Introduction
Non-Hodgkin lymphoma (NHL) is a form of lymphoid
malignancy that can manifest in lymph nodes and other lymphatic
organs (1). NHL is one of the 10
most common forms of cancer globally, with ~500,000 newly diagnosed
cases annually. There are ≥40 major subtypes of NHL with various
genetic, clinical, and morphological features. Diffuse large B cell
lymphoma (DLBCL) is the most common form of NHL, accounting for
30–40% of new cases. Rituximab, Cyclophosphamide, Epirubicin,
Vindesine, Prednisolone (R-CHOP) is the first-line standard of care
treatment for these patients and can achieve a 60% overall survival
(OS) rate (2); however, up to 40%
of patients suffer from treatment-refractory or relapsed disease
with associated high mortality rate (3,4).
Chemotherapeutic drugs can cause extensive DNA damage within both
tumor and healthy cells (5), such
that even patients that survive chemotherapy suffer significant
systemic toxicity and renal failure in a dose-dependent manner
(6).
A number of alternative therapeutic regimens have
been developed to overcome the aforementioned limitations and
toxicity of standard R-CHOP therapy, including oncolytic Vaccinia
virus (OVV) and gene therapies (7–9). OVV
approaches allow for direct tumor cell targeting, whereas gene
therapy allows precise genetic manipulation in target cells. A
previous OVV and gene therapy-based study have focused on the
induction of apoptosis within tumor cells (10). However, apoptosis is a complex
process and certain tumor cells are resistant to apoptotic cell
death (11), limiting the potential
utility of correlative therapeutic strategies. In addition to
apoptotic death, cells can also undergo autophagic death. Under
normal physiological conditions, autophagy is a process whereby
cells respond to stress and maintain homeostasis and proper protein
folding by degrading intracellular protein aggregates and
organelles (12,13). Beclin1 is a key regulator of
autophagy that facilitates co-localization of other autophagic
proteins to promote autophagosome formation and maturation in
mammalian cells (14). Beclin1 has
been found to function as a tumor suppressor gene in breast,
ovarian and other types of cancer (15,16).
The specific degree of autophagy is important: Moderate levels of
autophagy improve cell survival under stressful conditions, whereas
excessive autophagy triggers cell death (17–19).
Chemotherapeutic drugs cause extensive DNA damage, but tumor cells
can develop resistance to such damage by modulating intracellular
DNA repair capability (20).
Increasing autophagic activity within tumor cells can lead to the
degradation of these organelles and proteins associated with DNA
repair, potentially increasing their susceptibility to chemotherapy
(21–23).
The present study investigated a potential
therapeutic strategy aimed at increasing intratumoral Beclin1
expression levels as a means of inducing autophagic cell death,
based on previous research (24–26).
OVV-Beclin1 was developed as a therapeutic approach to induce NHL
cell death and increasing sensitivity to chemotherapeutic
treatment. Levels of the autophagosome marker LC3B in NHL cells
were assessed via western blotting following OVV-Beclin1 treatment
to confirm efficacy. Tumor inhibitory effects of OVV-Beclin1
treatment were confirmed via MTT and colony formation assays, as
well as an in vivo murine model system.
Materials and methods
Human samples, cell lines and
reagents
Pathological biopsy and paracancerous tissue samples
were obtained from 10 patients with NHL that underwent fine-needle
aspiration and biopsy at Zhejiang Provincial People's Hospital
(Hangzhou, China) from January 2015 to December 2016. All samples
were collected in a manner consistent with Chinese law and the
study was approved by the Ethics Committee of Zhejiang People's
Hospital (approval no. 2019KY232), and all patients provided
written informed consent. As DLBCL tumors are invasive,
paracancerous tissue samples were selected from the same region as
the primary tumor (distance, >5 cm).
OCI-LY3 and Pfeiffer cells were obtained from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China), and
were grown in RPMI-1640 (HyClone; GE Healthcare Life Sciences)
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin. Additionally, 293 cells were obtained from
the Institute of Clinical Medicine of Zhejiang Provincial People's
Hospital and maintained in DMEM containing 10% FBS, 2 mM
L-glutamine (both Gibco; Thermo Fisher Scientific, Inc.) and
penicillin/streptomycin. Cells were cultured in a humidified 5%
CO2 incubator at 37°C.
Anti-Beclin1 (1:1,000; clone 2A4; cat. no. 4122),
anti-LC3A/B (1:1,000; clone D3U4C; cat. no. 13118) and anti-P62
(1:1,000; clone Ser403; cat. no. 39786) were obtained from Cell
Signaling Technology, Inc., while anti-caspase-3 (1:500; cat. no.
ab13847) was from Bioworld Technology. Anti-PARP (1:500; cat. no.
100984-T46) was purchased Sino Biological, Inc. and anti-β-actin
(1:1,000; cat. no. ER62585) was from Huangzhou HuaAn Biotechnology
Co., Ltd. Acridine orange stain was obtained from Vacutainer
(Becton-Dickinson and Company). For R-CHOP, Rituximab was from
Roche Diagnostics GmbH, Cyclophosphamide was from Baxter Oncology
GmbH, Epirubicin was from Shenzhen Main Luck Pharmaceuticals, Inc.,
Vindesine was from Yangtze River Pharmaceutical Group Co., Ltd. and
Prednisolone was from Pfizer, Inc.
Immunohistochemistry (IHC) and
hematoxylin and eosin (H&E) staining
Samples from patients with DLCBL (n=10; median age:
70; range: 55–81 years) were fixed with 10% formalin at room
temperature for >24 h, embedded in paraffin and cut into 4-µm
sections. Sections were deparaffinized with xylene, rehydrated
using an ethanol gradient (100, 95, 90, 80 and 70%) and antigen
recovery was performed using sodium citrate buffer at 121°C and 80
kPa. Endogenous peroxidase activity was quenched using a 3%
H2O2 solution to treat samples for 15 min at
room temperature. Following three washes in PBS, sections were
stained with anti-Beclin1 (1:200) overnight at 4°C. Sections were
then washed and stained with appropriate horseradish peroxidase
(HRP)-labeled secondary antibodies for 30 min at room temperature,
as previously described (16).
Sections were again washed with PBS, stained for 15 min with
3′3′-diaminobenzidine at room temperature, and then counterstained
with hematoxylin at room temperature for 4 min. Sections were
examined via light microscopy (Shanghai Laika Microscope Co., Ltd.;
magnification, ×400) and were then assessed with Image-Pro Plus
software (Image-Pro Express 6.0; Media Cybernetics, Inc.). A total
of five fields of view per sample was analyzed. After fixing the
tissue as aforementioned, the tissue was dyed with hematoxylin for
~10 min, rinsed with PBS three times (3 min each), washed with
running water, dyed with eosin for 2 min, rinsed with PBS three
times (3 min each), dehydrated with alcohol and xylene and finally
sealed (all at room temperature), observed and photographed under a
light microscope.
OVV-Beclin1 preparation and
characterization
A homologous recombination approach was used for OVV
and OVV-Beclin1 preparation, as previously described (27–29).
The complete Beclin1 gene sequence was amplified via PCR from the
plasmid (cat. no. HG11162-ACG; Sino Biological, Inc.) with the
following primers: Forward,
5′-CCGGAATTCACCATGGAAGGGTCTAAGACGTCCAAC-3′ and reverse,
5′-ACGCGTCGACTTATCATTTGTTATAAAATTGTGAGG-3′. The PCR conditions were
as follows: Initial denaturation, 95°C, 3 min; denaturation, 95°C,
45 sec; annealing, 55°C, 45 sec; elongation, 72°C, 90 sec (30
cycles) and final extension, 72°C, 5 min. This sequence was then
inserted into the pCB plasmid via molecular cloning. The sequence
was verified, after which pCB or pCB-Beclin1 underwent homologous
recombination with wild-type Vaccinia virus in 293A cells, as
previously described (28). When
significant infection and associated suppression effects were
evident, as previously described (30), these 293 cell cultures were expanded
and used to harvest prepared virus particles. Viral particles were
then purified by repeatedly freezing and thawing (freezing
temperature, −80°C; thawing temperature, 37°C; 3 cycles at 15 min
each). Cell samples followed by ultracentrifugation (750 × g; 4°C;
10 min). The median tissue culture infective dose for these OVV
particles was then measured.
Acridine orange staining
Acridine orange was used to stain acidic
autophagosomes within NHL cells, as previously described (31,32).
Briefly, these cells were treated with PBS, OVV, or OVV-Beclin1
(MOI, 50) for 48 h at 37°C and were then stained with acridine
orange for 15 min. Cell fluoresce was then assessed via
fluorescence microscopy (magnification, ×400; excitation, 560 nm;
emission, 645 nm) as previously described) (32).
Western blotting
Proteins in OCI-LY3 cells and murine tumor tissue
were extracted using RIPA (Beyotime Institute of Biotechnology)
buffer containing protease inhibitors, after which a BCA assay kit
was used to measure protein levels in these samples. Equal amounts
of protein (10 µg) were then separated via SDS-PAGE (10–15%), and
samples were transferred to PVDF membranes and probed at 4°C
overnight with appropriate primary antibodies. Membranes were
blocked with ~5% milk in TBST (0.05% Tween-20) for 1 h at room
temperature. Blots were then probed at room temperature for 1 h
with secondary HRP-conjugated antibody (1:4,000; HuaAn
Biotechnology Co., Ltd) for 1 h. Protein bands were detected via
enhanced chemiluminescence detection system (Thermo Fisher
Scientific, Inc.) and quantification was performed using ImageJ
software (1.48v; National Institutes of Health).
MTT assay
OCI-LY3 cells were seeded in 96-well plates
(2×104 cells per well) and cultured at 37°C for 12 h.
PBS was used as the control. In order to assess cell death, cells
were treated with the apoptosis inhibitor z-VAD and/or the
autophagy inhibitor bafilomycin A1 (BAF1; both 10 µM; both Selleck
Chemicals). Following incubation at 37°C for 72 h, 20 µl MTT
reagent (Promega Corporation) was added to each well and cultured
for 4 h at 37°C with 5% CO2. Finally, the absorbance was
detected at a wavelength of 490 nm using a Microplate Reader Model
550 nm. (Bio-Rad Laboratories, Inc.).
Colony formation assay and growth
curves
OCI-LY3 cells were infected with OVV-green
fluorescent protein (GFP) or OVV-Beclin1(MOI=50 and 100) in a
6-well plate for 12 h at 37°C, and the cell density is
105/well. After which they were washed twice in PBS,
seeded in 24-well plates (density, 104/ml) in a solution
containing 2.7% methylcellulose and 200 µl FBS and cultured for 10
days at 37°C, after which colonies (>50 cells) were counted via
light microscopic examination. (magnification, ×10).
Murine xenograft experiments
Female nude mice (n=25; age, 5 weeks; weight, 23.5
g) were raised in the Experimental Animal Center of the Chinese
Academy of Sciences (Shanghai, China) received a subcutaneous right
flank injection of 1×107 OCI-LY3 cells. Mice received
standard diet and water ad libitum and were housed under
specific pathogen-free conditions. Beginning on day 9 after
injection of OCY-LY3 cells, when tumors were ~100 mm3,
tumor volume was measured and animals were randomized into five
treatment groups (n=5/group): PBS (100 µl), OVV (108
PFU/animal; intratumoral injection), OVV-Beclin1 (108
PFU/animal; intratumoral injection), R-CHOP (Rituximab, 375.0;
Cyclophosphamide, 750.0; Epirubicin, 50.0; Vindesine, 1.4;
Prednisolone, 15.0 mg/m2; caudal vein injection) and
R-CHOP + OVV-Beclin1 (administered with the same dosing strategies
as in the R-CHOP-alone and OVV-Beclin1-alone groups). For mice
administered R-CHOP therapy, Rituximab (2.74 mg) was administered
on day 1 (the day on which tumor volume reached ~100
mm3) CHO (5.40, 0.36 and 0.01 mg, respectively) was
administered on day 2 (the day after R-CHOP therapy) and
Prednisolone (0.11 mg) administered on days 2–5. For animals
treated with both OVV-Beclin1 and R-CHOP, OVV-Beclin1 was
administered prior to R-CHOP as aforementioned. Following
treatment, tumor volume was monitored every other day using
calipers and calculated as follows: 0.5 × length ×
width2. After 30 days, mice were sacrificed via cervical
dislocation. Tumors were harvested, fixed with 10% formalin,
paraffin-embedded and used for downstream analysis as
aforementioned. The Animal Center of Zhejiang Chinese Medical
University (Hangzhou, China) approved the present study, which was
performed in accordance with appropriate guidelines and regulations
(33).
Statistical analysis
SPSS 17.0 software (SPSS, Inc.) was used for
statistical analysis. Data are presented as the mean ± SD of three
independent repeats. Continuous data were compared via unpaired
two-tailed Student's t-tests or two-way ANOVA followed by Tukey's
multiple comparisons post hoc test as appropriate. The Kaplan-Meier
approach and log-rank test were used to compare survival outcomes
and Prism (version. no. 8.0.2; GraphPad Software, Inc.) was used to
construct figures. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics of patients
with NHL
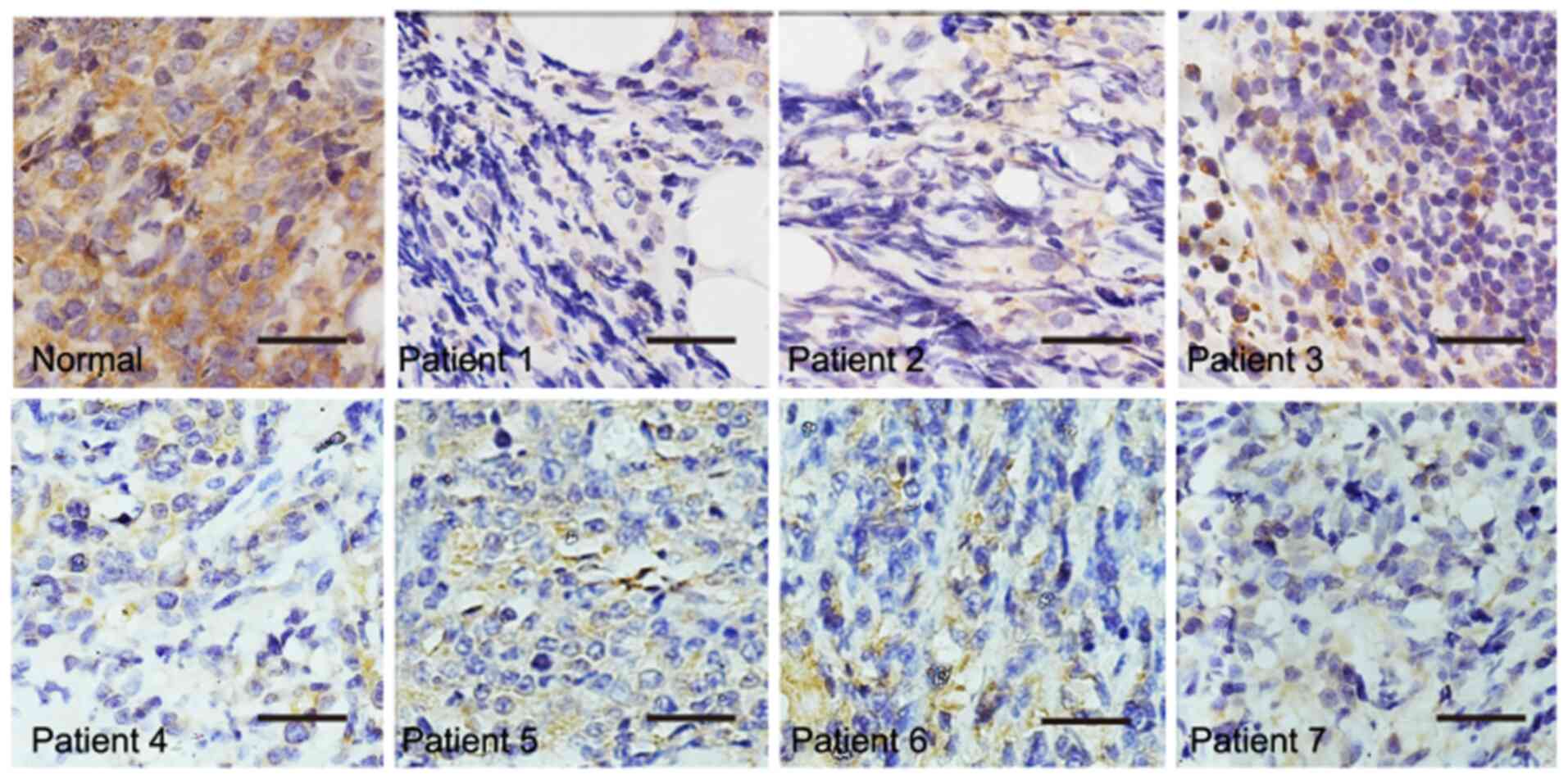
The clinical characteristics of patients with NHL
are shown in Table I. Beclin1
expression levels in these NHL tumor tissue samples were assessed
via IHC, revealing notably decreased Beclin1 levels in tumor cells
compared with normal control cells (Fig. 1). Beclin1 staining (brown) was very
weak in NHL tissue, suggesting that Beclin1 downregulation may be
associated with NHL progression.
 | Table I.Clinical characteristics of patients
with non-Hodgkin lymphoma. Beclin1 levels were analyzed in 10
randomly selected patients with DLBCL, revealing no association
between Beclin1 levels and patient sex, age or immunophenotype. |
Table I.
Clinical characteristics of patients
with non-Hodgkin lymphoma. Beclin1 levels were analyzed in 10
randomly selected patients with DLBCL, revealing no association
between Beclin1 levels and patient sex, age or immunophenotype.
| Patient | Sex/age, years | Classification | Immunohistochemical
staining results |
|---|
| 1 | Female/81 | GCB | CD3(−), CD20(+),
CD45(−), CD10(+), PAX5(+) |
| 2 | Male/55 | Non-GCB | PAX5(++), CD45(−),
CD10(−), BCL-6(+), MUMI(+) |
| 3 | Male/62 | Non-GCB | CD3(−), CD20(+),
CD45(−), CD10(−), BCL-6(−), MUMI(−) |
| 4 | Male/64 | GCB | CD3(−), CD20(+),
CD45(−), CD10(−), BCL-6(+), MUMI(−) |
| 5 | Male/77 | Non-GCB | CD3(−), CD20(+),
CD45(−), CD10(−), BCL-6(+), MUMI(+) |
| 6 | Female/67 | Non-GCB | CD3(−), CD20(+),
CD45(−), CD10(−), BCL-6(+), MUMI(+) |
| 7 | Female/76 | Non-GCB | CD3(−), CD20(+),
CD45(−), CD10(−), BCL-6(+), MUMI(+) |
| 8 | Male/55 | Non-GCB | CD3(−), CD20(+),
CD45(−), CD10(−), BCL-6(−), MUMI(+) |
| 9 | Female/51 | Non-GCB | CD3(−), CD20(+),
CD45(−), CD10(−), BCL-6(−), MUMI(+) |
| 10 | Female/63 | Non-GCB | CD3(−), CD20(+),
CD45(−), CD10(−), BCL-6(+), MUMI(+) |
Assessment of OVV-Beclin1 infectivity
in NHL cells
OVV-Beclin1 was generated via homologous
recombination (Fig. 2A). OVV
infectivity was assessed using an OVV-GFP construct, which was used
to infect the OCI-LY3 and Pfeiffer NHL cell lines. These cells were
readily infected by this virus, with a dose-dependent increase in
the frequency of GFP-positive cells from 30 to 60% on day 2
post-infection as viral MOI rose from 10 to 50 (Fig. 2B and C). Autophagic changes in cells
were assessed. Western blotting demonstrated that the OVV-Beclin1
treatment was associated with notably increased Beclin1 and LC3B
levels and decreased those of P62 (Fig.
2D), suggesting that cytotoxicity was associated with increased
induction of autophagy. In order to confirm that the OVV-Beclin1
virus was capable of inducing autophagy in NHL cells, Beclin1
levels in these cells were measured via western blotting;
OVV-Beclin1 led to a dose-dependent increase in levels of this
protein in NHL cell preparations (MOI=10-50). By contrast,
mock-infected cells exhibited relatively low levels of Beclin1
protein. Other autophagy-associated protein levels in these cells
were assessed, including levels of LC3B, which forms during
autophagy and is associated with the membrane of autophagosomes,
and SQSTM1(P62), which is a ubiquitin-binding protein that exhibits
decreased expression as autophagy progresses (34). Measurements of these proteins in
OVV-Beclin1-infected NHL cells (Fig.
2E) further confirmed that the virus was able to effectively
induce autophagy within NHL cells following infection. This
indicates that these cells were highly sensitive to OVV-Beclin1
infection. Infection with a 50 or 100 MOI dose of OVV-Beclin1
notably inhibited cell viability, as demonstrated by colony
formation assay. In order to ensure the best infection effect,
virus titer was tested during the experiment. The results showed
that after 48 h infection, the virus titer was significantly higher
than at 24 h (Fig. 2F and G).
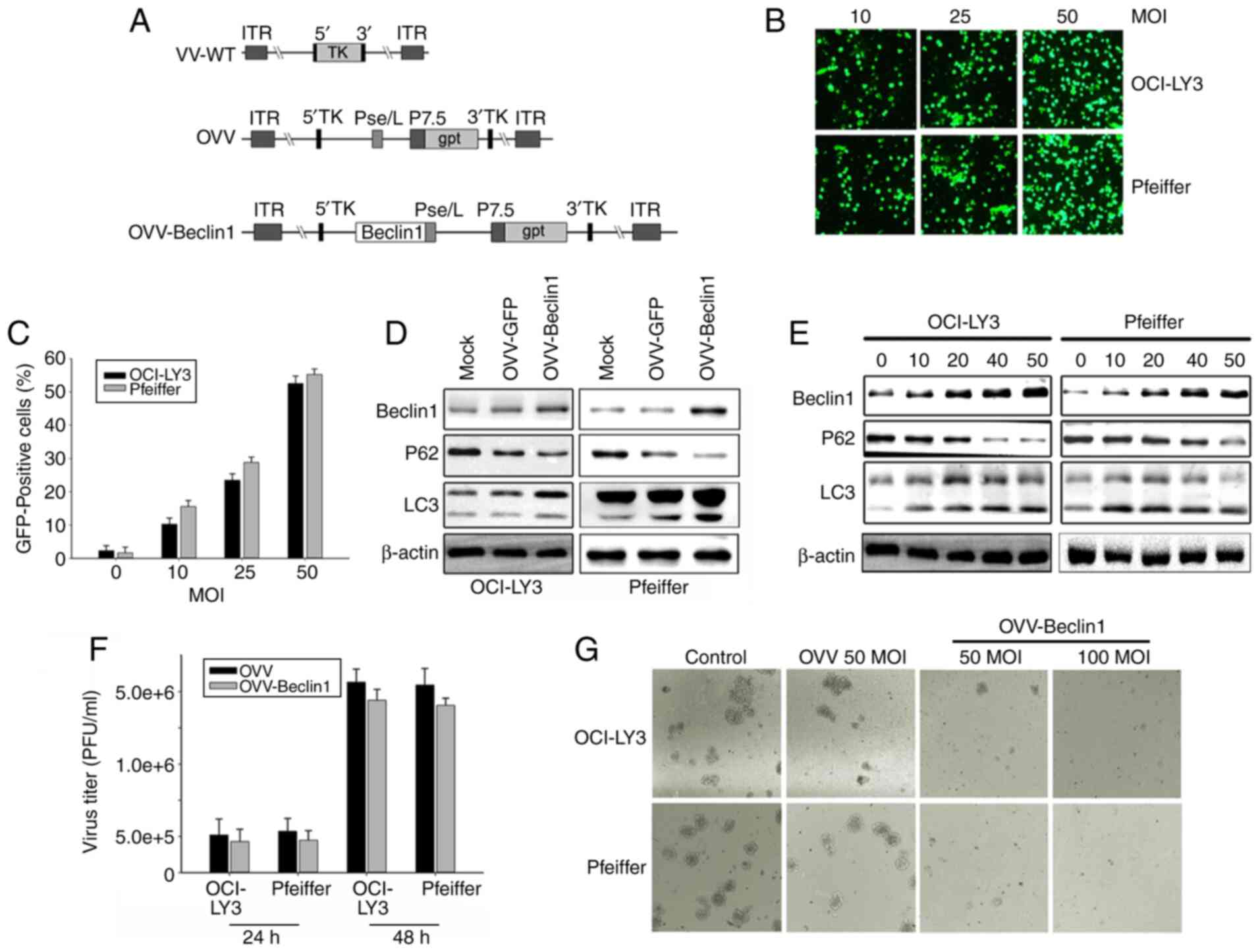 | Figure 2.OVV-Beclin1 infects NHL cells. (A)
Structure of OVV-Beclin1. OVV and OVV-Beclin1 were prepared via
homologous recombination using VV-WT; Beclin1 expression cassette
was introduced into the TK region of the virus. (B) OCI-LY3 and
Pfeiffer cells were infected with OVV-GFP (MOI=10, 25 or 50) and
analyzed via fluorescence microscopy after 24 h (magnification,
×200). (C) Quantitative analysis of fluorescence microscopy
results. (D) OCI-LY3 cells were treated with OVV or OVV-Beclin1
(MOI=50); after 48 h, expression levels of Beclin1, LC3 and actin
were measured via western blotting. (E) Western blotting was used
to quantify levels of Beclin-1, LC3A/B, and P62 in NHL cells at 48
h post-OVV-Beclin1 infection. Actin was used as a loading control.
(F) Determination of virus titer to determine the time of optimal
infection. Detection of virus titers after infection 24 and 48 h.
(G) OVV-Beclin1 inhibits NHL cell proliferation. Compared with the
OVV group, OVV-Beclin1 significantly inhibited frontal growth of
NHL cells. The inhibitory effect was greater as MOI increased from
50 to 100. OVV, oncolytic Vaccinia virus; NHL, non-Hodgkin
lymphoma; WT, wild-type; TK, thymidine kinase; Pse/L, synthetic
early/late promoter; P7.5, VV early-late promoter; gpt,
mycophenolic acid resistance gene; ITR, inverted terminal repeat;
GFP, green fluorescent protein. |
OVV-Beclin1 induces autophagic cell
death in NHL
Having demonstrated that the OVV-Beclin1 was capable
of efficiently infecting NHL cells and inducing autophagy, it was
next determined whether this virus drove autophagic cell death.
Levels of apoptosis-associated proteins were measured via western
blotting; expression levels were not significantly altered
following OVV-Beclin1 infection (Fig.
3A). In further support of this result, treatment of NHL cells
with the apoptosis inhibitor z-VAD did not prevent
OVV-Beclin1-induced cell death (Fig.
3B), indicating that death occurred in a caspase-independent
manner. During autophagy, acidic autophagosomes form and interact
with microtubule-associated protein LC3B (35). Autophagic vesicles were therefore
detected via staining with acid-sensitive acridine orange (Fig. 3C). These autophagosomes contained
protein, debris and organelles such as mitochondria, confirming
successful OVV-Beclin1-mediated autophagic induction. Treating
these cells with the autophagy inhibitor BAF1 prevented
OVV-Beclin1-induced cell death. Together, these findings confirmed
the ability of OVV-Beclin1 to induce autophagy in NHL cells, with
BAF1 inhibiting this process (Fig.
3D).
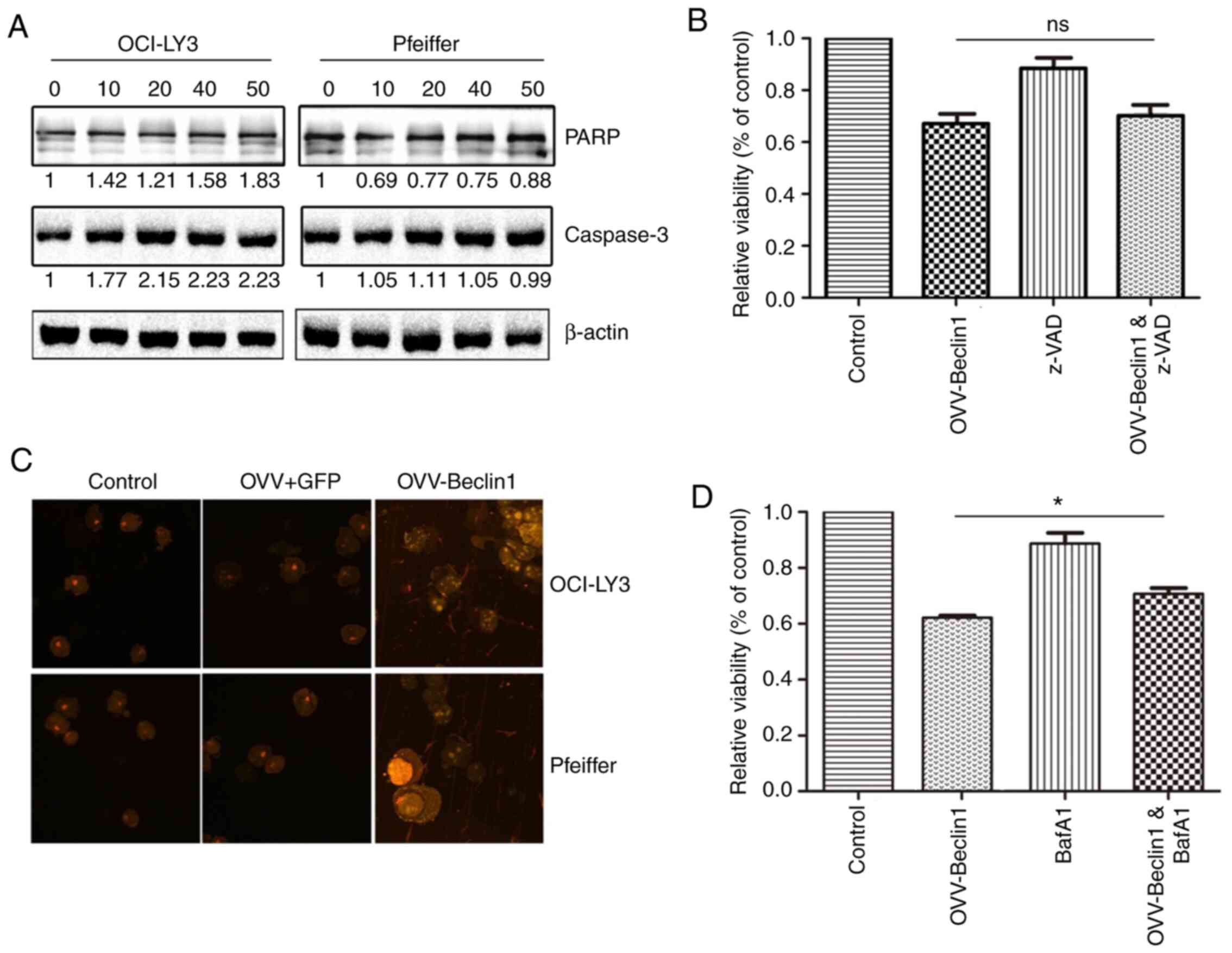 | Figure 3.OVV-Beclin1 induces autophagic death
of NHL cells. (A) Levels of apoptosis-associated proteins (PARP and
caspase-3) were assessed via western blotting using OCI-LY3 and
Pfeiffer cells infected with OVV-Beclin1 (MOI=0, 10, 25 or 50).
Actin was used as a loading control. (B) OCI-LY3 cells were treated
with OVV-Beclin1 in the presence or absence of z-VAD (10 µM) for 72
h, after which MTT assay was used to measure viability. (C)
Autophagosomes in NHL cells were stained using acridine orange
following treatment with PBS, OVV or OVV-Beclin1 (MOI=50) for 48 h.
Finally, fluorescence was observed under a microscope
(magnification, ×400). (D) OCI-LY3 cells were infected with
OVV-Beclin1 (MOI=50) in the presence or absence of 10 µM BafA1
Viability was assessed via MTT assay. *P<0.05. OVV, oncolytic
Vaccinia vaccine; NHL, non-Hodgkin lymphoma; PARP, poly(ADP-ribose)
polymerase; ns, not significant; BafA1, bafilomycin A1. |
OVV-Beclin1 and R-CHOP exhibit
synergistic efficacy in vivo
The aforementioned results were next assessed in
vivo to determine whether OVV-Beclin1 in combination with
R-CHOP may be a viable treatment for NHL. A murine NHL xenograft
model was established by subcutaneously implanting BALB/c nude mice
with OCI-LY3 cells. After tumors had grown to ~100 mm3,
animals were randomized into 5 treatment groups (PBS, OVV,
OVV-Beclin1, R-CHOP and OVV-Beclin1 + R-CHOP). OVV-Beclin1 was
administered intratumorally every third day over the course of
three treatment cycles and PBS and R-CHOP were injected via the
caudal vein. Tumor volume in these mice was then monitored ≥3 times
per week, and animals exhibiting signs of terminal disease or
excessive tumor growth were euthanized (Fig. 4A). Mice treated with PBS or OVV
exhibited larger tumors than mice treated with OVV-Beclin1, R-CHOP
or OVV-Beclin1 + R-CHOP, suggesting that OVV-mediated Beclin1
expression significantly suppressed tumor growth and final tumor
weight (Fig. 4B-C). Compared with
OVV-Beclin1- or R-CHOP-alone, combined OVV-Beclin1 + R-CHOP
treatment significantly prolonged survival (Fig. 4D).
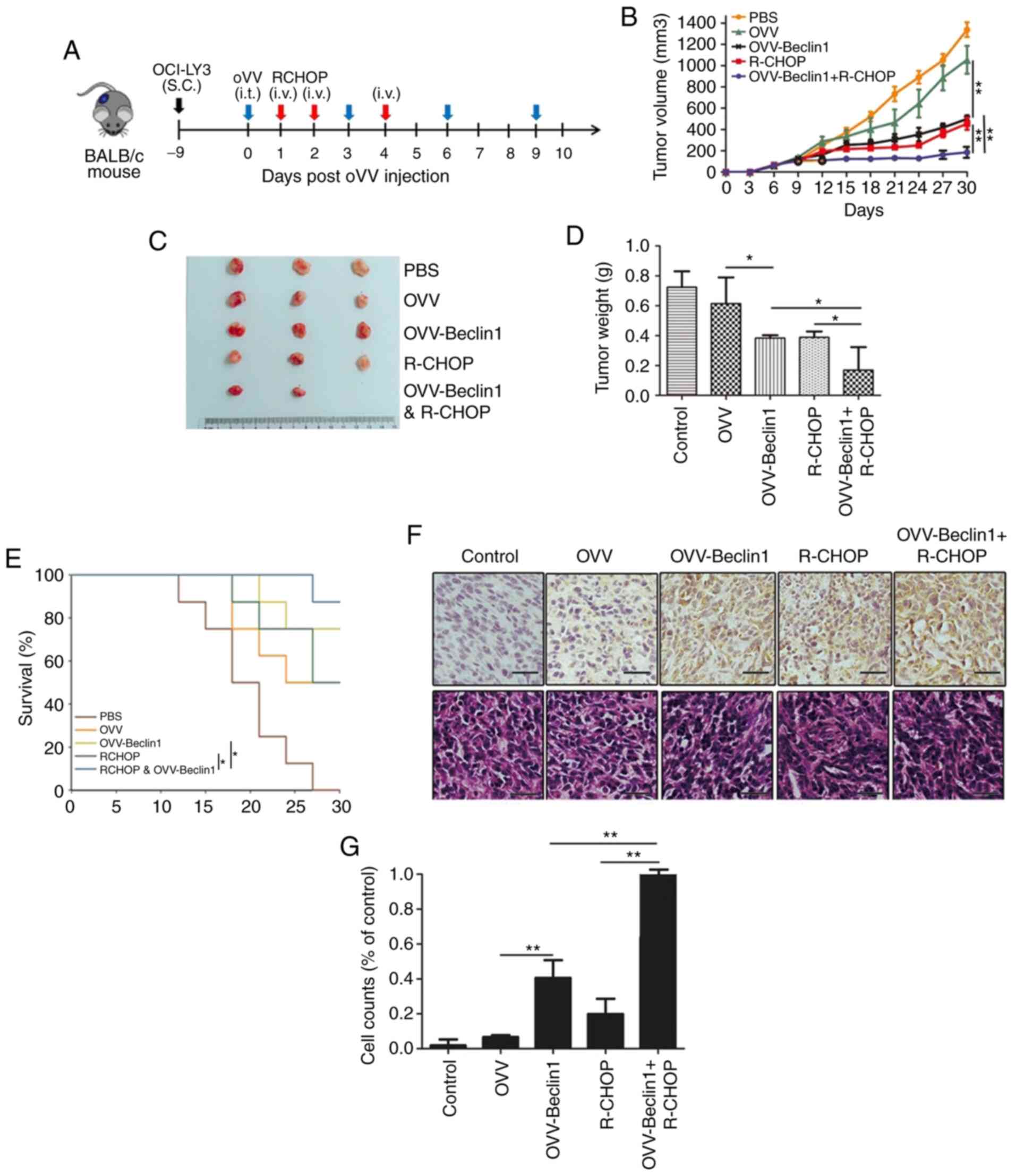 | Figure 4.Combination OVV-Beclin1 and R-CHOP
inhibits tumor growth in vivo. (A) Establishment of mouse
model. A murine non-Hodgkin lymphoma xenograft model was
established by implanting BALB/c nude mice with OCI-LY3 cells. Blue
arrow, i.t.; red arrow, i.v.; black arrow, S.C. (B) Tumor growth in
a murine diffuse large B cell lymphoma xenograft model system was
monitored for 30 days. (C) Representative tumor images (n=5). (D)
Tumor weight was measured. (E) Murine survival was monitored; mice
treated using OVV-Beclin1 exhibited greater survival vs. other
treatment groups (P=0.0072). (F) Tumor sections from mice were used
for Beclin1 staining; positive cells stained brown. Scale bar, 50
µm. (G) Beclin1-positive cells relative to PBS control animals were
counted. Data are presented as the mean ± SD. *P<0.05 and
**P<0.01. OVV, oncolytic Vaccinia virus; R-CHOP, Rituximab,
Cyclophosphamide, Epirubicin, Vindesine, Prednisolone; i.t.,
intratumoral; i.v., intravenous; S.C., subcutaneous. |
These results suggested that OVV-Beclin1 treatment
was able to confer significant survival benefits. In order to
confirm that Beclin1 levels were altered, Beclin1 levels in murine
tumor tissue were assessed via IHC. This analysis revealed that
tumors from the PBS, OVV and R-CHOP groups exhibited less
pronounced Beclin1 staining compared with the OVV-Beclin1 and
OVV-Beclin1 + R-CHOP groups. This was confirmed this by calculating
the frequency of Beclin1-positive cells per unit area relative to
that in the PBS control group. H&E staining was performed to
observe the changes of cells in tumor tissue samples: Necrosis was
highest in the OVV-Beclin1 + R-CHOP, indicating that the
combination of OVV-Beclin1 and OVV-Beclin1 and R-chop exerted a
cytotoxic effect (Fig. 4E and
F).
Discussion
A previous study (36) has demonstrated the value of OVV
approaches to cancer treatment, as these viruses exhibit superior
tumor selectivity and decreased toxicity compared with traditional
therapies (37–39). OVV is harmless in normal cells but
can effectively kill target tumor cells (40). The OVV genome is large, stable and
contains a number of non-essential genes, making it an ideal vector
for the delivery of recombinant genes into tumor cells.
Tumor-specific OVV strains can be produced by deleting specific
virulence genes from the viral genome. The OVV genome is replicated
in the cytoplasm of cells, and thus has no significant risk of
genomic integration (41). In the
present study, the Beclin1 gene was inserted into the OVV genome in
a stable manner and these OVV-Beclin1 particles were used to infect
NHL cells. Once expressed in tumor cells, Beclin1 induces autophagy
and increases the sensitivity of these cells to
chemotherapy-induced cell death (42). Beclin1-mediated autophagy can
further lead to the autophagic degradation of proteins and
organelles essential for chemotherapy resistance, rendering tumor
cells more susceptible to DNA damage and death (Fig. 5).
While a previous study focused on the development of
therapeutic approaches capable of inducing apoptotic cell death
(43), other modes of cell death
also serve as an effective anticancer modality. However, induction
of autophagic death is not always successful, underscoring the
importance of tailoring cancer treatments to specific targets and
disease types (44,45). Previous studies have detected
Beclin1 downregulation in human esophageal, breast, cervical, lung
and liver cancer (46,47). The upregulation of Beclin1 can
induce excessive autophagy in leukemia cells, leading to their
death (6). Here, the functional
role of Beclin1 in NHL was investigated; Beclin1 was downregulated
in this cancer type suggesting that these cells may be sensitive to
autophagic induction. These findings are consistent with work by
Huang et al (48)
demonstrating that 85% of patients with DLBCL exhibited decreased
Beclin1 protein levels following R-CHOP treatment.
The present study developed an OVV-Beclin1 vector
which could be used to infect NHL tumor cells and induce excessive
autophagy, culminating in cell death. Our previous research showed
that OVV-Bclin1 exhibits good therapeutic effects on leukemia and
myeloma via dissolving tumor cells and inducing autophagy death
(30). Additionally, previous work
has shown that Beclin1 upregulation in DLBCL increases PI3KC3
expression and associated phosphorylated-AKT1 activation. Beclin1
interacts with PI3KC3 and then with autophagy-related 12, 5 and 16L
and LC3B to facilitate the gradual expansion and maturation of
autophagosomes within the cell (49). Autophagy has been found to serve a
role in R-CHOP resistance in patients with lymphoma, suggesting
that inducing autophagy may be a valuable therapeutic strategy
(30).
The present study was able to confirm that
OVV-Beclin1 induced autophagic death of NHL cells, indicated by
detection of autophagosome formation in these cells.
OVV-Beclin1-treated cells did not undergo apoptotic death and
dose-dependent increases in virus-induced cytotoxicity were not
associated with increases in apoptotic staining or protein levels.
At low levels, the induction of autophagy can help tumor cells
resist treatment by degrading damaged organelle and proteins.
However, excessive autophagy can lead to unrestrained destruction
of functional proteins and organelles, compromising cell viability
and leading to tumor cell death (50). Here, OVV-Beclin1-induced autophagic
death also resulted in the release of viral particles that can then
infect proximal tumor cells, leading to further cell death and
enhanced R-CHOP efficacy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Medical Technology Plan Project (grant nos. WKJ-ZJ-1709 and
2020KY052), National Science Foundation of China (grant nos.
81570198 and 81602706) and Zhejiang Provincial Natural Science
Foundation of China (grant nos. LY19H160037 and LY17H160062).
Availability of data and materials
All data generated or analyzed in this study are
included in this published article.
Authors' contributions
SW, YW, WL and XT designed the study. SX, WF, CY, HP
and YW performed the experiments and collected and analyzed data.
SX, WF and HP wrote, reviewed and revised the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with ethical standards of the Institutional Animal Use and Care
Committee of the Zhejiang Provincial People's Hospital, and ethical
approval (approval no. A20190029) was obtained prior to the
commencement of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NHL
|
non-Hodgkin lymphoma
|
|
OVV
|
oncolytic Vaccinia virus
|
|
DLBCL
|
diffuse large B cell lymphoma
|
|
R-CHOP
|
Rituximab, Cyclophosphamide,
Epirubicin, Vindesine, Prednisolone
|
References
|
1
|
Howlader N, Morton L, Feuer E, Besson C
and Engels E: Contributions of subtypes of non-hodgkin lymphoma to
mortality trends. Cancer Epidemiol Biomarkers Prev. 25:174–179.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coccaro N, Anelli L, Zagaria A, Perrone T,
Specchia G and Albano F: Molecular complexity of diffuse large
b-cell lymphoma: Can it be a roadmap for precision medicine?
Cancers. 12:1852020. View Article : Google Scholar
|
|
3
|
Camicia R, Winkler HC and Hassa PO: Novel
drug targets for personalized precision medicine in
relapsed/refractory diffuse large B-cell lymphoma: A comprehensive
review. Mol Cancer. 14:2072015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu-Monette ZY, Wu L, Visco C, Tai YC,
Tzankov A, Liu Wm, Montes-Moreno S, Dybkaer K, Chiu A, Orazi A, et
al: Mutational profile and prognostic significance of TP53 in
diffuse large B-cell lymphoma patients treated with R-CHOP: Report
from an international DLBCL rituximab-CHOP consortium program
study. Blood. 120:3986–3996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee D, Lee D, Hwang Y, Seo H, Lee S and
Kwon J: The bromodomain inhibitor PFI-3 sensitizes cancer cells to
DNA damage by targeting SWI/SNF. Mol Cancer Res. 18:02892020.
|
|
6
|
Wechman SL, Pradhan AK, DeSalle R, Das SK,
Emdad L, Sarkar D and Fisher PB: New insights into beclin-1:
Evolution and pan-malignancy inhibitor activity. Adv Cancer Res.
137:77–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roy DG and Bell JC: Cell carriers for
oncolytic viruses: Current challenges and future directions.
Oncolytic Virother. 2:47–56. 2013.PubMed/NCBI
|
|
8
|
Aitken AS, Roy DG and Bourgeois-Daigneault
MC: Taking a stab at cancer; oncolytic virus-mediated anti-cancer
vaccination strategies. Biomedicines. 5:32017. View Article : Google Scholar
|
|
9
|
Willmon C, Harrington K, Kottke T,
Prestwich R, Melcher A and Vile R: Cell carriers for oncolytic
viruses: Fed ex for cancer therapy. Mol Ther. 17:1667–1676. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amin AD, Peters TL, Li L, Rajan SS,
Choudhari R, Puvvada SD and Schatz JH: Diffuse large B-cell
lymphoma: Can genomics improve treatment options for a curable
cancer? Cold Spring Harb Mol Case Stud. 3:a0017192017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang F, Wang BR and Wang YG: Role of
autophagy in tumori-genesis, metastasis, targeted therapy and drug
resistance of hepatocellular carcinoma. World J Gastroenterol.
24:4643–4651. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Denton D, Nicolson S and Kumar S: Cell
death by autophagy: Facts and apparent artefacts. Cell Death
Differ. 19:87–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Puri P and Chandra A: Autophagy modulation
as a potential therapeutic target for liver diseases. J Clin Exp
Hepatol. 4:51–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galluzzi L, Pietrocola F, Bravo-San Pedro
JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J,
Gewirtz DA, Karantza V, et al: Autophagy in malignant
transformation and cancer progression. EMBO J. 34:856–880. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Correa RJM, Valdes YR, Shepherd TG and
DiMattia GE: Beclin-1 expression is retained in high-grade serous
ovarian cancer yet is not essential for autophagy induction in
vitro. J Ovarian Res. 8:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hasui K, Wang J, Jia X, Tanaka M, Nagai T,
Matsuyama T and Eizuru Y: Enhanced autophagy and reduced expression
of cathepsin d are related to autophagic cell death in epstein-barr
virus-associated nasal natural killer/T-cell lymphomas: An
immunohistochemical analysis of beclin-1, LC3, mitochondria (AE-1),
and cathepsin D in nasopharyngeal lymphomas. Acta Histochem
Cytochem. 44:119–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guaman-Ortiz LM, Orellana MIR and
Ratovitski EA: Natural compounds as modulators of non-apoptotic
cell death in cancer cells. Curr Genom. 18:132–155. 2017.
View Article : Google Scholar
|
|
18
|
Shen HM and Codogno P: Autophagic cell
death: Loch ness monster or endangered species? Autophagy.
7:457–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sui X, Chen R, Wang Z, Huang Z, Kong N,
Zhang M, Han W, Lou F, Yang J, Zhang Q, et al: Autophagy and
chemotherapy resistance: A promising therapeutic target for cancer
treatment. Cell Death Dis. 4:e8382013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Derenzini E, Agostinelli C, Imbrogno E,
Iacobucci I, Casadei B, Brighenti E, Righi S, Fuligni F, Di Rorà
AGL, Ferrari A, et al: Constitutive activation of the DNA damage
response pathway as a novel therapeutic target in diffuse large
B-cell lymphoma. Oncotarget. 6:6553–6569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Czarny P, Pawlowska E, Bialkowska-Warzecha
J, Kaarniranta K and Blasiak J: Autophagy in DNA damage response.
Int J Mol Sci. 16:2641–2662l. 2014. View Article : Google Scholar
|
|
22
|
Zhang D, Tang B, Xie X, Xiao YF, Yang SM
and Zhang JW: The interplay between DNA repair and autophagy in
cancer therapy. Cancer Biol Ther. 16:1005–1013. 2014. View Article : Google Scholar
|
|
23
|
Mizushima N.; Autophagy, : Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rojas JJ and Thorne SH: Theranostic
potential of oncolytic vaccinia virus. Theranostic. 2:363–373.
2012. View Article : Google Scholar
|
|
25
|
Quirin C, Rohmer S, Fernández-Ulibarri I,
Behr M, Hesse A, Engelhardt S, Erbs P, Enk AH and Nettelbeck DM:
Selectivity and efficiency of late transgene expression by
transcriptionally targeted oncolytic adenoviruses are dependent on
the transgene insertion strategy. Hum Gene Ther. 22:389–404. 2007.
View Article : Google Scholar
|
|
26
|
Bastin D, Walsh SR, Saigh MA and Wan Y:
Capitalizing on cancer specific replication: Oncolytic viruses as a
versatile platform for the enhancement of cancer immunotherapy
strategies. Biomedicines. 4:212016. View Article : Google Scholar
|
|
27
|
Haddad D: Genetically engineered vaccinia
viruses as agents for cancer treatment, imaging, and transgene
delivery. Front Oncol. 7:962017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Buijs PR, Verhagen JH, van Eijck CH and
van den Hoogen BG: Oncolytic viruses: From bench to bedside with a
focus on safety. Hum Vaccin Immunother. 11:1573–1584. 2007.
View Article : Google Scholar
|
|
29
|
Deng L, Fan J, Ding Y, Zhang J and Huang
B: Oncolytic efficacy of thymidine kinase-deleted vaccinia virus
strain guang9. Oncotarget. 8:40533–40543. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lei W, Wang S, Xu N, Chen Y, Wu G, Zhang
A, Chen X, Tong Y and Qian W: Enhancing therapeutic efficacy of
oncolytic vaccinia virus armed with beclin-1, an autophagic gene in
leukemia and myeloma. Biomed Pharmacother. 125:1100302020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tong Y, You L, Liu H, Li L, Meng H, Qian Q
and Qian W: Potent antitumor activity of oncolytic adenovirus
expressing beclin-1 via induction of autophagic cell death in
leukemia. Oncotarget. 4:860–874. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu HC, Hou DR, Liu CY, Lin CS, Shiau CW,
Cheng AL and Chen KF: Cancerous inhibitor of protein phosphatase 2A
mediates bortezomib-induced autophagy in hepatocellular carcinoma
independent of proteasome. PLoS One. 8:e557052013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Diaz SL: Conducting and reporting animal
experimentation: Quo vadis? Eur J Neurosci. 52:3493–3498. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nguyen T, Shaid S, Vakhrusheva O, Koschade
SE, Klann K, Thölken M, Baker F, Zhang J, Oellerich T, Sürün D, et
al: Loss of the selective autophagy receptor p62 impairs murine
myeloid leukemia progression and mitophagy. Blood. 133:168–179.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Araveti PB and Srivastava A: Curcumin
induced oxidative stress causes autophagy and apoptosis in bovine
leucocytes transformed by. Cell Death Discov. 5:1002019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Islam SM, Lee B, Jiang F, Kim E, Ahn S and
Hwang TH: Engineering and characterization of oncolytic vaccinia
virus expressing truncated herpes simplex virus thymidine kinase.
Cancers. 12:2282020. View Article : Google Scholar
|
|
37
|
Walsh S, Bastin D, Chen L, Nguyen A,
Storbeck CJ, Lefebvre C, Stojdl D, Bramson JL, Bell JC and Wan Y:
Type I IFN blockade uncouples immunotherapy-induced antitumor
immunity and autoimmune toxicity. J Clin Invest. 129:518–530. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang B and Cheng P: Improving antitumor
efficacy via combinatorial regimens of oncolytic virotherapy. Mol
Cancer. 19:1582020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bourgeois-Daigneault M, Roy DG, Aitken AS,
El Sayes E, Martin NT, Varette O, Falls T, St-Germain LE, Pelin A,
Lichty BD, et al: Neoadjuvant oncolytic virotherapy before surgery
sensitizes triple-negative breast cancer to immune checkpoint
therapy. Sci Transl Med. 10:4222018. View Article : Google Scholar
|
|
40
|
Guo ZS, Lu B, Guo Z, Giehl E, Feist M, Dai
E, Liu W, Storkus WJ, He Y, Liu Z and Bartlett DL: Vaccinia
virus-mediated cancer immunotherapy: Cancer vaccines and
oncolytics. J Immunother Cancer. 7:62019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu Z, Ge Y, Wang H, Ma C, Feist M, Ju S,
Guo ZS and Bartlett DL: Modifying the cancer-immune set point using
vaccinia virus expressing re-designed interleukin-2. Nat Commun.
9:46822018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hill SM, Wrobel L and Rubinsztein DC:
Post-translational modifications of Beclin 1 provide multiple
strategies for autophagy regulation. Cell Death Differ. 26:617–629.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Place DE and Kanneganti TD: Cell
death-mediated cytokine release and its therapeutic implications. J
Exp Med. 216:1474–1486. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Torii S, Honda S, Murohashi M, Yamaguchi H
and Shimizu S: Autophagy involvement in oncogenesis. Cancer Sci.
111:3993–3999. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Levy JM and Thorburn A: Autophagy in
cancer: Moving from understanding mechanism to improving therapy
responses in patients. Cell Death Differ. 27:843–857. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu G, Pei F, Yang F, Li L, Amin AD, Liu
S, Buchan JR and Cho WC: Role of autophagy and apoptosis in
non-small-cell lung cancer. Int J Mol Sci. 18:3672017. View Article : Google Scholar
|
|
47
|
Zhang J, Yang S, Wang K, Huang Y, Yang N,
Yang Z, Zheng Z and Wang Y: Crocin induces autophagic cell death
and inhibits cell invasion of cervical cancer SiHa cells through
activation of PI3K/AKT. Ann Transl Med. 8:11802020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang JJ, Zhu YJ, Lin TY, Jiang WQ, Huang
HQ and Li ZM: Beclin 1 expression predicts favorable clinical
outcome in patients with diffuse large B-cell lymphoma treated with
R-CHOP. Hum Pathol. 42:1459–1466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang Z, Zhu S, Zhang G and Liu S:
Inhibition of autophagy enhances the anticancer activity of
bortezomib in B-cell acute lymphoblastic leukemia cells. Am J
Cancer Res. 5:639–650. 2015.PubMed/NCBI
|
|
50
|
Zhu Y, Jia H, Gao G, Pan GY, Jiang YW, Li
P, Zhou N, Li C, She C, Ulrich NW, et al: Mitochondria-acting
nanomicelles for destruction of cancer cells via excessive
mitophagy/autophagy-driven lethal energy depletion and
phototherapy. Biomaterials. 232:1196682020. View Article : Google Scholar : PubMed/NCBI
|