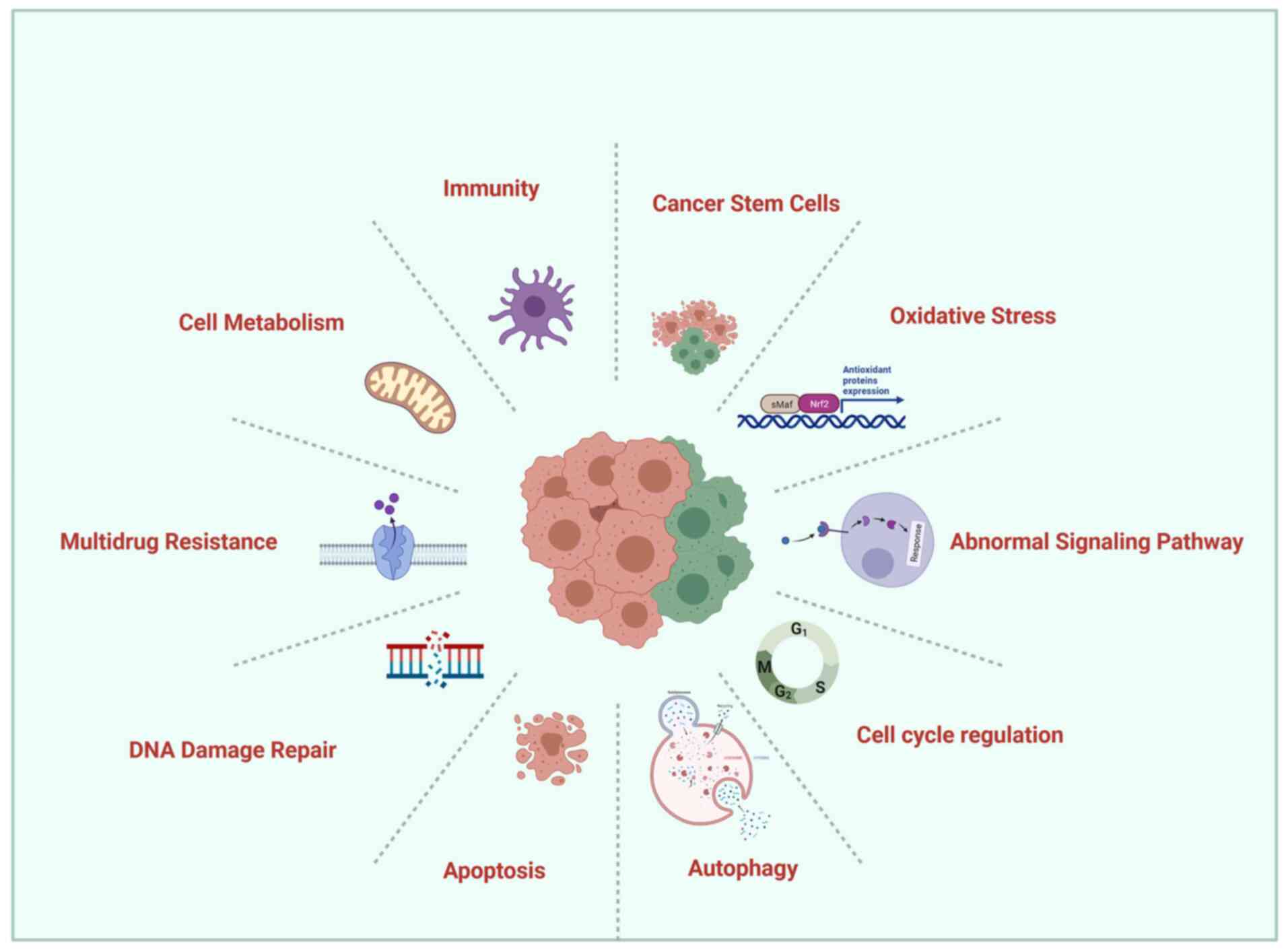
Ovarian cancer is one of the most common malignant
tumors of the reproductive organs and has the highest mortality
rate among all gynecological malignancies. At diagnosis, ~70% of
patients present with advanced disease and most are resistant to
platinum-based chemotherapy, resulting in a low five-year survival
rate (1,2). Ovarian cancer can be subdivided into
two main histological subtypes: Epithelial ovarian cancers (EOCs),
which account for ~90% of ovarian cancers, and non-EOCs, which
account for ~10% of ovarian cancers (3). Epithelia cancers include serous
[high-grade serous carcinoma (HGSC) and low-grade serous carcinoma
(LGSC)], endometrioid (high-grade endometrioid carcinoma and
low-grade endometrioid carcinoma), clear-cell and mucinous
carcinomas (2,4). LGSCs usually contain KRAS and BRAF
mutations (5,6), whereas most HGSCs have TP53 mutations
and exhibit severe aneuploidy genome aberrations. Clear cell
carcinoma is characterized by mutations in the ARID1A, PIK3CA, PTEN
and KRAS genes, while endometrioid carcinoma, similar to its
uterine counterpart, has mutations in ARID1A, CTNNB1, and PTEN, as
well as microsatellite instability (7,8).
Approximately 80% of patients with ovarian cancer
are treated with cytoreductive surgery followed by adjuvant
chemotherapy with carboplatin and paclitaxel or cisplatin and
paclitaxel (2,9). However, ~70% of patients with this
treatment regimen will relapse (10) and the recurring cancer is often
resistant to standard platinum-based chemotherapy. In patients with
advanced cancer, mortality is usually due to acquisition of drug
resistance. Cisplatin is one of the most effective chemotherapy
drugs for ovarian cancer, but resistance to cisplatin is common.
Patient recurrence more than 6 months after front-line
platinum-based therapy is considered platinum-sensitive, whereas
platinum-resistant recurrence occurs after less than 6 months
(2,11,12).
During the six months after the completion of major platinum-based
chemotherapy, disease progression is usually closely related to
platinum resistance. Due to its significant impact on patient
survival time and quality, improving the response to platinum is an
important challenge. At present, the standard treatment for
platinum-resistant or refractory ovarian cancer is pegylated
liposomal adriamycin, weekly paclitaxel and topotecan, but the
efficacy of this regimen is limited (13).
To provide a thorough understanding of the mechanism
of drug resistance, in the present review, the important molecular
mechanisms involved in the response of ovarian cancer patients to
platinum-based chemotherapy, as well as methods to circumvent these
mechanisms that have been studied extensively by clinical and
laboratory researchers are explored (Fig. 1).
A systematic literature search was conducted via
electronic search engine PubMed for eligible studies published
until December 31, 2021. The keywords for searches were as follows:
‘Ovarian cancer’, ‘chemotherapy resistance’, ‘chemoresistance’.
Furthermore, the referenes in retrieved articles were also manually
reviewed for potentially relevant studies.
Since the ovary is deep in the pelvic cavity and
small in size, it is difficult to detect early ovarian cancer due
to a lack of typical symptoms. Upon surgery, the tumor is confined
to the ovary in less than 30% of patients with ovarian cancer
(14); in most patients, the tumor
has spread to the pelvic and abdominal organs. Although progress
has been made in surgical techniques and chemotherapy drugs in
recent years, the mortality rate of ovarian cancer has not
decreased significantly. Chemotherapy MDR is the main cause of
treatment failure (15). MDR is a
drug-resistant phenotype in which cancer cells are simultaneously
resistant to multiple drugs with different molecular targets and
without obvious structural similarities (16). Overcoming MDR is a top priority in
clinical and research oncology but remains elusive. In the present
study, the latest literature on the main mechanisms of MDR were
summarized and several new MDR reversal strategies were evaluated,
including more effective and specific P-glycoprotein (P-gp)
inhibitors.
Membrane-bound adenosine triphosphate
(ATP)-dependent active drug efflux pumps can significantly decrease
the intracellular concentration of the drug and thus the efficacy
of treatment. P-gp uses the energy of ATP hydrolysis to transport
various structural and functional drugs out of the cell. P-gp and
multidrug-resistance-associated protein (MRP) are two main membrane
proteins known to cause MDR in cancer. Inhibiting these proteins is
a strategy to sensitize cancer cells to chemotherapy (17). Overexpression of P-gp can also lead
to the development of MDR in human tumors (including ovarian
cancer). Therefore, many years of extensive research have focused
on overcoming P-gp-based MDR. To date, three generations of P-gp
modulators have been developed. The second-generation P-gp
modulator, valspodar, has exhibited the capacity to modulate
ovarian cancer resistance in phase I, II and III clinical trials
(18).
As MDR is one of the most studied mechanisms of
ovarian cancer drug resistance in the early stages of the research
process, DDR is one of the most important mechanisms of ovarian
cancer drug resistance. At present, the standard treatment for
advanced ovarian cancer is surgical cytoreduction, followed by
platinum and taxane-based chemotherapy. Current research focuses on
new agents, particularly those that target the DDR pathway
(19,20). A comprehensive understanding of the
process of DDR in ovarian cancer and its working principle may
promote future research on treatments and drug resistance.
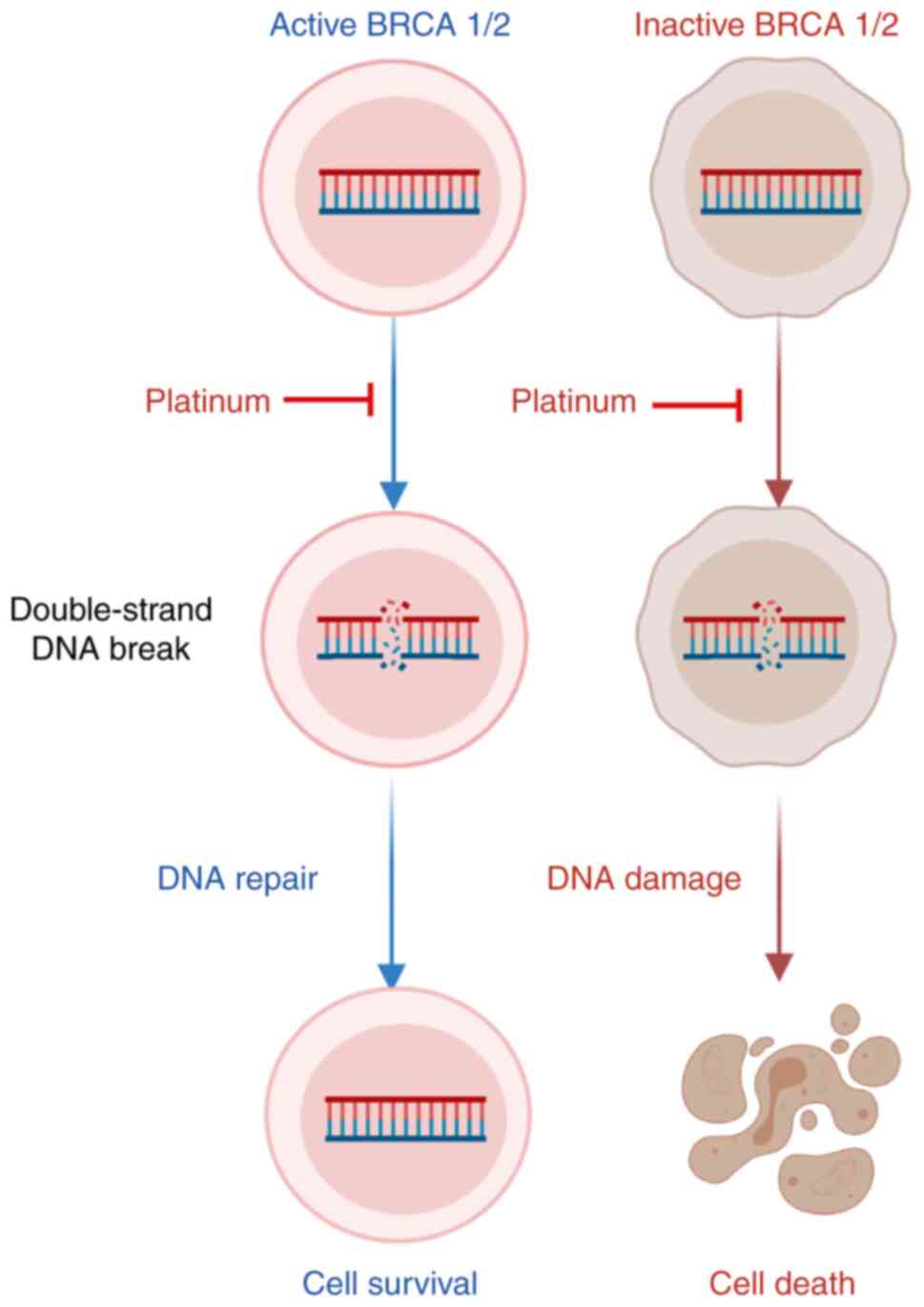
Studies have revealed that more than 50% of HGSCs
have homologous recombination repair (HR) pathway defects (21–23),
which are mainly related to genetic and epigenetic changes in HR
pathway genes. The tumor suppressors BRCA1 and BRCA2, which encode
proteins involved in DDR via homologous recombination, have been
associated with an increased risk of ovarian cancer (24). Mutations in BRCA1/2 are associated
with high sensitivity to DNA-damaging agents, including
poly-(ADP-ribose) polymerase (PARP) inhibitors and platinum
(25–27). Patients with BRCA mutations have an
improved overall response to platinum therapy, which is associated
with a longer survival rate for ovarian cancer (28,29).
Compared with patients who do not carry the mutation, patients with
the mutation are less likely to have disease progression within 6
months after the end of the main treatment (28). Therefore, patients with BRCA1/2
mutations are more likely to have longer progression-free survival
(PFS) than patients without mutations.
Concurrently, the study found that upon the first
relapse of platinum-sensitive or platinum-resistant patients, the
response rates for second-line platinum-based or nonplatinum-based
chemotherapy were higher in patients carrying mutations than in
those who did not (28). In
BRCA-mutant cancers, BRCA reversion mutations that restore protein
function are the key resistance mechanism of platinum-based
chemotherapy (30).
Reversion mutations that may be caused by
DNA-damaging chemotherapy or genome instability are base
substitutions or insertions/deletions. Such mutations are usually
close to the main protein truncation mutation and restore the open
reading frame (ORF) of the gene to allow the production of
functional protein, transforming tumor cells from HR defective to
proficient. A total of 18% of platinum-refractory cancers and 13%
of platinum-resistant cancers have BRCA mutations in circulating
cell-free DNA (cfDNA) before treatment, compared with 2% of
platinum-sensitive cancers (31).
Before treatment, patients with no BRCA reversion mutations in
cfDNA had significantly longer PFS after treatment with rucaparib
compared with patients with reversion mutations (32).
In cancer, the restoration of HR function promotes
drug resistance by repairing DNA damage induced by PARP inhibitors
and/or platinum-based chemotherapy, destroying the basis of
synthetic lethality and ultimately promoting cell survival
(33). Reversion mutations in
multiple HR pathway genes, including BRCA1, BRCA2, RAD51C, RAD51D
(34) and PALB2, cause acquired
resistance to platinum-based chemotherapies and PARP inhibitors
(32).
Limited DNA end resection is the key to impaired HR
in BRCA1-mutant cells. A loss-of-function CRISPR screen identified
dynein light chain 1 protein (DYNLL1) as a factor responsible for
platinum resistance in BRCA-defective patients with HGSC by
facilitating DNA end resection (35). After platinum-based chemotherapy
for BRCA1 mutant ovarian cancer, low expression of DYNLL1 was
significantly associated with poor PFS.
Strengthening DNA repair pathways is one of the ways
that cancer cells resist the DNA-damaging effects of platinum
(Fig. 2). Inhibiting these DNA
repair pathways may restore the sensitivity of cancer cells to
platinum. This is precisely the objective of several drugs under
development. PARP inhibitors disrupt the mechanism by which damaged
parts of DNA are removed and the drug trabectedin binds directly to
and damages DNA (36). TOP1
initiates the DNA relaxation by cleaving one DNA strand. This in
turn generates TOP1 cleavage complexes (TOP1ccs). The selective
trapping of TOP1ccs by topotecan stabilizes TOP1ccs which
covalently attach to the 3′-end of the breaks. These stalled
TOP1ccs lead to lethal DNA double-strand breaks when they produce
collisions with DNA replication (37). The drug topotecan blocks the action
of the enzyme TOP1, thereby helping to cause DNA damage, improving
the sensitivity of chemotherapy and is already licensed to treat
recurrent ovarian cancer (38). As
apical kinases, ATM (recruited to double-strand breaks) and ATR
(recruited to single-stranded DNA) regulate the DNA damage
response, and ATR inhibitors may restore platinum sensitivity for
the treatment of patients with recurrent BRCA1/2 mutant ovarian
cancer (39,40).
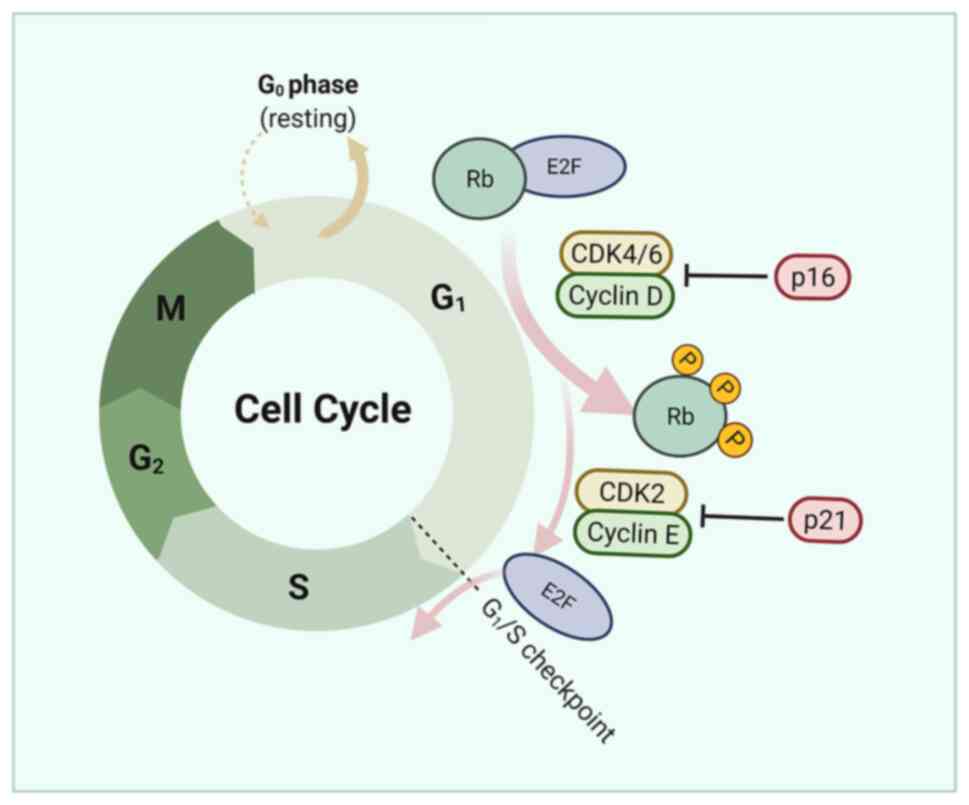
Cyclin-dependent kinase (CDKs) are proteins required
for appropriate progression of the cell cycle and also play a
central role in regulating DDR (44). CCNE1 is essential for CDK2
activation and its overexpression can lead to premature entry into
the S phase, abrogating DNA repair during the G1 phase and leading
to increased levels of replication stress. Checkpoint kinase 1 and
2 (CHK1 and CHK2) are responsible for regulating DNA replication
and DNA damage response (45).
Thus, CCNE1 overexpression may increase sensitivity to CHK1
inhibition (46). Promising
targeted strategies using WEE1 kinase inhibitors, CHK1 inhibitors
and CDK2 inhibitors are under review in clinical trials examining
biomarkers (47). The combination
of the CDK2 inhibitor dinaciclib and the AKT inhibitor MK2206
exhibited a selective synergistic effect in a CCNE1-expanded cell
line in a xenograft model (42).
Approximately 60% of patients with platinum-resistant or refractory
diseases receive clinical benefit from the CHK1 and CHK2 inhibitor,
prexasertib (LY2606368) (48).
Recently, a study reported that the CCNE1-overexpressing HGSC model
is markedly sensitive to combinations of cell cycle checkpoint
kinase and immune checkpoint inhibitors (29).
CCNE1 and RB1 are cyclins related to cell cycle
transition in the G1-S phase. Tumors with increased CCNE1 copy
number are more resistant to platinum therapy, while RB1 loss is
associated with high sensitivity to platinum therapy (49,50).
Studies have shown that loss of RB1 protein expression is
associated with longer OS and PFS (47,50,51).
ATM/ATR kinases play a central role in coordinating
the DDR. Blocking the activity of key CDKs can signal DNA damage
and cause cell cycle arrest. The combination of ATR inhibitor and
PARP inhibitor (PARPi) has a synergistic effect in reducing the
survival rate and colony formation in BRCA1/2 mutant
PARPi/platinum-resistant cell models that are resistant to PARPi
and platinum or exhibit de novo resistance (46).
CDK6 can bind to and phosphorylate FOXO3, thereby
inducing the expression of ATR. CDK6 regulates ATR through FOXO3 to
control platinum-induced cell death (52). In a model of advanced
platinum-resistant tumors, silencing or pharmacological inhibition
of CDK6 increased the sensitivity of EOC cells to platinum without
affecting RB1 phosphorylation but increased platinum-induced DNA
damage by increasing apoptosis (52). Notably, compared with other models,
CDK6 is less involved in regulating G1-S transition and
proliferation in EOC. When platinum and CDK6 inhibitor PD0332991
are combined, platinum induces significant cell cycle arrest in the
S phase (52), while CDK6
inhibition induces more apoptosis (Fig. 3).
Metabolic reprogramming is emerging as a proposed
molecular mechanism of cisplatin resistance (53). Accumulating evidence has suggested
that the metabolism of tumor tissues differs from that of matched
normal tissues and metabolic reprogramming is likely to be an
important cause of treatment resistance (54,55).
Metabolic reprogramming involves a series of metabolic alterations
involving all major pathways from glucose metabolism to glutamine
and lipids as well as mitochondrial (mt) alterations (56). The pentose phosphate pathway (PPP)
is an important component of glucose metabolism that uses
glucose-6-phosphate as the primary substrate (57). The products of PPP biosynthesis are
ribonucleotides and nicotinamide adenine dinucleotide phosphate;
the latter is essential for reductive biosynthesis (57).
Overexpression and higher enzyme activity of
glucose-6-phosphate dehydrogenase (G6PD) can increase cisplatin
resistance. G6PD and transketolase have been identified as possible
targets to overcome cisplatin resistance (53). The enzymes that regulate glycolysis
flow are transcriptionally regulated by three major transcription
factors: p53, hypoxia-inducible factor-1 (HIF-1) and Myc (58). HIF-1, a major hypoxia-induced
transcription factor, promotes a dissociation of glycolysis and the
tricarboxylic acid cycle (59).
HIF-1 allows adaptation to hypoxia by increasing glucose transport,
glycolysis and lactate production. In addition to stimulating
glycolysis, HIF-1 inhibits the function of the mt respiratory chain
in a variety of ways. Inhibition of HIF-1 may redirect aerobic
glycolysis toward mt oxidative phosphorylation, which can sensitize
cells to cisplatin through overproduction of reactive oxygen
species (ROS), leading to apoptosis; the cisplatin response in
ovarian cancer cells can be improved by targeting HIF-1-regulated
cancer metabolism (60). Studies
have demonstrated that metformin can modulate cell growth and
metabolism by inhibiting mt activity, AMP/ATP balance disturbance
and AMPK activation and can partially reverse platinum resistance
in the PDX model (61–63). This provides a new direction for
reducing resistance.
Cell metabolism induces the production of ROS; a
variety of chemotherapeutic drugs, including cisplatin, also induce
the production of large amounts of ROS in tumor cells. The
effectiveness of chemotherapy depends on the induction of oxidative
stress. Increased ROS can cause oxidative DNA damage, leading to
genomic instability and promoting cellular apoptosis, senescence or
autophagy. To withstand oxidative stress, cells activate the
transcription factor Nrf2, the major regulator of antioxidant
responsive element-mediated genes (64).
Activation of the Nrf2 pathway is involved in the
development of ovarian cancer and platinum resistance (65). Thus, targeting redox regulation is
a promising strategy to overcome drug resistance (66). In line with this, Nrf2 inhibition
is expected to increase chemotherapy sensitivity. Combining Nrf2
inhibition with chemotherapy enhances cytotoxic effects but
produces side effects such as chemotherapy-induced myelosuppression
(67). Additionally, Nrf2
inhibition results in enhanced sensitivity toward ROS-induced DNA
damage, whereas PARP inhibitors inhibit this DNA repair pathway
(68). Furthermore, PARP
inhibitors increase not only DNA damage but also ROS (69). Studies have revealed that combined
treatment with Nrf2 inhibitors and PARP inhibitors improves
therapeutic efficacy, particularly in BRCA1 mutant cancer cells and
no severe side effects are expected (68,69).
Mitochondria are important sites of redox activity.
Notably, compared with cisplatin-sensitive HGSC cells,
cisplatin-resistant HGSC cells have a lower mt content and lower
levels of mtROS, which induce cell death (70). The principle of anticancer
treatment with chemotherapeutic drugs is usually to disrupt cell
integrity by destroying nuclear DNA (nDNA) to induce cell death. In
addition, mtDNA, similar to nDNA, is greatly affected by cisplatin.
Therefore, mtDNA damage is evident in cisplatin-treated cells
(71,72). Furthermore, the ATP synthase
inhibitor oligomycin A can block mt function and prevent the
induction of mtROS during cisplatin treatment, thereby reducing
cisplatin-induced apoptosis (70).
In fact, several oxidative stress-related genes,
such as ARHGEF6, TXNRD1, GLA, GSTZ1 (73), thioredoxin (12), E26 oncogene homolog 1 (74,75)
and ALDH (76), have been linked
to chemoresistance in ovarian cancer. Increasing ROS through
pharmacological methods may render ovarian cancer cells sensitive
to cisplatin and overcome drug resistance. An ALDH inhibitor named
CM37, has been revealed to increase intracellular ROS levels in
ovarian cancer cells, leading to DNA damage and inhibition of cell
survival and proliferation (76).
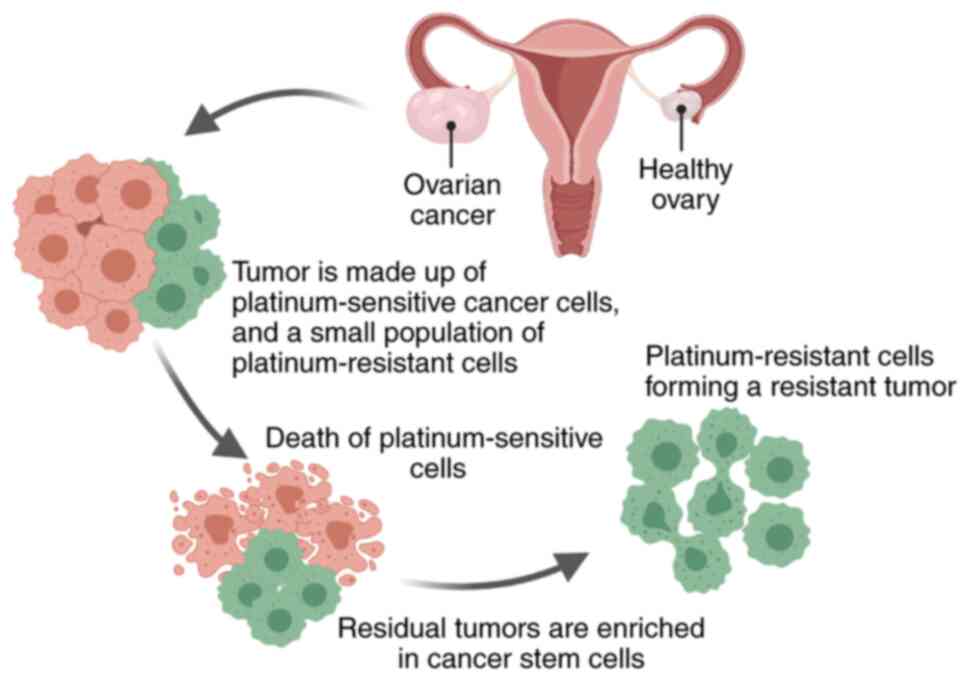
The current oncology hypothesis proposes that only a
small percentage of cancer cells can spread into the tumor. These
cells are called tumor promoter cells or CSCs and have pluripotent
properties similar to those of normal stem cells (77,78).
Previous studies have revealed that CSCs are a unique cell
population that causes tumor recurrence and metastasis, leading to
the formation of new tumors (79,80).
CSCs also exist in ovarian tumors and are resistant to chemotherapy
(81,82) Therefore, the development of new
therapies for CSCs aims to improve the lives of patients with
cancer, particularly those with metastatic disease, and to avoid
the recurrence of chemotherapy-resistant tumors.
One understudied mechanism of chemoresistance is the
persistence of quiescent cancer cells that are not eliminated by
chemotherapy. According to previous studies, these residual tumors
are enriched in CSCs (83), which
are more resistant to chemotherapy (Fig. 4) (74,84,85).
There are already several surface markers for ovarian CSC
identification, including MyoD, CD44, CD117 (86), CD133 (87), ALDH (76) and nuclear factor of activated T
cells, cytoplasmic 4 (NFATC4) (88,89).
CD44 is a cell-surface glycoprotein of the
hyaluronate receptor that plays a role in tumor stemness,
recurrence and drug resistance in ovarian cancer. Compared with
cells cultured under differentiation conditions, isolated
CD44+/CD117+ ovarian CSCs could completely
regenerate the original tumor phenotype in mice and were more
resistant to cisplatin and paclitaxel (90). The presence of
ALDH+CD133+ CSC-like cells in primary ovarian
tumors is associated with shorter disease-free survival and OS. In
addition, compared with parental cells, these cells exhibit
enhanced chemoresistance to the human primary ovarian tumor
phenotype (89,91). The aforementioned studies revealed
that CSCs are closely related to the chemoresistance of ovarian
cancer. Metformin treatment significantly reduces the stemness of
cancer by reducing ALDH+CD133+ CSCs in
patients with ovarian cancer (92). Phase III clinical trials have shown
that metformin is a favorable adjunct in the treatment of EOC.
Studies have demonstrated that activating the
PI3K/Akt/mTOR signaling pathway can enhance the expression of
epithelial-mesenchymal transition (EMT) and CSC markers in
chemoresistant EOC cells. Accordingly, the PI3K inhibitor BEZ235
renders EOC cells sensitive to cisplatin by inhibiting the
expression of EMT and CSC markers (91). NFATC4 is enriched in ovarian
CSC-like cells, which leads to chemotherapy resistance by
downregulating MYC at an early stage, helping cells enter a
quiescent state (88). The
aforementioned studies revealed that NFATC4 is a significant
therapeutic target for ovarian cancer that is worthy of in-depth
study.
There is increasing evidence that the immune
response may affect the prognosis of patients with ovarian cancer.
For patients with recurrent ovarian cancer, the immune system can
be activated to identify and attack cancer cells to prevent
recurrence. The tumor microenvironment is a potential factor for
recurrence and chemotherapy resistance (93). Among them, the presence of
tumor-infiltrating lymphocytes, particularly CD8+
tumor-infiltrating lymphocytes, often indicates an improved
prognosis (94). In a study of
patients receiving platinum-based chemotherapy, the 5-year OS rate
of patients whose tumors contained significant T-cell infiltration
was 38%, but the 5-year OS rate of patients whose tumors contained
very few T cells was only 4.5% (95). CXCL9, CCL21 and CCL22 were more
highly expressed in tumors with significant T-cell infiltrates than
in tumors with few T cells (96,97).
Several studies have shown that high
tumor-associated macrophage (TAM) density is closely related to
poor prognosis and resistance to treatment in patients with ovarian
cancer (98,99). The mechanism of drug resistance is
as follows: i) Macrophages promote tumor polarization; ii)
macrophages affect the pro-survival signaling pathways; and iii)
macrophages upregulate MDR genes in cancer cells (10). In various ovarian cancer cell lines
treated with cisplatin or carboplatin, macrophages are induced to
differentiate into M2 macrophages through increased IL-10
production and enhanced activation of the STAT3 signaling factor
(100,101). The understanding of the
involvement of TAMs in tumor progression and chemoresistance
provided by these studies has revealed new opportunities for the
development of ovarian cancer therapies (10,99).
In addition, the immune checkpoint protein
programmed death ligand (PD-L1) is often expressed by ovarian tumor
cells, and PD-1 is a receptor often expressed by tumor-infiltrating
lymphocytes. Studies have found that the interaction between PD-1
and PD-L1 is a key therapeutic target for reactivating the immune
response against a variety of cancers. Therefore, blocking the
PD-1/PD-L1 interaction with an antibody against the PD-L1 molecule
is a new therapeutic opportunity for patients with advanced
platinum-resistant ovarian cancer (102–104). The human immunoglobulin G1
monoclonal antibody avelumab has a wild-type Fc region that blocks
PD-L1. Avelumab has shown antitumor activity in patients with
relapsed or refractory ovarian cancer who have progressed after
platinum-based chemotherapy (13).
The effectiveness of chemotherapy strongly depends
on the ability of ovarian cancer cells to undergo drug-induced
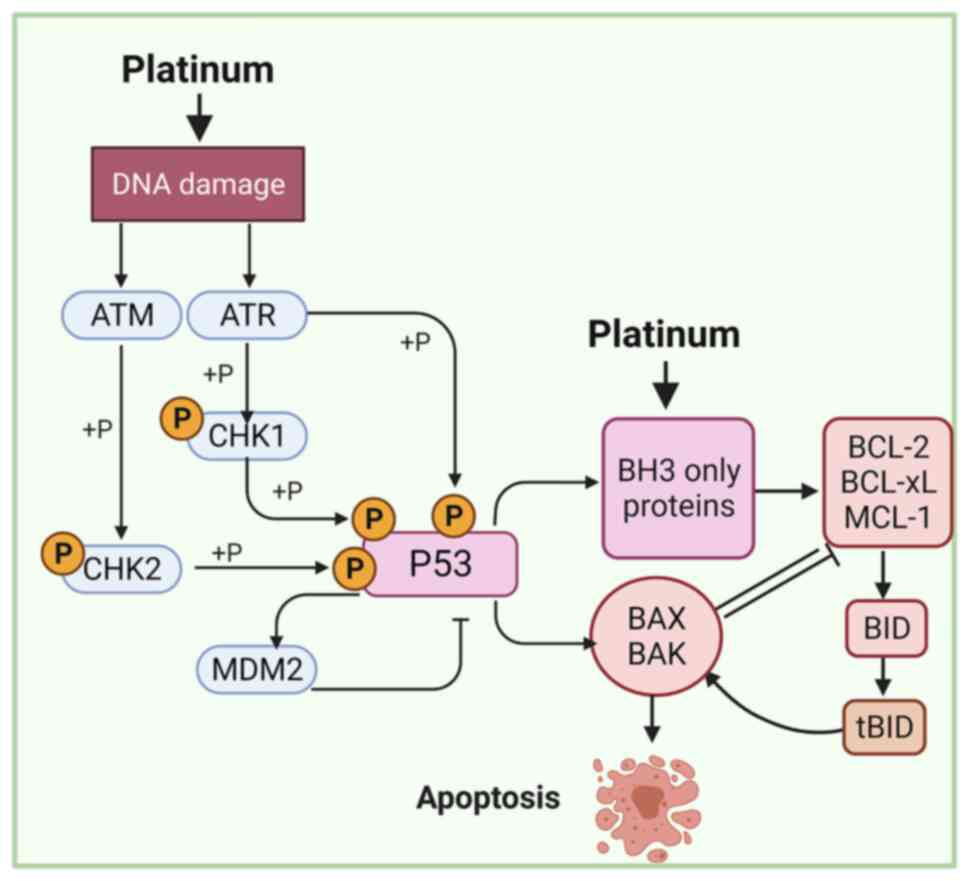
apoptosis (Fig. 5) (105–107). Platinum-based chemotherapeutics,
such as carboplatin and cisplatin, are alkylating agents that bind
to DNA to produce intra- and interstrand crosslinks, thereby
inducing DNA damage that culminates in mitochondria-mediated
apoptosis (108,109). The mt apoptotic pathway is
controlled by proapoptotic (e.g., PUMA, Bim, Bid, Bad, Bik, Bax,
Noxa and Bmf) and antiapoptotic (e.g., Bcl-2 Bcl-w, Mcl-1, Bfl-1
and Bcl-xL) proteins of the Bcl-2 family (110). Inhibition of apoptosis-related
proteins in ovarian cancer cells increases cisplatin resistance and
PI3K/mTOR inhibits the induction of pro-apoptotic and
anti-apoptotic Bcl-2 family proteins, which are associated with
treatment fragility in ovarian cancer cells (111).
ARID1A mutation is a known genetic driver of ovarian
cancer. Notably, ARID1A mutations are found in more than 50% of
ovarian clear cell carcinomas and 30% of ovarian endometrioid
carcinomas (116,117). ARID1A and TP53 mutations are
typically mutually exclusive in ovarian cancer (118). ARID1A mutations lead to
upregulation of HDAC6, which in turn inactivates the
apoptosis-promoting function of p53, indicating that drug
inhibition of HDAC6 is an effective treatment strategy for cancers
with ARID1A mutations (119).
On the one hand, autophagy protects cells from
genotoxic stress to prevent tumorigenesis and carcinogenic
transformation. On the other hand, autophagy can be used as a
survival strategy for cancer cells to overcome the stress caused by
chemotherapy, radiotherapy or other treatments (120,121). Previous studies have demonstrated
that autophagy in ovarian cancer cells can be induced by cisplatin
through ubiquitin-binding protein p62 (SQSTM1) or HMGB1 (122,123). Autophagic flux in
cisplatin-resistant ovarian cancer cells is caused by cisplatin. At
present, the cytoprotective function of autophagy in cancer cells
is considered a potential chemotherapy resistance mechanism.
Most studies have suggested that targeting
autophagy-related molecules may increase the chemosensitivity of
cancer cells (124,125). Knockdown of ATG7 and ATG14 can
inhibit basic autophagy in ovarian cancer cells and promote cell
death induced by cisplatin (126). As a negative regulator of
autophagy, mTOR inhibitors are used in combination with cisplatin
to make cancer cells sensitive to chemotherapy. However, certain
studies have suggested that chemosensitivity can be promoted by
autophagy (127,128). The enhanced sensitivity of
autophagy to cisplatin depends on different mechanisms, and dormant
autophagic cancer cells are still susceptible to cisplatin-based
chemotherapy.
A total of 10% of serous ovarian cancer is LGSC,
which is characterized by early onset (median age 46 years), slow
growth and poor response to chemotherapy (4,129).
BRAF and KRAS hotspot mutations are found in ~2/3 of patients with
LGSC. Furthermore, almost all patients with LGSC harbor a mutation
predicted to induce ERK activation (5). Notably, RAS and PI3K participate in
intensive cross-talk to regulate each other and coregulate
downstream functions (130).
Therefore, blocking only one pathway will induce compensatory
signaling in the other pathway, ultimately leading to treatment
failure and relapse (131).
PI3K/Akt/mTOR signaling plays an important role in
regulating the cell cycle, quiescence and proliferation. Various
somatic mutations in PTEN, Akt1 and mTOR have been identified in
ovarian cancer and can induce enhanced PI3K/Akt/mTOR signaling
(132,133). Excessive activation of
PI3K/Akt/mTOR signaling is associated to chemoresistance and cancer
metastasis and inhibition of PI3K/Akt/mTOR signaling can restore
the sensitivity of chemotherapy-resistant ovarian cancer cells to
chemotherapy drugs (134).
Furthermore, combination treatment using RAS and PI3K inhibitors in
ovarian cancer cell lines carrying activated oncogenic
KRASG12D and deletion of two copies of the PTEN gene is
a promising strategy for tumors that are rapidly resistant to
targeted therapy alone (135). In
addition, Wnt receptor Frizzled 7 (FZD7) is expressed in tumors and
platinum-resistant cells. Knockdown of FZD7 reduces spheroid
formation, increases sensitivity to platinum, and delays tumor
occurrence (83).
As a cell surface transmembrane glycoprotein, the
folate receptor (FR) promotes the unidirectional transport of
folates into the cell. FR has limited distribution, and aberrant
overexpression of FR is a characteristic of numerous epithelial
tumors, including non-small cell lung, endometrial and ovarian
cancer. Specifically, ~80% of EOC tumors constitutively express FR
(136). In addition, the increase
in receptor expression may be a negative prognostic factor for the
chemotherapy response of this malignant tumor (137). Preclinical studies have revealed
that folate-conjugated vintafolide (EC145) (138) and mirvetuximab soravtansine
(IMGN853) (139) are well
tolerated and active against platinum-resistant ovarian cancer, and
response rates and PFS are encouraging.
Despite advances in chemotherapy, the 5-year
survival rate of patients with ovarian cancer remains less than
50%, mainly due to chemotherapy resistance (22). Both primary resistance (patients do
not respond at all to treatment and the disease progresses) and
acquired resistance (patients eventually develop acquired
resistance after an initial response) to platinum are associated
with a severely negative prognosis for EOC patients. For these
patients, a thorough understanding of their resistance mechanisms
and active drugs is an urgent unmet clinical need (140). The resistance of ovarian cancer
cells to chemotherapeutic mechanisms is rather complex and includes
MDR, DDR, cell metabolism, oxidative stress, cell cycle regulation,
CSCs, immunity, apoptotic pathways, autophagy and abnormal
signaling pathways. Therefore, a single mechanism cannot fully
explain the resistance of ovarian cancer cells to treatment.
Numerous new strategies are being studied to try to
overcome this chemical resistance, including combining
platinum-based chemotherapy with new molecularly targeted drugs,
such as bevacizumab or olaparib. Combining the vascular endothelial
growth factor A-neutralizing antibody bevacizumab with chemotherapy
has been revealed to reduce or slow the growth of advanced EOC, but
this combination does not appear to extend survival (11). Olaparib is a PARP inhibitor that is
only used for cancer patients with BRCA gene mutations since the
drug only works on cells where the BRCA pathway is blocked
(36). However, only a small
percentage of patients with ovarian cancer have mutations in the
BRCA gene (141). More detailed
mechanistic insights and the development of biomarkers,
particularly non-invasive biomarkers, are required to accurately
select patients for therapy and facilitate the evaluation of
therapeutic efficacy in real time.
Not applicable.
The present study was supported by the West China Second
University Hospital Clinical Research Fund (grant no. KL105) and
the Sichuan Provincial Key Research and Development Projects
(grant. no. 2020YFS0081).
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
JM designed the review and edited the manuscript. LY
and HJX wrote the manuscript. HJX, YYL, XXL, and XW collected and
analyzed data. Data authentication is not applicable. All authors
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matulonis UA, Sood AK, Fallowfield L,
Howitt BE, Sehouli J and Karlan BY: Ovarian cancer. Nat Rev Dis
Primers. 2:160612016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orr B and Edwards RP: Diagnosis and
treatment of ovarian cancer. Hematol Oncol Clin North Am.
32:943–964. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gershenson DM, Bodurka DC, Lu KH, Nathan
LC, Milojevic L, Wong KK, Malpica A and Sun CC: Impact of age and
primary disease site on outcome in women with low-grade serous
carcinoma of the ovary or peritoneum: Results of a large
single-institution registry of a rare tumor. J Clin Oncol.
33:2675–2682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grisham RN, Sylvester BE, Won H, McDermott
G, DeLair D, Ramirez R, Yao Z, Shen R, Dao F, Bogomolniy F, et al:
Extreme outlier analysis identifies occult mitogen-activated
protein kinase pathway mutations in patients with low-grade serous
ovarian cancer. J Clin Oncol. 33:4099–4105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chui MH, Chang JC, Zhang Y, Zehir A,
Schram AM, Konner J, Drilon AE, Da Cruz Paula A, Weigelt B and
Grisham RN: Spectrum of BRAF mutations and gene rearrangements in
ovarian serous carcinoma. JCO Precis Oncol. 5:PO.21.00055.
2021.PubMed/NCBI
|
|
7
|
Davidson B and Tropé CG: Ovarian cancer:
Diagnostic, biological and prognostic aspects. Women's Health
(Lond). 10:519–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reavis HD and Drapkin R: The tubal
epigenome-An emerging target for ovarian cancer. Pharmacol Ther.
210:1075242020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coleman RL, Duska LR, Ramirez PT, Heymach
JV, Kamat AA, Modesitt SC, Schmeler KM, Iyer RB, Garcia ME, Miller
DL, et al: Phase 1–2 study of docetaxel plus aflibercept in
patients with recurrent ovarian, primary peritoneal, or fallopian
tube cancer. Lancet Oncol. 12:1109–1117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nowak M and Klink M: The Role of
Tumor-associated macrophages in the progression and chemoresistance
of ovarian cancer. Cells. 9:12992020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aghajanian C, Blank SV, Goff BA, Judson
PL, Teneriello MG, Husain A, Sovak MA, Yi J and Nycum LR: OCEANS: A
randomized, double-blind, placebo-controlled phase III trial of
chemotherapy with or without bevacizumab in patients with
platinum-sensitive recurrent epithelial ovarian, primary
peritoneal, or fallopian tube cancer. J Clin Oncol. 30:2039–2045.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cruz IN, Coley HM, Kramer HB, Madhuri TK,
Safuwan NA, Angelino AR and Yang M: Proteomics analysis of ovarian
cancer cell lines and tissues reveals drug resistance-associated
proteins. Cancer Genomics Proteomics. 14:35–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Disis ML, Taylor MH, Kelly K, Beck JT,
Gordon M, Moore KM, Patel MR, Chaves J, Park H, Mita AC, et al:
Efficacy and safety of avelumab for patients with recurrent or
refractory ovarian cancer: Phase 1b results from the JAVELIN solid
tumor trial. JAMA Oncol. 5:393–401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moufarrij S, Dandapani M, Arthofer E,
Gomez S, Srivastava A, Lopez-Acevedo M, Villagra A and Chiappinelli
KB: Epigenetic therapy for ovarian cancer: Promise and progress.
Clin Epigenetics. 11:72019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren F, Shen J, Shi H, Hornicek FJ, Kan Q
and Duan Z: Novel mechanisms and approaches to overcome multidrug
resistance in the treatment of ovarian cancer. Biochim Biophys
Acta. 1866:266–275. 2016.PubMed/NCBI
|
|
16
|
Chen AM, Zhang M, Wei D, Stueber D,
Taratula O, Minko T and He H: Co-delivery of doxorubicin and Bcl-2
siRNA by mesoporous silica nanoparticles enhances the efficacy of
chemotherapy in multidrug-resistant cancer cells. Small.
5:2673–2677. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zalewski M, Kulbacka J, Saczko J,
Drag-Zalesinska M and Choromanska A: Valspodar-modulated
chemotherapy in human ovarian cancer cells SK-OV-3 and MDAH-2774.
Bosn J Basic Med Sci. 19:234–241. 2019.PubMed/NCBI
|
|
18
|
Baekelandt M, Lehne G, Tropé CG, Szántó I,
Pfeiffer P, Gustavssson B and Kristensen GB: Phase I/II trial of
the multidrug-resistance modulator valspodar combined with
cisplatin and doxorubicin in refractory ovarian cancer. J Clin
Oncol. 19:2983–2993. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gee ME, Faraahi Z, McCormick A and
Edmondson RJ: DNA damage repair in ovarian cancer: Unlocking the
heterogeneity. J Ovarian Res. 11:502018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sengupta D, Mukhopadhyay A and Sengupta K:
Emerging roles of lamins and DNA damage repair mechanisms in
ovarian cancer. Biochem Soc Trans. 48:2317–2333. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ledermann JA, Drew Y and Kristeleit RS:
Homologous recombination deficiency and ovarian cancer. Eur J
Cancer. 60:49–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Christie EL and Bowtell DDL: Acquired
chemotherapy resistance in ovarian cancer. Ann Oncol. 28 (Suppl
8):viii13–viii15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karakashev S, Fukumoto T, Zhao B, Lin J,
Wu S, Fatkhutdinov N, Park PH, Semenova G, Jean S, Cadungog MG, et
al: EZH2 inhibition sensitizes CARM1-high, homologous recombination
proficient ovarian cancers to PARP Inhibition. Cancer Cell.
37:157–167.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moschetta M, George A, Kaye SB and
Banerjee S: BRCA somatic mutations and epigenetic BRCA
modifications in serous ovarian cancer. Ann Oncol. 27:1449–1455.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li
Q, Tian R, Bowman-Colin C, Li Y, Greene-Colozzi A, Iglehart JD, et
al: Telomeric allelic imbalance indicates defective DNA repair and
sensitivity to DNA-damaging agents. Cancer Discov. 2:366–375. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du Y, Yamaguchi H, Wei Y, Hsu JL, Wang HL,
Hsu YH, Lin WC, Yu WH, Leonard PG, Lee GR IV, et al: Blocking
c-Met-mediated PARP1 phosphorylation enhances anti-tumor effects of
PARP inhibitors. Nat Med. 22:194–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brown JS, O'Carrigan B, Jackson SP and Yap
TA: Targeting DNA repair in cancer: Beyond PARP inhibitors. Cancer
Discov. 7:20–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alsop K, Fereday S, Meldrum C, DeFazio A,
Emmanuel C, George J, Dobrovic A, Birrer MJ, Webb PM, Stewart C, et
al: BRCA mutation frequency and patterns of treatment response in
BRCA mutation-positive women with ovarian cancer: A report from the
Australian Ovarian Cancer Study Group. J Clin Oncol. 30:2654–2663.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iyer S, Zhang S, Yucel S, Horn H, Smith
SG, Reinhardt F, Hoefsmit E, Assatova B, Casado J, Meinsohn MC, et
al: Genetically defined syngeneic mouse models of ovarian cancer as
tools for the discovery of combination immunotherapy. Cancer
Discov. 11:384–407. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Domchek SM: Reversion mutations with
clinical use of PARP inhibitors: Many genes, many versions. Cancer
Discov. 7:937–939. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pietragalla A, Arcieri M, Marchetti C,
Scambia G and Fagotti A: Ovarian cancer predisposition beyond BRCA1
and BRCA2 genes. Int J Gynecol Cancer. 30:1803–1810. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin KK, Harrell MI, Oza AM, Oaknin A,
Ray-Coquard I, Tinker AV, Helman E, Radke MR, Say C, Vo LT, et al:
BRCA reversion mutations in circulating tumor DNA predict primary
and acquired resistance to the PARP inhibitor rucaparib in
high-grade ovarian carcinoma. Cancer Discov. 9:210–219. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Norquist B, Wurz KA, Pennil CC, Garcia R,
Gross J, Sakai W, Karlan BY, Taniguchi T and Swisher EM: Secondary
somatic mutations restoring BRCA1/2 predict chemotherapy resistance
in hereditary ovarian carcinomas. J Clin Oncol. 29:3008–3015. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kondrashova O, Nguyen M, Shield-Artin K,
Tinker AV, Teng NNH, Harrell MI, Kuiper MJ, Ho GY, Barker H, Jasin
M, et al: Secondary somatic mutations restoring RAD51C and RAD51D
associated with acquired resistance to the PARP inhibitor rucaparib
in high-grade ovarian carcinoma. Cancer Discov. 7:984–998. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He YJ, Meghani K, Caron MC, Yang C, Ronato
DA, Bian J, Sharma A, Moore J, Niraj J, Detappe A, et al: DYNLL1
binds to MRE11 to limit DNA end resection in BRCA1-deficient cells.
Nature. 563:522–526. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Penson RT, Valencia RV, Cibula D, Colombo
N, Leath CA III, Bidziński M, Kim JW, Nam JH, Madry R, Hernández C,
et al: Olaparib versus nonplatinum chemotherapy in patients with
platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2
mutation (SOLO3): A randomized phase III trial. J Clin Oncol.
38:1164–1174. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marzi L, Szabova L, Gordon M, Weaver Ohler
Z, Sharan SK, Beshiri ML, Etemadi M, Murai J, Kelly K and Pommier
Y: The indenoisoquinoline TOP1 inhibitors selectively target
homologous recombination-deficient and schlafen 11-positive cancer
cells and synergize with olaparib. Clin Cancer Res. 25:6206–6216.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yan S, Xuan J, Brajanovski N, Tancock MRC,
Madhamshettiwar PB, Simpson KJ, Ellis S, Kang J, Cullinane C,
Sheppard KE, et al: The RNA polymerase I transcription inhibitor
CX-5461 cooperates with topoisomerase 1 inhibition by enhancing the
DNA damage response in homologous recombination-proficient
high-grade serous ovarian cancer. Br J Cancer. 124:616–627. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yap TA, O'Carrigan B, Penney MS, Lim JS,
Brown JS, de Miguel Luken MJ, Tunariu N, Perez-Lopez R, Rodrigues
DN, Riisnaes R, et al: Phase I trial of first-in-class ATR
Inhibitor M6620 (VX-970) as monotherapy or in combination with
carboplatin in patients with advanced solid tumors. J Clin Oncol.
38:3195–3204. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Konstantinopoulos PA, Cheng SC, Wahner
Hendrickson AE, Penson RT, Schumer ST, Doyle LA, Lee EK, Kohn EC,
Duska LR, Crispens MA, et al: Berzosertib plus gemcitabine versus
gemcitabine alone in platinum-resistant high-grade serous ovarian
cancer: A multicentre, open-label, randomised, phase 2 trial.
Lancet Oncol. 21:957–968. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakayama N, Nakayama K, Shamima Y,
Ishikawa M, Katagiri A, Iida K and Miyazaki K: Gene amplification
CCNE1 is related to poor survival and potential therapeutic target
in ovarian cancer. Cancer. 116:2621–2634. 2010.PubMed/NCBI
|
|
42
|
Au-Yeung G, Lang F, Azar WJ, Mitchell C,
Jarman KE, Lackovic K, Aziz D, Cullinane C, Pearson RB, Mileshkin
L, et al: Selective targeting of cyclin E1-Amplified high-grade
serous ovarian cancer by cyclin-dependent kinase 2 and AKT
Inhibition. Clin Cancer Res. 23:1862–1874. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Etemadmoghadam D, Weir BA, Au-Yeung G,
Alsop K, Mitchell G, George J; Australian Ovarian Cancer Study
Group, ; Davis S, D'Andrea AD, Simpson K, et al: Synthetic
lethality between CCNE1 amplification and loss of BRCA1. Proc Natl
Acad Sci USA. 110:19489–19494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Campbell GJ, Hands EL and Van de Pette M:
The Role of CDKs and CDKIs in murine development. Int J Mol Sci.
21:53432020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Angius G, Tomao S, Stati V, Vici P, Bianco
V and Tomao F: Prexasertib, a checkpoint kinase inhibitor: From
preclinical data to clinical development. Cancer Chemother
Pharmacol. 85:9–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim H, Xu H, George E, Hallberg D, Kumar
S, Jagannathan V, Medvedev S, Kinose Y, Devins K, Verma P, et al:
Combining PARP with ATR inhibition overcomes PARP inhibitor and
platinum resistance in ovarian cancer models. Nat Commun.
11:37262020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gorski JW, Ueland FR and Kolesar JM: CCNE1
amplification as a predictive biomarker of chemotherapy resistance
in epithelial ovarian cancer. Diagnostics (Basel). 10:2792020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gralewska P, Gajek A, Marczak A and
Rogalska A: Participation of the ATR/CHK1 pathway in replicative
stress targeted therapy of high-grade ovarian cancer. J Hematol
Oncol. 13:392020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Garsed DW, Alsop K, Fereday S, Emmanuel C,
Kennedy CJ, Etemadmoghadam D, Gao B, Gebski V, Garès V, Christie
EL, et al: Homologous recombination DNA repair pathway disruption
and retinoblastoma protein loss are associated with exceptional
survival in high-grade serous ovarian cancer. Clin Cancer Res.
24:569–580. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
da Costa AABA, do Canto LM, Larsen SJ,
Ribeiro ARG, Stecca CE, Petersen AH, Aagaard MM, de Brot L,
Baumbach J, Baiocchi G, et al: Genomic profiling in ovarian cancer
retreated with platinum based chemotherapy presented homologous
recombination deficiency and copy number imbalances of CCNE1 and
RB1 genes. BMC Cancer. 19:4222019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shi M, Whorton AE, Sekulovski N, Paquet M,
MacLean JA, Song Y, Van Dyke T and Hayashi K: Inactivation of
TRP53, PTEN, RB1, and/or CDH1 in the ovarian surface epithelium
induces ovarian cancer transformation and metastasis. Biol Reprod.
102:1055–1064. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dall'Acqua A, Sonego M, Pellizzari I,
Pellarin I, Canzonieri V, D'Andrea S, Benevol S, Sorio R, Giorda G,
Califano D, et al: CDK6 protects epithelial ovarian cancer from
platinum-induced death via FOXO3 regulation. EMBO Mol Med.
9:1415–1433. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Giacomini I, Ragazzi E, Pasut G and
Montopoli M: The pentose phosphate pathway and its involvement in
cisplatin resistance. Int J Mol Sci. 21:9372020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Morandi A and Indraccolo S: Linking
metabolic reprogramming to therapy resistance in cancer. Biochim
Biophys Acta Rev Cancer. 868:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhao Y, Butler EB and Tan M: Targeting
cellular metabolism to improve cancer therapeutics. Cell Death Dis.
4:e5322013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Butler EB, Zhao Y, Muñoz-Pinedo C, Lu J
and Tan M: Stalling the engine of resistance: Targeting cancer
metabolism to overcome therapeutic resistance. Cancer Res.
73:2709–2717. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tennant DA, Durán RV and Gottlieb E:
Targeting metabolic transformation for cancer therapy. Nat Rev
Cancer. 10:267–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yeung SJ, Pan J and Lee MH: Roles of p53,
MYC and HIF-1 in regulating glycolysis-the seventh hallmark of
cancer. Cell Mol Life Sci. 65:3981–3999. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Icard P, Shulman S, Farhat D, Steyaert JM,
Alifano M and Lincet H: How the Warburg effect supports
aggressiveness and drug resistance of cancer cells? Drug Resist
Updat. 38:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ai Z, Lu Y, Qiu S and Fan Z: Overcoming
cisplatin resistance of ovarian cancer cells by targeting
HIF-1-regulated cancer metabolism. Cancer Lett. 373:36–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ricci F, Brunelli L, Affatato R, Chilà R,
Verza M, Indraccolo S, Falcetta F, Fratelli M, Fruscio R,
Pastorelli R and Damia G: Overcoming platinum-acquired resistance
in ovarian cancer patient-derived xenografts. Ther Adv Med Oncol.
11:17588359198395432019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Urpilainen E, Puistola U, Boussios S and
Karihtala P: Metformin and ovarian cancer: The evidence. Ann Transl
Med. 8:17112020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kim TH, Suh DH, Kim MK and Song YS:
Metformin against cancer stem cells through the modulation of
energy metabolism: Special considerations on ovarian cancer. Biomed
Res Int. 2014:1327022014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Itoh K, Tong KI and Yamamoto M: Molecular
mechanism activating Nrf2-Keap1 pathway in regulation of adaptive
response to electrophiles. Free Radic Biol Med. 36:1208–1213. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu X, Han LY, Zhang XX and Wang L: The
study of Nrf2 signaling pathway in ovarian cancer. Crit Rev
Eukaryot Gene Expr. 28:329–336. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gentric G, Kieffer Y, Mieulet V, Goundiam
O, Bonneau C, Nemati F, Hurbain I, Raposo G, Popova T, Stern MH, et
al: PML-regulated mitochondrial metabolism enhances
chemosensitivity in human ovarian cancers. Cell Metab.
29:156–173.e10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lister A, Nedjadi T, Kitteringham NR,
Campbell F, Costello E, Lloyd B, Copple IM, Williams S, Owen A,
Neoptolemos JP, et al: Nrf2 is overexpressed in pancreatic cancer:
Implications for cell proliferation and therapy. Mol Cancer.
10:372011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
van der Wijst MG, Huisman C, Mposhi A,
Roelfes G and Rots MG: Targeting Nrf2 in healthy and malignant
ovarian epithelial cells: Protection versus promotion. Mol Oncol.
9:1259–1273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hou D, Liu Z, Xu X, Liu Q, Zhang X, Kong
B, Wei JJ, Gong Y and Shao C: Increased oxidative stress mediates
the antitumor effect of PARP inhibition in ovarian cancer. Redox
Biol. 17:99–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kleih M, Böpple K, Dong M, Gaissler A,
Heine S, Olayioye MA, Aulitzky WE and Essmann F: Direct impact of
cisplatin on mitochondria induces ROS production that dictates cell
fate of ovarian cancer cells. Cell Death Dis. 10:8512019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Podratz JL, Knight AM, Ta LE, Staff NP,
Gass JM, Genelin K, Schlattau A, Lathroum L and Windebank AJ:
Cisplatin induced mitochondrial DNA damage in dorsal root ganglion
neurons. Neurobiol Dis. 41:661–668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang Z, Schumaker LM, Egorin MJ, Zuhowski
EG, Guo Z and Cullen KJ: Cisplatin preferentially binds
mitochondrial DNA and voltage-dependent anion channel protein in
the mitochondrial membrane of head and neck squamous cell
carcinoma: Possible role in apoptosis. Clin Cancer Res.
12:5817–5825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang J, Yang L, Xiang X, Li Z, Qu K and
Li K: A panel of three oxidative stress-related genes predicts
overall survival in ovarian cancer patients received platinum-based
chemotherapy. Aging (Albany NY). 10:1366–1379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Verschoor ML and Singh G: Ets-1 regulates
intracellular glutathione levels: Key target for resistant ovarian
cancer. Mol Cancer. 12:1382013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wilson LA, Yamamoto H and Singh G: Role of
the transcription factor Ets-1 in cisplatin resistance. Mol Cancer
Ther. 3:823–832. 2004.PubMed/NCBI
|
|
76
|
Nwani NG, Condello S, Wang Y, Swetzig WM,
Barber E, Hurley T and Matei D: A Novel ALDH1A1 inhibitor targets
cells with stem cell characteristics in ovarian cancer. Cancers
(Basel). 11:5022019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Beck B and Blanpain C: Unravelling cancer
stem cell potential. Nat Rev Cancer. 13:727–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Carnero A, Garcia-Mayea Y, Mir C, Lorente
J, Rubio IT and LLeonart ME: The cancer stem-cell signaling network
and resistance to therapy. Cancer Treat Rev. 49:25–36. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Maugeri-Saccà M, Vigneri P and De Maria R:
Cancer stem cells and chemosensitivity. Clin Cancer Res.
17:4942–4947. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hu L, McArthur C and Jaffe RB: Ovarian
cancer stem-like side-population cells are tumourigenic and
chemoresistant. Br J Cancer. 102:1276–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang S, Balch C, Chan MW, Lai HC, Matei
D, Schilder JM, Yan PS, Huang TH and Nephew KP: Identification and
characterization of ovarian cancer-initiating cells from primary
human tumors. Cancer Res. 68:4311–4320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang Y, Zhao G, Condello S, Huang H,
Cardenas H, Tanner EJ, Wei J, Ji Y, Li J, Tan Y, et al: Frizzled-7
identifies platinum-tolerant ovarian cancer cells susceptible to
ferroptosis. Cancer Res. 81:384–399. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen J, Cao X, An Q, Zhang Y, Li K, Yao W,
Shi F, Pan Y, Jia Q, Zhou W, et al: Inhibition of cancer stem cell
like cells by a synthetic retinoid. Nat Commun. 9:14062018.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Muñoz-Galván S, Felipe-Abrio B,
Verdugo-Sivianes EM, Perez M, Jiménez-García MP, Suarez-Martinez E,
Estevez-Garcia P and Carnero A: Downregulation of MYPT1 increases
tumor resistance in ovarian cancer by targeting the Hippo pathway
and increasing the stemness. Mol Cancer. 19:72020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Keyvani V, Farshchian M, Esmaeili SA, Yari
H, Moghbeli M, Nezhad SK and Abbaszadegan MR: Ovarian cancer stem
cells and targeted therapy. J Ovarian Res. 12:1202019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Baba T, Convery PA, Matsumura N, Whitaker
RS, Kondoh E, Perry T, Huang Z, Bentley RC, Mori S, Fujii S, et al:
Epigenetic regulation of CD133 and tumorigenicity of
CD133+ ovarian cancer cells. Oncogene. 28:209–218. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cole AJ, Iyengar M, Panesso-Gómez S,
O'Hayer P, Chan D, Delgoffe GM, Aird KM, Yoon E, Bai S and
Buckanovich RJ: NFATC4 promotes quiescence and chemotherapy
resistance in ovarian cancer. JCI Insight. 5:e1314862020.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Silva IA, Bai S, McLean K, Yang K,
Griffith K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds
RK, et al: Aldehyde dehydrogenase in combination with CD133 defines
angiogenic ovarian cancer stem cells that portend poor patient
survival. Cancer Res. 71:3991–4001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li SS, Ma J and Wong AST: Chemoresistance
in ovarian cancer: Exploiting cancer stem cell metabolism. J
Gynecol Oncol. 29:e322018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Deng J, Bai X, Feng X, Ni J, Beretov J,
Graham P and Li Y: Inhibition of PI3K/Akt/mTOR signaling pathway
alleviates ovarian cancer chemoresistance through reversing
epithelial-mesenchymal transition and decreasing cancer stem cell
marker expression. BMC Cancer. 19:6182019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Brown JR, Chan DK, Shank JJ, Griffith KA,
Fan H, Szulawski R, Yang K, Reynolds RK, Johnston C, McLean K, et
al: Phase II clinical trial of metformin as a cancer stem
cell-targeting agent in ovarian cancer. JCI Insight.
5:e1332472020.PubMed/NCBI
|
|
93
|
Bogani G, Lopez S, Mantiero M, Ducceschi
M, Bosio S, Ruisi S, Sarpietro G, Guerrisi R, Brusadelli C,
Dell'Acqua A, et al: Immunotherapy for platinum-resistant ovarian
cancer. Gynecol Oncol. 158:484–488. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Nelson BH: The impact of T-cell immunity
on ovarian cancer outcomes. Immunol Rev. 222:101–116. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang L, Conejo-Garcia JR, Katsaros D,
Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H,
Schlienger K, Liebman MN, et al: Intratumoral T cells, recurrence,
and survival in epithelial ovarian cancer. N Engl J Med.
348:203–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Shen GH, Ghazizadeh M, Kawanami O, Shimizu
H, Jin E, Araki T and Sugisaki Y: Prognostic significance of
vascular endothelial growth factor expression in human ovarian
carcinoma. Br J Cancer. 83:196–203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen CA, Cheng WF, Lee CN, Chen TM, Kung
CC, Hsieh FJ and Hsieh CY: Serum vascular endothelial growth factor
in epithelial ovarian neoplasms: Correlation with patient survival.
Gynecol Oncol. 74:235–240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Germano G, Frapolli R, Belgiovine C,
Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M,
Pasqualini F, et al: Role of macrophage targeting in the antitumor
activity of trabectedin. Cancer Cell. 23:249–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
An Y and Yang Q: Tumor-associated
macrophage-targeted therapeutics in ovarian cancer. Int J Cancer.
149:21–30. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liang R, Chen X, Chen L, Wan F, Chen K,
Sun Y and Zhu X: STAT3 signaling in ovarian cancer: A potential
therapeutic target. J Cancer. 11:837–848. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lamichhane P, Karyampudi L, Shreeder B,
Krempski J, Bahr D, Daum J, Kalli KR, Goode EL, Block MS, Cannon MJ
and Knutson KL: IL10 Release upon PD-1 blockade sustains
immunosuppression in ovarian cancer. Cancer Res. 77:6667–6678.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wan C, Keany MP, Dong H, Al-Alem LF,
Pandya UM, Lazo S, Boehnke K, Lynch KN, Xu R, Zarrella DT, et al:
Enhanced efficacy of simultaneous PD-1 and PD-L1 immune checkpoint
blockade in high-grade serous ovarian cancer. Cancer Res.
81:158–173. 2021.PubMed/NCBI
|
|
103
|
Kalim M, Iqbal Khan MS and Zhan J:
Programmed cell death ligand-1: A dynamic immune checkpoint in
cancer therapy. Chem Biol Drug Des. 95:552–566. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Constantinidou A, Alifieris C and Trafalis
DT: Targeting programmed cell death −1 (PD-1) and Ligand (PD-L1): A
new era in cancer active immunotherapy. Pharmacol Ther. 194:84–106.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Fraser M, Leung B, Jahani-Asl A, Yan X,
Thompson WE and Tsang BK: Chemoresistance in human ovarian cancer:
The role of apoptotic regulators. Reprod Biol Endocrinol. 1:662003.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Janzen DM, Tiourin E, Salehi JA, Paik DY,
Lu J, Pellegrini M and Memarzadeh S: An apoptosis-enhancing drug
overcomes platinum resistance in a tumour-initiating subpopulation
of ovarian cancer. Nat Commun. 6:79562015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan
JA, Tammareddi A, Moore Vdel G, Deng J, Anderson KC, Richardson P,
Tai YT, et al: Pretreatment mitochondrial priming correlates with
clinical response to cytotoxic chemotherapy. Science.
334:1129–1133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Baekelandt M, Kristensen GB, Nesland JM,
Tropé CG and Holm R: Clinical significance of apoptosis-related
factors p53, Mdm2, and Bcl-2 in advanced ovarian cancer. J Clin
Oncol. 17:20611999. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Baekelandt M, Holm R, Nesland JM, Tropé CG
and Kristensen GB: Expression of apoptosis-related proteins is an
independent determinant of patient prognosis in advanced ovarian
cancer. J Clin Oncol. 18:3775–3781. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Binju M, Amaya-Padilla MA, Wan G,
Gunosewoyo H, Suryo Rahmanto Y and Yu Y: Therapeutic inducers of
apoptosis in ovarian cancer. Cancers (Basel). 11:17862019.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zervantonakis IK, Iavarone C, Chen HY,
Selfors LM, Palakurthi S, Liu JF, Drapkin R, Matulonis U, Leverson
JD, Sampath D, et al: Systems analysis of apoptotic priming in
ovarian cancer identifies vulnerabilities and predictors of drug
response. Nat Commun. 8:3652017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Reles A, Wen WH, Schmider A, Gee C,
Runnebaum IB, Kilian U, Jones LA, El-Naggar A, Minguillon C,
Schönborn I, et al: Correlation of p53 mutations with resistance to
platinum-based chemotherapy and shortened survival in ovarian
cancer. Clin Cancer Res. 7:2984–2997. 2001.PubMed/NCBI
|
|
113
|
Lee JM, Nair J, Zimmer A, Lipkowitz S,
Annunziata CM, Merino MJ, Swisher EM, Harrell MI, Trepel JB, Lee
MJ, et al: Prexasertib, a cell cycle checkpoint kinase 1 and 2
inhibitor, in BRCA wild-type recurrent high-grade serous ovarian
cancer: A first-in-class proof-of-concept phase 2 study. Lancet
Oncol. 19:207–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chui MH, Momeni Boroujeni A, Mandelker D,
Ladanyi M and Soslow RA: Characterization of TP53-wildtype
tubo-ovarian high-grade serous carcinomas: Rare exceptions to the
binary classification of ovarian serous carcinoma. Mod Pathol.
34:490–501. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lavarino C, Pilotti S, Oggionni M, Gatti
L, Perego P, Bresciani G, Pierotti MA, Scambia G, Ferrandina G,
Fagotti A, et al: p53 gene status and response to
platinum/paclitaxel-based chemotherapy in advanced ovarian
carcinoma. J Clin Oncol. 18:3936–3945. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Jones S, Wang TL, Shih IeM, Mao TL,
Nakayama K, Roden R, Glas R, Slamon D, Diaz LA Jr, Vogelstein B, et
al: Frequent mutations of chromatin remodeling gene ARID1A in
ovarian clear cell carcinoma. Science. 330:228–231. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y,
Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, et
al: ARID1A mutations in endometriosis-associated ovarian
carcinomas. N Engl J Med. 363:1532–1543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Guan B, Wang TL and Shih IeM: ARID1A, a
factor that promotes formation of SWI/SNF-mediated chromatin
remodeling, is a tumor suppressor in gynecologic cancers. Cancer
Res. 71:6718–6727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Bitler BG, Wu S, Park PH, Hai Y, Aird KM,
Wang Y, Zhai Y, Kossenkov AV, Vara-Ailor A, Rauscher FJ III, et al:
ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nat Cell
Biol. 19:962–973. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Saha S, Panigrahi DP, Patil S and Bhutia
SK: Autophagy in health and disease: A comprehensive review. Biomed
Pharmacother. 104:485–495. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Hu YL, Jahangiri A, Delay M and Aghi MK:
Tumor cell autophagy as an adaptive response mediating resistance
to treatments such as antiangiogenic therapy. Cancer Res.
72:4294–4299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yu H, Su J, Xu Y, Kang J, Li H, Zhang L,
Yi H, Xiang X, Liu F and Sun L: p62/SQSTM1 involved in cisplatin
resistance in human ovarian cancer cells by clearing ubiquitinated
proteins. Eur J Cancer. 47:1585–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang Y, Cheng Y, Ren X, Zhang L, Yap KL,
Wu H, Patel R, Liu D, Qin ZH, Shih IM and Yang JM: NAC1 modulates
sensitivity of ovarian cancer cells to cisplatin by altering the
HMGB1-mediated autophagic response. Oncogene. 31:1055–1064. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Huang Z, Zhou L, Chen Z, Nice EC and Huang
C: Stress management by autophagy: Implications for
chemoresistance. Int J Cancer. 139:23–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Follo C, Cheng Y, Richards WG, Bueno R and
Broaddus VC: Inhibition of autophagy initiation potentiates
chemosensitivity in mesothelioma. Mol Carcinog. 57:319–332. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
He J, Yu JJ, Xu Q, Wang L, Zheng JZ, Liu
LZ and Jiang BH: Downregulation of ATG14 by EGR1-MIR152 sensitizes
ovarian cancer cells to cisplatin-induced apoptosis by inhibiting
cyto-protective autophagy. Autophagy. 11:373–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Shteingauz A, Boyango I, Naroditsky I,
Hammond E, Gruber M, Doweck I, Ilan N and Vlodavsky I: Heparanase
enhances tumor growth and chemoresistance by promoting autophagy.
Cancer Res. 75:3946–3957. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ashrafizadeh M, Zarrabi A, Orouei S,
Kiavash Hushmandi, Hakimi A, Amirhossein Zabolian, Daneshi S,
Samarghandian S, Baradaran B and Najafi M: MicroRNA-mediated
autophagy regulation in cancer therapy: The role in
chemoresistance/chemosensitivity. Eur J Pharmacol. 892:1736602021.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Sun C, Fang Y, Yin J, Chen J, Ju Z, Zhang
D, Chen X, Vellano CP, Jeong KJ, Ng PK, et al: Rational combination
therapy with PARP and MEK inhibitors capitalizes on therapeutic
liabilities in RAS mutant cancers. Sci Transl Med. 9:eaal51482017.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Chandarlapaty S, Sawai A, Scaltriti M,
Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK,
Baselga J and Rosen N: AKT inhibition relieves feedback suppression
of receptor tyrosine kinase expression and activity. Cancer Cell.
19:58–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Aksamitiene E, Kiyatkin A and Kholodenko
BN: Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt
pathways: A fine balance. Biochem Soc Trans. 40:139–146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Gewinner C, Wang ZC, Richardson A,
Teruya-Feldstein J, Etemadmoghadam D, Bowtell D, Barretina J, Lin
WM, Rameh L, Salmena L, et al: Evidence that inositol polyphosphate
4-phosphatase type II is a tumor suppressor that inhibits PI3K
signaling. Cancer Cell. 16:115–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Carpten JD, Faber AL, Horn C, Donoho GP,
Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage
S, et al: A transforming mutation in the pleckstrin homology domain
of AKT1 in cancer. Nature. 448:439–444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Choi HJ, Heo JH, Park JY, Jeong JY, Cho
HJ, Park KS, Kim SH, Moon YW, Kim JS and An HJ: A novel PI3K/mTOR
dual inhibitor, CMG002, overcomes the chemoresistance in ovarian
cancer. Gynecol Oncol. 153:135–148. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Kim MJ, Lee SJ, Ryu JH, Kim SH, Kwon IC
and Roberts TM: Combination of KRAS gene silencing and PI3K
inhibition for ovarian cancer treatment. J Control Release.
318:98–108. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Gupta S, Nag S, Aggarwal S, Rauthan A and
Warrier N: Maintenance therapy for recurrent epithelial ovarian
cancer: Current therapies and future perspectives-a review. J
Ovarian Res. 12:1032019. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Lorusso PM, Edelman MJ, Bever SL, Forman
KM, Pilat M, Quinn MF, Li J, Heath EI, Malburg LM, Klein PJ, et al:
Phase I study of folate conjugate EC145 (Vintafolide) in patients
with refractory solid tumors. J Clin Oncol. 30:4011–4016. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Naumann RW, Coleman RL, Burger RA,
Sausville EA, Kutarska E, Ghamande SA, Gabrail NY, Depasquale SE,
Nowara E, Gilbert L, et al: PRECEDENT: A randomized phase II trial
comparing vintafolide (EC145) and pegylated liposomal doxorubicin
(PLD) in combination versus PLD alone in patients with
platinum-resistant ovarian cancer. J Clin Oncol. 31:4400–4406.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Moore KN, Martin LP, O'Malley DM,
Matulonis UA, Konner JA, Perez RP, Bauer TM, Ruiz-Soto R and Birrer
MJ: Safety and activity of mirvetuximab soravtansine (IMGN853), a
folate receptor alpha-targeting antibody-drug conjugate, in
platinum-resistant ovarian, fallopian tube, or primary peritoneal
cancer: A Phase I expansion study. J Clin Oncol. 35:1112–1118.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Holmes D: Ovarian cancer: Beyond
resistance. Nature. 527:S2172015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Huber D, Seitz S, Kast K, Emons G and
Ortmann O: Use of oral contraceptives in BRCA mutation carriers and
risk for ovarian and breast cancer: A systematic review. Arch
Gynecol Obstet. 301:875–884. 2020. View Article : Google Scholar : PubMed/NCBI
|