Introduction
Arsenic trioxide (ATO; As2O3)
is a well-known toxin, that occurs in the environment and is also a
common medicine against a wide variety of solid tumors, such as
acute promyelocytic leukemia. However, the adverse drug reactions
of ATO severely limit its clinical application (1). ATO is water-soluble and can be
absorbed readily into the bloodstream. As a site of first-pass
metabolism, the liver is exposed to ATO, at high levels, first and
then ATO is distributed for systemic circulation among other organs
and tissues (2). The liver is a
principal detoxification organ for ATO, as it undergoes reduction
and oxidative methylation in the hepatocytes and is then excreted
out. Furthermore, ATO binds to biological ligands containing sulfur
groups and enhances reactive oxygen species (ROS) (3). The liver is the primary organ
subjected to ATO effects which is damaged by oxidative stress and
an abnormal liver function manifests as elevated serum enzymes
(4). Several studies have reported
a hypothesis that ATO could promote oxidative stress, apoptosis and
inflammation (5,6). The transcription factor nuclear factor
erythroid-2 related factor 2 (Nrf2)/antioxidant response element
(ARE) signaling pathway has been considered as the major cellular
defense against oxidative stress (7,8) and is
considered to be a novel therapeutic target to treat liver disease
(9). Stimulated by oxidative
stress, Nrf2 dissociates from Kelch-like ECH-related protein1
(Keap1) in the cytoplasm and transfers to the nucleus, where it
exhibits its antioxidant action and upregulates various
cytoprotective proteins (10). The
downstream targets of Nrf2 include Heme oxygenase-1 (HO-1), NADPH
quinine oxidoreductase-1 (NQO1), γ-glutamylcysteine synthetase
(γ-GCS) and superoxide dismutase (SOD), which are stimulated by
Nrf2 and then exert their antioxidative effects (10,11).
Therefore, antioxidants, particularly Nrf2 inhibitors, may play
important roles in preventing ATO damage or intoxication.
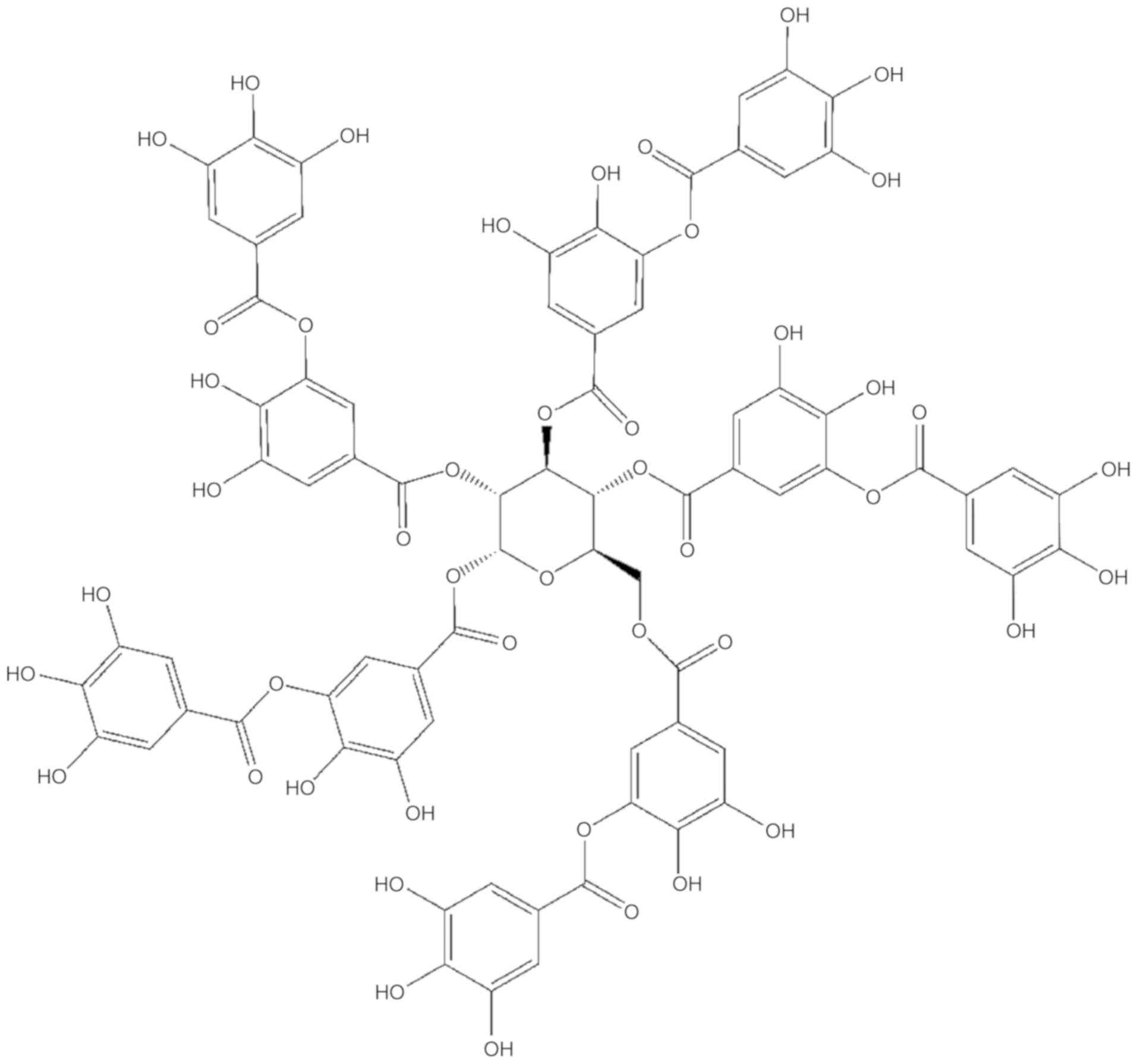
Tannic acid (TA;
C76H52O46) (Fig. 1) is a light-brown natural compound,
and is found in high amounts in various fruits, vegetables and
medicinal plants (12). TA can
chelate with metal ions and interact with biological macromolecules
such as proteins, alkaloids, and polysaccharides. The structure of
pyrogallol can be easily oxidized to a quinone structure, which
provides TA with a supply of hydrogen and becomes oxidation
resistant. Previous reports have shown that TA has multiple
pharmacological activities, such as antioxidative,
anti-inflammatory, antibacterial, anticarcinogenic and
antimutagenic properties (13,14).
Our previous studies have shown that TA exerts its protective
effects against ATO-induced nephrotoxicity through NF-κB and Nrf2
pathways (15) and carbon
tetrachloride and acetaminophen-induced hepatotoxicity (16,17).
In addition, other experimental evidence also shows that TA plays
an important role in the treatment of some hepatotoxicity and
liverish conditions (18) and
stimulates the role of the Nrf2-Keap1 signaling pathway in phase II
and the gene expression of antioxidation proteins in HepG2 cells
(19). At the same time, based on
the protective effect of TA on acetaminophen-induced hepatic
toxicity in mice, which was found in our previous study (17), we hypothesize that TA could improve
ATO-induced hepatic damage through the Keap1-Nrf2/ARE signaling
pathway. However, to date, confirmation of this hypothesis has not
been carried out.
In the present study, an ATO-induced liver injury
model in rats was established and the protective effects of TA on
oxidative stress and hepatic toxicity were subsequently
investigated. The regulation of TA in the Nrf2 signaling pathway
was also studied, as well as its downstream targets to determine
the protective effect of TA on ATO-induced hepatic toxicity and the
underlying mechanisms related to the Keap1-Nrf2/ARE signaling
pathway. Therefore, the present study suggests that TA may suppress
ATO-induced hepatic toxicity by activating the Keap1-Nrf2/ARE
signaling pathway, providing a possible therapeutic drug for
clinical treatment of ATO-induced hepatotoxicity.
Materials and methods
Drugs and reagents
TA, at manufacturer's standard and analytical
purity, was obtained from Jinbei Fine Chemical Co., Ltd. ATO was
purchased from Shuanglu Medicine Factory (Beijing, China). Alanine
aminotransferase (ALT), aspartate transaminase (AST), superoxide
dismutase (SOD), malondialdehyde (MDA), catalase (CAT) and
glutathione peroxidase (GSH-Px) kits were purchased from Nanjing
Jiancheng Bioengineering Institute. Antibodies against Bax (cat.
no. AF0120), Bcl-2 (cat. no. AF6139) were purchased from Affinity
Biosciences. Antibodies against IL-1β (cat. no. BS6067), TNF-α
(cat. no. BS6000), Lamin B1 (cat. no. AP6001), NQO1 (cat. no.
BS90961) and γ-GCS (cat. no. BS90566) were purchased from Bioworld
Technology. Antibodies against caspase-3 (cat. no. 19677-1-AP),
Keap1 (cat. no. 90503-2-AP), HO-1 (product code 10701-1-AP) and
Nrf2 (product code 16396-1-AP) were purchased from Proteintech
Group, Inc. Antibodies against IL-6 (product code ab9324) was
obtained from Abcam Biotechnology, and β-actin (product code
CST3700) was purchased from Cell Signaling Technology. Unless
otherwise indicated, the remaining chemicals were provided by Sigma
Chemical Co.
Animals and experimental protocol
Fifty male adult Sprague-Dawley rats (weight, 200±20
g; age, 12-week-old), purchased from the Experimental Animal Center
of Hebei Medical University, were raised under standard conditions
at room temperature, 45–55% relative humidity and a 12-h light-dark
cycle. All operations were approved by the Animal Experiment Ethics
Committee of Hebei University of Chinese Medicine (approval no.
DWLL2016001).
A total of 50 rats were evenly divided into five
groups: Control group (normal saline), ATO group (5 mg/kg ATO),
L-TA group (20 mg/kg TA + 5 mg/kg ATO), H-TA group (40 mg/kg TA + 5
mg/kg ATO) and TA group (40 mg/kg TA). Based on the literature and
our preliminary experiment, 20 or 40 mg/kg of TA were used for the
animal experiment (20). TA was
administered orally 1 h prior to ATO intraperitoneal treatment once
a day. During the treatment, the weight and behavior of rats were
monitored daily. The entire course lasted for 10 days (21), and the humane endpoint in our
program was defined as weight loss >10%, anorexia or lethargy.
It is worth noting that all 50 rats remained alive during this
period and were euthanized. The animals were anesthetized using
sodium pentobarbital (50 mg/kg), 24 h after the last treatment
following which, blood sampling from the femoral artery (~5–7 ml
each sample) in the rat were quickly collected. The euthanasia of
the rats was carried out by overdose with intraperitoneal injection
of sodium pentobarbital (200 mg/kg) and was confirmed by observing
the absence of respiration and heartbeat, and liver tissues were
quickly collected for further analysis.
Histological analysis
In order to evaluate the histopathological
manifestations in the liver, in the experimental groups of the
rats, the liver tissues were obtained, immobilized overnight with
10% neutral buffered formalin, dehydrated, embedded in paraffin and
cut into sections. Slices were stained with hematoxylin and eosin
(H&E) and observed using a Leica DM4000B microscope (Solms,
Germany).
Colorimetric analysis
To obtain the serum from the blood, the samples were
centrifuged at 2,200 × g for 10 min at 37°C. Serum ALT, AST, SOD,
MDA, CAT and GSH-Px levels were measured according to the
manufacturer's instructions. Finally, the absorbance was measured
using a Varioskan LUX Multimode Reader (Thermo Fisher Scientific,
Inc.).
Fluorescence microscopy
To enable evaluation of the level of ROS in the
liver, the liver tissues were stained with 10 nM dihydroethidium
(DHE) at room temperature for 30 min and then observed using an
optical microscope (DM5000B; Leica, Germany) at a condition of
Ex/Em=518 nm/605 nm. The intensity of ROS, from the digital images
was analyzed by Image-Pro Plus 6.0 (Media Cybernetics).
Western blot analysis
Liver tissues were homogenized and lysed using a
RIPA lysis buffer (Solarbio, China) to obtain proteins. Protein
concentrations were determined using BCA kits. SDS-PAGE gel (10%)
was used to separate the equal amounts of the protein (30 µg) and
then the protein was electrotransferred onto a PVDF membrane. The
membrane was subsequently incubated with the primary antibodies
(dilution 1:1,000) overnight at 4°C, and then incubated with the
secondary antibodies (dilution 1:3,000) at 37°C for 90 min.
Immunoreactive proteins were detected using an enhanced
chemiluminescence light detection kit (ZSGBBIO, China). Following
which, the gray value of the blots was determined using Tanon Gis
software (ver. 4.00; Tanon).
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling
(TUNEL) assay
TUNEL assay was used to detect hepatocyte apoptosis
in the liver sections. 4,6-Diamino-2-phenylindole (DAPI, 1 mg/ml)
was used for staining for 10 min, and DAB was subsequently used
also for staining. Following which, the slices were re-stained with
hematoxylin, dehydrated using alcohol hydrochloride, blocked with a
neutral gel and observed using a light microscope (magnification,
×400). Cells stained brown in the nucleus were considered
positive.
Statistical analysis
Statistical analysis was performed using the
Statistical Package for Social Sciences software (v16.0; SPSS,
Inc.). Data are expressed as the mean ± SEM. Statistical
differences were assessed using one-way analysis of variance
(ANOVA) followed by the Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of TA on liver histological
changes in ATO-treated rats
H&E-stained sections of the livers were observed
using an optical microscope, around the structures of the hepatic
central vein area (Fig. 2A) and
hepatic duct area (Fig. 2B). The
control rats demonstrated normal histological structure. The
central vein was radiating and arranged by the hepatic cord or
plate. The hepatic duct area is composed of the interlobular
artery, interlobular vein and interlobular bile duct. Liver
histological sections in the ATO group showed inflammatory cell
infiltration, hepatic sinus dilatation and congestion, mild atrophy
of hepatocytes and a mild narrow hepatic plate around the central
vein. Furthermore, the pathological findings regarding the portal
tract included edema of hepatocytes, cytoplasmic vacuolization,
lipid droplets of varying sizes in the cytoplasm and apoptosis with
chromatic agglutination and karyopyknosis. ATO-induced rats treated
with 20 or 40 mg/kg TA showed improved histological structure of
the livers. Observation of the liver sections in these two groups
suggested that TA markedly prevented liver steatosis, congestion
and hepatocyte apoptosis. The liver histological sections in the TA
group were similar compared with that in the control group,
suggesting that 40 mg/kg TA had few noxious side effects in the
rats.
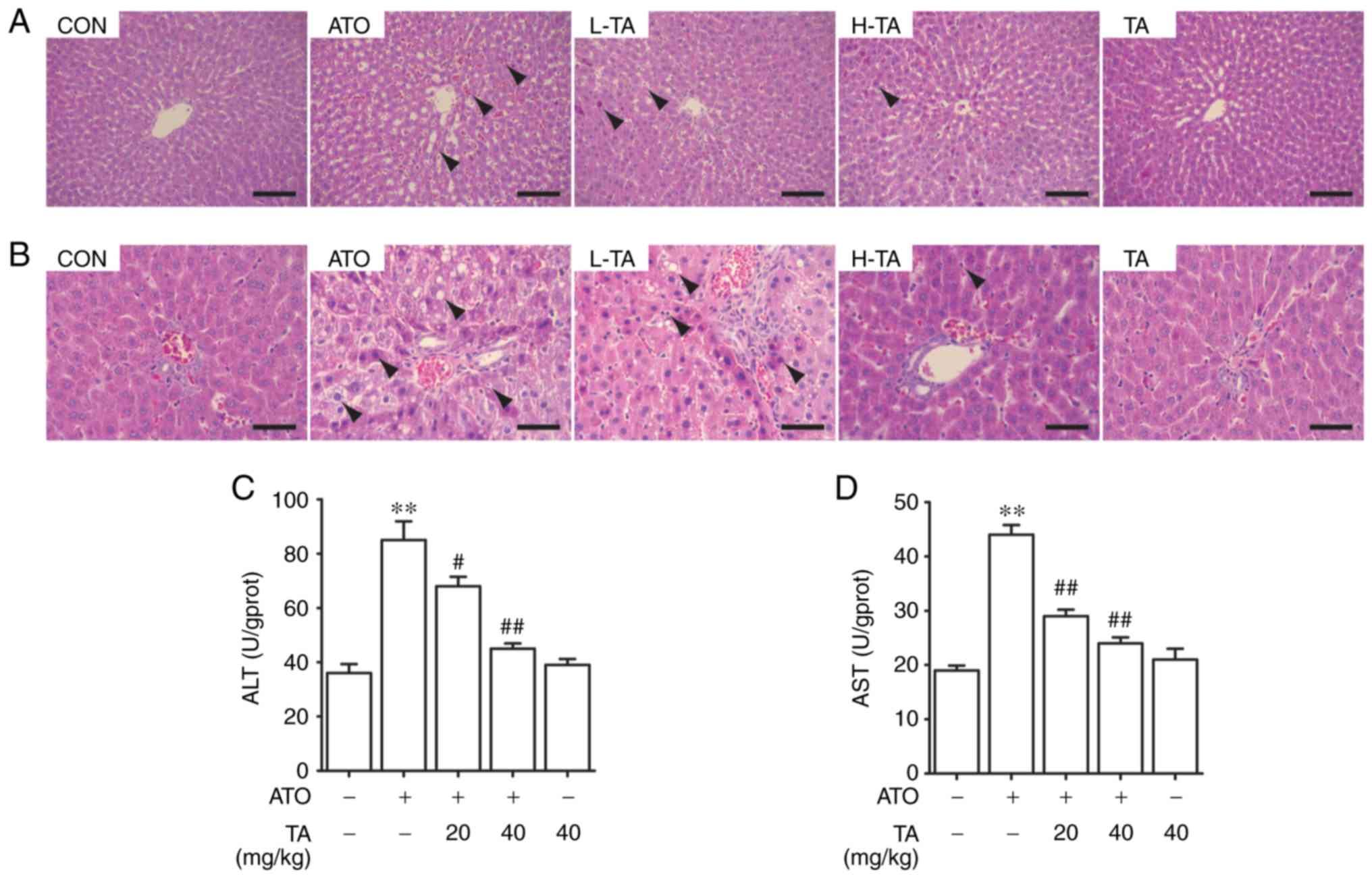 | Figure 2.Effects of TA on hepatic damage in
ATO-exposed rats. Representative sections of H&E staining in
the (A) hepatic central vein area (magnification ×200) and (B)
hepatic duct area (magnification ×400) in rats. Arrows indicate the
lesion area of the liver. Scale bar, 50 µm. (C) ALT and (D) AST
levels of serum in rats. All results are presented as the mean ±
SEM (n=4). **P<0.01 vs. the control group;
#P<0.05, ##P<0.01 vs. the ATO group.
TA, tannic acid; ATO, arsenic trioxide; ALT, alanine
aminotransferase; AST, aspartate transaminase; H&E, hematoxylin
and eosin. Groups included: Control group (CON) (normal saline),
ATO group (5 mg/kg ATO), L-TA group (20 mg/kg TA + 5 mg/kg ATO),
H-TA group (40 mg/kg TA + 5 mg/kg ATO) and TA group (40 mg/kg
TA). |
Effects of TA on the activation of ALT
and AST levels in ATO-treated rats
ALT and AST are considered to be circulating liver
function markers. The effect of TA on the levels of ALT and AST in
the control and ATO-treated rats is represented in Fig. 2C and D. Compared with that in the
control group, ATO-induced levels of ALT and AST in rats were
significantly increased in the serum (P<0.01), while rats that
received only 40 mg/kg TA showed non-significant changes in the
levels of the serum liver function markers (P>0.05). Treatment
of the ATO-induced rats with 20 or 40 mg/kg TA significantly
ameliorated the increased levels of ALT and AST levels in the serum
(P<0.05 or P<0.01).
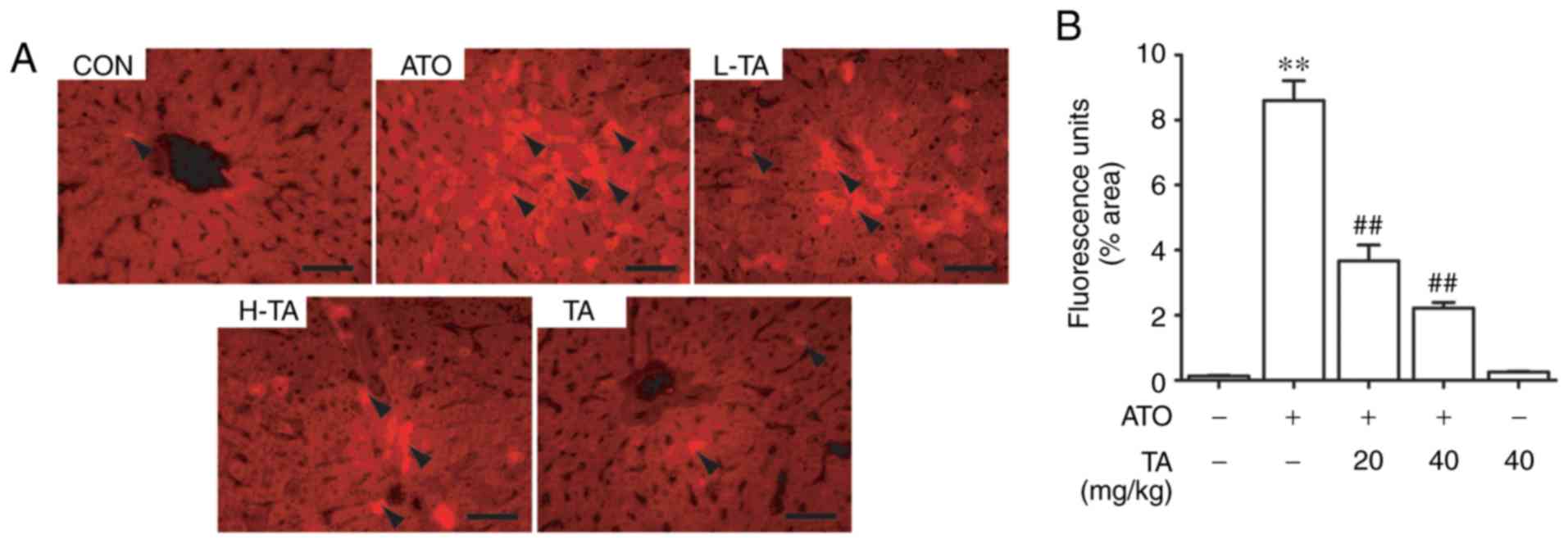
Effects of TA on ROS in ATO-treated
rats
The effect of TA on ROS fluorescence intensity in
the control and treated rats is represented in Fig. 3A. The intensity induced by ATO was
significantly higher than that in the control group (P<0.01).
However, TA effectively removed ROS induced by ATO (P<0.01).
Rats that received only 40 mg/kg TA showed non-significant changes
when compared to the control group. The semi-quantitative and
statistical results of each group are shown in Fig. 3B.
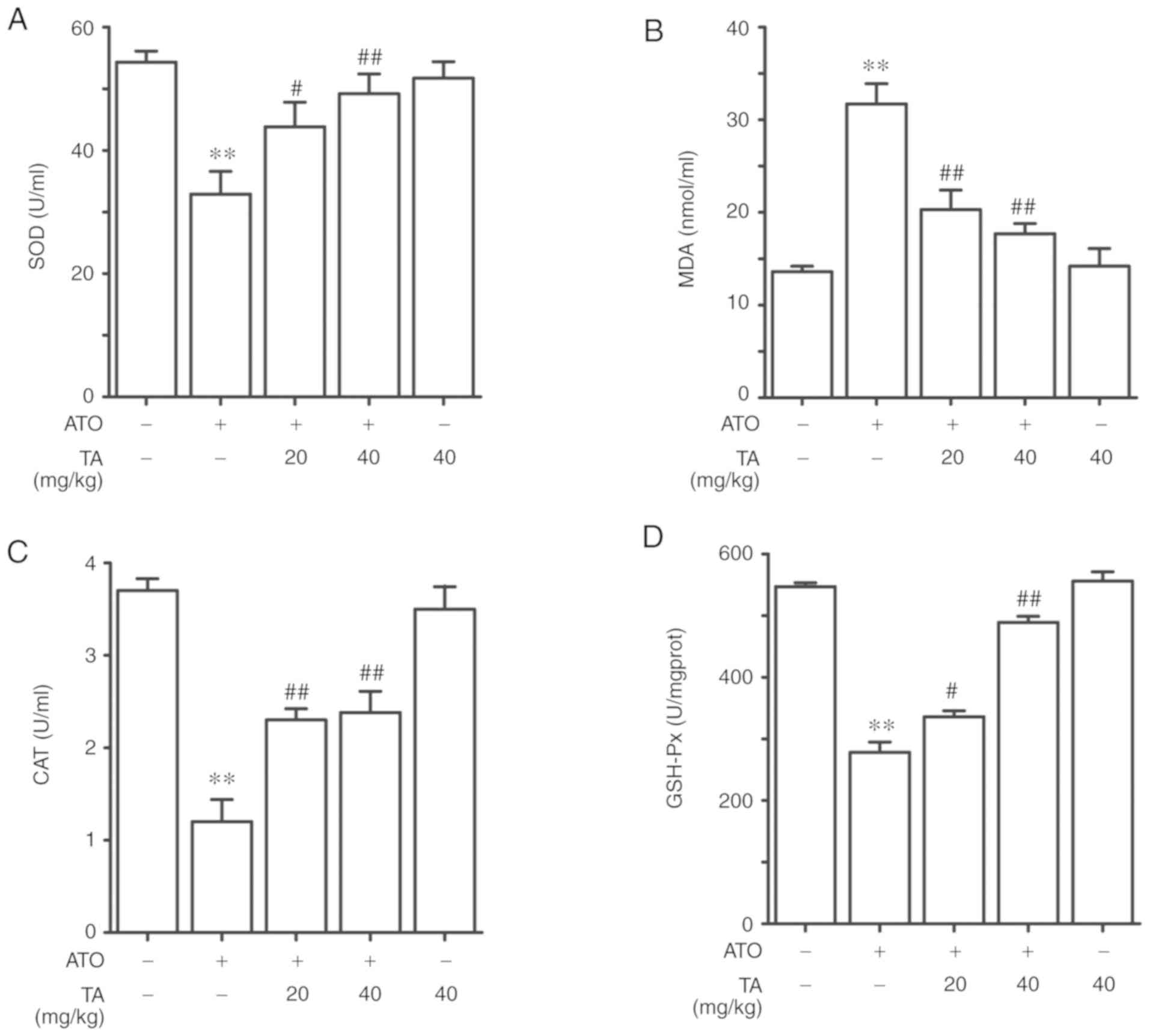
Effects of TA on the activities of
antioxidant enzymes in ATO-treated rats
ATO-exposed rats showed a marked decrease in the
levels of SOD, CAT and GSH-Px in the serum as shown in Fig. 4A-D (P<0.01). TA rejuvenated the
levels of SOD, CAT and GSH-Px in ATO-induced rats when supplemented
at 20 or 40 mg/kg (P<0.05 or P<0.01). On the other hand, the
lipid peroxidation marker MDA in the ATO-induced rats was
significantly increased when compared to the control group
(P<0.01). Treatment with 20 or 40 mg/kg TA significantly
decreased the MDA content in ATO-induced rat livers (P<0.01).
When compared against the control group, rats that received only 40
mg/kg TA showed non-significant changes in the serum SOD, MDA, CAT
and GSH-Px levels (P>0.05).
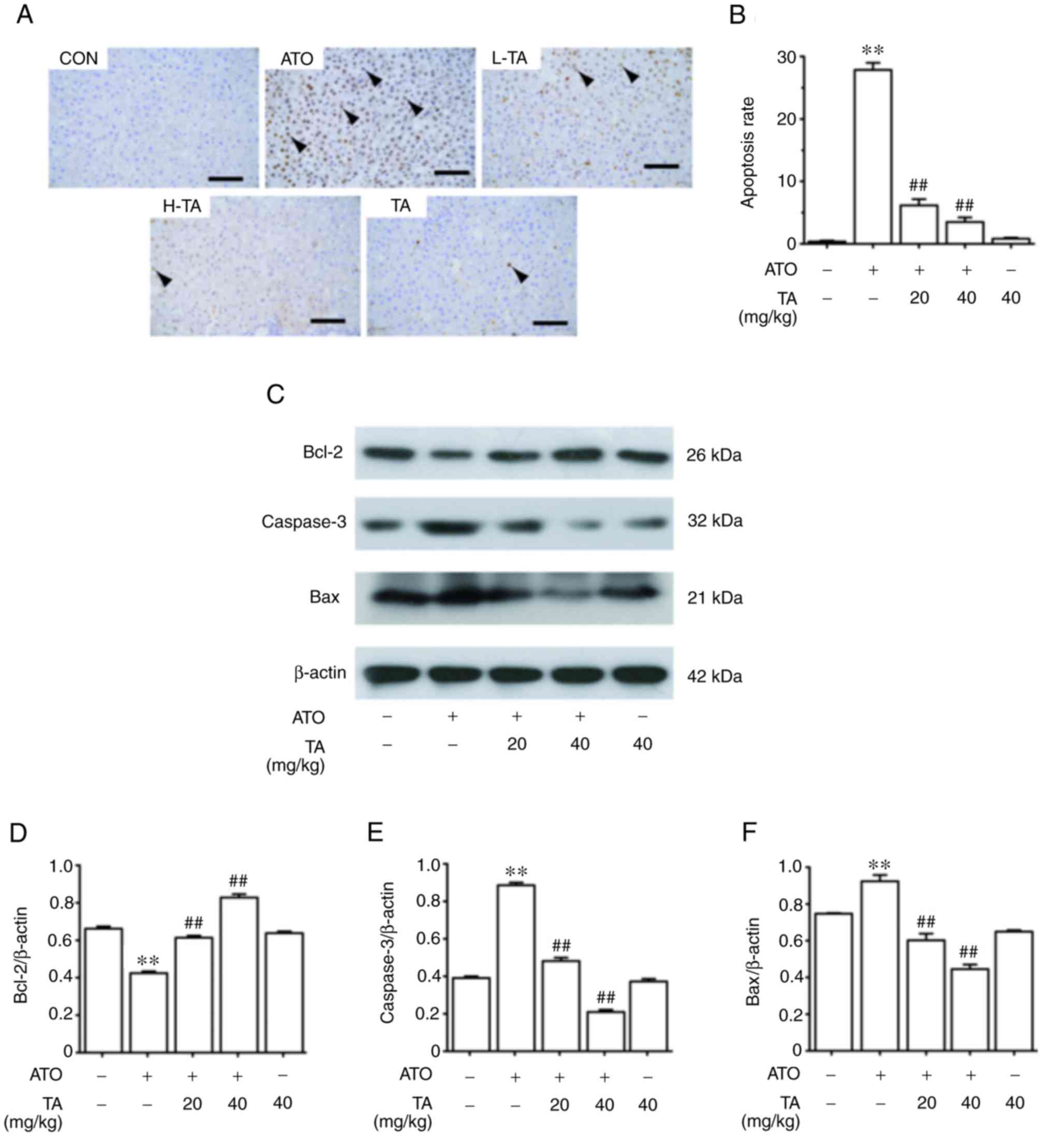
Effects of TA on apoptosis in
ATO-treated rats
The effect of TA on apoptosis in the control and
ATO-treated rats is represented in Fig.
5A. The apoptosis cells induced by ATO were significantly more
than that of the control group (P<0.01). However, TA effectively
removed apoptosis induced by ATO (P<0.01). The semi-quantitative
and statistical results of each group are shown in Fig. 5B. As shown in Fig. 5C-F, ATO-induced rats showed a marked
decrease in the protein expression level of Bcl-2 and an increase
in Bax and caspase-3 levels (P<0.01). TA rejuvenated Bcl-2 and
attenuated Bax and caspase-3 of ATO-induced rats when supplemented
at 20 or 40 mg/kg (P<0.01). Rats which only received 40 mg/kg TA
showed non-significant changes in the apoptosis rate, Bax, Bcl-2
and caspase-3 expression levels when compared to the control group
(P>0.05).
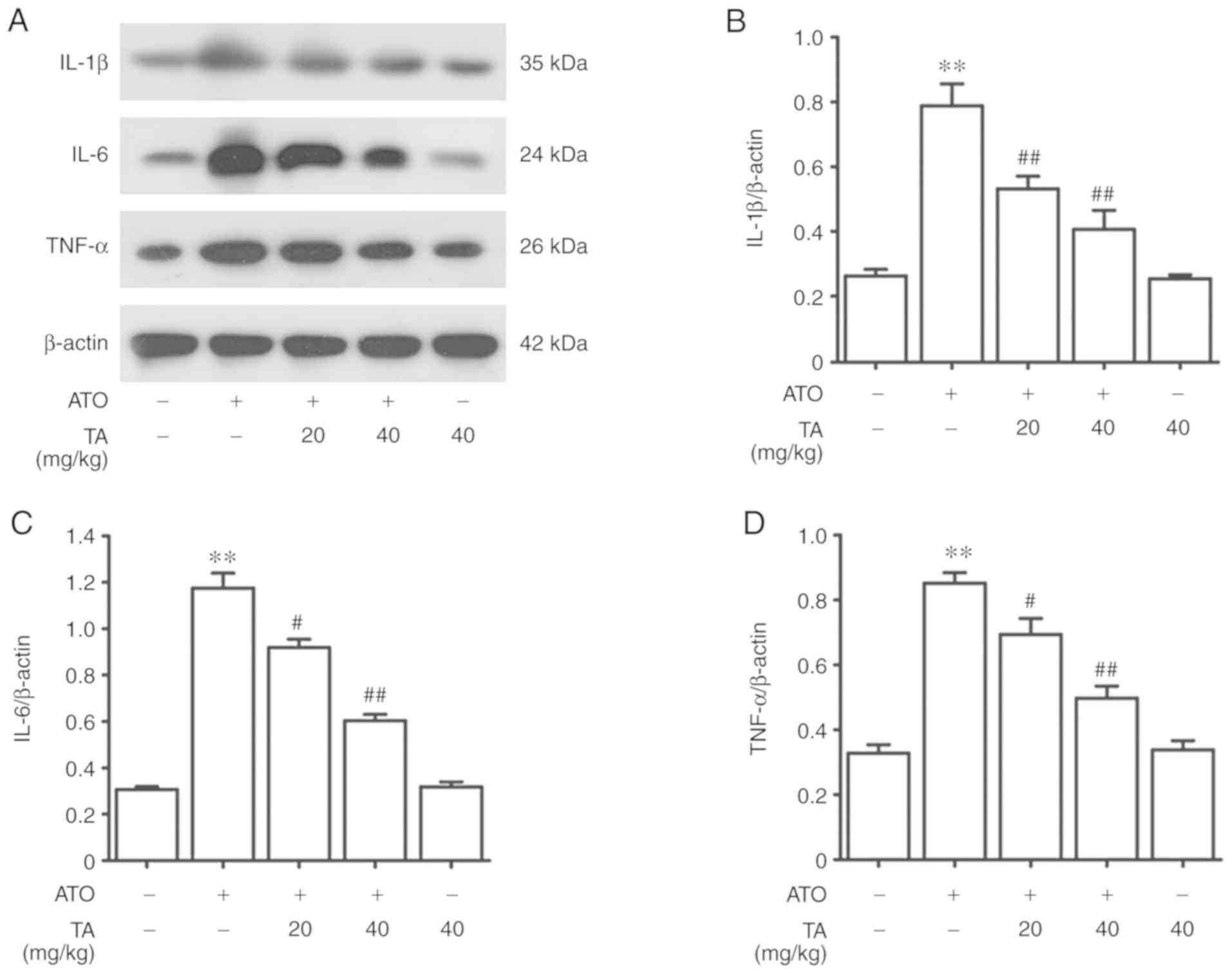
Effects of TA on inflammation in
ATO-treated rats
ATO-induced rats showed a marked increase in the
protein expression levels of interleukin (IL)-1β, IL-6 and tumor
necrosis factor (TNF)-α as shown in Fig. 6A-D (P<0.01). Treatment with 20 or
40 mg/kg TA significantly reduced the protein expression levels of
IL-1β, IL-6 and TNF-α in the rat livers in the ATO group
(P<0.01). Rats that received only 40 mg/kg TA showed
non-significant effect compared against the control group
(P>0.05).
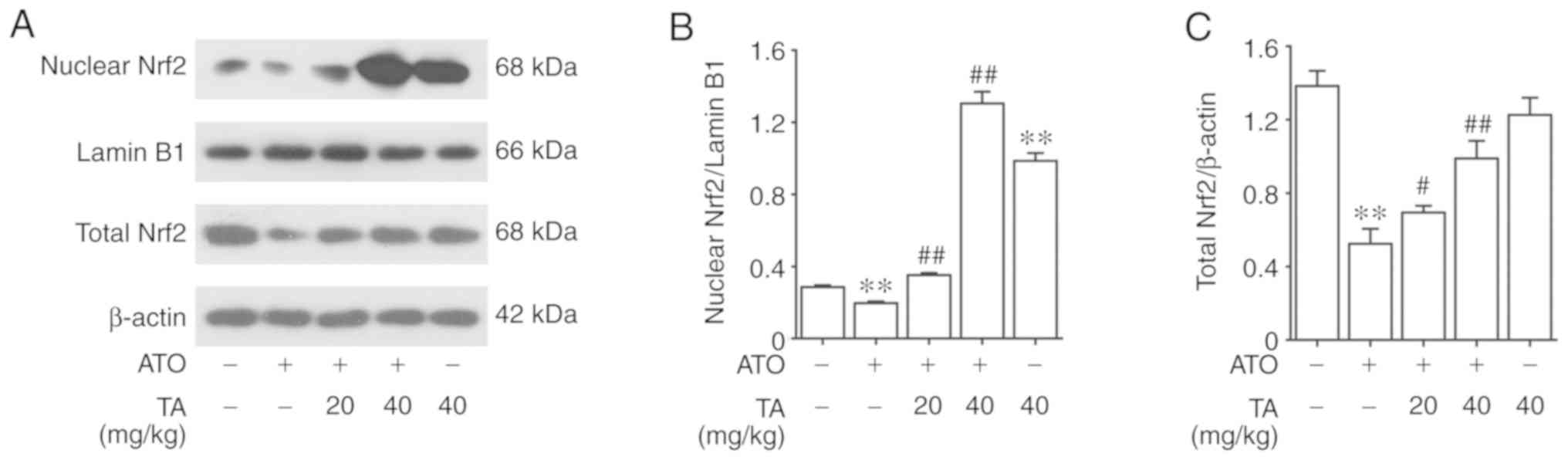
Effects of TA on Nrf2 activation in
ATO-treated rats
The effect of TA on Nrf2 protein expression in
control and ATO-treated rats is represented in Fig. 7A. Nrf2 was expressed only in the
cytoplasm of the control group and the expression level was higher
than that of the ATO group. Following treatment with 20 or 40 mg/kg
TA, Nrf2 expression was increased in both the nucleus and the
cytoplasm, prompting that TA led to Nrf2 translocation from the
cytoplasm into the nucleus. As shown in Fig. 7B and C by western blot analysis, ATO
decreased the cytoplasmic Nrf2 (P<0.01) while TA with 20 or 40
mg/kg regained it (P<0.01). Furthermore, TA treatment
significantly activated nuclear Nrf2 (P<0.01), which was
inhibited by ATO (P<0.01). Rats that received only 40 mg/kg TA
also showed significantly activated nuclear Nrf2 compared with the
control group (P<0.01).
Effects of TA on the expression levels
of downstream targets in the Nrf2 signaling pathway in the
ATO-treated rats
The protein expression level of Keap1 in ATO-induced
rats increased significantly as shown in Fig. 8A and B (P<0.01). Treatment with
20 or 40 mg/kg TA significantly reduced the expression level of
Keap1 in the livers of ATO-induced rats (P<0.01). On the
contrary, ATO-induced rats showed a marked decrease in the protein
expression levels of HO-1, NQO1 and γ-GCS (P<0.05 or P<0.01)
(Fig. 8A and C-E). Treatment with
20 or 40 mg/kg TA fortified the expression levels of HO-1, NQO1 and
γ-GCS in the livers of ATO-induced rats (P<0.01). Compared
against the control group, rats that only received 40 mg/kg TA
showed non-significant changes in the protein expressions of Keap1,
HO-1 and γ-GCS (P>0.05); however, showed significantly activated
expression of NQO1 (P<0.01).
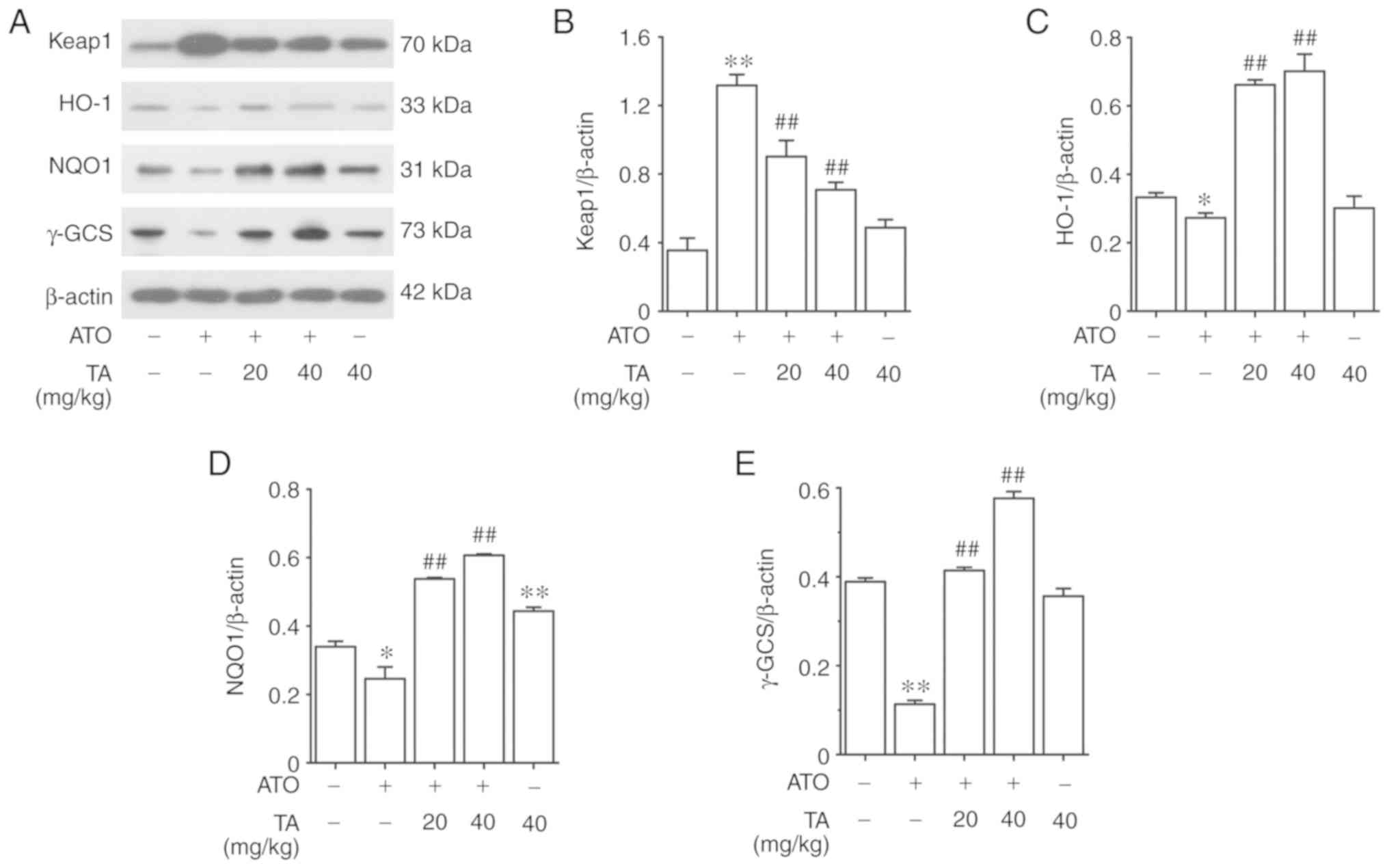 | Figure 8.Effects of TA on the expression
levels of downstream targets in the Nrf2 pathway in ATO-treated
rats. (A) The hepatic protein expression levels of (B) Keap1, (C)
HO-1, (D) NQO1 and (E) γ-GCS were measured by western blot
analysis. All results are presented as the mean ± SEM (n=4).
*P<0.05, **P<0.01 vs. control group; ##P<0.01
vs. ATO group. TA, tannic acid; ATO, arsenic trioxide. Nrf2,
nuclear factor erythroid 2-related factor 2; Keap1, Kelch-like
ECH-associated protein 1; HO-1, heme oxygenase-1; NQO1, NADPH
quinine oxidoreductase-1, γ-GCS, γ-glutamylcysteine synthetase. |
Discussion
Arsenic, as a pollutant and human carcinogen,
produces a complex effect in organisms, including oxidative stress,
apoptosis and inflammation (22).
The cytotoxicity of arsenic trioxide (ATO) may limit its clinical
application in cancer treatment. Thus, it makes sense to search for
an appropriate antidote to ATO. Previous studies have investigated
the relationship between complex mixtures containing tannic acid
(TA) and arsenic compounds, such as aqueous extract of green tea
leaves (23). It is reasonable to
guess that TA may have a protective effect against ATO-induced
liver injury. Accordingly, the present study focused on the
assessment of the hepatic changes induced by ATO, as well as the
therapeutic effect and underlying mechanisms of TA in rats.
It was found that ATO significantly induced liver
injury in the present study, which was manifested in the formation
of an abnormal histological central vein area and portal tract
structure and increased circulating ALT and AST levels. Due to the
fact that ALT and AST typically enter the bloodstream following an
injury to the structural integrity of liver cells, the levels of
ALT and AST in the serum have been generally regarded as
representative indicators of hepatic damage (24). TA treatment exhibited hepatic
protective effects as manifested by the improvement of pathological
damage and a decrease in the levels of ALT and AST. Previous
studies have shown that TA exhibited the same impact trend for
these indicators in carbon tetrachloride or iron-overload-induced
hepatotoxicity (16,25). All the aforementioned evidence
suggests that TA could be an effective natural compound for
relieving various types of liver damage.
Oxidative stress, apoptosis and inflammation are
common features of numerous diseases and play important roles in
tumorigenesis (26,27). Oxidative impairment following ATO
exposure if significantly reflected by increased ROS and MDA and
decreased SOD, CAT and GSH-Px (28,29).
The Bcl gene family is a critical regulatory gene in the regulation
of apoptosis. Both Bax and Bcl-2 belong to the Bcl family and play
important roles in the regulation of apoptosis pathways. Therefore,
Bcl-2 and Bax are often used to define the degree of apoptosis
(30,31). Moreover, as a cell death protease,
caspase-3 plays a significant role in cell apoptosis (29,32).
Activation of apoptotic pathways finally results in the activation
of caspase-3 (32). Caspase-3 is an
‘effector’ protease in the apoptosis cascade and is one of the main
executors of apoptosis (29,32).
Caspase-3 activation can alter cell morphology and degrade DNA,
sequentially triggering apoptosis. Accompanied by the production of
a large amount of ROS, the permeability of the mitochondrial
membrane increases significantly. Ultimately the apoptosis cascade
reaction of caspases is triggered (33). Excessive oxidative stress evoked by
ATO triggers some signaling pathways containing the activation of
caspase-3 resulting in hepatic cell apoptosis. Consistent with
previous researches (34–36), ATO promotes the apoptosis of hepatic
cells, decreases significantly Bcl-2 expression and elevates Bax
and caspase-3 expression levels as demonstrated in the present
study, which confirmed the occurrence of apoptosis in the
hepatotoxic rats. Interestingly, ATO-induced changes in Bax, Bcl-2
and caspase-3 expression levels were approximately restored to
normal levels following treatment TA, ultimately decreasing the
number of TUNEL-positive cells in the liver tissue. Taken together,
the results suggest that TA inhibits the apoptosis of hepatic cells
by increasing Bcl-2 expression and decreasing Bax and caspase-3
expression as confirmed in our experiments. In the present study,
we observed that ATO stimulated IL-1β, IL-6 and TNF-α protein
expression levels, which aggravated inflammation. At the same time,
it has been reported that Nrf2 improves the removal of ROS for
cellular defense and plays a protective role against oxidative
stress (37). Nrf2 controls Bcl-2
expression and apoptotic cell death with implications on the
survival of cells (38).
Furthermore, it has been evidenced that the activation of Nrf2
prevents proinflammatory cytokines, such as IL-1β and IL-6
(39). In the present study, it was
found that TA alleviated oxidative stress, apoptosis and
inflammation induced by ATO (Fig.
9). Therefore, we further investigated whether ATO and TA have
effects on the Nrf2 pathway.
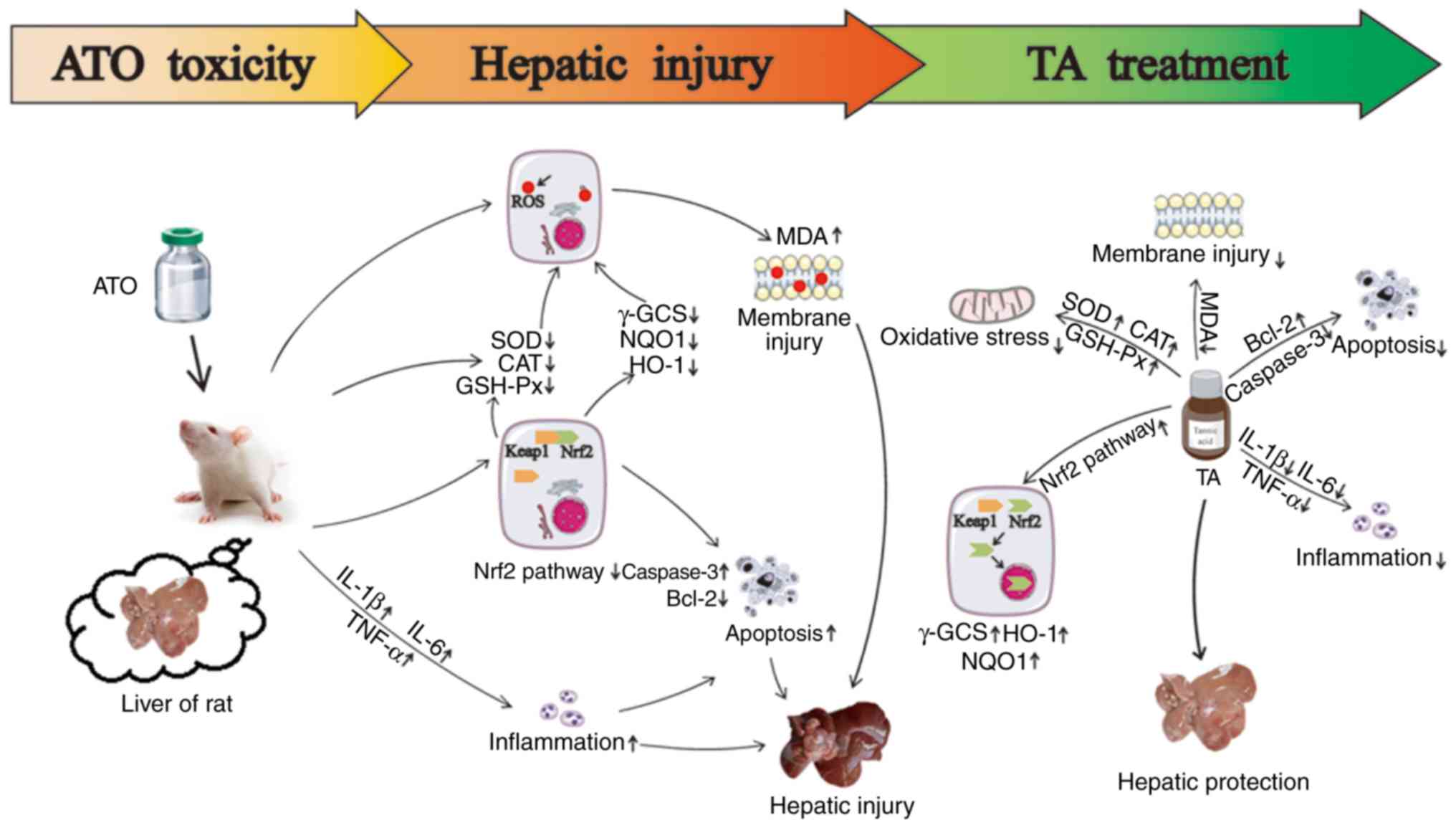 | Figure 9.Effects of TA treatment on
ATO-induced hepatic injury. TA stimulates the levels of SOD, CAT
and GSH-Px, attenuates the levels of MDA and ROS. TA is capable of
suppression of the expression levels of caspase-3, Bax, IL-1β,
IL-6, TNF-α and Keap1, and activates expression levels of Bcl-2,
nuclear Nrf2, total Nrf2, HO-1, NQO1 and γ-GCS. The protective
effect of TA on ATO-induced hepatic injury may be associated with
the attenuation of hepatic oxidative stress, apoptosis and
inflammation by activating the Keap1-Nrf2/ARE pathway. TA, tannic
acid; ATO, arsenic trioxide; SOD, superoxide dismutase; CAT,
catalase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde;
ROS, reactive oxygen species; IL, interleukin; TNF, tumor necrosis
factor; Nrf2, nuclear factor erythroid 2-related factor 2; Keap1,
Kelch-like ECH-associated protein 1; HO-1, heme oxygenase-1; NQO1,
NADPH quinine oxidoreductase-1, γ-GCS, γ-glutamylcysteine
synthetase; ARE, antioxidant response element. |
Nrf2 is a key transcription factor that regulates
gene expression of a series of anti-oxidant proteins and
detoxifying enzymes. Keap1, a redox-regulated substrate adaptor
protein for a cullin3-dependent ubiquitin ligase complex, senses
electrophilic or oxidative stresses and then arrests ubiquitination
of Nrf2, leading to Nrf2 activation (40). In addition to this canonical
pathway, one Nrf2 target p62/SQSTM1 (p62) competitively binds to
Keap1 to activate Nrf2. p62 is a stress-inducible intracellular
protein, which is known to regulate various signal transduction
pathways involved in cell survival and cell death. p62 interacts
with p62, NBR1, ERK1, and atypical PKC (aPKC) through Phox and Bem1
(PB1)-mediated homooligomerization or heterooligomerization to
regulate the NF-κB signaling pathway (40).
The Nrf2-Keap1 signaling pathway is a considerable
antioxidant defense mechanism that activates an adaptive cellular
response against oxidative stress and participates in the toxicant
metabolic and detoxifying process (41). Keap1 is a repressor of Nrf2 activity
and plays a key role in its regulation. It was found that ATO
significantly inhibited Nrf2 while increasing Keap1. This finding
was consistent with the result that arsenic-induced reduction in
antioxidant enzymes was more pronounced in Nrf2-inhibited cells,
indicating that arsenic may inhibit the Nrf2 pathway (42,43).
The mechanism of arsenic in the inhibition of Nrf2 expression may
be related to the acceleration of Nrf2 degradation and the
promotion of Keap1 protein expression (44). These results revealed that TA
treatment exhibited a facilitating effect on the Nrf2 pathway in
increasing Nrf2 while inhibiting Keap1. Following evasion from
Keap1, Nrf2 translocates to the nucleus, and activates the
expression of a series of cytoprotective and antioxidative
protein-dependent genes such as HO-1, NQO1 and γ-GCS (45). HO-1 is an inducer of bilirubin and
carbon monoxide and thus provides protection against cell oxidative
injury (46). NQO1, a member of the
NAD(P)H dehydrogenase family, encodes a cytoplasmic 2-electron
reductase (47). Furthermore, γ-GCS
is a rate-limiting enzyme of glutathione biosynthesis, which can
detoxify certain deleterious xenobiotics through direct thiol
conjugation. In brief, these are all important metabolism and
antioxidant enzymes and play key roles in the control of signaling
processes (48). In the present
study, TA treatment increased the expression levels of HO-1, NQO1
and γ-GCS, which were inhibited by ATO. As suggested by the results
of the present study, an underlying mechanism of TA in relation to
antioxidant properties has been demonstrated as the guidance of
antioxidants and phase II detoxifying enzymes by Nrf2
activation.
There are other issues which should be discussed in
regards to this previous experiment. The dosage of TA used in this
study was based on our previous experiments (15–17,20),
in which there were no significant changes in liver morphology,
enzyme activities and in the expression levels of the majority of
proteins. Our previous reseach on TA against ATO-induced
nephrotoxicity hypothesized that TA may play a protective role
through the NF-κB/Nrf2 pathway by determining the protein levels of
NF-κB, Nrf2 and Keap1 (15). In the
present research, we systematically studied the protective effect
and mechanism of TA on liver injury induced by ATO. In addition to
Nrf2 and Keap1, we also investigated the influence of TA on the
protein expression levels of HO-1, NQO1, and γ-GCS in this
signaling pathway. Additionally, dimercaptopropanol and its
derivatives have been found to be used to clinically relieve acute
intoxication (49); however, it was
not set as a positive control drug in the present study,
considering that the amount of ATO was still a small dose and could
not cause acute poisoning. Interestingly, arsenic has been reported
to activate the Nrf2-Keap1 signaling pathway in some types of
cancer cells, due to mutations in Nrf2, which can be beneficial for
cancer cell growth and self-protection (50). Based on the large number of previous
studies and the results from the present study, we believe that the
inhibitory effect of ATO on the Keap1-Nrf2/ARE signaling pathway in
non-cancerous rats is reasonable. However, the regulation of TA on
the Keap1-Nrf2 pathway in cancer cells needs further
investigation.
In summary, TA, as a valuable dietary component or
medicinal material, could mitigate the hepatic damage of ATO by
inhibiting oxidative stress, apoptosis and inflammation, and the
mechanisms that may be associated with the activation of the
Keap1-Nrf2/ARE signaling pathway. The results of the present study
could further reveal the molecular mechanisms of the beneficial
effects of TA on ATO-induced liver injury.
Acknowledgements
Not applicable.
Funding
This work was supported by the Research Foundation
of Administration of Traditional Chinese Medicine of Hebei
Province, China (no. 2020188).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC and XC contributed to the design of the
experiments. ML, YX and YL conducted the experiments and obtained
the data. JZ, JS and XH analyzed the data. ML and PL wrote the
manuscript. PL, JZ and LC contributed to the manuscript revisions.
All authors read the manuscript and approved the final version of
the manuscript.
Ethics approval and consent to
participate
All operating procedures regarding experimental
animals were approved by the Ethics Committee for Animal
Experiments of Hebei University of Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim PG, Bridgham K, Chen EC, Vidula MK,
Pozdnyakova O, Brunner AM and Fathi AT: Incident adverse events
following therapy for acute promyelocytic leukemia. Leuk Res Rep.
9:79–83. 2018.PubMed/NCBI
|
|
2
|
Benramdane L, Accominotti M, Fanton L,
Malicier D and Vallon JJ: Arsenic speciation in human organs
following fatal arsenic trioxide poisoning-a case report. Clin
Chem. 45:301–306. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
You BR and Park WH: Arsenic trioxide
induces human pulmonary fibroblast cell death via increasing ROS
levels and GSH depletion. Oncol Rep. 28:749–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abhilash S, Siviyasankar R, Binu P, Arathi
P and Harikumaran Nair R: Different administration patterns of
docosahexaenoic acid in combating cytotoxic manifestations due to
arsenic trioxide (acute promyelocytic leukemia drug) induced redox
imbalance in hepatocytes. Prostaglandins Other Lipid Mediat.
136:64–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahmed S, Mahabbat-e Khoda S, Rekha RS,
Gardner RM, Ameer SS, Moore S, Ekstrom EC, Vahter M and Raqib R:
Arsenic-associated oxidative stress, inflammation, and immune
disruption in human placenta and cord blood. Environ Health
Perspect. 119:258–264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng Y, Yamaguchi H, Tian C, Lee MW, Tang
H, Wang HG and Chen Q: Arsenic trioxide (As(2)O(3)) induces
apoptosis through activation of Bax in hematopoietic cells.
Oncogene. 24:3339–3347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bao J, Ding R, Zou L, Zhang C, Wang K, Liu
F, Li P, Chen M, Wan JB, Su H, et al: Forsythiae fructus inhibits
B16 melanoma growth involving MAPKs/Nrf2/HO-1 mediated
anti-oxidation and anti-inflammation. Am J Chin Med. 44:1043–1061.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu C, Xu H, Fu S, Chen Y, Chen Q, Cai Z,
Zhou J and Wang Z: Sulforaphane ameliorates bladder dysfunction
through activation of the Nrf2-ARE pathway in a rat model of
partial bladder outlet obstruction. Oxid Med Cell Longev.
2016:75982942016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirotsu Y, Katsuoka F, Funayama R,
Nagashima T, Nishida Y, Nakayama K, Engel JD and Yamamoto M:
Nrf2-MafG heterodimers contribute globally to antioxidant and
metabolic networks. Nucleic Acids Res. 40:10228–10239. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu D, Duan X, Dong D, Bai C, Li X, Sun G
and Li B: Activation of the Nrf2 pathway by inorganic arsenic in
human hepatocytes and the role of transcriptional repressor Bach1.
Oxid Med Cell Longev. 2013:9845462013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao HJ, Li MJ, Zhang MP, Wei MK, Shen LP,
Jiang M and Zeng T: Allyl methyl trisulfide protected against
acetaminophen (paracetamol)-induced hepatotoxicity by suppressing
CYP2E1 and activating Nrf2 in mouse liver. Food Funct.
10:2244–2253. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nepka C, Sivridis E, Antonoglou O,
Kortsaris A, Georgellis A, Taitzoglou I, Hytiroglou P,
Papadimitriou C, Zintzaras I and Kouretas D: Chemopreventive
activity of very low dose dietary tannic acid administration in
hepatoma bearing C3H male mice. Cancer Lett. 141:57–62. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gray JP, Mishin V, Heck DE and Laskin JD:
Dietary tannic acid stimulates production of reactive oxygen
intermediates and growth factor and inflammatory gene expression in
human colon tumor cells. Cancer Rese. 65:12232005.
|
|
14
|
Ninan N, Forget A, Shastri VP, Voelcker NH
and Blencowe A: Antibacterial and anti-inflammatory pH-responsive
tannic acid-carboxylated agarose composite hydrogels for wound
healing. ACS Appl Mater Interfaces. 8:28511–28521. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin W, Xue Y, Xue Y, Han X, Song Q, Zhang
J, Li Z, Cheng J, Guan S, Sun S and Chu L: Tannic acid ameliorates
arsenic trioxide-induced nephrotoxicity, contribution of NF-κB and
Nrf2 pathways. Biomed Pharmacother. 126:1100472020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chu X, Wang H, Jiang YM, Zhang YY, Bao YF,
Zhang X, Zhang JP, Guo H, Yang F, Luan YC and Dong YS: Ameliorative
effects of tannic acid on carbon tetrachloride-induced liver
fibrosis in vivo and in vitro. J Pharmacol Sci. 130:15–23. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Song Q, Han X, Zhang Y, Zhang Y,
Zhang X, Chu X, Zhang F and Chu L: Multi-targeted protection of
acetaminophen-induced hepatotoxicity in mice by tannic acid. Int
Immunopharmacol. 47:95–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung MY, Song JH, Lee J, Shin EJ, Park
JH, Lee SH, Hwang JT and Choi HK: Tannic acid, a novel histone
acetyltransferase inhibitor, prevents non-alcoholic fatty liver
disease both in vivo and in vitro model. Mol Metab. 9:34–48. 2019.
View Article : Google Scholar
|
|
19
|
Krajka-Kuźniak V, Paluszczak J, Szaefer H
and Baer-Dubowska W: The activation of the Nrf2/ARE pathway in
HepG2 hepatoma cells by phytochemicals and subsequent modulation of
phase II and antioxidant enzyme expression. J Physiol Biochem.
71:227–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Cui L, Han X, Zhang Y, Zhang X,
Chu X, Zhang F, Zhang Y and Chu L: Protective effects of tannic
acid on acute doxorubicin-induced cardiotoxicity: Involvement of
suppression in oxidative stress, inflammation, and apoptosis.
Biomed Pharmacother. 93:1253–1260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hemmati AA, Olapour S, Varzi HN, Khodayar
MJ, Dianat M, Mohammadian B and Yaghooti H: Ellagic acid protects
against arsenic trioxide-induced cardiotoxicity in rat. Hum Exp
Toxicol. 37:412–419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh AP, Goel RK and Kaur T: Mechanisms
pertaining to arsenic toxicity. Toxicol Int. 18:87–93. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Messarah M, Saoudi M, Boumendjel A,
Kadeche L, Boulakoud MS and Feki AE: Green tea extract alleviates
arsenic-induced biochemical toxicity and lipid peroxidation in
rats. Toxicol Ind Health. 29:349–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vermeulen NP, Bessems JG and Van De Straat
R: Molecular aspects of paracetamol-induced hepatotoxicity and its
mechanism-based prevention. Drug Metab Rev. 24:367–407. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Basu T, Panja S, Shendge AK, Das A and
Mandal N: A natural antioxidant, tannic acid mitigates
iron-overload induced hepatotoxicity in swiss albino mice through
ROS regulation. Environ Toxicol. 33:603–618. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahmed SM, Luo L, Namani A, Wang XJ and
Tang X: Nrf2 signaling pathway: Pivotal roles in inflammation.
Biochim Biophys Acta Mol Basis Di. 1863:585–597. 2017. View Article : Google Scholar
|
|
27
|
Lushchak IV: Free radicals, reactive
oxygen species, oxidative stress and its classification. Chem Biol
Interact. 224:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sumedha NC and Miltonprabu S: Diallyl
trisulfide ameliorates arsenic-induced hepatotoxicity by abrogation
of oxidative stress, inflammation, and apoptosis in rats. Hum Exp
Toxicol. 34:506–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Adedara IA, Adebowale AA, Atanda OE,
Fabunmi AT, Ayenitaju AC, Rocha JBT and Farombi EO: Selenium abates
reproductive dysfunction via attenuation of biometal accumulation,
oxido-inflammatory stress and caspase-3 activation in male rats
exposed to arsenic. Environ Pollut. 254:113079–113090. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Imam F, Al-Harbi NO, Al-Harbi MM, Ansari
MA, Al-Asmari AF, Ansari MN, Al-Anazi WA, Bahashwan S, Almutairi
MM, Alshammari M, et al: Apremilast prevent doxorubicin-induced
apoptosis and inflammation in heart through inhibition of oxidative
stress mediated activation of NF-κB signaling pathways. Pharmacol
Rep. 70:993–1000. 2018. View Article : Google Scholar
|
|
31
|
Dash SK, Chattopadhyay S, Ghosh T, Dash
SS, Tripathy S, Das B, Bag BG, Das D and Roy S: Self-assembled
betulinic acid protects doxorubicin induced apoptosis followed by
reduction of ROS-TNF-α-caspase-3 activity. Biomed Pharmacother.
72:144–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oliveira PJ, Santos MS and Wallace KB:
Doxorubicin-induced thiol-dependent alteration of cardiac
mitochondrial permeability transition and respiration. Biochemistry
(Mosc). 71:194–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu W, Gao FF, Li Q, Lv JW, Wang Y, Hu PC,
Xiang QM and Wei L: Protective effect of Astragalus polysaccharides
on liver injury induced by several different chemotherapeutics in
mice. Asian Pac J Cancer Prev. 15:10413–10420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu Z, Zhang Y, Song T, Song Q, Zhang Y,
Zhang X, Han X, Zhang J and Chu L: Magnesium isoglycyrrhizinate
ameliorates doxorubicin-induced acute cardiac and hepatic toxicity
via antio-xidant and anti-apoptotic mechanisms in mice. Exp Ther
Med. 15:1005–1012. 2018.PubMed/NCBI
|
|
36
|
Miltonprabu S, Sumedha NC and Senthilraja
P: Diallyl trisulfide, a garlic polysulfide protects against
As-induced renal oxidative nephrotoxicity, apoptosis and
inflammation in rats by activating the Nrf2/ARE signaling pathway.
Int Immunopharmacol. 50:107–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Singh S, Vrishni S, Singh BK, Rahman I and
Kakkar P: Nrf2-ARE stress response mechanism: A control point in
oxidative stress-mediated dysfunctions and chronic inflammatory
diseases. Free Radic Res. 44:1267–1288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Niture SK and Jaiswal AK: Nrf2 protein
up-regulates antiapoptotic protein Bcl-2 and prevents cellular
apoptosis. J Biol Chem. 287:9873–9886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kobayashi EH, Suzuki T, Funayama R,
Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi
H, Nakayama K and Yamamoto M: Nrf2 suppresses macrophage
inflammatory response by blocking proinflammatory cytokine
transcription. Nat Commun. 7:116242016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Katsuragi Y, Ichimura Y and Komatsu M:
Regulation of the Keap1-Nrf2 pathway by p62/SQSTM1. Oxidative
Toxicology. Johnson J, Puga A, Wallace KB and Zhang D: 1. Current
Opinion in Toxicology; pp. 54–61. 2016
|
|
41
|
Shi L, Hao Z, Zhang S, Wei M, Lu B, Wang Z
and Ji L: Baicalein and baicalin alleviate acetaminophen-induced
liver injury by activating Nrf2 antioxidative pathway: The
involvement of ERK1/2 and PKC. Biochem Pharmacol. 150:9–23. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shafik NM and El Batsh MM: Protective
effects of combined selenium and punica granatum treatment on some
inflammatory and oxidative stress markers in arsenic-induced
hepatotoxicity in rats. Biol Trace Elem Res. 169:121–128. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vineetha RC, Archana V, Binu P, Arathi P
and Nair RH: L-ascorbic acid and α-Tocopherol reduces
hepatotoxicity associated with arsenic trioxide chemotherapy by
modulating Nrf2 and Bcl2 transcription factors in chang liver
cells. Nutr Cancer. 70:684–696. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ugun-Klusek A, Tatham MH, Elkharaz J,
Constantin-Teodosiu D, Lawler K, Mohamed H, Paine SM, Anderson G,
John Mayer R, Lowe J, et al: Continued 26S proteasome dysfunction
in mouse brain cortical neurons impairs autophagy and the
Keap1-Nrf2 oxidative defence pathway. Cell Death Dis. 8:e25312017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baird L and Dinkova-Kostova AT: The
cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol.
85:241–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takahashi T, Morita K, Akagi R and Sassa
S: Heme oxygenase-1: A novel therapeutic target in oxidative tissue
injuries. Curr Med Chem. 11:1545–1561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu D, Hu L, Xia X, Song J, Li L, Song E
and Song Y: Tetrachlorobenzoquinone induces acute liver injury,
up-regulates HO-1 and NQO1 expression in mice model: The protective
role of chlorogenic acid. Environ Toxicol Pharmacol. 37:1212–1220.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu X, Chen K, Zhu L, Liu H, Ma T, Xu Q
and Xie T: Soyasaponin Ab protects against oxidative stress in
HepG2 cells via Nrf2/HO-1/NQO1 signaling pathways. J Functional
Foods. 45:110–117. 2018. View Article : Google Scholar
|
|
49
|
Aaseth J, Skaug MA, Cao Y and Andersen O:
Chelation in metal intoxication-Principles and paradigms. J Trace
Elem Med Biol. 31:260–266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lau A, Whitman SA, Jaramillo MC and Zhang
DD: Arsenic-mediated activation of the Nrf2-Keap1 antioxidant
pathway. J Biochem Mol Toxicol. 27:99–105. 2013. View Article : Google Scholar : PubMed/NCBI
|