Introduction
To date, glioblastoma (GBM) is still the most common
malignant brain tumour of glial descent in adults (1,2).
Even with the combined standard of care therapy comprising of three
major elements: Surgical removal of the tumour, followed by
chemoradiation and subsequent adjuvant chemotherapy with
temozolomide (TMZ), median survival is still limited to little over
a year (2–5). To address this major issue, several
new therapeutic approaches have been developed in recent years,
showing promising results in preclinical and clinical studies
(6–8). A number of these novel therapeutic
approaches target the tumour microenvironment (6,9–11),
that includes endothelial cells, pericytes, immune cells and
particularly tumour-associated microglia/macrophages which make up
to 30–50% of the tumour mass (12–16).
They secrete soluble factors such as growth factors and chemokines
and support tumour growth e.g., by initiating the formation of new
blood vessels, the proliferation of tumour cells and by expressing
immuno-suppressive molecules (12–15,17–20).
For instance, VEGF is one of the most important proangiogenic
growth factors in GBM (21,22).
Nevertheless, targeting VEGF-mediated angiogenesis in GBM has not
significantly improved patient survival (7,23).
Apart from growth factors such as VEGF, chemokines and their
respective receptors are crucial to numerous tumour-supporting
processes and therefore are relevant targets for new therapeutic
approaches in GBM (6). Chemokine
signalling axes, for instance the CXCR2 signalling pathway, have
been investigated in preclinical in vitro and in vivo
experimental models as well as in GBM patient ex vivo
samples (11,18,19,24).
As CXCR2 is expressed by glioma cells as well as endothelial cells
and overexpression of its ligands CXCL2 and IL8 is associated with
a reduced patient survival (11,18,25–28),
this signalling pathway is a feasible target for GBM therapy. In
this regard, SB225002 (SB), a commonly used small molecule
CXCR2-antagonist, inhibits CXCR2 downstream signalling (11,18,24,29).
In a previous study by the authors, it was demonstrated that
anti-CXCR2 therapy with SB led to a reduced vessel density in an
immunocompetent orthotopic mouse model in vivo. In addition,
in that study, a diminished proliferation of glioma and murine
endothelial cells was observed (11). Furthermore, SB also decreased
angiogenesis in a 3D spheroid-based angiogenesis model utilizing
primary human brain endothelial cells in vitro (18). Moreover, SB is known to inhibit
CXCR2 signalling-mediated vascular mimicry in vivo (24).
Emerging evidence suggests that combined therapeutic
approaches are superior to single therapies due to GBM
heterogeneity and various resistance mechanisms (2–4).
Based on the studies aforementioned, combining CXCR2-antagonisation
with the standard-of-care TMZ therapy appears promising. In another
previous study by the authors, it was demonstrated that a
combination therapy in vivo, consisting of TMZ and SB,
reduced tumour volume in immunocompetent mice (19). However, little is known about the
molecular changes during treatment with the combination therapy.
While proliferation was significantly reduced by the combination
therapy, gene expression of proangiogenic pathways and pro- and
antiapoptotic genes were not significantly altered within the
tumour lysates (19).
Nevertheless, certain tendencies were observed and the reduced
tumour volume is likely based on changes in gene and protein
expression (19). However, to take
this promising approach from mouse experiments to a potential
therapeutic approach in humans, more research is warranted. As
tumour growth is dependent on the formation of new blood vessels
and CXCR2 is widely expressed by endothelial cells, these cells are
highly relevant for GBM therapy (25,27,30).
Therefore, the aim of the present study was to elucidate the
outcome of combined TMZ and SB treatment in vitro. The
tumour microenvironment was mimicked in GBM patients with CXCL2 and
IL8 oversupply (18) in comparison
to normal culturing conditions and the effect of this treatment
strategy on gene and protein expression of primary human
endothelial cells was assessed.
Materials and methods
Culture of human endothelial
cells
Human umbilical vein endothelial cells (HUVECs) were
obtained from PromoCell GmbH and cultured in endothelial cell
growth medium 2 (ECGM2; cat. no. C-22111) containing supplements
(supplement mix for ECGM2; cat. no. C-39216; both PromoCell GmbH)
and 0.1 mg/ml gentamicin in 75 mm2 cell culture flasks
(Falcon®, Corning, Inc.). The cells were incubated at
37°C until they reached 90% confluency. For sub-culture of HUVECs
the PromoCell Detach Kit was used following the instructions of the
manufacturer. Cells from passages 3–4 were used for the experiments
(Fig. 1A).
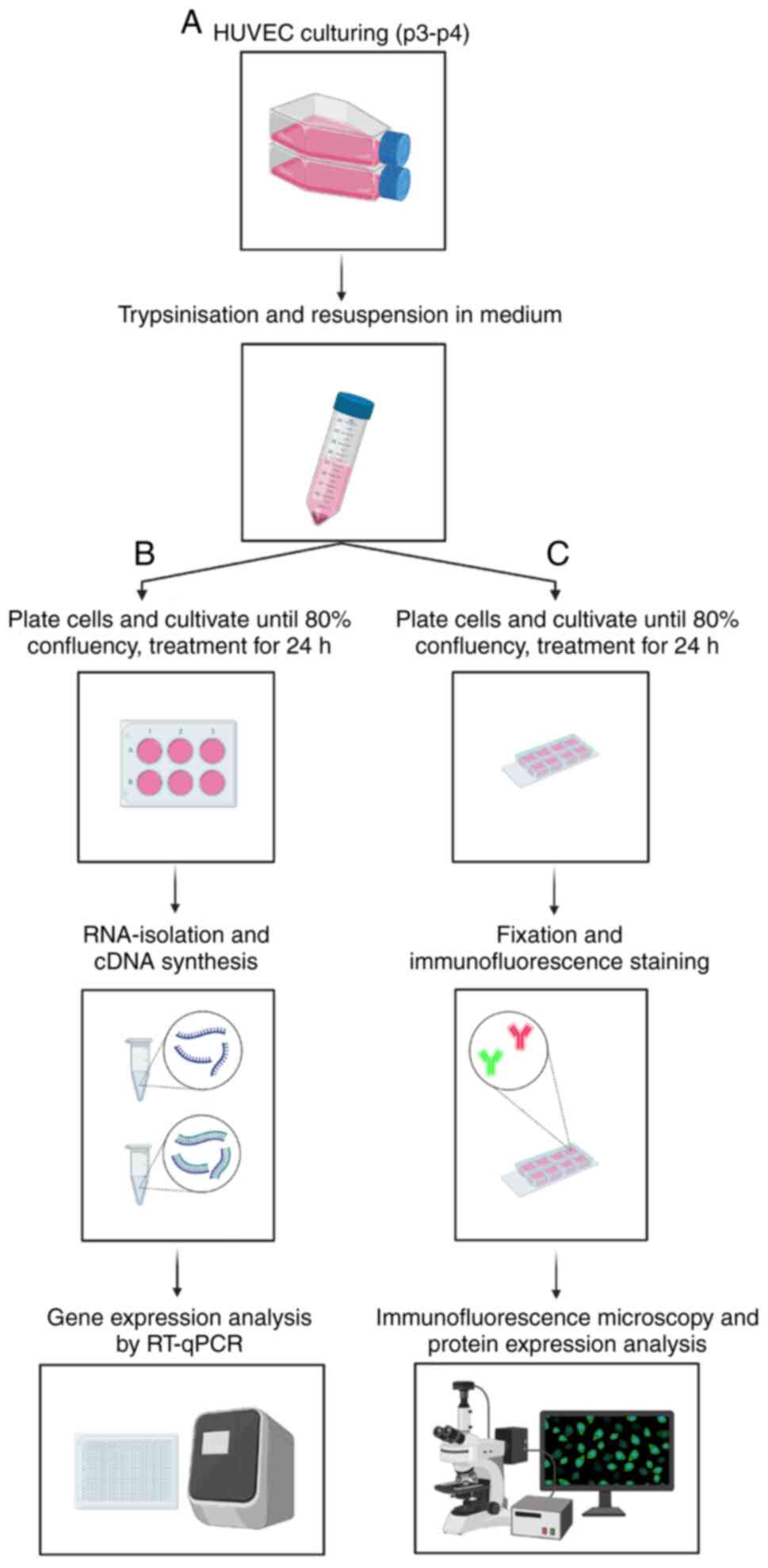 | Figure 1.Methodological setup. (A) Cells were
cultured and passaged. (B) HUVECs were seeded into 6-well plates
and cultured until they reached 80% confluency. The cells were then
starved for 4 h in 0.1% FCS in ECBM2, followed by treatment with 25
ng/ml CXCL2 and IL8 and/or 10 µM TMZ and/or 0.03 µM SB for 24 h.
Subsequently, RNA-isolation was performed followed by quantitative
PCR for BAX, BCL2, VEGFR1, VEGFR2, CXCR1, CXCR2, VEGF, CXCL2 and
IL8. (C) HUVECs were seeded into 8-well glass bottom plates and
cultured until they reached 80% confluency. The cells were then
starved for 4 h in 0.1% FCS in ECBM2, followed by treatment with 25
ng/ml CXCL2 and IL8 and/or 10 µM TMZ and/or 0.03 µM SB for 24 h.
Immunofluorescence staining for the cell nucleus (DAPI), the
cytoskeleton (Phalloidin), VEGFR2 and CXCR2 was carried out,
followed by immunofluorescence microscopy. This figure was created
using Biorender.org. HUVECs, human umbilical vein endothelial
cells; FCS, fetal calf serum; ECBM2, endothelial cell basal medium
2; CXCL2, C-X-C motif chemokine ligand 2; IL8, interleukin 8; TMZ,
temozolomide; SB, SB225002; VEGFR1, vascular endothelial growth
factor receptor 1; VEGFR2, vascular endothelial growth factor
receptor 2; CXCR1, C-X-C motif chemokine receptor 1; CXCR2, C-X-C
motif chemokine receptor 2; VEGF, vascular endothelial growth
factor. |
Treatment with TMZ and SB
HUVECs were seeded at 0.6×105 and
cultured on 6-well plates or 8-well-glass-bottom-µ-slides (both
Sarstedt®; SARSTEDT AG & Co. KG) until they reached
80% confluency. Cells were starved for 4 h in 0.1% fetal calf serum
(FCS) in endothelial cell basal medium 2 (ECBM2) (cat. no. C-22211;
PromoCell GmbH). Subsequently, the cells were treated with a
cocktail of 25 ng/ml CXCL2/IL8 (recombinant human CXCL2
(carrier-free), recombinant human CXCL8 (carrier-free); BioLegend,
Inc.) and/or 10 µM TMZ (Temodal®; MSD; Merck & Co.,
Inc.) and/or 0.03 µM SB (SB225002; Tocris Bioscience) for 24 h as
described below. A concentration of 25 ng/ml for CXCL2 and IL8 was
selected as our previous study showed a significant effect on cells
treated with that concentration (18). Furthermore, previous in
vitro studies also revealed an effect of SB at 0.03 µM
(11,18,31).
To elucidate the efficacy of combined treatment with TMZ and SB,
HUVECs were cultured in six different treatment conditions: i)
Control, ii) STIM (stimulation by CXCL2 and IL8), iii) TMZ + SB
(treatment with the combination therapy), iv) STIM + TMZ (treatment
by CXCL2/IL8 and additional TMZ), v) STIM + SB (treatment by
CXCL2/IL8 and additional SB), and vi) STIM + TMZ + SB (treatment by
CXCL2/IL8 and additional TMZ + SB).
RNA isolation and reverse
transcription-quantitative (RT-q)PCR
HUVECs were detached using cell scrapers
(Corning®; Corning, Inc.) after application of 300 µl
lysis buffer (PureLink RNA Mini Kit; Invitrogen; Thermo Fisher
Scientific, Inc.) with 1% 2-mercaptoethanol per well. The PureLink
RNA Mini Kit was used to isolate RNA according to the corresponding
protocol. RNA concentration was measured with a plate photometer
(Infinite M200; Tecan Group, Ltd.) and RNA quality was assessed
using Agilent 2100 Bioanalyzer prior to eradication of DNA
contamination. cDNA synthesis was carried out with 500 ng RNA using
the PrimeScript™ RT reagent Kit with gDNA Eraser (cat.
no. RR047A; Takara Bio, Inc.) according to the manufacturer's
instructions. The cDNA quantity was measured by photometry. RT-qPCR
was performed for BAX, BCL2, VEGF, VEGFR1, VEGFR2, IL8, CXCL2,
CXCR1 and CXCR2 using triplicates in a 10-µl reaction
volume and the TB Green™ Premix Ex Taq™ Kit (Takara Bio, Inc.).
18S was used as the reference gene. Primer sequences were
designed with Primer BLAST by the National Center for Biotechnology
Information, U.S. National Library of Medicine and purchased from
TIB MOLBIOL (Table I). RT-qPCR was
performed with the Quant Studio 6 Flex System (Thermo Scientific
Scientific, Inc.). The thermocycling conditions were as follows:
Initial denaturation at 95°C for 30 sec; denaturation at 95°C for 5
sec; annealing and elongation, each at 60°C for 30 and 60 sec,
respectively; and the hold stage at 4°C. The number of performed
cycles was 40. Target expression levels were normalized to
18S mRNA. The relative quantification method
2−ΔΔCq was used for analyses (32). Accordingly, fold change of target
gene expression (relative expression level) was calculated in
relation to the target gene expression of the control group
(32) (Fig. 1B).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer
orientation | Sequence 5′ →
3′ | Tm (°C) | Fragment size
(bp) |
|---|
| h18Sa | Forward |
GGCCCTGTAATTGGAATGAGTC | 59 | 146 |
|
| Reverse |
CCAAGATCCAACTACGAGCTT | 58 |
|
| hVEGFR1 | Forward |
CAGGCCCAGTTTCTGCCATT | 60 | 82 |
|
| Reverse |
TTCCAGCTCAGCGTGGTCGTA | 63 |
|
| hVEGFR2 | Forward |
CATGTACGGTCTATGCCATTCCTC | 61 | 73 |
|
| Reverse |
TTGGCGCACTCTTCCTCCAAC | 63 |
|
| hVEGFAa | Forward |
TGCAGATTATGCGGATCAAACC | 59 | 81 |
|
| Reverse |
TGCATTCACATTTGTTGTGCTGTAG | 61 |
|
| hCXCR1 | Forward |
GCAGCTCCTACTGTTGGACA | 60 | 84 |
|
| Reverse |
GCCCTACCCCACAGAAAGTC | 60 |
|
| hCXCR2 | Forward |
GGTGTCCTACAGGTGAAAAG | 55 | 85 |
|
| Reverse |
TGTCACTCTCCATGTTAAAA | 52 |
|
| hCXCL2 | Forward |
TCCCTTGGACATTTTATGTCTTTC | 57 | 89 |
|
| Reverse |
TCTCTGCTCTAACACAGAGGGA | 60 |
|
| hIL8a | Forward |
CTGAGAGTGATTGAGAGTGG | 55 | 113 |
|
| Reverse |
ACAACCCTCTGCACCCAGTT | 62 |
|
| hBAX | Forward |
GCCCTTTTGCTTCAGGGTTT | 59 | 121 |
|
| Reverse |
TGAGACACTCGCTCAGCTTC | 60 |
|
| hBCL2 | Forward |
TGCGGCCTCTGTTTGATTTC | 59 | 120 |
|
| Reverse |
GGCAGGCATGTTGACTTCAC | 60 |
|
Immunofluorescence staining
HUVECs were cultured to 80% confluency on
8-well-glass-bottom-µ-slides (Sarstedt®; SARSTEDT AG
& Co. KG) as aforementioned and then washed with PBS and fixed
with 4% paraformaldehyde (PFA) at room temperature for 20 min. The
fixed cells were blocked in 1% Casein/PBS for 30 min. Primary
antibodies rabbit anti-CXCR2 (1:200; product code ab14935; Abcam)
or rabbit anti-VEGFR2 (1:200; product no. 2479S; Cell Signaling
Technology, Inc.) combined with
AlexaFluor™488-Phalloidin (1:200; cat. no. A12379;
Thermo Fischer Scientific, Inc.) were applied in 0.5% Casein/PBS
for 2 h at room temperature. Sections were then washed and treated
with the secondary antibody (1:200; Cy™3 donkey
anti-rabbit; code no. 711-165-152, lot no. 122296; Jackson
ImmunoResearch Europe, Ltd.) for 1.5 h at room temperature. All
sections were mounted with DAPI-containing medium (Dianova GmbH)
and sealed with cover slips. Images were acquired using the same
exposure time for every condition, with a 20X magnifying objective
on a fluorescence microscope (Zeiss Axio Observer Z1; Zeiss
MicroImaging GmbH). ImageJ 1.53c (available from: http://imagej.nih.gov/ij; National Insitutes of
Health; accessed 28th June 2020) was used to analyse images
(Fig. 1C).
Statistical analyses
Statistical analyses were performed using GraphPad
Prism Software (v9.1.1; GraphPad Software, Inc.) expressed as the
mean ± standard deviation (SD). If not indicated otherwise, all
experiments were carried out at least three times. Groups were
compared by one-way ANOVA with Bonferroni correction. A P-value
≤0.05 was considered to indicate a statistically significant
difference.
Results
Treatment with TMZ and/or SB leads to
morphological changes in HUVECs
To analyse the effect of the combination therapy
consisting of TMZ and SB, HUVECs were treated as described in the
previous section. In brief, six treatment conditions were utilized:
i) Control, ii) STIM, iii) TMZ + SB, iv) STIM + TMZ, v) STIM + SB,
and vi) STIM + TMZ + SB. The optimal concentration of the reagents
for the experiments were based on previous experiments (11,18,31),
as aforementioned. During culture, images by phase contrast
microscopy were obtained regularly to investigate morphological
changes. Alterations in cell morphology of HUVECs were observed in
all groups treated with SB or TMZ (Fig. 2C-F). While cells in the control and
STIM group (Fig. 2A and B)
exhibited the typical long and thin phenotype, treatment with SB
led to a rather rounded cell morphology (Fig. 2C, E and F) (18). Furthermore in the groups treated
with either TMZ, SB or a combination of both, there were more
detached cells and more cell debris (Fig. 2C-F). Therefore, these observations
led us to speculate whether treatment with TMZ and/or SB triggered
apoptosis.
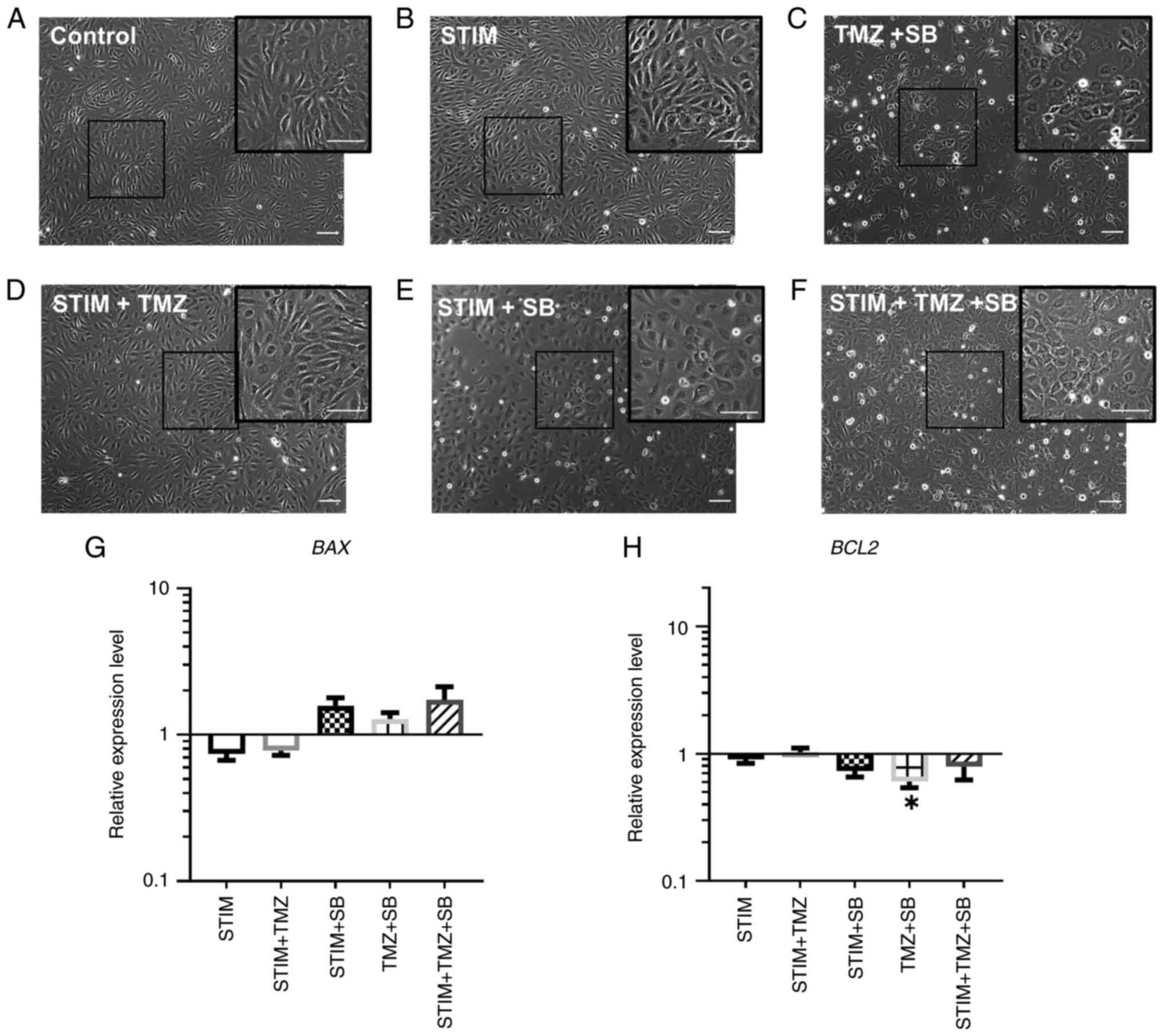 | Figure 2.Combination therapy with TMZ and SB
leads to morphological changes in HUVECs and downregulation of
antiapoptotic BCL2. (A-H) HUVECs were stimulated with a cocktail of
25 ng/ml CXLC2 and 25 ng/ml IL8 combined with 10 µM TMZ, 0.03 µM SB
or both for 24 h as indicated. Analysis of mRNA expression
regarding BAX and BCL2 expression was then performed. Gene
expression was analysed using the relative quantification method
(ΔΔCq) and compared to normal culturing conditions (control).
Accordingly, expression of each target in the control group was set
to 1. Medium containing 0.1% FCS/1% DMSO was used as the control.
(A-F) Representative images by phase contrast microscopy are shown
with more attached cells and debris in groups which included
treatment with SB, TMZ or both; scale bar, 100 µm. (G and H)
Changes of relative expression levels are shown for BAX and BCL2 on
a logarithmic scale. P-values indicated in the graph are in
comparison to the control group. Other significant P-values for BAX
expression: STIM vs. STIM + TMZ + SB, P=0.0253; STIM+ TMZ vs. STIM
+ TMZ + SB, P=0.0341. Other significant P-values for BCL2
expression: STIM + TMZ vs. STIM + SB, P=0.0259. Data represents
multiple experiments with similar results (n=9/condition out of
three independent experiments). *P<0.05; one-way ANOVA
(Bonferroni correction); bar graphs represent the mean ± standard
deviation. STIM, stimulation with 25 ng/ml CXCL2 and IL8; TMZ,
temozolomide; SB, SB225002; HUVECs, human umbilical vein
endothelial cells; CXCL2, C-X-C motif chemokine ligand 2; IL8,
interleukin 8; FCS, fetal calf serum; DMSO, dimethyl sulfoxide. |
Combination therapy induces
downregulation of anti-apoptotic BCL2
It is known that SB inhibits proliferation and leads
to apoptosis in leukaemia cells in vitro (29,31).
Therefore, investigating the effect of SB in combination with TMZ
on primary human endothelial cells was of special interest. Thus,
HUVECs were cultured and RNA was extracted for qPCR to evaluate the
expression level of two different genes involved in apoptosis:
BAX (proapoptotic) and BCL2 (antiapoptotic). The
expression of the proapoptotic molecule BAX was enhanced by
the combination therapy (TMZ + SB) in the simulated CXCL2/IL8
oversupply environment compared to the only simulated oversupply
group (Fig. 2G). Interestingly,
BAX expression was only minimally altered by the combination
therapy in standard culturing conditions (Control vs. TMZ + SB)
(Fig. 2G). Notably, antiapoptotic
BCL2 was significantly downregulated after combined
treatment with TMZ and SB (Fig.
2H). Within the simulated oversupply group (STIM) gene
expression of BAX and BCL2 was not altered.
Collectively, apoptosis was induced in all treatment groups
receiving SB, however none reached the level of significance and
BCL2 was significantly downregulated by treatment with TMZ
and SB.
CXCR2 gene and protein expression is
altered differently by the combination therapy
As SB has been shown to i) affect the CXCR2
signalling pathway in vitro and in vivo (11,18,19)
and ii) CXCR2 ligands, CXCL2 and IL8, are highly effective
alternative proangiogenic molecules (18), the therapy-induced changes in gene
and protein expression of proangiogenic pathways in vitro
were investigated (Fig. 3). Apart
from the standard proangiogenic receptors of VEGF, VEGFR1
and VEGFR2 (18,33–35),
HUVECs express the receptors for CXCL2 and IL8, CXCR1
and CXCR2 (18,25–28).
Moreover, CXCL2 and IL8 are overexpressed in
approximately one third of GBM patients, and overexpression is
associated with a reduced overall survival (18). The analysis of the alternative
proangiogenic receptors CXCR1 and CXCR2 revealed that
the expression of the proangiogenic receptor CXCR1 was not
significantly changed in HUVECs. By contrast, CXCR2 was
upregulated by the combination therapy and by SB alone within and
outside of the mimicked oversupply with CXCL2/IL8 (Fig. 3A and B). For instance, the
combination therapy with TMZ and SB in the simulated oversupply
environment significantly enhanced the expression of CXCR2.
Under normal culturing conditions, combining TMZ and SB enhanced
the expression of CXCR2 (Fig.
3B). In summary, CXCR2 was significantly highly
expressed in all groups receiving treatment with SB alone or in
combination with TMZ.
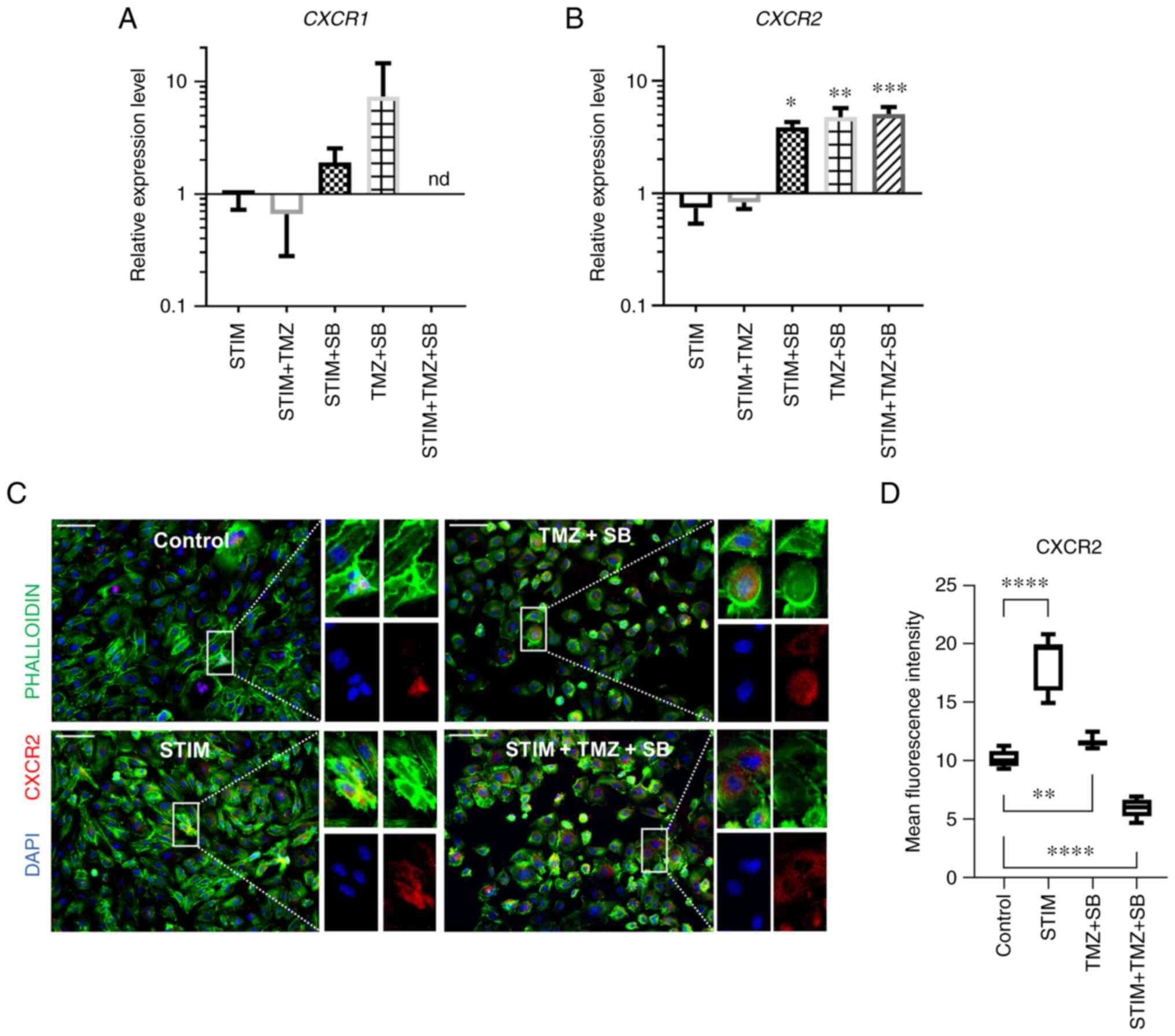 | Figure 3.Combination therapy with TMZ and SB
alters CXCR2 gene and protein expression in HUVECs. (A-D) HUVECs
were stimulated with a cocktail of 25 ng/ml CXLC2 and 25 ng/ml IL8
combined with 10 µM TMZ, 0.03 µM SB or both for 24 h as indicated.
(A and B) Analysis of mRNA expression regarding CXCR1 and CXCR2.
Expression of the proangiogenic receptor CXCR1 was not
significantly changed. By contrast, CXCR2 was upregulated by the
combination therapy and by SB alone within and outside of the
mimicked oversupply environment. Gene expression was analysed using
the relative quantification method (2-ΔΔCq) and compared to normal
culturing conditions (control). Accordingly, expression of each
target in the control group was set to 1. Medium containing 0.1%
FCS/1%DMSO was used as the control. P-values indicated in the graph
are in comparison to the control group. Other significant P-values
for CXCR2 expression: STIM vs. STIM + SB, P=0.0170; STIM vs. TMZ +
SB, 0.0007; STIM vs. STIM + TMZ + SB, P=0.0002; STIM + TMZ vs. STIM
+ TMZ, P=0.0035; STIM + TMZ vs. STIM + TMZ + SB, P=0.0013. Data
represents multiple experiments with similar results (n=9/condition
out of three independent experiments). (C and D) Immunofluorescence
staining of the cell nuclei (DAPI in blue), cytoskeleton
(PHALLOIDIN in green) and CXCR2 (in red). Representative images of
CXCR2 (C) in all four conditions, captured with the same exposure
time; Scale bar, 100 µm. (D) Boxplots depicting mean intensity
values under normal culturing conditions and in simulated
oversupply with and without combination therapy with TMZ and SB.
Mean intensity measurements revealed significant differences
between the treatment groups. In contrast to gene expression CXCR2
protein was significantly downregulated by the combination therapy
in the mimicked oversupply environment. (A-D) Medium containing
0.1% FCS/1% DMSO was used as the control. P-values indicated in the
graph are in comparison to the control group. Other significant
P-values for CXCR2 protein expression: STIM vs. TMZ + SB,
P<0.0001; STIM vs. STIM + TMZ + SB, P<0.0001; TMZ + SB vs.
STIM + TMZ + SB, P<0.0001; (n >10 images/condition).
*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. A, B
and D, one-way ANOVA (Bonferroni correction); bar graphs represent
the mean ± standard deviation. STIM, stimulation with 25 ng/ml
CXCL2 and IL8; TMZ, temozolomide; SB, SB225002; CXCR2, C-X-C motif
chemokine receptor 2; HUVECs, human umbilical vein endothelial
cells; CXCL2, C-X-C motif chemokine ligand 2; IL8, interleukin 8;
CXCR1, C-X-C motif chemokine receptor 1; FCS, fetal calf serum;
DMSO, dimethyl sulfoxide. |
As CXCR2 expression was affected by the
combination therapy and thus appeared to be more relevant than
CXCR1, the protein expression of CXCR2 was then evaluated
using immunofluorescence staining. In the mimicked CXCL2/IL8
oversupply environment, CXCR2 protein expression was significantly
increased (Fig. 3C and D).
Furthermore, the combination therapy led to an enhanced CXCR2
protein expression under normal culturing conditions and a
distinctively decreased CXCR2 expression under mimicked CXCL2/IL8
oversupply (Fig. 3C and D). In
summary, any treatment group receiving SB (STIM + SB, TMZ + SB and
STIM + TMZ + SB) exhibited an upregulation of CXCR2 at the gene
expression level while at the protein expression level CXCR2 was
differentially regulated. STIM and TMZ + SB exhibited an
upregulation of CXCR2 whereas the combination therapy in the
mimicked oversupply environment led to a downregulation of CXCR2 at
the protein level.
Expression of standard proangiogenic
receptors, VEGFR1 and VEGFR2, is unaltered by combination
therapy
VEGFR signalling is important for tumour
angiogenesis and has been widely studied (21,36).
It is known as the standard proangiogenic signalling in health and
in disease (21,37). Furthermore, previous studies
indicate a crosstalk between CXCL2/IL8 and VEGF signalling
(38–40). Therefore, investigating changes in
VEGFR signalling was of special interest. Treatment with TMZ, SB or
a combination of both had no effect on the expression of
VEGFR2, however VEGFR1 expression was significantly
reduced by SB in the mimicked oversupply environment with CXCL2 and
IL8 and by the combination therapy under normal culturing
conditions (Fig. 4A and B). The
next aim was to evaluate the effect of the combination therapy on
the protein expression of the most important proangiogenic VEGF
receptor, VEGFR2 (7). Treatment of
HUVECs with combined TMZ and SB in vitro was repeated, the
cells were fixed and immunofluorescence staining for VEGFR2 was
performed (Fig. 4C and D). At the
protein level VEGFR2 was strongly upregulated by the combination
therapy in the mimicked CXCL2/IL8 oversupply environment group and
in the mimicked oversupply environment alone. However, VEGFR2
expression was unaltered by the combination therapy under normal
culturing conditions (Fig. 4D).
Thus, VEGFR2 was differentially regulated at the gene and protein
expression levels.
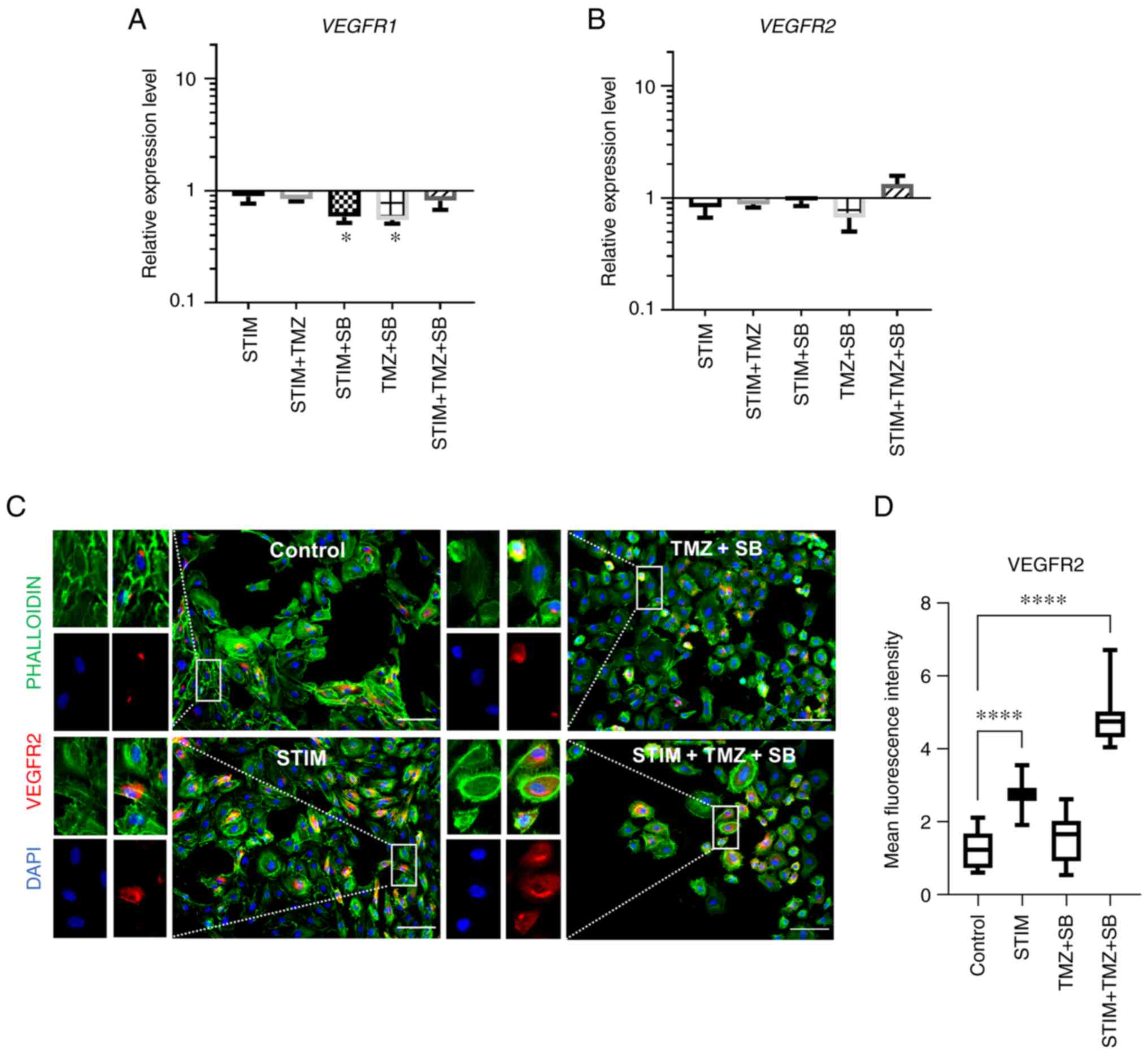 | Figure 4.Combination therapy with TMZ and SB
does not alter VEGFR2 gene expression but changes protein
expression of VEGFR2 in HUVECs. (A-D) HUVECs were stimulated with a
cocktail of 25 ng/ml CXLC2 and 25 ng/ml IL8 combined with 10 µM
TMZ, 0.03 µM SB or both for 24 h as indicated. (A and B) Analysis
of mRNA expression regarding VEGFR1 and VEGFR2. Expression of the
proangiogenic receptor VEGR1 and VEGFR2 was not significantly
changed in any group. Gene expression was analysed using the
relative quantification method (ΔΔCq) and compared to normal
culturing conditions (control). Accordingly, expression of each
target in the control group was set to 1. Medium containing 0.1%
FCS/1% DMSO was used as the control. Data represents multiple
experiments with similar results (n=9/condition out of three
independent experiments). P-values indicated in the graph are in
comparison to the control group. (C and D) Immunofluorescence
staining of the cell nuclei (DAPI in blue), cytoskeleton
(PHALLOIDIN; green) and VEGFR2 (red). Representative images of
VEGFR2 (C) in all four conditions, captured with the same exposure
time; Scale bar, 100 µm. (D) Boxplots depicting mean intensity
values under normal culturing conditions and in simulated
oversupply with and without combination therapy with TMZ and SB.
Mean intensity measurements revealed significant differences
between the treatment groups. In contrast to gene expression,
VEGFR2 was significantly upregulated by the combination therapy in
a mimicked oversupply environment. (A-D) Medium containing 0.1%
FCS/1% DMSO was used as the control. P-values indicated in the
graph are in comparison to the control group. Other significant
P-values for VEGFR2 protein expression: STIM vs. TMZ + SB,
P<0.0001; STIM vs. STIM + TMZ + SB, P<0.0001; TMZ + SB vs.
STIM + TMZ + SB, P<0.0001; (n >10 images/condition).
*P<0.05, ****P<0.0001. A, B and D, one-way ANOVA (Bonferroni
correction); bar graphs represent the mean ± standard deviation.
STIM, stimulation with 25 ng/ml CXCL2 and IL8; TMZ, temozolomide;
SB, SB225002; VEGFR2, vascular endothelial growth factor receptor
2; HUVECs, human umbilical vein endothelial cells; CXCL2, C-X-C
motif chemokine ligand 2; IL8, interleukin 8; VEGFR1, vascular
endothelial growth factor receptor 1; FCS, fetal calf serum; DMSO,
dimethyl sulfoxide. |
Expression of alternative
proangiogenic molecules is altered by the combination therapy
As gene and protein expression of the important
proangiogenic receptors VEGFR1/2 and CXCR1/2 were differently
affected by the combination therapy, the changes in the expression
of the associated ligands were evaluated. Expression of VEGF, CXCL2
and IL8 was not changed by the mimicked CXCL2/IL8 oversupply
environment (Fig. 5A-C).
Interestingly, CXCL2 and IL8 gene expression were altered by the
treatment with SB (Fig. 5A and B).
However, under normal culturing conditions only IL8 expression was
induced by the combination therapy (TMZ + SB), although not
reaching the level of significance (Fig. 5B). VEGF gene expression on the
other hand was not altered by SB and TMZ alone or combined
(Fig. 5C). Therefore, combination
therapy with TMZ and SB under normal culturing conditions as well
as in a mimicked oversupply environment mainly affected IL8
expression while the gene expression of CXCL2 and VEGF was
unaltered in human primary endothelial cells. However, CXCL2
expression was upregulated by sole treatment with the CXCR2
antagonist SB.
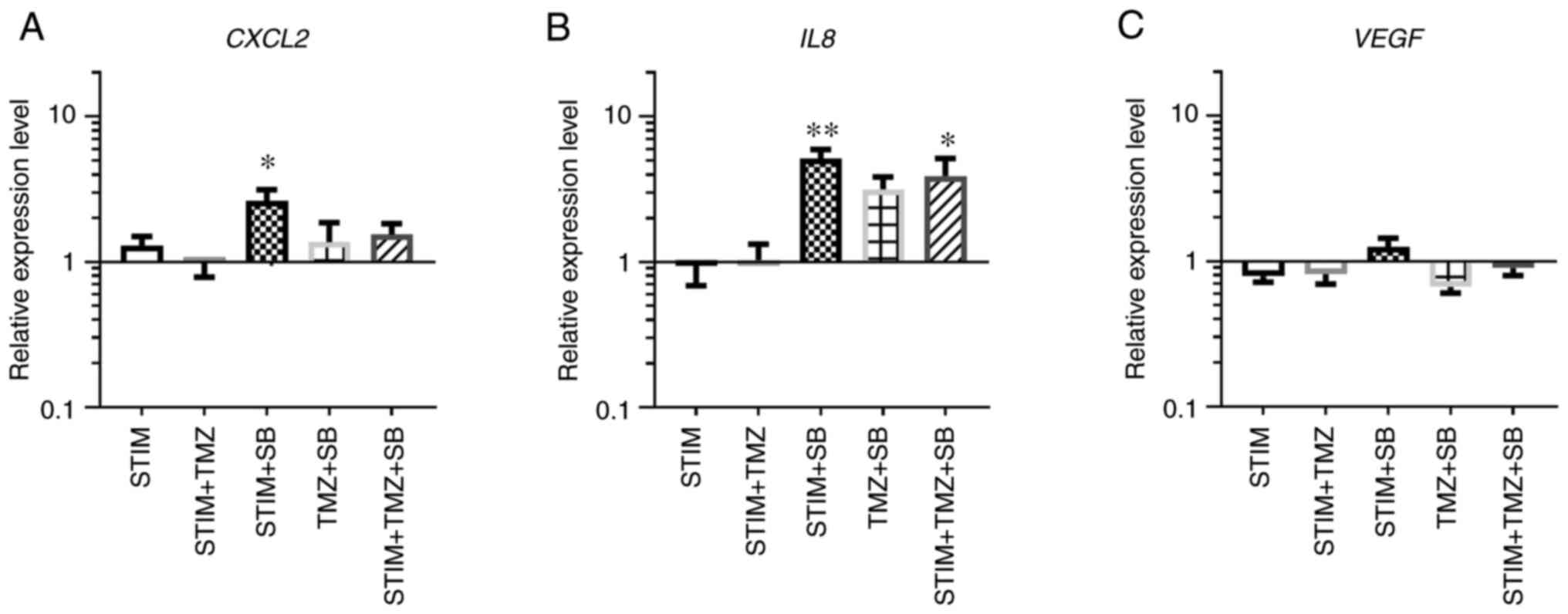 | Figure 5.RNA expression of proangiogenic
mediators is altered by treatment with TMZ and SB in human
endothelial cells. (A-C) HUVECs were stimulated with a cocktail of
25 ng/ml CXLC2 and 25 ng/ml IL8 combined with 10 µM TMZ, 0.03 µM SB
or both for 24 h. Analysis of mRNA expression regarding the
indicated genes are depicted. Gene expression was analysed using
the relative quantification method (ΔΔCq) and compared to normal
culturing conditions (control). Accordingly, expression of each
target in the control group was set to 1. Medium containing 0.1%
FCS/1% DMSO was used as the control. Changes of relative expression
levels are shown for CXCL2, IL8 and VEGF on a logarithmic scale.
P-values indicated in the graph are in comparison to the control
group. Other significant P-values for CXCL2 expression: STIM + TMZ
vs. STIM + SB, P=0.0353. Other significant P-values for IL8
expression: STIM vs. STIM + SB, P=0.0048; STIM + TMZ vs. STIM + SB,
P=0.0036. Data represents multiple experiments with similar results
(n=9/condition out of three independent experiments). *P<0.05,
**P<0.01. A-C, one-way ANOVA (Bonferroni correction); bar graphs
represent the mean ± standard deviation. TMZ, temozolomide; SB,
SB225002; HUVECs, human umbilical vein endothelial cells; STIM,
stimulation with 25 ng/ml CXCL2 and IL8; CXCL2, C-X-C motif
chemokine ligand 2; IL8, interleukin 8; FCS, fetal calf serum;
DMSO, dimethyl sulfoxide; VEGF, vascular endothelial growth
factor. |
Discussion
The data of the present study revealed that
treatment with SB and TMZ led to morphological changes of primary
human endothelial cells (HUVECs) and downregulated antiapoptotic
BCL2 in vitro. In addition, gene expression of the
alternative proangiogenic CXCL2/IL8/CXCR2 signalling pathway was
altered by the combination therapy, while the VEGF/VEGFR1/2 pathway
was only mildly affected. Furthermore, the data revealed that gene
and protein expression of these two proangiogenic pathways were
differentially regulated.
Resistance towards conventional GBM therapies
requires new therapeutic approaches. Due to GBM heterogeneity,
single treatments are destined to fail and combination therapies
can target the tumour more effectively (6,41).
In a previous study by the authors, it was demonstrated that single
treatment with SB reduced the tumour burden, vessel density and
infiltration of tumour-associated microglia/macrophages in an
orthotopic immunocompetent mouse model, but failed to cure the mice
(11). A follow-up study with a
combination therapy consisting of TMZ and SB revealed promising
first insights after just one cycle of combined treatment with TMZ
and SB (19). The combination
therapy was tolerated well in vivo and led to decreased
tumour volumes in an orthotopic GBM mouse model (19). However, little is known about the
molecular changes that the combination therapy may evoke in
specified niches. In the present study, it was determined that
protein and gene expression are differentially regulated in
vitro. Notably, primary human endothelial cells exhibited a
significant downregulation of antiapoptotic BCL2 and a
tendency to upregulate proapoptotic BAX after treatment with
TMZ + SB compared with TMZ alone in a mimicked CXCL2 and IL8
oversupply environment, which would be beneficial if these findings
could be translated to GBM tumours. This proapoptotic role of SB
has also been reported in other studies (29,31).
Furthermore, antitumoural effects have been shown in numerous
tumour entities in vitro and in vivo, which can be
explained by its proapoptotic functions (11,29,42–45).
However, in the previous study performed by the authors (19), gene expression analyses of murine
gliomas treated with the combination therapy did not exhibit an
upregulation of BAX within the tumour in contrast to the
findings in the present study. There, RNA was isolated from the
tumour bulk whereas the present study specifically focused on
endothelial cells (19).
Furthermore, these differences could be explained by the abundant
expression of CXCR2 in endothelial cells while glioma cells express
CXCR2 to a lesser extent 11,18,25,46).
Several studies indicate that there is a crosstalk
between CXCL2/IL8 and VEGF signalling (38–40).
Through different mechanisms this crosstalk can lead to an
upregulation of BCL2 and subsequent upregulation of IL8 in
endothelial cells (39). As
revealed by the data in the present study, SB alone and in
combination with TMZ led to a significant downregulation of BCL2
and therefore may weaken the effect of this crosstalk between the
two proangiogenic signalling pathways. Nevertheless, IL8 was
upregulated in all treatment groups receiving the antagonist
compared with the normal culturing conditions in vitro. This
effect has been described before for SB in breast cancer and glioma
cells in vitro (11,47).
TMZ alone did not have any significant effect on all the targeted
genes in the present study. Notably, IL8 and CXCL2 were regulated
differently, even though they mediate their functions through the
same receptor, CXCR2. This poses the question whether they have
complementary functions. While IL8 has been studied extensively,
less is known about CXCL2 (18,30,48).
In a previous study by the authors, it was demonstrated that CXCL2
and IL8 were equally potent initiators of angiogenesis in primary
brain endothelial cells while both molecules were less potent in
HUVECs (18). Furthermore, in a
previous analysis of 38 patients with matched primary and recurrent
GBM tumours, performed by the authors, it was revealed that CXCL2
was expressed by all patients in the primary tumour while IL8 was
only expressed by 43% (19).
However, IL8 was significantly upregulated to 67.5% in recurrent
GBM tumours (19). This should be
considered and further research with brain-derived endothelial
cells is warranted to verify the results.
Furthermore, while gene expression of VEGFR2
was not altered, protein expression was significantly upregulated
in the STIM + TMZ + SB group. This raises the question of whether
combination therapy could lead to an unexpected increase in
angiogenesis. Nevertheless, in the previous study by the authors,
VEGFR2 expression was also unchanged by the combination
therapy in vivo and vascular parameters e.g., vessel density
and vessel size were not increased (19). However, VEGFR2 protein expression
was not analysed in vivo (19). With regard to VEGFR2
upregulation, it is important to highlight that VEGF is not the
only signalling pathway relevant for angiogenesis in GBM. In the
present study, CXCR2 expression was downregulated, supporting the
theory of crosstalk between these two major proangiogenic
signalling pathways, which may explain the differences in gene and
protein expression underlining the importance of
post-transcriptional processing. The impact of post-transcriptional
processing as one reason for differences in mRNA and protein
expression has been previously described in detail (49–51).
Cheng et al suggest that these differences may be
time-dependent and therefore, measuring gene and protein expression
at the same time-point could lead to different results (49). Furthermore, the impact of other
post-translational steps is controversially discussed in literature
but could contribute to the differences in gene and protein
expression (49,51).
Although the present study specifically focused on
primary endothelial cells, it could function as a foundation for
the development of a new GBM treatment protocol. The combination
therapy was well tolerated in our previous in vivo study in
an immunocompetent mouse model. Apart from inducing angiogenesis,
proliferation and migration in endothelial cells as well as in
glioma cells, CXCR2 signalling is known for its important role in
vascular mimicry and the trans-differentiation of glioma cells into
endothelial-like cells (18,24,46,52,53).
Therefore, the molecular changes by the combination therapy may be
similar in glioma cells. GBM is a very heterogeneous tumour and it
is likely that this combination therapy would only target a
subgroup of tumour cells (54–56).
However, to date, a therapy that targets all tumour cells as well
as the tumour microenvironment has yet to be discovered. In this
regard combination therapies with SB appear promising. For
instance, therapeutic approaches combining anti-CXCR4 therapy,
CXCR4 is a different important chemokine receptor in GBM, with the
well-established treatment options such as TMZ and/or radiotherapy
are currently being investigated in clinical phase I and II studies
and show encouraging results (57)
(NCT03746080).
As aforementioned, the significance of the data may
be limited, as the experiments were carried out with primary human
endothelial cells. As demonstrated in a previous study performed by
the authors, primary endothelial cells from the periphery may
behave differently to primary brain endothelial cells (18). Furthermore, tumour cells,
especially glioma cells may also behave differently. However,
previous studies have shown that endothelial and glioma cell gene
expression is similarly altered by SB in vitro, therefore,
the combination therapy could have similar effects (11). However, it is unknown whether this
can also be applied to an in vivo setting. Furthermore,
additional research is warranted as protein expression was analysed
solely by immunofluorescence staining. Other techniques may deliver
more insight into the functional changes within the investigated
pathways. A step forward could be to investigate the effect of the
combination therapy in glioma organoids in vitro.
In conclusion, the data of the present study
revealed that the combination therapy consisting of SB and TMZ
altered the gene expression of antiapoptotic BCL2 and the
CXCR2 signalling pathway in primary endothelial cells. Furthermore,
the combination therapy led to differential gene and protein
expression of the proangiogenic receptors CXCR2 and VEGFR2 in
vitro. The data provides first insights into the molecular
changes of two major proangiogenic pathways during treatment with
TMZ and SB on primary endothelial cells, which should be considered
in future studies.
Acknowledgements
We would like to acknowledge the support that GA
received by the Lydia Rabinowitsch funding within the framework of
the advancement of women in science. GA is a participant of the
BIH-Charité Clinician Scientist Program funded by the Charité
Universitätsmedizin Berlin and the Berlin Institute of Health
(Berlin, Germany). We would also like to thank Dr Ran Xu
(Department of Neurosurgery, Charité-Universitätsmedizin Berlin,
Corporate Member of Freie Universität Berlin, Humboldt-Universität
zu Berlin, Berlin) for providing the Phalloidin staining
reagent.
Funding
The present study was supported by the Berliner
Krebsgesellschaft (BKG; grant no. ACFF201518). RMU was funded by a
doctoral scholarship from the BKG. The present scientific data was
obtained during the doctoral thesis of RMU at
Charité-Universitätsmedizin (Berlin, Germany).
Availability of data and materials
The datasets used and analysed during the present
study are available from the corresponding author upon request.
Authors' contributions
RMU was involved in the conceptualisation of the
project and acquisition of the funding. RMU carried out the
experiments, curated, visualised and analysed the data and drafted
the manuscript. CJ was involved in the interpretation of data, as
well as reviewed and edited the manuscript. PV interpreted the
data, was responsible for funding and supervision including
reviewing and editing the manuscript. SB and GA were involved in
the conceptualisation and supervision of the project, they curated
the data and verified the data analysis as well as reviewed and
edited the manuscript. In addition, GA was involved in the
acquisition of the funding. All authors confirm the authenticity of
all the raw data and have read and approved the final
manuscript.
Ethics approval and consent to
participate
An ethical standards statement was provided by the
supplier (PromoCell). Permission to publish the collected data was
obtained from the Ethics Committee at Charité-Universitätsmedizin
Berlin (EA1/090/22).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Cioffi G, Gittleman H, Patil N,
Waite K, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: primary brain and other central nervous system tumors
diagnosed in the United States in 2012–2016. Neuro Oncol. 21 (Suppl
5):v1–v100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laperriere N, Zuraw L and Cairncross G;
Cancer Care Ontario Practice Guidelines Initiative Neuro-Oncology
Disease Site Group, : Radiotherapy for newly diagnosed malignant
glioma in adults: A systematic review. Radiother Oncol. 64:259–273.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sueoka H, Hirano T, Uda Y, Iimuro Y,
Yamanaka J and Fujimoto J: Blockage of CXCR2 suppresses tumor
growth of intrahepatic cholangiocellular carcinoma. Surgery.
155:640–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marenco-Hillembrand L, Wijesekera O,
Suarez-Meade P, Mampre D, Jackson C, Peterson J, Trifiletti D,
Hammack J, Ortiz K, Lesser E, et al: Trends in glioblastoma:
Outcomes over time and type of intervention: A systematic evidence
based analysis. J Neurooncol. 147:297–307. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Urbantat RM, Vajkoczy P and Brandenburg S:
Advances in chemokine signaling pathways as therapeutic targets in
glioblastoma. Cancers (Basel). 13:29832021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shibuya M: Vascular endothelial growth
factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A
crucial target for anti- and pro-angiogenic therapies. Genes
Cancer. 2:1097–1105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stupp R, Taillibert S, Kanner A, Read W,
Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K,
et al: Effect of tumor-treating fields plus maintenance
temozolomide vs maintenance temozolomide alone on survival in
patients with glioblastoma: A randomized clinical trial. JAMA.
318:2306–2316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang B, Li X, Li Y, Zhang J, Zong Z and
Zhang H: Current immunotherapies for glioblastoma multiforme. Front
Immunol. 11:6039112021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sampson JH, Maus MV and June CH:
Immunotherapy for brain tumors. J Clin Oncol. 35:2450–2456. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Acker G, Zollfrank J, Jelgersma C,
Nieminen-Kelhä M, Kremenetskaia I, Mueller S, Ghori A, Vajkoczy P
and Brandenburg S: The CXCR2/CXCL2 signalling pathway-an
alternative therapeutic approach in high-grade glioma. Eur J
Cancer. 126:106–115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahir BK, Engelhard HH and Lakka SS: Tumor
development and angiogenesis in adult brain tumor: Glioblastoma.
Mol Neurobiol. 57:2461–2478. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takano S, Yamashita T and Ohneda O:
Molecular therapeutic targets for glioma angiogenesis. J Oncol.
2010:3519082010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szulzewsky F, Pelz A, Feng X, Synowitz M,
Markovic D, Langmann T, Holtman IR, Wang X, Eggen BJ, Boddeke HW,
et al: Glioma-associated microglia/macrophages display an
expression profile different from M1 and M2 polarization and highly
express Gpnmb and Spp1. PLoS One. 10:e01166442015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hambardzumyan D, Gutmann DH and Kettenmann
H: The role of microglia and macrophages in glioma maintenance and
progression. Nat Neurosci. 19:20–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brandenburg S, Blank A, Bungert AD and
Vajkoczy P: Distinction of microglia and macrophages in
glioblastoma: Close relatives, different tasks? Int J Mol Sci.
22:1942020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blank A, Kremenetskaia I, Urbantat RM,
Acker G, Turkowski K, Radke J, Schneider UC, Vajkoczy P and
Brandenburg S: Microglia/macrophages express alternative
proangiogenic factors depending on granulocyte content in human
glioblastoma. J Pathol. 253:160–173. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Urbantat RM, Blank A, Kremenetskaia I,
Vajkoczy P, Acker G and Brandenburg S: The CXCL2/IL8/CXCR2 pathway
is relevant for brain tumor malignancy and endothelial cell
function. Int J Mol Sci. 22:26342021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Urbantat RM, Jelgersma C, Brandenburg S,
Nieminen-Kelhä M, Kremenetskaia I, Zollfrank J, Mueller S, Rubarth
K, Koch A, Vajkoczy P and Acker G: Tumor-associated
microglia/macrophages as a predictor for survival in glioblastoma
and temozolomide-induced changes in CXCR2 signaling with new
resistance overcoming strategy by combination therapy. Int J Mol
Sci. 22:111802021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brandenburg S, Turkowski K, Mueller A,
Radev YT, Seidlitz S and Vajkoczy P: Myeloid cells expressing high
level of CD45 are associated with a distinct activated phenotype in
glioma. Immunol Res. 65:757–768. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chaudhry IH, O'Donovan DG, Brenchley PE,
Reid H and Roberts IS: Vascular endothelial growth factor
expression correlates with tumour grade and vascularity in gliomas.
Histopathology. 39:409–415. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Norden AD, Drappatz J and Wen PY:
Antiangiogenic therapy in malignant gliomas. Curr Opin Oncol.
20:652–661. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Angara K, Borin TF, Rashid MH, Lebedyeva
I, Ara R, Lin PC, Iskander A, Bollag RJ, Achyut BR and Arbab AS:
CXCR2-expressing tumor cells drive vascular mimicry in
antiangiogenic therapy-resistant glioblastoma. Neoplasia.
20:1070–1082. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murdoch C, Monk PN and Finn A: Cxc
chemokine receptor expression on human endothelial cells. Cytokine.
11:704–712. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Addison CL, Daniel TO, Burdick MD, Liu H,
Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A and Strieter RM:
The CXC chemokine receptor 2, CXCR2, is the putative receptor for
ELR+ CXC chemokine-induced angiogenic activity. J Immunol.
165:5269–5277. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brat DJ, Bellail AC and Van Meir EG: The
role of interleukin-8 and its receptors in gliomagenesis and
tumoral angiogenesis. Neuro Oncol. 7:122–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hillyer P, Mordelet E, Flynn G and Male D:
Chemokines, chemokine receptors and adhesion molecules on different
human endothelia: Discriminating the tissue-specific functions that
affect leucocyte migration. Clin Exp Immunol. 134:431–441. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Vasconcellos JF, Laranjeira AB, Leal
PC, Bhasin MK, Zenatti PP, Nunes RJ, Yunes RA, Nowill AE, Libermann
TA, Zerbini LF and Yunes JA: SB225002 induces cell death and cell
cycle arrest in acute lymphoblastic leukemia cells through the
activation of GLIPR1. PLoS One. 10:e01347832015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li A, Dubey S, Varney ML, Dave BJ and
Singh RK: IL-8 directly enhanced endothelial cell survival,
proliferation, and matrix metalloproteinases production and
regulated angiogenesis. J Immunol. 170:3369–3376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goda AE, Koyama M, Sowa Y, Elokely KM,
Yoshida T, Kim BY and Sakai T: Molecular mechanisms of the
antitumor activity of SB225002: A novel microtubule inhibitor.
Biochem Pharmacol. 85:1741–1752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gale NW and Yancopoulos GD: Growth factors
acting via endothelial cell-specific receptor tyrosine kinases:
VEGFs, angiopoietins, and ephrins in vascular development. Genes
Dev. 13:1055–1066. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Waltenberger J, Claesson-Welsh L, Siegbahn
A, Shibuya M and Heldin CH: Different signal transduction
properties of KDR and Flt1, two receptors for vascular endothelial
growth factor. J Biol Chem. 269:26988–26995. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Imoukhuede PI and Popel AS: Quantification
and cell-to-cell variation of vascular endothelial growth factor
receptors. Exp Cell Res. 317:955–965. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ferrara N: The role of VEGF in the
regulation of physiological and pathological angiogenesis. EXS.
209–231. 2005.PubMed/NCBI
|
|
38
|
Nör JE, Christensen J, Liu J, Peters M,
Mooney DJ, Strieter RM and Polverini PJ: Up-regulation of Bcl-2 in
microvascular endothelial cells enhances intratumoral angiogenesis
and accelerates tumor growth. Cancer Res. 61:2183–2188.
2001.PubMed/NCBI
|
|
39
|
Karl E, Zhang Z, Dong Z, Neiva KG, Soengas
MS, Koch AE, Polverini PJ, Núñez G and Nör JE: Unidirectional
crosstalk between Bcl-xL and Bcl-2 enhances the angiogenic
phenotype of endothelial cells. Cell Death Differ. 14:1657–1666.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Strieter RM, Burdick MD, Mestas J,
Gomperts B, Keane MP and Belperio JA: Cancer CXC chemokine networks
and tumour angiogenesis. Eur J Cancer. 42:768–778. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ghosh D, Nandi S and Bhattacharjee S:
Combination therapy to checkmate glioblastoma: Clinical challenges
and advances. Clin Transl Med. 7:332018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang B, Hendricks DT, Wamunyokoli F and
Parker MI: A growth-related oncogene/CXC chemokine receptor 2
autocrine loop contributes to cellular proliferation in esophageal
cancer. Cancer Res. 66:3071–3077. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Matsuo Y, Campbell PM, Brekken RA, Sung B,
Ouellette MM, Fleming JB, Aggarwal BB, Der CJ and Guha S: K-Ras
promotes angiogenesis mediated by immortalized human pancreatic
epithelial cells through mitogen-activated protein kinase signaling
pathways. Mol Cancer Res. 7:799–808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu M, Berk R and Kosir MA: CXCL7-mediated
stimulation of lymphangiogenic factors VEGF-C, VEGF-D in human
breast cancer cells. J Oncol. 2010:9394072010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Saintigny P, Massarelli E, Lin S, Ahn YH,
Chen Y, Goswami S, Erez B, O'Reilly MS, Liu D, Lee JJ, et al: CXCR2
expression in tumor cells is a poor prognostic factor and promotes
invasion and metastasis in lung adenocarcinoma. Cancer Res.
73:571–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hasan T, Caragher SP, Shireman JM, Park
CH, Atashi F, Baisiwala S, Lee G, Guo D, Wang JY, Dey M, et al:
Interleukin-8/CXCR2 signaling regulates therapy-induced plasticity
and enhances tumorigenicity in glioblastoma. Cell Death Dis.
10:2922019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Erin N, Nizam E, Tanrıöver G and Köksoy S:
Autocrine control of MIP-2 secretion from metastatic breast cancer
cells is mediated by CXCR2: A mechanism for possible resistance to
CXCR2 antagonists. Breast Cancer Res Treat. 150:57–69. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Koch AE, Polverini PJ, Kunkel SL, Harlow
LA, DiPietro LA, Elner VM, Elner SG and Strieter RM: Interleukin-8
as a macrophage-derived mediator of angiogenesis. Science.
258:1798–1801. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cheng Z, Teo G, Krueger S, Rock TM, Koh
HW, Choi H and Vogel C: Differential dynamics of the mammalian mRNA
and protein expression response to misfolding stress. Mol Syst
Biol. 12:8552016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vogel C and Marcotte EM: Insights into the
regulation of protein abundance from proteomic and transcriptomic
analyses. Nat Rev Genet. 13:227–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Buccitelli C and Selbach M: mRNAs,
proteins and the emerging principles of gene expression control.
Nat Rev Genet. 21:630–644. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sharma I, Singh A, Siraj F and Saxena S:
IL-8/CXCR1/2 signalling promotes tumor cell proliferation, invasion
and vascular mimicry in glioblastoma. J Biomed Sci. 25:622018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Baisiwala S, Auffinger B, Caragher SP,
Shireman JM, Ahsan R, Lee G, Hasan T, Park C, Saathoff MR,
Christensen AC and Ahmed AU: Chemotherapeutic stress induces
transdifferentiation of glioblastoma cells to endothelial cells and
promotes vascular mimicry. Stem Cells Int. 2019:61074562019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lauko A, Lo A, Ahluwalia MS and Lathia JD:
Cancer cell heterogeneity & plasticity in glioblastoma and
brain tumors. Semin Cancer Biol. 82:162–175. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Perrin SL, Samuel MS, Koszyca B, Brown MP,
Ebert LM, Oksdath M and Gomez GA: Glioblastoma heterogeneity and
the tumour microenvironment: Implications for preclinical research
and development of new treatments. Biochem Soc Trans. 47:625–638.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Qazi MA, Vora P, Venugopal C, Sidhu SS,
Moffat J, Swanton C and Singh SK: Intratumoral heterogeneity:
Pathways to treatment resistance and relapse in human glioblastoma.
Ann Oncol. 28:1448–1456. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Thomas RP, Nagpal S, Iv M, Soltys SG,
Bertrand S, Pelpola JS, Ball R, Yang J, Sundaram V, Lavezo J, et
al: Macrophage exclusion after radiation therapy (MERT): A first in
human phase I/II trial using a CXCR4 inhibitor in glioblastoma.
Clin Cancer Res. 25:6948–6957. 2019. View Article : Google Scholar : PubMed/NCBI
|



















