Introduction
Colorectal cancer (CRC) is the third most common
cancer and the second leading cause of cancer-related death
worldwide (1,2). The incidence of CRC continues to
increase in developing countries and is also increasing in
individuals younger than 50 years (3). A worldwide health burden, CRC exhibits
molecular heterogeneity, which contributes to the variable clinical
outcomes (4). The identification of
prognostic and predictive biomarkers for early diagnosis,
prevention, and targeted therapy is a major challenge in CRC
treatment. Progress has been made in the discovery of key oncogenic
markers and transcriptional modifications for decades (5,6); for
example, RAS mutation testing has shown beneficial effects
in predicting the clinical outcome of anti-EGFR therapy in patients
with CRC, and immunohistochemical analysis of DNA mismatch repair
proteins (MLH1, MSH2, MSH6 and PMS2) has been shown to have
predictive value in CRC and is recommended for clinical use.
However, the molecular mechanisms of CRC pathogenesis remain
unclear. More effective and sensitive biomarkers are still urgently
needed for defining therapeutic targets and personalized
therapeutic regimens in CRC.
N6-methyladenosine
(m6A), a reversible posttranscriptional RNA
modification, is installed by methyltransferase like 3 (METTL3) in
cooperation with methyltransferase like 14 (METTL14) and Wilms
tumor 1-associated protein (WTAP) (7). m6A RNA modification is
erased by fat mass and obesity-associated protein (FTO) and the RNA
demethylase alkB homolog 5 (ALKBH5) (7). The YT521-B homology (YTH) domain
family proteins (YTHDF1, YTHDF2, YTHDF3, and YTHDC1) have been
identified as m6A readers (7). The functional effects of
m6A RNA modification include RNA splicing, degradation,
and translation and RNA-protein interaction (8–10).
This type of RNA modification is critical for circadian clock
regulation, self-renewal and cell fate transition (11,12).
Accumulating studies have demonstrated that m6A
modification plays regulatory roles in various malignancies
(13–15). METTL3 promotes glioblastoma
progression and enhances cancer stem cell (CSC) self-renewal
(15), and METTL3 upregulation
appears to be critical for cancer cell growth, survival and
invasion in lung cancer (16).
Moreover, METTL3 dysfunction exerts critical effects in
gastrointestinal tract cancers. Elevated METTL3 expression augments
tumor proliferation and liver metastasis by increasing the RNA
m6A level in gastric cancer (17,18),
and METTL3 is involved in cancer progression and therapeutic
resistance in pancreatic cancer (19). However, the roles of m6A
and METTL3 in CRC still need to be demonstrated, and this knowledge
may provide insight into the development of new therapeutic
strategies.
Accumulating evidence indicates that the activity of
suppressor of cytokine signaling (SOCS) family proteins correlates
with progression and poor prognosis in various cancers (20,21).
SOCS2, a member of the SOCS family, is well defined as a
transcriptional repressor in multiple proliferation-related
pathways and acts as a tumor suppressor in multiple malignancies.
Previous studies have reported downregulation of SOCS2 in breast
and ovarian cancers (22,23). In addition, SOCS2 has been reported
to inhibit metastasis in prostate cancer and hepatocellular
carcinoma (24,25). However, the molecular mechanisms
underlying the role of SOCS2 in tumorigenesis, including CRC
tumorigenesis, are still obscure.
Leucine-rich repeat-containing G protein-coupled
receptor 5 (LGR5) is characterized as a CSC biomarker and is
essential for the maintenance of stemness properties (26,27).
Previously, LGR5 upregulation was found in CRC and was found to be
positively correlated with the pathological grade and invasiveness
of CRC (28). Thus, LGR5 is
considered a promising new therapeutic target for CRC. Although
LGR5 has been revealed to be associated with the prognosis and
progression of CRC, the upstream regulation of LGR5 in CRC is
unclear.
Here, we report that METTL3 overexpression promotes
tumor cell proliferation in CRC. Mechanistically, METTL3 induces
SOCS2 mRNA instability via m6A modification. Aberrant
overexpression of LGR5 is caused by SOCS2 instability in CRC, and
this effect maintains the high stemness and robust cell
proliferation observed in CRC.
Materials and methods
Clinical sample collection
Fresh colon tumor tissues and matched adjacent
normal colon tissues (>5 cm from the tumor border) (n=24 pairs)
were collected from patients with CRC after surgical resection at
the Department of Gastroenterological Surgery, Sun Yat-Sen Memorial
Hospital, Sun Yat-Sen University, between June and August 2018.
Samples were immediately frozen in liquid nitrogen and stored at
−80°C until analysis. Tumor tissues and adjacent normal colon
tissues were confirmed by postoperative pathological diagnosis. All
patients recruited in the study were confirmed by pathological
diagnosis. The exclusion criteria were as follows: i) Patients with
a metastatic colon tumor originating in another organ; ii) CRC
patients who had already received chemotherapy and/or radiotherapy;
and iii) patients with CRC in situ or a benign tumor
confirmed by pathological diagnosis. The clinicopathological
features of the patients (sex, age, tumor size, pathological type
and TNM stage) were collected. All enrolled patients and their
respective guardians provided written consent, and the study
strictly adhered to the guidelines of the Institutional Review
Board Committee of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen
University (Guangzhou, Guangdong, China) (Approval no. 58, 2016
record for Ethics).
Cancer database analysis
CRC-related data with entries defined as colorectal
adenocarcinoma were downloaded from The Cancer Genome Atlas (TCGA;
http://www.cbioportal.org). Expression levels of
METTL3, SOCS2 and LGR5 in normal colon tissues were compared with
those in CRC tissues. To comprehensively identify the gene
expression profile in CRC, we utilized the Oncomine database, an
online public cancer database of DNA and RNA sequences, to collect
transcriptional expression data for METTL3, SOCS2 and LGR5 in CRC
using the ‘Gene summary view’ and ‘Dataset view’ (https://www.oncomine.org/resource/login.html).
Transcriptional expression of METTL3, SOCS2 and LGR5 in CRC samples
was compared with that in normal colorectal epithelium samples
using Student's t-test. A total of 12 datasets were found.
Statistically significant differences and fold changes were defined
as P<0.05 and ≥2, respectively.
Cytoscape literature mining was performed with the
search term ‘METTL3’ to query the whole NCBI database (https://www.ncbi.nlm.nih.gov/pubmed) by default
parameters. The software automatically retrieved all articles in
PubMed mentioning METTL3. The reported correlations of METTL3 with
other genes were assembled and visualized with Cytoscape (29).
Colon cancer cell line culture
SW480 cells (a colon cancer cell line) were obtained
from the American Type Culture Collection (ATCC, USA) and cultured
in the recommended medium [L-15 medium supplemented with 10% fetal
bovine serum (FBS), 100 µg/ml streptomycin, and 100 U/ml
penicillin]. Cells were maintained in a humidified incubator
without CO2 at 37°C.
CRISPR-Cas9-mediated knockout of
METTL3
CRISPR/Cas9 was applied to stably knock out the
METTL3 gene (NCBI GeneID 56339) to generate stable cell
lines. In brief, SW480 cells were transfected with the METTL3
CRISPR/Cas9 and HDR plasmids (sc-404029, Santa Cruz Biotechnology,
Inc.). Colonies were selected, and western blotting was used to
detect METTL3 protein expression. The clones with the expected
knockdown of METTL3 were further validated by qPCR and Sanger
sequencing.
Cell transfection
To transiently knock down METTL3 expression, siRNA
transfection was used. Two siRNAs targeting METTL3 (si-METTL3#1 and
si-METTL3#2) were synthesized by GenePharma Corporation. The siRNA
sequences were as follows: si-METTL3#1, 5′-GGUGACUGCUCUUUCCUUATT-3′
and 3′-UAAGGAAAGAGCAGUCACCTT-5′; si-METTL3#2,
5′-GCUACCUGGACGUCAGUAUTT-3′ and 5′-AUACUGACGUCCAGGUAGCTT-3′. The
negative control siRNA sequences were as follows: si-Ctrl,
5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′. The
siRNA targeting SOCS2 (si-SOCS2) was obtained from Santa Cruz
Biotechnology, Inc. The pCMV6 plasmid containing the full-length
SOCS2 sequence (FULL-SOCS2) was purchased from Origene
(RC203163).
Cells were cultured in 6-well plates
(1×105 cells per well) overnight prior to the
experiment. The following day, siRNA (80 µM) was transfected into
cells with Lipofectamine RNAiMAX (5 µl per well) (Thermo Fisher
Scientific, Inc.). For plasmid transfection, cells were seeded at a
density of 1×105 cells per well in 6-well plates.
Twenty-four hours later, Lipofectamine 2000 (5 µl per well; Thermo
Fisher Scientific, Inc.) was used to transfect cells with the
plasmid (2.5 µg per well) following the manufacturer's
instructions. Cells were harvested 48 h after transfection for RNA
and protein detection.
Cell proliferation assays
For the MTS assay, cells were seeded in 96-well
plates at a density of 1×103 cells per well and cultured
for 4 days. The absorbance at 490 nm was measured every 24 h after
incubation with 20 µl of MTS reagent (Promega, Corp.) for 2 h.
A cell counting assay was utilized to assess the
cell proliferation ability. Cells (1×104 cells per well)
were plated in a 12-well plate. On days 1, 2, 3, and 4 after
seeding, the plated cells were trypsinized and then stained by
trypan blue staining. The total number of cells was counted under
Olympus BX63 microscope (Olympus).
For the colony formation assay, cells
(1×104 cells per well) were seeded in 6-well plates. On
days 7, 10 and 14 after seeding, colonies in each well were fixed
with 4% paraformaldehyde and stained with 1% crystal violet. The
colony-forming units were recorded and photographed by Olympus BX63
microscope and image software (cellSens Dimension; Olympus).
Tumorsphere formation assay
To investigate the tumorsphere formation capacity,
cells were resuspended in tumorsphere-complete medium [DMEM/F12
supplemented with 20 ng/ml human epidermal growth factor (EGF), 10
ng/ml fibroblast growth factor-basic (bFGF), 5 µg/ml insulin, 0.4%
BSA, and 1X B27] and were then plated in an ultra-low attachment
6-well plate. The medium was supplemented every other day. The
spheroid structures were photographed and counted by Olympus BX63
microscope and image software (cellSens Dimension; Olympus) on days
7 and 14 after plating.
Total RNA isolation and qPCR
detection
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and treated with a
DNA-free DNase Treatment & Removal I Kit (Thermo Fisher
Scientific, Inc.) to remove any remaining DNA. A total of 500 ng of
RNA was used for reverse transcription, which was carried out by
using Reverse Transcriptase Buffer and SuperScript II Reverse
Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA
(25 ng) was subjected to qPCR for each gene of interest with SYBR
Green reagent (Bio-Rad Laboratories, Inc.). qPCR was performed
using a Bio-Rad CFX96 platform with the following settings: Initial
denaturation at 95°C for 30 sec, followed by 40 cycles of
denaturation at 95°C for 5 sec, annealing at 60°C for 30 sec, and
elongation at 72°C for 30 sec. Fluorescent signals were detected
after each cycle. Each sample was run in triplicate. The relative
mRNA expression levels were calculated using the 2−ΔΔCq
(cycle threshold) method (30) and
normalized to GAPDH. The primer sequences are listed in Table SI.
Western blot analysis
Cells were harvested and lysed with M-PER mammalian
protein extraction reagent (Thermo Fisher Scientific, Inc.).
Following protein quantification, proteins in lysed samples (30 µg
per well) were separated on a 10% acrylamide gel and were then
transferred to PVDF membranes (Millipore). Membranes were incubated
with the following primary antibodies overnight at 4°C: Anti-METTL3
(dilution 1:1,000, 15073-1-AP, Proteintech), anti-SOCS2 (dilution
1:1,000, 2779, Cell Signaling Technology, Inc.), anti-LGR5
(dilution 1:1,000, sc-135238, Santa Cruz Biotechnology, Inc.), and
anti-β-actin (dilution 1:1,000, sc-130656, Santa Cruz
Biotechnology, Inc.). Positive signals were detected using ECL
reagent (Bio-Rad Laboratories, Inc.) in a FluorChem FC2 Imaging
System (Alpha Innotech). ImageJ software version 1.51 (National
Institutes of Health, USA) was used to measure the band
intensities. β-actin was used as the internal reference.
m6A quantification
Total RNA extracted from cells was purified and
quantified. The isolated total RNA was subjected to mRNA
purification using a Dynabeads mRNA Purification Kit (Thermo Fisher
Scientific, Inc.) to deplete rRNA and noncoding RNAs. A total of
200 ng of mRNA from each sample was used, and m6A
quantification was carried out by using an EpiQuik m6A
Methylation Quantification kit (Colorimetric, Epigentek) according
to the manufacturer's instructions.
m6A-RNA
immunoprecipitation-qPCR (MeRIP-qPCR)
MeRIP-qPCR was performed as previously reported
(31). In brief, mRNA was isolated,
purified and chemically shredded into ~100-nt fragments using
Ambion fragmentation reagent (Thermo Fisher Scientific, Inc.). mRNA
fragments (200 ng) were denatured at 65°C for 5 min and incubated
with 20 µl of Magna ChIP Protein A+G Magnetic Beads (2923270,
Millipore) conjugated to 1 µg of anti-m6A polyclonal
antibody (ab208577, Abcam) or mouse control IgG (sc-2025, Santa
Cruz Biotechnology, Inc.) in 1X IPP buffer (15 mM NaCl, 10 mM
Tris-HCl and 0.1% NP-40). mRNA was incubated with
m6A-bound beads with rotation at 4°C for 3 h in IPP
buffer. The beads were washed twice with 1X IPP buffer, twice with
low-salt buffer (50 mM NaCl, 10 mM Tris-HCl and 0.1% NP-40), twice
with high-salt buffer (500 mM NaCl, 10 mM Tris-HCl and 0.1% NP-40)
and once with 1X IPP buffer. RNA was eluted from the beads with 50
µl of RLT buffer and purified through Qiagen RNeasy columns
(Qiagen) according to the manufacturer's recommendation. Total RNA
was finally eluted in 100 µl of RNase-free water. The relative
abundances of RNAs of interest were measured using qPCR and
normalized to the input level.
RNA stability
Cells (2×104 cells per well) were plated
in 12-well plates. After overnight incubation, cells were treated
with actinomycin D (ACD; A9415, Sigma-Aldrich; Merck KGaA) at a
final concentration of 10 µg/ml and incubated in a humidified
incubator without CO2 at 37°C. Total RNA was extracted
at different time points after actinomycin D treatment (0, 1, 2, 4
and 8 h). Following total RNA extraction and quantification, the
expression levels of specific genes of interest were quantified
using qPCR.
Demethylation treatment with
3-deazaadenosine (DAA)
The global methylation inhibitor DAA (D8296,
Sigma-Aldrich; Merck KGaA) was used to inhibit RNA methylation
(11,32). After seeding, cells were treated
with 10 µg/ml DAA for 48 h. Cells were then collected for mRNA and
protein analysis.
Luciferase assay
The LGR5 promoter region sequence (−2 kb~+100 b) was
obtained from National Center for Biotechnology Information (NCBI).
The LGR5 promoter was cloned using specific primers and was then
ligated into the pGL3 vector digested with XhoI and
SacI. The pGL3-LGR5 promoter and the FULL-SOCS2 plasmid were
cotransfected into SW480 cells. Twenty-four hours after
transfection, cells were collected with luciferase lysis buffer
(E3971, Promega Corp.). Luciferase Assay Reagent (E1483, Promega
Corp.) was used to measure luciferase activity after
cotransfection. β-Gal activity was measured as an internal
transfection control.
Statistical analysis
Each experiment was performed at least three
independent times. All experimental data are presented as the mean
± SEM values. Student's t-test or one-way ANOVA was conducted for
evaluating intergroup differences. The correlation of protein
expression and clinicopathological features was analyzed by
Fisher's exact test or Chi-square test. Linear regression analysis
was used to analyze the correlation between two genes. Statistical
analysis was performed using GraphPad Prism 5 (GraphPad Software).
P<0.05 was considered to indicate a statistically significant
difference.
Results
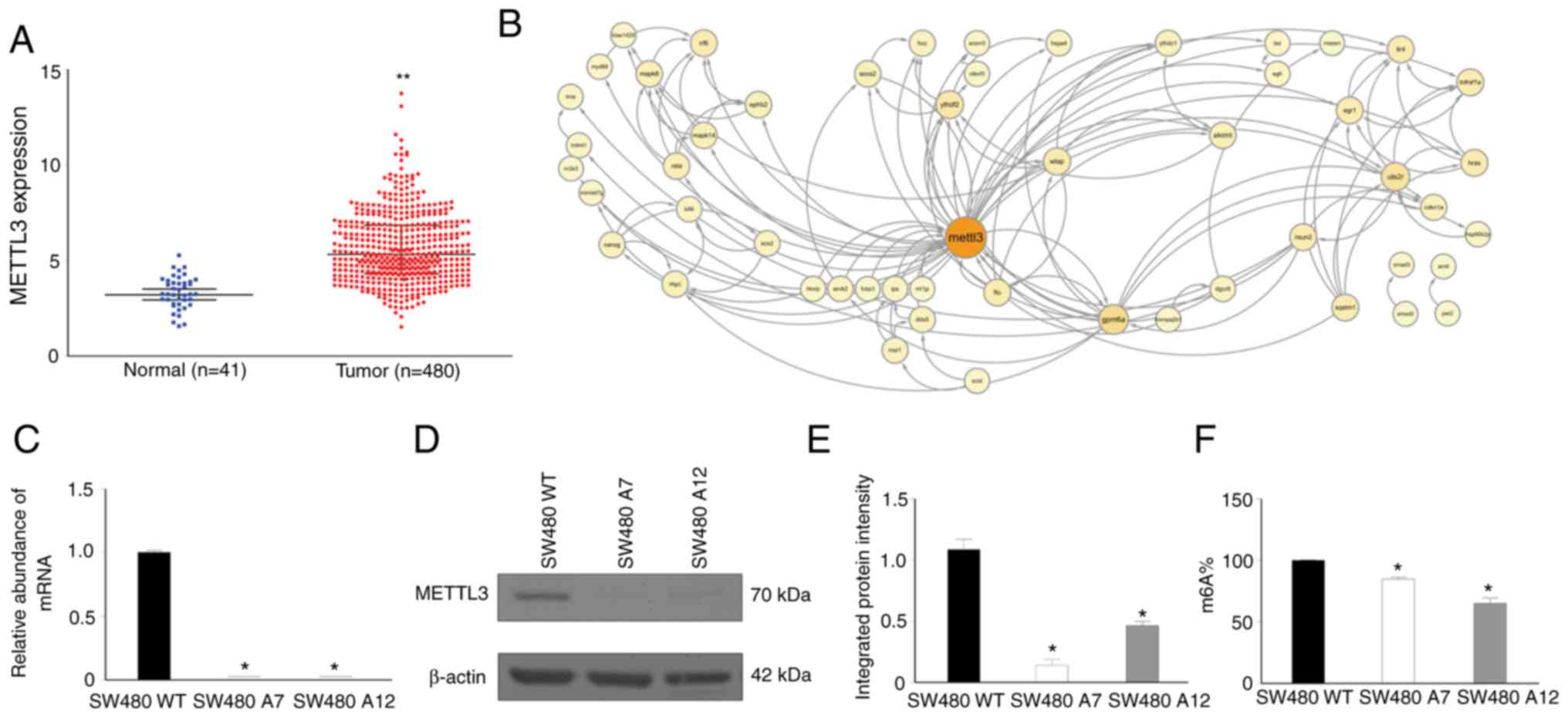
METTL3 expression is increased in
CRC
To determine the expression pattern of METTL3 in
CRC, we queried the TCGA database. A total of 480 colon cancer
samples and 41 normal colon tissues were found. Compared to normal
colon tissues, colon cancer samples showed an elevated METTL3 RNA
level (P<0.05; Fig. 1A).
To explore the potential role of METTL3 expression
in CRC tumorigenesis, we conducted literature mining via Cytoscape
network analysis and identified 52 candidate genes that may
interact with METTL3 (Fig. 1B).
Both METTL3 and METTL14 have been reported to act coordinately to
maintain m6A modification (7). Cytoscape analysis of METTL14 revealed
the same group of genes found for METTL3, suggesting that METTL3
and METTL14 were considered a single complex in the Cytoscape
analysis. Those candidates genes were further subjected to Gene
Ontology analysis (Table SIIA) via
the Database for Annotation, Visualization and Integrated Discovery
(DAVID) tool (https://david.ncifcrf.gov/). Our analysis revealed
that METTL3 is mainly involved in the following three bioprocesses:
Cell proliferation, inflammatory response and RNA processing. Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway analysis indicated
that these candidate genes are closely associated with multiple
diseases, including acute myeloid leukemia, CRC and prostate cancer
(Table SIIB). These results imply
a potential role for METTL3 in tumor progression and the regulation
of inflammation, cell proliferation and RNA processing.
Establishment of stable
METTL3-knockout (METTL3-KO) colon cancer cell lines
We used CRISPR-Cas9 to generate stable METTL3-KO
colon cancer cell lines in SW480 cells. Two lines of METTL3-KO
cells, SW480 A7 and SW480 A12, were finally obtained through
antibiotic selection, and the absence of METTL3 expression in these
cell lines was confirmed by qPCR and western blotting (Fig. 1C-E). No qPCR product was detected
using the specific primer sets covering the targeted region of
METTL3; in addition, the western blot results indicated the
knockout of the METTL3 protein in METTL3-KO SW480 cells. Moreover,
to verify METTL3 inhibition in METTL3-KO cells, global
m6A levels were measured using the EpiQuik
m6A Methylation Quantification kit. The overall
m6A level was evidently decreased in METTL3-KO SW480
cells when compared with the parental cells (Fig. 1F).
METTL3-KO elicits an inhibitory effect
on SW480 cell proliferation
Given the potential function of METTL3 in regulating
cell proliferation implied by the above bioinformatic analysis
results, we screened the differential expression profiles of
proliferation-related genes in METTL3-KO SW480 cells vs. the
control SW480 (SW480 WT) cells to determine whether downregulation
of METTL3 modulates cell proliferation in CRC. Ki67, LGR5, PCNA,
SOX2 and Cyclin B1 have been reported to be positively
correlated with cell proliferation (33–36).
All five of these genes were significantly downregulated in the
METTL3-KO SW480 cells. On the other hand, two antiproliferative
genes, SOCS2 and P21, were upregulated (Fig. 2A).
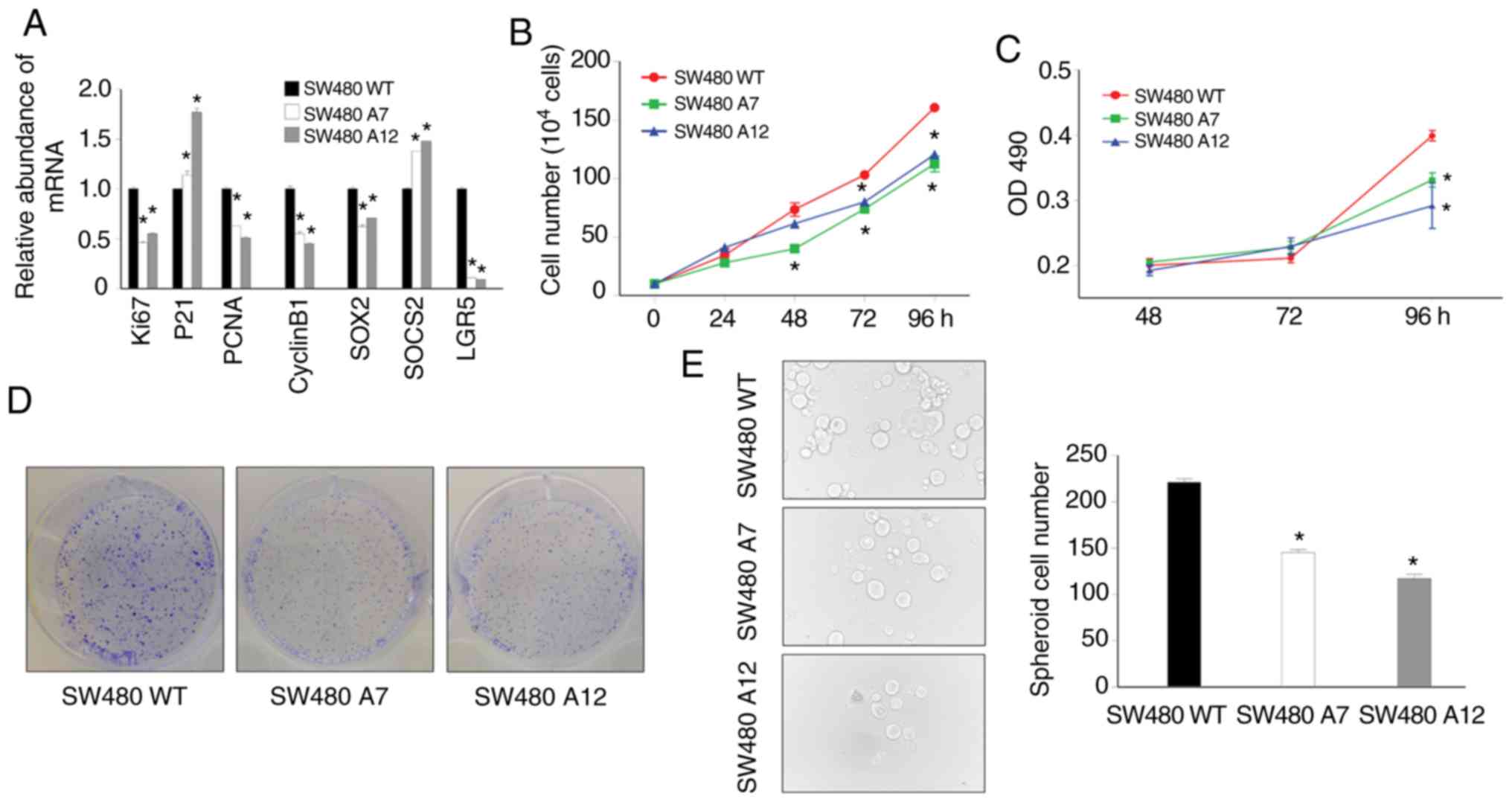 | Figure 2.Inhibition of cell proliferation in
METTL3-KO SW480 cells. (A) METTL3-KO SW480 cells showed
dysregulation of several proliferation-related genes (*P<0.05,
compared with the SW480 WT cells). (B) MTS assay results validated
the decreased proliferation of METTL3-KO cells (*P<0.05,
compared with the SW480 WT cells). (C) METTL3-KO cells showed a
reduced proliferation rate, as measured by cell counting
(*P<0.05, compared with the SW480 WT cells). (D) METTL3-KO cells
showed a lower colony formation rate than control cells at 7 days
after seeding. (E) Tumorigenic ability of METTL3-KO SW480 cells was
reduced (*P<0.05, compared with the SW480 WT cells). SOCS2,
suppressor of cytokine signaling 2; METTL3, methyltransferase like
3; LGR5, leucine-rich repeat-containing G protein-coupled receptor
5; SOX2, SRY-box transcription factor 2; PCNA, proliferating cell
nuclear antigen; KO, knockout; SW480 A7 and SW480 A12, METTL3-KO
SW480 cells. |
We then examined the effects of METTL3 knockout on
SW480 cell proliferation and stemness. Both METTL3-KO lines, SW480
A7 and SW480 A12, showed a decrease in cell proliferation in the
MTS assay (Fig. 2B). In addition,
the growth of METTL3-KO cells was markedly inhibited, as measured
by cell counting and colony formation assays (Fig. 2C and D). Moreover, we performed a
tumorsphere formation assay to observe stemness properties arising
from METTL3 knockout. Spheroid formation can be used to estimate
the percentage of CSCs present in a population of tumor cells.
Spheroid formation by METTL3-KO SW480 cell lines was significantly
inhibited, suggesting the loss of stemness of these cells (Fig. 2E). Collectively, these data indicate
that METTL3 may play an important role in regulating SW480 cell
self-renewal.
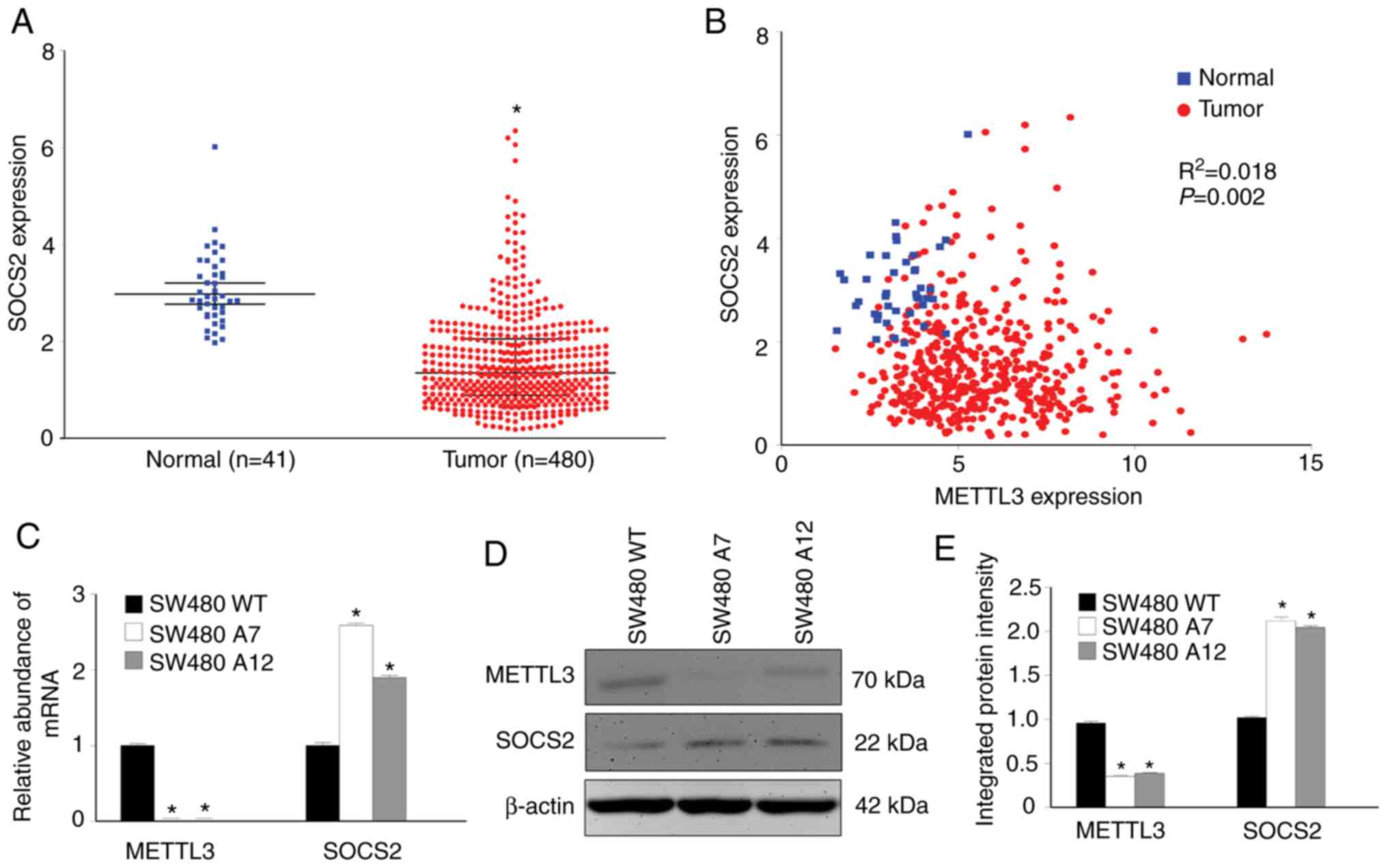
SOCS2 RNA levels are negatively
correlated with METTL3 expression levels in CRC
To identify the downstream regulatory effectors
controlling the inhibition of cell proliferation in METTL3-KO
cells, we sought to determine whether METTL3 regulates the
expression of SOCS2 to modulate the transcription of those
proliferation-related genes. A previous study indicated a potential
correlation between the expression levels of SOCS2 and METTL3 in
hepatocellular cancer (37). We
utilized the TCGA database to evaluate SOCS2 expression in CRC. In
the same set of TCGA samples used for METTL3 analysis, we found a
significant decrease in the SOCS2 RNA level in CRC (Fig. 3A). The correlation between the
expression levels of METTL3 and SOCS2 was studied by linear
regression analysis of data from the TCGA database. A significant
inverse association between the expression levels of METTL3 and
SOCS2 in CRC was identified (P<0.05; Fig. 3B), and the R2 coefficient
was small (0.018). We considered that the correlation between
METTL3 and SOCS2 in these TCGA data might not be strong but was
significant. The actual correlation still needs in-depth
investigation.
The correlation between the levels of SOCS2 and
METTL3 in CRC samples prompted us to investigate whether this
association holds in SW480 cells. We measured the SOCS2 expression
levels in METTL3-KO SW480 cells and compared them to those in
control SW480 cells. Consistent with the pattern in the TCGA data,
knockout of METTL3 was accompanied by SOCS2 upregulation (Fig. 3C-E). Our data thus imply that METTL3
may control SOCS2 expression in CRC.
METTL3 controls SOCS2 expression by
modulating methylation-mediated SOCS2 RNA degradation
To explore the underlying molecular mechanisms by
which METTL3 controls SOCS2 expression, we sought to determine
whether SOCS2 is a direct substrate of METTL3 for methylation. We
first verified whether METTL3 maintains the methylation status of
SOCS2 mRNA in SW480 cells. The MeRIP-qPCR results revealed a
significant decrease in SOCS2 RNA m6A methylation in
METTL3-KO SW480 cells compared with the control SW480 WT cells
(Fig. 4A). To evaluate the effect
of m6A modification on SOCS2 expression, we used the
global methylation inhibitor DAA to block RNA methylation and
quantified SOCS2 mRNA and protein expression in SW480 cells. The
abundance of SOCS2 mRNA was increased in the DAA-treated SW480
cells in a DAA dose-dependent manner (P<0.05; Fig. 4B). Consistent with the results from
METTL3-KO cells, DAA treatment increased the SOCS2 protein content
(P<0.05; Fig. 4C and D). Since
DAA globally inhibits all methyltransferase reactions, we used
MeRIP-qPCR to confirm its demethylation of SOCS2 mRNA. A
significant decrease in m6A-modified SOCS2 mRNA was
detected in SW480 cells after DAA intervention (P<0.05; Fig. 4E).
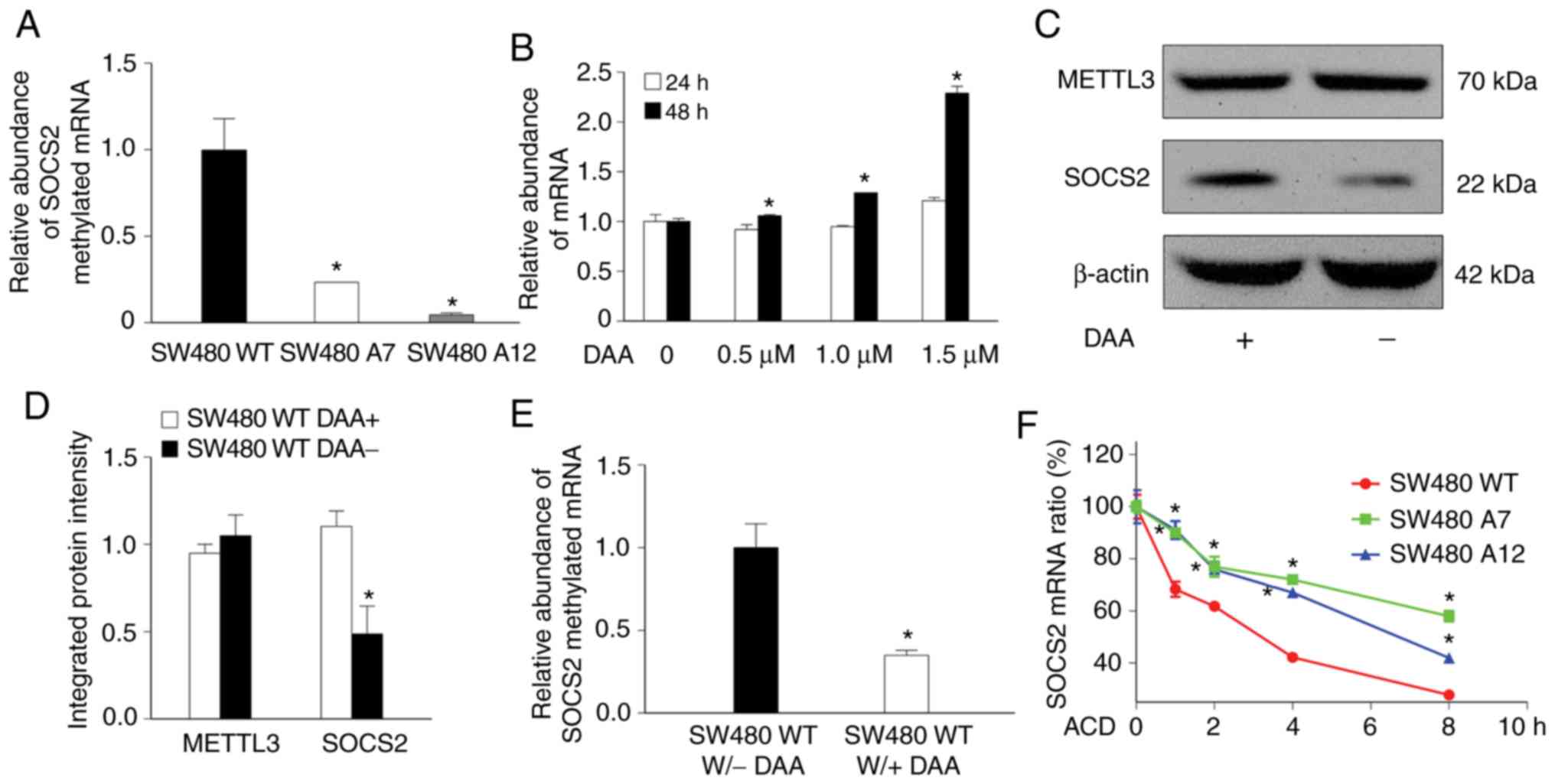 | Figure 4.Methylation status of SOCS2 mRNA in
SW480 cells. (A) Comparison of the SOCS2 mRNA methylation status
between SW480 WT and METTL3-KO SW480 cells via MeRIP-qPCR
(*P<0.05, compared with the SW480 WT cells). (B) Dose-dependent
increase in SOCS2 mRNA levels in SW480 cells in response to
demethylation treatment with DAA (*P<0.05, compared with
non-treated group). (C and D) SOCS2 protein expression was
upregulated after demethylation treatment with DAA (*P<0.05,
compared with the SW380 WT DAA-group). (E) DAA appreciably
decreased the m6A level in SOCS2 mRNA (*P<0.05,
compared with the SW380 WT DAA-group). (F) METTL3-KO increased
SOCS2 mRNA stability, showing a decreased mRNA decay rate
(*P<0.05, compared with the SW480 WT cells). SOCS2, suppressor
of cytokine signaling 2; METTL3, methyltransferase like 3; KO,
knockout; SW480 A7 and SW480 A12, METTL3-KO SW480 cells; DAA,
3-deazaadenosine. |
A previous study showed that RNA m6A
methylation exerts its effects via modulation of RNA stability
(8). To investigate this
possibility, we carried out an RNA stability assay. New RNA
synthesis was blocked with actinomycin D, and the decay rate of
SOCS2 mRNA was observed to be lower in METTL3-KO SW480 cells than
in control SW480 cells (P<0.05; Fig.
4F), suggesting that knockout of METTL3 significantly enhanced
SOCS2 mRNA stability. Collectively, our findings revealed that
METTL3 controls SOCS2 expression by modulating its mRNA
stability.
Changes in SOCS2 expression impact
LGR5 expression and disrupt colon cancer cell proliferation
To define whether SOCS2 is an effector regulating
colon cancer cell proliferation, we monitored the effects of
modulating SOCS2 expression on SW480 cell proliferation. Treatment
of cells with a SOCS2-specific siRNA and transfection with the
FULL-SOCS2 plasmid effectively downregulated and upregulated SOCS2
expression, respectively (Fig. 5A-C and
E-G). Accordingly, a significant increase and a marked decrease
in the cell number were detected in siRNA-treated SW480 cells and
SOCS2-overexpressing SW480 cells, respectively (Fig. 5D and H).
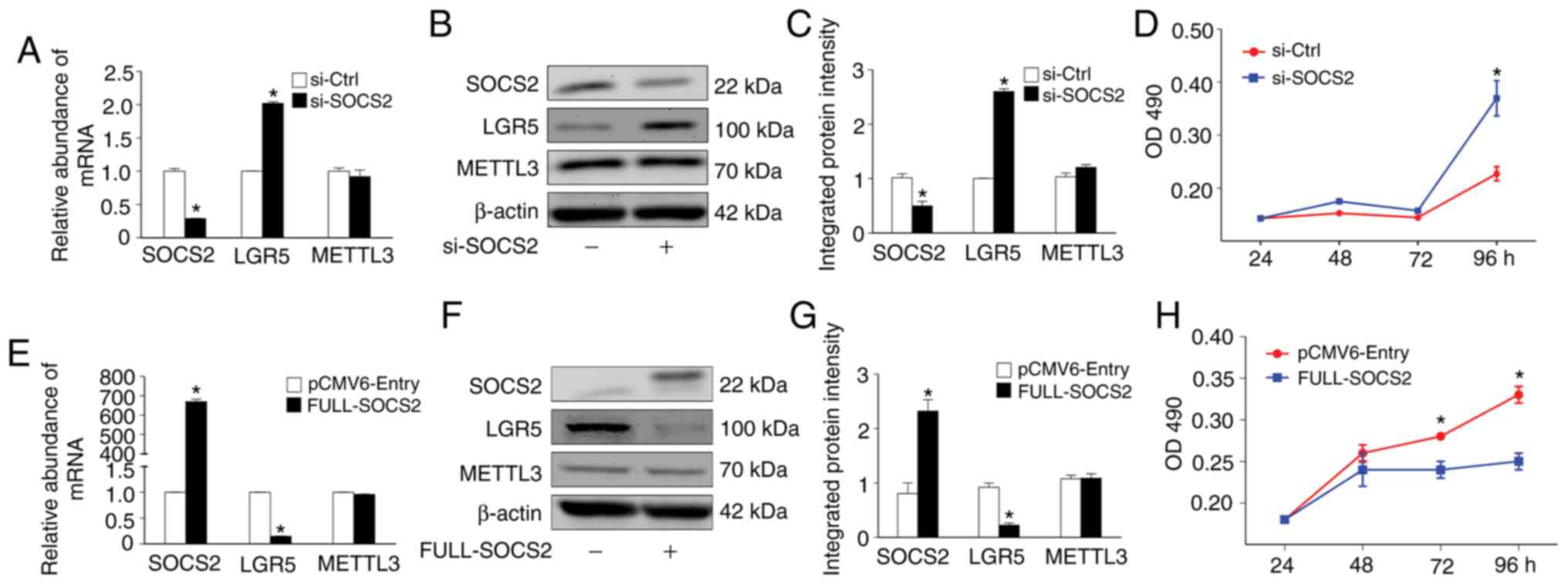 | Figure 5.Modulation of SOCS2 expression alters
the proliferation ability of SW480 cells. (A-C) siRNA (si-SOCS2)
was used to successfully knock down SOCS2 expression in SW480
cells. Knockdown of SOCS2 had minor impact on METTL3 expression but
significantly increased LGR5 expression in SW480 cells (*P<0.05,
compared with the si-Ctrl group). (D) Knockdown of SOCS2 enhanced
SW480 cell proliferation, as determined by an MTS assay
(*P<0.05, compared with the si-Ctrl group). (E-G) Transfection
of the pCMV6-SOCS2 plasmid upregulated SOCS2 expression and
decreased LGR5 expression in SW480 cells (*P<0.05, compared with
the pCMV6-Entry group). SOCS2 overexpression had minor impact on
METTL3 expression (P>0.05, compared with the pCMV6-Entry group).
(H) SOCS2 overexpression decreased SW480 cell proliferation, as
determined by an MTS assay (*P<0.05, compared with the
pCMV6-Entry group). SOCS2, suppressor of cytokine signaling 2;
METTL3, methyltransferase like 3; LGR5, leucine-rich
repeat-containing G protein-coupled receptor 5. |
Previous studies have demonstrated that SOCS2 acts
as a negative feedback regulator in multiple signaling pathways.
Dysregulation of SOCS2 crucially modulates the cell cycle
progression and apoptosis of cancer cells (38–40).
Recent research advances suggest that CSCs may be the origin of
colon tumorigenesis and tumor growth maintenance (41). As an important CSC marker, LGR5 may
enhance the stemness and chemoresistance development of tumor cells
(26,27,42).
Hence, we measured the abundance of LGR5 in SW480 cells with SOCS2
downregulation or overexpression. SOCS2 knockdown via siRNA
significantly elevated both the mRNA and protein expression levels
of LGR5 in the SW480 cells (P<0.05; Fig. 5A-C). Correspondingly, SOCS2
overexpression appreciably suppressed LGR5 expression (P<0.05;
Fig. 5E-G). Taken together, these
results suggest that SOCS2 may regulate colon cancer cell
proliferation via LGR5.
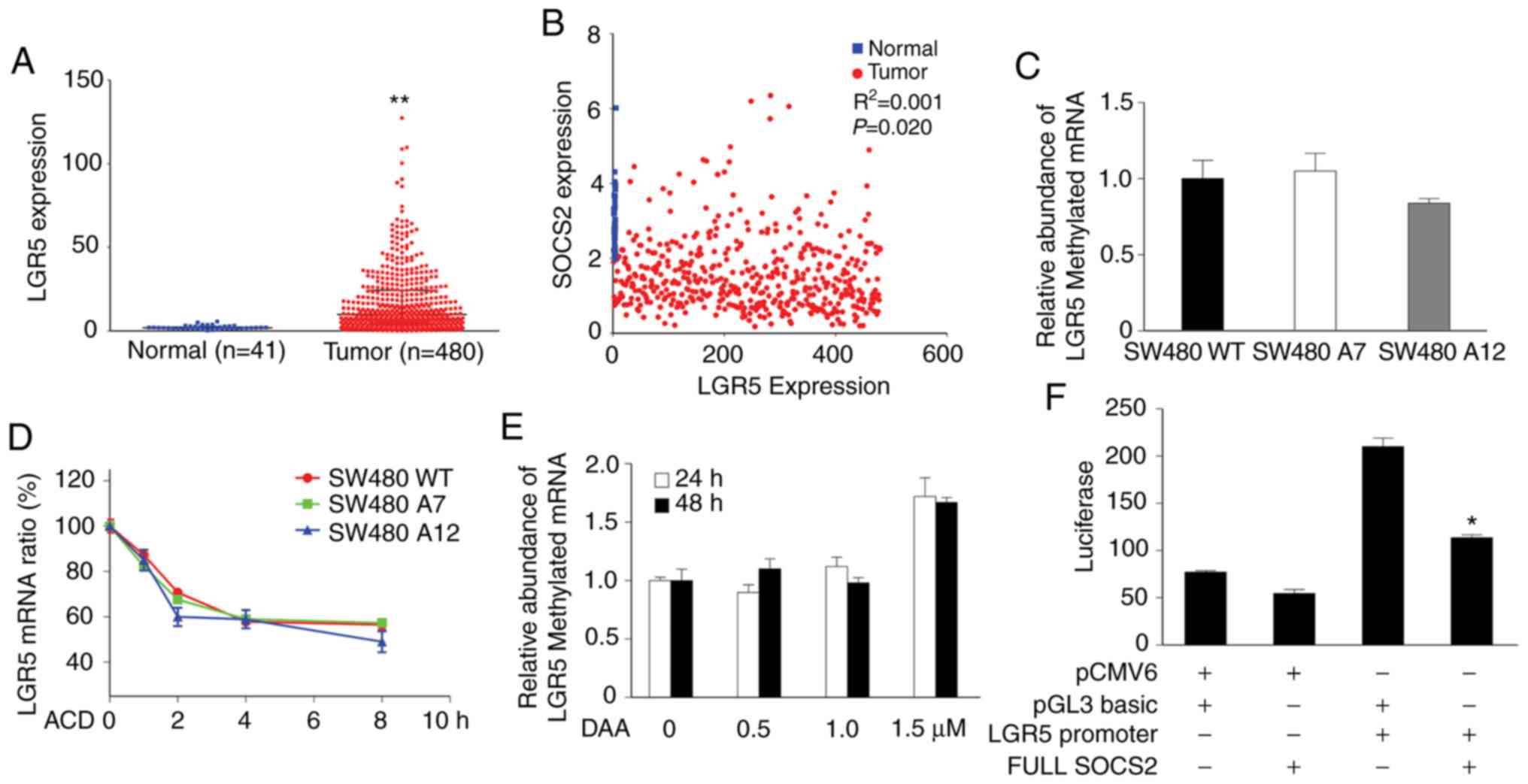
SOCS2 suppresses LGR5 promoter
activity
First, we explored the association of LGR5 and SOCS2
in data from the TCGA database. In the same set of TCGA samples
used in the present study, we found a distinct increase in the LGR5
RNA level in CRC (Fig. 6A).
Although the R2 coefficient was too small to be
considered to indicate a strong correlation, linear regression
analysis implied a potential negative correlation between the
expression levels of SOCS2 and LGR5 in CRC (P<0.05; Fig. 6B). This finding prompted us to
investigate the mechanism underlying the correlation between SOCS2
and LGR5. To exclude direct regulatory effects of METTL3 on LGR5
expression, MeRIP-qPCR was applied. The m6A methylation
level of LGR5 did not differ between METTL3-KO SW480 cells and
control SW480 WT cells (P>0.05; Fig.
6C), nor did METTL3 knockout impact the stability of LGR5 mRNA
in SW480 cells (P>0.05; Fig.
6D). Furthermore, DAA treatment had little impact on the
stability of LGR5 mRNA in SW480 cells (P>0.05; Fig. 6E). These data did not support a
direct modulatory effect of METTL3 on LGR5, indicating the
necessity of SOCS2 for the regulation of LGR5 by METTL3. SW480
cells were cotransfected with the FULL-SOCS2 plasmid or the empty
vector (as the control) and the luciferase reporter vector
containing the LGR5 promoter region, and luciferase activity was
then measured. Our luciferase activity assay results showed that
SOCS2 overexpression effectively repressed LGR5 promoter activity
in SW480 cells, suggesting a suppressive role of SOCS2 in
modulating LGR5 transcription (P<0.05; Fig. 6F).
Measurement of SOCS2 and METTL3
expression and their correlation in colon cancer tumorigenesis
To rule out the compensatory effect of other
molecules on SOCS2 and LGR5 expression in stable METTL3-KO SW480
cells, we used two separate siRNAs (si-METTL3#1 and si-METTL3#2) to
knockdown METTL3 expression in SW480 cells. Transient inhibition of
METTL3 via either siRNA resulted in significantly increased SOCS2
expression but significantly decreased LGR5 expression at both the
mRNA and protein levels (P<0.05; Fig. 7A-C).
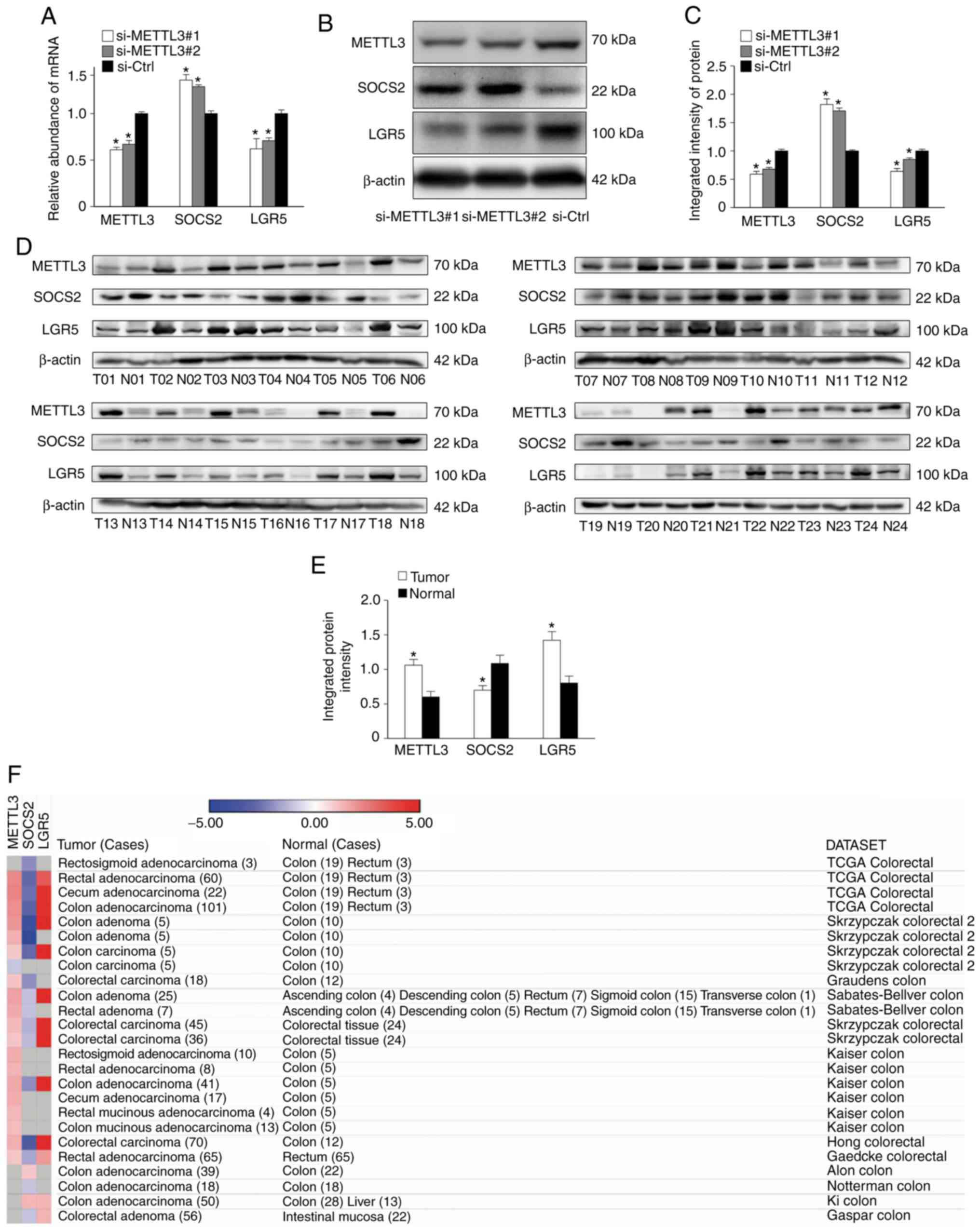 | Figure 7.Correlations among METTL3, SOCS2 and
LGR5 expression levels in CRC. (A-C) Knockdown of METTL3 using
siRNA (si-METTL3#1 and si-METTL3#2) significantly upregulated SOCS2
expression but downregulated LGR5 expression in SW480 cells
(*P<0.05, compared with the si-Ctrl group). (D and E) Western
blotting analysis of 24 paired tissues collected from CRC patients
showed high protein levels of METTL3 and LGR5 and a relatively low
protein level of SOCS2 in colon cancer (T, colon tumor samples; N,
adjacent normal colon tissues; *P<0.05, compared to Normal
tissues). (F) Visualization of expression profiles of METTL3, SOCS2
and LGR5 in CRC through the Oncomine database. METTL3 and LGR5 were
upregulated in CRC datasets. In contrast, SOCS2 was downregulated
in CRC datasets. CRC, colorectal cancer; SOCS2, suppressor of
cytokine signaling 2; METTL3, methyltransferase like 3; LGR5,
leucine-rich repeat-containing G protein-coupled receptor 5. |
Moreover, we collected 24 paired samples (i.e.,
tumor tissues and paired adjacent normal colon tissues) from
patients with CRC in the Department of Gastroenterology Surgery,
Sun Yat-Sen Memorial Hospital. The protein expression levels of
METTL3, SOCS2 and LGR5 were measured in the 24 paired CRC samples
and histologically normal adjacent tissues using western blotting.
The expression levels of METTL3 and LGR5 were increased in colon
tumor samples compared to the paired adjacent colon tissues (fold
change ≥1.5) in 19 (79.2%) and 20 (83.3%) cases, respectively. In
contrast, the level of SOCS2 was lower in the tumor samples (fold
change ≤0.5) in 18 (75.0%) cases (Fig.
7D and Table I). Additionally,
we analyzed the correlations between the expression levels of these
three genes and clinicopathological features (sex, age, TNM stage).
Upregulation of METTL3 or LGR5 was not correlated with sex, age,
tumor size, or TNM stage (P>0.05; Table I). Consistent with this result,
downregulation of SOCS2 was not associated with these clinical
features (P>0.05; Table I).
These results demonstrated that in general, the expression levels
of METTL3 and LGR5 are increased in CRC, whereas that of SOCS2 is
decreased.
 | Table I.Association between patient
characteristics and gene expression in 24 paired CRC cases. |
Table I.
Association between patient
characteristics and gene expression in 24 paired CRC cases.
|
|
| Expression of
METTL3a | Fisher's exact
test | Expression of
SOCS2a | Fisher's exact
test | Expression of
LGR5a | Fisher's exact
test |
|---|
|
|
|
|
|
|
|
|
|
|---|
|
| No. of patients n
(%) | Low | High | P-value | Low | High | P-value | Low | High | P-value |
|---|
| Total, n (%) | 24 | 5 (20.8) | 19 (79.2) |
| 18 (75.0) | 6 (25.0) |
| 4 (16.7) | 20 (83.3) |
|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
Male | 12 (50.0) | 2 | 10 | 0.64 | 8 | 4 | 0.64 | 2 | 10 | >0.99 |
|
Female | 12 (50.0) | 4 | 8 |
| 10 | 2 |
| 2 | 10 |
|
| Mean age
(years) | 61±11.64 |
|
|
|
|
|
|
|
|
|
|
≤60 | 10 (41.7) | 1 | 9 | 0.36 | 7 | 3 | 0.67 | 3 | 7 | 0.27 |
|
>60 | 14 (58.3) | 4 | 10 |
| 11 | 3 |
| 1 | 13 |
|
| Tumor size
(cm) |
|
|
|
|
|
|
|
|
|
|
| ≤5 | 10 (41.7) | 3 | 7 | 0.61 | 8 | 2 | >0.99 | 2 | 8 | >0.99 |
|
>5 | 14 (58.3) | 2 | 12 |
| 10 | 4 |
| 2 | 12 |
|
| Pathologic
type |
|
|
|
|
|
|
|
|
|
|
|
Adenocarcinoma | 24 | 5 | 19 |
| 18 | 6 |
| 4 | 20 |
|
|
Other | 0 |
|
|
|
|
|
|
|
|
|
| pT grade |
|
|
|
|
|
|
|
|
|
|
| Ta,
Tis, T1 | 2 (8.3) | 0 | 2 | 1.00 | 2 | 0 | >0.99 | 1 | 1 | 0.31 |
|
T2-T4 | 22 (91.7) | 5 | 17 |
| 16 | 6 |
| 3 | 19 |
|
| pN grade |
|
|
|
|
|
|
|
|
|
|
| N0 | 13 (54.2) | 1 | 12 | 0.14 | 10 | 3 | >0.99 | 1 | 12 | 0.30 |
| N1,
N2 | 11 (45.8) | 4 | 7 |
| 8 | 3 |
| 3 | 8 |
|
| pM grade |
|
|
|
|
|
|
|
|
|
|
| M0 | 18 (75.0) | 4 | 14 | 1.00 | 13 | 5 | 0.36 | 2 | 16 | 0.25 |
| M1 | 6 (25.0) | 1 | 5 |
| 3 | 3 |
| 2 | 4 |
|
| TNM staging |
|
|
|
|
|
|
|
|
|
|
| I | 5 (20.8) | 0 | 5 | 0.61b | 3 | 2 | 0.77b | 1 | 4 | 0.33b |
| II | 7 (29.2) | 2 | 5 |
| 5 | 2 |
| 0 | 7 |
|
|
III | 7 (29.2) | 2 | 5 |
| 6 | 1 |
| 1 | 6 |
|
| IV | 5 (20.8) | 1 | 4 |
| 4 | 1 |
| 2 | 3 |
|
To further evaluate the clinical value of our
findings, we searched the expression profiles of METTL3, SOCS2 and
LGR5 in CRC through the Oncomine database (43–52).
Regarding METTL3, analysis confirmed that METTL3 was upregulated in
CRC in 13/20 datasets (P<0.05; Fig.
7F and Table SIII). In
contrast, analysis revealed that SOCS2 was downregulated in CRC in
13/19 datasets (P<0.05; Fig. 7
and Table SIII). Accordingly, LGR5
expression was significantly increased in all 13 CRC datasets
(P<0.05; Fig. 7F and Table SIII).
Discussion
The present study indicates that methyltransferase
like 3 (METTL3) is generally upregulated in colorectal cancer
(CRC), contributing to the maintenance of m6A
modification in cancer cells. As a major m6A writer,
METTL3 accelerates suppressor of cytokine signaling 2 (SOCS2) mRNA
decay to maintain a high leucine-rich repeat-containing G
protein-coupled receptor 5 (LGR5) expression level in colon cancer
cells, resulting in enhanced tumorigenicity. Thus, our results
provide additional evidence that aberrant METTL3 expression may be
involved in tumorigenesis. As the most prevalent internal
modification of RNA, m6A modification regulates many
biological processes of RNA, including decay, splicing, translation
and transport (53). Maintenance of
m6A RNA methylation is crucial for embryonic stem cell
pluripotency (54,55). Emerging evidence indicates that the
cellular status of m6A modification may impact the
pathological processes of various types of cancers, including acute
myeloid leukemia, glioblastoma, lung cancer and liver cancer
(56). METTL3 is often upregulated
in malignancies and elicits potentially oncogenic effects by
epigenetically silencing the expression of specific genes, such as
ADAM19 and SOCS2 (15,37).
Previous studies demonstrated that the expression level of METTL3
can critically alter the cell cycle and apoptosis processes in
hepatic cancer and acute myeloid leukemia (37,57).
Knockdown of METTL3 increases chemosensitivity and radiosensitivity
in pancreatic cancer (19).
Having confirmed an elevated expression level of
METTL3 in CRC tissues from the TCGA database, we generated
METTL3-KO colon cancer (SW480) cell lines. Our m6A
quantification analysis results confirmed that CRISPR/Cas9-mediated
METTL3 knockout resulted in inhibition of both METTL3 expression
and m6A modification in the cells. Functionally,
knockout of METTL3 significantly reduced the cell proliferation
ability. METTL3 acts as a key m6A methyltransferase and
thus may promote cell proliferation via the installation of
m6A on key genes critical to cell growth (58–60).
Indeed, our data suggest that elevated expression of METTL3
accelerates SOCS2 mRNA decay to maintain a high level of LGR5 and
thus promotes the proliferation of colon cancer cells.
SOCS2, a member of the SOCS family, regulates
multiple cytokine-induced intracellular signaling pathways
essential to numerous biological processes, including immune
responses (61,62). For example, SOCS2 can act as a
downstream factor of the JAK/STAT pathway, regulating signaling
activity via negative feedback (63). Accumulating evidence suggests a
carcinostatic effect of SOCS2 in numerous cancers. Disruption of
SOCS2 promotes colon tumorigenesis in Apc (Min/+) mice (64). On the other hand, overexpression of
SOCS2 has an antiproliferative effect on Caco-2 colon cancer cells
(65). A meta-analysis integrating
more than 600 CRC patients and normal samples from different
datasets revealed that SOCS2 expression is markedly reduced in CRC
and may be a novel diagnostic biomarker for CRC (66). Indeed, the JAK/STAT signaling
pathway is often dysregulated in CRC (67). Our TCGA database analysis results
confirmed the downregulation of SOCS2 in CRC. Furthermore, our data
supported the hypothesis that SOCS2 may play an antioncogenic role
in colon malignancies. We observed a negative association between
SOCS2 expression and the cell proliferation ability in colon cancer
cells. Knockdown of SOCS2 increased the proliferation of colon
cancer cells, while overexpression of SOCS2 effectively inhibited
cell proliferation.
The molecular mechanisms underlying the
dysregulation of SOCS2 and SOCS2-mediated cell proliferation in CRC
are still unclear. Our data revealed a negative correlation between
METTL3 and SOCS2 in clinical CRC tissues and colon cancer cells.
Moreover, knockdown of METTL3 in colon cancer cells increased the
expression of SOCS2. Given that METTL3 is the predominant
m6A RNA methyltransferase and that mRNA stability is
confirmed as a cellular biological processes regulated by
m6A modification, we speculated that METTL3 may control
SOCS2 expression through modulation of SOCS2 RNA stability. The
results of our MeRIP-qPCR and RNA decay experiments revealed that
METTL3 may directly install m6A on SOCS2 mRNA and
decrease its stability. Inhibition of global demethylation with
DAA, a global methylation inhibitor, further confirmed the effect
of RNA methylation on SOCS2 expression. The effect of DAA on SOCS2
expression was dose-dependent and showed a positive correlation
between m6A demethylation and SOCS2 expression.
One pathological hallmark of cancer cells is their
potential for uncontrolled self-renewal (68). Targeting biomarkers of significance
in self-renewal may be an effective strategy to reverse
tumorigenesis. LGR5, which can maintain continuous self-renewal of
the intestinal epithelium, is considered a cancer stem cell (CSC)
marker in CRC (69). Cancer cells
with LGR5 expression in CRC tissues possess an enhanced
self-renewal capacity and have been referred to as colon CSCs
(26,27). A meta-analysis revealed that tumor
tissues from patients with CRC often show upregulated LGR5
expression and that an increased level of LGR5 could be a
prognostic factor for CRC (70).
Consistent with these results, the results of our TCGA database
analysis showed a higher LGR5 expression level in CRC tissues than
on their normal counterparts; this finding was further confirmed by
our Oncomine database analysis in a larger clinical cohort.
Moreover, we collected clinical CRC tissue samples from our
hospital to assess the protein expression profiles of METTL3, SOCS2
and LGR5. METTL3 and LGR5 were highly expressed in our CRC samples.
Although SOCS2 showed highly heterogeneous expression, the
statistical analysis results supported the downregulation of SOCS2
in our CRC samples. Accordingly, the decreased SOCS2 expression
level was negatively correlated with the elevated LGR5 expression
level and increased tumor spheroid formation rate in colon cancer
cell lines, consistent with a previous meta-analysis (55). Alteration of either METTL3 or SOCS2
expression can cause reciprocal alterations in the LGR5 expression
level in SW480 cells. Interestingly, METTL3 does not appear to
directly regulate LGR5 mRNA stability through m6A
methylation but instead appears to maintain LGR5 expression through
suppression of SOCS2. However, several limitations should be
resolved to fully reveal the precise mechanisms of METTL3
facilitating CRC tumorigenicity. Notably, the expression of METTL3,
SOCS2 and LGR5 in our CRC samples was not significantly correlated
with the clinicopathological features of the patients (sex, age,
tumor size, pathological type and TNM stage). This statistically
insignificant correlation may be due to the small enrolled sample
size. Moreover, the present study did not measure the gene
expression of METTL3, SOCS2 and LGR5 in CRC samples. Both a
comprehensive study with a larger sample size and a multicenter
clinical study would be conducted to fully evaluate the therapeutic
potential of METTL3 for CRC.
In conclusion, our study revealed elevated METTL3
expression in CRC. Upregulation of METTL3 was associated with
decreased expression of SOCS2 and promoted tumor cell proliferation
via induction of LGR5. To our knowledge, our finding provides the
first characterization of the underlying mechanism of
METTL3/m6A-mediated posttranscriptional modification in
CRC tumorigenesis, suggesting the therapeutic potential of
targeting this axis in CRC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from the
Nebraska Cancer and Smoking Disease Research Program (LB595) and
the National Natural Science Foundation of China (nos. 81370475 and
81970464). The content is solely the responsibility of the authors
and does not necessarily represent the official views of the
Nebraska Cancer and Smoking Disease Research Program or the
National Natural Science Foundation of China.
Availability of data and materials
All data generated or analyzed in the present study
(and its supplementary information files) are fully available
without restrictions.
Authors' contributions
JX, QC, TY and XC conceived the project. JX, TC,
KT, AG and RL carried out the experiments. JX, AG, QC, TY and XC
analyzed and interpreted the data. JX, NWM and XC wrote the study.
NWM and RL participated in the bioinformatics research. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
All enrolled patients and their respective
guardians provided written consent prior to the use of clinical
samples and pathological features for research purposes. The
overall protocol strictly adhered to the guidelines of the
Institutional Review Board Committee of Sun Yat-Sen Memorial
Hospital, Sun Yat-Sen University (approval no. 58, 2016 record for
Ethics).
Patient consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to
declare.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
m6A
|
N6-methyladenosine
|
|
METTL3
|
methyltransferase like 3
|
|
METTL14
|
methyltransferase like 14
|
|
WTAP
|
Wilms tumor 1-associated protein
|
|
FTO
|
fat mass and obesity-associated
protein
|
|
ALKBH5
|
RNA demethylase alkB homolog 5
|
|
YTH
|
YT521-B homology
|
|
CSC
|
cancer stem cell
|
|
SOCS
|
suppressor of cytokine signaling
|
|
LGR5
|
leucine-rich repeat-containing G
protein-coupled receptor 5
|
|
TCGA
|
The Cancer Genome Atlas
|
|
MeRIP-qPCR
|
m6A-RNA
immunoprecipitation-qPCR
|
|
ACD
|
actinomycin D
|
|
DAA
|
3-deazaadenosine
|
|
METTL3-KO
|
METTL3-knockout
|
References
|
1
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prasetyanti PR, van Hooff SR, van
Herwaarden T, de Vries N, Kalloe K, Rodermond H, van Leersum R, de
Jong JH, Franitza M, Nürnberg P, et al: Capturing colorectal cancer
inter-tumor heterogeneity in patient-derived xenograft (PDX)
models. Int J Cancer. 144:366–371. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mahasneh A, Al-Shaheri F and Jamal E:
Molecular biomarkers for an early diagnosis, effective treatment
and prognosis of colorectal cancer: Current updates. Exp Mol
Pathol. 102:475–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sepulveda AR, Hamilton SR, Allegra CJ,
Grody W, Cushman-Vokoun AM, Funkhouser WK, Kopetz SE, Lieu C,
Lindor NM, Minsky BD, et al: Molecular biomarkers for the
evaluation of colorectal cancer: Guideline from the American
society for clinical pathology, college of American Pathologists,
Association for Molecular Pathology, and the American Society of
Clinical Oncology. J Clin Oncol. 35:1453–1486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang S, Sun C, Li J, Zhang E, Ma Z, Xu W,
Li H, Qiu M, Xu Y, Xia W, et al: Roles of RNA methylation by means
of N6-methyladenosine (m6A) in human cancers.
Cancer Lett. 408:112–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han
D, Fu Y, Parisien M, Dai Q, Jia G, et al:
N6-methyladenosine-dependent regulation of messenger RNA stability.
Nature. 505:117–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng G, Dahl JA, Niu Y, Fedorcsak P,
Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, et al: ALKBH5
is a mammalian RNA demethylase that impacts RNA metabolism and
mouse fertility. Mol Cell. 49:18–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao X, Yang Y, Sun BF, Shi Y, Yang X,
Xiao W, Hao YJ, Ping XL, Chen YS, Wang WJ, et al: FTO-dependent
demethylation of N6-methyladenosine regulates mRNA splicing and is
required for adipogenesis. Cell Res. 24:1403–1419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fustin JM, Doi M, Yamaguchi Y, Hida H,
Nishimura S, Yoshida M, Isagawa T, Morioka MS, Kakeya H, Manabe I,
et al: RNA-methylation-dependent RNA processing controls the speed
of the circadian clock. Cell. 155:793–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Li Y, Toth JI, Petroski MD, Zhang
Z and Zhao JC: N6-methyladenosine modification destabilizes
developmental regulators in embryonic stem cells. Nat Cell Biol.
16:191–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang S, Zhao BS, Zhou A, Lin K, Zheng S,
Lu Z, Chen Y, Sulman EP, Xie K, Bögler O, et al: m6A
demethylase ALKBH5 maintains tumorigenicity of glioblastoma
stem-like cells by sustaining FOXM1 expression and cell
proliferation program. Cancer Cell. 31:591–606.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Weng H, Su R, Weng X, Zuo Z, Li C,
Huang H, Nachtergaele S, Dong L, Hu C, et al: FTO plays an
oncogenic role in acute myeloid leukemia as a
N6-methyladenosine RNA demethylase. Cancer Cell.
31:127–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun
G, Lu Z, Huang Y, Yang CG, et al: m6A RNA methylation
regulates the self-renewal and tumorigenesis of glioblastoma stem
cells. Cell Rep. 18:2622–2634. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin S, Choe J, Du P, Triboulet R and
Gregory RI: The m(6)A methyltransferase METTL3 promotes translation
in human cancer cells. Mol Cell. 62:335–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Chen C, Ding Q, Zhao Y, Wang Z,
Chen J, Jiang Z, Zhang Y, Xu G, Zhang J, et al: METTL3-mediated
m6A modification of HDGF mRNA promotes gastric cancer
progression and has prognostic significance. Gut. 69:1193–1205.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yue B, Song C, Yang L, Cui R, Cheng X,
Zhang Z and Zhao G: METTL3-mediated N6-methyladenosine modification
is critical for epithelial-mesenchymal transition and metastasis of
gastric cancer. Mol Cancer. 18:1422019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taketo K, Konno M, Asai A, Koseki J,
Toratani M, Satoh T, Doki Y, Mori M, Ishii H and Ogawa K: The
epitranscriptome m6A writer METTL3 promotes chemo- and
radioresistance in pancreatic cancer cells. Int J Oncol.
52:621–629. 2018.PubMed/NCBI
|
|
20
|
Wu M, Song D, Li H, Yang Y, Ma X, Deng S,
Ren C and Shu X: Negative regulators of STAT3 signaling pathway in
cancers. Cancer Manag Res. 11:4957–4969. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang M, Zhang WW, Liu P, Yu W, Liu T and
Yu J: Dysregulation of SOCS-mediated negative feedback of cytokine
signaling in carcinogenesis and its significance in cancer
treatment. Front Immunol. 8:702017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sutherland KD, Lindeman GJ, Choong DY,
Wittlin S, Brentzell L, Phillips W, Campbell IG and Visvader JE:
Differential hypermethylation of SOCS genes in ovarian and breast
carcinomas. Oncogene. 23:7726–7733. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Slattery ML, Lundgreen A, Hines LM,
Torres-Mejia G, Wolff RK, Stern MC and John EM: Genetic variation
in the JAK/STAT/SOCS signaling pathway influences breast
cancer-specific mortality through interaction with cigarette
smoking and use of aspirin/NSAIDs: The Breast Cancer Health
Disparities Study. Breast Cancer Res Treat. 147:145–158. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Das R, Gregory PA, Fernandes RC, Denis I,
Wang Q, Townley SL, Zhao SG, Hanson AR, Pickering MA, Armstrong HK,
et al: MicroRNA-194 promotes prostate cancer metastasis by
inhibiting SOCS2. Cancer Res. 77:1021–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vitali C, Bassani C, Chiodoni C, Fellini
E, Guarnotta C, Miotti S, Sangaletti S, Fuligni F, De Cecco L,
Piccaluga PP, et al: SOCS2 controls proliferation and stemness of
hematopoietic cells under stress conditions and its deregulation
marks unfavorable acute leukemias. Cancer Res. 75:2387–2399. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Sousa e Melo F, Kurtova AV, Harnoss JM,
Kljavin N, Hoeck JD, Hung J, Anderson JE, Storm EE, Modrusan Z,
Koeppen H, et al: A distinct role for Lgr5+ stem cells
in primary and metastatic colon cancer. Nature. 543:676–680. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimokawa M, Ohta Y, Nishikori S, Matano
M, Takano A, Fujii M, Date S, Sugimoto S, Kanai T and Sato T:
Visualization and targeting of LGR5+ human colon cancer
stem cells. Nature. 545:187–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stanisavljević L, Myklebust MP, Leh S and
Dahl O: LGR5 and CD133 as prognostic and predictive markers for
fluoropyrimidine-based adjuvant chemotherapy in colorectal cancer.
Acta Oncol. 55:1425–1433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li HB, Tong J, Zhu S, Batista PJ, Duffy
EE, Zhao J, Bailis W, Cao G, Kroehling L, Chen Y, et al:
m6A mRNA methylation controls T cell homeostasis by
targeting the IL-7/STAT5/SOCS pathways. Nature. 548:338–342. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karoopongse E, Yeung C, Byon J,
Ramakrishnan A, Holman ZJ, Jiang PY, Yu Q, Deeg HJ and Marcondes
AM: The KDM2B-let-7b-EZH2 axis in myelodysplastic syndromes as a
target for combined epigenetic therapy. PLoS One. 9:e1078172014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jing N, Huang T, Guo H, Yang J, Li M, Chen
Z and Zhang Y: LncRNA CASC15 promotes colon cancer cell
proliferation and metastasis by regulating the
miR4310/LGR5/Wnt/β-catenin signaling pathway. Mol Med Rep.
18:2269–2276. 2018.PubMed/NCBI
|
|
34
|
Gopalan V, Ebrahimi F, Islam F, Vider J,
Qallandar OB, Pillai S, Lu CT and Lam AK: Tumour suppressor
properties of miR-15a and its regulatory effects on BCL2 and SOX2
proteins in colorectal carcinomas. Exp Cell Res. 370:245–253. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alcantara-Flores E, Brechu-Franco AE,
Garcia-Lopez P, Rocha-Zavaleta L, Lopez-Marure R and
Martinez-Vazquez M: Argentatin B inhibits proliferation of prostate
and colon cancer cells by inducing cell senescence. Molecules.
20:21125–21137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gong S, Xu D, Zhu J, Zou F and Peng R:
Efficacy of the MEK inhibitor cobimetinib and its potential
application to colorectal cancer cells. Cell Physiol Biochem.
47:680–693. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen M, Wei L, Law CT, Tsang FH, Shen J,
Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, et al: RNA
N6-methyladenosine methyltransferase-like 3 promotes liver cancer
progression through YTHDF2-dependent posttranscriptional silencing
of SOCS2. Hepatology. 67:2254–2270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao X, Zhang W and Ji W: miR-196b is a
prognostic factor of human laryngeal squamous cell carcinoma and
promotes tumor progression by targeting SOCS2. Biochem Biophys Res
Commun. 501:584–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Y, Zhang Z, Wang N, Chen J, Zhang X,
Guo M, John ZL and Wang Q: Suppressor of cytokine signalling-2
limits IGF1R-mediated regulation of epithelial-mesenchymal
transition in lung adenocarcinoma. Cell Death Dis. 9:4292018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chhabra Y, Wong HY, Nikolajsen LF,
Steinocher H, Papadopulos A, Tunny KA, Meunier FA, Smith AG,
Kragelund BB, Brooks AJ, et al: A growth hormone receptor SNP
promotes lung cancer by impairment of SOCS2-mediated degradation.
Oncogene. 37:489–501. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hatano Y, Fukuda S, Hisamatsu K, Hirata A,
Hara A and Tomita H: Multifaceted Interpretation of Colon Cancer
Stem Cells. Int J Mol Sci. 18:14462017. View Article : Google Scholar
|
|
42
|
Chen X, Wei B, Han X, Zheng Z, Huang J,
Liu J, Huang Y and Wei H: LGR5 is required for the maintenance of
spheroid-derived colon cancer stem cells. Int J Mol Med. 34:35–42.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Skrzypczak M, Goryca K, Rubel T, Paziewska
A, Mikula M, Jarosz D, Pachlewski J, Oledzki J and Ostrowski J:
Modeling oncogenic signaling in colon tumors by multidirectional
analyses of microarray data directed for maximization of analytical
reliability. PLoS One. 5:e130912010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Graudens E, Boulanger V, Mollard C,
Mariage-Samson R, Barlet X, Gremy G, Couillault C, Lajemi M,
Piatier-Tonneau D, Zaborski P, et al: Deciphering cellular states
of innate tumor drug responses. Genome Biol. 7:R192006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sabates-Bellver J, Van der Flier LG, de
Palo M, Cattaneo E, Maake C, Rehrauer H, Laczko E, Kurowski MA,
Bujnicki JM, Menigatti M, et al: Transcriptome profile of human
colorectal adenomas. Mol Cancer Res. 5:1263–1275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kaiser S, Park YK, Franklin JL, Halberg
RB, Yu M, Jessen WJ, Freudenberg J, Chen X, Haigis K, Jegga AG, et
al: Transcriptional recapitulation and subversion of embryonic
colon development by mouse colon tumor models and human colon
cancer. Genome Biol. 8:R1312007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hong Y, Downey T, Eu KW, Koh PK and Cheah
PY: A ‘metastasis-prone’ signature for early-stage mismatch-repair
proficient sporadic colorectal cancer patients and its implications
for possible therapeutics. Clin Exp Metastasis. 27:83–90. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gaedcke J, Grade M, Jung K, Camps J, Jo P,
Emons G, Gehoff A, Sax U, Schirmer M, Becker H, et al: Mutated KRAS
results in overexpression of DUSP4, a MAP-kinase phosphatase, and
SMYD3, a histone methyltransferase, in rectal carcinomas. Genes
Chromosomes Cancer. 49:1024–1034. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alon U, Barkai N, Notterman DA, Gish K,
Ybarra S, Mack D and Levine AJ: Broad patterns of gene expression
revealed by clustering analysis of tumor and normal colon tissues
probed by oligonucleotide arrays. Proc Natl Acad Sci USA.
96:6745–6750. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Notterman DA, Alon U, Sierk AJ and Levine
AJ: Transcriptional gene expression profiles of colorectal adenoma,
adenocarcinoma, and normal tissue examined by oligonucleotide
arrays. Cancer Res. 61:3124–3130. 2001.PubMed/NCBI
|
|
51
|
Ki DH, Jeung HC, Park CH, Kang SH, Lee GY,
Lee WS, Kim NK, Chung HC and Rha SY: Whole genome analysis for
liver metastasis gene signatures in colorectal cancer. Int J
Cancer. 121:2005–2012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gaspar C, Cardoso J, Franken P, Molenaar
L, Morreau H, Moslein G, Sampson J, Boer JM, de Menezes RX and
Fodde R: Cross-species comparison of human and mouse intestinal
polyps reveals conserved mechanisms in adenomatous polyposis coli
(APC)-driven tumorigenesis. Am J Pathol. 172:1363–1380. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dominissini D, Moshitch-Moshkovitz S,
Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K,
Jacob-Hirsch J, Amariglio N, Kupiec M, et al: Topology of the human
and mouse m6A RNA methylomes revealed by m6A-seq. Nature.
485:201–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang D, Qiao J, Wang G, Lan Y, Li G, Guo
X, Xi J, Ye D, Zhu S, Chen W, et al: N6-methyladenosine
modification of lincRNA 1281 is critically required for mESC
differentiation potential. Nucleic Acids Res. 46:3906–3920. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bertero A, Brown S, Madrigal P, Osnato A,
Ortmann D, Yiangou L, Kadiwala J, Hubner NC, de Los MI, Sadee C, et
al: The SMAD2/3 interactome reveals that TGFβ controls
m6A mRNA methylation in pluripotency. Nature.
555:256–259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Deng X, Su R, Weng H, Huang H, Li Z and
Chen J: RNA N(6)-methyladenosine modification in cancers: Current
status and perspectives. Cell Res. 28:507–517. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vu LP, Pickering BF, Cheng Y, Zaccara S,
Nguyen D, Minuesa G, Chou T, Chow A, Saletore Y, MacKay M, et al:
The N6-methyladenosine (m6A)-forming enzyme
METTL3 controls myeloid differentiation of normal hematopoietic and
leukemia cells. Nat Med. 23:1369–1376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Visvanathan A, Patil V, Arora A, Hegde AS,
Arivazhagan A, Santosh V and Somasundaram K: Essential role of
METTL3-mediated m6A modification in glioma stem-like
cells maintenance and radioresistance. Oncogene. 37:522–533. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang
Z, Liu Y, Zhang X, Zhang W and Ye L: HBXIP-elevated
methyltransferase METTL3 promotes the progression of breast cancer
via inhibiting tumor suppressor let-7g. Cancer Lett. 415:11–19.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Barbieri I, Tzelepis K, Pandolfini L, Shi
J, Millan-Zambrano G, Robson SC, Aspris D, Migliori V, Bannister
AJ, Han N, et al: Promoter-bound METTL3 maintains myeloid leukaemia
by m6A-dependent translation control. Nature.
552:126–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dimitriou ID, Clemenza L, Scotter AJ, Chen
G, Guerra FM and Rottapel R: Putting out the fire: Coordinated
suppression of the innate and adaptive immune systems by SOCS1 and
SOCS3 proteins. Immunol Rev. 224:265–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Palmer DC and Restifo NP: Suppressors of
cytokine signaling (SOCS) in T cell differentiation, maturation,
and function. Trends Immunol. 30:592–602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Letellier E and Haan S: SOCS2:
Physiological and pathological functions. Front Biosci (Elite Ed).
8:189–204. 2016.PubMed/NCBI
|
|
64
|
Newton VA, Ramocki NM, Scull BP, Simmons
JG, McNaughton K and Lund PK: Suppressor of cytokine signaling-2
gene disruption promotes Apc(Min/+) tumorigenesis and activator
protein-1 activation. Am J Pathol. 176:2320–2332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Miller ME, Michaylira CZ, Simmons JG, Ney
DM, Dahly EM, Heath JK and Lund PK: Suppressor of cytokine
signaling-2: A growth hormone-inducible inhibitor of intestinal
epithelial cell proliferation. Gastroenterology. 127:570–581. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Letellier E, Schmitz M, Baig K, Beaume N,
Schwartz C, Frasquilho S, Antunes L, Marcon N, Nazarov PV, Vallar
L, et al: Identification of SOCS2 and SOCS6 as biomarkers in human
colorectal cancer. Br J Cancer. 111:726–735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Slattery ML, Lundgreen A, Kadlubar SA,
Bondurant KL and Wolff RK: JAK/STAT/SOCS-signaling pathway and
colon and rectal cancer. Mol Carcinog. 52:155–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Barker N, van Es JH, Kuipers J, Kujala P,
van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H,
Peters PJ, et al: Identification of stem cells in small intestine
and colon by marker gene Lgr5. Nature. 449:1003–1007. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jiang Y, Li W, He X, Zhang H, Jiang F and
Chen Z: Lgr5 expression is a valuable prognostic factor for
colorectal cancer: Evidence from a meta-analysis. BMC Cancer.
16:122016. View Article : Google Scholar : PubMed/NCBI
|