Introduction
Prostate cancer is one of the most common malignant
neoplasms in the male genitourinary system (1,2). Current
treatments for prostate cancer include surgical resection,
chemoradiotherapy, and endocrine therapy (3,4). However,
advanced metastatic prostate cancer patients treated by these
methods still have a poor prognosis and are prone to relapse.
Moreover, they tend to show resistance to radiation and
chemotherapy drugs (5–7). Therefore, studying the pathogenesis of
prostate cancer and identifying key drug targets are urgent
concerns (8,9).
MicroRNAs (miRNAs/miRs) are a class of
single-stranded noncoding RNAs 18–25 nucleotides long (10,11).
miRNAs play an important role in tumor cell proliferation,
apoptosis, metastasis, invasion, and drug resistance (12). Current preliminary studies have found
that miR-367-3p-5p plays an important role in the occurrence and
development of many types of tumors (13,14).
miR-367-3p can act as a tumor-suppressor gene and participate in
cell proliferation, migration, and apoptosis. However, the specific
mechanism of these actions is unknown.
The Ras superfamily of small GTPases includes more
than 60 different proteins. Rab is the largest member of the Ras
superfamily (15). Studies suggest
that Rab23 regulates endosomal pathways that are related to the
biogenesis of lysosomal-related organelles (15–17). Rab23
may also play a role in in vivo transport and mitochondrial
dynamics (18). Rab23 controls
mitochondrial division by interacting with the mitochondrial
fission factor Drp1 (19,20) and alters mitochondrial morphology,
thus promoting the necrosis or apoptosis of primary neurons
(21). However, the role of Rab23 in
the development of prostate cancer has rarely been reported. The
discovery of the Hedgehog/Gli signaling pathway began with the
development of Drosophila embryos. Its function involves
cell proliferation and differentiation and tissue development.
Numerous studies have shown that this pathway is associated with a
variety of tumors, including lung, breast, and prostate cancers.
Inhibition of this pathway may be a new target for tumor prevention
and treatment.
Rab23, a key tumor-related protein, is closely
related to the growth activity of prostate cells (22,23).
However, whether miR-367-3p can target the expression of the Rab23
gene and affect the proliferation, invasion, and migration of
prostate cancer cells has not yet been reported. In this study,
bioinformatics and molecular biology techniques were used to
investigate the biological functions and molecular mechanisms of
miR-367-3p in the occurrence and development of prostate cancer.
This study provides a scientific basis for the development of drugs
targeting miR-367-3p for the treatment of prostate cancer.
Materials and methods
Patient tissue collection
Prostate cancer tissues and para-cancer tissues
(>2 cm from the surgical edge) confirmed by pathology after
urologic surgery at The First Affiliated Hospital of Jinan
University from September 2018 to March 2020 were selected. All 10
patients were pathologically confirmed and were not previously
treated with chemoradiotherapy. Benign prostatic hyperplasia and
other urinary problems were ruled out. Pathological grading was
based on Gleason scoring in accordance with the World Health
Organization histopathological classification standard for prostate
cancer (24,25). Among the samples, 10 were from cases
of prostate cancer and 10 were from adjacent tissues. All tissues
were stored in a refrigerator at −80°C. The patients ranged in age
from 49 to 73 years with an average age of 62.3±8.9 years. This
study was performed at The First Affiliated Hospital of Jinan
University and approved by the Ethics Committee of The First
Affiliated Hospital of Jinan University. It was in line with the
Declaration of Helsinki. All patients provided signed informed
consent.
Cell culture
Human prostate cancer cell lines BPH-1, DU145, PC3,
TRAMP-C2, and normal prostate cell line (RWPE-1) were purchased
from the American Type Culture Collection (ATCC, Washington, DC,
America). The cells were cultured in RPMI-1640 medium containing
10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific,
Inc.) and incubated in an incubator (Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2. The cell culture was digested
with 0.25% trypsin. After counting and digestion, cells in
logarithmic growth phase were inoculated into a 6-well plate at the
density of 2×105 cells per well.
Cell transfection
The cells were transfected with miR-367-3p mimics
and mimic-NC (NC: Non-targeting), and were divided into the
miR-367-3p mimic group (miR-367-3p upregulated group,
5′-UAGCUUAUCAGACUGAUGUUGA-3′ and 5′-AACAUCAGUCUGAUAAGCUAUU-3′) and
mimic-NC control group (NC, 5′-UUCUCCGAACGUGUCACGUTT−3′ and
5′-ACGUGACACGUUCGGAGAATT−3′). Meanwhile, the cells were transfected
with miR-367-3p inhibitor and inhibitor NC, which were divided into
the miR-367-3p inhibitor group (the group with downregulated
miR-367-3p expression, 5′-UCAACAUCAGUCUGAUAAGCUA-3′) and inhibitor
NC control group (5′-GUGGAUAUUGUUGCCAUCA-3′). In addition, the
cells were also transfected with si-NC, si-Rab23, vector-NC and
vector-Rad23. Transient transfection of cells was performed
according to the instructions for Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.). After transfection, cell RNA and proteins were
extracted for real-time quantitative PCR (qPCR) and western blot
experiments.
BrdU experiment
The prostate cancer cells were inoculated into a
12-well plate. A day later, BrdU was added at a concentration of 10
µM. The experiment was performed 24 h later. The cells were washed
with PBS three times for 3 min each time and fixed with 4%
paraformaldehyde for 20 min. The cells were washed with PBS three
times for 3 min each time. HCl (2 M) was added at 37°C for 30 min.
The cells were washed three times with 0.1 mol/l boric acid
solution and then passed through a Triton X-100 permeable membrane
for 20 min. The cells were blocked with 5% BSA serum at 37°C for 30
min. BrdU primary antibody was added at the dilution of 1:1,200.
The cells were incubated at 4°C overnight, mixed with red
fluorescent secondary antibody (1:200), and incubated for 45 min.
They were stained with DAPI for 1 min. An anti-quenching agent was
added to block the plate. The cells were imaged and counted under a
fluorescence microscope (magnification, ×200, Nikon, Japan).
Wound healing assay
Vertical wounds were scratched into prostate cancer
cells by using a 100-µl pipette tip. The cell culture medium was
discarded. The wounded plate was flushed with PBS three times.
After the scratch, the cells were cultured in serum-free medium.
The cultured cells were photographed at 0 and 24 h. Image Pro PLUS
6.0 software (Media Cybernetics) was used to analyze and calculate
the cell migration distance (26).
The migration distance of each group was represented by the ratio
of the migration distance and original scratch distance (9).
Transwell experiment
Cells were selected from each group 48 h after
transfection. After trypsin digestion, Matrigel-Transwell chambers
(Millipore, USA) were inoculated with 5×104 cells per
well (27). Serum-free DMEM medium
was used for culture in the laboratory. A total of 500 µl of 10%
FBS culture medium was added to the lower chamber. After 24 h, the
upper layer without invaded cells was wiped with a cotton swab and
fixed with 4% poly(methanol) (Sigma-Aldrich; Merck KGaA) for 15
min. The invasive cells were stained with 1% crystal violet for 5
min and washed with PBS for three times. Five fields were randomly
selected under a Nikon Eclipse TE2000-U fluorescence microscope
(magnification, ×100, Nikon) to observe and count and record the
number of invasive cells.
Double luciferase reporter gene
The target gene of Rab23 for miR-367-3p was
predicted by using TagetScan (http://www.targetscan.org/vert_71/) bioinformatics
software. The Rab23 mRNA 3′-untranslated region (UTR) fragment
containing the miR-367-3p binding site and the Rab23 3′-UTR
mutation fragment mutated at the miR-367-3p binding site were
cloned into pmIR-Reporter Luciferase Vector (designed by Shanghai
Gemma Biological Co.). The recombinant plasmids were named Rab23-WT
and Rab23-MUT. DU145 cells in the logarithmic growth phase were
collected and inoculated into a 6-well plate at a density of
1×106 cells per plate. After 90% cell confluence, the
miR-367-3p mimic and recombinant plasmid were cotransfected with
Lipofectamine™ 2000 for backup in accordance with the
manufacturer's specifications (Invitrogen; Thermo Fisher
Scientific, Inc.). After cotransfection for 24 h, reporter cell
lysis buffer was added for 10 min at room temperature in accordance
with the instructions of the dual-luciferase reporter assay kit. A
total of 50 µl of firefly luciferase assay reagent was added.
Relative light units (RLUs) were detected after blending. After 10
min, 100 µl of Renilla luciferase assay reagent was added.
The RLU of the internal reference plasmid pRL-TK was measured after
blending, and relative luciferase activity was calculated.
qRT-PCR
The mirVana miRNA separation kit and the TaqMan
miRNA kit were purchased from Applied Biosystems. Reverse
transcription kits (Prime Script™ RT Reagent Kit with gDNA Eraser)
and real-time PCR kits (SYBR Premix II ExTaq™) were procured from
TaKaRa (Japan). After digestion and counting, well-grown cells were
inoculated into a 10-cm Petri dish at the density of
1×106 cells per plate and incubated at 37°C and 5%
CO2 under saturated humidity. The cells were collected
when their confluence reached 90%. The SYBR Green II fluorescent
dye method and an IQ5™ Real-Time PCR Detection System (Bio-Rad
Laboratories, Inc.) were used for data analysis. The primer
sequences were as follows: U6 F, 5′-CACTGTTCCACCCCTCAGAGC−3′ and R,
5′-GCCACTTGTCGGCGATAAGG-3′ and GAPDH F, 5′-ATATCGCTGCGCTGGTCGTC-3′
and R, 5′-AGGATGGCGTGAGGGAGAsGC-3′. The reaction conditions were
94°C (15 min), 94°C (30 sec), 60°C (30 sec), and 72°C (30 sec) for
a total of 40 cycles with a final extension at 72°C for 8 min.
miRNA results for U6 were corrected. Rab23 mRNA expression was
corrected on the basis of GAPDH expression. Relative expression was
determined through the 2−ΔΔCq method (28). Three independent replications were
conducted.
Western blot analysis
The cells were collected through centrifugation and
resuspended with RIPA (50 mmol/l Tris-HCl, pH 7.5, 150 mmol/l NaCl,
1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS). They were
ultrasonicated at 12,000 r/min and centrifuged at 4°C for 10 min.
Total protein concentration was determined in accordance with the
instructions of the BCA kit. SDS-PAGE was performed with 30 µg of
each sample. Protein was transferred to a PVDF membrane and blocked
with 5% skimmed milk at room temperature for 1 h. The samples were
incubated with the primary antibodies of Gli1 (ab217326, 1:1,000
dilution; Abcam), Gli2 (ab277800, 1:1,000, Abcam), and Rab23
(ab230200, 1:1,000, Abcam) separately. GAPDH was used as an
internal reference. The samples were kept at 4°C overnight. The
membranes were washed with TBST and incubated with secondary
horseradish peroxidase-conjugated antibody for 1 h at room
temperature. Relative protein expression after ECL development was
analyzed by using QuantityOne software (v4.6.7) (Bio-Rad
Laboratories, Inc.) after internal reference correction.
Immunohistochemical analysis
The tumor tissues were fixed with 40% formalin
(volume fraction). The tissues (approximately 2-mm in thickness)
were cut into the appropriate sizes, embedded, and microwaved with
citrate buffer (pH=6.0) for antigen repair. The tissues were
blocked with normal goat serum for 20 min and incubated overnight
with the primary antibody (Rab23, ab230200, 1:100 dilution; Abcam)
at 4°C then with the corresponding secondary antibody. The samples
were then subjected to DAB color development treatment, hematoxylin
redyeing, dehydration, and sealing.
Xenograft model
A total of 12 male SPF-grade BALB/C nude mice with
weights of 15–20 g/mouse and ages of 4–6 weeks were used in the
experiment. Six mice in each group were inoculated with PC3-miR-NC
and PC3-miR-367 prostate cancer cells under the armpit at the
injection volume of approximately 0.2 ml/piece. The animal
experiments were performed in Feb. 2019. The tumor volume was
measured with a micron caliper when the tumor became visible (100
mm3) after inoculation. The living conditions of nude
mice were observed daily. The length and width of the tumor were
measured. All nude mice were sacrificed at the end of the third
week of the experiment. Euthanasia method was as follows. The mice
were put into a euthanasia chamber without pre-filled
CO2. Next, the cylinder was opened and 100% carbon
dioxide was added. The filling rate was about 15%
CO2/min of the chamber volume. After 10 min, the nude
mice were examined for death. The surviving mice continued to be
treated with CO2 for 5 mins. When the animals were
determined to be not moving, breathing, and the pupils were
dilated. The CO2 was closed and another 2 min passed to
confirm the animal death. The exfoliated transplanted tumor was
weighed. And the maximum diameter of the tumor tissue we observed
was no more than 1.3 cm. Tumor volume (TV) was calculated in
accordance with the following formula: TV (mm3)=0.5 ×
long diameter (mm) × short diameter2 (mm2).
Experimental animal welfare followed the guidelines for Welfare
Ethics Review (GB/T 35892-2018). Animal experiments were approved
by the Ethics Committee of The First Affiliated Hospital of Jinan
University (Guangzhou, Guangdong, China) (no. IRB-JN-2019-023).
Statistical analysis
Each experiment was repeated independently three
times. SPSS 17.0 statistical software (SPSS Inc.) was used to
analyze relevant data (29). The
results are expressed as mean ± SD. Comparison between groups was
performed by the unpaired Student's t-test. One-way ANOVA followed
by Tukey's multiple comparison tests were selected for multiple
group comparisons. Pearson correlation coefficient was used to
analyze coexpression correlation. Here, P<0.05 was indicative of
a statistically significant result (30).
Results
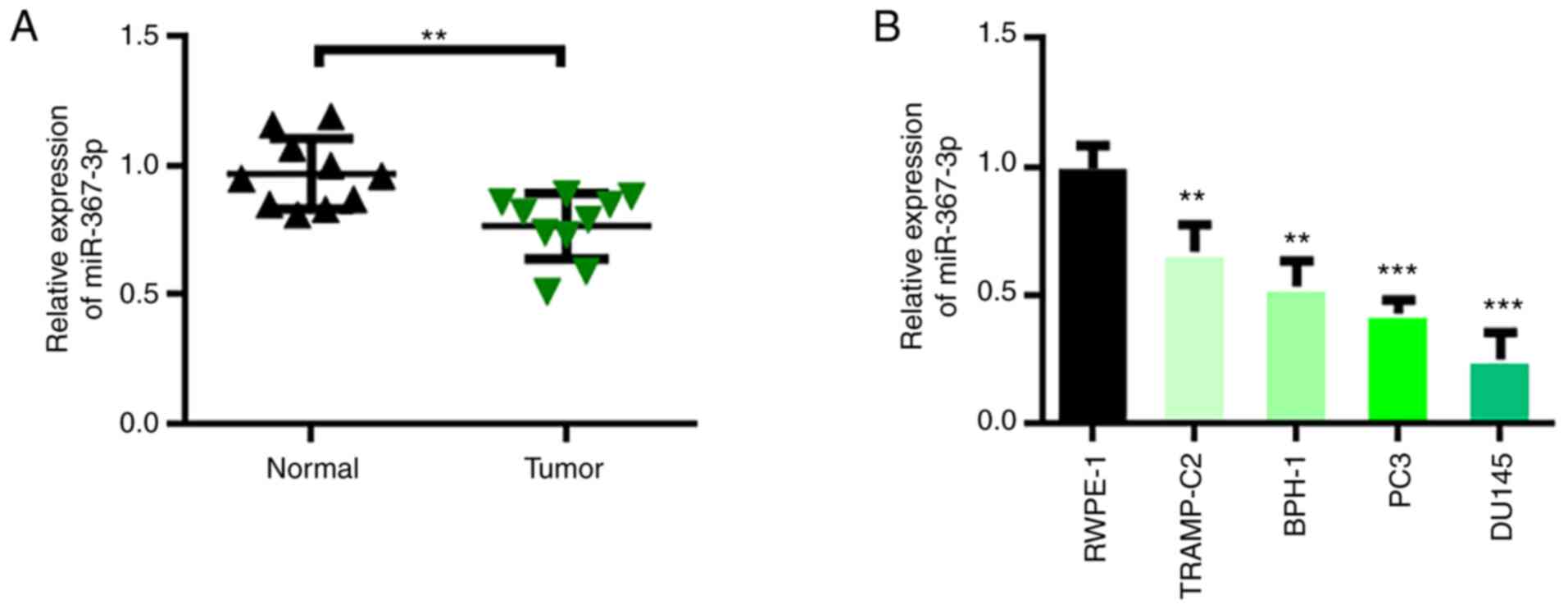
miR-367-3p is downregulated in
prostate cancer
The role of miR-367-3p in prostate cancer can be
inferred by detecting its expression level. We collected 10 pairs
of prostate cancer tumor tissues and adjacent control tissues. The
expression of miR-367-3p was detected through qRT-PCR. The
experimental results showed that miR-367-3p expression in prostate
cancer tumor tissues was downregulated relative to that in the
adjacent control tissues (Fig. 1A).
Furthermore, the expression levels of miR-367-3p in normal human
prostate epithelial cells (RWPE-1) and prostate cancer cells
(TRAMP-C2, BPH-1, PC3, and DU145) were analyzed. The experimental
results showed that compared with that in RWPE-1 cells, miR-367-3p
expression in BPH-1, DU145, PC3, and TRAMP-C2 cells was
downregulated and was the lowest in the DU145 and PC3 cells
(Fig. 1B). Therefore, the PC3 and
DU145 cells were selected for subsequent experiments.
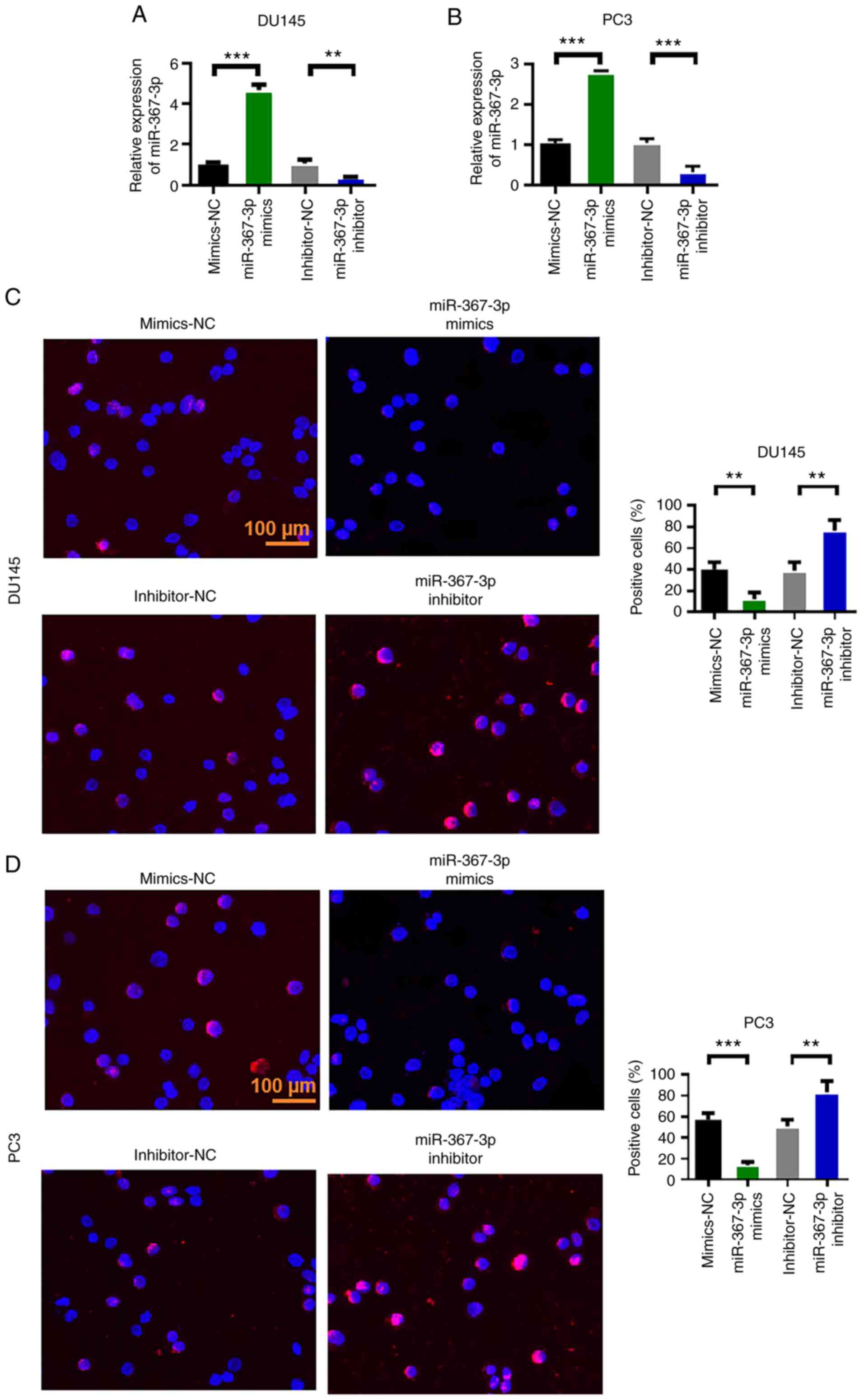
Overexpression of miR-367-3p inhibits
the malignant evolution of DU145 and PC3 in prostate cancer
After analyzing the expression level of miR-367-3p
in prostate cancer tissues and cell lines, the effects of
miR-367-3p on the malignant evolution of prostate cancer cells were
evaluated through cell proliferation, wound healing and Transwell
assays. First, we measured the expression efficiency of miR-367-3p
in DU145 and PC3 cells. The experimental results showed that
miR-367-3p mimics upregulated the expression of miR-367-3p, whereas
the miR-367-3p inhibitor inhibited the expression of miR-367-3p
(Fig. 2A and B). Subsequently, the
proliferation capability of the cells was measured via the BrdU
assay. The experimental results showed that the overexpression of
miR-367-3p inhibited the proliferation of DU145 and PC3 cells,
whereas the inhibition of miR-367-3p enhanced the proliferation of
DU145 and PC3 cells (Fig. 2C and
D).
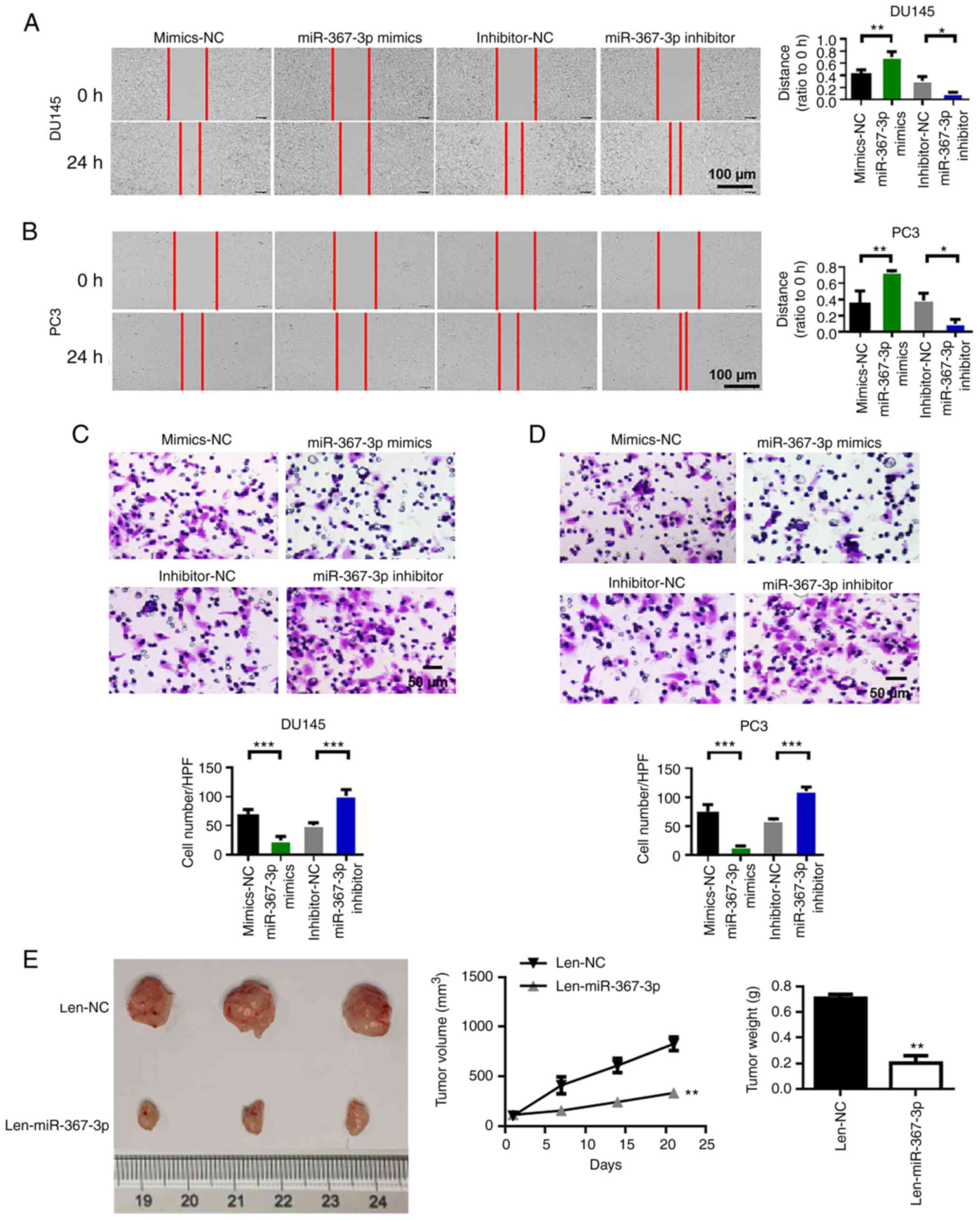
Overexpression of miR-367-3p inhibits
the migration, invasion and proliferation
We performed wound healing experiments to evaluate
the migratory capability of prostate cancer cells. The results
showed that the overexpression of miR-367-3p inhibited the
migration of DU145 and PC3 cells, whereas the inhibition of
miR-367-3p increased the migration of DU145 and PC3 cells (Fig. 3A and B). Subsequently, we performed
the invasion assay to evaluate the invasive capability of prostate
cancer cells. We found that the invasive capability of the DU145
and PC3 cells in the miR-367-3p mimic group was significantly lower
than that in the blank control group. Compared with that of the
cells in the inhibitor group, the invasion capability of DU145 and
PC3 cells in the miR-367-3p inhibitor group was enhanced (Fig. 3C and D). We performed animal
experiments to verify the antitumor effect of miR-367-3p. The
results of the animal experiments showed that miR-367-3p inhibited
tumor growth and reduced tumor weight (Fig. 3E).
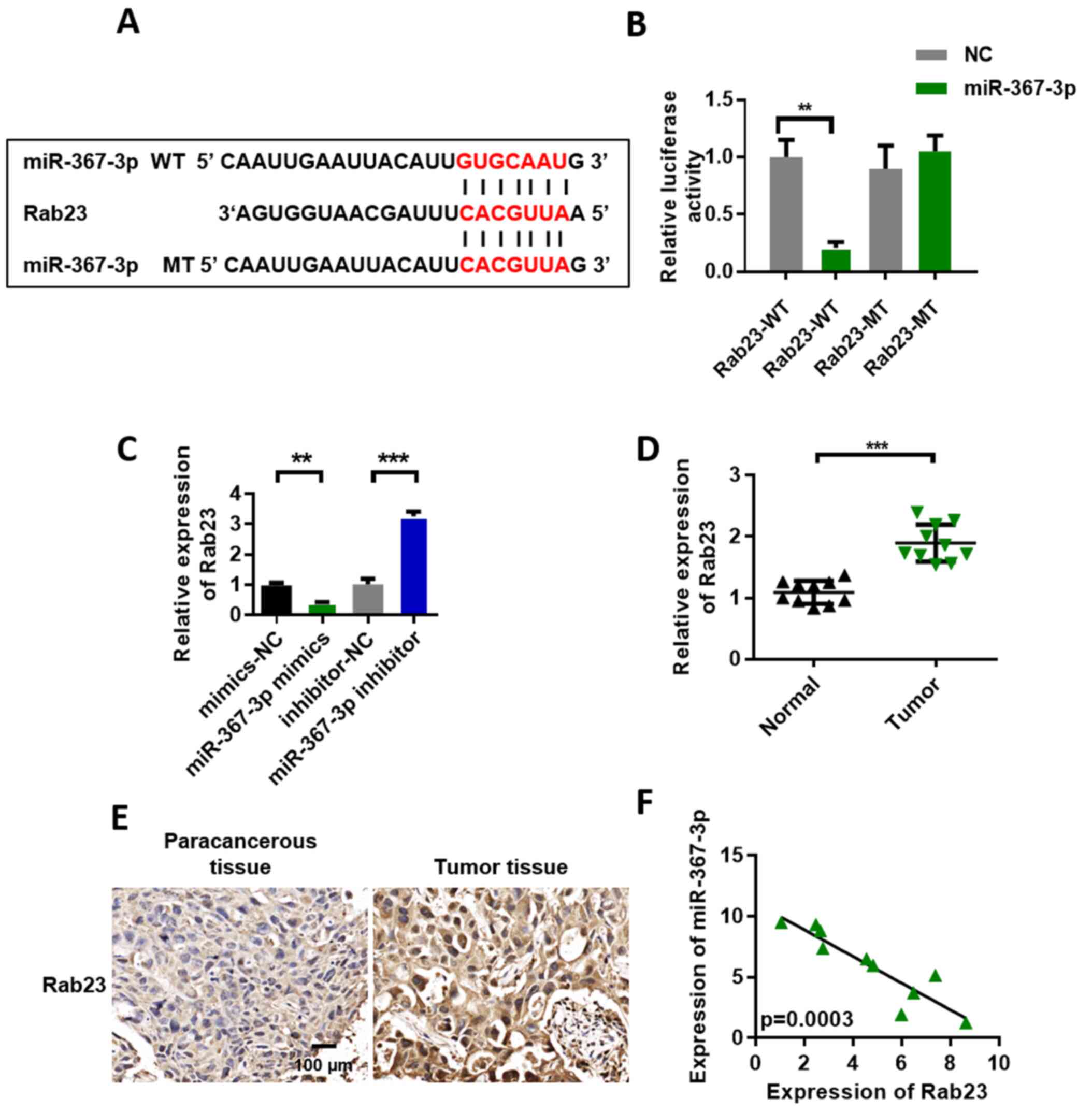
Rab23 may be a target of
miR-367-3p
We used TargetScan to predict the target genes of
miR-367-3p to investigate the function of miR-367-3p. The
prediction results showed that Rab23 may be a target gene of
miR-367-3p. Fig. 4A shows the binding
site information of Rab23 and miR-367-3p. We conducted the
luciferase reporter gene experiment to further verify our
conjecture in DU145 cell lines. The experimental results showed
that luciferase activity in the miR-367-3p mimic + Rab23-WT
(wild-type) group was lower than that in the mimic-NC + Rab23-WT
group. However, after the mutation of the Rab23 binding site, the
miR-367-3p mimics could no longer inhibit the luciferase activity
of Rab23 (Fig. 4B). The qRT-PCR
results showed that Rab23 expression in the miR-367-3p mimic group
was significantly decreased compared with that in the miR-NC group.
However, Rab23 expression was significantly upregulated in the
miR-367-3p inhibitor group (Fig. 4C).
Furthermore, we analyzed the expression level of Rab23 in prostate
cancer tissues. The experimental results showed that Rab23 was
upregulated in the prostate cancer tissues (Fig. 4D). In addition, we quantified the
expression of Rab23 in the para-cancer control and tumor tissues.
Our immunohistochemical test results showed that Rab23 was highly
expressed in the prostate cancer group relative to that in the
control group (Fig. 4E). miR-367-3p
and Rab23 coexpression levels were highly and negatively correlated
(Fig. 4F).
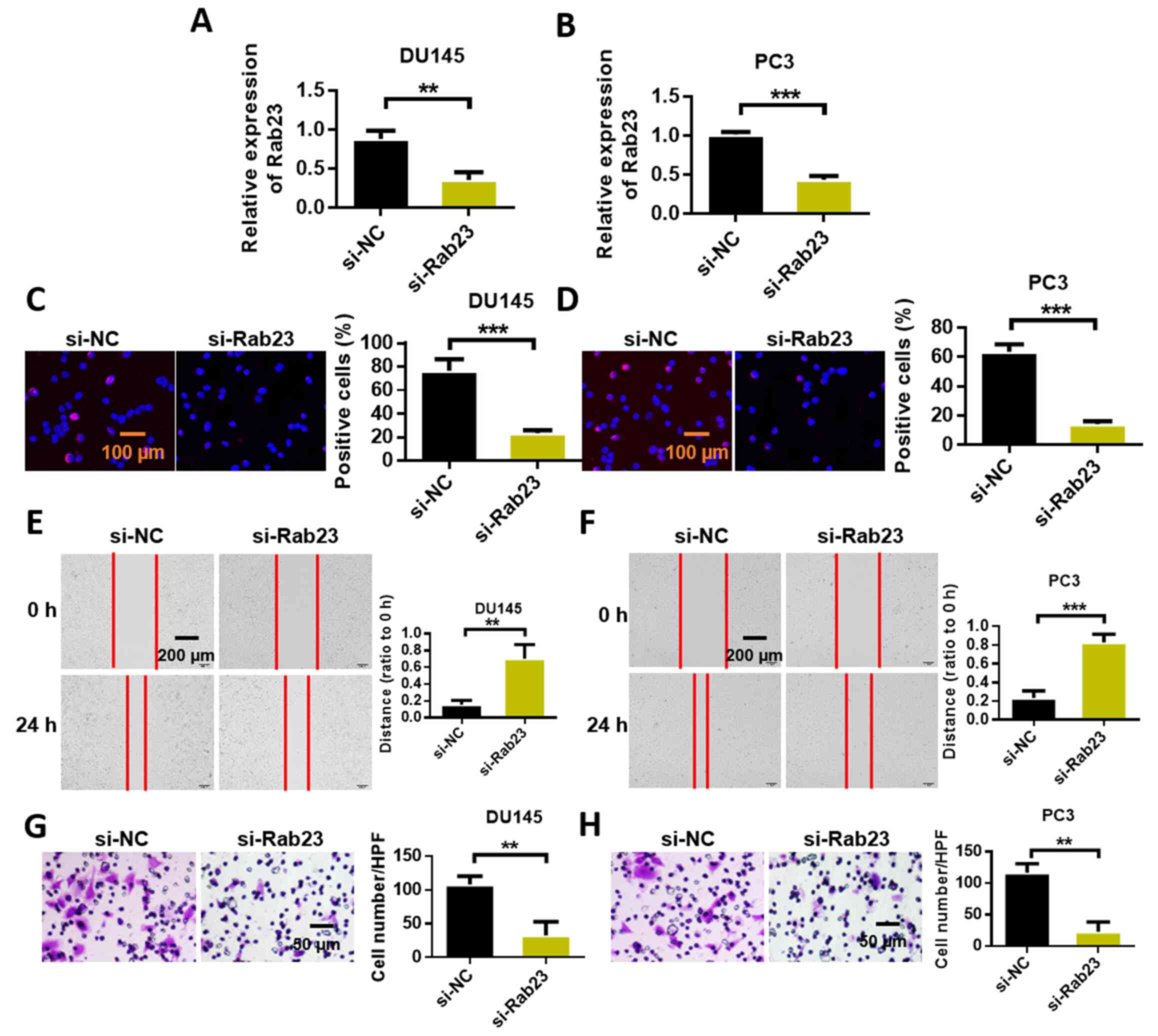
Rab23 knockdown inhibits the malignant
evolution of prostate cancer cells
We studied the effect of Rab23 on the malignant
evolution of prostate cancer cells through Transwell, wound
healing, and cell proliferation assays. First, the expression
efficiency of Rab23 in DU145 and PC3 cells was detected. The
experimental results showed that siRNA Rab23 reduced the expression
of Rab23 (Fig. 5A and B).
Subsequently, the proliferation capability of the cells was
measured via the BrdU assay. The experimental results showed that
Rab23 knockdown inhibited the proliferation of DU145 and PC3 cells
(Fig. 5C and D). We performed wound
healing experiments to evaluate the migratory capability of
prostate cancer cells. The results showed that the knockdown of
Rab23 significantly inhibited the migration of DU145 and PC3 cells
(Fig. 5E and F). Subsequently, we
conducted the invasion assay to evaluate the invasive capability of
prostate cancer cells. We found that the invasive capability of
DU145 and PC3 cells in the Rab23 knockout group was significantly
decreased compared with that in the blank control group (Fig. 5G and H).
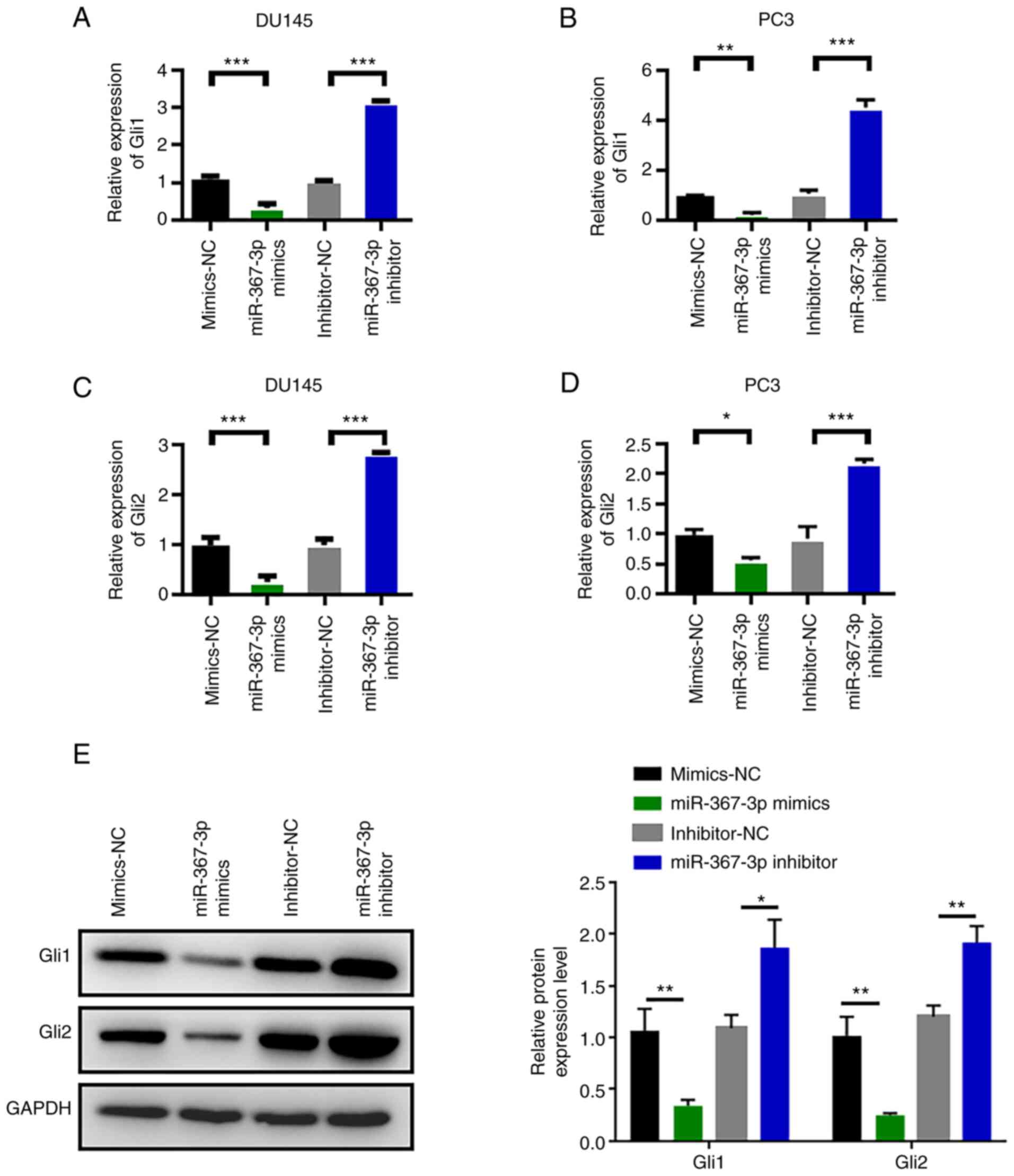
miR-367-3p downregulates Rab23
expression and inhibits the Hedgehog signaling pathway
Next, we examined the effects of miR-367-3p on the
Hedgehog signaling pathway. RT-PCR results showed that Gli1 and
Gli2 mRNAs were expressed in the prostate cancer cell lines DU145
and PC3. Expression levels were statistically analyzed in
accordance with the ratio of the absorbance value of the amplified
products. The expression of Gli1 mRNA in DU145 and PC3 cells was
significantly decreased after transfection with miR-367-3p mimics
and was statistically different than that in the normal control
group (Fig. 6A and B). After
transfection with miR-367-3p mimics, the expression of Gli2 mRNA in
DU145 and PC3 cells was also significantly decreased (Fig. 6C and D). Changes in the expression
levels of Gli1 and Gli2 were detected through western blot analysis
and are shown in Fig. 6E. The
experimental results showed that miR-367-3p mimics inhibited the
expression of Gli1 and Gli2. However, Gli1 and Gli2 were
upregulated after treatment with the miR-367-3p inhibitor. The
above experimental results indicated that miR-367-3p downregulated
Rab23 expression and inhibited the Hedgehog signaling pathway.
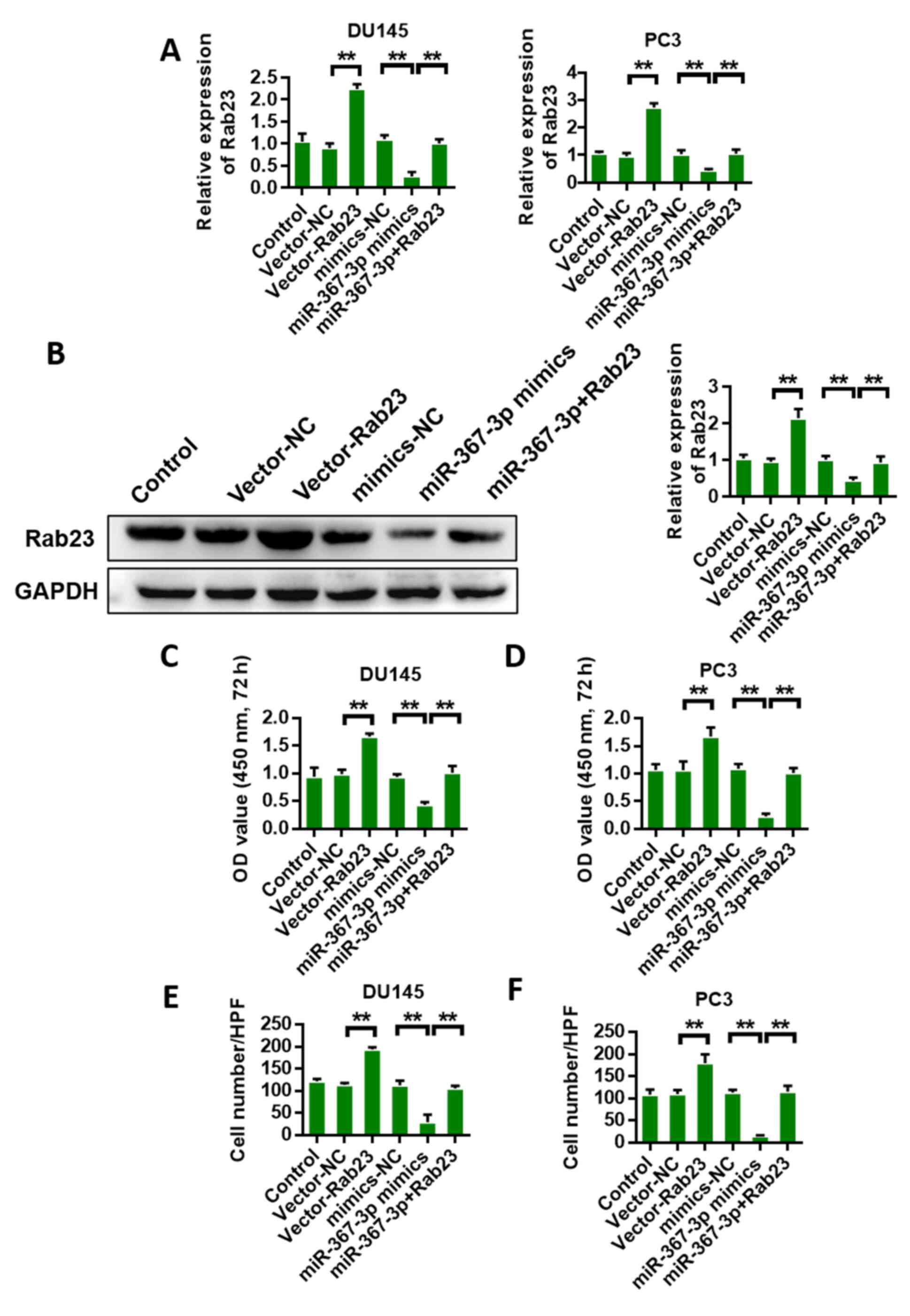
Overexpression of Rab23 reverses the
anticancer effect of miR-367-3p
Rab23 was overexpressed, and the anticancer effect
of miR-367-3p was analyzed again to validate the interaction
between Rab23 and miR-367-3p. First, we detected Rab23 expression
in DU145 and PC3 cells. The results of qRT-PCR and western blot
analyses showed that miR-367-3p mimics could inhibit the expression
of Rab23, whereas Rab23 overexpression could reverse the inhibition
of miR-367-3p (Fig. 7A and B). CCK-8
assay results of DU145 and PC3 cells showed that Rab23
overexpression could promote cell proliferation, whereas miR-367-3p
mimics could inhibit cell proliferation. The overexpression of
Rab23 reversed the miR-367-3p mimic-mediated inhibition of
proliferation (Fig. 7C and D). The
invasion detection results of DU145 and PC3 cells showed that the
overexpression of Rab23 could promote cell invasion, whereas
miR-367-3p mimics could inhibit cell invasion. Rab23 overexpression
reversed the miR-367-3p mimic-mediated inhibition and invasion of
the DU145 and PC3 cells (Fig. 7E and
F).
Discussion
MicroRNA (miRNAs/miRs) are short noncoding RNAs that
can perform post-transcriptional gene silencing by degrading target
genes and inhibiting target gene translation (31–33). The
occurrence of malignant tumors is closely related to changes in
miRNA expression profiles (34–36). A
large number of studies have found that miRNAs play an important
regulatory role in the differentiation, apoptosis, metastasis, drug
resistance, metabolism, and other biological behaviors of tumor
cells (37–40).
miRNAs are associated with the occurrence and
development of prostate cancer (41).
An in-depth understanding of miRNA regulatory pathways in prostate
cancer can improve our understanding of the pathogenesis of this
disease. miR-367 is a member of many miRNA families, and the
relationship between miR-367 and prostate cancer has been rarely
reported. The expression level of miR-367 in non-small cell lung
cancer (NSCLC) (42), renal cell
carcinoma (43) and other tumor
tissues was specifically increased, playing a role in promoting
cancer growth. In gastric cancer (44), miR-367 displays low expression and
plays a role in cancer inhibition. miR-367 was found to promote
tumor growth by inhibiting FBXW7 in NSCLC (45). These results indicate that miR-367
expression patterns and potential effects are different for
specific types of tumors.
The present study investigated the effects of
miR-367 on the proliferation, invasion and migration of prostate
cancer cells as well as the potential mechanisms, aiming to provide
a new basis for the application of miR-367 in the diagnosis and
treatment of prostate cancer. In this study, we found that the
expression of miR-367-3p was down-regulated in prostate cancer
tissues. The overexpression of miR-367-3p inhibited the
proliferation, invasion, and migration of prostate cancer cells.
Inhibition of miR-367-3p promoted the proliferation, migration, and
invasion of prostate cancer cells.
We found that Rab23 was highly expressed in prostate
cancer tissues and prostate cancer cells. Studies have shown that
Rab23 is the downstream target gene of miR-200b. miR-200b was found
to act as a tumor-suppressor gene by altering the expression of
Rab23 (46). The downstream signaling
pathway of Rab23 includes the Hedgehog (Hh) signaling pathway. In
recent years, several studies have found that the Hh signaling
pathway is closely related to the occurrence and development of
tumors. The overactivation of this pathway or the dysfunction of
key regulatory factors in this pathway may lead to the excessive or
abnormal proliferation of cells, which may eventually lead to the
occurrence of tumors (47–49). Hh signaling pathways involve many
molecules, such as Smoothened and Gli transcription factors (Gli1,
Gli2, and Gli3) (47). In this study,
we found that the expression levels of miR-367-3p and Rab23 were
negatively correlated. Meanwhile, the overexpression of miR-367-3p
significantly inhibited the protein expression of Rab23. These
results suggest that Rab23 is the target of miR-367-3p. Studies on
cell phenotypes showed that when miR-367-3p was upregulated, cell
activity was inhibited, and cell invasion and migration
capabilities were significantly reduced. At the same time, Rab23
partially reversed the inhibitory effect of miR-367-3p
overexpression on cell activity, invasion, and migration. The
results of this study suggest that miR-367-3p inhibits the
malignant phenotype of tumor cells by inhibiting Rab23. We also
found that miR-367-3p regulated the expression of Gli1 and Gli2 in
the Hh pathway through Rab23. As transcription factors, Gli
downstream genes include a variety of genes (cyclin D1 and D2,
Hes1, FoxM1, PdgfRa, Igf2, Wnts, and N-Myc) that are related to
cell proliferation and differentiation, which may be a key factor
leading to tumorigenesis via this pathway (50–55). The
gene that maintains cell growth is Bcl2. Genes that promote cell
self-renewal include Bmi1 and Nanog (56–58). VEGF
is an angiogenesis-related gene. Epithelial stromal transformation
genes include Snail1, Sip1, Elk1, and Msx2 (59–61) and
invasion genes. All these results suggest that miR-367-3p may play
an anticancer role by regulating the activation of pathways and the
expression of downstream genes.
In conclusion, the expression level of miR-367-3p
was found to be decreased in prostate cancer tumor tissues. Further
experiments confirmed that Rab23 is a target of miR-367-3p. The
overexpression of miR-367-3p inhibited the Hedgehog pathway by
inhibiting the expression of Rab23 and finally inhibiting the
growth, invasion, and migration of prostate cancer cells.
Therefore, miR-367-3p is a new target for the development of drugs
for prostate cancer treatment. This study provides a new idea for
the development of drugs targeting miR-367-3p for the treatment of
prostate cancer.
Acknowledgements
Not applicable.
Funding
Funding was provided by the Guangzhou Municipal
Science and Technology Program (no. 201804010453).
Availability of data and materials
The data used to support the findings of this study
are available from the corresponding author upon request.
Authors' contributions
PT participated in the design of this study. WD
analyzed and interpreted the data. DL carried out the study and
collected important background information. WD and JX carried out
literature search, data acquisition, and manuscript editing. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study, both for the use of human tissues and
animals, was approved by the Ethics Committee of The First
Affiliated Hospital of Jinan University (Guangzhou, Guangdong,
China) (no. IRB-JN-2019-023). All patients provided signed written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nabhan C: Sipuleucel-T immunotherapy for
castration-resistant prostate cancer. N Engl J Med. 363:1966–1968.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schröder FH, Hugosson J, Roobol MJ,
Tammela TLJ, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H,
Zappa M, et al: Screening and prostate-cancer mortality in a
randomized European study. N Engl J Med. 360:1320–1328. 2009.
View Article : Google Scholar
|
|
3
|
Tomlins SA, Rhodes DR, Perner S,
Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J,
Kuefer R, et al: Recurrent fusion of TMPRSS2 and ETS transcription
factor genes in prostate cancer. Science. 310:644–648. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tomlins SA, Rhodes DR, Perner S,
Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J,
Kuefer R, et al: Recurrent fusion of TMPRSS2 and ETS transcription
factor genes in prostate cancer. J Urol. 175:17072006. View Article : Google Scholar
|
|
5
|
Raimondi A, Sepe P, Claps M, Maccauro M,
Aliberti G, Pagani F, Apollonio G, Randon G, Peverelli G, Seregni
E, et al: Safety and activity of radium-223 in metastatic
castration-resistant prostate cancer: The experience of Istituto
Nazionale dei Tumori. Tumori. 106:406–412. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pernar CH, Ebot EM, Wilson KM and Mucci
LA: The epidemiology of prostate cancer. Cold Spring Harb Perspect
Med. 8:a0303612018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nelson WG, De Marzo AM and Isaacs WB:
Prostate cancer. N Engl J Med. 349:366–381. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xi X, Liu N, Wang Q, Chu Y, Yin Z, Ding Y
and Lu Y: ACT001, a novel PAI-1 inhibitor, exerts synergistic
effects in combination with cisplatin by inhibiting PI3K/AKT
pathway in glioma. Cell Death Dis. 10:7572019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong W, Yang W, Qin Y, Gu W, Xue Y, Tang
Y, Xu H, Wang H, Zhang C, Wang C, et al: 6-Gingerol stabilized the
p-VEGFR2/VE-cadherin/β-catenin/actin complex promotes microvessel
normalization and suppresses tumor progression. J Exp Clin Cancer
Res. 38:2852019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mahn R, Heukamp LC, Rogenhofer S, Ruecker
AV, Müller SC and Ellinger JR: Circulating microRNAs (miRNA) in
serum of patients with prostate cancer. Urology. 77:1265.e9–e16.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xi X, Chu Y, Liu N, Wang Q, Yin Z, Lu Y
and Chen Y: Joint bioinformatics analysis of underlying potential
functions of hsa-let-7b-5p and core genes in human glioma. J Transl
Med. 17:1292019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SJ, Ha JW and Zhang BT: Constructing
higher-order miRNA-mRNA interaction networks in prostate cancer via
hypergraph-based learning. BMC Syst Biol. 7:472013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu Z, Xu Y, Zhao J, Liu Q, Feng W, Fan J
and Wang P: miR-367 promotes epithelial-to-mesenchymal transition
and invasion of pancreatic ductal adenocarcinoma cells by targeting
the Smad7-TGF-β signalling pathway. Br J Cancer. 112:1367–1375.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Campayo M, Navarro A, Viñolas N, Diaz T,
Tejero R, Gimferrer JM, Molins L, Cabanas ML, Ramirez J, Monzo M
and Marrades R: Low miR-145 and high miR-367 are associated with
unfavourable prognosis in resected nonsmall cell lung cancer. Eur
Respir J. 41:1172–1178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shinde SR and Maddika S: Post
translational modifications of Rab GTPases. Small GTPases. 9:49–56.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prashar A, Schnettger L, Bernard EM and
Gutierrez MG: Rab GTPases in immunity and inflammation. Front Cell
Infect Microbiol. 7:4352017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Solano-Collado V, Rofe A and Spanò S:
Rab32 restriction of intracellular bacterial pathogens. Small
GTPases. 9:216–223. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao Y, Wilson GR, Stephenson SEM, Bozaoglu
K, Farrer MJ and Lockhart PJ: The emerging role of Rab GTPases in
the pathogenesis of Parkinson's disease. Mov Disord. 33:196–207.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ortiz-Sandoval CG, Hughes SC, Dacks JB and
Simmen T: Interaction with the effector dynamin-related protein 1
(Drp1) is an ancient function of Rab32 subfamily proteins. Cell
Logist. 4:e9863992014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rybnicek J, Samtleben S, Herrera-Cruz MS
and Simmen T: Expression of a T39N mutant Rab32 protein arrests
mitochondria movement within neurites of differentiated SH-SY5Y
cells. Small GTPases. 11:289–292. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haile Y, Deng X, Ortiz-Sandoval C, Tahbaz
N, Janowicz A, Lu JQ, Kerr BJ, Gutowski NJ, Holley JE, Eggleton P,
et al: Rab32 connects ER stress to mitochondrial defects in
multiple sclerosis. J Neuroinflammation. 14:192017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin Z, Li JW, Wang Y, Chen T, Ren N, Yang
L, Xu W, He H, Jiang Y, Chen X, et al: Abnormal miRNA-30e
expression is associated with breast cancer progression. Clin Lab.
62:121–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shibata D, Mori Y, Cai K, Zhang L, Yin J,
Elahi A, Hamelin R, Wong YF, Lo WK, Chung TK, et al: RAB32
hypermethylation and microsatellite instability in gastric and
endometrial adenocarcinomas. Int J Cancer. 119:801–806. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alexandratou E, Yova D, Gorpas D, Maragos
P, Agrogiannis G and Kavantzas N: Texture analysis of tissues in
Gleason grading of prostate cancer. Int Soc Opt Photon.
6859:6859042008.
|
|
25
|
Ozkan TA, Eruyar AT, Cebeci OO, Memik O,
Ozcan L and Kuskonmaz I: Interobserver variability in Gleason
histological grading of prostate cancer. Scand J Urol. 50:420–424.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Francisco JS, Moraes HP and Dias EP:
Evaluation of the Image-Pro Plus 4.5 software for automatic
counting of labeled nuclei by PCNA immunohistochemistry. Braz Oral
Res. 18:100–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong W, Hou H, Liu T, Su S, Xi X, Liao Y,
Xie R, Jin G, Liu X, Zhu L, et al: Cartilage oligomeric matrix
protein promotes epithelial-mesenchymal transition by interacting
with transgelin in colorectal cancer. Theranostics. 10:8790–8806.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T))method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ong MHA and Puteh F: Quantitative data
analysis: Choosing between SPSS, PLS, and AMOS in social science
research. Int Interdiscip J Sci Res. 3:14–25. 2017.
|
|
30
|
Zhong W, Sun B, Gao W, Qin Y, Zhang H,
Huai L, Tang Y, Liang Y, He L, Zhang X, et al: Salvianolic acid A
targeting the transgelin-actin complex to enhance vasoconstriction.
EBioMedicine. 37:246–258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al: Sipuleucel-T immunotherapy for castration-resistant
prostate cancer. N Engl J Med. 363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, Shu K, Yao J, et al: Is it
necessary to perform pelvic lymph node dissection in patients with
high-and very high-risk prostate cancer treated with radical
prostatectomy? -a retrospective single-center study. J Clin Urol
(in Chinese) 2018. https://xueshu.baidu.com/usercenter/paper/show?paperid=9a69f7a8534cf198bff20528e96f7190&site=xueshu_se
|
|
34
|
Zhang W, Zang J, Jing X, Sun Z, Yan W,
Yang D, Shen B and Guo F: Identification of candidate miRNA
biomarkers from miRNA regulatory network with application to
prostate cancer. J Transl Med. 12:662014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schaefer A, Jung M, Miller K, Lein M,
Kristiansen G, Erbersdobler A and Jung K: Suitable reference genes
for relative quantification of miRNA expression in prostate cancer.
Exp Mol Med. 42:749–758. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sanders I, Holdenrieder S,
Walgenbach-Brünagel G, von Ruecker A, Kristiansen G, Müller SC and
Ellinger J: Evaluation of reference genes for the analysis of serum
miRNA in patients with prostate cancer, bladder cancer and renal
cell carcinoma. Int J Urol. 19:1017–1025. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mikolajczyk SD, Catalona WJ, Evans CL,
Linton HJ, Millar LS, Marker KM, Katir K, Amirkhan A and
Rittenhouse HG: Proenzyme forms of prostate-specific antigen in
serum improve the detection of prostate cancer. Clin Chemistry.
50:1017–1025. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Messina M, Kucuk O and Lampe JW: An
overview of the health effects of isoflavones with an emphasis on
prostate cancer risk and prostate-specific antigen levels. J AOAC
Int. 89:1121–1134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tímár J: Molecular pathology of prostate
cancer. Magy Onkol. 63:5–9. 2019.(In Hu).
|
|
40
|
Xiao T, Zhong W, Zhao J, Qian B, Liu H,
Chen S, Qiao K, Lei Y, Zong S, Wang H, et al: Polyphyllin I
suppresses the formation of vasculogenic mimicry via
Twist1/VE-cadherin pathway. Cell Death Dis. 9:9062018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Majid S, Dar AA, Saini S, Shahryari V,
Arora S, Zaman MS, Chang I, Yamamura S, Tanaka Y, Chiyomaru T, et
al: miRNA-34b inhibits prostate cancer through demethylation,
active chromatin modifications, and AKT pathways. Clin Cancer Res.
19:73–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu J, Wu W, Wang J, Huang C, Wen W, Zhao
F, Xu X, Pan X, Wang W, Zhu Q and Chen L: miR-367 promotes the
proliferation and invasion of non-small cell lung cancer via
targeting FBXW7. Oncol Rep. 37:1052–1058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ding D, Zhang Y, Wen L, Fu J, Bai X, Fan
Y, Lin Y, Dai H, Li Q, Zhang Y and An R: miR-367 regulates cell
proliferation and metastasis by targeting metastasis-associated
protein 3 (MTA3) in clear-cell renal cell carcinoma. Oncotarget.
8:63084–63095. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bin Z, Dedong H, Xiangjie F, Hongwei X and
Qinghui Y: The microRNA-367 inhibits the invasion and metastasis of
gastric cancer by directly repressing Rab23. Genet Test Mol
Biomarkers. 19:69–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xiao G, Gao X, Sun X, Yang C, Zhang B, Sun
R, Huang G, Li X, Liu J, Du N, et al: miR-367 promotes tumor growth
by inhibiting FBXW7 in NSCLC. Oncol Rep. 38:1190–1198. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu Q, Tang H, Liu X, Liao Y, Li H, Zhao
Z, Yuan X and Jiang W: miR-200b as a prognostic factor targets
multiple members of RAB family in glioma. Med Oncol. 31:8592014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin EH, Kao YR, Lin CA, Kuo TY, Yang SP,
Hsu CF, Chou TY, Ho CC and Wu CW: Hedgehog pathway maintains cell
survival under stress conditions, and drives drug resistance in
lung adenocarcinoma. Oncotarget. 7:24179–24193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Harris LG, Pannell LK, Singh S, Samant RS
and Shevde LA: Increased vascularity and spontaneous metastasis of
breast cancer by hedgehog signaling mediated upregulation of cyr61.
Oncogene. 31:3370–3380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Atwood SX, Li M, Lee A, Tang JY and Oro
AE: GLI activation by atypical protein kinase C ι/λ regulates the
growth of basal cell carcinomas. Nature. 494:484–488. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dahmane N, Lee J, Robins P, Heller P and
Ruiz I Altaba A: Activation of the transcription factor Gli1 and
the Sonic hedgehog signalling pathway in skin tumours. Nature.
389:876–881. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kenney AM and Rowitch DH: Sonic hedgehog
promotes G(1) cyclin expression and sustained cell cycle
progression in mammalian neuronal precursors. Mol Cell Biol.
20:9055–9067. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mullor JL, Dahmane N, Sun T and Ruiz I
Altaba A: Wnt signals are targets and mediators of Gli function.
Curr Biol. 11:769–773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Teh MT, Wong ST, Neill GW, Ghali LR,
Philpott MP and Quinn AG: FOXM1 is a downstream target of Gli1 in
basal cell carcinomas. Cancer Res. 62:4773–4780. 2002.PubMed/NCBI
|
|
54
|
Kenney AM, Cole MD and Rowitch DH: Nmyc
upregulation by sonic hedgehog signaling promotes proliferation in
developing cerebellar granule neuron precursors. Development.
130:15–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ingram WJ, McCue KI, Tran TH, Hallahan AR
and Wainwright BJ: Sonic Hedgehog regulates Hes1 through a novel
mechanism that is independent of canonical Notch pathway
signalling. Oncogene. 27:1489–1500. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Leung C, Lingbeek M, Shakhova O, Liu J,
Tanger E, Saremaslani P, Van Lohuizen M and Marino S: Bmi1 is
essential for cerebellar development and is overexpressed in human
medulloblastomas. Nature. 428:337–341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Clement V, Sanchez P, de Tribolet N,
Radovanovic I and Ruiz I Altaba A: HEDGEHOG-GLI1 signaling
regulates human glioma growth, cancer stem cell self-renewal, and
tumorigenicity. Curr Biol. 17:165–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Stecca B and Ruiz i Altaba A: A GLI1-p53
inhibitory loop controls neural stem cell and tumour cell numbers.
EMBO J. 28:663–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li Y, Zhang H, Litingtung Y and Chiang C:
Cholesterol modification restricts the spread of Shh gradient in
the limb bud. Proc Natl Acad Sci USA. 103:6548–6553. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ohta H, Aoyagi K, Fukaya M, Danjoh I, Ohta
A, Isohata N, Saeki N, Taniguchi H, Sakamoto H, Shimoda T, et al:
Cross talk between hedgehog and epithelial-mesenchymal transition
pathways in gastric pit cells and in diffuse-type gastric cancers.
Br J Cancer. 100:389–398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Varnat F, Duquet A, Malerba M, Zbinden M,
Mas C, Gervaz P and Ruiz i Altaba A: Human colon cancer epithelial
cells harbour active HEDGEHOG-GLI signalling that is essential for
tumour growth, recurrence, metastasis and stem cell survival and
expansion. EMBO Mol Med. 1:338–351. 2009. View Article : Google Scholar : PubMed/NCBI
|