Introduction
According to clinical statistics, the number of new
cases and cancer-related deaths each year remain high, bringing a
heavy burden to health care and economy systems worldwide (1,2).
Tumorigenesis and development of cancer are influenced by various
factors, such as genes, the environment and current treatment
strategies; thus, it is extremely challenging to identify new
targets and antitumor drugs (3). It
has been revealed that the immune evasion of cancer, a process
through which cancer cells evade the immune surveillance system of
the body, is involved in tumorigenesis and cancer progression
(4). Therefore, immunotherapies based
on the immune evasion of cancer have been developed; as typical
examples, treatments targeting the anti-program death-1 and
anti-program death-ligand 1 pathways have been widely utilized in
various types of cancer, such as lung cancer, lymphoma, melanoma
and liver cancer (5).
A previous study demonstrated that the knockdown of
serine proteinase inhibitor B9 (serpin B9) led to the granzyme B
(GrB)-dependent death of lung cancer cells, and serpin B9
inhibitors not only increased the apoptosis of lung cancer cells,
but also inhibited tumour growth and prolonged the survival time of
mice with lung cancer (6). Serpin B9,
as a promising target molecule of antitumour treatment, has been
revealed to protect cancer cells by enhancing immune evasion; it
has thus attracted increased attention from scholars. Therefore,
the aim of the present review was to summarise the current
knowledge on the role of serpin B9 in cancer and provide
information on its discovery, structure, functions and association
with cancer.
Serpin superfamily
Serpin is a superfamily of protease inhibitors with
a common source and highly homologous structural sequence, yet with
highly differentiated functions, which was first discovered in
human plasma and obtained through separation and purification
(7–10). Serpin is widely found in the natural
world, including in bacteria, fungi, plants and animals (11). Serpin, as a single-chain protein, is
usually composed of 350–400 amino acids, with a molecular weight
between 40–100 kDa (8). Thus far,
this superfamily has been identified in >1,000 species, and is
divided into 16 subgroups (A-P) in terms of different origin
(11). All serpins share a conserved
tertiary structure made of three β-sheets, eight or nine α-helices
and a reactive centre loop (RCL), which contains 17 amino acids and
is located between the A and C β-sheets (11). The RCL is the main action site located
in the protease recognition domain close to the C-terminus of
serpin (12) and determines the
specificity of serpin inhibitory functions (13,14).
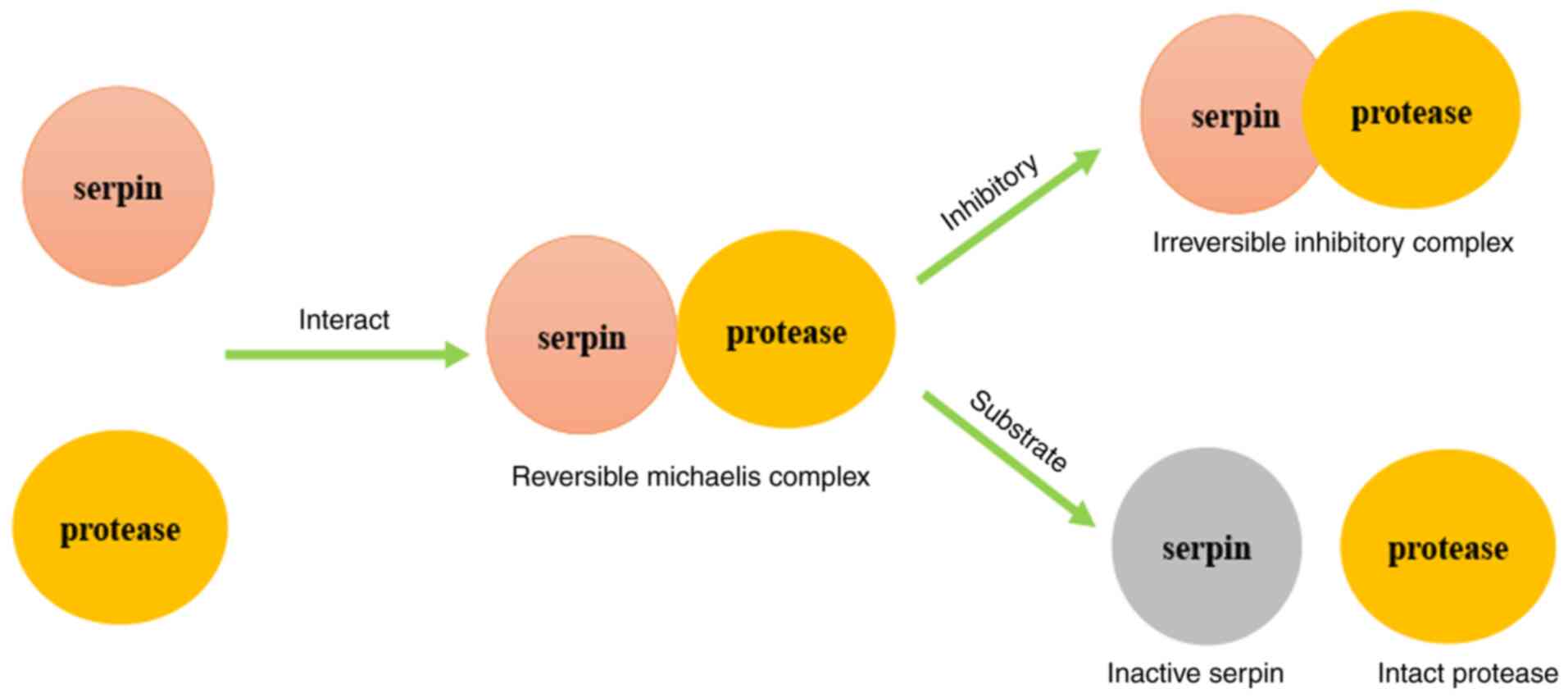
Previous research has demonstrated that there are two types of
serpin inhibitory mechanisms, termed the inhibitory pathway and the
substrate pathway (Fig. 1). At first,
serpin and protease interact and form a compound named the
reversible Michaelis complex. Subsequently, according to the
stoichiometry of inhibition (SI), which is divided by 1, the
complex undergoes different pathways. When the SI is close to 1,
the irreversible inhibitory complex is formed through inhibitory
pathway; when the SI is >1, cleaved and inactivated serpin and
intact protease remain through the substrate pathway (15). However, the pathway of serpin B3 and
serpin B4, which also leads to a decreased function of protease,
has not yet been elucidated (10,11).
An increasing number of studies have demonstrated
that serpin inhibits proteases associated with inflammation, the
complement system, blood coagulation, immunity and other processes,
in various pathophysiological processes and diseases (16–18). An
example of this is the role of serpin members in cancer. Specific
serpins are associated with the development or inhibition of
tumours, thus rendering them valuable as diagnostic or therapeutic
targets in cancer. Serpin B2, also known as plasminogen activator
inhibitor type 2 (PAI-2), is a regulator of thrombolysis in the
body (19). The inhibition of PAI-2
has been revealed to be involved in decreasing the migration,
proliferation and metastasis of cancer cells via the modulation of
the function of urokinase-type plasminogen activator receptor,
which suggests that PAI-2 may become a biomarker for cancer
diagnosis or a target for cancer treatment (20). Serpin B3, initially known as squamous
cell carcinoma antigen-1, is also a member of the serpin
superfamily (21). It has been found
that serpin B3 is involved in chronic liver injury, which is
considered as having a high risk to develop into liver cancer,
including the inhibition of liver cell apoptosis, the induction of
epithelial-to-mesenchymal transition, and increasing liver cell
proliferation and invasiveness (22).
Serpin F1 [pigment epithelium-derived factor (PEDF)] has been
revealed to be upregulated in certain tumour cells, such as breast
cancer cells (23,24). Further studies have indicated that,
compared with the normal expression of PEDF in tumours, the
decreased intra-tumoural expression of PEDF is related to a higher
microvessel density, a more metastatic phenotype and a poorer
clinical outcome of breast cancer; thus, PEDF may be a valuable
prognostic biomarker for cancer (23,24). An
increasing number of studies have thus demonstrated that serpin
plays a significant role in cancer. Therefore, it may be a
promising biomarker for early diagnosis, an important predictor of
prognosis and a potential therapeutic target. In the serpin
superfamily, serpin B9 is receiving increased attention from
scholars.
Serpin B9 structure and functions
The clade B serpins have 13 members of the B
subgroup in humans, namely serpin B1-serpin B13, which lack signal
peptides and are primarily located intracellularly (25,26).
Serpin B9 is a single-chain protein consisting of 376 amino acids
with a molecular weight of 42 kDa, which was first screened from
the human placental cDNA library and whose genes are located at
chromosome 6p25, containing seven exons and six introns (27–29).
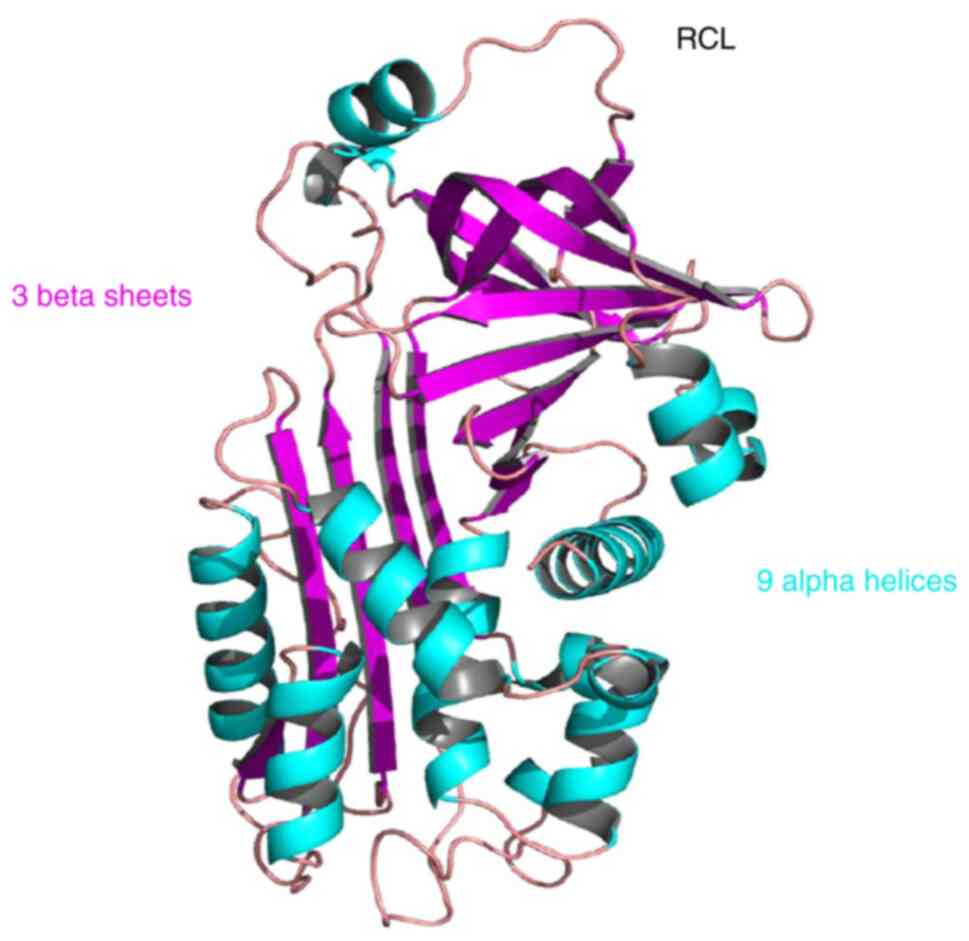
Serpin B9 has a highly conserved tertiary structure, which includes
nine α-helices, three β-sheets and an RCL at the carboxyl end
(15) (Fig.
2). The RCL of serpin B9 has a specific PI-PI′ site which
consists of a highly conserved cysteine pair, and the amino acid
residues at this site determine the specificity of protease
inhibitors (30).
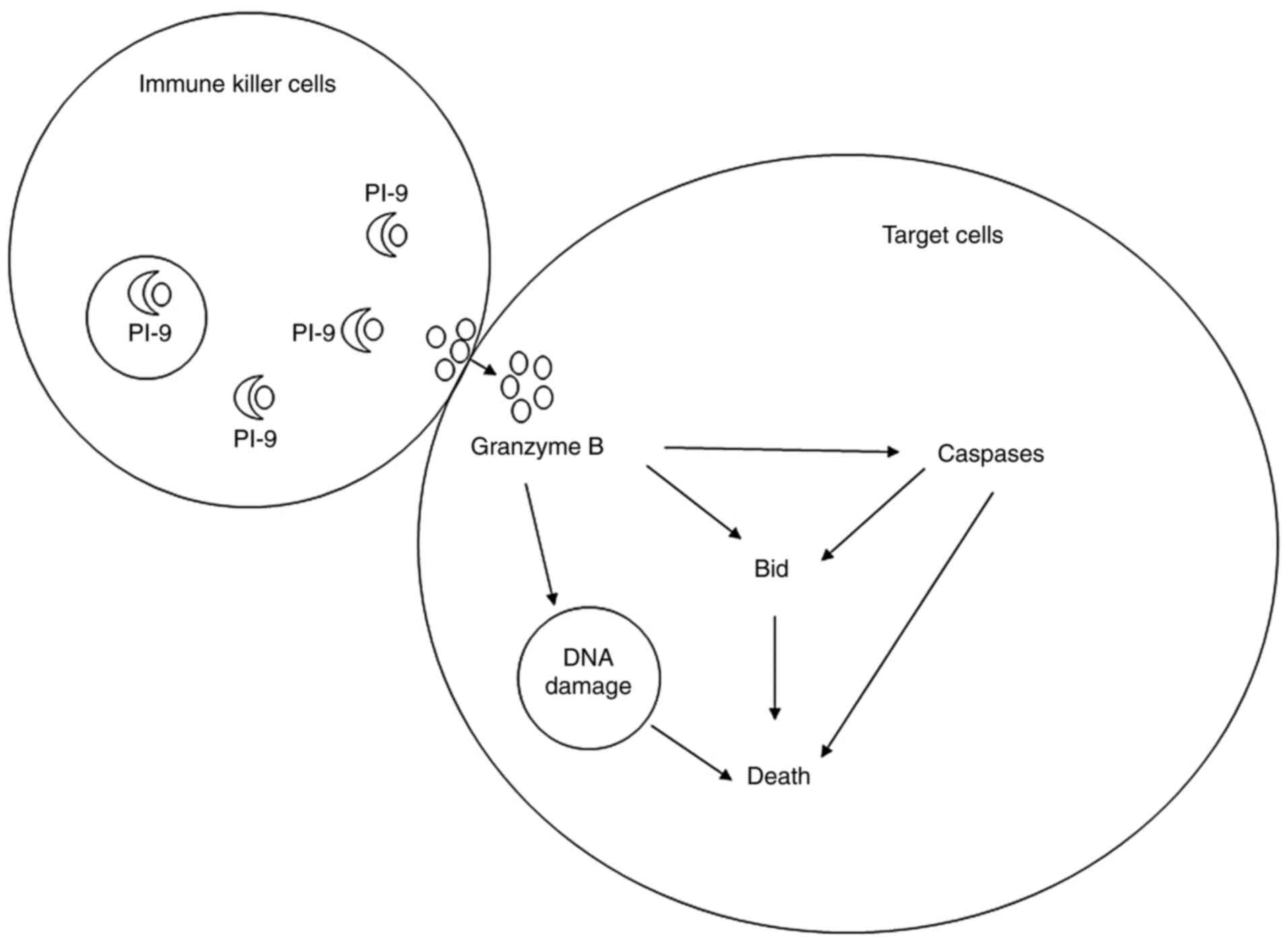
As previously described, serpin B9 plays a role in a
number of processes in the body by inhibiting GrB (6). GrB is a caspase-like serine protease
released by immune killer cells to kill target cells, such as
virus-infected cells and tumour cells (31). Serpin B9, alternatively known as
protease inhibitor 9 (PI-9), inhibits multi-pathway killing induced
by GrB and exerts a cytoprotective effect (32) (Fig. 3).
Cytotoxic T lymphocytes (CTLs) release GrB to injure other cells,
while secreted GrB may sometimes be endocytosed by CTLs or
accidently released from endosomes (33). In order to protect themselves, CTLs
have been revealed to express serpin B9, which protects them from
self-inflicted injury (28). Apart
from the protective role of serpin B9 in CTLs, it has been
demonstrated that serpin B9 exerts the same protective effect on
dendritic cells (DC), natural killer (NK) cells and memory cells
(34–36). Moreover, studies have indicated that
serpin B9 is a marker of antigen cross-presenting DCs and may be
involved in the regulation of antigen presentation (37,38).
Serpin B9 is widely expressed in various human tissues, including
the blood, heart, lung and liver, and particularly in the placenta,
testes, ovaries, eyes and other immunocompromised parts, inhibiting
immune killing and exerting a cytoprotective effect (39). Thus, it is suggested that serpin B9
principally functions by regulating the immune system.
A previous study reported that serpin B is
associated with immune and inflammatory processes in chronic
inflammatory diseases, such as atherosclerotic lesions (40). Since then, a large number of studies
have focused on the association between serpin B9 and diseases. For
example, GrB-positive T lymphocytes have been revealed to cause
graft injury by inducing the apoptosis of renal tubular cells
(41). However, a high expression of
serpin B9 in renal tubular epithelial cells serves as a protective
barrier for avoiding acute and chronic rejection (41–43).
Moreover, other researchers have demonstrated that serpin B9 is
involved in atherosclerosis and diabetes, while circulating serpin
B9 mRNA levels have been revealed to be significant and negatively
related to the severity of atherosclerotic coronary artery diseases
(44). Serpin B9 mRNA expression is
also evidently lower in patients with diabetes than in healthy
control subjects without diabetes (44). In addition, serpin B9 has been
revealed to be involved in the pathophysiological processes of
viral infections (45), autoimmune
diseases (46), pre-eclampsia
(47), cancer (48) and celiac disease (49).
Serpin B9 in cancer
In previous years, an increasing number of studies
have demonstrated that serpin B9 plays an important role in cancer
(50–52). With the increasing research on serpin,
the hypothesis that serpin B9 protects tumour cells has emerged
(53). According to various studies,
the prognostic role of serpin B9 has been described. A high level
of serpin B9 was demonstrated to indicate a poor prognosis of
patients with uveal melanoma at the genetic and molecular level
through bioinformatics methods (54).
Another study on 105 patients with hepatocellular carcinoma
demonstrated that the expression of serpin B9 in hepatocellular
carcinoma was significantly and positively associated with the
differentiation, node metastasis stage and size of the tumour
(55). Moreover, further Cox
regression analysis demonstrated that a high expression of serpin
B9 in hepatocellular carcinoma was an independent predictor of poor
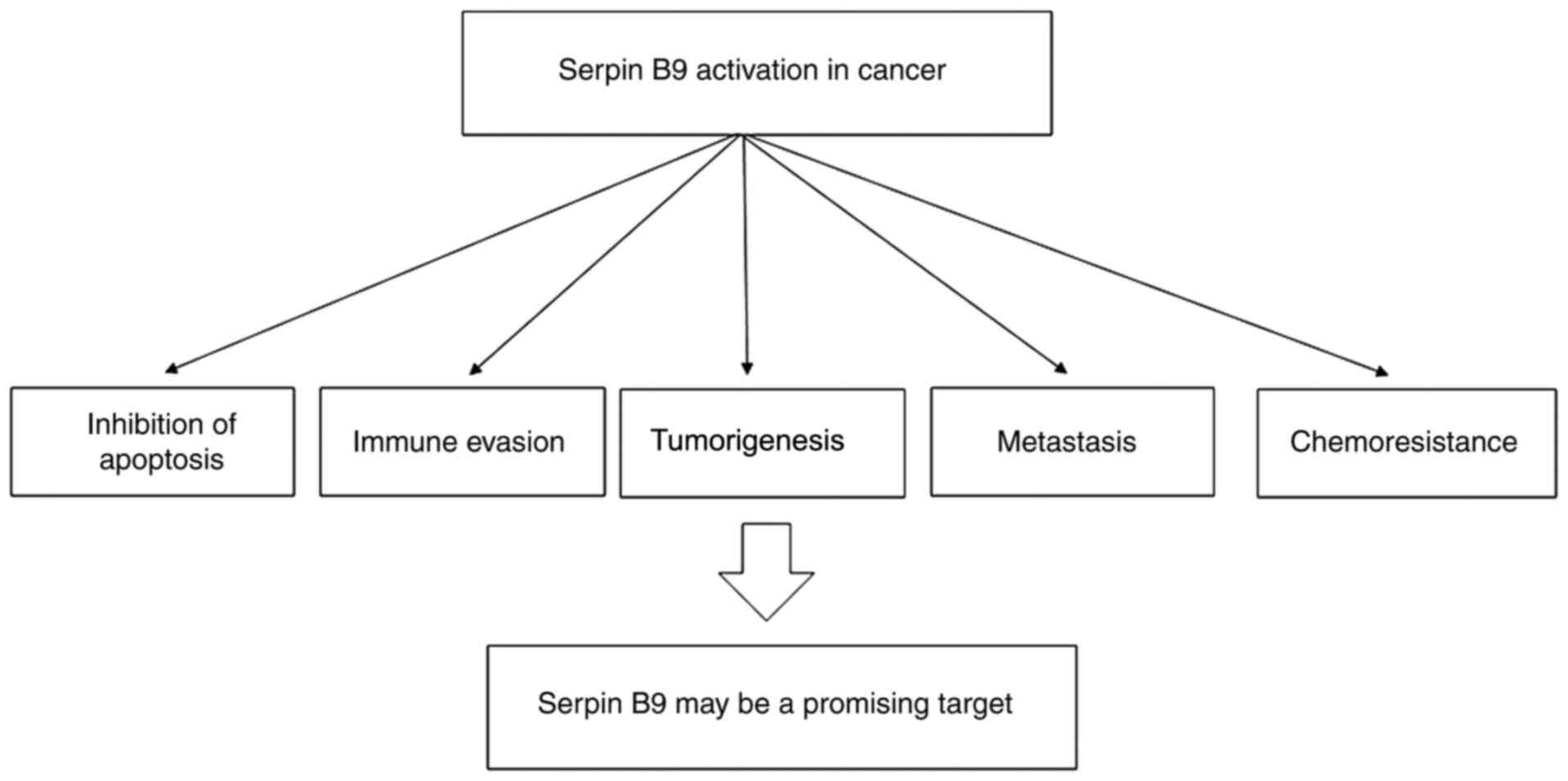
prognosis and was related to a shorter overall survival (55). Current studies have found that serpin
B9 regulates tumour apoptosis, immune evasion, tumorigenesis,
metastasis and drug resistance, and may participate in maintaining
the stemness of cancer stem cells (6,56–60); thus, it may be a potential novel
molecular target for tumour therapy (Fig.
4).
Apoptosis
The association between serpin B9 and the apoptosis
of cancer cells has been widely investigated, and varying results
have been obtained. Recent research has demonstrated that a
GrB-dependent apoptosis of lung cancer cells was observed following
the genetic ablation of serpin B9 in lung cancer cells (6). Another study focusing on lung
adenocarcinoma presented a similar outcome, in that upregulating
the expression of serpin B9 through oestrogen and cigarette side
stream smoke particulate matter was accompanied by the promotion of
resistance to GrB-mediated apoptosis (61). In patients with non-small cell lung
cancer undergoing surgery, the level of serpin B9 mRNA expression
was revealed to be upregulated in the less-differentiated lung
adenocarcinomas, and primary cells derived from surgical specimens
with a higher expression of serpin B9 tended to have lower
apoptosis (62). In addition, serpin
B9 has been shown to be expressed in ~3/4 of epithelial cancer cell
lines and in all leukemic cell lines, and has been confirmed to
protect them from apoptosis (63).
These aforementioned studies have suggested that serpin B9 inhibits
the apoptosis of cancer cells. However, studies on liver cancer
have described differential results, which should not be ignored.
In a previous study, when upregulating serpin B9 by transient
transfection in HepG2 cells, researchers found that a high
expression of serpin B9 was associated with decreased apoptosis,
which was similar to the findings of previous research (64). Another study used pcDNA3.1-serpin B9
and small interfering (si)RNA-serpin B9 to regulate its expression
and detected changes in cell proliferation and apoptosis using MTT
assay, colony formation assay and flow cytometry, respectively
(55). The results of that study
demonstrated that the apoptosis of HepG2 cells was markedly
decreased following the silencing of serpin B9 using siRNA and was
significantly increased following serpin B9 overexpression, which
indicated that serpin B9 enhanced the apoptosis of HepG2 cells
(55). This result is contradictory
to the conclusion that serpin B9 inhibited the apoptosis of cancer
cells according to aforementioned studies. However, further Cox
regression analysis revealed that serpin B9 expression was an
independent factor of poor prognosis of patients with
hepatocellular carcinoma (55). The
contradictory results in liver cancer have not yet been elucidated,
which may be related to specific tumour types. Collectively, the
majority of researchers have demonstrated that serpin B9 inhibits
the apoptosis of cancer cells; however, further studies are
required in order to clarify the role of serpin B9 in different
types of cancer.
Apoptotic mechanisms
GrB mediates the apoptosis of cancer cells through a
variety of mechanisms, and serpin B9 protects tumour cells mainly
by inhibiting GrB (65). GrB induces
the death of NK/T-cell lymphoma cells through aspartase-dependent
and non-aspartase-dependent mechanisms, which in turn leads to
extensive tumour necrosis (66).
However, the upregulation of serpin B9 has been revealed to protect
tumours by reversing this pathophysiological process (66). In certain tumor necrosis factor
(TNF)-sensitive cancer cell lines, serpin B9 has been revealed to
inhibit TNF and TNF-related apoptosis-inducing ligand- and Fas
ligand-mediated apoptosis (67).
Serpin B9 functions as regulator of pro-apoptotic apical caspases
(67). In addition to directly
inhibiting the apoptotic effect of GrB, serpin B9 has been
demonstrated to cooperate with other protease inhibitors to resist
the killing effect of GrB. A number of colon carcinoma cell lines
have been found to exhibit resistance to membranolysis, which is
dependent on serpin B9; however, the expression of an associated
serpin known as serine protease inhibitor involved in cytotoxicity
inhibition (SPI-CI) is required (68). SPI-CI is a novel immune evasion
molecule that functions in association with serpin B9 to protect
cancer cells from CTL-mediated death (68). Although the mechanisms involved in
serpin B9 are complex, scholars have reached a consensus on the
anti-apoptotic effect of serpin B9 on cancer cells.
Immune evasion
The immune system plays a dual role in cancer,
acting as a double-edged sword. On the one hand, it prevents cancer
cells from undergoing immune killing, while on the other hand, it
protects the body with the immune elimination of tumour cells
(69). The immune evasion of cancer
occurs though the normal host immune surveillance system; tumours
still occur, develop, metastasize and invade (4). Aimed at immune evasion, scholars have
explored immunotherapy, which has been applied in almost all
tumours, such as lung and liver cancer, lymphoma, melanoma and skin
cancer (70,71). As aforementioned, serpin B9
participates in the pathophysiological process of diseases by
regulating the immune system. Therefore, a number of scholars are
interested in the association between serpin B9 and tumour immune
evasion. In a previous study describing the expression of serpin B9
in vivo in human neoplastic cells, serpin B9 was revealed to
be involved in protecting neoplastic cells from immune elimination
by CTLs (72). As previously
demonstrated, serpin B9 not only protected serpin B9-positive
classical Hodgkin lymphoma cells from immune attack by GrB, but
also resisted immunotherapy, which was used to enhance the killing
effects of GrB, suppressing the immune killing effect or rendering
it invalid (56). In order to clarify
the association between serpin B9 and immune evasion, a cancer cell
line expressing serpin B9 was previously engineered and exhibited
an obvious nonexponential survival time distribution, which was
confirmed by evidence that two or more immune attacks were likely
required to kill serpin B9-expressing cells (73). A similar result was revealed in breast
cancer cell line studies. Oestrogen has been shown to induce an
increase in the expression of serpin B9 in breast cancer cells,
thus preventing immune surveillance, providing a double advantage
for breast cancer (74). In addition,
in different types of lymphoma, the overexpression of serpin B9 in
lymphoma cells has been revealed to trigger an immune disorder in
the body and to facilitate the malignant transformation of normal
cells (72). Clinical studies have
demonstrated that serpin B9 is involved in enhancing immune evasion
and this effect is associated with the tumour stage and a poor
prognosis as the level of serpin B9 increases (65,75). In
addition, there were other studies describing the association
between tumour staging and serpin B9. A previous study detected
serpin B9 expression in lung cancer cell lines; the study obtained
primary lung cancer cells from the curative lung resection of
patients with cancer and assessed the influence of conditioned
media for lung cancer cell lines on GrB expression and the
cytotoxic activity of CD8+ T-cells (65). The results of that study indicated
that an increased serpin B9 enhanced the immune evasion of cancer,
which was accompanied by the upgrading of the lung cancer stage
(65). Moreover, the strong
association of serpin B9 with a poor prognosis by promoting immune
evasion has been previously demonstrated by the survival analysis
of Hodgkin's lymphoma and anaplastic large-cell lymphoma (76). Thus, collectively, there is evidence
to indicate that serpin B9 enhances cancer immune evasion.
Tumorigenesis
The association between tumorigenesis and serpin B9
has been investigated by a number of studies. In patients
undergoing kidney transplantation, skin squamous cell carcinoma is
a severe post-operative complication (77). It has been revealed that, compared
with patients without skin squamous cell carcinoma, patients with
skin squamous cell carcinoma following transplantation exhibit a
significantly increased serpin B9 expression; in addition, a high
DNA methylation of serpin B9 circulating T cells has been detected
in patients with recurrent cutaneous squamous cell carcinoma
(57). These results indicated that a
high level of serpin B9 is a high-risk factor of skin squamous cell
carcinoma in patients undergoing kidney transplantation (57). A previous study focused on primary
malignant brain tumours in children and provided intuitive evidence
that serpin B9 is acquired in 29% of cancer during tumorigenesis
(78). Similar results have been
revealed in placental tissues, which have a low immunity. The
serpin B9 expression level in trophoblasts of placental tissues in
different gestational stages has been demonstrated to differ
significantly (79). Compared with
the placenta from early pregnancy, the levels of serpin B9 in
trophoblasts of partial and complete hydatidiform moles were
significantly higher, and the level of serpin B9 in choriocarcinoma
cell lines was the highest (79).
These results indicated that serpin B9 expression in trophoblasts
was consistent with the corresponding immune evasion, and the
upregulation of serpin B9 expression in gestational trophoblasts
may contribute to tumorigenesis. Therefore, serpin B9 may have a
potential biomarker role in precancerous lesions (80,81). In
patients with prostate cancer, serpin B9 has been identified
consistently in high-grade prostatic intraepithelial neoplasia and
atrophic lesions, which indicates that early prostatic inflammation
may trigger an increase in serpin B9 expression (80). A study on liver cells provided direct
evidence. The knockdown of serpin B9 by siRNA blocked the ability
of oestrogen, inhibiting cytolytic lymphocyte-mediated immune
surveillance to protect newly transformed cells (81). This result provided a novel mechanism
for an oestrogen-serpin B9-mediated increase in tumour incidence.
Thus, a number of studies have clarified that serpin B9 is
intensively involved in tumorigenesis.
Proliferation
At present, only a few studies have focused on the
role of serpin B9 in tumour proliferation. In cancer mouse models,
when the tumour and host are both deficient in serpin B9, the
maximal inhibition of tumour development is detected and serpin B9
inhibition exerts a suppressive effect on tumour growth (6). However, other studies have described
conflicting results. In a study on nasal NK/T-cell lymphoma, the
expression of serpin B9 was analysed through immunohistochemistry
and the overall survival time of patients was followed-up (82). Nasal NK/T-cell lymphoma is a rare
Epstein-Barr virus-related malignancy with a poor outcome (83). It has been demonstrated that the
absence of serpin B9 and a low apoptotic index are associated with
a poor outcome, indicating that a lack of serpin B9 expression in
cancer cells may be associated with certain mechanisms related to
tumour progression (82). In
addition, another study on liver cancer obtained similar results.
In HepG2 hepatoblastoma cells, cell proliferation detected using
MTT and colony formation assays was decreased following the
upregulation of serpin B9, and was markedly increased following the
siRNA interference of serpin B9, which suggested that serpin B9
inhibited the progression of liver cancer (55). These studies are contradictory and
there is no clear conclusion regarding the role of serpin B9 in the
proliferation of cancer.
Metastasis
The majority of malignant tumours cannot be
eradicated, and metastasis and recurrence are the main causes of
cancer-related mortality (84).
According to previous studies, the expression of serpin B9 in
tumours tends to promote tumour metastasis. The decreased level of
serpin B9 in liver cancer cells is associated with the reduced
liver metastasis of colorectal, lung and pancreatic cancer, which
indicates that serpin B9 expression in tumours may promote
metastasis (85). Another study on
lymph node metastasis also supported this conclusion. A total of
152 tumour samples and clinical features from 152 patients who
underwent resection for colorectal carcinoma were collected and
analysed (58). The results
demonstrated that GrB was positively related to a positive outcome
of colorectal carcinoma; conversely, the level of serpin B9
indicated lymph node metastasis and a poor prognosis (58). Of note, in the same cancer cell line,
serpin B9 appeared to be involved in metastasis to different sites.
Compared with breast cancer cell lines which specifically
metastasize to other parts, lung-specific metastatic cells have
been revealed to significantly overexpress serpin B9 (86). This result provided a new perspective
for the investigation of the association between serpin B9 and
metastasis. In addition, the findings of the following study should
be noted. Specifically, the high expression of serpin B9 in liver
NK cells and sinusoidal endothelial cells protected the liver
microenvironment from the destruction of the highly active
perforin/GrB, maintaining the liver microenvironment stability for
killing metastatic cancer cells (87). That study clarified the antitumour
effect of serpin B9 in normal tissues. Although serpin B9 in normal
tissues was not the main part of the discussion of the present
review, the complexity of serpin B9 in tumour metastasis is
suggested.
Resistance to chemotherapy
To date, chemoresistance remains the main cause of
ineffective tumour treatment (88).
Previous studies have demonstrated that serpin B9 is involved in
chemoresistance (59,89). A study on locally advanced rectal
cancer patients demonstrated that serpin B9 analysed by
immunoblotting was negatively associated with the response to
neoadjuvant chemotherapy before surgery, indicating that a high
level of serpin B9 was related to a poor chemotherapeutic effect
(59). In a study on 244 children
with acute lymphoblastic leukaemia, compared with patients without
minimal residual disease, patients with minimal residual disease
had a higher serpin B9 expression (89). The high level of serpin B9 may
indicate the chemoresistance of cancer. However, there are a few
studies which have demonstrated varying results. Previously, in
order to evaluate the role of serpin B9 in cancer, three stably
transfected HeLa cell lines expressing wild-type serpin B9 and one
cell line expressing the inactive mutant serpin B9 were constructed
(90). The results revealed that the
high level of serpin B9 in wild-type cell lines protected tumour
cells from NK cell-mediated killing; however, it did not influence
etoposide-induced apoptosis (90).
This result did not support the promoting role of serpin B9 in the
chemoresistance of cancer; thus, further more in-depth
investigations are warranted to explore the association between
serpin B9 and drug resistance.
Radiotherapy
Studies on the role of serpin B9 in tumour
radiotherapy are limited. Radiotherapy not only stimulates the
immune response to clear tumour cells, but also induces tumour drug
resistance (91). The following
studies may provide some insight on this matter. In a previous
study, after cancer cells were treated with ionizing radiation,
serpin B9 expression was upregulated through type I interferon, to
protect cancer cells from T cell-mediated cytotoxicity, which
suggested that radiotherapy protected cancer cells through serpin
B9 (92). Another study conducted on
235 male clean-up workers who were exposed to radiation during the
Chernobyl accident in 1986–1987 indicated that the expression of
serpin B9 was decreased by radiation in a dose-dependent manner,
which suggested that radiation may reduce the tumour protective
effects of serpin B9 (93). These
results were contradictory, although they indicated the
‘double-edged sword’ effect of radiation. However, to date, to the
best of our knowledge, there is no study available exploring the
association between serpin B9 and radioresistance, which may become
a future research hotspot.
Immunotherapy
Given the regulatory role of serpin B9 in the cancer
immune system, a number of studies have focused on the association
between immunotherapy and serpin B9. According to the expression of
serpin B9 in various human and murine tumours, and its role in
resistance to CTL-mediated killing with the inhibition of the
perforin/GrB pathway, Medema et al (94) identified that serpin B9 was an
important parameter determining the success of T-cell-based
immunotherapeutic modalities aimed at solid cancer. Moreover,
research on classical Hodgkin lymphoma has demonstrated similar
findings, in that the overexpression of serpin B9 was described in
the most classical Hodgkin lymphoma-derived cell lines to protect
them against the GrB attack for resisting GrB-based immunotherapies
(56). The ‘double-edged sword’
effect of the immune system in cancer is presented in the
association between serpin B9 and immunotherapies. As previously
demonstrated, in oesophageal cancer and gastric cancer cells, when
the expression level of serpin B9 is decreased, the apoptosis of
invariant NK T (iNKT) cells is increased and the effect of iNKT
cell-based immunotherapies is decreased, which suggests that serpin
B9 enhances the effects of iNKT cell immunotherapies (95). In addition, the expression of serpin
B9 in a specific site has been revealed to cooperate with another
immunotherapy for an enhancing effect. At present, genetically
modified DCs are regarded as a promising immunotherapy for the
treatment of cancer (96). Through
introducing serpin B9 expression vectors into embryonic stem (ES)
cells and subsequently inducing differentiation to DCs, the genetic
modification of ES-DCs overexpressing serpin B9 enhances the
capacity to prime antigen-specific CTL in semi-allogeneic recipient
mice to assist immunotherapy (97).
Thus, the expression of serpin B9 in different sites exerts varying
protective or killing effects on cancer, based on the
cytoprotective effect of serpin B9. For this reason, strategies
aimed at maximizing the killing effect on tumours by regulating the
level and expression sites of serpin B9 may become future study
hotpots.
Cancer stem cells (CSCs)
CSCs are a small part of immature cells in tumours,
which have a strong capacity of self-renewal, multidirectional
differentiation and infinite proliferation, and are intensively
involved in the tumorigenesis, development, metastasis and drug
resistance of cancer (98,99). Therefore, it is meaningful to study
the association between serpin B9 and CSCs. Studies have revealed
that bone mesenchymal stem cells and embryonic stem cells highly
express serpin B9, which protects them from the killing effect of
GrB released by CTLs (100,101). This indicates that serpin B9
protects normal stem cells from apoptosis; however, there are a few
studies focusing on whether serpin B9 participates in maintaining
the stemness of stem cells. Studies on serpin B9 in CSCs provide
some insight. In tertiary breast cancer cell spheres, which exhibit
high levels of stemness markers (Nanog, Oct3/4 and Sox2) and a
self-renewal capacity, high levels of C-X-C motif chemokine
receptor (CXCR)4 and phosphorylated p38 have been detected
(60). CXCR4 is a specific receptor
for C-X-C motif chemokine ligand 12 (CXCL12), which has been
identified to be involved in maintaining the stemness of CSCs and
to participate in the chemotaxis of CSCs (102). Another study demonstrated that
activating the CXCR4/phosphorylated p38 axis increased the level of
serpin B9 for interfering with the immune surveillance system,
which enhanced the proliferation and self-renewal ability of breast
cancer stem cells (60). In addition,
Hjelmeland et al (103)
demonstrated that the expression of hypoxia inducible factor
(HIF)-2α in glioma was significantly upregulated in the acidic
tumour microenvironment, and serpin B9, as a specific target gene
of HIF-2α, not only directly interacted with the target gene
promoter, but also promoted the transcription of important stem
cell factors through epigenetic modification in brain glial stem
cells. This indicated that serpin B9 plays an important role in
maintaining the stemness of CSCs, providing a novel target for
future targeted CSC therapy.
Conclusion and future perspectives
Studies have indicated that serpin B9, as a serine
protease inhibitor, mainly inhibits the immune killing effect of
GrB to protect tumour cells. According to numerous studies, the
expression of serpin B9 in tumours decreases apoptosis, enhances
immune evasion and promotes tumorigenesis, metastasis and
chemoresistance, and is even involved in maintaining the stemness
of CSCs. In addition, clinical studies have demonstrated that a
high level of serpin B9 was associated with the occurrence and a
poor prognosis of cancer, which could possibly become a predictor
of ineffective chemotherapy and immunotherapy. Collectively, serpin
B9 may be a potential novel biomarker and target for tumour
therapy.
However, a number of issues regarding serpin B9
remain to be addressed. Firstly, in terms of tumour proliferation
and radiotherapy, the results of current research are inconclusive
and even contradictory. Secondly, to the best of our knowledge,
there is no study available focusing on the association between
tumour angiogenesis and serpin B9. Thirdly, although several
studies have indicated the protective role of serpin B9 in cancer,
the exact mechanisms remain elusive due to the lack of
comprehensive research, particularly in terms of CSCs. Fourthly,
the influence of serpin B9 expression in normal tissues on tumours
remains unclear. Finally, the strategy of targeting serpin B9 to
enhance the tumour-killing effect of chemotherapy or immunotherapy
remains indistinct and unstable. Therefore, further in-depth
studies are required to clarify the association between serpin B9
and tumours.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81660493) and the
Natural Science Foundation of Jiangxi Province (grant nos.
20171BAB205053 and 20202ACBL206019).
Availability of data and materials
Not applicable.
Authors' contributions
WJW, JW and XQY developed and designed the idea and
wrote the manuscript. CC, XFX, CO, JW and WJW performed the
literature review and graphing. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng RM, Zong YN, Cao SM and Xu RH:
Current cancer situation in China: Good or bad news from the 2018
Global Cancer Statistics? Cancer Commun (Lond). 39:222019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hausman DM: What Is Cancer? Perspect Biol
Med. 62:778–784. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vinay DS, Ryan EP, Pawelec G, Talib WH,
Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, et
al: Immune evasion in cancer: Mechanistic basis and therapeutic
strategies. Semin Cancer Biol. 35 (Suppl):S185–S198. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qin A, Coffey DG, Warren EH and Ramnath N:
Mechanisms of immune evasion and current status of checkpoint
inhibitors in non-small cell lung cancer. Cancer Med. 5:2567–2578.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang L, Wang YJ, Zhao J, Uehara M, Hou Q,
Kasinath V, Ichimura T, Banouni N, Dai L, Li X, et al: Direct tumor
killing and immunotherapy through Anti-SerpinB9 therapy. Cell.
183:1219–1233.e18. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lucas A, Yaron JR, Zhang L and Ambadapadi
S: Overview of serpins and their roles in biological systems.
Methods Mol Biol. 1826:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh P and Jairajpuri MA: Structure
function analysis of serpin super-family: ‘a computational
approach’. Protein Pept Lett. 21:714–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Law RH, Zhang Q, McGowan S, Buckle AM,
Silverman GA, Wong W, Rosado CJ, Langendorf CG, Pike RN, Bird PI
and Whisstock JC: An overview of the serpin superfamily. Genome
Biol. 7:2162006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Gent D, Sharp P, Morgan K and
Kalsheker N: Serpins: Structure, function and molecular evolution.
Int J Biochem Cell Biol. 35:1536–1547. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gatto M, Iaccarino L, Ghirardello A, Bassi
N, Pontisso P, Punzi L, Shoenfeld Y and Doria A: Serpins, immunity
and autoimmunity: Old molecules, new functions. Clin Rev Allergy
Immunol. 45:267–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marijanovic EM, Fodor J, Riley BT,
Porebski BT, Costa M, Kass I, Hoke DE, McGowan S and Buckle AM:
Reactive centre loop dynamics and serpin specificity. Sci Rep.
9:38702019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olson ST and Gettins PG: Regulation of
proteases by protein inhibitors of the serpin superfamily. Prog Mol
Biol Transl Sci. 99:185–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rashid Q, Kapil C, Singh P, Kumari V and
Jairajpuri MA: Understanding the specificity of serpin-protease
complexes through interface analysis. J Biomol Struct Dyn.
33:1352–1362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaiserman D and Bird PI: Control of
granzymes by serpins. Cell Death Differ. 17:586–595. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pike RN, Bottomley SP, Irving JA, Bird PI
and Whisstock JC: Serpins: Finely balanced conformational traps.
Iubmb Life. 54:1–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huntington JA, Read RJ and Carrell RW:
Structure of a serpin-protease complex shows inhibition by
deformation. Nature. 407:923–926. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huntington JA: Serpin structure, function
and dysfunction. J Thromb Haemost. 9 (Suppl 1):S26–S34. 2011.
View Article : Google Scholar
|
|
19
|
Zheng D, Chen H, Davids J, Bryant M and
Lucas A: Serpins for diagnosis and therapy in cancer. Cardiovasc
Hematol Disord Drug Targets. 13:123–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Utaijaratrasmi P, Vaeteewoottacharn K,
Tsunematsu T, Jamjantra P, Wongkham S, Pairojkul C, Khuntikeo N,
Ishimaru N, Sirivatanauksorn Y, Pongpaibul A, et al: The
microRNA-15a-PAI-2 axis in cholangiocarcinoma-associated
fibroblasts promotes migration of cancer cells. Mol Cancer.
17:102018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gettins PG: Serpin structure, mechanism,
and function. Chem Rev. 102:4751–4804. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pontisso P: Role of SERPINB3 in
hepatocellular carcinoma. Ann Hepatol. 13:722–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hong H, Zhou T, Fang S, Jia M, Xu Z, Dai
Z, Li C, Li S, Li L, Zhang T, et al: Pigment epithelium-derived
factor (PEDF) inhibits breast cancer metastasis by down-regulating
fibronectin. Breast Cancer Res Treat. 148:61–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou D, Cheng SQ, Ji HF, Wang JS, Xu HT,
Zhang GQ and Pang D: Evaluation of protein pigment
epithelium-derived factor (PEDF) and microvessel density (MVD) as
prognostic indicators in breast cancer. J Cancer Res Clin Oncol.
136:1719–1727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Silverman GA, Whisstock JC, Askew DJ, Pak
SC, Luke CJ, Cataltepe S, Irving JA and Bird PI: Human clade B
serpins (ov-serpins) belong to a cohort of evolutionarily dispersed
intracellular proteinase inhibitor clades that protect cells from
promiscuous proteolysis. Cell Mol Life Sci. 61:301–325. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Izuhara K, Ohta S, Kanaji S, Shiraishi H
and Arima K: Recent progress in understanding the diversity of the
human ov-serpin/clade B serpin family. Cell Mol Life Sci.
65:2541–2553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eyre HJ, Sun J, Sutherland GR and Bird P:
Chromosomal mapping of the gene (PI9) encoding the intracellular
serpin proteinase inhibitor 9 to 6p25 by fluorescence in situ
hybridization. Genomics. 37:406–408. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun J, Bird CH, Sutton V, McDonald L,
Coughlin PB, De Jong TA, Trapani JA and Bird PI: A cytosolic
granzyme B inhibitor related to the viral apoptotic regulator
cytokine response modifier A is present in cytotoxic lymphocytes. J
Biol Chem. 271:27802–27809. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sprecher CA, Morgenstern KA, Mathewes S,
Dahlen JR, Schrader SK, Foster DC and Kisiel W: Molecular cloning,
expression, and partial characterization of two novel members of
the ovalbumin family of serine proteinase inhibitors. J Biol Chem.
270:29854–29861. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bird CH, Sutton VR, Sun J, Hirst CE, Novak
A, Kumar S, Trapani JA and Bird PI: Selective regulation of
apoptosis: The cytotoxic lymphocyte serpin proteinase inhibitor 9
protects against granzyme B-mediated apoptosis without perturbing
the Fas cell death pathway. Mol Cell Biol. 18:6387–6398. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Trapani JA and Sutton VR: Granzyme B:
Pro-apoptotic, antiviral and antitumor functions. Curr Opin
Immunol. 15:533–543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X,
Li Z, Traugh N, Bu X, Li B, et al: Signatures of T cell dysfunction
and exclusion predict cancer immunotherapy response. Nat Med.
24:1550–1558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang M, Park SM, Wang Y, Shah R, Liu N,
Murmann AE, Wang CR, Peter ME and Ashton-Rickardt PG: Serine
protease inhibitor 6 protects cytotoxic T cells from self-inflicted
injury by ensuring the integrity of cytotoxic granules. Immunity.
24:451–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ansari AW, Temblay JN, Alyahya SH and
Ashton-Rickardt PG: Serine protease inhibitor 6 protects iNKT cells
from self-inflicted damage. J Immunol. 185:877–883. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Andrew KA, Simkins HM, Witzel S, Perret R,
Hudson J, Hermans IF, Ritchie DS, Yang J and Ronchese F: Dendritic
cells treated with lipopolysaccharide up-regulate serine protease
inhibitor 6 and remain sensitive to killing by cytotoxic T
lymphocytes in vivo. J Immunol. 181:8356–8362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lovo E, Zhang M, Wang L and
Ashton-Rickardt PG: Serine protease inhibitor 6 is required to
protect dendritic cells from the kiss of death. J Immunol.
188:1057–1063. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mangan MS, Vega-Ramos J, Joeckel LT,
Mitchell AJ, Rizzitelli A, Roediger B, Kaiserman D, Weninger WW,
Villadangos JA and Bird PI: Serpinb9 is a marker of antigen
cross-presenting dendritic cells. Mol Immunol. 82:50–56. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Classen CF, Bird PI and Debatin KM:
Modulation of the granzyme B inhibitor proteinase inhibitor 9
(PI-9) by activation of lymphocytes and monocytes in vitro and by
Epstein-Barr virus and bacterial infection. Clin Exp Immunol.
143:534–542. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bladergroen BA, Strik MC, Bovenschen N,
van Berkum O, Scheffer GL, Meijer CJ, Hack CE and Kummer JA: The
granzyme B inhibitor, protease inhibitor 9, is mainly expressed by
dendritic cells and at immune-privileged sites. J Immunol.
166:3218–3225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Young JL, Sukhova GK, Foster D, Kisiel W,
Libby P and Schönbeck U: The serpin proteinase inhibitor 9 is an
endogenous inhibitor of interleukin 1beta-converting enzyme
(caspase-1) activity in human vascular smooth muscle cells. J Exp
Med. 191:1535–1544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rowshani AT, Florquin S, Bemelman F,
Kummer JA, Hack CE and Ten BI: Hyperexpression of the granzyme B
inhibitor PI-9 in human renal allografts: A potential mechanism for
stable renal function in patients with subclinical rejection.
Kidney Int. 66:1417–1422. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rowshani AT, Strik MC, Molenaar R, Yong
SL, Wolbink AM, Bemelman FJ, Hack CE and Ten BI: The granzyme B
inhibitor SERPINB9 (protease inhibitor 9) circulates in blood and
increases on primary cytomegalovirus infection after renal
transplantation. J Infect Dis. 192:1908–1911. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lau A, Khan K, Pavlosky A, Yin Z, Huang X,
Haig A, Liu W, Singh B, Zhang ZX and Jevnikar AM: Serine protease
inhibitor-6 inhibits granzyme B-mediated injury of renal tubular
cells and promotes renal allograft survival. Transplantation.
98:402–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sanad EF, Hamdy NM, El-Etriby AK, Sebak SA
and El-Mesallamy HO: Peripheral leucocytes and tissue gene
expression of granzyme B/perforin system and serpinB9: Impact on
inflammation and insulin resistance in coronary atherosclerosis.
Diabetes Res Clin Pract. 131:132–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Heutinck KM, Kassies J, Florquin S, Ten
BI, Hamann J and Rowshani AT: SerpinB9 expression in human renal
tubular epithelial cells is induced by triggering of the viral
dsRNA sensors TLR3, MDA5 and RIG-I. Nephrol Dial Transplant.
27:2746–2754. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
van der Burgh R, Meeldijk J, Jongeneel L,
Frenkel J, Bovenschen N, van Gijn M and Boes M: Reduced
serpinB9-mediated caspase-1 inhibition can contribute to
autoinflammatory disease. Oncotarget. 7:19265–19271. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kaartokallio T, Cervera A, Kyllönen A,
Laivuori K, Kere J and Laivuori H: Gene expression profiling of
pre-eclamptic placentae by RNA sequencing. Sci Rep. 5:141072015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ten BR, Meijer CJ, Dukers DF, Kummer JA,
Bladergroen BA, Vos W, Hack CE, Ossenkoppele GJ and Oudejans JJ:
Expression levels of apoptosis-related proteins predict clinical
outcome in anaplastic large cell lymphoma. Blood. 99:4540–4546.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pohjanen VM, Kokkonen TS, Arvonen M,
Augustin MA, Patankar M, Turunen S, Vähäsalo P and Karttunen TJ:
Decreased expression of protease inhibitor 9, a granzyme B
inhibitor, in celiac disease: A potential mechanism in enterocyte
destruction and villous atrophy. Int J Immunopathol Pharmacol.
26:897–905. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vycital O, Pitule P, Hosek P, Kriz T,
Treska V and Liska V: Expression of serpin B9 as a prognostic
factor of colorectal cancer. Anticancer Res. 39:6063–6066. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
McCorkle JR, Leonard MK, Kraner SD,
Blalock EM, Ma D, Zimmer SG and Kaetzel DM: The metastasis
suppressor NME1 regulates expression of genes linked to metastasis
and patient outcome in melanoma and breast carcinoma. Cancer
Genomics Proteomics. 11:175–194. 2014.PubMed/NCBI
|
|
52
|
Ferreri AJ, Govi S, Pileri SA and Savage
KJ: Anaplastic large cell lymphoma, ALK-negative. Crit Rev Oncol
Hematol. 85:206–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bots M, Offringa R and Medema JP: Does the
serpin PI-9 protect tumor cells? Blood. 107:4974–4975, 4975. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Luo H and Ma C: Identification of
prognostic genes in uveal melanoma microenvironment. PLoS One.
15:e2422632020. View Article : Google Scholar
|
|
55
|
Zhou B, Chen E, Chen J, Zhang J, Zhang N
and Chen Z: Overexpression of proteinase inhibitor 9 is associated
with poor prognosis in human hepatocellular carcinoma and with
proliferation and apoptosis in HepG2 cells in vitro. Int J Clin Exp
Pathol. 12:3719–3727. 2019.PubMed/NCBI
|
|
56
|
Schiffer S, Hansen HP, Hehmann-Titt G,
Huhn M, Fischer R, Barth S and Thepen T: Efficacy of an adapted
granzyme B-based anti-CD30 cytolytic fusion protein against
PI-9-positive classical Hodgkin lymphoma cells in a murine model.
Blood Cancer J. 3:e1062013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Peters FS, Peeters A, van den Bosch T,
Mooyaart AL, van de Wetering J, Betjes M, Baan CC and Boer K:
Disrupted regulation of serpinB9 in circulating T cells is
associated with an increased risk for post-transplant skin cancer.
Clin Exp Immunol. 197:341–351. 2019.PubMed/NCBI
|
|
58
|
Vycital O, Dubova M, Palek R, Hosek P,
Branzovsky J, Treska V, Daum O and Liska V: The impact of immune
interaction on the metastatic infiltration of colorectal carcinoma
to lymph nodes. Anticancer Res. 38:4159–4167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Repetto O, De Re V, De Paoli A, Belluco C,
Alessandrini L, Canzonieri V and Cannizzaro R: Identification of
protein clusters predictive of tumor response in rectal cancer
patients receiving neoadjuvant chemo-radiotherapy. Oncotarget.
8:28328–28341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lauricella M, Carlisi D, Giuliano M,
Calvaruso G, Cernigliaro C, Vento R and D'Anneo A: The analysis of
estrogen receptor-α positive breast cancer stem-like cells unveils
a high expression of the serpin proteinase inhibitor PI-9: Possible
regulatory mechanisms. Int J Oncol. 49:352–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kuo LC, Cheng LC, Lee CH, Lin CJ, Chen PY
and Li LA: Estrogen and cigarette sidestream smoke particulate
matter exhibit ERα-dependent tumor-promoting effects in lung
adenocarcinoma cells. Am J Physiol Lung Cell Mol Physiol.
313:L477–L490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Rousalova I, Krepela E, Prochazka J,
Cermak J and Benkova K: Expression of proteinase
inhibitor-9/serpinB9 in non-small cell lung carcinoma cells and
tissues. Int J Oncol. 36:275–283. 2010.PubMed/NCBI
|
|
63
|
Tanaka K, Harashima N, Niiya F, Miyagi Y,
Hida N, Ochi M, Imai N, Harada M, Itoh K and Shichijo S: Serine
proteinase inhibitor 9 can be recognized by cytotoxic T lymphocytes
of epithelial cancer patients. Jpn J Cancer Res. 93:198–208. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kannan-Thulasiraman P and Shapiro DJ:
Modulators of inflammation use nuclear factor-kappa B and activator
protein-1 sites to induce the caspase-1 and granzyme B inhibitor,
proteinase inhibitor 9. J Biol Chem. 277:41230–41239. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Soriano C, Mukaro V, Hodge G, Ahern J,
Holmes M, Jersmann H, Moffat D, Meredith D, Jurisevic C, Reynolds
PN and Hodge S: Increased proteinase inhibitor-9 (PI-9) and reduced
granzyme B in lung cancer: Mechanism for immune evasion? Lung
Cancer. 77:38–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ko YH, Park S, Jin H, Woo H, Lee H, Park C
and Kim K: Granzyme B leakage-induced apoptosis is a crucial
mechanism of cell death in nasal-type NK/T-cell lymphoma. Lab
Invest. 87:241–250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kummer JA, Micheau O, Schneider P,
Bovenschen N, Broekhuizen R, Quadir R, Strik MC, Hack CE and
Tschopp J: Ectopic expression of the serine protease inhibitor PI9
modulates death receptor-mediated apoptosis. Cell Death Differ.
14:1486–1496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bots M, Kolfschoten IG, Bres SA, Rademaker
MT, de Roo GM, Krüse M, Franken KL, Hahne M, Froelich CJ, Melief
CJ, et al: SPI-CI and SPI-6 cooperate in the protection from
effector cell-mediated cytotoxicity. Blood. 105:1153–1161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lakshmi NB, Eshvendar RK, Shantikumar S
and Ramakrishna S: Immune system: A double-edged sword in cancer.
Inflamm Res. 62:823–834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Riley RS, June CH, Langer R and Mitchell
MJ: Delivery technologies for cancer immunotherapy. Nat Rev Drug
Discov. 18:175–196. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Helmy KY, Patel SA, Nahas GR and Rameshwar
P: Cancer immunotherapy: Accomplishments to date and future
promise. Ther Deliv. 4:1307–1320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bladergroen BA, Meijer CJ, Ten BR, Hack
CE, Muris JJ, Dukers DF, Chott A, Kazama Y, Oudejans JJ, van Berkum
O and Kummer JA: Expression of the granzyme B inhibitor, protease
inhibitor 9, by tumor cells in patients with non-Hodgkin and
Hodgkin lymphoma: A novel protective mechanism for tumor cells to
circumvent the immune system? Blood. 99:232–237. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Choi PJ and Mitchison TJ: Quantitative
analysis of resistance to natural killer attacks reveals stepwise
killing kinetics. Integr Biol (Camb). 6:1153–1161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jiang X, Ellison SJ, Alarid ET and Shapiro
DJ: Interplay between the levels of estrogen and estrogen receptor
controls the level of the granzyme inhibitor, proteinase inhibitor
9 and susceptibility to immune surveillance by natural killer
cells. Oncogene. 26:4106–4114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jiang X, Patterson NM, Ling Y, Xie J,
Helferich WG and Shapiro DJ: Low concentrations of the soy
phytoestrogen genistein induce proteinase inhibitor 9 and block
killing of breast cancer cells by immune cells. Endocrinology.
149:5366–5373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Muris JJ, Meijer CJ, Cillessen SA, Vos W,
Kummer JA, Bladergroen BA, Bogman MJ, MacKenzie MA, Jiwa NM,
Siegenbeek VHL, et al: Prognostic significance of activated
cytotoxic T-lymphocytes in primary nodal diffuse large B-cell
lymphomas. Leukemia. 18:589–596. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tang CH, Sue YM, Hsieh WT, Wang YH and
Wang CC: Increased risk of cutaneous squamous cell carcinoma in
organ transplant recipients and patients on chronic dialysis: A
cancer registry-based study in taiwan. Acta Derm Venereol.
99:1275–1281. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Vermeulen JF, van Hecke W, Spliet WG,
Villacorta HJ, Fisch P, Broekhuizen R and Bovenschen N: Pediatric
primitive neuroectodermal tumors of the central nervous system
differentially express granzyme inhibitors. PLoS One.
11:e1514652016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Buzza MS, Hosking P and Bird PI: The
granzyme B inhibitor, PI-9, is differentially expressed during
placental development and up-regulated in hydatidiform moles.
Placenta. 27:62–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ray M, Hostetter DR, Loeb CR, Simko J and
Craik CS: Inhibition of Granzyme B by PI-9 protects prostate cancer
cells from apoptosis. Prostate. 72:846–855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jiang X, Orr BA, Kranz DM and Shapiro DJ:
Estrogen induction of the granzyme B inhibitor, proteinase
inhibitor 9, protects cells against apoptosis mediated by cytotoxic
T lymphocytes and natural killer cells. Endocrinology.
147:1419–1426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bossard C, Belhadj K, Reyes F,
Martin-Garcia N, Berger F, Kummer JA, Brière J, Baglin AC, Cheze S,
Bosq J, et al: Expression of the granzyme B inhibitor PI9 predicts
outcome in nasal NK/T-cell lymphoma: Results of a Western series of
48 patients treated with first-line polychemotherapy within the
Groupe d'Etude des Lymphomes de l'Adulte (GELA) trials. Blood.
109:2183–2189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tse E and Kwong YL: NK/T-cell lymphomas.
Best Pract Res Clin Haematol. 32:253–261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zeeshan R and Mutahir Z: Cancer
metastasis-tricks of the trade. Bosn J Basic Med Sci. 17:172–182.
2017.PubMed/NCBI
|
|
85
|
Milette S, Hashimoto M, Perrino S, Qi S,
Chen M, Ham B, Wang N, Istomine R, Lowy AM, Piccirillo CA and Brodt
P: Sexual dimorphism and the role of estrogen in the immune
microenvironment of liver metastases. Nat Commun. 10:57452019.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Selicharova I, Sanda M, Mladkova J, Ohri
SS, Vashishta A, Fusek M, Jiracek J and Vetvicka V: 2-DE analysis
of breast cancer cell lines 1833 and 4175 with distinct metastatic
organ-specific potentials: Comparison with parental cell line
MDA-MB-231. Oncol Rep. 19:1237–1244. 2008.PubMed/NCBI
|
|
87
|
Vermijlen D, Luo D, Froelich CJ, Medema
JP, Kummer JA, Willems E, Braet F and Wisse E: Hepatic natural
killer cells exclusively kill splenic/blood natural
killer-resistant tumor cells by the perforin/granzyme pathway. J
Leukoc Biol. 72:668–676. 2002.PubMed/NCBI
|
|
88
|
Zheng HC: The molecular mechanisms of
chemoresistance in cancers. Oncotarget. 8:59950–59964. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sullivan EM, Jeha S, Kang G, Cheng C,
Rooney B, Holladay M, Bari R, Schell S, Tuggle M, Pui CH and Leung
W: NK cell genotype and phenotype at diagnosis of acute
lymphoblastic leukemia correlate with postinduction residual
disease. Clin Cancer Res. 20:5986–5994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Cunningham TD, Jiang X and Shapiro DJ:
Expression of high levels of human proteinase inhibitor 9 blocks
both perforin/granzyme and Fas/Fas ligand-mediated cytotoxicity.
Cell Immunol. 245:32–41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Baskar R, Lee KA, Yeo R and Yeoh KW:
Cancer and radiation therapy: Current advances and future
directions. Int J Med Sci. 9:193–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen J, Cao Y, Markelc B, Kaeppler J,
Vermeer JA and Muschel RJ: Type I IFN protects cancer cells from
CD8+ T cell-mediated cytotoxicity after radiation. J
Clin Invest. 129:4224–4238. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ilienko IM, Golyarnik NA, Lyaskivska OV,
Belayev OA and Bazyka DA: Expression of biological markers induced
by ionizing radiation at the late period after exposure in a wide
range of doses. Probl Radiac Med Radiobiol. 23:331–350. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Medema JP, de Jong J, Peltenburg LT,
Verdegaal EM, Gorter A, Bres SA, Franken KL, Hahne M, Albar JP,
Melief CJ and Offringa R: Blockade of the granzyme B/perforin
pathway through overexpression of the serine protease inhibitor
PI-9/SPI-6 constitutes a mechanism for immune escape by tumors.
Proc Natl Acad Sci USA. 98:11515–11520. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Melo AM, Conroy MJ, Foley EK, Dockry É,
Breen EP, Reynolds JV, Lysaght J and Doherty DG: CD1d expression
and invariant natural killer T-cell numbers are reduced in patients
with upper gastrointestinal cancers and are further impaired by
commonly used chemotherapies. Cancer Immunol Immunother.
69:969–982. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Senju S, Hirata S, Motomura Y, Fukuma D,
Matsunaga Y, Fukushima S, Matsuyoshi H and Nishimura Y: Pluripotent
stem cells as source of dendritic cells for immune therapy. Int J
Hematol. 91:392–400. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fukuma D, Matsuyoshi H, Hirata S, Kurisaki
A, Motomura Y, Yoshitake Y, Shinohara M, Nishimura Y and Senju S:
Cancer prevention with semi-allogeneic ES cell-derived dendritic
cells. Biochem Biophys Res Commun. 335:5–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kuşoğlu A and Biray AÇ: Cancer stem cells:
A brief review of the current status. Gene. 681:80–85. 2019.
View Article : Google Scholar
|
|
99
|
Erkisa M, Karakas D and Ulukaya E: Cancer
stem cells: Root of the evil. Crit Rev Oncog. 24:69–87. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
El HN, Heathcote D, Moore R, Yang S, Azzi
J, Mfarrej B, Atkinson M, Sayegh MH, Lee JS, Ashton-Rickardt PG and
Abdi R: Mesenchymal stem cells express serine protease inhibitor to
evade the host immune response. Blood. 117:1176–1183. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Abdullah Z, Saric T, Kashkar H, Baschuk N,
Yazdanpanah B, Fleischmann BK, Hescheler J, Krönke M and Utermöhlen
O: Serpin-6 expression protects embryonic stem cells from lysis by
antigen-specific CTL. J Immunol. 178:3390–3399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lourenco S, Teixeira VH, Kalber T, Jose
RJ, Floto RA and Janes SM: Macrophage migration inhibitory
factor-CXCR4 is the dominant chemotactic axis in human mesenchymal
stem cell recruitment to tumors. J Immunol. 194:3463–3474. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hjelmeland AB, Wu Q, Heddleston JM,
Choudhary GS, MacSwords J, Lathia JD, McLendon R, Lindner D, Sloan
A and Rich JN: Acidic stress promotes a glioma stem cell phenotype.
Cell Death Differ. 18:829–840. 2011. View Article : Google Scholar : PubMed/NCBI
|