Introduction
Oral squamous cell carcinoma (OSCC) is the most
common malignant neoplasm of the oral cavity (1). Despite recent advances in screening
and treatment, the current 5-year survival rate of OSCC is ~50%,
which is generally attributed to either distant metastasis or
locoregional recurrence (2–4). Molecular targeted inhibitors, such as
cetuximab, were previously considered to be potentially effective
measures; however, the long-term clinical results have been mixed
or unfavorable, with a large number of patients ultimately
exhibiting resistance (5).
Therefore, revealing the molecular mechanisms underlying the
carcinogenesis of OSCC, which may provide a basis for other
therapeutic targets, is necessary.
Aberrant expression of Ras-related proteins has been
frequently reported in various types of human cancer (6). Dysregulation of Ras-related proteins
often leads to a loss of control of cell proliferation, adhesion
and migration, which affects disease progression and prognosis
(6,7). The Ras-related protein Rab31, also
termed Rab22B, is a member of the Rab family (8,9)
involved in the organization of the trans-Golgi network and
transport carrier formation (10).
Previous studies have reported that high Rab31 expression levels
are associated with cancer. For example, expression profiling
analyses have identified Rab31 as one of 11 genes robustly
upregulated in estrogen receptor-positive compared with estrogen
receptor-negative breast cancer (11). Additionally, Rab31 has been reported
to promote the proliferation of cancer and normal cells (12,13).
The potential effects of the Rab family on head and
neck cancer (HNC) have been identified in a number of studies with
contradictory results. Rab25, a member of the Rab11 subfamily, was
reported to regulate cellular invasion and metastasis in HNC
(14). Similar inhibitory effects
of Rab25 were confirmed in another study, which demonstrated
decreased expression in advanced metastatic oral and oropharyngeal
cancer (15). By contrast, Rab5a,
Rab9 and Rab14 were observed in several studies to exert
cancer-promoting functions, thus serving as oncogenes rather than
tumor suppressors (16–18). Rab5a can upregulate the expression
of various cell cycle-associated proteins and regulate the activity
of a number of signaling pathways, such as the ERK/MMP signaling
pathway (16). The vast
discrepancies among Rab family proteins indicate the complex
mechanisms linking Rab and HNC. Despite being a core member of the
Rab family, the potential functions of Rab31 in OSCC remain
unknown. Therefore, the present study aimed to investigate the
expression of Rab31 in human OSCC tissues and analyze the
associations between Rab31 expression levels and the
clinicopathological characteristics of patients with OSCC, as well
as determine the functional roles of Rab31 in OSCC by in
vitro and in vivo experiments.
Materials and methods
Ethics statement
All experiments in the present study were approved
by the Independent Ethics Committee and Animal Experimental Ethical
Inspection of the Shanghai Ninth People's Hospital, Shanghai Jiao
Tong University School of Medicine and performed at the Shanghai
Ninth People's Hospital. The approval numbers were SH9H-2019-T117-1
for human tissues and HKDL-2017-304 for animal studies. All
experiments were performed in 2019.
OSCC samples
A total of 54 human OSCC and 16 normal oral mucosa
samples were obtained from patients with OSCC and healthy
individuals, respectively, at the Shanghai Ninth People's Hospital
between January 2014 and December 2017. Each patient provided
informed written consent for enrollment in the current study. The
median follow-up duration was 31 months with no patients lost to
the follow-up. The most affected sites among the OSCC samples were
the tongue (n=31, 57.4%), followed by the floor of mouth (n=14,
25.9%), bucca (n=6, 11.1%) and upper gingiva (n=3, 5.6%). Healthy
tissue was taken from the tongue (n=7, 43.8%), bucca (n=5, 31.2%),
or lower gingiva (n=4, 25.0%). Histological diagnosis, pathological
grading and Tumor-Node-Metastasis (TNM) staging of the samples were
performed by three independent pathologists based on the 8th
edition of the American Joint Committee on Cancer (AJCC) staging
system (19).
Antibodies
The primary antibodies used in the current study
were as follows: Rabbit anti-Rab31 [1:200 for immunohistochemistry
(IHC) and immunofluorescence (IF); cat. no. ab230881; Abcam],
rabbit anti-Ki-67 (1:200 for IHC; cat. no. ab15580; Abcam), rabbit
anti-Survivin [1:200 for IHC, 1:1,000 for western blotting (WB);
cat. no. 2808], rabbit anti-cyclin D1 (1:1,000 for WB; cat. no.
2978), rabbit anti-B-cell lymphoma 2 (Bcl2; 1:1,000 for WB; cat.
no. 2978), rabbit anti-E-cadherin (1:200 for IHC, 1:1,000 for WB;
cat. no. 3195) and rabbit anti-N-cadherin (1:200 for IHC, 1:1,000
for WB; cat. no. 3116) (all from Cell Signaling Technology,
Inc.).
IHC and IF staining
The OSCC samples were fixed 10% neutral buffered
formalin at room temperature for 12 h. Fixed paraffin-embedded
tissues were cut into 4-µm sections. For IHC staining, antigen
retrieval was performed with citrate buffer (pH 6.0) under 121°C
for 90 sec. Following incubation with 0.3% hydrogen peroxide and 5%
normal goat serum (cat. no. 5425; Cell Signaling Technology. Inc.)
for 30 min at 4°C, the sections were incubated with primary
antibodies at 4°C overnight. The following day, the sections were
incubated with biotinylated goat anti-rabbit or anti-mouse serum
IgG antibodies (Cell Signaling Technology, Inc, #7074 and #7076),
followed by incubation with horseradish peroxidase (HRP)-conjugated
streptavidin-biotin for 30 min at room temperature. A
3,3′-Diaminobenzidine Staining kit (Fuzhou Maixin Biotech Co.,
Ltd.) was used at room temperature for 5 min for section staining,
followed by counterstaining with hematoxylin 90 sec. For IF, the
incubation with primary antibodies was as aforementioned;
subsequently, the sections were incubated at room temperature for 1
h with a secondary Alexa Fluro 594 anti-rabbit antibody (cat. no.
A-11037; 1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.,) and
stained with 4′,6′-diamidino-2-phenylindole (DAPI; Jackson
ImmunoResearch Laboratories, Inc.) Following 1-h incubation, images
were captured using a fluorescence microscope (Olympus Corporation)
at ×100 magnification in 10 random fields with ≥500 cells, and the
number of cells with different staining intensities was counted by
two pathologists. When no observable staining was present, the
membrane or nuclear staining was incomplete or faint in ≤10% of
cancer cells, the sample was defined as Rab31-negative.
Cell culture
The human OSCC cell lines SCC-4, SCC-9, SCC-25 and
CAL27 were maintained in Dulbecco's modified Eagle's medium
(DMEM)/F12 (HyClone; Cytiva) with 10% fetal bovine serum (FBS;
HyClone; Cytiva). Cells were cultured at 37°C in a humidified
atmosphere with 5% CO2. Primary human oral epithelial
cells (HOECs) were cultured as follows: Fresh oral mucosa tissues
were obtained from healthy adults undergoing third molar
extraction. Written consent was obtained from these healthy adults
for this study. The obtained tissues were washed with PBS without
calcium or magnesium three times, cut into 0.3-cm2
pieces, incubated with 2 µg/ml Dispase solution (MilliporeSigma)
for 18 h at 4°C and trypsinized with 0.125% trypsin and 0.01 mM
EDTA. The cell suspension was centrifuged at room temperature at
300 × g for 10 min, and the cells were cultured with keratinocyte
serum-free medium (Invitrogen; Thermo Fisher Scientific, Inc.) with
25 µg/ml bovine pituitary extract (Cell Applications, Inc.) and 0.2
µg/ml EGF recombinant protein (Invitrogen; Thermo Fisher
Scientific, Inc.).
Establishment of Rab31-knockdown OSCC
cell lines
Short hairpin (sh)RNA sequences (shRNA1 and shRNA2)
specific for Rab31 and a negative control shRNA were designed and
synthesized by Shanghai GenePharma Co., Ltd. The sequences were as
follows: shRNA1,
5′-CCGGTTATGTGTATGGGATTCTAAACTCGAGTTTAGAATCCCATACACATAATTTTTG-3′;
shRNA2,
5′-GTACCGGAGTGCGACCTCTCAGATATTACTCGAGTAATATCTGAGAGGTCGCACTTTTTTTG-3′;
and control shRNA, 5′-TTCTCCGAACGTGTCACGT-3′. For the construction
of human Rab31-specific shRNA plasmids, the two shRNA sequences
were separately inserted at the BamHI/EcoRI
restriction sites of a pGLVU6/Puro lentiviral vector (Shanghai
GenePharma Co., Ltd). The lentiviral expression vectors and
packaging plasmids were co-transduced into 293T cells (American
Type Culture Collection), a highly transfectable derivative of
human embryonic kidney 293 cells, according to the lentiviral
vector manufacturer's instructions. Briefly, 1.6×104
cells were added to fresh medium in a 96-well plate; 5 ml
(1×108 TU/ml) of lentiviral particles were added, and
the plates were incubated for 18 h at 37°C in a humidified
incubator with 5–7% CO2. The 293T cells were transfected
with the pGLVU6 vector with the packaging plasmids pGag/Pol, pRev,
and pVSV-G using RNAi-Mate (Shanghai GenePharma Co., Ltd.). The
viral particles were harvested at 48 h post-transfection.
Subsequently, 5 ml (1×108 TU/ml) of lentiviral particles
per 1.6×104 cells) were incubated with SCC-4 or SCC-25
cells for 10 h at 37°C in a humidified incubator with 5–7%
CO2 in the presence of 6 µg/ml polybrene
(MilliporeSigma) (20).
Rab31-knockdown cells were selected with 2 µg/ml puromycin at 37°C
in a humidified incubator (R&D Systems, Inc.); the medium was
replaced with fresh puromycin-containing medium every 3–4 days
until resistant colonies were identified.
Colony formation assays
SCC-4 and SCC-25 cells transduced with the negative
control or Rab31-specific shRNA were seeded in 6-well plates at a
density of 2,000 cells/well at 37°C in a humidified incubator with
5–7% CO2. One week later, the cell colonies were fixed
with 4% formaldehyde for 15 min at room temperature and stained
with 0.05% crystal violet at room temperature for 20 min
(Sigma-Aldrich; Merck KGaA). The numbers of colonies were then
counted and recorded under a bright-field microscope (EVOS FL Auto
Imaging System, v1.6 software; Thermo Fisher Scientific, Inc.).
Cell proliferation assay
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) assay was used to assess the proliferative
ability of OSCC cells. Briefly, 300 SCC-4 or SCC-25 cells per well
transduced with the negative control or Rab31-specific shRNA were
seeded in a 96-well plate. After 5 days, the cells were incubated
with a CCK-8 solution for 4 h at 37°C in a humidified
incubator with 5–7% CO2. The plates were analyzed with a
plate reader at a wavelength of 450 nm.
Flow cytometry
The SCC-4 and SCC-25 cells (1×106
cells/well) transfected with or without shRNA targeting Rab31 were
used for flow cytometry analyses. The cells were induced with
Camptothecin stock solution (cat. no. C-9911; Sigma-Aldrich; Merck
KGaA). The cells were incubated for 4–6 h at 37°C, and
Annexin V/propidium iodide (PI) (BD Pharmingen, Cat. no. 556570; BD
Biosciences) staining was performed according to the manufacturer's
instructions. The cells were washed twice and resuspended in 1X
binding buffer at 1×106 cells/ml. Subsequently, 5 µl
Annexin V and 5 PI were added and incubated for 15 min at 25°C in
the dark. The late apoptotic cells were counted with a BD
FACSCalibur flow cytometer (BD Biosciences) using FlowJo version
7.6 software (BD Biosciences).
Enzyme-linked immunosorbent assay
The protein level of MMP9 in the supernatants of
SCC-4 and SCC-25 cell cultures (transfected with the negative
control or Rab31-specific shRNA) was quantified using the Human
MMP-9 Quantikine ELISA kit (cat. no. DMP900; R&D Systems, Inc.)
according to the manufacturer's instructions. Briefly, SCC-4 and
SCC-25 cells transfected with or without shRNA targeting Rab31 were
cultured in DMEM containing 10% FBS. The cells were washed with PBS
three times and cultured with serum-free DMEM for 24 h at 37°C. The
supernatants were harvested for analysis by centrifugation at 300 ×
g for 10 min at 4°C. The optical density was measured at 450 nm,
and the protein concentration was determined by comparing the
relative absorbance of the samples with a standard curve.
Cell invasion assay
Costar Transwell inserts (8-µm pore size; Corning,
Inc.) coated with a matrix gel (BD Pharmingen; BD Biosciences) at
37°C for 30 min were used for the cell invasion assay. SCC-4 and
SCC-25 cells transduced with negative control or Rab31-specific
shRNA were seeded in the upper chamber at a density of
2×104 cells/well in FBS-free DMEM/F12; medium and 10%
FBS were added to the lower chamber. Following incubation at 37°C
for 24 h, the cells in the upper chamber were removed with a cotton
swab. The cells in the lower chamber were fixed with 5% glutaric
dialdehyde for 15 min at room temperature and stained with 0.1%
crystal violet for 20 min. The invasive cells were imaged and
counted under an Olympus IX73 inverted microscope at ×400
magnification in five fields per sample using ImageJ 1.48 software
(National Institutes of Health).
In vivo experiments
Female athymic BALB/c nude mice (weight, 18–20 g;
age, 5–6 weeks) used in the current study were purchased from Hunan
Silaike Jingda Laboratory Animal Co., Ltd. The experiments were
performed according to the institutional guidelines of The Public
Experimental Platform Center of Zhejiang. Nude mice were housed in
sterile laminar flow cabinets under specific pathogen-free
conditions (humidity, 50%; temperature, 25°C; light cycle, 12 h
light/12 h dark; ad libitum food and water). SCC-25 cells
(1×106) were suspended 1 ml DMEM for the injection. The
SCC-25 cells transduced with control (n=5) or Rab31-specific (n=5)
shRNA were inoculated into the right flank of nude mice. Tumor
volumes was measured with a caliper every other day and calculated
according to the following formula: Volume = (width2 ×
length)/2. On day 28, the mice were sacrificed using CO2
inhalation by adjusting the air displacement rate at 10–30%/min.
Exposure to CO2 was continued for at least 5 min after
respiratory arrest. Euthanasia was confirmed by cervical
dislocation, and the tumors were collected.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted from human OSCC or normal
oral epithelial cells and mouse tumors using TRIzol®
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. First-strand cDNA was synthesized
using 1 µg total RNA and a RevertAid First Strand cDNA Synthesis
kit (Fermentas; Thermo Fisher Scientific, Inc.). qPCR was performed
on a ViiA 7 Real-Time PCR system (Thermo Fisher Scientific, Inc.)
with the following thermocycling conditions: 2 min at 95°C,
followed by 40 cycles of 10 sec at 95°C, 30 sec at 60°C and 30 sec
at 72°C. All experiments were performed in triplicate. The relative
RNA expression levels were calculated using the 2−ΔΔCq
method (21). β-actin was used as
the internal control. The primer sequences are listed in Table I.
 | Table I.Primer sequences used in the present
study. |
Table I.
Primer sequences used in the present
study.
| Gene | Sequence
(5′→3′) |
|---|
| Rab31 | F:
TGTCTTCTCGGGGACACGGGA |
|
| R:
CACGATGTTCTCTGGGCCATGCTC |
| Cyclin D1 | F:
CCGCCTCACACGCTTCCTCTC |
|
| R:
TCCTCCTCGGCGGCCTTGGGG |
| E-cadherin | F:
CCCATCAGCTGCCCAGAAAATGAA |
|
| R:
CTGTCACCTTCAGCCATCCTGTTT |
| N-cadherin | F:
CGAGCCGCCTGCGCTGCCAC |
|
| R:
CGCTGCTCTCCGCTCCCCGC |
| Bcl-2 | F:
TTCTTTGAGTTCGGTGGGGTC |
|
| R:
TGCATATTTGTTTGGGGCAGG |
| Survivin | F:
TGCCTGGCAGCCCTTTCTCA |
|
| R:
TGGCACGGCGCACITTCTTC |
| β-actin | F:
ATCACCATTGGCAATGAGCG |
|
| R:
ATCACCATTGGCAATGAGCG |
Protein extraction and WB
Total protein was extracted from cells or mouse
tumors using cell lysis buffer (Pierce; Thermo Fisher Scientific,
Inc.). Protein quantification was conducted using a Bradford assay;
20 µg of protein was loaded per lane, separated by SDS-PAGE (10%)
and transferred to polyvinylidene difluoride membranes (Merck
KGaA). The membranes were incubated with primary antibodies
overnight at 4°C. The following day, the membranes were washed with
1X PBS + 0.1% Tween-20 and incubated with an HRP-conjugated
anti-rabbit IgG secondary antibody (1:3,000; cat. no. 7074S; Cell
Signaling Technology, Inc.) for 60 min at room temperature. An
enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.) was
used for protein visualization. β-actin (1:2,000; cat. no. 84575;
Cell Signaling Technology, Inc.) was used as the loading
control.
Statistical analysis
Data are presented as the mean ± SEM. GraphPad Prism
5.0 software (GraphPad Software, Inc.) was used to analyze data.
Student's t test or one-way ANOVA with Tukey's post hoc test was
used for statistical comparisons. The associations between Rab31
expression levels in normal and cancerous tissues, and those with
different pathological characteristics were analyzed by Fisher's
exact test. Kaplan-Meier survival analysis and log-rank test were
performed using SPSS 20.0 (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Rab31 is expressed at high levels in
human OSCC tissues
To investigate the expression of Rab31 in OSCC
tissues, 54 human OSCC and 16 normal oral mucosa tissue specimens
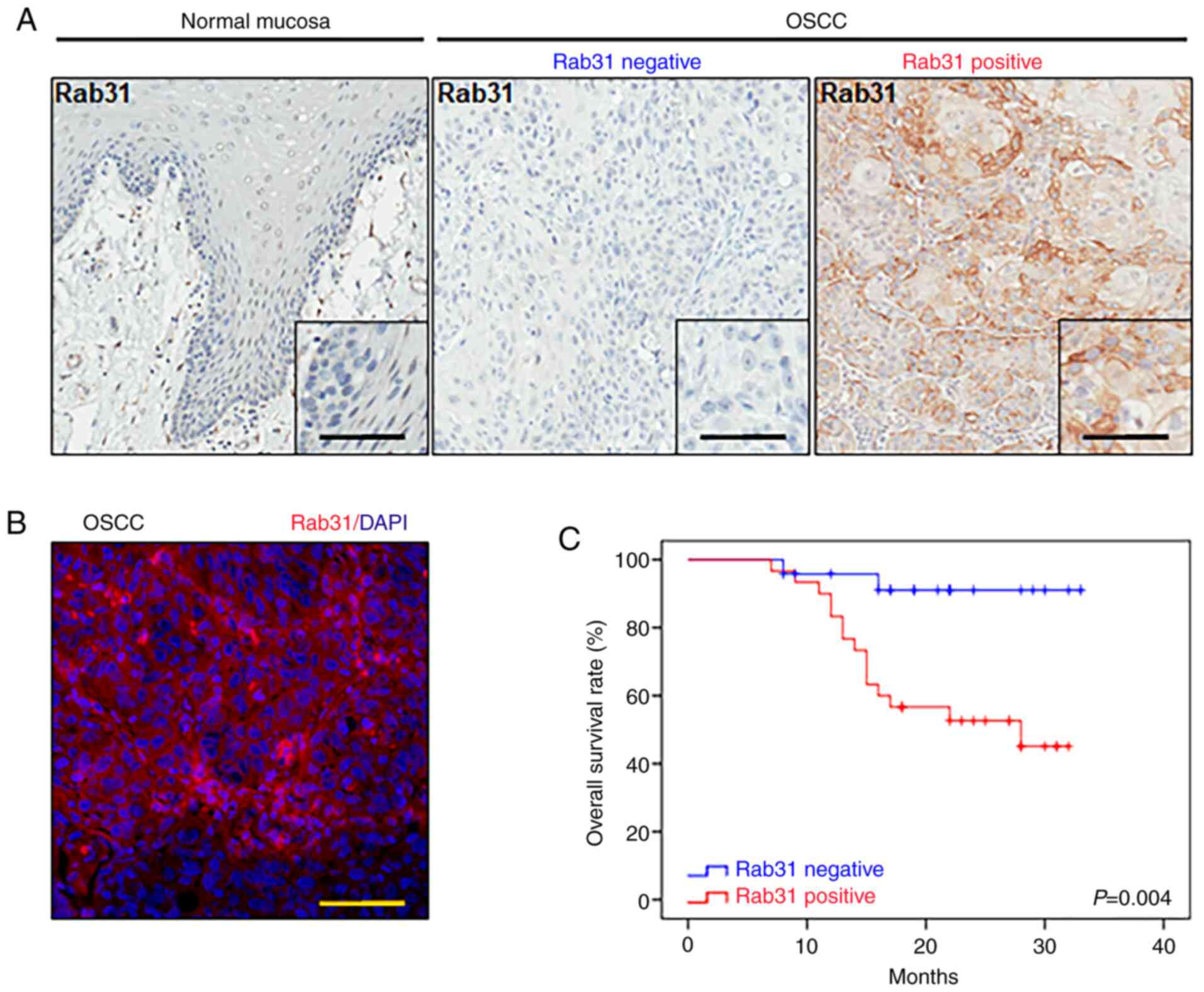
were used in the current study. Representative images of Rab31 IHC
staining (normal mucosa, Rab31-negative OSCC and Rab31-positive
OSCC) are presented in Fig. 1A. The
results of IF demonstrated that Rab31 was mainly located in the
membrane and cytoplasm of cancerous epithelial cells (Fig. 1B). Among the OSCC samples, 55.55%
(30/54) were positive for Rab31, and the expression levels of Rab31
were significantly higher in human OSCC tissues compared with the
normal oral mucosa samples (P=0.002; Table II).
 | Table II.Rab31 expression levels in human OSCC
and healthy mucosa samples. |
Table II.
Rab31 expression levels in human OSCC
and healthy mucosa samples.
|
|
| Rab31 |
|
|---|
|
|
|
|
|
|---|
| Tissue samples | Total | Negative | Positive |
P-valuea |
|---|
| Mucosa | 16 | 14 | 2 |
|
| OSCC | 54 | 24 | 30 | 0.002 |
Rab31 expression is associated with a
high pathological grade and poor prognosis in human OSCC
To determine the relationship between Rab31 and the
clinicopathological characteristics of patients with OSCC, the
associations between Rab31 expression levels and patient TNM stage,
pathological grade and prognosis were analyzed. Due to the limited
number of cases with poor differentiation (n=4), pathological
grades were compared between grades I and II/III. In addition,
since no T4 stage cases were included in the present study, T1 and
T2 were considered early-stage OSCC. and T3 was considered
late-stage OSCC for the comparison. The results demonstrated that
positive expression of Rab31 was associated with a high
pathological grade (P=0.016), whereas no significant associations
were observed between Rab31 expression levels and tumor size
(P=0.363) or lymph node involvement (P=0.799) (Table III). Notably, Kaplan-Meier
analysis with the log-rank test revealed that patients with
positive expression of Rab31 presented with shorter survival times
compared with patients who were negative for Rab31 expression
(P=0.004; Fig. 1C). These results
suggested that Rab31 may be associated with a poor prognosis of
patients with OSCC.
 | Table III.Associations between patient
clinicopathological characteristics and Rab31 expression levels |
Table III.
Associations between patient
clinicopathological characteristics and Rab31 expression levels
|
|
| Rab31 |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Total | Negative | Positive |
P-valuea |
|---|
| Grade |
|
|
| 0.016 |
| I | 12 | 9 | 3 |
|
|
II+III | 42 | 15 | 27 |
|
| Tumor stage |
|
|
| 0.363 |
|
T1+T2 | 45 | 21 | 24 |
|
| T3 | 9 | 3 | 6 |
|
| Lymph node
involvement |
|
|
| 0.799 |
|
Negative | 35 | 16 | 19 |
|
|
Positive | 19 | 8 | 11 |
|
Establishment of Rab31-knockdown OSCC
cell lines
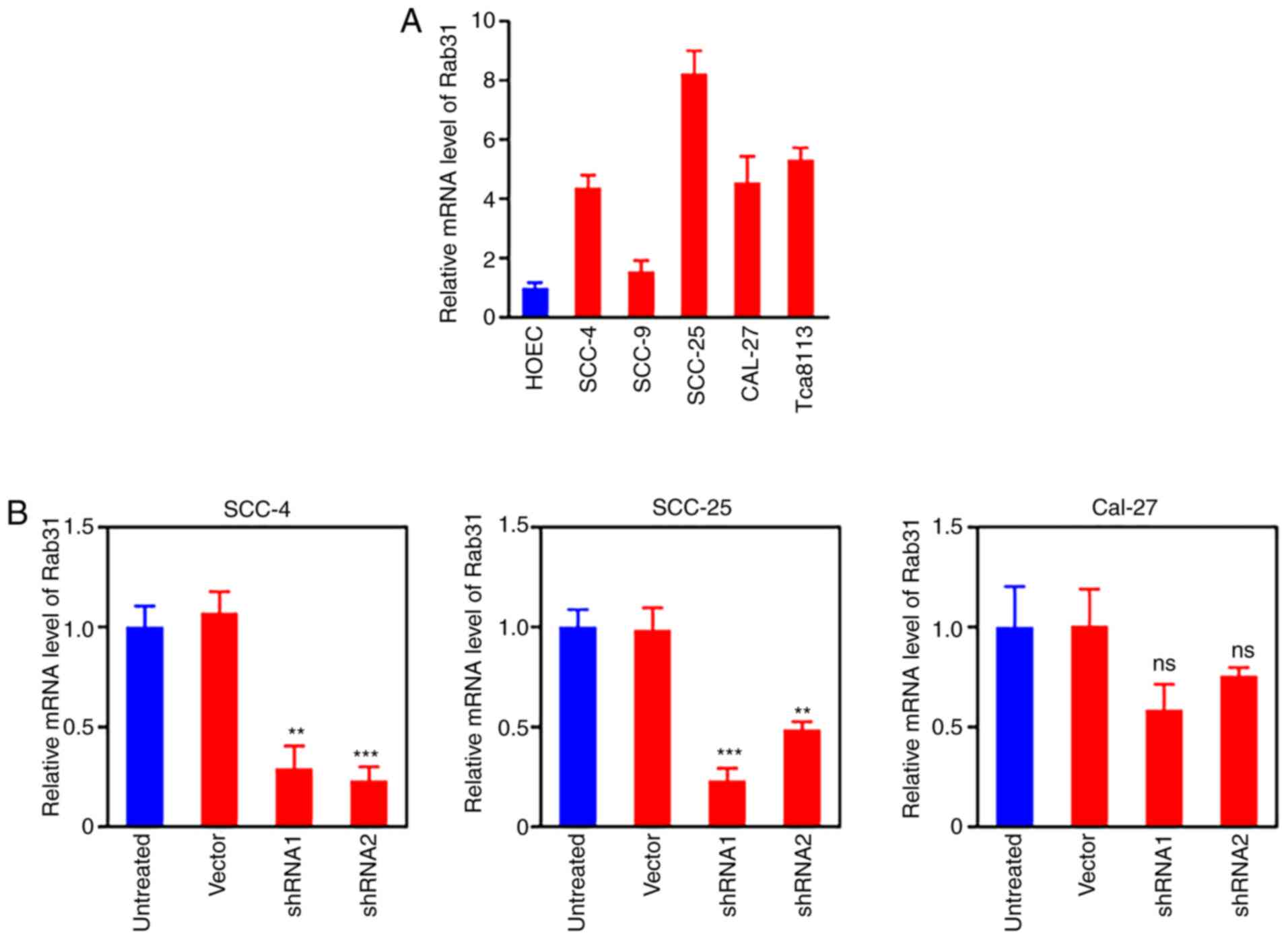
To assess the expression levels of Rab31 in cultured
OSCC cells, the mRNA expression levels of Rab31 were determined in
four OSCC cell lines (SCC-4, SCC-9, SCC25, and CAL-27); primary
cultured HOECs were used as the normal control (Fig. 2A). The SCC-9 cells exhibited no
notable increase in Rab31 expression levels compared with those in
the normal HOECs. To establish Rab31-knockdown OSCC cell lines, two
shRNAs (shRNA1 and shRNA2) were transduced into CAL-27, SCC-4 and
SCC-25 cells. The selection of SCC-4 and SCC-25 cell lines was
based on the successful Rab 31 knockdown, which was not observed in
CAL-27 cells. Both shRNA1 and shRNA2 effectively downregulated the
mRNA expression levels of Rab31 in SCC-4 and SCC-25 cells compared
with those in the control groups; although shRNA2 appeared to be
highly effective in the SCC-4 cell line, the results for SCC-25
were not satisfactory (Fig. 2B).
Thus, to achieve efficient knockdown in both cell lines, shRNA1
against Rab31 (sh-Rab31) was selected for use in further
experiments.
Silencing Rab31 inhibits OSCC cell
proliferation and induces apoptosis
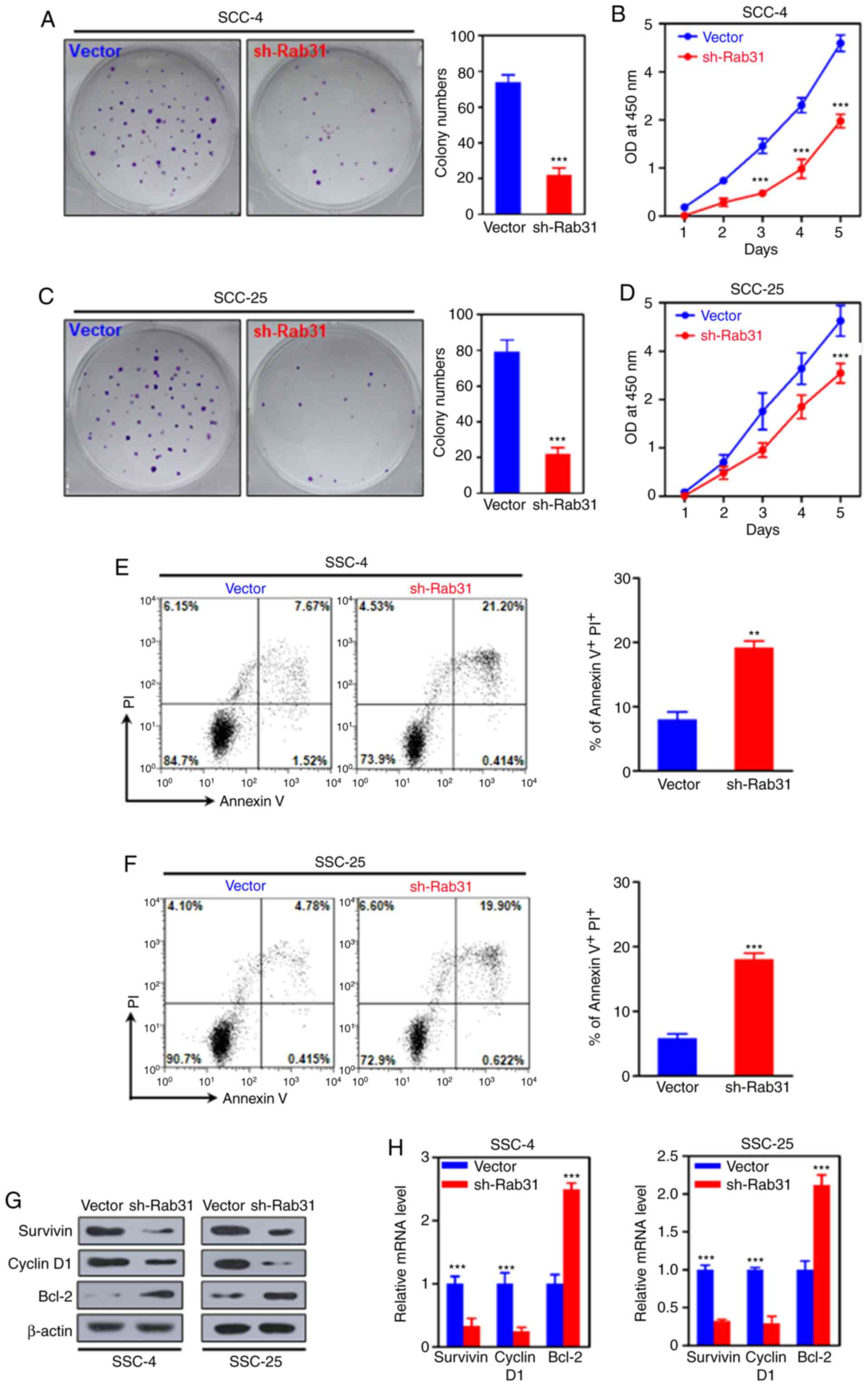
The effects of Rab31 on OSCC cell proliferation were
next assessed. Compared with those in the corresponding control
groups, silencing Rab31 significantly decreased the colony numbers
and suppressed the proliferation of SCC-4 and SCC-25 cells
(Fig. 3A-D). Additionally, the
results of the flow cytometry assays revealed that Rab31 knockdown
resulted in increased percentages of apoptotic SCC-4 (control,
7.67% vs. sh-Rab31, 21.20%; P<0.001) and SCC-25 (control, 4.78%
vs. sh-Rab31, 19.90%; P<0.001) cells (Fig. 3E and F). Subsequent WB and RT-qPCR
analyses demonstrated that Rab31 silencing reduced the expression
levels of Survivin and cyclin D1, but increased the expression of
Bcl-2 at the protein and mRNA levels compared with those in the
control cells (Fig. 3G and H).
These results suggested that Rab31 was associated with cell
survival, the cell cycle and apoptosis.
Knockdown of Rab31 expression
suppresses the invasive ability of OSCC cells
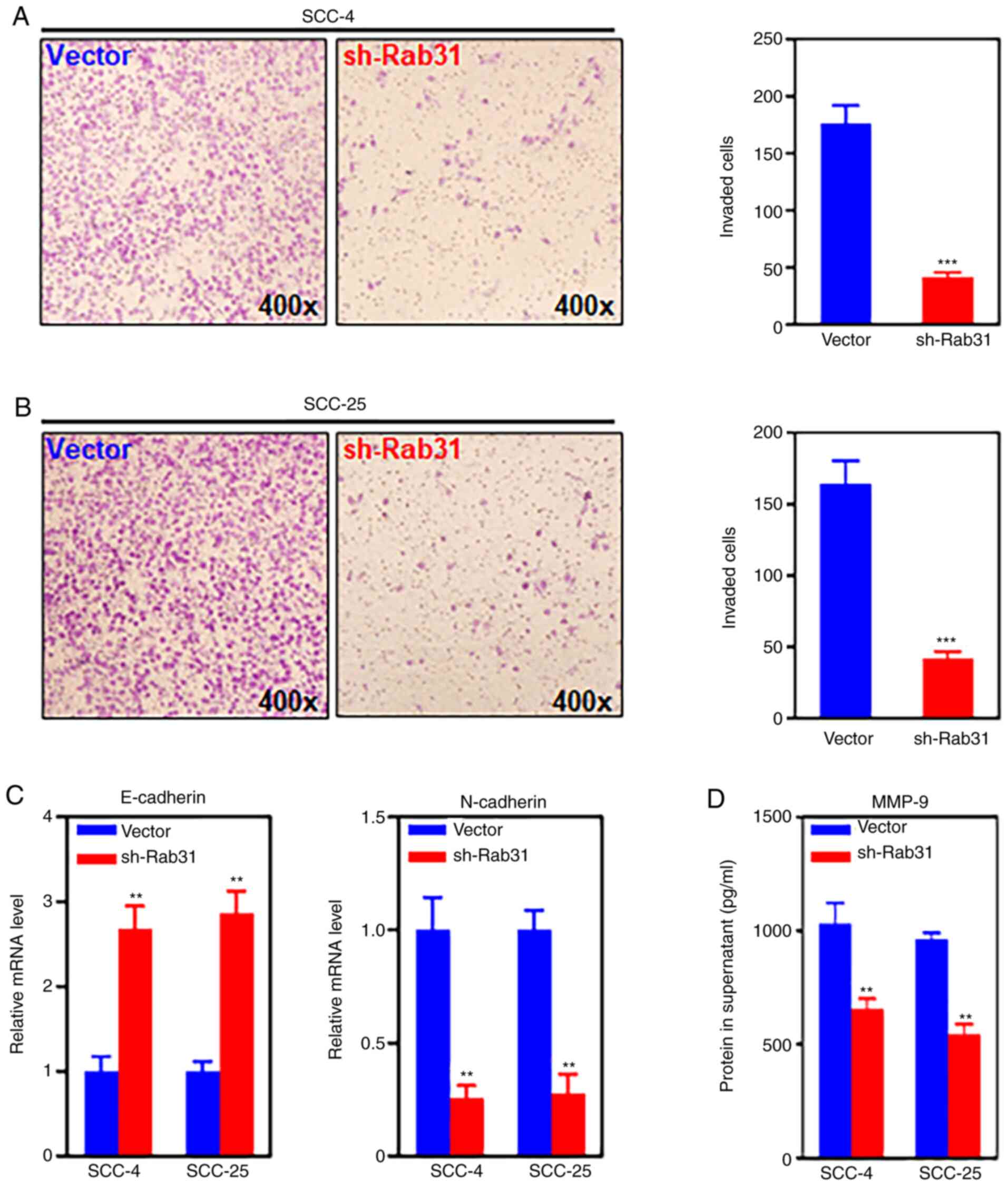
In the current study, the effects of Rab31 silencing
on the invasive ability of OSCC cell lines were assessed using a
Transwell invasion assay. The results demonstrated that knockdown
of Rab31 expression significantly reduced the invasive capability
of SCC-4 and SCC-25 cells compared with that in the control groups
(Fig. 4A and B). Additionally, the
mRNA expression levels of two crucial markers of the
epithelial-mesenchymal transition (EMT) were detected by RT-qPCR;
the results revealed that compared with those in the control
groups, Rab31 silencing significantly upregulated the mRNA
expression levels of E-cadherin and downregulated the levels of
N-cadherin (Fig. 4C). These results
suggested a link between Rab31 and the EMT. The protein secretion
of matrix metalloproteinase (MMP-9), an indispensable enzyme
involved in tumor cell invasion, was detected by ELISA. Rab31
knockdown significantly decreased the MMP-9 protein levels in the
supernatants of SCC-4 and SCC-5 cell cultures compared with those
in the supernatants of the control-transfected cells (Fig. 4D).
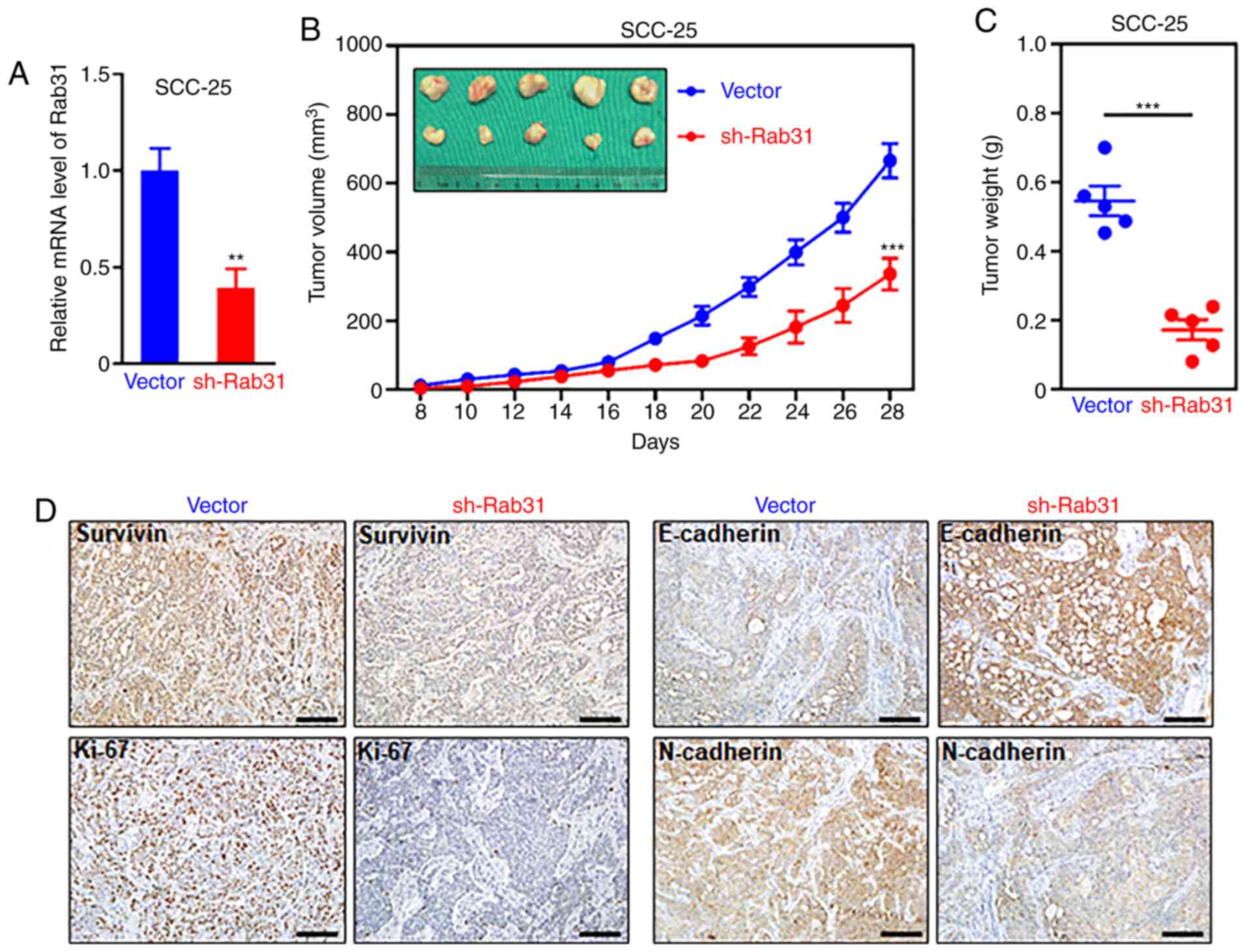
Silencing Rab31 suppresses the growth
of xenograft tumors
To determine the effects of Rab31 on OSCC cells
in vivo, control (n=5) or sh-Rab31-transfected (n=5) SCC-25
cells were inoculated into nude mice. The knockdown efficiency of
sh-Rab31 in tumor tissues was tested and compared with that of the
control vector (Fig. 5A). The tumor
growth curves demonstrated that Rab31 silencing significantly
suppressed tumor growth in vivo (Fig. 5B). On day 28, the mice were
euthanized, and the tumors were harvested for further analysis. The
results revealed that the tumor weight of the shRab31-treated group
was significantly lower compared with that of the control group
(Fig. 5C). IHC staining results
demonstrated that compared with the control group, Rab31 silencing
decreased the expression levels of survivin, Ki-67 and N-cadherin,
but upregulated those of E-cadherin (Fig. 5D). These observations were in
accordance with the results of the in vitro analyses.
Discussion
Rab31 is known to serve a number of roles in cancer
progression (22); however, its
role in human OSCC remains poorly understood. The current study
examined the expression of Rab31 in resected OSCC tissue specimens.
The results demonstrated that Rab31 expression was upregulated in
human OSCC tissue samples compared with those from healthy
subjects; further analysis identified the relationship between
Rab31 expression and a poor prognosis in patients with OSCC.
Knockdown of Rab31 expression inhibited cancer cell proliferation
and induced apoptosis compared with those observed in the negative
control-transfected cells. In addition, the results demonstrated
that silencing Rab31 reduced the invasive ability of OSCC cells.
Notably, in vivo experimental analysis revealed that
knockdown of Rab31 expression suppressed tumor growth in
Rab31-specific shRNA-transfected xenografts compared with that in
the control group.
High expression levels of Rab31 have been reported
in various types of cancer, including breast and ovarian cancer,
glioblastoma and hepatocellular carcinoma (11,22–25).
The results of the present study demonstrated a significant
increase in Rab31 expression levels in OSCC samples compared with
those in normal oral mucosa samples. In addition, OSCC cell lines
presented with higher protein and mRNA expression levels of Rab31
compared with those in the normal oral mucosa cells. These results
were consistent with those of previous studies. Rab31 mRNA
expression levels have been previously identified to be elevated in
a cisplatin-resistant OSCC cell line via Affymetrix microarray
analysis (26). Additionally,
differential gene expression profiling revealed Rab31 to be among a
number of genes exhibiting upregulated expression by cDNA
microarray analysis in a pingyangmycin-resistant cell line compared
with non-resistant cell lines (27). Furthermore, the expression of Rab31
has been associated with the clinicopathological characteristics of
certain types of cancer. In hepatocellular carcinoma, high
expression levels of Rab31 have been reported to be a predictive
factor for a poor prognosis, which is independent of advanced TNM
staging and intrahepatic metastasis (24). Additionally, high Rab31 mRNA
expression levels have been reported to be associated with distant
metastasis-free and overall survival in a multivariate analysis of
breast cancer (28); however, no
associations have been identified between Rab31 expression levels
and overall or progression-free survival in advanced ovarian cancer
(29). The results of the present
study suggested that positive expression of Rab31 may be associated
with a high pathological grade and poor prognosis in patients with
OSCC.
The present study revealed the potential role and
the underlying mechanism of Rab31 in OSCC. In the present study,
knockdown of Rab31 expression attenuated OSCC cell colony formation
and proliferation rates compared with those in the control cells,
as determined by colony formation and CCK-8 proliferation assays.
Cyclin D1 and Survivin serve pivotal roles in tumor cell
proliferation (30). The results of
the present study demonstrated that Rab31 silencing suppressed the
protein and mRNA expression levels of cyclin D1 and Survivin. Bcl-2
has been reported to be a key mediator of the apoptotic response to
anticancer treatment (31). The
results of the current study demonstrated that knockdown of Rab31
expression resulted in the induction of Bcl-2 expression. A
previous study has reported that silencing Rab31 expression
suppresses the proliferation and induced apoptosis in MHCC97 cells,
whereas ectopic expression of Rab31 leads to an increase in the
Bcl-2/Bax ratio by activating the phosphoinositide 3-kinase/protein
kinase B (AKT) signaling pathway (23). The indirect interaction between Rab
family proteins and Bcl-2 are further supported by another study in
gastric cancer, which has demonstrated that inhibition of Rab9
expression leads to increased levels of Bax and decreased levels of
Bcl-2, triggering apoptosis (20).
In addition, overexpression of Rab31 decreases the adhesion and
invasion and promotes the proliferation of breast cancer cells,
which results in a transition from an invasive to a proliferative
phenotype (12). The results of the
present study also demonstrated that Rab31 silencing inhibited the
invasive ability of OSCC cell lines. E-cadherin and N-cadherin are
markers of the EMT (32). During
the EMT process, epithelial cells obtain migratory and invasive
properties, and lose cell-cell adhesion, accompanied by an increase
in N-cadherin and a decrease in E-cadherin expression levels
(33). The present study
demonstrated that Rab31 silencing downregulated the mRNA expression
levels of N-cadherin and upregulated those of E-cadherin. These
results were in agreement with those of a study by Zhang et
al (32) on Rab3D in esophageal
cancer cells involving the PI3K/Akt signaling pathway, in which
knockdown of Rab3D significantly suppressed esophageal cancer cell
migration and invasion and accordingly altered EMT-related markers,
including the upregulation E-cadherin and downregulation of
N-cadherin expression levels. However, the positive in vitro
results did not correspond to the negative in vivo results
in the present study, as no significant associations were observed
between lymph node metastasis and Rab31 expression in human
tissues. These negative results, to the best of our knowledge, may
have occurred due to the design of the pathological sample study
(comparisons among different patients rather than tissues from the
same patient) and the limited sample size of the current study. On
the other hand, Rab31 is also regulated by MUC1 proteins and the
interaction of u-plasminogen activator receptor with the
cation-independent mannose 6-phosphate receptor complex (22), which may affect the expression and
functions of Rab31. Such contradictory in vitro and in
vivo results have also been observed in a study of breast
cancer (12). Notably, when Rab31
was overexpressed in breast cancer cell lines, it enhanced the cell
proliferation, diminished adhesion mediated through several
extracellular matrix components, and attenuated invasion in
vitro; however, when breast cancer cells moderately
overexpressing Rab31 were xenografted into nude mice, they
exhibited significantly reduced lung metastasis compared with that
observed in control cell xenografts (12). The present results are consistent
with those of the aforementioned study, suggesting a complicated
molecular loop system regulating the oncogenic potential of
Rab31.
OSCC cells exhibit high levels of MMP-9, and
targeting MMPs by selective gelatinase peptides inhibits the
invasion of oral tongue squamous cell carcinoma (33,34).
Rab27a, Rab27b and Rab27 effector proteins are involved in assembly
at fusion sites before and during the exocytosis of MMP-9, and
these components are lost from sites of exocytosis when MMP-9 is
released (35). A direct
relationship between Rab family proteins and MMP-9 release has been
observed and speculated in a study Stephens et al (36). In the present study, the protein
levels of MMP-9 in the supernatant obtained from OSCC cells were
measured by ELISA; knockdown of Rab31 expression significantly
reduced MMP-9 secretion compared with that in the
control-transfected cells. Previous studies have focused on other
types of cancer and reported similar results. For example, in
glioblastoma and cervical cancer, Rab31 serves a crucial role in
cell proliferation by promoting G1/S transition and regulating the
expression levels of cell cycle-related proteins, such as cyclins
D1, A and B1 (23,30). In addition, Rab31-induced cervical
cancer cell proliferation and migration require the phosphorylation
of AKT and extracellular signal-regulated kinase 1/2 (23). Rab31 knockdown in cervical cancer
cells decreases the protein and mRNA expression levels of
N-cadherin and upregulates those of E-cadherin (23). Taken together, these studies
indicate the essential roles of Rab31 in the proliferation and
migration of human OSCC.
In conclusion, upregulation of Rab31 expression
levels was detected in OSCC tissues compared with those from
healthy subjects, and it was associated with a high pathological
grade and short survival. The results of the present study also
demonstrated that Rab31 contributed to the proliferation and
invasion of OSCC cells. In addition, Rab31 knockdown induced
apoptosis in OSCC cells in vitro and suppressed xenograft
tumor growth in vivo. These results suggested that Rab31 may
be exploited for the development of a therapeutic agent for
OSCC.
Acknowledgements
Not applicable.
Funding
This work was supported by The National Natural
Science foundation Project (grant no. 81570949), The Excellent
Subject Leader Plan of Shanghai Municipal Commission of Health and
Family Planning (grant no. 2017BR019), The Youth Project of
Shanghai Municipal Commission of Health and Family Planning (grant
no. 20164Y0067), The Science and Technology Commission of Shanghai
Municipality, Natural Science Grant (grant no. 19ZR1430000) and The
Hospital Innovation Project (grant no. CK2019004).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YH and CM conceived and designed the study, and
contributed to data interpretation, obtaining the patient
information and revising the manuscript. CM acquired the
pathological specimens. XL and FZ performed most of the
experiments. XL and FZ confirm the authenticity of all the raw
data. ZL, XT, YH and JJ conducted a part of the in vivo and
in vitro transfection experiments. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All patients' samples were used under written
approval by the patients. All experiments in the present study were
approved by the Independent Ethics Committee and Animal
Experimental Ethical Inspection of the Shanghai Ninth People's
Hospital, Shanghai Jiao Tong University School of Medicine
(Shanghai, China) and performed at the Shanghai Ninth People's
Hospital (approval nos. SH9H-2019-T117-1 for human tissues and
HKDL-2017-304 for animal studies).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi S and Myers JN: Molecular
pathogenesis of oral squamous cell carcinoma: Implications for
therapy. J Dent Res. 87:14–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hunter KD, Parkinson EK and Harrison PR:
Profiling early head and neck cancer. Nat Rev Cancer. 5:127–135.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang YJ, Zhang ZF, Fan SH, Zhuang J, Shan
Q, Han XR, Wen X, Li MQ, Hu B, Sun CH, et al: MicroRNA-433 inhibits
oral squamous cell carcinoma cells by targeting FAK. Oncotarget.
8:100227–100241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonner JA, Harari PM, Giralt J, Cohen RB,
Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, et al:
Radiotherapy plus cetuximab for locoregionally advanced head and
neck cancer: 5-year survival data from a phase 3 randomised trial,
and relation between cetuximab-induced rash and survival. Lancet
Oncol. 11:21–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chia WJ and Tang BL: Emerging roles for
Rab family GTPases in human cancer. Biochim Biophys Acta.
1795:110–116. 2009.PubMed/NCBI
|
|
7
|
Recchi C and Seabra MC: Novel functions
for Rab GTPases in multiple aspects of tumour progression. Biochem
Soc Trans. 40:1398–1403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen D, Guo J, Miki T, Tachibana M and
Gahl WA: Molecular cloning of two novel rab genes from human
melanocytes. Gene. 174:129–134. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klopper TH, Kienle N, Fasshauer D and
Munro S: Untangling the evolution of Rab G proteins: Implications
of a comprehensive genomic analysis. BMC Biol. 10:712012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ng EL, Wang Y and Tang BL: Rab22B's role
in trans-Golgi network membrane dynamics. Biochem Biophys Res
Commun. 361:751–757. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abba MC, Hu Y, Sun H, Drake JA, Gaddis S,
Baggerly K, Sahin A and Aldaz CM: Gene expression signature of
estrogen receptor alpha status in breast cancer. BMC Genomics.
6:372005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grismayer B, Solch S, Seubert B, Kirchner
T, Schäfer S, Baretton G, Schmitt M, Luther T, Krüger A, Kotzsch M
and Magdolen V: Rab31 expression levels modulate tumor-relevant
characteristics of breast cancer cells. Mol Cancer. 11:622012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pu SY, Yu Q, Wu H, Jiang JJ, Chen XQ, He
YH and Kong QP: ERCC6L, a DNA helicase, is involved in cell
proliferation and associated with survival and progress in breast
and kidney cancers. Oncotarget. 8:42116–42124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amornphimoltham P, Rechache K, Thompson J,
Masedunskas A, Leelahavanichkul K, Patel V, Molinolo A, Gutkind JS
and Weigert R: Rab25 regulates invasion and metastasis in head and
neck cancer. Clin Cancer Res. 19:1375–1388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clausen MJ, Melchers LJ, Mastik MF,
Slagter-Menkema L, Groen HJ, Laan BF, van Criekinge W, de Meyer T,
Denil S, van der Vegt B, et al: RAB25 expression is epigenetically
downregulated in oral and oropharyngeal squamous cell carcinoma
with lymph node metastasis. Epigenetics. 11:653–663. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang D, Lu C and Ai H: Rab5a is
overexpressed in oral cancer and promotes invasion through ERK/MMP
signaling. Mol Med Rep. 16:4569–4576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bao Q, Liao X, Li R and Ding N: KCNQ1OT1
promotes migration and inhibits apoptosis by modulating
miR-185-5p/Rab14 axis in oral squamous cell carcinoma. Dev Growth
Differ. 61:466–474. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu Y, Shi F, Wang M and Ding J: Knockdown
of Rab9 suppresses the progression of gastric cancer through
regulation of Akt signaling pathway. Technol Cancer Res Treat.
19:15330338209159582020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pollaers K, Hinton-Bayre A, Friedland PL
and Farah CS: AJCC 8th Edition oral cavity squamous cell carcinoma
staging-Is it an improvement on the AJCC 7th Edition? Oral Oncol.
82:23–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Bao W, Jiang F, Che Q, Chen Z,
Wang F, Tong H, Dai C, He X, Liao Y, et al: Mutant p53 (p53-R248Q)
functions as an oncogene in promoting endometrial cancer by
up-regulating REGγ. Cancer Lett. 360:269–279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chua CE and Tang BL: The role of the small
GTPase Rab31 in cancer. J Cell Mol Med. 19:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pan Y, Zhang Y, Chen L, Liu Y, Feng Y and
Yan J: The Critical Role of Rab31 in cell proliferation and
apoptosis in cancer progression. Mol Neurobiol. 53:4431–4437. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sui Y, Zheng X and Zhao D: Rab31 promoted
hepatocellular carcinoma (HCC) progression via inhibition of cell
apoptosis induced by PI3K/AKT/Bcl-2/BAX pathway. Tumour Biol.
36:8661–8670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kotzsch M, Kirchner T, Soelch S, Friedrich
K, Baretton G, Magdolen V and Luther T: Inverse association of
rab31 and mucin-1 (CA15-3) antigen levels in estrogen
receptor-positive (ER+) breast cancer tissues with
clinicopathological parameters and patients' prognosis. Am J Cancer
Res. 7:1959–1970. 2017.PubMed/NCBI
|
|
26
|
Zhang P, Zhang Z, Zhou X, Qiu W, Chen F
and Chen W: Identification of genes associated with cisplatin
resistance in human oral squamous cell carcinoma cell line. BMC
Cancer. 6:2242006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng G, Zhou M, Ou X, Peng B, Yu Y, Kong
F, Ouyang Y and He Z: Identification of carbonic anhydrase 9 as a
contributor to pingyangmycin-induced drug resistance in human
tongue cancer cells. FEBS J. 277:4506–4518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kotzsch M, Sieuwerts AM, Grosser M, Meye
A, Fuessel S, Meijer-van Gelder ME, Smid M, Schmitt M, Baretton G,
Luther T, et al: Urokinase receptor splice variant
uPAR-del4/5-associated gene expression in breast cancer:
Identification of rab31 as an independent prognostic factor. Breast
Cancer Res Treat. 111:229–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kotzsch M, Dorn J, Doetzer K, Schmalfeldt
B, Krol J, Baretton G, Kiechle M, Schmitt M and Magdolen V: mRNA
expression levels of the biological factors uPAR, uPAR-del4/5, and
rab31, displaying prognostic value in breast cancer, are not
clinically relevant in advanced ovarian cancer. Biol Chem.
392:1047–1051. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Z, Cui J, Yu Q, Wu X, Pan A and Li L:
Evaluation of CCND1 amplification and CyclinD1 expression: diffuse
and strong staining of CyclinD1 could have same predictive roles as
CCND1 amplification in ER positive breast cancers. Am J Transl Res.
8:142–153. 2016.PubMed/NCBI
|
|
31
|
Zhang W, Liu Y, Li YF, Yue Y, Yang X and
Peng L: Targeting of survivin pathways by YM155 inhibits cell death
and invasion in oral squamous cell carcinoma cells. Cell Physiol
Biochem. 38:2426–2437. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Kong R and Sun L: Silencing of
Rab3D suppresses the proliferation and invasion of esophageal
squamous cell carcinoma cells. Biomed Pharmacother. 91:402–407.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Henriques AC, de Matos FR, Galvao HC and
Freitas Rde A: Immunohistochemical expression of MMP-9 and VEGF in
squamous cell carcinoma of the tongue. J Oral Sci. 54:105–111.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heikkila P, Suojanen J, Pirila E, Väänänen
A, Koivunen E, Sorsa T and Salo T: Human tongue carcinoma growth is
inhibited by selective antigelatinolytic peptides. Int J Cancer.
118:2202–2209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bobrie A, Krumeich S, Reyal F, Recchi C,
Moita LF, Seabra MC, Ostrowski M and Théry C: Rab27a supports
exosome-dependent and -independent mechanisms that modify the tumor
microenvironment and can promote tumor progression. Cancer Res.
72:4920–4930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stephens DC, Osunsanmi N, Sochacki KA,
Powell TW, Taraska JW and Harris DA: Spatiotemporal organization
and protein dynamics involved in regulated exocytosis of MMP-9 in
breast cancer cells. J Gen Physiol. 151:1386–1403. 2019. View Article : Google Scholar : PubMed/NCBI
|