Introduction
Cellular junctions are critical for maintaining
cellular architecture and are comprised of several different cell
adhesion molecules (CAM) (1). Thus,
the four major CAM families are selectins, cadherins, integrins,
and the immunoglobulin CAM superfamily (2). The extracellular domain of selectins
consists of a calcium-dependent lectin domain, an epidermal growth
factor (EGF)-like domain, a domain homologous to EGF, and two to
nine consensus repeats. Selectins also contain a hydrophobic
transmembrane domain and a short cytoplasmic tail. The cadherins
are calcium-dependent glycoproteins that have an extracellular
domain CAM with three to five internal repeats, along with a
single-span transmembrane domain and an intracellular domain
(2). Integrins are composed of two
or more noncovalently associated membrane-spanning subunits labeled
α and β (3). The specific
combination of α and β subunits confers specificity for different
extracellular ligands and their concomitant intracellular signaling
events, and each represents a significant receptor family within
the context of interaction with the extracellular matrix (3). The Ig-CAMs are calcium-independent,
with an extracellular domain comprising a ligand-binding region of
four to six Ig-like repeats, one to five fibronectin-like repeats,
a transmembrane domain, and an intracellular component (1). While these families predominate,
numerous CAMs do not share any structural similarities with them,
one of which is epithelial cell adhesion molecule (EpCAM) (4).
EpCAM was discovered approximately 40 years ago and
was one of the first identified human tumor-associated biomarkers
(5). These days, EpCAM is also
considered as a marker for tumor-initiating cells (6). EpCAM is a transmembrane,
calcium-independent, homophilic, intercellular adhesion
glycoprotein. It has three distinct domains, extracellular,
transmembrane, and intracellular. In addition to cell adhesion,
EpCAM functions include cell signaling, differentiation,
proliferation, and migration (4).
EpCAM has been implicated in carcinogenesis and is expressed
robustly in numerous human epithelial cancers, including cancers of
the lung, colon, breast, ovary, cervix, and oral cavity, making it
a promising target for cancer diagnosis and therapy (7–9).
Oral cancers account for approximately 2% of all
cancer cases worldwide (10). More
than 350,000 individuals are diagnosed with oral cancer each year,
and it is ultimately fatal in nearly half of all cases (11,12).
Of the defined histological types of oral cancers, >90% of
patients develop oral squamous cell carcinoma (OSCC) on the lips or
within the oral cavity (13). The
most effective current treatments for OSCC vary. Stage-I and -II
OSCCs are treated with surgery or radiotherapy; advanced stage-III
and -IV disease are treated with a combination of excision,
radiation, and chemotherapy (14).
Typically, chemotherapeutic regimens include cisplatin as a
first-line agent; it is often combined with docetaxel or
5-fluorouracil (15,16). Other anticancer drugs, such as
paclitaxel, methotrexate, and carboplatin, have also been used for
OSCCs (17). Effective
molecular-targeting drugs, however, including antibody therapies,
remain lacking.
In the present study, we developed a set of new
anti-EpCAM monoclonal antibodies (mAbs), using Cell-Based
Immunization and Screening (CBIS) methods (18). We then tested whether these
anti-EpCAM mAbs induced antibody-dependent cellular cytotoxicity
(ADCC), complement-dependent cytotoxicity (CDC), or other antitumor
activity against oral cancers in a murine xenograft model.
Materials and methods
Plasmids
The Genome Network Project clone IRAK021G03 (EpCAM)
was provided by the RIKEN BioResource Research Center through the
National BioResource Project of the MEXT and AMED agencies of Japan
(19–22). EpCAM DNA plus a C-terminal PA tag
that is recognized by the anti-PA tag mAb (NZ-1), was subcloned
into a pCAG-Ble vector (FUJIFILM Wako Pure Chemical
Corporation).
Cell lines
P3X63Ag8U.1 (P3U1) and CHO-K1 cells were obtained
from the American Type Culture Collection (ATCC). CHO/EpCAM was
established by transfecting pCAG/EpCAM-PA into CHO-K1 cells using
the Neon Transfection System (Thermo Fisher Scientific, Inc.). A
few days after transfection, cells positive for anti-EpCAM mAb
(clone 9C4; cat. no. 324202; BioLegend, Inc.) were sorted using a
cell sorter (SH800; Sony Biotecnology Corp.), and stable
transfectants were cultured at 37°C for 14 days on media containing
0.5 mg/ml zeocin (InvivoGen). OSCC cell lines, including SAS
(tongue) and HSC-2 (oral cavity), were obtained from the Japanese
Collection of Research Bioresources Cell Bank. P3U1, CHO-K1 and
CHO/EpCAM were cultured in RPMI-1640 media (Nacalai Tesque, Inc.).
SAS and HSC-2 were cultured in Dulbecco's modified Eagle's medium
(DMEM; Nacalai Tesque, Inc.). The media were supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific
Inc.), 100 U/ml of penicillin (Nacalai Tesque, Inc.), 100 µg/ml
streptomycin (Nacalai Tesque, Inc.), and 0.25 µg/ml amphotericin B
(Nacalai Tesque, Inc.), and incubated at 37°C for 14 days in a
humidified atmosphere containing 5% CO2.
Antibodies
Purified mouse IgG (cat. no. I8765) and mouse
IgG2a (cat. no. M7769) were purchased from
Sigma-Aldrich; Merck KGaA. Anti-EpCAM mAbs were purified using
Protein G-Sepharose (GE Healthcare Bio-Sciences).
Animals
Thirty-eight 5-week-old female BALB/c nude mice
(mean weight, 15±3 g) were purchased from Charles River
Laboratories, Inc. All animal experiments were performed in
accordance with institutional guidelines and regulations to
minimize animal suffering and distress in the laboratory. The
Institutional Committee for experiments of the Institute of
Microbial Chemistry (Permit no. 2019-066) approved the animal
studies for ADCC and antitumor activity. Mice were maintained in a
specific pathogen-free environment on an 11-h light/13-h dark cycle
at a temperature of 23±2°C and 55±5% humidity with food and water
supplied ad libitum throughout the experiments. Mice were
monitored for health and weight every 1 or 4 days. Experiments on
mice were conducted in three or fewer weeks. Weight loss exceeding
25% or tumor size exceeding 3,000 mm3 were identified as
humane endpoints for euthanasia. At humane and experimental
endpoints, mice were euthanized by cervical dislocation, and death
was verified by validating respiratory and cardiac arrest.
Hybridoma production
We used a CBIS method (18) to develop mAbs against EpCAM.
Briefly, one BALB/c mouse was intraperitoneally (i.p.) immunized
with CHO/EpCAM cells (1×108) along with Imject Alum
adjuvant (Thermo Fisher Scientific, Inc.). The procedure included
three additional immunizations, followed by a final booster
injection administered i.p. two days before spleen cell harvesting.
Spleen cells were then fused with P3U1 cells using PEG1500 (Roche
Diagnostics). Hybridomas were grown at 37°C for 10 days in
RPMI-1640 media with HAT Supplement (50×) (cat. no. 21060017;
Thermo Fisher Scientific, Inc.) for selection. Culture supernatants
were screened using flow cytometry.
Flow cytometry
Cells (2×105 cells/ml) were harvested
after brief exposure to 0.25% trypsin in 1 mM
ethylenediaminetetraacetic acid (EDTA; Nacalai Tesque, Inc.). After
washing with 0.1% bovine serum albumin (BSA; Nacalai Tesque, Inc.)
in phosphate-buffered saline (PBS; Nacalai Tesque, Inc.), cells
were treated with 1 µg/ml of anti-EpCAM mAbs for 30 min at 4°C, and
then with Alexa Fluor 488-conjugated anti-mouse IgG (1:1,000;
product no. 4408; Cell Signaling Technology, Inc.). Fluorescence
data were collected using an EC800 Cell Analyzer (Sony
Biotechnology Corp.).
Western blot analyses
Cell pellets were resuspended in PBS with 1% Triton
X-100 (cat. no. 168-11805; FUJIFILM Wako Pure Chemical Corporation)
and 50 µg/ml aprotinin (product no. 03346-84; Nacalai Tesque,
Inc.). Protein concentration was determined by BCA method. Cell
lysates were boiled in sodium dodecyl sulfate sample buffer
(Nacalai Tesque, Inc.). The samples (10 µg/lane) were then
electrophoresed on 5-20% polyacrylamide gels (Nacalai Tesque, Inc.)
and transferred onto polyvinylidene difluoride (PVDF) membranes
(Merck KGaA). After blocking with 4% skim milk (Nacalai Tesque,
Inc.) for 1 h, the membrane was incubated with an anti-EpCAM mAb (5
µg/ml) or anti-β-actin (1 µg/ml; clone AC-15; cat. no. A5441;
Sigma-Aldrich; Merck KGaA) for 1 h, followed by incubation with
HRP-conjugated anti-mouse immunoglobulins (cat. no. P0260; Agilent
Technologies, Inc.) or anti-rat IgG (cat. no. A9542; Sigma-Aldrich;
Merck KGaA) at a 1:2,000 dilution for 1 h. The membrane was
developed using the ImmunoStar LD Chemiluminescence Reagent
(FUJIFILM Wako Pure Chemical Corporation) and a Sayaca-Imager (DRC
Co., Ltd.). All western blot procedures were performed at room
temperature.
Immunohistochemical analyses
Histologic sections 4-µm thick of an oral cancer
tissue array (cat. no. OR481; US Biomax, Inc.) were autoclaved
directly in EnVision FLEX Target Retrieval Solution, High pH
(Agilent Technologies, Inc.) for 20 min. Sections were then
incubated with 10 µg/ml of an anti-EpCAM mAb for 1 h at room
temperature and treated using an Envision+ kit (Agilent
Technologies, Inc.) for 30 min according to the manufacturer's
instructions. The color was developed using 3, 3′-diaminobenzidine
tetrahydrochloride (DAB; Agilent Technologies Inc.) for 2 min at
room temperature, and sections were then counterstained with
hematoxylin (FUJIFILM Wako Pure Chemical Corporation) at room
temperature for 5 min. Hematoxylin and eosin (H&E) staining
(FUJIFILM Wako Pure Chemical Corporation) was performed using
consecutive tissue sections at room temperature for 5 min. Leica
DMD108 (Leica Microsystems GmbH) was used to examine the sections
and obtain images (×100 and ×400).
Determination of the binding
affinity
Cells (2×105 cells/ml) were suspended in
100 µl of serially diluted anti-EpCAM mAb (6 ng/ml-100 µg/ml),
followed by the addition of Alexa Fluor 488-conjugated anti-mouse
IgG (1:200; cat. no. 4408; Cell Signaling Technology, Inc.).
Fluorescence data were collected using an EC800 Cell Analyzer (Sony
Biotechnology Corp.). The dissociation constant
(KD) was calculated by fitting binding isotherms
to built-in, one-site binding models in GraphPad Prism 6 (GraphPad
Software, Inc.).
ADCC
ADCC inducement by EpCAM was assayed as follows. Six
female five-week-old BALB/c nude mice (mean weight, 15±3 g) were
purchased from Charles River Laboratories, Inc. After euthanasia by
cervical dislocation, spleens were removed aseptically, and
single-cell suspensions were obtained by forcing spleen tissues
through a sterile cell strainer (product no. 352360; BD Falcon;
Corning, Inc.) with a syringe. Erythrocytes were lysed with a
10-sec exposure to ice-cold distilled water. The splenocytes were
washed with DMEM and resuspended in DMEM with 10% FBS; this
preparation was designated as effector cells. The target tumor
cells were labeled with 10 µg/ml Calcein-AM (Thermo Fisher
Scientific, Inc.) and resuspended in the same medium. The target
cells were transferred to 96-well plates, at 2×104
cells/well, and mixed with effector cells at an effector-to-target
ratio of 50:1, along with 100 µg/ml of anti-EpCAM antibodies or
control mouse IgG2a. After a 4-h incubation at 37°C,
Calcein-AM release into the supernatant was measured for each well.
Fluorescence intensity was assessed using a microplate reader
(Power Scan HT; BioTek Instruments, Inc.) with an excitation
wavelength of 485 nm and an emission wavelength of 538 nm.
Cytolytic activity was measured as a percentage of lysis and
calculated using the equation: Percentage of lysis (%) =
(E-S)/(M-S) ×100, where E is the fluorescence measured in combined
cultures of target and effector cells, S is the spontaneous
fluorescence of the target cells, and M is the maximum fluorescence
measured after lysis of all cells with buffer containing 0.5%
Triton X-100, 10 mM Tris-HCl (pH 7.4), and 10 mM EDTA.
CDC
CDC inducement by EpCAM was assayed as follows.
Target cells were labeled with 10 µg/ml Calcein-AM (Thermo Fisher
Scientific, Inc.) and resuspended in medium. Target cells were
plated in 96-well plates, at 2×104 cells/well, and 10%
rabbit complement (Low-Tox-M rabbit complement; Cedarlane
Laboratories), 100 µg/ml of anti-EpCAM antibodies, or control IgG
(mouse IgG2a) was added to each well. After 4 h of
incubation at 37°C, Calcein-AM release into the supernatant was
measured for each well. Fluorescence intensity was calculated as
described in the ADCC section above.
Antitumor activity of anti-EpCAM mAbs
in xenografts of oral cancers
Thirty-two five-week-old female BALB/c nude mice
were purchased from Charles River Laboratories Japan, Inc. After a
two-week acclimation period, these mice were used in experiments at
seven weeks of age (mean weight, 16±2 g). HSC-2 and SAS cells (0.3
ml of 1.33×108 cells/ml in DMEM) were mixed with 0.5 ml
BD Matrigel Matrix Growth Factor Reduced (BD Biosciences), and 100
µl of this suspension (5×106 cells) was injected
subcutaneously into the left flank of each animal. On day 1
post-inoculation, 100 µg of an anti-EpCAM mAb or control mouse IgG
in 100 µl PBS was injected i.p. Additional antibody inoculations
were performed on days 8 and 14. Seventeen days or eighteen days
after cell implantation, all mice were euthanized by cervical
dislocation, and tumor diameters and volumes were measured and
recorded.
Statistical analyses
All data are expressed as mean ± SEM. Statistical
analysis was conducted with one-way ANOVA and Tukey's multiple
comparisons tests for ADCC and CDC, one-way ANOVA and Sidak's
multiple comparisons tests for tumor volume and mouse weight, and
Welch's t-test for tumor weight. All calculations were performed
with GraphPad Prism 7 (GraphPad Software, Inc.). A P-value of
<0.05 was considered to indicate a statistically significant
difference.
Results
Development and characterization of
anti-EpCAM mAbs
Anti-EpCAM mAbs were developed by immunizing a
single mouse with CHO/EpCAM cells. Hybridomas were cultured, and
supernatants positive for CHO/EpCAM and negative for CHO-K1 were
selected by flow cytometry. Further screening using western blot
and immunohistochemical assays were performed for validation,
resulting in EpMab-16 (IgG2a, kappa) identification and
establishment.
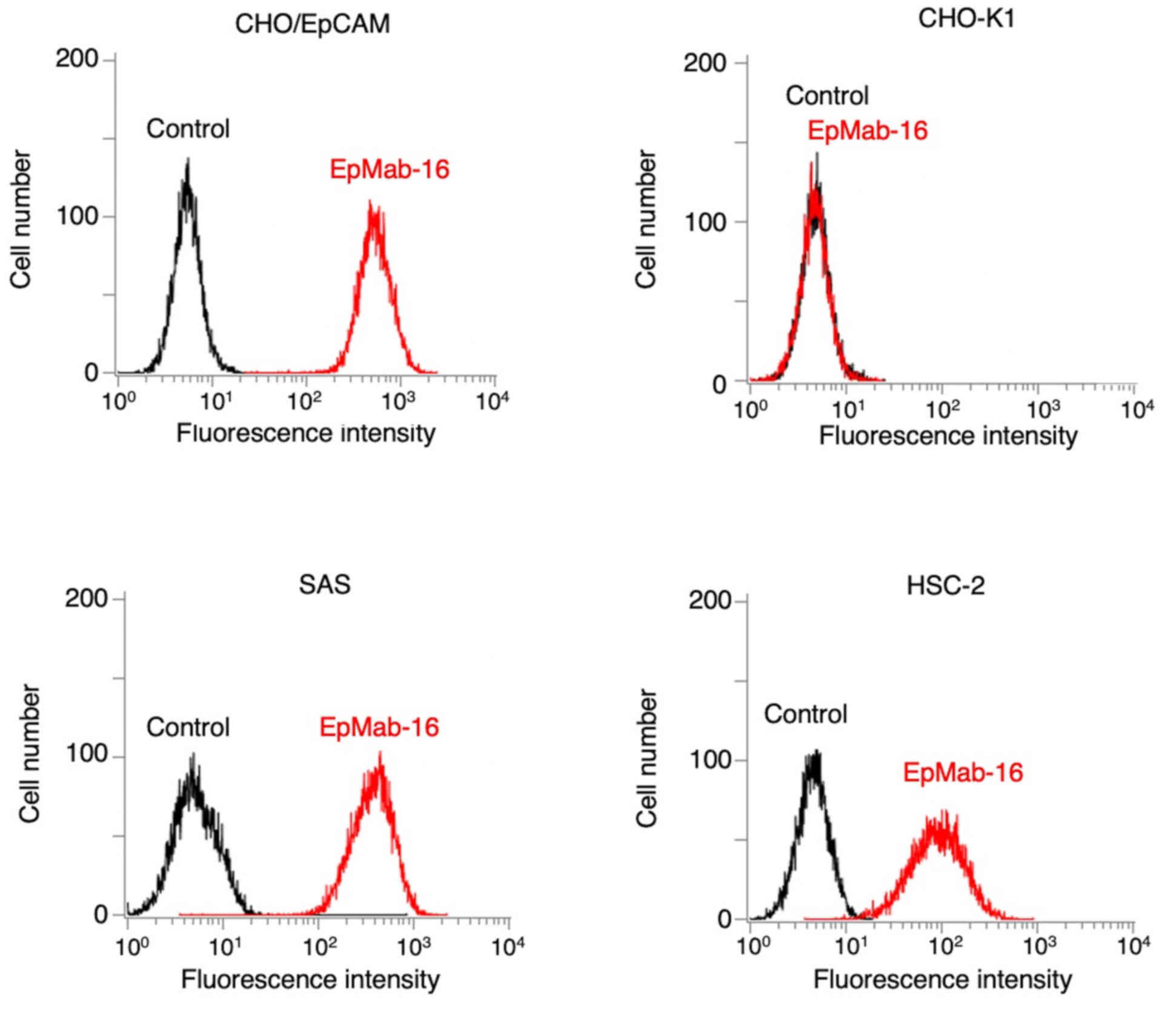
Flow cytometry was then used to assess the
sensitivity of EpMab-16 in CHO/EpCAM and OSCC cell lines (SAS and
HSC-2). EpMab-16 reacted with CHO/EpCAM cells but not with CHO-K1
cells (Fig. 1). EpMab-16 also
reacted with SAS and HSC-2 cells (Fig.
1). Flow cytometric data indicated that EpMab-16 was highly
sensitive and highly specific for EpCAM.
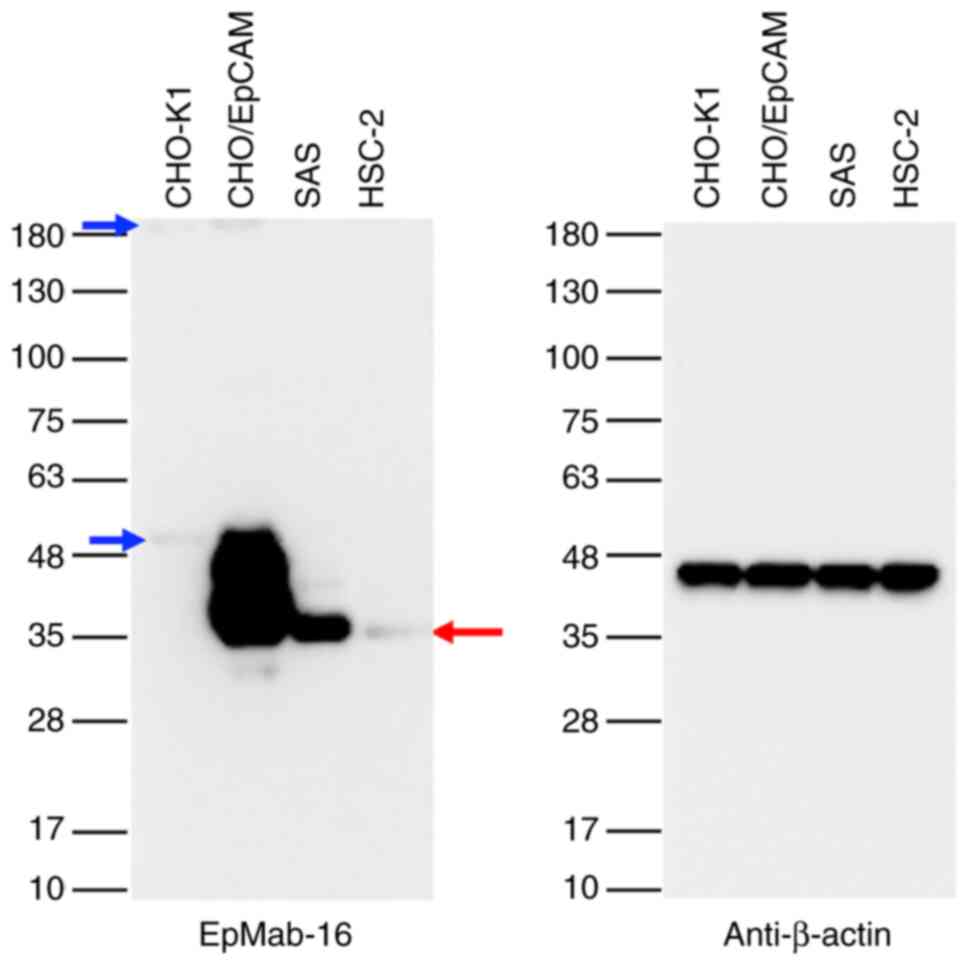
Then a western blot assay was conducted to delineate
the sensitivity of EpMab-16 further. Lysates of CHO/EpCAM, SAS, and
HSC-2 cells were probed, and the results revealed that EpMab-16
detected 35 kDa band of EpCAM strongly in cell lysates from
CHO/EpCAM, whereas it detected EpCAM moderately in cell lysates
from SAS, and faintly from HSC-2 cells, indicating that EpMab-16
could detect both exogenous and endogenous EpCAM (Fig. 2).
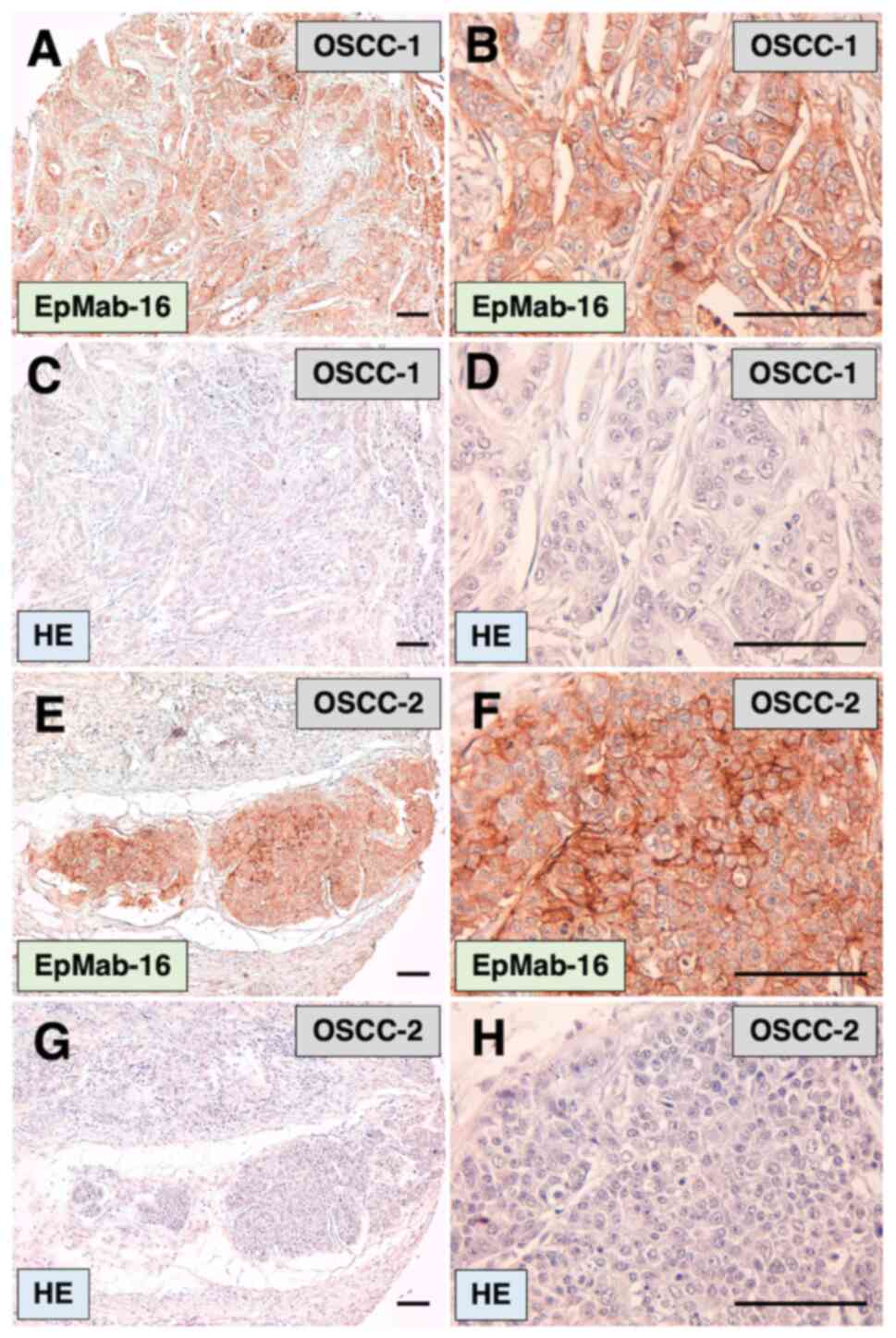
Immunohistochemical analysis results revealed that
EpMab-16 detected membrane antigens in oral cancer tissues
(Fig. 3A, B, E and F). Among 38
OSCC cases in the oral cancer tissue array, 6 cases (16%) were
stained by EpMab-16. In this staining, antigen retrieval using
EnVision FLEX Target Retrieval Solution High pH was performed. The
signal was lower for citrate buffer (pH 6.0) antigen retrieval
(data not shown), such that antigen retrieval using high pH was
deemed essential for immunostaining using EpMab-16. Hematoxylin and
eosin (H&E) staining was performed using consecutive OSCC
tissues (Fig. 3C, D, G and H).
Results indicated that EpMab-16 detected EpCAM in
immunohistochemical analysis on formalin-fixed paraffin-embedded
(FFPE) tissues effectively.
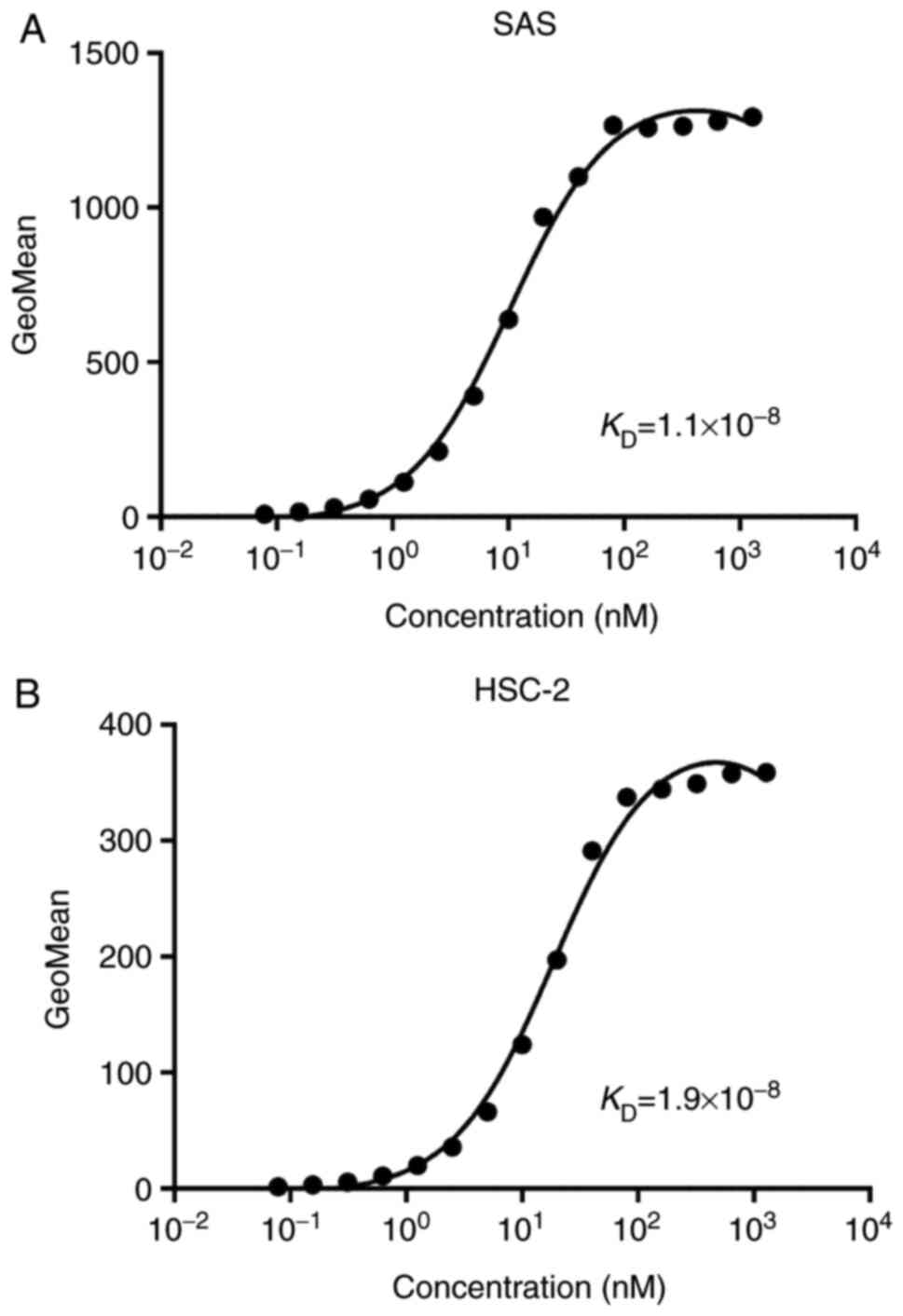
Then a kinetic analysis of the interactions of
EpMab-16 with SAS and HSC-2 oral cancer cell lines was then
conducted using flow cytometry. The KD for
EpMab-16 in SAS cells was 1.1×10−8 M, and the
KD for EpMab-16 against HSC-2 cells was
1.9×10−8 M (Fig. 4),
indicating a moderate binding affinity of EpMab-16 against oral
cancer cells.
ADCC and CDC activities of EpMab-16 in
oral cancer cell lines
We then examined whether EpMab-16 (mouse
IgG2a) induced ADCC and CDC antitumor action in
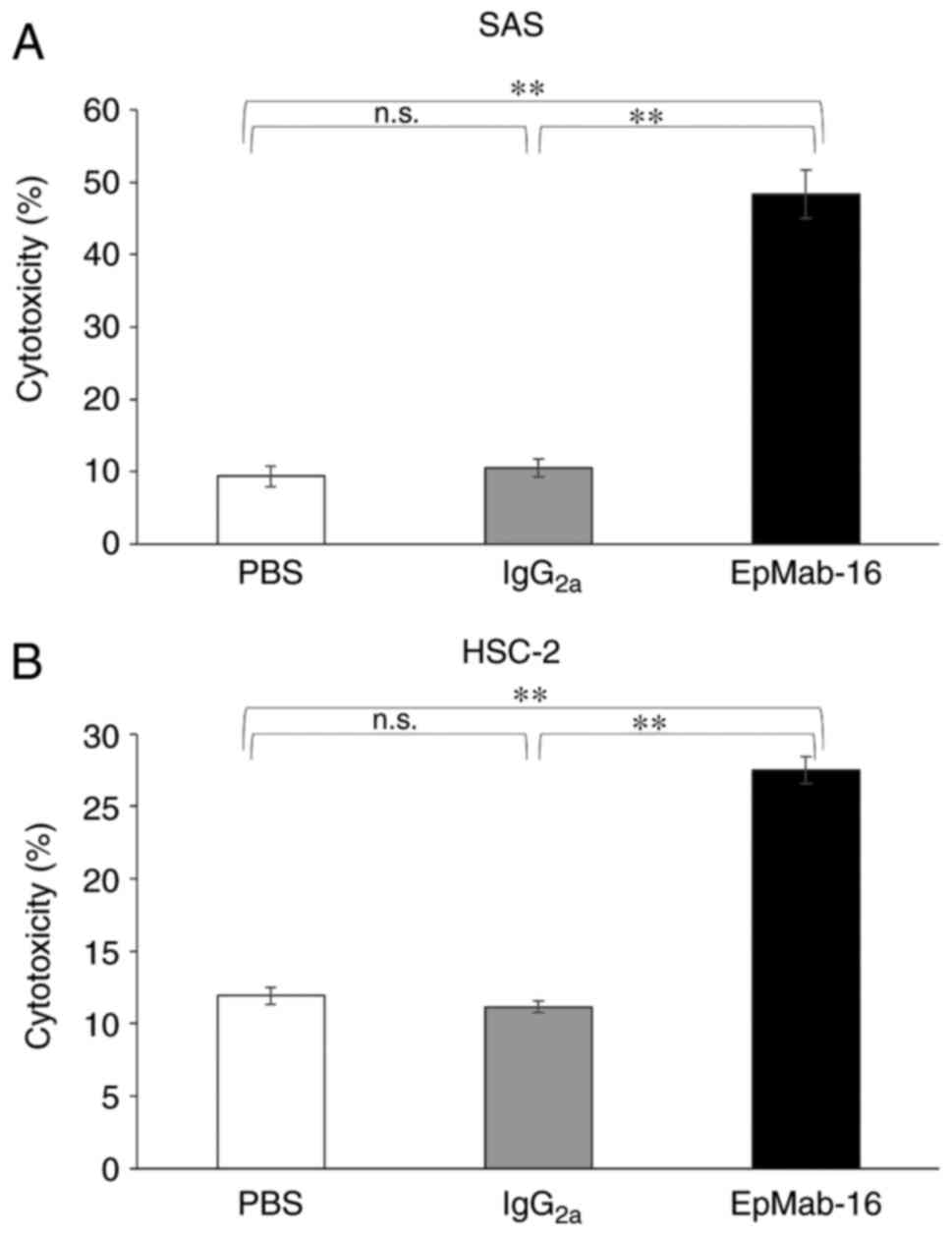
EpCAM-expressing SAS and HSC-2 oral cancer cell lines. EpMab-16
exhibited higher ADCC (48% cytotoxicity) in SAS cells than that of
control mouse IgG2a (11% cytotoxicity; P<0.01) or
control PBS (9.4% cytotoxicity; P<0.01) treatment (Fig. 5A). Similarly, EpMab-16 exhibited
higher ADCC (27% cytotoxicity) in HSC-2 cells than that of control
mouse IgG2a (11% cytotoxicity; P<0.01) or control PBS
treatment (12% cytotoxicity; P<0.01) (Fig. 5B).
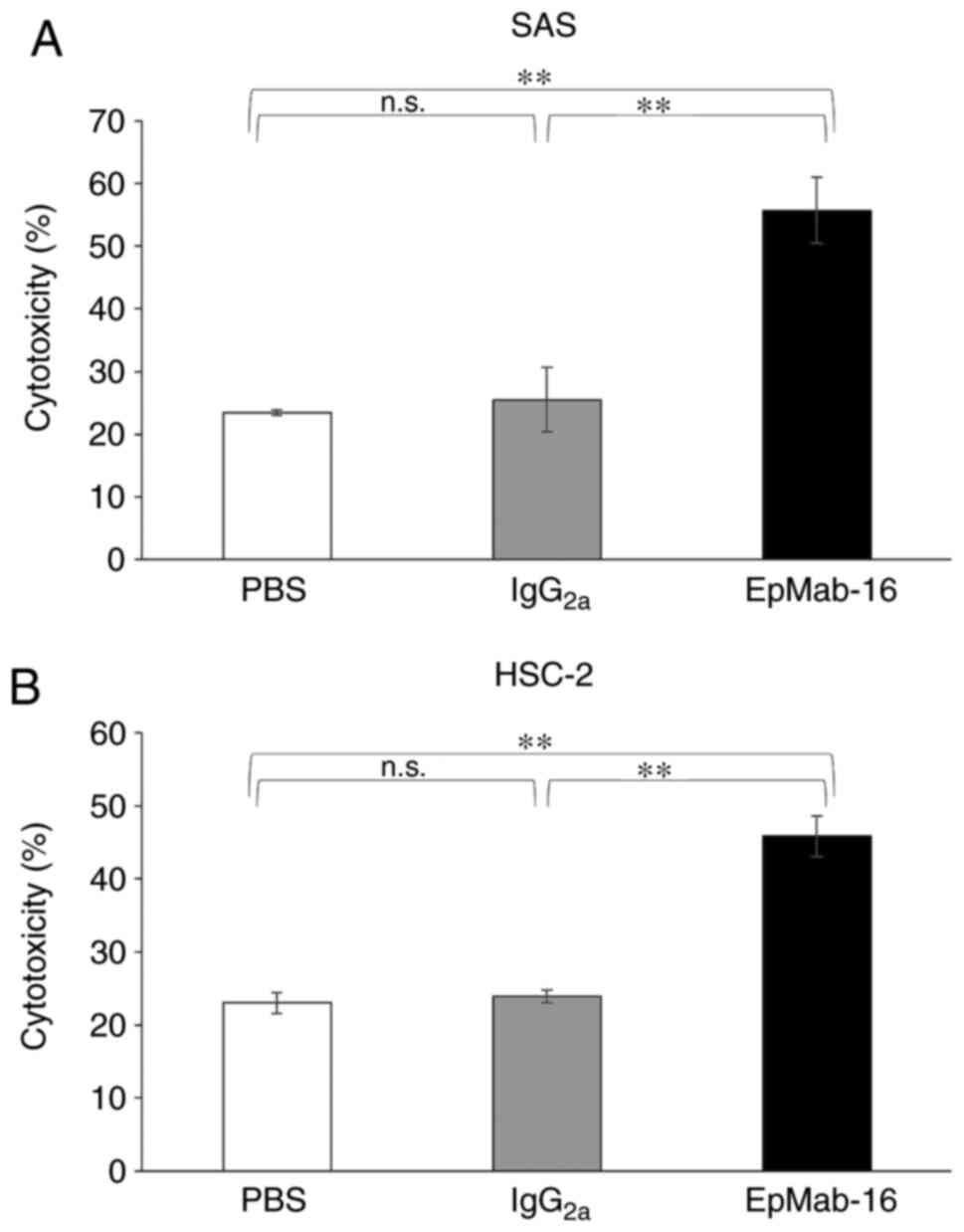
EpMab-16 was also associated with more robust CDC
activity (56% cytotoxicity) in SAS cells than control mouse
IgG2a (26% cytotoxicity; P<0.01) or control PBS
treatment (23% cytotoxicity; P<0.01) (Fig. 6A). Similarly, EpMab-16-treated cells
exhibited more CDC activity (46% cytotoxicity) in HSC-2 cells than
control mouse IgG2a (24% cytotoxicity; P<0.01) or
control PBS treatment (23% cytotoxicity; P<0.01) (Fig. 6B). These favorable ADCC and CDC
activity results indicated that EpMab-16 may induce strong
antitumor action against oral cancer cells in vivo as well
as in vitro.
EpMab-16 antitumor effect in mouse
xenografts of SAS oral cancer cells
On days 1, 8, and 14 after SAS cell injections into
the mice, EpMab-16 (100 µg) or control mouse IgG (100 µg) were
injected i.p. in SAS xenograft model mice. Tumor formation was
observed in mice from treatment and control groups. Tumor volume
was measured on days 7, 10, 14, and 17 after SAS cell injection.
EpMab-16-treated mice exhibited significantly less tumor growth on
day 10 (P<0.05), day 14 (P<0.05) and day 17 (P<0.01)
compared with IgG-treated control mice (Fig. 7A). Tumor volume reduction by
EpMab-16 treatment was 37% as of day 17. Tumors from
EpMab-16-treated mice weighed significantly less than tumors from
IgG-treated control mice (20% reduction, P<0.05; Fig. 7B). Resected tumors on day 17 are
presented in Fig. 7C. Total body
weights did not significantly differ between the treatment and
control groups (Fig. S1). These
results indicated that EpMab-16 reduced the growth of SAS
xenografts, but did not altogether eliminate them.
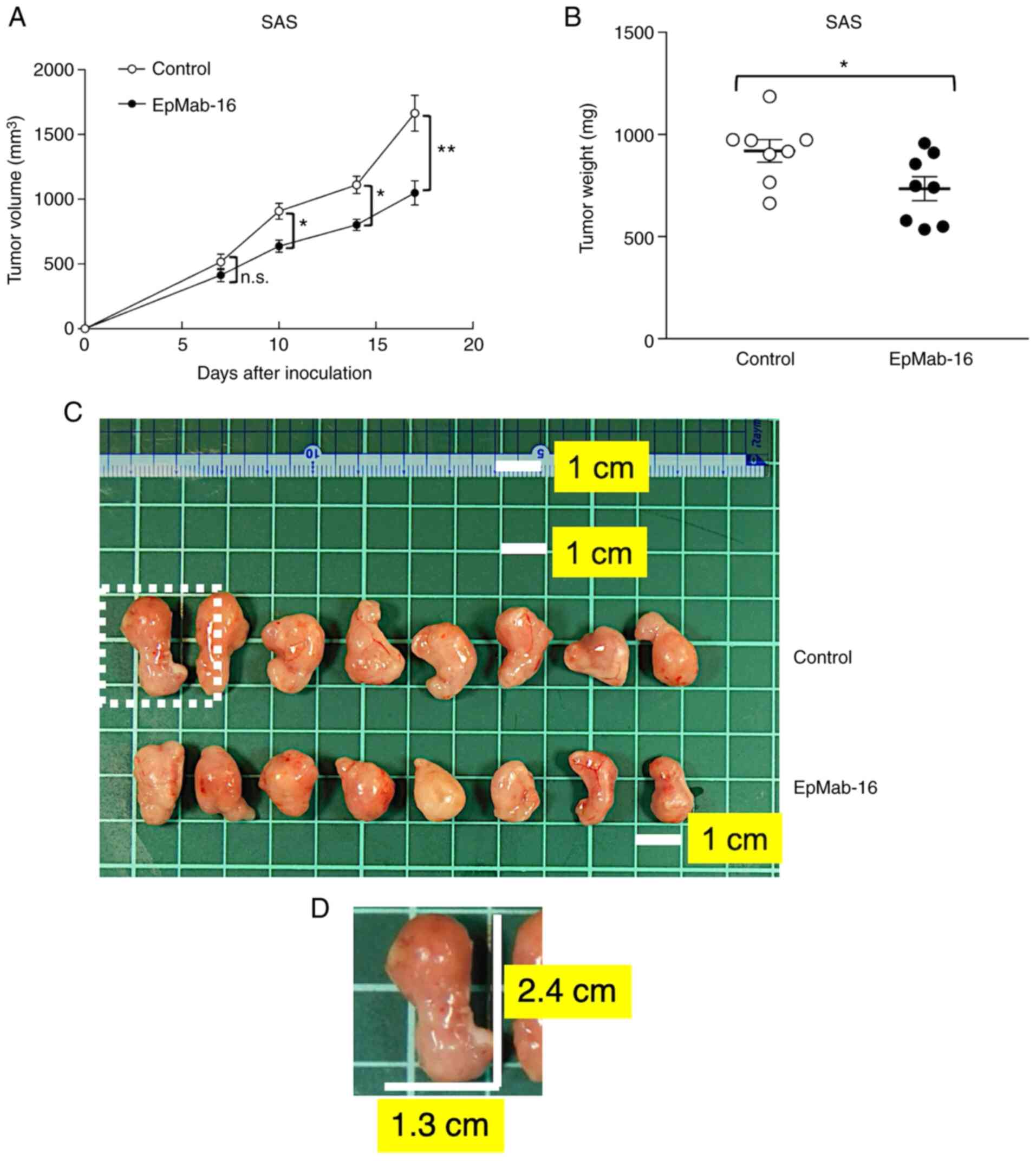 | Figure 7.Antitumor activity of an anti-EpCAM
mAb in SAS xenografts. (A) Tumor volume of SAS xenografts. SAS
cells (5×106 cells) were injected subcutaneously into
the left flank, and 100 µg of EpMab-16 or control mouse IgG in 100
µl PBS was injected i.p. into treatment and control mice,
respectively. Additional antibodies were injected on days 8 and 14.
The tumor volume was measured on days 7, 10, 14, and 17. Values
represent the mean ± SEM. *P<0.05 and **P<0.01 (determined by
ANOVA and Sidak's multiple comparisons tests) (B) Tumor weights of
SAS xenografts. Tumors of SAS xenografts were resected from
EpMab-16 and control mouse IgG groups. The tumor weight on day 17
was measured from excised xenografts. Values represent the mean ±
SEM. *P<0.05 (determined by Welch's t-test). (C) Resected tumors
of SAS xenografts from EpMab-16 and control mouse IgG groups on day
17. The tumor in the dotted region was the largest tumor in this
experiment. The white square grids under the tumors are 1×1 cm
each. Scale bar, 1 cm. (D) A magnified image of the largest tumor
in the dotted region of C. The vertical and horizontal lengths for
SAS cells were 2.4 and 1.3 cm, respectively (estimated tumor
volume, 2028 mm3). All mice possessed one tumor each.
EpCAM, epithelial cell adhesion molecule; mAb, monoclonal antibody;
PBS, phosphate-buffered saline; SEM, standard error of the mean;
n.s., not significant. |
EpMab-16 antitumor effect in mouse
xenografts of HSC-2 oral cancer cells
On days 1, 8, and 14 after HSC-2 cell injections,
EpMab-16 (100 µg) or control mouse IgG (100 µg) were injected i.p.
into HSC-2 ×enograft model mice. Tumor formation was observed in
mice in the treatment and control groups. Tumor volume was measured
on days 7, 10, 14, and 18 after HSC-2 cell injection. The
EpMab-16-treated mice exhibited significantly less tumor growth on
day 18 (P<0.01) than the IgG-treated control mice (Fig. 8A). Tumor volume reduction by
EpMab-16 treatment was 33% as of day 18. Tumors from
EpMab-16-treated mice weighed significantly less than tumors from
IgG-treated control mice (21% reduction, P<0.05; Fig. 8B). Resected tumors on day 18 are
presented in Fig. 8C. Total body
weights did not significantly differ between the treatment and
control groups (Fig. S1). These
results indicated that EpMab-16 reduced the growth of HSC-2
×enografts, although it did not eliminate them.
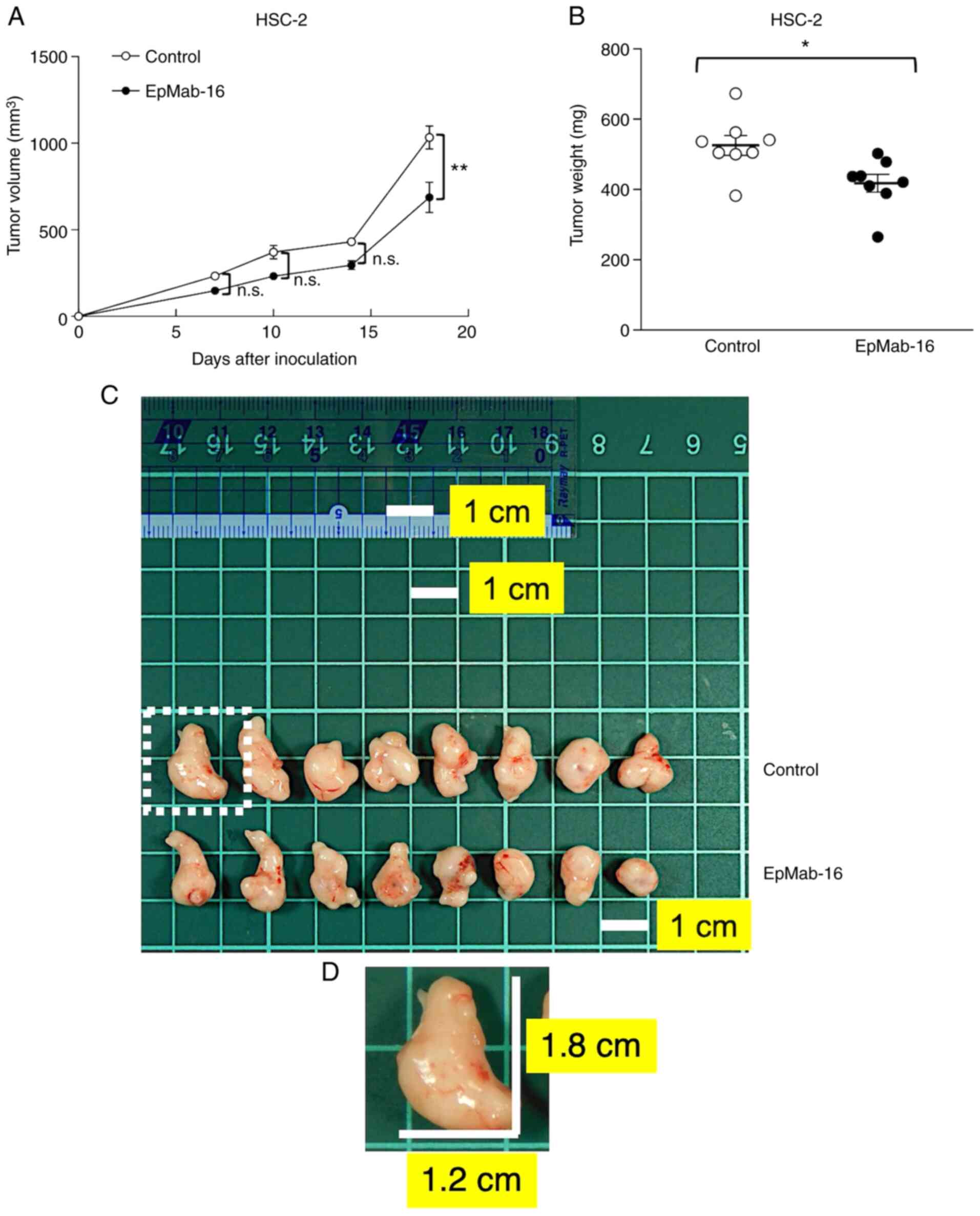 | Figure 8.Antitumor activity of an anti-EpCAM
mAb in HSC-2 ×enografts. (A) Tumor volume of HSC-2 ×enografts.
HSC-2 cells (5×106 cells) were injected subcutaneously
into the left flank, and 100 µg of EpMab-16 or control mouse IgG in
100 µl PBS was injected i.p. into treatment and control mice,
respectively. Additional antibodies were then injected on days 8
and 14. The tumor volume was measured on days 7, 10, 14, and 18.
Values represent the mean ± SEM. **P<0.01 (determined by ANOVA
and Sidak's multiple comparisons tests). (B) Tumor weights of HSC-2
×enografts. Tumors of HSC-2 ×enografts were resected from EpMab-16
and control mouse IgG groups. The tumor weight on day 18 was
measured from excised xenografts. Values represent the mean ± SEM.
*P<0.05 (determined by Welch's t-test). (C) Resected tumors of
HSC-2 ×enografts from EpMab-16 and control mouse IgG groups on day
18. The tumor in the dotted region was the largest tumor in this
experiment. The white square grids under the tumors are 1×1 cm
each. Scale bar, 1 cm. (D) A magnified image of the largest tumor
in the dotted region of C. The vertical and horizontal lengths for
HSC-2 cells were 1.8 and 1.2 cm, respectively (estimated tumor
volume, 1296 mm3). All mice possessed one tumor each.
EpCAM, epithelial cell adhesion molecule; mAb, monoclonal antibody;
PBS, phosphate-buffered saline; SEM, standard error of the mean;
n.s., not significant. |
Discussion
EpCAM has been reported to be overexpressed in
several cancers (23–27). EpCAM is overexpressed in 55–75% of
ovarian cancers (24), and is
overexpressed in over 90% of ovarian cancer metastatic lesions
(25). Overexpression of EpCAM has
been revealed to be correlated with poor prognosis, therapeutic
failure and early tumor recurrence in hepatocellular carcinoma
(HCC) patients (26). EpCAM has
also been revealed to play important roles in proliferation,
apoptosis, and metastasis during breast cancer progression
(27). Therefore, EpCAM should be a
promising novel therapeutic target.
In the present study, it was investigated whether
anti-EpCAM mAbs could be useful for treating oral cancers via ADCC
and CDC activity. We first developed a sensitive and specific
anti-EpCAM mAb (EpMab-16, mouse IgG2a), which exhibited
favorable potential in flow cytometry, western blot and
immunohistochemical analyses. The present results also suggested
that EpMab-16 had diagnostic efficacy in FFPE tissues because
pathological diagnosis utilizes FFPE tissues. It was also
demonstrated that EpMab-16 was associated with strong ADCC and CDC
activity against SAS and HSC-2 OSCC cell lines in vitro. The
ADCC and CDC activity of EpMab-16 against OSCC was more pronounced
in SAS cells than HSC-2 cells, presumably because the EpCAM
expression level and binding affinity are greater in SAS than in
HSC-2. ADCC and CDC activities of EpMab-16 against Caco-2, a colon
cancer cell line were also assessed, and values were 44 and 49%,
respectively (data not shown), suggesting that EpMab-16 could
induce observable ADCC and CDC in other EpCAM-expressing cancers in
addition to the oral cancer cell lines assessed in this study.
It was then investigated whether EpMab-16 exerted
antitumor function against OSCC xenografts in vivo. Although
EpMab-16 significantly reduced not only the growth of SAS and HSC-2
×enografts, but also the tumor weight of SAS and HSC-2 ×enografts,
the tumor reduction was not sufficient to eliminate them. We
previously investigated whether podocalyxin (PODXL) could be a
therapeutic target in OSCC using anti-PODXL mAbs developed by
converting an anti-PODXL mAb of IgG1 subclass (PcMab-47)
into a mouse IgG2a-type mAb (47-mG2a) to
increase ADCC (28). This was
further developed into 47-mG2a-f, a core
fucose-deficient variant of 47-mG2a, also to increase
its ADCC. In vivo analyses demonstrated that
47-mG2a-f, but not 47-mG2a, exerted antitumor
activity in SAS and HSC-2 ×enograft models at a dose of 100
µg/mouse/week administered three times, indicating that a core
fucose-deficient anti-PODXL mAb could be profitable for
antibody-based therapy against PODXL-expressing OSCCs (28). We thereby anticipate that developing
a core fucose-deficient variant of EpMab-16 will reveal a similar
enhancement of ADCC activity in a future study.
Results of this study and our previous work, which
includes the development of mAbs effective in SAS and HSC-2
×enografts against epidermal growth factor receptor (EGFR) (clone
EMab-17, mouse IgG2a) (29), human epidermal growth factor
receptor 2 (HER2) (clone H2Mab-19, mouse
IgG2b) (30), and
cancer-specific mAb (CasMab) against podoplanin (PDPN) (31), suggest that the targeting of several
molecules, such as PODXL, EGFR, HER2, PDPN, as well as EpCAM, could
be an effective therapy to cure OSCCs. In the future,
cancer-specific anti-EpCAM mAbs may also be developed that can
reduce the adverse effects of traditional antibody therapy.
Supplementary Material
Supporting Data
Acknowledgements
We would like to thank Ms. Saori Handa, Ms. Saki
Okamoto, and Mr. Yu Komatsu (Department of Antibody Drug
Development, Tohoku University Graduate School of Medicine) for
technical assistance of in vitro experiments, and Ms. Akiko
Harakawa (Institute of Microbial Chemistry (BIKAKEN), Numazu,
Microbial Chemistry Research Foundation) for technical assistance
of animal experiments.
Funding
This research was supported in part by the Japan
Agency for Medical Research and Development (AMED) under grant nos.
JP20am0401013 (to YK), JP20am0101078 (to YK), and JP20ae0101028 (to
YK), and by the Japan Society for the Promotion of Science (JSPS)
Grants-in-Aid for Scientific Research (KAKENHI) grant no. 17K07299
(to MKK), grant nos. 19K07705 (to YK), and 20K16322 (to MS).
Availability of data and materials
The datasets used and/or analyzed during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
HHo, JT, TO, TN, MS TA, YS, and MY performed the
experiments. MKK analyzed the experimental data. MK, HHa, and YK
designed the present study and wrote the manuscript. All the
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal studies for ADCC and the antitumor activity
were approved by the Institutional Committee for experiments of the
Institute of Microbial Chemistry (Permit no. 2019-066).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
|
BSA
|
bovine serum albumin
|
|
CBIS
|
Cell-Based Immunization and
Screening
|
|
CDC
|
complement-dependent cytotoxicity
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
FBS
|
fetal bovine serum
|
|
mAb
|
monoclonal antibody
|
|
OSCC
|
oral squamous cell carcinoma
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Okegawa T, Pong RC, Li Y and Hsieh JT: The
role of cell adhesion molecule in cancer progression and its
application in cancer therapy. Acta Biochim Pol. 51:445–457. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petruzzelli L, Takami M and Humes HD:
Structure and function of cell adhesion molecules. Am J Med.
106:467–476. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maheshwari G, Brown G, Lauffenburger DA,
Wells A and Griffith LG: Cell adhesion and motility depend on
nanoscale RGD clustering. J Cell Sci. 113:1677–1686.
2000.PubMed/NCBI
|
|
4
|
Trzpis M, McLaughlin PM, de Leij LM and
Harmsen MC: Epithelial cell adhesion molecule: More than a
carcinoma marker and adhesion molecule. Am J Pathol. 171:386–395.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herlyn M, Steplewski Z, Herlyn D and
Koprowski H: Colorectal carcinoma-specific antigen: Detection by
means of monoclonal antibodies. Proc Natl Acad Sci USA.
76:1438–1442. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Imrich S, Hachmeister M and Gires O: EpCAM
and its potential role in tumor-initiating cells. Cell Adh Migr.
6:30–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sen S and Carnelio S: Expression of
epithelial cell adhesion molecule (EpCAM) in oral squamous cell
carcinoma. Histopathology. 68:897–904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baeuerle PA and Gires O: EpCAM (CD326)
finding its role in cancer. Br J Cancer. 96:417–423. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Went P, Vasei M, Bubendorf L, Terracciano
L, Tornillo L, Riede U, Kononen J, Simon R, Sauter G and Baeuerle
PA: Frequent high-level expression of the immunotherapeutic target
Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer.
94:128–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tota JE, Anderson WF, Coffey C, Califano
J, Cozen W, Ferris RL, St John M, Cohen EE and Chaturvedi AK:
Rising incidence of oral tongue cancer among white men and women in
the United States, 1973–2012. Oral Oncol. 67:146–152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hussein AA, Helder MN, de Visscher JG,
Leemans CR, Braakhuis BJ, de Vet HCW and Forouzanfar T: Global
incidence of oral and oropharynx cancer in patients younger than 45
years versus older patients: A systematic review. Eur J Cancer.
82:115–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rivera C: Essentials of oral cancer. Int J
Clin Exp Pathol. 8:11884–11894. 2015.PubMed/NCBI
|
|
14
|
Güneri P and Epstein JB: Late stage
diagnosis of oral cancer: Components and possible solutions. Oral
Oncol. 50:1131–1136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vokes EE: Induction chemotherapy for head
and neck cancer: Recent data. Oncologist. 15 (Suppl 3):S3–S7. 2010.
View Article : Google Scholar
|
|
16
|
Marcazzan S, Varoni EM, Blanco E, Lodi G
and Ferrari M: Nanomedicine, an emerging therapeutic strategy for
oral cancer therapy. Oral Oncol. 76:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Furness S, Glenny AM, Worthington HV,
Pavitt S, Oliver R, Clarkson JE, Macluskey M, Chan KK and Conway
DI: Interventions for the treatment of oral cavity and
oropharyngeal cancer: Chemotherapy. Cochrane Database of Systematic
Reviews. CD0063862011.PubMed/NCBI
|
|
18
|
Itai S, Fujii Y, Nakamura T, Chang YW,
Yanaka M, Saidoh N, Handa S, Suzuki H, Harada H, Yamada S, et al:
Establishment of CMab-43, a sensitive and specific anti-CD133
monoclonal antibody, for immunohistochemistry. Monoclon Antib
Immunodiagn Immunother. 36:231–235. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ota T, Suzuki Y, Nishikawa T, Otsuki T,
Sugiyama T, Irie R, Wakamatsu A, Hayashi K, Sato H, Nagai K, et al:
Complete sequencing and characterization of 21,243 full-length
human cDNAs. Nat Genet. 36:40–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Otsuki T, Ota T, Nishikawa T, Hayashi K,
Suzuki Y, Yamamoto J, Wakamatsu A, Kimura K, Sakamoto K, Hatano N,
et al: Signal sequence and keyword trap in silico for selection of
full-length human cDNAs encoding secretion or membrane proteins
from oligo-capped cDNA libraries. DNA Res. 12:117–126. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kimura K, Wakamatsu A, Suzuki Y, Ota T,
Nishikawa T, Yamashita R, Yamamoto J, Sekine M, Tsuritani K,
Wakaguri H, et al: Diversification of transcriptional modulation:
Large-scale identification and characterization of putative
alternative promoters of human genes. Genome Res. 16:55–65. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Itoh M, Yasunishi A, Imamura K,
Kanamori-Katayama M, Suzuki H, Suzuki M, Carninci P, Kawai J and
Hayashizaki Y: Constructing ORFeome resources with removable
termination codons. Biotechniques. 41:44–46, 48 passim. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jing Y, Zhou L, Chen J, Xu H, Sun J, Cai
M, Jiang J, Gao J and Wang H: Quantitatively mapping the assembly
pattern of EpCAM on cell membranes with peptide probes. Anal Chem.
92:1865–1873. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vorobyeva A, Konovalova E, Xu T, Schulga
A, Altai M, Garousi J, Rinne SS, Orlova A, Tolmachev V and Deyev S:
Feasibility of imaging EpCAM expression in ovarian cancer using
radiolabeled DARPin Ec1. Int J Mol Sci. 21:33102020. View Article : Google Scholar
|
|
25
|
van den Brand D, van Lith SAM, de Jong JM,
Gorris MAJ, Palacio-Castañeda V, Couwenbergh ST, Goldman MRG,
Ebisch I, Massuger LF, Leenders WPJ, et al: EpCAM-binding DARPins
for targeted photodynamic therapy of ovarian cancer. Cancers
(Basel). 12:17622020. View Article : Google Scholar
|
|
26
|
Pandit H, Li Y, Zheng Q, Guo W, Yu Y, Li S
and Martin RCG: Carcinogenetic initiation contributed by EpCAM+
cancer cells in orthotopic HCC models of immunocompetent and
athymic mice. Oncotarget. 11:2047–2060. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang D, Yang L, Liu X, Gao J, Liu T, Yan
Q and Yang X: Hypoxia modulates stem cell properties and induces
EMT through N-glycosylation of EpCAM in breast cancer cells. J Cell
Physiol. 235:3626–3633. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Itai S, Ohishi T, Kaneko MK, Yamada S, Abe
S, Nakamura T, Yanaka M, Chang YW, Ohba SI, Nishioka Y, et al:
Anti-podocalyxin antibody exerts antitumor effects via
antibody-dependent cellular cytotoxicity in mouse xenograft models
of oral squamous cell carcinoma. Oncotarget. 9:22480–22497. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takei J, Kaneko MK, Ohishi T, Kawada M,
Harada H and Kato Y: A novel anti-EGFR monoclonal antibody
(EMab-17) exerts antitumor activity against oral squamous cell
carcinomas via antibody-dependent cellular cytotoxicity and
complement-dependent cytotoxicity. Oncol Lett. 19:2809–2816.
2020.PubMed/NCBI
|
|
30
|
Takei J, Kaneko MK, Ohishi T, Kawada M,
Harada H and Kato Y: H(2)Mab-19, an anti-human epidermal growth
factor receptor 2 monoclonal antibody exerts antitumor activity in
mouse oral cancer xenografts. Exp Ther Med. 20:846–853. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaneko MK, Nakamura T, Kunita A, Fukayama
M, Abe S, Nishioka Y, Yamada S, Yanaka M, Saidoh N, Yoshida K, et
al: ChLpMab-23: Cancer-specific human-mouse chimeric
anti-podoplanin antibody exhibits antitumor activity via
antibody-dependent cellular cytotoxicity. Monoclon Antib
Immunodiagn Immunother. 36:104–112. 2017. View Article : Google Scholar : PubMed/NCBI
|