrochloride, dihydrate and hydrogen peroxide. The measurements
were repeated in duplicate. The lowest concentration of HDGF
detected by this system was 30 pg/ml. The colour intensity of each
solution after development was quantified using a SpectraMax PLUS
384 microplate reader (Molecular Devices, Sunnyvale, CA).
Semi-quantitative real-time reverse
transcription-polymerase chain reaction (RT-qPCR)
Isolation of total RNA, synthesis of first-strand
cDNA and PCR were performed as described previously (8) with slight modifications. Briefly,
total RNAs from EBC-1, A549 and Lu99 cells were isolated using the
TRIzol reagent (Enzo Life Sciences Inc.). First-strand cDNA was
synthesised from total RNA (1.25 µg) using the PrimeScript RT
Reagent Kit (Takara Bio, Inc.). PCR was performed with the
synthesised cDNA products using TaqMan Gene Expression Master Mix
(Thermo Fisher Scientific, Inc.) for each target gene. All
reactions were carried out in triplicate. The sequences of the PCR
primer pairs and fluorogenic probes used for HDGF,
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 18S-rRNA, VEGF-A,
brain-derived neurotrophic factor (BDNF), FGF-2 and FGF-5 are
available on the Thermo Fisher Scientific website (http://www.thermofisher.com, HDGF assay ID:
Hs00610314_m1; GAPDH assay ID: Hs99999905_m1; 18S-rRNA assay ID:
Hs99999901_s1; VEGF-A assay ID: Hs00900054_m1; BDNF assay ID:
Hs00380947_m1; FGF-2 assay ID: Hs00266645_m1; FGF-5 assay ID:
Hs00170454_m1). The PCR products were analysed using the ABI 7500
real-time PCR system (Thermo Fisher Scientific. Inc.) and
quantified by employing the 2−ΔΔCq quantification method
(9). As internal controls, the
expression levels of HDGF mRNA in EBC-1 cells cultured with or
without FBS were normalised to corresponding expression levels of
GAPDH mRNA, and those of HDGF, VEGF-A, BDNF, FGF-2 and FGF-5 mRNAs
in the NSCLC cell lines were normalised to the corresponding
expression levels of 18S-rRNA.
Gene microarray analysis
Isolation of total RNA from EBC-1 and Lu99 cells was
performed as described above. The degrees of RNA cross-linking and
RNA degradation were analysed by electrophoresis using the Agilent
2100 Bioanalyzer (Agilent Technologies). Microarray analysis was
performed with the 3D-Gene Human Oligo Chip 25k (Toray Industries
Inc.). For efficient hybridisation, this microarray was designed
with a columnar structure to stabilise spot morphologies and to
enable micro-bead agitation. Total RNA (0.5 µg) isolated from EBC-1
and Lu99 cells was amplified using the Amino Allyl MessageAMP II
aRNA Amplification Kit (Applied Biosystems). Amplified RNAs derived
from EBC-1 and Lu99 cells (10 µg) were labeled with Cyanine 5 (Cy5)
and Cy3, respectively. Purified Cy5- and Cy3-labeled aRNA pools
(each 1 µg) were individually mixed with hybridisation buffer, and
hybridised at 37°C for 16 h. The hybridisation was performed
according to the manufacturer's instructions (www.3d-gene.com). The hybridisation signals were
obtained using the 3D-Gene Scanner (Toray Industries Inc.) and
processed using the 3D-Gene Extraction software (Toray Industries
Inc.). The signals detected for each gene were normalised using
global normalisation method.
Three-dimensional culture of Lu99
cells
Lu99 cells were trypsinised and spun down, the
culture supernatants were aspirated, and the cell pellets were
mixed with 0.258% type I collagen gels as described above. Collagen
gel (42 or 350 µl) was added to each well of 96- or 24-well culture
plates, respectively, and the plates were incubated at 37°C for 3 h
to allow the collagen gel to solidify. The abovementioned
tube-induction medium was added to each well for a total of 120 µl
(96-well plates) or 1 ml (24-well plates) at cell densities of
0–5×106 cells/ml for cell viability analysis or
collecting 3D culture supernatants derived from Lu99 cells. Lu99
cells in 3D culture were incubated in 24-well culture plates for 24
h, each supernatant was then collected, and 500 µl of each
supernatant was stratified in tube-induction medium to induce tube
formation in sandwich culture described above. Cell viability was
determined using the Cell Counting Kit-8 (Dojindo, Japan) as
described previously (8). Cell
viability assays in 3D culture were performed in triplicate.
Protein concentrations in 3D culture supernatant derived from Lu99
cells cultured at a cell density of 2×106 cells/ml (Lu99
supernatant) were determined using the Bradford method.
Quantification and statistical
analysis
All data are presented as means ± standard errors of
the means (SEMs, n=3) of three independent experiments. Differences
in mean values among groups for multiple comparisons were subjected
to one-way factorial ANOVA and subsequent Dunnett's test or Tukey's
test, and were considered significant when P-values were <0.05
(*P<0.05, **P<0.01, ***P<0.005 and ****P<0.001) as
indicated in the figures and legends by the relevant symbol.
Statistical analyses were performed using JMP Pro 13 (SAS Institute
Inc., Japan).
Results
Serum-free culture supernatant derived
from a human NSCLC cell line, EBC-1, induces tube formation
To identify angiogenic factors in NSCLC, we
performed serum-free culture in this study, based on our previous
study where angiogenic factors were identified on human
mesothelioma cells (8). As shown in
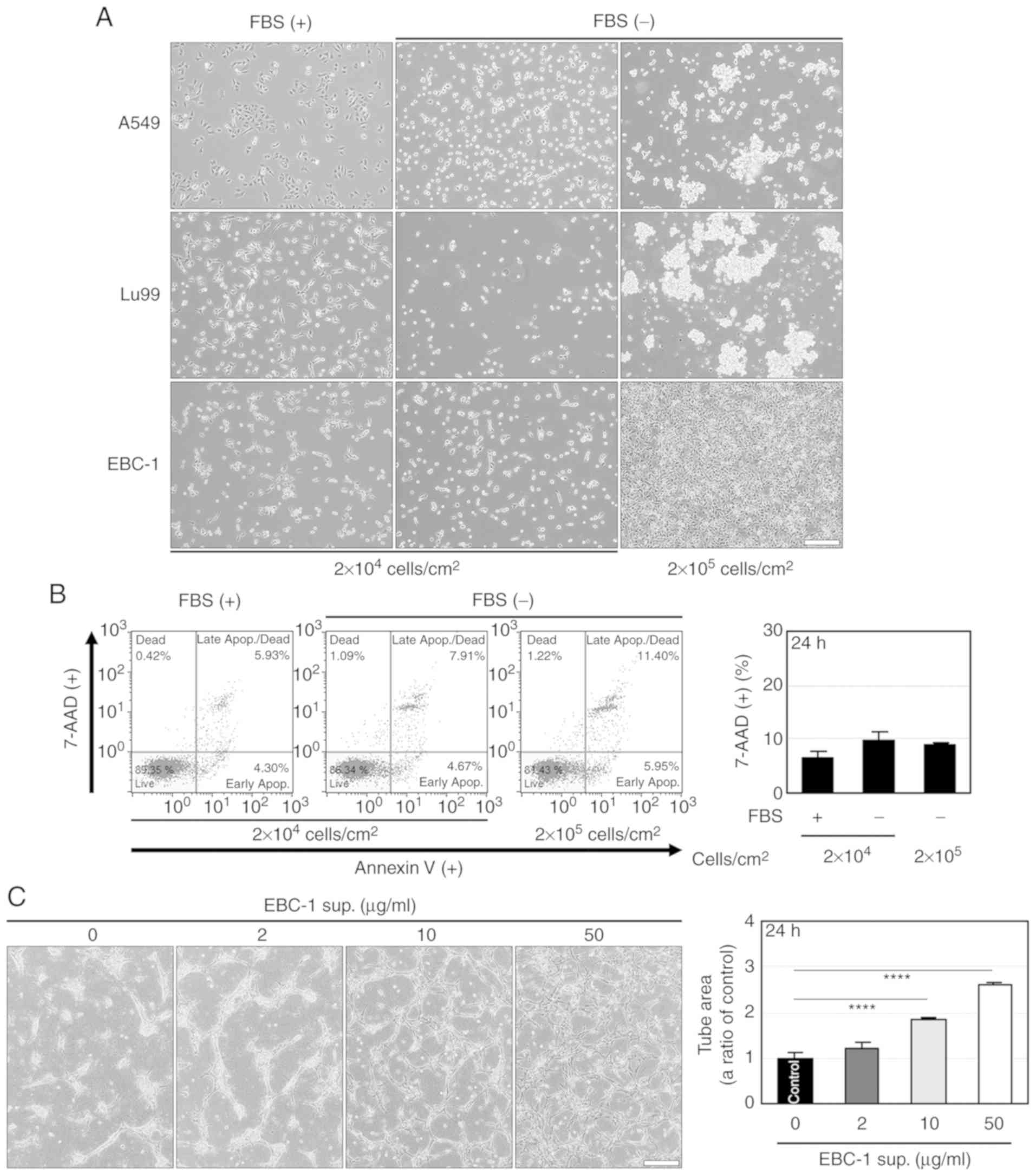
Fig. 1A, spheroid-like aggregation
and cell shrinkage-like cell death were observed at 24 h after
serum-free culture in human NSCLC cell lines, A549 and Lu99.
Meanwhile, the serum-free culture induced neither the aggregation
nor cell death in another NSCLC cell line, EBC-1 (Fig. 1A and B). These results indicate that
EBC-1 cells can adapt to serum-free culture.
We examined the effects of serum-free culture
supernatant derived from EBC-1 cells (EBC-1 supernatant) on
angiogenesis. EBC-1 supernatant induced the tube formation of
HUVECs (Fig. 1C) and increased the
cell viability of HUVECs in 3D cultures (Fig. S1A) for 24 h in
concentration-dependent manners. The numbers of HUVECs in monolayer
cultures were significantly increased by EBC-1 supernatant after
48-h or 72-h incubations, but not after a 24-h incubation (Fig. S1B). These results indicate that
EBC-1 supernatant induces tube formation and suggest that the
increase in cell viability of HUVECs by EBC-1 supernatant is due to
suppressed cell death rather than to promotion of cell
proliferation.
EBC-1 supernatant-induced tube
formation is mediated by both VEGF-dependent and -independent
pathways
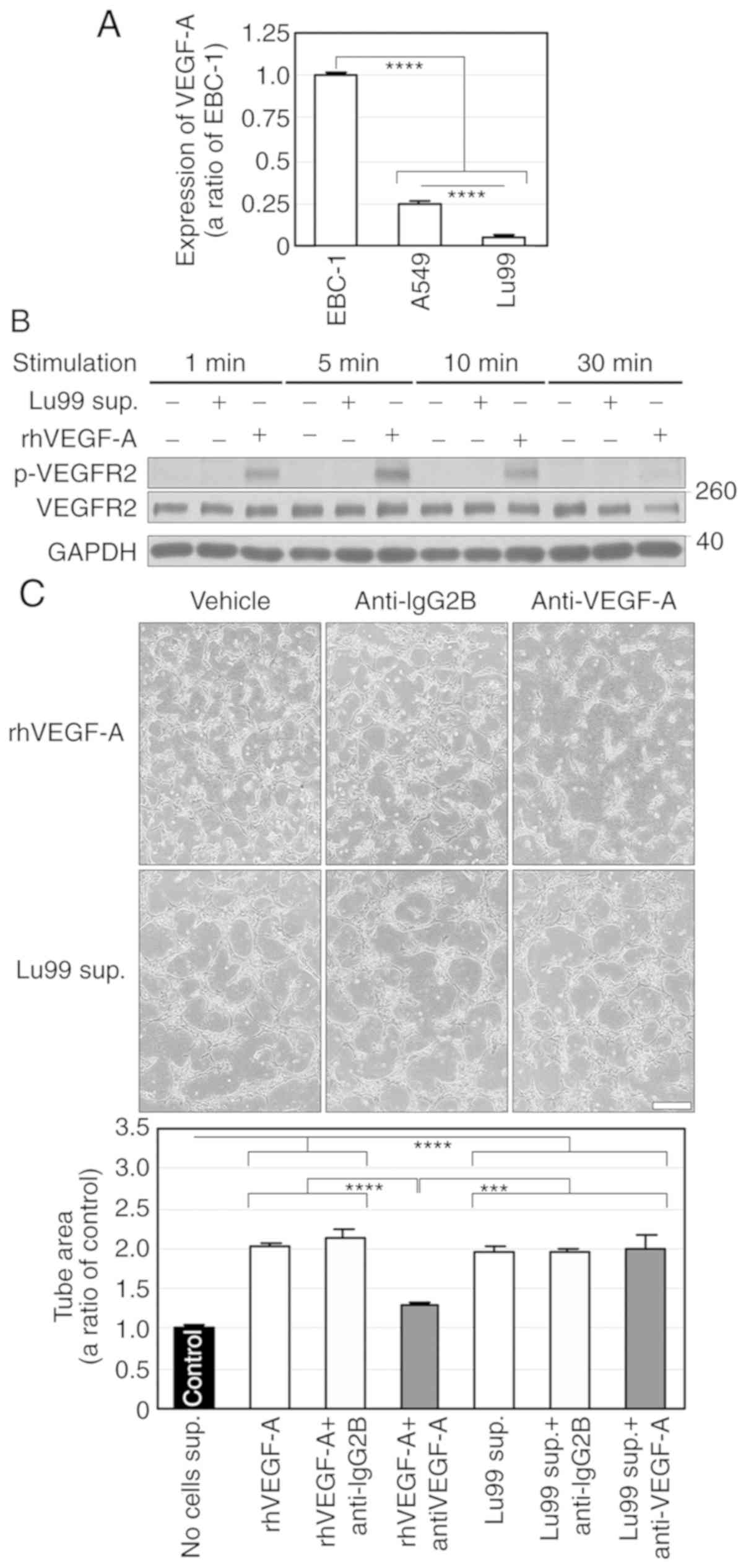
We then examined VEGFR2 phosphorylation in HUVECs
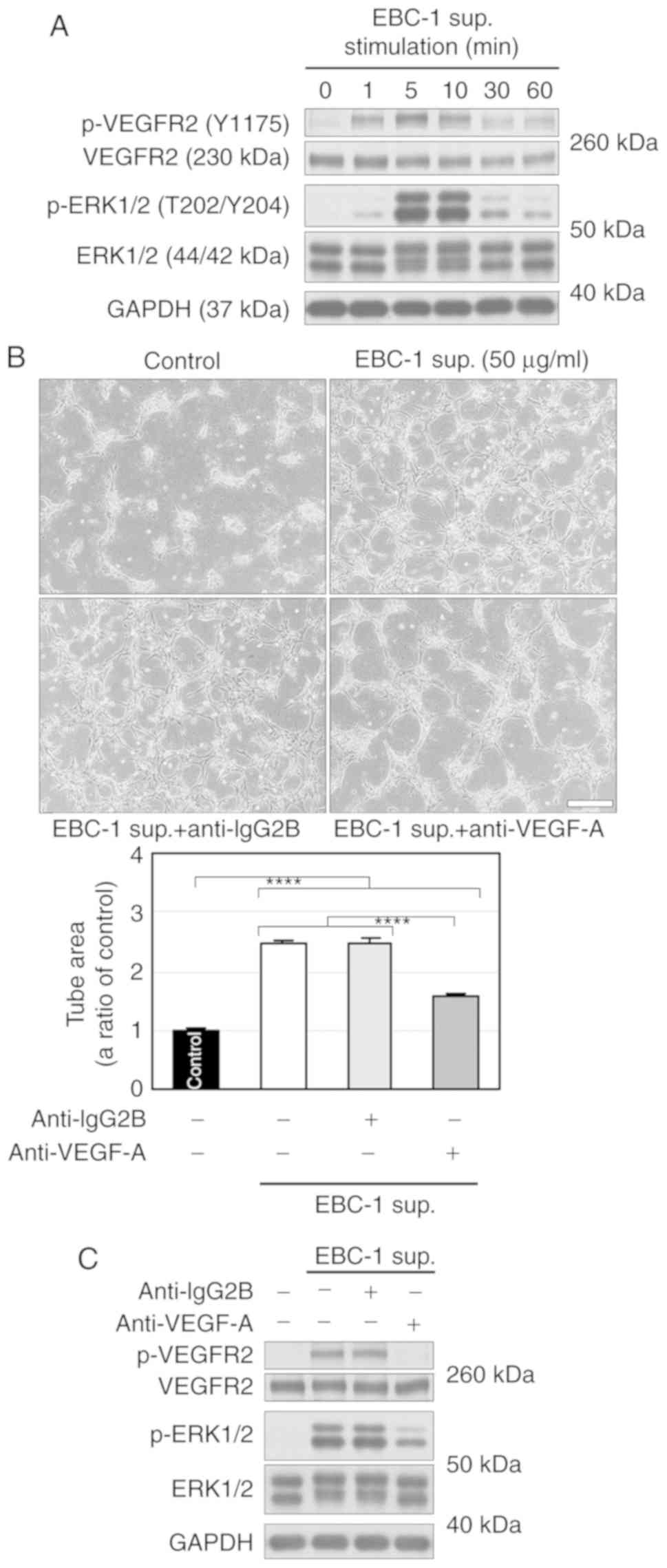
treated with EBC-1 supernatant. EBC-1 supernatant transiently
phosphorylated VEGFR2 and ERK1/2 (Fig.
2A). An anti-VEGF-A antibody (10 µg/ml) significantly, but not
completely, suppressed EBC-1 supernatant-induced tube formation
(Fig. 2B). In addition, the
antibody markedly suppressed the phosphorylation of VEGFR2, but not
completely that of ERK1/2 (Fig.
2C). We also performed RNAi using a siRNA targeting VEGF-A
(siVEGF-A) in EBC-1 cells. Treatment of EBC-1 cells with siVEGF-A
did not induce cell death more significantly than those with
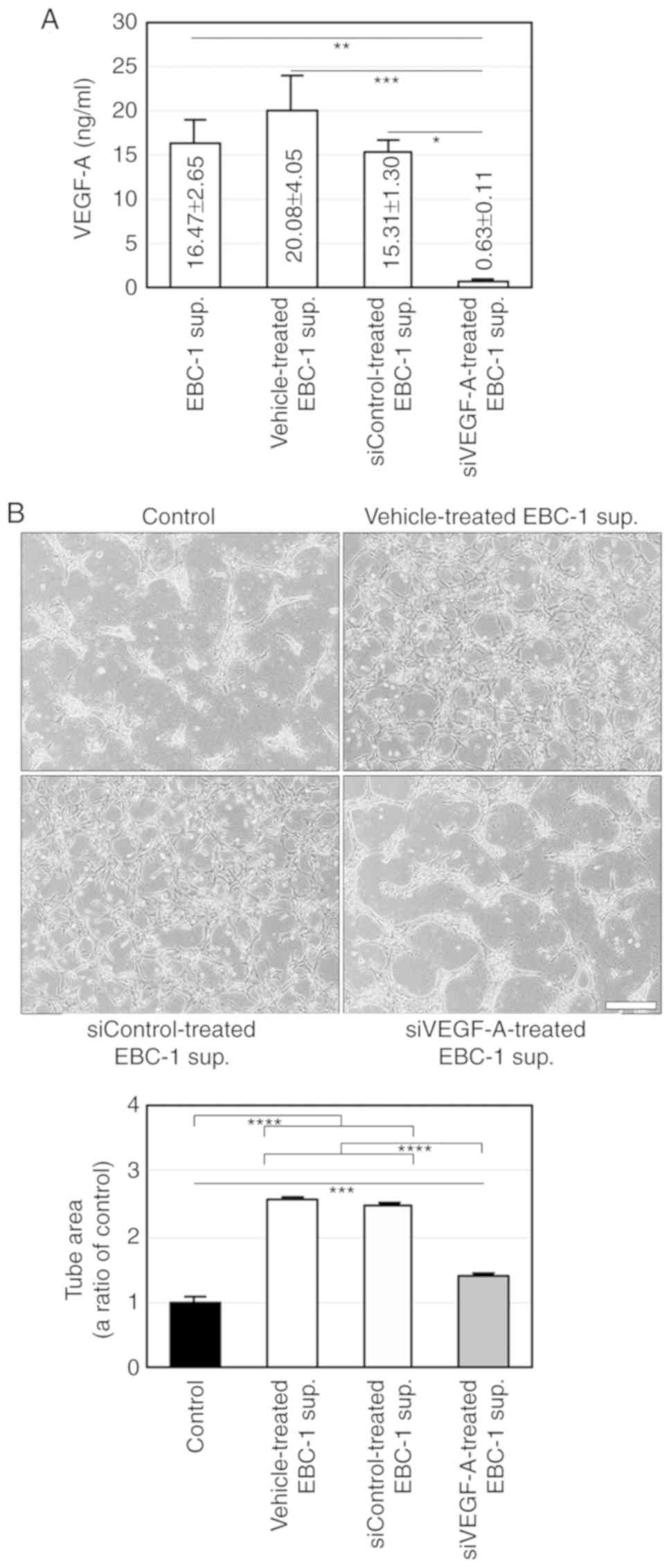
vehicle or control siRNA (siControl, Fig. S2). ELISA for VEGF-A showed that the
mean VEGF-A concentration in the supernatant (50 µg/ml) was
16.47±2.65 ng/ml (Fig. 3A). We
previously reported that the anti-VEGF-A antibody (10 µg/ml)
completely abrogated tube formation and VEGFR2 phosphorylation
induced by VEGF-A (30 ng/ml) (8).
The result of ELISA for VEGF-A and our previous findings indicate
that the anti-VEGF-A antibody completely inhibited VEGF-dependent
tube formation. ELISA also showed that siVEGF-A significantly
abrogated VEGF-A secretion (Fig.
3A). Furthermore, the serum-free culture supernatant derived
from EBC-1 cells transfected with siVEGF-A significantly, but not
completely, suppressed tube formation (Fig. 3B). These results suggest that EBC-1
supernatant-induced tube formation is regulated by both
VEGF-dependent and -independent pathways.
VEGF and HDGF regulate EBC-1
supernatant-induced tube formation
To explore humoral factors that regulate EBC-1
supernatant-induced tube formation independently of VEGF-A, we
analysed proteins in the supernatant by using mass spectrometry
(MS) and identified 1,007 proteins (Table SIII), most related to adhesion,
cytoskeletal and nuclear proteins, indicating that there were
microvesicles such as exosomes in the EBC-1 supernatant (10,11).
Among the proteins, interleukin-8 (IL-8) (12), macrophage migration inhibitory
factor (MIF) (13), galectin-1
(14), MK (15), IL-18 (16), galectin-3 (17), HDGF (18), osteopontin (OPN) (19), connective tissue growth factor
(CTGF) (20) and GRN (21) are known to be involved in
angiogenesis besides VEGF-A. We examined the involvements of IL-8,
MIF, galectin-1, IL-18, galectin-3, OPN and CTGF in EBC-1
supernatant-induced tube formation using commercially available
neutralising antibodies corresponding to each of the above proteins
or their receptors. None of the antibodies suppressed EBC-1
supernatant-induced tube formation (Fig. S3), suggesting that IL-8, MIF,
galectin-1, IL-18, galectin-3, OPN and CTGF are not directly
involved in EBC-1 supernatant-induced tube formation.
We then performed RNAi using siRNAs targeting MK
(siMK), HDGF (siHDGF) and GRN (siGRN) in EBC-1 cells and
investigated the involvements of these three proteins in EBC-1
supernatant-induced tube formation. Each siRNA and siControl did
not induce cell death in EBC-1 cells compared to vehicle in the
24-h serum-free cultures (Fig.
S4A). We also confirmed that each siRNA abrogated secretion of
the protein corresponding to the target gene (Fig. 4A). The supernatant of EBC-1 cells
transfected with HDGF siRNA (siHDGF) significantly, but not
completely, suppressed tube formation compared to those treated
with vehicle or other siRNAs (Fig.
4B).
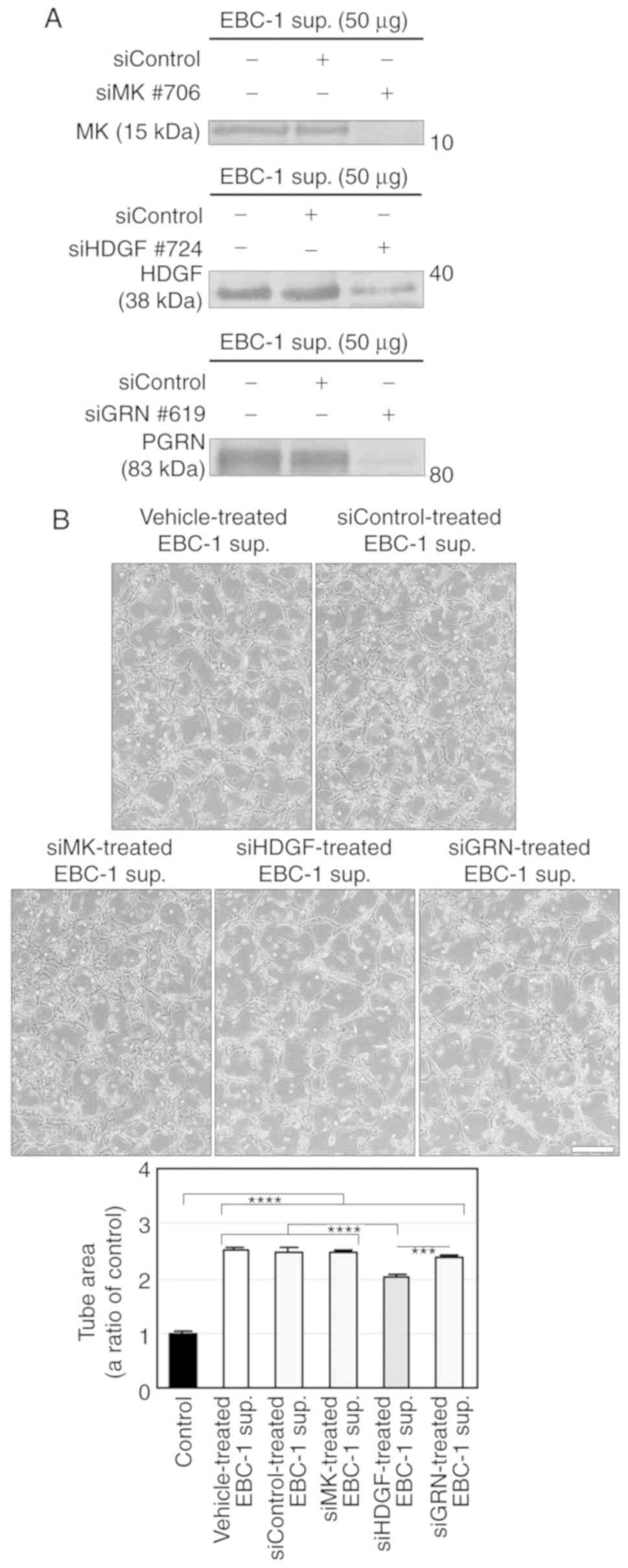 | Figure 4.HDGF, but not MK and GRN, is involved
in EBC-1 supernatant-induced tube formation. (A) Knockdown of MK,
HDGF and GRN by RNAi in the EBC-1 supernatant (sup.). Serum-free
culture supernatants (50 µg) of EBC-1 cells transfected with
vehicle, siControl, siMK (#706), siHDGF (#724) or siGRN (#619) were
used for western blotting. (B) HDGF, but not MK and GRN, is
involved in EBC-1 supernatant-induced tube formation. HUVECs
sandwiched between two layers of collagen were incubated with
serum-free culture supernatants (50 µg/ml) of EBC-1 cells
transfected with vehicle, siControl, siMK (#706), siHDGF (#724) or
siGRN (#619) for 24 h. ***P<0.005 and ****P<0.001. Each assay
was performed in three independent experiments and representative
images are shown. Data represent the means ± SEMs of three
independent experiments. Statistically significant differences were
determined by using one-way factorial ANOVA-Tukey's test. Scale
bar, 100 µm. HDGF, hepatoma-derived growth factor; MK, midkine;
GRN, granulin; HUVECs, human umbilical vein endothelial cells. |
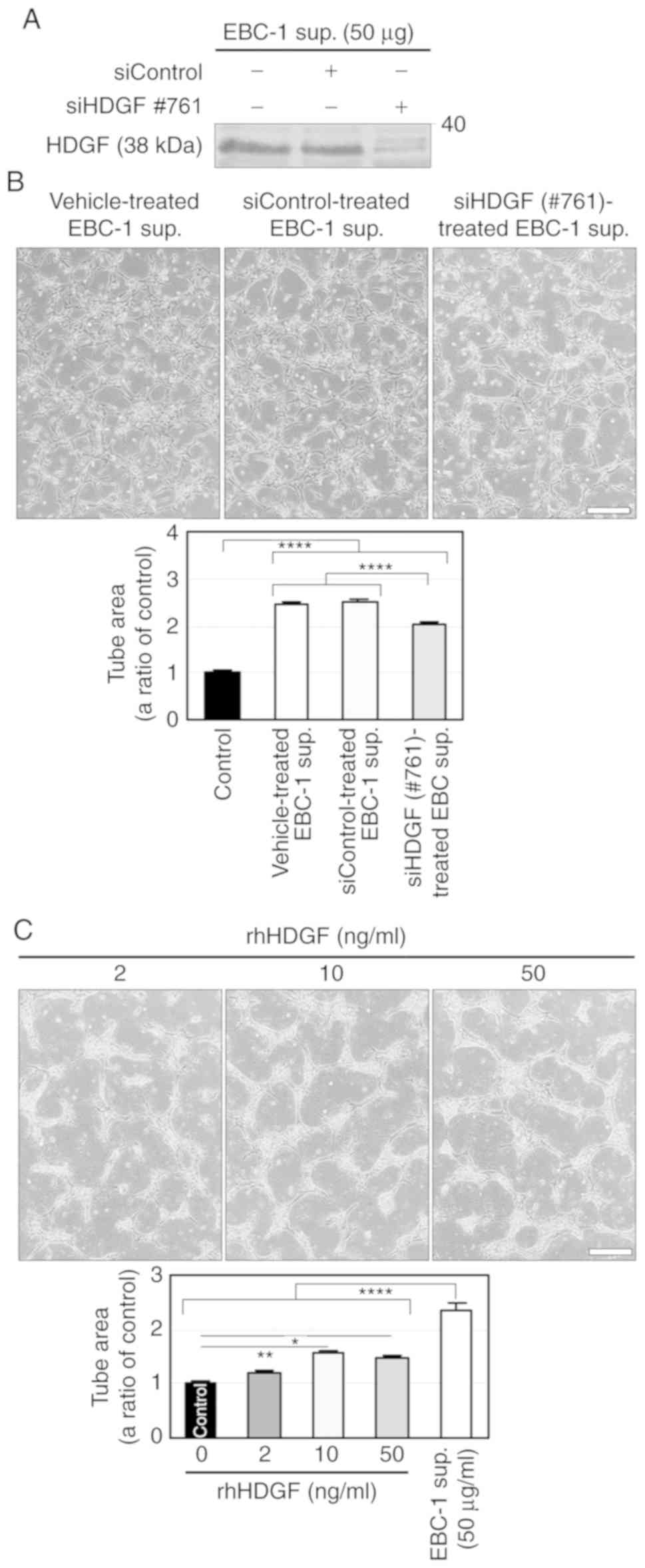
We further investigated the involvement of HDGF in
EBC-1 supernatant-induced tube formation using another siHDGF
(#761). siHDGF#761 did not induce cell death in EBC-1 cells
compared to vehicle and siControl (Fig. S4B) and abrogated HDGF secretion in
24-h serum-free cultures (Fig. 5A).
Similar to the result shown in Fig.
4B, the supernatant of EBC-1 cells transfected with siHDGF#761
significantly, but not completely, suppressed tube formation
compared to those treated with vehicle or siControl (Fig. 5B). We also investigated the effect
of HDGF on tube formation using rhHDGF. rhHDGF (10 ng/ml)
significantly induced tube formation, but much less potently than
did the EBC-1 supernatant (Fig.
5C).
ELISA for HDGF showed that the mean HDGF
concentration in EBC-1 supernatant (50 µg/ml) was 1.67±0.26 ng/ml
(Table SIV), while HDGF mRNA was
expressed in the EBC-1 cells independently of the presence of FBS
(Fig. 6A). In addition, the
expression levels of HDGF mRNA in A549 and Lu99 cells were
significantly higher than that in the EBC-1 cells (Fig. 6B). Furthermore, additions of the
anti-VEGF-A antibody to the supernatants of EBC-1 cells transfected
with siHDGFs (#724 or #761) suppressed tube formation more
significantly than those of the antibody to the supernatants of the
cells treated with vehicle or siControl (Fig. 6C). These results indicate that VEGF
mainly regulates EBC-1 supernatant-induced tube formation and HDGF
enhances VEGF-dependent tube formation.
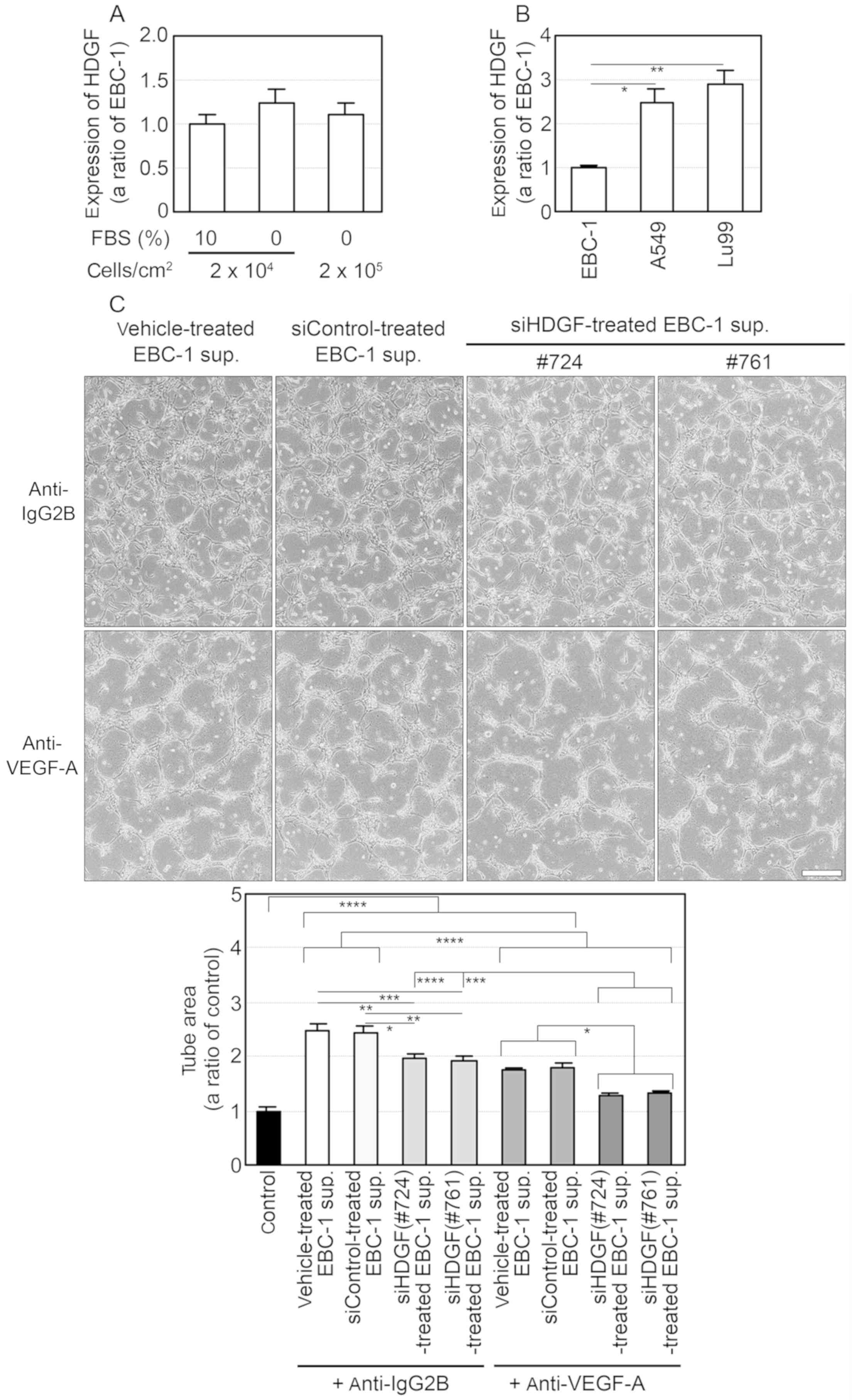 | Figure 6.VEGF-A and HDGF regulate tube
formation induced by the EBC-1 supernatant (sup.). (A and B)
Expression of HDGF mRNA in human NSCLC cells. Each total RNA was
isolated from EBC-1 cells at the indicated cell densities with or
without 10% FBS (A) and from EBC-1, A549 and Lu99 cells incubated
with 10% FBS (B) for 24 h. Synthesis of first-strand cDNA and PCR
were then performed as described in Materials and methods. The
expression levels of HDGF mRNA were normalised to the corresponding
levels of GAPDH mRNA (A) and those of 18S-rRNA (B) as an internal
control. *P<0.05 and **P<0.01 (B). (C) VEGF-A and HDGF in
EBC-1 supernatant induce tube formation. HUVECs sandwiched between
two layers of collagen were incubated with serum-free culture
supernatants (50 µg/ml) of EBC-1 cells transfected with vehicle,
siControl, siHDGF (#724) or siHDGF (#761) together with the mouse
monoclonal anti-IgG2B (10 µg/ml) or the anti-VEGF-A antibody.
*P<0.05, **P<0.01, ***P<0.005 and ****P<0.001. Each
assay was performed in three independent experiments and
representative images are shown. Data represent the means ± SEMs of
three independent experiments. Statistically significant
differences were determined by using one-way factorial ANOVA with
Dunnett's test (A and B) and Tukey's test (C) Scale bar, 100 µm.
VEGF, vascular endothelial growth factor; NSCLC, non-small cell
lung cancer; HDGF, hepatoma-derived growth factor; HUVECs, human
umbilical vein endothelial cells. |
Three-dimensional culture supernatant
derived from Lu99 cells induces HDGF- and VEGF-independent tube
formation
To determine whether HDGF is involved in
angiogenesis induced by the other NSCLC cell lines, we established
a novel culture method; NSCLC cells were embedded in collagen gel
and cultured three-dimensionally in tube-induction medium
containing FBS (Fig. 7A). The 3D
culture supernatants derived from Lu99 cells incubated at
0–5×106 cells/ml for 24 h significantly induced tube
formation in a cell density-dependent manner, whereas no
significant differences were noted in tube formation when using
different cell densities (2-5×106 cells/ml; Fig. 7B). Thus, we used the supernatant
derived from Lu99 cells cultured at a cell density of
2×106 cells/ml (Lu99 supernatant) in subsequent
experiments.
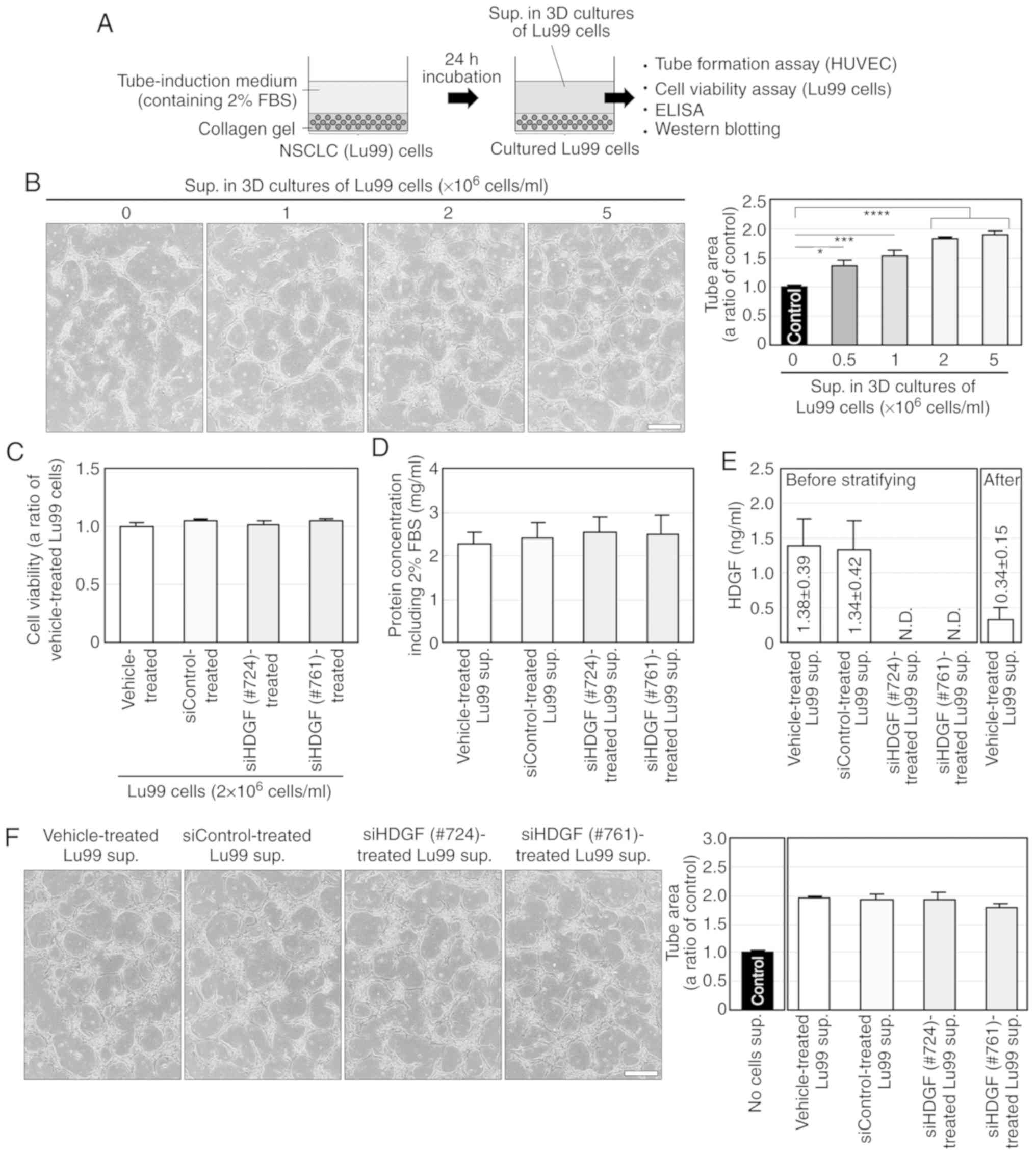 | Figure 7.Lu99 supernatant (sup.) induces
HDGF-independent tube formation. (A) Procedure of preparation and
use of the supernatant derived from Lu99 cells in 3D culture. Lu99
cells embedded in collagen were three-dimensionally incubated with
tube-induction medium containing 2% FBS for 24 h, and then Lu99
supernatant was collected as described in Materials and methods.
(B) Lu99 supernatant induces tube formation in a
cell-density-dependent manner. HUVECs sandwiched between two layers
of collagen were incubated with tube-induction medium, in which 3D
culture supernatants derived from Lu99 cells cultured at
0–5×106 cells/ml were stratified, for 24 h as described
in Materials and methods. *P<0.05, ***P<0.005 and
****P<0.001. (C) Lu99 cell viability in 3D culture was not
decreased by HDGF knockdown. Lu99 cells transfected with vehicle,
siControl, siHDGF (#724) or siHDGF (#761) were three-dimensionally
embedded in collagen and incubated in tube-induction medium for 24
h (a total of 96-h incubations after starting the siRNA
transfections) as described in Materials and methods. (D) Protein
concentration in Lu99 supernatants. After 24-h incubations in 3D
cultures of Lu99 cells transfected with vehicle, siControl, siHDGF
(#724) or siHDGF (#761), the culture supernatants derived from
these cells were collected and protein concentrations in the
supernatants were determined using the Bradford method as described
in Materials and methods. (E) ELISA for HDGF in Lu99 supernatant.
The levels of HDGF protein in 3D culture supernatants of Lu99 cells
transfected with vehicle, siControl, siHDGF (#724) or siHDGF (#761)
and that after stratifying vehicle-treated Lu99 supernatant in
tube-induction medium were measured using the Human HDGF ELISA Kit
as described in Materials and methods. N.D., not detectable. (F)
HDGF is not directly involved in Lu99 supernatant-induced tube
formation. HUVECs sandwiched between two layers of collagen were
incubated with tube-induction medium, in which 3D culture
supernatants of Lu99 cells transfected with vehicle, siControl,
siHDGF (#724) or siHDGF (#761) were stratified, for 24 h. Each
assay was performed in three independent experiments and
representative images are shown. Data represent the means ± SEMs of
three independent experiments. Statistically significant
differences were determined by using one-way factorial
ANOVA-Tukey's test. Scale bar, 100 µm. HDGF, hepatoma-derived
growth factor; HUVECs, human umbilical vein endothelial cells. |
We performed RNAi in Lu99 cells using siHDGFs (#724
or #761) and investigated the involvement of HDGF in Lu99
supernatant-induced tube formation. Both siHDGFs (#724 or #761) had
little effects on cell viability of Lu99 cells in 3D cultures
(Fig. 7C) and on protein
concentrations in Lu99 supernatants (Fig. 7D), and markedly abrogated HDGF
secretion (Fig. 7E) compared to
treatments with vehicle and siControl. However, the supernatants
derived from Lu99 cells transfected with siHDGFs did not suppress
tube formation (Fig. 7F), although
the HDGF concentrations in EBC-1 and Lu99 supernatants were almost
the same (Table SIV and Fig. 7E). The reason why HDGF was not
directly involved in Lu99 supernatant-induced tube formation was
presumed to be due to a decrease in HDGF concentration by
stratification of the Lu99 supernatant in the tube-induction medium
(Fig. 7E). These results suggest an
involvement of more potent angiogenic factors than HDGF in Lu99
supernatant-induced tube formation.
We also investigated the expression levels of VEGF-A
mRNA in EBC-1, A549 and Lu99 cells. Intriguingly, VEGF-A mRNA
expression in Lu99 cells was significantly weaker than those in
EBC-1 and A549 cells (Fig. 8A). The
Lu99 supernatant did not induce VEGFR2 phosphorylation (Fig. 8B). In addition, the anti-VEGF-A
antibody failed to suppress Lu99 supernatant-induced tube formation
(Fig. 8C). These results indicate
that the Lu99 supernatant induces HDGF- and VEGF-independent tube
formation.
FGF-2 regulates Lu99
supernatant-induced tube formation
To explore the humoral factor that regulates Lu99
supernatant-induced tube formation, we comprehensively analysed
mRNA expression in Lu99 and EBC-1 cells by using gene microarray,
and thereby identified 61 mRNAs expressed in Lu99 cells, but not in
EBC-1 cells (Table SV). Among
these mRNA-encoded proteins, brain-derived neurotrophic factor
(BDNF) (22), FGF-2 (23) and FGF-5 (24) are secreted extracellularly and are
known to be involved in angiogenesis. The expression levels of
BDNF, FGF-2 and FGF-5 mRNAs were significantly higher in Lu99 cells
than in EBC-1 and A549 cells (Fig.
9A). We then examined the involvements of BDNF, FGF-2 and FGF-5
in Lu99 supernatant-induced tube formation using commercially
available neutralising antibodies corresponding to each of the
above proteins. Consequently, Lu99 supernatant-induced tube
formation was not suppressed by antibodies to BDNF and FGF-5, but
was markedly inhibited by that to FGF-2 (Fig. 9B). As expected, rhFGF-2 (≥3 ng/ml)
induced tube formation as potently as did Lu99 supernatant
(Fig. S5). We further confirmed
the involvement of FGF-2 in Lu99 supernatant-induced tube formation
using another monoclonal anti-FGF-2 neutralising antibody. Similar
to the result shown in Fig. 9B, the
antibody significantly inhibited Lu99 supernatant-induced tube
formation (Fig. 9C). These results
indicate that FGF-2 regulates HDGF- and VEGF-independent tube
formation induced by Lu99 supernatant.
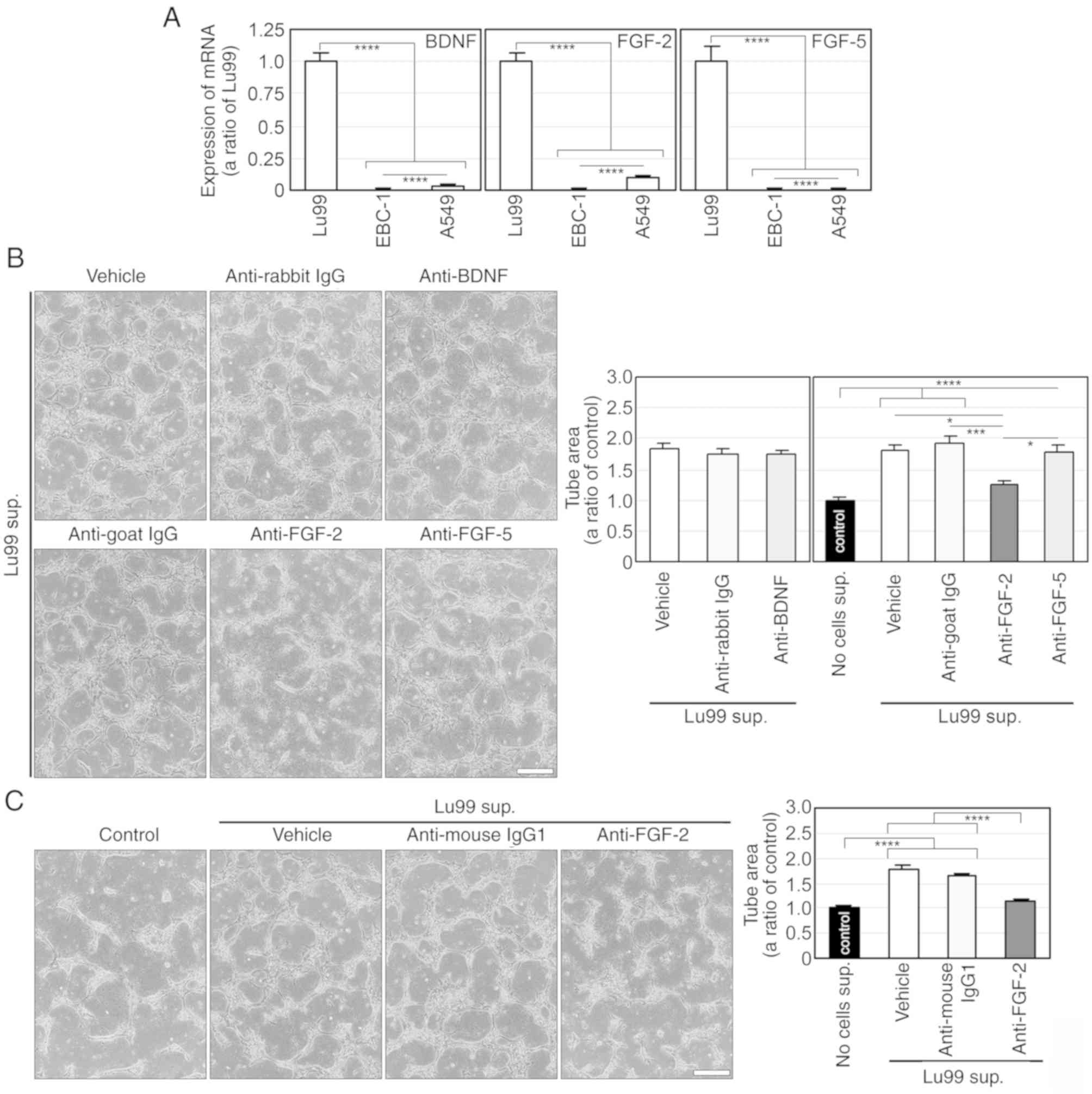 | Figure 9.FGF-2 regulates HDGF- and
VEGF-independent tube formation induced by the Lu99 supernatant
(sup.). (A) mRNA expressions of BDNF, FGF-2 and FGF-5 in human
NSCLC cells. Total RNA was isolated from Lu99, EBC-1 and A549 cells
incubated with 10% FBS for 24 h, and then synthesis of first-strand
cDNA and PCR was performed. Each expression level of BDNF, FGF-2
and FGF-5 mRNAs was normalised to the corresponding levels of
18S-rRNA as an internal control. ****P<0.001. (B) FGF-2, but not
BDNF and FGF-5, is involved in Lu99 supernatant-induced tube
formation. HUVECs sandwiched between two layers of collagen were
incubated with tube-induction medium alone and that together with
the rabbit polyclonal anti-IgG (10 µg/ml), the anti-BDNF, the goat
polyclonal anti-IgG (10 µg/ml), the anti-FGF-2 or the anti-FGF-5
antibody, in which Lu99 supernatants were stratified, for 24 h.
*P<0.05, ***P<0.005 and ****P<0.001. (C) FGF-2 in Lu99
supernatant induces HDGF- and VEGF-independent tube formation.
HUVECs sandwiched between two layers of collagen were incubated
with tube-induction medium alone and that together with the mouse
monoclonal anti-IgG1 (10 µg/ml) or the anti-FGF-2 antibody, in
which Lu99 supernatants were stratified, for 24 h.
****P<0.001. Each assay was performed in three
independent experiments and representative images are shown. Data
represent the means ± SEMs of three independent experiments.
Statistically significant differences were determined by using
one-way factorial ANOVA-Tukey's test. Scale bar, 100 µm. FGF-2,
fibroblast growth factor-2; HDGF, hepatoma-derived growth factor;
VEGF, vascular endothelial growth factor; BDNF, brain-derived
neutrophic factor; FGF-2, fibroblast growth factor-2; FGF-5,
fibroblast growth factor-5; HUVECs, human umbilical vein
endothelial cells. |
Discussion
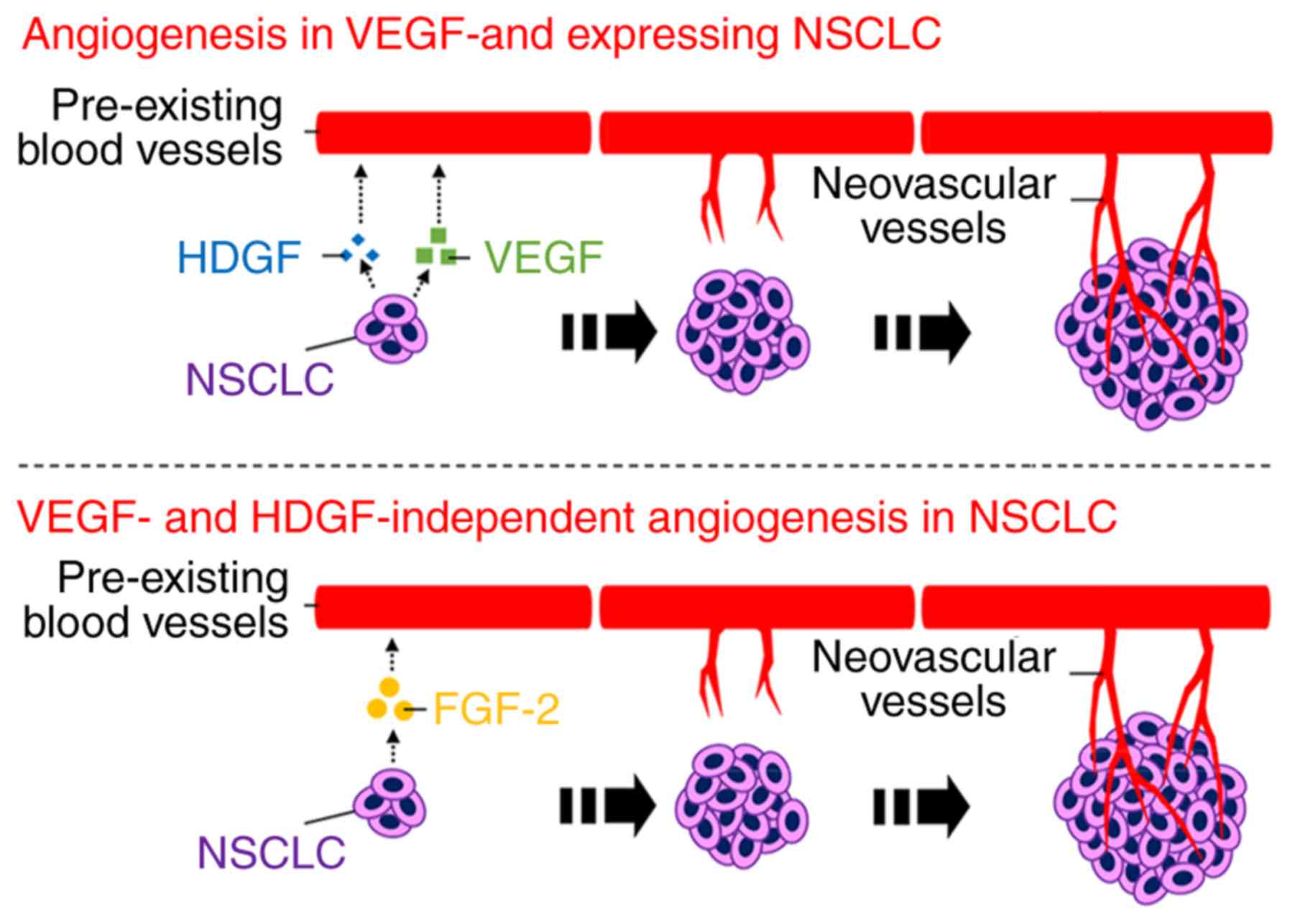
In the present study, we demonstrated for the first
time that hepatoma-derived growth factor (HDGF) enhances
angiogenesis in non-small cell lung cancer (NSCLC) cells expressing
vascular endothelial growth factor (VEGF) and that fibroblast
growth factor-2 (FGF-2) is a VEGF-independent angiogenic factor in
VEGF-downregulated NSCLC cells (Fig.
10). Our established serum-free and 3D culture methods revealed
the direct involvement of HDGF and FGF-2 in NSCLC-induced
angiogenesis. These novel findings may prove to be useful in
developing antiangiogenic therapy to neutralise HDGF or FGF-2 in
NSCLC.
HDGF is a secretory heparin-binding growth factor
purified from conditioned medium in which a human hepatoma cell
line (HuH-7) was cultured (25) and
involved in tumour-associated events such as tumourigenesis,
metastasis and angiogenesis (18).
HDGF is also known to be expressed endogenously in endothelial
cells and to induce angiogenesis exogenously (26). It has been shown that numerous NSCLC
cell lines and primary NSCLC specimens express HDGF (27,28)
and that patients with NSCLC expressing high HDGF present with a
poor prognosis compared with those expressing low HDGF (28,29).
Recently, downregulation of microRNAs, which target multiple genes
containing HDGF, was reported to promote tumour growth and invasion
of primary cells or cell lines including A549 in NSCLC (30–32).
Glioblastoma stem-like cells, but not normal neural stem cells,
were shown to express HDGF to directly induce tumour angiogenesis
(33). Compared to wild-type
hepatocellular carcinoma cells, tumour growth of their clone which
had downregulated HDGF in vivo was also suppressed by
inhibiting tumour angiogenesis rather than cell growth (34). While VEGF overexpression in NSCLC
patients has been associated with a poor prognosis (23), no significant association has been
found between the microvascular density in lesions and VEGF-A level
in the blood of patients with advanced NSCLC (35). In addition to these reports, our
findings show obvious evidence regarding the direct involvement of
HDGF in human NSCLC cells and enhancement of VEGF-dependent
angiogenesis by HDGF.
We performed serum-free culture with A549, Lu99 and
EBC-1 cells and found that only EBC-1 cells could adapt to the
culture. Consequently, cell death and HDGF mRNA expression in EBC-1
cells were little influenced regardless of whether FBS was present
or absent, but the possibility of alteration of the cell condition
in the serum-free culture cannot be completely excluded. In
addition, it was extremely difficult to confirm whether VEGF and
HDGF function as angiogenic factors in A549 and Lu99 cells, as
these cell lines could not adapt to the serum-free culture. Thus,
we established a novel 3D culture method, which enabled culture
supernatant, containing high concentrations of humoral factors
derived from NSCLC cells, to be utilised without FBS condensation
and cell contamination. By using the novel 3D culture method, we
clarified that the Lu99 supernatant induced HDGF- and
VEGF-independent tube formation and that FGF-2 regulated Lu99
supernatant-induced tube formation. FGF-2, also known as basic FGF,
belongs to the FGF family which consists of 23 FGF heparin-binding
polypeptides. FGF-2 is physiologically and pathologically an
important regulator of cell growth, survival and differentiation
such as development, tumourigenesis and angiogenesis (36). FGF-2 overexpression in operable
NSCLC patients was found to be a prognostic indicator of poor
survival (23,37,38),
whereas stromal FGF-2 in patients with NSCLC receiving
postoperative radiotherapy was found to be a positive prognostic
factor for survival (39).
Recently, a humanised anti-FGF-2 antibody produced by Wang et
al was reported to reduce tumour growth of a NSCLC cell line
(NCI-H460) and microvessel density in nude mice (40). The implication of FGF-2 for
prognosis in NSCLC was controversial in these reports; however,
based on these reports and our present study, FGF-2 overexpression
in NSCLC cells is thought to induce tumour angiogenesis.
To determine the involvement of FGF-2 in Lu99
supernatant-induced tube formation, we transfected Lu99 cells with
FGF-2 siRNA (siFGF-2). siFGF-2 did abrogate expression of FGF-2 (18
kDa) and its splicing variants (22, 22.5 and 24 kDa) in Lu99 cell
lysate (Fig. S6A). It has been
shown that FGF-2 proteins including the variants are lacking
secretory signal peptide (41). The
variants have both N- and C-terminal nuclear localisation signals
(NLSs), but 18 kDa FGF-2 has only C-terminal NLS, and translocation
of FGF-2 into the nucleus requires both NLSs, which means
transportation of the splicing variants, but not 18 kDa FGF-2, into
the nucleus (42). Intriguingly,
FGF-2 in the Lu99 supernatant did not remain monomeric but instead
formed oligomers, and their molecular weights differed from those
of rhFGF-2 dimers and oligomers; siFGF-2 did not suppress FGF-2
secretion in the Lu99 supernatant (Fig. S6B). In addition to phosphoinositol
4,5-bisphosphate-dependent FGF-2 oligomerisation concomitant with
membrane insertion (41,43), disassembly of membrane-inserted
FGF-2 oligomers at the outer leaflet, mediated by cell-surface
heparan sulphates, was recently reported (44). Type I collagen is also known to
function as a FGF-2 reservoir (45). We therefore speculated that FGF-2
bound to heparan sulphates on the surface of Lu99 cells was
retained after FGF-2 knockdown in Lu99 cells and that the residual
FGF-2 was gradually transported to the extracellular space with the
peptide chains of the heparan sulphates partially bound and then
complexed with collagen in 3D culture. In fact, we tried to measure
the FGF-2 concentration in Lu99 supernatant using the Human bFGF
ELISA kit (RayBiotech, Inc.), but the kit, in which the anti-FGF-2
antibodies recognise rhFGF-2 monomer produced by Escherichia
coli, failed to detect FGF-2 in the Lu99 supernatant. These
results posed an issue that accurate measurements for FGF-2 of
patients with NSCLC by ELISA need the anti-FGF-2 antibody to
recognise not only FGF-2 monomer but also its oligomer. We also
found no involvement of FGF-2 in Lu99 cell proliferation in
vitro, but further studies are needed to confirm whether FGF-2
inhibition by its neutralising antibodies and multi-tyrosine kinase
inhibitors targeting FGF receptors suppresses tumour growth of
NSCLC cells including A549 cells, in which serum-free culture could
not be adapted and FGF-2 expression was extremely lower than in
Lu99 cells, through in vivo assays to test the
anti-angiogenic effect. Furthermore, future studies are needed to
develop the experimental system for immunostaining of our 3D
cultures to further evaluate the findings in the present study.
In conclusion, we demonstrated for the first time
that HDGF enhances VEGF-dependent angiogenesis and that FGF-2 is a
VEGF-independent angiogenic factor in human NSCLC cells. We hope
that anti-angiogenic-antibody therapy targeting HDGF or FGF-2 can
provide a novel treatment option for patients with NSCLC resistant
to bevacizumab and ramucirumab.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Uehara Memorial
Foundation and Japan Society for the Promotion of Science KAKENHI
Grant-in-Aid (grant. no. 18K07278).
Availability of data and materials
The data that support the findings of this study are
available from the corresponding author upon reasonable
request.
Authors' contributions
RE proposed the study, conducted the experiments and
analysed the data. RE and IW designed the experiments and edited
the manuscript. Both authors read and approved the final manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
7-AAD
|
7-amino-actinomycin D
|
|
ANOVA
|
analysis of variance
|
|
BDNF
|
brain-derived neutrophic factor
|
|
Cy
|
cyanine
|
|
CTGF
|
connective tissue growth factor
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
ERK1/2
|
extracellular-regulated kinase 1/2
|
|
FBS
|
fetal bovine serum
|
|
FGF
|
fibroblast growth factor
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
GF
|
growth factor
|
|
GRN
|
granulin
|
|
HDGF
|
hepatoma-derived growth factor
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
IL
|
interleukin
|
|
MIF
|
macrophage migration inhibitory
factor
|
|
MK
|
midkine
|
|
MS
|
mass spectrometry
|
|
NSCLC
|
non-small cell lung cancer
|
|
OPN
|
osteopontin
|
|
PBS
|
phosphate-buffered saline
|
|
PCR
|
polymerase chain reaction
|
|
rhGF
|
recombinant human growth factor
|
|
RNAi
|
RNA interference
|
|
SEMs
|
standard errors of the means
|
|
siRNA
|
small interfering RNA
|
|
3D
|
three-dimensional
|
|
VEGF
|
vascular endothelial growth factor
|
|
VEGFR2
|
vascular endothelial growth factor
receptor 2
|
References
|
1
|
Inoue M, Sawada N, Matsuda T, Iwasaki M,
Sasazuki S, Shimazu T, Shibuya K and Tsugane S: Attributable causes
of cancer in Japan in 2005-systematic assessment to estimate
current burden of cancer attributable to known preventable risk
factors in Japan. Ann Oncol. 23:1362–1369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Villaruz LC and Socinski MA: The role of
anti-angiogenesis in non-small-cell lung cancer: An update. Curr
Oncol Rep. 17:262015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dvorak HF: Vascular permeability
factor/vascular endothelial growth factor: A critical cytokine in
tumor angiogenesis and a potential target for diagnosis and
therapy. J Clin Oncol. 20:4368–4380. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ebos JM and Kerbel RS: Antiangiogenic
therapy: Impact on invasion, disease progression, and metastasis.
Nat Rev Clin Oncol. 8:210–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoh K, Hosomi Y, Kasahara K, Yamada K,
Takahashi T, Yamamoto N, Nishio M, Ohe Y, Koue T, Nakamura T, et
al: A randomized, double-blind, phase II study of ramucirumab plus
docetaxel vs placebo plus docetaxel in Japanese patients with stage
IV non-small cell lung cancer after disease progression on
platinum-based therapy. Lung Cancer. 99:186–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eguchi R, Nakano T and Wakabayashi I:
Progranulin and granulin-like protein as novel VEGF-independent
angiogenic factors derived from human mesothelioma cells. Oncogene.
36:714–722. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ung TH, Madsen HJ, Hellwinkel JE, Lencioni
AM and Graner MW: Exosome proteomics reveals transcriptional
regulator proteins with potential to mediate downstream pathways.
Cancer Sci. 105:1384–1392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bosque A, Dietz L, Gallego-Lleyda A,
Sanclemente M, Iturralde M, Naval J, Alava MA, Martínez-Lostao L,
Thierse HJ and Anel A: Comparative proteomics of exosomes secreted
by tumoral Jurkat T cells and normal human T cell blasts unravels a
potential tumorigenic role for valosin-containing protein.
Oncotarget. 7:29287–29305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keeley EC, Mehrad B and Strieter RM: CXC
chemokines in cancer angiogenesis and metastases. Adv Cancer Res.
106:91–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chesney JA and Mitchell RA: 25 Years On: A
retrospective on migration inhibitory factor in tumor angiogenesis.
Mol Med. 21 (Suppl 1):S19–S24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thijssen VL and Griffioen AW: Galectin-1
and −9 in angiogenesis: A sweet couple. Glycobiology. 24:915–920.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kadomatsu K, Bencsik P, Gorbe A, Csonka C,
Sakamoto K, Kishida S and Ferdinandy P: Therapeutic potential of
midkine in cardiovascular disease. Br J Pharmacol. 171:936–944.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Palma G, Barbieri A, Bimonte S, Palla M,
Zappavigna S, Caraglia M, Ascierto PA, Ciliberto G and Arra C:
Interleukin 18: Friend or foe in cancer. Biochim Biophys Acta.
1836:296–303. 2013.PubMed/NCBI
|
|
17
|
Funasaka T, Raz A and Nangia-Makker P:
Galectin-3 in angiogenesis and metastasis. Glycobiology.
24:886–891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bao C, Wang J, Ma W, Wang X and Cheng Y:
HDGF: A novel jack-of-all-trades in cancer. Future Oncol.
10:2675–2685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai J, Peng L, Fan K, Wang H, Wei R, Ji G,
Cai J, Lu B, Li B, Zhang D, et al: Osteopontin induces angiogenesis
through activation of PI3K/AKT and ERK1/2 in endothelial cells.
Oncogene. 28:3412–3422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramazani Y, Knops N, Elmonem MA, Nguyen
TQ, Arcolino FO, van den Heuvel L, Levtchenko E, Kuypers D and
Goldschmeding R: Connective tissue growth factor (CTGF) from basics
to clinics. Matrix Biol 68–69. 44–66. 2018. View Article : Google Scholar
|
|
21
|
He Z and Bateman A: Progranulin
(granulin-epithelin precursor, PC-cell-derived growth factor,
acrogranin) mediates tissue repair and tumorigenesis. J Mol Med
(Berl). 81:600–612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kermani P and Hempstead B: Brain-derived
neurotrophic factor: A newly described mediator of angiogenesis.
Trends Cardiovasc Med. 17:140–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Farhat FS, Tfayli A, Fakhruddin N, Mahfouz
R, Otrock ZK, Alameddine RS, Awada AH and Shamseddine A:
Expression, prognostic and predictive impact of VEGF and bFGF in
non-small cell lung cancer. Crit Rev Oncol Hematol. 84:149–160.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghassemi S, Vejdovszky K, Sahin E,
Ratzinger L, Schelch K, Mohr T, Peter-Vorosmarty B, Brankovic J,
Lackner A, Leopoldi A, et al: FGF5 is expressed in melanoma and
enhances malignancy in vitro and in vivo. Oncotarget.
8:87750–87762. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakamura H, Kambe H, Egawa T, Kimura Y,
Ito H, Hayashi E, Yamamoto H, Sato J and Kishimoto S: Partial
purification and characterization of human hepatoma-derived growth
factor. Clin Chim Acta. 183:273–284. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Everett AD, Narron JV, Stoops T, Nakamura
H and Tucker A: Hepatoma-derived growth factor is a pulmonary
endothelial cell-expressed angiogenic factor. Am J Physiol Lung
Cell Mol Physiol. 286:L1194–L1201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren H, Chu Z and Mao L: Antibodies
targeting hepatoma-derived growth factor as a novel strategy in
treating lung cancer. Mol Cancer Ther. 8:1106–1112. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren H, Tang X, Lee JJ, Feng L, Everett AD,
Hong WK, Khuri FR and Mao L: Expression of hepatoma-derived growth
factor is a strong prognostic predictor for patients with
early-stage non-small-cell lung cancer. J Clin Oncol. 22:3230–3237.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iwasaki T, Nakagawa K, Nakamura H, Takada
Y, Matsui K and Kawahara K: Hepatoma-derived growth factor as a
prognostic marker in completely resected non-small-cell lung
cancer. Oncol Rep. 13:1075–1080. 2005.PubMed/NCBI
|
|
30
|
Zhao WY, Wang Y, An ZJ, Shi CG, Zhu GA,
Wang B, Lu MY, Pan CK and Chen P: Downregulation of miR-497
promotes tumor growth and angiogenesis by targeting HDGF in
non-small cell lung cancer. Biochem Biophys Res Commun.
435:466–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ke Y, Zhao W, Xiong J and Cao R:
Downregulation of miR-16 promotes growth and motility by targeting
HDGF in non-small cell lung cancer cells. FEBS Lett. 587:3153–3157.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo H, Li W, Zheng T and Liu Z: MiR-195
targets HDGF to inhibit proliferation and invasion of NSCLC cells.
Tumour Biol. 35:8861–8866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thirant C, Galan-Moya EM, Dubois LG, Pinte
S, Chafey P, Broussard C, Varlet P, Devaux B, Soncin F, Gavard J,
et al: Differential proteomic analysis of human glioblastoma and
neural stem cells reveals HDGF as a novel angiogenic secreted
factor. Stem Cells. 30:845–853. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Enomoto H, Nakamura H, Liu W, Iwata Y,
Nishikawa H, Takata R, Yoh K, Hasegawa K, Ishii A, Takashima T, et
al: Down-regulation of HDGF inhibits the growth of hepatocellular
carcinoma cells in vitro and in vivo. Anticancer Res. 35:6475–6479.
2015.PubMed/NCBI
|
|
35
|
Bacic I, Karlo R, Zadro AS, Zadro Z,
Skitarelic N and Antabak A: Tumor angiogenesis as an important
prognostic factor in advanced non-small cell lung cancer (Stage
IIIA). Oncol Lett. 15:2335–2339. 2018.PubMed/NCBI
|
|
36
|
Akl MR, Nagpal P, Ayoub NM, Tai B, Prabhu
SA, Capac CM, Gliksman M, Goy A and Suh KS: Molecular and clinical
significance of fibroblast growth factor 2 (FGF2 /bFGF) in
malignancies of solid and hematological cancers for personalized
therapies. Oncotarget. 7:44735–44762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu M, Hu Y, He J and Li B: Prognostic
value of basic fibroblast growth factor (bFGF) in lung cancer: A
systematic review with meta-analysis. PLoS One. 11:e01473742016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu MM, Hu Y, Gao GK, Han Y, Shi GL and Li
BL: Basic fibroblast growth factor shows prognostic impact on
survival in operable non-small cell lung cancer patients. Thorac
Cancer. 6:450–457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Andersen S, Donnem T, Al-Saad S, Al-Shibli
K, Busund LT and Bremnes RM: Angiogenic markers show high
prognostic impact on survival in marginally operable non-small cell
lung cancer patients treated with adjuvant radiotherapy. J Thorac
Oncol. 4:463–471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang S, Qin Y, Wang Z, Xiang J, Zhang Y,
Xu M, Li B, Xia Y, Zhang P and Wang H: Construction of a human
monoclonal antibody against bFGF for suppression of NSCLC. J
Cancer. 9:2003–2011. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
La Venuta G, Zeitler M, Steringer JP,
Muller HM and Nickel W: The startling properties of fibroblast
growth factor 2: How to exit mammalian cells without a signal
peptide at hand. J Biol Chem. 290:27015–27020. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sorensen V, Nilsen T and Wiedlocha A:
Functional diversity of FGF-2 isoforms by intracellular sorting.
Bioessays. 28:504–514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Muller HM, Steringer JP, Wegehingel S,
Bleicken S, Munster M, Dimou E, Unger S, Weidmann G, Andreas H,
Garcia-Saez AJ, et al: Formation of disulfide bridges drives
oligomerization, membrane pore formation, and translocation of
fibroblast growth factor 2 to cell surfaces. J Biol Chem.
290:8925–8937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dimou E, Cosentino K, Platonova E, Ros U,
Sadeghi M, Kashyap P, Katsinelos T, Wegehingel S, Noe F,
Garcia-Saez AJ, et al: Single event visualization of unconventional
secretion of FGF2. J Cell Biol. 218:683–699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kanematsu A, Marui A, Yamamoto S, Ozeki M,
Hirano Y, Yamamoto M, Ogawa O, Komeda M and Tabata Y: Type I
collagen can function as a reservoir of basic fibroblast growth
factor. J Control Release. 99:281–292. 2004. View Article : Google Scholar : PubMed/NCBI
|