Pancreatic cancer (PC) is the seventh leading cause
of cancer-associated mortality worldwide (1) and it is predicted to become the second
leading cause of cancer-related death in Western countries in the
next decades (2). An up-to-date
report estimated 57,600 new cases of PC in 2020. Among all cancer
types, the 5-year survival rate is the lowest for all stages of
cancers of the pancreas combined (9%) (3). The 5-year overall survival (OS) for
metastatic PC is even poorer, namely as low as 2% (4,5), with a
median survival expectancy of <1 year with current treatments
(2). PC is hyperaggressive and
evolves from non-invasive precursor lesions. Therefore, only minor
symptoms may be noticed in the early stage. The lack of specific
risk factors makes early detection a formidable challenge. The
tumor grows along with genetic and epigenetic alterations. Delays
in diagnosis lead to poor prognosis. Furthermore, no consensus has
been reached regarding the optimal therapy. Focusing on early
detection of metastatic PC at least prolongs the survival, improves
life quality and reduces treatment-associated toxicities.
Metastatic PC is defined as stage IV cancer and it
is not possible to completely remove it by surgery. Recent research
has shifted the focus on prevention and early detection. However,
effective and feasible screening strategies may provide accurate
identification of at-risk individuals. Extensive studies have
managed to determine signaling pathways, biomarkers or their
combinations that may be accurate in detecting PC or predicting
tumor metastasis (4,5). The intricate communication among these
elements and the low prevalence of PC make such attempts
challenging.
At diagnosis, it is common to detect that PC has
already metastasized to a certain extent, so that curative surgery
is impossible. Tumor cell migration has been a long-standing
obstacle for disease management. At first, the tumor spreads
confined to the pancreas. Subsequently, it spreads to adjacent
organs, blood vessels that surround the pancreas or to other parts,
but still within the abdomen. Much more aggressive types spread to
distant organs such as the liver, lungs or bones (6). While it is rarely observed, it may also
spread to the brain (7). Tumor cells
travel to other body regions through the blood or lymphatic system.
Therefore, the early identification of signs of such migration is
paramount to the treatment course and improve OS outcomes. Accurate
staging of PC is essential for the course of therapy. The diagnosis
of PC is made based on pathological results that are combined with
imaging. Commonly used imaging modalities for tumor assessment
include computed tomography (8),
magnetic resonance imaging (MRI), endoscopic ultrasound (EUS),
positron emission tomography (PET) and magnetic resonance
cholangiopancreatography. Furthermore, advanced techniques such as
cinematic rendering and radiomics may be applied. Among all of
those imaging techniques, CT and MRI are first-line diagnostic
modalities for suspected PC. Both of them are capable of evaluating
resectability. While most cases of PC have metastasis at the
time-point of diagnosis, early detection of PC metastases via
imaging features is still feasible.
The use of CT has undergone a revolution. A large
number of studies have applied different combinations of CT to
determine whether this improves the accuracy and sensitivity.
PET/CT is an advanced technique. It provides 3-dimensional imaging
based on the detection of radiation from the emission of positrons.
PET/CT helps with observing molecular activities and is thus
promising in the early detection of cancers and prediction of
treatment response. It is inarguably of prognostic value in
patients with PC, regardless of metastases (21–23). A
study evaluated the application of PET/CT for presurgical tumor
staging of PC and concluded that its utility is rather limited
(24). Hu et al (25) examined 19 patients with metastatic PC
and determined that 18FDG-PET/CT is useful in the early
detection of metastases. A meta-analysis evaluated the sensitivity
and accuracy of CT, 18FDG-PET and
18FDG-PET/CT, respectively, analyzing 11 eligible
articles and 5 types of cancers (head and neck cancer, lung cancer,
melanoma, sarcomas and colorectal cancer). The study determined
that the integration of CT with PET performed best in assessing
distant metastases (26). However, PC
was not included in this analysis, possibly due to its low
prevalence. It may be possible to perform another meta-analysis for
PC metastases with the accumulation of cases in the near future.
Furthermore, a slightly modified version of PET/CT, namely
PET/contrast-enhanced (CE)-CT, was reported to have a diagnostic
accuracy rate of 80% in the evaluation of staging in resectable PC,
and surprisingly, it was as high as 94% for distant metastasis of
PC, while it was low for lymph node (LN) metastasis (only 42%)
(27).
Multidetector CT is the most widely available and
validated imaging modality for staging and diagnosis of PC
(28). Thin-section arterial and
venous phase imaging allows evaluation of distant metastases
(17,29) but requires a timely re-examination
within a short period of time (30).
MRI, on the other hand, provided additional
information on the stage or presence of small liver metastases that
may otherwise be missed on CT. It is used when CT imaging lacks
clarity or when CT is not applicable for patients. MRI has a
slightly higher sensitivity compared with CT (83–94 vs. 76–96%,
respectively) (31–33). The reported accuracy in determining
tumor resectability ranges from 73 to 87% for CT and from 70 to 80%
for MRI (33). In terms of pancreatic
surveillance for individuals at high risk, MRI is preferred due to
its higher accuracy in detecting sub-centimeter pancreatic cysts
and low ionizing radiation (34–36).
Machine learning, not surprisingly, paves a road for
the automatic extraction of imaging features to classify and
discriminate. Unsupervised training to ‘read’ scans effectively
identifies foci that may otherwise be easily ignored or wrongly
interpreted by radiologists. Automatic identification of the
pancreas achieved high accuracy, with a reported Dice-Sørensen
coefficient ranging from 71 to 82% with CT data (41,42), and
up to 83% with MR data (43,44).
Apart from all of these merits mentioned above, a
standardized reporting template for radiologists is required.
Previously, the Society of Abdominal Radiology and the American
Pancreatic Association adopted one template, emphasizing the
importance of a complete, accurate and reproducible radiology
report together with high-quality imaging. A repeat workup with
tailored pancreas protocol multidetector CT angiography is
beneficial for ruling out the possibility of a tumor (45). Whether early detection may achieve
high accuracy and sensitivity through machine learning warrants
evaluation of mass data and accurate algorithms. Furthermore, the
smart utilization of imaging modalities to screen at-risk
individuals should be promoted. A reported 3 and 20% of CT and MRI
scans identified pancreatic cysts that are likely to develop into
PC (46,47), emphasizing the importance of a
thorough interpretation of scanning images during screening.
Tissue biopsy is the gold standard for the diagnosis
of primary or metastatic diseases, but it is invasive. Liquid
biopsy, a technique that has been used in lung cancer and breast
cancer, is of high diagnostic value. Its clinical diagnostic value
has now also been demonstrated for PC, including high-risk cohort
surveillance, disease staging and longitudinal monitoring of tumor
evolution and progression in response to treatment, as well as
analyses to provide genomic and molecular information on potential
pancreatic ductual adenocarcinoma (PDAC) (48). Liquid biopsies are minimally invasive
and have an improved ability to represent tumor heterogeneity and
nonsolid biological tissue, including circulating tumor cells
(CTCs), primarily circulating tumor DNA (ctDNA) and exosomes
secreted by cancer or normal cells, from all tumor sources,
including metastatic sites (49).
CTCs are cells derived from primary and secondary
tumors that may enter the vascular system at an early stage and
seed to distant organs (50). The
content is determined by enrichment and a combination of multiple
detection methods (51). CTC has an
important role in the transfer cascade reaction, but its
limitations are low sensitivity, rarity and high heterogeneity
(49). The number of CTCs may vary
over time and space, with blood passing through the portal vein to
the liver immediately after leaving the pancreas and large CTCs and
clusters may become trapped. Furthermore, the blood flow in
pancreatic malignancies is 60% less than that in normal pancreatic
tissue, so that peripheral blood CTCs may not be the best choice
for diagnosis and prognosis of PC, but portal vein samples may be
more representative of the CTC population (48). CTC detection tends to increase with
tumor staging and is useful for the diagnosis of PC, but does not
provide any relevant prognostic information (51).
ctDNA, also known as prenatal cell-free DNA, is the
DNA released into the plasma by CTCs, the primary tumor or
secondary tumor depositing necrotic or apoptotic cells as a result
of cell death, but it is difficult to obtain effectively and is
able to reflect the tumor load of patients with solid pancreatic
tumor (49–51). The specificity of ctDNA was reported
to be much higher than that of CTC and its sensitivity was slightly
lower than that of CTC (49). CtDNA
detection is based on KRAS mutations, but KRAS mutations are
present not only in PDAC, but also in various other types of
malignancies and even in chronic pancreatitis (CP) (50). Therefore, its relatively low
specificity should be considered. The detection of ctDNA requires
ultra-sensitive techniques and a large amount of plasma (12). At present, there is no reliable
clinical evidence for its role in detecting early cancer.
Exosomes are small vesicles released from the plasma
membrane by almost all cells, including cancer cells (50). Exosomes provide substrates for
molecular profiling of circulating nucleic acids (such as exosomal
DNA and exosomal RNA) and may also transfer a variety of
biologically active molecules [such as proteins, lipids and
pathogenic microRNAs (miRNAs/miRs) or mRNAs] from donor cells to
recipient cells (48). Pancreatic
cells have a strong exocrine function, leading to a high content of
exosomes in peripheral blood with easy detection and high
sensitivity (49). Since it enters
the circulation at an early stage of cancer development, it may be
used as a biomarker for early disease detection and tumor
surveillance. Ariston Gabriel et al (52) illustrated that exosomes act as a
carrier of miRNAs and other markers, including miR-196a, miR-1246,
miR-191, miR-21, miR-451a, miR-16a and miR-196a, carbohydrate
antigen 19-9 (CA19-9), miR-483-3p, miR-1246, miR-4644, miR-4525,
miR-451a, miR-21, miR-155, miR196a, miR-1246, miR-4644, miR-3976,
miR-4306, CD44v6, Tspan8, EpCAM, MET, CD104, exmiR-21, miR-17-5p,
miR-10b, miR-550, miR-10b, miR-21, miR-30c and miR-181a, as well as
low miR-let7a, which may be employed as diagnostic markers for
PC.
The role of liquid biopsy in the early diagnosis of
PDAC is theoretically promising as a standard of care for early
diagnosis, molecular stratification, prognosis and predictive
utility of PC, and for longitudinal monitoring of the effect of
treatment of established disease (48). However, the data available so far
appear contradictory and the true role of certain factors remains
to be elucidated. One of the major limitations is the lack of
standardized testing methods (50).
Current technologies are frequently time-consuming, inherently
limited in terms of processing and analysis, labor-intensive and
potentially costly (48). Therefore,
large-scale validation studies are required prior to clinical
application (50).
ATAC sequencing (ATAC-seq) uses an overactive TN5
transposon to assess DNA accessibility, simultaneously cleaves DNA
and inserts sequencing splices, preferentially in open chromatin
regions. DNA sequencing libraries rich in DNA super-accessible
regions are being generated and subjected to high-throughput
sequencing. The readings are then aligned with the assembled genome
to identify areas marked by high-density aligned reads. ATAC-seq,
similar to other methods of chromatin accessibility analysis,
provides a static assessment of the chromatin structure and reveals
local and super-accessible regions. This method has proved to be
valuable for high-throughput identification of active cis
regulatory elements in a variety of cell types.
To simplify the assessment of chromatin
accessibility signatures to the point of clinical utility, Dhara
et al (53) developed a
microarray approach termed ‘ATAC-array’, where the accessible
regions from the differential chromatin accessibility signatures
were arrayed on glass slides and then hybridized with
fluorescent-labeled ATAC libraries. Applying this method to the
original ATAC-seq library and the patient-derived organ-like
independent library, they determined the characteristics of
chromatin accessibility and transcription factors (TF), such as
ZKSCAN1 and HNF1b, which are significantly related to the prognosis
of PDAC, providing a novel chromatin-based prognostic paradigm for
accurate oncological practice. The ATAC-array technique may be
combined with nuclear localization of HNF1B by
immunohistochemistry, which provides a simple and achievable
prediction of the beneficial and detrimental epigenetic status of
the disease for clinical work. However, whether poor patterns of
chromatin accessibility contribute to the selection of patients
with PDAC for epigenetic ‘reprogramming’ therapy remains to be
determined.
A deep understanding of the mechanisms of tumor
metastases is paramount for disease management. Over the years,
scientific researchers have made efforts to discover signaling
pathways that may explain the pattern of metastasis during tumor
progression. The goal itself is laudable but difficult to achieve
and progress is slow. In the present review, it is not possible to
cover all of the known signaling pathways. Instead, it was decided
to focus on epithelial-mesenchymal transition (EMT), a mechanism
that has received extensive attention from researchers, in addition
to TANK binding kinase-1 (TBK1)-nuclear factor κB (NF-κB), an
emerging signaling pathway that may be a potential therapeutic
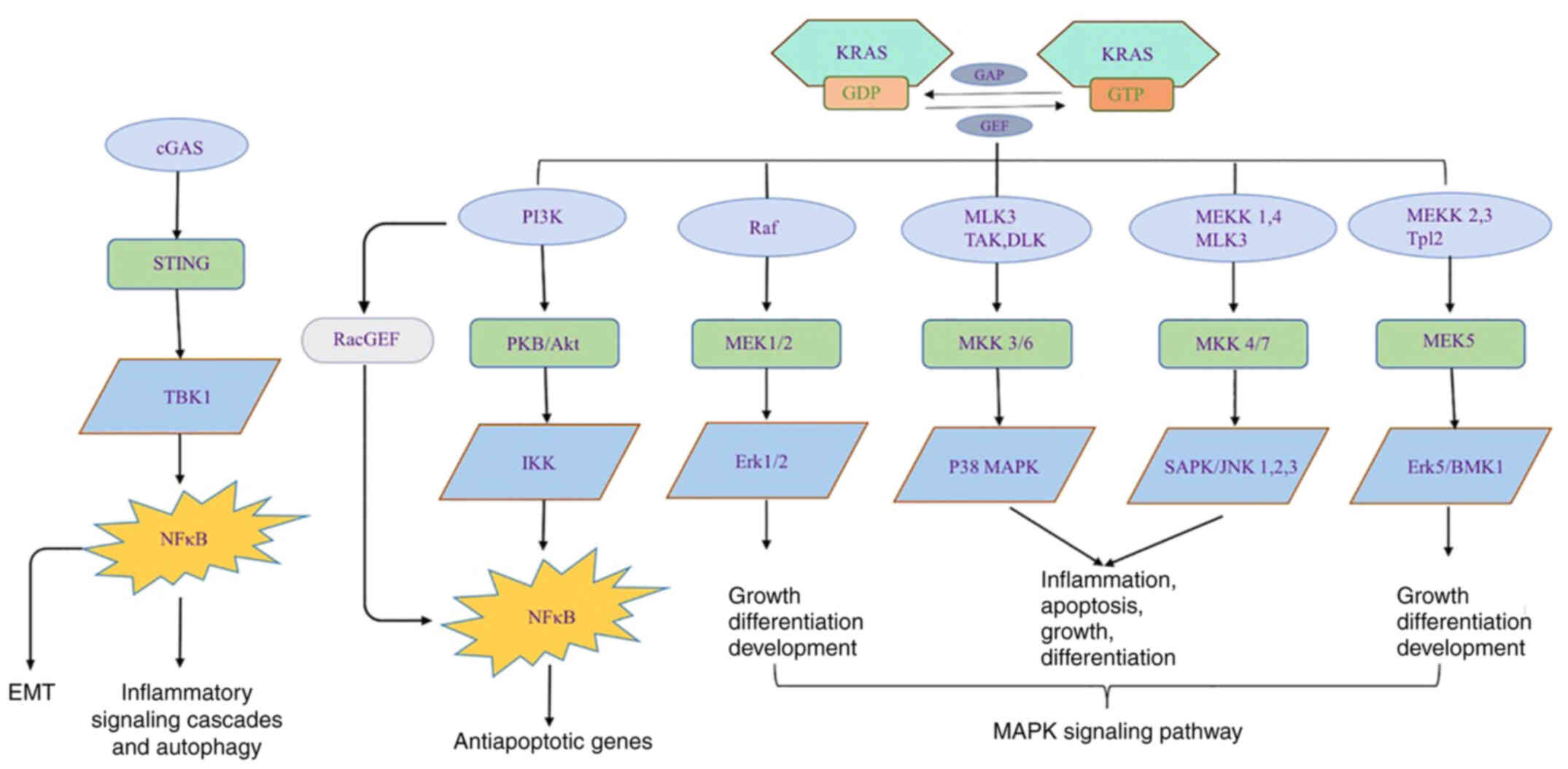
target candidate, as well as KRAS, as the major contributors of PC.
Various relevant signaling pathways are illustrated in Fig. 1.
EMT is a physiological process that allows cancer
cells to undergo morphological and genetic changes through
epithelial-mesenchymal transformation, which underlies the highly
metastatic function of cancer cells and contributes to their
invasion and drug resistance. EMT is triggered by a variety of
tumor microenvironmental factors, including cytokines, growth
factors and chemotherapy drugs (54).
EMT is a potential biomarker for early prognosis, so it is
necessary to determine the effect of EMT on the metastatic process
of cancer cells. Sannino et al (55) identified that BCL9L is a key modulator
for invasion and metastasis of PC cells and reduced BCL9L
expression delayed the response to TGF-β-induced EMT, which was
associated with the loss of proliferation, migration and invasion
of PC cells. In a study by Ye and Weinberg (56), TGF-β-induced E-cadherin expression in
control Panc-1 cells was significantly downregulated and
accompanied by the expression of mesenchymal genes (SNAI2, VIM) in
PC cells, although not significantly. However, this EMT response
was significantly reduced in cells that did not express the BCL9L
gene (55,57). When the expression level of E-cadherin
increased, the expression level of the mesenchymal gene SNA12
decreased. Therefore, the expression of the BCL9L gene has a
decisive role in inhibiting PC cells undergoing EMT in vivo
and may effectively counteract PDAC invasion and metastasis by
triggering EMT in addition to the classical WNT signaling pathway
(55).
The NF-κB family functions as regulators of cell
proliferation, differentiation, immune responses, inflammation,
invasion and metastasis. The activation of NF-κB is determined by
proinflammatory cytokine paracrine loops (58,59). Among
those cytokines, IL-1α was indicated to activate NF-κB in
metastatic PC cell lines, which in turn induced invasion mainly of
the liver. NF-κB directly regulates EMT-TFs. In line with this,
suppression of NF-κB by dehydroxymethylepoxyquinomicin degraded
EMT-TF expression in PC (60). TBK1
modulates inflammatory signaling cascades and autophagy. Although
the number of relevant studies is low, Labelle et al
(61) reported that when NF-κB is
activated synergistically by platelet-derived TGF-β and direct
platelet-tumor cell contact, it transforms the cells into an
aggressive phenotype and enhances metastasis in vivo.
Inhibiting the activation of NF-κB and the expression of TGF-β may
effectively reduce tumor metastasis. Therefore, the metastasis of
tumor cells is mainly through signals derived from platelets
outside the tumor in vivo. However, this experiment was
performed in mouse models of colon cancer and breast cancer and
thus, numerous experiments are still required to verify this
conclusion (Fig. 1).
KRAS is one of the four major driver genes in PC.
KRAS protein is a small GTPase per se. The functions of KRAS
include endocytosis/exocytosis, survival, proliferation, invasion
and transformation. When bound to GTP, KRAS is activated. KRAS
protein interacts with >80 downstream effector proteins and
signaling pathways, such as PI3K-AKT-mTOR, MAPK-MAPK kinase (MEK)
or rapidly accelerated fibrosarcoma-MEK-ERK (51). Nuclear TFs are also activated (such as
ELK, JUN and MYC), leading to stimulation of cell differentiation,
proliferation, migration, transformation, adhesion and
survival.
KRAS mutation is an early event in tumorigenesis. An
activating point mutation of KRAS of oncogene on codon 12 (exon 2)
is observed in the majority of PC cases. The existence of a KRAS
mutation predicts poor prognosis in PDAC. Mutated KRAS contributes
to tumor growth and metastasis in several ways. First, oncogenic
KRAS secrete chemokines to activate T cells, B cells and
macrophages, which drive the inflammatory response and tumor
growth. Furthermore, the Warburg effect was observed during
tumorigenesis, with an increase in glucose uptake and a shift from
mitochondrial oxidative phosphorylation to aerobic glycolysis.
Finally, high levels of lactate and reactive oxygen species are
produced as a result of KRAS mutation (62).
Given that the oncogenic point mutation of KRAS is a
frequent event during PDAC, the identification of this mutation in
biological fluids and tumor tissues may prove useful in the
diagnosis as well as in the prognostic evaluation and therapeutic
decision-making. In the past 20 years, evaluation of KRAS mutation
testing in patients with PDAC has been discussed in depth. A
meta-analysis of mutated KRAS detection in pancreatic juice
reported a pooled sensitivity of 59% (95% CI: 54–64%) and a
specificity of 87% (95% CI: 84–89%) (63). In addition to pancreatic juice, these
studies have involved EUS-fine-needle aspiration samples and, more
recently, circulating cell-free tumour DNA. The latter is part of
the concept of liquid biopsy that also involves the search for
CTCs, exosomes or miRNA. Various test methods are being performed
on these samples to detect gene mutations, among which PCR is
popular. However, the accuracy of PCR is challenged by poor
specimens or complex cellular background. The adoption of digital
PCR is complimentary, achieving high sensitivity in the presence of
a noisy background (64).
Despite extensive efforts to explore specific
biomarkers of cancers, the origins of tumor metastasis have
remained to be fully elucidated. It is hypothesized that the
metastatic cascade results from an epigenetically altered
transcriptional output of the oncogenic signals (65). While a vast number of hypotheses have
been postulated, the present review focuses on 7 biomarkers that
were determined to regulate tumor cell migration in PC. These are
neutrophil extracellular traps (NETs), prostate cancer-associated
transcript-1 (PCAT-1), F-box/LRR-repeat protein 7 (FBXL7), CA19-9,
pentraxin 3 (PTX3), tumor stroma and non-coding RNAs.
Since the discovery of NETs, their role has been
widely debated. NETs have beneficial physiological consequences by
strengthening the host defense. However, uncontrolled NETs are
destructive and associated with cancer metastasis (66). Thus, the function of NETs in tumor
progression may always heat a discussion. It was reported that
chloroquine (67) represses NETs
(67) and slows cancer progression
(66). Murthy et al (68) further demonstrated the role of CQ in
impeding NET formation. In their model, the severity of acute
pancreatitis was decreased by CQ, thus improving survival by
inhibiting NETs. In vivo culture of neutrophils performed by
Hiroki et al (69) revealed
that HMGB1 derived from NETs potentiates the degree of malignancy
of cancer cells. Inhibition of HMGB1 by thrombomodulin inhibited
NETs, hence impeding PC metastasis to the liver. However, the study
of NETs is mostly performed using in vitro or murine models.
Therefore, further investigations are required to determine whether
these results are translatable to humans.
Long noncoding RNAs (lncRNAs) are vital to tumor
progression. Multiple lncRNAs have various pro-oncogenic functions
in PC. For instance, HOTAIR, MALAT-1, ENST00000480739 and AFAP1-AS1
regulate cell invasion (70–73). The latter three lncRNAs are also
promoters of cancer cell migration (74).
Using reverse transcription-quantitative PCR
analysis, it was determined that upregulation of PCAT-1 inhibited
the mRNA and protein expression of RBM5. In other words, knocking
down PCAT-1 suppresses tumor cell migration and invasion (75). However, studies on PCAT-1 are
currently scarce. Further investigation is required to fully
explain the molecular mechanisms that drive tumor cell
dissemination during cancer progression.
CA19-9 is a controversial biomarker. It lacks
specificity in detecting PC. False-positive CA19-9 may be observed
in obstructive jaundice even if successfully drained (76). Serum of patients that have biliary
infection, inflammation or obstruction may test positive for CA19-9
(77,78). It was indicated to be associated with
lymph node metastasis and unfavorable survival outcome in patients
with colon cancer (79).
In the American Association of Clinical Oncology
guidelines, CA19-9 is not recommended as a substitute for imaging
for post-operative evaluation (11).
CA19-9 is not a robust screening tool for PC and previous studies
reported a low positive predictive value ranging from 0.5 to 0.9%
(80,81). However, it may be utilized for
screening at-risk individuals (82).
New international guidelines for managing populations at high risk
for developing familial PC recommend that CA19-9 testing should be
performed when suspected, regardless of its uncertain diagnostic
value (83).
In fact, screening of at-risk individuals has gained
interest from researchers recently. Extensive studies have
identified risk factors for PC. A meta-analysis concluded that
diabetes mellitus is both an early manifestation and consequence of
PC with a summary relative risk of 1.94 (95% CI, 1.66-2.27)
(84). Long-standing pancreatitis is
proclaimed to be a strong risk factor (85). Though rarely observed, PC may result
from mucinous pancreatic cysts (IPMNs) and mucinous cystic
neoplasms (86).
The diagnostic value of CA19-9 for PC has been
argued to be satisfactory. According to a previous evaluation,
multiple tumor markers did not perform better than the single use
of CA19-9 (87). However, this
requires further confirmation.
Despite all the controversies, the present review
favors the utilization of CA19-9 in the screening, diagnosis,
prognosis and surveillance of PC. While there is currently no
consensus regarding the cut-off value of CA19-9 during these steps,
nor any clear understanding of the relationship between CA19-9 and
PC, it is likely to be beneficial to monitor CA19-9 together with
other approaches.
The SCF E3 ubiquitin ligase family controls abundant
protein degradation through the proteasome system and is pivotal in
tumorigenesis and progression. However, the knowledge regarding
their role in PC metastasis remains limited. Low FBXL7 mRNA and
protein levels were observed in PC metastasis. Defects in the
FBXL7-mediated degradation of c-SRC increase cell migration and
invasion and the expression of EMT markers (93). Insignificant FBXL7 expression predicts
poor survival. Previous work unveiled the anti-metastatic role of
ubiquitin ligase subunit FBXL7 in pancreatic carcinoma using
decitabine (a Food and Drug Association-approved DNA-methylase
inhibitor to reduce metastasis) (94). FBXL7 promotes cancer cell invasion and
metastasis through regulation of EMT, while EMT may be mediated by
c-SRC. In numerous types of solid tumor, c-SRC expression levels
are rather high and correlated with metastasis. FBXL7 was observed
to coordinate c-SRC degradation and to further suppress the reduced
EMT and tumor cell migration (93).
Collectively, FBXL7 may be a candidate target for PC therapy.
PTX3 belongs to the pentachlorobenzene toxin family
and is synthesized in numerous cell types, such as endothelial
cells, macrophages and monocytes. It has been reported that serum
PTX3 is an important and specific biomarker for early infection
(95). It helps with the diagnosis of
PDAC and distinguish it from non-cancerous conditions such as
intraductal papillary mucinous tumors or chronic pancreatitis (CP).
PTX3 levels in blood samples from patients with PDAC, healthy
volunteers and subjects with other non-cancerous diseases of the
pancreas were measured by ELISA and patients with PDAC had
significantly higher serum levels of PTX3 than patients with
intraductal papillary myxoma or CP, and the sensitivity and
specificity of PTX3 in detecting PDAC were better than those of
serum CA19-9 and carcinoembryonic antigen. Goulart et al
(96) advocated that PTX3 is a
putative stromal-derived biomarker for PDAC, which warrants further
testing in larger, prospective, multi-center cohorts and within
clinical trials targeting stroma.
A dense stroma that blocks therapeutic agents is a
typical hallmark of PC and this subsequently facilitates
chemoresistance. Stroma depletion is an option to enhance
therapeutic effects, which, in turn, hinders the stroma's role in
tumor metastasis. Thus, it has been proposed to reshape tumor
stroma to alter the communication between cancer cells and stromal
compartments, eventually improving survival outcomes. Stromal-based
therapies heavily rely on multiple elements of stroma, such as the
extracellular matrix (ECM), immune cells, carcinoma-associated
fibroblasts, blood and the lymphatic vasculature. It was argued
that effects of ECM remodeling are not as promising as expected due
to the heterogeneity of the tumor microenvironment (97). However, ECM alterations induce changes
in the intra-tumor vasculature (98).
In other words, such intricate interaction makes manipulation even
better. Changes in either of them may affect stromal performance
during cancerous progression and alter the outcome of malignancy.
Theoretically, stroma depletion is a promising potential means of
PC treatment. However, several clinical studies indicated that the
combination of stromal depletion and chemotherapy was not
beneficial (99–103). Of note, several studies using mouse
models of PC exhibited undesired adverse effects, including
cachexia, weight loss, hypoxia, increased immunosuppression and
vascular density, loss of vascular integrity, an enhanced cancer
stem cell-like phenotype and acidosis (103–106).
To conclude, the stromal alteration strategy enhances the efficacy
of therapeutic agent delivery but prior to its implementation,
suppression of its side effects must be achieved first.
Non-coding RNAs are RNA molecules that are
transcribed from genomes that do not code for proteins. They may be
divided into two categories. In the first category, the role of the
non-coding RNAs is to ensure that the basic biological functions
are being performed and they are called constitutive noncoding
RNAs. The other category is that of the macro-control noncoding
RNAs (regulatory non-coding RNAs). Non-coding RNA participates in
processes of various cellular functions, such as EMT, cell cycle
control, apoptosis and autophagy (107).
miRNAs as a class of small non-coding RNAs regulate
gene expression at the post-transcriptional level by binding to the
3′untranslated region of their target mRNAs. Altered expression of
miRNAs has been indicated to be involved in the regulation of
crucial pathological processes in tumorigenesis, progression and
metastasis of PC (Table I). As a
potential non-invasive biomarker for numerous cancer types, miRNA
may be used as a diagnostic and prognostic marker for PC (108,109).
Khan et al (110)
demonstrated a significant upregulation of miR-215-5p, miR-122-5p
and miR-192-5p, while the levels of miR-30b-5p and miR-320b were
significantly lower in serum samples from patients with PDAC as
compared to those from subjects with CP and healthy controls (HC).
Receiver operating characteristic analysis indicated that these 5
miRNAs are able to distinguish PDAC from both CP and HC. Hence,
this panel may serve as a non-invasive biomarker for the early
detection of PDAC.
miRNAs may also be used as prognostic biomarkers. It
was reported that high expression of miR-212 and miR-675 and low
expression of miR-148a, miR-187 and let-7g in non-microanatomical
carcinoma tissues from patients undergoing PC surgery was able to
predict OS, and high expression of miR-155, miR-155, miR-203,
miR-210 and miR-222 in pancreatic tumors was associated with a low
survival rate (111,112). Furthermore, low expression of miR-7
was reported to be associated with poor prognosis and to accelerate
tumor progression in PC (113).
The goal of early detection of metastatic PC is
laudable. Obstacles are the relatively low prevalence of PC (and
even smaller subpopulations), resulting in a less feasible
screening protocol for the general population. Biomarkers for early
detection remain to be validated. Unveiling the roles of signaling
pathways in PC may be insufficient for the timely diagnosis of PC.
Novel combinations with imaging modalities with state-of-the-art
robust algorithms may clearly determine the anatomical structure
and pathological changes for this disease.
Not applicable.
This work was supported by the National Natural
Science Foundation of China (grant no. 82073833), Chengdu Science
and Technology Bureau focus on research and development support
plan (grant no. 2019-YF09-00097-SN), the popular scientific
research project of Sichuan Health Commission (grant no. 20PJ171)
and Yunnan education program (grant no. SYSX202036).
Data sharing is not applicable.
XC and FX drafted the manuscript, FL drew the
figure, and QX and XW collected the references and extracted the
necessary data. All authors read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Rawla P, Sunkara T and Gaduputi V:
Epidemiology of pancreatic cancer: Global trends, etiology and risk
factors. World J Oncol. 10:10–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neoptolemos JP, Kleeff J, Michl P,
Costello E, Greenhalf W and Palmer DH: Therapeutic developments in
pancreatic cancer: Current and future perspectives. Nat Rev
Gastroenterol Hepatol. 15:333–348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer Statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malvezzi M, Bertuccio P, Levi F, La
Vecchia C and Negri E: European cancer mortality predictions for
the year 2013. Ann Oncol. 24:792–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang W, Zhang J, Wang H, Gao P, Singh M,
Shen K and Fang N: Angiotensin II downregulates catalase expression
and activity in vascular adventitial fibroblasts through an
AT1R/ERK1/2-dependent pathway. Mol Cell Biochem. 358:21–29. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasaki T, Sato T, Nakai Y, Sasahira N,
Isayama H and Koike K: Brain metastasis in pancreatic cancer: Two
case reports. Medicine (Baltimore). 98:e142272019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dang Z, Xu WH, Lu P, Wu N, Liu J, Ruan B,
Zhou L, Song WJ and Dou KF: MicroRNA-135a inhibits cell
proliferation by targeting Bmi1 in pancreatic ductal
adenocarcinoma. Int J Biol Sci. 10:733–745. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tempero MA, Malafa MP, Al-Hawary M, Asbun
H, Bain A, Behrman SW, Benson AB III, Binder E, Cardin DB, Cha C,
et al: Pancreatic adenocarcinoma, version 2.2017, NCCN Clinical
Practice Guidelines in Oncology. J Natl Compr Canc Netw.
15:1028–1061. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tempero MA, Malafa MP, Chiorean EG, Czito
B, Scaife C, Narang AK, Fountzilas C, Wolpin BM, Al-Hawary M, Asbun
H, et al: Pancreatic adenocarcinoma, version 1.2019. J Natl Compr
Canc Netw. 17:202–210. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sohal DPS, Kennedy EB, Cinar P, Conroy T,
Copur MS, Crane CH, Garrido-Laguna I, Lau MW, Johnson T,
Krishnamurthi S, et al: Metastatic pancreatic cancer: ASCO
Guideline Update. J Clin Oncol. Aug 5–2020.(Epub ahead of print).
doi: 10.1200/JCO.20.01364. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mellby LD, Nyberg AP, Johansen JS, Wingren
C, Nordestgaard BG, Bojesen SE, Mitchell BL, Sheppard BC, Sears RC
and Borrebaeck CA: Serum biomarker signature-based liquid biopsy
for diagnosis of early-stage pancreatic cancer. J Clin Oncol.
36:2887–2894. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee ES and Lee JM: Imaging diagnosis of
pancreatic cancer: A state-of-the-art review. World J
Gastroenterol. 20:7864–7877. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singhi AD, Koay EJ, Chari ST and Maitra A:
Early detection of pancreatic cancer: Opportunities and challenges.
Gastroenterology. 156:2024–2040. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garces-Descovich A, Beker K,
Jaramillo-Cardoso A, James Moser A and Mortele KJ: Applicability of
current NCCN Guidelines for pancreatic adenocarcinoma
resectability: Analysis and pitfalls. Abdom Radiol (NY).
43:314–322. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zins M, Matos C and Cassinotto C:
Pancreatic adenocarcinoma staging in the Era of preoperative
chemotherapy and radiation therapy. Radiology. 287:374–390. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong JC and Lu DS: Staging of pancreatic
adenocarcinoma by imaging studies. Clin Gastroenterol Hepatol.
6:1301–1308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sah RP, Sharma A, Nagpal S, Patlolla SH,
Sharma A, Kandlakunta H, Anani V, Angom RS, Kamboj AK, Ahmed N, et
al: Phases of metabolic and soft tissue changes in months preceding
a diagnosis of pancreatic ductal adenocarcinoma. Gastroenterology.
156:1742–1752. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Danai LV, Babic A, Rosenthal MH, Dennstedt
EA, Muir A, Lien EC, Mayers JR, Tai K, Lau AN, Jones-Sali P, et al:
Altered exocrine function can drive adipose wasting in early
pancreatic cancer. Nature. 558:600–604. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng SH, Cheng YJ, Jin ZY and Xue HD:
Unresectable pancreatic ductal adenocarcinoma: Role of CT
quantitative imaging biomarkers for predicting outcomes of patients
treated with chemotherapy. Eur J Radiol. 113:188–197. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mohamed E, Needham A, Psarelli E, Carroll
M, Vinjamuri S, Sanghera B, Wong WL, Halloran C and Ghaneh P:
Prognostic value of (18)FDG PET/CT volumetric parameters in the
survival prediction of patients with pancreatic cancer. Eur J Surg
Oncol. 46:1532–1538. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Im HJ, Oo S, Jung W, Jang JY, Kim SW,
Cheon GJ, Kang KW, Chung JK, Kim EE and Lee DS: Prognostic value of
metabolic and volumetric parameters of preoperative FDG-PET/CT in
patients with resectable pancreatic cancer. Medicine (Baltimore).
95:e36862016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu D, Wang L, Zhang H, Chen J, Wang Y,
Byanju S and Liao M: Prognostic value of 18F-FDG-PET/CT parameters
in patients with pancreatic carcinoma: A systematic review and
meta-analysis. Medicine (Baltimore). 96:e78132017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim MJ, Lee KH, Lee KT, Lee JK, Ku BH, Oh
CR, Heo JS, Choi SH and Choi DW: The value of positron emission
tomography/computed tomography for evaluating metastatic disease in
patients with pancreatic cancer. Pancreas. 41:897–903. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu S, Zhang J, Zuo C, Cheng C, Liu Q and
Sun G: (18)F-FDG-PET/CT findings in pancreatic metastasis. Radiol
Med. 120:887–898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao G, Gong B and Shen W: Meta-analysis of
the additional value of integrated 18FDG PET-CT for tumor distant
metastasis staging: Comparison with 18FDG PET alone and CT alone.
Surg Oncol. 22:195–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Asagi A, Ohta K, Nasu J, Tanada M, Nadano
S, Nishimura R, Teramoto N, Yamamoto K, Inoue T and Iguchi H:
Utility of contrast-enhanced FDG-PET/CT in the clinical management
of pancreatic cancer: Impact on diagnosis, staging, evaluation of
treatment response, and detection of recurrence. Pancreas.
42:11–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Callery MP, Chang KJ, Fishman EK,
Talamonti MS, William Traverso L and Linehan DC: Pretreatment
assessment of resectable and borderline resectable pancreatic
cancer: Expert consensus statement. Ann Surg Oncol. 16:1727–1733.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bae JS, Kim JH, Joo I, Chang W and Han JK:
MDCT findings predicting post-operative residual tumor and survival
in patients with pancreatic cancer. Eur Radiol. 29:3714–3724. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raman SP, Reddy S, Weiss MJ, Manos LL,
Cameron JL, Zheng L, Herman JM, Hruban RH, Fishman EK and Wolfgang
CL: Impact of the time interval between MDCT imaging and surgery on
the accuracy of identifying metastatic disease in patients with
pancreatic cancer. AJR Am J Roentgenol. 204:W37–W42. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ichikawa T, Haradome H, Hachiya J,
Nitatori T, Ohtomo K, Kinoshita T and Araki T: Pancreatic ductal
adenocarcinoma: Preoperative assessment with helical CT versus
dynamic MR imaging. Radiology. 202:655–662. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen FM, Ni JM, Zhang ZY, Zhang L, Li B
and Jiang CJ: Presurgical evaluation of pancreatic cancer: A
comprehensive imaging comparison of CT versus MRI. Am J Roentgenol.
206:526–535. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sheridan MB, Ward J, Guthrie JA, Spencer
JA, Craven CM, Wilson D, Guillou PJ and Robinson PJ: Dynamic
contrast-enhanced MR imaging and dual-phase helical CT in the
preoperative assessment of suspected pancreatic cancer: A
comparative study with receiver operating characteristic analysis.
AJR Am J Roentgenol. 173:583–590. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Canto MI, Harinck F, Hruban RH, Offerhaus
GJ, Poley JW, Kamel I, Nio Y, Schulick RS, Bassi C, Kluijt I, et
al: International Cancer of the Pancreas Screening (CAPS)
Consortium summit on the management of patients with increased risk
for familial pancreatic cancer. Gut. 62:339–347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pereira SP, Oldfield L, Ney A, Hart PA,
Keane MG, Pandol SJ, Li D, Greenhalf W, Jeon CY, Koay EJ, et al:
Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol.
5:698–710. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Canto MI, Hruban RH, Fishman EK, Kamel IR,
Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen
G, et al: Frequent detection of pancreatic lesions in asymptomatic
high-risk individuals. Gastroenterology. 142:796–804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mizumoto T, Toyama H, Terai S, Mukubou H,
Yamashita H, Shirakawa S, Nanno Y, Sofue K, Kido M, Ajiki T and
Fukumoto T: Prediction of lymph node metastasis in pancreatic
neuroendocrine tumors by contrast enhancement characteristics.
Pancreatology. 17:956–961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leng KM, Wang ZD, Zhao JB, Cui YF and
Zhong XY: Impact of pancreatic margin status and lymph node
metastases on recurrence after resection for invasive and
noninvasive intraductal papillary mucinous neoplasms of the
pancreas: A meta-analysis. Dig Surg. 29:213–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mohamed A, Ayav A, Belle A, Orry X,
Chevaux JB and Laurent V: Pancreatic cancer in patients with
chronic calcifying pancreatitis: Computed tomography findings-a
retrospective analysis of 48 patients. Eur J Radiol. 86:206–212.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Riviere DM, van Geenen EJM, van der Kolk
BM, Nagtegaal ID, Radema SA, van Laarhoven CJHM and Hermans JJ:
Improving preoperative detection of synchronous liver metastases in
pancreatic cancer with combined contrast-enhanced and
diffusion-weighted MRI. Abdom Radiol (NY). 44:1756–1765. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Farag A, Le Lu, Roth HR, Liu J, Turkbey E
and Summers RM: A Bottom-up approach for pancreas segmentation
using cascaded superpixels and (Deep) image patch labeling. IEEE
Trans Image Process. 26:386–399. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Karasawa K, Oda M, Kitasaka T, Misawa K,
Fujiwara M, Chu C, Zheng G, Rueckert D and Mori K: Multi-atlas
pancreas segmentation: Atlas selection based on vessel structure.
Med Image Anal. 39:18–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gou S, Lee P, Hu P, Rwigema JC and Sheng
K: Feasibility of automated 3-dimensional magnetic resonance
imaging pancreas segmentation. Adv Radiat Oncol. 1:182–193. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chu LC, Goggins MG and Fishman EK:
Diagnosis and detection of pancreatic cancer. Cancer J. 23:333–342.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Al-Hawary MM, Francis IR, Chari ST,
Fishman EK, Hough DM, Lu DS, Macari M, Megibow AJ, Miller FH,
Mortele KJ, et al: Pancreatic ductal adenocarcinoma radiology
reporting template: Consensus statement of the society of abdominal
radiology and the american pancreatic association.
Gastroenterology. 146:291–304.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Laffan TA, Horton KM, Klein AP,
Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK and
Hruban RH: Prevalence of unsuspected pancreatic cysts on MDCT. AJR
Am J Roentgenol. 191:802–807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee KS, Sekhar A, Rofsky NM and Pedrosa I:
Prevalence of incidental pancreatic cysts in the adult population
on MR imaging. Am J Gastroenterol. 105:2079–2084. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu Y, Zhang H, Chen N, Hao J, Jin H and
Ma X: Diagnostic value of various liquid biopsy methods for
pancreatic cancer: A systematic review and meta-analysis. Medicine
(Baltimore). 99:e185812020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kamyabi N, Bernard V and Maitra A: Liquid
biopsies in pancreatic cancer. Expert Rev Anticancer Ther.
19:869–878. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kunovsky L, Tesarikova P, Kala Z, Kroupa
R, Kysela P, Dolina J and Trna J: The use of biomarkers in early
diagnostics of pancreatic cancer. Can J Gastroenterol Hepatol.
2018:53898202018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sefrioui D, Blanchard F, Toure E, Basile
P, Beaussire L, Dolfus C, Perdrix A, Paresy M, Antonietti M,
Iwanicki-Caron I, et al: Diagnostic value of CA19.9, circulating
tumour DNA and circulating tumour cells in patients with solid
pancreatic tumours. Br J Cancer. 117:1017–1025. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ariston Gabriel AN, Wang F, Jiao Q, Yvette
U, Yang X, Al-Ameri SA, Du L, Wang YS and Wang C: The involvement
of exosomes in the diagnosis and treatment of pancreatic cancer.
Mol Cancer. 19:1322020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dhara S, Chhangawala S, Chintalapudi H,
Askan G, Aveson V, Massa AL, Zhang L, Torres D, Makohon-Moore AP,
Lecomte N, et al: Pancreatic cancer prognosis is predicted by an
ATAC-array technology for assessing chromatin accessibility. Nat
Commun. 12:30442021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sannino G, Armbruster N, Bodenhöfer M,
Haerle U, Behrens D, Buchholz M, Rothbauer U, Sipos B and Schmees
C: Role of BCL9L in transforming growth factor-β (TGF-β)-induced
epithelial-to-mesenchymal-transition (EMT) and metastasis of
pancreatic cancer. Oncotarget. 7:73725–73738. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ye X and Weinberg RA:
Epithelial-mesenchymal plasticity: A central regulator of cancer
progression. Trends Cell Biol. 25:675–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Oberstein PE and Olive KP: Pancreatic
cancer: Why is it so hard to treat? Therap Adv Gastroenterol.
6:321–337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Carbone C and Melisi D: NF-κB as a target
for pancreatic cancer therapy. Expert Opin Ther Targets. 16 (Suppl
2):S1–S10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pires BR, Mencalha AL, Ferreira GM, de
Souza WF, Morgado-Díaz JA, Maia AM, Corrêa S and Abdelhay ES:
NF-kappaB is involved in the regulation of EMT genes in breast
cancer cells. PLoS One. 12:e01696222017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Labelle M, Begum S and Hynes RO: Direct
signaling between platelets and cancer cells induces an
epithelial-mesenchymal-like transition and promotes metastasis.
Cancer Cell. 20:576–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Buscail L, Bournet B and Cordelier P: Role
of oncogenic KRAS in the diagnosis, prognosis and treatment of
pancreatic cancer. Nat Rev Gastroenrerol Hepatol. 17:153–168. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang J, Li S, Li J, Wang F, Chen K, Zheng
Y, Wang J, Lu W, Zhou Y, Yin Q, et al: A meta-analysis of the
diagnostic value of detecting K-ras mutation in pancreatic juice as
a molecular marker for pancreatic cancer. Pancreatology.
16:605–614. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dong L, Wang S, Fu B and Wang J:
Evaluation of droplet digital PCR and next generation sequencing
for characterizing DNA reference material for KRAS mutation
detection. Sci Rep. 8:96502018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Patel SA and Vanharanta S: Epigenetic
determinants of metastasis. Mol Oncol. 11:79–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Thålin C, Hisada Y, Lundström S, Mackman N
and Wallén H: Neutrophil extracellular traps: Villains and targets
in arterial, venous, and cancer-associated thrombosis. Arterioscler
Thromb Vasc Biol. 39:1724–1738. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Pastushenko I, Brisebarre A, Sifrim A,
Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D,
Moers V, Lemaire S, et al: Identification of the tumour transition
states occurring during EMT. Nature. 556:463–468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Murthy P, Singhi AD, Ross MA, Loughran P,
Paragomi P, Papachristou GI, Whitcomb DC, Zureikat AH, Lotze MT,
Zeh Iii HJ and Boone BA: Enhanced neutrophil extracellular trap
formation in acute pancreatitis contributes to disease severity and
is reduced by chloroquine. Front Immunol. 10:282019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kajioka H, Kagawa S, Ito A, Yoshimoto M,
Sakamoto S, Kikuchi S, Kuroda S, Yoshida R, Umeda Y, Noma K, et al:
Targeting neutrophil extracellular traps with thrombomodulin
prevents pancreatic cancer metastasis. Cancer Let. 497:1–13. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang K, Sun X, Zhou X, Han L, Chen L, Shi
Z, Zhang A, Ye M, Wang Q, Liu C, et al: Long non-coding RNA HOTAIR
promotes glioblastoma cell cycle progression in an EZH2 dependent
manner. Oncotarget. 6:537–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jiao F, Hu H, Han T, Yuan C and Wang L,
Jin Z, Guo Z and Wang L: Long noncoding RNA MALAT-1 enhances stem
cell-like phenotypes in pancreatic cancer cells. Int J Mol Sci.
16:6677–6693. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sun YW, Chen YF, Li J, Huo YM, Liu DJ, Hua
R, Zhang JF, Liu W, Yang JY, Fu XL, et al: A novel long non-coding
RNA ENST00000480739 suppresses tumour cell invasion by regulating
OS-9 and HIF-1alpha in pancreatic ductal adenocarcinoma. Br J
Cancer. 111:2131–2141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ye Y, Chen J, Zhou Y, Fu Z, Zhou Q, Wang
Y, Gao W, Zheng S, Zhao X, Chen T and Chen R: High expression of
AFAP1-AS1 is associated with poor survival and short-term
recurrence in pancreatic ductal adenocarcinoma. J Transl Med.
13:1372015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Huang X, Zhi X, Gao Y, Ta N, Jiang H and
Zheng J: LncRNAs in pancreatic cancer. Oncotarget. 7:57379–57390.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang Y, Jiang XM, Feng ZX, Li XL and Zhang
WL: Long noncoding RNA PCAT-1 accelerates the metastasis of
pancreatic cancer by repressing RBM5. Eur Rev Med Pharmacol Sci.
23:7350–7355. 2019.PubMed/NCBI
|
|
76
|
Marrelli D, Caruso S, Pedrazzani C, Neri
A, Fernandes E, Marini M, Pinto E and Roviello F: CA19-9 serum
levels in obstructive jaundice: Clinical value in benign and
malignant conditions. Am J Surg. 198:333–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Shang X, Song C, Du X, Shao H, Xu D and
Wang X: The serum levels of tumor marker CA19-9, CEA, CA72-4, and
NSE in type 2 diabetes without malignancy and the relations to the
metabolic control. Saudi Med J. 38:204–208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yoshida H, Onda M, Tajiri T, Mamada Y,
Taniai N, Mineta S, Hirakata A, Futami R, Arima Y, Inoue M, et al:
Infected hepatic cyst. Hepatogastroenterology. 50:507–509.
2003.PubMed/NCBI
|
|
79
|
Zhai H, Huang J, Yang C, Fu Y and Yang B:
Serum CEA and CA19-9 levels are associated with the presence and
severity of colorectal neoplasia. Clin Lab. 64:351–356. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ballehaninna UK and Chamberlain RS: The
clinical utility of serum CA 19-9 in the diagnosis, prognosis and
management of pancreatic adenocarcinoma: An evidence based
appraisal. J Gastrointest Oncol. 3:105–119. 2012.PubMed/NCBI
|
|
81
|
Tempero MA, Arnoletti JP, Behrman S,
Ben-Josef E, Benson AB III, Berlin JD, Cameron JL, Casper ES, Cohen
SJ, Duff M, et al: Pancreatic adenocarcinoma. J Natl Compr Canc
Netw. 8:972–1017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kimmey MB, Bronner MP, Byrd DR and
Brentnall TA: Screening and surveillance for hereditary pancreatic
cancer. Gastrointest Endosc. 56 (Suppl 4):S82–S86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Goggins M, Overbeek KA, Brand R, Syngal S,
Del Chiaro M, Bartsch DK, Bassi C, Carrato A, Farrell J, Fishman
EK, et al: Management of patients with increased risk for familial
pancreatic cancer: Updated recommendations from the International
Cancer of the Pancreas Screening (CAPS) Consortium. Gut. 69:7–17.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ben Q, Xu M, Ning X, Liu J, Hong S, Huang
W, Zhang H and Li Z: Diabetes mellitus and risk of pancreatic
cancer: A meta-analysis of cohort studies. Eur J Cancer.
47:1928–1937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kirkegård J, Mortensen FV and
Cronin-Fenton D: Chronic pancreatitis and pancreatic cancer risk: A
systematic review and Meta-analysis. Am J Gastroenterol.
112:1366–1372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gaiser RA, Halimi A, Alkharaan H, Lu L,
Davanian H, Healy K, Hugerth LW, Ateeb Z, Valente R, Fernández Moro
C, et al: Enrichment of oral microbiota in early cystic precursors
to invasive pancreatic cance. Gut. 68:2186–2194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Duraker N, Hot S, Polat Y, Höbek A,
Gençler N and Urhan N: CEA, CA 19-9, and CA 125 in the differential
diagnosis of benign and malignant pancreatic diseases with or
without jaundice. J Surg Oncol. 95:142–147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Rieser CJ, Zenati M, Hamad A, Al Abbas AI,
Bahary N, Zureikat AH, Zeh HJ III and Hogg ME: CA19-9 on
postoperative surveillance in pancreatic ductal adenocarcinoma:
Predicting recurrence and changing prognosis over time. Ann Surg
Oncol. 25:3483–3491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Azizian A, Rühlmann F, Krause T, Bernhardt
M, Jo P, König A, Kleiß M, Leha A, Ghadimi M and Gaedcke J: CA19-9
for detecting recurrence of pancreatic cancer. Sci Rep.
10:13322020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ferrone CR, Finkelstein DM, Thayer SP,
Muzikansky A, Fernandez-delCastillo C and Warshaw AL: Perioperative
CA19-9 levels can predict stage and survival in patients with
resectable pancreatic adenocarcinoma. J Clin Oncol. 24:2897–2902.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Berger AC, Garcia M Jr, Hoffman JP, Regine
WF, Abrams RA, Safran H, Konski A, Benson AB III, MacDonald J and
Willett CG: Postresection CA 19-9 predicts overall survival in
patients with pancreatic cancer treated with adjuvant
chemoradiation: A prospective validation by RTOG 9704. J Clin
Oncol. 26:5918–5922. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Jia F, Liu M, Li X, Zhang F, Yue S and Liu
J: Relationship between S100A4 protein expression and pre-operative
serum CA19.9 levels in pancreatic carcinoma and its prognostic
significance. World J Surg Oncol. 17:1632019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Moro L, Simoneschi D, Kurz E, Arbini AA,
Jang S, Guaragnella N, Giannattasio S, Wang W, Chen YA, Pires G, et
al: Epigenetic silencing of the ubiquitin ligase subunit FBXL7
impairs c-SRC degradation and promotes epithelial-to-mesenchymal
transition and metastasis. Nat Cell Biol. 22:1130–1142. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Moro L and Pagano M: Epigenetic
suppression of FBXL7 promotes metastasis. Mol Cell Oncol.
7:18336982020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Baumert M, Surmiak P, Szymkowiak M and
Janosz A: The assessment of Pentraxin 3: A novel biomarker in early
detection of infection in newborns. Biomed Res Int.
2021:66386222021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Goulart MR, Watt J, Siddiqui I, Lawlor RT,
Imrali A, Hughes C, Saad A, ChinAleong J, Hurt C, Cox C, et al:
Pentraxin 3 is a stromally-derived biomarker for detection of
pancreatic ductal adenocarcinoma. NPJ Precis Oncol. 5:612021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Laklai H, Miroshnikova YA, Pickup MW,
Collisson EA, Kim GE, Barrett AS, Hill RC, Lakins JN, Schlaepfer
DD, Mouw JK, et al: Genotype tunes pancreatic ductal adenocarcinoma
tissue tension to induce matricellular fibrosis and tumor
progression. Nat Med. 22:497–505. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Vennin C, Murphy KJ, Morton JP, Cox TR,
Pajic M and Timpson P: Reshaping the tumor stroma for treatment of
pancreatic cancer. Gastroenterology. 154:820–838. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kim EJ, Sahai V, Abel EV, Griffith KA,
Greenson JK, Takebe N, Khan GN, Blau JL, Craig R, Balis UG, et al:
Pilot clinical trial of hedgehog pathway inhibitor GDC-0449
(vismodegib) in combination with gemcitabine in patients with
metastatic pancreatic adenocarcinoma. Clin Cancer Res.
20:5937–5945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Catenacci DV, Junttila MR, Karrison T,
Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M,
Rajdev L, et al: Randomized Phase Ib/II study of gemcitabine plus
placebo or vismodegib, a hedgehog pathway inhibitor, in patients
with metastatic pancreatic cancer. J Clin Oncol. 33:4284–4292.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ko AH, LoConte N, Tempero MA, Walker EJ,
Kate Kelley R, Lewis S, Chang WC, Kantoff E, Vannier MW, Catenacci
DV, et al: A phase I study of FOLFIRINOX Plus IPI-926, a hedgehog
pathway inhibitor, for advanced pancreatic adenocarcinoma.
Pancreas. 45:370–375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ramanathan RK, McDonough S, Philip PA,
Hingorani SR, Lacy J, Kortmansky JS, Thumar J, Chiorean EG, Shields
AF, Behl D, et al: Phase IB/II randomized study of FOLFIRINOX plus
pegylated recombinant human hyaluronidase versus FOLFIRINOX alone
in patients with metastatic pancreatic adenocarcinoma: SWOG S1313.
J Clin Oncol. 37:1062–1069. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wang WQ, Liu L, Xu JZ and Yu XJ:
Reflections on depletion of tumor stroma in pancreatic cancer.
Biochim Biophys Acta Rev Cancer. 1871:267–272. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Özdemir BC, Pentcheva-Hoang T, Carstens
JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C,
Novitskiy SV, et al: Depletion of carcinoma-associated fibroblasts
and fibrosis induces immunosuppression and accelerates pancreas
cancer with reduced survival. Cancer Cell. 28:831–833. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lee JJ, Perera RM, Wang H, Wu DC, Liu XS,
Han S, Fitamant J, Jones PD, Ghanta KS, Kawano S, et al: Stromal
response to Hedgehog signaling restrains pancreatic cancer
progression. Proc Natl Acad Sci USA. 111:E3091–E3100. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Roberts KJ, Kershner AM and Beachy PA: The
stromal niche for epithelial stem cells: A template for
regeneration and a brake on malignancy. Cancer Cell. 32:404–410.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lin Z, Lu S, Xie X, Yi X and Huang H:
Noncoding RNAs in drug-resistant pancreatic cancer: A review.
Biomed Pharmacother. 131:1107682020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther. 1:150042016.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Khan IA, Rashid S, Singh N, Rashid S,
Singh V, Gunjan D, Das P, Dash NR, Pandey RM, Chauhan SS, et al:
Panel of serum miRNAs as potential non-invasive biomarkers for
pancreatic ductal adenocarcinoma. Sci Rep. 11:28242021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Greither T, Grochola LF, Udelnow A,
Lautenschlager C, Wurl P and Taubert H: Elevated expression of
microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated
with poorer survival. Int J Cancer. 126:73–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Schultz NA, Andersen KK, Roslind A,
Willenbrock H, Wojdemann M and Johansen JS: Prognostic microRNAs in
cancer tissue from patients operated for pancreatic cancer-five
microRNAs in a prognostic index. World J Surg. 36:2699–2707. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ye ZQ, Zou CL, Chen HB, Jiang MJ, Mei Z
and Gu DN: MicroRNA-7 as a potential biomarker for prognosis in
pancreatic cancer. Dis Markers. 2020:27821012020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kumarswamy R, Volkmann I and Thum T:
Regulation and function of miRNA-21 in health and disease. RNA
Biol. 8:706–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Liu R, Zhang H, Wang X, Zhou L, Li H, Deng
T, Qu Y, Duan J, Bai M, Ge S, et al: The miR-24-Bim pathway
promotes tumor growth and angiogenesis in pancreatic carcinoma.
Oncotarget. 6:43831–43842. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang L, Jamaluddin MS, Weakley SM, Yao Q
and Chen C: Roles and mechanisms of microRNAs in pancreatic cancer.
World J Surg. 35:1725–1731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Habbe N, Koorstra JB, Mendell JT,
Offerhaus GJ, Ryu JK, Feldmann G, Mullendore ME, Goggins MG, Hong
SM and Maitra A: MicroRNA miR-155 is a biomarker of early
pancreatic neoplasia. Cancer Biol Ther. 8:340–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Huang X, Ding L, Bennewith KL, Tong RT,
Welford SM, Ang KK, Story M, Le QT and Giaccia AJ:
Hypoxia-inducible mir-210 regulates normoxic gene expression
involved in tumor initiation. Mol Cell. 35:856–867. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wu X, Wang Y, Yu T, Nie E, Hu Q, Wu W, Zhi
T, Jiang K, Wang X, Lu X, et al: Blocking MIR155HG/miR-155 axis
inhibits mesenchymal transition in glioma. Neurooncology.
19:1195–1205. 2017.
|
|
120
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Luthra R, Singh RR, Luthra MG, Li YX,
Hannah C, Romans AM, Barkoh BA, Chen SS, Ensor J, Maru DM, et al:
MicroRNA-196a targets annexin A1: A microRNA-mediated mechanism of
annexin A1 downregulation in cancers. Oncogene. 27:6667–6678. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ma Y, Yu S, Zhao W, Lu Z and Chen J:
miR-27a regulates the growth, colony formation and migration of
pancreatic cancer cells by targeting Sprouty2. Cancer Lett.
298:150–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Du J, Zheng X, Cai S, Zhu Z, Tan J, Hu B,
Huang Z and Jiao H: MicroRNA-506 participates in pancreatic cancer
pathogenesis by targeting PIM3. Mol Med Rep. 12:5121–5126. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Chen Z, Chen LY, Dai HY, Wang P, Gao S and
Wang K: miR-301a promotes pancreatic cancer cell proliferation by
directly inhibiting Bim expression. J Cell Biochem. 113:3229–3235.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Fu Y, Liu X, Chen Q, Liu T, Lu C, Yu J,
Miao Y and Wei J: Downregulated miR-98-5p promotes PDAC
proliferation and metastasis by reversely regulating MAP4K4. J Exp
Clin Cancer Res. 37:1302018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Chang W, Liu M, Xu J, Fu H, Zhou B, Yuan T
and Chen P: MiR-377 inhibits the proliferation of pancreatic cancer
by targeting Pim-3. Tumour Biol. 37:14813–14824. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Fu XF, Zhao HC, Yang CL, Chen CZ, Wang K,
Gao F, Tian YZ and Zhao HL: MicroRNA-203-3p inhibits the
proliferation, invasion and migration of pancreatic cancer cells by
downregulating fibroblast growth factor 2. Oncol Lett. 22:6262021.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Liu G, Ji L, Ke M, Ou Z, Tang N and Li Y:
miR-125a-3p is responsible for chemosensitivity in PDAC by
inhibiting epithelial-mesenchymal transition via Fyn. Biomed
Pharmacother. 106:523–531. 2018. View Article : Google Scholar : PubMed/NCBI
|