Introduction
Currently, immunotherapy is considered a standard
care for human cancer and contributes to improving the outcome
following diagnosis. Programmed death-1 (PD-1) blockade serves a
crucial role to improve a survival time in cancer treatment.
Although exploratory studies have been performed to discover an
optimal predictive marker of PD-1 blockade treatment, there are no
established markers associated with the efficacy of PD-1 blockade
aside from programmed death ligand-1 (PD-L1) expression in tumor
specimens (1,2). The expression of PD-L1 has been
identified as a predictive marker for certain types of human
neoplasm, such as non-small cell lung cancer (NSCLC). Since PD-L1
expression is not completely accurate biomarker for predicting the
efficacy of immune checkpoint inhibitors (ICIs), novel predictors
of PD-1 blockade should be identified to improve treatment outcome.
Aside from PD-L1 expression, there are numerous useful markers for
predicting the efficacy of ICIs, such as tumor mutation burden
(TMB), microsatellite instability-high/mismatch repair-deficient
(MSI-H/dMMR), major histocompatibility complex molecules and T cell
receptor (2). PD-1 blockade is
associated with improved response and prolonged survival in
patients with advanced NSCLC harboring high TMB and metastatic
colorectal cancer with MSI-H/Dmmr (2). However, it is difficult to predict
the response and outcome of PD-1 blockade using current biomarkers.
Therefore, the discovery of new biomarkers for ICIs treatment is
necessary to improve the outcome of patients with cancer who
receive PD-1 therapy.
Recently, a combination of antiangiogenic agents and
ICIs such as pembrolizumab plus ramucirumab has emerged as an
effective treatment for cancer (3). Proangiogenic factors, such as
vascular endothelial growth factor (VEGF), cause an
immunosuppressive tumor microenvironment, which increases the
proliferation of FOXP3 and myeloid-derived suppressor cells
(3,4). Based on preclinical data, the
addition of angiogenic inhibitors to ICIs has been successful in
treatment of several types of human cancer, including
hepatocellular and clear cell renal carcinoma and non-squamous
NSCLC (4–7). As a combination of ICIs,
multi-targeted or selective VEGF receptors (VEGFRs), tyrosine
kinase inhibitors and anti-VEGF monoclonal antibodies provide a
significant survival benefit compared with treatment with single
agents (5–8). The combination of bevacizumab and
atezolizumab with chemotherapy has been approved for advanced NSCLC
(7). Moreover, a phase I expansion
study of a combination of ramucirumab (anti-VEGFR2 monoclonal
antibody) and pembrolizumab was performed in patients with
previously untreated advanced NSCLC with PD-L1 expression ≥1%
(9). The objective response rate
(ORR; 42.3 vs. 27.2%) and median progression-free survival (PFS;
9.3 vs. 5.4 months) for combination of VEGFR2 inhibitor with
pembrolizumab are greater than those for pembrolizumab monotherapy
(9,10). A potential increase in activity of
tumor immune cells by inhibiting VEGFR2 has been suggested
(8,9); however, it is uncertain whether
VEGFR2 expression in tumor cells is a useful biomarker for
predicting the efficacy of PD-1 blockade. Furthermore, ramucirumab,
a VEGFR2 inhibitor, has been clinically identified as a standard
treatment for patients with previously treated NSCLC and inhibition
of VEGFR2 plays a crucial role in the suppression of tumor growth
(11). A recent study reported
that VEGFR2 expression is associated with worse prognosis in
patients with surgically resected NSCLC (12). As the combination of certain
therapeutic agents with PD-1 blockade is known to be effective for
patients with advanced NSCLC, immunotherapy in addition to VEGF
inhibitor is expected to be an effective treatment for advanced
NSCLC (13,14). Further studies are needed to
elucidate the prognostic significance of VEGFR2 expression as a
predictor of PD-1 blockade.
The present clinicopathological study aimed to
elucidate the predictive role of VEGFR2 expression in patients with
advanced NSCLC who received PD-1 blockade as a first-line
treatment, based on correlation with the number of
tumor-infiltrating lymphocytes (TILs) in intratumoral and stromal
tissue.
Materials and methods
Patients
A total of 207 patients with advanced or metastatic
NSCLC were treated with pembrolizumab monotherapy as the first-line
treatment at our institution (Saitama Medical University, Hidaka,
Japan) from May 2017 to March 2021. The inclusion criteria was as
follows; having a therapeutic history of first-line pembrolizumab
and enough tumor tissue for immunohistochemistry. Of these, 99
patients did not have sufficient tumor specimens for
immunohistochemistry before pembrolizumab treatment. Thus, a total
of 108 patients (nmale=86, nfemale=22; age range 37–85 years), was
eligible for the study. Of these, 32 patients were analyzed as
training cohort for investigation and 76 patients were evaluated as
validation cohort. The patients who received first-line
pembrolizumab from May 2017 to November 2018 were registered as
training cohort, whereas, those from December 2018 to March 2021
were allocated as validation cohort. Clinical data such as age,
sex, performance status (PS), and smoking history were extracted
from medical records. The present study was approved by the
Institutional Ethics Committee of the International Medical Center
of Saitama Medical University (approval no. 19-075). The
requirement for written informed consent for use of human tissue
was waived by the ethics committee of Saitama Medical University
owing to the retrospective nature of the study.
Therapeutic schedule and
evaluation
For first-line monotherapy in all patients, 200
mg/day pembrolizumab was administered intravenously. Physical
examination, complete blood count, biochemical testing such as
liver and renal dysfunction and electrolytes, and adverse events
were measured by the chief physician. Toxicity was graded based on
the Common Terminology Criteria for Adverse Events, version 4.0
(15). Tumor response was examined
according to Response Evaluation Criteria in Solid Tumors version
1.1 (16). Objective response rate
(ORR) and disease control rate (DCR) were assessed. DCR was defined
as the percentage of complete response (CR), partial response (PR)
and stable disease (SD).
Immunohistochemical staining
Immunohistochemical staining was performed as
previously described (17). VEGFR2
(1:100; Cell Signaling Technology, Inc.; cat #2472) was scored
according to the stained tumor area (biopsy and surgical sample) as
follows: 1, ≤10; 2, 11–24; 3, 25–49 and 4, ≥50% staining. High and
low expression were defined by scores of 1–3 and 4, respectively,
for VEGFR2, as previously described (17). The sections were evaluated using a
light microscope (×200 and ×400 magnification) in a blinded fashion
by at least two authors. In the case of discrepancies, both
investigators evaluated the slides simultaneously until they
reached a final consensus. The investigators were blinded to
patient outcome.
Multiplex immunohistochemistry (mIHC;
OPAL™) staining, image acquisition and data
analysis
Tumor specimens were formalin-fixed and
paraffin-embedded (fixative concentration, 10%; temperature, room;
duration 30 min.). Then, three sections with the largest area of
viable tumor cells were selected. Afterwards, 5-µm-thick sections
of tissue were deparaffinized and rehydrated using xylene and
ethanol for mIHC staining. Next, slides were treated with 0.3%
hydrogen peroxide in methanol for 30 min to block endogenous
peroxidase activity. To expose antigens, sections were autoclaved
in 10 mmol/l sodium citrate buffer (pH 6.0) for 121°C and 20 min,
followed by microwave treatment at 98°C for 15 min and cooled for
30 min. After rinsing in 0.05 M tris-buffered saline containing
0.1% Tween 20, sections were incubated at 4°C overnight with mouse
monoclonal CD4 (Leica Biosystems; clone 4B12; 1:100; high pH
retrieval; cat. NCL-L-CD4-368), CD8 (Dako; Agilent Technologies,
Inc.; clone C8/144B; 1:150 high pH retrieval; cat. M7103), FOXP3
(Abcam; 1:50; clone 236A/E7; pH 6 retrieval) (cat. Ab20034) and pan
cytokeratin (Abcam; clone AE1/AE3; 1:100; pH 6) (cat. Ab27988).
Blocking reagent (Antibody Diluent, Akoya) was incubated at room
temperature, 10 min. Secondary antibody (2 drops of Opal Polymer
HRP, Akoya) (cat. NEL811001KT) was incubated for room temperature.
10 min. using Akoya: NEL 811001KT opal-7-color Manual IHC KIT (opal
Polymer HRP MS+Rb). Chromogen detection reagent for HRP/DAB was
Akoya: NEL 811001KT opal-7-color Manual IHC KIT (1Xplus
Amplification Diluent) and counterstain was incubated for room
temperature, 5 min. using Spectral DAPI solution.
Immunofluorescence signals were visualized using
OPAL 7-color IHC kit (Akoya Biosciences, Inc.) tyramide signal
amplification dyes 520, 540, 570, 620, 650 and 690 and
counterstained with Spectral DAPI. The Mantra imaging platform
(Akoya Biosciences, Inc.) was used for imaging with Mantra snap
software (version 1.0.3) for data acquisition (http://www.perkinelmer.com). Color separation, tissue
and cell segmentation and cell phenotyping were performed using
inForm® Software v2.5.1 (Akoya Biosciences, Inc.) to
extract image data. Slides were evaluated for the presence of TILs
in the tumor and stroma. mIHC staining and data analysis were
performed as previously described (18).
All slides were scanned at 20× magnification to
achieve high-powered imaging at a resolution of 0.5 µm/pixel using
Phenochart (Akoya Biosciences, Inc.). High-powered imaging was used
to assess intratumoral area with lymphocytic infiltrate, tumor
margin and stromal area. An algorithm was designed based on pattern
recognition of pan cytokeratin-positive (tumor) and -negative areas
(stroma). Cell segmentation was performed on all cells
counterstained with DAPI. TIL distribution scoring for was
performed on the 20× pre-scanned images of each patient. A total of
three high-powered images of tumor parenchyma and stroma with
highest TIL density were selected to grade TIL density,
OPAL-positive TIL count and percentage. Multiple images (3 images)
from the tumor and stroma were quantified. The cell count of TILs
was determined by normalizing to 1,000 cells after counting all
cells Images were analyzed on inForm 2.5.1 software (Akoya
Biosciences, MA). Type of microscope was Mantra2 multispectral
microscopy (Akoya Biosciences, Marlborough, MA) with magnification:
20× objective. Fluorescence images were acquired on Mantra2
multispectral microscopy (Akoya Biosciences, Marlborough, MA) with
20× objective.
Statistical analysis
Student's t (unpaired t) and χ2 test were
used for continuous and categorical variables, respectively.
P<0.05 was considered to indicate a statistically significant
difference. Correlation between TIL measurement and variables were
analyzed using Pearson's rank test. Tumor PD-L1 expression was
counted as a tumor proportional score and classified as high or low
based on median expression value. Median TIL count in the tumor and
stroma was used to define high and low expression. Progression-free
survival (PFS) was defined as the time from initial ICI treatment
to disease progression or death. Overall survival (OS) was defined
as the time from initial ICI treatment to death from any cause.
Kaplan-Meier method was used to estimate survival as a function of
time and survival differences were analyzed using log-rank test.
Univariate and multivariate analyses were performed using logistic
regression. All statistical analyses were performed using GraphPad
Prism (v.8.0; GraphPad Software, Inc.) and JMP 14.0 (SAS Institute,
Inc.).
Results
Patient demographics according to
VEGFR2 expression in training and validation cohorts
Patient demographic according to VEGFR2 expression
in training cohort and validation cohort are shown in Table I. In the training cohort of 32
patients, PS was 0, 1, 2, and 3 in 10 (31.2%), 14 (43.8%), 7
(21.8%) and 1 (3.2%) patients, respectively. A histology of
adenocarcinoma (AC) and non-AC was observed in 17 (53.1%) and 15
(46.9%) patients, respectively. In the validation cohort of 76
patients (nmale=86, nfemale=22; median age, 70 years; age range,
37–85 years), smoking history was observed in 96 (88.8%) patients.
PS was 0, 1, 2 and 3 in 37 (34.2%), 53 (49.1%), 12 (11.1%) and 6
(5.6%) patients, respectively. Histological types of AC and non-AC
(squamous cell carcinoma and other) were identified in 61 (56.5%),
22 (20.4%), and 25 (23.1%) patients, respectively. Regarding PD-L1
expression, 62 (57.4%) and 46 (42.6%) displayed levels ≥50% and
<50%, respectively.
 | Table I.Characteristics according to VEGFR2
in patients receiving pembrolizumab. |
Table I.
Characteristics according to VEGFR2
in patients receiving pembrolizumab.
|
| Training
cohort | Validation
cohort |
|---|
|
|
|
|
|---|
| Characteristic | All patients
(n=32) | High (n=11) | Low (n=21) | P-value | All patients
(n=76) | High (n=19) | Low (n=57) | P-value |
|---|
| Age, <75/≥75
years | 20/12 | 8/3 | 12/9 | 0.467 | 42/34 | 14/5 | 28/29 | 0.069 |
| Sex,
male/female | 23/8 | 11/0 | 12/ 8 | 0.028a | 62/14 | 17/2 | 45/12 | 0.496 |
| ECOG PS 0–1
/2–3 | 24/8 | 9/2 | 15/6 | 0.680 | 61/15 | 13/6 | 48/9 | 0.182 |
| Smoking (BI),
<900/≥900 | 10/22 | 4/7 | 6/15 | 0.702 | 37/39 | 12/7 | 25/32 | 0.188 |
| Histology,
AC/Non-AC | 17/15 | 6/5 | 11/10 | >0.999 | 41/35 | 6/13 | 35/22 | 0.033a |
| Brain meta,
yes/no | 14/18 | 5/6 | 9/12 | >0.999 | 22/54 | 7/12 | 15/42 | 0.394 |
| Bone meta |
|
|
|
|
|
|
|
|
|
Yes/No | 14/18 | 6/5 | 8/13 | 0.465 | 21/55 | 3/16 | 18/39 | 0.242 |
| Response |
|
|
|
|
|
|
|
|
|
PR/Non-PR | 12/20 | 3/8 | 9/12 | 0.467 | 25/51 | 5/14 | 20/37 | 0.579 |
| PD-L1 |
|
|
|
|
|
|
|
|
| 1-49
/50–100% | 11/21 | 4/7 | 7/14 | >0.999 | 20/56 | 3/16 | 17/40 | 0.367 |
| Prior RT |
|
|
|
|
|
|
|
|
|
Yes/No | 13/19 | 3/8 | 10/11 | 0.450 | 28/48 | 5/14 | 23/34 | 0.411 |
| G3/4 irAE |
|
|
|
|
|
|
|
|
|
Yes/No | 7/25 | 1/10 | 6/15 | 0.374 | 16/60 | 2/17 | 14/43 | 0.329 |
| Albumin |
|
|
|
|
|
|
|
|
|
High/Low | 16/16 | 4/7 | 12/9 | 0.457 | 46/30 | 11/8 | 35/22 | 0.792 |
| CRP |
|
|
|
|
|
|
|
|
|
High/Low | 17/15 | 6/5 | 11/10 | >0.999 | 33/43 | 9/10 | 24/33 | 0.791 |
VEGFR2 was highly expressed in lung
cancer and closely correlated with histology of non-AC
Immunohistochemical examination was performed on all
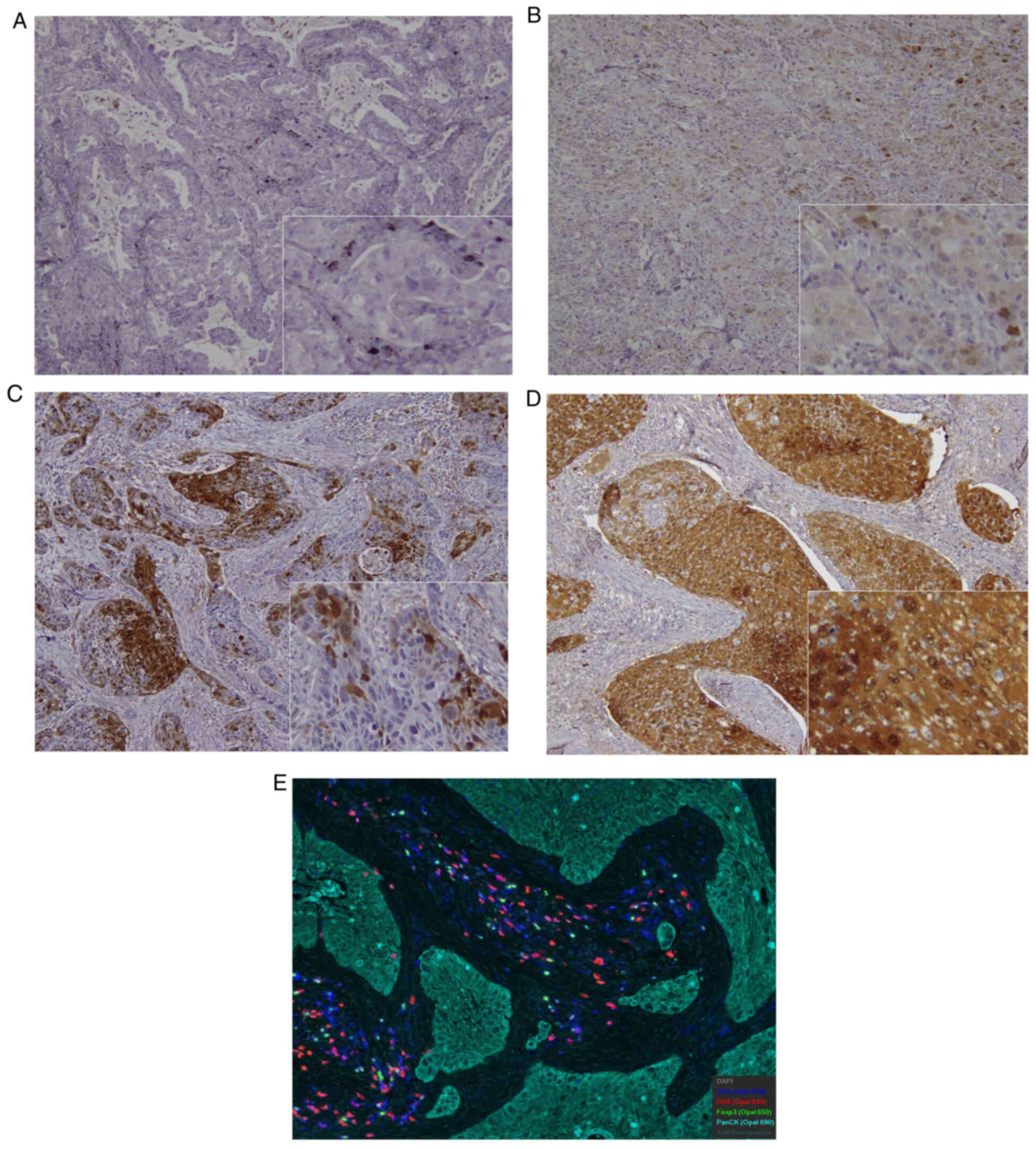
tumor specimens. Representative images of VEGFR2, CD4, CD8 and
FOXP3 expression are shown in Fig.
1. Immunostaining for VEGFR2 was performed on cell membranes
and cytoplasm of the tumor specimens. The percentages of high
expression of VEGFR2 in training and validation cohort were 34.3%
(11/32) and 25.0% (19/76), respectively. The incidence of scoring
1, 2, 3, and 4 was 9 (28.1%), 7 (21.9%), 5 (15.6%), and 11 (34.4%)
for training cohort, respectively, and 18 (23.7%), 16 (21.1%), 23
(30.2%), and 19 (25.0%) for validation cohort, respectively. High
VEGFR2 expression was significantly associated with sex in the
training cohort and histological type in the non-AC group for
validation cohort (Table I).
Table II shows ORR
and disease control rate (DCR) according to VEGFR2 expression
levels. ORR and DCR were 38.7 and 67.7 for training cohort and 31.2
and 79.1% for validation cohort. No statistically significant
difference in ORR of the patients between high and low VEGFR2
expression was observed in the training (27.2 vs. 45.0) and
validation (31.2 vs. 35.7%) cohorts. In training cohort, patients
with high VEGFR2 expression yielded a significantly lower DCR than
those with low VEGFR2 expression (36.3 vs. 85.0) but not in
validation cohort (75.0 vs. 80.3%).
 | Table II.ORR and DCR. |
Table II.
ORR and DCR.
| Response | Training cohort
(n=32) | Validation cohort
(n=76) |
|---|
| CR | 0.000 | 0.000 |
| PR | 12.000 | 25.000 |
| SD | 9.000 | 32.000 |
| PD | 10.000 | 15.000 |
| NE | 1.000 | 4.000 |
| ORR, % | 38.700 | 34.700 |
High VEGFR2 expression was closely
associated with positive FOXP3, but not CD4 and CD8
In training cohort (n=32), median cell count for
CD4, CD8, and FOXP3/1,000 cells was 1.4 (range, 0–126), 2.7 (0–166)
and 4.7 (0–65) in intratumoral sites, respectively, and 7.4
(0–214), 16.9 (0–212) and 9.5 (0–116) in stromal sites,
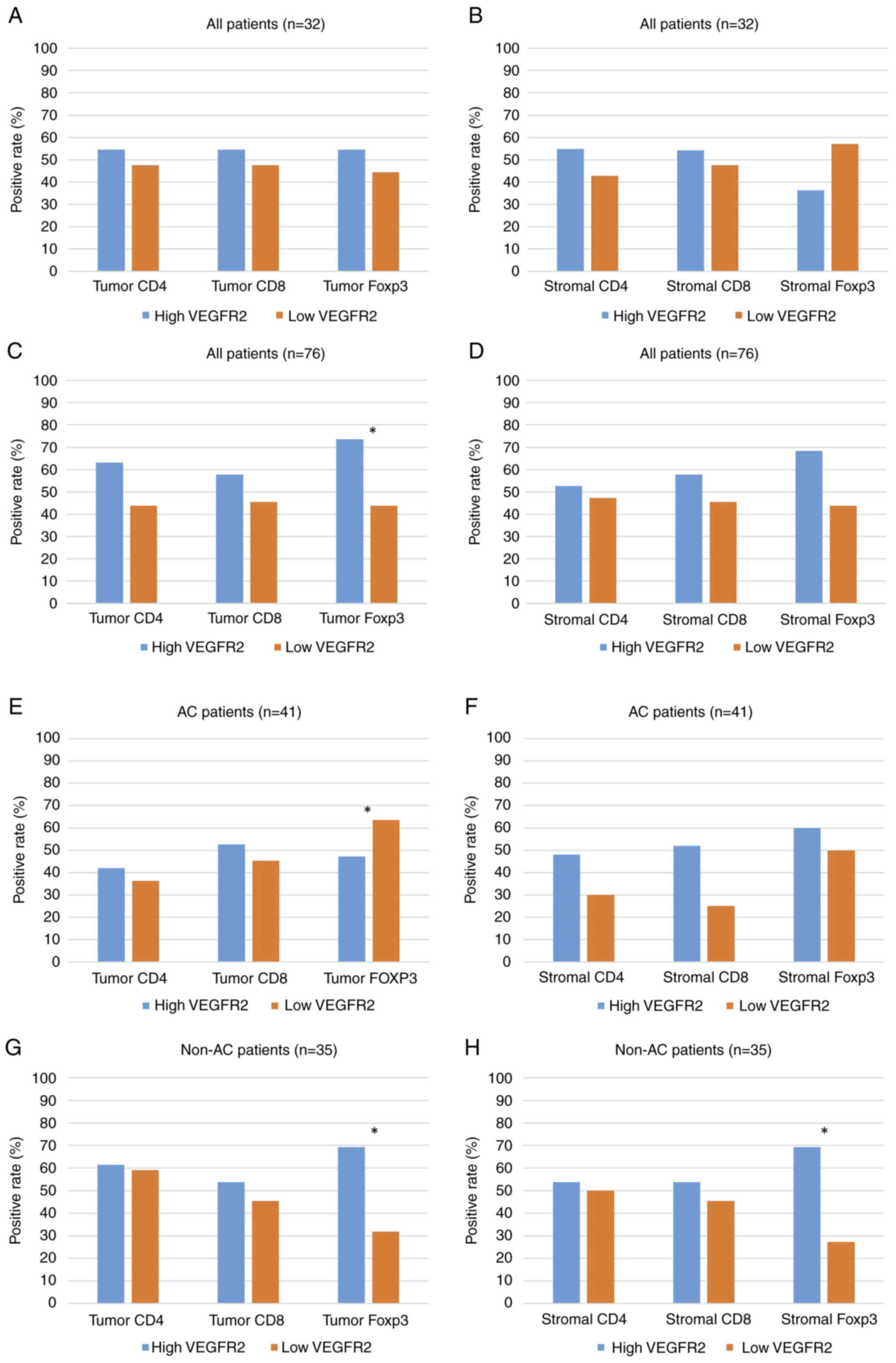
respectively (data not shown). No statistically significant
difference in positive percentage of CD4, CD8, and FOXP3 TILs was
observed between high and low VEGFR2 expression in intratumoral
(Fig. 2A) and stromal sites
(Fig. 2B). For validation cohort,
median cell counts for CD4, CD8 and FOXP3 TILs/1,000 cells were 3.1
(range, 0–589), 9.2 (0–221) and 5.9 (0–658) in intratumoral
lesions, respectively, and 6.7 (0–442), 19.7 (0–363) and 8.6
(0–205) in the stromal lesions, respectively. Positive rate of
intratumoral, but not stromal, FOXP3 (Fig. 2C and D) was significantly
associated with high VEGFR2 expression in all patients. Positive
FOXP3 expression exhibited a significant association with high
VEGFR2 expression in intratumoral, but not stromal, lesions in
patients with AC (Fig. 2E and F).
A statistically significant association was observed between high
VEGFR2 expression and positive intratumoral/stromal FOXP3 in
patients with non-AC (Fig. 2G and
H).
High VEGFR2 expression was
significantly associated with worse outcome
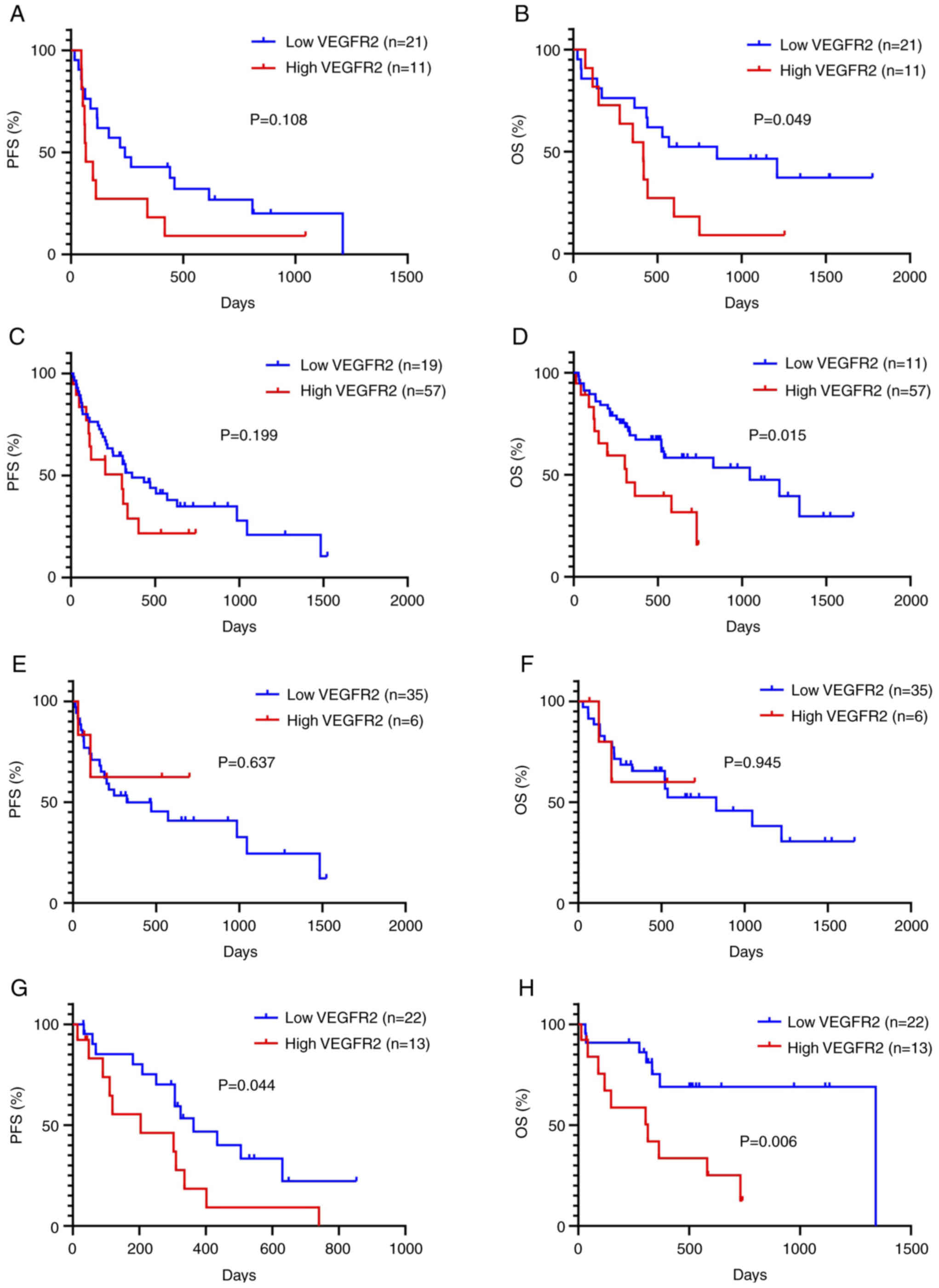
Kaplan-Meier curves based on expression of VEGFR2
were constructed for all patients (Fig. 3). In training cohort, median PFS
and OS were 143 and 485 days, respectively. A total of 27 patients
experienced tumor recurrence and 22 died due to progressive disease
(data not shown). Patients with high VEGFR2 expression showed a
significantly worse OS, but not PFS, than those with low VEGFR2
expression (Fig. 3A and B).
Univariate and multivariate survival analyses were performed
according to VEGFR2 expression in validation cohort. The median PFS
and OS were 324 and 731 days, respectively. A total of 47 patients
experienced tumor recurrence and 37 died as a result of progressive
disease (Table II). Univariate
analysis revealed PS for PFS and PS and VEGFR2 as significant
predictor for OS. PS, VEGFR2 and VEGFC were selected for subsequent
multivariate analysis. Multivariate analysis confirmed that PS was
an independent prognostic factor for PFS and PS and VEGFR2 were
identified as significant predictors of OS (Table III). A sub-analysis revealed that
high expression of VEGFR2 was significantly associated with shorter
PFS and OS in 35 patients without AC but not in 41 patients with AC
(Fig. 3C and D).
 | Table III.Univariate and multivariate survival
analysis in 76 patients with pembrolizumab treatment. |
Table III.
Univariate and multivariate survival
analysis in 76 patients with pembrolizumab treatment.
|
| Progression-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variable | MST, days | P-value | HR | 95% CI | P-value | MST, days | P-value | HR | 95% CI | P-value |
|---|
| Age, <75/≥75
years | 402/307 | 0.380 |
|
|
| 731/829 | 0.881 |
|
|
|
| Sex,
male/female | 324/258 | 0.558 |
|
|
| 581/829 | 0.454 |
|
|
|
| ECOG PS,
0–1/2–3 | 336/203 | 0.027a | 1.462 | 0.991-2.067 | 0.046a | 829/203 |
<0.001a | 1.813 | 1.247-2.571 | 0.002a |
| Smoking (BI),
<900/≥900 | 336/324 | 0.610 |
|
|
| 731/1046 | 0.928 |
|
|
|
| Histology,
AC/non-AC | 470/310 | 0.282 |
|
|
| 829/731 | 0.745 |
|
|
|
| Brain metastasis,
yes/no | 470/307 | 0.564 |
|
|
| 731/1046 | 0.663 |
|
|
|
| Bone metastasis,
yes/no | 216/402 | 0.116 |
|
|
| 537/829 | 0.307 |
|
|
|
| Prior RT,
yes/no | 363/307 | 0.731 |
|
|
| 522/731 | 0.887 |
|
|
|
| CRP, high/low | 303/336 | 0.451 |
|
|
| 522/731 | 0.186 |
|
|
|
| Albumin,
high/low | 363/307 | 0.251 |
|
|
| 829/521 | 0.073 |
|
|
|
| PD-L1 expression,
1–49/50–100% | 324/307 | 0.927 |
|
|
| 581/731 | 0.974 |
|
|
|
| VEGFR2,
high/low | 303/363 | 0.199 | 1.251 | 0.873-1.736 | 0.221 | 314/1046 | 0.015a | 1.592 | 1.088-2.281 | 0.017a |
Discussion
To the best of our knowledge, the present
clinicopathological study is the first to evaluate the prognostic
significance of angiogenic markers in patients with advanced NSCLC
who received first-line pembrolizumab monotherapy. There was an
association between high VEGFR2 expression and regulatory T
lymphocytes in tumor specimens with advanced NSCLC and high VEGFR2
expression was an independent marker for predicting worse OS in
patients who received first-line pembrolizumab monotherapy,
particularly in those with non-AC. VEGFR2 was highly expressed in
patients with NSCLC and played a negative role in the efficacy of
PD-1 blockade treatment. VEGFR2 expression was associated with the
immunosuppressive tumor environment and tended to be resistant to
PD-1 blockade treatment in patients with NSCLC with non-AC
histology. However, the reason for this phenomenon remains unclear.
The present study explored the clinical significance of VEGFR2
expression by training cohort. High VEGFR2 expression was
associated with poor OS following PD-1 blockade administration. A
significant association between high VEGFR2 expression and worse
outcome following pembrolizumab treatment was confirmed by
validation cohort. However, high VEGFR2 expression was associated
with high FOXP3 in validation cohort, whereas there was not
significant relationship between the expression of VEGFR2 and FOXP3
in training cohort. The association of TILs with VEGFR2 may be weak
in human tumor specimens. Further investigation is warranted to
elucidate the therapeutic significance of VEGFR2 inhibitors in
addition to PD-1 blockade in patients with NSCLC with high FOXP3
levels in tumor specimens.
A review reported that the synergistic effects
between VEGFR2-targeting therapy and immunotherapy in previous
studies (3,4,19)
and suppression of VEGFR2 in T cells decreases infiltration of
regulatory T cells (Tregs) into tumor tissue (19). Experimental studies have
demonstrated that VEGFR2 is selectively expressed by
FOXP3high but not FOXP3low Treg (20) and blockade of VEGF is associated
with inhibition of the immunosuppressive phenotype of
VEGFR2+ myeloid cells and increased T cell activation
(21). The aforementioned reports
suggest that inhibition of VEGFR2 enhances the efficacy of ICIs in
patients with cancer (19–21). Recently, Shibaki et al
(22) demonstrated that high serum
VEGF is significantly associated with worse prognosis following
PD-1 blockade treatment in 235 patients with advanced NSCLC. To the
best of our knowledge, however, no studies have reported the
association between VEGF expression in tumor specimens and the
efficacy of PD-1 blockade. VEGF-ligand antibodies exhibits
non-specific staining within tumor tissue, whereas, VEGFR2 is
clearly stained in small tissues such as biopsy samples. Here,
FOXP3 increased in tumor tissue when VEGFR2 was highly expressed
and VEGFR2 mobilized FOXP3 entry into intratumoral lesions with
non-AC histology. Histological analysis showed that expression of
VEGF was not associated with the mobilization of FOXP3 TILs in AC
tumor tissue, whereas VEGFR2 increased FOXP3 TILs infiltration into
non-AC intratumoral and stromal tissue. The reason for this
difference is unclear. Survival analysis demonstrated that high
VEGFR2 expression was associated with worse outcomes in patients
with non-AC. Considering the potential to increase FOXP3 by
upregulating VEGFR2 (20,21), the present study suggested an
association between FOXP3 and VEGFR2 expression in tumor
specimens.
Clinical studies reporting the efficacy of
pembrolizumab with VEGFR2 inhibitor have been performed in patients
with different types of cancer (23–25).
It has been reported that lenvatinib (a multikinase inhibitor of
VEGFR2, VEGFR2 and VEGFR3) + pembrolizumab exhibits anti-tumor
activity (ORR, 39.6%) in patients with advanced endometrial cancer
(23) and ramucirumab in
combination with pembrolizumab yields favorable antitumor activity
in patients with advanced gastric or gastro-esophageal junction AC
and urothelial carcinoma (ORR, 7 and 13%, respectively) (24), but ramucirumab + pembrolizumab
shows limited clinical activity (ORR, 4%) in patients with advanced
biliary tract cancer (25). The
aforementioned studies showed that the synergistic efficacy of
VEGFR2 inhibitor in addition to pembrolizumab may be different
based on histological or cancer type. Bevacizumab + atezolizumab is
a standard first-line treatment for patients with advanced
hepatocellular carcinoma (5).
Reckamp et al (26)
performed a phase II study to evaluate the efficacy of ramucirumab
+ pembrolizumab compared with investigator's choice of care in
patients with advanced NSCLC who previously received chemotherapy +
PD-1 blockade. Even following resistance to prior ICI, ramucirumab
with pembrolizumab improved OS compared with standard care, with
ORR of 22% (26). The results of
the aforementioned study suggested that modulation of tumor immune
microenvironment by antiangiogenic drug promotes resensitization to
PD-1 blockade in patients with advanced NSCLC, although the
mechanism remains unclear (26).
Thus, inhibition of VEGFR2 may serve a key role in the improvement
of immune microenvironment.
The expression of PD-L1 within tumor cells is a
useful marker for predicting the efficacy of ICI treatment in
patients with advanced NSCLC (10,13,14).
It has been reported that ICI therapy is also effective for
patients with NSCLC with negative PD-L1 expression and ~20% of
patients are expected to achieve long term survival (27,28).
Therefore, PD-L1 expression does not predict efficacy and outcome
of ICI therapy, and is not suitable for an optimal biomarker to
PD-1 blockade treatment.
The present study had several limitations. First,
the sample size was relatively limited; thus, the results may have
been biased. For immunohistochemistry, only 108 of 207 patients
were available. For molecular targeting therapy, the majority of
biopsy samples are used for detection of genetic alterations. Thus,
more than half of patients were not eligible because of inadequate
or unavailable tumor tissue. Base-line testing for molecular
characteristics is important for subsequent therapy. Recent
research reported that TMB, POLE mutation and alterations in DNA
damage repair genes could affect the efficacy of ICI treatment,
loss of serine/threonine kinase 11/liver kinase B1 induces primary
resistance to PD-1 blockade in patients with KRAS mutant
lung AC and epidermal growth factor receptor (EGFR)
mutations are associated with low response rate to PD-1 blockade
(2,29–32).
Patients with TP53 and KRAS mutant exhibit greater
PFS than those with wild-type TP53 and KRAS) treated
with pembrolizumab (33). Here,
patient molecular profiling before ICI treatment was not adequately
investigated. Thus, further study is warranted to evaluate detailed
molecular profiling before ICI therapy. Second, it was difficult to
evaluate the expression of VEGFR2 in immune cells in stromal
tissue. Immune cells, such as lymphocytes, may serve a key role in
VEGFR2-mediated resistance to immunotherapy (20,21).
It is difficult to detect expression of VEGFR2 for
immunohistochemistry is inadequate for detection of stromal immune
cells. Here, VEGF-A, B and D as VEGF-ligand markers and VEGFR1 and
VEGFR3 as VEGF receptor markers were immunohistochemically
examined. Non-specific staining for these markers was wholly
observed in the tumor specimens, regardless of clones and methods
(data not shown). For immunohistochemical staining of small
samples, such as a transbronchial lung biopsy, use of VEGF or VEGFR
antibodies with non-specific staining should be avoided (34). Therefore, VEGFR2 was selected for
accurate immunohistochemistry and other VEGF antibodies were not
used.
In conclusion, high expression of VEGFR2 was
identified as a significant prognostic marker for predicting worse
OS following first-line pembrolizumab monotherapy in patients with
advanced NSCLC, particularly in those with non-AC. VEGFR2 may serve
a crucial role in predicting the efficacy of PD-1 blockade
monotherapy. Moreover, VEGFR2 expression in tumor specimens was
associated with levels of regulatory T cells. Further investigation
is required to elucidate the therapeutic significance of VEGFR2
inhibition in addition to PD-1 blockade based on expression of
FOXP3.
Acknowledgements
The authors would like to thank Ms Kozue Watanabe,
Ms. Chieko Ono, Ms. Saki Toita, Ms. Hiroko Noguchi, Mr. Joji
Shiotani and Ms. Koko Kodaira in Saitama Medical University, Hidaka
city, Japan for assistance in preparing the manuscript.
Funding
The present study was supported by the Japan Society for the
Promotion of Science (grant nos. 20K08118 and 21K07627).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Author's contributions
KKa, OY and TK conceived the study and wrote the
manuscript. KH, YM, AS, HI, KKo and MY collected and analyzed data.
KKa, HI and HK interpreted data. KKa, OY, TK and HK revised the
manuscript. All authors have read and approved the final
manuscript. KKa and HI confirm the authenticity of all the raw
data
Ethics approval and consent to
participate
The present study was approved (19–075) by the Institutional Ethics
Committee of the International Medical Center of Saitama Medical
University, Hidaka city, Japan. The Ethical Committee waived the
need to obtain written informed consent for the use of human
tissues to participate from the patients owing to the retrospective
nature of the study.
Patient consent for publication
Not applicable.
Competing interests
KKa has received research grants and a speaker
honorarium from Ono Pharmaceutical Company, Boehringer Ingelheim,
Chugai Pharmaceutical, Taiho Pharmaceutical, Eli Lilly Japan and
AstraZeneca. AM and OY received a speaker honorarium from Eli
Lilly, Taiho Pharmaceutical, Pfizer, Chugai Pharmaceutical and
AstraZeneca. HK has received research grants and a speaker
honorarium from Ono Pharmaceutical Company, Bristol-Myers Company,
Boehringer Ingelheim, MSD, Daiichi Sankyo Company, Chugai
Pharmaceutical, Taiho Pharmaceutical, Merck Biopharma Company, Eli
Lilly Japan and AstraZeneca. HI has received research grants and a
speaker honorarium from Ono Pharmaceutical Company, AstraZeneca and
Bristol-Myers Company.
References
|
1
|
Garon EB, Hellmann MD, Rizvi NA, Carcereny
E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L,
et al: Five-year overall survival for patients with advanced
non-small-cell lung cancer treated with pembrolizumab: Results from
the phase I KEYNOTE-001 study. J Clin Oncol. 37:2518–2527. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma K, Jin Q, Wang M, Li X and Zhang Y:
Research progress and clinical application of predictive biomarker
for immune checkpoint inhibitors. Expert Rev Mol Diagn. 19:517–529.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song Y, Fu Y, Xie Q, Zhu B, Wang J and
Zhang B: Antiangiogenic agents in combination with immune
checkpoint inhibitors: A promising strategy for cancer treatment.
Front Immunol. 11:19562020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Padda SK and Reckamp LK: Combination of
immunotherapy and antiangiogenic therapy in cancer-a rational
approach. J Thorac Oncol. 16:178–182. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim YY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rini BI, Plimack ER, Stus V, Gafanov R,
Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B,
et al: Pembrolizumab plus axitinib versus sunitinib for advanced
renal-cell carcinoma. N Engl J Med. 380:1116–1127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for first-line treatment
of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motzer RJ, Penkov K, Haanen J, Rini B,
Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S,
Uemura M, et al: Avelumab plus axitinib versus sunitinib for
advanced renal-cell carcinoma. N Engl J Med. 380:1103–1115. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herbst RS, Arkenau HT, Bendell J,
Arrowsmith E, Wermke M, Soriano A, Penel N, Santana-Davila R,
Bischoff H, Chau I, et al: Phase 1 expansion cohort of ramucirumab
plus pembrolizumab in advanced treatment-naïve NSCLC. J Thorac
Oncol. 16:289–298. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1 expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomized,
open-label, controlled, phase 3 Trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garon EB, Ciuleanu TE, Arrieta O, Prabhash
K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel
J, et al: Ramucirumab plus docetaxel versus placebo plus docetaxel
for second-line treatment of stage IV non-small-cell lung cancer
after disease progression on platinum-based therapy (REVEL): A
multicenter, double-blind, randomised phase 3 Trial. Lancet.
384:665–673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bilguun EO, Kaira K, Kawabata-Iwakawa R,
Rokudai S, Shimizu K, Yokobori T, Oyama T, Shirabe K and Nishiyama
M: Distinctive roles of syntaxin binding protein 4 and its action
target, TP63, in lung squamous cell carcinoma: A theranostic study
for the precision medicine. BMC Cancer. 20:9352020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paz-Ares L, Luft A, Vicente D, Tafreshi A,
Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et
al: Pembrolizumab plus chemotherapy for squamous non-small-cell
lung cancer. N Engl J Med. 379:2040–2051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
CTCAEv 4.0, URL. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumour:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Imai H, Kaira K, Hashimoto K, Nitanda H,
Taguchi R, Yanagihara A, Umesaki T, Yamaguchi O, Mouri A, Kawasaki
T, et al: Tumor immunity is related to 18F-FDG uptake in
thymic epithelial tumor. Cancer Med. 10:6317–6326. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Halse H, Colebatch AJ, Petrone P,
Henderson MA, Mills JK, Snow H, Westwood JA, Sandhu S, Raleigh JM,
Behren A, et al: Multiplex immunohistochemistry accurately defines
the immune context of metastatic melanoma. Sci Rep. 8:111582018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu P, Hu C, Hui K and Jiang X: The role
and significance of VEGFR2 regulatory T cells in tumor immunity.
Onco Targets Ther. 10:4315–4319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki H, Onishi H, Wada J, Yamasaki A,
Tanaka H, Nakano K, Morisaki T and Katano M: VEGFR2 is selectively
expressed by FOXP3high CD4+ Treg. Eur J Immunol. 40:197–203. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Huang H, Coleman M, Ziemys A,
Gopal P, Kazumi S and Brekken R: VEGFR2 activity on myeloid cells
mediates immune-suppression in the tumor environment. JCI Insight.
6:e1507352021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shibaki R, Murakami S, Shinno Y, Matsumoto
Y, Yoshida T, Goto Y, Kanda S, Horinouchi H, Fujiwara T, Yamamoto
N, et al: Predictive value of serum VEGF levels for elderly
patients or for patients with poor performance status receiving
anti-PD-1 antibody therapy for advanced non-small cell lung cancer.
Cancer Immunol Immunother. 69:1229–1236. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Makker V, Rasco D, Vogelzang NJ, Brose MS,
Cohn AL, Mier J, Simone CD, Hyman DM, Stepan DE, Dutcus CE, et al:
Lenvatinib plus pembrolizumab in patients with advanced endometrial
cancer: An interim analysis of a multicenter, open-label,
single-arm, phase 2 trial. Lancet Oncol. 20:711–718. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herbest RS, Arkenau HT, Santana-Davila R,
Calvo E, Paz-Ares L, Cassier PA, Bendell J, Penel N, Krebs MG,
Martin-Leberal J, et al: Ramucirumab plus pembrolizumab in patients
with previously treated advanced non-small-cell lung cancer,
gastro-oesophageal cancer, or urothelial carcinomas (JVDE): A
multicohort, non-randomised, open-label, phase 1a/b trial. Lancet
Oncol. 20:1109–1123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arkenau HT, Martin-Liberal J, Calvo E,
Penel N, Krebs MG, Herbst RS, Walgren RA, Widau RC, Mi G, Jin J, et
al: Ramucirumab plus pembrolizumab in patients with previously
treated advanced or metastatic biliary tract cancer: Nonrandomized,
open-label, phase I trial (JVDF). Oncologist. 23:1407–e136. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reckamp KL, Radman MW, Dragnew KH,
Minichiello K, Villaruz LC, Faller B, Baghdadi TA, Hines S,
Everhart L, Highleyman L, et al: Phase II randomized study of
ramucirumab and pembrolozumab versus standard of care in advanced
non-small-cell lung cancer previously treated with
immunotherapy-Lung-MAP S1800A. J Clin Oncol. 40:2295–2306. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paz-Ares L, Ciuleanu TE, Cobo M, Schenker
M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E,
Juan-Vidal O, et al: First-line nivolumab plus ipilimumab combined
with two cycles of chemotherapy in patients with non-small-cell
lung cancer (CheckMate 9LA): An international, randomised,
open-label, phase 3 trial. Lancet Oncol. 22:198–211. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paz-Ares LG, Ramalingam SS, Ciuleanu TE,
Lee JS, Urban L, Caro RB, Park K, Sakai H, Ohe Y, Nishio M, et al:
First-line Nivolumab plus Ipilimumab in advanced NSCLC: 4-year
outcomes from the randomized, open-label, phase 3 CheckMate 227
Part 1 Trial. J Thorac Oncol. 17:289–308. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma X, Riaz N, Samstein RM, Lee M, Makarov
V, Valero C, Chowell D, Kuo F, Hoen D, Fitzgerald CWR, et al:
Functional landscapes of POLE and POLD1 mutations in checkpoint
blockade-dependent antitumor immunity. Nat Genet. 54:996–1012.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun H, Liu SY, Zhou JY, Xu JT, Zhang HK,
Yan HH, Huan JJ, Dai PP, Xu CR, Su J, et al: Specific TP53 subtype
as biomarker for immune checkpoint inhibitors in lung
adenocarcinoma. EBioMedicine. 60:1029902020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
West HJ, McCleland M, Cappuzzo F, Reck M,
Mok TS, Jotte RM, Nishio M, Kim E, Morris S, Zou W, et al: Clinical
efficacy of atezolizumab plus bevacizumab and chemotherapy in
KRAS-mutated non-small cell lung cancer with STK11, KEAP1, or TP53
comutations: Subgroup results from the phase III IMpower150 trial.
J Immunother Cancer. 10:e0030272022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Skoulidis F, Li BT, Dy GK, Price TJ,
Falchook GS, Wolf J, Italiano A, Schuler M, Borghaei H, Barlesi F,
et al: Sotorasib for Lung Cancers with KRAS p.G12C Mutation.
N Engl J Med. 384:2371–2381. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Frost N, Kollmeier J, Vollbrecht C, Grah
C, Matthes B, Pultermann D, von Laffert M, Lüders H, Olive E, Raspe
M, et al: KRASG12C/TP53 co-mutations identify long-term
responders to first line palliative treatment with pembrolizumab
monotherapy in PD-L1 high (≥50%) lung adenocarcinoma. Transl Lung
Cancer Res. 10:737–752. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ohtaki Y, Kaira K, Yajima T, Erkhem-Ochir
B, Kawashima O, Kamiyoshihara M, Igai H, Onozato R, Ibe T, Kosaka
T, et al: Comprehensive expression analysis of
chemosensitivity-related markers in large cell neuroendocrine
carcinoma of the lung. Thorac Cancer. 12:2666–2679. 2021.
View Article : Google Scholar : PubMed/NCBI
|