Glioma is a malignancy that originates from glial
cells. According to the World Health Organization (WHO) in 2016,
gliomas can be classified into four grades, of which grade I and II
are low-grade, whereas grade III and IV are high-grade (1). In 2021, WHO updated the molecular
biomarkers of different tumor types, recommended the use of Arabic
numerals for scoring, emphasized the importance of grading within
tumor types, and brought more benefits and meaningful guidance for
clinical practice (2).
Glioblastoma (GBM) accounts for ~60% of all high-grade gliomas.
High-grade gliomas can be observed in individuals of all ages, but
are particularly common in men (3–5).
Low-grade gliomas will typically relapse after initial treatment.
Following recurrence, low-grade gliomas can transform into
high-grade gliomas (6–8). The incidence of glioma accounts for
~40% of all malignancies in the central nervous system tumors,
which is highly invasive and infiltrative (9). In addition, it has high recurrence
rates and low 5-year survival rates, which poses a serious threat
to human health (10). The median
survival time of patients with glioma is only 15 months (11), whereas the 5-year survival rate is
<10% (12). The etiology and
mechanism underlying the pathogenesis of glioma remain unclear, but
it has been demonstrated to show complex molecular characteristics,
such as increases in chromosomes 7 and 19, loss of chromosomes 10
and 13, amplifications of EGFR and mouse double minute 2 homolog,
mutations in PTEN, neurofibromatosis type 1, platelet-derived
growth factor receptor α1, isocitrate dehydrogenase 1 and deletion
of cyclin dependent kinase inhibitor 2A (13,14).
Ionizing radiation exposure is also considered to be one of the
causes of glioma (14,15).
At present, the standard treatment option for glioma
is surgical resection combined with radiotherapy and chemotherapy
(16). However, the long-term
therapeutic efficacy is limited, where the main reasons are as
follows: i) Due to the invasiveness of this malignancy, local
resection of the tumor only removes the primary tumor but cannot
remove the surrounding tissues where the tumor has already invaded
(17); ii) the chemotherapeutic
agent cannot reach the tumor due to the blood-brain barrier (BBB),
which causes systemic toxicity and side effects, reducing the
maximum efficacy (18,19); and iii) tumor cells develop
internal resistance to the agent (20), thus exerting varying degrees of
resistance to different types of chemotherapeutic drugs, minimizing
the effect of chemotherapy. Therefore, major obstacles remain
before glioma can be completely cured (20). The invasiveness of GBM is caused by
the signal pathway of tyrosine kinase receptor (21). Tyrosine kinase inhibitors (TKIs)
and signal pathways (PI3K/AKT, Wnt/β-catenin, Hedgehog and NF-κB)
serve important roles in the progression of GBM (22–24).
TKIs selectively inhibit tumor growth and regulate cell
proliferation, migration, apoptosis and angiogenic factors by
activating intracellular signal transduction (25). Treatment strategies for glioma
includes surgery, radiotherapy, chemotherapy, targeting and
immunotherapy (25,26). However, chemotherapy agents when
applied individually exert limited therapeutic effects (3). As such, different combinations of
chemotherapeutic agents can be applied together to overcome drug
resistance and reduce their toxic adverse effects (27). Drug delivery systems, such as
nanoparticles, liposomes and polymer micelles can all transport
chemotherapeutic drugs through the BBB to improve efficacy and
enhance the targeting effects (27,28).
Therefore, chemotherapy remains to be a basic component of
multimodal therapeutic method for malignant glioma treatment
(29). Postoperative application
of chemotherapeutic drugs in patients with glioma serves an
important role in preventing postoperative recurrence. Considering
the existence of the BBB in the central nervous system, the ideal
chemotherapeutic drug should be small in molecular weight,
lipophilic, non-protein, and able to spread to the brain stroma
through the BBB (30). The main
obstacle to effective glioma chemotherapy is currently drug
resistance development and the occurrence of toxic side effects.
Therefore, there is demand for the development of novel agents and
drug delivery systems to alleviate these two challenges for
clinical application. A systematic literature search for eligible
studies through the electronic search engine PubMed was performed.
The keywords for searches were as follows: ‘Glioma’, ‘glioblastoma’
and ‘chemotherapeutic drugs’. Studies published in the past 10
years were searched using these keywords. The prevent review
discusses the potential mechanism and application of various
chemotherapeutic agents for glioma treatment.
The unique stability and solubility of TMZ render it
viable for oral administration and gives it the ability to pass
through the BBB. It can be activated without liver metabolism and
can spontaneously transform into the active alkylating agent
monomethyl triazene
5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide (MITC) under
physiological conditions (67,68).
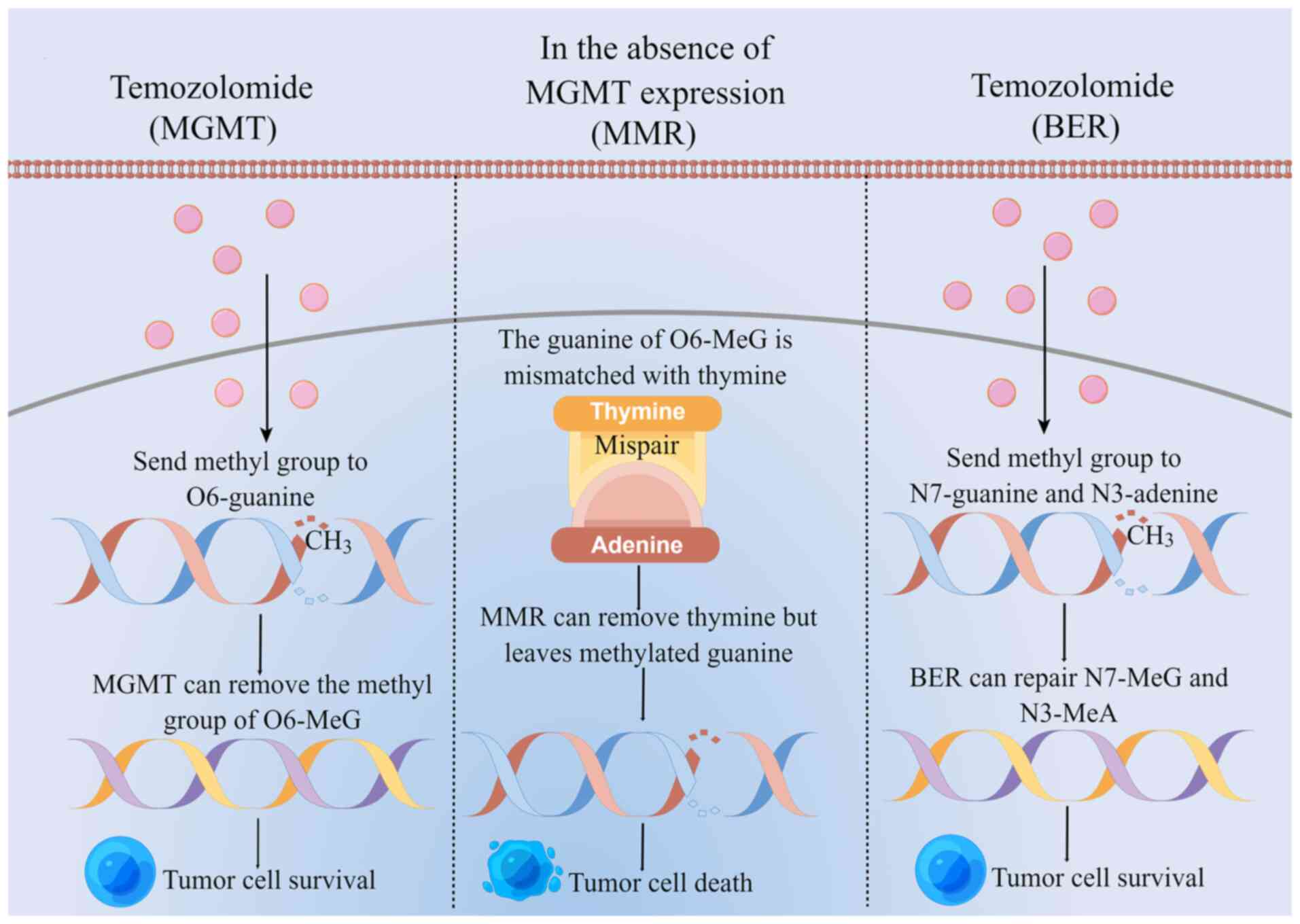
The main mechanism of TMZ for treating glioma is to first deliver
its methyl group to tumor cell DNA, causing DNA methylation and DNA
damage in tumor cells. This in turn inhibits tumor cell
proliferation, while inducing cell apoptosis (62,69).
TMZ destroys the DNA of tumor cells by delivering the active methyl
groups to O6 and
N7 position of guanine and the
N3 position of adenine to form cytotoxic
O6-methylguanine
(O6-MeG),
N7-methylguanine
(N7-MeG) and
N3-methyladenine
(N3-MeA) (70). By contrast, cytotoxicity induced by
TMZ is mainly caused by O6-MeG.
TMZ-resistant DNA repair systems mainly include
O6-methylguanine-DNA methyltransferase
(MGMT), mismatch repair (MMR) and base excision repair (BER)
(Fig. 1) (62). MGMT is a DNA repair protein that
removes the methyl group from O6-MeG and
binds it to its own cysteine residue, so that the tumor cells
survive (71,72). In the absence of MGMT expression,
O6-MeG mismatches with thymine, where MMR
removes thymine leaving behind the methylated guanine, which
reduces the efficiency of DNA mismatch repair and produces double
strand breaks, in turn inducing eventual cell death. BER can repair
N7-MeG and N3-MeA
so that the tumor cells survive (73–75).
MGMT is the most important factor for TMZ resistance in GBM and can
be regulated by various factors, including transcription factors,
epigenetic elements (such as methylation, phosphorylation, histone
acetylation and microRNA expression from the MGMT promoter), the
protein kinase A, and the MAPK/JNK and NF-κB signaling pathways
(76). Both
O6-benzylguanine
(O6-BG) and
O6-(4-bromophenyl) guanine
(O6-BTG) are MGMT inhibitors. Although
both held promise as a treatment option for glioma, they can cause
damage to normal tissues, causing adverse events (71). Hu et al (37) revealed that receptor-interacting
protein 2 (RIP2) could activate the NF-κB signaling pathway,
upregulate the expression of MGMT and increase the resistance of
glioma cells to TMZ. By using NF-κB and MGMT inhibitors combined
with TMZ on TMZ-resistant glioma cell lines T98G and U87MG in
addition to RIP2-overexpressing cells, it was found that MGMT
expression was downregulated whereas the inhibition of NF-κB/MGMT
signaling can improve TMZ sensitivity. The RIP2/NF-κB/MGMT
signaling pathway serves an important role in mediating TMZ
resistance. Poly (ADP-ribose) polymerase (PARP) is an enzyme that
regulates BER. PARP1-mediated BER/single strand break repair is a
key process for the repair of N7-MeG and
N3-MeA. Wu et al (38) previously documented that the PARP
inhibitor talazoparib (25 nM) significantly inhibited the
proliferation of MGMT-positive glioma globular-forming cells
induced by TMZ. In addition, the protein expression levels of MGMT
and PARP activation induced by TMZ were decreased. PARP can
regulate the activity of MGMT through BER, whereas PARP inhibitors
can improve the sensitivity of TMZ for the treatment of gliomas.
The in vitro results from a study by Rahman et al
(39) demonstrated that
pre-treatment with 10 nM 26S proteasome inhibitor BTZ combined with
TMZ could significantly inhibit the proliferation of glioma cells
while reducing the expression levels of MGMT, MGMT mRNA and nuclear
phosphorylation/activation of the p65 subunit. Subsequent in
vivo experiments revealed that BTZ + TMZ treatment could
significantly reduce the tumor volume and prolong the survival time
of the mice (the median survival time was 114 days). BTZ + TMZ was
therefore proposed to consume MGMT and block NF-κB/p65 signaling
transduction to improve the sensitivity of TMZ (39). Riluzole is a metabolic glutamate
receptor 1 (mGluR1) inhibitor. It has been previously reported that
riluzole can inhibit the progression of GBM by inhibiting the
PI3K/AKT/mTOR signaling pathway. PI3K inhibitors can also inhibit
the expression of MGMT by inhibiting the PI3K/AKT/NF-κB and
mGluR1/PI3K/AKT/mTOR signaling pathways. TMZ combined with riluzole
was found to significantly inhibit the proliferation of the
MGMT-positive cell lines T98G and GL261, but not in the
MGMT-negative cell line U87. In addition, riluzole was also able to
downregulate MGMT expression induced by TMZ. TMZ combined with
riluzole could significantly reduce the average maximum
cross-sectional area and volume of the tumors in mice injected with
GL261 cells, suggesting TMZ + riluzole as a potential treatment
(77). The expression level of
MGMT is associated with the efficacy of treatment. Overexpression
of MGMT is an important mechanism underlying TMZ resistance.
Understanding this mechanism while developing novel treatment
methods would be beneficial for improving the sensitivity to
TMZ.
Nitrosourea-based chemotherapeutic drugs, including
nimustine (ACNU), carmustine (BCNU), lomustine (CCNU) and
ranimustine can pass through the BBB. ACNU is a second-line
chemotherapeutic agent for the treatment of gliomas (78,79).
Nitrosourea-based chemotherapeutic agents act by inducing
cytotoxicity in malignant gliomas, where they can induce tumor cell
death and changes in biological behavior. These drugs mainly target
tumor cell DNA by first alkylating the bases of tumor cell DNA,
which then form inter-chain crosslinks. This causes high
cytotoxicity, induces DNA double-strand break and prevents DNA
replication and transcription to finally result in cell apoptosis
and necrosis (80). Nitrosourea
chemotherapeutic drugs have myelosuppression and hematological
toxicity (80,81). Due to the limited efficacy of
chemotherapeutic drugs when applied alone, previous studies that
investigated the effects of multiple combination therapies revealed
that the resultant efficacy is superior compared with that in
chemotherapy alone (30,80,82).
O6-alkylguanine-DNA alkyltransferase
(AGT)/MGMT is the main component that determines the efficacy and
drug resistance of nitrosourea. O6-BG is
an inhibitor of AGT that can effectively inhibit the activity of
AGT. By contrast, O6-BTG can exert
superior inhibitory effects on AGT, and has since entered clinical
trial testing, but it lacks a specific target and induces serious
myelosuppressive toxicity (83,84).
Tumor cell death is dependent on the inhibition of glycolysis. The
glycolysis inhibitor 3-bromopyruvate combined with BCNU has been
shown to significantly enhance the cytotoxicity of BCNU while
reducing intracellular ATP and glutathione levels in human glioma
cell lines SF763 and SF126. Glycolysis inhibitors are expected to
become an effective agent for reversing nitrosourea resistance
(84). Rezaei et al
(40) previously treated U373
glioma cells with microRNA (miR or miRNA)-181a combined with BCNU.
The results showed that miR-181a mediated downregulation of AKT1,
suggesting that miRNA can inhibit glioma cell proliferation by
regulating the PI3K/AKT signaling pathway. In addition, miR-181a
significantly reduced the semi-inhibitory concentration of BCNU
from 169.8 µM in BCNU alone to 83.25 µM in the combined treatment
group. The combined use of miR-181a and BCNU was also shown to
significantly inhibit cell migration compared with that in BCNU
alone. In addition, miR-181a could potentiate apoptosis induced by
BCNU (40). Hyperbaric oxygen is a
non-invasive form of therapy that can inhibit glioma cell
proliferation and inflammatory cell infiltration. Lu et al
(41) used hyperbaric oxygen
combined with ACNU to treat mice injected with the human glioma SU3
stem cells, which significantly inhibited tumor growth and reduced
tumor volume, where the tumor inhibition rate was ≤76.29%. The
tumor weight of the mice treated with combined therapy was found to
be significantly lower compared with that in mice treated with
either drug alone, suggestive of a synergistic effect. Subsequent
tumor histopathological results showed that after hyperbaric oxygen
combined with ACNU treatment, the degree of necrosis and
inflammatory cell infiltration were reduced. However, toxicity
associated with nitrosourea-based chemotherapy and resistance to
nitrosourea are key factors that minimize their efficacy. To
address the toxicity issue, Yi et al (42) used the synthesized BCNU and loaded
them into metal nanoparticles prior to treating glioma U251 cells,
which was found to inhibit proliferation while significantly
decreasing its survival rate. In the mouse model, this
nano-compound was shown to readily deliver the drugs to the brain
without causing damage to the normal brain tissue. In addition, it
tended to preferentially accumulate in glioma cell masses, to a
greater degree compared with the accumulation of BCNU alone. The
results of subsequent wound healing assays in vivo revealed
that the synthesis of BCNU and metal nanoparticles is beneficial to
wound tissue regeneration and promote wound healing, implicating
them to be another potential treatment option (42). Nitrosourea chemotherapeutic drugs
have been applied for the treatment of gliomas before the emergence
of TMZ (81). Over the past
decade, previous studies have shown that nitrosourea chemotherapy
can be used for the remedial treatment of TMZ resistance (85).
Platinum chemotherapeutic agents include cisplatin,
carboplatin and oxaliplatin. It has been reported that the exposure
of glioma cells to the platinum family of chemotherapeutic drugs
can induce tumor cell death. The potential mechanism underlying
this effect may be the induction of single-strand or double-strand
breaks in the DNA (86). Cisplatin
and carboplatin are broad-spectrum agents that cannot be taken
orally. Instead, they need to be injected intravenously because
they are not soluble in water and are not stable. In addition, they
are polar macromolecules, meaning that they cannot pass through the
BBB easily (87). This results in
low antitumor efficacy, high risks of systemic toxicity and drug
resistance. Cisplatin therapy can cause nephrotoxicity and
gastrointestinal toxicity, while carboplatin therapy can cause
myelosuppression and ototoxicity (88). After carboplatin and cisplatin are
injected intravenously, the permeability of BBB to carboplatin and
cisplatin is limited, leading to the uneven distribution of these
drugs to the tumor area, where the antitumor effect is poor.
However, although arterial infusion can greatly increase the
accumulation of drugs in the tumor cell masses, it is considered to
be highly toxic (89). To improve
therapeutic efficacy and reduce systemic toxicity, chemotherapeutic
drugs can be packaged into liposomes, peptide/protein conjugates,
polymers, polymer micelles and nanoparticles (90). Transferrin receptor (TR) is a
potential biological vehicle for drug delivery in the brain.
Furthermore, OX26 is a mouse monoclonal antibody that can target TR
in rats. Ashrafzadeh et al (43) previously packaged the OX26
monoclonal antibody into the targeted polyethylene glycol cisplatin
liposomes (TPL-Cispt). Results from the cell uptake assay in
vitro showed that TPL-Cispt delivery resulted in significantly
higher cell uptake, specifically increasing uptake by C6 cells
1.43-fold. Subsequent results from in vivo experiments
showed that the mean survival time (MST) of rats treated with
TPL-Cispt was 45 days, which represents a 1.7-fold increase. In
addition, the toxicity normally induced by cisplatin was shown to
be reduced. Therefore, polyethylene glycol liposomes carrying
cisplatin was concluded to improve the treatment efficacy of brain
tumors with reduced toxicity. However, lipid nanoparticles are
typically large in diameter and adhere to the extracellular matrix,
meaning that they cannot penetrate brain tumors efficiently. Zhang
et al (44) previously
manufactured a type of brain-penetrating nanoparticles
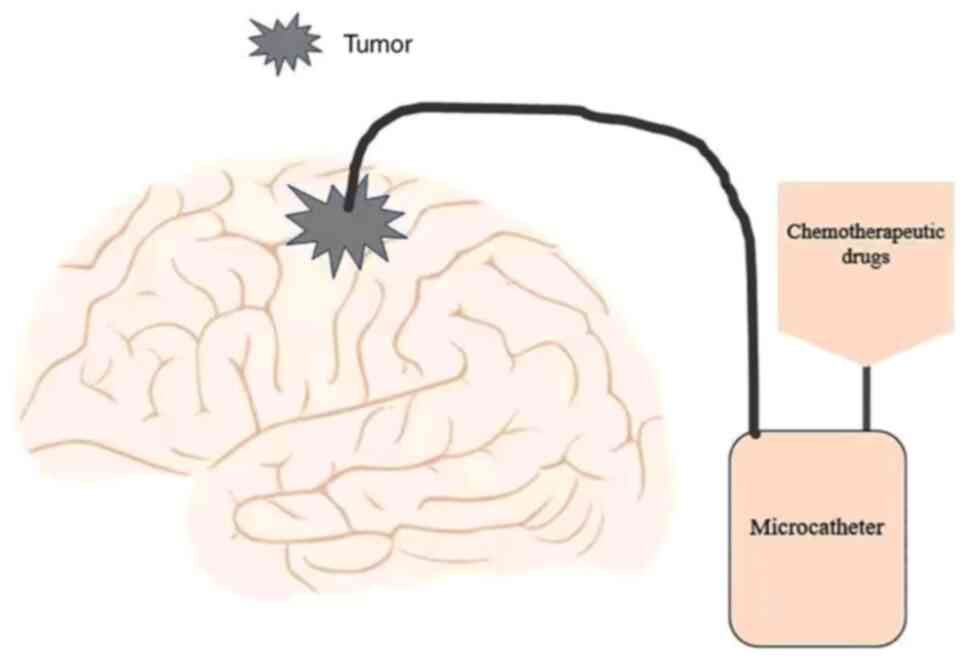
encapsulating cisplatin and delivered it by convection-enhanced
delivery (CED). CED (Fig. 2) is a
minimally invasive, locally targeted drug delivery method. Using
stereotactic technology, the therapeutic agents in the catheter are
directly injected into the tumor around the BBB to achieve the
purpose of accurate targeted therapy (91–93).
The diffusion rate of brain-penetrating nanoparticles containing
cisplatin in healthy rats and rats injected with F98 glioma cells
was found to be significantly higher compared with that in rats
treated with cisplatin alone (44). In addition, the distribution volume
of brain-penetrating nanoparticles containing cisplatin in the
brain was found to be 14 times higher compared with that in the
cisplatin-alone group. After the brain-penetrating nanoparticles
containing cisplatin and cisplatin alone were delivered separately
through CED, the distribution volume of brain-penetrating
nanoparticles containing cisplatin in the brain was found to be 29
times higher compared with that in the cisplatin-alone group. In
terms of survival, the targeted delivery of brain-penetrating
nanoparticles of cisplatin using CED can significantly prolong the
survival time of rats with superior tolerance (44). In conclusion, although
platinum-based chemotherapeutic agents are highly effective
especially in vitro, the BBB and occurrence of systemic
toxicity limit their efficacy in treating glioma, which require
optimization using alternative drug delivery systems.
DOX is an anthracycline antibiotic chemotherapy
agent and is one of the most extensively used antitumor drugs
(94). Its main mechanism of
action is by quickly entering the nuclei of tumor cells and
inhibiting the proliferation of tumor cells by binding to DNA and
blocking DNA replication (95,96).
DOX has been found to inhibit the proliferation of glioma cells.
After the glioma cells were treated with 10 and 100 nM of DOX for
7, 10 and 14 days, the cell survival rate was decreased in a dose-
and time-dependent manner, which was the lowest at the dosage of
100 nM DOX for 14 days. However, DOX treatment combined with TMZ
conferred no synergistic effects (97). DOX cannot pass through the BBB
easily, which limits its use in gliomas. However, exosomes can
cross the BBB and serve as a potential carrier for the delivery of
chemotherapeutic drugs for glioma. Thakur et al (45) previously loaded DOX into exosomes
derived from SF7761 glioma stem cells and U251 glioma cells using a
microfluidic device, before treating SF7761 and U251 cells with
DOX-containing exosomes. The results showed that DOX-containing
exosomes can inhibit the proliferation of cells more effectively
compared with that treated with DOX alone. Over the past number of
years, to improve the permeability of BBB to chemotherapeutic
drugs, studies into novel drug delivery systems have accelerated.
Meng et al (46) used a
borneol-modified nano-micelle delivery system to encapsulate and
deliver DOX. Results from proliferation experiments in vitro
showed that borneol-modified DOX nano-micelles inhibited the
proliferation and migration of C6 glioma cells while inducing C6
cell apoptosis to a higher degree, where the magnitude of these
inhibitory effects was greater compared with those mediated by DOX
alone. The results of tumor experiments in vivo revealed
that borneol-modified DOX nano-micelles significantly reduced the
tumor volume, bleeding and necrosis in mice compared with those
treated with DOX alone. There were no clear side effects following
measurements of the weight of the mice. In addition,
borneol-modified DOX nano-micelles significantly improved the
transport efficiency of DOX across the BBB, which was rapidly
accumulated in the brain tissues. However, large doses or long-term
use of DOX can cause cardiomyocyte toxicity, ultimately leading to
heart failure. To minimize this toxic adverse effect, it has been
proposed that DOX liposomes can be used for targeted delivery,
where the combination of DOX liposomes with other drugs conferred
superior antitumor activity (94,96).
Vincristine can mediate cardioprotective effects against
DOX-induced cardiomyocyte toxicity and chemical and hypoxic
oxidative stress. T7 is a heptapeptide ligand of the transferrin
receptor that can bypass the BBB and target glioma directly.
DA7R is a D-peptide ligand of VEGF receptor 2
overexpressed on angiogenesis, which has exhibited promising homing
characteristics towards glioma. Zhang et al (27) previously applied T7- and
DA7R dipeptide-modified liposomes to co-deliver DOX and
vincristine into mouse glioma tumors, which significantly reduced
the tumor volume, reduced the tumor proliferation rate and
significantly prolonged the median survival time (34 days).
Although DOX confers anti-glioma effects, it cannot pass through
BBB at its effective therapeutic dose range. Therefore, it is
necessary to develop novel drug delivery systems into the brain to
improve its efficacy (98,99).
Vincristine is a type of drug that functions by
destroying the stability of microtubules and interferes with the
metabolism of tumor cells by acting on the tubulin cytoskeleton of
tumor cells (100). Therefore,
anti-angiogenesis and antitumor activities (47) have been reported. Park et al
(47) reported that vincristine
can inhibit glioma cell proliferation in a dose-dependent manner
while inhibiting glioma angiogenesis under hypoxic conditions,
which was proposed to be mediated at least in part by the
inhibition of hypoxia-inducible factor-1α (HIF-1α). Vincristine is
a microtubule-targeting drug that is also commonly used in gliomas.
Microtubule-associated protein 2 (MAP2) is a
microtubule-stabilizing protein. Knocking down MAP2 expression can
significantly inhibit the migration and survival of glioma cells
in vitro, in addition to inducing apoptosis and increasing
the sensitivity of glioma cells to vincristine (101). Vincristine has a large molecular
weight and therefore cannot pass through the BBB easily, which can
cause peripheral neuropathy and systemic toxicity (102). The existence of BBB and the
blood-brain tumor barrier (BBTB) render the accurate targeting of
chemotherapeutic drugs challenging. Fu et al (48) designed a peptide nanoparticle
carrier dual-modified with T7 and reticulon 4 receptor (NGR) based
on encapsulated solid lipid nanoparticles from red blood cells
(T7/NGR-RBCSLNs) to encapsulate vincristine for the treatment of
gliomas. T7 was found to penetrate the BBB and target glioma cells.
NGR is a polypeptide ligand of CD13 that is overexpressed during
angiogenesis and has demonstrated promising homing characteristics
towards glioma. In addition, it can penetrate the BBTB and target
glioma cells. The results of cytotoxicity testing in vitro
showed that T7/NGR-RBCSLNs encapsulated with vincristine could
significantly increase the toxic effects of vincristine on C6
cells. Subsequent tumor experiments in vivo found that the
tumor in the T7/NGR-RBCSLNs-encapsulated vincristine group was
significantly reduced, where the relative tumor inhibition rate was
the highest and the MST was significantly prolonged (36 days).
Vincristine combined with the double-targeting delivery
(T7/NGR-RBCSLNs) showed the optimal anti-glioma effects in
vivo and in vitro in the brain. The efficacy conferred
by treatment with only a single chemotherapeutic drug is limited
whereas treatment with a combination of drugs can improve efficacy
without worsening systemic toxicity. Therefore, overcoming drug
resistance using other drug delivery systems is required to enhance
the efficacy of chemotherapy. Wu et al (49) used solid nano-lipid particles and
nano-lipid carriers (NLCS) to enclose vincristine and TMZ.
Subsequent results from the in vitro cytotoxicity assay
showed that the inhibitory effect of NLCS-vincristine + TMZ on U87
glioma cells was the most potent, which was significantly higher
compared with that mediated by either drug alone. The results of
this previous tumor study in vivo showed that
NLCS-encapsulated vincristine and TMZ significantly inhibited tumor
growth, where the tumor inhibition rate was >80%. These results
were consistent both in vivo and in vitro. This
dual-drug administration provides a novel idea for the clinical
intervention of glioma.
Topotecan is a water-soluble, semisynthetic
camptothecin analog that has a small molecular weight and can pass
through the BBB (103). It has
been approved for use in different types of tumors as topoisomerase
I inhibitors by Serwer et al (104). It is toxic to glial cells and
relatively non-toxic to normal brain tissue (105). Topotecan can form stable
topoisomerase I and DNA cleavage complexes in vivo and in
vitro. During the process of DNA replication in tumor cells,
topoisomerase I can be embedded in DNA, resulting in DNA damage and
fragmentation, which finally promote the death of tumor cells
(104). Topotecan has significant
reported antitumor effects according to various preclinical studies
(106). Small ubiquitin-like
modifier (SUMO) modification is a unique form of protein
post-translational modification that is key for the maintenance of
cell function under stress or adverse conditions. However, blocking
SUMO-1-3 binding in glioma cells can inhibit DNA synthesis,
proliferation and survival of glioma cells. Topotecan (1 and 10 µM)
significantly inhibited SUMO binding in glioma cells, decreased
cyclin-dependent kinase 6 and HIF-1α expression and changed the
cell cycle and metabolic profile (103). At present, the most important
strategy used to treat glioma is to maximize surgical resection
combined with radiotherapy and chemotherapy. However, the adverse
effects caused by radiotherapy and chemotherapy are typically
severe. Radiosensitization drugs can improve the efficacy of
radiotherapy and reduce its side effects. Koosha et al
(50) reported the
radiosensitization effects of topotecan combined with A966492 on
U87 glioma cell spheres. A966492 is a new type of PARP inhibitor,
that can cross the BBB. Compared with radiation alone, topotecan
combined with A966492 and 6 mV X-ray radiation significantly
increased the lethal effects of radiation on U87 glioma cell
spheres. Chemotherapeutic drugs can induce systemic toxicity, while
local chemotherapy can reduce systemic exposure. Sharon and
Rubinstein (51) divided the dose
of topotecan into three equal parts and changed it daily during the
72-h incubation with U87 glioma cells to simulate slow and
continuous administration. The relative survival rate of cells was
significantly decreased, from 35 to 7%. After CaCl2 was
added, the relative survival rate of cells was decreased even
further, significantly from 28 to 1.9%. Topotecan mediated
synergistic effects with CaCl2. Co-loading of
CaCl2 into topotecan poly (lactic-co-glycolic acid)
microspheres increased the toxic effects of topotecan on U87 cells,
where the cell viability decreased from 72 to 27%. Topotecan has
not been routinely used in clinical practice due to toxicity and
related pharmacokinetic shortcomings. Drug delivery systems, such
as poly (lactic-co-glycolic acid) microspheres, are increasingly
being used as the blueprint to develop novel cancer treatments,
because this technology can regulate the biological distribution
and target accumulation of chemotherapeutic drugs, thereby reducing
their toxicity.
In recent years, patients with glioma who received a
single chemotherapy regimen are particularly at risk of drug
resistance and relapse. In a previous clinical study of 27 patients
treated with TMZ combined with cisplatin, the progression-free
survival (PFS) was improved (107). In another clinical trial, TMZ
combined with bevacizumab was used to treat 30 patients with
recurrent GBM, which yielded a median overall survival (OS) of 51
weeks and a PFS of 52% at 6 months, suggesting that this treatment
may be effective for patients with recurrent GBM (108). Herrlinger et al (109) previously compared the effects of
CCNU combined with TMZ and standard TMZ therapy on the survival
time of patients with GBM and MGMT methylation. The results showed
that the OS and median survival time in the CCNU + TMZ treatment
group were significantly higher compared with those in the standard
TMZ group (109). However, in the
study performed by Kim et al (110), the efficacy of procarbazine
combined with CCNU for the treatment of patients with recurrent GBM
and MGMT methylation was not optimistic due to its toxicity.
Although the combination of chemotherapeutic drugs together does
exert certain curative effects, drug resistance and toxicity limit
efficacy. Therefore, additional prospective studies are required to
evaluate the efficacy of various combinations of chemotherapeutic
drugs for the treatment of gliomas. A number of clinical trials
(Table II) that are evaluating
the targeted treatment of GBM using chemotherapeutic drugs and
other combination therapies can be found in https://clinicaltrials.gov. Amongst these studies,
one of them is the evaluation of the combination of
O6-BG and TMZ for the treatment of newly
diagnosed GBM (ClinicalTrials.gov identifier no. NCT00272870).
Glioma is the most common malignancy in the central
nervous system with high recurrence rates and poor prognosis. Drug
resistance is the main complication obstructing glioma chemotherapy
and provides a significant challenge for improving the therapeutic
effects. The development of novel agents and the use of drug
resistance induction media and molecular signaling pathway
inhibitors are some of the methods of overcoming the resistance of
glioma to chemotherapeutic drugs. The combined use of drugs and
nanoparticles along with other carriers has been reported to reduce
drug resistance and toxicity. Therefore, it is of importance to
develop novel drug delivery methods for improving drug solubility,
prolonging circulation time, enhancing the targeted effects and
improving the efficacy of chemotherapeutic drugs for clinical
application. As information is being constantly accumulated
regarding the physiology of glioma, the comprehensive application
of multiple treatment methods for glioma and the treatment
mechanism of chemotherapy drugs, chemotherapeutic agents serve an
increasingly important role for the treatment of glioma.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 12071075), the Guangdong Medical
Research Fund (grant no. A2020278) and Summit Program of Foshan
(grant no. 2019C016).
Not applicable.
YSZ wrote the manuscript. YSZ, NC and LCW collected
and analyzed data. JBH and WW critically revised the manuscript.
All authors read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan AC, Ashley DM, López GY, Malinzak M,
Friedman HS and Khasraw M: Management of glioblastoma: State of the
art and future directions. CA Cancer J Clin. 70:299–312. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nayak L and Reardon DA: High-grade
gliomas. Continuum (Minneap Minn). 23:1548–1563. 2017.PubMed/NCBI
|
|
5
|
Miller KD, Ostrom QT, Kruchko C, Patil N,
Tihan T, Cioffi G, Fuchs HE, Waite KA, Jemal A, Siegel RL and
Barnholtz-Sloan JS: Brain and other central nervous system tumor
statistics, 2021. CA Cancer J Clin. 71:381–406. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han T, Zuo Z, Qu M, Zhou Y, Li Q and Wang
H: Comprehensive analysis of inflammatory response-related genes,
and prognosis and immune infiltration in patients with low-grade
glioma. Front Pharmacol. 12:7489932021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogino H, Taylor JW, Nejo T, Gibson D,
Watchmaker PB, Okada K, Saijo A, Tedesco MR, Shai A, Wong CM, et
al: Randomized trial of neoadjuvant vaccination with tumor-cell
lysate induces T cell response in low-grade gliomas. J Clin Invest.
132:e1512392022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi S, Yu Y, Grimmer MR, Wahl M, Chang SM
and Costello JF: Temozolomide-associated hypermutation in gliomas.
Neuro Oncol. 20:1300–1309. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Faulkner H, Arnaout O, Hoshide R, Young
IM, Yeung JT, Sughrue ME and Teo C: The surgical resection of
brainstem glioma: Outcomes and prognostic factors. World Neurosurg.
146:e639–e650. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong CY, Hong S, Zheng DW, Huang QX, Liu
FS, Zhong ZL and Zhang XZ: Multifunctionalized gold sub-nanometer
particles for sensitizing radiotherapy against glioblastoma. Small.
17:e20065822021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poon MTC, Sudlow CLM, Figueroa JD and
Brennan PM: Longer-term (≥2 years) survival in patients with
glioblastoma in population-based studies pre- and post-2005: A
systematic review and meta-analysis. Sci Rep. 10:116222020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Jiang Y, Wei D, Singh P, Yu Y, Lee
T, Zhang L, Mandl HK, Piotrowski-Daspit AS, Chen X, et al:
Nanoparticle-mediated convection-enhanced delivery of a DNA
intercalator to gliomas circumvents temozolomide resistance. Nat
Biomed Eng. 5:1048–1058. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang K, Wu Z, Zhang H, Zhang N, Wu W, Wang
Z, Dai Z, Zhang X, Zhang L, Peng Y, et al: Glioma targeted therapy:
Insight into future of molecular approaches. Mol Cancer. 21:392022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carrillo JA and Munoz CA: Alternative
chemotherapeutic agents: Nitrosoureas, cisplatin, irinotecan.
Neurosurg Clin N Am. 23297–306. (ix)2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi H, Sun S, Xu H, Zhao Z, Han Z, Jia J,
Wu D, Lu J, Liu H and Yu R: Combined delivery of temozolomide and
siPLK1 using targeted nanoparticles to enhance temozolomide
sensitivity in glioma. Int J Nanomedicine. 15:3347–3362. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mehta S and Lo Cascio C: Developmentally
regulated signaling pathways in glioma invasion. Cell Mol Life Sci.
75:385–402. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao M, van Straten D, Broekman MLD, Préat
V and Schiffelers RM: Nanocarrier-based drug combination therapy
for glioblastoma. Theranostics. 10:1355–1372. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haumann R, Videira JC, Kaspers GJL, van
Vuurden DG and Hulleman E: Overview of current drug delivery
methods across the blood-brain barrier for the treatment of primary
brain tumors. CNS Drugs. 34:1121–1131. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Y, Yang X, Mei S, Sun Y and Li J:
Acquisition of temozolomide resistance by the rat C6 glioma cell
line increases cell migration and side population phenotype. Oncol
Rep. 42:2355–2362. 2019.PubMed/NCBI
|
|
21
|
Liu S, Shi W, Zhao Q, Zheng Z, Liu Z, Meng
L, Dong L and Jiang X: Progress and prospect in tumor treating
fields treatment of glioblastoma. Biomed Pharmacother.
141:1118102021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Velásquez C, Mansouri S, Mora C, Nassiri
F, Suppiah S, Martino J, Zadeh G and Fernández-Luna JL: Molecular
and clinical insights into the invasive capacity of glioblastoma
cells. J Oncol. 2019:17407632019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muir M, Gopakumar S, Traylor J, Lee S and
Rao G: Glioblastoma multiforme: Novel therapeutic targets. Expert
Opin Ther Targets. 24:605–614. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng F and Guo D: MET in glioma:
Signaling pathways and targeted therapies. J Exp Clin Cancer Res.
38:2702019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Desjardins A, Rich JN, Quinn JA,
Vredenburgh J, Gururangan S, Sathornsumetee S, Reardon DA, Friedman
AH, Bigner DD and Friedman HS: Chemotherapy and novel therapeutic
approaches in malignant glioma. Front Biosci. 10:2645–2668. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lapointe S, Perry A and Butowski NA:
Primary brain tumours in adults. Lancet. 392:432–446. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Zhai M, Chen Z, Han X, Yu F, Li
Z, Xie X, Han C, Yu L, Yang Y and Mei X: Dual-modified liposome
codelivery of doxorubicin and vincristine improve targeting and
therapeutic efficacy of glioma. Drug Deliv. 24:1045–1055. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
d'Angelo M, Castelli V, Benedetti E,
Antonosante A, Catanesi M, Dominguez-Benot R, Pitari G, Ippoliti R
and Cimini A: Theranostic nanomedicine for malignant gliomas. Front
Bioeng Biotechnol. 7:3252019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brandes AA, Bartolotti M, Tosoni A and
Franceschi E: Nitrosoureas in the management of malignant gliomas.
Curr Neurol Neurosci Rep. 16:132016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stupp R: Drug development for glioma: Are
we repeating the same mistakes? Lancet Oncol. 20:10–12. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu Y, Wang L, Han J, Wang A, Chu L, Xi X,
Kan R, Sha C and Sun K: Synthesis and characterization of a series
of temozolomide esters and its anti-glioma study. J Pharm Sci.
110:3431–3438. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karachi A, Dastmalchi F, Mitchell DA and
Rahman M: Temozolomide for immunomodulation in the treatment of
glioblastoma. Neuro Oncol. 20:1566–1572. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao H, Li X, Wang F, Zhang Y, Xiong Y and
Yang Q: Phytochemical-mediated glioma targeted treatment: Drug
resistance and novel delivery systems. Curr Med Chem. 27:599–629.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mirabdaly S, Elieh Ali Komi D, Shakiba Y,
Moini A and Kiani A: Effects of temozolomide on U87MG glioblastoma
cell expression of CXCR4, MMP2, MMP9, VEGF, anti-proliferatory
cytotoxic and apoptotic properties. Mol Biol Rep. 47:1187–1197.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Gao S, Wang W and Liang J:
Temozolomide inhibits cellular growth and motility via targeting
ERK signaling in glioma C6 cells. Mol Med Rep. 14:5732–5738. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Da Ros M, Iorio AL, De Gregorio V,
Fantappiè O, Laffi G, de Martino M, Pisano C, Genitori L and Sardi
I: Aldoxorubicin and temozolomide combination in a xenograft mice
model of human glioblastoma. Oncotarget. 9:34935–34944. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu YH, Jiao BH, Wang CY and Wu JL:
Regulation of temozolomide resistance in glioma cells via the
RIP2/NF-κB/MGMT pathway. CNS Neurosci Ther. 27:552–563. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu S, Li X, Gao F, de Groot JF, Koul D and
Yung WKA: PARP-mediated PARylation of MGMT is critical to promote
repair of temozolomide-induced O6-methylguanine DNA damage in
glioblastoma. Neuro Oncol. 23:920–931. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rahman MA, Gras Navarro A, Brekke J,
Engelsen A, Bindesbøll C, Sarowar S, Bahador M, Bifulco E, Goplen
D, Waha A, et al: Bortezomib administered prior to temozolomide
depletes MGMT, chemosensitizes glioblastoma with unmethylated MGMT
promoter and prolongs animal survival. Br J Cancer. 121:545–555.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rezaei T, Hejazi M, Mansoori B, Mohammadi
A, Amini M, Mosafer J, Rezaei S, Mokhtarzadeh A and Baradaran B:
microRNA-181a mediates the chemo-sensitivity of glioblastoma to
carmustine and regulates cell proliferation, migration, and
apoptosis. Eur J Pharmacol. 888:1734832020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu Z, Ma J, Liu B, Dai C, Xie T, Ma X, Li
M, Dong J, Lan Q and Huang Q: Hyperbaric oxygen therapy sensitizes
nimustine treatment for glioma in mice. Cancer Med. 5:3147–3155.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yi S, Yang F, Jie C and Zhang G: A novel
strategy to the formulation of carmustine and bioactive
nanoparticles co-loaded PLGA biocomposite spheres for targeting
drug delivery to glioma treatment and nursing care. Artif Cells
Nanomed Biotechnol. 47:3438–3447. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ashrafzadeh MS, Akbarzadeh A, Heydarinasab
A and Ardjmand M: In vivo glioblastoma therapy using targeted
liposomal cisplatin. Int J Nanomedicine. 15:7035–7049. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang C, Nance EA, Mastorakos P, Chisholm
J, Berry S, Eberhart C, Tyler B, Brem H, Suk JS and Hanes J:
Convection enhanced delivery of cisplatin-loaded brain penetrating
nanoparticles cures malignant glioma in rats. J Control Release.
263:112–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thakur A, Sidu RK, Zou H, Alam MK, Yang M
and Lee Y: Inhibition of glioma cells' proliferation by
doxorubicin-loaded exosomes via microfluidics. Int J Nanomedicine.
15:8331–8343. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Meng L, Chu X, Xing H, Liu X, Xin X, Chen
L, Jin M, Guan Y, Huang W and Gao Z: Improving glioblastoma
therapeutic outcomes via doxorubicin-loaded nanomicelles modified
with borneol. Int J Pharm. 567:1184852019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park KJ, Yu MO, Park DH, Park JY, Chung YG
and Kang SH: Role of vincristine in the inhibition of angiogenesis
in glioblastoma. Neurol Res. 38:871–879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fu S, Liang M, Wang Y, Cui L, Gao C, Chu
X, Liu Q, Feng Y, Gong W, Yang M, et al: Dual-modified novel
biomimetic nanocarriers improve targeting and therapeutic efficacy
in glioma. ACS Appl Mater Interfaces. 11:1841–1854. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu M, Fan Y, Lv S, Xiao B, Ye M and Zhu X:
Vincristine and temozolomide combined chemotherapy for the
treatment of glioma: A comparison of solid lipid nanoparticles and
nanostructured lipid carriers for dual drugs delivery. Drug Deliv.
23:2720–2725. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Koosha F, Neshasteh-Riz A, Takavar A,
Eyvazzadeh N, Mazaheri Z, Eynali S and Mousavi M: The combination
of A-966492 and Topotecan for effective radiosensitization on
glioblastoma spheroids. Biochem Biophys Res Commun. 491:1092–1097.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sharon GR and Rubinstein A: Controlling
the release rate of topotecan from PLGA spheres and increasing its
cytotoxicity towards glioblastoma cells by co-loading with calcium
chloride. Int J Pharm. 602:1206162021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim MM, Umemura Y and Leung D: Bevacizumab
and glioblastoma: Past, present, and future directions. Cancer J.
24:180–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sonoda Y, Kanamori M, Deen DF, Cheng SY,
Berger MS and Pieper RO: Overexpression of vascular endothelial
growth factor isoforms drives oxygenation and growth but not
progression to glioblastoma multiforme in a human model of
gliomagenesis. Cancer Res. 63:1962–1968. 2003.PubMed/NCBI
|
|
54
|
Grossman R, Brastianos H, Blakeley JO,
Mangraviti A, Lal B, Zadnik P, Hwang L, Wicks RT, Goodwin RC, Brem
H and Tyler B: Combination of anti-VEGF therapy and temozolomide in
two experimental human glioma models. J Neurooncol. 116:59–65.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang R, Saito R, Shibahara I, Sugiyama S,
Kanamori M, Sonoda Y and Tominaga T: Temozolomide reverses
doxorubicin resistance by inhibiting P-glycoprotein in malignant
glioma cells. J Neurooncol. 126:235–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang X, Ni Q, Wang Y, Fan H and Li Y:
Synergistic anticancer effects of formononetin and temozolomide on
glioma C6 cells. Biol Pharm Bull. 41:1194–1202. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ni Q, Fan Y, Zhang X, Fan H and Li Y: In
vitro and in vivo study on glioma treatment enhancement by
combining temozolomide with calycosin and formononetin. J
Ethnopharmacol. 242:1116992019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Vengoji R, Macha MA, Nimmakayala RK,
Rachagani S, Siddiqui JA, Mallya K, Gorantla S, Jain M, Ponnusamy
MP, Batra SK and Shonka N: Afatinib and temozolomide combination
inhibits tumorigenesis by targeting EGFRvIII-cMet signaling in
glioblastoma cells. J Exp Clin Cancer Res. 38:2662019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tang JH, Yang L, Chen JX, Li QR, Zhu LR,
Xu QF, Huang GH, Zhang ZX, Xiang Y, Du L, et al: Bortezomib
inhibits growth and sensitizes glioma to temozolomide (TMZ) via
down-regulating the FOXM1-survivin axis. Cancer Commun (Lond).
39:812019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu P, Wang H, Pan H, Chen J and Deng C:
Anlotinib combined with temozolomide suppresses glioblastoma growth
via mediation of JAK2/STAT3 signaling pathway. Cancer Chemother
Pharmacol. 89:183–196. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Thomas AA and Rauschkolb PK: Tumor
treating fields for glioblastoma: Should it or will it ever be
adopted? Curr Opin Neurol. 32:857–863. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ortiz R, Perazzoli G, Cabeza L,
Jimenez-Luna C, Luque R, Prados J and Melguizo C: Temozolomide: An
updated overview of resistance mechanisms, nanotechnology advances
and clinical applications. Curr Neuropharmacol. 19:513–537. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Peng Y, Huang J, Xiao H, Wu T and Shuai X:
Codelivery of temozolomide and siRNA with polymeric nanocarrier for
effective glioma treatment. Int J Nanomedicine. 13:3467–3480. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Song S, Mao G, Du J and Zhu X: Novel RGD
containing, temozolomide-loading nanostructured lipid carriers for
glioblastoma multiforme chemotherapy. Drug Deliv. 23:1404–1408.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Afzalipour R, Khoei S, Khoee S,
Shirvalilou S, Jamali Raoufi N, Motevalian M and Karimi MR:
Dual-targeting temozolomide loaded in folate-conjugated magnetic
triblock copolymer nanoparticles to improve the therapeutic
efficiency of rat brain gliomas. ACS Biomater Sci Eng. 5:6000–6011.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Costagliola di Polidoro A, Zambito G,
Haeck J, Mezzanotte L, Lamfers M, Netti PA and Torino E:
Theranostic design of angiopep-2 conjugated hyaluronic acid
nanoparticles (Thera-ANG-cHANPs) for dual targeting and boosted
imaging of glioma cells. Cancers (Basel). 13:5032021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yang Z, Wei D, Dai X, Stevens MFG,
Bradshaw TD, Luo Y and Zhang J: C8-substituted imidazotetrazine
analogs overcome temozolomide resistance by inducing DNA adducts
and DNA damage. Front Oncol. 9:4852019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hotchkiss KM and Sampson JH: Temozolomide
treatment outcomes and immunotherapy efficacy in brain tumor. J
Neurooncol. 151:55–62. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sorribes IC, Handelman SK and Jain HV:
Mitigating temozolomide resistance in glioblastoma via DNA
damage-repair inhibition. J R Soc Interface. 17:201907222020.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Butler M, Pongor L, Su YT, Xi L, Raffeld
M, Quezado M, Trepel J, Aldape K, Pommier Y and Wu J: MGMT status
as a clinical biomarker in glioblastoma. Trends Cancer. 6:380–391.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yu W, Zhang L, Wei Q and Shao A:
O6-methylguanine-DNA methyltransferase (MGMT): Challenges and new
opportunities in glioma chemotherapy. Front Oncol. 9:15472020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lang F, Liu Y, Chou FJ and Yang C:
Genotoxic therapy and resistance mechanism in gliomas. Pharmacol
Ther. 228:1079222021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Daniel P, Sabri S, Chaddad A, Meehan B,
Jean-Claude B, Rak J and Abdulkarim BS: Temozolomide induced
hypermutation in glioma: Evolutionary mechanisms and therapeutic
opportunities. Front Oncol. 9:412019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tomar MS, Kumar A, Srivastava C and
Shrivastava A: Elucidating the mechanisms of temozolomide
resistance in gliomas and the strategies to overcome the
resistance. Biochim Biophys Acta Rev Cancer. 1876:1886162021.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ohba S, Yamashiro K and Hirose Y:
Inhibition of DNA repair in combination with temozolomide or
dianhydrogalactiol overcomes temozolomide-resistant glioma cells.
Cancers (Basel). 13:25702021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu Y, Du Z, Xu Z, Jin T, Xu K, Huang M,
Wang S, Zheng Y, Liu M and Xu H: Overexpressed GNA13 induces
temozolomide sensitization via down-regulating MGMT and p-RELA in
glioma. Am J Transl Res. 13:11413–11426. 2021.PubMed/NCBI
|
|
77
|
Yamada T, Tsuji S, Nakamura S, Egashira Y,
Shimazawa M, Nakayama N, Yano H, Iwama T and Hara H: Riluzole
enhances the antitumor effects of temozolomide via suppression of
MGMT expression in glioblastoma. J Neurosurg. 134:701–710. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kaina B and Christmann M: DNA repair in
personalized brain cancer therapy with temozolomide and
nitrosoureas. DNA Repair (Amst). 78:128–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Berte N, Piée-Staffa A, Piecha N, Wang M,
Borgmann K, Kaina B and Nikolova T: Targeting homologous
recombination by pharmacological inhibitors enhances the killing
response of glioblastoma cells treated with alkylating drugs. Mol
Cancer Ther. 15:2665–2678. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Weller M and Le Rhun E: How did lomustine
become standard of care in recurrent glioblastoma? Cancer Treat
Rev. 87:1020292020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lombardi G, Farina P, Della Puppa A,
Cecchin D, Pambuku A, Bellu L and Zagonel V: An overview of
fotemustine in high-grade gliomas: From single agent to association
with bevacizumab. Biomed Res Int. 2014:6985422014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rudà R, Touat M and Soffietti R: Is
chemotherapy alone an option as initial treatment for low-grade
oligodendrogliomas? Curr Opin Neurol. 33:707–715. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ge Y, Lai X, Li J, Yu R, Zhuang Z, Sun G,
Cui X, Zhang N, Zhao L, Upadhyaya P and Zhong R: NBGNU: A
hypoxia-activated tripartite combi-nitrosourea prodrug overcoming
AGT-mediated chemoresistance. Future Med Chem. 11:269–284. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sun X, Sun G, Huang Y, Zhang S, Tang X,
Zhang N, Zhao L, Zhong R and Peng Y: Glycolytic inhibition by
3-bromopyruvate increases the cytotoxic effects of
chloroethylnitrosoureas to human glioma cells and the DNA
interstrand cross-links formation. Toxicology. 435:1524132020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yamamuro S, Takahashi M, Satomi K, Sasaki
N, Kobayashi T, Uchida E, Kawauchi D, Nakano T, Fujii T, Narita Y,
et al: Lomustine and nimustine exert efficient antitumor effects
against glioblastoma models with acquired temozolomide resistance.
Cancer Sci. 112:4736–4747. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Rottenberg S, Disler C and Perego P: The
rediscovery of platinum-based cancer therapy. Nat Rev Cancer.
21:37–50. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yang W, Barth RF, Huo T, Nakkula RJ,
Weldon M, Gupta N, Agius L and Grecula JC: Radiation therapy
combined with intracerebral administration of carboplatin for the
treatment of brain tumors. Radiat Oncol. 9:252014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shi M, Fortin D, Sanche L and Paquette B:
Convection-enhance-ment delivery of platinum-based drugs and
Lipoplatin(TM) to optimize the concomitant effect with radiotherapy
in F98 glioma rat model. Invest New Drugs. 33:555–563. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Charest G, Sanche L, Fortin D, Mathieu D
and Paquette B: Optimization of the route of platinum drugs
administration to optimize the concomitant treatment with
radiotherapy for glioblastoma implanted in the Fischer rat brain. J
Neurooncol. 115:365–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
da Ros M, Iorio AL, Lucchesi M, Stival A,
de Martino M and Sardi I: The use of anthracyclines for therapy of
CNS tumors. Anticancer Agents Med Chem. 15:721–727. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Nwagwu CD, Immidisetti AV, Jiang MY,
Adeagbo O, Adamson DC and Carbonell AM: Convection enhanced
delivery in the setting of high-grade gliomas. Pharmaceutics.
13:5612021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Han Y and Park JH: Convection-enhanced
delivery of liposomal drugs for effective treatment of glioblastoma
multiforme. Drug Deliv Transl Res. 10:1876–1887. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
El Demerdash N, Kedda J, Ram N, Brem H and
Tyler B: Novel therapeutics for brain tumors: Current practice and
future prospects. Expert Opin Drug Deliv. 17:9–21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mizuta Y, Tokuda K, Guo J, Zhang S,
Narahara S, Kawano T, Murata M, Yamaura K, Hoka S, Hashizume M and
Akahoshi T: Sodium thiosulfate prevents doxorubicin-induced DNA
damage and apoptosis in cardiomyocytes in mice. Life Sci.
257:1180742020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Du K, Xia Q, Heng H and Feng F:
Temozolomide-doxorubicin conjugate as a double intercalating agent
and delivery by apoferritin for glioblastoma chemotherapy. ACS Appl
Mater Interfaces. 12:34599–34609. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Liu J, Zhang Y, Chen T, Chen H, He H, Jin
T, Wang J and Ke Y: Environmentally self-adaptative nanocarriers
suppress glioma proliferation and stemness via codelivery of
shCD163 and doxorubicin. ACS Appl Mater Interfaces. 12:52354–52369.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Horescu C, Elena Cioc C, Tuta C, Sevastre
AS, Tache DE, Alexandru O, Artene SA, Danoiu S, Dricu A and Stefana
Oana P: The effect of temozolomide in combination with doxorubicin
in glioblastoma cells in vitro. J Immunoassay Immunochem.
41:1033–1043. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Maksimenko O, Malinovskaya J, Shipulo E,
Osipova N, Razzhivina V, Arantseva D, Yarovaya O, Mostovaya U,
Khalansky A, Fedoseeva V, et al: Doxorubicin-loaded PLGA
nanoparticles for the chemotherapy of glioblastoma: Towards the
pharmaceutical development. Int J Pharm. 572:1187332019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Muniswamy VJ, Raval N, Gondaliya P, Tambe
V, Kalia K and Tekade RK: ‘Dendrimer-Cationized-Albumin’ encrusted
polymeric nanoparticle improves BBB penetration and anticancer
activity of doxorubicin. Int J Pharm. 555:77–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Li GZ, Hu YH, Li DY, Zhang Y, Guo HL, Li
YM, Chen F and Xu J: Vincristine-induced peripheral neuropathy: A
mini-review. Neurotoxicology. 81:161–171. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yang L, Xu X and Zheng J:
Microtubule-associated protein 2 knockdown sensitizes glioma cells
to vincristine treatment. Neuroreport. 31:197–204. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
De Witt M, Gamble A, Hanson D, Markowitz
D, Powell C, Al Dimassi S, Atlas M, Boockvar J, Ruggieri R and
Symons M: Repurposing mebendazole as a replacement for vincristine
for the treatment of brain tumors. Mol Med. 23:50–56. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bernstock JD, Ye D, Gessler FA, Lee YJ,
Peruzzotti-Jametti L, Baumgarten P, Johnson KR, Maric D, Yang W,
Kögel D, et al: Topotecan is a potent inhibitor of SUMOylation in
glioblastoma multiforme and alters both cellular replication and
metabolic programming. Sci Rep. 7:74252017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Serwer LP, Noble CO, Michaud K, Drummond
DC, Kirpotin DB, Ozawa T, Prados MD, Park JW and James CD:
Investigation of intravenous delivery of nanoliposomal topotecan
for activity against orthotopic glioblastoma xenografts. Neuro
Oncol. 13:1288–1295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Upadhyayula PS, Spinazzi EF, Argenziano
MG, Canoll P and Bruce JN: Convection enhanced delivery of
topotecan for gliomas: A single-center experience. Pharmaceutics.
13:392020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kaiser MG, Parsa AT, Fine RL, Hall JS,
Chakrabarti I and Bruce JN: Tissue distribution and antitumor
activity of topotecan delivered by intracerebral clysis in a rat
glioma model. Neurosurgery. 47:1391–1399. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang Y, Kong X, Guo Y, Wang R and Ma W:
Continuous dose-intense temozolomide and cisplatin in recurrent
glioblastoma patients. Medicine (Baltimore). 96:e62612017.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Badruddoja MA, Pazzi M, Sanan A, Schroeder
K, Kuzma K, Norton T, Scully T, Mahadevan D and Ahmadi MM: Phase II
study of bi-weekly temozolomide plus bevacizumab for adult patients
with recurrent glioblastoma. Cancer Chemother Pharmacol.
80:715–721. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Herrlinger U, Tzaridis T, Mack F,
Steinbach JP, Schlegel U, Sabel M, Hau P, Kortmann RD, Krex D,
Grauer O, et al: Lomustine-temozolomide combination therapy versus
standard temozolomide therapy in patients with newly diagnosed
glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A
randomised, open-label, phase 3 trial. Lancet. 393:678–688. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kim SH, Yoo H, Chang JH, Kim CY, Chung DS,
Kim SH, Park SH, Lee YS and Yang SH: Procarbazine and CCNU
chemotherapy for recurrent glioblastoma with MGMT promoter
methylation. J Korean Med Sci. 33:e1672018. View Article : Google Scholar : PubMed/NCBI
|