Introduction
Osteosarcoma (OS) is the most common type of primary
malignant bone tumor and it primarily affects children and
adolescents (1). The outcome of
patients with OS may improve through a combination of surgery and
chemotherapy, and the 5-year survival rate has increased to 60–70%
over the past 30 years (2,3). However, a considerable number of
patients are either not sensitive to chemotherapy or develop drug
resistance to the currently available chemotherapeutic regimens
(4). Therefore, it is crucial to
elucidate the mechanisms underlying the development of
chemoresistance in these patients and to develop new strategies for
the treatment of OS.
Numerous studies have demonstrated that cancer stem
cells (CSCs) are not only the cause of relapse and metastasis, but
also contribute to chemoresistance in tumors (5–8). OS
stem cells (OSCs) have recently been identified as a subset of CSCs
using a distinct set of stem cell markers, including CD133,
aldehyde dehydrogenase 1 (ALDH1), CD117/Stro-1 and CD271 (9–12).
Additional stem cell markers, such as octamer-binding transcription
factor 4 (Oct-4), sex determining region Y-box 2 (Sox 2) and Nanog,
have also been recommended for distinguishing OSC populations from
other cells (13–15). OS sarcosphere cells exhibit CSC
characteristics and display high expression levels of markers
associated with stem cell self-renewal, tumorigenicity, and
multiple drug resistance (16).
The heat shock protein (Hsp)90 inhibitor, 17-AAG
(tanespimycin), interferes with the binding of ATP to Hsp90 and
results in the proteasome-mediated degradation of Hsp90 client
protein complexes (17,18). 17-AAG significantly enhances the
cytotoxicity of etoposide in human colon cancer HCT116 cells
(19). As known Hsp90 client
proteins are required for the maintenance of self-renewal, this
requirement for self-renewal may be exploited by treatment with
17-AAG. Indeed, several studies have demonstrated that 17-AAG can
effectively target CSCs (20,21).
However, little is known on the effects of 17-AAG on OSCs, and the
molecular mechanisms underlying its antitumor activity remain to be
determined.
The Hedgehog signaling pathway plays a key role in
the development of several CSCs, such as glioblastoma stem cells,
CD34+ leukemic cells and gastric CSCs (22–24).
The Hedgehog signaling pathway is primarily dependent on the Gli
transcription factor family (Gli1-3), which are its downstream
effectors. Specifically, Gli1 is the principal transcriptional
effector that regulates gene expression in response to Hedgehog
signaling activation (25).
However, its clinical significance and biological function in OS
chemoresistance remains unclear. In the present study, the human OS
cell lines MG-63 and Saos-2 were examined for their expression of
the putative stem cell markers CD133, ALDH1, CD117/Stro-1 and
CD271. The efficacy of 17-AAG in inhibiting stem cell-like
properties and chemoresistance in OS cells was then investigated.
The aim of the present study was to explore how 17-AAG interacts
with OSCs and to determine the mechanism underlying 17-AAG-mediated
suppression of stemness. Furthermore, it was investigated whether
one of the mechanisms underlying the action of 17-AAG was the
induction of glycogen synthase kinase (GSK) 3β
inactivation-mediated repression of the Hedgehog pathway, which is
crucial for the development of OS. The findings of the present
study may provide insight into acquired drug resistance and
indicate novel treatment strategies to prevent or overcome this
resistance, which may improve the prognosis and survival of
patients with OS.
Materials and methods
Cell culture and reagents
The MG-63 and Saos-2 human OS cell lines were
purchased from the China Center for Type Culture Collection. MG-63
cells were maintained in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc), 1% L-glutamine and 1% penicillin-streptomycin
sulfate (Thermo Fisher Scientific, Inc.). Saos-2 cells were
cultured in McCoy's 5A medium (Gibco; Thermo Fisher Scientific,
Inc.), supplemented with 10% FBS, 1% L-glutamine and 1%
penicillin-streptomycin sulfate. 17-AAG was purchased from Selleck
Chemicals. Methotrexate and cisplatin were purchased from Pfizer,
Inc.
Serum-free medium (SFM) was comprised of DMEM/F12,
supplemented with 20 µl/ml B27 and 20 ng/ml basic fibroblast growth
factor (bFGF) (all from Gibco; Thermo Fisher Scientific, Inc.).
OSCs were isolated from two cell lines using SFM and were assessed
for their ability to form sphere-like cell aggregates in <7
days. These cells were collected and disassociated with 2.5%
trypsin, and viable cells were counted using trypan blue
(Sigma-Aldrich; Merck KGaA) exclusion. All cultures were maintained
in a 37°C incubator with 5% CO2.
Sphere formation assay
MG-63 and Saos-2 cells were plated in each well (500
cells/well) of Ultra-Low Attachment 24-well plates (Corning, Inc.)
with 0.8% methyl cellulose (Sigma-Aldrich; Merck KGaA) supplemented
with 20 µl/ml B27, 20 ng/ml bFGF, 10 ng/ml epidermal growth factor,
1% L-glutamine and 1% penicillin-streptomycin sulfate (all from
Thermo Fisher Scientific, Inc.). Every 3 days, each well was
examined under a light microscope (IX71; Olympus Corporation).
Flow cytometry and
fluorescence-activated cell sorting (FACS)
Cells were detached into single-cell suspensions
using trypsin-EDTA, followed by staining of 1×106 cells
in 500 µl PBS/0.5% BSA (Sigma-Aldrich; Merck KGaA) with
fluorescence-labeled primary antibodies (1–5 µl), including
CD133-PE (1:10; cat. no. 130-112-195; Miltenyi Biotec GmbH),
Aldeflour™ kit (cat. no. 01700; Stemcell Technologies, Inc.) and
CD271-fluorescein isothiocyanate (1:400; cat. no. 746728; BD
Biosciences) at 4°C for 60 min. After washing, labeled cells were
analyzed and sorted immediately using a BD FACS AriaIII system (BD
Biosciences). A blank control without labeling was used to
delineate any unstained populations and analyzed by FlowJo 7.6.1.
(FlowJo LLC).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was prepared and detected as previously
described (26). First-strand cDNA
was synthesized using a RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. RT-qPCR analysis was performed using a PCR mixture
containing 7.5 µM of each primer and FastStart Universal SYBR Green
Master (Rox) (Roche Diagnostics GmbH). Amplifications were
performed at 95°C for 15 sec and at 60°C for 60 sec for 40 cycles
using a StepOnePlus Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Each sample was examined in
triplicate, and GAPDH was used as the internal control. The primer
sequences are listed in Table I.
The gene expression was quantified using the 2−ΔΔCq
method (27).
 | Table I.Primers used for quantitative PCR
analysis. |
Table I.
Primers used for quantitative PCR
analysis.
| Gene | Sequences
(5′-3′) | Product size
(bp) |
|---|
| Sox 2 | F:
ATGCACCGCTACGACGTGA | 198 |
|
| R:
CCTGGAGTGGGAGGAAGAGGTA |
|
| Oct-4 | F:
AGGATGTGGTCCGAGTGTGGTT | 191 |
|
| R:
GTACAGTGCAGTGAAGTGAGGGCT |
|
| Nanog | F:
GAGAAGAGTGTCGCAAAAAAGGA | 163 |
|
| R:
TGAGGTTCAGGATGTTGGAGAGT |
|
| Gli1 | F:
CCAAGCACCAGAATCGGACC | 140 |
|
| R:
TTTGGTCACATGGGCGTCAG |
|
| Hsp90 | F:
TTCACTGTGCGTGCTGACCAT | 289 |
|
| R:
TGTCATCCTCCTCATCTGAACCC |
|
| Ptch1 | F:
TGGAACGAGGACAAAGCGG | 202 |
|
| R:
AGGCATAGGCGAGCATGAGTAA |
|
| Smo | F:
TCCTGCGTCATCATCTTTGTCA | 267 |
|
| R:
CGCACGGTATCGGTAGTTCTT |
|
| GAPDH | F:
GGAAGCTTGTCATCAATGGAAATC | 168 |
|
| R:
TGATGACCCTTTTGGCTCCC |
|
Immunofluorescence assay
After cells were treated with vehicle or 17-AAG,
cell samples were fixed with 4% paraformaldehyde for 15 min at room
temperature and then permeabilized with 0.02% Triton X-100 for 5
min. Subsequently, the cells were incubated with primary antibodies
against Hsp90 (1:100; cat. no. ab13492; Abcam) or Sox 2 (1:100;
cat. no. ab92494; Abcam) at 4°C overnight. On the following day,
the samples were incubated with secondary antibody of Alexa
Fluor® 594-conjugated AffiniPure Donkey Anti-Mouse IgG
(H+L) (1:250; cat. no. 715-585-150; Jackson ImmunoResearch
Laboratories, Inc.) and Alexa Fluor® 488-conjugated
AffiniPure Goat Anti-Rabbit IgG (H+L) (1:250; cat. no. 111-545-144;
Jackson ImmunoResearch Laboratories, Inc.) at room temperature for
1 h. Finally, the nuclei were counterstained with DAPI at room
temperature for 30 min. Immunofluorescence was detected using a
laser scanning confocal microscope (FV1000; Olympus
Corporation).
Cell viability analysis
OS cells were plated at a density of
5×103 cells per well in a 96-well plate. After 24 h, the
cells were treated with 0.05, 0.5, 1, 10, or 50 µM 17-AAG for 72 h.
Inhibition of cell proliferation was analyzed using a Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.), according to
the manufacturer's protocol. The plates were incubated for an
additional 2 h, and the absorbance was then measured at 450 nm.
Experiments were performed in triplicate.
Cell transfection
The small interfering RNAs against the following
genes were designed and synthesized by Guangzhou RiboBio Co., Ltd.:
GSK3β sense, 5′-CUCAAGAACUGUCAAGUAATT-3′ and antisense,
5′-UUACUUGACAGUUCUUGAGTT-3′. The siRNA was transfected into OS
cells when they reached a confluence of 30–50% in 6-well plates
using Lipofectamine® 2000 according to the
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.) for 72 h.
Western blot analysis
Cells were lysed in RIPA lysis buffer containing
protease inhibitor and phosphatase inhibitor cocktails (Thermo
Fisher Scientific, Inc.). Nuclear protein fractions were isolated
using a Nuclear Protein Extraction kit according to the
manufacturer's protocol (Thermo Fisher Scientific, Inc.). Equal
amounts of protein (40 µg per lane) were resolved on 10% SDS-PAGE
gels and transferred to PVDF membranes (EMD Millipore). The
membranes were incubated with primary antibody overnight at 4°C.
The membranes were then washed with TBST (Tris-buffered saline with
0.05% Tween 20) and incubated with horseradish
peroxidase-conjugated secondary antibodies. The primary antibodies
used were Hsp90 (cat. no. ab13495), Ptch1 (cat. no. ab53715), Smo
(cat. no. ab38686), Gli1 (cat. no. ab49314) (all from Abcam),
β-actin (cat. no. 3700S), Akt (cat. no. 2920S), phosphorylated (p-)
Akt (cat. no. 4060S), GSK-3β (cat. no. 9315S), and p-GSK-3β (cat.
no. 9323S) (all from Cell Signaling Technology, Inc.) at a dilution
of 1:1,000. Peroxidase-conjugated Affinipure goat anti-mouse (cat.
no. 115-035-044; 1:5,000; Jackson ImmunoResearch Laboratories,
Inc.) and anti-rabbit (cat. no. 111-035-003; 1:5,000; Jackson
ImmunoResearch Laboratories, Inc.) secondary antibodies, were
incubated with the membranes for 1 h at room temperature. Proteins
were then visualized using SuperSignal™ West Femoto Maximum
Sensitivity Substrate (Thermo Fisher Scientific, Inc.).
Statistical analysis
All numerical data are presented as the mean ±
standard deviation of three independent experiments, each performed
in triplicate. SPSS (version 13.0; SPSS, Inc.) and GraphPad Prism
version 5.0 (GraphPad Software, Inc.) were used for statistical
analysis. Data were analyzed using a two-tailed Student's t-test or
one-way analysis of variance followed by Tukey's post hoc test,
unless otherwise specified. P<0.05 was considered to indicate a
statistically significant difference.
Results
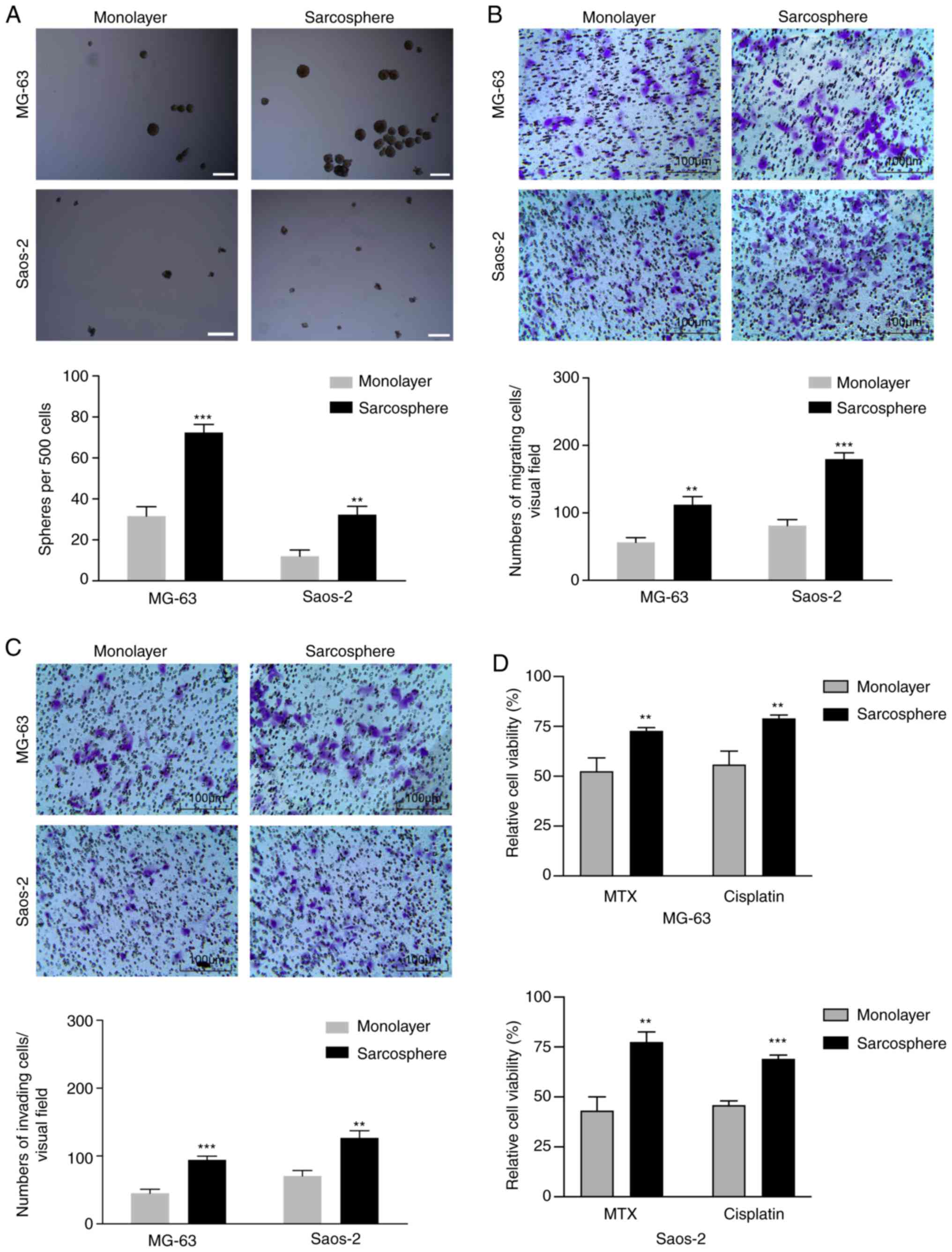
OS sarcosphere cells exhibit notable
stem cell-like properties and resistance to chemotherapy
Under serum-free conditions, CSCs form spheres that
possess notable similarities to endogenous CSCs in human tumor
tissues. Therefore, a sphere formation assay was performed to
determine CSC self-renewal capacities. The self-renewal capacities
of OS sarcosphere cells derived from the MG-63 and Saos-2 cell
lines were determined 1–2 weeks following disaggregation of
sarcosphere cells with accutase and culture in SFM. The results
demonstrated that sarcosphere cells derived from MG-63 and Saos-2
cells formed significantly more spherical colonies after 7 days of
incubation in SFM, compared with the monolayer cells (Fig. 1A). To investigate the migratory and
invasive abilities of sarcosphere and monolayer cells from the
MG-63 and Saos-2 cell lines, the cells were cultured in Transwell
chambers. After 24 h of incubation, the number of sarcosphere cells
from MG-63 and Saos-2 cells cultured on Transwell inserts was
significantly higher compared with the number derived from
monolayer cells (Fig. 1B). A Boyden
chamber coated with Matrigel was used to determine changes in cell
invasion ability after 24 h of incubation. Compared with the
monolayer cells, the sarcosphere cells from the MG-63 and Saos-2
cell lines exhibited markedly increased invasion abilities
(Fig. 1C).
Previous studies have demonstrated that CSCs are
more resistant to chemotherapy compared with other OS cell
populations (28,29). Two commonly used chemotherapies for
the treatment of OS, cisplatin and methotrexate, were used to
examine the sensitivity of monolayer and sarcosphere cells derived
from the MG-63 and Saos-2 cell lines. The viability of monolayer
cells was reduced by 45–57% when exposed to methotrexate at 50
nmol/l, whereas methotrexate decreased the viability of sarcosphere
cells by only 20–30%. Monolayer cells from MG-63 and Saos-2 cells
were also sensitive to cisplatin at 625 nmol/l, with a reduction in
cell viability of 40–55%, whereas sarcosphere cells were relatively
resistant to cisplatin, with cell viability decreasing by only
20–32% (Fig. 1D). These data
indicated that OS sarcosphere cells possess CSC characteristics and
are resistant to chemotherapy.
The expression of Hsp90 is positively
associated with stem cell related-genes and CSC markers in OS
sarcosphere cells
To determine the CSC characteristics of OS
sarcosphere cells, RT-qPCR was employed to analyze the expression
of Hsp90 and stem cell-related genes in MG-63- and Saos-2-derived
monolayer and sarcosphere cells. The results demonstrated that
sarcosphere cells from MG-63 and Saos-2 cells exhibited higher
expression levels of Hsp90 and the stem cell markers Sox 2, Nanog
and Oct-4 compared with the respective monolayer cells (Fig. 2A). Furthermore, immunofluorescence
double-staining revealed that Hsp90 and Sox 2 were co-expressed in
sarcosphere cells from the MG-63 and Saos-2 cell lineages (Fig. 2B).
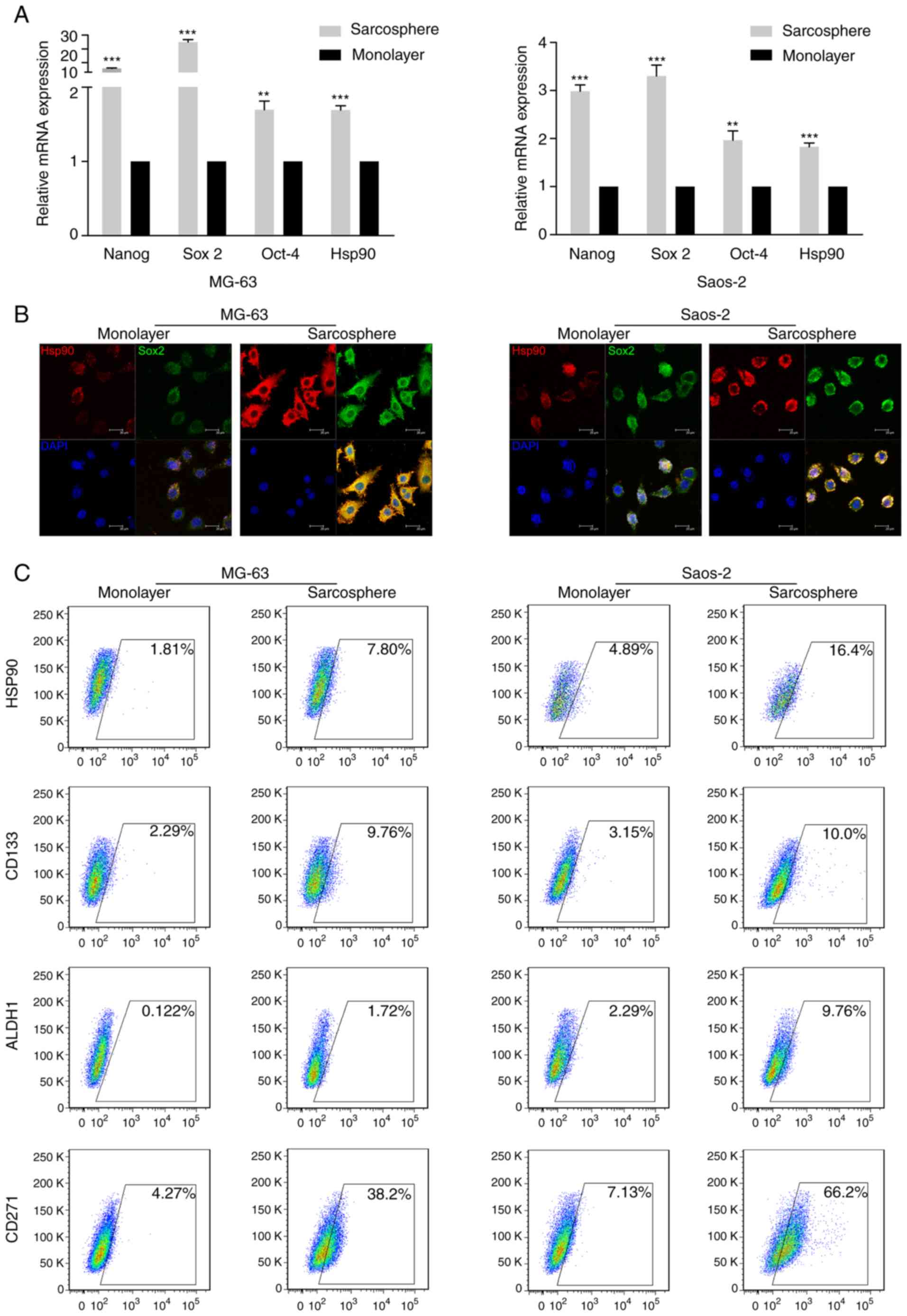 | Figure 2.Hsp90 is positively associated with
the expression of stem cell-related genes and CSC markers in
osteosarcoma sarcosphere cells. (A) mRNA expression levels of Hsp90
and the stem cell markers Sox 2, Oct-4 and Nanog in sarcosphere and
monolayer cells derived from MG-63 and Saos-2 cells. (B) Double
staining for Hsp90 and Sox 2 using immunofluorescence in
sarcosphere cells derived from MG-63 and Saos-2 cells. Scale bar,
50 µm. (C) Analysis of Hsp90, CD133, ALDH1 and CD271 expression in
sarcosphere and monolayer cells derived from MG-63 and Saos-2 cells
Hsp, heat shock protein; CSC, cancer stem cell; Sox 2, sex
determining region Y-box 2; Oct-4, octamer-binding transcription
factor 4; ALDH1, aldehyde dehydrogenase 1. **P<0.01,
***P<0.001. |
To explore the association between Hsp90 and CSC
markers in OSCs, the MG-63 and Saos-2 OS cell lines were cultured
in SFM for 1–2 weeks, and flow cytometry analysis was performed to
assess the expression of Hsp90 and CSC markers (CD133, ALDH1 and
CD271). The results demonstrated that sarcosphere cells from the
MG-63 and Saos-2 cell lines exhibited notably higher expression
levels of Hsp90, CD133, ALDH1 and CD271 compared with monolayer
cells (Fig. 2C).
Collectively, the data presented above suggest that
Hsp90 expression is positively associated with the expression of
stem cell-related genes and CSC markers in OS sarcosphere
cells.
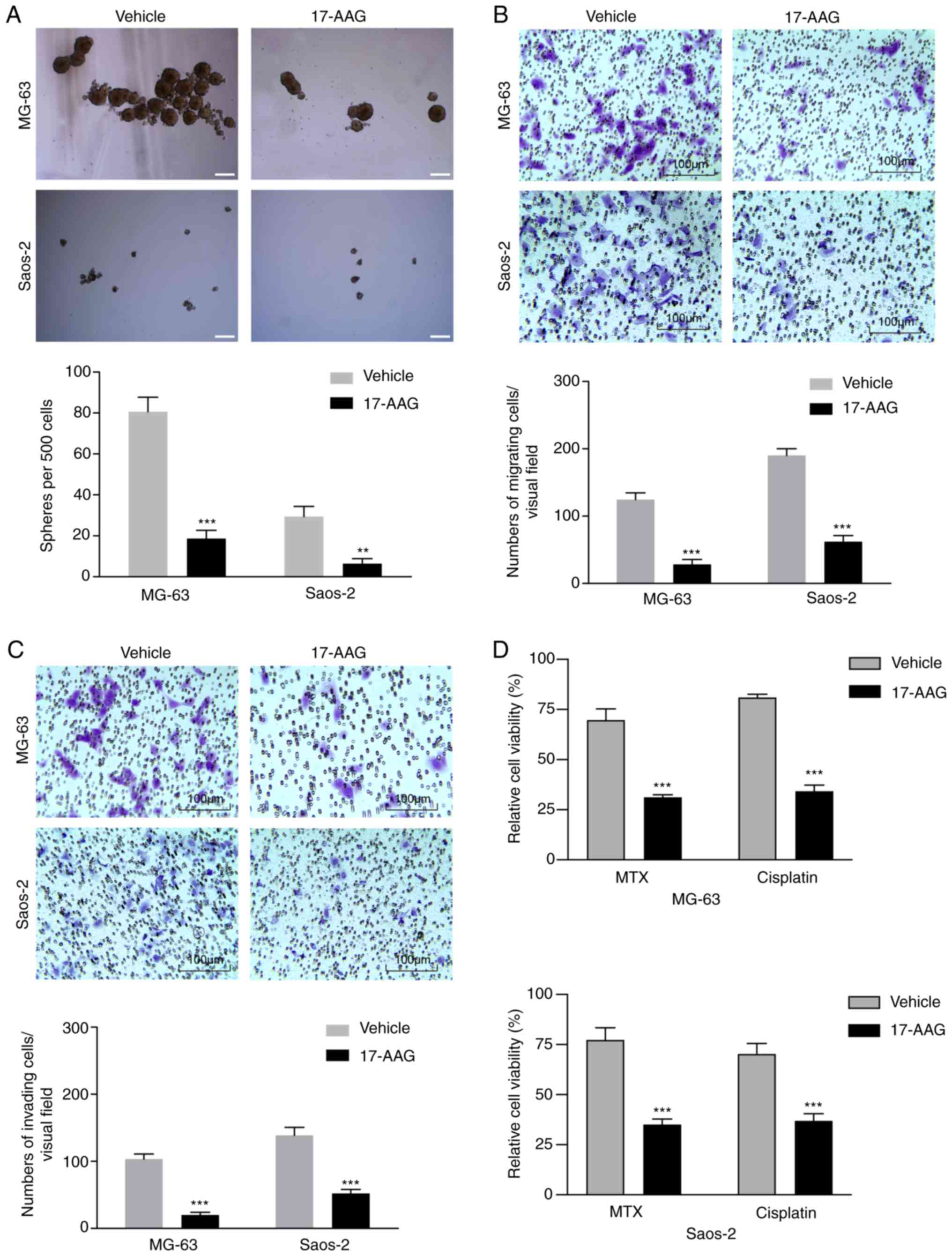
17-AAG suppress stem cell-like
properties and chemoresistance in OS sarcosphere cells
To investigate whether 17-AAG could suppress stem
cell-like properties in OS cells, a sphere formation assay was
performed. 17-AAG treatment significantly reduced the number of
spheres formed by sarcosphere cells from the MG-63 and Saos-2 cell
lines (Fig. 3A). Furthermore, the
effects of 17-AAG on the migration and invasion of sarcosphere
cells derived from MG-63 and Saos-2 cells were assessed. 17-AAG
reduced the migration of sarcosphere cells by 67.4–77.3% and the
invasion of sarcosphere cells by 62.5–80.6% (Fig. 3B and C).
To determine whether 17-AAG suppressed
chemoresistance in OS sarcosphere cells, the synergistic effects of
17-AAG with other commonly used chemotherapeutics were assessed.
Compared with the reduction of viability by 22.8–31.4% with
methotrexate chemotherapy alone, the viability of sarcosphere cells
derived from MG-63 and Saos-2 cells was reduced by 54.7–61.5% when
treated with 50 nmol/l 17-AAG. Additionally, compared with the
reduction of viability by 19.1–29.8% with cisplatin chemotherapy
alone, the viability of sarcosphere cells from MG-63 and Saos-2
cells was reduced by 47.5–50.9% when the cells were treated with
17-AAG (Fig. 3D).
These data suggest that 17-AAG suppressed CSC
characteristics and chemotherapy resistance in OS sarcosphere
cells.
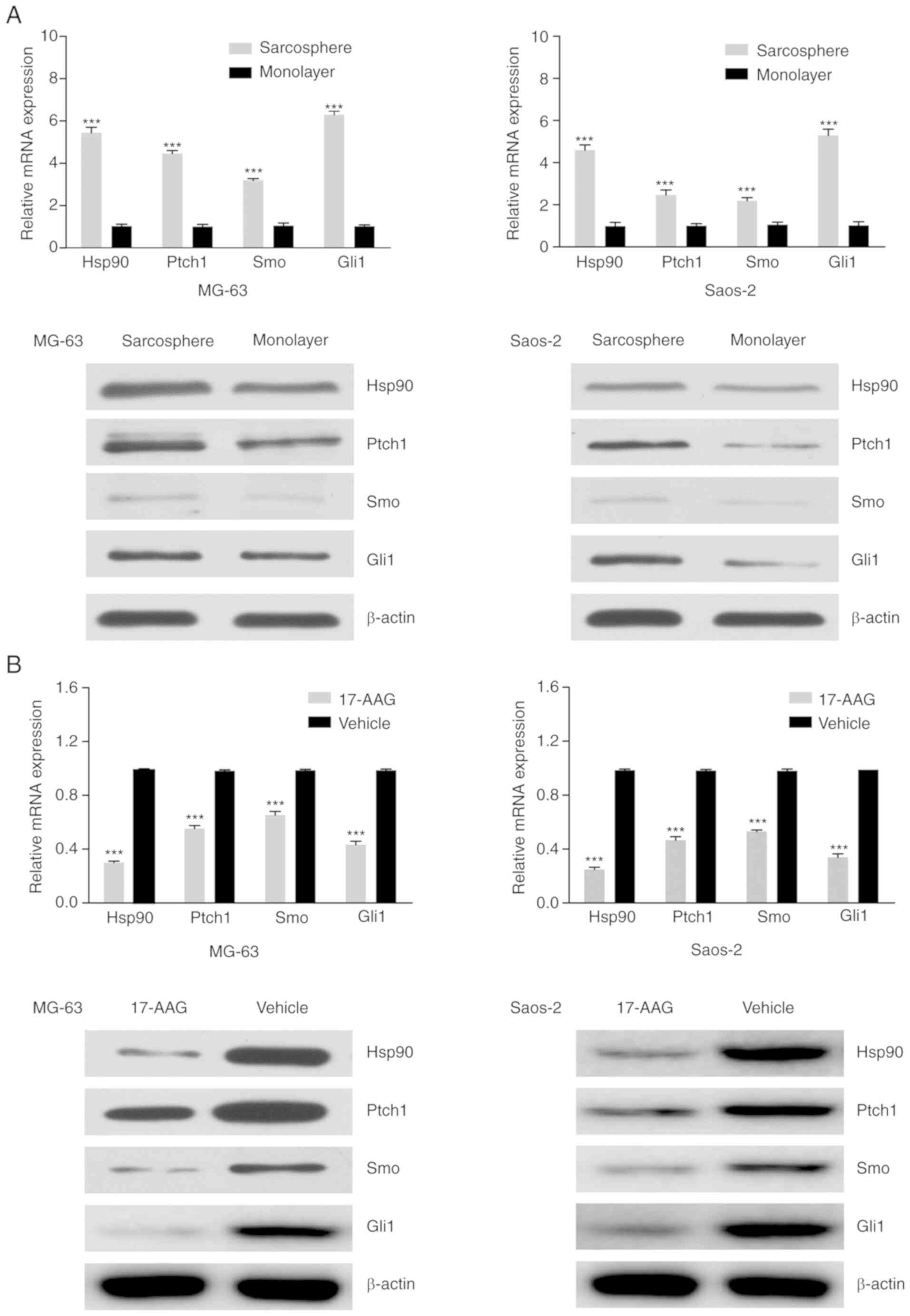
17-AAG inhibits OSC characteristics
and chemoresistance in OS sarcosphere cells through the
downregulation of the Hedgehog signaling pathway
Inhibition of the Hedgehog signaling pathway can
suppress CSC characteristics and reverse chemotherapy resistance.
To elucidate the mechanisms underlying the anti-OSC effects induced
by 17-AAG, RT-qPCR analysis and western blotting were used to
assess the expression levels of the Hedgehog signaling pathway
proteins Hsp90, Ptch1, Smo and Gli1, which were all notably higher
in sarcosphere cells derived from MG-63 and Saos-2 cells compared
with the respective monolayer cells (Fig. 4A). 17-AAG treatment of sarcosphere
cells from MG-63 and Saos-2 cells significantly reduced the
expression of Hsp90, Pcth1, Smo and Gli1 compared with the vehicle
group (Fig. 4B). Taken together,
these results demonstrated that 17-AAG inhibits OS stem cell-like
properties and chemoresistance through the repression of the
Hedgehog signaling pathway.
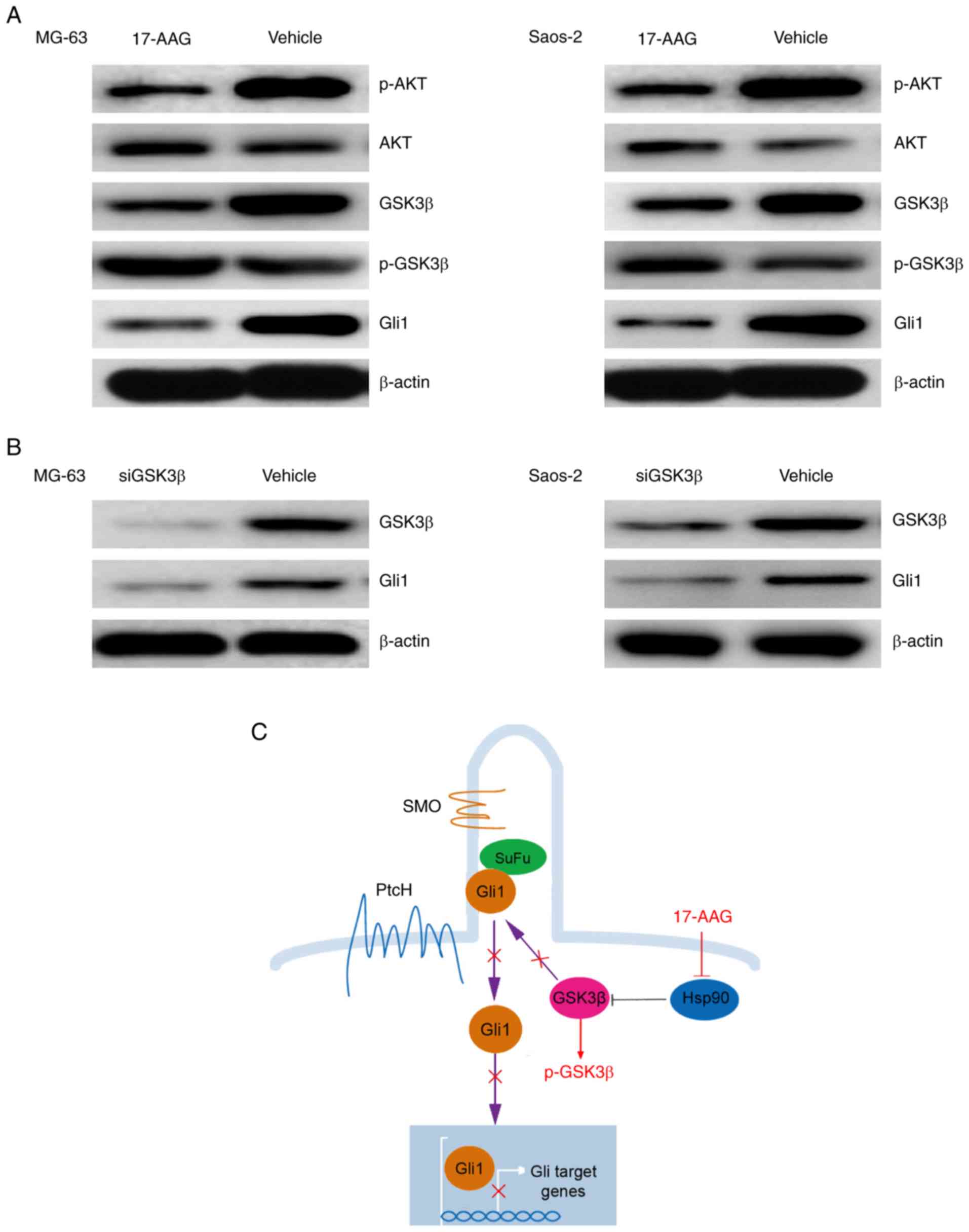
17-AAG suppresses Gli1 expression and
activation mainly by blocking GSK3β activity
To gain insights into the molecular mechanism by
which 17-AAG suppresses the Hedgehog pathway, we focused on Gli1,
as it has been demonstrated that it plays a key role in
GSK3β-mediated tumor malignant behaviors (30). Therefore, it was hypothesized that
GSK3β activity was involved in 17-AAG-induced Gli1 repression in OS
sarcospheres. To verify this hypothesis, sarcosphere cells from the
MG-63 and Saos-2 cell lines were treated with 17-AAG, and western
blotting demonstrated that the expression of Gli1 and p-Akt was
decreased, whereas the expression of p-GSK3β was increased
(Fig. 5A). Furthermore, OS
sarcosphere cells from MG-63 and Saos-2 cells were transfected with
siRNAs targeting GSK3β, and the results revealed that blocking
GSK3β downregulated Gli1 expression (Fig. 5B). Thus, the inhibition of the Gli1
signaling pathway induced by 17-AAG primarily involved the
repression of GSK3β activity.
Discussion
According to the OSC hypothesis, tumors of various
origins are driven and maintained by a small fraction of OSCs
(31). Recent studies have
demonstrated that OSCs are associated with chemoresistance, relapse
and metastasis of tumors (32,33).
Current chemotherapeutics for the treatment of OS primarily target
proliferating tumor cells, which can significantly reduce tumor
bulk, but exert minimal cytotoxic effects on OSCs. This results in
tumor recurrence and lung metastasis, emphasizing the urgent need
to develop new methods for directly targeting OSCs. Recent studies
have reported that 17-AAG is a promising chemopreventive agent
against CSCs in glioma and leukemia (34,35).
However, the research based on the chemotherapeutic activity of
17-AAG is in its early stages, and the underlying molecular
mechanisms and genetic drivers controlling OSC phenotypes remain
largely undefined at present.
In the present study, a previously unrecognized role
for 17-AAG as an Hsp90 inhibitor in the regulation of the OS cell
stemness associated with drug resistance was identified.
Furthermore, it was demonstrated that the Hedgehog signaling
pathway was involved in 17-AAG-mediated OS cell stemness. First, a
subpopulation of OSCs was isolated from the cell lines using a
sphere formation culture assay, and these cells were shown to
exhibit upregulated expression of a panel of stem cell-related
genes and proteins compared with the respective monolayer cells,
including CD133, ALDH1 and CD271, as well as Oct-4, Sox 2 and
Nanog, all of which are known embryonic stem cell markers that are
essential for the pluripotency and self-renewal of embryonic stem
cells (36). The expression levels
of Hsp90, Oct-4, Sox 2 and Nanog were increased in the sarcosphere
cells, and these cells also exhibited increased self-renewal
capacity, chemoresistance, and invasive and metastatic abilities,
all of which were attenuated following Hsp90 inhibition using
17-AAG. OSCs were also eliminated through 17-AAG-mediated Hsp90
inhibition. Thus, it is possible that Hsp90 acts as an oncogene in
OS, and the present study highlights its potential as a target for
therapeutic intervention in OS.
Hsp90 is a molecular chaperone that plays a key role
in the stabilization and function of several proteins that are
dysregulated in various types of cancer, such as steroid receptors,
transcription factors and kinases (37–39).
By assessing the potential mechanisms of action of Hsp90 in
regulating the stem-like properties and chemoresistance of
sarcosphere cells, it was demonstrated that increased Hsp90
expression resulted in increased levels of Hedgehog signaling
pathway-associated proteins. The Hedgehog signaling pathway is also
considered to be crucially involved in the development and
progression of several types of cancer, and its activity has been
demonstrated to be increased and associated with cancer growth and
drug resistance (40–43). Activated Gli proteins, primarily
Gli1, translocate into the nucleus and stimulate the transcription
of Hedgehog signaling pathway target genes, including Gli1, Smo and
several other survival-promoting molecules (44–46).
17-AAG reduced the expression of Hsp90 and Gli1 in sarcosphere
cells. These findings indicate that Gli1 is a significant
prognostic marker and that 17-AAG inhibits stem cell-like
properties and chemoresistance by inactivating the Hedgehog
pathway.
Based on the results of the present study, it is
suggested that 17-AAG exerts antitumor effects through its ability
to modulate Gli1 expression and activation by blocking GSK3β.
However, further study is required to elucidate how 17-AAG inhibits
GSK3β activity and represses the Hedgehog/Gli1 pathway. 17-AAG
inhibited activation of GSK3β by increasing its phosphorylation
level. Gli1 has been reported to be activated by several kinases,
such as AKT, MAPK/ERK and mTOR/S6K1 (39,47).
In the present study, an association between GSK3β and Gli1 was
demonstrated. As several kinases play a key role in the survival
and growth of cancer cells, it has been reported that the activity
of GSK3β may promote tumor growth in OS, and therapeutic targeting
of GSK3β may be an effective approach to the treatment of OS
(48). The Hedgehog signaling
pathway is regulated by GSK-3β, and the activity of this pathway is
reduced when GSK-3β is suppressed (40). GSK-3β is a binding partner of
suppressor of fused homolog (SuFu). SuFu suppresses Gli activity by
sequestering Gli in the cytoplasm (49–51).
To further determine the potential anti-OSC molecular mechanism of
action of 17-AAG, the effect of inhibiting Hsp90 on the stability
and activity of mature GSK3β were assessed. The results revealed
that 17-AAG inhibited the activation of GSK3β by increasing its
phosphorylation level, and the expression of Gli1 was
downregulated, suggesting an association between GSK3β and Gli1
(Fig. 5C). Thus, it may be inferred
that 17-AAG inhibited GSK3β through its ability to affect Gli1
expression and activation by blocking GSK3β. However, how 17-AAG
inhibits the activity of GSK3β and whether this inhibition is
direct requires further investigation. Overall, 17-AAG has emerged
as an effective strategy for targeting OSCs. To prolong drug
circulation and reduce the toxicity of 17-AAG, improved delivery
methods of 17-AAG, such as nanoparticles, liposomes, micelles and
carbon nanomaterials, are required, and 17-AAG may prove to be of
value as OS treatment in the future (52–54).
In summary, inhibition of OSC properties and
chemoresistance by 17-AAG was found to be mediated through
repression of the Hedgehog pathway, which suggests that 17-AAG may
be a promising therapeutic agent for the treatment of OS. The
present study not only identified the Smo/Gli1 axis as a critical
regulator of OSC properties, but also demonstrated that GSK3β may
be a novel therapeutic target as well as a significant prognostic
marker for the clinical treatment of OS.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant nos. 51973021 and 51932002), the
Beijing Municipal Health Commission (grant nos. BMC2018-4 and
BMC2019-9), the Natural Science Foundation of Beijing JiShuiTan
Hospital (grant no. ZR-201906) and the CAMS Innovation Fund for
Medical Sciences (grant no. 2017-I2M-3-005).
Availability of data and materials
All datasets generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
XS, YR, DFC designed the experiments; RZ and YSJ
carried out the experiments; XS, RZ, YSJ, HQ, LC, RXW, CW and HQL
were involved in data acquisition, analysis and interpretation; XS
and YR drafted the manuscript and revised it critically for
important intellectual content. All the authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Whelan JS and Davis LE: Osteosarcoma,
chondrosarcoma, and chordoma. J Clin Oncol. 36:188–193. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schiavone K, Garnier D, Heymann MF and
Heymann D: The heterogeneity of osteosarcoma: The role played by
cancer stem cells. Adv Exp Med Biol. 1139:187–200. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown HK, Tellez-Gabriel M and Heymann D:
Cancer stem cells in osteosarcoma. Cancer Lett. 386:189–195. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan GN, Lv YF and Guo QN: Advances in
osteosarcoma stem cell research and opportunities for novel
therapeutic targets. Cancer Lett. 370:268–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clevers H: The cancer stem cell: Premises,
promises, and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shackleton M, Quintana E, Fearon ER and
Morrison SJ: Heterogeneity in cancer: Cancer stem cells versus
clonal evolution. Cell. 138:822–829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujiwara T, Katsuda T, Hagiwara K, Kosaka
N, Yoshioka Y, Takahashi RU, Takeshita F, Kubota D, Kondo T,
Ichikawa H, et al: Clinical relevance and therapeutic significance
of microRNA-133a expression profiles and functions in malignant
osteosarcoma-initiating cells. Stem Cells. 32:959–973. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Honoki K, Fujii H, Kubo A, Kido A, Mori T,
Tanaka Y and Tsujiuchi T: Possible involvement of stem-like
populations with elevated ALDH1 in sarcomas for chemotherapeutic
drug resistance. Oncol Rep. 24:501–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adhikari AS, Agarwal N, Wood BM, Porretta
C, Ruiz B, Pochampally RR and Iwakuma T: CD117 and Stro-1 identify
osteosarcoma tumor-initiating cells associated with metastasis and
drug resistance. Cancer Res. 70:4602–4612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian J, Li X, Si M, Liu T and Li J: CD271+
osteosarcoma cells display stem-like properties. PLoS One.
9:e985492014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qi XT, Li YL, Zhang YQ, Xu T, Lu B, Fang
L, Gao JQ, Yu LS, Zhu DF, Yang B, et al: KLF4 functions as an
oncogene in promoting cancer stem cell-like characteristics in
osteosarcoma cells. Acta Pharmacol Sin. 40:546–555. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee YH, Yang HW, Yang LC, Lu MY, Tsai LL,
Yang SF, Huang YF, Chou MY, Yu CC and Hu FW: DHFR and MDR1
upregulation is associated with chemoresistance in osteosarcoma
stem-like cells. Oncol Lett. 14:171–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong J, Bi B, Zhang L and Gao K: GLIPR1
inhibits the proliferation and induces the differentiation of
cancer-initiating cells by regulating miR-16 in osteosarcoma. Oncol
Rep. 36:1585–1591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qu H, Xue Y, Lian W, Wang C, He J, Fu Q,
Zhong L, Lin N, Lai L, Ye Z and Wang Q: Melatonin inhibits
osteosarcoma stem cells by suppressing SOX9-mediated signaling.
Life Sci. 207:253–264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maloney A and Workman P: HSP90 as a new
therapeutic target for cancer therapy: The story unfolds. Expert
Opin Biol Ther. 2:3–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neckers L: Hsp90 inhibitors as novel
cancer chemotherapeutic agents. Trends Mol Med. 8 (4
Suppl):S55–S61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barker CR, Hamlett J, Pennington SR,
Burrows F, Lundgren K, Lough R, Watson AJ and Jenkins JR: The
topoisomerase II-Hsp90 complex: A new chemotherapeutic target? Int
J Cancer. 118:2685–2693. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
White PT, Subramanian C, Zhu Q, Zhang H,
Zhao H, Gallagher R, Timmermann BN, Blagg BS and Cohen MS: Novel
HSP90 inhibitors effectively target functions of thyroid cancer
stem cell preventing migration and invasion. Surgery. 159:142–152.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HB, Lee SH, Um JH, Kim MJ, Hyun SK,
Gong EJ, Oh WK, Kang CD and Kim SH: Sensitization of
chemo-resistant human chronic myeloid leukemia stem-like cells to
Hsp90 inhibitor by SIRT1 inhibition. Int J Biol Sci. 11:923–934.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nanta R, Shrivastava A, Sharma J, Shankar
S and Srivastava RK: Inhibition of sonic hedgehog and PI3K/Akt/mTOR
pathways cooperate in suppressing survival, self-renewal and
tumorigenic potential of glioblastoma-initiating cells. Mol Cell
Biochem. 454:11–23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kobune M, Takimoto R, Murase K, Iyama S,
Sato T, Kikuchi S, Kawano Y, Miyanishi K, Sato Y, Niitsu Y and Kato
J: Drug resistance is dramatically restored by hedgehog inhibitors
in CD34+ leukemic cells. Cancer Sci. 100:948–955. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoon C, Park DJ, Schmidt B, Thomas NJ, Lee
HJ, Kim TS, Janjigian YY, Cohen DJ and Yoon SS: CD44 expression
denotes a subpopulation of gastric cancer cells in which Hedgehog
signaling promotes chemotherapy resistance. Clin Cancer Res.
20:3974–3988. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mastrangelo E and Milani M: Role and
inhibition of GLI1 protein in cancer. Lung Cancer (Auckl). 9:35–43.
2018.PubMed/NCBI
|
|
26
|
Pan Y, Shu X, Sun L, Yu L, Sun L, Yang Z
and Ran Y: miR-196a-5p modulates gastric cancer stem cell
characteristics by targeting Smad4. Int J Oncol. 50:1965–1976.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmittgen TD, Zakrajsek BA, Mills AG,
Gorn V, Siner MJ and Reed MW: Quantitative reverse transcription
polymerase chain reaction to study mRNA decay: Comparison of end
point and realtime methods. Anal Biochem. 285:194–204. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martins-Neves SR, Paiva-Oliveira DI,
Wijers-Koster PM, Abrunhosa AJ, Fontes-Ribeiro C, Bovee JV,
Cleton-Jansen AM and Gomes CM: Chemotherapy induces stemness in
osteosarcoma cells through activation of Wnt/β-catenin signaling.
Cancer Lett. 370:286–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu L, Fan Z, Fang S, Yang J, Gao T, Simoes
BM, Eyre R, Guo W and Clarke RB: Cisplatin selects for stem-like
cells in osteosarcoma by activating notch signaling. Oncotarget.
7:33055–33068. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trnski D, Sabol M, Gojević A, Martinić M,
Ozretić P, Musani V, Ramić S and Levanat S: GSK3β and Gli3 play a
role in activation of Hedgehog-Gli pathway in human colon
cancer-Targeting GSK3β downregulates the signaling pathway and
reduces cell proliferation. Biochim Biophys Acta. 1852:2574–2584.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Izadpanah S, Shabani P, Aghebati-Maleki A,
Baghbanzadeh A, Fotouhi A, Bisadi A, Aghebati-Maleki L and
Baradaran B: Prospects for the involvement of cancer stem cells in
the pathogenesis of osteosarcoma. J Cell Physiol. 235:4167–4182.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu B, Ma W, Jha RK and Gurung K: Cancer
stem cells in osteosarcoma: Recent progress and perspective. Acta
Oncol. 50:1142–1150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Basu-Roy U, Basilico C and Mansukhani A:
Perspectives on cancer stem cells in osteosarcoma. Cancer Lett.
338:158–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sauvageot CM, Weatherbee JL, Kesari S,
Winters SE, Barnes J, Dellagatta J, Ramakrishna NR, Stiles CD, Kung
AL, Kieran MW and Wen PY: Efficacy of the HSP90 inhibitor 17-AAG in
human glioma cell lines and tumorigenic glioma stem cells. Neuro
Oncol. 11:109–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Newman B, Liu Y, Lee HF, Sun D and Wang Y:
HSP90 inhibitor 17-AAG selectively eradicates lymphoma stem cells.
Cancer Res. 72:4551–4561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Amini S, Fathi F, Mobalegi J,
Sofimajidpour H and Ghadimi T: The expressions of stem cell
markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3,
Dppa4, and Esrrb in bladder, colon, and prostate cancer, and
certain cancer cell lines. Anat Cell Biol. 47:1–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sarto C, Binz PA and Mocarelli P: Heat
shock proteins in human cancer. Electrophoresis. 21:1218–1226.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Staufer K and Stoeltzing O: Implication of
heat shock protein 90 (HSP90) in tumor angiogenesis: A molecular
target for anti-angiogenic therapy? Curr Cancer Drug Targets.
10:890–897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zuehlke A and Johnson JL: Hsp90 and
co-chaperones twist the functions of diverse client proteins.
Biopolymers. 93:211–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao Z, Jia Q, Wu MS, Xie X, Wang Y, Song
G, Zou CY, Tang Q, Lu J, Huang G, et al: Degalactotigonin, a
natural compound from solanum nigrum L., inhibits growth and
metastasis of osteosarcoma through GSK3β inactivation-mediated
repression of the hedgehog/Gli1 pathway. Clin Cancer Res.
24:130–144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stecca B, Mas C, Clement V, Zbinden M,
Correa R, Piguet V, Beermann F and Ruiz I Altaba A: Melanomas
require HEDGEHOG-GLI signaling regulated by interactions between
GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci USA.
104:5895–5900. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Y, Ding Q, Yen CJ, Xia W, Izzo JG,
Lang JY, Li CW, Hsu JL, Miller SA, Wang X, et al: The crosstalk of
mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 21:374–387. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Das S, Harris LG, Metge BJ, Liu S, Riker
AI, Samant RS and Shevde LA: The hedgehog pathway transcription
factor GLI1 promotes malignant behavior of cancer cells by
up-regulating osteopontin. J Biol Chem. 284:22888–22897. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiang J and Hui CC: Hedgehog signaling in
development and cancer. Dev Cell. 15:801–812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ruiz I Altaba A: Gli proteins and Hedgehog
signaling: Development and cancer. Trends Genet. 15:418–425. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ng JM and Curran T: The Hedgehog's tale:
Developing strategies for targeting cancer. Nat Rev Cancer.
11:493–501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Katoh Y and Katoh M: Integrative genomic
analyses on GLI1: Positive regulation of GLI1 by Hedgehog-GLI,
TGFbeta-Smads, and RTK-PI3K-AKT signals, and negative regulation of
GLI1 by Notch-CSL-HES/HEY, and GPCR-Gs-PKA signals. Int J Oncol.
35:187–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tang QL, Xie XB, Wang J, Chen Q, Han AJ,
Zou CY, Yin JQ, Liu DW, Liang Y, Zhao ZQ, et al: Glycogen synthase
kinase-3β, NF-KB signaling, and tumorigenesis of human
osteosarcoma. J Nat Cancer Inst. 104:749–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hur EM and Zhou FQ: GSK3 signaling in
neural development. Nat Rev Neurosci. 11:539–551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Beurel E, Grieco SF and Jope RS: Glycogen
synthase kinase-3 (GSK3): Regulation, actions, and diseases.
Pharmacol Ther. 148:114–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Riobó NA, Lu K, Ai X, Haines GM and
Emerson CP Jr: Phosphoinositide 3-kinase and Akt are essential for
Sonic Hedgehog signaling. Proc Natl Acad Sci USA. 103:4505–4510.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Feng X, Xu W, Li Z, Song W, Ding J and
Chen X: Immunomodulatory Nanosystems. Adv Sci (Weinh).
6:19001012019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yin F, Wang Z, Jiang Y, Zhang T, Wang Z,
Hua Y, Song Z, Liu J, Xu W, Xu J, et al: Reduction-responsive
polypeptide nanomedicines significantly inhibit progression of
orthotopic osteosarcoma. Nanomedicine. 23:1020852020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ding J, Chen J, Gao L, Jiang Z, Zhang Y,
Li M, Xiao Q, Lee SS and Chen X: Engineered nanomedicines with
enhanced tumor penetration. Nano Today. 29:1008002019. View Article : Google Scholar
|