Introduction
Oesophageal cancer (EC) has a very high prevalence
and mortality worldwide (1).
Oesophageal squamous cell carcinoma (ESCC) is the main histological
type of EC in developing countries and accounts for more than 90%
of cancer-related deaths in China (2,3).
Early detection is a prerequisite for effective cancer treatment,
but when patients are diagnosed with ESCC, most often have tumours
at medium or late stages with high metastatic potential, which
leads to poor prognosis and 5-year survival rates (4). Thus, there is an urgent need to
identify and characterize molecular candidates that can serve as
biomarkers for early diagnosis and monitoring the treatment of
ESCC.
Growth and survival are major events in the
initiation and progression of human cancers. In this process,
metallopeptidases may play an important role by promoting cell
proliferation and reducing apoptosis due to their critical function
in protein metabolism (5,6). In fact, the dysregulation of
metallopeptidases is causally associated with the development of
various diseases ranging from cancer, inflammation, and microbial
infection to neurodegenerative diseases and cardiovascular
disorders (7). As a
metallopeptidase, dipeptidyl peptidase III (DPP3) is involved in
the progression of diseases such as cancer development, oxidative
stress and inflammation (8,9). A
number of studies have demonstrated that DPP3 elevation is
associated with carcinogenesis of the breast, lung, endometrium,
ovary and brain and may be a potential target in tumour therapy
(10–15). In a genome-wide expression array
analysis, an elevation of DPP3 in ESCC tissues was also found (data
not shown). However, the role of DPP3 expression in ESCC is not
fully understood. In the present study, the differential expression
of DPP3 between tumour and normal tissues from ESCC patients was
evaluated. Furthermore, the aberrant regulation of DPP3 expression
associated with cellular functions and oesophageal carcinogenesis
was studied using DPP3-depleted EC cells and xenograft modelling to
understand its role in ESCC progression and analyse its potential
as a molecular target in the early diagnosis and treatment of
ESCC.
Materials and methods
Human tissue specimens
The present study was approved (approval no.
2210226-138) and monitored by the Ethics Committee of the First
Affiliated Hospital of Xinjiang Medical University (Urumqi, China).
All procedures were followed in accordance with the Helsinki
Declaration of 1975, as revised in 2000. Informed consent was
obtained from all donors, and the data were analysed anonymously
throughout the study. A total of 93 patients diagnosed with EC were
enrolled in the present study according to the criteria of the
World Health Organization and the Chinese Medical Association. The
average age of the patients was 62 years (age range, 43–78 years).
None of the patients received chemoradiotherapy or radiotherapy
prior to surgery. The patients were followed-up by outpatient,
inpatient, and telephone monitoring. The average follow-up period
was 49 months and ended in May 2022. Clinicopathological
characteristics of patients are provided in Table II.
 | Table II.DPP3 expression associated with lymph
node metastasis of oesophageal cancer. |
Table II.
DPP3 expression associated with lymph
node metastasis of oesophageal cancer.
|
|
| Expression level of
DPP3 |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics of all patients | Total number
(93) | Low (51) | High (42) | P-value |
|---|
| Age, years |
|
|
| 0.925 |
|
<65 | 46 | 25 | 21 |
|
|
≥65 | 47 | 26 | 21 |
|
| Sex |
|
|
| 0.324 |
|
Male | 75 | 43 | 32 |
|
|
Female | 18 | 8 | 10 |
|
| Tumor size (long
diameter) |
|
|
| 0.989 |
| ≤5
cm | 51 | 28 | 23 |
|
| >5
cm | 42 | 23 | 19 |
|
| T Infiltrate |
|
|
| 0.738 |
| T1 | 6 | 2 | 4 |
|
| T2 | 18 | 11 | 7 |
|
| T3 | 66 | 36 | 30 |
|
| T4 | 3 | 2 | 1 |
|
| Lymph node
metastasis (N) |
|
|
| 0.039 |
| N0 | 42 | 26 | 16 |
|
| N1 | 22 | 14 | 8 |
|
| N2 | 18 | 9 | 9 |
|
| N3 | 11 | 2 | 9 |
|
| Stage |
|
|
| 0.681 |
| I | 13 | 6 | 7 |
|
| II | 33 | 22 | 11 |
|
|
III | 44 | 20 | 24 |
|
| IV | 3 | 3 | 0 |
|
| Lymphatic
metastasis |
|
|
| 0.214 |
| Lymph
node-positive | 51 | 25 | 26 |
|
| Lymph
node-negative | 42 | 26 | 16 |
|
| Grade |
|
|
| 0.448 |
| I | 11 | 6 | 5 |
|
| II | 53 | 27 | 26 |
|
|
III | 29 | 18 | 11 |
|
For immunohistochemistry analysis, 186
formalin-fixed and paraffin-embedded EC and adjacent non-tumour
specimens from the tumour periphery were obtained from the specimen
bank in the Pathology department after a case review by two
experienced pathologists at the First Affiliated Hospital of
Xinjiang Medical University. The clinical staging of the patients
was based on the guidelines established by the American Joint
Committee on Cancer using TNM staging, from the seventh or eighth
edition. Tumour specimens were collected from cancer patients who
underwent radical thoracic surgery for EC at clinical stages I–IV
from December 2017 to June 2018.
Immunohistochemistry (IHC)
Paraffin-embedded tissues were sectioned into 3-µm
slices. The tissue slices were conventionally stained by the
streptavidin peroxidase-conjugated (S-P) method. In brief, the
tissue was immersed in xylene and ethanol in turn dewaxed and
rehydrated. The slices were boiled in 10 mM sodium citrate buffer
(pH 6.0) and maintained for 10 min. After that, the slices were
cooled and soaked in distilled water for cleaning. The primary
antibody against DPP3 (1:50; cat. no. PA5-35038; Thermo Fisher
Scientific Inc.) was incubated at room temperature for 2 h. The
incubation was continued overnight at 4°C in a humidified chamber
and IHC kits containing biotin-labelled secondary antibodies
(1:200; cat. no. PV-6000; Zhongshan Golden Bridge Biotechnology
Co., Ltd.) in accordance with the manufacturer's recommended
procedures. All tissue slices were stained with DAB solution as
well as haematoxylin.
The staining intensity was scored under a light
microscope by two experienced pathologists. Briefly, the positively
stained area and intensity were scored using the following
criteria: positively stained area (1, <5%; 2, 5–30%; 3, 30–70%;
4, >70%) and staining intensity (0, no staining; 1, weak; 2,
moderate; 3, strong) were multiplied for each observer and then
averaged. An overall score (0 to 12) was calculated: an overall
score of 0 to 5 indicated total loss or weak expression, and 6 to
12 indicated strong expression of the protein.
Cell culture
The EC9706, KYSE450, EC9706 and TE-1 human ESCC cell
lines were obtained from Shanghai Cell Collection (Shanghai,
China). The cells were cultured in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% foetal bovine serum (FBS) and
1% penicillin/streptomycin (all from Biological Industries, Inc.)
in a 37°C incubator filled with 5% CO2.
DPP3 depletion by lentiviral short
hairpin (sh)RNA expression
For depletion of DPP3 expression in EC cells, three
different shRNA fragments targeting the mRNA sequence coding for
the DPP3 protein were designed. The shRNA target sequences were as
follows: DPP3-shRNA-1, CTTCAAAGAGGTCGATGGAGA; DPP3-shRNA-2,
CCGAGGAGAATTTGAAGGTTT; and DPP3-shRNA-3, GCTGGAGAAAGCCAAGGCCTA. The
aforementioned shRNAs were synthesized (Shanghai GeneChem, Co.,
Ltd.) and ligated into the BR-V108 lentiviral vector, which
expresses both a shRNA fragment and enhanced green fluorescent
protein (EGFP) as a reporter gene. The RNA interference target
sequence of negative control was designed: shCtrl,
TTCTCCGAACGTGTCACGT. DPP3-depleted EC cells were created by
liposomal transfection of 293T cells (Procell Life Science &
Technology Co., Ltd.,) with the constructs to produce virus for 24
h in a six-well plate, followed by infection of Eca-109 and TE-1 EC
cells with the virus at virus titres of 1×108 TU/ml and
20 µg/ml polybrene (Sigma-Aldrich; Merck KGaA) for 72 h in
accordance with the manufacturer's recommendation. The efficacy of
viral expression was estimated by determining the EGFP-derived
fluorescence with flow cytometry (Guava easyCyte HT; EMD
Millipore). The data were analysed with FlowJo software (version
7.6.1; FlowJo LLC).
RNA extraction and quantitative
analysis by reverse transcription-quantitative (RT-q) PCR
Total RNA was isolated from cultured cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA was synthesized from extracted mRNA by reverse
transcription (RT) using the Revert Aid First Strand cDNA Synthesis
kit (Thermo Fisher Scientific, Inc.) according to the manufacturer
using a standard procedure. The cDNA was analysed by qPCR using the
QuantiNova SYBR Green PCR Kit (Qiagen GmbH) and a primer pair
specific to the mRNA sequence coding for DPP3 (forward,
5′-TGAGTGCCAAGTTTGAGCG-3′ and reverse,
5′-AGCGAAGGTGAGAACATCCAG-3′), setting the mRNA coding for
glyceraldehyde-3-phosphate dehydrogenate (GAPDH; forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′) as a control. The thermocycling
conditions suitable for DPP3 mRNA were 95°C for 1 min, followed by
45 cycles of denaturation at 95°C for 10 sec and synthesis at 60°C
for 30 sec. All reactions were performed in triplicate, and the
expression level of target genes was quantified by the
2−ΔΔCq method (16).
Western blotting
Protein extracts were prepared by cell lysis using
radioimmunoprecipitation assay buffer (RIPA, Beijing Solarbio
Science & Technology Co., Ltd.). Protein concentration was
determined using the BCA protein assay kit (Thermo Fisher
Scientific, Inc.), and 20 mg protein extract was separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) followed by transfer onto a polyvinylidene fluoride
(PVDF) microporous membrane (MilliporeSigma). The membrane was
blocked by 5% non-fat milk at room temperature for 2 h and
incubated with rabbit primary antibodies against DPP3 (1:1,000;
cat. no. PA5-35038; Invitrogen; Thermo Fisher Scientific, Inc.),
and GAPDH (1:3,000; cat. no. AP0063; Bioworld Technology, Inc.) at
4°C overnight was used as an internal control. After incubation
with goat anti-rabbit secondary antibody (1:3,000; cat. no. A0208;
Beyotime Institute of Biotechnology) at room temperature for 2 h,
the blots were visualized by Amersham ECL plus TM Western Blot
system (cat. no. AI600; Cytiva) and the density of the protein band
was analyzed by ImageJ (v1.8.0; National Institutes of Health).
Cell growth assay
The effect of DPP3 depletion on the proliferation of
EC cells was analysed. The cells (2,000 cells/well) were inoculated
in the logarithmic phase into 96-well plates with a culture volume
of 100 µl in each well, and three wells were set as a group for
testing. The cell growth per day was determined in a 5-day interval
by counting cells using Celigo Imaging Cellometer (Nexcelom).
Wound healing assay
The cells were inoculated into 96-well plates
(~3×104 cells/well) and cultured in DMEM containing 10%
FBS at 37°C and 5% CO2 for 24 h. After achieving an
appropriate cell attachment, scrape wounds were generated with a 96
Wounding Replicator (V&P Scientific, Inc.). The cells were
washed and subsequently cultured with low-serum medium (0.5% FBS).
The wound healing process was then captured by microscopy using an
inverted fluorescence microscope (IX73; Olympus Corporation) at
intervals of 0, 24, and 48 h/0, 4, and 8 h. The percentage of cell
migration was calculated utilizing Image-Pro Plus software (version
6.0; Media Cybernetics, Inc.).
Cell migration
Chambers were put in a 24-well plate. The cells at a
density of 5×105 cells per well were seeded in
serum-free DMEM in the upper chamber of the Transwell chambers
(Corning, Inc.) and incubated for 24 to 48 h at 37°C, while 600 µl
medium containing 30% FBS was added to the lower chamber. After 24
h, cells remaining on the upper surface of the chamber were removed
by cotton swabs. The penetrated cells were fixed with 4%
paraformaldehyde and stained with 0.1% crystal violet for 30 min at
room temperature. The number of penetrating cells was counted under
a light microscope (Olympus Corporation) within the scope of 5
random fields.
Detection of the cell cycle and
apoptosis
For cell cycle analysis, the DPP3-depleted cells and
controls were collected in precooled phosphate buffered saline
(PBS) solution, immobilized in 75% precooled ethanol for at least 1
h and stained with propidium iodide (PI, 2 mg/ml; MilliporeSigma)
for 15 min at room temperature without light, followed by flow
cytometric analysis (Guava easyCyte HT; EMD Millipore) in
accordance with the manufacturer's protocols. Cellular apoptosis
was detected using the Annexin V Apoptosis Detection Kit APC (cat.
no. 88-8007, Thermo Fisher Scientific, Inc.) on flow cytometry, and
the data were analysed with FlowJo software (version 7.6.1; FlowJo
LLC).
Animal model
The animal experiment was approved (approval no.
20210301-194) and monitored by the Animal Ethics Committee of the
First Affiliated Hospital of Xinjiang Medical University (Urumqi,
China). A total of 20 female, 4- to 6-week-old BALB/c nude mice
weighing 13–15 g were purchased from Charles River Laboratories
(Beijing, China) and raised in a specific pathogen-free (SPF)
environment for the xenograft model. The mice were raised in animal
individually ventilated cage (IVC cages) with room temperature of
24°C and relative humidity of 70%. Mice could drink filtered tap
water and commercial feed ad libitum under a strict 12-h
light/dark cycle. The animal laboratory was cleaned twice one day
and sterilized with ultraviolet light for 1 h every week. The
experimental animals were provided with humane care in accordance
with the institutional animal care guidelines (17). Humane endpoints were in place where
animals would be sacrificed if they had lost 20% weight or
exhibited 10% weight loss alongside hypotrichosis, anorexia or
decreased vitality decreases.
Antibody array analysis
The expression of apoptosis-related proteins was
detected in cell lysates from DPP3-depleted cells and controls
using a Human Apoptosis Antibody Array kit (cat. no. ab134001;
Abcam) that can detect 43 apoptosis-related proteins of human
origin. The pixel densities of each protein spot were determined by
using ImageJ software (v1.8.0; National Institutes of Health).
Statistical analysis
Statistical analyses were performed using SPSS
(version 21.0; IBM Corp.) and GraphPad Prism (version 8.0.1;
GraphPad Software Inc.) software. Data were presented as the mean ±
standard deviation (SD). Chi-squared test and the Mann-Whitney U
test was used to analyse the differences between the two groups and
multiple groups. Patient survival was analysed using Kaplan-Meier
survival analysis and the log-rank test. The Spearman correlation
method was used to calculate the correlation between DPP3 and lymph
node metastasis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical significance of DPP3
expression in oesophageal carcinogenesis
To evaluate the role of DPP3 expression during the
development of oesophageal carcinoma, tumour tissues and their
adjacent normal tissues from the tumour periphery were analysed by
IHC. The data showed strong DPP3 staining in the cytoplasm and
nucleus of EC cells and weak expression in most of the controls
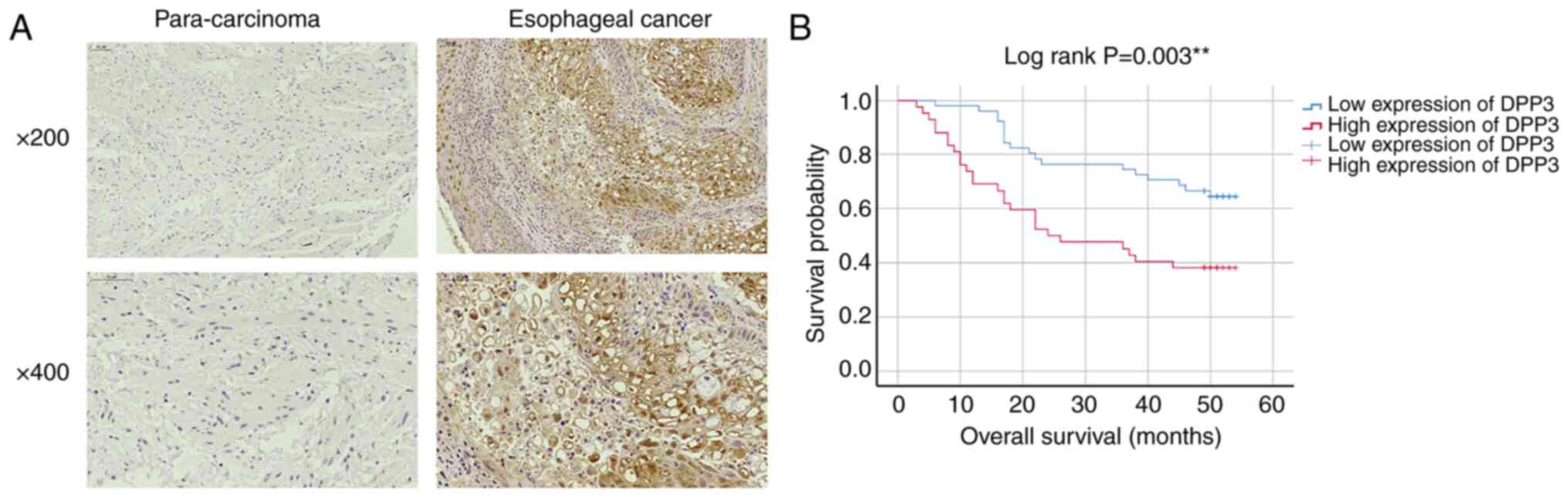
(Fig. 1A). Statistical analysis
confirmed a significantly higher DPP3 expression level in tumour
tissues than in normal control tissues (Table I; P<0.05). In addition, DPP3
expression was positively correlated with lymph node metastasis and
decreased overall survival (Tables
II and III and Fig. 1B; P<0.05), but no difference was
found for age, sex, tumour size, T infiltrate, differential stage,
lymphoid positive number or grade (P>0.05).
 | Table I.Analysis of DPP3 expression in
oesophageal cancer by immunohistochemistry. |
Table I.
Analysis of DPP3 expression in
oesophageal cancer by immunohistochemistry.
| Expression level of
DPP3 expression | Tumor specimens
(%) | Normal controls
(%) | P-value |
|---|
| Weak | 51 (54.8) | 83 (89.2) | <0.001 |
| Strong | 42 (45.2) | 10 (10.8) |
|
 | Table III.Relationship between expression of
dipeptidyl peptidase III and lymph node metastasis in patients with
oesophagus cancer analyzed by spearman rank correlation
analysis. |
Table III.
Relationship between expression of
dipeptidyl peptidase III and lymph node metastasis in patients with
oesophagus cancer analyzed by spearman rank correlation
analysis.
| Tumour
characteristics | Index |
|---|
| Lymph node | Spearman
correlation | 0.215 |
| metastasis (N) | Significance
(double-tail) | 0.038 |
|
| N | 93 |
Depletion of DPP3 in EC cells by
lentiviral expression of shRNA targeting DPP3 mRNA
DPP3 had a relatively higher expression level in
Eca-109 and TE-1 cells compared with the other four EC cell lines,
KYSE450, Eca-109, TE-1 and EC9706, among which Eca-109 and TE-1
were chosen for subsequent investigations (Fig. 2A). A total of 3 shRNA fragments
targeting DPP3 mRNA were then screened for the efficiency of DPP3
depletion, and an almost identical pattern and level of DPP3
depletion was confirmed by RT-qPCR, among which DPP3-shRNA-3 was
used in the follow-up experiments (Fig. 2B). The transfection efficiency was
confirmed by detection of the GFP simultaneously expressed by the
shDPP3 construct that contains shRNA targeting DPP3 mRNA and the
shCtrl as normal control (Fig.
2C). RT-qPCR and western blot analyses demonstrated a
significant decrease in DPP3 expression after shRNA transfection in
Eca-109 and TE-1 cells (Fig. 2D and
E).
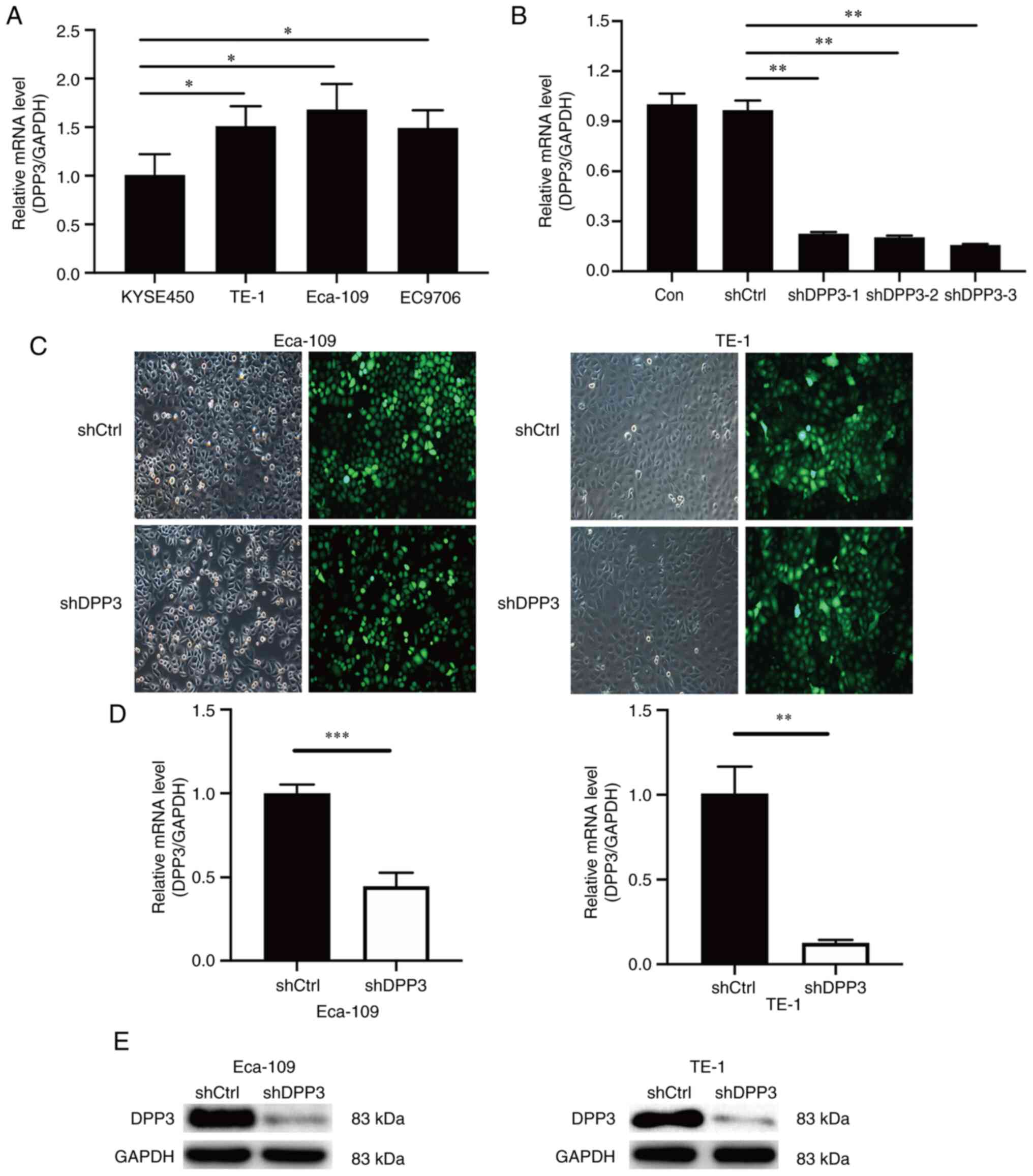 | Figure 2.Effect of DPP3 depletion by
lentiviral expression of shRNA targeting DPP3 mRNA in Eca109 and
TE-1 EC cells. (A) Baseline expression of DPP3 in Eca-109, KYSE450,
EC9706 and TE-1 EC cells measured by RT-qPCR. (B) RT-qPCR detection
of DPP3 depletion by lentiviral expression of shRNAs, shDPP3-1,
shDPP3-2 and shDPP3-3, targeting DPP3 mRNA. (C) Assessment of
lentiviral infection by microscopic detection of green fluorescent
protein simultaneously expressed with shRNA in Eca-109 and TE-1
cells. (D and E) Detection of DPP3 mRNA and protein expression by
(D) RT-qPCR analyses and (E) western blotting, respectively, after
DPP3 depletion in Eca-109 and TE-1 cells. Data are presented as the
mean ± SD of 3 independent experiments performed in triplicate.
*P<0.05, **P<0.01 and ***P<0.01. DPP3, dipeptidyl
peptidase III; shRNA, short hairpin RNA; EC, oesophageal cancer;
RT-qPCR, reverse transcription-quantitative PCR. |
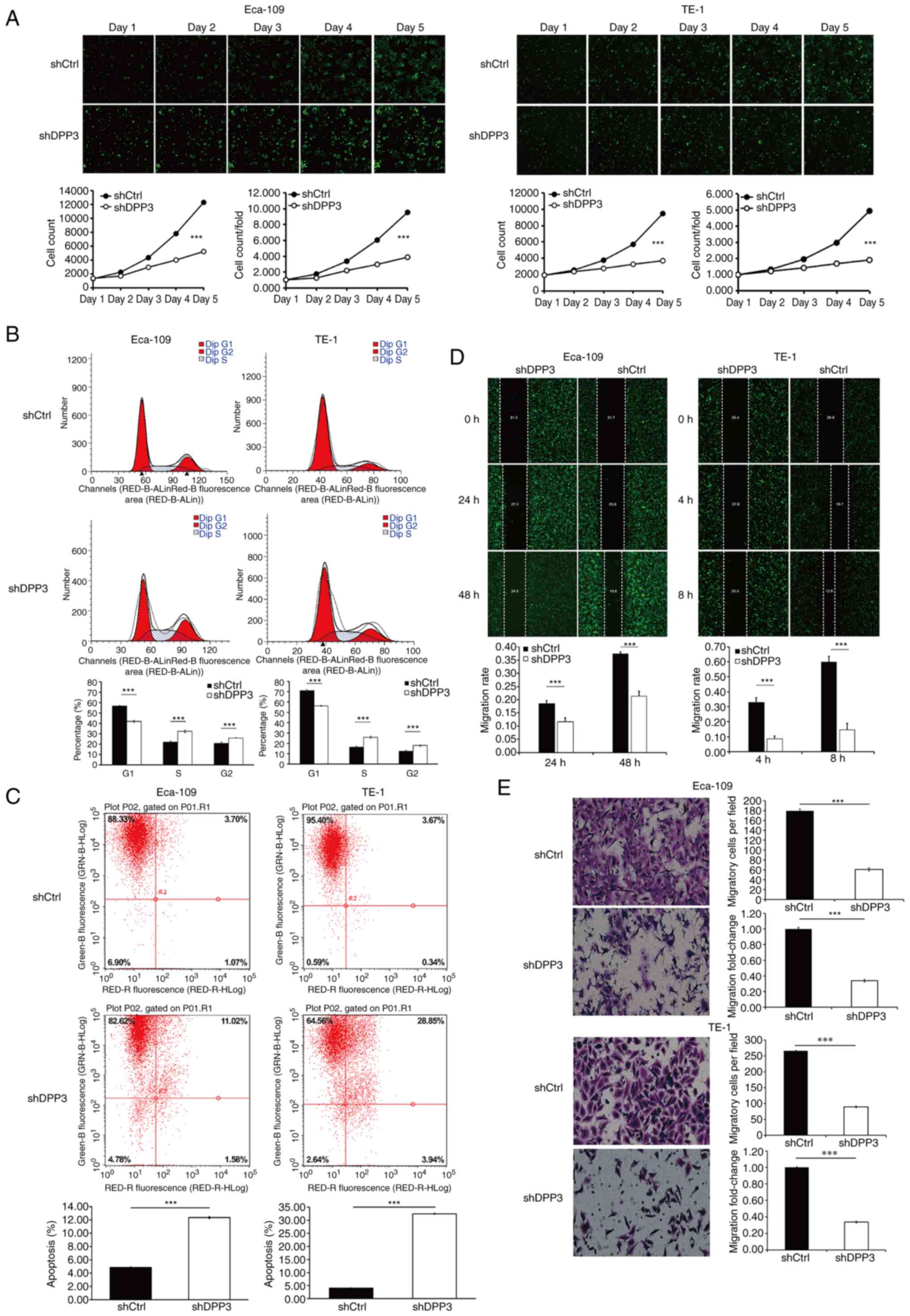
Impact of DPP3 depletion on the
cellular function of EC
Fluorescence microscopy, flow cytometry, wound
healing assays and Transwell assays were applied to detect changes
in cellular function after expression of the shRNA targeting DPP3
mRNA in Eca109 and TE-1 EC cells (Fig.
3). The data showed a remarkable decrease in cell proliferation
and cell cycle retention in the S and G2 phases and increased
apoptosis as well as the inhibition of cell migration after
depletion of DPP3 in EC cells.
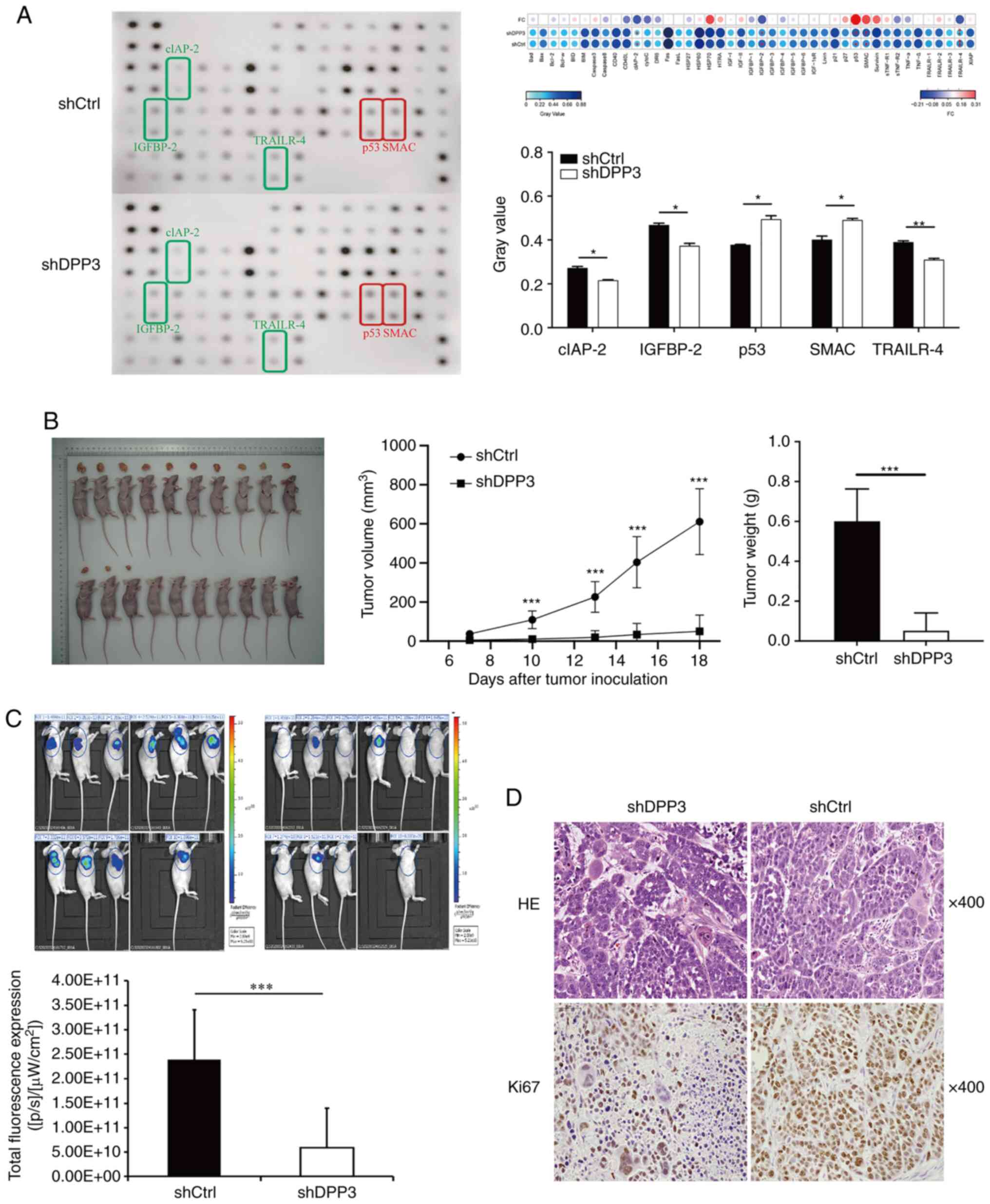
Changes in the protein expression
profile related to apoptosis after DPP3 depletion
As aforementioned, cellular apoptosis was
significantly increased in EC cells after depletion of DPP3
expression. Accordingly, the protein expression profile associated
with apoptosis was detected using an antibody array recognizing 43
human proteins functionally related to apoptosis signalling. The
data demonstrated an increase in the expression of p53 and SMAC,
which are proapoptotic proteins, and a decrease in clAP-2, IGFBP-2
and TRAILR-4, which are antiapoptotic proteins (Fig. 4A).
Impact of DPP3 depletion on the growth
of tumour xenografts from EC cells
The role of DPP3 expression in tumorigenesis in
vivo was studied by analyses of tumour xenografts generated by
intracutaneous injection of nude mice with Eca109 EC cells before
and after DPP3 depletion (shCtrl vs. shDPP3). DPP3 depletion
resulted in a rapid decrease in tumour weight, tumour growth,
tumour size and fluorescent expression in animals injected with
DPP3-depleted EC cells compared with controls (Fig. 4B and C). IHC analysis confirmed a
reduced expression of Ki-67 protein in tumour xenografts from
DPP3-depleted EC cells compared with controls (Fig. 4D). These results suggested that
DPP3 depletion in EC cells may not only inhibit cell proliferation
and promote apoptosis in vitro but also inhibit tumour
growth in vivo.
Discussion
Mammalian dipeptidyl peptidases (DPPs) consist of 8
members, DPP1 (cathepsin-C), DPP2 (DPP7), DPP3, DPP4 (CD26), DPP6,
DPP8, DPP9, and DPP10, and play a role in oligopeptide N-terminal
processing and degradation of bioactive peptides (18). However, the roles of most DPPs
family members in physiological functions and pathological
conditions remain largely unclear (19). The present study, to the best of
our knowledge, is the first to clarify the role of DPP3 in EC.
Knockdown of DPP3 gene expression inhibited cell proliferation, and
an increase in the number of floating cells was observed in the
culture medium of EC cells. Subsequent studies showed that the
decreased expression of DPP3 played a key role in cell apoptosis.
The present results are consistent with existing studies. Recent
studies have reported that DPP3 is associated with apoptosis in
colon cancer cells and hippocampal neurons (20,21).
DPP3 belongs to the DPPs family, among which DPP4 has been the most
reported. A recent study reported that silencing DPP4 can inhibit
cell proliferation and enhance cell apoptosis (22). Therefore, considering the present
study, the effect of apoptosis may be most important after DPP
reduction. Nevertheless, certain studies have reported that DPP8/9
inhibitors can induce pyroptosis in cell experiments (23–25).
Ferroptosis can be inhibited by blocking DPP4 activity (26). However, as derived from the present
study and previous studies, the effect of apoptosis after DPPs
reduction may be the most important (19).
DPP3 is a zinc-dependent metallopeptidase. Unlike
other metallopeptidases, DPP3 harbours a similar but unique
catalytic motif, HEXXGH. The two His residues of this motif
contribute to coordinate Zn2+ ion binding (27). Multiple studies have confirmed the
role of Zn2+ in the catalytic activity of DPP III, where
it binds in a 1:1 stoichiometry (DPPIII: Zn2+). A marked
increase in its activity with sexual maturity (28), histological aggressiveness of the
tumour (13), and an increase
(11.6-fold) in human retroplacental serum compared with control
serum (29) have been observed.
The significant upregulation of DPP3 in EC and its association with
malignant behaviours such as lymph node metastasis were
demonstrated. This is consistent with previous reports on the
elevation of DPP3 in several human cancers, particularly in
aggressive ovarian and endometrial cancers (12,13).
Similarly, as a matrix metallopeptidase (MMPs) in the
metallopeptidase family, they are characterized by their ability to
degrade the extracellular matrix and their dependence upon
Zn2+ binding for proteolytic activity. The imbalance
between MMPs, particularly MMP-2 and MMP-9, as well as their
inhibitors, TIMP-1 and TIMP-2, may facilitate tumour progression
(30). Most of these peptidases
require Zn2+ for their catalytic activity and, with
increasing activity (within certain limits), may increase tumour
aggressiveness.
DPP3 is mainly localized in the cytosol, but a few
studies have found its membranous activity, which is consistent
with our IHC results (31,32). The present results suggested that
high DPP3 expression is significantly associated with poor
prognosis, and it has been reported that DPP3 is overexpressed in
ER-positive breast cancer and associated with poor survival
(33). DPP3 has substrate
specificity for several bioactive peptides (19). Degradation by DPP3 may prevent
peptide leakage from necrotic cells and block antigen presentation
to the immune system, which can be utilized by tumour cells for
immune evasion (34). Thus, this
may provide evidence for the role of DPP3 expression in the
survival and metastasis of oesophageal carcinoma.
Using analyses of DPP3-depleted EC cells and tumour
xenografts, it was showed that DPP3 elevation had an impact on cell
functions during oesophageal carcinogenesis, including the
inhibition of cell cycle arrest and apoptosis, the increase in cell
proliferation and migration in vitro, and the promotion of
tumour growth and survival in vivo. This may further explain
why DPP3 is involved in the progression and development of EC and
has oncogene-like features. The upregulation of DPP3 expression is
positively correlated with increased expression of NRF2 in lung
cancer, indicating a possible link between DPP3 and NRF2 in
malignant tumours (11). A few
studies have demonstrated oxidative stress-induced binding of DPP3
to Keap1 that prevents Keap1-mediated degradation of NRF2, leading
to increased NRF2 nuclear translocation (10,11,35).
DPP3 elevation may result in increased NRF2 downstream expression
of cytoprotective genes associated with aggressive cancer
phenotypes (11,35). This may contribute to increased
cell proliferation and migration and decreased cell cycle control
and apoptosis of EC cells in vitro, leading to tumour growth
and progression in vivo.
Antibody array analysis revealed that DPP3 depletion
may cause the downregulation of antiapoptotic proteins such as
clAP-2, IGFBP-2, and TRAILR-4 and the upregulation of the
proapoptotic proteins P53 and SMAC. Apoptosis is a tightly
regulated cellular process, and faulty regulation of apoptosis is a
hallmark of human cancers. TNF-related apoptosis-inducing ligand
(TRAIL) is a type II transmembrane protein that binds its receptors
TRAIL-R1 and TRAIL-R2, which in turn recruit downstream adaptor
proteins via their intracellular death domain (DD) and activate the
intrinsic apoptotic pathway, triggering selective neoplastic cell
apoptosis. This is regulated by non-functional TRAIL receptors,
TRAIL-R3 and TRAIL-R4, which are devoid of a cytoplasmic tail or
carry a truncated intracellular DD, respectively, and block
TRAIL-mediated apoptosis (36).
TRAILR4 can also induce non-apoptotic signalling mediated by the
NF-kB and AKT pathways that is correlated with its overexpression
in malignant tumour phenotypes (37,38).
In human cancer cells, the TRAIL-R4 expression level is also
positively correlated with TRAIL resistance, and its downregulation
leads to reduced tumorigenic potential or apoptosis (39,40).
Cellular inhibitor of apoptosis (cIAP2) plays a role in degrading
caspases by linking them to ubiquitin molecules and supports cell
survival by preventing cellular apoptosis of cancer cells (41). cIAP-2 is upregulated in malignant
tumours and promotes the proliferation and invasion of tumour cells
through the activation of the NF-κB signalling pathway (42). Second mitochondria-derived
activator of caspases (SMAC) is an endogenous antagonist of cIAP1,
cAIP2 and XIAP and promotes apoptosis by binding IAPs and
preventing the inhibition of caspases 3, 7 and 9 (43). SMAC is normally sequestered within
the mitochondria and is released into the cytoplasm upon cell death
stimuli, thereby overcoming anti-apoptotic actions caused by the
IAPs (44). In line with the
pro-apoptotic role of SMAC, its expression is downregulated in EC
cells and tumour specimens from patients with EC (45). Insulin-like growth factor binding
protein 2 (IGFBP-2) is a member of the IGF system and apoptosis
suppressor (46). IGFBP-2 is
elevated in tumour specimens from patients and plays a role in
tumour cell proliferation, migration, invasion, angiogenesis, and
epithelial to mesenchymal transition by integrating a series of
signalling pathways (47). Nuclear
IGFBP-2 itself functions as a tumour enhancer by directly targeting
multiple oncogene promoters (48).
P53 is a well-known tumour suppressor that negatively regulates
cell proliferation and promotes cell differentiation by inducing
cell cycle arrest and apoptosis (49). These studies provided evidence for
the role of DPP3 in the upregulation of the antiapoptotic proteins
clAP-2, IGFBP-2 and TRAILR-4 and the downregulation of the
proapoptotic proteins SMAC and p53, which contribute to tumour
initiation and progression.
There are certain limitations to the present study
that should be taken into consideration when interpreting the
results. The population included in the clinical study was
recruited from a single centre, and there may be more effective
genes to prevent EC. The present study focused on apoptosis and did
not explore other cell death modes. In addition, the effect of DPP3
as an enzyme on tumour cell activity was not further verified.
Therefore, further basic studies are needed to verify the function
of DPP3 in EC.
In conclusion, DPP3 expression was significantly
upregulated in EC tissues compared with adjacent non-tumour
tissues. In addition, high DPP3 expression was significantly
correlated with poor prognosis. In cultured cells with DPP3
depletion, fewer cells showed cellular proliferation and migration.
By contrast, more cells underwent cell cycle arrest and apoptosis.
Consistently, tumour growth and invasion were inhibited in a
xenograft model of DPP3 depletion. Mechanistically, DPP3 depletion
was associated with the upregulation of proapoptotic proteins and
the downregulation of antiapoptotic proteins. In conclusion, DPP3
is involved in the progression and development of EC and is a
potential prognostic and therapeutic target for EC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82260471), the Xinjiang Uygur
Autonomous Region Graduate Scientific Research Innovation Project
(grant no. XJ2022G154), and the State Key Laboratory of
Pathogenesis, Prevention and Treatment of High Incidence Diseases
in Central Asia Fund (grant no. SKL-HIDCA-2022-SG4).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
J-KL and AA performed most experiments and wrote the
manuscript. H-TY carried out the data collection and analysis. L-XX
participated in the in vivo study. AT and YN participated in
the in vitro study. GB performed certain of the experiments
in this study, drafted the work and revised it critically for
important intellectual content, as well as provided experimental
technical support. ME designed the overall study, supervised the
experiments, and analysed the results. J-KL, AA and ME confirm the
authenticity of all the raw data. All authors commented on previous
versions of the manuscript. All authors read and approved the final
version of the manuscript and agree to take responsibility and be
accountable for the contents of the article.
Ethics approval and consent to
participate
Human tissue studies and animal experiments in the
present study were approved (approval nos. 2210226-138 and
20210301-194, respectively) and monitored by the Ethics Committee
of the First Affiliated Hospital of Xinjiang Medical University
(Urumqi, China). Human tissue studies were conducted in accordance
with the 1964 Helsinki Declaration and its later amendments of
comparable ethical standards. Informed consent was obtained from
all patients or their legal guardian, and the data were analysed
anonymously throughout the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu K, Zhao T, Wang J, Chen Y, Zhang R,
Lan X and Que J: Etiology, cancer stem cells and potential
diagnostic biomarkers for esophageal cancer. Cancer Lett.
458:21–28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Y, Yin D, Li L, Deng YC and Tian W:
Screening aberrant methylation profile in esophageal squamous cell
carcinoma for Kazakhs in Xinjiang area of China. Mol Biol Rep.
42:457–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang VE, Grandis JR and Ko AH: New
strategies in esophageal carcinoma: Translational insights from
signaling pathway and immune checkpoints. Clin Cancer Res.
22:4283–4290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lowther WT and Matthews BW:
Metalloaminopeptidases: Common functional themes in disparate
structural surroundings. Chem Rev. 102:4581–4608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saghatelian A, Jessani N, Joseph A,
Humphrey M and Cravatt BF: Activity-based probes for the proteomic
profiling of metalloproteases. Proc Natl Acad Sci USA.
101:10000–10005. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cerdà-Costa N and Gomis-Rüth FX:
Architecture and function of metallopeptidase catalytic domains.
Protein Sci. 23:123–144. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Menale C, Robinson LJ, Palagano E, Rigoni
R, Erreni M, Almarza AJ, Strina D, Mantero S, Lizier M, Forlino A,
et al: Absence of dipeptidyl peptidase 3 increases oxidative stress
and causes bone loss. J Bone Miner Res. 34:2133–2148. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jha S, Taschler U, Domenig O, Poglitsch M,
Bourgeois B, Pollheimer M, Pusch LM, Malovan G, Frank S, Madl T, et
al: Dipeptidyl peptidase 3 modulates the renin-angiotensin system
in mice. J Biol Chem. 295:13711–13723. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu K, Alcivar AL, Ma J, Foo TK, Zywea S,
Mahdi A, Huo Y, Kensler TW, Gatza ML and Xia B: NRF2 induction
supporting breast cancer cell survival is enabled by oxidative
stress-induced DPP3-KEAP1 interaction. Cancer Res. 77:2881–2892.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hast BE, Goldfarb D, Mulvaney KM, Hast MA,
Siesser PF, Yan F, Hayes DN and Major MB: Proteomic analysis of
ubiquitin ligase KEAP1 reveals associated proteins that inhibit
NRF2 ubiquitination. Cancer Res. 73:2199–2210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simaga S, Babić D, Osmak M, Ilić-Forko J,
Vitale L, Milicić D and Abramić M: Dipeptidyl peptidase III in
malignant and non-malignant gynaecological tissue. Eur J Cancer.
34:399–405. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simaga S, Babić D, Osmak M, Sprem M and
Abramić M: Tumor cytosol dipeptidyl peptidase III activity is
increased with histological aggressiveness of ovarian primary
carcinomas. Gynecol Oncol. 91:194–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh R, Sharma MC, Sarkar C, Singh M and
Chauhan SS: Transcription factor C/EBP-β mediates downregulation of
dipeptidyl-peptidase III expression by interleukin-6 in human
glioblastoma cells. FEBS J. 281:1629–1641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prajapati SC and Chauhan SS: Human
dipeptidyl peptidase III mRNA variant I and II are expressed
concurrently in multiple tumor derived cell lines and translated at
comparable efficiency in vitro. Mol Biol Rep. 43:457–462. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Couto M and Cates C: Laboratory guidelines
for animal care. Methods Mol Biol. 1920:407–430. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mulvihill EE and Drucker DJ: Pharmacology,
physiology, and mechanisms of action of dipeptidyl peptidase-4
inhibitors. Endocr Rev. 35:992–1019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sato A and Ogita H: Pathophysiological
implications of dipeptidyl peptidases. Curr Protein Pept Sci.
18:843–849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tong Y, Huang Y, Zhang Y, Zeng X, Yan M,
Xia Z and Lai D: DPP3/CDK1 contributes to the progression of
colorectal cancer through regulating cell proliferation, cell
apoptosis, and cell migration. Cell Death Dis. 12:5292021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren X, Yu J, Guo L and Ma H:
Dipeptidyl-peptidase 3 protects oxygen-glucose
deprivation/reoxygenation-injured hippocampal neurons by
suppressing apoptosis, oxidative stress and inflammation via
modulation of Keap1/Nrf2 signaling. Int Immunopharmacol.
96:1075952021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu X, Chen S, Xie C, Li Z, Wu Z and You Z:
DPP4 gene silencing inhibits proliferation and
epithelial-mesenchymal transition of papillary thyroid carcinoma
cells through suppression of the MAPK pathway. J Endocrinol Invest.
44:1609–1623. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohnson DC, Taabazuing CY, Okondo MC, Chui
AJ, Rao SD, Brown FC, Reed C, Peguero E, de Stanchina E, Kentsis A
and Bachovchin DA: DPP8/DPP9 inhibitor-induced pyroptosis for
treatment of acute myeloid leukemia. Nat Med. 24:1151–1156. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taabazuing CY, Okondo MC and Bachovchin
DA: Pyroptosis and apoptosis pathways engage in bidirectional
crosstalk in monocytes and macrophages. Cell Chem Biol.
24:507–514.e4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cui C, Tian X, Wei L, Wang Y, Wang K and
Fu R: New insights into the role of dipeptidyl peptidase 8 and
dipeptidyl peptidase 9 and their inhibitors. Front Pharmacol.
13:10028712022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J,
Zhong M, Yuan H, Zhang L, Billiar TR, et al: The tumor suppressor
p53 limits ferroptosis by blocking DPP4 activity. Cell Rep.
20:1692–1704. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Prajapati SC and Chauhan SS: Dipeptidyl
peptidase III: A multifaceted oligopeptide N-end cutter. FEBS J.
278:3256–3276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vanha-Perttula T: Dipeptidyl peptidase III
and alanyl aminopeptidase in the human seminal plasma: Origin and
biochemical properties. Clin Chim Acta. 177:179–195. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimamori Y, Watanabe Y and Fujimoto Y:
Purification and characterization of dipeptidyl aminopeptidase III
from human placenta. Chem Pharm Bull (Tokyo). 34:3333–3340. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Groblewska M, Siewko M, Mroczko B and
Szmitkowski M: The role of matrix metalloproteinases (MMPs) and
their inhibitors (TIMPs) in the development of esophageal cancer.
Folia Histochem Cytobiol. 50:12–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hashimoto J, Yamamoto Y, Kurosawa H,
Nishimura K and Hazato T: Identification of dipeptidyl peptidase
III in human neutrophils. Biochem Biophys Res Commun. 273:393–397.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mazzocco C, Fukasawa KM, Raymond AA and
Puiroux J: Purification, partial sequencing and characterization of
an insect membrane dipeptidyl aminopeptidase that degrades the
insect neuropeptide proctolin. Eur J Biochem. 268:4940–4949. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choy TK, Wang CY, Phan NN, Khoa Ta HD,
Anuraga G, Liu YH, Wu YF, Lee KH, Chuang JY and Kao TJ:
Identification of dipeptidyl peptidase (DPP) family genes in
clinical breast cancer patients via an integrated bioinformatics
approach. Diagnostics (Basel). 11:12042021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gamrekelashvili J, Kapanadze T, Han M,
Wissing J, Ma C, Jaensch L, Manns MP, Armstrong T, Jaffee E, White
AO, et al: Peptidases released by necrotic cells control CD8+ T
cell cross-priming. J Clin Invest. 123:4755–4768. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Kern JT, Walker JR, Johnson JA,
Schultz PG and Luesch H: A genomic screen for activators of the
antioxidant response element. Proc. Natl Acad Sci USA.
104:5205–5210. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sanlioglu AD, Korcum AF, Pestereli E,
Erdogan G, Karaveli S, Savas B, Griffith TS and Sanlioglu S: TRAIL
death receptor-4 expression positively correlates with the tumor
grade in breast cancer patients with invasive ductal carcinoma. Int
J Radiat Oncol Biol Phys. 69:716–723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
von Karstedt S, Montinaro A and Walczak H:
Exploring the TRAILs less travelled: TRAIL in cancer biology and
therapy. Nat Rev Cancer. 17:352–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sanlioglu AD, Dirice E, Elpek O, Korcum
AF, Ozdogan M, Suleymanlar I, Balci MK, Griffith TS and Sanlioglu
S: High TRAIL death receptor 4 and decoy receptor 2 expression
correlates with significant cell death in pancreatic ductal
adenocarcinoma patients. Pancreas. 38:154–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lalaoui N, Morlé A, Mérino D, Jacquemin G,
Iessi E, Morizot A, Shirley S, Robert B, Solary E, Garrido C and
Micheau O: TRAIL-R4 promotes tumor growth and resistance to
apoptosis in cervical carcinoma HeLa cells through AKT. PLoS One.
6:e196792011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sanlioglu AD, Dirice E, Aydin C, Erin N,
Koksoy S and Sanlioglu S: Surface TRAIL decoy receptor-4 expression
is correlated with TRAIL resistance in MCF7 breast cancer cells.
BMC Cancer. 5:542005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Labbé K, McIntire CR, Doiron K, Leblanc PM
and Saleh M: Cellular inhibitors of apoptosis proteins cIAP1 and
cIAP2 are required for efficient caspase-1 activation by the
inflammasome. Immunity. 35:897–907. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang XJ, Chen ZW, Zhao JF, Liao CX, Cai
QH and Lin J: cIAP2 via NF-κB signalling affects cell proliferation
and invasion in hepatocellular carcinoma. Life Sci. 266:1188672021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morrish E, Brumatti G and Silke J: Future
therapeutic directions for smac-mimetics. Cells. 9:4062020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Beug ST, Conrad DP, Alain T, Korneluk RG
and Lacasse EC: Combinatorial cancer immunotherapy strategies with
proapoptotic small-molecule IAP antagonists. Int J Dev Biol.
59:141–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu Y, Zhou L, Huang J, Liu F, Yu J, Zhan
Q, Zhang L and Zhao X: Role of Smac in determining the
chemotherapeutic response of esophageal squamous cell carcinoma.
Clin Cancer Res. 17:5412–5422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Russo VC, Azar WJ, Yau SW, Sabin MA and
Werther GA: IGFBP-2: The dark horse in metabolism and cancer.
Cytokine Growth Factor Rev. 26:329–346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yau SW, Azar WJ, Sabin MA, Werther GA and
Russo VC: IGFBP-2-taking the lead in growth, metabolism and cancer.
J Cell Commun Signal. 9:125–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li T, Forbes ME, Fuller GN, Li J, Yang X
and Zhang W: IGFBP2: Integrative hub of developmental and oncogenic
signaling network. Oncogene. 39:2243–2257. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rodriguez J, Herrero A, Li S, Rauch N,
Quintanilla A, Wynne K, Krstic A, Acosta JC, Taylor C, Schlisio S
and von Kriegsheim A: PHD3 regulates p53 protein stability by
hydroxylating proline 359. Cell Rep. 24:1316–1329. 2018. View Article : Google Scholar : PubMed/NCBI
|