Introduction
Osteosarcoma (OS) is the most common type of
malignant bone tumor worldwide, particularly among young adults and
children (1). Currently, surgical
treatment and the use of chemotherapeutic drugs are the main
treatment strategies for OS. However, the rate of early metastasis
of OS is high, presenting considerable challenges to its successful
treatment (2). OS pulmonary
metastasis of is often an important factor contributing to a
decreased patient survival rate. The emergence of neoadjuvant
chemotherapy has improved the patient survival rate; however, drug
resistance and chemotherapeutic side-effects have become a main
issue of concern in patients treated using these neoadjuvant
therapies (3,4). Thus, the identification of safe and
effective novel therapeutics is mandatory in order to address the
aforementioned challenges.
Schisandrin B (Sch B), is an active dibenzooctadiene
lignin that can be isolated from the fruit of Schisandra
chinensis (a traditional Chinese herb) (5). In previously published studies, Sch B
was demonstrated to play a crucial role in the treatment of
cardiovascular and neurodegenerative diseases. Of particular
interest was the anti-tumorigenic role attributed to Sch B,
including the regulation of cell cycle arrest, apoptosis, reactive
oxygen species production and autophagy (6). Treatment with Sch B has been reported
to lead to the G0/G1 phase arrest of
HCCC-9810 human cholangiocarcinoma cells, and to induce apoptosis
via the upregulation of Bax, cleaved caspase-3, cleaved caspase-9
and cleaved poly(ADP-ribose) polymerase (PARP) levels (7). Furthermore, Sch B has been reported
to inhibit prostate cancer cell proliferation and to promote DU145
and LNCaP cell apoptosis via S phase cell cycle arrest. Moreover,
the apoptotic process induced by Sch B in LNCaP cells has been
shown to be associated with the Sch B-induced oxidative stress
increase, androgen receptor inhibition, and PI3K/AKT and
STAT3/Janus kinase 2 phosphorylation (8). The anticancer potential of Sch B in
triple-negative breast cancer (TNBC) has also been reported. Sch B
has been reported to inhibit TNBC growth by inducing cell cycle
arrest and triggering apoptotic death, with the mediation of the
aforementioned inhibitory activities via the suppression of STAT3
phosphorylation and nuclear translocation (9). Additionally, Sch B has been reported
to suppress malignant glioma cell (U251 and U87) migration and
invasion via the PI3K/Akt/mTOR/MMP-9 signaling pathway (10). Sch B has also been demonstrated to
exert anticancer effects on lung adenocarcinoma A549 cells by
inhibiting A549 cell proliferation in a concentration-dependent
manner and inducing cell cycle arrest at the
G0/G1 phase (11).
The present study investigated the anticancer
effects of Sch B on human OS cells (143B, MG63, Saos2 and U2OS) and
the related mechanisms in vitro and in vivo. The
143B, MG63, Saos2 and U2OS cell lines were used herein, which are
commonly used cell lines in OS research (12,13).
Materials and methods
Cells and cell culture
The OS cell lines (143B, CRL-8303; MG63, CRL-1427;
Saos2, HTB-85l; and U2OS, HTB-96) and normal cells (HEB, CRL-8621;
and HS5, CRL-11882) used were provided by Professor Tongchuan He
(University of Chicago, USA), and were originally obtained from the
American Type Culture Collection. The cells were cultured in DMEM
(HyClone; Cytiva), supplemented with 10% FBS (Shanghai ExCell
Biology, Inc.) and 1% penicillin-streptomycin (100 IU/ml; Hyclone;
Cytiva). The OS cells were cultured in 90- or 60-mm cell culture
dishes, in a humidified incubator at 37°C with 5% CO2.
The cells to be passaged were washed with PBS (Gibco; Thermo Fisher
Scientific, Inc.) and treated with 0.25% trypsin-EDTA (Thermo
Fisher Scientific, Inc.) for subsequent subculturing.
Drug preparations
Sch B (purity ≥98%) was purchased from Chengdu
Herburify Co., Ltd. (http://www.herbpurify.com/). A total of 20 mg Sch B
was dissolved in 1.5 ml DMSO (SigmaAldrich; Merck KGaA). Sch B was
stored at −80°C and used for subsequent use in in vitro cell
experiments by diluting immediately prior to application. For the
in vivo experiments, 200 mg Sch B were dissolved in 40 ml
carboxymethyl cellulose (SigmaAldrich; Merck KGaA) and stored at
−80°C following ultrasonication overnight.
Crystal violet assay
The OS cells (143B, MG63, Saos2 and U2OS) were
seeded into 24 well-plates at a concentration of 2.5×104
cells/well. The OS cells were first treated with various
concentrations of Sch B (0, 20, 40, 60, 80 or 100 µM), in order to
determine the effective concentration range (data not shown). The
cells (at 50% confluency) were then treated with various
concentrations of Sch B (0, 20, 40, 60 or 80 µM) or DMSO (vehicle
control). Following treatment for 24, 48 and 72 h, the cells were
washed with 4°C PBS three times and fixed with 4% paraformaldehyde
for 20 min at 25°C. After removing the 4% paraformaldehyde, crystal
violet dye (Beyotime Institute of Biotechnology) was added to the
24-well plate for 3 min in the dark at 25°C. Subsequently, the
excess crystal violet dye was washed with double distilled water.
The 24 well-plates were dried and imaged using an Epson Perfection
V200 Photo (Epson). For quantification analysis, 200 µl 20% acetic
acid solution were added to each well to dissolve the crystal
violet for 10 min with constant shaking in the dark at 25°C. The
absorbance was measured at 595 nm in a multifunctional enzyme
labeled detector (BioTek_Synergy4; Gene Company, Ltd.).
MTT assay
MTT powder was purchased from Sigma-Aldrich; Merck
KGaA. The OS cells were seeded in 96-well plates (3×103
cells/well). OS cells were first treated with various
concentrations of Sch B (0, 20, 40, 60, 80 or 100 µM) to determine
the effective concentration range (data not shown). The OS cells
(at 50% confluency) were treated with various concentrations of Sch
B (0, 20, 40, 60 or 80 µM) or DMSO. Normal cells were treated in
the same manner, but with a larger range of concentrations (0–200
µM). Following 24, 48 and 72 h treatment, 10 µl of 5 mg/ml MTT
solution (Sigma-Aldrich; Merck KGaA) were added to each well of the
96-well plate and incubated for 4 h at 37°C. The solution was then
removed using a 1-ml syringe and 100 µl DMSO were added to each
well. Following a 15-min agitation at 25°C, the absorbance in the
wells was detected at 492 nm using a multifunctional enzyme labeled
detector (BioTek_Synergy4 Gene Company, Ltd.). The following
formula was used to determine the IC50 values:
lgIC=Xm-I[P-(3-Pm-Pn)/4], where Xm is the maximum drug
concentration, I is the maximum drug concentration/adjacent drug
concentration, P is the sum of positive reaction rates, Pm is the
maximum positive reaction rate and Pn is the minimum positive
reaction rate.
Colony formation assay
The 143B cells were seeded into 6-well plates (200
cells/well). Considering the high cell density of the colony
formation assay and the purpose of the experiment, the OS cells
were first treated with lower concentrations of Sch B (0, 3, 5, 7,
9, 11, 13, 15, 17 or 19 µM) to determine the effective
concentration range (data not shown). The cells were then treated
with various concentrations of Sch B (0, 3, 5, 7 or 9 µM) or DMSO
when clusters of 2–3 cells could be observed under a light
microscope (ECLIPSE Ti, Nikon Corporation, White Light, ×100
magnification). Following 2 weeks of culture, all cells were
stained with crystal violet (Beyotime Institute of Biotechnology)
as aforementioned, and dried and imaged using an Epson Perfection
V200 Photo (Epson). The number of colonies were counted, and a
cluster of cells was considered a colony if it consisted of >50
cells.
Wound healing assay
The 143B and MG63 were seeded into 6-well plates to
evaluate the changes in the cell migratory capability in Sch
B-treated cells. When the cells had grown to 90% confluency, a
sterile pipette tip was used to create a wound in the cell
monolayer. The floating cells were washed using PBS. Serum-starved
medium (HyClone; Cytiva) was used during the wound healing assay.
Considering the high cell density required for the wound healing
assay, the OS cells were first treated with large concentrations of
Sch B (0, 30, 50, 70, 90, 110, 130, 150, 170 or 190 µM) to
determine the effective concentration range before formal
experiments (data not shown). All cells were treated with various
concentrations of Sch B (0, 30, 50, 70 or 90 µM) or DMSO, and
incubated for 24 h at 37°C. The wound width was recorded using an
inverted microscope (ECLIPSE Ti; Nikon Corporation, White Light,
×100 magnification) after 0, 12 and 24 h. The following formula was
used to determine the healing ability of the treated cells as a
percentage: [(Start scratch width-end scratch width)/(start scratch
width)] ×100.
Transwell assays
Transwell chamber inserts (8-µm pores;
MilliporeSigma) were placed in 24-well plates. For the migration
assay, 2.5×104 cells were resuspended in 400 µl FBS-free
medium (HyClone; Cytiva) and added to the upper chamber. The OS
cells were first treated with various concentrations of Sch B (0,
20, 40, 60, 80 or 100 µM) to determine the effective concentration
range (data not shown). Subsequently, media (HyClone; Cytiva)
supplemented with 10% FBS as a chemotactic agent with various Sch B
concentrations (0, 20, 40, 60 or 80 µM) were placed in the lower
compartment. For the invasion assay, 30 µl Matrigel (BD
Biosciences) diluted with 270 µl DMEM was placed in each filter (50
µl per) to create the matrix adhesive film. Subsequently, all
remaining experimental steps were performed in the same manner as
those described above for the migration assay. Following 24 h of
incubation at 37°C, the cells that had not invaded or migrated were
removed using a cotton swab. Filters were stained with crystal
violet as aforementioned and subsequently wiped with a cotton swab
for the removal of the excess dye. Finally, the number of cells
which had migrated or invaded were imaged (ECLIPSE Ti; Nikon
Corporation) and counted in a random field of view (magnification,
White Light, ×100 magnification).
Hoechst 33258 staining assay
The OS cells were first treated with various
concentrations of Sch B (0, 20, 40, 60, 80 or 100 µM) to determine
the effective concentration range (data not shown). The OS cells
were seeded into 6-well plates and treated with various
concentrations of Sch B (0, 20, 40, 60 or 80 µM) or DMSO when the
cell density reached 50%. Following 24 h of treatment, the cells
were fixed with 4% paraformaldehyde for 20 min, washed with cold
PBS, and stained with 10 ng/ml Hoechst 33258 (Beijing Solarbio
Science & Technology Co., Ltd.) for 10 min in the dark at 25°C.
Finally, the apoptotic cells were imaged (ECLIPSE Ti; Nikon
Corporation) and counted in a random field of view (magnification,
fluorescence, ×100 magnification).
Flow cytometric analysis
The OS cells were cultured in 60-mm dishes. The
cells were treated with various concentrations of Sch B (0, 20, 40,
60 or 80 µM) or DMSO when the cell density reached 50%, for 24 h.
The cells in each dish were digested with trypsin and placed in 500
µl PBS for 1 min at 25°C, stained with annexin V-FITC/PI (Beyotime
Institute of Biotechnology) and analyzed using a flow cytometer (BD
Biosciences) to detect the apoptotic rate at each stage and
analyzed using FlowJo software (version 10.4.0; BD Biosciences).
For determining the cell cycle distribution, the cells were
digested with trypsin and placed in 500 µl 70% ethanol solution
overnight at 4°C, with the subsequent application of all previously
described experimental procedures. A CytoFLEX flow cytometer
(Beckman Coulter, Inc.) was used to detect the cell cycle
distribution.
Network pharmacology analysis
Firstly, the 3D chemical structure of Sch B was
obtained using PubChem (https://pubchem.ncbi.nlm.nih.gov/), and the drug
target of Sch B was then obtained using the PharmMapper (http://www.lilab-ecust.cn/pharmmapper/),
CTD (http://ctdbase.org/) and STITCH databases
(http://stitch.embl.de/). The OS target genes were
then collected from the GeneCards database (https://genecards.weizmann.ac.il/v3/), and the drug
disease intersection genes were finally obtained. The protein
interaction network was constructed using the STRING database
(https://string-db.org/) (14), and the Sch B OS target protein
regulatory network was visualized using Cytoscape (15). The node degree value was calculated
and the key genes were screened. The target gene Gene Ontology (GO)
functional annotations and Kyoto Encyclopedia of Genes and Genomes
(KEGG) signaling pathway enrichment analysis were performed using R
and R studio (version 3.4.0) (16–20).
Western blot analysis
The 143B cells cultured in 90-mm dishes were treated
with Sch B (0, 40, 60 or 80 µM) or DMSO for 24 h. For protein
extraction, the cells were washed with cold PBS three times and 1
ml pre-cooled lysate was added (Wuhan Boster Biological Technology,
Ltd.) to lyse the cells. After boiling with 1X SDS loading buffer
for 5 min, the protein concentration was measured using BCA protein
assays (Beyotime Institute of Biotechnology). For electrophoresis,
35 µg protein samples were loaded on 10% SDS gels and resolved
using SDS-PAGE. When the target protein was ~1 cm away from the
lower edge of the gel, electrophoresis was terminated, and the
resolved proteins were transferred to PVDF membranes
(MilliporeSigma). The membranes were blocked in 5% BSA (Beijing
Solarbio Science & Technology Co., Ltd.) for 30 min at 37°C,
and the membranes were incubated with specific primary antibodies
[β-actin (cat. no. 3700), proliferating cell nuclear antigen (PCNA;
cat. no. 13110) β-catenin (cat. no. 8480), c-Myc (cat. no. 18583),
cyclin D1 (cat. no. 55506), GSK-3β (cat. no. 12456), Bcl-2 (cat.
no. 15071), Bad (cat. no. 9268), Bax (cat. no. 14796), caspase-3
(cat. no. 9662), cleaved caspase-3 (cat. no. 9661), PARP (cat. no.
9532), cleaved-PARP (cat. no. 5625), MMP-2 (cat. no. 40994), MMP-7
(cat. no. 3801), MMP-9 (cat. no. 13667), vimentin (cat. no. 5741),
E-cadherin (cat. no. 14472), N-cadherin (cat. no. 13116), cyclin E
(cat. no. 4129), Akt (cat. no. 4685), p-Akt (Ser473; cat. no.
4060), phosphorylated (p-)Akt (Thr308; cat. no. 4070); dilution
1:1,000 for all the aforementioned antibodies; all from Cell
Signaling Technology, Inc.] at 4°C overnight in 5% BSA (Cell
Signaling Technology, Inc.). The secondary antibodies used
(anti-mouse IgG cat. no. 7076, anti-rabbit IgG, cat. no. 7074;
dilution 1:1,000; both from Cell Signaling Technology, Inc.) was
selected based on the source of the primary antibody, and the PVDF
membranes were incubated with the secondary antibodies at room
temperature for 1 h. The membranes were then washed with
TBS-Tween-20 three times, 10 min each time, and an enhanced
chemiluminescent kit (MilliporeSigma) was used to visualize the
proteins with a ChemiDoc MP Imaging system and Image Lab Software
(version 5.2.1; Bio-Rad Laboratories, Inc.). All bands presented
together were probed on the same membrane in order for the proteins
to share the same loading control.
Rescue assay
The 143B cells were treated with the Wnt signaling
pathway agonist (BML284; cat. no. S8178) and Akt phosphorylation
activator (SC79; cat. no. S7863) purchased from Selleck Chemicals
in combination with Sch B. Subsequently, the effects of BML284 and
SC79 were observed using crystal violet, wound healing and
Transwell assays, as well as flow cytometric analysis.
Establishment of an orthotopic OS
tumor animal model
In the present study, all experimental procedures
involving animals were reviewed and approved by the Ethics
Committee of the First Affiliated Hospital of Chongqing Medical
University (approval no. 2020-503), and experiments were performed
in accordance with the guidelines of the Declaration of Helsinki
(21). A total of 15 BALB/c nude
mice (female, 28 days old, weighing 16.5-18.5 g) were purchased
from Beijing Huafukang Biotechnology Co., Ltd. The mice were reared
under specific pathogen-free conditions and allowed to acclimatize
for 1 week without any treatment. The environment was maintained at
a constant temperature of 24°C and at 55% humidity, with a 12 h
light/dark cycle. All the mice had free access to food and water.
143B cells (2.5×106) in 60 µl PBS were injected into the
tibial plateau of the mice through the proximal tibia. At 3 days
after the injection of the tumor cells, the mouse vital signs were
observed. Afterwards, 15 mice were randomly divided into five
groups, with 3 mice per group. One group was selected as the
control group, and the remaining four groups were administered
various doses of Sch B (25, 50 or 75 mg/kg) or 0.5% sodium
carboxymethyl cellulose by intra-gastric administration every other
day. The mouse weight and tumor size (length and width) were
recorded prior to each administration. Mouse survival was judged by
observing the respiratory movements and heartbeat of the mice.
Mouse death was verified when no breathing and heartbeat were
observed for >3 min. The animal survival rate was evaluated for
up to 40 days. The experimental animals were euthanized when
obvious cachexia, infection and excessive tumor volume were
observed. However, there were no animals that required euthanasia
during the experiment due to the reasons stated above. In addition,
no animals died during the experiment. Following 15 treatments, all
mice were euthanized by CO2 with a flow rate of 30%
volume displacement/min. Tumor tissue and lungs were removed and
fixed in 4% paraformaldehyde. The maximum observed length and width
of the tumors were 18 and 16 mm, respectively, and the tumor volume
did not exceed 2,500 mm3.
H&E staining and
immunohistochemistry
Tumor and lung tissues were fixed with 4%
paraformaldehyde at 4°C for 72 h, and embedded in paraffin.
Paraffinembedded tumor samples were cut into 5-µm-thick sections.
The wax on the section surface was removed by heating and soaking
in various concentrations of alcohol (100, 90, 80 and 70%). The OS
tumor sections and lung tissues were then stained with H&E
(Beyotime Institute of Biotechnology) at 37°C for 1 min, to
visualize tumor tissue lung metastasis. The same embedded sections
and dewaxing methods used for the tumor and lung tissues in H&E
staining were used also to perform immunohistochemistry. For
immunohistochemistry, the OS tumor tissue sections were incubated
with specific histochemical antibodies [PCNA (cat. no. 13110),
Bcl-2 (cat. no. 15071), Vimentin (cat. no. 5741), p-Akt (Ser473;
cat. no. 4060) and β-catenin (cat. no. 8480); dilution for all
antibodies, 1:100; Cell Signaling Technology, Inc.] at 4°C
overnight, and probed with HRPconjugated secondary antibody
(anti-rabbit IgG cat. no. ab6721; dilution 1:350; Abcam) at 37°C
for 30 min, and then imaged under a light microscope (ECLIPSE Ti,
Nikon Corporation, magnification, ×200 and ×400).
Statistical analysis
All experiments were repeated three times to
eliminate errors. All data are presented as the mean ± standard
deviation and were analyzed using GraphPad Prism version 5.0
(GraphPad Software, Inc.). A one-way ANOVA followed by a Tukey's
post hoc test was used for conducting comparisons between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
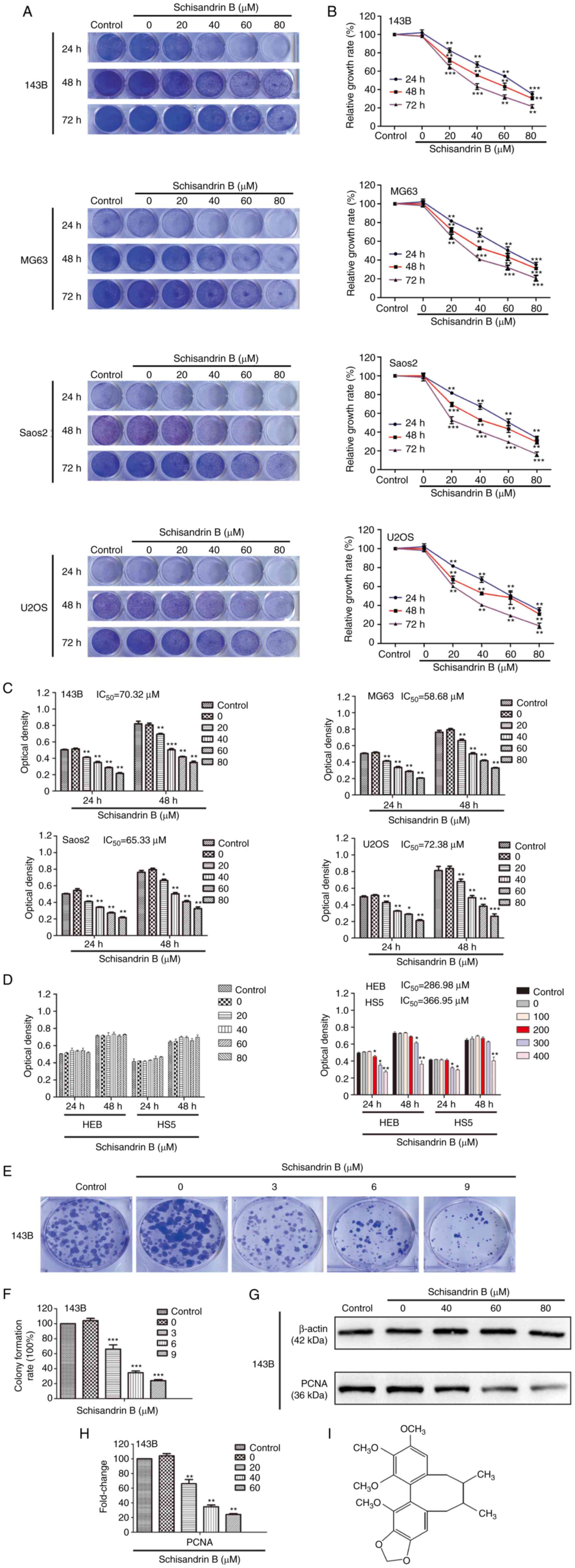
Sch B inhibits the proliferation of OS
cells
Firstly, the inhibitory effects of Sch B on OS cell
(143B, MG63, Saos2 and U2OS) proliferation were observed using
crystal violet staining, and quantitative analysis demonstrated
that the inhibitory effects of Sch B on OS cell proliferation were
concentration-dependent (Fig. 1A and
B). An MTT assay was used to further verify the effects of Sch
B on OS cell viability and evaluate its toxic effects on normal
cells from the perspective of safety. Following 48 h of treatment,
the IC50 values in the OS cells were 70.32 µM (143B
cells), 58.68 µM (MG63 cells), 65.33 µM (Saos2 cells) and 72.38 µM
(U2OS cells) (Fig. 1C), whereas
for the normal cells (HEB and HS5 cells), these values were 286.98
and 366.95 µM respectively, which further illustrated the safety of
the use of Sch B (Fig. 1D). In
addition, it was found that Sch B inhibited the colony formation
ability of the 143B cells at very low concentrations (3, 6 and 9
µM), which indicated that the OS cells were highly sensitive to Sch
B, suggesting that it has the ability to inhibit the initiation of
OS (Fig. 1E and F). Moreover, Sch
B decreased the protein expression levels of PCNA (Fig. 1G and H), which is a recognized
marker of cell proliferation. Together, these results indicated
that Sch B exerted satisfactory OS cell proliferation inhibitory
effects, whilst it did not exert notable toxic effects on normal
cells at therapeutic concentrations (0–100 µM).
Sch B inhibits the migration and
invasion of OS cells
OS metastasis has been associated with a poor
prognosis (3,4). Therefore, in the present study, the
effects of Sch B on OS cell migration were assessed using wound
healing and Transwell assays. In the wound healing assay, the
healing rate of the different groups at different time periods was
calculated to analyze the inhibitory effects of Sch B on OS cell
migration. Compared with the control and DMSO groups, Sch B
significantly inhibited OS cell migration in a
concentration-dependent manner (Fig.
2A-F). The prominent invasive ability of OS often leads to the
destruction of surrounding tissues, which gives rise to the
hematogenous metastasis of OS. Herein, Matrigel Transwell invasion
assays revealed that Sch B suppressed OS cell invasive potential as
well (Fig. 2G and H). However,
considering that Sch B can effectively inhibit cell proliferation,
when the cells exhibit different proliferative abilities under the
different experimental conditions, this may affect the study of
cell migration and invasion. Therefore, the expression levels of
well-known markers of tumor invasion and metastasis were also
assessed. Epithelial-mesenchymal transition (EMT) is a biological
process in which epithelial cells transform into cells with
mesenchymal phenotype through specific procedures (22). EMT is important for tumor invasion
and metastasis. The expression of the EMT-related proteins,
N-cadherin, Snail and Vimentin, was observed using western blot
analysis, and was found to be decreased by Sch B, whereas the
opposite effect was observed as regards E-cadherin expression
(Fig. 2I and J). MMPs can degrade
almost all types of protein components in the extracellular matrix;
thus, increasing attention has been given due to their role in
destroying the histological barrier (23). Sch B also significantly decreased
the expression of MMP-2, MMP-7 and MMP-9 (Fig. 2I and J). Therefore, Sch B may
effectively suppress OS cell migration and invasion.
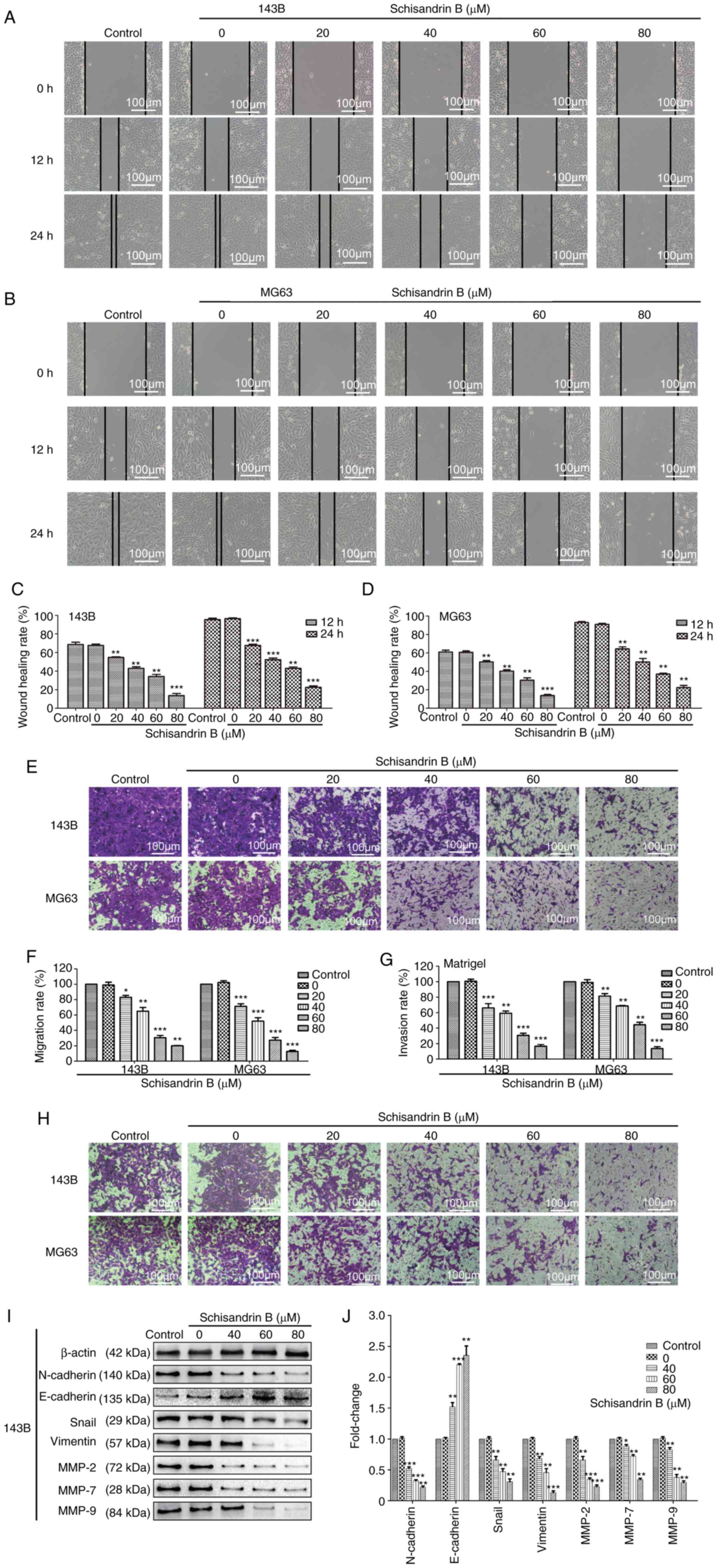 | Figure 2.(A-D) Wound healing, and (E and F)
Transwell migration assays revealed that Sch B inhibited the
migration of 143B and MG63 OS cells. (G and H) Matrigel Transwell
invasion assays revealed that Sch B suppressed 143B and MG63 OS
cell invasive potential. (I and J) Western blot analysis was used
to detect the effects of Sch B on the expression of migration- and
invasion-related proteins, including MMP-2, MMP-7, MMP-9,
N-cadherin, E-cadherin Snail and Vimentin. *P<0.05, **P<0.01,
***P<0.001 vs. control group; n=3. Sch B, Schisandrin B; OS,
osteosarcoma; MMP, matrix metalloproteinase. |
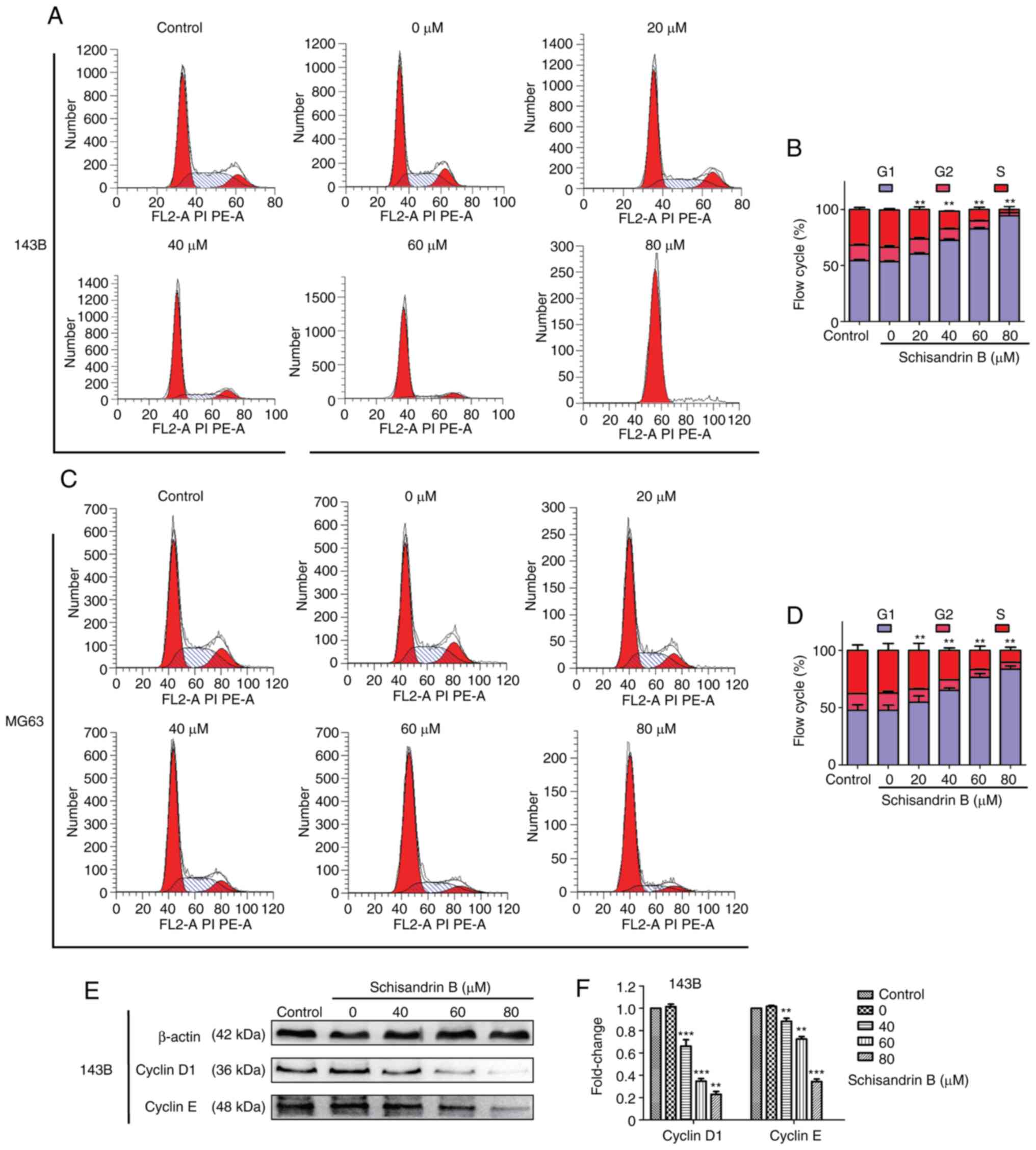
Sch B induces the cell cycle arrest
and apoptosis of OS cells
Through flow cytometry analysis, it was revealed
that Sch B hindered OS cell mitosis progression in the
G1 phase (Fig. 3A-D).
Therefore, the expression levels of ccyclin D1 and cyclin E were
detected using western blot analysis, and it was demonstrated that
the cyclin levels, which promote cell cycle progression from the
G1 to the S phase, decreased in a
concentration-dependent manner (Fig.
3E and F). Following Hoechst 33258 staining, it was observed
under a fluorescence microscope that following treatment with Sch
B, nuclear pyknosis, nuclear fragmentation and a large number of
apoptotic bodies were present in OS cells (Fig. 4A). In addition, flow cytometric
analysis for apoptosis, it was demonstrated that Sch B
significantly increased the OS cell apoptotic rate, particularly
that of late apoptosis (Fig.
4B-E). In order to explore the molecular mechanisms underlying
the promoting effects of Sch on the apoptosis of OS cells, the
expression of a large number of apoptosis-related proteins was
assessed. It was observed that Sch B increased the expression of
certain apoptosis-promoting proteins, including the Bcl-2 protein
family members Bad and Bax. However, Sch B decreased Bcl-2
expression. Moreover, the expression of the apoptotic biomarkers,
cleaved caspase-3, PARP and cleaved PARP was increased following
treatment with Sch B, and the expression of caspase-3 decreased
(Fig. 4F and G).
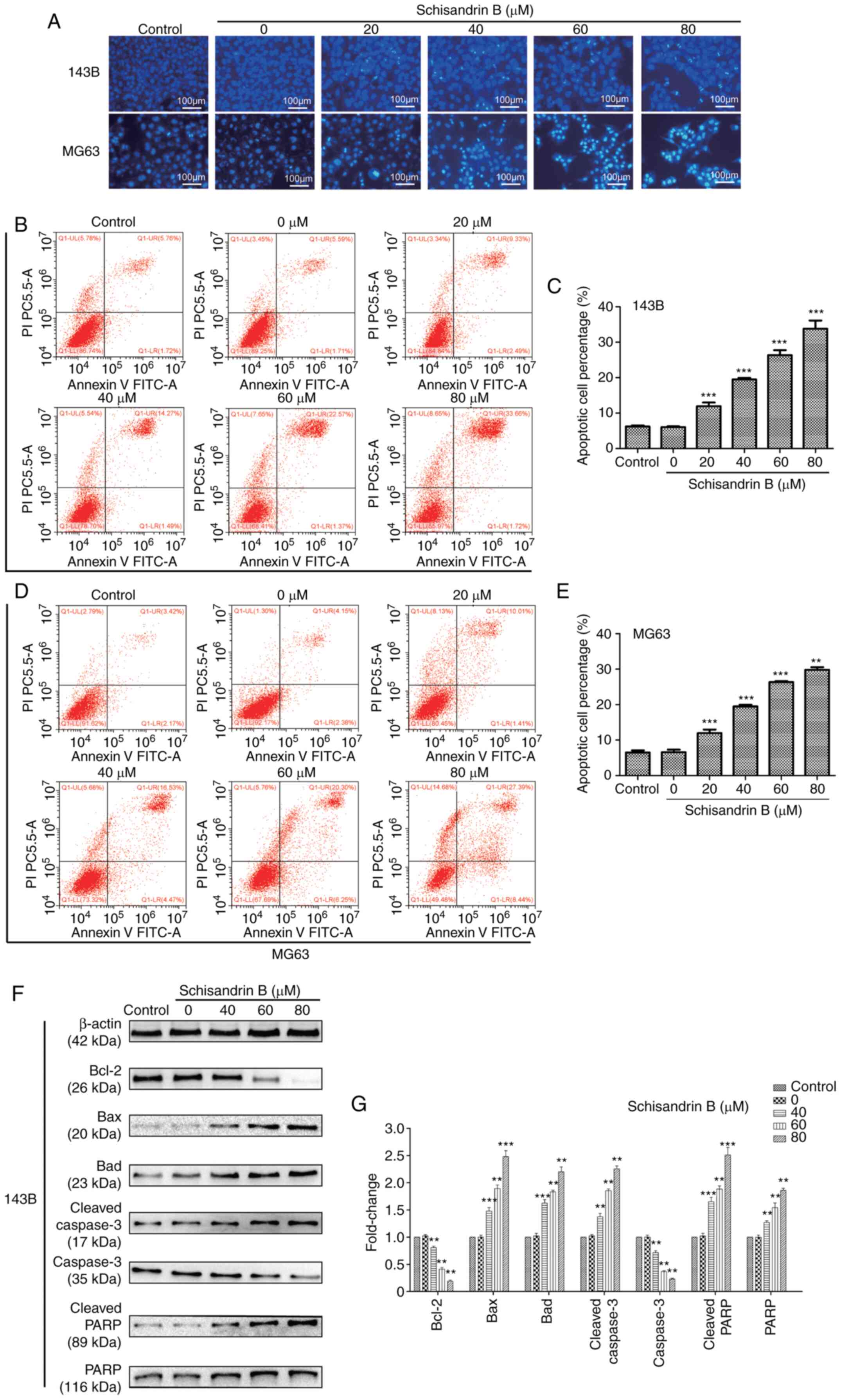 | Figure 4.(A-E) Hoechst 33258 staining revealed
that Sch B promoted OS cell apoptosis in a concentration-dependent
manner and flow cytometric analysis of apoptosis revealed that Sch
B significantly increased the apoptotic rate of OS cells,
particularly the proportion of cells in late apoptosis. (F and G)
Expression of Bad, Bax, Bcl-2, caspase-3, cleaved caspase-3, PARP
and cleaved PARP were detected using western blot analysis.
**P<0.01, ***P<0.001 vs. control group; n=3. Sch B,
Schisandrin B; OS, osteosarcoma; PARP, poly(ADP-ribose)
polymerase. |
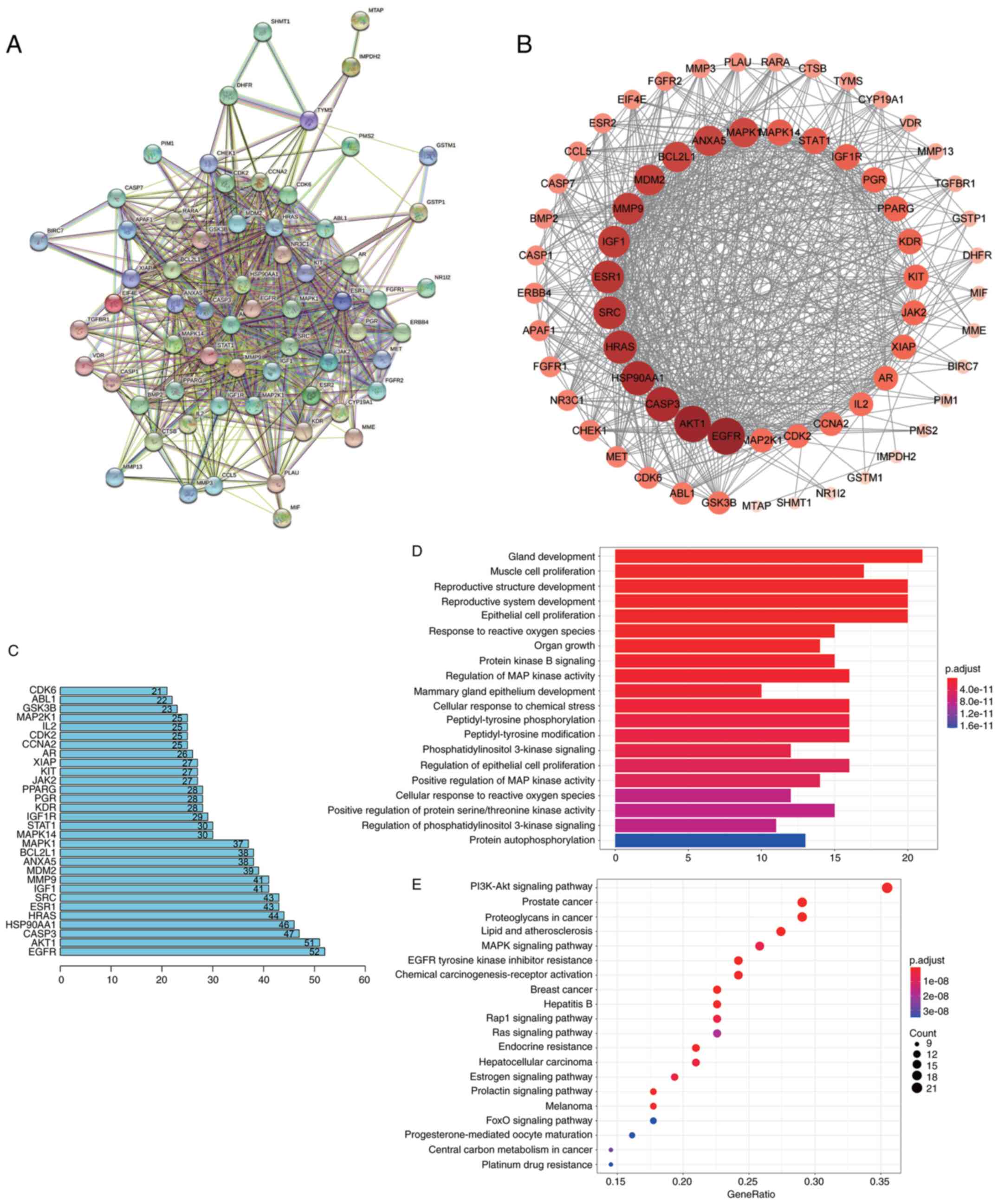
Sch B exerts its effects on OS cells
via the Wnt/β-catenin and PI3K/Akt signaling pathways
In order to examine the mechanisms underlying the
inhibitory effects of Sch B on OS cells, the drug-disease gene
interactions of Sch B and OS were determined using network
pharmacological analysis (Fig.
5A), and the target protein network was visualized (Fig. 5B), so as to screen the top 30 key
genes (Fig. 5C). Then the relevant
signal pathways were determined by GO function annotation and KEGG
pathway enrichment analysis (Fig. 5D
and E). It was found that the core targets may be located in
the Wnt/β-catenin and PI3K/Akt signaling pathways.
Subsequently, the changes in key targets in related
signaling pathways were assessed using western blot analysis. It
was observed that Sch B primarily altered the key targets of the
Wnt/β-catenin and PI3K/Akt signaling pathways in OS cells. The
Wnt/β-catenin signaling pathway plays a crucial role in early
development, organ formation, tissue regeneration and other
physiological processes during animal embryonic development. If the
key proteins in this signaling pathway mutate, this may culminate
in an abnormal signal activation, possibly inducing cancer
occurrence (24). In the present
study, the components and downstream targets of the Wnt/β-catenin
signaling pathway were examined using western blot analysis. The
results revealed that compared with the control group, the
expression of β-catenin, the core member of the Wnt/β-catenin
signaling pathway, was decreased, whereas that of GSK-3β, a
negative regulator of the Wnt/β-catenin signaling pathway, was
significantly increased in the OS cells treated with Sch B. In
addition, the expression of c-Myc, the target gene of Wnt, was also
significantly decreased (Fig. 6A and
B). As an important signal transduction pathway, the PI3K/Akt
signaling pathway plays a key role in inhibiting the apoptosis and
promoting the proliferation of cells by affecting the activation of
downstream effector molecules (25,26).
It is closely related to the occurrence and development of various
human tumors (27). Herein,
western blot analysis revealed that the expression levels of the
PI3K/Akt signaling pathway-related proteins, Akt, p-Akt (Ser473)
and p-Akt (Thr308), were all decreased in the OS cells following
treatment with Sch B (Fig. 6A and
B). In order to further confirm that the changes in the protein
expression levels of the targets in the related signaling pathways
were involved in the effects of Sch B on OS cells, the cells were
treated with the Wnt signaling pathway agonist, BML284, and the Akt
phosphorylation activator, SC79, in combination with Sch B. Both
BML284 and SC79 partially reversed the anticancer effects of Sch B
on OS cells (Fig. 6C-K). Taken
together, the anticancer effects of Sch B on OS cells were
demonstrated to be related to Wnt/β-catenin and PI3K/Akt signaling
pathway inhibition.
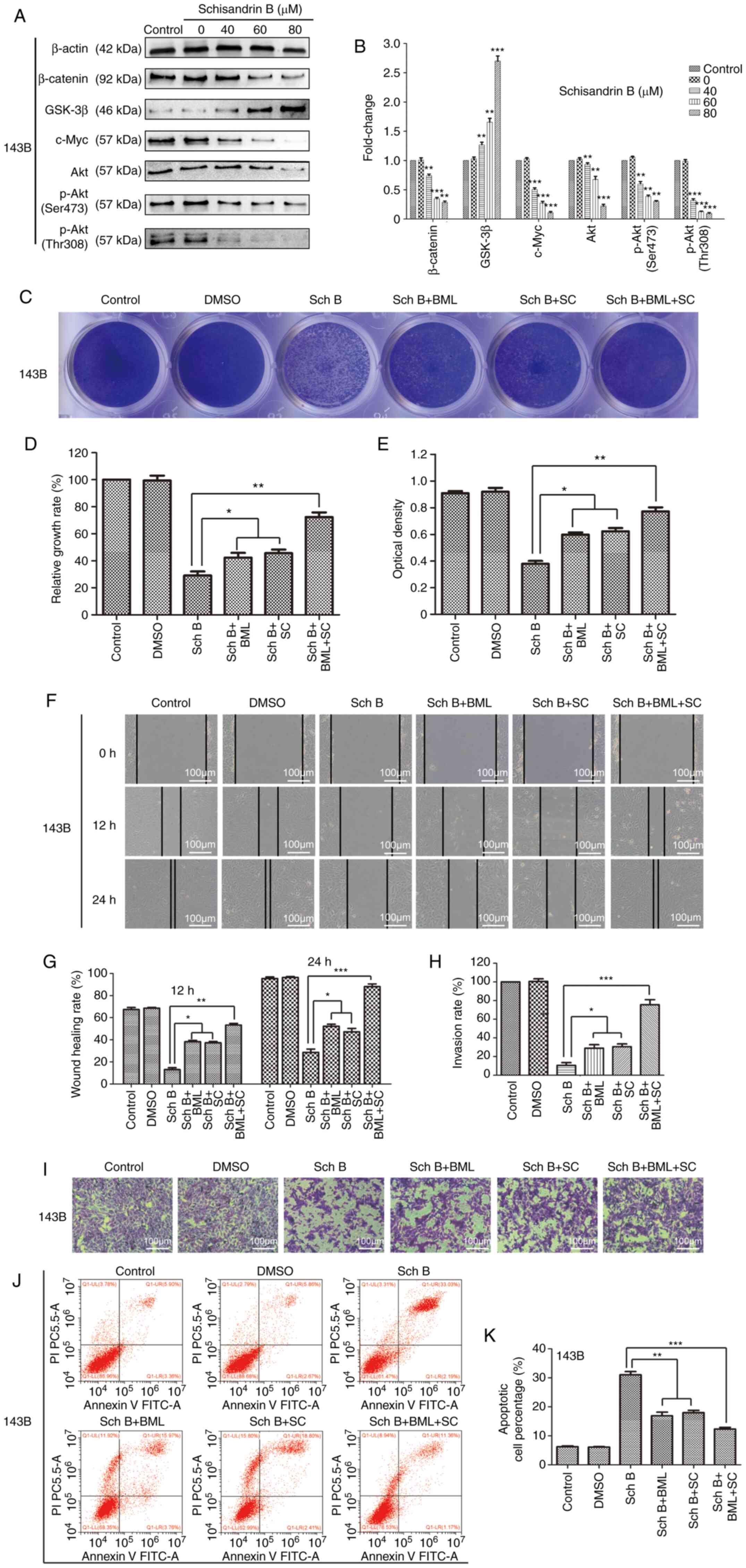 | Figure 6.Sch B exerts its effects on OS cells
through the regulation of the Wnt/β-catenin and PI3K/Akt signaling
pathways. The Wnt signaling pathway agonist, BML284, and the Akt
phosphorylation activator, SC79, partially reversed the antitumor
effects of Sch B. (A and B) Western blot analysis was used to
detect the expression of related proteins, including β-catenin,
GSK-3β, c-Myc, Akt, p-Akt (Ser473) and p-Akt (Thr308) in 143B OS
cells treated with Sch B. *P<0.05, **P<0.01 and ***P<0.001
vs. the control group. n=3. (C-K) The Wnt signaling pathway
agonist, BML284, and the Akt phosphorylation activator, SC79,
partially reversed the inhibitory effects of Sch B on 143B OS cell
proliferation, migration and invasion, and suppressed the high rate
of apoptosis induced by Sch B. *P<0.05, **P<0.01,
***P<0.001 vs. the Sch B group; n=3. Sch B, Schisandrin B; OS,
osteosarcoma; p-, phosphorylated. |
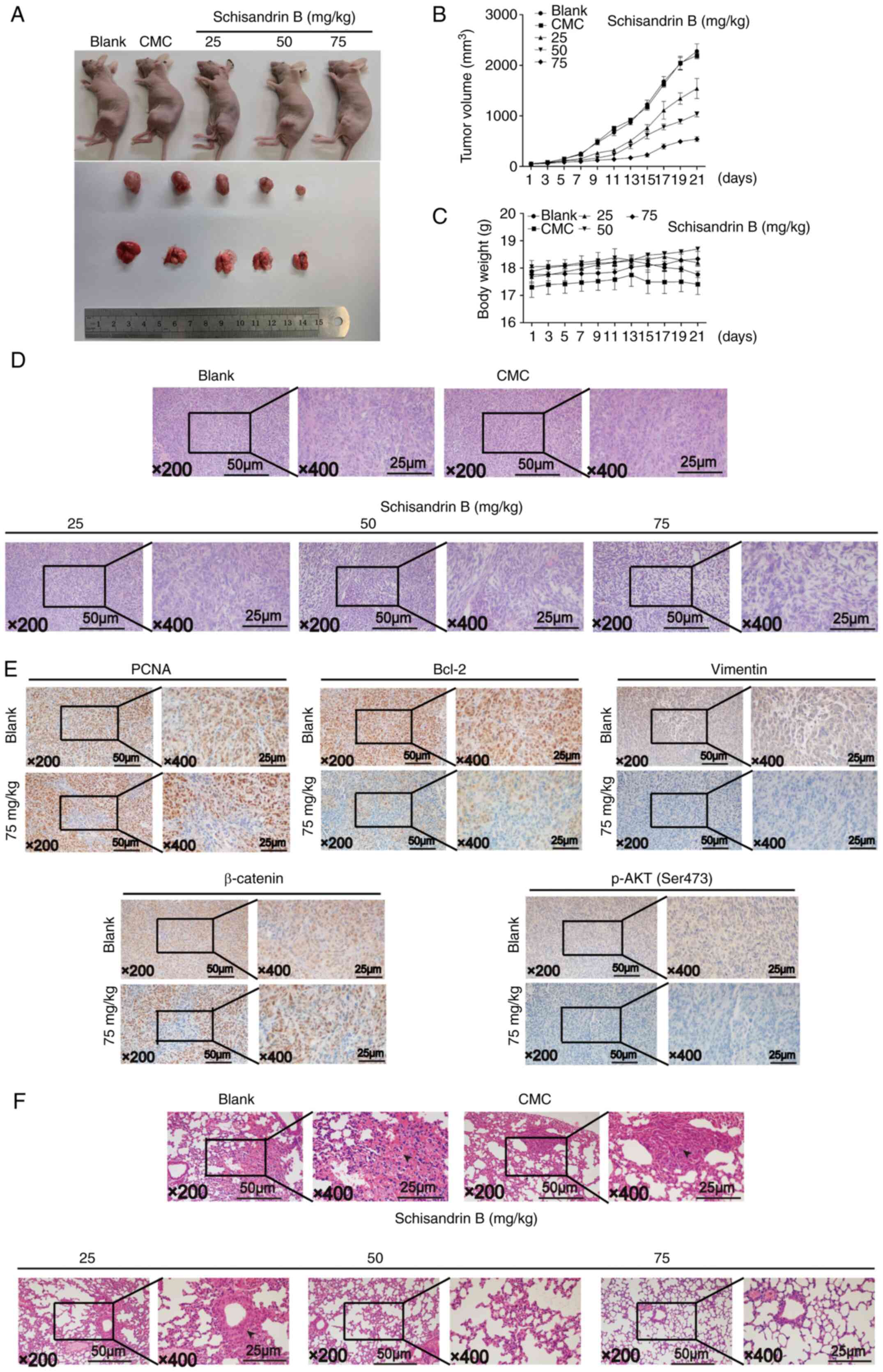
Sch B inhibits tumor growth and lung
metastasis in vivo
An in vivo OS model was established to
further examine the effects of Sch B on the growth and metastasis
of OS. The results revealed that Sch B inhibited OS growth in a
dose-dependent manner, and the body weight of the mice in each
group did not change significantly during the administration period
(Fig. 7A-C). Using H&E
staining, it was demonstrated that compared with the control group,
the tumor nuclear heterogeneity in the treatment group was reduced;
conversely, nuclear fragmentation and nucleolysis were increased.
In addition, the tumor tissue in the treatment group became
significantly more detached (Fig.
7D). Immunohistochemical results demonstrated that the
expression levels of PCNA, Bcl-2, Vimentin, β-catenin and p-Akt
(ser308) were significantly decreased when treated with a high Sch
B concentration, in accordance with the in vitro results
(Fig. 7E). As regards OS
metastasis inhibition in vivo, dense and heterogeneous cell
masses were observed in the mouse lung tissue in the control group,
suggesting the lung metastasis of OS; however, similar cell masses
could not be observed in the high Sch B concentration treatment
group. Sch B effectively reduced the tumor mass in the lungs
according to lung H&E staining (Fig. 7F). Thus, these results suggested
that Sch B inhibited OS growth and lung metastasis in
vivo.
Discussion
OS is well known for its high malignancy capacity
and early occurrence of distant metastasis. The reported high OS
incidence in children and adolescents is accompanied by severe
burdens for families and society (1). At present, surgery combined with
chemotherapy is the primary treatment option for OS, including
neoadjuvant chemotherapy prior to surgery and adjuvant chemotherapy
following surgery (28). Although
the emergence of neoadjuvant chemotherapy has improved the 5-year
survival rate of patients with OS, the use of common
chemotherapeutic drugs, including adriamycin, methotrexate and
cisplatin results in severe side-effects, such as congestive heart
failure induced by adriamycin interfering with the structure and
function of mitochondria in tumor cells, greatly affecting the
quality of life of patients (29,30).
In addition, the development of resistance to chemotherapeutic
drugs in the treatment of OS also brings additional challenges to
the treatment of OS (30).
Therefore, it is of utmost importance to identify novel and safe
drugs for the treatment of OS.
The characteristics of multi-targeting traditional
Chinese medicine extracts with fewer and less severe side-effects
in the treatment of tumors has attracted increased attention
(32). Amongst these agents, Sch B
has been shown to exert therapeutic effects in a variety of
malignant tumors, and recent reports have demonstrated that Sch B
may inhibit osteoclast formation and function, highlighting Sch B
as a potential drug for OS treatment (7–11,33).
In the present study, it was revealed that Sch B exerted inhibitory
effects on OS in vitro and in vivo via Wnt/β-catenin
and PI3K/Akt signaling pathway regulation; SchB inhibited the
proliferation, migration and invasion, and promoted the apoptosis
of OS cells; it also inhibited the tumor growth and reduced the
lung metastasis of OS in vivo.
The inhibition of excessive tumor cell proliferation
is the primary issue required to be addressed for the treatment of
malignant tumors. In the present study, by conducting a series of
cell proliferation-related experiments, it was demonstrated that
Sch B inhibited the proliferation of OS cells in a
concentration-dependent manner, and it had no obvious toxic effects
on normal cells at the therapeutic concentrations used (0–100 µM).
For the normal cells (HEB and HS5 cells), the IC50
values were 286.98 and 366.95 µM respectively. In addition, colony
formation assays, which were used to detect the sensitivity of OS
cells to Sch B, revealed that Sch B inhibited OS cell colony
production at a concentration far lower than the one required for
the inhibition of OS cell proliferation, thus suggesting that OS
cells are highly sensitive to Sch B, and that it may have the
potential to inhibit OS initiation (34,35).
Furthermore, PCNA plays a central role in promoting DNA
replication. It provides a molecular platform, promoting a variety
of protein-protein and protein-DNA interactions at the replication
fork (36). In OS, the
proliferation of OS cells is necessary for rapid progression,
whether in the primary site or in the metastatic site. Compared
with other signaling pathways, PCNA is an indispensable factor in
DNA replication, which is unlikely to be bypassed by resistance
mechanisms frequently acquired in chemotherapy treated tumors.
Therefore, the inhibition of PCNA is considered to be a feasible
anticancer strategy (37). In the
present study, the results of western blot analysis revealed that
Sch B effectively decreased PCNA expression in OS cells, further
highlighting the inhibitory effects of Sch B on OS cell
proliferation.
OS has a high mortality rate due to its high rate of
metastasis, mainly referring to lung metastasis. Although
neoadjuvant chemotherapy improves the 5-year survival rate of
patients with localized OS from 20% to >65%, the outcome of
patients with metastases remains poor (38). In the present study, using wound
healing and Transwell assays, it was revealed that Sch B
effectively inhibited OS cell migration and invasion. EMT is the
basic process required by the initial and subsequent events in the
process of embryogenesis (39). It
has been previously reported that when cancer stem cells begin
undergoing EMT, the cancer-derived mesenchymal cells are invasive,
and these cells thus exhibit metastatic activity (40). Snail has been reported to play a
key role in the regulation of EMT, being considered a key
regulatory node in the transcriptional activation of EMT (41). Snail has been also demonstrated to
inhibit E-cadherin transcription, which encodes epithelial
cell-specific proteins, further promoting cell adhesion junction
degradation (42). During this
process, E-cadherin is replaced by N-cadherin, and epithelial cells
have been reported to obtain the characteristics of high
mesenchymal cell fluidity, thus being able to provide increased
flexibility in cell migration and invasion (43). Furthermore, it has been previously
reported that the cytokeratin cytoskeleton may be transformed into
a Vimentin-dominated cytoskeleton, including also the morphological
characteristics of mesenchymal cells (44,45).
EMT has been reported to be involved in the invasion and migration
of tumor cells through a dense collagen-rich extracellular matrix
surrounding the tumor. MMPs inhibit or enhance tumor invasion by
destroying the dynamic balance of extracellular matrix (46). Western blotting revealed that Sch B
could inhibit OS cell migration and invasion by inhibiting EMT. In
addition, it has been demonstrated that Sch B inhibits
osteoclastogenesis (33). In
primary bone cancer and metastasis, the process of bone remodeling
creates a favorable environment for the establishment and
progression of tumors. In this environment, osteoclasts mediate the
destruction of inorganic bone components and collagen dissolution,
providing an opportunity for distant metastasis of primary OS,
particularly for hematogenous metastasis (47). Therefore, Sch B may inhibit OS
metastasis not only by reducing migration- and invasion-related OS
cell protein expression, but by also participating in physiological
bone remodeling process hijacked by OS.
Cell cycle arrest is considered to be an important,
however closely related to apoptosis factor, affecting cell
proliferation. Cell cycle arrest leads to apoptosis by increasing
the frequency and/or degree of DNA damage, or by reducing the
ability of DNA damage repair mechanisms in cells. In this process,
cell cycle arrest has dual significance: Not only does it provide a
window of opportunity for the repair of damaged DNA, but it also
increases the time and probability for the repair system of DNA to
be exposed to exogenous harmful stimuli (if the harmful factor is
toxic to DNA and/or its repair system). The result of the contest
between these two factors ultimately determines the final fate of
cells (48–50). In the present study, using flow
cytometric analysis, it was revealed that Sch B blocked OS cell
cycle progression in the G1 phase, and western blot
analysis revealed that the expression of cyclin D1 and cyclin E
decreased in a concentration-dependent manner. Cyclin D1 modulates
the transition from the G1 to the S phase through its
action as an allosteric regulator of CDK4 and CDK6. The upregulated
expression of cyclin D1 culminates in unchecked cellular
proliferation, thereby promoting tumor growth (51). Cyclin E, a regulatory subunit of
CDK2, has been considered to be integral for the initiation of DNA
replication at the G1/S checkpoint (52). Therefore, the mechanisms through
which Sch B blocks OS cell cycle progression in the G1
phase were fund to involve the reduction in cyclin D1 and cyclin E
expression. Similarly, flow cytometric analysis demonstrated that
Sch B promoted OS cell apoptosis in a concentration-dependent
manner, particularly in the late stages. In mammals, two primary
pathways have evolved for activating the caspase cascade, namely,
the mitochondrial pathway (intrinsic pathway) and the death
receptor pathway (extrinsic pathway). In the endogenous apoptotic
pathway, mitochondrial outer membrane permeability is primarily
regulated by Bcl-2 family members, including anti-apoptotic protein
(Bcl-2) and pro-apoptotic proteins (Bax and Bad) (53). When subjected to a decrease in
Bcl-2 and an increase in Bax and Bad levels, mitochondria have been
revealed to be stimulated to release cytochrome c,
subsequently activating caspase-3, and ultimately leading to
apoptosis. The most important substrate of caspase-3 is PARP. The
activation of caspase-3 leads to the over-activation of PARP,
consuming intracellular NAD+, reducing intracellular ATP
levels and eventually resulting in cell death (54). Herein, using western blot analysis,
it was shown that the expression levels of cleaved caspase-3, PARP
and cleaved PARP were increased in OS cells treated with Sch B, and
the expression level of caspase-3 decreased. In addition, amongst
the Bcl-2 family member proteins, the expression of the
anti-apoptotic protein, Bcl-2 decreased, whereas that of the
pro-apoptotic proteins, Bax and Bad, increased. It was thus
revealed that Sch B blocked OS cell cycle progression in the
G1 phase and promoted apoptosis.
The Wnt pathway has been reported to be important
for the control of embryonic bone development, bone integrity and
postnatal bone regeneration. In addition, The Wnt signaling pathway
has been shown to be closely associated with OS (55). Four Wnt signaling pathways have
been described thus far: The Wnt/β-catenin pathway, the
Wnt/Ca2+ pathway, the Wnt/planar cell polarity (Wnt/PCP)
pathway and the Wnt/protein kinase A (Wnt/PKA) pathway. The
Wnt/β-catenin pathway has been widely studied, particularly with
regard to its association with tumor development. When cells are
not stimulated by a Wnt signal, most of the β-catenin in the
cytoplasm binds with cadherin protein on the cell membrane to make
it adhere to the cytoskeletal protein actin and participate in cell
adhesion. In the presence of the appropriate Wnt ligands, the
binding of Wnt to its receptor complex leads to the activation of
intracellular protein Disheveled (DVL). DVL can stabilize the free
state of β-catenin protein in the cytoplasm by inhibiting the
degradation activity of the β-catenin degradation complex formed by
GSK3-β and other proteins. After the stable accumulation of
β-catenin in the cytoplasm entering the nucleus, it binds to the
TCF/LEF transcription factor family members and initiates the
transcription of downstream target genes (including -Myc and Cyclin
D1), ultimately leading to abnormal cell proliferation and cell
cycle transition, and in turn promoting tumor formation (55–57).
In the present study, it was demonstrated that Sch B also inhibited
the PI3K/Akt signaling pathway to play an inhibitory role in OS.
Akt, also known as protein kinase B, is a 57-kDa serine/threonine
kinase and is the central node of several signaling pathways, and
has often been reported to be deregulated in numerous types of
human cancer (58). The upstream
activator phosphoinositide-dependent kinase 1 phosphorylates Akt at
Thr308, resulting in partial activation of Akt. Ser473 is
phosphorylated by the mechanistic target of rapamycin complex 2,
which can stimulate the complete enzymatic activity of Akt
(59). Therefore, Akt can be
dephosphorylated and its activation reduced, preventing all
downstream signal transduction events regulated by Akt, and thus
regulating cell proliferation, differentiation, apoptosis and
migration. In the present study, Sch B downregulated the expression
of p-Akt (Ser473) and p-Akt (Thr308) in OS cells concurrently,
which was different from the previous single inhibition of p-Akt
isoforms (60,61).
In the present study, all cells were treated when
the cell density reached 50%; although tumor cells have a strong
clonal growth ability, they still have a certain population. This
is due to the interdependence between tumor cells. If these
dependencies are eliminated or weakened, the activity of cancer
cell proliferation and growth may be affected. When the cell
density is ~50%, it suggests that the tumor cells are in the
exponential growth period and have a strong proliferation and
growth activity. Therefore, the addition of drugs for treatment at
this time can best reflect the inhibitory ability of drugs on the
proliferation and growth of tumor cells. In order to avoid the
interference factor of the influence of culture medium on tumor
cells, a control group with only culture medium was used; neutral
medium was used to minimize the effect of medium acidity on tumor
cells.
Through the establishment of an OS mouse model, the
effects of Sch B on OS in vivo were examined. The results
demonstrated that Sch B effectively inhibited tumor growth and the
occurrence of lung metastasis in OS.
Furthermore, drug-disease gene interactions of Sch B
and OS were determined using network pharmacological analysis,
followed by relevant signal pathway determination using GO
functional annotation and KEGG pathway enrichment analysis. The key
target changes in related signal pathways were then assessed, and
it was demonstrated that Sch B primarily altered the key targets of
the Wnt/β-catenin and PI3K/Akt signaling pathways. In addition, in
order to further confirm that the changes of the targets in the
related signal pathways were involved in the effects of Sch B on OS
cells, the cells were treated with the Wnt signaling pathway
agonist, BML284, and the Akt phosphorylation activator, SC79, in
combination with Sch B. Both BML284and SC79 partially reversed the
inhibitory effects of Sch B on OS, this being the main novelty, to
the best of our knowledge, of the present study.
In conclusion, the present study revealed that Sch B
may be used in the treatment of OS in vitro and in
vivo, and its potential anti-OS mechanism may be mediated
through the inhibition of the Wnt/β-catenin and PI3K/Akt signaling
pathways. Therefore, it is suggested that Sch B may possibly serve
as a novel drug target in the clinical treatment of OS. In future
studies, the authors aim to further examine the effects of Sch B
combined with cytotoxic drugs, as well as the internal mechanisms
of these effects.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81873998).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (XL, JL, YW, JC, YH, SY, TT, NW, JZ, CY
and MW) made substantial contributions to the study design. XL and
JL critically revised the manuscript for important intellectual
content. YW drafted the manuscript, and agreed to be accountable
for the work in ensuring that questions related to the integrity of
any part of the work are appropriately investigated and resolved.
YW and XL confirm the authenticity of all the raw data. YW, CY, NW
and JZ performed the experiments and acquired the data. NW, TT, SY,
YH and JC analyzed and interpreted the data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki, and approved by the
Ethics Committee of the First Affiliated Hospital of Chongqing
Medical University (approval no. Research ethics 2020-503; date of
approval, September 29, 2020).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Papakonstantinou E, Stamatopoulos A,
Athanasiadis DI, Kenanidis E, Potoupnis M, Haidich AB and Tsiridis
E: Limb-salvage surgery offers better five-year survival rate than
amputation in patients with limb osteosarcoma treated with
neoadjuvant chemotherapy. A systematic review and meta-analysis. J
Bone Oncol. 25:1003192020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meazza C and Scanagatta P: Metastatic
osteosarcoma: A challenging multidisciplinary treatment. Expert Rev
Anticancer Ther. 16:543–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hattinger CM, Patrizio MP, Magagnoli F,
Luppi S and Serra M: An update on emerging drugs in osteosarcoma:
Towards tailored therapies? Expert Opin Emerg Drugs. 24:153–171.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leong PK and Ko KM: Schisandrin B: A
double-edged sword in nonalcoholic fatty liver disease. Oxid Med
Cell Longev. 2016:61716582016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nasser MI, Zhu S, Chen C, Zhao M, Huang H
and Zhu P: A comprehensive review on Schisandrin B and its
biological properties. Oxid Med Cell Longev. 2020:21727402020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang X, Wang S, Mu Y and Zheng Y:
Schisandrin B inhibits cell proliferation and induces apoptosis in
human cholangiocarcinoma cells. Oncol Rep. 36:1799–1806. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nasser MI, Han T, Adlat S, Tian Y and
Jiang N: Inhibitory effects of Schisandrin B on human prostate
cancer cells. Oncol Rep. 41:677–685. 2019.PubMed/NCBI
|
|
9
|
Dai X, Yin C, Guo G, Zhang Y, Zhao C, Qian
J, Wang O, Zhang X and Liang G: Schisandrin B exhibits potent
anticancer activity in triple negative breast cancer by inhibiting
STAT3. Toxicol Appl Pharmacol. 358:110–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang Y, Zhang Q, Bao J, Du C, Wang J,
Tong Q and Liu C: Schisandrin B suppresses glioma cell metastasis
mediated by inhibition of mTOR/MMP-9 signal pathway. Biomed
Pharmacother. 74:77–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv XJ, Zhao LJ, Hao YQ, Su ZZ, Li JY, Du
YW and Zhang J: Schisandrin B inhibits the proliferation of human
lung adenocarcinoma A549 cells by inducing cycle arrest and
apoptosis. Int J Clin Exp Med. 8:6926–6936. 2015.PubMed/NCBI
|
|
12
|
Pautke C, Schieker M, Tischer T, Kolk A,
Neth P, Mutschler W and Milz S: Characterization of osteosarcoma
cell lines MG-63, Saos-2 and U-2 OS in comparison to human
osteoblasts. Anticancer Res. 24:3743–3748. 2004.PubMed/NCBI
|
|
13
|
Jiao Y, Guo Y, Fan Y, Wang R, Li X, Wu H,
Meng Z, Yang X, Cui Y, Liu H, et al: Triggering of apoptosis in
osteosarcoma 143B cell line by carbon quantum dots via the
mitochondrial apoptotic signal pathway. Biomed Res Int.
2020:28462972020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47(D1):D607–D613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. Nat
Genet. 25:25–29. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
The Gene Ontology Consortium, . The gene
ontology resource: 20 years and still GOing strong. Nucleic Acids
Res. 47:D330–D338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanehisa M: Post-genome Informatics.
Oxford University Press; Oxford: 2000
|
|
19
|
R Core Team: R: A language and environment
for statistical computing. R Foundation for Statistical Computing;
Vienna, Austria: 2012
|
|
20
|
R Studio Team, . R Studio: Integrated
Development for R. R Studio, Inc.; Boston, MA: 2015
|
|
21
|
Stockhausen K: The Declaration of
Helsinki: Revising ethical research guidelines for the 21st
century. Med J Aust. 172:252–253. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rohani MG and Parks WC: Matrix remodeling
by MMPs during wound repair. Matrix Biol. 44–46. 113–121.
2015.PubMed/NCBI
|
|
24
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia P and Xu XY: PI3K/Akt/mTOR signaling
pathway in cancer stem cells: From basic research to clinical
application. Am J Cancer Res. 5:1602–1609. 2015.PubMed/NCBI
|
|
26
|
Jafari M, Ghadami E, Dadkhah T and
Akhavan-Niaki H: PI3k/AKT signaling pathway: Erythropoiesis and
beyond. J Cell Physiol. 234:2373–2385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ritter J and Bielack SS: Osteosarcoma. Ann
Oncol. 21 Suppl 7:vii320–vii325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Armstrong J and Dass CR: Doxorubicin
action on mitochondria: Relevance to osteosarcoma therapy? Curr
Drug Targets. 19:432–438. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harrison DJ, Geller DS, Gill JD, Lewis VO
and Gorlick R: Current and future therapeutic approaches for
osteosarcoma. Expert Rev Anticancer Ther. 18:39–50. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hagleitner MM, Coenen MJ, Gelderblom H,
Makkinje RR, Vos HI, de Bont ES, van der Graaf WT, Schreuder HW,
Flucke U, van Leeuwen FN, et al: A first step toward personalized
medicine in osteosarcoma: Pharmacogenetics as predictive marker of
outcome after chemotherapy-based treatment. Clin Cancer Res.
21:3436–3441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang
Z and Han J: The advantages of using traditional Chinese medicine
as an adjunctive therapy in the whole course of cancer treatment
instead of only terminal stage of cancer. Biosci Trends. 9:16–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Fang Z, Song C, Kang H, Guo Q,
Dong Y, Zhang Y, Peng R, Guan H and Li F: Schisandrin B inhibits
osteoclastogenesis and protects against ovariectomy-induced bone
loss. Front Pharmacol. 11:11752020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gruber M, Handle F and Culig Z: The stem
cell inhibitor salinomycin decreases colony formation potential and
tumor-initiating population in docetaxel-sensitive and
docetaxel-resistant prostate cancer cells. Prostate. 80:267–273.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kume K and Nishizuka SS: Colony lysate
arrays for proteomic profiling of drug-tolerant persisters of
cancer cell. Anal Chem. 89:8626–8631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mailand N, Gibbs-Seymour I and
Bekker-Jensen S: Regulation of PCNA-protein interactions for genome
stability. Nat Rev Mol Cell Biol. 14:269–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang SC: PCNA: A silent housekeeper or a
potential therapeutic target? Trends Pharmacol Sci. 35:178–186.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cui J, Dean D, Hornicek FJ, Chen Z and
Duan Z: The role of extracelluar matrix in osteosarcoma progression
and metastasis. J Exp Clin Cancer Res. 39:1782020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McCabe EM and Rasmussen TP: lncRNA
involvement in cancer stem cell function and epithelial-mesenchymal
transitions. Semin Cancer Biol. 75:38–48. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Skrzypek K and Majka M: Interplay among
SNAIL transcription factor, MicroRNAs, long non-coding RNAs, and
circular RNAs in the regulation of tumor growth and metastasis.
Cancers (Basel). 12:2092020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mendonsa AM, Na TY and Gumbiner BM:
E-cadherin in contact inhibition and cancer. Oncogene.
37:4769–4780. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ramkumar N, Omelchenko T, Silva-Gagliardi
NF, McGlade CJ, Wijnholds J and Anderson KV: Crumbs2 promotes cell
ingression during the epithelial-to-mesenchymal transition at
gastrulation. Nat Cell Biol. 18:1281–1291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Paolillo M and Schinelli S: Extracellular
matrix alterations in metastatic processes. Int J Mol Sci.
20:49472019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Torzilli PA, Bourne JW, Cigler T and
Vincent CT: A new paradigm for mechanobiological mechanisms in
tumor metastasis. Semin Cancer Biol. 22:385–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nørregaard KS, Jürgensen HJ, Gårdsvoll H,
Engelholm LH, Behrendt N and Søe K: Osteosarcoma and metastasis
associated bone degradation-A tale of osteoclast and malignant cell
cooperativity. Int J Mol Sci. 22:68652021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ogrodnik M, Salmonowicz H, Jurk D and
Passos JF: Expansion and cell-cycle arrest: Common denominators of
cellular senescence. Trends Biochem Sci. 44:996–1008. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Goel S, DeCristo MJ, McAllister SS and
Zhao JJ: CDK4/6 inhibition in cancer: Beyond cell cycle arrest.
Trends Cell Biol. 28:911–925. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pack LR, Daigh LH and Meyer T: Putting the
brakes on the cell cycle: Mechanisms of cellular growth arrest.
Curr Opin Cell Biol. 60:106–113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Montalto FI and De Amicis F: Cyclin D1 in
cancer: A molecular connection for cell cycle control, adhesion and
invasion in tumor and stroma. Cells. 9:E26482020. View Article : Google Scholar
|
|
52
|
Caruso JA, Duong MT, Carey JP, Hunt KK and
Keyomarsi K: Low-molecular-weight cyclin E in human cancer:
Cellular consequences and opportunities for targeted therapies.
Cancer Res. 78:5481–5491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
D'Amelio M, Cavallucci V and Cecconi F:
Neuronal caspase-3 signaling: Not only cell death. Cell Death
Differ. 17:1104–1114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu P, Cai X, Zhang W, Li Y, Qiu P, Lu D
and He X: Flavonoids of Rosa roxburghii Tratt exhibit
radioprotection and anti-apoptosis properties via the
Bcl-2[Ca(2+)]/Caspase-3/PARP-1 pathway. Apoptosis. 21:1125–43.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cai Y, Cai T and Chen Y: Wnt pathway in
osteosarcoma, from oncogenic to therapeutic. J Cell Biochem.
115:625–631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Danieau G, Morice S, Rédini F, Verrecchia
F and Royer BB: New insights about the Wnt/β-Catenin signaling
pathway in primary bone tumors and their microenvironment: A
promising target to develop therapeutic strategies? Int J Mol Sci.
20:37512019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
McQueen P, Ghaffar S, Guo Y, Rubin EM, Zi
X and Hoang BH: The Wnt signaling pathway: Implications for therapy
in osteosarcoma. Expert Rev Anticancer Ther. 11:1223–1232. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Revathidevi S and Munirajan AK: Akt in
cancer: Mediator and more. Semin Cancer Biol. 59:80–91. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang Q, Jiang W and Hou P: Emerging role
of PI3K/AKT in tumor-related epigenetic regulation. Semin Cancer
Biol. 59:112–124. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen Y, Li H, Zhang W, Qi W, Lu C, Huang
H, Yang Z, Liu B and Zhang L: Sesamin suppresses NSCLC cell
proliferation and induces apoptosis via Akt/p53 pathway. Toxicol
Appl Pharmacol. 387:1148482020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liang XX, Wang RY, Guo YZ, Cheng Z, Lv DY,
Luo MH, He A, Luo SX and Xia Y: Phosphorylation of Akt at Thr308
regulates p-eNOS Ser1177 during physiological conditions. FEBS Open
Bio. 11:1953–1964. 2021. View Article : Google Scholar : PubMed/NCBI
|