Introduction
Ovarian cancer is generally diagnosed at an advanced
stage, and is thus the leading cause of mortality in women
diagnosed with gynecologic cancer (1). Despite the progress in systemic
therapies in the past few years, the long-term prognosis of
patients with ovarian cancer remains poor. Understanding the
molecular mechanisms that are involved in ovarian cancer
progression may lead to the development of more effective cancer
treatments.
Aberrant lncRNA expression has been identified in
numerous types of diseases, including cancer (2). Long noncoding RNAs (lncRNAs) are
transcripts of >200 nucleotides without protein-coding functions
(2). LncRNAs play a role in
regulating cancer cell proliferation, differentiation, invasion,
and metastasis (3,4). Additionally, epithelial-mesenchymal
transition (EMT) has been increasingly investigated in various
types of cancer. Epithelial cells lose apical-basal polarity,
cell-cell adhesion, and acquire migration properties to transform
into invasive mesenchymal cells during EMT (5,6).
However, it remains unclear whether EMT is involved in the
lncRNA-mediated progression of epithelial ovarian cancer (EOC).
HOXA cluster antisense RNA 3 (HOXA-AS3) belongs to
the family of homeobox (HOX) genes characterized by the presence of
highly conserved homeodomains, which are essential for embryonic
development and tumorigenesis. Several studies have investigated
the role of HOXA-AS3 in the carcinogenesis of gliomas and lung
cancers (7–9). Although HOXA-AS3 has been demonstrated
to be associated with the progression of other types of cancer,
little is known about its involvement in the molecular pathways of
ovarian cancer cells.
In the present study, the role and underlying
mechanisms of lncRNAs in ovarian cancer were investigated with a
focus on HOXA-AS3, which is highly expressed in ovarian cancer.
Materials and methods
Patient specimens
Ovarian cancer tissue and matched benign tissue
specimens were collected from 130 patients who underwent surgery at
the Department of Obstetrics and Gynecology, Severance Hospital
(Seoul, Korea), between January 2005 and December 2017. The
inclusion criteria was as follows: i) Aged ≥19 years; ii)
pathologically confirmed EOC; and iii) underwent primary treatment,
either primary debulking surgery followed by platinum-based
postoperative adjuvant chemotherapy or platinum-based neoadjuvant
chemotherapy followed by interval debulking surgery and
postoperative adjuvant chemotherapy. In addition, patients were
excluded from the present study, according to the following
criteria: i) Were immunocompromised or pregnant; ii) did not
receive platinum-based combination chemotherapy; iii) had
synchronous double primary cancers; and iv) were lost to follow-up
before reaching six months of progression-free survival (PFS)
without evidence of disease recurrence. The clinicopathological
characteristics of the patients (age range, 32–78 years; mean age,
51±12.4 years) are presented in Table
I. All tissue samples were immediately frozen in liquid
nitrogen and transferred to a −80°C deep freezer. Patient follow-up
information and survival were determined based on the medical
records. The study was approved (approval ethics code 4-2021-1394)
by the Institutional Review Board of Severance Hospital, Yonsei
University College of Medicine. Individual patient consent was
waived for the present study because it was a retrospective study
which involved no risk to the subjects.
 | Table I.HOXA-AS3 expression and
clinicopathological characteristics of patients with ovarian
cancer. |
Table I.
HOXA-AS3 expression and
clinicopathological characteristics of patients with ovarian
cancer.
|
|
| HOXA-AS3
expression |
|
|---|
|
|
|
|
|
|---|
| Parameters | Total (n=100) | Low (n=50) | High (n=50) | P-value |
|---|
| Age (years), mean ±
SD | 51.3±12.4 | 52.1±10.1 | 50.4±11.7 | 0.617 |
| Stage, n (%) |
|
|
|
|
| I | 21 (21.0) | 16 (32.0) | 5 (10.0) | 0.05 |
| II | 16 (16.0) | 11 (22.0) | 5 (10.0) |
|
|
III | 48 (48.0) | 18 (36.0) | 30 (60.0) |
|
| IV | 15 (15.0) | 5 (10.0) | 10 (20.0) |
|
| Grade, n (%) |
|
|
|
|
| 1 | 13 (13.0) | 9 (18.0) | 4 (8.0) | 0.323 |
| 2 | 35 (35.0) | 17 (34.0) | 18 (36.0) |
|
| 3 | 52 (52.0) | 24 (48.0) | 28 (56.0) |
|
| Histological type,
n (%) |
|
|
|
|
|
Serous | 64 (64.0) | 31 (62.0) | 33 (66.0) | 0.710 |
|
Endometrioid | 15 (15.0) | 6 (12.0) | 9 (18.0) |
|
| Clear
cell | 14 (14.0) | 9 (18.0) | 5 (10.0) |
|
|
Mucinous | 4 (4.0) | 2 (4.0) | 2 (4.0) |
|
|
Others | 3 (3.0) | 2 (4.0) | 1 (2.0) |
|
| Lymph node
metastasis, n (%) | 42 (42.0) | 15 (30.0) | 27 (54.0) | 0.015 |
| Recurrence, n
(%) | 58 (58.0) | 23 (46.0) | 35 (70.0) | 0.015 |
| CA 125 ≥200, n
(%) | 68 (68.0) | 31 (62.0) | 37 (74.0) | 0.198 |
Cell culture
Human Ovarian Surface Epithelial Cells (cat. no.
7310; Sciencell Research Laboratories, Inc.) were cultured using
OepiCM, which consisted of basal medium, 5 ml of Ovarian Epithelial
Cell Growth Supplement (OEpiCGS; cat. no. 7352) and 1%
penicillin/streptomycin solution (cat. no. 0503), and incubated at
37°C in a humidified atmosphere containing 5% CO2. Human
EOC cell line A2780 (cat. no. 93112519) was purchased from the
European Collection of Cell Cultures (ECACC; Sigma-Aldrich; Merck
KGaA) and cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS) and 1% penicillin/streptomycin (pen/strep; all
from Gibco; Thermo Fisher Scientific, Inc.). OVCA429 and OVCA433
were provided by the Korea Gynecologic Cancer Bank through the Bio
and Medical Technology Development Program of the Ministry of
Science of Korea, Information and Communication Technology and
Future Planning (10,11). These cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM; Welgene, Inc.) containing
10% FBS and 1% pen/strep in an incubator at 37°C with 5%
CO2.
Small interfering RNA (siRNA)
transfection
siRNAs targeting HOXA-AS3 (si-HOXA-AS3-651 sense,
5′-UCUAUUCUCCAAGGGAAATT-3′ and antisense,
5′-UUUCCCUUGCGAGAAUAGATT-3′; si-HOXA-AS3-728 sense,
5′-GGGCCGAACAACUCAUAAATT-3′and antisense,
5′-UUUAUGAGUUGUUCGGCCCTT-3′; si-HOXA-AS3-3507 sense,
5′-GCACAGAAUCUCAACUUUATT-3′ and antisense,
5′-UAAAGUUGAGAUUCUGUGCTT-3′; all from Bioneer Corporation), HOXA3
(sense, 5′-GGUAGAUUCAUAGAAUAUAAC-3′ and antisense,
5′-GUUAUAUUCUAUGAAUCUACC-3′; cat. no. sc-38675; Santa Cruz
Biotechnology, Inc.) and negative control siRNA (siNC; cat. no.
SN-1011; Bioneer Corporation) were used. The target sequence for
HOXA-AS3 was as follows: 5′-GGUAGAUUCAUAGAAUAUAAC-3′. Both OVCA429
and OVCA433 cells were cultured to 80% confluency, and the
transfection was performed at a concentration of 30 nM of siRNAs in
6-well plates at 1×105 cells/well using Lipofectamine™
3000 Transfection Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) in Opti-MEM I Reduced Serum Medium (Gibco; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions at
37°C for 48 h. The time interval between transfection and
subsequent experimentation was 48 h.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cancerous and
non-cancerous specimens and cell lines using TRIzol®
reagent (cat. no. 15596026; Invitrogen; Thermo Fisher Scientific,
Inc.). RNA concentration and quality were determined using a
NanoDrop ND-2000 spectrophotometer (cat. no. ND-2000; NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). Total RNA (1 µg) was
reverse transcribed into first-strand cDNA using a reverse
transcription reagent kit (TaqMan™ Reverse Transcription Reagents;
cat. no. N8080234; Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. RT-qPCR was carried out
in 96-well blocks with the StepOnePlus™ Real-Time PCR System (cat.
no. 4376600; Applied Biosystems; Thermo Fisher Scientific, Inc.)
using SensiFAST™ SYBR® Hi-ROX (cat. no. BIO-73001;
Meridian Bioscience, Inc.) in a reaction volume of 20 µl. The
following PCR program was used: An initial denaturation at 95°C for
2 min, followed by 40 cycles of 5 sec at 95°C and 60°C for 15 sec.
18S rRNA and GAPDH were used as internal controls. The PCR primer
sequences were as follows: 18S rRNA sense,
5′-GTAACCCGTTGAACCCCATT-3′ and antisense,
5′-CCATCCAATCGGTAGTAGCG-3′; GAPDH sense, 5′-ACCCACTCCTCCACCTTTGA-3′
and antisense, 5′-CTGTTGCTGTAGCCAAATTCGT-3′; HOXA-AS3 sense,
5′-TTCATCCGCTGCATCCAAGG-3′ and antisense,
5′-AGAGAGGTGTCTGAAGCGCT-3′; HOXA3 sense, 5′-CAGCTCATGAAACGGTCTGC-3′
and antisense, 5′-GAGCTGTCGTAGTAGGTCGC-3′; HOXA4 sense,
5′-ATAACGGAGGGGAGCCTAAG-3′ and antisense,
5′-GCTCAGACAAACAGAGCGTG-3′; HOXA5 sense, 5′-CCAGATCTACCCCTGGATGC-3′
and antisense, 5′-ACTTCATTCTCCGGTTTTGGAAC-3′; HOXA6 sense,
5′-AAGCACTCCATGACGAAGGC-3′ and antisense,
5′-GTCTGGTAGCGCGTGTAGGT-3′. Changes in the expression levels were
analyzed using the 2−ΔΔCq method (12).
Cell viability assay
HOXA-AS3 siRNA-transfected cells were seeded into
96-well flat-bottomed plates at a density of 5×103 cells
per well in 200 µl of culture medium. Cell proliferation was
evaluated using the Cell Counting Kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc.) in accordance with the manufacturer's
instructions. The cells were incubated overnight to allow cell
attachment and recovery. Subsequently, the cells were transfected
with siHOXA-AS3 for 24, 48, or 72 h. A total of 20 µl of CCK-8
solution was added to each well of the plate and incubated for an
additional 1 h. The absorbance was measured at 450 nm using a
microplate reader. Three independent experiments were performed in
triplicate.
Colony formation assay
To assess clonogenic ability, cells (OVCA429 and
OVCA433) were incubated for 3 days in 12-well plates at a low
density (5×102 cells per well) in a complete medium for
14 days. The medium was changed to culture medium every 3 days. The
plates were washed with phosphate-buffered saline (PBS), fixed with
ice-cold methanol for 20 min at room temperature, and stained with
0.1% crystal violet (Sigma-Aldrich; Merck KGaA) for 10 min at room
temperature. The total number of colonies were counted manually
using a light microscope (Olympus Corporation), and a colony was
defined as 50 cells or more. Each experiment was performed on three
independent occasions.
Wound healing assay
Cells (OVCA429 and OVCA433) were seeded into 6-well
culture plates with serum-enriched medium and allowed to grow to
90% confluency. The serum-containing medium was removed, and the
cells were serum-starved overnight. When the cells reached 100%
confluence, artificial wounds were created by scratching the
monolayer with a 200-µl pipette tip. After scratching, floating
cells were washed with serum-free medium and replenished with fresh
medium. Wound healing was investigated at 0, 24, and 48 h, and
images were captured using a light microscope (Olympus Corporation)
and quantified using ImageJ software (version 1.8.0_172; National
Institutes of Health). Each experiment was repeated three
times.
Western blotting
Total protein was isolated from ovarian cancer cell
lines (OVCA429 and OVCA433) using radioimmunoprecipitation assay
lysis and extraction buffer (Thermo Fisher Scientific, Inc.), and
protein concentrations were quantified using the Pierce BCA Protein
Assay Kit (Thermo Fisher Scientific, Inc.). Protein samples (20 µg)
were separated on 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis gels and then transferred to polyvinylidene
difluoride membranes (MilliporeSigma). The membranes were blocked
with 5% skimmed milk in 1X Tris-buffered saline and 0.1%
Tween®−20 (TBST) buffer for 1 h at room temperature and
incubated with the primary antibody overnight at 4°C with gentle
rocking. The membrane was washed three times with 1X TBST buffer,
followed by incubation with horseradish peroxidase-conjugated
secondary antibodies (1:5,000; cat. nos. 7074 and 7076; Cell
Signaling Technology, Inc.) for 2 h at room temperature. The
primary antibodies used in this study were as follows: Anti-HOXA3
(1:1,000; cat. no. sc-374237; Santa Cruz Biotechnology, Inc.),
anti-β-catenin (1:1,000; cat. no. 2698S), anti-E-cadherin (1:1,000;
cat. no. 3195), anti-AKT (1:1,000; cat. no. 9272), anti-vimentin
(1:1,000; cat. no. 5741; all Cell Signaling Technology, Inc.), and
β-actin antibody (1:5,000; cat. no. A5316; Sigma-Aldrich; Merck
KGaA). The bands were visualized using an enhanced
chemiluminescence solution (Pierce™ ECL Western Blotting Substrate;
cat. no. 32106; Thermo Scientific™, Inc.) and analyzed using ImageJ
software (version 1.8.0_172; National Institutes of Health).
Immunofluorescence analysis
OVCA429 and OVCA433 cells were cultured at a density
of 5×103 cells per confocal dish (SPL Life Sciences,
Co., Ltd.). After incubation, the dishes were washed with PBS and
fixed in 4% formaldehyde for 20 min at room temperature. The cells
were then rinsed twice with PBS and blocked with blocking buffer
(10% normal donkey serum; cat. no. 017-000-121; Jackson
ImmunoResearch Laboratories, Inc.; and 0.3% Triton X-100) for 45
min at room temperature. The dishes were incubated with a primary
antibody, overnight at 4°C, followed by incubation with the
appropriate secondary fluorescence-labeled antibody for 1 h at room
temperature. Nuclei were counterstained with DAPI (cat. no. D1306;
Invitrogen; Thermo Fisher Scientidic, Inc.) for 5 min at room
temperature. The images were visualized using a confocal microscope
(Zeiss AG). The primary antibodies used in this study were as
follows: Anti-HOXA3 (1:100; cat. no. ab230879; Abcam), anti-tubulin
(1:100; cat. no. 2144; Cell Signaling Technology, Inc.). The
secondary antibodies used in this study were as follows:
Anti-rabbit Alexa Fluor 488 (1:100; cat. no. A-11008; Invitrogen;
Thermo Fisher Scientific, Inc.) and Rhodamine Red anti-mouse
(1:200; cat. no. 715-295-151; Jackson ImmunoResearch Laboratories,
Inc.).
Statistical analysis
Statistical analyses were performed using SPSS
version 25 for Windows (IBM Corp.). The Kolmogorov-Smirnov test was
used to validate standard normal-distributional assumptions. Data
are expressed as the mean ± standard deviation. Multiple
comparsions among groups was performed using one-way ANOVA with
Bonferroni correction and for comparisons between groups paired
Student's t-test was used. Two-tailed P-values of <0.05 were
considered to indicate a statistically significant difference. PFS
and overall survival (OS) were calculated by Kaplan-Meier analysis
using the log-rank test to determine significance. Statistical
significance was set at P<0.05.
Results
Upregulation of HOXA-AS3 is associated
with poor prognosis in EOC tissues and cells
To explore the function of HOXA-AS3 in ovarian
cancer, HOXA-AS3 expression in ovarian cancer cell tissues and
cells (A2780, OVCA429, and OVCA433) was detected by RT-qPCR. The
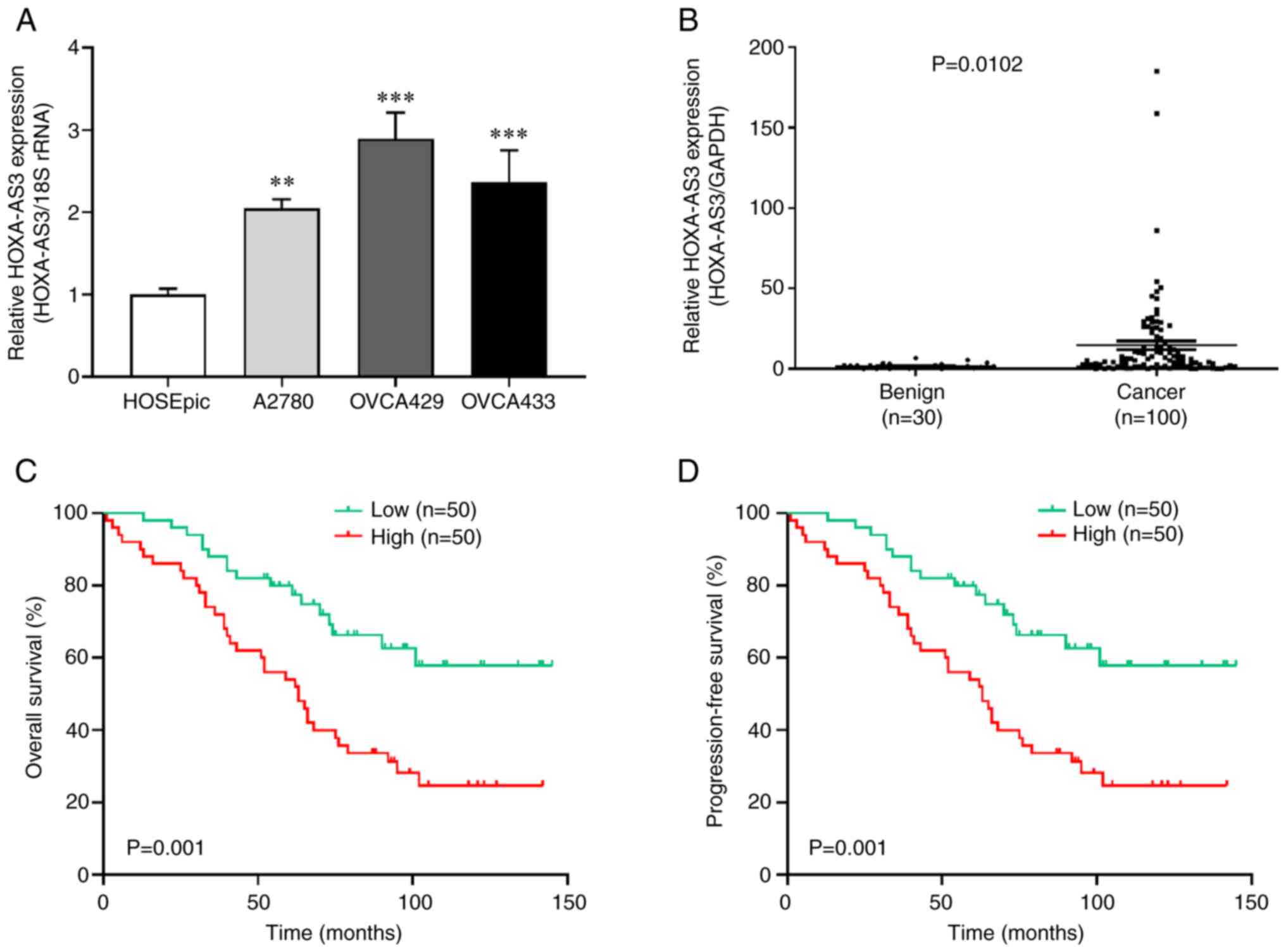
results revealed that the expression of HOXA-AS3 was higher in
ovarian cancer cells than in non-cancerous cells (Fig. 1A). Among them, HOXA-AS3 levels were
higher in OVCA429 and OVCA433 cells and lowest in the A2780 cell
line. For this reason, OVCA429 and OVCA433 cells were selected for
further experiments. In addition, benign tissues and human ovarian
surface epithelial cells were used as controls. The results
revealed that lncRNA HOXA-AS3 expression was significantly higher
in ovarian cancer tissue (n=100) than in the control (n=30)
(Fig. 1B). The median relative
expression levels of HOXA-AS3 were used to determine the cut-off
values of the high and low HOXA-AS3 expression groups. Kaplan-Meier
analysis indicated that patients with high HOXA-AS3 expression had
worse OS and PFS compared to those with low HOXA-AS3 expression
(Fig. 1C and D). The clinical
characteristics of patients who were in the low (n=50) and high
(n=50) HOXA-AS3 expression groups were also compared.
Clinicopathological data, such as age, stage, grade, histological
type, lymph node metastasis, recurrence, and cancer antigen (CA125)
levels, were compared between the low and high expression groups
(Table I). The results indicated
that high expression of lncRNA HOXA-AS3 was associated with poor
prognostic factors including lymph node metastasis and recurrence
in patients with ovarian cancer.
Knockdown of HOXA-AS3 inhibits cell
proliferation in ovarian cancer
To further investigate the biological function of
HOXA-AS3 in ovarian cancer cell development and progression, the
expression of HOXA-AS3 was knocked down in OVCA429 and OVCA433
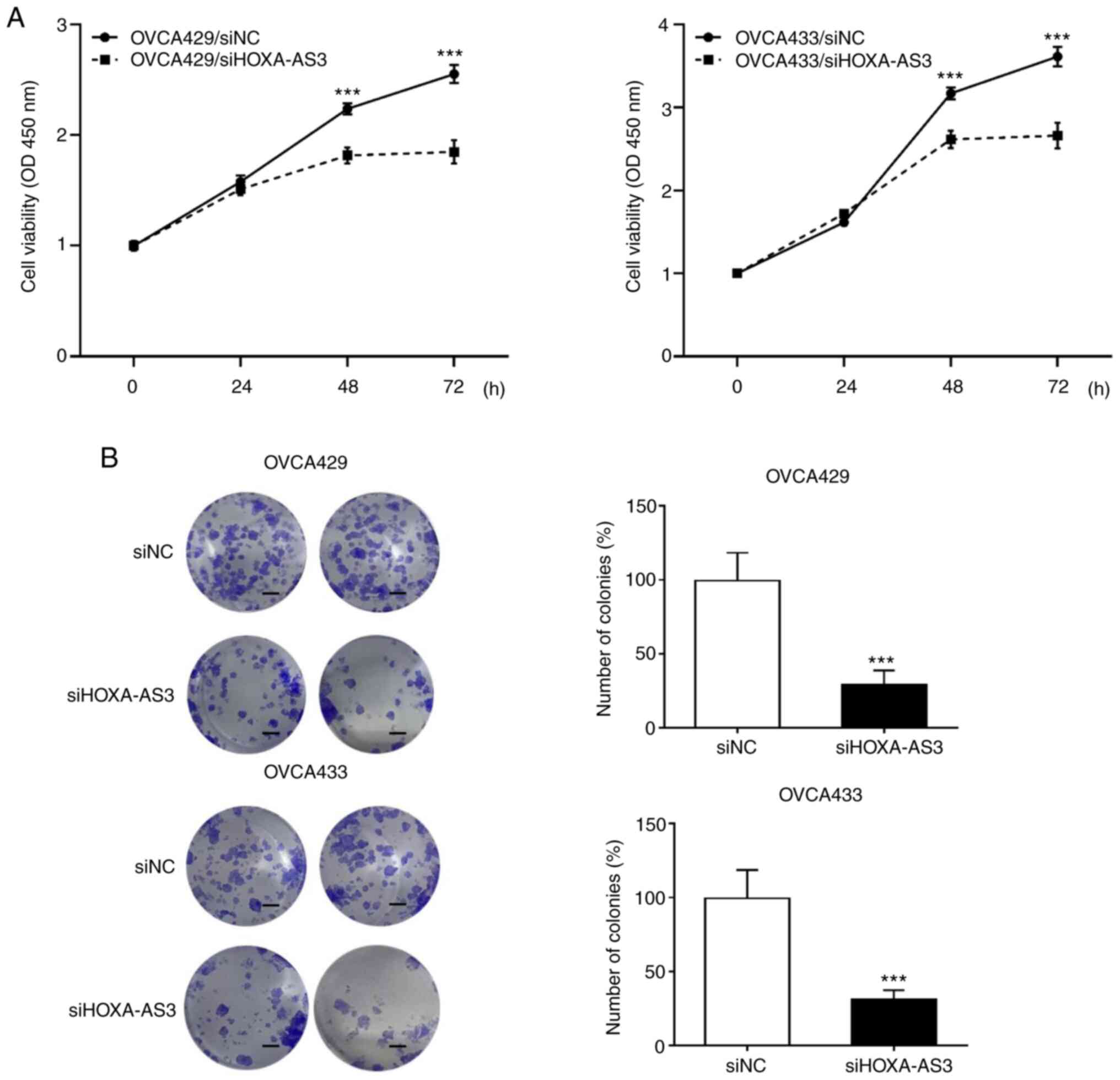
cells by transfecting siRNA. The CCK-8 assay was performed to
determine whether the knockdown of HOXA-AS3 suppressed cell
proliferation in ovarian cancer cells. The results revealed that
HOXA-AS3 knockdown significantly decreased the proliferation of
OVCA429 and OVCA433 cells (Fig.
2A). In addition, the colony formation assay demonstrated that
the cell proliferation rate decreased following HOXA-AS3 knockdown
(Fig. 2B). Taken together, these
results revealed that the knockdown of HOXA-AS3 significantly
hindered ovarian cancer cell proliferation.
HOXA-AS3 promotes ovarian cancer cell
migration
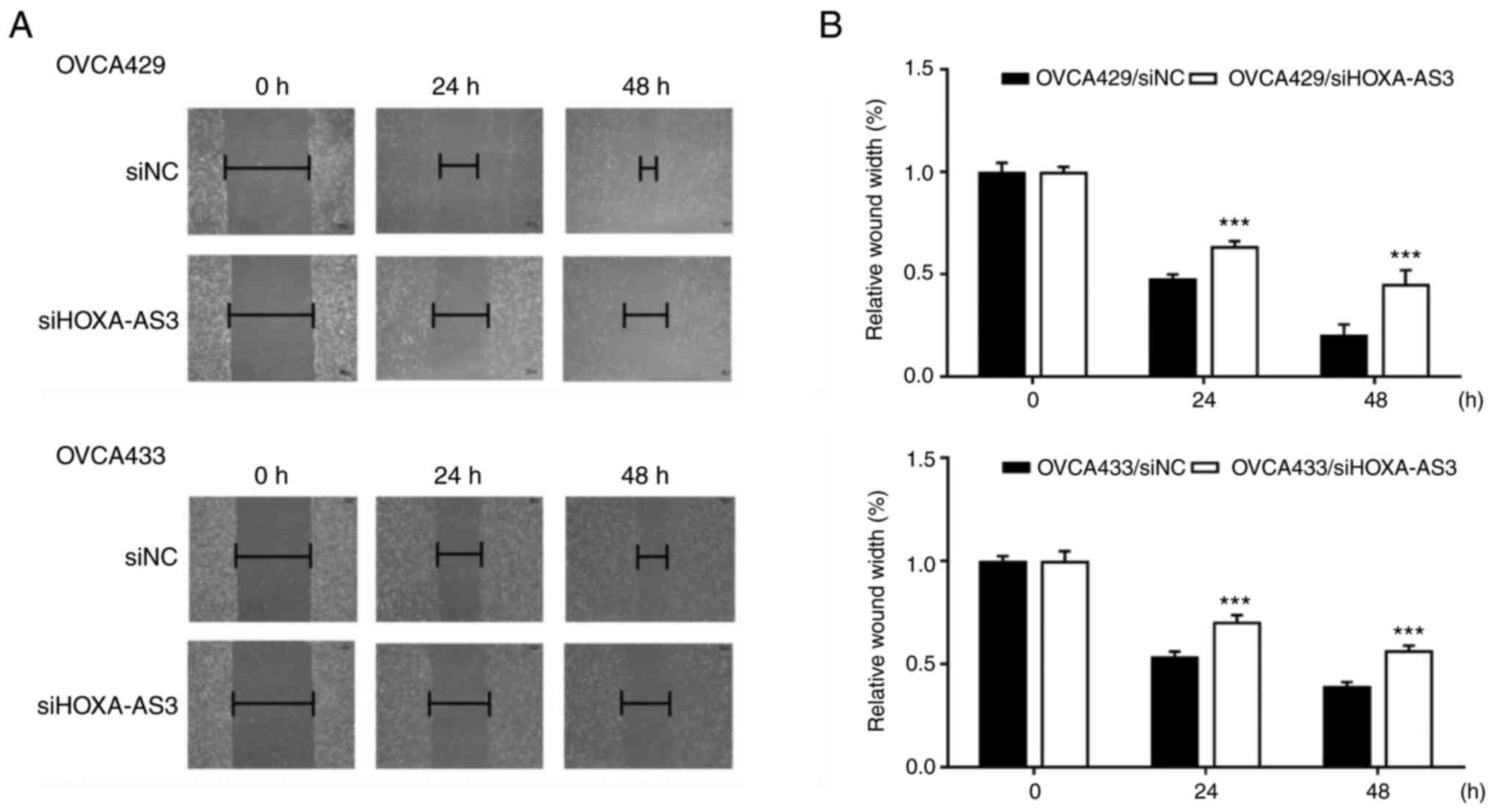
Wound healing assays were performed to investigate
the effect of HOXA-AS3 on ovarian cancer cell migration. The
results showed that HOXA-AS3 knockdown reduced the migration
ability of OVCA429 and OVCA433 cells (Fig. 3A and B), indicating that HOXA-AS3
promoted cell migration in ovarian cancer. Silencing of HOXA-AS3 in
OVCA429 and OVCA433 cell lines reduced scratch closure by 50 and
60%, respectively, compared with control cell lines at 48 h.
Collectively, these results revealed that the knockdown of HOXA-AS3
significantly hindered cell migration.
HOXA-AS3 regulates the EMT signaling
pathway in ovarian cancer cells
Numerous studies have revealed that the EMT
signaling pathway plays a critical role in the modulation of cell
proliferation and metastasis of cancers (5,6). To
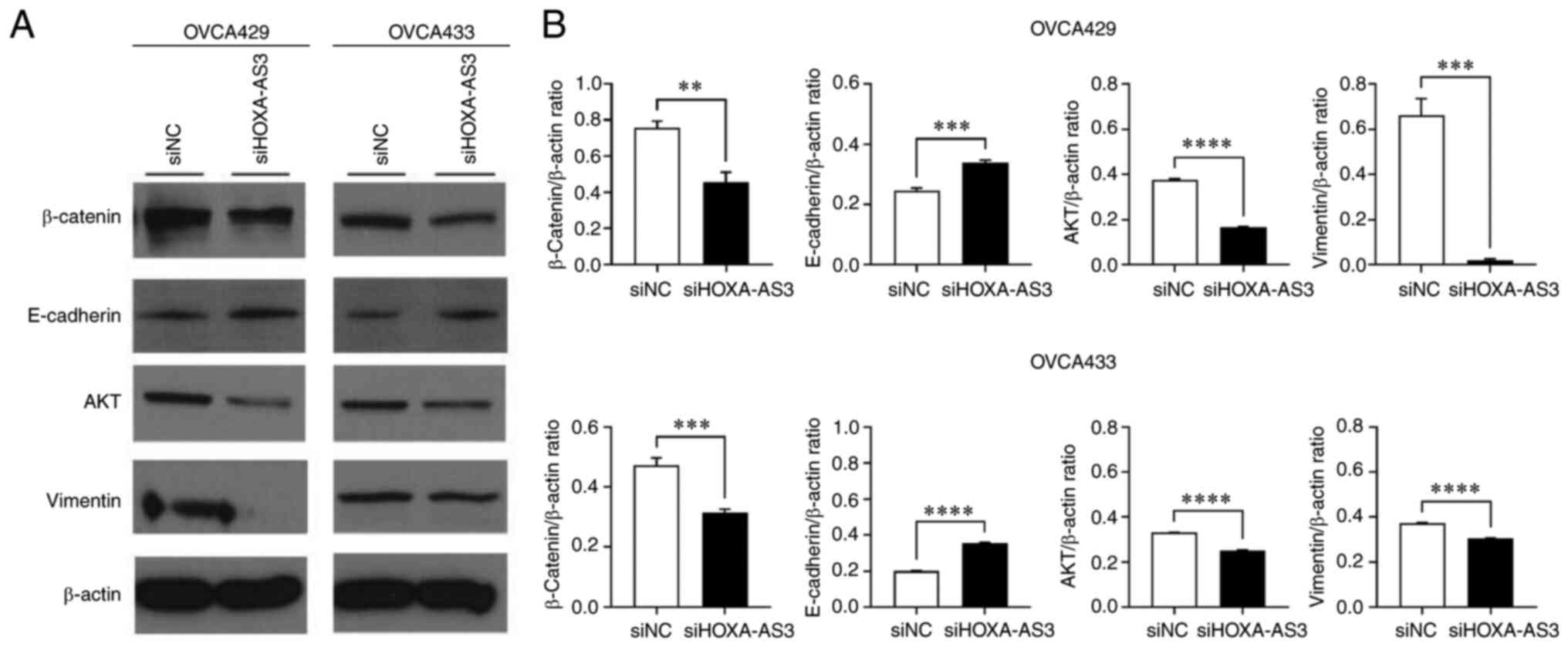
evaluate the role of HOXA-AS3 in EMT signaling, western blotting
and RT-qPCR were performed (Fig.
4). The results of the western blot analysis demonstrated that
HOXA-AS3 knockdown caused significant upregulation of E-cadherin
and downregulation of β-catenin, AKT and vimetin in OVCA429 and
OVCA433 cells compared with the cells in the siNC group (Fig. 4A and B). The mRNA levels of
β-catenin, AKT, and vimentin were downregulated, and E-cadherin was
upregulated following the knockdown of HOXA-AS3 in ovarian cancer
(Fig. 4B). These results indicated
that knockdown of HOXA-AS3 suppressed ovarian cancer cell
proliferation and migration by inhibiting EMT signaling.
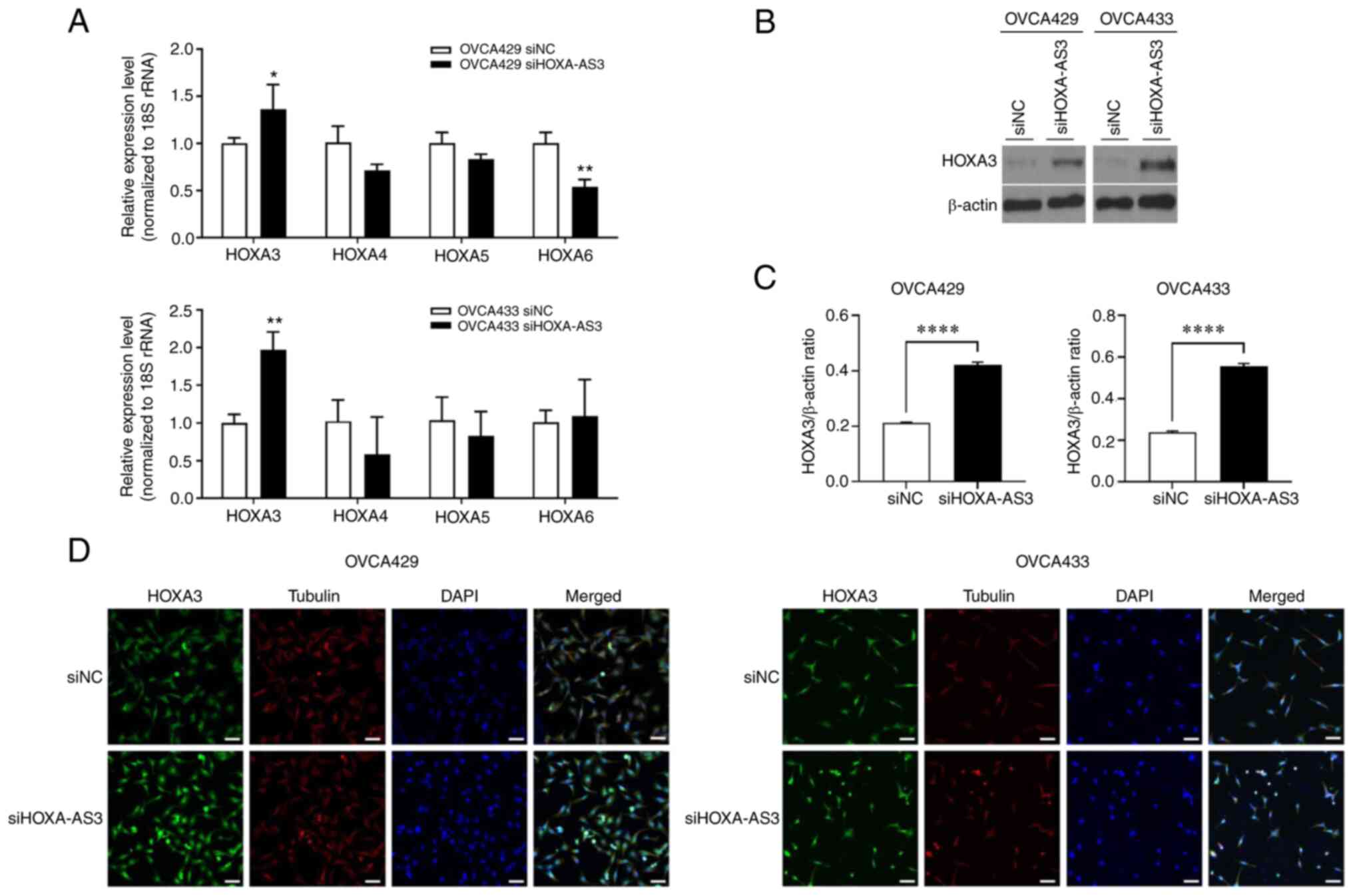
HOXA-AS3 regulates HOXA3 mRNA and
protein
The knockdown of HOXA-AS3 upregulated HOXA3
expression at both the mRNA and protein levels, and HOXA-AS3
interacted with HOXA3 in non-small-cell lung carcinoma cell lines
(9). To evaluate the association
between HOXA-AS3 and HOXA3 in ovarian cancer, RT-qPCR and western
blot analyses were performed. The results revealed that knockdown
of HOXA-AS3 upregulated HOXA3 expression at the mRNA and protein
levels in ovarian cancer (Fig.
5A-C). In addition, immunofluorescence microscopy revealed
increased HOXA3 expression in the HOXA-AS3-knockdown ovarian cancer
cells (Fig. 5D). These results
revealed that knockdown of HOXA-AS3 upregulated HOXA3 expression in
ovarian cancer cells.
Discussion
The present study aimed to explore the functional
role of HOXA-AS3 in ovarian cancer cell lines and its effect on the
clinical characteristics of patients. In the present study, the
knockdown of HOXA-AS3 expression was associated with decreased cell
growth and migration in ovarian cancer cells. The effect of
HOXA-AS3 on tumor progression may be mediated by genes involved in
cell migration, invasion, and the EMT signaling pathway. The
findings suggest that HOXA-AS3 may be utilized as a biomarker and
therapeutic target for ovarian cancer.
Notably, lncRNAs have been receiving increasing
attention for their possible role in cancer progression, including
tumorigenesis, metastasis, and drug resistance (3,13,14).
In particular, lncRNA HOXA-AS3, located on chromosome 7p15.2, was
revealed to be correlated with the progression of several types of
cancer, including glioma and lung cancer (7,9).
HOXA-AS3 was highly expressed in glioma tissues and cell lines, and
upregulated HOXA-AS3 expression was correlated with a poor
prognosis in patients with glioma (7).
In addition, HOXA-AS3 was revealed to act as an
oncogene in glioma by increasing cell proliferation, promoting cell
migration, and inhibiting apoptosis (15). HOXA-AS3 was also significantly
overexpressed in lung adenocarcinoma tissues and A549 cells.
Knockdown of HOXA-AS3 was demonstrated to inhibit cancer cell
proliferation, migration, and invasion (8). A previous study on lung cancer
suggested that HOXA-AS3 was associated with cisplatin resistance
in vitro and in vivo. In particular, HOXA-AS3 induced
cisplatin resistance by interacting with HOXA3 in non-small cell
lung carcinoma cells (9). Another
interesting study on the underlying mechanism of HOXA-AS3 indicated
that HOXA-AS3 acts as an epigenetic switch that determines the
lineage specification of mesenchymal stem cells interacting with
EZH2 and is involved in H3K27me3 deposition on RUNX2, which is a
key osteogenic transcription factor gene (16).
lncRNAs potentially regulate target genes through
various mechanisms, including transcriptional and
post-transcriptional processing, chromatin remodeling, protein
functioning and localization, and intercellular signaling (17–19).
In the present study, HOXA3, which encodes for
highly conserved transcription factors that are important for
physiological functions, including early embryonic development, and
thymus and parathyroid differentiation, was investigated (20,21).
HOXA3 has various functions in the immune and nervous systems, such
as promoting the differentiation of hematopoietic precursor cells
into myeloid cells, regulation of macrophage activation, and
prevention of aberrant neuronal identity and behavior (22–24).
Particularly in tumors, HOXA3 has been reported to promote colon
cancer formation by regulating the EGFR/Ras/Raf/MEK/ERK signaling
pathway (25). In addition, DNA
hypermethylation of HOXA3 has been identified as a potent biomarker
for lung cancer (26). However, the
role of HOXA3 in ovarian cancer has yet to be elucidated. In the
present study, the association between HOXA-AS3 and HOXA3 was
determined in ovarian cancer cell lines for the first time, to the
best of our knowledge, and it was demonstrated that HOXA-AS3 is
associated with cancer progression.
A previous study reported that HOXA3 induces
migration and angiogenesis of endothelial and epithelial cells in
response to injury (27). In
addition, HOXA3 was reported to be an important modulator of EMT in
a mouse model of idiopathic pulmonary fibrosis (7). EMT has been revealed to play an
important role in tumor progression in numerous types of
malignancies, including breast, lung, colon, pancreatic, and
ovarian cancers (28–30). Therefore, the EMT pathway is a
possible target for anticancer treatment (31,32).
Furthermore, suppression of HOXA-AS3 downregulated β-catenin, AKT,
vimentin, and upregulated E-cadherin expression levels, indicating
that HOXA-AS3 knockdown advanced the progression of ovarian cancer
cells by inducing EMT.
The present study has some limitations. Further
research is required to fully elucidate the mechanism of
HOXA-AS3-mediated ovarian cancer cell proliferation and migration.
First, additional studies are needed to investigate other possible
mechanisms and signaling pathways that may be involved in the
process by which HOXA-AS3 induces cancer cell proliferation and
migration to ovarian cancer cells. In addition, correlations
between HOXA-AS3/HOXA3 expression and detailed clinical variables,
including oncologic outcomes in ovarian cancer patients, should be
explored. Lastly, the mechanism by which HOXA-AS3 affects the EMT
pathway should be studied in detail to provide a deeper
understanding of the underlying molecular process of
HOXA-AS3-mediated cancer progression.
Collectively, the results of the present study
revealed that lncRNA HOXA-AS3 is associated with the motility and
invasiveness of ovarian cancer cells. Specifically, lncRNA HOXA-AS3
promoted ovarian cancer progression by inducing cell migration and
invasion via upregulation of EMT signaling pathway-related genes.
Thus, lncRNA HOXA-AS3 may be a potential therapeutic target and
prognostic marker for ovarian cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by a research grant from Yongin
Severance Hospital, Yonsei University College of Medicine.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
KJE and YTK conceived and designed the study. JIK
performed the experiments. KJE, DWL, EJN, HM, SWK and JIK acquired
and analyzed the data. KJE and YTK wrote the manuscript. DWL, EJN,
HM and SWK revised the study critically for important intellectual
content. KJE, JIK and YTK confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved (approval ethics code
4-2021-1394) by the Institutional Review Board of Severance
Hospital, Yonsei University College of Medicine. Individual patient
consent was waived for the present study because it was a
retrospective study which involved no risk to the subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HOXA-AS3
|
HOXA cluster antisense RNA 3
|
|
EMT
|
epithelial-mesenchymal transition
|
|
lncRNAs
|
long noncoding RNAs
|
|
siRNA
|
small interfering RNA
|
|
siNC
|
negative control siRNA
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
CCK-8
|
Cell Counting Kit-8
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Shin W, Won YJ, Yoo CW, Lim J and Lim MC:
Incidence trends for epithelial peritoneal, ovarian, and fallopian
tube cancer during 1999–2016: A retrospective study based on the
Korean National Cancer Incidence Database. J Gynecol Oncol.
31:e562020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guttman M, Donaghey J, Carey BW, Garber M,
Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, et al:
lincRNAs act in the circuitry controlling pluripotency and
differentiation. Nature. 477:295–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim HJ, Eoh KJ, Kim LK, Nam EJ, Yoon SO,
Kim KH, Lee JK, Kim SW and Kim YT: The long noncoding RNA HOXA11
antisense induces tumor progression and stemness maintenance in
cervical cancer. Oncotarget. 7:83001–83016. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eoh KJ, Paek J, Kim SW, Kim HJ, Lee HY,
Lee SK and Kim YT: Long non-coding RNA, steroid receptor RNA
activator (SRA), induces tumor proliferation and invasion through
the NOTCH pathway in cervical cancer cell lines. Oncol Rep.
38:3481–3488. 2017.PubMed/NCBI
|
|
5
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes to chemoresistance. Nature. 527:472–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu F, Zhang C, Cai J, Yang F, Liang T, Yan
X, Wang H, Wang W, Chen J and Jiang T: Upregulation of long
noncoding RNA HOXA-AS3 promotes tumor progression and predicts poor
prognosis in glioma. Oncotarget. 8:53110–53123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Liu Y, Yan L, Zhang M, Yu X, Du
W, Wang S, Li Q, Chen H, Zhang Y, et al: Increased levels of the
long noncoding RNA, HOXA-AS3, promote proliferation of A549 cells.
Cell Death Dis. 9:7072018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin S, Zhang R, An X, Li Z, Fang C, Pan B,
Chen W, Xu G and Han W: LncRNA HOXA-AS3 confers cisplatin
resistance by interacting with HOXA3 in non-small-cell lung
carcinoma cells. Oncogenesis. 8:602019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yim GW, Lee DW, Kim JI and Kim YT: Long
Non-coding RNA LOC285194 promotes epithelial ovarian cancer
progression via the apoptosis signaling pathway. In Vivo.
36:121–131. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eoh KJ, Kim HJ, Lee JW, Kim LK, Park SA,
Kim HS, Kim YT and Koo PJ: E2F8 induces cell proliferation and
invasion through the Epithelial-mesenchymal transition and notch
signaling pathways in ovarian cancer. Int J Mol Sci. 21:58132020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim HJ, Lee DW, Yim GW, Nam EJ, Kim S, Kim
SW and Kim YT: Long non-coding RNA HOTAIR is associated with human
cervical cancer progression. Int J Oncol. 46:521–530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun H, Chen J, Qian W, Kang J, Wang J,
Jiang L, Qiao L, Chen W and Zhang J: Integrated long non-coding RNA
analyses identify novel regulators of epithelial-mesenchymal
transition in the mouse model of pulmonary fibrosis. J Cell Mol
Med. 20:1234–1246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Z, Yang S, Zhou Q, Wang G, Song J, Li
Z, Zhang Z, Xu J, Xia K and Chang Y: Emerging role of
exosome-derived long non-coding RNAs in tumor microenvironment. Mol
Cancer. 17:822018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marcucci F, Stassi G and De Maria R:
Epithelial-mesenchymal transition: A new target in anticancer drug
discovery. Nat Rev Drug Discov. 15:311–325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Y, Cheung BB, Atmadibrata B, Marshall
GM, Dinger ME, Liu PY and Liu T: The regulatory role of long
noncoding RNAs in cancer. Cancer Lett. 391:12–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohana P, Bibi O, Matouk I, Levy C, Birman
T, Ariel I, Schneider T, Ayesh S, Giladi H, Laster M, et al: Use of
H19 regulatory sequences for targeted gene therapy in cancer. Int J
Cancer. 98:645–650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizrahi A, Czerniak A, Levy T, Amiur S,
Gallula J, Matouk I, Abu-Lail R, Sorin V, Birman T, de Groot N, et
al: Development of targeted therapy for ovarian cancer mediated by
a plasmid expressing diphtheria toxin under the control of H19
regulatory sequences. J Transl Med. 7:692009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gofrit ON, Benjamin S, Halachmi S,
Leibovitch I, Dotan Z, Lamm DL, Ehrlich N, Yutkin V, Ben-Am M and
Hochberg A: DNA based therapy with diphtheria toxin-A BC-819: A
phase 2b marker lesion trial in patients with intermediate risk
nonmuscle invasive bladder cancer. J Urol. 191:1697–1702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanna N, Ohana P, Konikoff FM, Leichtmann
G, Hubert A, Appelbaum L, Kopelman Y, Czerniak A and Hochberg A:
Phase 1/2a, dose-escalation, safety, pharmacokinetic and
preliminary efficacy study of intratumoral administration of BC-819
in patients with unresectable pancreatic cancer. Cancer Gene Ther.
19:374–381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hasenpusch G, Pfeifer C, Aneja MK, Wagner
K, Reinhardt D, Gilon M, Ohana P, Hochberg A and Rudolph C:
Aerosolized BC-819 inhibits primary but not secondary lung cancer
growth. PLoS One. 6:e207602011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lavie O, Edelman D, Levy T, Fishman A,
Hubert A, Segev Y, Raveh E, Gilon M and Hochberg A: A phase 1/2a,
dose-escalation, safety, pharmacokinetic, and preliminary efficacy
study of intraperitoneal administration of BC-819 (H19-DTA) in
subjects with recurrent ovarian/peritoneal cancer. Arch Gynecol
Obstet. 295:751–761. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sidi AA, Ohana P, Benjamin S, Shalev M,
Ransom JH, Lamm D, Hochberg A and Leibovitch I: Phase I/II marker
lesion study of intravesical BC-819 DNA plasmid in H19 over
expressing superficial bladder cancer refractory to bacillus
Calmette-Guerin. J Urol. 180:2379–2383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smaldone MC and Davies BJ: BC-819, a
plasmid comprising the H19 gene regulatory sequences and diphtheria
toxin A, for the potential targeted therapy of cancers. Curr Opin
Mol Ther. 12:607–616. 2010.PubMed/NCBI
|
|
27
|
Zhu XX, Yan YW, Chen D, Ai CZ, Lu X, Xu
SS, Jiang S, Zhong GS, Chen DB and Jiang YZ: Long non-coding RNA
HoxA-AS3 interacts with EZH2 to regulate lineage commitment of
mesenchymal stem cells. Oncotarget. 7:63561–63570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen YG, Satpathy AT and Chang HY: Gene
regulation in the immune system by long noncoding RNAs. Nat
Immunol. 18:962–972. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang C, Li X, Zhao H and Liu H: Long
non-coding RNAs: Potential new biomarkers for predicting tumor
invasion and metastasis. Mol Cancer. 15:622016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng WX, Koirala P and Mo YY:
LncRNA-mediated regulation of cell signaling in cancer. Oncogene.
36:5661–5667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kyba M: Modulating the malignancy of Hox
proteins. Blood. 129:269–270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Monterisi S, Lo Riso P, Russo K, Bertalot
G, Vecchi M, Testa G, Di Fiore PP and Bianchi F: HOXB7
overexpression in lung cancer is a hallmark of acquired stem-like
phenotype. Oncogene. 37:3575–3588. 2018. View Article : Google Scholar : PubMed/NCBI
|