Gastric cancer (GC) is one of the most common cancer
type in China, and newly diagnosed cases increased from 403,000 in
2015 to 479,000 in 2020 (1). It
remains the fourth leading cause of cancer mortality worldwide
(2). A large portion of patients
with GC do not respond to conventional therapies or are at a higher
risk for recurrent disease after they received those interventions,
such as perioperative chemotherapy or chemotherapy alone (3,4) and
anti-HER2 agent trastuzumab in combination with chemotherapy
(5).
Over the last decade, the tumor microenvironment
(TME) has become a hot topic for overcoming treatment failure or
resistance to current therapies for advanced cancer, including GC
(6–8). Various studies have evidenced
promising outcomes in patients with GC through targeting
angiogenesis, immune suppression and normalization of the
tumor-permissive environment (9–15). The
Xiaotan Sanjie decoction, a traditional Chinese medicine (TCM)
regimen, is composed of 11 traditional medicinal components
(16), formulated according to the
phlegm syndrome theory (17).
Phlegm syndrome, a TCM term, is used to diagnose patients with
diseases caused by phlegm (harmful products originated from
dysfunctional body fluid circulation) characterized by symptoms and
signs including lack of appetite, nausea, vomiting, feel of heavy
body, oily looking skin on the face and a pale tongue (18,19).
Phlegm syndrome is prevalent in patients with GC (17). Previous preclinical studies have
shown that the Xiaotan Sanjie decoction has the ability to regulate
levels of some cytokines, growth factors and receptors, such as
interleukin-8 (IL-8) (20),
transforming growth factor-β (TGF-β) (21), TGF-β receptor 2 (TGFBR2) (22) and CD44V6 (23) in the TME of GC. The Xiaotan Sanjie
decoction shows favorable antitumor activities (24) and clinical outcomes (25) in patients with GC.
The TME may constitute the biological basis of
phlegm syndrome in patients with GC. We hypothesized that TME in GC
may redefine the phlegm syndrome by way of current biomolecular
approaches. A literature review was performed to explore the
association between phlegm syndrome and the TME, and what roles
treatment with the Xiaotan Sanjie decoction played in the
management of patients with GC in regard to target components,
including cancer-associated fibroblasts (CAFs), extracellular
matrix (ECM) and the erratic tumor vasculature within the TME. A
preliminary literature search for the Xiaotan Sanjie decoction and
TME related articles was performed on the PubMed (https://pubmed.ncbi.nlm.nih.gov/), ScienceDirect
(https://www.sciencedirect.com/), Google
Scholar (https://scholar.google.com/) and CNKI
(https://www.cnki.net/). The following key search
terms and phrases were used in combination or separately: ‘Xiaotan
Sanjie’, ‘Jinlongshe’ (hospital prepared formulation of Xiaotan
Sanjie decoction), ‘phlegm’, ‘phlegm syndrome’, ‘gastric cancer’,
‘tumor microenvironment’, ‘cancer-associated fibroblasts’,
‘extracellular matrix’, ‘transforming growth factor-β’, ‘tumor
vasculature’ and ‘angiogenesis’. This may promote translation of
TCM theories into modern molecular landscape.
For >2,000 years, TCM has established a tradition
of interpreting diseases and making therapeutic decisions (26). TCM syndrome (pronounced ‘Zheng’ in
Chinese), a TCM term, is a combination of the presentation,
pathogenesis, site and development tendency of a disease at a
specific stage during its course) is based on the gathering of
general manifestations of patients through inspection, hearing,
inquiry and taking pulse, which is the essential diagnostic process
by which TCM physicians describe signs and symptoms and make a
treatment plan (27,28). ‘Phlegm syndrome’ is an important
type of ‘syndrome’ in TCM. Zhenheng Zhu, a famous TCM physician
from the Yuan dynasty (1,279–1,368 AC), said that 9/10 diseases
were caused by phlegm (29). TCM
has two general types of phlegm: Internal and external (nasal
discharge or sputum from respiratory tract) (30). In TCM it is considered that the
internal phlegm will arise when the body's fluid metabolism is
disturbed by intrinsic or extrinsic factors, such as qi
deficiency, qi stagnation, stagnancy of dampness, climatic
factors, emotional changes and improper diet (31). The internal phlegm may accumulate in
Zang and Fu viscera to disrupt the normal functions of the organs
and systems or circulate via meridians and collaterals to distant
organs from its source (30). The
characteristics of phlegm syndrome can be observed in numerous
disorders, including poor general status, respiratory diseases,
gastrointestinal disorders, cardiovascular diseases, overweight,
neurological dysfunctions and cancer (31).
Numerous major risk factors can be observed in
patients with phlegm syndrome and GC concurrently. Improper dietary
behaviors take prominent part in the development of phlegm syndrome
and GC, such as excessive consumption of pickled or salted food or
roasted and greasy foods (17).
Overeating itself is also a cause of phlegm (32). H. pylori infection increases
the risk of GC; moreover, salted food consumption may further
promote H pylori infection, and jointly participate in the
development of GC (33). Aging,
obesity and chronic inflammation are all commonly found in patients
with phlegm syndrome and in patients with GC (32).
GC is characterized by heterogeneous pathology in
regard to anatomical location and histological subtypes (33). GC may be detected in almost all
parts of the stomach, especially in the lower region (34). GC cells may move from their original
site to distant body parts by way of the blood or lymphatic vessels
with advancing stages of the disease in patients with GC (35). A broad range of locations should be
noted in the diagnostic process, including the abdominal cavity,
liver, supraclavicular lymph node, ovaries and umbilical region and
Blumer shelf (a mass in the perirectal pouch) (36). Correspondingly, phlegm syndrome
presents as a dynamic evolutionary process similar to GC
development (32). Phlegm can also
be categorized into substantial and insubstantial. Substantial
phlegm is visible and palpable, such as scrofula and nodules in the
skin and muscles (29). When
accumulating, the phlegm is substantial; when dispersing, the
phlegm is insubstantial (32).
Phlegm arising in the spleen can easily extend to or accumulate in
almost all other parts of the body (31), which is referred to as ‘phlegm evil
flowing’ in TCM (32). Phlegm may
play an important role in the pathogenesis and metastases of GC.
Accumulation of internal phlegm transforms from an insubstantial to
a substantial phlegmatic nodule in the stomach, and a phlegmatic
nodule may create a vicious phlegmatic environment in which it will
release more phlegm to relocate to other parts of the body
(32).
The Xiaotan Sanjie decoction is prepared to relieve
symptoms and signs of phlegm syndrome in patients with GC. The
Xiaotan Sanjie decoction is mainly composed of Pinelliae
rhizome, Arisaematis rhizoma, Scorpio, Scolopendra, baked
Endothelium corneum gigeriae galli, prepared Glycyrrhiza
uralensis Fisch and other natural products (Table I). Pinelliae rhizoma and
Arisaematis rhizoma are the dominant ingredients to dry
dampness and resolve phlegm, prevent nausea and vomiting, relieve
pain and dissolve lumps and resolve masses. Scorpio,
Scolopendra and baked Endothelium corneum gigeriae galli are
second principal medicinal components to activate the meridians,
relieve pain and eliminate phlegmatic nodules. The prepared
Glycyrrhiza uralensis Fisch plays a coordinator role to
direct other medicines to the sites where affected by phlegm, to
reduce toxicity and to improve flavor (37). All of these natural products work
synergistically together to improve overall physical status of the
patients with GC, especially in patients with advanced disease
(17). TCM usages, active compounds
and antitumor effects of each component in the Xiaotan Sanjie
decoction are described and summarized in a non-exhaustive list in
Table I.
Malfunctioned vascular structures and ECM create a
hostile metabolic and mechanical TME that is characterized by
hypoxia, low pH and high interstitial pressure (38). Tumors become infiltrated with immune
and inflammatory cells (39–41),
blood endothelial cells (42),
lymphatic endothelial cells (6),
CAFs (43), the ECM (44) and bone marrow-derived mesenchymal
stem cells (8) within the stroma.
Crosstalk exists between tumor-associated stromal cells and tumor
cells through signaling molecules to promote tumor invasion,
metastasis, immunosuppression and to induce treatment resistance
(45). Regulation of the active
interaction between tumor cells and tumor-associated stromal cells
(that interfere with IL-6 mediated crosstalk between tumor cells
and CAFs) in the TME has shown promising antitumor effects in GC
(46). The Xiaotan Sanjie
decoction, a decoction that contains a plethora of phytochemical
compounds (47–49), vitamins (50), and peptides (51–53)
has demonstrated multiple actions on various soluble molecules,
cytokines and growth factors released by parenchyma and stroma
cells of GC. The Xiaotan Sanjie decoction shows activities on
desmoplastic reactions (21,54,55),
ECM formation and degradation (56,57),
and tumor blood supply (58–61)
through regulating these elements in the TME over the course of the
GC progression.
CAFs are a predominant stromal constituent within
the TME and play a prominent part in tumor progression (62). The high proportion of CAFs was
identified as a predictor of poor outcome in patients with GC in a
meta-analysis (63). The fibroblast
activation protein α (FAP) is highly expressed on CAFs and is
rarely detected in normal stomach tissue. FAP upregulation has been
reported as an indication for a worse prognosis in GC and has a
significant effect on GC development, immunosuppression and drug
resistance to immune checkpoint inhibitors (64). The Xiaotan Sanjie decoction
downregulates FAP protein and mRNA expression in GC MKN-45 cells
xenografted in nude mice (65).
TGF-β is a ubiquitous, pleiotropic cytokine that
plays an important role in cancer development (66). Activation of the TGF-β signaling
pathway is involved in gastric carcinogenesis in earlier and later
stages; in addition, elevated serum TGF-β1 protein levels are
predictors of lymph metastases and dissemination in the peritoneal
cavity after gastrectomy (67).
TGF-β plays an indispensable role in activation of resident
quiescent fibroblasts, and differentiation of bone marrow-derived
mesenchymal stem cells and adipose tissue-derived stem cells into
CAFs (68–70). Inhibition of TGF-β signaling
interrupts the differentiation of human MSCs to CAFs and abrogates
their pro-tumorigenic function (71). TGF-β signaling includes a
coordinated interaction between TGF-β receptor (TGFBR)1 and TGFBR2,
which has been thoroughly described in the literature (66,72).
TGF-β shows antitumor activities in the early stage and activity as
a tumor promoter in the later stage of various cancer types, such
as hepatocellular carcinoma, prostate cancer and GC (73,74).
The antitumor activities of TGF-β are compromised by the loss or
reduction of TGF-β receptor expression or of downstream signaling
targets while the cancer progresses to an advanced stage (75,76).
TGFBR2 gene is a putative tumor suppressor, and loss of function of
TGFBR2 is closely correlated to progression in patients with GC
(77–80). In an experimental study, the
expression level of TGF-β was downregulated in serum of Xiaotan
Sanjie decoction-treated nude mice with orthotopically transplanted
GC tumors (21). The Xiaotan Sanjie
decoction has been observed to exert antitumor effects by
upregulating TGFBR2 in vivo and in vitro (22). Previous studies have suggested that
the aqueous extracts of Fritillariae cirrhosae bulbus
(54) and Sinapis semen
(55), two herbal medicinal
components of the Xiaotan Sanjie decoction, downregulate the
activity of the TGF-β/SMAD signaling pathway. The aqueous extracts
of scorpion (an animal medicinal product used in the
decoction)-medicated serum significantly alleviates the
TGF-β1-induced epithelial-mesenchymal transition (EMT) process
(81). We hypothesize that the
Xiaotan Sanjie decoction has the ability to decrease the formation
of CAFs by interrupting the TGF-β signaling pathway.
Activated CAFs can secrete soluble molecules,
including the upregulation of IL-6 in dysplastic stomach tissue,
and is associated with GC development (46). IL-6 is a major mediator in
cross-talking between tumor cells and CAFs in the TME (82). Notably, interruption of this
interaction by genetic modification of IL-6 expression inhibits
gastric tumor growth (46). In a
Xiaotan Sanjie decoction-treated S180 tumor-bearing mice model, the
expression levels of IL-6 decrease significantly in the tumor and
adjacent tissues (83). Bioactive
research has revealed that isolated compounds extracted from
Galli gigerii endothelium corneum (84) and Glycyrrhiza uralensis Fisch
(85), another two natural products
in the Xiaotan Sanjie decoction, have the ability to downregulate
expression of TNFα, IL-1 and IL-6.
The tumor-derived IL-8, also secreted by CAFs,
actively participates in vascularity and tumorigenesis in gastric
carcinoma cell lines in vitro (86). A meta-analysis concludes that IL-8
expression might be a poor prognosticator for GC (87). Overexpression of IL-8 located in
CAFs is associated with resistance to cisplatin in patients with GC
via NF-κB activation and ABCB1 upregulation (88). Ju et al (20) examined expression levels of IL-8 and
its receptors in gastric tissue in S180 ×enograft-bearing mice
treated using the Xiaotan Sanjie decoction. The IL-8 protein level
was observed to be markedly decreased in tumor xenografts and
neighboring gastric tissue. In another study, the Xiaotan Sanjie
decoction has shown positive effects on inhibiting progression and
metastatic behavior of SGC-7901 GC cells through downregulation of
IL-8 (89). The Xiaotan Sanjie
decoction, in combination with platinum-based chemotherapy may be
able to achieve predictable benefits for patients with GC in the
clinical setting.
An altered ECM, which is the supporting structure of
the TME, has a notable impact on the aggressiveness of cancer cell
(6,42). CAFs play predominant roles in the
production of structural macromolecules of ECM (such as collagen,
fibronectin and laminin) as well as in the secretion of enzymes
(such as lysyl hydroxylases and metalloproteinases) to degrade
these structural components (90).
TGF-β is an enhancer in the process of constructing a stiffened ECM
by the CAFs (91,92). As previously discussed, the Xiaotan
Sanjie decoction has been shown to downregulate activities of CAFs
and to interfere with TGF-β signaling pathway (21,22,54,55).
Therefore, the recipe may alleviate the excessive deposition of
components of ECM, such as collagen, fibronectin and laminin, as
well as prevent degradation of structural macromolecules of the
ECM.
Hyaluronic acid (HA), an important component in the
ECM, is expressed in numerous cancer types, including GC (93). A HA-positive tumor is a predictor of
advanced disease and poor survival rate (94,95).
HA plays an important role in limiting the delivery of therapeutic
agents to tumor tissue (96,97).
Hyaluronidase is associated with favorable antitumor effects in GC
by degrading HA within the TME (98). Hyaluronidase activities have been
found in venom extracted from Scorpion and Scolopendra, two natural
animal products used in the Xiaotan Sanjie decoction (99,100).
CD44, a receptor for HA, collagen, fibronectin and
growth factors, is a multifunctional receptor involved in cell-cell
and cell-ECM interactions (101).
High expression of CD44 variants on GC cells is associated with
local tumor growth and metastatic spread in patients with GC
(102). The HA-CD44 interaction
has been suggested to induce uncontrolled proliferation, migration
and drug resistance in various tumor types including metastatic
breast tumor, ovarian tumor and GC in cellular studies (103). An animal study found significant
differences in CD44V6 expression between the Xiaotan Sanjie
decoction group and control group in a MKN-45 GC nude mouse model
(23). Another study further
confirmed the link between downregulation of expression of CD44V6
and the Jinlongshe formulae (Hospital-prepared Xiaotan Sanjie
decoction) treatment (104).
Destruction of ECM and basement membrane barriers is
a prerequisite for the metastasis of GC (105). The Xiaotan Sanjie decoction has
shown the ability to prevent MMPs from degrading the ECM and its
basement membrane in a MKN-45 GC nude mouse model (56). In an animal study, Sprague-Dawley
rats were used to prepare the Xiaotan Sanjie decoction drug serum.
The drug serum significantly inhibited the proliferative,
metastatic and invasive ability of the human GC cell line SGC-7901.
Protein and gene expression levels of MMP-9 were downregulated in
this experiment (57).
Increased vascularity and neoangiogenesis provide
oxygen and nutrients for tumor growth and expansion with advancing
cancer stages (15). VEGF and its
receptor (VEGFR) are important elements to form a new blood vessel
network. Activated CAFs serve as an important VEGF promoter that
establish an abnormal vascular condition in the TME (106). In a study on stomach biopsy of 20
cases with GC, the effects of the Xiaotan Sanjie decoction on
microvessel density (MVD) and VEGF-A/VEGFR-2 activities were
evaluated in histopathological samples of GC and adjacent tissue.
At 6 months, MVD and VEGF-A/VEGFR-2 expression levels were
significantly decreased in samples from patients treated with the
Xiaotan Sanjie decoction (58).
Animal studies have also shown that the Xiaotan Sanjie decoction
downregulates expression of VEGF, kinase insert domain receptor,
VEGF-D protein and mRNA in comparison with 5-fluorouracil group in
tumor xenografts mice to inhibit tumor metastasis (59,60).
Xiaotan Sanjie decoction has also shown inhibition of the formation
of vasculogenic mimicry, a distinct tumor microcirculation model
that does not depend on endothelial cells (61).
The Xiaotan Sanjie decoction has been used to treat
GC with good efficacy and safety profile for >20 years (17,32).
The Xiaotan Sanjie decoction improves quality of life more compared
with chemotherapy in patients with advanced GC who had undergone
the subtotal or total gastrectomy proceedure (107–109). The Xiaotan Sanjie decoction has
shown satisfactory outcomes in clinical studies that evaluated the
effects of the decoction in combination of chemotherapy or other
Chinese medicinal formulations on quality of life and overall
survival in patients with advanced GC (110–113). In a clinical study, among patients
with stage III and IV GC, those who were treated with the Xiaotan
Sanjie decoction in combination with chemotherapy (Etoposide,
Calcium Folinate and 5-Fluorouracil) had longer overall survival
and 3-year survival compared with those who received chemotherapy
only (110). In another study,
efficacy of Xiaotan Sanjie decoction combined with Huangqi
injection and Huachansu injection (both are traditional Chinese
patent medicine) were observed in patients with advanced GC. The
results showed that TCM can improve Karnofsky performance score, NK
cell activity and CD3 and CD4 cells counts more compared with those
of chemotherapy (5-Fluorouracil, Oxaliplatin, Vincristine,
Cisplatin, Mitomycin) (111). The
Xiaotan Sanjie decoction has been shown to have a well-established
safety profile, provide an enhanced quality of life, improve tumor
response and prolong survival time and Karnofsky performance score
in patients with GC (112,113). Therefore, Xiaotan Sanjie decoction
may be a promising candidate for use in collaboration with
conventional treatment regimens as an alternative method to delay
cancer progression.
The TME in GC is a complex ecosystem that consists
of newly formed blood and lymphatic vessels and diversified stromal
cell types embedded in a modified ECM (8). Targeting the TME has revealed
promising survival outcomes by inhibition of VEGF signaling pathway
or PD1 signaling pathway in patients with GC (9–11).
However, there is a gap between the complete understanding of the
underlying molecular mechanisms of a plethora of interconnected
molecules and pathways in the TME in GC and exactly how metastatic
GC leads to death. More pivotal molecular pathways or cell types
need to be revealed in the TME in GC to develop innovative
therapeutic regimens.
For thousand years, Chinese physicians have used
traditional natural medicine to treat a plethora of diseases,
including GC. TCM formulations have notable effects on multiple
targets and seldomly cause the occurrence of undesirable effects
(26,28). In addition, they may provide
long-term benefits for patients with GC (19). The therapeutic effect of TCM
formulations on tumors has been demonstrated in multiple pathways,
providing reasonable evidence for the ability to restore abnormal
TME in GC back into balance (32).
For >20 years, models have been established based on phlegm
syndrome theory for GC (17). The
biochemical properties of the TCM term ‘phlegm’ in GC may be partly
described in modern medical language by way of a series of
preclinical studies.
Contaminated phlegm is likely to be an important
pathological product in the development of GC (114). The hypothesis regarding symptoms,
signs and molecular profiles stems from the clinical and
experimental observation that the TME in GC can share commonalities
with phlegm syndrome (17,32,114).
A TCM decoction, Xiaotan Sanjie decoction, has been formulated to
treat the phlegm syndrome in patients with GC (17,32).
As discussed, the Xiaotan Sanjie decoction has shown activities on
CAFs (21,54,55,65),
ECM (23,56,57,99,100,104) and the tumor blood supply (58–61)
within the TME during tumorigenesis, growth and migration of GC in
preclinical studies.
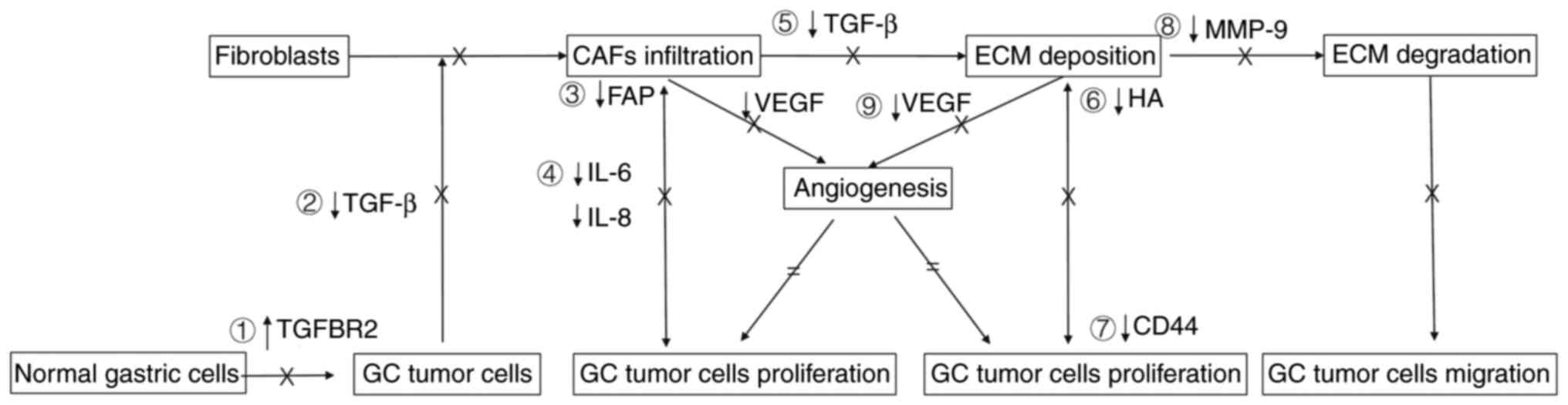
The Xiaotan Sanjie decoction inhibits GC initiation
by upregulating TGFBR2 to normalize the antitumor action of the
TGF-β/SMAD signaling pathway (22).
At subsequent stages, the Xiaotan Sanjie decoction has revealed the
ability to decrease the formation of CAFs through interruption of
the expression of TGF-β (21). In
the single-component studies, the aqueous extracts of
Fritillariae cirrhosae bulbus (54) and Sinapis semen (55), two herbal medicinal compositions of
Xiaotan Sanjie decoction, suppress the protumorigenic activity of
TGF-β/SMAD signaling pathway. In addition, the aqueous extracts of
scorpion-medicated serum significantly alleviates the
TGF-β1-induced EMT process (81).
The Xiaotan Sanjie decoction interferes with crosstalk between CAFs
and tumor cells by downregulating IL-6 (83) and IL-8 (20,89) to
inhibit tumor proliferation. Isolated compounds extracted from
Galli gigerii endothelium corneum (84) and Glycyrrhiza uralensis Fisch
(85), another two natural products
in the Xiaotan Sanjie decoction, have the ability to downregulate
expression of IL-6. Furthermore, the Xiaotan Sanjie decoction
alleviates the development of deposition of ECM by blocking the
activities of CAFs. The Xiaotan Sanjie decoction interrupts HA-CD44
interaction between ECM and tumor cells by degrading HA (99,100)
and downregulating expression of CD44V6 (23,104).
The Xiaotan Sanjie decoction has also been revealed to potentially
target angiogenesis by downregulating the expression level of VEGF
to exert antitumor effects (58–60).
During advanced stages, the Xiaotan Sanjie decoction has
demonstrated effects on GC tumor cell migration by preventing the
degradation of the ECM and its basement membrane (56,57).
The possible mechanisms of antitumor activities of the Xiaotan
Sanjie decoction are summarized in Fig.
1.
The Xiaotan Sanjie decoction improves survivorship
and quality of life in patients with advanced GC in clinical
studies. However, it is difficult to compare these clinical
outcomes to studies conducted to observe efficacy and safety of
synthetic drugs or biological products in patients with GC because
the sample size of clinical studies using the decoction is
relatively small. Well-designed, prospective, large-scale clinical
trials should be conducted to find the most appropriate niche of
the Xiaotan Sanjie decoction in the treatment of patients with GC.
However, a preliminary conclusion may be drawn that the Xiaotan
Sanjie decoction targets CAFs, ECM and angiogenesis in the TME, and
thereby the decoction prepared a preferable physical condition for
chemoradiation therapy or immune therapy. Advanced technologies,
including synthetic biology and scalable spatial analysis of the
TME (115,116), are enabling novel methods for
finding and exploiting the medicinal value of natural products.
Therefore, this has the potential to define the conclusive role of
the Xiaotan Sanjie decoction in the treatment of GC.
Not applicable.
This project was supported by grants from the National Natural
Science Foundation of China (grant no. 82074168) and the Science
and Technology Support Program of Shanghai Science and Technology
Commission (grant no. 19401930400).
Not applicable.
PKW and XQY developed the concept, designed the
study and revised the manuscript. DZS collected the literature and
wrote the draft. All authors have read and approved the final
manuscript. Data sharing is not applicable.
Not applicable.
Not applicable.
Dr Da-Zhi Sun, ORCID ID:
0000-0002-7195-4931cz2016sdz.
The authors declare that they have no competing
interests.
|
1
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Batran SE, Homann N, Pauligk C, Goetze
TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, et al:
Perioperative chemotherapy with fluorouracil plus leucovorin,
oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus
cisplatin and epirubicin for locally advanced, resectable gastric
or gastro-oesophageal junction adenocarcinoma (FLOT4): A
randomised, phase 2/3 trial. Lancet. 393:1948–1957. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sounni NE and Noel A: Targeting the tumor
microenvironment for cancer therapy. Clin Chem. 59:85–93. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and Metastasis. Nat Med.
19:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oya Y, Hayakawa Y and Koike K: Tumor
microenvironment in gastric cancers. Cancer Sci. 111:2696–2707.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry
DR, et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T,
Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al: Safety and
efficacy of pembrolizumab monotherapy in patients with previously
treated advanced gastric and gastroesophageal junction cancer:
Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 4:e1800132018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng YB, Cao FY, Liu KJ, Gan HF, He XB
and Tong SL: Value of normalization window of tumor vasculature in
neoadjuvant chemotherapy for patients with unresectable gastric
cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 15:55–58. 2012.(In
Chinese). PubMed/NCBI
|
|
14
|
Sasaki A, Nakamura Y, Togashi Y, Kuno H,
Hojo H, Kageyama S, Nakamura N, Takashima K, Kadota T, Yoda Y, et
al: Enhanced tumor response to radiotherapy after PD-1 blockade in
metastatic gastric cancer. Gastric Cancer. 23:893–903. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jain RK: Normalizing tumor
microenvironment to treat cancer: Bench to bedside to biomarkers. J
Clin Oncol. 31:2205–2218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan B, Liu L, Zhao Y, Xiu LJ, Sun DZ, Liu
X, Lu Y, Shi J, Zhang YC, Li YJ, et al: Xiaotan Sanjie decoction
attenuates tumor angiogenesis by manipulating Notch-1-regulated
proliferation of gastric cancer stem-like cells. World J
Gastroenterol. 20:13105–13118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi J and Wei PK: The phlegm theory of
gastric cancer. Zhong Xi Yi Jie He Xue Bao. 9:581–587. 2011.(In
Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
State Administration of Traditional
Chinese Medicine, . Criteria for Diagnosis and Evaluation of
Therapeutic Effect on Diseases and Syndromes in Traditional Chinese
Medicine. Nanjing University Press; Nanjing: pp. 9–11. 1994
|
|
19
|
Sun DZ, Jiao JP, Ju DW, Ye M, Zhang X, Xu
JY, Lu Y, He J, Wei PK and Yang MH: Tumor interstitial fluid and
gastric cancer metastasis: An experimental study to verify the
hypothesis of ‘tumor-phlegm microenvironment’. Chin J Integr Med.
18:350–358. 2012.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ju DW, Wei PK, Lin HM, Sun DZ, Yu S and
Xiu LJ: Effects of Xiaotan Sanjie Decoction on expressions of
interleukin-8 and its receptors in gastric tumor xenografts and
gastric tissue adjacent to the tumor in mice. Zhong Xi Yi Jie He
Xue Bao. 8:74–79. 2010.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu X, Zhao Y, Wei P, Chen M, Jing J, Zhong
L, et al: Effects of Xiaotan Sanjie decoction on serum expression
levels of IL-8 and TGF-β in gastric tumor xenografts in nude mice.
Chin Arch Tradit Chin Med. 36:2382–2385. 2018.(In Chinese).
https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2018&filename=ZYHS201810021&v=8GUmiMviuOMeRswXcUcanzXDS%25mmd2Bixowvul5WOa%25mmd2BraRlfuEWfwqoBiKhPNA6nOO6Ea
|
|
22
|
Ye M, Jiao J, Zhang X, et al: Xiaotan
Sanjie decoction inhibit MKN-45 human gastric cancer cells by
upregulating TGFβRII in vivo and in vitro. Chin J Clin. 11:596–601.
2017.(In Chinese). https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2017&filename=ZLYD201704014&v=SSyny61gLr%25mmd2BD7csH5ElmOXxnrufLy1TFMCFLEq%25mmd2BKRZiigitzRn%25mmd2FLZPmCZon7uwML
|
|
23
|
Wang J, Wei P, Li Y and Xu L: Effects of
Xiaotan Sanjie decoction on expression of CD44V6 in MKN-45 human
gastric cancer nude mouse model. J Chengdu Univ Tradit Chin Med.
27:20–21. 2001.(In Chinese).
|
|
24
|
Gui MW, Wei PK, Lu Y, Guo W, Qin ZF and
Sun DZ: Effects of Xiaotan Sanjie Decoction-containing serum on
proliferation and apoptosis of human gastric cancer cells MKN-45.
Zhong Xi Yi Jie He Xue Bao. 8:250–255. 2010.(In Chinese).
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Xiu LJ, Jiao JP, Zhao J, Zhao Y, Lu
Y, Shi J, Li YJ, Ye M, Gu YF, et al: Traditional Chinese medicine
integrated with chemotherapy for stage IV non-surgical gastric
cancer: A retrospective clinical analysis. J Integr Med.
15:469–475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun DZ, Li SD, Liu Y, Zhang Y, Mei R and
Yang MH: Differences in the origin of philosophy between Chinese
Medicine and Western medicine: Exploration of the holistic
advantages of Chinese medicine. Chin J Integr Med. 19:706–711.
2013.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
World Health Organization, . WHO
International Standard Terminologies on Traditional Medicine in the
Western Pacific Region, World Health Organization, Western Pacific
Region. 2007.https://asiantherapies.org/wp-content/uploads/2021/10/WHO-Terminology-Manual-1.pdf
|
|
28
|
Lu A, Jiang M, Zhang C and Chan K: An
integrative approach of linking traditional Chinese medicine
pattern classification and biomedicine diagnosis. J Ethnopharmacol.
141:549–556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clavey S: Phlegm: Aetiology and
symptomatology. In: Fluid Physiology and Pathology in Traditional
Chinese Medicine. Churchill Livingstone Elsevier; London: pp.
265–312. 2003
|
|
30
|
Meng Q: Basic Thoery of Traditional
Chinese Medicine. China Press of Traditional Chinese Medicine;
Beijing: 2005
|
|
31
|
Greenwood MT: Dysbiosis, Spleen Qi,
phlegm, and complex difficulties. Med Acupunct. 29:128–137. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yue X and Wei P: Phlegm as the target of
gastric cancer. Ti Erh Chun I Ta Hsueh Hsueh Pao. 39:1297–1301.
2018.
|
|
33
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guner A and Yildirim R: Surgical
management of metastatic gastric cancer: Moving beyond the
guidelines. Transl Gastroenterol Hepatol. 4:582019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Andreoli TE: Cecil essentials of medicine.
Elsevier; Philadelphia, PA: pp. 440–441. 2010
|
|
37
|
Shi J, Qin Z, Wang X, et al: Clinical
observation of TCM treatment of dispersing phlegm and eliminating
stagnation on postoperative gastric carcinoma. Chin J Info Tradit
Chin Med. 18:14–17. 2011.(In Chinese). https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2011&filename=XXYY201110010&v=WYxc3H1bWPb2I7WrEYpn3%25mmd2Bq7b0lH46oPvMqfiqxL2Kfg7dCZ%25mmd2FVE4dYKAbMYdugZR
|
|
38
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gajewski TF, Meng Y, Blank C, Brown I,
Kacha A, Kline J and Harlin H: Immune resistance orchestrated by
the tumor microenvironment. Immunol Rev. 213:131–145. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fox JG and Wang TC: Inflammation, atrophy,
and gastric cancer. J Clin Invest. 117:60–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bockerstett KA and DiPaolo RJ: Regulation
of gastric carcinogenesis by inflammatory cytokines. Cell Mol
Gastroenterol Hepatol. 4:47–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun H, Wang X, Wang X, Xu M and Sheng W:
The role of cancer-associated fibroblasts in tumorigenesis of
gastric cancer. Cell Death Dis. 13:8742022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Noël A, Gutiérrez-Fernández A, Sounni NE,
Behrendt N, Maquoi E, Lund IK, Cal S, Hoyer-Hansen G and López-Otín
C: New and paradoxical roles of matrix metalloproteinases in the
tumor microenvironment. Front Pharmacol. 3:1402012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bussard KM, Mutkus L, Stumpf K,
Gomez-Manzano C and Marini FC: Tumor-associated stromal cells as
key contributors to the tumor microenvironment. Breast Cancer Res.
18:842016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kinoshita H, Hirata Y, Nakagawa H,
Sakamoto K, Hayakawa Y, Takahashi R, Nakata W, Sakitani K, Serizawa
T, Hikiba Y, et al: Interleukin-6 mediates epithelial-stromal
interactions and promotes gastric tumorigenesis. PLoS One.
8:e609142013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bai J, Qi J, Yang L, Wang Z, Wang R and
Shi Y: A comprehensive review on ethnopharmacological,
phytochemical, pharmacological and toxicological evaluation, and
quality control of Pinellia ternata (Thunb.) Breit. J
Ethnopharmacol. 298:1156502022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Song S, Huang W, Lu X, Liu J, Zhou J, Li Y
and Shu P: A network pharmacology study based on the mechanism of
Citri Reticulatae Pericarpium-Pinelliae Rhizoma in the
treatment of gastric cancer. Evid Based Complement Alternat Med.
2021:66675602021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ayeka PA, Bian Y, Githaiga PM and Zhao Y:
The immunomodulatory activities of licorice polysaccharides
(Glycyrrhiza uralensis Fisch.) in CT 26 tumor-bearing mice.
BMC Complement Altern Med. 17:5362017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Park A, Yang Y, Lee Y, Jung H, Kim TD, Noh
JY, Lee S and Yoon SR: Aurantii Fructus Immaturus enhances
natural killer cytolytic activity and anticancer efficacy in vitro
and in vivo. Front Med (Lausanne). 9:9736812022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ding J, Chua PJ, Bay BH and
Gopalakrishnakone P: Scorpion venoms as a potential source of novel
cancer therapeutic compounds. Exp Biol Med (Maywood). 239:387–393.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rapôso C: Scorpion and spider venoms in
cancer treatment: State of the art, challenges, and perspectives. J
Clin Transl Res. 3:233–249. 2017.PubMed/NCBI
|
|
53
|
Zhao H, Li Y, Wang Y, Zhang J, Ouyang X,
Peng R and Yang J: Antitumor and immunostimulatory activity of a
polysaccharide-protein complex from Scolopendra subspinipes
mutilans L. Koch in tumor-bearing mice. Food Chem Toxicol.
50:2648–2655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bokhari AA and Syed V: Inhibition of
transforming growth factor-β (TGF-β) signaling by Scutellaria
baicalensis and Fritillaria cirrhosa Extracts in
endometrial cancer. J Cell Biochem. 116:1797–1805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cao S, Zheng B, Chen T, Chang X, Yin B,
Huang Z, Shuai P and Han L: Semen Brassicae ameliorates hepatic
fibrosis by regulating transforming growth factor-β1/Smad, nuclear
factor-κB, and AKT signaling pathways in rats. Drug Des Devel Ther.
12:1205–1213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xiao Y, Wei P, Li Y, He J, Xu L and Qin Z:
Influence of Xiaotan Sanjie decoction on the expression of matrix
metalloproteinase and inhibitor in Gastric Carcinoma. Hubei J
Tradit Chin Med. 34:8–10. 2005.
|
|
57
|
Ju D, Xiu L, Sun D, Lu Y and Wei P:
Effects of Xiaotan Sanjie decoction on invasive ability of human
gastric cancer cell line SGC-7901. Chin J Woman Child Health Res.
27:343–344. 2016.(In Chinese).
|
|
58
|
Tang J, Wei P and Zhang Y: Effects of
Xiaotan Sanjie decoction on microvessel density and expression
level of VEGF-A/VEGFR-2. World J Integr Tradit Western Med.
10:346–349. 2015.
|
|
59
|
Xu L, Su X, Chen Y and Wei P: Xiaotan
Sanjie decoction downregulate expression level of VEGF, KDRmRNA in
orthotopic human gastric carcinoma in nude mice. World Chin J
Digest. 12:32004.(In Chinese).
|
|
60
|
Pang B, Wei P, Mao Z and Li Y: Effects of
Xiaotan Sanjie Decoction on expression levels of VEGF-D in MKN45
×enografts in nude mice. Chin J Inf Tradit Chin Med. 18:42–44.
2011.(In Chinese).
|
|
61
|
Zhou W, Li YJ and Wei PK: Effects of
xiaotan sanjie decoction on vasculogenic mimicry of human gastric
cancer xenografts in nude mice. Zhongguo Zhong Xi Yi Jie He Za Zhi.
31:532–536. 2011.(In Chinese). PubMed/NCBI
|
|
62
|
Kumar V, Ramnarayanan K, Sundar R,
Padmanabhan N, Srivastava S, Koiwa M, Yasuda T, Koh V, Huang KK,
Tay ST, et al: Single-cell atlas of lineage states, tumor
microenvironment, and subtype-specific expression programs in
gastric cancer. Cancer Discov. 12:670–691. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gu J, Wang C, Xu X, Zhao L, Zhou J, Bai C
and Sun Z: Immunohistochemical detection of cancer-associated
fibroblasts in gastrointestinal cancer as a potential prognostic
biomarker of survival: Meta-analysis. Transl Cancer Res.
9:6629–6638. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wen X, He X, Jiao F, Wang C, Sun Y, Ren X
and Li Q: Fibroblast activation Protein-a-positive fibroblasts
promote gastric cancer progression and resistance to immune
checkpoint blockade. Oncol Res. 25:629–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yu Z, Zhao Y, Hu X, Shao Z, Jiang Z, Lu Y,
Xiao Z, Pang B, et al: Effects of Xiaotan Sanjie decoction on
expression of fibroblast activation protein α in gastric cancer
MKN-45 cells xenografted in nude mice. Chin Arch Tradit Chin Med.
28:331–334. 2010.(In Chinese). https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2010&filename=ZYHS201002050&v=CTYLMbKoZXwaH0%25mmd2FV8SFqFzODQhR2nxC%25mmd2FPoPnpAkNeO7fQrS0ZGsv7vUfMkTs49D0
|
|
66
|
French R, Feng Y and Pauklin S: Targeting
TGFβ signaling in cancer: Toward context-specific strategies.
Trends Cancer. 6:538–540. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Saito H, Tsujitani S, Oka S, Kondo A,
Ikeguchi M, Maeta M and Kaibara N: An elevated serum level of
transforming growth factor-beta 1 (TGF-beta 1) significantly
correlated with lymph node metastasis and poor prognosis in
patients with gastric carcinoma. Anticancer Res. 20:4489–4493.
2000.PubMed/NCBI
|
|
68
|
Evans RA, Tian YC, Steadman R and Phillips
AO: TGF-β1-mediated fibroblast-myofibroblast terminal
differentiation-the role of smad proteins. Exp Cell Res.
282:90–100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang L, Chang N, Liu X, Han Z, Zhu T, Li
C, Yang L and Li L: Bone marrow-derived mesenchymal stem cells
differentiate to hepatic myofibroblasts by transforming growth
factor-β1 via sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P
receptor axis. Am J Pathol. 181:85–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jotzu C, Alt E, Welte G, Li J, Hennessy
BT, Devarajan E, Krishnappa S, Pinilla S, Droll L and Song YH:
Adipose tissue derived stem cells differentiate into
carcinoma-associated fibroblast-like cells under the influence of
tumor derived factors. Cell Oncol (Dordr). 34:55–67. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Calon A, Tauriello DV and Batlle E:
TGF-beta in CAF-mediated tumor growth and metastasis. Semin Cancer
Biol. 25:15–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Piek E, Heldin CH and Ten Dijke P:
Specificity, diversity, and regulation in TGF-beta superfamily
signaling. FASEB J. 13:2105–2124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Massagué J, Seoane J and Wotton D: Smad
transcription factors. Gene Dev. 19:2783–2810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Elliott RL and Blobe GC: Role of
transforming growth factor Beta in human cancer. J Clin Oncol.
23:2078–2093. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pickup M, Novitskiy S and Moses HL: The
roles of TGFβ in the tumour microenvironment. Nat Rev Cancer.
13:788–799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bierie B and Moses HL: TGF-β and cancer.
Cytokine Growth Factor Rev. 17:29–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Park K, Kim SJ, Bang YJ, Park JG, Kim NK,
Roberts AB and Sporn MB: Genetic changes in the transforming growth
factor beta (TGF-beta) type II receptor gene in human gastric
cancer cells: Correlation with sensitivity to growth inhibition by
TGF-beta. Proc Natl Acad Sci USA. 91:8772–8776. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nadauld LD, Garcia S, Natsoulis G, Bell
JM, Miotke L, Hopmans ES, Xu H, Pai RK, Palm C, Regan JF, et al:
Metastatic tumor evolution and organoid modeling implicate TGFBR2
as a cancer driver in diffuse gastric cancer. Genome Biol.
15:4282014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kim SH, Lee SH, Choi YL, Wang LH, Park CK
and Shin YK: Extensive alteration in the expression profiles of
TGFB pathway signaling components and TP53 is observed along the
gastric dysplasia-carcinoma sequence. Histol Histopathol.
23:1439–1452. 2008.PubMed/NCBI
|
|
80
|
Pak KH, Kim DH, Kim H, Lee DH and Cheong
JH: Differences in TGF-β1 signaling and clinicopathologic
characteristics of histologic subtypes of gastric cancer. BMC
Cancer. 16:602016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yan YQ, Xie J, Wang JF, Shi ZF, Zhang X,
Du YP and Zhao XC: Scorpion inhibits epithelial-mesenchymal
transition and metastasis of hepatocellular carcinoma. Exp Biol Med
(Maywood). 243:645–654. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Karakasheva TA, Lin EW, Tang Q, Qiao E,
Waldron TJ, Soni M, Klein-Szanto AJ, Sahu V, Basu D, Ohashi S, et
al: IL-6 mediates cross-talk between tumor cells and activated
fibroblasts in the tumor microenvironment. Cancer Res.
78:4957–4970. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ju D, Wei P, Sun D, Lin H and Yu S:
Effects of Xiaotan Sanjie decoction on IL-6 gene expression in
tumor and adjacent tissues of S180 tumor-bearing mice model. Chin J
Integr Dig. 15:284–288. 2008.(In Chinese).
|
|
84
|
Li S, Zheng M, Zhang Z, Peng H, Dai W and
Liu J: Galli gigeriae endothelium corneum: Its intestinal barrier
protective activity in vitro and chemical composition. Chin Med.
16:222021.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yu JY, Ha JY, Kim KM, Jung YS, Jung JC and
Oh S: Anti-Inflammatory activities of licorice extract and its
active compounds, glycyrrhizic acid, liquiritin and liquiritigenin,
in BV2 cells and mice liver. Molecules. 20:13041–13054. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kitadai Y, Takahashi Y, Haruma K, Naka K,
Sumii K, Yokozaki H, Yasui W, Mukaida N, Ohmoto Y, Kajiyama G, et
al: Transfection of interleukin-8 increases angiogenesis and
tumorigenesis of human gastric carcinoma cells in nude mice. Br J
Cancer. 81:647–653. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang Z, Hou Y, Yao Z, Zhan Y, Chen W and
Liu Y: Expressivity of Interleukin-8 and gastric cancer prognosis
susceptibility: A systematic review and meta-analysis. Dose
Response. 19:155932582110371272021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhai J, Shen J, Xie G, Wu J, He M, Gao L,
Zhang Y, Yao X and Shen L: Cancer-associated fibroblasts-derived
IL-8 mediates resistance to cisplatin in human gastric cancer.
Cancer Lett. 454:37–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shi J and Wei PK: Xiaotan Sanjie decoction
inhibits interleukin-8-induced metastatic potency in gastric
cancer. World J Gastroenterol. 21:1479–1487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lynch MD and Watt FM: Fibroblast
heterogeneity: Implications for human disease. J Clin Investig.
128:26–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Voloshenyuk TG, Landesman ES, Khoutorova
E, Hart AD and Gardner JD: Induction of cardiac fibroblast lysyl
oxidase by TGF-β1 requires PI3K/Akt, Smad3, and MAPK signaling.
Cytokine. 55:90–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Najafi M, Farhood B and Mortezaee K:
Extracellular matrix (ECM) stiffness and degradation as cancer
drivers. J Cell Biochem. 120:2782–2790. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Huang J, Zhang L, Wan D, Zhou L, Zheng S,
Lin S and Qiao Y: Extracellular matrix and its therapeutic
potential for cancer treatment. Signal Transduct Target Ther.
6:1532021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Balazs EA, Laurent TC and Jeanloz RW:
Nomenclature of hyaluronic acid. Biochem J. 235:9031986. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Setälä LP, Tammi MI, Tammi RH, Eskelinen
MJ, Lipponen PK, Agren UM, Parkkinen J, Alhava EM and Kosma VM:
Hyaluronan expression in gastric cancer cells is associated with
local and nodal spread and reduced survival rate. Br J Cancer.
79:1133–1138. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Eikenes L, Tufto I, Schnell EA, Bjørkøy A
and De Lange Davies C: Effect of collagenase and hyaluronidase on
free and anomalous diffusion in multicellular spheroids and
xenografts. Anticancer Res. 30:359–368. 2010.PubMed/NCBI
|
|
97
|
Jacobetz MA, Chan DS, Neesse A, Bapiro TE,
Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, et
al: Hyaluronan impairs vascular function and drug delivery in a
mouse model of pancreatic cancer. Gut. 62:112–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhao R, Cui Y, Zheng Y, Li S, Lv J, Wu Q,
Long Y, Wang S, Yao Y, Wei W, et al: Human Hyaluronidase PH20
potentiates the antitumor activities of Mesothelin-specific CAR-T
cells against gastric cancer. Front Immunol. 12:6604882021.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wright RP, Chan TK, Honetschlager L,
Howell DE and Odell GV: Enzymes and toxins of the scorpion venom
Palamneus gravimanus. Toxicon. 15:197–205. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
González-Morales L, Pedraza-Escalona M,
Diego-Garcia E, Restano-Cassulini R, Batista CV, Gutiérrez Mdel C
and Possani LD: Proteomic characterization of the venom and
transcriptomic analysis of the venomous gland from the Mexican
centipede Scolopendra viridis. J Proteomics. 111:224–237. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Naor D, Sionov RV and Ish-Shalom D: CD44:
Structure, function, and association with the malignant process.
Adv Cancer Res. 71:241–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen Y, Fu Z, Xu S, Xu Y and Xu P: The
prognostic value of CD44 expression in gastric cancer: A
meta-analysis. Biomed Pharmacother. 68:693–697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Misra S, Heldin P, Hascall VC, Karamanos
NK, Skandalis SS, Markwald RR and Ghatak S: Hyaluronan-CD44
interactions as potential targets for cancer therapy. FEBS J.
278:1429–1443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang S, Wei P, He J, Xiao Y, Chen G, Gu
J, et al: Effects of Jinlongshe formula on tumor proliferation and
metastases and expression of cell adhesion molecules in MKN-45
human gastric cancer nude mouse model. Tumor. 25:519–524.
2006.https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2006&filename=ZZLL200606006&v=s1YASjhB7tsvRqd%25mmd2F%25mmd2BMg%25mmd2Fq8XnD2Vn96oB%25mmd2BffZf4A4Bh7qvD40ECTEqijOPzzUCyqD
|
|
105
|
Lukaszewicz-Zając M, Mroczko B and
Szmitkowski M: Gastric cancer- The role of matrix
metalloproteinases in tumor progression. Clin Chim Acta.
412:1725–1730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Fukumura D, Xavier R, Sugiura T, Chen Y,
Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK and Seed B:
Tumor induction of VEGF promoter activity in stromal cells. Cell.
94:715–725. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhao Y, Wang X, Lu Y, Liu X, Xiu L, Yue X,
et al: Xiaotan Sanjie decoction on quality of life in patients with
advanced gastric cancer. Ti Erh Chun I Ta Hsueh Hsueh Pao.
37:1333–1337. 2016.(In Chinese). https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2017&filename=DEJD201611003&v=kQr6y0QoJCwsnabe8RyD3I5ziphgCCMRnPcPGOv3wTJP8Apz2l2jK77cElywgR16
|
|
108
|
Wu F, Qin Z, Zhang C, Yan B, Shen W and
Wei P: Effects of Jinlongshe formulae on quality of life in
patients with gastric cancer after gastric resection. Chin J Integr
Tradit Western Med Digest. 20:289–292. 2012.(In Chinese).
|
|
109
|
Shi J, Qin Z, Wang X, Zhang Y, Zhang C,
Wang D, et al: Xiaotan Sanjie decoction in patients with gastric
cancer after gastric resection. Chin J Info Tradit Chin Med.
18:14–17. 2011.(In Chinese). https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2011&filename=XXYY201110010&v=WYxc3H1bWPb2I7WrEYpn3%25mmd2Bq7b0lH46oPvMqfiqxL2Kfg7dCZ%25mmd2FVE4dYKAbMYdugZR
|
|
110
|
Li X and Wei P: Jinlongshe formulae in
advanced gastric cancer. Hubei J Tradit Chin Med. 23:3–5. 2001.(In
Chinese).
|
|
111
|
Xu L, Chen Y, Liu Y, Qin Z, Li J, Shi J,
et al: Efficacy of Xiaotan Sanjie decoction combined with Huangqi
injection and Huachansu injection in patients with stage IV gastric
cancer. J Chengdu Univ Tradit Chin Med. 28:7–9. 2005.(In Chinese).
https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7F1IFNsBV5UtixLZQlfT5Bp4RioHTO7MFcAQH76h6XVw4s4ZTzR4S80OigHx_bgBm&uniplatform=NZKPT
|
|
112
|
Sun DZ, Jiao JP, Zhang X, Xu JY, Ye M, Xiu
LJ, Zhao Y, Lu Y, Liu X, Zhao J, et al: Therapeutic effect of
Jinlongshe granule on quality of life of stage IV gastric cancer
patients using EORTC QLQ-C30: A double-blind placebo-controlled
clinical trial. Chin J Integr Med. 21:579–586. 2015.(In Chinese).
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Liu X, Xiu LJ, Jiao JP, Zhao J, Zhao Y, Lu
Y, Shi J, Li YJ, Ye M, Gu YF, et al: Traditional Chinese medicine
integrated with chemotherapy for stage IV non-surgical gastric
cancer: A retrospective clinical analysis. J Integr Med.
15:469–475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sun DZ, Ju DW, He J, Lu Y, Wu F, Li C and
Wei PK: Tumor interstitial fluid and postoperative recurrence of
tumors: An experimental study for verifying hypothesis of
‘Tumor-phlegm Microenvironment’. Chin J Integr Med. 16:435–441.
2010.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Koelzer VH, Sirinukunwattana K, Rittscher
J and Mertz KD: Precision immunoprofiling by image analysis and
artificial intelligence. Virchows Archiv. 474:511–522. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lin L and Wang LV: The emerging role of
photoacoustic imaging in clinical oncology. Nat Rev Clin Oncol.
19:365–384. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Qi CY, Wang J, Wu X, He SR, Zhang Q, Wu JH
and Zhao CB: Botanical, traditional use, phytochemical, and
toxicological of Arisaematis rhizoma. Evid Based Complement
Alternat Med. 2021:90555742021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang Y, Li T, Zhao Y, Zhang J and Liu H:
Contents of some metabolites in the peel and flesh of the medicinal
mushroom Wolfiporia cocos (F.A. Wolf) Ryvarden et
Gilb. (Higher Basidiomycetes). Int J Med Mushrooms.
14:79–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Bian C, Xie N and Chen F: Preparation of
bioactive water-soluble pachyman hydrolyzed from sclerotial
polysaccharides of Poria cocos by hydrolase. Polym J.
42:256–260. 2010. View Article : Google Scholar
|
|
120
|
Wagner H, Püls S, Barghouti T, Staudinger
A and Melchart D: Chromatographic Fingerprint Analysis of Herbal
Medicines. Springer; Vienna: pp. 31–44. 2018
|
|
121
|
Duan L, Guo L, Liu K, Liu EH and Li P:
Characterization and classification of seven citrus herbs by liquid
chromatography-quadrupole time-of-flight mass spectrometry and
genetic algorithm optimized support vector machines. J Chromatogr
A. 1339:118–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Hao EW, Su ZX, Gong YL, Du ZC, Yang X,
Huang CT, Hou XT and Deng JG: Analysis on application law of
dampness-removing traditional Chinese medicines in treatment of
coronavirus disease 2019. Chin Herb Med. 13:518–524. 2021.(In
Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Song L, Xiong P, Zhang W, Hu H, Tang S,
Jia B and Huang W: Mechanism of Citri Reticulatae
Pericarpium as an anticancer agent from the perspective of
flavonoids: A review. Molecules. 27:56222022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Moon JY and Cho SK: Nobiletin Induces
protective autophagy accompanied by ER-stress mediated apoptosis in
human gastric cancer SNU-16 cells. Molecules. 21:9142016.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Dong Y, Cao A, Shi J, Yin P, Wang L, Ji G,
Xie J and Wu D: Tangeretin, a citrus polymethoxyflavonoid, induces
apoptosis of human gastric cancer AGS cells through extrinsic and
intrinsic signaling pathways. Oncol Rep. 31:1788–1794. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yu W, Xie X, Yu Z, Jin Q and Wu H:
Mechanism of hesperidin-induced apoptosis in human gastric cancer
AGS cells. Trop J Pharm Res. 50:2363–2369. 2019.
|
|
127
|
Xiong Q, Li X, Zhou R, Hao H, Li S, Jing
Y, Zhu C, Zhang Q and Shi Y: Extraction, characterization and
antioxidant activities of polysaccharides from E. corneum
gigeriae galli. Carbohydr Polym. 108:247–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chen T, Zhong F, Yao C, Chen J, Xiang Y,
Dong J, Yan Z and Ma Y: A systematic review on traditional uses,
sources, phytochemistry, pharmacology, pharmacokinetics, and
toxicity of Fritillariae Cirrhosae Bulbus. Evid Based
Complement Alternat Med. 2020:15365342020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wang D, Chen X, Atanasov AG, Yi X and Wang
S: Plant resource availability of medicinal Fritillaria
species in traditional producing regions in Qinghai-Tibet plateau.
Front Pharmacol. 8:5022017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Li R, Zhang Y, Wang Y, Huang K, Yang Q,
Zhang T, Xie K, Li J and Zhao Q: Aqueous extract of Fritillariae
cirrhosae induces cellular apoptosis through activation of
STATs-mediated immunomodulation. J Ethnopharmacol. 261:1123382020.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wang X, Zhang H, Chen L, Shan L, Fan G and
Gao X: Liquorice, a unique ‘guide drug’ of traditional Chinese
medicine: A review of its role in drug interactions. J
Ethnopharmacol. 150:781–790. 2013. View Article : Google Scholar : PubMed/NCBI
|