Introduction
Ovarian cancer (OC) is a type of gynaecological
malignant tumour and is the eighth leading cause of
cancer-associated mortality in women worldwide (1). The high mortality rate associated
with OC may be due to its late detection, as the vast majority of
patients with OC are diagnosed at an advanced stage (2), and thus encounter recurrence and drug
resistance. Although a significant proportion of patients with
advanced disease can achieve a complete response with tumour
resection and adjuvant chemotherapy, the majority of patients will
relapse within 2 years (2,3). The 5-year survival rate of patients
with advanced OC is ~40% in the United States (4,5). The
combination of paclitaxel (PTX) and carboplatin is the first-line
medication for postoperative treatment in patients with OC. PTX can
bind to tubulin proteins and enhance their polymerization to induce
mitotic G2/M phase arrest, which results in cell cycle
arrest (6). However, PTX
application is limited because of its serious side effects and
multidrug resistance in tumour cells (7,8).
Over the past decade, there has been little improvement in the cure
rate for advanced OC; therefore, it is necessary to develop
effective therapeutics to overcome chemoresistance.
In recent years, adjuvant chemotherapy with
traditional Chinese medicine has been widely considered.
Schisandra chinensis is a perennial woody vine of the
Magnoliaceae family, which is commonly used as a traditional
Chinese medicine. It is commonly called Wuweizi or ‘five-flavour
fruit’ in China. Fructus Schizandra has a high medicinal value
because of its protective effect on a number of organs, including
the heart, liver, kidney, nervous system and gastrointestinal tract
(9). The active ingredient of
Schisandra chinensis, Schisandrin B, has been used to treat
human lung carcinoma cells (10)
and murine breast cancer 4T1 cells (11). In addition, it has been
demonstrated that the major bioactive constituents of Fructus
Schizandra can significantly enhance the doxorubicin-induced
apoptosis of cancer cells, such as SMMC7721, a human hepatic
carcinoma cell line, and MCF-7, a human breast cancer cell line
(12). Crude extracts of S.
chinensis have been shown to reverse multidrug resistance and
improve the sensitivity of cancer cells to chemotherapeutic drugs
by inhibiting the expression of P-glycoprotein and protein kinase C
(13). Furthermore, previous
studies have indicated that an effective component of
Schisandra, Gomisin A (GA), serves a role in reversing drug
resistance (14), inhibiting
proliferation, and exerting antioxidant and anti-inflammatory
activities (15). In addition, as
one of the major bioactive constituents of Fructus Schizandra, GA
has been shown to possess anticancer and antiangiogenic activity
(16). Although GA has exhibited
its inhibitory effects against various tumour cell lines, such as
melanoma (17) and colorectal
cancer (18) cell lines, its
cytotoxicity in OC cells has not yet been evaluated. Furthermore,
the benefit of the combination of GA and PTX on OC cells is
unknown.
It has previously been reported that PTX can promote
the production of reactive oxygen species (ROS) (19). ROS are a series of chemical
compounds containing oxygen produced during cellular metabolism,
including superoxide anion, hydroxyl radical and hydrogen peroxide;
these chemicals can act on lipids, proteins and DNA (20). Oxidative stress induced by
potential ROS causes irreversible cell damage and death; however,
it has also been suggested that ROS can initiate carcinogenesis and
help tumour cells survive (21).
Radiation, tobacco and xenobiotics have long been some of the
best-known sources of ROS and are associated with tumour-induced
events (22). Therefore, the
present study hypothesized that GA could enhance the sensitivity of
OC cells to PTX by reducing ROS production.
In the present study, the human ovarian cancer cell
lines SKOV3 and A2780 were used to investigate the effects of an
effective component of Schisandra, GA, with PTX in
vitro and in vivo. The present study assessed whether GA
could increase the antitumor effects of PTX. Moreover, by observing
the changes in ROS in cells, the possible mechanism underlying the
effects of GA-induced ROS inhibition on PTX sensitivity was
discussed.
Materials and methods
Chemicals and reagents
GA (purity ≥98%), PTX (purity ≥98%), and N-acetyl
cysteine (NAC; purity ≥98%) were purchased from Shanghai Yuanye
Bio-Technology Co., Ltd. An MTT reagent kit and dimethyl sulfoxide
(DMSO) were purchased from MilliporeSigma. Wright-Giemsa stain
solution, and haematoxylin and eosin (H&E) Staining Kit were
purchased from Beijing Solarbio Science & Technology Co., Ltd.
Propidium iodide (PI), RNase-A, Triton X-100, ROS DCFH-DA assay kit
(cat. no. S0033S), and radioimmunoprecipitation assay (RIPA) lysis
buffer were purchased from Beyotime Institute of Biotechnology. An
Annexin V-FITC/PI cell apoptosis detection kit was purchased from
Beijing TransGen Biotech Co., Ltd. Dihydroethidium (DHE; cat. no.
KGAF019) assay kit was purchased from Nanjing KeyGen Biotech Co.,
Ltd. Antibodies against epidermal growth factor receptor (EGFR;
cat. no. 18986-1-AP), PI3K (cat. no. 20584-1-AP), Akt (cat. no.
10176-2-AP), phosphorylated (p)-Akt (cat. no. 66444-1-Ig), β-actin
(cat. no. 20536-1-AP), cyclin-dependent kinase 4 (CDK4; cat. no.
11026-1-AP), cyclin B1 (cat. no. 28603-1-AP), GAPDH (cat. no.
10494-1-AP), matrix metallopeptidase 2 (MMP-2; cat. no.
10373-2-AP), Nrf2 (cat. no. 16396-1-AP), Keap-1 (cat. no.
10503-2-AP), NQO1 (cat. no. 11451-1-AP), and Ki-67 (cat. no.
27309-1-AP) were purchased from Wuhan Sanying Biotechnology.
Cell culture
SKOV3 and A2780 cell lines were obtained from the
Prostate Disease Prevention and Control Centre, Basic Medical
College of Jilin University; the SKOV3 (cat. no. CL-0215) and A2780
(cat. no. CL-0013) cell lines were originally purchased from
Procell Life Science & Technology Co., Ltd. The SKOV3 cells
were cultured in Iscove's modified Dulbecco's medium (HyClone;
Cytiva) and the A2780 cells were cultured in Dulbecco's modified
Eagle's medium (HyClone; Cytiva) both supplemented with 10% foetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in a humidified
atmosphere containing 5% CO2.
Animals
A total of 20 female BALB/c-Nu mice (age, 6 weeks;
weight, 16–20 g) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd., and were reared under specific
pathogen-free conditions at a temperature of 22±1°C and 45–55%
humidity. Mice were maintained under a 12-h light/dark cycle and
were provided with ad libitum access to food and water. The
feeding and handling of experimental animals followed the
regulations of Jilin University and the in vivo experimental
protocol was approved by the Experimental Animal Ethics Committee
of Jilin University Second Hospital (approval no. 2018106;
Changchun, China).
A2780 cells (2×106 cells) were
subcutaneously injected into the right back of each mouse. When the
volume reached 100–150 mm3, the mice were distributed
into four groups (n=5 mice/group) as follows: i) Control group, in
which mice were intraperitoneally injected with 200 µl PBS once
every 2 days; ii) GA group, in which mice were intraperitoneally
injected with 200 µl GA (10 mg/kg) once every 2 days; iii) PTX
group, in which mice were intraperitoneally injected with 200 µl
PTX (10 mg/kg) once every 2 days; and iv) PTX combined with GA
group, in which mice received the same dose of each drug as the
monotherapy group. PTX and GA were dissolved in saline. For each
mouse, a volume of 200 µl solution containing the drug was injected
each time.
Tumour volume was calculated daily as follows:
Volume=L × W2/2, where L and W represent the length and
width of the tumour, respectively. In addition, the body weight of
each mouse was calculated daily. No animals were withdrawn from the
study. After 14 days of treatment, the mice were euthanized by
cervical dislocation under inhalation of excess ether (mice were
anaesthetized until their muscles were completely relaxed and their
eyelid reflexes disappeared, the ether was then removed and mice
were sacrificed by cervical dislocation), and the tumours were
excised and weighed. The heart, liver, spleen, lung and kidney were
also removed for further analysis. The specific humane endpoints
used to determine euthanasia were when the tumours were >2,000
mm3, tumour diameter was >20 mm, or body weight loss
was >20%. Death was verified by observation of the cessation of
breath and heartbeat, and areflexia.
MTT assay
The in vitro cytotoxicity of GA, NAC and PTX
in SKOV3 and A2780 cells was measured using the MTT assay, as
previously described (23).
Briefly, the cells were cultured in 96-well plates at a density of
3,000 cells/well. SKOV3 and A2780 cells were treated with 0.04 µΜ
GA, 16 µg/ml PTX or their combination (GA + PTX group) for 6 and 24
h at 37°C. In addition, for additional experiments, SKOV3 and A2780
cells were incubated with 0.5 mM NAC for 1 h, and then 16 µg/ml PTX
was added to the NAC + PTX group for 6 and 24 h at 37°C. After
being exposed to the drugs, MTT (5 mg/ml) was added to each well.
After incubated at 37°C for 4 h, DMSO was added to dissolve the
precipitate. The absorbance of each well was measured the optical
density at 490 nm (OD490). All experiments were repeated three
times. The inhibition rates were calculated as following:
Inhibition rate (%)=(ODc-Odt)/ODc, where ODt and ODc represent the
OD490 values of the treatment group and control group,
respectively.
Colony formation
SKOV3 and A2780 cells were seeded into 6-well plates
at 300 cells/well and were treated with GA, PTX, NAC, NAC + PTX or
GA + PTX for 2 weeks at 37°C. The drug concentrations were the same
as those used prior to the MTT assay, and these concentrations were
used for the subsequent experiments in vitro. After
treatment, the cells were fixed with 4% paraformaldehyde for 15 min
at 37°C and washed three times with PBS. Finally, the cells were
stained with Wright-Giemsa stain solution according to the
manufacturer's protocols. The colonies containing >50 cells were
counted. The cell clonality rates were calculated as following:
Cell clonality (%)=colony number/seeded cells number.
Cell cycle and apoptosis assays
SKOV3 and A2780 cells were seeded into 6-well plates
at 4×105 cells/well and were divided into different
groups with the indicated treatments for 24 h at 37°C, after which
cell cycle progression and apoptosis were analysed. For the cell
cycle assay, after being harvested, the cells were fixed in 70%
precooled ethanol overnight at −20°C and then suspended in PBS
containing 50 µg/ml PI, 100 µg/ml RNase-A and 0.2% Triton X-100 for
30 min at 4°C in the dark. The stained cells were analysed by a
FACScan flow cytometer (BD Biosciences), and the results were
analysed by Modfit LT 5 software (Verity Software House, Inc.).
For apoptosis detection. all cells, both floating
and adherent, were harvested. Subsequently, the cells were stained
with 5 µl Annexin V-FITC and 5 µl PI for 15 min at room temperature
in the dark according to the manufacturer's instructions. The
stained cells were analysed by a FACScan flow cytometer and the
results were analysed using BD Diva 3 software (BD
Biosciences).
Wound scratch assay and Transwell
invasion assay
For the wound scratch assay, SKOV3 and A2780 cells
were seeded into 12-well plates at 3×105 cells/well.
When the cells reached 80–90% confluence, a sterile 200-µl pipette
tip was used to scratch the cell monolayer. The wounds were
observed at 0 and 48 h after being treated with GA, NAC, PTX, NAC +
PTX or GA + PTX in serum-free medium at 37°C. Cell images were
captured by an optical microscope (Motic Incorporation, Ltd.).
Semi-quantification of the wound scratch assay was conducted by
measuring the gap distance. Migration rate (%) was calculated as
follows: (0 h gap distance-48 h gap distance)/0 h gap distance.
For the invasion assay, 24-well Transwell chambers
(pore size, 8.0 µm; cat. no. 3422; Corning, Inc.) were used to
analyse the invasive ability of the cells in each treatment group.
The chamber was coated with Matrigel (BD Biosciences) according to
the manufacturer's instructions. A total of 3×104
cells/well were seeded into the upper chamber with 100 µl
serum-free media-based drugs (0.04 µΜ GA, 16 µg/ml PTX and 0.5 mM
NAC), whereas 600 µl 10% FBS-containing media-based drug was added
into the lower chamber. After 24 h of treatment at 37°C, the
chambers were removed and stained with Wright-Giemsa staining
solution as aforementioned for colony formation. Images were
captured by an optical microscope (Motic Incorporation, Ltd.).
Western blot analysis
Briefly, cells were collected after different
treatments and maintained on ice. The cells were resuspended in
RIPA lysis buffer, according to the manufacturer's instructions,
for protein extraction, and protein concentrations were quantified
using the bicinchoninic acid method. Western blot analysis was
conducted as previously described (24). A total of 20 µg proteins were
loaded per lane into a 4–15% Precast Tris-Glycine Gel (TransGen
Biotech Co., Ltd.); the proteins were then separated
electrophoretically and transferred to PVDF membranes. The PVDF
membranes were blocked with 5% skim milk at room temperature for 30
min, followed by incubation with the primary antibodies overnight
at 4°C. The membranes were then incubated with HRP-tagged goat
anti-rabbit and anti-mouse antibodies secondary antibodies
(1:10,000; cat. nos. SA00001-2 and SA00001-1; Proteintech Group,
Inc.) for 1 h at room temperature. Finally, the bands were
visualized using the ECL method (BeyoECL Moon kit; cat. no.
P0018FS; Beyotime Institute of Biotechnology) on a ChemiScope 6000
Exp chemiluminescence imaging system (Clinx Science Instruments
Co., Ltd.) and were semi-quantified with ImageJ software (Version
2.1.0/1.53c; National Institutes of Health). The following
dilutions of primary antibodies were used: Anti-EGFR (1:3,000),
anti-PI3K (1:500), anti-Akt (1:5,000), anti-p-Akt (1:5,000),
anti-β-actin (1:3,000), anti-CDK4 (1:4,000), anti-cyclin B1
(1:4,000), anti-GAPDH (1:10,000), anti-MMP-2 (1:500), anti-Nrf-2
(1:3,000), anti-Keap-1 (1:5,000) and anti-NQO1 (1:4,000).
DHE staining
Cells were plated into 6-well cell culture plates at
a density of 4×105 cells/well were treated according to
the aforementioned methods. After being washed with PBS twice, the
cells were incubated with 2 ml medium containing 10% FBS and 5 µM
DHE for 30 min at 37°C in the dark and were then visualized under a
fluorescence microscope (Motic Incorporation, Ltd.). Red staining
indicating oxidative stress was semi-quantified (ROS-positive
staining) using ImageJ software (Version 2.1.0/1.53c).
DCHF-DA staining
Cells were plated on 6-well cell culture plates at a
density of 4×105 cells/well and were then treated with
GA, NAC, PTX, NAC + PTX, and GA + PTX. All of the cells were
harvested in medium supplemented with 10% FBS, centrifuged at 300 ×
g for 5 min at room temperature, and resuspended in PBS containing
10 µM DCHF-DA. Cells were maintained at 37°C in the dark for 30
min. Subsequently, the cells were pelleted by centrifugation at 300
× g for 5 min at room temperature and resuspended in preheated PBS.
The cells were finally analysed using a FACScan flow cytometer and
the results were analysed by BD Diva 3 software.
Immunohistochemical (IHC) and H&E
staining
IHC staining and H&E staining were conducted as
previously described (25).
Briefly, tumour, heart, liver, spleen, lung and kidney tissues were
fixed in 10% formalin at room temperature overnight, embedded in
paraffin and then cut into 4-µm consecutive sections. For H&E
staining, following deparaffinization and rehydration, the sections
were stained with H&E, according to the manufacturer's
protocol. Images were captured under an optical microscope (Motic
Incorporation, Ltd.). For IHC staining, following deparaffinization
and rehydration, the slides were heated in a microwave oven at 97°C
for 20 min with EDTA retrieval buffer (Beijing Solarbio Science
& Technology Co., Ltd.) for epitope retrieval. Nonspecific
blinding was conducted with PBS containing 2% BSA (cat. no. AR1006;
Boster Biological Technology) for 2 h at room temperature.
Subsequently, the sections were incubated with anti-Ki-67
(1:6,000), anti-p-Akt (1:200), anti-MMP-2 (1:200) and anti-Nrf2
(1:200) primary antibodies at 4°C overnight. Slides were then
incubated with HRP-conjugated goat anti-rabbit IgG (1:200) and goat
anti-Mouse IgG (1:200) secondary antibodies, for 30 min at room
temperature, and were then visualized with a DAB Detection Kit
(Thermo Fisher Scientific, Inc.). Images were captured under an
optical microscope (Motic Incorporation, Ltd.) and were
semi-quantified with ImageJ software (Version 2.1.0/1.53c).
Routine blood examination
Whole blood samples (200 µl) were collected from
retro-orbital venous sinus of each mouse after inhalation of ether
at the final day of the experiment. The white blood cell (WBC)
count, red blood cell (RBC) count, haemoglobin (HGB) count and
blood platelet (PLT) count were analysed using a haematology
analyser (Shinova).
Statistical analysis
All data are presented as the mean ± SD. One-way
ANOVA followed by Bonferroni's post hoc test was used for
comparisons between multiple groups. All experiments were repeated
three times. ANOVA was performed using SPSS (version 26; IBM Corp.)
software and graphs were constructed using GraphPad Prism 5
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
GA enhances the
proliferation-inhibiting effect of PTX on OC cells
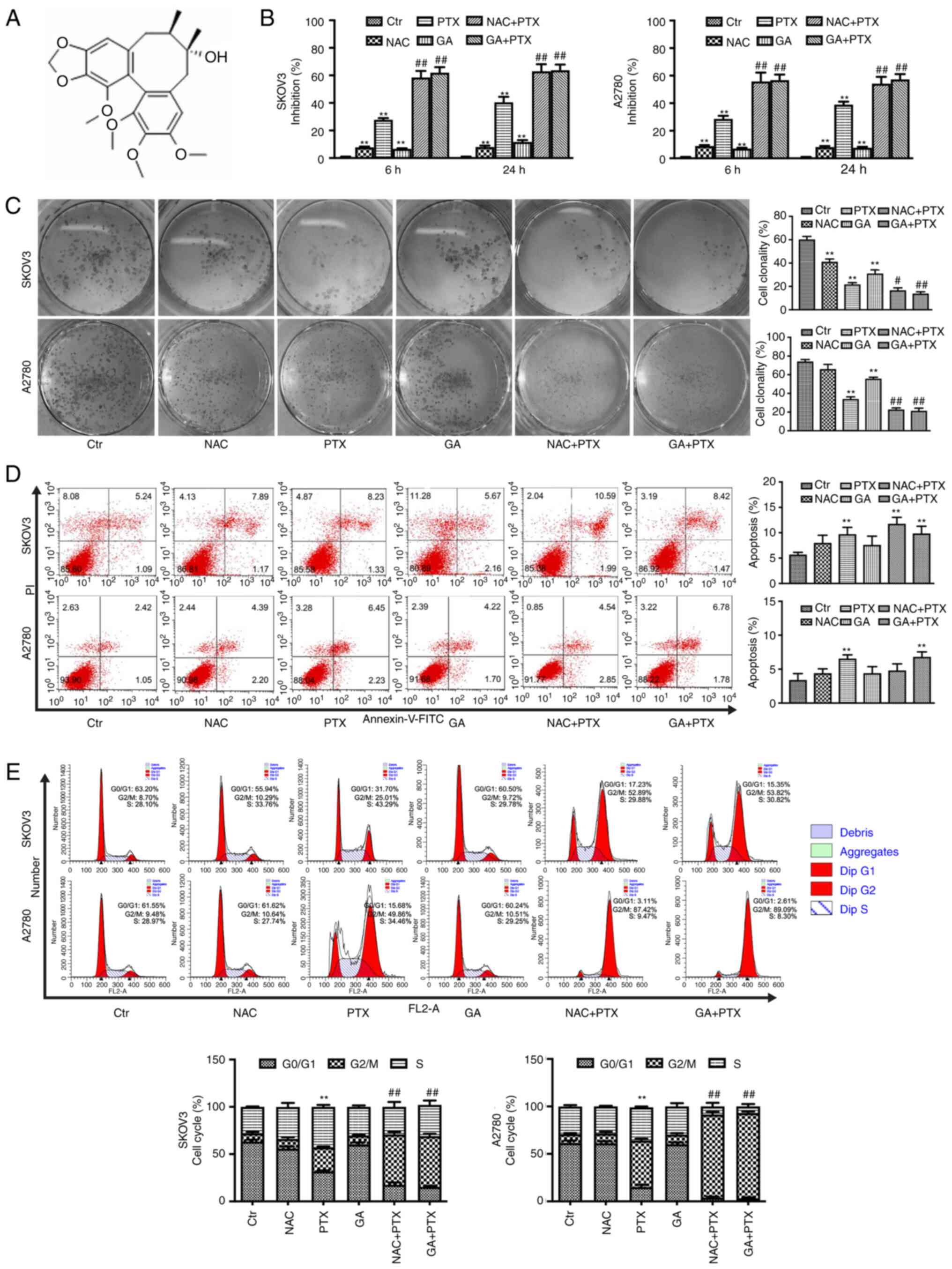
The structure of GA is shown in Fig. 1A. The inhibitory effects of GA and
PTX on two OC cell lines, SKOV3 and A2780, were detected by MTT
assay. The results showed that the inhibition rates of SKOV3 and
A2780 cells treated with GA + PTX were significantly increased
compared with the inhibition rates of the PTX group at 6 and 24 h
(Fig. 1B). These data indicated
that GA could reinforce the sensitivity of SKOV3 and A2780 cells to
PTX. The antioxidant effect of GA has been widely confirmed
(26). To identify whether a
GA-induced reduction in ROS levels was associated with the
inhibition of cell proliferation, GA was replaced with NAC. The MTT
results showed that the inhibition rates of SKOV3 and A2780 cells
treated with NAC + PTX were almost identical to the inhibition
rates of the GA + PTX group. To verify the effect of GA and PTX on
the proliferation of OC cells, a colony formation assay was
performed (Fig. 1C). Treatment
with GA or NAC combined with PTX resulted in a significantly
reduced colony formation rate in both OC cell lines compared with
that in the PTX group.
To gain insight into the mechanism by which GA
enhances the sensitivity of SKOV3 and A2780 cells to PTX, flow
cytometry was used to detect the rate of apoptosis (Fig. 1D). The results revealed that
apoptotic rate was very similar between the PTX group and both
combined groups in both cell lines. The apoptotic rate of the GA +
PTX group was significantly increased compared with that in control
group in both SKOV3 and A2780 cells, whereas the apoptotic rate of
the NAC + PTX group was only increased compared with the control
group in SKOV3 cells. These results indicated that the apoptotic
process may not be involved in the mechanism by which GA mediates
the enhancement of sensitivity to PTX in OC cells.
In addition, to investigate whether the mechanism
was related to the cell cycle, PI staining was employed to observe
cell cycle progression. According to the results of flow cytometric
analysis (Fig. 1E), the treatment
of both cell lines with PTX significantly increased the proportion
of cells in the G2/M phase of the cell cycle and
decreased the proportion of cells in the
G0/G1 phase of the cell cycle. In addition,
the two combination treatments, GA + PTX and NAC + PTX, enhanced
the accumulation of cells in the G2/M phase compared
with PTX treatment alone.
To explore the mechanism underlying the effects of
NAC, GA and PTX on G2/M phase arrest, western blot
analysis was performed to measure the expression levels of the cell
cycle-regulating proteins cyclin B1 and CDK4. CDK4 has been
reported to play an important role in regulating cell
G1, as CDK4 mediates the progression of cells from
G1 to S phase (27),
and cyclin B1 regulates the G2/M phase (28). In the present study, the expression
levels of CDK4 in the control, NAC, PTX, NAC + PTX, and GA + PTX
groups in both cells and GA group in A2780 cell were consistent
with the proportion of cells in G1 phase, in that the
lower the proportion of cells in G1 phase, the lower the
expression of CDK4 detected. However, in the GA group of SKOV3
cells, the proportion of cells in G1 phase showed no
difference with that of the control group, whereas the expression
of CDK4 was significantly decreased compared with that in the
control group. It was hypothesized that these results were caused
by the higher sensitivity of SKOV3 cells to GA than A2780 cells,
and that the change in molecular level (CDK4 protein) needed more
time to cause a change in the reduction of the proportion of cells
in G1 phase. The expression of cyclin B1 was
significantly upregulated following treatment with PTX alone in
A2780 cells, but not in SKOV3 cells, and it was hypothesized that
these results were associated with the low-dose ROS stimulation
produced by PTX. Compared with in the PTX group, the expression
levels of cyclin B1 and CDK4 were significantly attenuated in the
groups treated with NAC + PTX and GA + PTX (Fig. 2A and C).
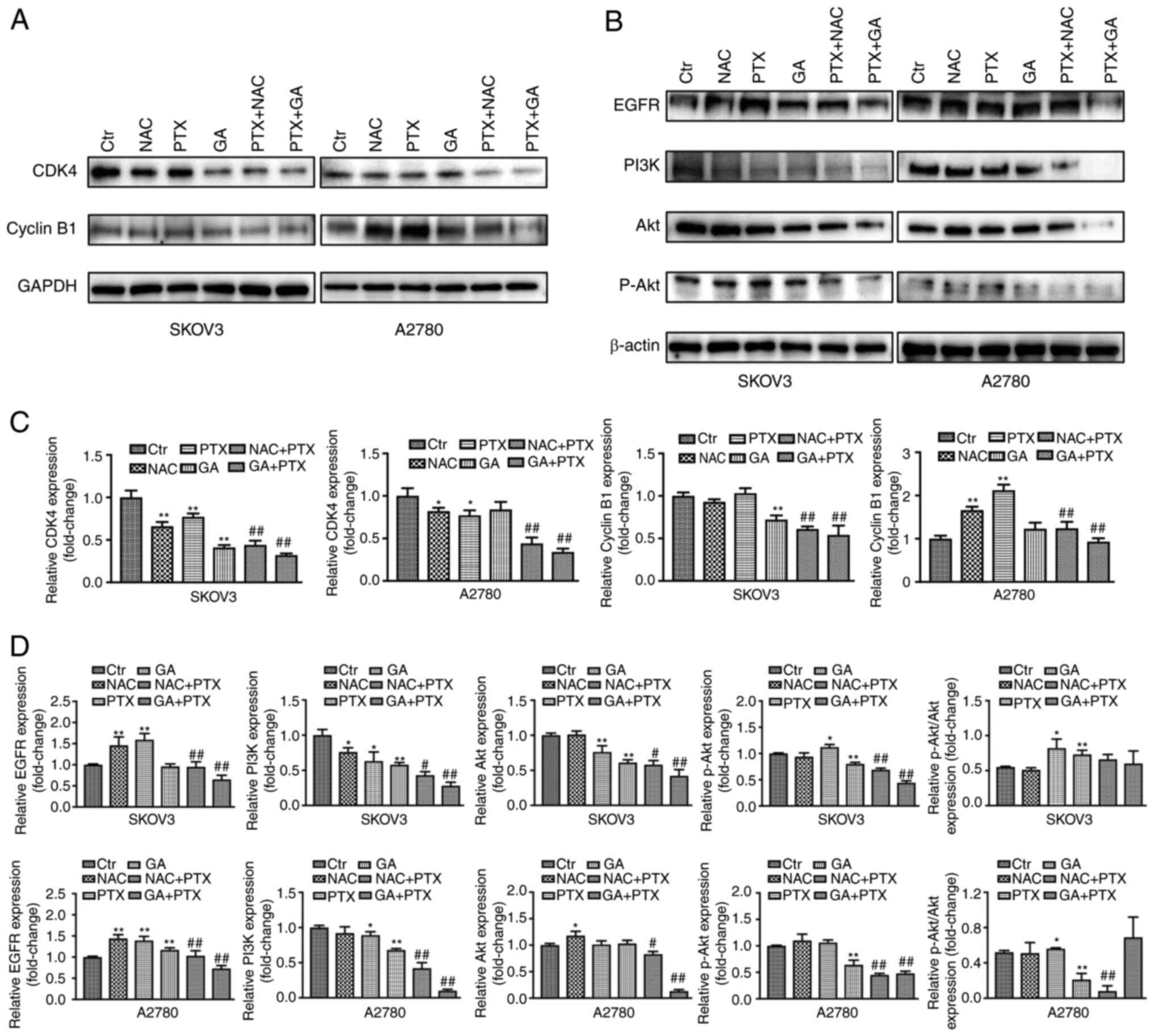 | Figure 2.Effects of GA and PTX treatment on
protein expression in ovarian cancer cells. Expression levels of
(A) CDK4 and cyclin B1, and (B) EGFR, PI3K, Akt and p-Akt were
detected by western blotting. (C and D) Semi-quantification of the
western blot analysis. Data are expressed as the mean ± SD from
three independent experiments. *P<0.05,
**P<0.01 vs. Ctrl; #P<0.05,
##P<0.01 vs. PTX. Ctr, control; EGFR, epidermal
growth factor receptor; GA, Gomisin A; NAC, N-acetyl cysteine; p,
phosphorylated; PTX, paclitaxel. |
In addition, the PI3K/Akt signalling pathway was
analysed by western blotting. The situation of cyclin B1 also
occurred in the PI3K/AKT pathway. Although PTX downregulated the
expression levels of PI3K, the low-dose ROS stimulation produced by
PTX upregulated the expression levels of p-AKT, which is an
important downstream protein of the PI3K pathway. Similarly, the
expression levels of PI3K, AKT and p-AKT were downregulated in the
NAC + PTX and GA + PTX groups. Subsequently, the ratio of p-Akt/Akt
was assessed in each group. The ratio of p-Akt/Akt in the PTX group
was significant increased compared with that in the control group.
Although there was no difference in the p-Akt/Akt ratio between the
PTX group and the GA + PTX group, total Akt was markedly lower in
the GA + PTX group, which resulted in a higher ratio of p-Akt/Akt
in the GA + PTX group (Fig. 2B and
D).
These data suggested that GA and PTX could block the
G2/M phase and induce cell cycle arrest in OC cells,
indicating that the inhibitory effect of PTX enhanced by GA was
mediated by blocking the cell cycle, but not apoptosis.
GA enhances the inhibitory effects of
PTX on OC cell invasion and migration
To clarify whether GA or NAC could increase the
inhibitory effects of PTX on SKOV3 and A22780 cell invasion and
migration, wound scratch and Transwell invasion assays were used to
detect the migration and invasion of both cell lines, respectively.
The migratory ability of both SKOV3 and A2780 cells was decreased
in the GA, NAC and PTX groups compared with that in the control
group. Following treatment with GA + PTX and NAC + PTX, the
migratory ability of SKOV3 and A2780 cells was further decreased
compared with that in cells treated with PTX or GA alone, and the
difference was statistically significant (Fig. 3A and C). Chambers coated with
Matrigel were used to mimic the extracellular matrix and simulate
the invasion process of tumour cells in vivo. The invasion
rate, relative to the control group, of SKOV3 and A2780 cells in
the GA + PTX treatment groups was significantly lower than the
relative invasion rate of both SKOV3 and A2780 cells in the PTX
group (Fig. 3B and D).
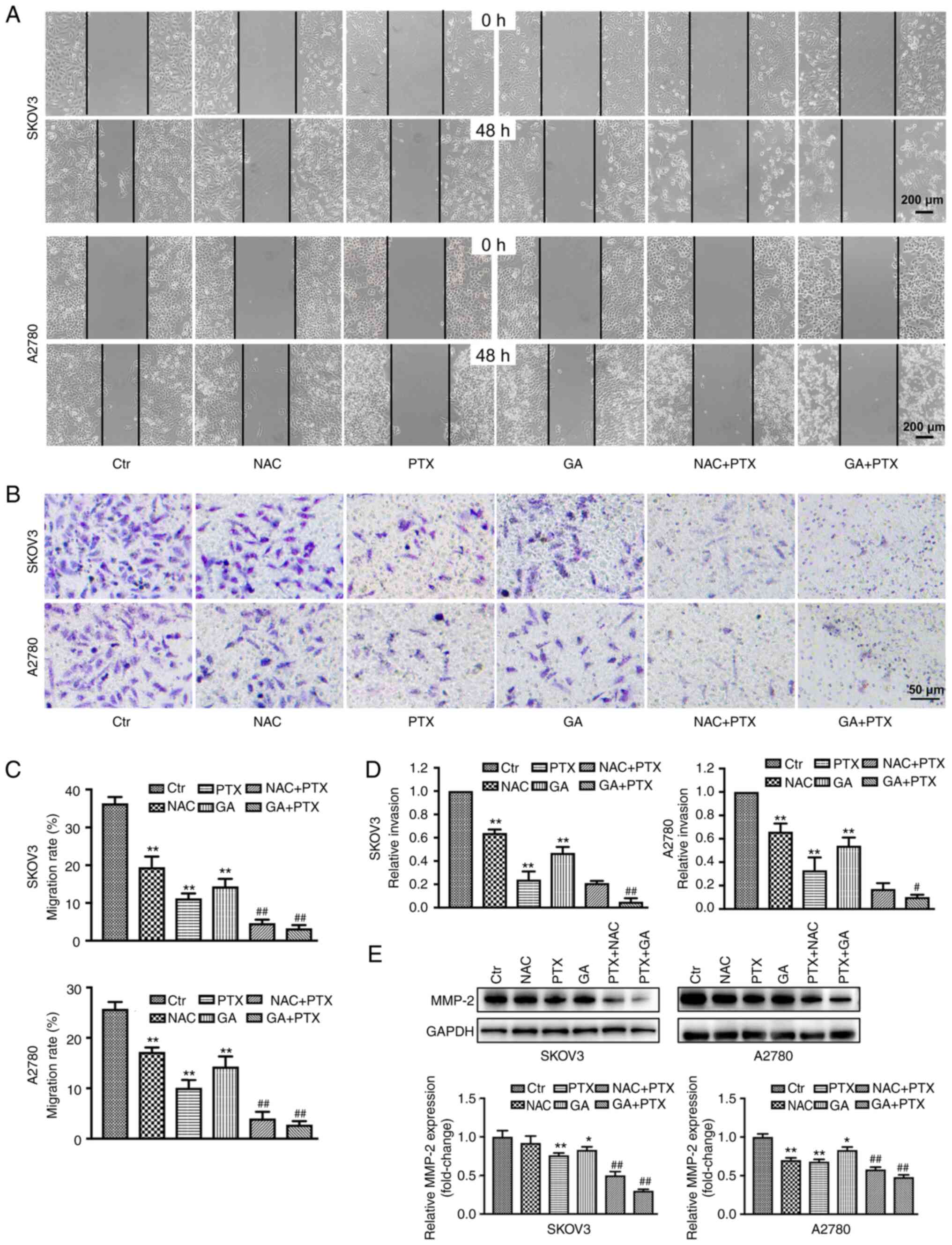 | Figure 3.GA enhances the inhibitory effect of
PTX on ovarian cancer cell migration and invasion. (A and C)
Migratory ability of SKOV3 and A2780 cells following different
treatments, as determined by the wound scratch assay. (B and D)
Invasive ability of SKOV3 and A2780 cells following different
treatments, as determined by Transwell assay. (E) Expression levels
of MMP-2, as detected by western blot analysis. Data are expressed
as the mean ± SD from three independent experiments.
*P<0.05, **P<0.01 vs. Ctrl;
#P<0.05, ##P<0.01 vs. PTX. Ctr,
control; GA, Gomisin A; NAC, N-acetyl cysteine; PTX, paclitaxel;
MMP-2, matrix metallopeptidase 2. |
Western blotting results showed that following
treatment with NAC + PTX or GA + PTX, the expression levels of
MMP-2 were downregulated compared with those in the PTX group
(Fig. 3E).
GA enhances the inhibitory effects of
PTX on OC cells by downregulating the levels of ROS
As shown in the aforementioned results, the NAC +
PTX group exhibited a similar inhibitory effect to the GA + PTX
group. To explore whether this was mediated by a GA-induced
reduction in ROS levels, ROS were detected by DHE staining. The PTX
group exhibited a marked increase in ROS production; ROS levels
were presented as fluorescence intensity relative to the control
group. However, the relative fluorescence intensity was decreased
in the GA + PTX and NAC + PTX groups compared with that in the PTX
group (Fig. 4A). In addition, flow
cytometry was employed to detect the ROS content in each group. The
results were in accordance with the DHE staining results (Fig. 4B and C). Furthermore, the
Keap-1/Nrf-2 signalling pathway was detected by western blotting
(Fig. 4D). Compared with in the
control group, the expression levels of Keap-1 were downregulated
in the PTX group, whereas they were upregulated in the GA + PTX and
NAC + PTX groups. The expression levels of Nrf-2 and NQO1 were
upregulated in the PTX group but were markedly downregulated in the
GA + PTX and NAC + PTX groups. These results indicated that GA
strengthened the sensitivity of SKOV3 and A2780 cells to PTX, which
was mediated by ROS inhibition regulated by GA.
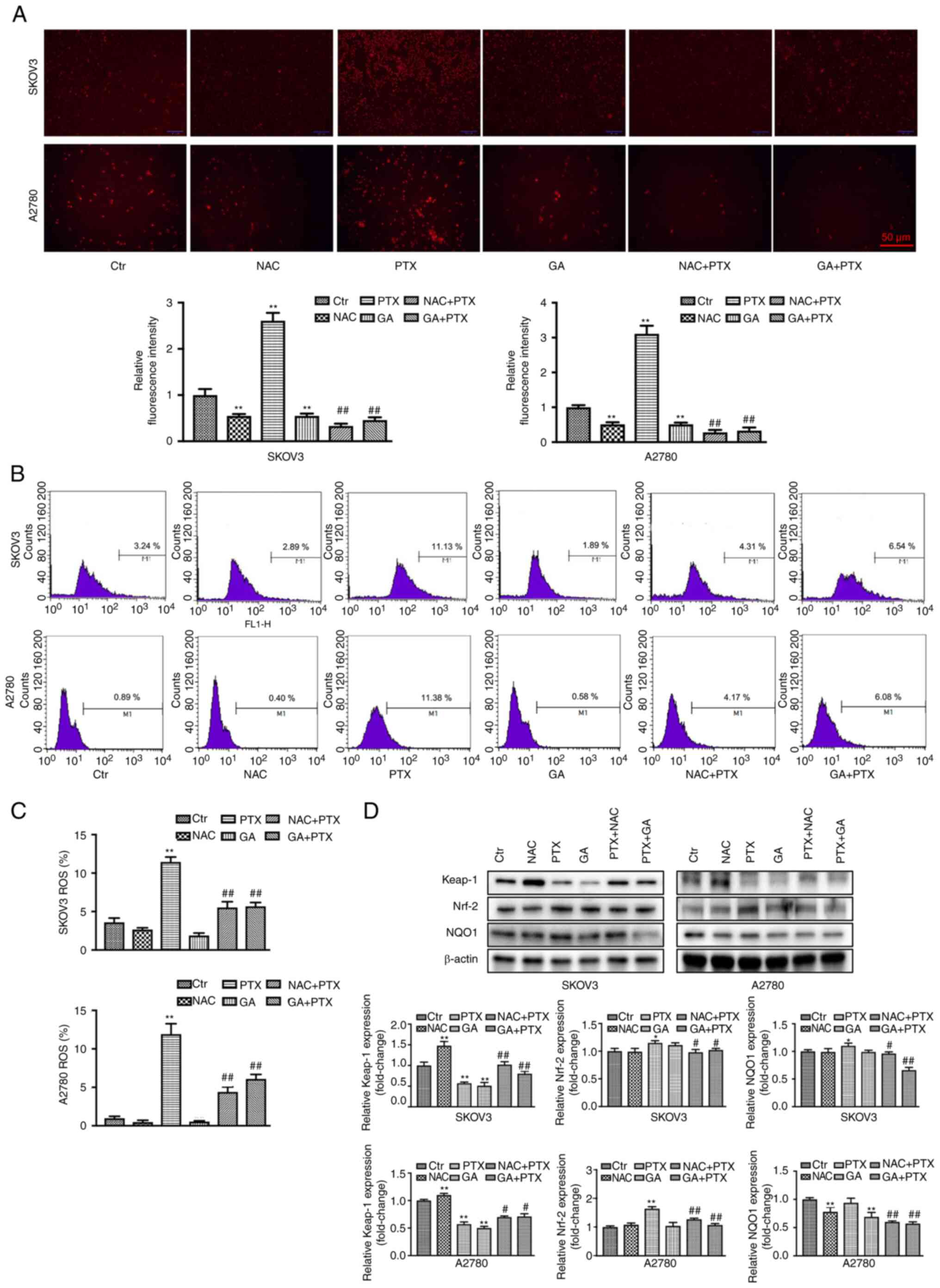 | Figure 4.GA enhances the inhibitory effect of
PTX by downregulating the levels of ROS. (A) DHE staining and
statistical analysis of ROS in SKOV3 and A2780 cells following
different treatments. (B and C) Flow cytometric and statistical
analysis of ROS levels in SKOV3 and A2780 cells following different
treatments, as determined by DCHF-DA staining. (D) Expression
levels of Keap-1, Nrf-2 and NQO1, as detected by western blot
analysis. Data are expressed as the mean ± SD from three
independent experiments. *P<0.05,
**P<0.01 vs. Ctrl; #P<0.05,
##P<0.01 vs. PTX. Ctr, control; DHE, dihydroethidium;
GA, Gomisin A; NAC, N-acetyl cysteine; PTX, paclitaxel; ROS,
reactive oxygen species. |
GA enhances the inhibitory effects of
PTX on mouse ovarian subcutaneous xenograft tumours
When tumour volume reached 100–150 mm3,
the mice were distributed into each group and the in vivo
antitumor effect study was initiated. Notably, there was no
significant difference in the mean graft volume among the four
groups at the start of the study. A direct visual representation of
the tumours is shown in Fig. 5A.
The suppressive effect of GA on tumour volume and weight was only
slightly smaller than that in the control group and no
statistically significant difference was observed in terms of
antitumor effect, which may be due to the low dose of GA used. By
contrast, the PTX group and the combined group exhibited an obvious
reduction in tumour growth compared with that in the control group.
Moreover, the combination group further enhanced the tumour
inhibitory effects of the PTX group.
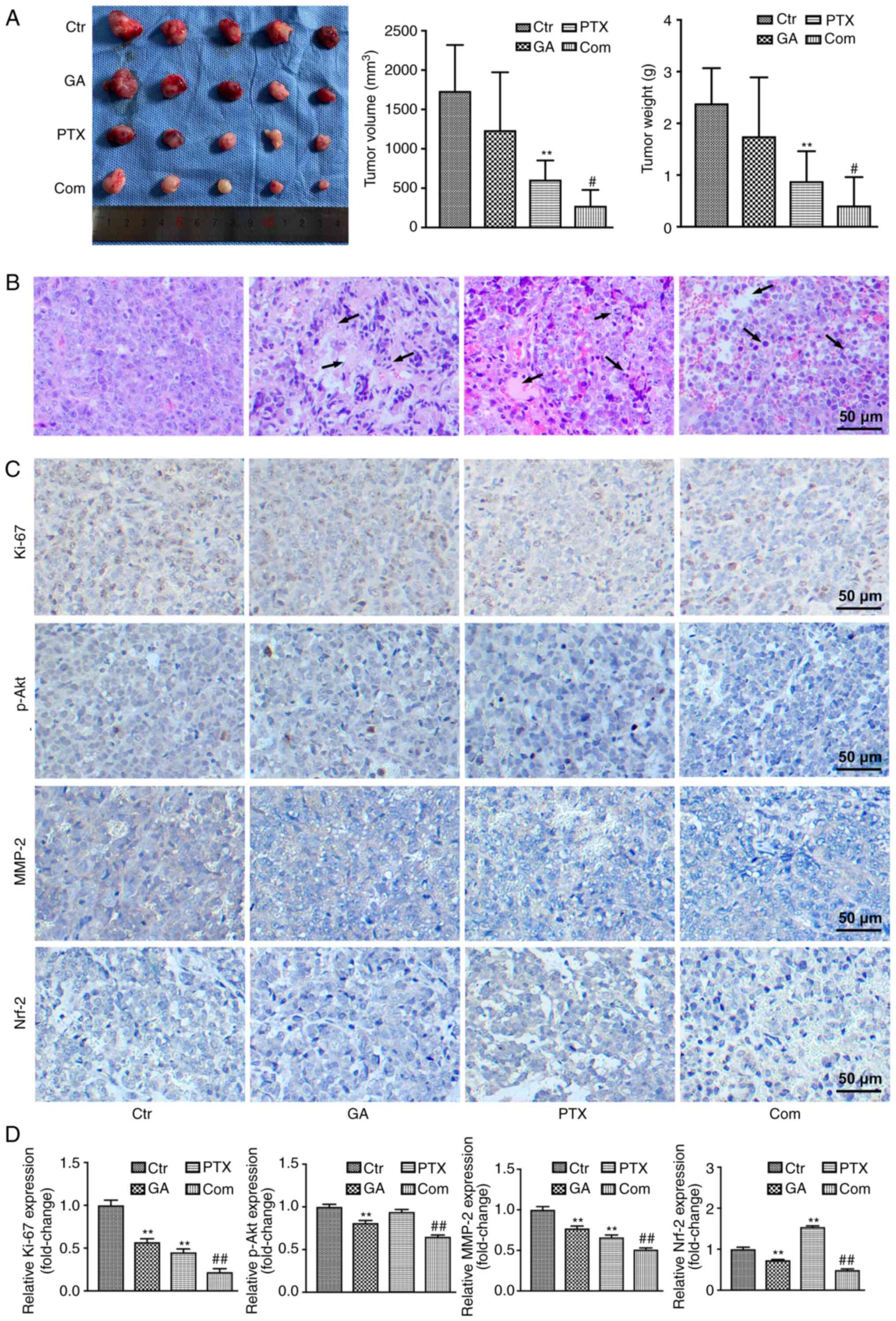 | Figure 5.GA enhances the inhibitory effect of
PTX on transplanted tumours. (A) Volume (left and middle panels)
and weight (right panel of transplanted tumours. (B) H&E
staining of transplanted tumours. H&E staining showed more
tumour necrosis and the tumour tissue was impaired with nuclear
pyknosis and fragmentation, with some nuclei disappearing (arrows),
in the GA, PTX and combined groups compared with in the control
group. (C) Immunohistochemical staining of Ki-67, p-Akt, MMP-2 and
Nrf-2. (D) Semi-quantification of immunohistochemical staining
analysis. **P<0.01 vs. Ctrl; #P<0.05,
##P<0.01 vs. PTX, Ctr, control; Com, combined; GA,
Gomisin A; p, phosphorylated; PTX, paclitaxel; MMP-2, matrix
metallopeptidase 2; H&E, haematoxylin and eosin. |
To further validate the antitumor activity of GA and
PTX, pathological analysis of tumour tissues was performed by
H&E staining (Fig. 5B). Tumour
necrosis was not obvious and most tissue structures were intact in
the control group. In the GA, PTX and combined groups, the tumour
tissue was impaired to varying degrees, and the pathological
differences were noticeable, with nuclei in the necrotic area
showing pyknosis and fragmentation, and with some nuclei
disappearing.
Immunohistochemical staining was further used to
detect the expression of the cell proliferation markers Ki-67 and
p-Akt, the cell migration marker MMP-2 and the ROS marker Nrf2
(Fig. 5C and D). The highest
Ki-67, MMP-2 and p-Akt expression levels were both detected in the
control group of A2780 tumour-bearing mice, whereas the lowest were
detected in the combined group. Nrf2 expression was markedly
upregulated in the PTX group compared with that in the control
group, whereas it was obviously downregulated in the GA and GA +
PTX groups; the expression of Nrf2 was much lower in the GA + PTX
than that in the GA group. The immunohistochemistry results for
Ki-67, p-Akt, MMP-2 and Nrf2 expression were consistent with those
of western blot analysis of in vitro results.
Additionally, biosafety assessments were performed
by H&E staining to detect histological changes in the heart,
liver, spleen, lung and kidney (Fig.
6A), and routine blood examinations of WBC, RBC, HGB and PLT
(Fig. 6B). The results of H&E
staining showed that no obvious pathological damage was detected,
and no significant differences were identified in the routine blood
examinations of each group.
 | Figure 6.Biosafety assessments of GA and PTX
treatments. (A) Haematoxylin and eosin staining of the histological
changes in the heart, liver, spleen, lung and kidney. (B) Routine
blood examinations of WBC, RBC, HGB and PLT counts. Ctr, control;
GA, Gomisin A; Com, combined; HGB, haemoglobin; PLT, platelet; PTX,
paclitaxel; RBC, red blood cell; WBC, white blood cell. |
Discussion
OC is a malignant tumour of the female reproductive
system, which is associated with a high mortality rate (29). Platinum-based chemotherapy combined
with PTX is the most important adjuvant therapy for OC. Although
PTX has strong antiangiogenic activity, the drug resistance of PTX,
which may be related to the production of ROS induced by it, limits
its clinical application to a certain extent (19). Notably, the antioxidant activity of
GA has been widely proven (13)
and the present study confirmed this. In recent years, GA has been
widely used in several research domains due to its minimal side
effects (30,31); however, the exact effect of GA on
human OC is unclear. In the present study, OC cells treated with GA
or PTX were observed, and the results provided a theoretical and
experimental basis for the practical application of PTX-based
combined chemotherapy in the clinic.
The sensitivity of OC cells to PTX is based on the
growth inhibition rate. Previous studies have shown that EGFR
overexpression in a number of solid tumours is associated with
tumour cell proliferation, angiogenesis, tumour invasion,
metastasis and inhibition of apoptosis (32,33).
The present study demonstrated that the expression of EGFR was
significantly decreased after GA combined with PTX treatment,
indicating that GA + PTX could significantly enhance the inhibition
of proliferation of the OC cell lines A2780 and SKOV3 compared with
PTX alone. As GA has an antioxidant effect, it was hypothesized
that GA could decrease the level of ROS produced by PTX. In
addition, a specific inhibitor of ROS, NAC, was used in the present
study. As an antioxidant, a high dose of NAC (5 mM) has previously
been shown to completely suppress ROS in cells, which could induce
cell protection (34). In the
present study, low-dose (0.5 mM) NAC only neutralized the ROS that
initiated cell proliferation, but did not entirely suppress ROS
levels. Additionally, the inhibitory effect of NAC was identified
in previous studies (35,36); therefore, NAC was used in the
present study to definitively identify whether the enhancement of
the sensitivity of OC cells to PTX was related to GA-induced ROS
reduction.
An increasing number of studies have reported that
ROS can have dual roles, with different doses having different
effects. High ROS concentrations inhibit cell survival, whereas low
ROS concentrations promote cell survival (37). ROS may benefit therapeutic
resistance in cancer (38). In
most tumours, the high level of ROS production caused by
chemotherapy or radiotherapy has been considered an antitumour
factor (39); however, a low level
of ROS is a key driver of tumour initiation. Immortal proliferation
of cancer cells can be initiated by a low ROS environment that can
regulate the cell cycle, autophagy and metastasis. Our previous
study also reported that low levels of ROS could attenuate the
antitumor effect of Raddeanin on SKOV3 cells (40). The detection of ROS implied that
the levels of ROS in the GA + PTX group were significantly
decreased compared with those in the PTX group, and the expression
levels of Nrf-2 and NQO1 were downregulated, indicating that SKOV3
and A2780 cells were subjected to a lower degree of oxidative
stress. These results suggested that low-dose ROS induced by PTX is
one of the mechanisms limiting the tumour suppressive effect of
PTX. Combining PTX with GA or NAC intensified the antiproliferative
effect of PTX, implying that the sensitivity of OC cells to PTX was
heightened when the cells lost the irritation of low-level ROS,
also suggesting that GA enhanced the sensitivity of A2780 and SKOV3
cells to PTX by inhibiting intracellular ROS generation.
To further explore the mechanism underlying the
antitumour effect of GA combined with PTX on OC cells, apoptosis
and proliferation were detected. Cell death via apoptosis is
required for homeostatic turnover of cells under physiological
conditions; however, it is usually inhibited in tumours. Thus,
apoptosis has been regarded as an essential mechanism of antitumor
effects in most treatment methods (41). In addition, the apoptosis-related
signalling pathway, the PI3K/Akt pathway, was evaluated (42). The results of the present study are
consistent with those of previous studies (43,44),
showing that PTX can exert its antitumour effect by promoting cell
apoptosis; however, there was no difference between PTX and the
combined treatment in terms of cell apoptosis. PTX is a drug that
inhibits mitosis (45). High doses
of PTX have been reported to promote apoptosis (43,44).
In the present study, low doses of PTX were used. Although the
percentage of apoptotic cells detected in response to PTX was
<10%, there was still a statistically significant difference
between the PTX and control groups. In addition, low-dose ROS
stimulation produced by PTX upregulated the expression of p-AKT,
which was significantly downregulated following treatment with GA.
This indicated that the PI3K/Akt pathway may be involved in the
mechanism by which GA mediates the enhancement of sensitivity to
PTX in OC cells. Subsequent studies should consider using a
PI3K/Akt inhibitor as a positive control to further assess
this.
In addition, the relationship between the PI3K/Akt
pathway and the oxidative stress-related Keap1/Nrf-2 pathway has
been extensively reported. Resveratrol has been shown to protect
porcine intestinal epithelial cells from oxidative stress through
the PI3K/Akt-mediated Nrf2 signalling pathway (46). The protective effect of
Chrysanthemum morifolium on cell oxidative damage has also
been reported to be related to the PI3K/Akt-mediated Nrf2/HO-1
signalling pathway (47). As an
upstream signalling pathway, the PI3K/Akt pathway serves a key role
in regulating Nrf-2/HO-1 protein expression. PI3K/Akt can
dissociate Nrf-2 from Keap1 and promote subsequent signal
transduction, thus inducing the activation of antioxidant
enzymes.
It has previously been reported that ROS regulate
tumours via multiple mechanisms, including apoptosis and the cell
cycle (48). The cell cycle
consists of mainly two phases, G1/S and G2/M,
is essential for cell survival and is modulated by a stream of
molecules. The two cell cycle phases are tightly controlled by
cyclins, CDKs and CDK inhibitors. The present study revealed that
PTX could inhibit A2780 and SKOV3 cells by inducing G2/M
phase arrest. Notably, the production of low levels of ROS could
promote the cell cycle process (20), which was in accordance with the
present finding that the ROS inhibitor GA or NAC could prolong the
G2/M phase arrest induced by PTX. CDKs are a group of
serine/threonine protein kinases, which drive the cell cycle
through the chemical action of serine/threonine proteins and act
synergistically with cyclin, which is an important factor in cell
cycle regulation. Among the CDK family members, CDK4 has an
important role in regulating G1 cells (27). Moreover, the decrease in cyclin B1
has been related to inactivation of the G2/M checkpoint
and G2/M phase arrest (28). As cyclin B1/CDK1 can modulate
mitochondrial activities in cell cycle progression and
proliferation, subsequent studies will fully consider evaluating
the expression of CDK1 to illustrate whether GA enhances the
therapeutic effect of PTX through its effects on mitochondrial
function.
All of these findings suggested that low levels of
ROS may promote OC cell proliferation via a cell cycle mechanism
and that the sensitivity of OC cells to PTX could be enhanced by
the GA-induced reduction in ROS, which may further block the cell
cycle compared with PTX alone. This model provides a strong
strategy to target ROS with GA in OC therapy.
In conclusion, the present study showed that GA
enhanced the inhibition rate of PTX by reducing the ROS levels in
human OC cells both in vivo and in vitro.
Furthermore, it was revealed that the mechanism was not related to
the apoptosis pathway. However, G2/M phase arrest was
associated with the mechanism. The present findings suggested that
the combination of GA and PTX may be a promising treatment for
OC.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Jilin Province of
Department Finance (grant nos. 2019SCZT040 and 2019SCZT050) and the
Jilin Province Science and Technology Department (grant no.
20200201589JC).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SHZ, JL and TWW conceived and designed the
experiments. TWW, CQZ, MYS, JYC and ZYY performed the experiments.
XMH, MMC and SW analysed the data. XMH, SW, MMC and ZYY wrote the
manuscript, critically revised the study for important intellectual
content, and performed language editing. JL and SHZ supervised the
study and were involved in project management. SHZ and TWW confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript, and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The animal experiments were approved by the
Institutional Animal Care and Use Committee of Jilin University
Second Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GA
|
Gomisin A
|
|
FS
|
Fructus Schizandra
|
|
ROS
|
reactive oxygen species
|
|
PTX
|
paclitaxel
|
|
NAC
|
N-acetyl cysteine
|
|
OC
|
ovarian cancer
|
|
DMSO
|
dimethyl sulfoxide
|
|
DHE
|
dihydroethidium
|
|
MMP-2
|
matrix metallopeptidase 2
|
|
CDKs
|
cyclin-dependent kinases
|
References
|
1
|
Merritt MA, Rice MS, Barnard ME, Hankinson
SE, Matulonis UA, Poole EM and Tworoger SS: Pre-diagnosis and
post-diagnosis use of common analgesics and ovarian cancer
prognosis (NHS/NHSII): A cohort study. Lancet Oncol. 19:1107–1116.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu J and Matulonis UA: New strategies in
ovarian cancer: Translating the molecular complexity of ovarian
cancer into treatment advances. Clin Cancer Res. 20:5150–5156.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
du Bois A, Kristensen G, Ray-Coquard I,
Reuss A, Pignata S, Colombo N, Denison U, Vergote I, Del Campo JM,
Ottevanger P, et al: Standard first-line chemotherapy with or
without nintedanib for advanced ovarian cancer (AGO-OVAR 12): A
randomised, double-blind, placebo-controlled phase 3 trial. Lancet
Oncol. 17:78–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Majidi A, Na R, Dixon-Suen S, Jordan SJ
and Webb PM: Common medications and survival in women with ovarian
cancer: A systematic review and meta-analysis. Gynecol Oncol.
157:678–685. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van der Hel OL, Timmermans M, van Altena
AM, Kruitwagen RFPM, Slangen BFM, Sonke GS, van de Vijver KK and
van der Aa MA: Overview of non-epithelial ovarian tumours:
Incidence and survival in the Netherlands, 1989–2015. Eur J Cancer.
118:97–104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zierhut C, Yamaguchi N, Paredes M, Luo JD,
Carroll T and Funabiki H: The cytoplasmic DNA sensor cGAS promotes
mitotic cell death. Cell. 178:302–315.e23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koshiyama M, Matsumura N, Imai S, Yamanoi
K, Abiko K, Yoshioka Y, Yamaguchi K, Hamanishi J, Baba T and
Konishi I: Combination of aprepitant, azasetron, and dexamethasone
as antiemetic prophylaxis in women with gynecologic cancers
receiving paclitaxel/carboplatin therapy. Med Sci Monit.
23:826–833. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren F, Shen J, Shi H, Hornicek FJ, Kan Q
and Duan Z: Novel mechanisms and approaches to overcome multidrug
resistance in the treatment of ovarian cancer. Biochim Biophys
Acta. 1866:266–275. 2016.PubMed/NCBI
|
|
9
|
Li Z, He X, Liu F, Wang J and Feng J: A
review of polysaccharides from Schisandra chinensis and
Schisandra sphenanthera: Properties, functions and
applications. Carbohydr Polym. 184:178–190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lv XJ, Zhao LJ, Hao YQ, Su ZZ, Li JY, Du
YW and Zhang J: Schisandrin B inhibits the proliferation of human
lung adenocarcinoma A549 cells by inducing cycle arrest and
apoptosis. Int J Clin Exp Med. 8:6926–6936. 2015.PubMed/NCBI
|
|
11
|
Xu Y, Liu Z, Sun J, Pan Q, Sun F, Yan Z
and Hu X: Schisandrin B prevents doxorubicin-induced chronic
cardiotoxicity and enhances its anticancer activity in vivo. PLoS
One. 6:e283352011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Shi S, Wang H, Li N, Su J, Chou G
and Wang S: A Homogeneous polysaccharide from fructus Schisandra
chinensis (Turz.) baill induces mitochondrial apoptosis through
the Hsp90/AKT signalling pathway in HepG2 cells. Int J Mol Sci.
17:10152016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chun JN, Cho M, So I and Jeon JH: The
protective effects of Schisandra chinensis fruit extract and
its lignans against cardiovascular disease: A review of the
molecular mechanisms. Fitoterapia. 97:224–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wan CK, Zhu GY, Shen XL, Chattopadhyay A,
Dey S and Fong WF: Gomisin A alters substrate interaction and
reverses P-glycoprotein-mediated multidrug resistance in HepG2-DR
cells. Biochem Pharmacol. 72:824–837. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Young Park J, Wook Yun J, Whan Choi Y, Ung
Bae J, Won Seo K, Jin Lee S, Youn Park S, Whan Hong K and Kim CD:
Antihypertensive effect of gomisin A from Schisandra
chinensis on angiotensin II-induced hypertension via
preservation of nitric oxide bioavailability. Hypertens Res.
35:928–934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qu HM, Liu SJ and Zhang CY: Antitumor and
antiangiogenic activity of Schisandra chinensis
polysaccharide in a renal cell carcinoma model. Int J Biol
Macromol. 66:52–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han YH, Mun JG, Jeon HD, Park J, Kee JY
and Hong SH: Gomisin A ameliorates metastatic melanoma by
inhibiting AMPK and ERK/JNK-mediated cell survival and metastatic
phenotypes. Phytomedicine. 68:1531472020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kee JY, Han YH, Mun JG, Park SH, Jeon HD
and Hong SH: Gomisin A suppresses colorectal lung metastasis by
inducing AMPK/p38-mediated apoptosis and decreasing metastatic
abilities of colorectal cancer cells. Front Pharmacol. 9:9862018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alexandre J, Hu Y, Lu W, Pelicano H and
Huang P: Novel action of paclitaxel against cancer cells: Bystander
effect mediated by reactive oxygen species. Cancer Res.
67:3512–3517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Sá Junior PL, Câmara DAD, Porcacchia
AS, Fonseca PMM, Jorge SD, Araldi RP and Ferreira AK: The roles of
ROS in cancer heterogeneity and therapy. Oxid Med Cell Longev.
2017:24679402017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harris IS and DeNicola GM: The complex
interplay between antioxidants and ROS in cancer. Trends Cell Biol.
30:440–451. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prasad S, Gupta SC and Tyagi AK: Reactive
oxygen species (ROS) and cancer: Role of antioxidative
nutraceuticals. Cancer Lett. 387:95–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Liu J, Wu S, Liu W, Xia Y, Zhao J,
Yang Y, Wang Y, Peng Y and Zhao S: Atrazine promoted epithelial
ovarian cancer cells proliferation and metastasis by inducing low
dose reactive oxygen species (ROS). Iran J Biotechnol.
19:e26232021.PubMed/NCBI
|
|
24
|
Chen J, Xia Y, Peng Y, Wu S, Liu W, Zhang
H, Wang T, Yang Z, Zhao S and Zhao L: Analysis of the association
between KIN17 expression and the clinical features/prognosis of
epithelial ovarian cancer, and the effects of KIN17 in SKOV3 cells.
Oncol Lett. 21:4752021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng Z, Wang Q, Yang X, Ren Y, Jiao S, Zhu
Q, Guo D, Xia K, Wang Y, Li C and Wang W: Qishen granule attenuates
cardiac fibrosis by regulating TGF-β/Smad3 and GSK-3β pathway.
Phytomedicine. 62:1529492019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takanche JS, Kim JE, Han SH and Yi HK:
Effect of gomisin A on osteoblast differentiation in high
glucose-mediated oxidative stress. Phytomedicine. 66:1531072020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goel S, DeCristo MJ, Watt AC, BrinJones H,
Sceneay J, Li BB, Khan N, Ubellacker JM, Xie S, Metzger-Filho O, et
al: CDK4/6 inhibition triggers anti-tumour immunity. Nature.
548:471–475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang B, Ni H, Zhou Z and Li Y: Parkin
enhances sensitivity of paclitaxel to NPC by arresting cell cycle.
Pathol Res Pract. 216:1527552020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahn JH, Lee HS, Lee JS, Lee YS, Park JL,
Kim SY, Hwang JA, Kunkeaw N, Jung SY, Kim TJ, et al: nc886 is
induced by TGF-β and suppresses the microRNA pathway in ovarian
cancer. Nat Commun. 9:11662018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han YH, Kee JY and Hong SH: Gomisin A
alleviates obesity by regulating the phenotypic switch between
white and brown adipocytes. Am J Chin Med. 49:1929–1948. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hao LJ, Lin RA, Chen LC, Wang JL, Chen IS,
Kuo CC, Chou CT, Chien JM and Jan CR: Action of the natural
compound gomisin a on Ca2+ movement in human prostate
cancer cells. Chin J Physiol. 65:151–157. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
da Cunha Santos G, Shepherd FA and Tsao
MS: EGFR mutations and lung cancer. Annu Rev Pathol. 6:49–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Talukdar S, Emdad L, Das SK and Fisher PB:
EGFR: An essential receptor tyrosine kinase-regulator of cancer
stem cells. Adv Cancer Res. 147:161–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ko YH, Jeong M, Jang DS and Choi JH:
Gomisin L1, a lignan isolated from Schisandra berries,
induces apoptosis by regulating NADPH oxidase in human ovarian
cancer cells. Life (Basel). 11:8582021.PubMed/NCBI
|
|
35
|
Kretzmann NA, Chiela E, Matte U, Marroni N
and Marroni CA: N-acetylcysteine improves antitumoural response of
Interferon alpha by NF-kB downregulation in liver cancer cells.
Comp Hepatol. 11:42012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu B, Huang X, Li Y, Liao W, Li M, Liu Y,
He R, Feng D, Zhu R and Kurihara H: JS-K, a nitric oxide donor,
induces autophagy as a complementary mechanism inhibiting ovarian
cancer. BMC Cancer. 19:6452019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mendes S, Sá R, Magalhães M, Marques F,
Sousa M and Silva E: The role of ROS as a double-edged sword in
(In)Fertility: The impact of cancer treatment. Cancers (Basel).
14:15852022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okon IS and Zou MH: Mitochondrial ROS and
cancer drug resistance: Implications for therapy. Pharmacol Res.
100:170–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma N, Liu P, He N, Gu N, Wu FG and Chen Z:
Action of gold nanospikes-based nanoradiosensitizers: Cellular
internalization, radiotherapy, and autophagy. ACS Appl Mater
Interfaces. 9:31526–31542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao F, Gao Y, Chu X, Chen J, Huang L,
Zhao J, Zhang J and Zhao S: ROS attenuates the antitumor effect of
Raddeanin on ovarian cancer cells Skov3. Int J Clin Exp Pathol.
10:8292–8302. 2017.PubMed/NCBI
|
|
41
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang S, Zhang E, Ruan H, Ma J, Zhao X,
Zhu Y, Xie X, Han N, Li J, Zhang H, et al: Actinomycin V induces
apoptosis associated with mitochondrial and PI3K/AKT pathways in
human CRC cells. Mar Drugs. 19:5992021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang TH, Wang HS and Soong YK:
Paclitaxel-induced cell death: Where the cell cycle and apoptosis
come together. Cancer. 88:2619–2628. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang CC, Li CG, Wang YF, Xu LH, He XH,
Zeng QZ, Zeng CY, Mai FY, Hu B and Ouyang DY: Chemotherapeutic
paclitaxel and cisplatin differentially induce pyroptosis in A549
lung cancer cells via caspase-3/GSDME activation. Apoptosis.
24:312–325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Manfredi JJ and Horwitz SB: Taxol: An
antimitotic agent with a new mechanism of action. Pharmacol Ther.
25:83–125. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhuang Y, Wu H, Wang X, He J, He S and Yin
Y: Resveratrol attenuates oxidative stress-induced intestinal
barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway.
Oxid Med Cell Longev. 2019:75918402019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hao Y, Li Y, Liu J, Wang Z, Gao B, Zhang Y
and Wang J: Protective effect of Chrysanthemum morifolium
cv. Fubaiju hot-water extracts against ARPE-19 cell oxidative
damage by activating PI3K/Akt-mediated Nrf2/HO-1 signaling pathway.
Front Nutr. 8:6489732021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ren Y, Geng R, Lu Q, Tan X, Rao R, Zhou H,
Yang X and Liu W: Involvement of TGF-β and ROS in G1 cell cycle
arrest induced by titanium dioxide nanoparticles under UVA
irradiation in a 3D spheroid model. Int J Nanomedicine.
15:1997–2010. 2020. View Article : Google Scholar : PubMed/NCBI
|