Introduction
Glioma is a type of aggressive tumor originating
from the neuroectoderm and accounts for 45–50% of all intracranial
tumors (1). According to the 2007 WHO
central nervous system tumor grading system, malignant gliomas,
including glioblastoma, anaplastic astrocytoma, anaplastic
oligodendrocyte tumor and anaplastic oligodendroglioma (WHO grade
III–IV) account for more than half of all gliomas (2). Malignant glioma is a solid tumor
composed of rapidly proliferating non-glial cells, and is
characterized by intra- and intertumor heterogeneity, resistance to
chemotherapy and poor prognosis (3).
Due to its characteristic malignant invasive growth, the
therapeutic approach has evolved from a single surgical approach to
a combination of surgical approaches with radiotherapy and
chemotherapy. However, the median survival of patients with
glioblastoma only increased from 12.1 with radiotherapy alone to
14.6 months with radiotherapy plus temozolomide, and the prognosis
of patients did not significantly improve (4).
Temozolomide (TMZ) is an oral DNA alkylating agent,
which inactivates O6-methylguanine-DNA alkyl transferase (5), forms a notch for substrate DNA, and
causes DNA double strand breaks and cell cycle arrest in the G2/M
phase, eventually leading to cell apoptosis (6). TMZ readily crosses the blood-brain
barrier; thus, it is one of the most effective chemotherapeutic
drugs for glioma (7). However, TMZ is
only ~45% effective against malignant glioma (8), and a significant proportion of gliomas
develop resistance to TMZ during treatment. The emergence of
chemotherapy resistance and subsequent disease recurrence have
become an urgent clinical problem in glioma (9). Most studies on TMZ resistance in gliomas
are focused on drug efflux mediated by ABC transporters, repair of
damaged DNA, changes in apoptotic pathways, cell cycle and cell
metabolism (10,11). All these mechanisms are closely
intertwined, and may be implicated in the development of
chemotherapy resistance (12).
However, other underlying mechanisms may also be involved in the
development of resistance. Thus, the exact molecular mechanisms
underlying resistance of glioma to chemotherapy remain to be
further elucidated.
Exosomes are lipid bilayer vesicles with a diameter
of 30–150 nm that are actively produced by almost all cells
(13,14). Exosomes are developed from early
endosomes, and usually express specific surface markers, including
CD9, CD63, TSG101, Alix and Flotillin-1 (15). Exosomes contain non-coding RNAs,
lipids and up to 7,000 proteins, and they mediate cell-cell
communication by delivering these substances to surrounding cells
(16). It was previously demonstrated
that exosomes play an important role in the migration, invasion and
chemotherapy resistance of cancer cells (17). Exosomes may alter the tumor
microenvironment and promote drug resistance through transferring
various drug resistance-related factors between drug-resistant and
drug-sensitive cells, and between drug-resistant cells and tumor
stromal cells (18–20). Most studies have focused on the role
of exosomal contents, but a few also reported that exosomal
membrane components may affect the recognition and uptake of
exosomes by recipient cells (21).
Exosomes interact with recipient cells through receptor-ligand
interaction, endocytosis and membrane fusion (22). Phagocytic cells, such as macrophages,
are more likely to endocytose exosomes (23). Non-phagocytic cells, such as
T-lymphocyte subsets, express receptors for binding with the
ligands on exosomes, thereby mediating membrane fusion and
delivering exosomal contents to recipient cells (24). In the latter case, exosomal membrane
components play an important role in the uptake of exosomes by
recipient cells. Recently, it was reported that connexins (Cxs) may
also be assembled into a hemi-channel in the membrane of exosomes
secreted by cells (25). Therefore,
it is crucial to investigate whether exosomal Cxs are involved in
the interaction between exosomes and recipient cells.
Cxs are transmembrane proteins composed of two
extracellular rings, an intracellular ring, an amino and a carboxyl
tail domain (26). More than 20 Cxs
have been identified in humans and mice (27). Cxs can oligomerize on the cell
membrane to form gap junction hemi-channels, which are docked and
assembled into gap junction channels to mediate gap junction
intercellular communication. Cx43 is one of the most common Cxs,
and is involved in tumor invasion, progression, and the resistance
of glioma cells to TMZ (28–30). It has also been reported that the
expression level of Cx43 in gliomas is higher compared with that in
the adjacent tissues (31), which
suggests the role of Cx43 in gliomagenesis. Moreover, upregulated
expression of Cx43 through epidermal growth factor
receptor-activated c-Jun N-terminal kinase/extracellular
signal-regulated kinase 1/2/activator protein-1 signaling axis has
been found in TMZ-resistant glioblastoma cell lines (32).
The present study was undertaken to investigate the
role of exosomal Cx43 in the resistance of glioma cells to TMZ and
cell migration. The expression of Cx43 was examined in
TMZ-resistant and TMZ-sensitive U251 cells (U251r and U251s,
respectively) as well as in their exosomes (sExo and rExo,
respectively). The effects of rExo on the IC50 of U251s
cells to TMZ, colony formation ability, Bcl-2 expression, cell
migration and Bax expression were also examined. Furthermore, it
was investigated whether pretreatment with the gap junction mimetic
peptide 37,43Gap27 would be able to inhibit the uptake
of rExo by U251s cells, and alleviate rExo-induced TMZ resistance,
colony formation and migration of U251s cells. The aim was to
elucidate the role of exosomal Cx43 in chemotherapy resistance and
migration of glioma cells, and determine whether it may serve as a
therapeutic target for glioblastoma in the future.
Materials and methods
Chemicals and reagents
Fetal bovine serum (FBS) was purchased from EVERY
GREEN (Biotech Co.). Dulbecco's modified Eagle's medium (DMEM),
DMSO, PMSF, crystal violet, 4% paraformaldehyde, anti-fluorescence
attenuation sealant, trypsin and penicillin-streptomycin solution
(P/S) were purchased from Beijing Solarbio Science & Technology
Co., Ltd. Tween-20 and TEMED were purchased from Sigma-Aldrich;
Merck KGaA. TMZ was obtained from MedChemExpress.
37,34Gap27 was purchased from Eurogentec. PrimeScript™
RT reagent Kit (cat. no. RR037A) with gDNA Eraser and QuantiNova™
SYBR®-Green PCR Kit (cat. no. RR420A) were obtained from
Takara Bio, Inc.
Cell culture
U251s and U251r cells were purchased from the
American Type Culture Collection. The human U251s and U251r cell
lines were grown in DMEM supplemented with 10% FBS and 100 µg/ml
P/S in a humidified incubator at 37°C with an atmosphere containing
5% CO2.
Reverse transcription-quantitative PCR
(RT-PCR)
Total RNA was isolated from cells using
Eastep® Super (Promega Corporation). Reverse
transcription was performed using PrimeScript™ RT reagent Kit
(Takara Bio, Inc.) at 37°C for 15 min, 85°C for 5 sec and then held
at 4°C. qPCR was performed using TB Green® Premix Ex
Tap™ (Takara Bio, Inc.) on CFX Connect™ Real-time System (Bio-Rad
Laboratories, Inc.) at 95°C for 30 sec, for 39 thermal cycles (95°C
for 5 sec and 60°C for 30 sec), with GAPDH serving as the internal
control. The sequences of the primers used for RT-PCR are presented
in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
|
| Sequences
(5′→3′) |
|---|
|
|
|
|---|
| Gene names | Forward | Reverse |
|---|
| Connexin 43 |
CAAAATCGAATGGGGAGGC |
GCTGGTCCACAATGGCTAGT |
| GAPDH |
GTCAAGGCTGAGAACGGGAA |
AAATGAGCCCCAGCCTTCTC |
Western blotting
Cells were harvested and lysed by RIPA lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd.) according to
the manufacturer's instructions. Protein concentrations were
determined with BCA Protein Assay Kit (Applygen Technologies,
Inc.). Protein samples (18 µg) were separated by 10% SDS-PAGE
(Bio-Rad Laboratories, Inc.) and transferred to PVDF membranes by
Trans-Blot SD type membrane transfer system (Bio-Rad Laboratories,
Inc.). After blocking with 5% skimmed milk for 1 h at room
temperature, proteins on the PVDF membranes were immunoblotted for
2 h at room temperature using primary antibodies for Cx43 (cat. no.
ab79010; Abcam, 1:1,000), Bax (cat. no. 2772; Cell Signaling
Technology, Inc., 1:1,000), Bcl-2 (cat. no. YT0407; ImmunoWay,
1:1,000), cleaved caspase-3 (cat. no. 9664; Cell Signaling
Technology, Inc., 1:1,000), TSG101 and CD63 (cat. no. DF8427 and
cat. no. AF5117; Affinity Biosciences, 1:1,000) and GADPH (cat. no.
70004; Affinity Biosciences, 1:2,000). After washing for 3 times,
the membranes were further incubated with HRP-conjugated goat
anti-mouse (cat. no. GAM007; MultiSciences Biotech Co., Ltd.,
1:2,000) or goat anti-rabbit (cat. no. BA1060; Boster Biological
Technology Co., Ltd., 1:2,000) antibodies at room temperature for 1
h. The chemiluminescence signals were detected with an enhanced
chemiluminescence assay kit (Beijing Kangwei Century Biotechnology
Co., Ltd.). Densitometric analysis was conducted with the Gel
Imaging System (Analytik Jena US LLC).
Isolation and identification of
exosomes
Exosomes were isolated by differential velocity
centrifugation. In brief, culture medium was initially subjected to
centrifugation at 300 × g for 10 min, 2,000 × g for 10 min and
10,000 × g for 30 min at 4°C. The supernatant was further subjected
to centrifugation at 100,000 × g for 70 min at 4°C, and pellets
rich in exosomes were resuspended in 1X PBS. To identify the
isolated exosomes, the particle size of exosomes was initially
determined by Zetasizer Nano ZS 90 particle size analyzer (Malvern
Panalytical), and the morphology of the exosomes was observed under
a transmission electron microscope (Leica Microsystems GmbH;
magnification, ×40,000). As described previously (33), 10 µl of sample was added to a 2-mm
copper mesh grid and the excess PBS was removed with a filter
paper. Then, 10 µl of phosphotungstate (20 g/l) was added to the
copper net for negative staining for 2 min and grilled under an
incandescent lamp for 5 min. The isolated samples were subjected to
10% SDS-PAGE, and western blotting was used to detect the marker
proteins TSG101 and CD63 on exosomes.
Exosome uptake experiment
To label exosomes, 10 µM of
dioctadecyloxacarbocyanine (Dio) membrane dye was added to the
exosomal suspension in a 1:1 ratio and incubated at 37°C in the
dark for 30 min, followed by washing with PBS and centrifugation at
100,000 × g for 70 min for 3 times to remove the free Dio dye. The
Dio-labeled exosomes were resuspended with sterile 1X PBS. The
exosome concentration was detected by the BCA Protein Assay. Three
concentrations of 25, 50 and 100 µg/ml were used in the cell
treatment experiments. The results revealed that the effect of rExo
at 25 µg/ml was weak, while rExo at 50 and 100 µg/ml markedly
affected Cx43 expression in U251 cells. Therefore, the
concentration of exosomes at 50 µg/ml was considered sufficient and
was used for the following cell treatment experiments. In the
control groups, the same volume of PBS was added to the cells. To
detect uptake of exosomes, U251s cells were incubated with 50 µg/ml
of Dio-labeled rExo or sExo for 30 min at 37°C in the dark. Then,
the cells were washed twice with PBS, and fixed with 4%
paraformaldehyde for 10 min on ice. The nuclei of U251s cells were
counterstained with 500 µl of Hoechst 33342 (10 µg/ml) for 5 min at
room temperature in the dark. Finally, the cells were washed three
times with PBS. The uptake of Dio-labeled exosomes by cells was
observed under an inverted fluorescence microscope (Leica
Microsystems GmbH; magnification, ×400).
Cell viability detection
The Cell Counting Kit-8 (CCK-8; Vazyme Biotech Co.,
Ltd.) assay was performed to evaluate the viability of U251s and
U251r cells according to the manufacturer's instructions. Briefly,
~4×103 cells were seeded on 96-well plates and cultured
in full growth medium. After incubation with TMZ at 0, 100, 200,
400, 600, 800, 1,000 and 1,200 µM for 72 h, 10 µl of CCK-8 was
added to each well, and cells were further incubated with CCK-8 for
~2 h. The absorbance at 450 nm was measured spectrophotometrically
using a microplate reader (Bio-Rad Laboratories, Inc.). Control and
blank wells were also detected. All assays were performed at least
three times.
Colony formation assay
U251s Cells were incubated with 1X PBS, 50 µg/ml of
sExo or rExo and rExo + 200 µM 37,43Gap27 for 48 h.
After incubation, cells were harvested and resuspended to a density
of 6×103/ml. a total of 50 µl cell suspension was
inoculated in a 6-well plate, and was further cultured for 10 days.
Then, the cells were washed with 1X PBS and fixed in 4%
paraformaldehyde for 15 min on ice. A total of 2 ml of crystal
violet solution (0.1%) was added to each well and the cells were
stained for 30 min at room temperature. After washing 3 times with
1X PBS, the number of colonies with >50 cells was counted.
Wound healing and Transwell
assays
The wound healing and Transwell assays were
performed as previously described (34). For the wound healing assay,
3×105 U251s cells were seeded on 35-mm culture dishes.
When the cell confluence reached ~90%, a scratch was made in
monolayer of cells with a 200-µl pipette tip. After removing
unattached cells, U251 cells were incubated with 1X PBS, 50 µg/ml
of sExo or rExo and rExo + 200 µM 37,43Gap27 for 48 h.
At 0, 24 and 48 h of incubation with the aforementioned reagents,
the cells were photographed for evaluation of wound closure under
an inverted microscope (DMi8; Leica Microsystems GmbH;
magnification, ×100). ImageJ software (1.52v; National Institutes
of Health) was used to calculate the area of migration. Transwell
chambers (Corning, Inc.) were used for Transwell assay. Briefly,
3×106 U251s cells incubated with 1X PBS, 50 µg/ml of
sExo or rExo and rExo + 200 µM 37,43Gap27 for 48 h were
inoculated in the upper chamber with 200 µl serum-free medium. The
upper chamber was then incubated in a 24-well plate chamber with
complete medium containing 10% FBS for 24 h. After removing the
cells on the top surface of the upper chamber, the migrated cells
on lower surface of the chamber were fixed in 4% paraformaldehyde
solution for 10 min at room temperature, then stained in 0.1%
crystal violet solution for 30 min at room temperature. The
migrated cells were photographed under an inverted microscope
(DMi8; Leica Microsystems GmbH) and counted in four random fields
at a magnification of ×200.
Statistical analysis
The data in the present study were analyzed with
GraphPad Prism v.5 (GraphPad Software, Inc.) and are presented as
the mean ± SD. The statistical significance of the differences
among multiple groups was analyzed by one-way ANOVA parametric
testing followed by Tukey's multiple comparisons test. In the case
of comparison between two groups, unpaired two-tailed Student's
t-test was performed. P<0.05 was considered to indicate
statistically significant differences.
Results
Cx43 expression is upregulated in
TMZ-resistant U251r cells
To investigate the role of Cx43 in the chemotherapy
resistance of glioblastoma cells, the mRNA and protein levels of
Cx43 were measured in U251r and U251s cells using RT-PCR and
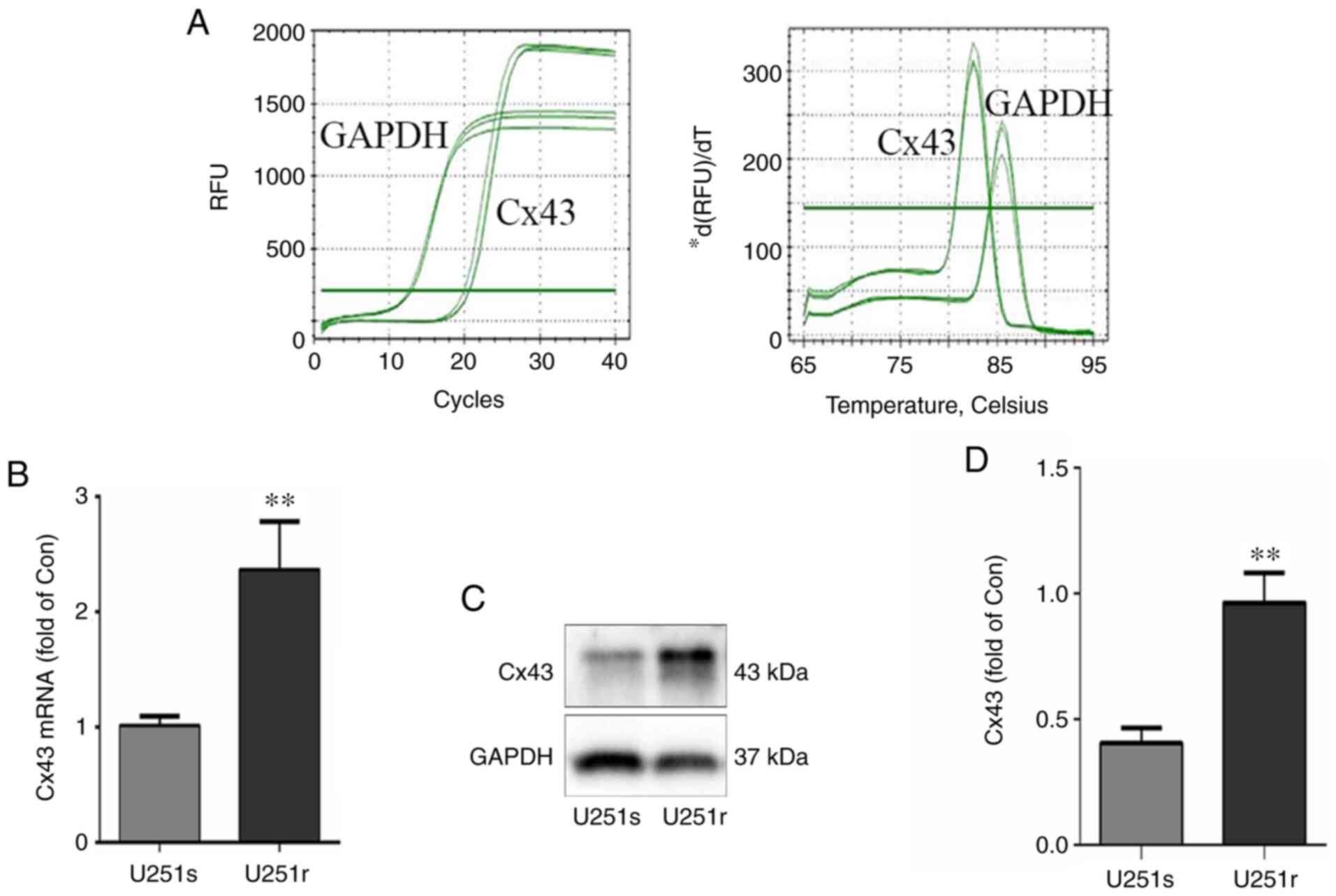
western blotting assays, respectively. As shown in Fig. 1A and B, the mRNA level of Cx43 in
U251r cells was significantly higher compared with that in U251s
cells. Similarly, the results of western blotting demonstrated that
the Cx43 protein level was also significantly higher in U251r cells
compared with that in U251s cells (Fig.
1C and D; P<0.01). These results are consistent with those
of previous studies (31,32), and suggest that Cx43 may be involved
in the resistance of glioblastoma cells to TMZ.
Isolation and identification of
exosomes from U251s and U251r cells
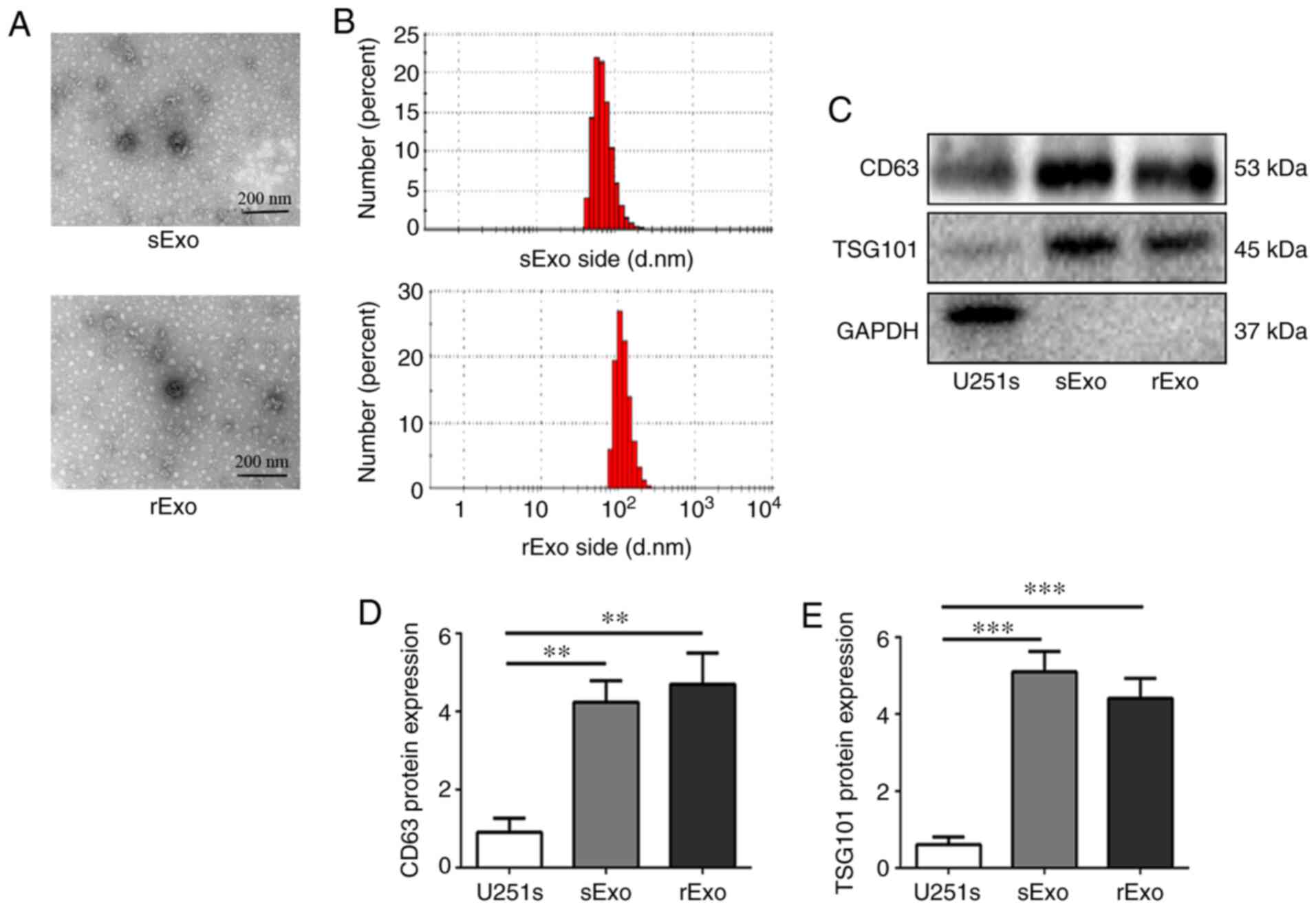
First, exosomes were isolated from U251s and U251r
cells by differential velocity centrifugation, as previously
described (35). After isolation, the
morphology of the vesicles was observed under a transmission
electron microscope (Fig. 2A). It has
been reported that exosomes are lipid bilayer vesicles with a
diameter of 30–150 nm. The samples isolated from U251s and U251r
cells were subjected to Zetasizer Nano ZS 90 particle size
analyzer. As shown in Fig. 2B, the
size distribution of the vesicles was concentrated in the range of
40–120 nm. Thus, the morphology and particle size distribution of
vesicles isolated from cells were found to be consistent with the
characteristics of exosomes. Furthermore, western blotting was used
to detect the expression of exosomal markers, including CD63 and
TSG101, in isolated samples. The results revealed that a strong
signal of CD63 and TSG101 was detected in samples isolated from
U251s and U251r cells, while the signal was notably weaker in
whole-cell lysate from U251s cells (Fig.
2C-E). These results indicate that the exosomes (sExo and rExo)
were successfully isolated from U251s and U251r cells,
respectively.
rExo upregulates Cx43 expression in
U251s cells and facilitates exosome uptake via Cx43
It has been reported that Cxs can be assembled into
a hemi-channel in the exosomal membrane (24). To explore the role of Cx43 in TMZ
resistance, the expression of Cx43 in sExo and rExo was detected by
western blotting. As shown in Fig. 3A and
C, the expression of Cx43 in rExo was significantly higher
compared with that in sExo (P<0.005). In addition, the effect of
sExo and rExo on Cx43 expression in glioma cells was examined.
U251s cells were incubated with 50 µg/ml sExo, rExo and PBS for 48
h. Of note, the expression of Cx43 in U251s cells incubated with
rExo was significantly increased, while there was no significant
change in the expression of Cx43 in U251s cells treated with PBS or
sExo (Fig. 3B and D). Next, the role
of Cx43 in exosome uptake by glioma cells was further explored.
U251s cells were incubated with Dio-sExo, Dio-rExo or Dio-rExo +
37,43Gap27. As shown in Fig.
3E, the uptake of sExo and rExo by U251s cells was observed.
Consistent with exosomal Cx43 expression level, the fluorescence
intensity of Dio-rExo in U251s cells was found to be stronger
compared with that of Dio-sExo groups. Moreover, the gap junction
mimetic peptide 37,43Gap27 directed against exosomal
Cx43 markedly alleviated the fluorescence intensity of Dio-rExo in
U251s cells. These results suggest that exosomal Cx43 not only
upregulates Cx43 expression in U251s cells, but also facilitates
exosome uptake via Cx43.
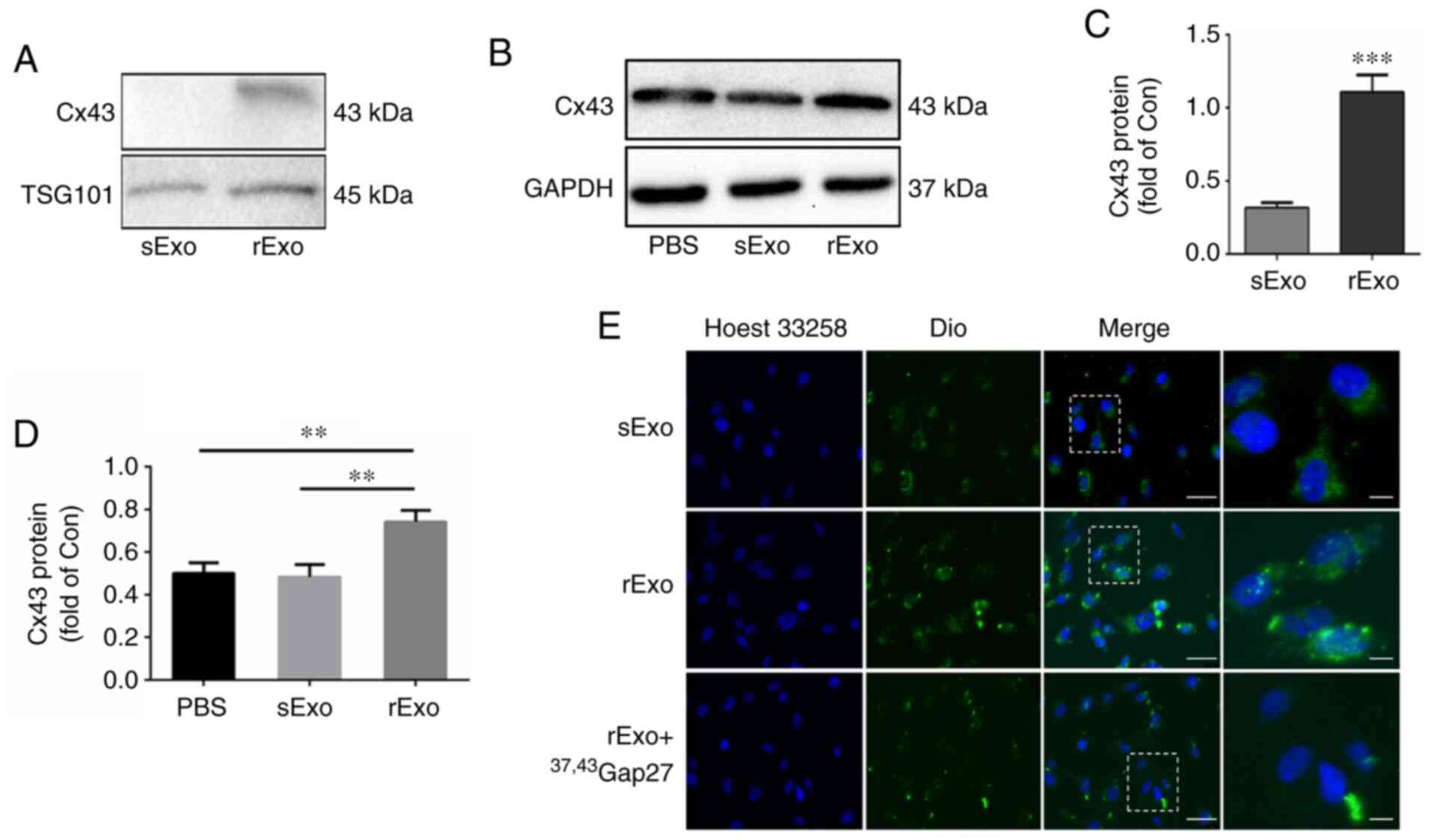 | Figure 3.Role of exosomal Cx43 in exosome
uptake by glioma cells. (A) Expression level of Cx43 in sExo and
rExo. GAPDH was used as endogenous reference. (B) Expression of
Cx43 in U251s cells after treatment with PBS, sExo and rExo for 48
h. GAPDH was used as endogenous reference. (C and D) The relative
expression level of Cx43 was indicated as the ratio of Cx43/GAPDH
in each group. Data represent the results of at least three
independent experiments for each condition. **P<0.01,
***P<0.001. (E) Exosome uptake in U251s cells after treatment
with Dio-sExo, Dio-rExo and Dio-rExo + 37,43Gap27. U251s
cells were incubated with Dio-stained sExo, rExo and Dio-rExo +
37,43Gap27 for 30 min, then were counterstained with
Hoechst 33342 (blue) for 5 min. Dio-stained exosome uptake (green)
in cells was observed under fluorescence microscope. Scale bar, 100
µm. Cx43, connexin 43; U251r, temozolomide-resistant U251 glioma
cells; U251s, temozolomide-sensitive U251 glioma cells; sExo,
exosomes of U251s cells; rExo, exosomes of U251r cells; Dio,
dioctadecyloxacarbocyanine. |
Exosomal Cx43 regulates TMZ resistance
and colony formation ability of U251s cells
The aforementioned experiments demonstrated that
exosomal Cx43 facilitated exosome uptake by U251s cells. Next, the
role of exosomal Cx43 in development of TMZ resistance was
examined. U251s cells were separately pretreated with PBS, sExo,
rExo or rExo + 37,43Gap27, followed by treatment with
0–800 µM TMZ. The results of the CCK-8 assay demonstrated that the
IC50 value of U251s cells to TMZ was ~220 µM in the PBS
and sExo groups, while the IC50 value of U251s cells to
TMZ was increased to 580 µM in the rExo group (Fig. 4A and B). Additionally,
37,43Gap27 significantly alleviated the rExo-induced
increase in the IC50 value of U251s cells to TMZ
(P<0.001). Furthermore, the effect of exosomal Cx43 on the
colony formation ability of U251s cells was examined. As shown in
Fig. 4C and D, the colony number in
the PBS and sExo groups was 25±6 and 38±4, respectively. By
contrast, rExo significantly increased the colony number to 76±6
(P<0.001), whereas treatment with rExo + 37,43Gap27
decreased the colony number to 51±7 (P<0.01). These results
suggest that exosomal Cx43 enhances TMZ resistance and colony
formation ability in glioma cells.
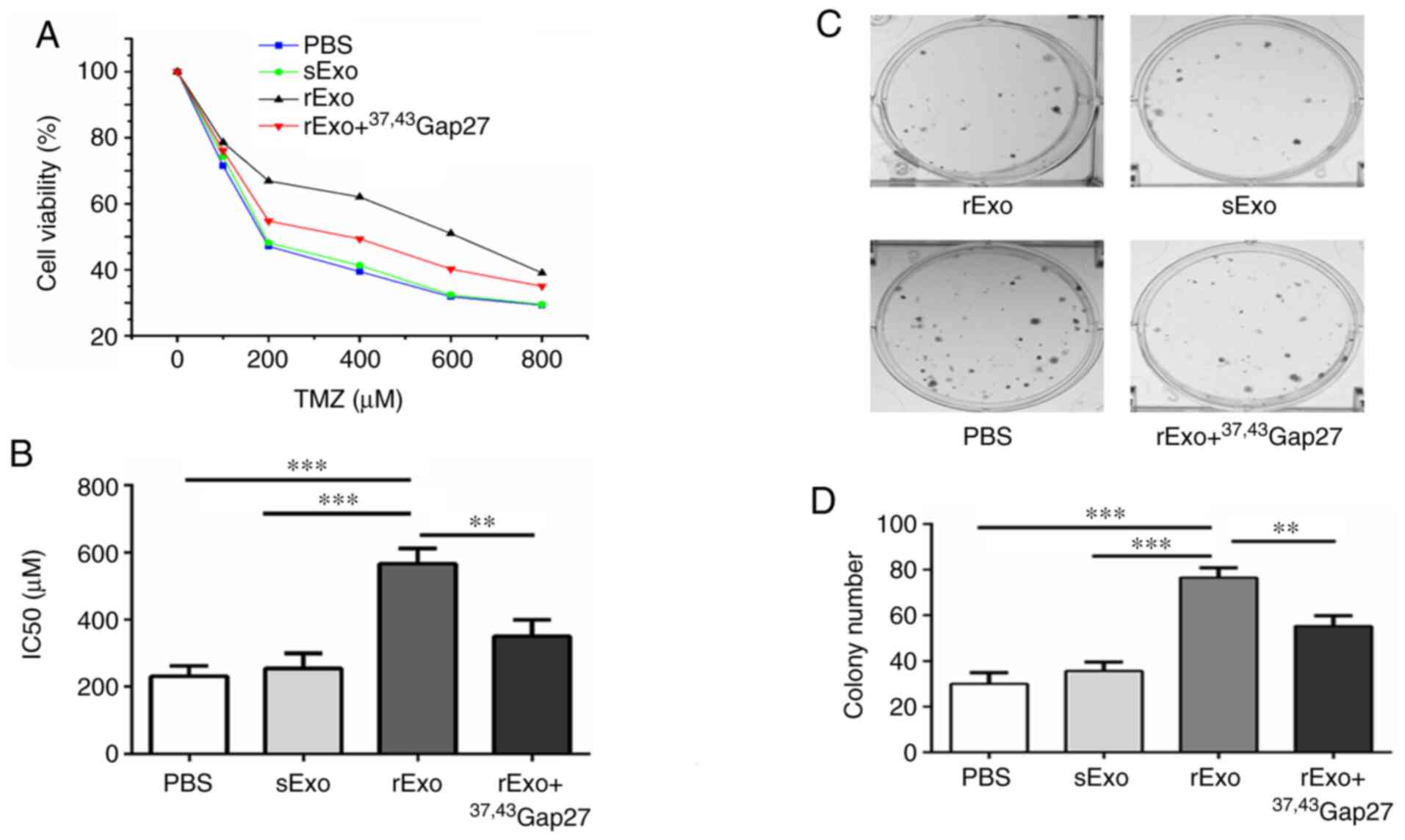 | Figure 4.Effect of exosomal Cx43 on TMZ
resistance and colony formation ability of U251s cells. (A) U251s
cell viability in each group was detected using Cell Counting Kit-8
assay at 72 h after treatment with TMZ, and the cell viability at
each TMZ concentration was plotted for the dose-response curve. (B)
IC50 values of U251s cells to TMZ in the PBS, sExo, rExo
and rExo + 37,43Gap27 groups. Data represent the mean of
at least three independent experiments for each condition.
**P<0.01, ***P<0.001. (C) Colony formation assay was
conducted in U251s cells treated with PBS, sExo, rExo and rExo +
37,43Gap27. The representative images showed the colony
morphology in each group. (D) Quantification of the number of
colonies for statistical analysis. Data represent the results of
three independent experiments for each group. **P<0.01,
***P<0.001. Cx43, connexin 43; TMZ, temozolomide; U251r,
TMZ-resistant U251 glioma cells; U251s, TMZ-sensitive U251 glioma
cells; sExo, exosomes of U251s cells; rExo, exosomes of U251r
cells. |
Exosomal Cx43 regulates the expression
of Bcl-2, Bax and cleaved caspase-3 in U251s cells
The antineoplastic effect of TMZ is mediated by
inducing intrinsic apoptosis and cell cycle arrest. Bcl-2 family
proteins and caspases are important regulators of the intrinsic
apoptosis pathway, which includes the pro-apoptotic (Bax),
anti-apoptotic (Bcl-2) members and the cleavage of caspase-3. It
has been reported that TMZ increases the Bax/Bcl-2 ratio in glioma
cells, and dexamethasone induces TMZ resistance by maintaining the
Bax/Bcl-2 ratio (36). Thus, effect
of rExo on the expression of Bcl-2, Bax and cleaved caspase-3 in
U251s cells was examined. Compared with the PBS and sExo groups,
the expression of Bax and cleaved caspase-3 was significantly
decreased and that of Bcl-2 was significantly increased in the rExo
group. 37,43Gap27 efficiently attenuated the
rExo-induced changes in the expression of Bax, Bcl-2 and cleaved
caspase-3 in U251s cells (Fig. 5A-C).
These results indicated that exosomal Cx43 may enhance TMZ
resistance through modulating the expression of pro-apoptotic and
anti-apoptotic proteins and activation of caspase signaling.
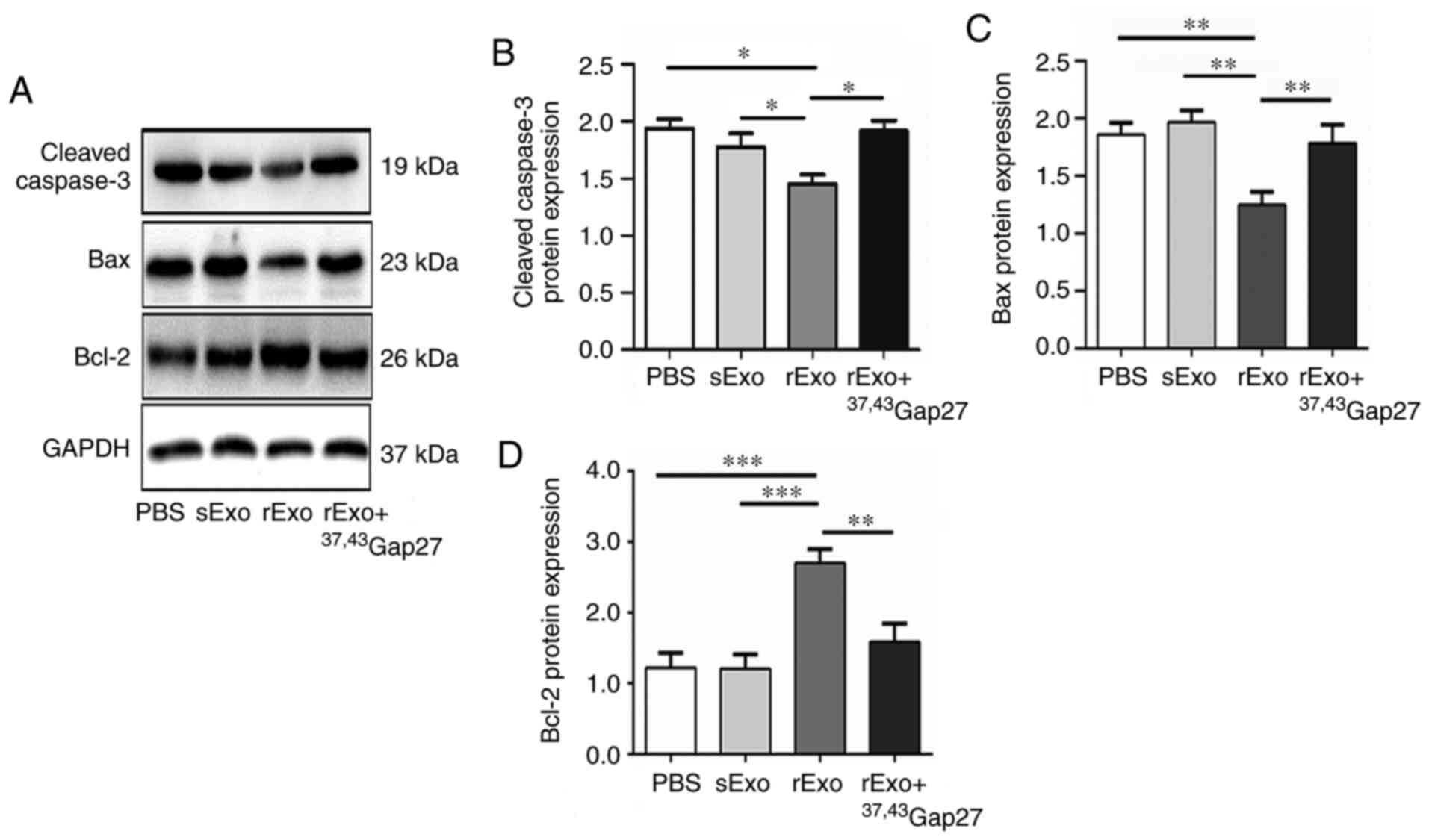 | Figure 5.Effect of exosomal Cx43 on the
expression of Bcl-2, Bax and cleaved caspase-3 in U251s cells. (A)
Western blotting was used to detect the expression level of Bax,
Bcl-2 and cleaved caspase-3 in U251s treated with PBS, sExo, rExo
and rExo + 37,43Gap27. GAPDH was used as endogenous
control. (B, C and D) Relative expression levels of Bax, Bcl-2 and
cleaved caspase-3 were indicated as the Bax/GAPDH, Bcl-2/GAPDH and
cleaved caspase-3/GAPDH ratios in each group. Data represent the
results of at least three independent experiments for each
condition. *P<0.05, **P<0.01, ***P<0.001. Cx43, connexin
43; U251r, temozolomide-resistant U251 glioma cells; U251s,
temozolomide-sensitive U251 glioma cells; sExo, exosomes of U251s
cells; rExo, exosomes of U251r cells. |
Exosomal Cx43 enhances the migration
ability of U251s cells
Glioma is a highly aggressive brain tumor. The
strong migratory and invasive ability of glioma cells are closely
associated with the poor prognosis of glioma. The present study
further examined the effect of exosomal Cx43 on the migration
ability of U251s cells. In the wound healing experiments, the
migration of U251s cells at 0, 24 and 48 h in each group was
photographed. As shown in Fig. 6A and
B, rExo significantly increased the wound closure at 24 and 48
h compared to the PBS and sExo groups. 37,43Gap27
efficiently inhibited rExo-induced wound closure in U251s cells.
Consistently, the results of the Transwell assay also demonstrated
that rExo enhanced the migration of U251s cells, whereas
37,43Gap27 significantly alleviated rExo-induced cell
migration (Fig. 6C and D). The
results of the wound healing and Transwell assays suggest that Cx43
in rExo is involved in the regulation of glioma cell migration.
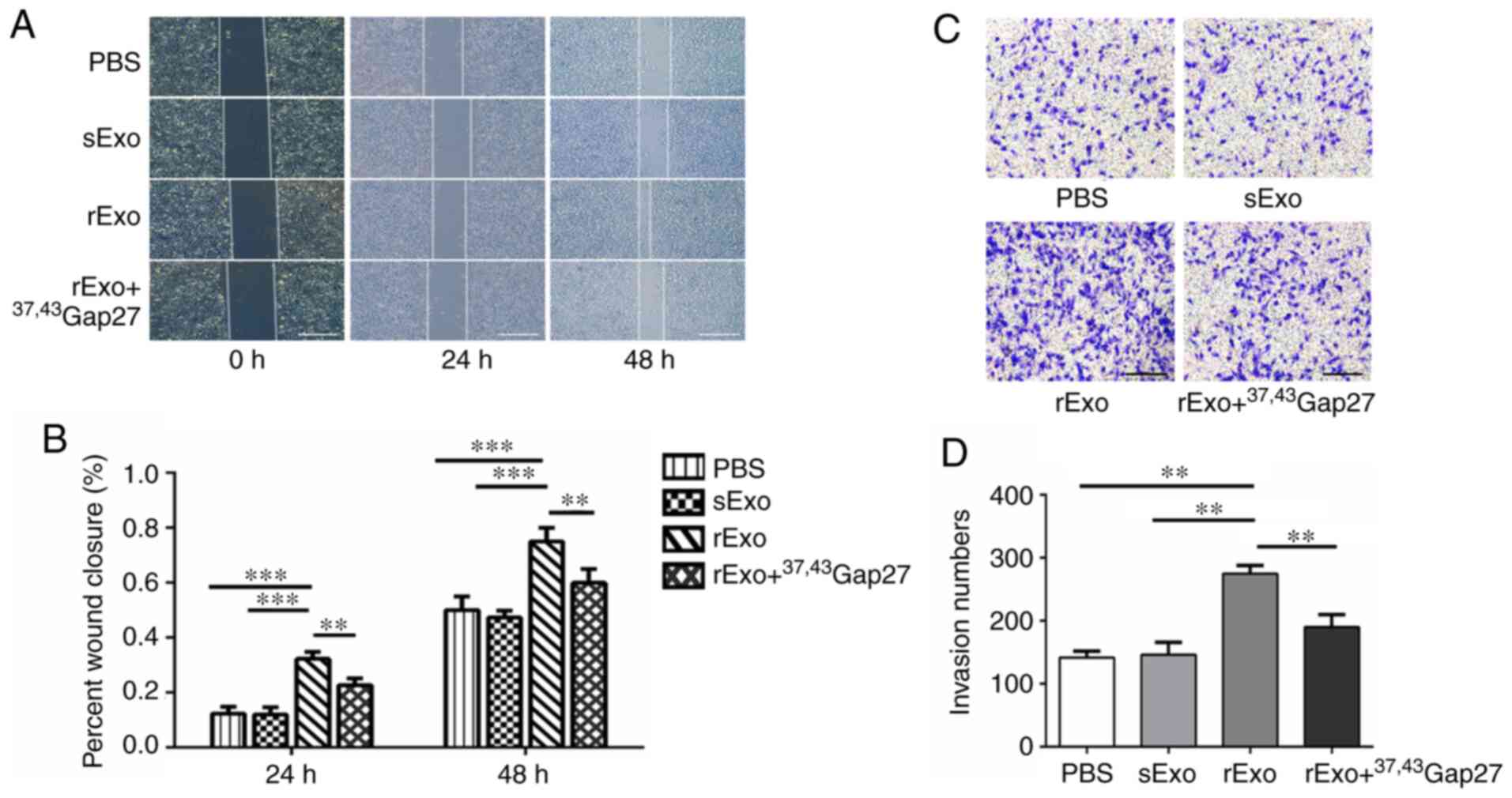 | Figure 6.Exosomal Cx43 enhances the migration
ability of U251s cells. (A) Wound healing experiments were
performed to evaluate the migration of U251s cells at 0, 24 and 48
h after treatment with PBS, sExo, rExo and rExo +
37,43Gap27. Scale bar, 200 µm. (B) Wound closure rate
was measured using ImageJ software for statistical analysis. Data
represent the results of at least three independent experiments for
each group. **P<0.01, ***P<0.001. (C) Transwell assay was
used to examine the migration of U251s cells after treatment with
PBS, sExo, rExo and rExo + 37,43Gap27. Scale bar, 200
µm. (D) The migrated U251s cells were counted in at least 9 fields
from three independent experiments for each treatment. **P<0.01.
Cx43, connexin 43; U251r, temozolomide-resistant U251 glioma cells;
U251s, temozolomide-sensitive U251 glioma cells; sExo, exosomes of
U251s cells; rExo, exosomes of U251r cells. |
Discussion
Glioma is one of the most common primary malignant
brain tumors. Although TMZ is widely used for the treatment of
primary and metastatic brain cancer, a considerable proportion of
gliomas are refractory to TMZ or gradually develop resistance,
which is the main reason for the failure of chemotherapy for glioma
(37). It has been demonstrated that
exosomes from glioma cells can promote tumor progression by
transferring oncogenic and immunomodulatory proteins and nucleic
acids between different cancer cell subsets or between cancer cells
and surrounding stromal cells (38),
which plays an important role in chemotherapy resistance. On the
other hand, treatment with neuropilin-1-targeted peptide
(RGE)-modified, superparamagnetic iron oxide nanoparticle (SPION)-
and curcumin (Cur)-loaded exosomes, named RGE-Exo-SPION/Cur, has
been reported to target glioma and prolong the survival time of
tumor-bearing mice (39). Thus, it is
necessary to further investigate the mechanisms underlying the role
of exosomes in the resistance of glioma cells to TMZ.
It has been reported that Cxs are assembled into a
hemi-channel in the exosomal membrane (24). The hemi-channel formed by Cxs on the
plasma membrane can promote communication between the cytoplasm and
the extracellular environment (40).
Cx43 is the most common gap junction protein and is highly
expressed in primary glioma cell lines (41). It has been reported that high
expression of Cx43 is associated with the development of resistance
of glioma cells to TMZ (31). In
addition, a Cx43 C-terminus (CT) mimetic peptide-dubbed αCT1 (a
selective inhibitor of Cx43 channels) combined with TMZ
significantly blocked the growth of human LN229/GSC tumors in mice
(41). Consistently, in the present
study, higher expression of Cx43 was detected in U251r cells
compared with that in U251s cells (Fig.
1). To explore the role of exosomal Cx43 in chemotherapy
resistance, exosomes were initially isolated from U251s and U251r
cells. Similar to parent cells, rExo expressed significantly higher
Cx43 levels than sExo (Fig. 3A and
C). Prior to incorporation, exosomes must dock and fuse with
recipient cells. Thus, it is necessary to examine the role of
exosomal Cx43 in exosome uptake by glioma cells. Although sExo and
rExo uptake was observed in U251s cells, rExo with higher Cx43
expression was more readily incorporated into cells compared with
sExo. In addition, the synthetic connexin 43 mimetic peptide
37,43Gap27 possesses conserved sequence homology to a
portion of the second extracellular loop leading into the fourth
transmembrane connexin segment, and is widely used as a specific
gap junction inhibitor directed against Cx43 (42,43). It
has been reported that 37,43Gap27 (100 nM-100 µM)
attenuated hemi-channel activity in adult keratinocytes and
fibroblasts sourced from juvenile foreskin. The expression level of
Cx43 was also decreased when JF and AK cells were exposed to
37,43Gap27 for 24 h (44).
In the present study, 37,43Gap27 directed against Cx43
markedly alleviated the uptake of rExo by U251s cells (Fig. 3E). These results suggest that exosomal
Cx43 regulates the process of incorporation of exosomes into
recipient cells. However, the mechanism underlying the role of
exosomal Cx43 in exosome uptake remains to be further elucidated.
It will be interesting to examine whether exosomal Cx43 cooperates
with other membrane component molecules to facilitate docking or
fusion with recipient cells. Next, the effect of exosomal Cx43 on
the sensitivity of glioma cells to TMZ was examined. Treatment with
rExo significantly increased the IC50 value of U251s
cells to TMZ and colony formation, whereas 37,43Gap27
efficiently inhibited the effect of rExo on U251s cells (Fig. 4A-D). TMZ induces DNA damage and cell
cycle arrest, which leads to an imbalance of Bcl-2 and Bax and
activates the intrinsic apoptosis pathway in glioma cells (45). It was previously demonstrated that
Cx43 reduces the sensitivity of human LN18 and LN229 glioma cells
to TMZ by reducing the Bax/Bcl-2 ratio and the release of
cytochrome c (29).
Overexpression of Cx43 promotes the migration of U251 cells and
inhibits apoptosis by upregulating Bcl-2 and downregulating the
expression of Bax and caspase-3 (46). Thus, the effect of rExo on the
expression of Bcl-2, Bax and cleaved caspase-3 in U251s cells was
examined. As expected, rExo increased the expression of Bcl-2 and
decrease Bax and cleaved caspase-3 in U251s cells, while
37,43Gap27 alleviated the rExo-induced changes in the
expression of Bax, Bcl-2 and cleaved caspase-3 in U251s cells
(Fig. 5A-C). There are at least two
plausible explanations for the role of exosomal Cx43 in the
resistance of glioma cells to TMZ. First, the exosomal Cx43 may
enhance TMZ resistance through modulating the expression of the
pro-apoptotic and anti-apoptotic proteins and inhibiting the
intrinsic apoptosis pathway (29,46).
Second, high expression of Cx43 in rExo may contribute to the
formation of gap junction channels between exosomes and recipient
cells, which facilitates transferring various TMZ
resistance-related factors from exosomes to U251s cells.
Furthermore, the role of exosomal Cx43 in the migration of glioma
cells was also examined. The results of the wound healing and
Transwell assays demonstrated that rExo promoted the migration of
U251s cells, and 37,43Gap27 efficiently alleviated
rExo-induced cell migration (Fig. 6).
In previous studies, gap junctions have been demonstrated to serve
as an important regulator of cell migration during tumor
metastasis, although some results are controversial. For example,
increased expression of Cx26 has been reported to enhance the
invasion of lung squamous cell carcinoma (47). However, overexpression of Cx26 and
Cx43 has also been found to slow down the migration of breast
cancer cells (48,49). By contrast, the present study
demonstrated that rExo with high Cx43 expression accelerated the
migration of U251s cells. One possible explanation is that
Cx43-assembled gap junction channels facilitate the delivery of
effector molecules promoting migration from exosomes to U251s
cells. Another explanation is that the response of glioma cells may
differ from that of breast cancer cells.
In conclusion, the results of the present study may
provide new insight into the role of exosomal Cx43 in the
resistance of glioma cells to TMZ and cell migration. Cx43 was
found to be highly expressed in TMZ-resistant U251r cells and rExo.
Furthermore, treatment with rExo enhanced the resistance of U251s
cells to TMZ, their colony formation and migration abilities, and
changeed the expression pattern of Bax and Bcl-2.
37,43Gap27 directed against exosomal Cx43 significantly
attenuated rExo-induced TMZ resistance, colony formation, cell
migration and alterations in the expression of Bax and Bcl-2. These
results indicate the important role of exosomal Cx43 in TMZ
resistance and migration of glioma cells, and suggest a promising
new strategy for preventing chemotherapy resistance and reducing
the aggressiveness of glioblastoma by targeting exosomal Cx43 in
the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81660014 and
81660159), the Key Program of the Natural Science Foundation of
Jiangxi Province (grant no. 20202ACB206001), the Key Research and
Development Program of Jiangxi Province (grant nos. 20192BBG70012
and 20192BBG70049), and the Research Fund for Key Laboratory of
Drug Targets and Drug Screening of Jiangxi Province (grant no.
20171BCD40007).
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
LPJ and XJH designed the current project. ZJY, LLZ,
QCB, LJG, RJT, XML and LHL performed the experiments. LPJ, XJH, TH,
MJW, ZJY and LLZ analyzed the data. ZJY, XJH and LLZ drafted the
manuscript. LPJ, XJH and TH supervised the study. LPJ and XJH have
seen and can confirm the authenticity of the raw data. All the
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Cx43
|
connexin 43
|
|
TMZ
|
temozolomide
|
|
U251r
|
TMZ-resistant U251 cells
|
|
U251s
|
TMZ-sensitive U251 cells
|
|
Exo
|
exosome
|
|
Dio
|
dioctadecyloxacarbocyanine
|
References
|
1
|
Lara-Velazquez M, Al-Kharboosh R,
Jeanneret S, Vazquez-Ramos C, Mahato D, Tavanaiepour D, Rahmathulla
G and Quinones-Hinojosa A: Advances in brain tumor surgery for
glioblastoma in adults. Brain Sci. 20:1662017. View Article : Google Scholar
|
|
2
|
Iwadate Y: Epithelial-mesenchymal
transition in glioblastoma progression. Oncol Lett. 11:1615–1620.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonavia R, Inda MM, Cavenee WK and Furnari
FB: Heterogeneity maintenance in glioblastoma: A social network.
Cancer Res. 71:4055–4060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. New Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alonso MM, Gomez-Manzano C, Bekele BN,
Yung WK and Fueyo J: Adenovirus-based strategies overcome
temozolomide resistance by silencing the O6-methylguanine-DNA
methyltransferase promoter. Cancer Res. 67:11499–11504. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Stevens MF and Bradshaw TD:
Temozolomide: Mechanisms of action, repair and resistance. Curr Mol
Pharmacol. 5:102–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Omar AI and Mason WP: Temozolomide: The
evidence for its therapeutic efficacy in malignant astrocytomas.
Core Evid. 4:93–111. 2010.PubMed/NCBI
|
|
8
|
Neyns B, Tosoni A, Hwu WJ and Reardon DA:
Dose-dense temozolomide regimens: Antitumor activity, toxicity, and
immunomodulatory effects. Cancer. 116:2868–2877. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garcia-Mayea Y, Mir C, Masson F, Paciucci
R and ME LL: Insights into new mechanisms and models of cancer stem
cell multidrug resistance. Semin Cancer Biol. 60:166–180. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Munoz JL, Walker ND, Mareedu S, Pamarthi
SH, Sinha G, Greco SJ and Rameshwar P: Cycling quiescence in
temozolomide resistant glioblastoma cells is partly explained by
microRNA-93 and −193-mediated decrease of cyclin D. Front
Pharmacol. 10:1342019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao X, Lu Y, Liu Y, Zhou Y, Song H, Zhang
W, Davis D, Cui J, Hao S, Jung J, et al: Combination of PARP
inhibitor and temozolomide to suppress chordoma progression. J Mol
Med (Berl). 97:1183–1193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ratajczak J, Wysoczynski M, Hayek F,
Janowska-Wieczorek A and Ratajczak MZ: Membrane-derived
microvesicles: Important and underappreciated mediators of
cell-to-cell communication. Leukemia. 20:1487–1495. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: Extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cocucci E and Meldolesi J: Ectosomes and
exosomes: Shedding the confusion between extracellular vesicles.
Trends Cell Biol. 25:364–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Azmi AS, Bao B and Sarkar FH: Exosomes in
cancer development, metastasis, and drug resistance: A
comprehensive review. Cancer Metastasis Rev. 32:623–642. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao GY, Cheng CC, Chiang YS, Cheng WT,
Liu IH and Wu SC: Exosomal miR-10a derived from amniotic fluid stem
cells preserves ovarian follicles after chemotherapy. Sci Rep.
6:231202016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanaka S, Hosokawa M, Ueda K and Iwakawa
S: Effects of decitabine on invasion and exosomal expression of
miR-200c and miR-141 in oxaliplatin-resistant colorectal cancer
cells. Biol Pharm Bull. 38:1272–1279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Brien K, Lowry MC, Corcoran C, Martinez
VG, Daly M, Rani S, Gallagher WM, Radomski MW, MacLeod RA and
O'Driscoll L: MiR-134 in extracellular vesicles reduces
triple-negative breast cancer aggression and increases drug
sensitivity. Oncotarget. 6:32774–32789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Santos JC, da Silva Lima N, Sarian LO,
Matheu A, Ribeiro ML and Derchain SFM: Exosome-mediated breast
cancer chemoresistance via miR-155 transfer. Sci Rep. 8:8292018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mulcahy LA, Pink RC and Carter DR: Routes
and mechanisms of extracellular vesicle uptake. J Extracell
Vesicles. 3:34022014. View Article : Google Scholar
|
|
22
|
Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ,
Chang LF, Zhou Q and Sui SF: Cellular internalization of exosomes
occurs through phagocytosis. Traffic. 11:675–687. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mincheva-Nilsson L and Baranov V: Cancer
exosomes and NKG2D receptor-ligand interactions: Impairing
NKG2D-mediated cytotoxicity and anti-tumour immune surveillance.
Semin Cancer Biol. 28:24–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soares AR, Martins-Marques T,
Ribeiro-Rodrigues T, Ferreira JV, Catarino S, Pinho MJ, Zuzarte M,
Anjo SI, Manadas B, Sluijter JP, et al: Gap junctional protein Cx43
is involved in the communication between extracellular vesicles and
mammalian cells. Sci Rep. 5:132432015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scemes E, Spray DC and Meda P: Connexins,
pannexins, innexins: Novel roles of ‘hemi-channels’. Pflugers Arch.
457:1207–1226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maes M, Decrock E, Cogliati B, Oliveira
AG, Marques PE, Dagli ML, Menezes GB, Mennecier G, Leybaert L,
Vanhaecke T, et al: Connexin and pannexin (hemi)channels in the
liver. Front Physiol. 4:4052014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aasen T, Mesnil M, Naus CC, Lampe PD and
Laird DW: Gap junctions and cancer: Communicating for 50 years. Nat
Rev Cancer. 16:775–788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sin WC, Crespin S and Mesnil M: Opposing
roles of connexin43 in glioma progression. Biochim Biophys Acta.
1818:2058–2067. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gielen PR, Aftab Q, Ma N, Chen VC, Hong X,
Lozinsky S, Naus CC and Sin WC: Connexin43 confers temozolomide
resistance in human glioma cells by modulating the mitochondrial
apoptosis pathway. Neuropharmacology. 75:539–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Theis M and Giaume C: Connexin-based
intercellular communication and astrocyte heterogeneity. Brain Res.
1487:88–98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Caltabiano R, Torrisi A, Condorelli D,
Albanese V and Lanzafame S: High levels of connexin 43 mRNA in high
grade astrocytomas. Study of 32 cases with in situ hybridization.
Acta Histochem. 112:529–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Munoz JL, Rodriguez-Cruz V, Greco SJ,
Ramkissoon SH, Ligon KL and Rameshwar P: Temozolomide resistance in
glioblastoma cells occurs partly through epidermal growth factor
receptor-mediated induction of connexin 43. Cell Death Dis.
5:e11452014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Helwa I, Cai J, Drewry MD, Zimmerman A,
Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer
WD, et al: A comparative study of serum exosome isolation using
differential ultracentrifugation and three commercial reagents.
PLoS One. 12:e01706282017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han XJ, Yang ZJ, Jiang LP, Wei YF, Liao
MF, Qian Y, Li Y, Huang X, Wang JB, Xin HB and Wan YY:
Mitochondrial dynamics regulates hypoxia-induced migration and
antineoplastic activity of cisplatin in breast cancer cells. Int J
Oncol. 46:691–700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Konadu KA, Huang MB, Roth W, Armstrong W,
Powell M, Villinger F and Bond V: Isolation of exosomes from the
plasma of HIV-1 positive individuals. J Vis Exp. 5:534952016.
|
|
36
|
Das A, Banik NL, Patel SJ and Ray SK:
Dexamethasone protected human glioblastoma U87MG cells from
temozolomide induced apoptosis by maintaining Bax:Bcl-2 ratio and
preventing proteolytic activities. Mol Cancer. 3:362004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fan QW, Cheng C, Hackett C, Feldman M,
Houseman BT, Nicolaides T, Haas-Kogan D, James CD, Oakes SA,
Debnath J, et al: Akt and autophagy cooperate to promote survival
of drug-resistant glioma. Sci Signal. 3:ra812010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chistiakov DA and Chekhonin VP:
Extracellular vesicles shed by glioma cells: Pathogenic role and
clinical value. Tumour Biol. 35:8425–8438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jia G, Han Y, An Y, Ding Y, He C, Wang X
and Tang Q: NRP-1 targeted and cargo-loaded exosomes facilitate
simultaneous imaging and therapy of glioma in vitro and in vivo.
Biomaterials. 178:302–316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Goodenough DA and Paul DL: Beyond the gap:
Functions of unpaired connexon channels. Nat Rev Mol Cell Biol.
4:285–294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Murphy SF, Varghese RT, Lamouille S, Guo
S, Pridham KJ, Kanabur P, Osimani AM, Sharma S, Jourdan J, Rodgers
CM, et al: Connexin 43 inhibition sensitizes chemoresistant
glioblastoma cells to temozolomide. Cancer Res. 76:139–149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ilvesaro J, Tavi P and Tuukkanen J:
Connexin-mimetic peptide gap 27 decreases osteoclastic activity.
BMC Musculoskelet Disord. 2:102001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Edwards G, Félétou M, Gardener MJ, Thollon
C, Vanhoutte PM and Weston AH: Role of gap junctions in the
responses to EDHF in rat and guinea-pig small arteries. Br J
Pharmacol. 128:1788–1794. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Faniku C, O'Shaughnessy E, Lorraine C,
Johnstone SR, Graham A, Greenhough S and Martin PE: The connexin
mimetic peptide gap27 and Cx43-knockdown reveal differential roles
for connexin43 in wound closure events in skin model systems. Int J
Mol Sci. 19:6042018. View Article : Google Scholar
|
|
45
|
Hombach-Klonisch S, Mehrpour M, Shojaei S,
Harlos C, Pitz M, Hamai A, Siemianowicz K, Likus W, Wiechec E,
Toyota BD, et al: Glioblastoma and chemoresistance to alkylating
agents: Involvement of apoptosis, autophagy, and unfolded protein
response. Pharmacol Ther. 184:13–41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu J, Yu M, Lin Z, Lue S, Zhang H, Zhao H,
Xu Y and Liu H: Effects of connexin43 overexpression on U251 cell
growth, migration, and apoptosis. Med Sci Monit. 23:2917–2923.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ito A, Koma Y, Uchino K, Okada T,
Ohbayashi C, Tsubota N and Okada M: Increased expression of
connexin 26 in the invasive component of lung squamous cell
carcinoma: Significant correlation with poor prognosis. Cancer
Lett. 234:239–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kalra J, Shao Q, Qin H, Thomas T,
Alaoui-Jamali MA and Laird DW: Cx26 inhibits breast MDA-MB-435 cell
tumorigenic properties by a gap junctional intercellular
communication-independent mechanism. Carcinogenesis. 27:2528–2537.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
McLachlan E, Shao Q, Wang HL, Langlois S
and Laird DW: Connexins act as tumor suppressors in
three-dimensional mammary cell organoids by regulating
differentiation and angiogenesis. Cancer Res. 66:9886–9894. 2006.
View Article : Google Scholar : PubMed/NCBI
|