Introduction
Fucoidan, a type of marine polysaccharide containing
substantial percentages of L-fucose and sulfate ester groups, is an
essential constituent of brown seaweed (1) and certain marine invertebrates (such
as sea urchins and sea cucumbers) (2–4). For
the past decade, fucoidan has been widely studied owing to its
various biological activities, including anticoagulant, antitumor,
antiviral, anti-inflammatory, immunomodulatory, and antioxidant
properties (5–7). Therefore, fucoidan has been considered
as a promising potential marine resource for the development of
medications or functional foods beneficial to human health.
Osteosarcoma (OS) is the most primary malignant bone
cancer commonly found in children and adolescents worldwide
(8). The frequent sites of OS are
the femur, the tibia and the humerus, and osteosarcoma represents
more than half of all bone cancers (8). The 5 year cumulative survival rate of
primary OS has been greatly improved by combining surgery with
multi-agent chemotherapy in the past decades (9,10).
However, almost 80% of OS patients eventually develop pulmonary
metastasis, which is the major cause of fatal outcomes (11,12).
Hence, new therapeutic approaches blocking the metastatic potential
are urgently required to improve the outcomes for OS patients.
Sea cucumber is a widely used traditional Chinese
medicine (TCM) with numerous healthy benefits (13). It is rich in a variety of bioactive
components, such as polysaccharide, polypeptide and saponins
(13–16). Sea cucumber fucoidan has been
reported to have anticoagulant, anti-hyperglycemic,
anti-inflammatory, and immunomodulatory activity (17–19).
Growing evidence demonstrates that fucoidan possesses marked
anticancer and anti-metastatic effects (20,21).
Unfortunately, the underlying mechanism that accounts for the
anti-metastatic effect of sea cucumber Cucumaria frondosa
fucoidan (Cf-Fuc) remains largely unknown and requires further
investigation. Therefore, in the present study the inhibitory
effect and mechanism of Cf-Fuc on the in vitro migration of
human bone osteosarcoma epithelial cells (U2OS) were examined. The
present findings may reveal the potential targets affected by C.
frondosa fucoidan and promising therapeutic implications in
treating osteosarcoma metastasis.
Materials and methods
Materials and chemicals
Dry sea cucumber C. frondosa was purchased
from a local market (Changchun, Jilin Province, China). Antibodies
against focal adhesion kinase (FAK) (product code ab131435),
phospho-FAK (product code ab81298), paxillin (product code ab32115)
and phospho-paxillin (product code ab4833) were purchased from
Abcam. Antibodies against p21-activated kinase 1 (PAK1) (cat. no.
sc-166887) and phospho-PAK1 (sc-135755) were purchased from Santa
Cruz Biotechnology, Inc. LIM domain kinase 1 (LIMK1) (product no.
3842), phospho-LIMK1 (product no. 3841), cofilin (product no. 5175)
and phospho-cofilin (product no. 3311) were obtained from Cell
Signaling Technology, Inc. ECL chemiluminescent detection reagent
and Glutathione Sepharose 4B affinity chromatography resin were
obtained from GE Healthcare Life Sciences. Fibronectin, DMSO, EDTA,
cysteine and TIRTC-conjugated phalloidin were purchased from
Sigma-Aldrich; Merck KGaA. Fetal bovine serum, penicillin,
streptomycin and Dulbecco's Modified Eagle's Medium (DMEM) were
purchased from Gibco Life Technologies; Thermo Fisher Scientific,
Inc. All other chemical reagents used in this study were analytical
grade.
Preparation of Cf-Fuc
The preparation of sea cucumber (C. frondosa)
fucoidan was performed according to a previous method (22) with some modifications. Briefly, the
dry body wall of the sea cucumber (200 g) was ground and degreased
by reflux extraction with anhydrous acetone at 60°C for 4 h. Then,
the sample was digested with 0.5% papain solution containing 5 mM
EDTA and 5 mM cysteine at 60°C for 10 h. Subsequently, the digested
sample was centrifuged at 8,000 × g for 15 min at 4°C.
Polysaccharides were precipitated from the supernatant with 200 ml
of 10% cetylpyridinium chloride solution at 4°C overnight. The
precipitate obtained by centrifugation was re-dissolved with 1.5 l
of 3 M NaCl:ethanol (100:15, v/v), and then 1 l of 95% ethanol was
added into the mixture. After centrifugation at 8,000 × g for 15
min at 4°C and removal of the precipitate chondroitin sulfate,
another 1.5 l of ethanol was added to the supernatant. The
precipitate collected by centrifugation was re-dissolved and
dialyzed (MWCO 1000 Da) against deionized water for 48 h. The
sample was lyophilized to obtain sea cucumber C. frondosa
fucoidan named Cf-Fuc. Cf-Fuc was composed of L-fucose and a
sulfate ester group. The purity of Cf-Fuc was determined as 98.6%
(total carbohydrate content 72.5% quantified by phenol-sulfuric
acid method (23); the sulfate
content 26.1% determined by BaCl2-gelatin method
(24).
Cell culture
U2OS cells were purchased from the Cell Bank of
Shanghai Institute of Biochemistry and Cell Biology. U2OS cells
were routinely cultured in DMEM supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin, incubated in a 5%
CO2 incubator at 37°C.
Cytotoxicity of Cf-Fuc
Cells (1×104) were seeded into each well
of 96-well plates. After incubation at 37°C for 24 h, Cf-Fuc was
administered to the cells at 50 and 100 µg/ml. After 24 h of
incubation at 37°C, the medium was replaced with 100 µl fresh
medium containing 0.5 mg/ml of MTT. Subsequently, the cells were
incubated at 37°C for another 4 h, and 150 µl of DMSO was added
after removal of the supernatant. The absorbance at 570 nm was
determined using a microplate reader.
Cell adhesion assay
U2OS cells were detached by trypsin digestion and
suspended in serum-free medium with or without Cf-Fuc (50 and 100
µg/ml) for 30 min. Then, the cells were seeded into a 96-well plate
coated with fibronectin (10 µg/ml), and incubated for 0.5, 1, 2 or
4 h. After washing with PBS, the adherent cells were fixed with 4%
paraformaldehyde at room temperature (RT) for 30 min, and stained
with 0.5% crystal violet at RT for 15 min. The dye, extracted with
33% acetic acid after washing with PBS, was quantified using a
microplate reader.
Transwell migration assay
U2OS cells incubated with or without Cf-Fuc (50 and
100 µg/ml) at 37°C for 4 h were then placed in 24-well Transwell™
(Corning Costar, Corning, NY) filter inserts (8 µm pore diameter).
DMEM containing 10% FBS as the chemoattractant was added in the
lower well. After incubation for 12 h, the non-migrated U2OS cells
inside the inserts were gently removed using cotton swab. The
migrated cells were then fixed and stained with 0.1% crystal violet
at RT for 15 min. Migrated cells were counted in five random fields
under a light microscope at ×400 magnification.
U2OS cell migration assay
U2OS cells were seeded into a CELLview cell culture
dish (Greiner Bio-One) pre-coated with fibronectin (10 µg/ml), then
incubated with or without Cf-Fuc (50 and 100 µg/ml) at 37°C for 4
h. U2OS cells were then placed in a 37°C heating chamber with
CO2 supply. Images were captured at 5 min intervals with
a CCD camera for 4 h. Image stacks were quantitatively analyzed and
wind-rose plots of tracked migration paths of U2OS cells were
plotted by NIH ImageJ software (version 1.30; National Institutes
of Health).
Determination of F-actin content
Six-well plates were pre-coated with fibronectin (10
µg/ml) at 37°C overnight, and then U2OS cells were seeded into each
well and incubated at 37°C for 2 h. Cells were further treated with
or without Cf-Fuc (50 and 100 µg/ml) for 0.5, 1, 2 or 4 h. After
being washed with PBS the cells were fixed and stained with
rhodamine phalloidin (0.1% Triton X-100, 3.7% formaldehyde, and 2
µM rhodamine phalloidin in PBS) for 1 h. Cells were incubated with
methanol to extract the dye and rhodamine fluorescence (excitation
wavelength: 530 nm, emission wavelength: 590 nm) was further
assessed with a fluorescence microplate reader (TECAN GENios; Tecan
Austria GmbH). F-actin content at time 0 was considered as 1;
F-actin content at 0.5, 1, 2 and 4 h are expressed as a relative
fold change of mean fluorescence intensity at time 0.
Ras-related C3 botulinum toxin
substrate 1 (Rac1) activation assay
Rac1 activation was determined according to a
previous method (25). Briefly,
U2OS cells were lysed with RIPA lysis buffer (Beyotime Institute of
Biotechnology) and the lysate supernatant was then incubated with
GST-PAK1 PBD fusion proteins immobilized on Glutathione Sepharose
4B affinity chromatography resin. After washing with lysis buffer,
the resin was boiled in Laemmli sample buffer. Then, Rac1 protein
bound to GST-PAK1 PBD as well as the endogenous total Rac1 were
analyzed by SDS-PAGE and immunoblotting.
Immunoblotting
Cells were harvested and lysed with RIPA lysis
buffer. Cell lysates were collected and the concentration of
proteins was quantified by BCA protein assay kit (Beyotime
Institute of Biotechnology). Equal amounts of protein (30 µg of
total protein per lane) were subjected to 10% SDS-PAGE and
transferred onto PVDF membranes. The membranes were blocked with 3%
BSA for 2 h at room temperature and then incubated with indicated
primary antibodies (1:1,000 dilution) at 4°C overnight.
HRP-conjugated secondary antibody (Beyotime Institute of
Biotechnology) was incubated at RT for another 1 h after washing
with TBST three times. Protein bands were subsequently visualized
using ECL Western blotting chemiluminescent detection reagent. The
band intensities for quantitative analysis were measured by
densitometric analysis using NIH ImageJ software (version 1.30;
National Institutes of Health).
Statistical analysis
Data were expressed as the mean ± SD. Significant
differences were determined by one-way ANOVA, followed by
Bonferroni post hoc test using GraphPad Prism 6 (GraphPad Software,
Inc.). P-values <0.05 were considered to indicate a
statistically significant difference.
Results
Cf-Fuc inhibits U2OS cell
adhesion
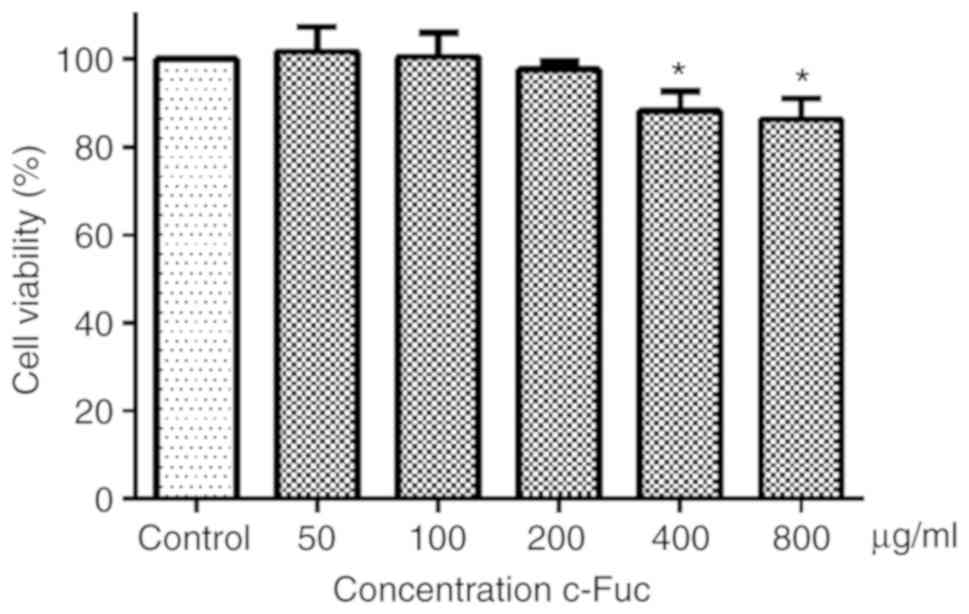
As revealed in Fig.
1, U2OS cell viability was not impacted by Cf-Fuc treatment at
concentrations of 50, 100, and 200 µg/ml for 24 h. When the
concentration of Cf-Fuc increased to ≥400 µg/ml, the U2OS cell
viability was significantly decreased compared to the control
group. Therefore, the non-cytotoxic concentrations of 50 and 100
µg/ml were selected for the following experiment to evaluate the
inhibitory effect of Cf-Fuc on U2OS cell adhesion and migration, in
case the phenotype presented in the present study was caused by
cytotoxicity.
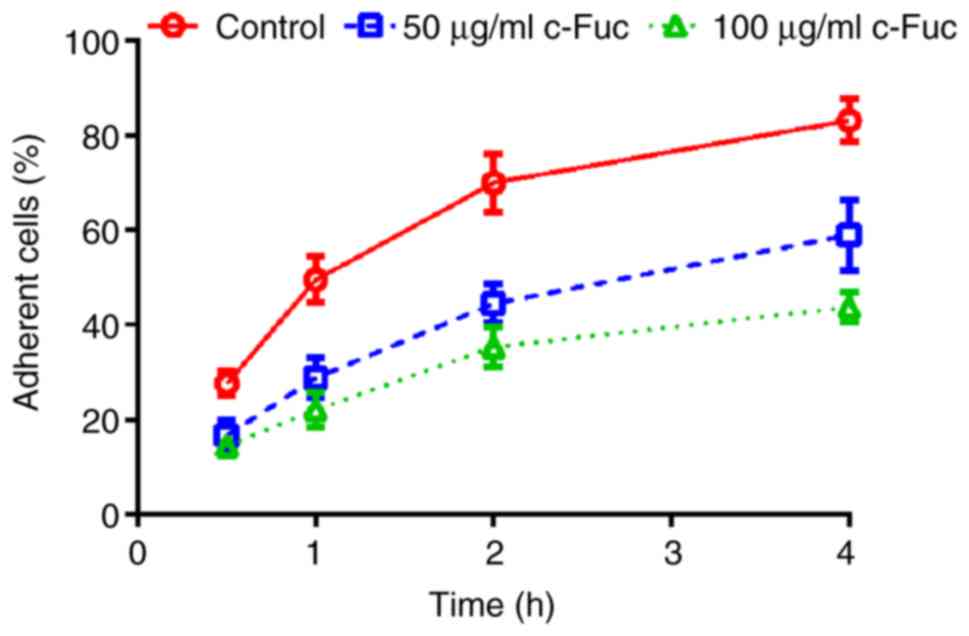
As revealed in Fig.
2, the adherent percentage of U2OS cells without treatment
gradually increased, and eventually reached 83.2% at 4 h. While,
the adherent percentage of Cf-Fuc-treated cells increased slowly,
and only reached 59.0 and 43.8% at 4 h, respectively, with the
Cf-Fuc concentration of 50 and 100 µg/ml, indicating that Cf-Fuc
treatment could inhibit U2OS cell adhesion.
Cf-Fuc impairs the migration capacity
of U2OS cells
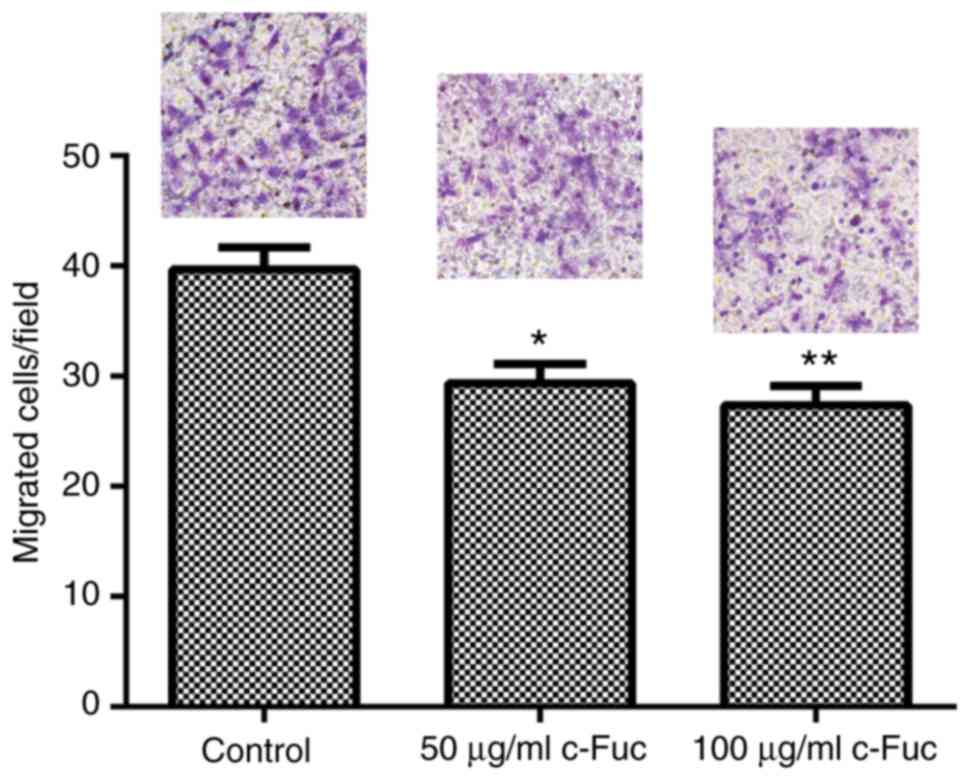
First, the inhibitory effect of Cf-Fuc on U2OS cell
migration was evaluated by Transwell assay. Cf-Fuc caused strong
inhibition on U2OS cell migration, leading to a significant
decrease in the number of migrated cells compared with the control
group (Fig. 3). Then, the migratory
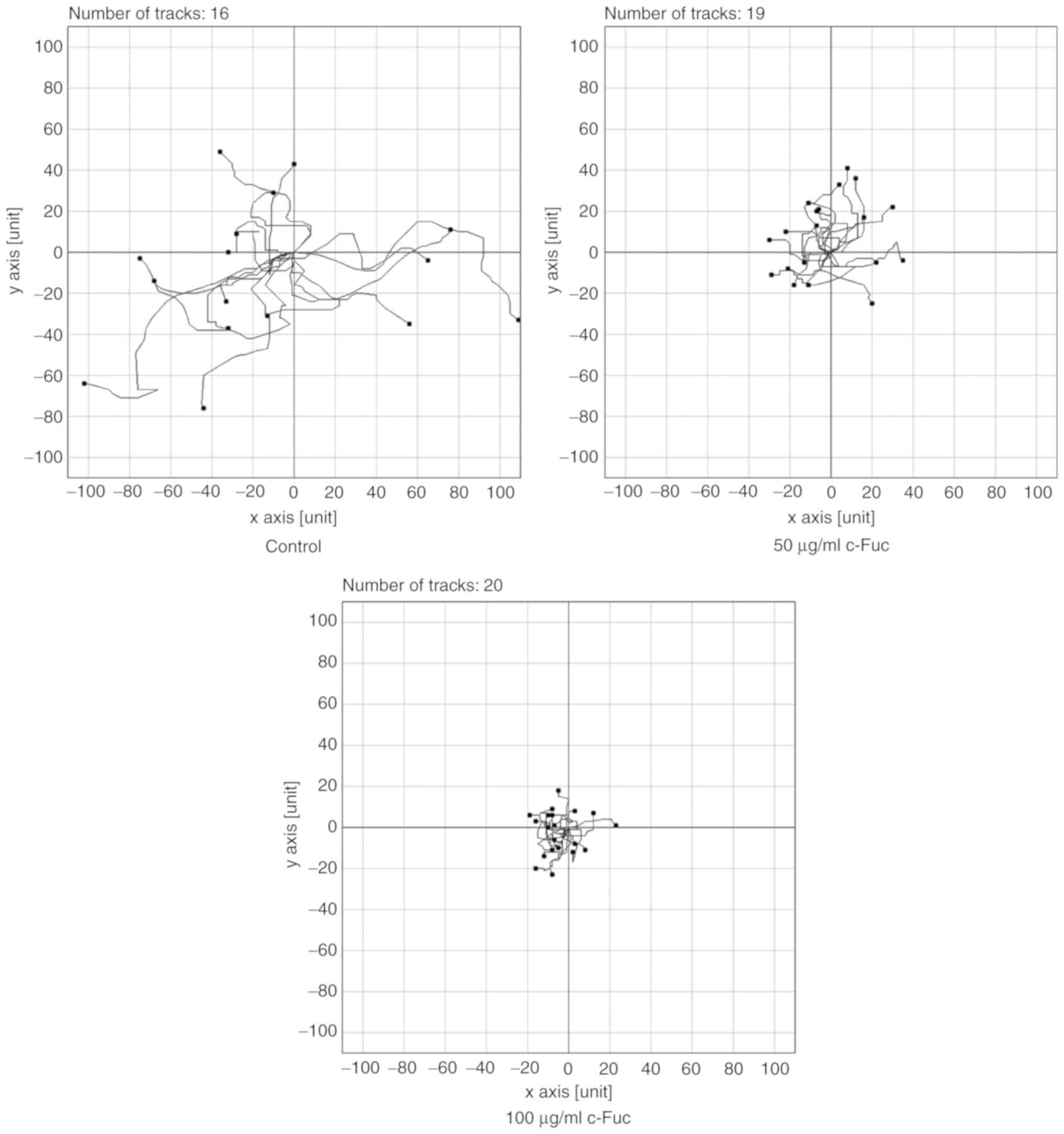
capacity of Cf-Fuc-treated U2OS cells was quantitatively analyzed
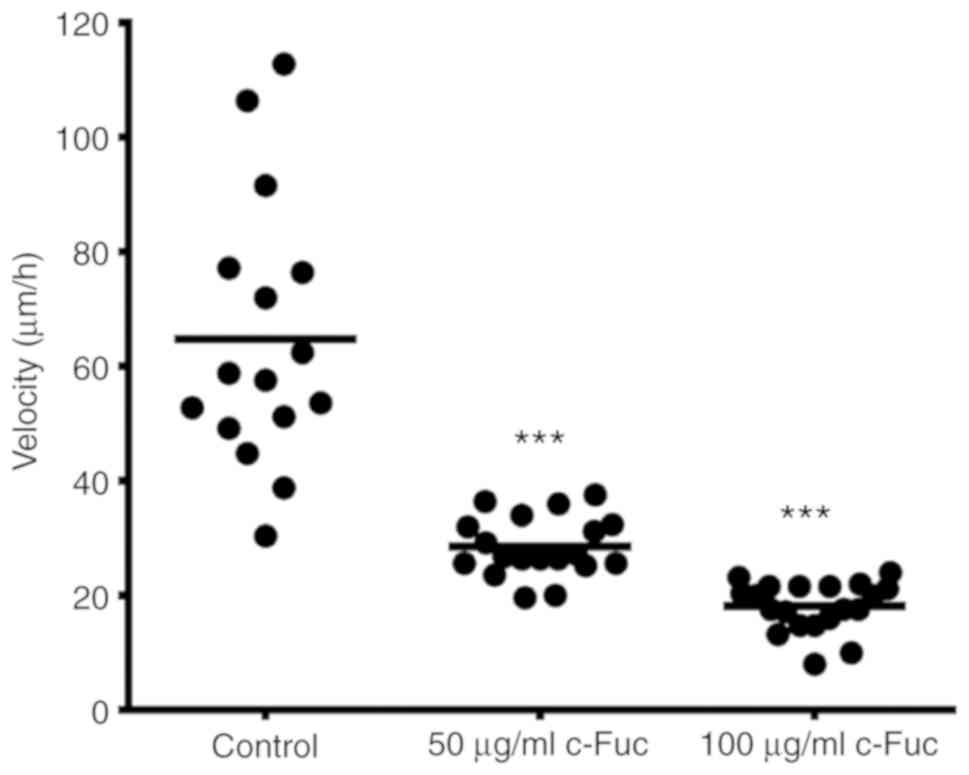
by NIH ImageJ. As revealed in Figs.
4 and 5, the control cells
exhibited a strong migration ability, and the velocity reached 64.8
µm/h. Notably, in comparison to the cells in the control group,
Cf-Fuc impaired the migratory capacity of U2OS cells. The migratory
distance of Cf-Fuc-treated cells significantly decreased and the
velocity was reduced to 28.5 and 18.1 µm/h, respectively, at
concentrations of 50 and 100 µg/ml.
Cf-Fuc inhibits the formation of
F-actin
Cytoskeleton remodeling is a critical event involved
in cancer metastasis (26). During
the adhesion and migration of cancer cells, G-actin polymerizes
into F-actin filaments, which acts as stress fiber of the
cytoskeleton and participates in the formation of lamellar
pseudopods required for osteosarcoma metastasis (27,28).
Therefore, the effect of Cf-Fuc on actin polymerization was further
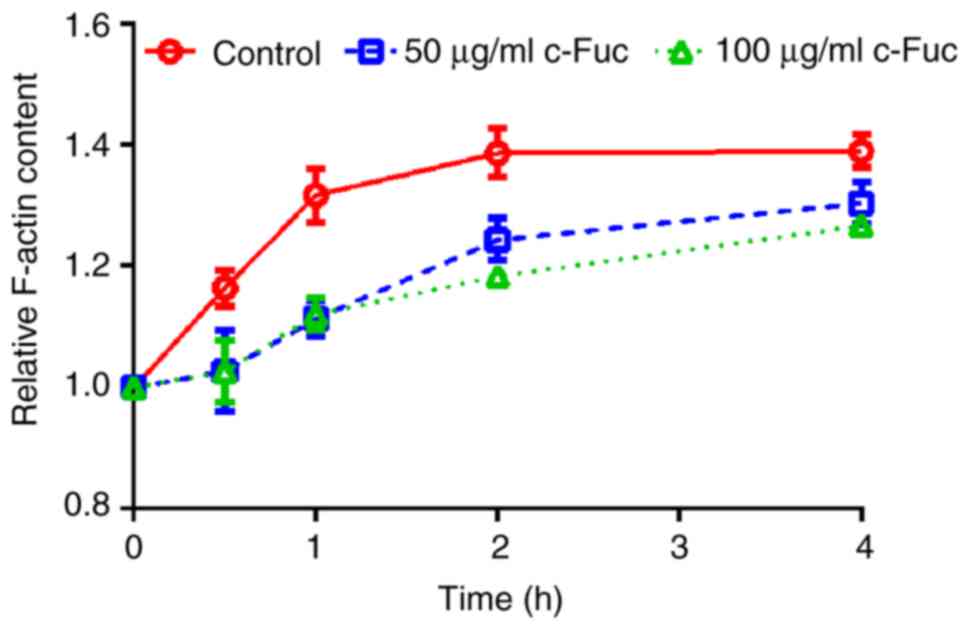
investigated. As revealed in Fig.
6, the actin polymerization of U2OS cells increased in a
time-dependent manner. However, F-actin content of Cf-Fuc-treated
cells was reduced compared to the control cells.
Cf-Fuc blocks the signaling
transduction of cell adhesion
The effect of Cf-Fuc on cell adhesion signaling of
U2OS cells was further examined. As revealed in Fig. 7A, Cf-Fuc treatment significantly
suppressed the phosphorylation of FAK (Fig. 7B) and paxillin (Fig. 7C) compared to the control group,
indicating that Cf-Fuc inhibited the U2OS cell migration possibly
by modulating the FAK/paxillin cell adhesion signaling axis.
Cf-Fuc impacts the cytoskeleton
remodeling signaling axis
Small GTPase Rac1 plays a crucial role in the
remodeling of the actin cytoskeleton. Whether Cf-Fuc could inhibit
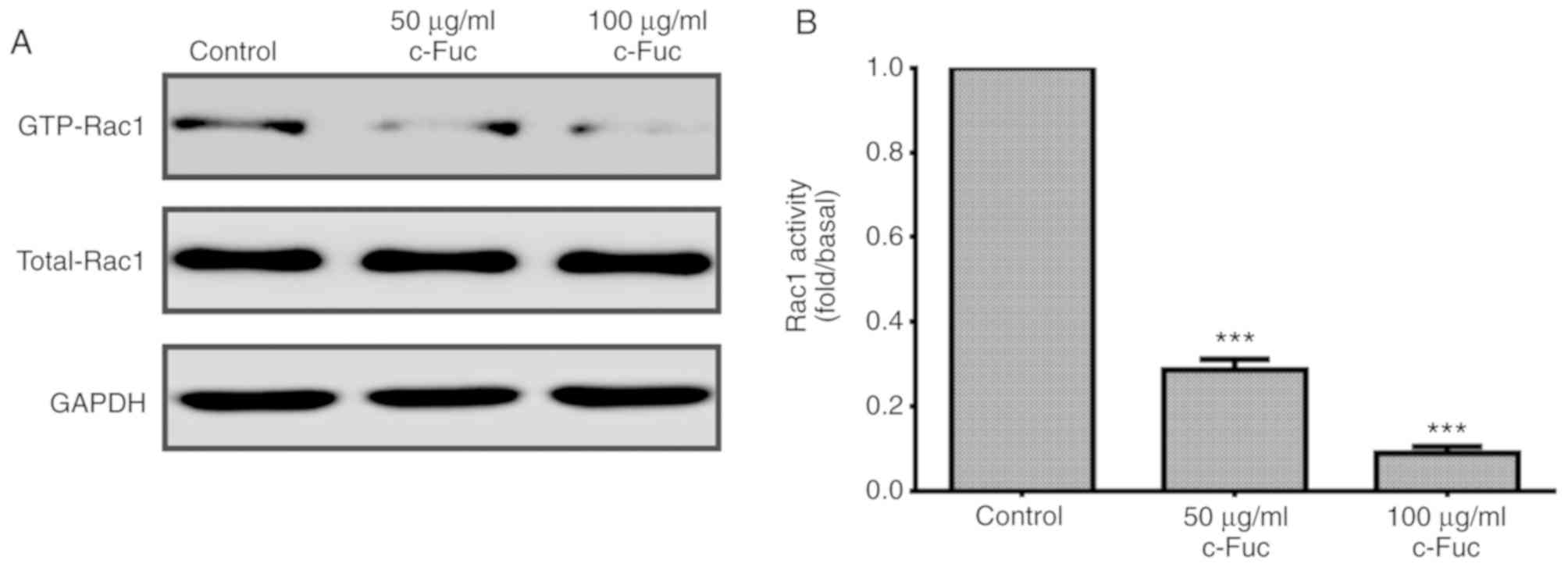
Rac1 activation in U2OS cells was subsequently examined. As
revealed in Fig. 8A, compared to
the control group, once treated with Cf-Fuc, the level of GTP-Rac1
(activated form of Rac1) was reduced by 72 and 91%, respectively,
at concentrations of 50 and 100 µg/ml (Fig. 8B).
The Rac1/PAK1/LIMK1/cofilin signaling axis could
regulate the assembly of the actin cytoskeleton and has been
revealed to be involved in cancer metastasis (29,30).
Therefore, to determine whether Cf-Fuc inhibited U2OS cell adhesion
and migration via the PAK1/LIMK1/cofilin signaling axis, the
phosphorylation levels of PAK1, LIMK1 and cofilin were further
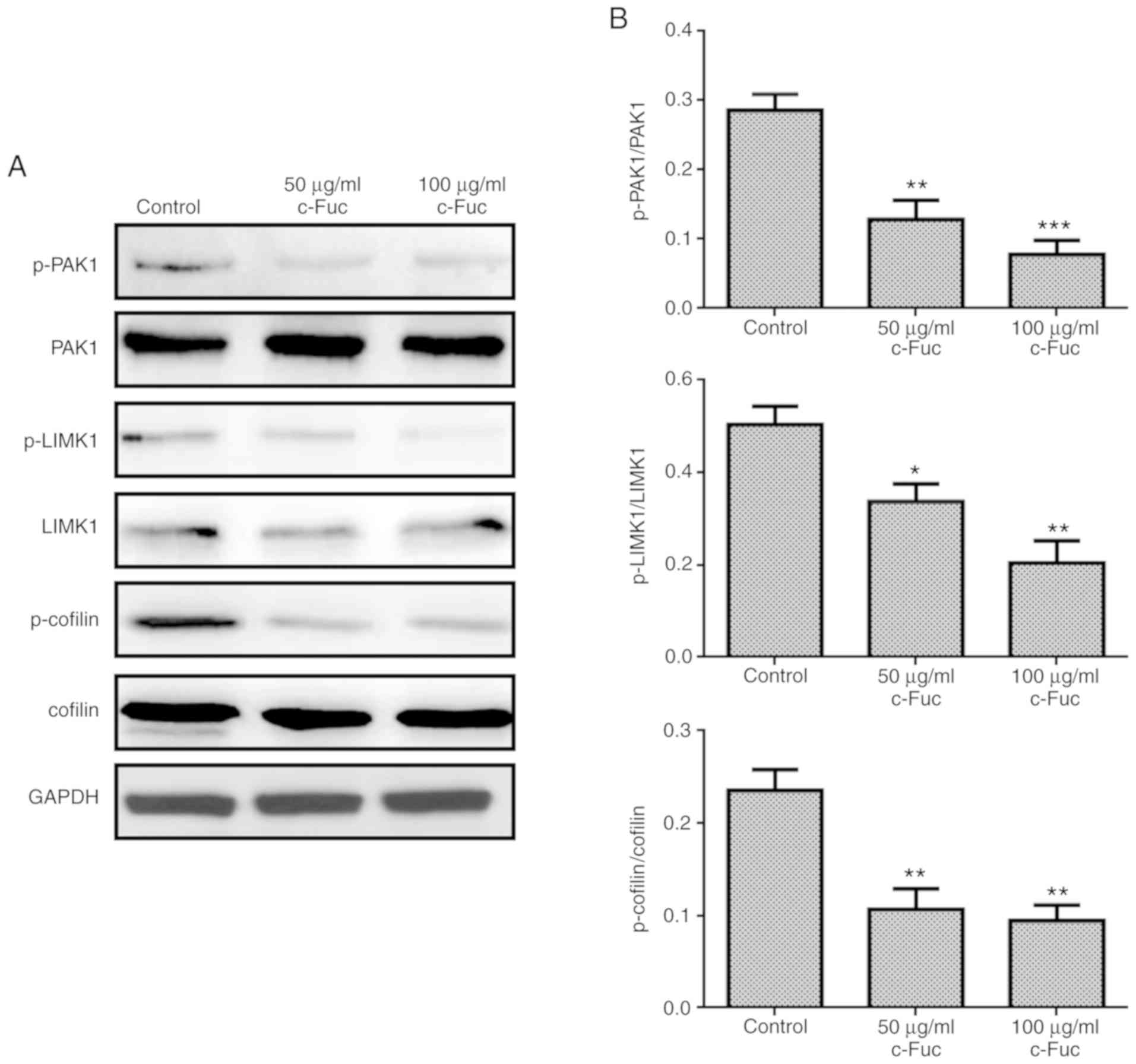
investigated. As revealed in Fig.
9, the phosphorylated forms of PAK1, LIMK1 and cofilin were
significantly inhibited by Cf-Fuc treatment at concentrations of 50
and 100 µg/ml compared to the control group. The results indicated
the potential molecular mechanism of Cf-Fuc in inhibiting U2OS cell
adhesion and migration by blocking the activation of the
Rac1/PAK1/LIMK1/cofilin signaling axis.
Discussion
Osteosarcoma (OS), a primary malignancy of bone, is
prone to early metastasis (8).
Currently, resection surgery and chemotherapy are standard
treatments for OS (9). However, OS
patients receive treatment that remains fundamentally unchanged
since the 1970s, and accumulative results from clinical trials for
OS treatment have proven largely disappointing (31). Outcomes of OS remain largely
unimproved; notably, metastasis remains the most fatal complication
of OS, and thus, the long-term survival rate of patients with OS is
low due to the high risk of metastasis (32,33).
Hence, novel approaches are urgently required to improve the
treatment of OS and prevent its metastasis. Compared with current
chemotherapy, marine natural active molecules exhibit less adverse
effects and improved anti-metastatic effects, and have been
considered as a promising strategy to treat cancer metastasis
(34,35).
Growing evidence reveals that polysaccharides from
marine resources possess extensive health benefits, such as
anti-metastatic ability (36,37).
Research on the underlying anti-metastatic mechanisms of
polysaccharides has revealed that multiple signaling pathways are
involved. Wang et al (38)
reported that a fucoidan derived from Undaria pinnatifida
sporophylls (Ups-fucoidan) exerted a concentration- and
time-dependent inhibitory effect on tumor metastasis in vivo
and inhibited mouse hepatocarcinoma Hca-F cell growth, migration,
invasion, and adhesion capabilities in vitro. Their study
revealed that Ups-fucoidan inhibited growth by downregulating
VEGFC/VEGFR3, c-MET, cyclin D1, CDK4, PI3K/Akt and ERK, and
suppressed adhesion and invasion by downregulating L-selectin and
upregulating TIMPs. Similarly, other sulfated polysaccharides
isolated from marine invertebrates (Strongylocentrotus
droebachiensis and Echinometra lucunter) were revealed
to attenuate tumor metastasis by a P-selectin-mediated mechanism
(39). Another study (40) also demonstrated that Undaria
pinnatifida fucoidan significantly inhibited the
hypoxia-induced expression, nuclear translocation and activation of
HIF-1α, the synthesis and secretion of VEGF-C and HGF, as well as
the invasion and lymphatic metastasis in a mouse hepatocarcinoma
Hca-F cell line. In the present study, our findings also provide
evidence that sulfated polysaccharide Cf-Fuc, isolated from sea
cucumber C. frondosa, could inhibit the migratory capacity
of U2OS cells in vitro, indicating that Cf-Fuc possesses
potential anti-metastatic capacity.
As an essential cell adhesion molecule in OS cells,
integrin plays important roles in mediating OS metastasis (41,42).
Therefore, integrin and its downstream signaling have been
considered as promising therapeutic targets for OS metastasis. It
is widely accepted that FAK is a crucial kinase in integrin
signaling that can phosphorylate multiple substrates, as well as a
scaffold protein for protein-protein interactions, regulating
adhesion and migration of cancer cells (43). Furthermore, paxillin is a
multifunctional adaptor protein phosphorylated by FAK during cell
migration, and performs a critical function in coordinating
integrin signaling involved in cancer metastasis (44). The present findings revealed that
Cf-Fuc could significantly reduce the number of adherent U2OS cells
and cause a significant decrease in the migration capacity of U2OS
cells. In addition, Cf-Fuc treatment inhibited the phosphorylation
of FAK and paxillin, and this may be the major reason for the
decrease in the adhesion and migration of U2OS cells.
Cancer metastasis is associated with increased
motility characteristics, such as an extensively cross-linked actin
network to form pseudopods, which requires appropriate cytoskeleton
remodeling (45). Rac1 is an
important member of the Rho small GTPase family which classically
regulates actin cytoskeleton rearrangement (45). Rac1 activation is correlated with
metastatic progression in many types of cancers (45). Growing evidence clearly reveals that
Rac1-dependent signaling activation can promote cancer cell
adhesion, invasion and metastasis in a variety of cancers,
including osteosarcoma (46). Rac1
initially activates PAK1 which further phosphorylates and activates
LIMK1. Activated LIMK1 further phosphorylates and inactivates
cofilin, leading to the growth of actin filaments and formation of
pseudopodia. Therefore, Rac-1 exhibits crucial roles in regulating
cancer invasion and metastasis. The present study determined that
Cf-Fuc could inhibit actin polymerization as revealed by the
decreased content of F-actin, and this may be due to the blockage
of the Rac1/PAK1/LIMK1/cofilin signaling axis, which impaired the
dynamic reorganization of the actin cytoskeleton. Further research
will be carried out to confirm the roles of Cf-Fuc on this pathway
during the cytoskeleton remodeling of OS metastasis by RNAi,
overexpression or inhibitor treatment, not only on U2OS cells, but
also on other OS cell lines such as MG-63 and Saos-2 cells to
support the present findings. In addition, considering fucoidans
are huge molecules with a complex structure, the bioactivity of
Cf-Fuc in the inhibition of adhesion and migration of U2OS cells
may not depend on the integral molecule, Cf-Fuc may contain some
essential motifs. Therefore, the degradation of Cf-Fuc, the
purification and characterization of the functional motif(s), as
well as its pharmacokinetics and the distribution traced by
radioactive isotope labeling in vivo, will become future
research directions. In addition, a blood compatibility test of
Cf-Fuc will be performed in our future research to confirm its
clinical potential.
In summary, Cf-Fuc significantly inhibited OS cell
adhesion and migration, reduced F-actin formation, and
downregulated adhesion signaling via suppression of the
phosphorylation of FAK and paxillin. Cf-Fuc also impaired Rac1
activation and the PAK1/LIMK1/cofilin signaling axis for
reorganization of the actin cytoskeleton, providing the potential
anti-metastatic mechanism of Cf-Fuc.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MZ, LC and YL designed and performed the
experiments, analyzed the data and wrote the manuscript. MC and SZ
also performed the experiments. DK designed, interpreted and funded
the study, and also wrote the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sudirman S, Ong AD, Chang HW and Kong ZL:
Effect of fucoidan on anterior cruciate ligament transection and
medial meniscectomy induced osteoarthritis in high-fat diet-induced
obese rats. Nutrients. 10:E6862018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Surayot U, Lee S and You S: Effects of
sulfated fucan from the sea cucumber stichopus japonicus on natural
killer cell activation and cytotoxicity. Int J Biol Macromol.
108:177–184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thinh PD, Ly BM, Usoltseva RV, Shevchenko
NM, Rasin AB, Anastyuk SD, Malyarenko OS, Zvyagintseva TN, San PT
and Ermakova SP: A novel sulfated fucan from Vietnamese sea
cucumber Stichopus variegatus: Isolation, structure and anticancer
activity in vitro. Int J Biol Macromol. 117:1101–1109. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mansour MB, Balti R, Yacoubi L, Ollivier
V, Chaubet F and Maaroufi RM: Primary structure and anticoagulant
activity of fucoidan from the sea cucumber holothuria polii. Int J
Biol Macromol. 121:1145–1153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luthuli S, Wu S, Cheng Y, Zhen X, Wu M and
Tong H: Therapeutic effects of fucoidan: A review on recent
studies. Mar Drugs. 17:E4872019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Xing M, Cao Q, Ji A, Liang H and
Song S: Biological activities of fucoidan and the factors mediating
its therapeutic effects: A review of recent studies. Mar Drugs.
17:E1832019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Y, Zheng Y, Wang J, Ma S, Yu Y, White
WL, Yang S, Yang F and Lu J: Fucoidan extracted from undaria
pinnatifida: Source for nutraceuticals/functional foods. Mar Drugs.
16:E3212018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kager L, Tamamyan G and Bielack S: Novel
insights and therapeutic interventions for pediatric osteosarcoma.
Future Oncol. 13:357–368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rickel K, Fang F and Tao J: Molecular
genetics of osteosarcoma. Bone. 102:69–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ram Kumar RM, Boro A and Fuchs B:
Involvement and clinical aspects of MicroRNA in osteosarcoma. Int J
Mol Sci. 17:E8772016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Otoukesh B, Boddouhi B, Moghtadaei M,
Kaghazian P and Kaghazian M: Novel molecular insights and new
therapeutic strategies in osteosarcoma. Cancer Cell Int.
18:1582018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khotimchenko Y: Pharmacological potential
of sea cucumbers. Int J Mol Sci. 19:13422018. View Article : Google Scholar
|
|
14
|
Kim JL, Park SH, Jeong S, Kim BR, Na YJ,
Jo MJ, Jeong YA, Yun HK, Kim DY, Kim BG, et al: Sea cucumber
(Stichopus japonicas) F2 enhanced TRAIL-Induced apoptosis via XIAP
ubiquitination and er stress in colorectal cancer cells. Nutrients.
11:E10612019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mondol MAM, Shin HJ, Rahman MA and Islam
MT: Sea cucumber glycosides: Chemical structures, producing species
and important biological properties. Mar Drugs. 15:E3172017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Attoub S, Arafat K, Khalaf T, Sulaiman S
and Iratni R: Frondoside a enhances the anti-cancer effects of
oxaliplatin and 5-fluorouracil on colon cancer cells. Nutrients.
10:E5602018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shang F, Mou R, Zhang Z, Gao N, Lin L, Li
Z, Wu M and Zhao J: Structural analysis and anticoagulant
activities of three highly regular fucan sulfates as novel
intrinsic factor xase inhibitors. Carbohydr Polym. 195:257–266.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Wang J, Zhao Y, Hu S, Shi D and
Xue C: Fucoidan from sea cucumber cucumaria frondosa exhibits
anti-hyperglycemic effects in insulin resistant mice via activating
the PI3K/PKB pathway and GLUT4. J Biosci Bioeng. 121:36–42. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Hu S, Jiang W, Song W, Cai L and
Wang J: Fucoidan from sea cucumber may improve hepatic inflammatory
response and insulin resistance in mice. Int Immunopharmacol.
31:15–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Weelden G, Bobinski M, Okla K, van
Weelden WJ, Romano A and Pijnenborg JMA: Fucoidan structure and
activity in relation to anti-cancer mechanisms. Mar Drugs.
17:E322019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Janakiram NB, Mohammed A and Rao CV: Sea
cucumbers metabolites as potent anti-cancer agents. Mar Drugs.
13:2909–2923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li S, Li J, Mao G, Wu T, Hu Y, Ye X, Tian
D, Linhardt RJ and Chen S: A fucoidan from sea cucumber
pearsonothuria graeffei with well-repeated structure alleviates gut
microbiota dysbiosis and metabolic syndromes in HFD-fed mice. Food
Funct. 9:5371–5380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jain VM, Karibasappa GN, Dodamani AS and
Mali GV: Estimating the carbohydrate content of various forms of
tobacco by phenol-sulfuric acid method. J Edu Health Promot.
6:902017. View Article : Google Scholar
|
|
24
|
DODGSON KS and PRICE RG: A note on the
determination of the ester sulphate content of sulphated
polysaccharides. Biochem J. 84:106–110. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Benard V, Bohl BP and Bokoch GM:
Characterization of rac and cdc42 activation in
chemoattractant-stimulated human neutrophils using a novel assay
for active GTPases. J Biol Chem. 274:13198–13204. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kedrin D, van Rheenen J, Hernandez L,
Condeelis J and Segall JE: Cell motility and cytoskeletal
regulation in invasion and metastasis. J Mammary Gland Biol
Neoplasia. 12:143–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lambrechts A, Van Troys M and Ampe C: The
actin cytoskeleton in normal and pathological cell motility. Int J
Biochem Cell Biol. 36:1890–1909. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ryan GL, Petroccia HM, Watanabe N and
Vavylonis D: Excitable actin dynamics in lamellipodial protrusion
and retraction. Biophy J. 102:1493–1502. 2012. View Article : Google Scholar
|
|
29
|
Ohashi K, Fujiwara S, Watanabe T, Kondo H,
Kiuchi T, Sato M and Mizuno K: LIM kinase has a dual role in
regulating lamellipodium extension by decelerating the rate of
actin retrograde flow and the rate of actin polymerization. J Biol
Chem. 286:36340–36351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mizuno K: Signaling mechanisms and
functional roles of cofilin phosphorylation and dephosphorylation.
Cell Signal. 25:457–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vos HI, Coenen MJ, Guchelaar HJ and Te Loo
DM: The role of pharmacogenetics in the treatment of osteosarcoma.
Drug Discov Today. 21:1775–1786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liao Z, Qiu M, Yang J, Yang Y, Zhu L, Yang
B, Bai X, Xing P, Zhang J, Xing R, et al: Outcomes of surgery
and/or combination chemotherapy for extraskeletal osteosarcoma: A
single-center retrospective study from China. Sci Rep. 9:48162019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hattinger CM, Fanelli M, Tavanti E, Vella
S, Ferrari S, Picci P and Serra M: Advances in emerging drugs for
osteosarcoma. Exp Opin Emerg Drugs. 20:495–514. 2015. View Article : Google Scholar
|
|
34
|
Yao Z, Han L, Chen Y, He F, Sun B, Kamar
S, Zhang Y, Yang Y, Wang C and Yang Z: Hedgehog signalling in the
tumourigenesis and metastasis of osteosarcoma, and its potential
value in the clinical therapy of osteosarcoma. Cell Death Dis.
9:7012018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Daw NC, Chou AJ, Jaffe N, Rao BN, Billups
CA, Rodriguez-Galindo C, Meyers PA and Huh WW: Recurrent
osteosarcoma with a single pulmonary metastasis: A
multi-institutional review. Br J Cancer. 112:278–282. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khan T, Date A, Chawda H and Patel K:
Polysaccharides as potential anticancer agents-A review of their
progress. Carbohydr Polym. 210:412–428. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Senthilkumar K and Kim SK: Anticancer
effects of fucoidan. Adv Food Nutr Res. 72:195–213. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang P, Liu Z, Liu X, Teng H, Zhang C, Hou
L and Zou X: Anti-Metastasis effect of fucoidan from undaria
pinnatifida sporophylls in mouse hepatocarcinoma Hca-F cells. PLoS
One. 9:e1060712014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Teixeira FCOB, Kozlowski EO, Micheli KVA,
Vilela-Silva ACES, Borsig L and Pavão MSG: Sulfated fucans and a
sulfated galactan from sea urchins as potent inhibitors of
selectin-dependent hematogenous metastasis. Glycobiology.
28:427–434. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Teng H, Yang Y, Wei H, Liu Z, Liu Z, Ma Y,
Gao Z, Hou L and Zou X: Fucoidan suppresses hypoxia-induced
lymphangiogenesis and lymphatic metastasis in mouse
hepatocarcinoma. Mar Drugs. 13:3514–3530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gvozdenovic A, Boro A, Meier D,
Bode-Lesniewska B, Born W, Muff R and Fuchs B: Targeting αvβ3 and
αvβ5 integrins inhibits pulmonary metastasis in an intratibial
xenograft osteosarcoma mouse model. Oncotarget. 7:55141–55154.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu XZ, Li CJ, Wu SJ, Shi X and Zhao JN:
Involvement of α5 integrin in survivin-mediated osteosarcoma
metastasis. Asian Pac J Trop Med. 9:478–483. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Panera N, Crudele A, Romito I, Gnani D and
Alisi A: Focal adhesion kinase: Insight into molecular roles and
functions in hepatocellular carcinoma. Int J Mol Sci. 18:E992017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
López-Colomé AM, Lee-Rivera I,
Benavides-Hidalgo R and López E: Paxillin: A crossroad in
pathological cell migration. J Hematol Oncol. 10:502017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yamaguchi H and Condeelis J: Regulation of
the actin cytoskeleton in cancer cell migration and invasion.
Biochim Biophy Acta. 1773:642–652. 2007. View Article : Google Scholar
|
|
46
|
Cao J, Wang Y, Dong R, Lin G, Zhang N,
Wang J, Lin N, Gu Y, Ding L, Ying M, et al: Hypoxia-Induced WSB1
promotes the metastatic potential of osteosarcoma cells. Cancer
Res. 75:4839–4851. 2015. View Article : Google Scholar : PubMed/NCBI
|