Introduction
Ubiquitination, an enzymatic post-translational
modification (PTM) process, plays an important role in multiple
cellular processes, including proteasome degradation of proteins,
cell cycle, transcriptional regulation, DNA repair and signal
transduction. The ubiquitin cascade reaction is carried out in
three steps: Ubiquitin is activated by ubiquitin activating enzyme
(UBE1) through ATP, supplied with energy, and transferred to the
cysteine sulfhydryl group of ubiquitin-binding enzyme (UBE2). Next,
ubiquitin conjugate is transferred to the target protein via
specific recognition by E3 ubiquitin protein ligase. Ubiquitin
binding enzymes, also known as UBE2 or ubiquitin carrier enzymes
under certain conditions, perform the second step of the ubiquitin
reaction.
UBE2 is a polygenic family, and its members are
diverse in terms of molecular weight, structure and function, but
they all contain a highly conserved ubiquitin domain called UBC
domain. According to whether it contains a terminal extension, E2
family members are divided into four categories: i) Class I
(containing only catalytic domain and requiring E3 for substrate
recognition); ii) class II (containing a N-terminal extension);
iii) class III (containing a C-terminal extension); and iv class IV
(containing N- and C-terminal extensions).
Ubiquitin-conjugating enzyme E2 T (UBE2T, also known
as E2 ubiquitin-conjugating enzyme T, FANCT, PIG50 and HSPC150)
belongs to the UBE2 superfamily, which plays a fundamental role in
the second step of ubiquitination. Following previous studies that
reported that UBE2T was involved in the regulation of DNA repair in
Fanconi anaemia (FA), it was recently molecularly identified as a
risk factor closely associated with oncogenesis, metastasis,
survival and prognosis in human patients or mammal animals with
cancer (1–11).
In humans and the majority of mammals UBE2T has been
described as exhibiting both oncogene activity and non-oncogene
functions. Therefore, it is currently unclear if UBE2T is a typical
oncogene or if it is involved in other functions besides
carcinogenesis, and whether it has cancer-inhibitory effects.
Similarly, it is not known at present whether UBE2T has any other
mechanism of action besides ubiquitin-dependent functions, how it
behaves in different cancer types and which is its effect on
chemotherapy/radiotherapy sensitivity. To answer these questions,
further research on UBE2T is required.
UBE2T
Ubiquitination of proteins can change their
localization, activity and/or stability. Ubiquitination requires
the synergy of ubiquitin-activating enzymes (E1s),
ubiquitin-binding enzymes (E2s) and ubiquitin ligases (E3s). E2s,
as a central player, contributes to the interaction with an E1
enzyme and ≥1 E3s (12,13). As the main components of the
ubiquitin-proteasome system (UPS), E2s and E3s are considered key
determinants of substrate recognition and ubiquitination.
To date, ~40 types of UBE2s have been reported,
which promote ubiquitin substrate binding, and regulate the
stability and binding of cancer promoters, suppressors and
regulators (14–16). Increasing evidence has verified that
E2s show dysregulation in various cancer types, as they are linked
to tumor-promoting processes and self-renewal abilities such as
dysregulation in DNA repair, cell cycle, apoptosis and oncogenic
signalling (10,17–22).
The dysfunctional status of E2 members is considered a potential
biomarker for diagnosis, prognosis and therapy in patients with
various cancer types (22–31).
The structures of the majority of E2 proteins
(full-length or UBC domains) have been confirmed, and the
topologies are mainly consistent with this canonical folding. In
few cases, including UBE2J, UBE2G, UBE2Q and UBE2R, the UBC domain
is decorated with functionally important insertions. UBE2K has
additional domains linked to the UBC domains, or forms part of
large multidomain proteins such as UBE2O or baculoviral IAP repeat
containing 6. In addition, the majority of E2s contain only a
single structural UBC domain, the α/β fold, but a large number of
them have short N- and/or C-terminal extensions that can confer
important E2-specific functions. Thus, E2s have a common fold that
may been applied to specific systems (14–16).
UBE2T (UniProt accession no. Q9NPD8) belongs to the
superfamily UBE2, which comprises protein-coding genes. Human UBE2T
is 22.521 kDa, similar to the majority of members of this family,
which have a molecular weight of 20–25 kDa. UBE2T is a protein with
197 amino acids and a basal isoelectric point of 7.78 that is
attached covalently to the Lys residues of the substrate during the
ubiquitination process (21,32).
UBE2T is composed of the core UBC folding and a C-terminal
extension (~40 residues). To a large extent, this extension is
unstructured, little conserved and has a negligible role in
fan-mediated FA group D2 (FANCD2) mono-ubiquitination in
vitro (33). The conserved
Lys91 located near the catalytic site of UBE2T is constitutively
monoubiquitinated in vivo and has been proposed to
negatively regulate E2 (34).
The importance of E2 function in disease is the role
of UBE2T in FA. A total of 20 genes, which are classified into two
types, are involved in the FA pathway. Certain type I proteins,
namely FANCA, -B, -C and -D2, as well as UBE2T together with
FA-associated proteins comprise an ‘upstream’ ubiquitin signalling
module. E2 has been recognized as a bona fide FA gene and is
alternatively named FANCT, and exhibits a strong affinity for the
RING domain of FANCL [dissociation constant (KD) ~450
nM] (33,35). A previous study determined the
structure for the E3-E2 pair, the FANCL RING domain (residues
299–373) and UBE2T (residues 1–153) to 2.4 Å resolution, and
indicated that not only the conserved hydrophobic residues Ile309
and Trp341, but also the FANCL-specific Tyr311 are important for
the FANCL-UBE2T interaction. The authors revealed that residues
involved in the electrostatic and hydrogen-bonding network observed
in the FANCL-UBE2T interface are highly variable (36).
UBE2T is the primary E2 of the FA pathway, and is
required for FANCD2 and FANCI activation. Deficiency of UBE2T,
which is necessary for FANCD2 and FANCI ubiquitination, causes the
FA-T subtype of FA (37). A
previous study carried out genetic engineering on derivatives of
red fluorescent protein (RFP), with a His6 tag on the N-terminus
and human wild-type ubiquitin (HisRFP ubiquitin) on the C-terminus,
and found that RFP with only a small part of the E2 enzymes E2B,
E2C, UBC5B, UBC4C, E2D4 and E2T directly ubiquitinated chimera. The
authors detected that E2T catalysed ubiquitin polymerization
through Lys27 of ubiquitin, which indicated that E2T contained a
region that interacted non-covalently with the attacking
ubiquitin-ubiquitin molecules to form a Lys-specific linkage, with
the ubiquitin binding to the active site through this interaction.
The authors also found that the β2-β3 loop of UBE2T contained a
unique basic residue that formed a salt bridge with Glu340 of
FANCL, while Arg60 in UBE2T was predominantly acidic (Asp/Glu) in
other ubiquitin E2s and served as a positive selector for the FANCL
RING-UBE2T pairing (33).
Large-scale E2-E3 interaction studies have suggested that UBE2T
interacts with ≥15 other E3s (33,38–40).
UBE2T appears to function with a limited group of homologous to the
E6-AP C-terminus E3s, and mainly supports multi-simple
ubiquitination. However, the proposed UBE2T binding ring E3s and
the FANCL ring domains are less conservative, and only 5 of them
may support a β2-β weak interaction of 3 rings (if any) (33). However, when presented with a
different set of E2s, FANCL could exclusively form a compound with
UBE2T, and only homologous E2-E3 could cause site specific
uni-ubiquitination of FANCD2 in vitro (36). The specific and stable FANCL-UBE2T
interaction provides insights into how uni-ubiquitination events
are regulated. Since both loading ubiquitin from E1s and unloading
it through E3s require overlapping UBE2T surfaces, low UBE2T
closure rates may limit the reload of ubiquitin, thereby inhibiting
continuous ubiquitination (33).
In addition, the allosteric ‘linchpin’ residue
(typically Arg) required for all RING/U-box-mediated ubiquitination
events are absent from FANCL. Moreover, the allosteric ‘linchpin’
residue (typically Arg) required for all RING/U-box-mediated
ubiquitination events is absent from FANCL (Ser363) (41).
It accepts ubiquitin in the E1 complex and catalyses
its covalent attachment to other proteins, thus catalysing
ubiquitination, and participates in mitomycin C (MMC)-induced DNA
repair. On the other hand, it acts as a specific E2 ubiquitin
binding enzyme of the FA complex by binding to the E3 ubiquitin
protein ligase FANCL and catalysing the mono-ubiquitination of
FANCD2 (which is a direct interaction and a key step in the DNA
damage pathway), thus mediating the mono-ubiquitination of FANCL
and FANCI (42–48). FA is a disease highly associated
with UBE2T, and its related pathways include DNA damage and protein
metabolism. Genome analysis of patient revealed a paternal 23-kb
deletion across the UBE2T locus (49). The Gln2 residue in helix1 of UBE2T
is not an integral part of the UBE2T-FANCL interaction surface.
However, the binding of Gln2Glu mutation to FANCL was not as
efficient, and reduced FANCD2 mono-ubiquitination by UBE2T-FANCL
in vitro (33). A c.180 +
5G>A splice donor site mutation initiated a frame shift and
premature stop codon, resulting in a truncated UBE2T protein with a
non-functional UBC domain (49).
The gene sequence from a FA patient revealed markedly low
frequencies of either AluY-mediated deletions or duplications of
UBE2T exons 2–6 (50). It has been
suggested that all the identified pathogenic alterations of UBE2T
are likely loss-of-function alleles, and the deficiency of UBE2T
protein is associated with FA. It has been reported that UBE2T and
FANCD2 access this subcellular fraction independently of the FA
core complex, and substrates FANCD2/FANCI independently accumulate
on chromatin during DNA replication or damage (34). Besides its interaction with
essential E2, FANCL also has a highly conserved RWD-like domain
(characterised by RING finger and WD repeat containing proteins and
DEAD-like helicases) that stimulates the UBE2T-mediated
uni-ubiquitination of FANCD2 (51).
Moreover, it has been shown to contribute to the
ubiquitination and degradation of breast cancer gene 1 (BRCA1)
(28,47,52–54).
It interacts with the BRCA1/BRCA1 associated RING domain 1 (BARD1)
complex in breast cancer (BrC) cells and causes the degradation of
BRCA1. It also impacts BRCA1 ubiquitination but not that of BARD1
(52). Importantly, UBE2N, UBE2C,
BE2H, UBE2B, UBE2W and UBE2F are also considered paralogues of
that. UBE2T appears to contribute promoting the response by acting
at different ubiquitin Lys residues. PTM was found for the UBE2T
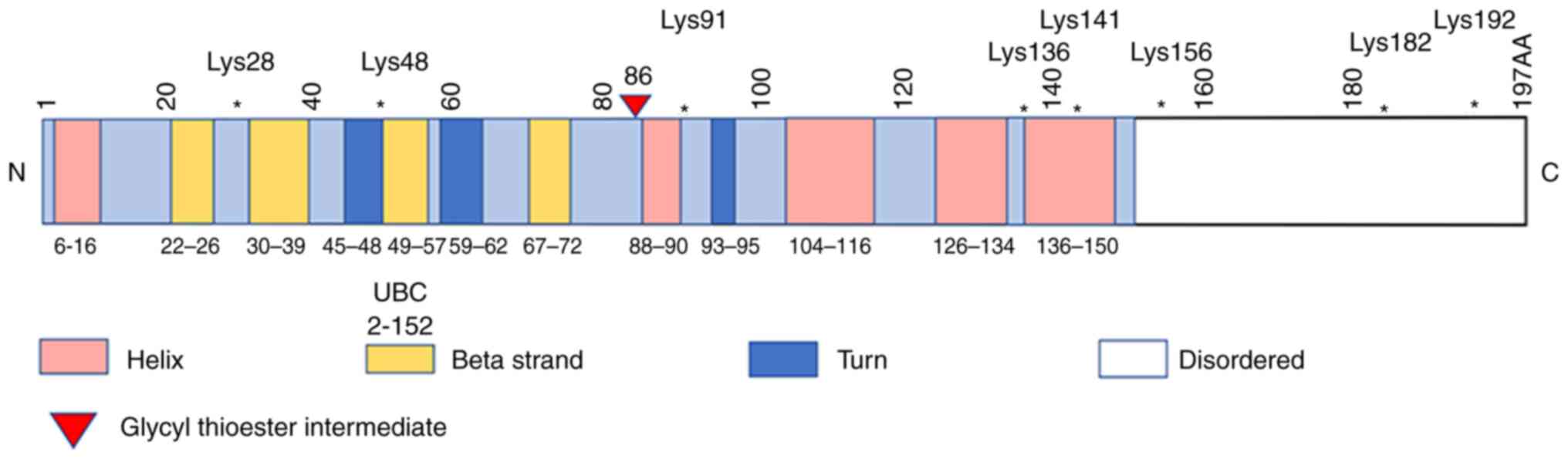
gene (Fig. 1), including
auto-ubiquitination, since it contains 8 Lys sites (namely Lys28,
48, 91, 136, 141, 156, 182 and 192). In addition, it also exhibits
two other modifications, namely sites of phosphorylation (Thr72,
Ser184 and His194) and acetylation (Lys191). UBE2T has been shown
to directly or indirectly interact with numerous proteins involved
in a wide range of cellular processes, including cell cycle, cell
proliferation, transcription and translation. Bioinformatic
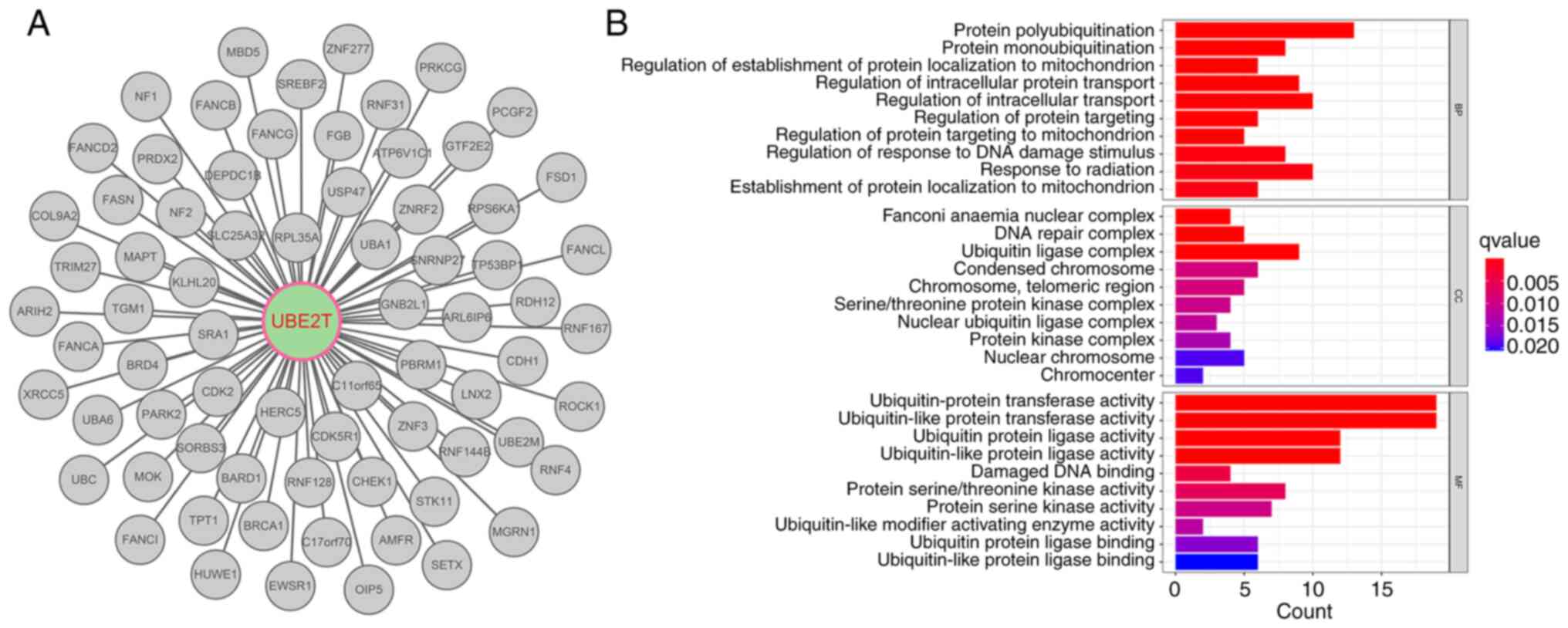
analyses using data from the Biological General Repository for
Interaction Datasets (https://thebiogrid.org/), Protein Interaction Network
Analysis 2 (https://omics.bjcancer.org/pina2012/) and STRING
(https://cn.string-db.org/) databases
found 73 interacting proteins for human UBE2T (Fig. 2A). Several of them are associated
with protein (poly/mono)-ubiquitination, regulation of
intracellular transport, regulation of intracellular protein
transport and response to radiation (Fig. 2B).
Expression of UBE2T in cancer
UBE2T not only regulates numerous biological
functions, particularly DNA repair, but also affects the
occurrence, development and prognosis of cancer by impacting
various cancer pathways characterized by oncogenes in multiple
cancer types (8,55,56).
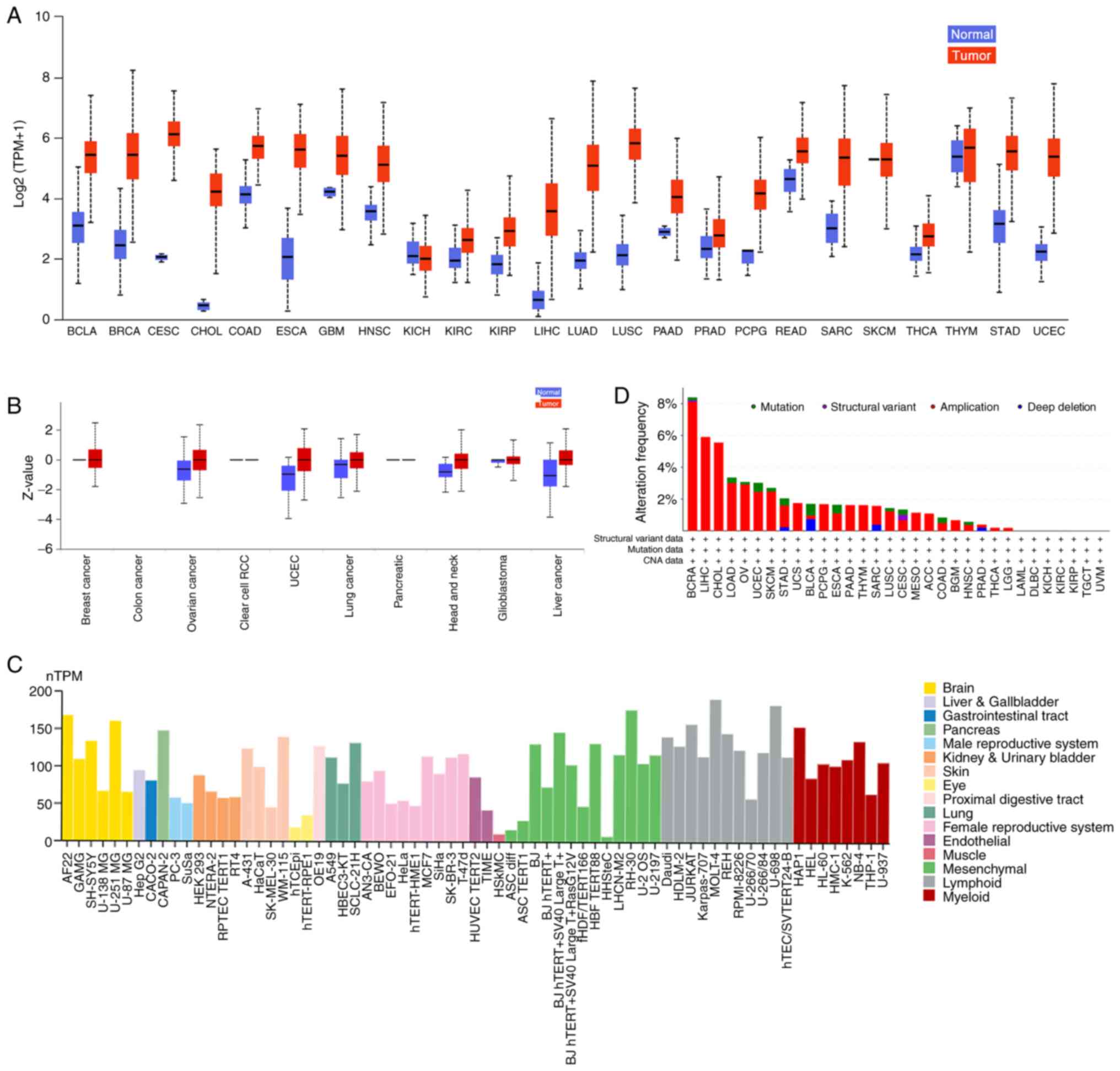
Aberrant UBE2T expression is frequently found in cancer tissue
samples and cell lines (Fig. 3A-C).
UBE2T overexpression promoted cell proliferation, which was
inhibited by UBE2T knockdown. Recently, research has focused on the
function of UBE2T in tumorigenesis and tumor progression (57). Previous studies on UBE2T included
BrC, bladder cancer (BC), cervical cancer, cholangiocarcinoma,
colorectal cancer (CRC), esophageal cancer, lung cancer,
hepatocellular carcinoma (HCC/liver HCC), melanoma, nasopharyngeal
carcinoma, osteosarcoma, ovarian cancer and pancreatic cancer
(PCA). The present study confirmed that high expression of UBE2T
occurred in multiple types of cancer, and revealed the specific
biological function and associated related cell signaling pathways
(Table I). Data obtained from The
Cancer Genome Atlas (TCGA, http://www.genome.gov/Funded-Programs-Projects/Cancer-Genome-Atlas)
database indicated that UBE2T amplification was a common phenomenon
in multiple types of cancer, although mutations have also been
recognised (Fig. 3D). Additionally,
overexpression of UBE2T is associated with poor survival in
patients with cancer (Fig. 4).
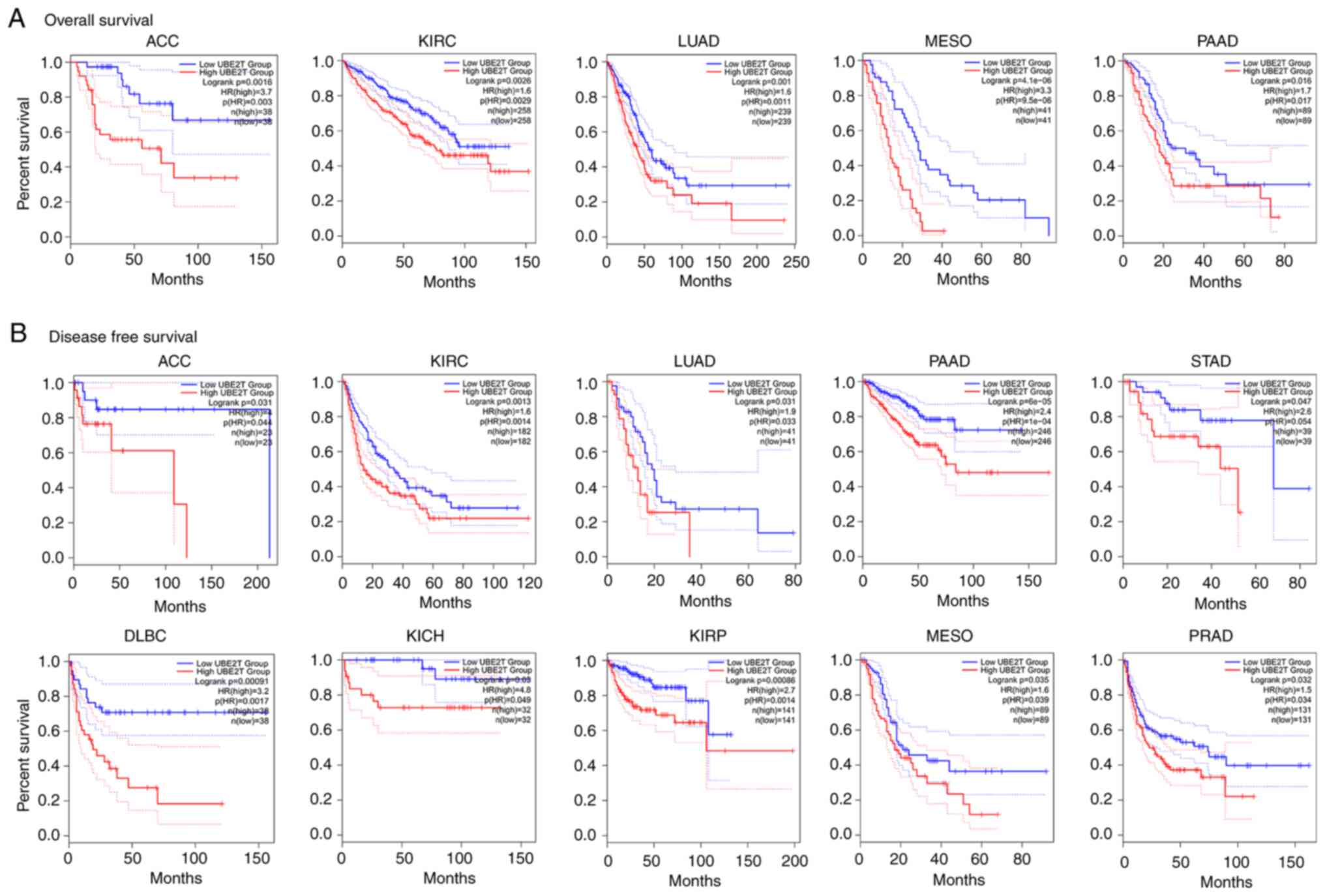 | Figure 4.Correlation between UBE2T expression
and survival prognosis of cancer in TCGA. The GEPIA2 tool was used
to perform (A) overall survival and (B) disease-free survival
analyses of different tumors in TCGA by UBE2T expression. The
Log-rank test was used for the survival analysis. Only TCGA cancers
with statistically significant differences between cohorts are
presented. UBE2T, human ubiquitin-conjugating enzyme E2 T; TCGA,
The Cancer Genome Atlas; ACC, adrenocortical cancer; DLBC, large
B-cell lymphoma; KICH, kidney chromophobe; KIRC, kidney clear cell
carcinoma; KIRP, kidney papillary cell carcinoma; LUAD, lung
adenocarcinoma; MESO, mesothelioma; PAAD, pancreatic
adenocarcinoma; PRAD, prostate adenocarcinoma; STAD, stomach
adenocarcinoma. |
 | Table I.The characteristic landscape of
aberrant UBE2T in different types of cancer. |
Table I.
The characteristic landscape of
aberrant UBE2T in different types of cancer.
| Tumor type | Alterations | Affected
functions | Pathways | Role | Target
validation | (Refs.) |
|---|
| HCC | Elevated | Proliferation, | miR-1322 | Downstream | Circ_0000291 | Wang et al
2022 (59) |
|
|
| apoptosis,
cycle, |
| target | knockdown |
|
|
|
| migration,
invasion, | miR-1305/Akt | Downstream | UBE2T | Wei et al
2019 (56) |
|
|
|
chemosensitivity, | miR-605-5p | target | knockdown |
|
|
|
| metastasis,
prognosis | miR-548c-3p | Downstream | circ_0090049 | Chen et al
2022 (3) |
|
|
| and metabolism | miR-212-5p | target | knockdown |
|
|
|
|
|
| Downstream | UBE2T | Ren et al
2021 (60) |
|
|
|
|
| target | knockdown/ |
|
|
|
|
|
|
| overexpress |
|
|
|
|
| CASC11 | Downstream | CASC11 | Chen et al
2021 (2) |
|
|
|
|
| target | knockdown |
|
|
|
|
| Akt/β-catenin | Critical | UBE2T | Zhu et al
2022 (58) |
|
|
|
|
| regulator | knockdown/ |
|
|
|
|
|
|
| overexpress |
|
|
|
|
| MAPK/ERK, | Critical | UBE2T | Lioulia et
al 2022 (61) |
|
|
|
| AKT/mTOR and | Regulator | knockdown/ |
|
|
|
|
| Wnt/β-catenin |
| overexpress |
|
|
|
|
| Mule/β-catenin | Regulator | UBE2T | Ho et al
2021 (62) |
|
|
|
|
|
| knockdown/ |
|
|
|
|
|
|
| overexpress |
|
|
|
|
| miR-543 | Downstream | UBE2T | Liu et al
2017 (8) |
|
|
|
|
| target | knockdown/ |
|
|
|
|
|
|
| overexpress |
|
|
|
|
| CHK1 | Regulator | UBE2T | Sun et al
2020 (64) |
|
|
|
|
|
| knockdown/ |
|
|
|
|
|
|
| overexpress |
|
|
|
|
| SENP1 | Downstream | SENP1 | Tao et al
2020 (55) |
|
|
|
|
| target | knockdown |
|
|
|
|
| cyclin B1, | Regulator | UBE2T | Liu et al
2019 (66) |
|
|
|
| CDK1 |
| knockdown/ |
|
|
|
|
|
|
| overexpress |
|
| ICC | Elevated | Proliferation, | mTOR | Downstream | UBE2T | Liu et al
2020 (110) |
|
|
| migration,
invasion, |
| target | knockdown/ |
|
|
|
| apoptosis and
cycle |
|
| overexpress |
|
| GC | Elevated | Proliferation, | E2F5 | Downstream | E2F5 | Li et al
2022 (69) |
|
|
| migration,
invasion, |
| target | knockdown |
|
|
|
| apoptosis,
prognosis, | CHPF | Regulator | E2F1 | Lin et al
2021 (70) |
|
|
| cycle and
metastasis |
|
| knockdown |
|
|
|
|
| MYC | Biomarker | MYC | Heitor da Silva
Maues et al |
|
|
|
|
|
| knockdown | 2020 (71) |
|
|
|
| Wnt/β-catenin | Upstream | UBE2T | Yu et al
2021 (72) |
|
|
|
|
| regulator | knockdown / |
|
|
|
|
|
|
| inhibitor |
|
|
|
|
|
|
| M435-1279 |
|
|
|
|
| EMT and Wnt/β- | Biomarker/ | UBE2T | Luo et al
2017 (9) |
|
|
|
| catenin | target | knockdown |
|
|
|
|
| EMT | Biomarker | UBE2T | Yu et al
2016 (73) |
|
|
|
|
|
| knockdown/ |
|
|
|
|
|
|
| overexpress |
|
| CRC | Elevated | Proliferation, | P53 | Biomarker/ | UBE2T | Wu et al
2020 (75) |
|
|
| apoptosis,
migration |
| target | knockdown/ |
|
|
|
| and invasion |
|
| overexpress |
|
| PCA | Elevated | Proliferation, | EMT | Biomarker/ | UBE2T | Zheng et al
2020 (76) |
|
|
| apoptosis,
migration |
| target | knockdown/ |
|
|
|
| and invasion |
|
| overexpress |
|
| LUAD | Elevated | Proliferation, | EMT | Regulator | UBE2T | Zhang et al
2022 (77) |
|
|
| prognosis,
migration, |
|
| knockdown/ |
|
|
|
| invasion,
apoptosis, |
|
| overexpress |
|
|
|
| autophagy and
cycle | FBLN5, | Biomarker/ | UBE2T | Li et al
2022 (78) |
|
|
|
| ERK/GSK3β | target | knockdown |
|
|
|
|
| Wnt/β-catenin | Regulator/ | UBE2T | Liu et al
2017 (85) |
|
|
|
|
| target | knockdown |
|
|
|
|
| PI3K/AKT | Biomarker/ | NEDD4L | Chen et al
2021 (86) |
|
|
|
|
| target | knockdown |
|
|
|
|
| P53/AMPK/ | Regulator | UBE2T | Zhu et al
2021 (87) |
|
|
|
| mTOR |
| knockdown/ |
|
|
|
|
|
|
| overexpress |
|
| NPC | Elevated | Proliferation, | AKT/GSK3β/ | Biomarker/ | UBE2T | Hu et al
2016 (6) |
|
|
| metastasis,
invasion | β-catenin | target | knockdown |
|
| Osteosarcoma | Elevated | Proliferation, | Cell cycle | Regulator | UBE2T | Shen et al
2019 (89) |
|
|
| migration,
invasion, |
|
| knockdown |
|
|
|
| apoptosis, cell
cycle | PI3K/AKT | Regulator/ | UBE2T | Wang et al
2016 (90) |
|
|
| and
radiosensitivity |
| target | knockdown |
|
| MM | Elevated | Proliferation, | miR-498 | Regulator/ | Not yet | Cao et al
2022 (91) |
|
|
| migration,
invasion, |
| target |
|
|
|
|
| apoptosis and |
|
|
|
|
|
|
| prognosis |
|
|
|
|
| BrC | Elevated | Proliferation, | miR-543 | Target | miR-543 | Li and Li 2021
(95) |
|
|
| migration,
glycolysis |
|
| overexpress |
|
|
|
| and invasion | PI3K/AKT | Target | UBE2T | Qiao et al
2022 (97) |
|
|
|
|
|
| knockdown/ |
|
|
|
|
|
|
| overexpress |
|
| OV | Elevated | Proliferation, | AKT/mTOR | Biomarker/ | UBE2T | Huang et al
2022 (7) |
|
|
| autophagy,
invasion |
| target | knockdown |
|
|
|
| and prognosis |
|
|
|
|
| Cervical | Elevated | Proliferation, | GRP78/FAK | Regulator | UBE2T | Liu et al
2021 (18) |
| cancer |
| migration,
invasion |
|
| knockdown/ |
|
|
|
| and prognosis |
|
| overexpress |
|
| PC | Elevated | Proliferation, | EMT | Target | UBE2T | Wen et al
2015 (99) |
|
|
| migration,
metastasis |
|
| knockdown/ |
|
|
|
| and invasion |
|
| overexpress |
|
| BC | Elevated | Proliferation, | Cell cycle | Biomarker/ | UBE2T | Gong et al
2016 (101) |
|
|
| migration, cell
cycle |
| target | knockdown |
|
|
|
| and apoptosis |
|
|
|
|
| RCC | Elevated | Proliferation, | miR-182-5p | Target | UBE2T | Wu et al
2022 (103) |
|
|
| migration,
metastasis |
|
| knockdown/ |
|
|
|
| invasion, cycle
and |
|
| overexpress |
|
|
|
| apoptosis | PI3K/Akt/mTOR | Regulator | UBE2T | Hao et al
2019 (5) |
| MuM | Elevated | Proliferation,
cycle | Homologous | Target | UBE2T | Alagpulinsa et
al |
|
|
|
|
|
|
| 2019 (1) |
|
|
| and
chemosensitivity | recombination |
| knockdown |
|
|
|
|
|
|
| knockdown |
|
| DLBC | Elevated | Proliferation | PI3K | Not yet | UBE2T | Derenzini et
al 2018 (106) |
|
|
|
|
|
| knockdown |
|
| RB | Elevated | Proliferation,
immune | Anaplasia | Biomarker | UBE2T | Wang et al
2022 (107) |
|
|
| infiltration,
prognosis |
|
| knockdown |
|
|
|
| cell cycle and |
|
|
|
|
|
|
| apoptosis |
|
|
|
|
| GBM | Elevated | Invasion, and | GRP78/EMT | Target | UBE2T | Huang et al
2020 (108) |
|
|
| migration |
|
| knockdown |
|
UBE2T in the digestive system
Liver
HCC is the most common type of primary liver cancer,
and UBE2T is upregulated and associated with adverse clinical
outcomes in patients. Experiments in vitro and in
vivo showed that UBE2T promoted the development of HCC,
including apoptosis, invasion, metastasis, metabolism migration and
proliferation (58,59).
Circular RNAs (circRNAs) exhibit important
regulatory functions in cancer biology. UBE2T is a target of
certain circRNAs in cancer. It was suggested that there was a
significant negative correlation between microRNA (miR)-1322 and
UBE2T, which can directly interact. The overexpression of miR-1322
resulted in a significant decrease in UBE2T protein in HCC cells
(59). Targeting UBE2T via miR-1305
disrupted the activation of the Akt signaling pathway, and
ultimately inhibited the formation and proliferation of liver
cancer stem cells, as well as tumorigenicity (56). Moreover, circ_0090049 regulated the
expression of UBE2T by regulating miR-605-5p or miR-548c-3p,
thereby promoting the proliferation of HCC cells (3). The expression level of miR-212-5p was
observed to be markedly lower in HCC than in adjacent tissues, and
was negatively correlated with the expression of UBE2T. miR-212-5p
inhibited the malignant phenotype in a UBE2T-dependent manner
(30,60).
It was found that cancer susceptibility 1 recruited
AlkB homolog 5 to UBE2T mRNA, reduced the m6A level of
UBE2T (a downstream target), enhanced the stability of UBE2T mRNA
and inhibited its binding with YTH N6-methyladenosine RNA binding
protein 2, thus causing UBE2T upregulation (2). It was found that UBE2T was positively
correlated with pyrimidine metabolism, the Akt/β-catenin cell
signaling pathway and ubiquitination mediated by Akt Lys63. Liquid
chromatography/mass spectrometry metabolomics results showed that
the key products of pyrimidine metabolism, which include several
key enzymes [namely carbamoyl-phosphate synthetase 2, aspartate
transcarbamylase, and dihydroorotase, dihydroorotate dehydrogenase
(DHODH) and uridine monophosphate synthetase] in pyrimidine
synthesis, were significantly increased in HCC cells overexpressing
UBE2T (58). UBE2T regulated
β-catenin nuclear translocation, which led to the subsequent
induction of the epithelial-mesenchymal transition (EMT) through
MAPK/ERK-dependent activation. Overexpression of UBE2T also
activated other EMT-related signaling pathways such as Akt/mTOR and
Wnt/β-catenin (61). Silencing
UBE2T reduced the percentages of CD47-, CD133- and CD90-positive
cells, while UBE2T overexpression exhibited increased percentages
of these markers (62).
Overexpression of UBE2T was negatively correlated
with prognosis and overall survival (OS) time, while it was
positively correlated with pathological grade and TNM stage of HCC
progression, which showed higher expression at all four stages
compared with that of non-cancer control samples (8,30,63).
The level of UBE2T was significantly higher in stages II and III
than in stage I (30). In addition,
it was confirmed to have notable advantages with HCC grade, HCC
satellite lesions and vascular invasion (63). miR-543 directly targeted UBE2T,
which was downregulated in HCC. Ectopic expression of UBE2T led to
the reduction of p53, p21 and Noxa, and promoted the degradation of
p53 protein by enhancing its ubiquitination (8).
Abnormal cell cycle progression and inhibition of
apoptosis are considered markers of cancer. UBE2T promoted
radioresistance, which led to DNA damage response (DDR), and
promoted G2/M arrest by enhancing checkpoint kinase 1
(CHK1) activation. It also enhanced CHK1 activation and promoted
G2/M arrest, while its knockdown impaired the activation
of CHK1 (64). Numerous genes are
controlled by UBE2T (65). The
carcinogenesis of small ubiquitin-like modifier (SUMO) specific
peptidase 1 was mediated by the deSUMOylation of UBE2T and the
UBE2T/Akt signaling pathway (55).
UBE2T regulated G2/M conversion via regulating CDK1 and
cyclin B1. Silencing UBE2T resulted in an increase in the
percentage of cells in G2/M phase and a decrease in the
percentage of cells in G1 phase, indicating
G2/M phase cell cycle arrest. By contrast, the
percentage of cells in the G2/M phase decreased after
UBE2T overexpression (66).
Bile duct, gallbladder, stomach, colon
and rectum
UBE2T is considered a useful biomarker for the
differential diagnosis of intrahepatic cholangiocarcinoma (CAA).
High expression of UBE2T can be an independent indicator of poor
prognosis, and is associated with shorter recurrence time and OS
(67). In gallbladder cancer, high
expression of UBE2T is significantly correlated with high risk,
which is considered an independent risk factor for patients with
gallbladder cancer (68).
Previous studies have shown that E2F family members
show an advantageous function in gastric cancer (GC). E2F5 was
significantly positively correlated with UBE2T in GC, which
promoted malignant progression by promoting UBE2T transcription,
while UBE2T overexpression reversed the effect of E2F5 depletion on
cell invasion and proliferation (69). Chondroitin polymerizing factor may
regulate E2F1 by affecting UBE2T-mediated E2F1 ubiquitination,
which may determine the status of GC (70). The level of UBE2T was negatively
correlated with the prognosis of patients with GC. High levels of
UBE2T were identified, which were associated with risk factors,
including early and late stages, lymph nodes, distant metastasis
and Helicobacter pylori. Overexpression of UBE2T showed that
markers of poor prognosis were higher than tubulin tyrosine
ligase-like family member 12 in diffuse GC, indicating a shorter
life span. In intestinal GC, however, the survival rate of patients
overexpressing CDC16 was significantly lower than that of patients
overexpressing UBE2T (71).
UBE2T is ubiquitinated by receptor for activated C
kinase 1 (RACK1), and its degradation (mediated by the
hyperactivation of the Wnt/β-catenin signaling pathway) promotes GC
progression, which indicates that UBE2T is the upstream regulator
of RACK1. M435-1279, an inhibitor of UBE2T, blocks the
UBE2T-mediated RACK1 degradation to inhibit the overactivation of
Wnt/β-catenin, thereby inhibiting the proliferation and progression
of GC (72). Similarly, in HCC,
UBE2T inhibition mediated by small interfering (si)RNA inhibits the
proliferation and colony formation of GC cells by promoting
G2/M phase cell cycle arrest and increasing apoptosis
(9,73). Bioinformatics analysis of clinical
samples found that cell cycle, DNA replication, mitotic
M-M/G1 phases and the ataxia-telangiectasia-mutated
signaling pathway were involved in CRC progression. The finding was
consistent with a previous study, which reported that higher UBE2T
expression levels were associated with poorer prognosis, and
silencing UBE2T could inhibit CRC cell proliferation (74). UBE2T is not only associated with
prognosis and clinical TNM stage, but also with N classification
and histological grade in CRC (75). Overexpression of UBE2T could promote
the proliferation, migration and invasion of CRC cells through the
ubiquitination of p53 (8). UBE2T
has been shown to promote the invasion of PCA and GC, which may be
regulated by EMT (73,76).
UBE2T in the respiratory system
Lung and larynx
Silencing UBE2T may regulate the stability of FANCI
by binding to FANCI, since upon silencing UBE2T, the FANCI protein
levels decreased without significant changes in its mRNA levels.
The ubiquitin content in A549 and H1299 cells were downregulated
after silencing UBE2T, which was also involved in FANCD2 (a
paralogue of FANCI) ubiquitination. Knockdown of UBE2T decreased
the mono-ubiquitination of FANCD2, while overexpression of UBE2T
increased it (77). Cell
proliferation, migration and invasion abilities decreased after
knocking down of UBE2T, while silencing UBE2T increased fibulin 5,
and inhibited the activation of phosphorylated (p)-ERK, p-GSK3β and
β-catenin (78).
According to the expression profile of non-small
cell lung cancer (NSCLC) obtained from the National Center for
Biotechnology Information-Gene Expression Omnibus database
(https://www.ncbi.nlm.nih.gov/geo/),
UBE2T is one of the differential genes, and its prognosis risk
ratio is the most remarkable. Its increase was identified as a
potential risk factor for pathological stage I lung adenocarcinoma
(LUAD) (79). Certain differential
genes, particularly UBE2T, showed similar survival risks (80). Elevated expression of these genes,
including UBE2T, was associated with poorer OS in patients with
NSCLC (81). The expression of
UBE2T in LUAD was correlated with late clinicopathological factors
(age, sex, clinical stage, and T and M classification). Survival
analysis also revealed a similar trend, where high expression of
UBE2T was associated with poor prognosis (82). UBE2T exhibited the strongest
protein-protein interaction with other 7 protein types based on
protein-protein interaction networks (83). According to data from cBioPortal
(https://www.cbioportal.org/), UBE2T was
amplified in ~7% of cases of NSCLC and was associated with disease
recurrence after surgical resection. No significant molecular
alterations or clear trends in clinical outcomes were observed in
these genes (84).
UBE2T impacts certain downstream genes of the
β-catenin and Wnt/β-catenin signalling pathways. In NSCLC cells
transfected with si-UBE2T, the protein levels of β-catenin, c-Myc
and cyclin D1 were significantly decreased, while the expression of
E-cadherin was significantly increased (85). UBE2T, a novel physiological
substrate of the E3 ubiquitin ligase NEDD4 like E3 ubiquitin
protein ligase (NEDD4L), targets the ubiquitination and degradation
of UBE2T, and leads to the inhibition of PI3K-Akt signalling,
thereby inhibiting LUAD progression (86). UBE2T stimulated autophagy, and
silencing it eliminated autophagy in LUAD cells. Blockade of p53
counteracted the inhibitory effect of UBE2T depletion on autophagy.
In addition, the AMPK/mTOR axis was activated during UBE2T-mediated
autophagy, while UBE2T promoted autophagy through the p53/AMPK/mTOR
signaling pathway (87).
UBE2T in pharynx, bones and skin
UBE2T overexpression increased β-catenin expression,
enhanced p-Akt and p-GSK3β, and promoted β-catenin nuclear
translocation (6). Transcription
and DNA copy number of UBE2T were significantly increased in
esophageal squamous cell carcinoma (ESCC). ESCC is usually caused
by base excision repair, cell cycle, DNA replication, FA, mismatch
repair or the p53 signaling pathway. The protein level of UBE2T was
significantly associated with disease-free survival, but not with
OS, in ESCC (88).
UBE2T is also highly expressed in human
osteosarcoma. Silencing UBE2T inhibited the proliferation of
osteosarcoma cells and induced cell arrest in the G2/M
phase (89). The effect of UBE2T
knockdown on the activity of the PI3K/Akt signaling pathway was
investigated. The protein levels of p-PI3K and p-Akt were
significantly downregulated in UBE2T-silenced MG63 cells compared
with those in the corresponding control cells. However, the total
protein levels of PI3K and Akt were hardly affected (90).
UBE2T is a novel target gene of miR-498, which can
directly bind to the 3′-untranslated region of UBE2T and inhibit
the level of UBE2T. Overexpression of UBE2T could reverse the
inhibitory effect of miR-498 on the progression of malignant
melanoma (MM) cells. High levels of UBE2T were associated with poor
prognosis in MM (91), and showed a
significant association with mitosis (92).
UBE2T in the breast and genital
system
The hormone positive (HR+) subtype (also
named luminal type) is the most common type of BrC. UBE2T is
associated with the survival rate, but HR+ BrC cells
showed dependence on UBE2C (93).
UBE2T was highly expressed in BrC, particularly in triple-negative
BrC (TNBC) and human epidermal growth factor receptor 2-positive
BrC. It was also significantly positively correlated with T helper
(Th)2 in all BrC subtypes. Its upregulation in different subtypes
led to Th1/Th2 imbalance, while polarization towards Th2 cells may
lead to disease progression (94).
miR-543 directly targeted UBE2T, and a negative correlation between
miR-543 and UBE2T was observed in BrC tissues. In addition,
overexpression of miR-543 disrupted the cancer-promoting effects of
UBE2T by inhibiting the activity of the ERK/MAPK signaling pathway,
thus inhibiting the viability, proliferation, migration and
invasion of BrC (95).
Various genes, including UBE2T, were found to be
associated with an unfavourable prognosis in each BrC subtype
(54). High expression of UBE2T
indicated a lower pathological complete remission rate in patients
with TNBC after neoadjuvant chemotherapy, and in patients with
luminal BrC with tumor recurrence within 5 years after endocrine
therapy or chemotherapy (96).
Compared with that in the normal breast epithelial cell line
MCF-10A, the expression of UBE2T was upregulated in BrC cells.
Overexpression of UBE2T promoted cell proliferation, migration,
invasion and glycolysis, while UBE2T knockdown showed the opposite
results (97).
In total, 8 highly connected hub genes, were
selected for further study (98).
UBE2T was significantly associated with BrC, and was involved in
cell cycle, DNA replication, p53 signaling pathway, prognosis of
patients with BrC and aggressive degrees (98). UBE2T was amplified in ~12% of breast
tumors, while in ovarian serous cystadenocarcinoma, no significant
molecular alterations or clear trends in clinical outcomes were
observed (84). Upon UBE2T
knockdown, Akt/mTOR inactivation activated autophagy in ovarian
cancer (OV) cells, causing UBE2T depletion and inhibiting EMT.
UBE2T upregulation could predict poor prognosis and promote the
malignant progression of OV (7).
UBE2T upregulation also showed a strong correlation with poor OS in
OV. Moreover, UBE2T was closely associated to specific immune
cells, which were mainly involved in cell cycle-related events, and
in Titin and p53 mutations. UBE2T copy number amplification and
hypomethylation may be responsible for its upregulation in OV
(57). UBE2T overexpression of
contributed to the proliferation and metastasis of cervical cancer
cells, and UBE2T-overexpressing cervical cancer cells exhibited
enhanced self-renewal ability. UBE2T promoted cervical cancer stem
cell characteristics and exerted carcinogenic effects by activating
the glucose regulated protein 78 (GRP78)/focal adhesion kinase
signaling pathway (18).
UBE2T in the urinary system
The expression of UBE2T and vimentin was positively
correlated with the metastatic ability of prostate cancer (PC).
Overexpression of UBE2T induced EMT, and promoted PC proliferation
and metastasis. It acted as an oncogene, at least in part through
cooperation with vimentin (99). In
addition, UBE2T and the LASSO regression analysis were used to
calculate the autophagy score of each patient in a previous study
(100).
UBE2T could be detected in the nucleus and cytoplasm
of cancer cells, but showed stronger expression in the nucleus.
UBE2T knockdown significantly decreased the proliferation ability
of BC cells. Moreover, silencing UBE2T induced cell cycle arrest at
the G2/M phase and increased apoptosis; thus, it may be
considered a potential biomarker and therapeutic target for BC
(101). Univariate Cox regression
results showed that it was potential ubiquitination-related
prognostic molecule, and it was verified to be associated with OS
(102). High expression of UBE2T
was positively correlated with advanced pathological stage, distant
metastasis, histological grade and maximum tumor diameter.
miR-182-5p exhibited inhibitory effects on the development,
proliferation, migration and invasion of clear cell renal cell
carcinoma (ccRCC) by targeting UBE2T (103). Gao et al (104) analyzed and screened 5 hypoxia gene
subsets by using the Gene Set Enrichment Analysis. UBE2T was one of
the genes, and the genomic characteristics were significantly
correlated with the survival rate of ccRCC. UBE2T was significantly
correlated with advanced tumor stage and high grade in RCC, and the
prognosis was poor with its high expression. Vimentin and
N-cadherin, which are markers of mesenchymal cells, were decreased
with UBE2T knockdown, while the levels of E-cadherin and
fibronectin were enhanced, indicating that the EMT process was
blocked (5).
UBE2T in miscellaneous systems
Myeloma and leukemia
UBE2T is often amplified, and this is frequently
found in multiple myeloma (MuM), where its increased copy number
and expression are associated with low survival. This indicated
that UBE2T was required for efficient DNA repair by homologous
recombination (1). UBE2T is a
meaningful indicator of MuM staging, particularly in the early
stage. Its expression increased with the deterioration of MuM
compared with stages I and II, while the expression of UBE2T in
stage III was significantly higher, as well as in patients with
relapse. UBE2T overexpression was associated with poor prognosis,
and amplification of 1q21 indicated poor results, revealing that
the UBE2T level increased with the increase of amplification
(11). Its strong association with
homologous recombination and the abundance of UBE2T in subgroups of
MuM suggest a central role for gain of chromosome 1q and
upregulation of UBE2T as potential drivers of MuM aggressiveness
(105). Clustered regularly
interspaced short palindromic repeats single guide RNA determined
the specific dependence of UBE2T on the ubiquitination mechanism
involved in acute myeloid leukaemia (46) Downregulation of UBE2T induced by
bromodomain and extra-terminal protein family inhibitor (BETi)
enhanced the levels of GSK3β S9 phosphorylation and β-catenin. BETi
decreased UBE2T levels and increased the phosphorylation of GSK3β
S9; however, combined depletion of UBE2T and UBE2C strengthened the
anti-proliferative effect of a PI3K inhibitor (106).
Eye, brain and nervous system
As one of the core genes, UBE2T was closely
associated with anaplastic grade, and its level was significantly
different in retinoblastoma cells (107). UBE2T and GRP78 have a dominant
association. UBE2T enhanced glioblastoma invasion and migration
abilities via GRP78. UBE2T could maintain the stability of GRP78,
but the level of EMT biomarkers alterations was significantly
different upon silencing UBE2T, which led to significant
overexpression of E-cadherin, and downregulation of N-cadherin and
vimentin (108). DEP domain
containing 1B affected baculoviral IAP repeat containing 5
ubiquitination via UBE2T, causing its deregulation and thus
regulating the progression of chordoma (109).
UBE2T-mediated signaling pathways in
cancer
EMT is an important biological process in which
malignant tumor cells derived from epithelial cells acquire the
abilities of migration and invasion. The related proteins mainly
involved in UBE2T research include β-catenin and E-cadherin, which
participate in the EMT-related Wnt/β-catenin signaling pathway
(5). For example, RACK1 inhibited
the degradation of UBE2T and decreased the stability of Wnt after
increasing the expression of UBE2T/β-catenin, promoted the EMT
process, and led to the infiltration and migration of cancer cells
(72). Ubiquitination of
mutator-like elements via UBE2T inhibited the degradation of
β-catenin protein and promoted the EMT process (62). Moreover, mTOR and p-Wnt could also
be activated by β-catenin, and promoted the EMT process (61).
Cell cycle regulation is common in cancer research
focused on UBE2T. UBE2T-mediated overexpression of ubiquitinated
p53 activated mouse double minute 2 homolog (MDM2), and both
PI3K/protein tyrosine phosphatase 3 and MDM2 could activate Akt,
and inhibit GSK3β and p21 (6,8). GSK3β
could inhibit cyclin D1 to increase the risk of carcinogenesis, and
p21 could activate cyclin D1 to regulate the cell cycle (9). It has been reported that β-catenin,
after activating c-Myc, can activate cyclin D1 to control the cell
cycle, and NEDD4L can inhibit the PI3K-Akt signaling pathway by
targeting the ubiquitination and degradation of UBE2T, thus
inhibiting the progression of cancer cells (86). UBE2T-related pathways usually lead
to changes in cell number in the G2/M phase (66,89).
In the autophagic phenotype, UBE2T could inhibit Akt
after deubiquitination of Lys63, and activate the mTOR signaling
pathway upon phosphorylation of Akt after inhibition, thus
activating cell autophagy (58). In
addition, UBE2T downregulated the p53 level, and activated the
AMPK/mTOR axis, suggesting that it promoted autophagy through
p53/AMPK/mTOR signaling (87).
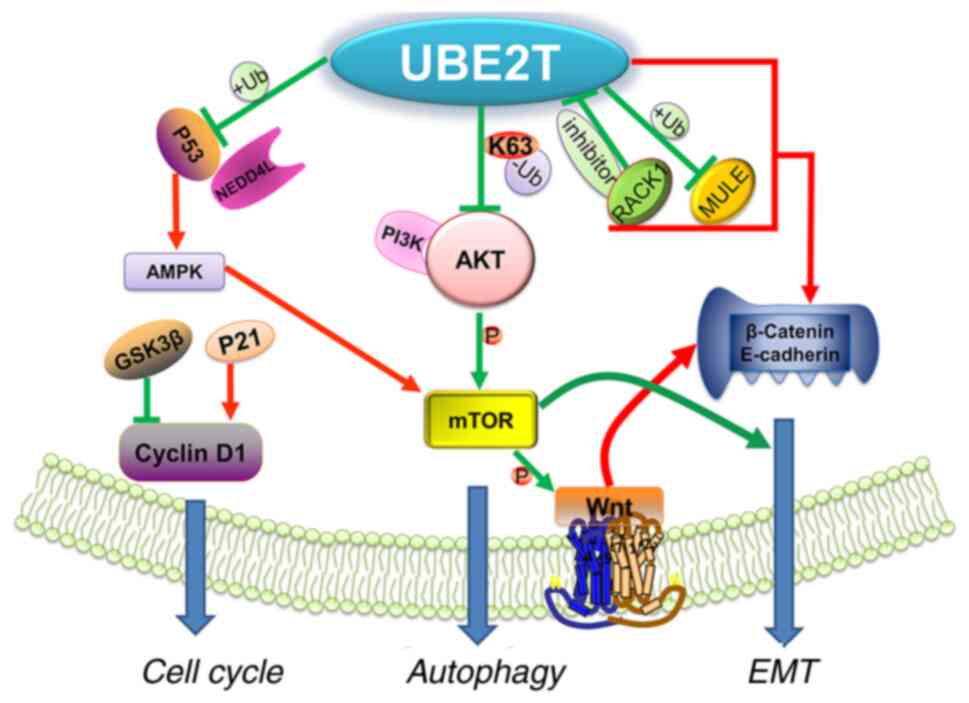
The aforementioned are the three common phenotypes
and related pathways described in the current study, but are not
limited to the aforementioned mechanisms (Fig. 5) (108), since there are also complex
regulatory associations and cross-links between different pathways,
which must be further evaluated in future studies (10).
Cancer therapies involving UBE2T
Chemosensitivity
Surgery remains the best treatment for localized
cancer, and is often combined with conventional radiotherapy and
chemotherapy. Previous evidence confirmed that palbociclib and
ribosciclib, two CDK4/6 inhibitors, suppressed the expression of
the ubiquitin-conjugating enzyme UBE2T in luminal BrC (93). Leflunomide, a DHODH inhibitor that
has been approved by the USA Food and Drug Administration for the
treatment of rheumatoid arthritis, decreased DHODH levels and
attenuated the DHODH upregulation induced by UBE2T, and reduced the
proliferation ability of UBE2T-overexpressing cells (58). Rapamycin (RAPA), a specific mTOR
inhibitor, was used to further investigate the UBE2T effect via the
EMT process and mTOR signaling in HuCCT1 and QBC939 cells. The
application of RAPA suppressed the proliferation, migration and
invasion abilities of cells, and effectively attenuated the effects
induced by UBE2T overexpression in CAA cells, namely EMT process
activation and mTOR downstream effectors enhancement (110).
Certain common chemotherapeutic drugs, including
cisplatin, paclitaxel, gemcitabine and docetaxel, were more
sensitive to cisplatin and gemcitabine in patients with ESCC in a
low-risk group based on TCGA-ESCC cohort analysis (88). Cisplatin stimulated autophagy in GC
(A549) cells, and overexpression of UBE2T further exacerbated
autophagy, involving cisplatin-induced protective autophagy.
Moreover, UBE2T overexpression counteracted the chemosensitivity of
A549 cells to cisplatin treatment, while inhibiting autophagy by
chloroquine reversed the UBE2T-induced cisplatin resistance
(87). Currently, treating tumors
such as NSCLC is becoming difficult because chemotherapeutic drugs
are ineffective due to resistance. It was found that the
hsa_circ_0092887-mediated miR-490-5p/UBE2T signaling axis may
contribute to paclitaxel-resistance intervention in NSCLC (111).
MOLM-13 cells treated with 5-azacytidine were also
significantly less sensitive to UBE2T inactivation (46). A previous study evaluated the effect
of UBE2T alteration on the chemoresistance of HCC by using annexin
V (62). UBE2T-silenced HCC cells
(MHCC-97L and PLC/PRF/5) exhibited enhanced cell death after
doxorubicin, sorafenib and lenvatinib treatment. By contrast,
UBE2T-overexpressing Huh7 cells exhibited reduced cell death when
treated with doxorubicin (62).
It has been found that M435-1279, a novel UBE2T
small molecule inhibitor, significantly inhibited the proliferation
of HGC27, AGS and MKN45 cells, while the small molecule
AG-690/12244866 significantly supressed HGC27 cell proliferation
but not AGS or MKN45 cell proliferation. M435-1279 exerted lower
cytotoxicity in GES-1 cells than AG-690/12244866, indicating that
M435-1279 may be a potential drug for the treatment of GC (72). UBE2T promoted the proliferation and
metastasis of nasopharyngeal carcinoma cells via modulating the
Akt/GSK3β/β-catenin axis. MK-2206 2HCl, as a specific inhibitor of
Akt, could block the pro-migration and -invasion effects of UBE2T
(6).
UBE2T knockdown not only decreased the viability of
MM1S and U266 cells in vitro (in MuM), but also apparently
reinforced the cytotoxicity of the DNA-damaging agents camptothecin
(0–40 nM), MMC (0–80 nM) and melphalan (0–20 µM). The
IC50 of these three agents in UBE2T-silenced MuM cell
lines was 2.7-11-fold lower than that in control cells (1). Wortmannin is an Akt inhibitor that
could significantly enhance the proliferation and invasion
abilities of MG63 cells when UBE2T was knocked out (90). Similarly, it could reverse the
increased phosphorylation of PI3K, Akt and mTOR induced by UBE2T
overexpression, and even attenuate the UBE2T
overexpression-mediated induced proliferation of 786-O cells
(5). In the bortezomib and
dexamethasone groups, there was no marked alteration in UBE2T in
MuM in either of the treatment responses, although triple drug
therapy (vincristine, adriamycin and dexamethasone) showed
favourable partial response as induction therapy followed by
autologous stem cell transplant as a maintenance therapy (11). Hydroxyurea and aphidicolin produced
more potent inhibiting effects in a concentration-dependent manner
on the proliferation of BrC cells transfected with UBE2T small
hairpin RNA. In addition, UBE2T suppression enhanced the
therapeutic benefits of drugs that functioned by inducing DNA
replication stress (4). BETi
enhanced lymphoma vulnerability to PI3K inhibitors by
downregulating UBE2T, which further enhanced the negative feedback
regulation of the PI3K signaling pathway (106).
It was found that several cell lines (HT29, DU145,
MCF-7, MDA-MD-468, U373, HCT116, HeLa and ME180) exposed to a
hypoxic environment (<0.02% O2) for 24 or 48 h were
more sensitive to treatment with 1 µg/ml MMC (112). Notably, the activation of the FA
pathway has been linked to chemotherapy resistance in several types
of cancer. A previous study identified a small-molecule inhibitor
of UBE2T/FANCL-mediated FANCD2 mono-ubiquitylation that sensitizes
cancer cell lines to the DNA cross-linking agent. Two compounds,
CU1 and CU2, were identified to sensitize osteosarcoma cells to a
more clinically relevant chemotherapeutic agent. However, CU1
exhibited strong cytotoxicity, while CU2 exhibited promising
selectivity in biochemical ubiquitylation assays and demonstrated
activity against the FA pathway in U2OS cells. Thus, it could be
used in clinical applications to sensitize cancer cells to DNA
cross-linking agents such as carboplatin or MMC (44).
Radiosensitivity
DDR is closely associated with radioresistance in
cancer cell lines. The survival fraction, as well as the volume and
weight of HCC tumors (xenografts originating from MHCC-97H cells),
were longer, larger and heavier after radiotherapy. In
UBE2T-overexpressing cells, there were fewer cells with >10
γH2AX foci, lower γH2AX levels after irradiation (IR), and improved
recovery back to the basal levels, compared with those in control
cells, which was indicative of a stronger DDR (64). Cantharidin is an inhibitor of
protein phosphatase 2 A, and has been demonstrated to be able to
arrest the cell cycle in the G2/M phase. It could
sensitize PC cells to radiotherapy, involving cell cycle
modulation, increased DNA damage and DDR suppression (113).
NSCLC (H1299) and osteosarcoma (U-2OS and MG-63)
cells with UBE2T knockdown were more sensitive to IR than control
cells, and A549 cells overexpressing UBE2T were more resistant to
radiotherapy (10,89). Radiation resistance could affect
prognosis in NSCLC, and UBE2T promoted radiation resistance in
vitro (0–10 Gy) and in vivo (10 Gy) via accelerating the
G2/M transition and inhibiting apoptosis. UBE2T could
contribute to radiation resistance via the ubiquitination of
forkhead box protein O1, and it could reverse radiation resistance
in NSCLC cells (10).
Osteosarcoma cell lines (simultaneously transfected
with siRNA-UBE2T or siRNA-control) were irradiated with 0, 2, 4, 6,
8 or 10 Gy and then incubated for 1–2 weeks. Silencing UBE2T could
significantly strengthen the effect of radiation in osteosarcoma.
UBE2T knockdown combined with X-ray IR could significantly reduce
the proliferation of osteosarcoma cells and the growth of
osteosarcoma (6 Gy), as well as inhibit metastasis, stimulate the
production of reactive oxygen species, and promote apoptosis
(89).
Conclusion
A growing number of studies have revealed that UBE2T
expression is upregulated in human pan-cancer. Recent evidence has
indicated the importance of UBE2T in tumorigenesis, proliferation,
migration, metastasis, drug resistance, radioresistance and poor
prognosis in cancer patients. The articles included in the present
review were focused on different tumor types, pathways and factors.
The purpose was to summarize the research gaps and identify topics
for further research. In the present review, it was discussed that
UBE2T activates the mono-ubiquitination of FANCD2 and has been
reported to be involved in cancer development. The roles of UBE2T
and associated enzymes in pan-cancer pathogenesis involving DNA
repair, cell cycle, apoptosis and oncogenic signaling were
reviewed. In addition, the roles of UBE2T in tumorigenesis,
progression and treatment, as well as the development of small
molecule modulators to regulate ubiquitination therapeutic
strategies were also discussed.
Increased transcription and translation of UBE2T has
been widely linked to pan-cancer. Numerous studies show serious
limitations, since despite the fact that numerous studies are
available, a considerable part of them derive from bioinformatics
analysis, and lack experiments to verify the screening information
from the datasets.
UBE2T-associated genes or pathways have not been
clearly recognized as drug targets or prognostic biomarkers,
including TPX2, CDC20, CDC20, CDC45, ANLN, ASPM, PRC1, CCNB2,
CCNB2, MELK, PRC1, TOP2A and KIAA0101. Regulating these
genes/proteins in cancer cell lines or mouse/rat models via
silencing, overexpression, inhibitor, chemosensitivity or
radiosensitivity may clarify their therapeutic value in cancer.
The majority of studies have focused on the
mechanism of UBE2T in the occurrence, development and prognosis of
cancer. Overexpression of UBE2T results in tumour growth and
deterioration, and by knocking out or overexpressing UBE2T,
cancer-suppressing functions can be activated or inactivated in
pan-cancer. However, the current data suggest that this modulation
may be specific, and exactly which genes/pathways are affected by
EMT, cell cycle and autophagy should be identified. Additionally,
chemosensitivity and radiosensitivity can regulate cancer cell fate
and status, and affect their functions in the pathological process.
Once these target mechanisms are elucidated, more precise and
efficient treatment therapies can be expected.
UBE2T is a typical oncogene that is involved in
chromosome instability syndromes and immunological disorder besides
carcinogenesis. It does not exhibit cancer-inhibitory effects. As a
disease-associated E2-conjugating enzyme, UBE2T, has been reported
to be more than just an intermediary of the ubiquitin signaling
pathway, by playing multifaceted roles in human pathology. UBE2T
insufficiency in tumors is expected to enhance the tumor growth
inhibitory effect of IR/chemical components.
Notably, a considerable part of the current data on
UBE2T in cancer are preclinical data from major databases and
clinical tissue samples. Limited experimental studies suggested
numerous potential roles for UBE2T, which remain to be elucidated.
Clinical data have highlighted the role of the UPS. Unexpectedly,
it was found that there were 16 clinical trials on ubiquitin
enzymes, which involved the inhibitor of ubiquitin-activating
enzyme MLN7243 (ClinicalTrials.gov Identifier NCT02045095) and the
ubiquitin-binding protein p62 (ClinicalTrials.gov Identifier NCT03925753). Novel E2s
therapies for cancer should be investigated in clinical trials, and
the identification of novel E2s as potential cancer drug targets,
as well as the development of new specific chemical probes of UBE2T
should be explored in future studies.
Acknowledgements
Not applicable.
Funding
The present study was funded by Research and development
projects in key areas of the Hunan Provincial Department of Science
and Technology (grant no. 2020SKC2009) and the Scientific research
project of Hunan Provincial Health Commission (grant no.
202112070150).
Availability of data and materials
Not applicable.
Authors' contributions
NM and XL proposed the concept and design. NM and
ZL acquired the data. JY and RX analyzed and interpreted the data
(e.g., statistical analysis, computational analysis). NM and XL
wrote the manuscript. LH supported administrative, technical, or
material support (i.e., reporting or organizing data, constructing
databases). XL supervised the study. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alagpulinsa DA, Kumar S, Talluri S,
Nanjappa P, Buon L, Chakraborty C, Samur MK, Szalat R, Shammas MA
and Munshi NC: Amplification and overexpression of E2 ubiquitin
conjugase UBE2T promotes homologous recombination in multiple
myeloma. Blood Adv. 3:3968–3972. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen F, Li M and Wang L: LncRNA CASC11
promotes hepatocellular carcinoma progression via upregulation of
UBE2T in a m(6)A-Dependent Manner. Front Oncol. 11:7726712021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Z, Jin P, Chen Z, Ye F, Ren Z, Ji T,
Li R and Yu L: The expression of circ_0090049 in hepatocellular
carcinoma and the molecular regulation mechanism of other
biological functions. Anticancer Drugs. 33:48–60. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dutta R, Guruvaiah P, Reddi KK, Bugide S,
Reddy Bandi DS, Edwards YJK, Singh K and Gupta R: UBE2T promotes
breast cancer tumor growth by suppressing DNA replication stress.
NAR Cancer. 4:zcac0352022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hao P, Kang B, Li Y, Hao W and Ma F: UBE2T
promotes proliferation and regulates PI3K/Akt signaling in renal
cell carcinoma. Mol Med Rep. 20:1212–1220. 2019.PubMed/NCBI
|
|
6
|
Hu W, Xiao L, Cao C, Hua S and Wu D: UBE2T
promotes nasopharyngeal carcinoma cell proliferation, invasion, and
metastasis by activating the AKT/GSK3β/β-catenin pathway.
Oncotarget. 7:15161–15172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang W, Huang H, Xiao Y, Wang L, Zhang T,
Fang X and Xia X: UBE2T is upregulated, predicts poor prognosis,
and promotes cell proliferation and invasion by promoting
epithelial-mesenchymal transition via inhibiting autophagy in an
AKT/mTOR dependent manner in ovarian cancer. Cell Cycle.
21:780–791. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu LP, Yang M, Peng QZ, Li MY, Zhang YS,
Guo YH, Chen Y and Bao SY: UBE2T promotes hepatocellular carcinoma
cell growth via ubiquitination of p53. Biochem Biophys Res Commun.
493:20–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo C, Yao Y, Yu Z, Zhou H, Guo L, Zhang
J, Cao H, Zhang G and Li Yand Jiao Z: UBE2T knockdown inhibits
gastric cancer progression. Oncotarget. 8:32639–32654. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin H, Wang X, Zhang X, Zeng Y, Xu Q, Wang
W, Zhou F and Zhou Y: UBE2T promotes radiation resistance in
non-small cell lung cancer via inducing epithelial-mesenchymal
transition and the ubiquitination-mediated FOXO1 degradation.
Cancer Lett. 494:121–131. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Zhang Y, Yang Z, Liu X, Yang P,
Wang J, Hu K, He X, Zhang X and Jing H: High expression of UBE2T
predicts poor prognosis and survival in multiple myeloma. Cancer
Gene Ther. 26:347–355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang SC and Ding JL: Ubiquitination and
SUMOylation in the chronic inflammatory tumor microenvironment.
Biochim Biophys Acta Rev Cancer. 1870:165–175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Damgaard RB: The ubiquitin system: From
cell signalling to disease biology and new therapeutic
opportunities. Cell Death Differ. 28:423–426. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stewart MD, Ritterhoff T, Klevit RE and
Brzovic PS: E2 enzymes: More than just middle men. Cell Res.
26:423–440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang SC, Zhang BX and Ding JL: E2-E3
ubiquitin enzyme pairing-partnership in provoking or mitigating
cancers. Biochim Biophys Acta Rev Cancer. 1877:1886792022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye Y and Rape M: Building ubiquitin
chains: E2 enzymes at work. Nat Rev Mol Cell Biol. 10:755–764.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng L, Meng T, Chen L, Wei W and Wang P:
The role of ubiquitination in tumorigenesis and targeted drug
discovery. Signal Transduct Target Ther. 5:112020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Ji W, Yue N and Zhou W:
Ubiquitin-conjugating enzyme E2T promotes tumor stem cell
characteristics and migration of cervical cancer cells by
regulating the GRP78/FAK pathway. Open Life Sci. 16:1082–1090.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Meng T, Cui S, Feng L, Liu D,
Pang Q and Wang P: Ubiquitination of nonhistone proteins in cancer
development and treatment. Front Oncol. 10:6212942020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang H, Fang T, Ji M, Wang JP, Song LL,
Zhang QY and Wu JS: UBE2S exerts oncogenic activities in urinary
bladder cancer by ubiquitinating TSC1. Biochem Biophys Res Commun.
578:7–14. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao B, Tsai YC, Jin B, Wang B, Wang Y,
Zhou H, Carpenter T, Weissman AM and Yin J: Protein engineering in
the ubiquitin system: Tools for discovery and beyond. Pharmacol
Rev. 72:380–413. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao R, Yu Z, Mao X, Zheng Y, Wang Y and
Zhou Y: Knockout of UBE2S inhibits the proliferation of gastric
cancer cells and induces apoptosis by FAS-mediated death receptor
pathway. Exp Cell Res. 419:1132932022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Z, Zhang M, Lu Y, Ding T, Liu Z, Liu
Y, Zhou Z and Wang L: Overexpressed lncRNA FTX promotes the cell
viability, proliferation, migration and invasion of renal cell
carcinoma via FTX/miR4429/UBE2C axis. Oncol Rep. 48:1632022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang GZ, Chen ZQ, Wu J, Shao TR, Zou C,
Ai YL and Lv XZ: Pan-Cancer analyses of the tumor microenvironment
reveal that ubiquitin-conjugating enzyme E2C Might Be a potential
immunotherapy target. J Immunol Res. 2021:92502072021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kariri Y, Toss MS, Alsaleem M, Elsharawy
KA, Joseph C, Mongan NP, Green AR and Rakha EA:
Ubiquitin-conjugating enzyme 2C (UBE2C) is a poor prognostic
biomarker in invasive breast cancer. Breast Cancer Res Treat.
192:529–539. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Presta I, Novellino F, Donato A, La Torre
D, Palleria C, Russo E, Malara N and Donato G: UbcH10 a major actor
in cancerogenesis and a potential tool for diagnosis and therapy.
Int J Mol Sci. 21:20412020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang YF, Chang YC, Tsai KW, Hung MH and
Kang BH: UBE2C triggers HIF-1α-glycolytic flux in head and neck
squamous cell carcinoma. J Cell Mol Med. 26:3716–3725. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan Y, Xiao WW, Xie WH, Li RZ and Gao YH:
Prognostic value of ubiquitin E2 UBE2W and its correlation with
tumor-infiltrating immune cells in breast cancer. BMC Cancer.
21:4792021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng X, Zheng W, Sheng Y and Ma H: UBE2B
promotes ovarian cancer growth via promoting RAD18 mediated ZMYM2
monoubiquitination and stabilization. Bioengineered. 13:8000–8012.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang CY and Yang M: Functions of three
ubiquitin-conjugating enzyme 2 genes in hepatocellular carcinoma
diagnosis and prognosis. World J Hepatol. 14:956–971. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang M, Liu Y, Yin Y, Sun Z, Wang Y,
Zhang Z, Li F and Chen X: UBE2S promotes the development of ovarian
cancer by promoting PI3K/AKT/mTOR signaling pathway to regulate
cell cycle and apoptosis. Mol Med. 28:622022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van Wijk SJ and Timmers HT: The family of
ubiquitin-conjugating enzymes (E2s): Deciding between life and
death of proteins. FASEB J. 24:981–993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alpi AF, Chaugule V and Walden H:
Mechanism and disease association of E2-conjugating enzymes:
Lessons from UBE2T and UBE2L3. Biochem J. 473:3401–3419. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alpi A, Langevin F, Mosedale G, Machida
YJ, Dutta A and Patel KJ: UBE2T, the Fanconi anemia core complex,
and FANCD2 are recruited independently to chromatin: A basis for
the regulation of FANCD2 monoubiquitination. Mol Cell Biol.
27:8421–8430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hodson C, Cole AR, Lewis LP, Miles JA,
Purkiss A and Walden H: Structural analysis of human FANCL, the E3
ligase in the Fanconi anemia pathway. J Biol Chem. 286:32628–32637.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hodson C, Purkiss A, Miles JA and Walden
H: Structure of the human FANCL RING-Ube2T complex reveals
determinants of cognate E3-E2 selection. Structure. 22:337–344.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rickman KA, Lach FP, Abhyankar A, Donovan
FX, Sanborn EM, Kennedy JA, Sougnez C, Gabriel SB, Elemento O,
Chandrasekharappa SC, et al: Deficiency of UBE2T, the E2 Ubiquitin
Ligase Necessary for FANCD2 and FANCI Ubiquitination, Causes FA-T
Subtype of Fanconi Anemia. Cell Rep. 12:35–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sheng Y, Hong JH, Doherty R, Srikumar T,
Shloush J, Avvakumov GV, Walker JR, Xue S, Neculai D, Wan JW, et
al: A human ubiquitin conjugating enzyme (E2)-HECT E3 ligase
structure-function screen. Mol Cell Proteomics. 11:329–341. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kar G, Keskin O, Nussinov R and Gursoy A:
Human proteome-scale structural modeling of E2-E3 interactions
exploiting interface motifs. J Proteome Res. 11:1196–1207. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Markson G, Kiel C, Hyde R, Brown S,
Charalabous P, Bremm A, Semple J, Woodsmith J, Duley S,
Salehi-Ashtiani K, et al: Analysis of the human E2 ubiquitin
conjugating enzyme protein interaction network. Genome Res.
19:1905–1911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pruneda JN, Littlefield PJ, Soss SE,
Nordquist KA, Chazin WJ, Brzovic PS and Klevit RE: Structure of an
E3:E2~Ub complex reveals an allosteric mechanism shared among
RING/U-box ligases. Mol Cell. 47:933–942. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lemonidis K, Arkinson C, Rennie ML and
Walden H: Mechanism, specificity, and function of FANCD2-FANCI
ubiquitination and deubiquitination. FEBS J. 289:4811–4829. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chaugule VK, Arkinson C, Rennie ML,
Kamarainen O, Toth R and Walden H: Allosteric mechanism for
site-specific ubiquitination of FANCD2. Nat Chem Biol. 16:291–301.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cornwell MJ, Thomson GJ, Coates J,
Belotserkovskaya R, Waddell ID, Jackson SP and Galanty Y:
Small-Molecule Inhibition of UBE2T/FANCL-Mediated Ubiquitylation in
the Fanconi Anemia Pathway. ACS Chem Biol. 14:2148–2154.
2019.PubMed/NCBI
|
|
45
|
Frost MG, Mazloumi Aboukheili AM, Toth R
and Walden H: Characterization of FANCL variants observed in
patient cancer cells. Biosci Rep. 40:BSR201913042020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang Z, Wu XS, Wei Y, Polyanskaya SA, Iyer
SV, Jung M, Lach FP, Adelman ER, Klingbeil O, Milazzo JP, et al:
Transcriptional Silencing of ALDH2 confers a dependency on fanconi
anemia proteins in acute myeloid leukemia. Cancer Discov.
11:2300–2315. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mehta PA and Ebens C: Fanconi Anemia.
GeneReviews((R)). Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace
SE, Bean LJH, Gripp KW and Amemiya A: (Seattle, WA).
1993.PubMed/NCBI
|
|
48
|
Yang Y, Guo T, Liu R, Ke H, Xu W, Zhao S
and Qin Y: FANCL gene mutations in premature ovarian insufficiency.
Hum Mutat. 41:1033–1041. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hira A, Yoshida K, Sato K, Okuno Y,
Shiraishi Y, Chiba K, Tanaka H, Miyano S, Shimamoto A, Tahara H, et
al: Mutations in the gene encoding the E2 conjugating enzyme UBE2T
cause Fanconi anemia. Am J Hum Genet. 96:1001–1007. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Virts EL, Jankowska A, Mackay C, Glaas MF,
Wiek C, Kelich SL, Lottmann N, Kennedy FM, Marchal C, Lehnert E, et
al: AluY-mediated germline deletion, duplication and somatic stem
cell reversion in UBE2T defines a new subtype of Fanconi anemia.
Hum Mol Genet. 24:5093–5108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Alpi AF, Pace PE, Babu MM and Patel KJ:
Mechanistic insight into site-restricted monoubiquitination of
FANCD2 by Ube2t, FANCL, and FANCI. Mol Cell. 32:767–777. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ueki T, Park JH, Nishidate T, Kijima K,
Hirata K, Nakamura Y and Katagiri T: Ubiquitination and
downregulation of BRCA1 by ubiquitin-conjugating enzyme E2T
overexpression in human breast cancer cells. Cancer Res.
69:8752–8760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ozmen Yaylaci A and Canbek M: The role of
ubiquitin signaling pathway on liver regeneration in rats. Mol Cell
Biochem. 478:131–147. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cabanas Morafraile E, Perez-Pena J,
Fuentes-Antras J, Manzano A, Pérez-Segura P, Pandiella A,
Galán-Moya EM and Ocaña A: Genomic Correlates of DNA damage in
breast cancer subtypes. Cancers (Basel). 13:21172021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tao Y, Li R, Shen C, Li J, Zhang Q, Ma Z,
Wang F and Wang Z: SENP1 is a crucial promotor for hepatocellular
carcinoma through deSUMOylation of UBE2T. Aging (Albany NY).
12:1563–1576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wei X, You X, Zhang J and Zhou C:
MicroRNA-1305 Inhibits the Stemness of LCSCs and tumorigenesis by
repressing the UBE2T-Dependent akt-signaling pathway. Mol Ther
Nucleic Acids. 16:721–732. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zou R, Xu H, Li F, Wang S and Zhu L:
Increased Expression of UBE2T Predicting Poor survival of
epithelial ovarian cancer: Based on comprehensive analysis of
UBE2s, Clinical Samples, and the GEO Database. DNA Cell Biol.
40:36–60. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhu Z, Cao C, Zhang D, Zhang Z, Liu L, Wu
D and Sun J: UBE2T-mediated Akt ubiquitination and Akt/beta-catenin
activation promotes hepatocellular carcinoma development by
increasing pyrimidine metabolism. Cell Death Dis. 13:1542022.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang F, Zhong S, Mao C, Jin J and Wang H:
Circ_0000291 contributes to hepatocellular carcinoma tumorigenesis
by binding to miR-1322 to up-regulate UBE2T. Ann Hepatol.
27:1007222022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ren X, Li A, Ying E, Fang J, Li M and Yu
J: Upregulation of ubiquitin-conjugating enzyme E2T (UBE2T)
predicts poor prognosis and promotes hepatocellular carcinoma
progression. Bioengineered. 12:1530–1542. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lioulia E, Mokos P, Panteris E and Dafou
D: UBE2T promotes β-catenin nuclear translocation in hepatocellular
carcinoma through MAPK/ERK-dependent activation. Mol Oncol.
16:1694–1713. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ho NPY, Leung CON, Wong TL, Lau EYT, Lei
MML, Mok EHK, Leung HW, Tong M, Ng IOL, Yun JP, et al: The
interplay of UBE2T and Mule in regulating Wnt/β-catenin activation
to promote hepatocellular carcinoma progression. Cell Death Dis.
12:1482021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhao X, Weng W, Jin M, Li S, Chen Q, Li B,
Zhou Z, Lan C and Yang Y: Identification of biomarkers based on
bioinformatics analysis: The expression of ubiquitin-conjugating
enzyme E2T (UBE2T) in the carcinogenesis and progression of
hepatocellular carcinoma. Med Sci Monit. 27:e9290232021.PubMed/NCBI
|
|
64
|
Sun J, Zhu Z, Li W, Shen M, Cao C, Sun Q,
Guo Z, Liu L and Wu D: UBE2T-regulated H2AX monoubiquitination
induces hepatocellular carcinoma radioresistance by facilitating
CHK1 activation. J Exp Clin Cancer Res. 39:2222020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Guo J, Wang M, Wang JP and Wu CX:
Ubiquitin-conjugating enzyme E2T knockdown suppresses
hepatocellular tumorigenesis via inducing cell cycle arrest and
apoptosis. World J Gastroenterol. 25:6386–6403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu LL, Zhu JM, Yu XN, Zhu HR, Shi X,
Bilegsaikhan E, Guo HY, Wu J and Shen XZ: UBE2T promotes
proliferation via G2/M checkpoint in hepatocellular carcinoma.
Cancer Manag Res. 11:8359–8370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yu H, Wang H, Dong W, Cao ZY, Li R, Yang
C, Cong WM, Dong H and Jin GZ: The diagnostic and prognostic value
of UBE2T in intrahepatic cholangiocarcinoma. PeerJ. 8:e84542020.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhu X, Li T, Niu X, Chen L and Ge C:
Identification of UBE2T as an independent prognostic biomarker for
gallbladder cancer. Oncol Lett. 20:442020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li L, Liu J and Huang W: E2F5 promotes
proliferation and invasion of gastric cancer through directly
upregulating UBE2T transcription. Dig Liver Dis. 54:937–945. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lin X, Han T, Xia Q, Cui J, Zhuo M, Liang
Y, Su W, Wang L, Wang L, Liu Z and Xiao X: CHPF promotes gastric
cancer tumorigenesis through the activation of E2F1. Cell Death
Dis. 12:8762021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Heitor da Silva Maues J, Ferreira Ribeiro
H, de Maria Maues Sacramento R, Maia de Sousa R, Pereira de Tommaso
R, Dourado Kovacs Machado Costa B, Cardoso Soares P, Pimentel
Assumpção P, de Fátima Aquino Moreira-Nunes C and Mário Rodriguez
Burbano R: Downregulated genes by silencing MYC pathway identified
with RNA-SEQ analysis as potential prognostic biomarkers in gastric
adenocarcinoma. Aging (Albany NY). 12:24651–24670. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yu Z, Jiang X, Qin L, Deng H, Wang J, Ren
W, Li H, Zhao L, Liu H, Yan H, et al: A novel UBE2T inhibitor
suppresses Wnt/beta-catenin signaling hyperactivation and gastric
cancer progression by blocking RACK1 ubiquitination. Oncogene.
40:1027–1042. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yu H, Xiang P, Pan Q, Huang Y, Xie N and
Zhu W: Ubiquitin-Conjugating Enzyme E2T is an independent
prognostic factor and promotes gastric cancer progression. Tumour
Biol. 37:11723–11732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Luo M and Zhou Y: Comprehensive analysis
of differentially expressed genes reveals the promotive effects of
UBE2T on colorectal cancer cell proliferation. Oncol Lett.
22:7142021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wu M, Li X, Huang W, Chen Y, Wang B and
Liu X: Ubiquitin-conjugating enzyme E2T(UBE2T) promotes colorectal
cancer progression by facilitating ubiquitination and degradation
of p53. Clin Res Hepatol Gastroenterol. 45:1014932021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zheng YW, Gao PF, Ma MZ, Chen Y and Li CY:
Role of ubiquitin-conjugating enzyme E2T in the carcinogenesis and
progression of pancreatic cancer. Oncol Lett. 20:1462–1468. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang J, Wang J, Wu J, Huang J, Lin Z and
Lin X: UBE2T regulates FANCI monoubiquitination to promote NSCLC
progression by activating EMT. Oncol Rep. 48:1392022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li Y, Yang X and Lu D: Knockdown of
ubiquitin-conjugating enzyme E2T (UBE2T) suppresses lung
adenocarcinoma progression via targeting fibulin-5 (FBLN5).
Bioengineered. 13:11867–11880. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Deng Y, Chen X, Huang C, Song J, Feng S,
Chen X and Zhou R: Screening and validation of significant genes
with poor prognosis in pathologic stage-I lung adenocarcinoma. J
Oncol. 2022:37940212022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen Y, Jin L, Jiang Z, Liu S and Feng W:
Identifying and validating potential biomarkers of early stage lung
adenocarcinoma diagnosis and prognosis. Front Oncol. 11:6444262021.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tu H, Wu M, Huang W and Wang L: Screening
of potential biomarkers and their predictive value in early stage
non-small cell lung cancer: A bioinformatics analysis. Transl Lung
Cancer Res. 8:797–807. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wu ZH, Zhang YJ and Sun HY: High ubiquitin
conjugating enzyme E2 T mRNA expression and its prognostic
significance in lung adenocarcinoma: A study based on the TCGA
database. Medicine (Baltimore). 99:e185432020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yao R, Chen X, Wang L, Wang Y, Chi S, Li
N, Tian X, Li N and Liu J: Identification of key protein-coding
genes in lung adenocarcinomas based on bioinformatic analysis.
Transl Cancer Res. 8:2829–2840. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Perez-Pena J, Corrales-Sanchez V, Amir E,
Pandiella A and Ocana A: Ubiquitin-conjugating enzyme E2T (UBE2T)
and denticleless protein homolog (DTL) are linked to poor outcome
in breast and lung cancers. Sci Rep. 7:175302017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu J and Liu X: UBE2T silencing inhibited
non-small cell lung cancer cell proliferation and invasion by
suppressing the wnt/β-catenin signaling pathway. Int J Clin Exp
Pathol. 10:9482–9488. 2017.PubMed/NCBI
|
|
86
|
Chen Y, Hong H, Wang Q, Li J, Zhang W,
Chen T and Li P: NEDD4L-induced ubiquitination mediating UBE2T
degradation inhibits progression of lung adenocarcinoma via
PI3K-AKT signaling. Cancer Cell Int. 21:6312021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhu J, Ao H, Liu M, Cao K and Ma J: UBE2T
promotes autophagy via the p53/AMPK/mTOR signaling pathway in lung
adenocarcinoma. J Transl Med. 19:3742021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang X, Liu Y, Leng X, Cao K, Sun W, Zhu J
and Ma J: UBE2T Contributes to the prognosis of esophageal squamous
cell carcinoma. Pathol Oncol Res. 27:6325312021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shen L, Zhao K, Li H, Ning B, Wang W, Liu
R, Zhang Y and Zhang A: Downregulation of UBE2T can enhance the
radiosensitivity of osteosarcoma in vitro and in vivo. Epigenomics.
11:1283–1305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wang Y, Leng H, Chen H, Wang L, Jiang N,
Huo X and Yu B: Knockdown of UBE2T inhibits osteosarcoma cell
proliferation, migration, and invasion by suppressing the PI3K/Akt
signaling pathway. Oncol Res. 24:361–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Cao W, Ni L, Li P, Wang QX, Li MM, Huang
SH and Dang NN: miR-498 Targets UBE2T to inhibit the proliferation
of malignant melanoma cells. Technol Cancer Res Treat.
21:153303382210824312022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Gorlov I, Orlow I, Ringelberg C, Hernando
E, Ernstoff MS, Cheng C, Her S, Parker JS, Thompson CL,
Gerstenblith MR, et al: Identification of gene expression levels in
primary melanoma associated with clinically meaningful
characteristics. Melanoma Res. 28:380–389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lin CY, Yu CJ, Liu CY, Chao TC, Huang CC,
Tseng LM and Lai JI: CDK4/6 inhibitors downregulate the
ubiquitin-conjugating enzymes UBE2C/S/T involved in the
ubiquitin-proteasome pathway in ER + breast cancer. Clin Transl
Oncol. 24:2120–2135. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xiao Y, Deng Z, Li Y, Wei B, Chen X, Zhao
Z, Xiu Y, Hu M, Alahdal M, Deng Z, et al: ANLN and UBE2T are
prognostic biomarkers associated with immune regulation in breast
cancer: A bioinformatics analysis. Cancer Cell Int. 22:1932022.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Li L and Li Q: miR-543 impairs breast
cancer cell phenotypes by targeting and suppressing
ubiquitin-conjugating enzyme E2T (UBE2T). Bioengineered.
12:12394–12406. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Fuentes-Antras J, Alcaraz-Sanabria AL,
Morafraile EC, Noblejas-López MDM, Galán-Moya EM, Baliu-Pique M,
López-Cade I, García-Barberán V, Pérez-Segura P, Manzano A, et al:
Mapping of genomic vulnerabilities in the post-translational
ubiquitination, SUMOylation and neddylation machinery in breast
cancer. Cancers (Basel). 13:8332021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Qiao L, Dong C and Ma B: UBE2T promotes
proliferation, invasion and glycolysis of breast cancer cells by
regualting the PI3K/AKT signaling pathway. J Recept Signal
Transduct Res. 42:151–159. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang Y, Li Y, Liu B and Song A:
Identifying breast cancer subtypes associated modules and
biomarkers by integrated bioinformatics analysis. Biosci Rep.
41:BSR202032002021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wen M, Kwon Y, Wang Y, Mao JH and Wei G:
Elevated expression of UBE2T exhibits oncogenic properties in human
prostate cancer. Oncotarget. 6:25226–25239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wen C, Ge Q, Dai B, Li J, Yang F, Meng J,
Gao S, Fan S and Zhang L: Signature for prostate cancer based on
autophagy-related genes and a nomogram for quantitative risk
stratification. Dis Markers. 2022:75989422022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Gong YQ, Peng D, Ning XH, Yang XY, Li XS,
Zhou LQ and Guo YL: UBE2T silencing suppresses proliferation and
induces cell cycle arrest and apoptosis in bladder cancer cells.
Oncol Lett. 12:4485–4492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Cai H, Chen H, Huang Q, Zhu JM, Ke ZB, Lin
YZ, Zheng QS, Wei Y, Xu N and Xue XY: Ubiquitination-Related
molecular subtypes and a novel prognostic index for bladder cancer
patients. Pathol Oncol Res. 27:16099412021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wu Y, Zhang C, Peng D, He S, Huang C, Qian
J, Zhu W, Feng N, Gong Y, Li X and Zhou L: MiR-182-5p inhibits the
tumorigenesis of clear cell renal cell carcinoma by repressing
UBE2T. Hum Cell. 35:542–556. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Gao J, Ye F, Han F, Wang X, Jiang H and
Zhang J: A novel radiogenomics biomarker based on hypoxic-gene
subset: Accurate survival and prognostic prediction of renal clear
cell carcinoma. Front Oncol. 11:7398152021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li C, Wendlandt EB, Darbro B, Xu H, Thomas
GS, Tricot G, Chen F, Shaughnessy JD Jr and Zhan F: Genetic
analysis of multiple myeloma identifies cytogenetic alterations
implicated in disease complexity and progression. Cancers (Basel).
13:5172021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Derenzini E, Mondello P, Erazo T,
Portelinha A, Liu Y, Scallion M, Asgari Z, Philip J, Hilden P,
Valli D, et al: BET Inhibition-Induced GSK3β feedback enhances
lymphoma vulnerability to PI3K Inhibitors. Cell Rep. 24:2155–2166.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang Z, Chen N, Liu C, Cao G, Ji Y, Yang W
and Jiang Q: UBE2T is a prognostic biomarker and correlated with
Th2 cell infiltrates in retinoblastoma. Biochem Biophys Res Commun.
614:138–144. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Huang P, Guo Y, Zhao Z, Ning W, Wang H, Gu
C, Zhang M, Qu Y, Zhang H and Song Y: UBE2T promotes glioblastoma
invasion and migration via stabilizing GRP78 and regulating EMT.
Aging (Albany NY). 12:10275–10289. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wang L, Tang L, Xu R, Ma J, Tian K, Liu Y,
Lu Y, Wu Z and Zhu X: DEPDC1B regulates the progression of human
chordoma through UBE2T-mediated ubiquitination of BIRC5. Cell Death
Dis. 12:7532021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu F, Zhu C, Gao P, Zheng S and Li C:
Ubiquitin-conjugating enzyme E2T regulates cell proliferation and
migration in cholangiocarcinoma. Anticancer Drugs. 31:836–846.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wang L, Zhang Z and Tian H:
Hsa_circ_0092887 targeting miR-490-5p/UBE2T promotes paclitaxel
resistance in non-small cell lung cancer. J Clin Lab Anal.
37:e247812023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ramaekers CH, van den Beucken T, Meng A,
Kassam S, Thoms J, Bristow RG and Wouters BG: Hypoxia disrupts the
Fanconi anemia pathway and sensitizes cells to chemotherapy through
regulation of UBE2T. Radiother Oncol. 101:190–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Xu MD, Liu SL, Zheng BB, Wu J, Wu MY,
Zhang Y, Gong FR, Tao M, Zhang J and Li W: The
radiotherapy-sensitization effect of cantharidin: Mechanisms
involving cell cycle regulation, enhanced DNA damage, and inhibited
DNA damage repair. Pancreatology. 18:822–832. 2018. View Article : Google Scholar : PubMed/NCBI
|