Introduction
Gallbladder cancer (GBC) is among the most common
and lethal types of human malignancy (1). However, classical chemotherapy is
rarely effective and drug resistance represents a significant
challenge in treating GBC, particularly advanced GBC cells, which
exhibit a strong resistance to apoptosis-inducing chemotherapeutic
drugs owing to the reprograming of apoptosis (2). Therefore, novel therapeutic targets
are required to increase the pro-apoptotic effects of therapeutic
drugs in GBC.
TNF-related apoptosis induced ligand (TRAIL)
mediates the extrinsic apoptotic pathway by binding to Death
Receptor 4 or 5 on the cell surface. Upon binding of ligands to
these receptors, they assemble into homomeric and heteromeric
complexes and recruit FADD and caspase-8, which trigger a
downstream caspase cascade (3).
TRAIL has shown notable efficacy as a therapeutic drug owing to its
high selectivity of inducing apoptosis (4). However, an increasing number of
studies have shown that certain GBC cells respond poorly to TRAIL
alone, and cancer cell death is not induced (5,6). There
are numerous causes underlying the development of resistance to
TRAIL in GBC cells. As caspase-8 mediates TRAIL-induced apoptosis,
the TRAIL resistance of GBC cells is related to downregulation of
caspase-8, which results in the reduced activity of the apoptotic
pathway (6). Of note, caspase-8
activity is reduced through c-FLIP in TRAIL-resistant GBC cells. In
addition, c-FLIP is reported to be abundant in TRAIL-resistant GBC
cells compared with TRAIL-sensitive cells. Furthermore, a decrease
of c-FLIP can overcome resistance in GBC cells to TRAIL (7). Thus, reducing the abundance of c-FLIP
or inhibiting its function may counter TRAIL resistance in GBC
cells.
Targeting the initiation of translation is a rapidly
emerging anti-tumor strategy in cancer treatment (8). Rocaglates are a class of natural
compounds extracted from Aglaia genus that possess potent
anti-neoplastic properties by targeting eukaryotic translation
initiation factor 4A (eIF4A) (9,10). In
particular, CR-1-31B (CR-31), is a synthetic rocaglate, which has
been shown to exhibit powerful inhibitory effects over eIF4A by
perturbing the interaction between eIF4A and RNA, sequentially
impeding initiation during protein synthesis (11). However, the exact anticancer effects
of rocaglate CR-31 in GBC remain to be determined.
In the present study, it was demonstrated that eIF4A
was abundant in GBC tissues and cell lines, and its inhibitor CR-31
significantly sensitized GBC cells to TRAIL-induced apoptosis via
the eIF4A-mediated translational downregulation of c-FLIP, in
addition to mediating the caspase cascade in vitro.
Furthermore, CR-31 strongly repressed the growth and enhanced the
apoptosis of a GBC xenograft mouse model. Thus, it was shown that
eIF4A may be a novel therapeutic target and that CR-31 may serve as
an adjuvant to TRAIL treatment, which may be valuable for the
management of GBC.
Materials and methods
CBC cell lines, chemicals, human GBC
samples and ethics
GBC-SD and SGC-996 cells were obtained from The Cell
Bank of Type Culture Collection of the Chinese Academy of Sciences
(Wuhan, China) and were cultured in RPMI-1640 medium supplemented
with 10% FBS, 100 µg/ml streptomycin and 100 U/ml penicillin (all
from Gibco; Thermo Fisher Scientific, Inc.). Normal human
intrahepatic biliary epithelial cells (HIBECs) were obtained from
ScienCell Research Laboratories, Inc. and were incubated using
epithelial cell medium (ScienCell Research Laboratories, Inc.).
Cells were incubated at 37°C with 5% CO2. TRAIL was
obtained from PeproTech, Inc. (cat. no. 310-04). Rocaglate CR-1-31B
was synthesized as previously described (12) and other reagents were obtained from
Sigma-Aldrich; Merck KGaA, unless otherwise stated.
Human GBC and paired normal gallbladder tissues were
collected from 42 patients during gallbladder resection for GBC (22
males and 20 females) between July 2015 and June 2018 at the Tongji
Hospital, Tongji Medical College, Huazhong University of Science
and Technology. The patients did not receive chemotherapy prior to
surgery. The use of human GBC tissues was approved by Tongji
Hospital Research Ethical Committee, and written informed consent
was obtained from all the patients.
Cell viability analysis
GBC-SD and SGC-996 cells were cultured in 96-well
plates in complete medium (Gibco; Thermo Fisher Scientific, Inc.)
with CR-31 (0–200 nM for 48 h) and CR-31 (100 nM) and/or TRAIL (100
ng/ml) for the indicated time intervals (0–24 h). An MTT assay kit
(cat. no. ab211091; Abcam) was used to assess cell viability.
Briefly, following removal of the medium, MTT (150 µl; 0.5 mg/ml)
was added to each well and then cultured for 2 h at 37°C in an
incubator. The medium was removed and DMSO (150 µl) was added to
each well and the plates were shaken. Absorbance at 570 nm was
analyzed using a microplate reader (BioTek Instruments, Inc.).
Results were normalized to DMSO-treated cells.
Colony formation assay
For the colony formation assays, cells were plated
in a 12-well plate and incubated for 12 h at 37°C with 5%
CO2, and then incubated with CR-31 (100 nM) and/or TRAIL
(100 ng/ml) for another 12 h at 37°C with 5% CO2. Cells
were washed with PBS, fixed using 4% paraformaldehyde for 15 min,
and subsequently stained using crystal violet for 15 min.
Flow cytometry analysis
Cells were treated with CR-31 (100 nM) or DMSO for
12 h, and incubated with TRAIL (100 ng/ml) for an additional 12 h
at 37°C with 5% CO2. An Annexin V/propidium iodide (PI)
kit (cat. no. v13242; Invitrogen; Thermo Fisher Scientific, Inc.)
was used to assess cell apoptosis, according to the manufacturer's
protocol. Flow cytometry was used to determine the proportion of
apoptotic cells. Flow cytometry was performed on a FACSCalibur
system (BD Biosciences).
Reverse transcription-quantitative
(RT-q)PCR
RT-qPCR was performed as described previously
(13). Briefly, total RNA was
extracted from GBC-SD cells using TRIzol® reagent (cat.
no. 15596018; Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol. A total of 1 µg total RNA was used
as the template for cDNA synthesis using a reverse transcription
kit (cat. no. K1691; Thermo Fisher Scientific, Inc.). Equal
quantities of cDNA were used for PCR analysis. The sequences of the
primers were: c-FLIP forward, 5′-CGCTCAACAAGAACCAGTG-3′and reverse,
5′-AGGGAAGTGAAGGTGTCTC-3′; and β-actin forward,
5′-AGTGTGACGTCGACATCCGC-3′ and reverse, 5′-GACTCGTCGTACTCCTGCTT-3′.
PCR primers were purchased from Sangon Biotech Co., Ltd.
Western blot analysis
Western blot analysis was performed as described
previously (13). Briefly, GBC-SD
and SGC-996 cells were washed twice with ice-cold PBS and then
lysed with ice-cold RIPA lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology) supplemented using EDTA-free protease
inhibitor cocktail (cat. no. 11836170001; Roche Diagnostics), and
followed by protein concentration determination with BCA assay.
Lysates were kept on ice for 30 min and then centrifuged at 14,000
× g for 20 min at 4°C. Equal amounts (20 µg) of proteins were
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
(SDS-PAGE) and then transferred onto polyvinylidene difluoride
(PVDF) membranes. The membranes were block with 5% bovine serum
albumin (BSA) in Tris-buffered saline Tween-20 for 2 h and then
incubated with primary antibodies at 4°C overnight. Immunoreactive
proteins were detected with horseradish peroxidase-conjugated
secondary antibodies. The primary antibodies used were: Caspase-8
(cat. no. 9746; Cell Signaling Technology, Inc.), caspase-3 (cat.
no. 9662; Cell Signaling Technology, Inc.), cleaved-caspase-3 (cat.
no. 9664; Cell Signaling Technology, Inc.), eIF4A (cat. no.
ab31217; Abcam), c-FLIP (cat. no. ab8421; Abcam) and α-tubulin
(cat. no. sc-5286; Santa Cruz Biotechnology, Inc.).
Immunofluorescence assay
The treated cells were fixed using 4% formaldehyde
for 15 min at room temperature, washed and permeabilized using 0.5%
Triton X-100 for 20 min at room temperature. The cells were treated
for 1 h using Alexa Fluro 647-labeled IgG (H + L) (cat. no.
ab150115; Abcam) and an anti-c-FLIP antibody (cat. no. ab8421;
Abcam). Nuclei were stained using DAPI. Cells were washed with PBS
and visualized using confocal laser scanning microscopy (Olympus
Corporation).
Knockdown of eIF4A
Highly pure small interfering (si)RNA non-targeting
control (CTRL; 5′-aacuuacgcugaguacuucga-3′) or siRNA eIF4A (Sangon
Biotech Co., Ltd.) were transfected into GBC-SD cells using
Lipofectamine® 3000 (cat. no. L3000008; Invitrogen,
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Briefly, 2.5 µl Lipofectamine 3000 and siRNA (50 nmol/l)
were added to the medium (100 µl), and then the mixture was added
to the cells, which had been washed. Cells were subsequently
cultured for 5 h at 37°C, after which, the medium was removed and
replaced with fresh medium for 48 h. Cells were treated using DMSO
or TRAIL for 12 h following transfection. After that, the cells
were used for subsequent experiments immediately.
In vivo BALB/c nude mice
GBC-SD cells (~2×106 cells) were
suspended in 100 µl 1:1 Matrigel/medium and then injected
subcutaneously into the right flank of male BALB/c nude mice (n=20;
age, 6-weeks old). The animals were housed and assayed under
conditions of control temperature (22±2°C), humidity (45–65%), and
artificial light (12-h light-dark cycle) with free access to rodent
chow and water. The mice were randomly separated into four equal
groups (n=5 per group): i) Control group [receiving vehicle (10%
DMSO and 90% olive oil)]; ii) TRAIL group [intraperitoneal
injection of TRAIL (80 µg/kg) in 60 µl olive oil]; iii) CR-31 group
[intraperitoneal injection of CR-31 (2 mg/kg) in 60 µl olive oil];
and iv) TRAIL/CR-31 group [intraperitoneal injection of TRAIL (80
µg/kg) and CR-31 (2 mg/kg) in 60 µl olive oil]. Treatment was
performed once every 2 days for 28 days. Body weight and tumor
volume were monitored on a weekly basis. Tumor volumes
(mm3) were calculated as follows: Volume=length ×
S2/2 (where S is the shortest diameter). After 28 days,
mice were anesthetized by intraperitoneal injection using 10%
chloral hydrate (350 mg/kg) and were sacrificed using cervical
dislocation. A comprehensive evaluation of death by respiratory,
heartbeat, pupil and nerve reflex of these mice was carried out and
recorded. Finally, tumor specimens were collected for
immunohistochemistry and TUNEL staining.
The mice were procured from HFK Bioscience Company.
Animal experiments were performed according to the Guidelines of
Laboratory Animals of Tongji Hospital, which is approved by the
National of Health (NIH publication 85–23 revised 1996).
Immunohistochemistry and TUNEL
assay
Immunohistochemistry was performed using
anti-cleaved-caspase-3 (cat. no. 9664; Cell Signaling Technology,
Inc.) and anti-eIF4A (cat. no. ab31217; Abcam) antibodies, as
previously described (14). Two
independent individuals examined the proportion of positive cells
in ≥5 fields of view (magnification, ×400), and tissues were scored
as follows: 0, negative; 1, <25% positive cells; 2, 25–50; 3,
50–75; and 4, >75%. Images were visualized and calculated using
a Nikon microscope (Nikon Corporation). TUNEL assays was performed
on paraffin-embedded tissue sections using a one-step TUNEL
apoptosis assay kit (Beyotime Institute of Biotechnology),
according to the manufacturer's protocol. Briefly, samples were
incubated with TUNEL reaction mixture for 1 h at 37°C in the dark
and then washed twice with PBS. Nuclei were counterstained with
DAPI (300 nM; cat. no. D8417; Sigma-Aldrich) for 10 min at room
temperature and mounting with antifade medium (cat. no. P0126;
Beyotime Institute of Biotechnology) and then washed twice with
PBS. The condensed or fragmented nuclei of apoptotic cells were
observed using fluorescence microscopy (Olympus Corporation)
(magnification, ×200) in 20 fields of vision.
Statistical analysis
All data were analyzed using GraphPad Prism version
8.4.2 (GraphPad Software, Inc.). All data are presented as the mean
± standard deviation. Student's t-test was used to compare
differences between two groups, and multiple groups were compared
with one-way or two-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
CR-31 sensitizes GBC cells to
TRAIL-mediated apoptosis
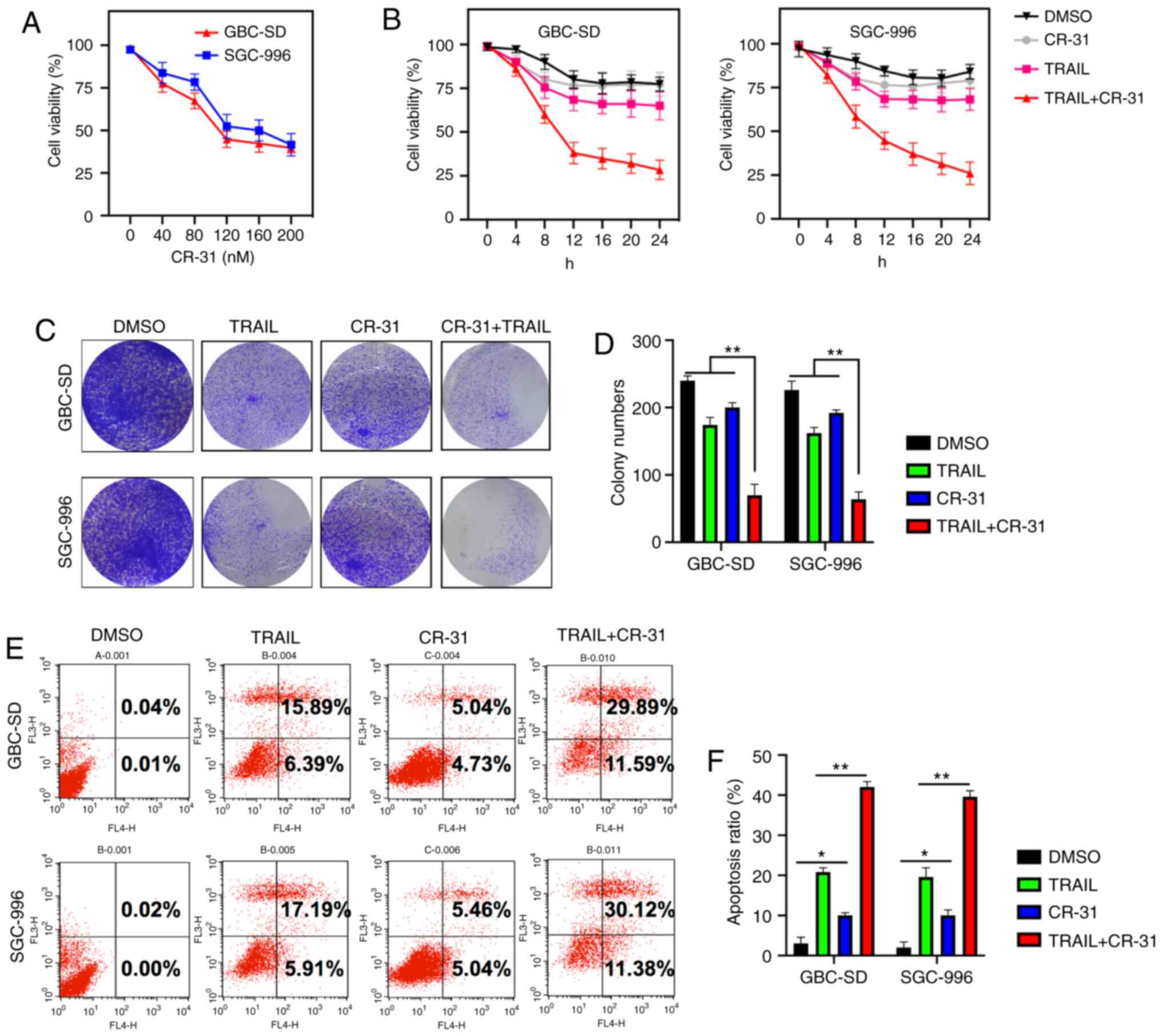
To assess whether CR-31 could sensitize GBC cells to
TRAIL-induced apoptosis, GBC-SD and SGC-996 cells were used, as
they exhibit strong chemoresistance to TRAIL (7). The treatment of GBC-SD and SGC-996
cells with CR-31 resulted in dose-dependent growth inhibition, with
an IC50 of ~100 nM (Fig.
1A). Furthermore, the efficacy of CR-31 on TRAIL-mediated
cytotoxicity was assessed. CR-31 and TRAIL alone were weakly
cytotoxic to GBC-SD and SGC-996 cells; however, the treatment of
GBC-SD and SGC-996 cells using CR-31 increased the cytotoxicity to
TRAIL (Fig. 1B). Moreover, although
CR-31 or TRAIL did not reduce the colony numbers, the combined
treatment with CR-31 and TRAIL significantly reduced the colony
formation of GBC-SD and SGC-996 cells, indicating a synergistic
effect (Fig. 1C and D). Similar
results were observed based on the Annexin V/PI assay in GBC-SD and
SGC-996 cells. TRAIL or CR-31 alone resulted in increased early
apoptosis and necrosis in GBC-SD (22.28 and 9.77%, respectively)
and SGC-996 cells (23.1 and 10.5%, respectively). However, combined
apoptosis and necrosis were increased in both GBC-SD (41.48%) and
SGC-996 cells (41.5%) (Fig. 1E and
F). Collectively, the results showed that CR-31 may highly
sensitize chemo-resistant GBC-SD and SGC-996 cells to TRAIL.
eIF4A may be a therapeutic target and
CR-31 can downregulate the expression of c-FLIP at the
translational level in GBC
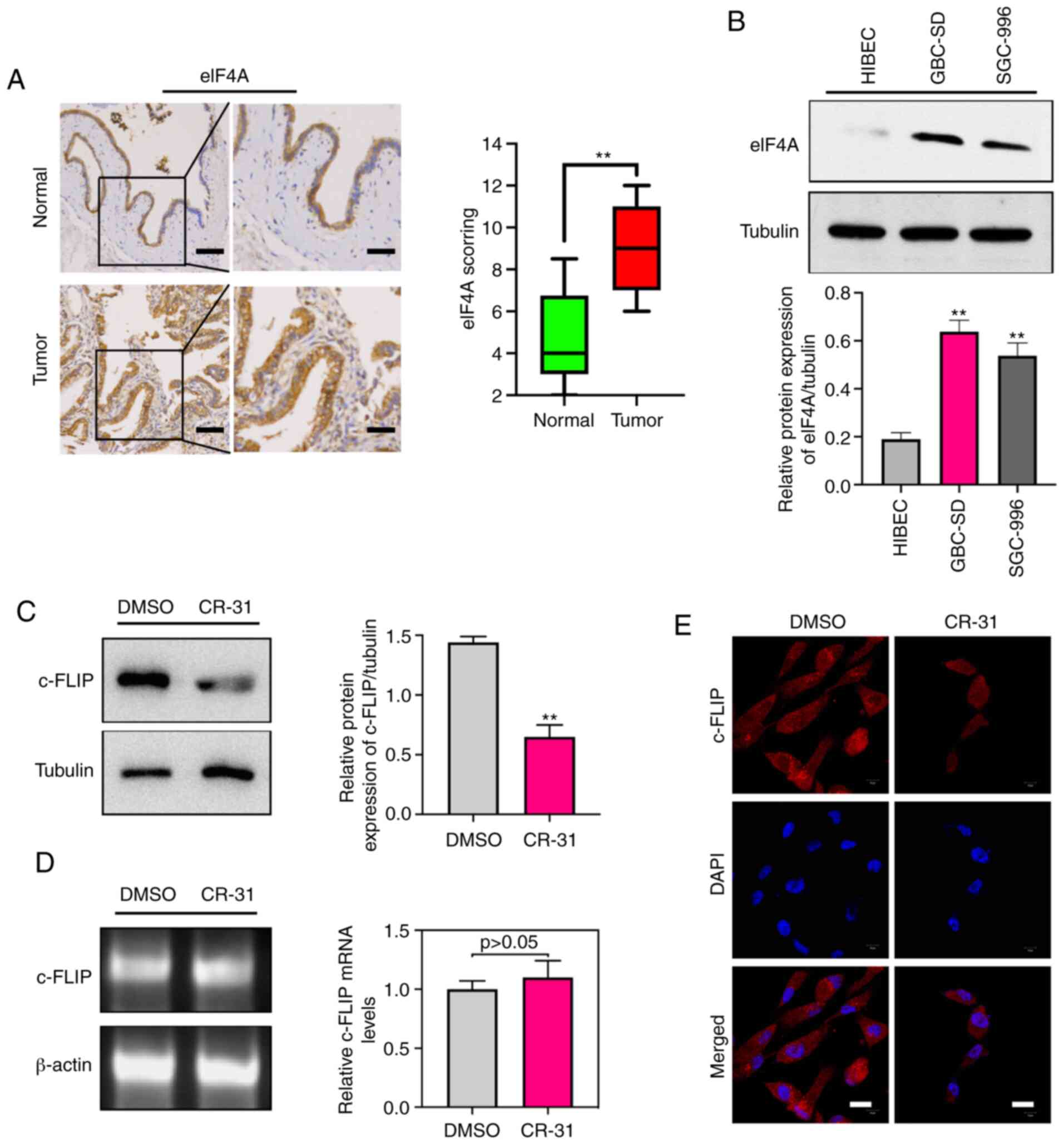
To investigate the clinical significance of eIF4A in
human samples of GBC, the abundance of eIF4A was detected by
immunohistochemistry in GBC and paired normal gallbladder tissues.
Immunohistochemistry analysis suggested that eIF4A abundance was
significantly higher in the 42 GBC samples compared with the paired
normal control tissues (Fig. 2A).
Furthermore, the eIF4A protein was strongly expressed in GBC-SD and
SGC-996 cells compared with the HIBECs (Fig. 2B). Increased expression of c-FLIP in
GBC cells has been shown to be associated with TRAIL resistance
(7), thus the effect of CR-31 on
the abundance of c-FLIP was determined. The results showed that
eIF4A and c-FLIP were abundantly expressed in GBC cells, and CR-31
treatment significantly decreased the levels of c-FLIP protein in
GBC-SD cells (Fig. 2C). Notably,
there were no significant changes in c-FLIP mRNA levels (Fig. 2D), suggesting a translational
mechanism of regulation of expression. Furthermore, confocal
microscopy showed that CR-31 resulted in a decrease in the plasma
localization of c-FLIP (Fig. 2E).
Collectively, the results showed that eIF4A may be a therapeutic
target and CR-31 can downregulate the abundance of c-FLIP at the
translational level in GBC.
Knockdown of eIF4A downregulates the
abundance of c-FLIP protein, mimicking the effects of CR-31 on
GBC-SD cells
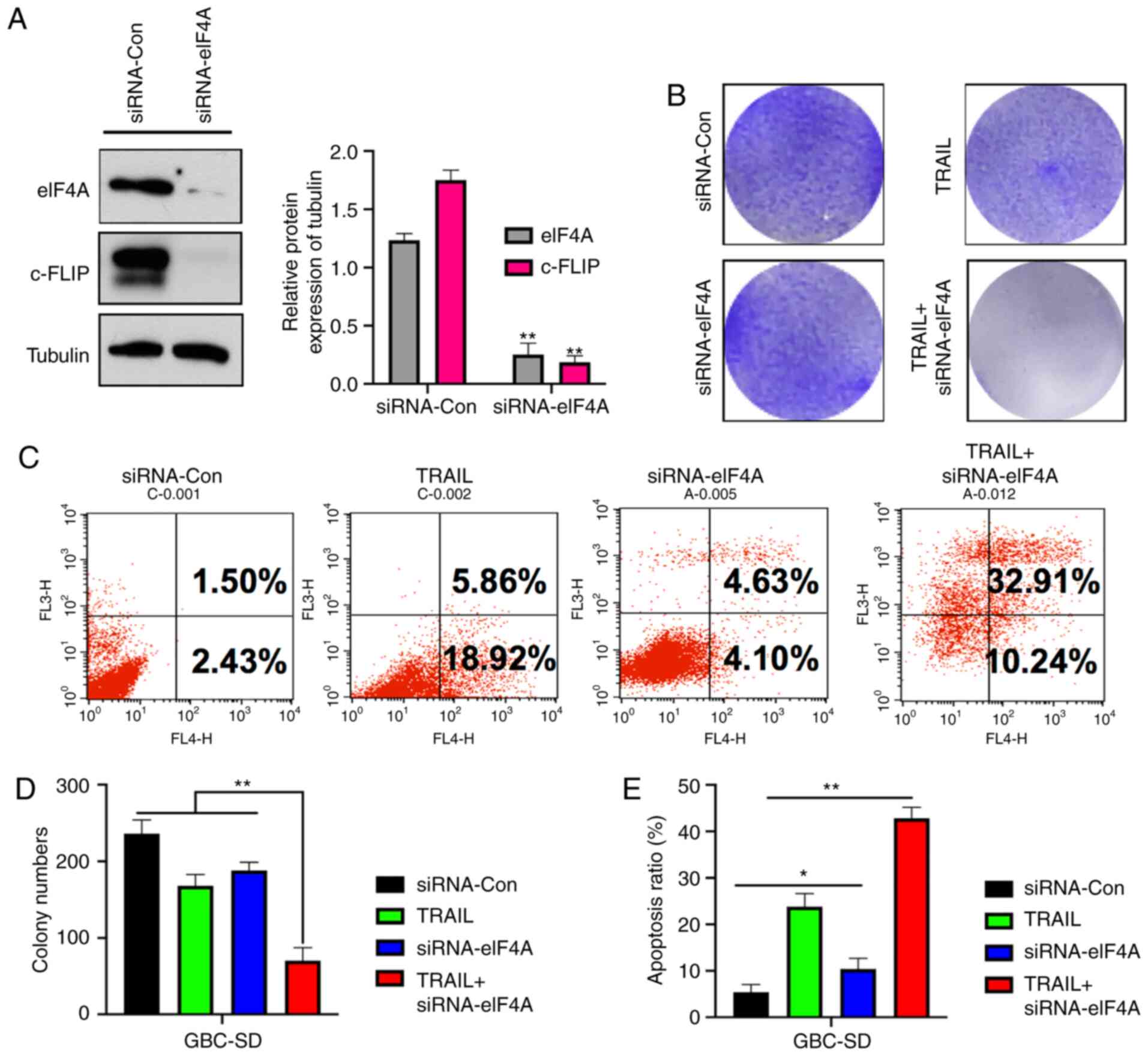
To assess the effect of knockdown of eIF4A on c-FLIP
expression and CR-31 treatment, an eIF4A-knockdown GBC-SD cell line
was established. siRNA-eIF4A resulted in a significant decrease in
both eIF4A and c-FLIP expression (Fig.
3A). Crystal violet staining showed eIF4A knockout GBC-SD cells
exhibited significantly reduced colony formation when treated with
TRAIL compared with the control group (Fig. 3B and D). Furthermore, the results of
Annexin V-PI apoptosis by flow cytometry showed the same effect;
the proportion of apoptotic and necrotic cells following TRAIL
treatment was 24.78 and 8.73%, respectively, in the
siRNA-eIF4A-transfected cells, and 43.15% in
siRNA-eIF4A-transfected cells treated with TRAIL (Fig. 3C and E), showing that knockdown of
eIF4A in GBC-SD cells resulted in similar effects to treatment with
CR-31.
CR-31 enhances TRAIL-mediated
apoptosis in a caspase-dependent manner
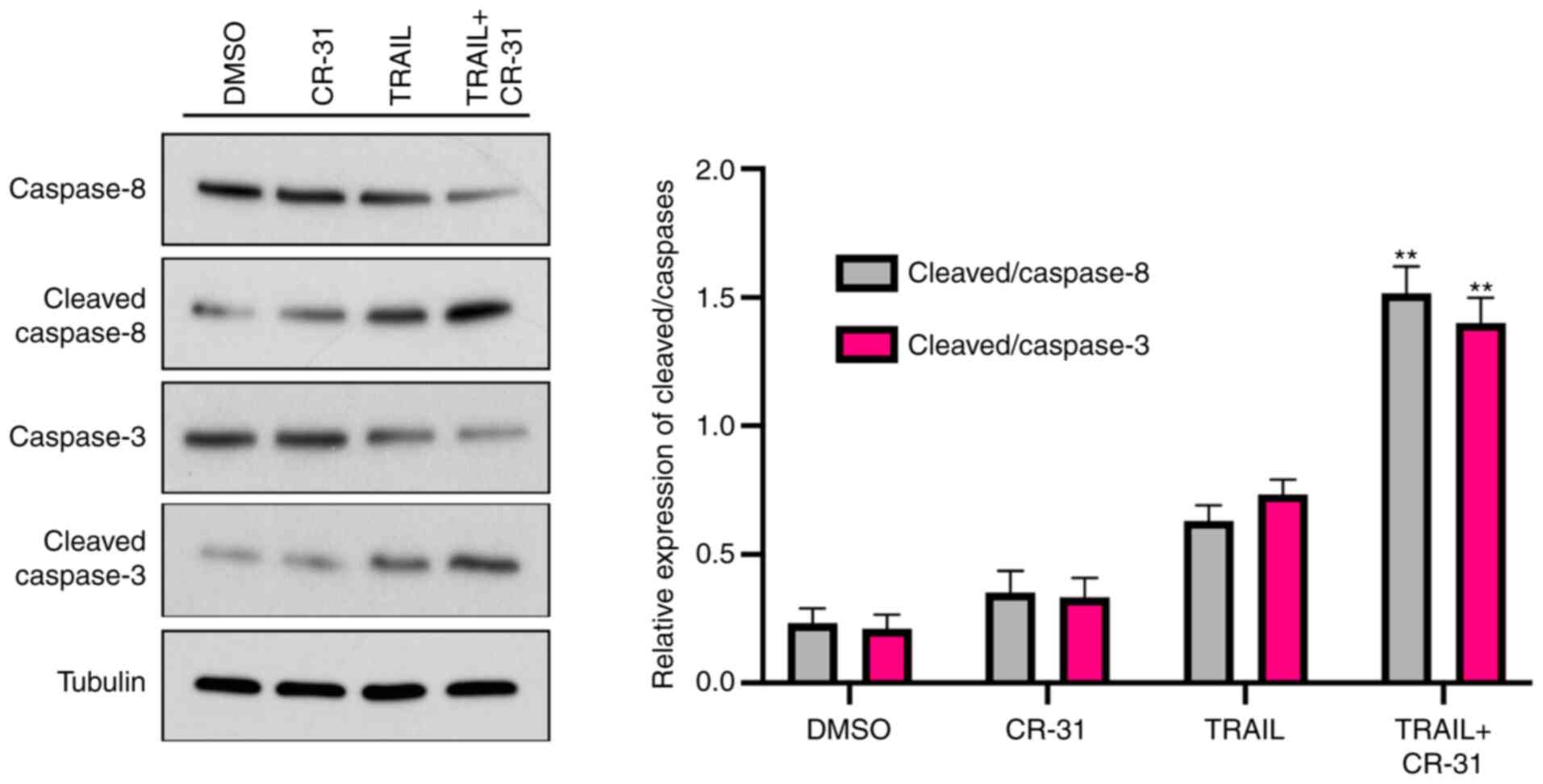
As TRAIL-mediated apoptosis is mediated through the
activation of the caspase cascade (15), whether caspase-8 and caspase-3
expression was increased in GBC-SD cells treated with TRAIL or
CR-31 alone was next determined. The results showed that the levels
of pro-caspase-8 were weakly decreased when treated with CR-31 or
TRAIL alone, along with an increase in cleaved-caspase-8 expression
(Fig. 4). Notably, when combined,
CR-31 and TRAIL significantly increased the levels of
cleaved-caspase-8 (Fig. 4).
Notably, TRAIL or CR-31 alone resulted in slightly increased levels
of cleaved-caspase-3 in GBC-SD cells (Fig. 4). However, CR-31 and TRAIL combined
significantly increased the levels of cleaved-caspase-3 (Fig. 4). Therefore, the results suggest
that CR-31 enhanced TRAIL-mediated apoptosis in a caspase-dependent
manner.
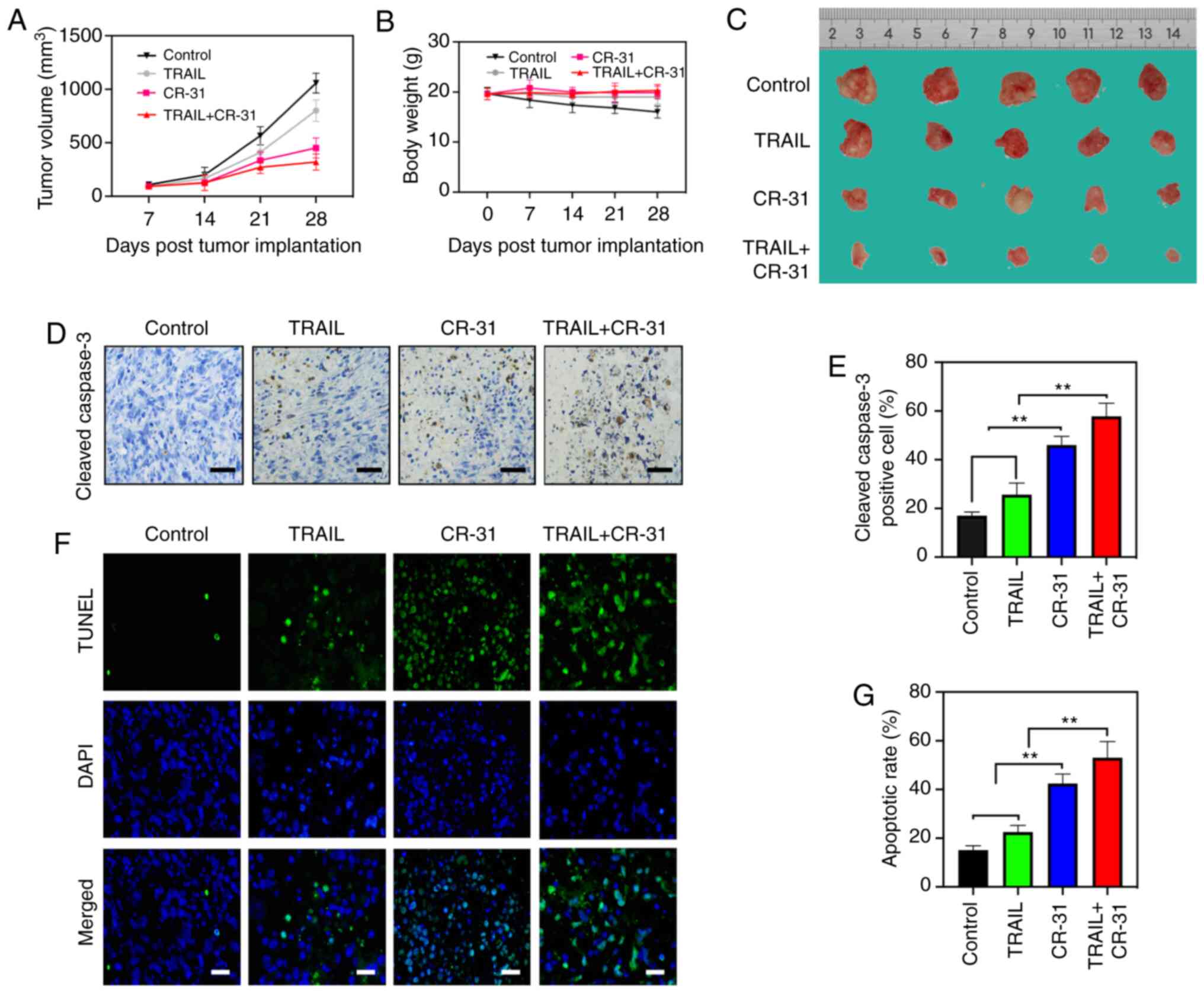
CR-31 administration reduces the
growth and initiates tumor apoptosis in a BALB/c nude mice model of
GBC
To evaluate the effects of CR-31 on TRAIL in
vivo, a BALB/c nude mice model of GBC was established using
GBC-SD cells. The tumor volume and weights of GBC-SD xenografts
were measured. Tumor weights after 4 weeks of treatment showed a
decreasing trend from control, to TRAIL to CR-31 to combination
treatment (Fig. 5A and C). However,
tumor weight did not exceed 10% of body weight of mice.
Interestingly, there was a notable reduction in tumor volumes
treated with CR-31 compared with those treated with TRAIL (Fig. 5A and C), and this may due to an
increase in the production of TRAIL from natural killer cells in
vivo (16). Notably, CR-31
neither resulted in the notable reduction of body weight nor showed
evidence of toxicity (Fig. 5B) and
the maximum percentage of weight loss did not exceed 8% of body
weigh of mice, indicating that CR-31 was safe in vivo. The
apoptotic effect of CR-31 and TRAIL were assessed in vivo
using cleaved-caspase-3 and TUNEL staining. Upregulated expression
of cleaved-caspase-3 were observed, and there was an increase in
apoptosis when treated with the combined treatment compared with
either TRAIL or CR-31 alone, which was in agreement with the in
vitro results (Fig. 5D-G).
Collectively, the results showed that CR-31 enhanced TRAIL-induced
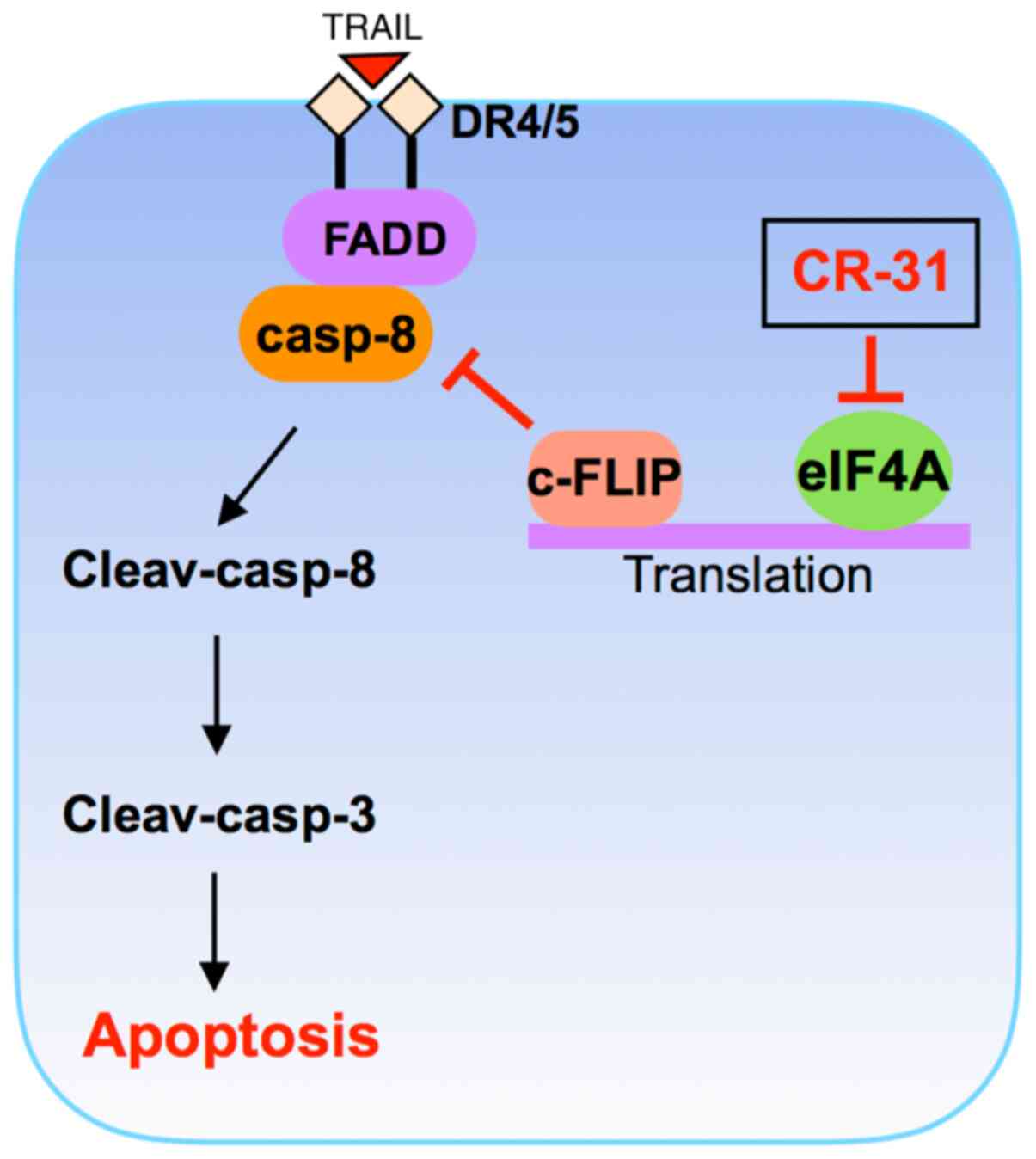
apoptosis of GBC xenograft tumors in vivo. Taking these
findings together, we conclude that CR-31 can enhance
TRAIL-triggered apoptosis by downregulating c-FLIP expression at
the translational level and then activating the caspase cascade in
TRAIL-resistant GBC cells (Fig.
6).
Discussion
GBC is one of the most malignant types of cancer and
is associated with a poor prognosis that is largely attributed to
late diagnosis and acquired drug resistance to traditional
chemotherapy regimens (17). In
particular, GBC cells exhibit significant resistance to TRAIL.
Therefore, there is a need to develop novel strategies for
overcoming TRAIL resistance in GBC cells. Recently, the effect of
increasing apoptosis through the use of natural compounds has been
described in GBC cells (18,19).
In the present study, it was shown that rocaglate CR-31, an
inhibitor of eIF4A, enhanced the TRAIL-mediated apoptosis of GBC
cells through the eIF4A-mediated translational downregulation of
c-FLIP.
As the cancer cell type may influence the response
to TRAIL, the highly TRAIL-resistant GBC cell lines, GBC-SD and
SGC-996 cells, were chosen (7).
Subsequently, the effect of CR-31 on GBC-SD and SGC-996 cells and
their sensitivity to TRAIL treatment was determined. Notably,
GBC-SD and SGC-996 cells were less responsive to TRAIL treatment.
However, TRAIL-resistant GBC-SD and SGC-996 cells treated with
CR-31 showed notably reduced growth. Therefore, the results suggest
that CR-31 has the potential to sensitize cells to TRAIL-mediated
cell death.
Targeting translation initiation may serve as a
promising anti-tumor strategy. In the present study, eIF4A was
shown to be highly abundant in GBC tissues and cell lines. In
addition, eIF4A inhibitor rocaglates CR-31 is currently the most
potent translation initiation inhibitor that functions via eIF4A,
and is well tolerated in vivo (20,21).
Moreover, inhibitors of eIF4A, such as hippuristanol, can induce
apoptosis of adult T-cell leukemia (22). Downregulating c-FLIP by targeting
the translation of c-FLIP may be a promising therapeutic strategy.
Therefore, whether CR-31 treatment attenuated the translation of
c-FLIP was determined. However, there was no significant change in
c-FLIP at the mRNA expression level, suggesting that the effect of
CR-31 on c-FLIP was at the translational level. The results showed
that eIF4A may be a valuable therapeutic target and CR-31 can
downregulate the translational abundance of c-FLIP and can
sensitize GBC cells to TRAIL.
Due to its specificity against cancer cells and
minimal toxicity on normal cells, TRAIL-based chemotherapy may
serve as a favorable strategy in the treatment of cancer (23). However, GBC shows resistance to
TRAIL-mediated apoptosis, suggesting that TRAIL alone is not
suitable for the treatment of GBC. In the present study, GBC-SD
cells were resistant to TRAIL; however, following the treatment
with CR-31, the cells became sensitized to TRAIL. These data
suggest that CR-31 may be used as an adjuvant in the TRAIL-based
chemotherapy of GBC. Moreover, CR-31 sensitizes TRAIL-mediated
apoptosis at nanomolar concentrations, suggesting the efficacy of
CR-31 was potent, but also safe on normal cells. Although the
mechanisms of TRAIL resistance in GBC cells remain to be
determined, emerging evidence has demonstrated that the activity of
death-inducing signaling complex (DISC)-recruited proteins
caspase-8 and c-FLIP influenced the sensibility of TRAIL-induced
cancer cell apoptosis (24). The
present study showed that CR-31 markedly activated caspase-8 in GBC
cells, following the upregulation of cleaved-caspase-3 in
vitro.
The formation of DISC is a critical initiating
process of the extrinsic signaling of apoptosis, activating the
caspase cascade, which then induces apoptotic death. c-FLIP
prevents the accumulation of caspase-8, resulting in the disruption
of DISC (25). Moreover, the
abundance of c-FLIP confers resistance in tumor cells to apoptotic
stimuli (26). Additionally, a low
abundance of c-FLIP increases the sensitivity of GBC cells to
chemotherapy (27,28). Therefore, these reports indicate
that c-FLIP may be a target of GBC. In the present study, it was
shown that CR-31 significantly reduced c-FLIP levels in GBC-SD
cells at nanomolar concentrations. In addition, CR-31 and TRAIL
combined notably increased cell death, indicating that CR-31
increased the sensitivity to TRAIL through downregulation of c-FLIP
in GBC-SD cells. Using siRNA specifically to knock down c-FLIP in
GBC-SD cells showed that the downregulation of c-FLIP in GBC-SD
resulted in a similar effect to CR-31 treatment.
In summary, it was shown that eIF4A was highly
abundant in GBC tissues and cell lines, and its inhibitor rocaglate
CR-31 enhanced TRAIL-mediated apoptosis by downregulating c-FLIP
expression at the translational level and then activating the
caspase cascade in TRAIL-resistant GBC cells, both in vitro
and in vivo. Therefore, the data indicate that eIF4A may be
a therapeutic target, and the present study highlights a
potentially valuable strategy, that is, the combination of
rocaglate CR-31 with TRAIL, for the treatment of GBC.
Acknowledgements
Not applicable.
Funding
The work was supported by the National Natural
Science Foundation of China (grant no. 81802427).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YC and YH performed the experimental work. LY
analyzed the experimental data. ZL designed this study and wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Medical Research, Tongji Hospital, Tongji Medical College, Huazhong
University of Science and Technology. Written informed consent for
publication was obtained from all participants (approval no.
20180302536). Also, this study was approved by the Ethics Committee
of Laboratory Animals, Tongji Hospital, Tongji Medical College,
Huazhong University of Science and Technology (approval no.
20180405482).
Patient consent for publication
Consent for publication was obtained from each
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baichan P, Naicker P, Devar JWS, Smith M,
Candy GP and Nweke E: Targeting gallbladder cancer: A pathway based
perspective. Mol Biol Rep. 47:2361–2369. 2020. View Article : Google Scholar
|
|
2
|
Maurya SK, Tewari M, Mishra RR and Shukla
HS: Genetic aberrations in gallbladder cancer. Surg Oncol.
21:37–43. 2012. View Article : Google Scholar
|
|
3
|
Henry CM and Martin SJ: Caspase-8 acts in
a non-enzymatic role as a scaffold for assembly of a
pro-inflammatory ‘FADDosome’ complex upon TRAIL stimulation. Mol
Cell. 65:715–729.e5. 2017. View Article : Google Scholar
|
|
4
|
Merino D, Kelly GL, Lessene G, Wei AH,
Roberts AW and Strasser A: BH3-mimetic drugs: Blazing the TRAIL for
new cancer medicine. Cancer Cell. 34:879–891. 2018. View Article : Google Scholar
|
|
5
|
Zhu W, Zhan D, Wang L, Ma D, Cheng M, Wang
H, Zhao J, Cai Y and Cheng Z: Proteasome inhibitor MG132
potentiates TRAIL-induced apoptosis in gallbladder carcinoma GBC-SD
cells via DR5-dependent pathway. Oncol Rep. 36:845–852. 2016.
View Article : Google Scholar
|
|
6
|
Srivastava K, Srivastava A and Mittal B:
Caspase-8 polymorphisms and risk of gallbladder cancer in a
northern Indian population. Mol Carcinog. 49:684–692. 2010.
|
|
7
|
Zong H, Yin B, Chen J, Ma B, Cai D and He
X: Over-expression of c-FLIP confers the resistance to
TRAIL-induced apoptosis on gallbladder carcinoma. Tohoku J Exp Med.
217:203–208. 2009. View Article : Google Scholar
|
|
8
|
Bhat M, Robichaud N, Hulea L, Sonenberg N,
Pelletier J and Topisirovic I: Targeting the translation machinery
in cancer. Nat Rev Drug Discov. 14:261–278. 2015. View Article : Google Scholar
|
|
9
|
Iwasaki S, Floor SN and Ingolia NT:
Rocaglates convert DEAD-box protein eIF4A into a sequence-selective
translational repressor. Nature. 534:558–561. 2016. View Article : Google Scholar
|
|
10
|
Chu J, Zhang W, Cencic R, Devine WG,
Beglov D, Henkel T, Brown LE, Vajda S, Porco JA Jr and Pelletier J:
Amidino-rocaglates: A potent class of eIF4A inhibitors. Cell Chem
Biol. 26:1586–1593.e3. 2019. View Article : Google Scholar
|
|
11
|
Langlais D, Cencic R, Moradin N, Kennedy
JM, Ayi K, Brown LE, Crandall I, Tarry MJ, Schmeing M, Kain KC, et
al: Rocaglates as dual-targeting agents for experimental cerebral
malaria. Pro Natl Acad Sci USA. 115:E2366–E2375. 2018. View Article : Google Scholar
|
|
12
|
Rodrigo CM, Cencic R, Roche SP, Pelletier
J and Porco JA: Synthesis of rocaglamide hydroxamates and related
compounds as eukaryotic translation inhibitors: Synthetic and
biological studies. J Med Chem. 55:558–562. 2012. View Article : Google Scholar
|
|
13
|
Luan Z, He Y, Alatar M, Chen Z and He F:
Targeting the prohibitin scaffold-CRAF kinase interaction in
RAS-ERK-driven pancreatic ductal adenocarcinoma. Mol Cancer.
13:382014. View Article : Google Scholar
|
|
14
|
Collisson EA, Trejo CL, Silva JM, Gu S,
Korkola JE, Heiser LM, Charles RP, Rabinovich BA, Hann B, Dankort
D, et al: A central role for RAF-MEK-ERK signaling in the genesis
of pancreatic ductal adenocarcinoma. Cancer Discov. 2:685–693.
2012. View Article : Google Scholar
|
|
15
|
Kong X, Luo J, Xu T, Zhou Y, Pan Z, Xie Y,
Zhao L, Lu Y, Han X, Li Z and Liu L: Plumbagin enhances
TRAIL-induced apoptosis of human leukemic Kasumi-1 cells through
upregulation of TRAIL death receptor expression, activation of
caspase-8 and inhibition of cFLIP. Oncol Rep. 7:3423–3432. 2017.
View Article : Google Scholar
|
|
16
|
Hayakawa Y, Screpanti V, Yagita H,
Grandien A, Ljunggren HG, Smyth MJ and Chambers BJ: NK cell TRAIL
eliminates immature dendritic cells in vivo and limits dendritic
cell vaccination efficacy. J Immunol. 172:123–129. 2004. View Article : Google Scholar
|
|
17
|
Rawla P, Sunkara T, Thandra KC and Barsouk
A: Epidemiology of gallbladder cancer. Clin Exp Hepatol. 5:93–102.
2019. View Article : Google Scholar
|
|
18
|
Wu K, Huang J, Lin N, Xu T, Cai W and Ye
Z: Antitumor effect of ginsenoside Rg3 on gallbladder cancer by
inducing endoplasmic reticulum stress-mediated apoptosis in
vitro and in vivo. Oncol Lett. 16:5687–5696. 2018.
|
|
19
|
Song X, Wang Z, Liang H, Zhang W, Ye Y, Li
H, Hu Y, Zhang Y, Weng H, Lu J, et al: Dioscin induces gallbladder
cancer apoptosis by inhibiting ROS-mediated PI3K/AKT signalling.
Int J Bio Sci. 13:782–793. 2017. View Article : Google Scholar
|
|
20
|
Chu J, Galicia-Vázquez G, Cencic R, Mills
JR, Katigbak A, Porco JA Jr and Pelletier J: CRISPR-mediated
drug-target validation reveals selective pharmacological inhibition
of the RNA helicase, eIF4A. Cell Rep. 15:2340–2347. 2016.
View Article : Google Scholar
|
|
21
|
Zhang W, Chu J, Cyr AM, Yueh H, Brown LE,
Wang TT, Pelletier J and Porco JA Jr: Intercepted retro-nazarow
reaction: Syntheses of amidino-rocaglate derivatives and their
biological evaluation as eIF4A inhibitors. J Am Chem Soc.
141:12891–12900. 2019. View Article : Google Scholar
|
|
22
|
Tsumuraya T, Ishikawa C, Machijima Y,
Nakachi S, Senba M, Tanaka J and Mori N: Effects of hippuristanol,
an inhibitor of eIF4A, on adult T-cell leukemia. Biochem Pharmacol.
81:713–722. 2011. View Article : Google Scholar
|
|
23
|
Nazim UM and Park SY: Attenuation of
autophagy flux by 6-shogaol sensitizes human liver cancer cells to
TRAIL-induced apoptosis via p53 and ROS. Int J Mol Med. 43:701–708.
2019.
|
|
24
|
Min KJ, Han MA, Kim S, Park JW and Kwon
TK: Osthole enhances TRAIL-mediated apoptosis through
downregulation of c-FLIP expression in renal carcinoma Caki cells.
Oncol Rep. 37:2348–2354. 2017. View Article : Google Scholar
|
|
25
|
Hughes MA, Powley IR, Jukes-Jones R, Horn
S, Feoktistova M, Fairall L, Schwabe JW, Leverkus M, Cain K and
MacFarlane M: Co-operative and hierarchical binding of c-FLIP and
caspase-8: A unified model defines how c-FLIP isoforms
differentially control cell fate. Mol Cell. 61:834–849. 2016.
View Article : Google Scholar
|
|
26
|
Huang Y, Yang X, Xu T, Kong Q, Zhang Y,
Shen Y, Wei Y, Wang G and Chang KJ: Overcoming resistance to
TRAIL-induced apoptosis in solid tumor cells by simultaneously
targeting death receptors, c-FLIP and IAPs. Int J Oncol.
49:153–163. 2016. View Article : Google Scholar
|
|
27
|
Zong H, Zhou H, Xiang Y and Wu G: miR-125b
suppresses cellular proliferation by targeting c-FLIP in
gallbladder carcinoma. Oncol Lett. 18:6822–6828. 2019.
|
|
28
|
Su W, Jiang X, Chen M, Huang M, Tang N,
Wang X, Li X, She F and Chen Y: cIAP1 promotes proliferation and
migration and prevents apoptosis in gallbladder cancer in vitro.
Biosci Rep. 39:BSR201822662019. View Article : Google Scholar
|