Introduction
Primary gastric lymphoma (PGL) is the most common
type of extranodal tissue lymphoma of non-Hodgkin's lymphoma,
accounting for 30–40% of all extranodal tissue lymphomas worldwide
(1). Furthermore, PGL accounts for
4–20% of all non-Hodgkin's lymphomas and ~5% of primary gastric
cancers (2). Gastric diffuse large
B-cell lymphoma (GDLBCL) is the most common type of PGL and has an
increasing incidence (3).
Currently, chemotherapy is the main method applied for GDLBCL
therapy (4). However, GDLBCL is
prone to metastasis and recurrence (5). Evaluation of the pathology of GDLBCL
and development of effective therapeutic methods are urgently
needed.
Immunotherapy reverses the immunosuppression induced
by cancer, which enhances the killing effect of immune cells toward
cancer cells. Because they enhance the anticancer effect of
adaptive immunity based on effector T cells, inhibitors of immune
checkpoints are widely used in cancer treatment, which has opened a
new era for immunotherapy (6,7).
Although programmed cell death protein 1 (PD-1) and programmed
death-ligand 1 (PD-L1) inhibitors have sustained anticancer effects
in certain neoplastic cases, most patients do not benefit from
immunotherapy due to the heterogeneity of immune responses and
cancer (8–10). The upregulated expression of PD-L1
is significantly associated with poor survival in DLBCL (11). Our previous study reported the
suppressive effect of the innate immune effector ISG12a on the
transcriptional activity of PD-L1, which indicated the inhibitory
effect of the PD-L1/PD-1 axis on natural killer (NK) cell-mediated
anticancer immunity (12).
Moreover, we previously reported that PD-L1 is a prognostic factor
for primary GDLBCL patients treated with rituximab,
cyclophosphamide, doxorubicin, vincristine, and prednisolone
(R-CHOP) (13). However, the
regulatory effect of PD-L1 on GDLBCL is still unclear.
Exosomes are a type of membranous vesicle with a
diameter of 40–200 nm with special expression of protein markers
such as cluster of differentiation 9 (CD9), cluster of
differentiation 63 (CD63), and tumor susceptibility gene 101
protein (14,15). Exosomes carry biologically active
molecules such as RNA and protein, transmitting signals between
cells and influencing the extracellular environment and cancer
biology (16,17). Cancer-derived extracellular vesicles
have been reported to be promising therapeutic targets and disease
biomarkers (18,19). Specifically, exosomal PD-L1 may
downregulate CD69 expression by effector T cells in HNSCC, suppress
the function of CD8 T cell reinvigoration in cancers, such as
melanoma, and inhibit CD8+ T cell proliferation and activation in
colon cancer, which influences the therapeutic efficiency of
clinical cancer (20–22). Interestingly, DLBCL-derived exosomes
induce apoptosis and upregulation of PD-1 in T cells in
vitro; however, dendritic cells (DCs) pulsed DLBCL-derived
exosomes stimulate the anti-lymphoma potency of T cells in
vivo (23). It is important to
elucidate the regulatory role and mechanism of exosomal PD-L1 in
GDLBCL.
Cell-intrinsic PD-L1 promotes the occurrence and
development of GDLBCL; however, the regulatory effect of exosomal
PD-L1 derived from GDLBCL cells on the immune microenvironment is
still unclear. Although T cells, natural killer (NK) cells,
macrophages and other immune cells are involved the in formation of
the tumor microenvironment, immuno-oncology, represented by the
inhibitory PD1/PD-L1 signaling, is mainly focused on enhancing
T-cell responses (24).
Specifically, myriad intricate regulatory mechanisms control T cell
function in the context of antitumor immunity (25). Interestingly, the CAR-T therapy for
GDLBCL has been performed globally with promising results (26,27).
It is of great clinical significance to evaluate the regulatory
relationship between T cells and GDLBCL. In the present study, the
regulatory role of exosomes derived from DLBCL cells with high
levels of PD-L1 were evaluated in tumor growth and T-cell
proliferation.
Materials and methods
Cell culture
The DLBCL cell lines U2932 (RRID: CVCL_1896) and
OCI-LY8 (RRID: CVCL_8803) were purchased from Nanjing Cobioer
Biotechnology Co., Ltd., the human gastric mucosal epithelial cell
line GES-1 (RRID: CVCL_EQ22) was kindly provided by the Institute
of Oncology, Nanhua University (Hengyang, China), and the human T
cell line H9 (RRID: CVCL_1240) was purchased from Shanghai Yiyan
Biotechnology Co., Ltd. U2932, OCI-LY8 and H9 cells were cultured
in RPMI 1640 medium (Thermo Fisher Scientific, Inc.) with 10% (v/v)
heat inactivated fetal bovine serum (FBS; Thermo Fisher Scientific,
Inc.), 100 units/ml penicillin (Thermo Fisher Scientific, Inc.) and
100 µg/ml streptomycin (Thermo Fisher Scientific, Inc.). H9 cells
were cultured in 75 cm2 tissue culture flasks at 37°C in
an incubator with 5% CO2 and 95% humidity, and the
culture medium was refreshed every 2–4 days. H9 cells were
centrifuged at 125 × g at room temperature for 5–10 min and
resuspended in fresh medium at 5×105 cells/ml to remove
cell debris and replace the medium. For good growth states, H9
cells were maintained at cell concentrations between
5×105 and 2×106 cells/ml. Passage of U2932
and OCI-LY8 cells was performed when cells reached 80–90%
confluence. U2932 and OCI-LY8 cells were sequentially centrifuged
at 125 × g at room temperature for 5 min, resuspended in 2 ml fresh
medium, seeded into a new 25 cm2 cell culture bottle
with 8 ml fresh culture medium. The human normal gastric mucosal
GES-1 cell line was cultured in Dulbecco's modified Eagle's medium
(Thermo Fisher Scientific, Inc.) with 10% (v/v) FBS, 100 U/ml
penicillin (Thermo Fisher Scientific, Inc.) and 100 µg/ml
streptomycin (Thermo Fisher Scientific, Inc.). The FBS used for
cultivation of all types of cells was ultracentrifuged at 100,000 ×
g and 4°C for 10 h to remove exosomes. All kinds of cells were
cultured at 37°C in a cell incubator with 5% CO2.
Plasmid construction
The total cellular RNA isolated using
TRIzol® regent (Thermo Fisher Scientific, Inc.) from
U2932 cells was used for synthesis of complementary DNA using the
PrimeScript™ RT reagent Kit with gDNA Eraser (Takara Biotechnology
Co., Ltd.). Sequences used for plasmid construction were obtained
using a high-fidelity PCR kit KOD-Plus-Neo (Toyobo Life Science)
and cloned into the p3×Flag-CMV-14 vector (MilliporeSigma). The
primers used for plasmid construction were as follows: forward,
5′-GGGGTACCATGAGGATATTTGCTGTCTTT-3′ and reverse,
5′-GCTCTAGACGTCTCCTCCAAATGTGTAGT-3′. Gene silencing plasmids for
PD-L1 were constructed using the pRNAT-U6.1/neo vector (GenScript)
according to the manufacturer's protocols. The target sequence used
for constructing the gene silencing plasmid shRNA-PD-L1 was
5′-GCATTTGCTGAACGCATTT-3′. The negative control (target sequence:
5′-ACTACCGTTGTTATAGGTG-3′) was constructed previously (12). All plasmids were amplified using
Escherichia coli DH5α competent cells and were sequenced by
Sangon Biotech Co., Ltd to confirm the correct construction of the
plasmid.
Cell transfection of plasmids
Cell transfection of plasmids was performed
according to the manufacturer's protocols. Briefly, a total of
1.5×106 cells at the logarithmic growth stage were
seeded into each well of a 12-well cell culture plate. A total of 1
µg plasmids and 4 µl Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) were mixed in 100 µl of Opti-MEMTM
medium (Thermo Fisher Scientific, Inc.), separately. After
incubation for 5 min at room temperature, plasmids and
Lipofectamine® 2000 were mixed together and incubated at
room temperature for 20 min. Then, 200 µl of the mixture was added
to each well of the 12-well cell culture plate. After transfection
at 37°C for 12 h, the culture medium was replaced with fresh
medium. The cells were used for further experiments 24–48 h after
transfection.
Extraction and characterization of
exosomes
Exosomes in the supernatants of cultured cells were
extracted by ultracentrifugation according to previously reported
protocols (28). Briefly, the
supernatants of cultured cells were centrifuged at 2,000 × g and
4°C for 20 min and then transferred into a centrifuge tube. The
supernatants were then centrifuged at 10,000 × g and 4°C for 30 min
and then transferred into new ultracentrifuge tubes. The
supernatants were then ultracentrifuged at 100,000 × g and 4°C for
70 min, and the remaining supernatants were discarded. The pellets
were resuspended in PBS and ultracentrifuged at 100,000 × g and 4°C
for 70 min. The pellets were considered to be the extracted
exosomes, which were resuspended in PBS or RIPA lysis buffer
(Thermo Fisher Scientific, Inc.) with proteinase inhibitors.
Exosomes in the plasma of GDLBCL patients and healthy individuals
were extracted using the Exoquick TC kit (System Biosciences, LLC)
according to the manufacturer's protocols. The extracted exosomes
were identified by nanoparticle tracking analysis (NTA) and
transmission electron microscopy (TEM) as previously reported
(29). Protein expression levels of
specific markers of exosomes, CD9 and CD63, were assessed using
immunoblotting.
Immunoblotting
The DLBCL cells were lysed using RIPA lysis buffer
(Thermo Fisher Scientific, Inc.) with proteinase inhibitors. After
incubation on ice for 15–30 min, lysates were centrifuged at 16,100
× g and 4°C for 15 min. After determination of the protein
concentration by the BCA method, 40 µg total protein was
sequentially run on 10% SDS-PAGE gels and transferred onto PVDF
membranes which were blocked with 5% skim milk at room temperature
for 1 h. Membranes were incubated with the primary antibodies at
4°C overnight, washed using TBST buffer with 1% Tween-20, and
incubated with the secondary antibodies at room temperature for 2
h. Protein bands were detected using the SuperSignal®
West Pico Chemiluminescent Substrate kit (Thermo Fisher Scientific,
Inc.). β-actin was used as the internal control for total protein,
and CD9 and CD63 were used as the internal controls for exosomes.
Densitometric analysis of protein bands was performed using
software Image Lab (version 5.2; Bio-Rad Laboratories, Inc.). The
antibodies used in the present study were as follows: Rabbit
anti-PD-L1 (1:1,000, clone E1L3N, cat. no. 13684, Cell Signaling
Technology, Inc.), mouse anti-β-actin (1:5,000, clone AC-15, A5441,
Sigma-Aldrich; Merck KGaA), mouse anti-Flag (1:5,000, clone M2,
F3165, MilliporeSigma), mouse anti-CD9 (1:200, clone C-4, sc-13118,
Santa Cruz Biotechnology, Inc.), mouse anti-CD63 (1:200, clone
MX-49.129.5, cat. no. sc-5275, Santa Cruz Biotechnology, Inc.),
goat anti-mouse IgG (HRP-linked, 1:5,000, AP124P, MilliporeSigma),
goat anti-rabbit IgG (HRP-linked, 1:5,000, AP132P,
MilliporeSigma).
MTT assay
Briefly, an average of 1×104 stably
transfected DLBCL cells in 100 µl of culture medium were seeded per
well of a 96-well cell culture plate. After culturing at 37°C for
24 h, 10 µl of 5 mg/ml MTT solution was added to the culture medium
and incubated at 37°C for 4 h. Then, the culture medium was
carefully removed and replaced with 150 µl of DMSO. The OD value at
490 nm was quantified using a Synergy HTX microplate reader (BioTek
Instruments, Inc.). Experiments were repeated three times.
Animal experiment
A total of 12 NOD-SCID mice (age, 6 weeks; average
weight, 20 g; Hunan Slack Jingda Experimental Animal Co., Ltd.)
were used to perform animal experiments. Mice were fed under
standard conditions (25°C and 50% humidity) with free access to
sterile feed and water in a pathogen-free environment with a 12 h
light/dark cycle at the animal care facility of Hunan Cancer
Hospital (Changsha, Hunan, China). The mice were randomly assigned
to two groups with six mice per group. An average of
5×106 U2932 cells in 200 µl of sterile PBS were
subcutaneously injected into all mice. Seven days after cell
injection, 100 µg of exosomes derived from U2932 cells stably
transfected with the p3×Flag vector or p3×Flag-PD-L1 in 100 µl of
sterile PBS were administered to the mice every four days through
the tail vein. Mice were sacrificed when they experienced a sharp
decrease in activity, water and diet intake, if the tumors formed
under the skin of mice were assessed to be about to reach 15 mm in
diameter in any dimension or if a total of 30 days after cell
injection was reached. The mice were sacrificed by cervical
dislocation immediately after isoflurane anesthesia (induction, 3%;
maintenance, 1%). The formed tumors were measured every two days
and recorded for further analysis.
Clinical specimens
All tissue specimens used in the present study were
obtained from Hunan Cancer Hospital with the informed consent of
patients and the present study was approved by the institutional
review boards of Hunan Cancer Hospital (approval no. 2021-012) and
was performed in accordance with the Declaration of Helsinki. The
blood samples of 26 GDLBCL patients were collected from Hunan
Cancer Hospital from January 2017 to December 2020. The inclusion
criteria used were as follows: i) the stomach was the primary site,
which may have been accompanied by lymph node metastasis in the
gastric drainage area; and ii) the pathological diagnosis was
DLBCL. Samples that failed to meet both of the above inclusion
criteria were excluded. A total of 10 males and 16 females, aged
30–69 years old with a median age of 51.2 years were included. The
patients were not treated with antineoplastic therapy before
samples were taken. The Lugano stage (2016) (30) of patients was stages I–II in 15
cases and stages III–IV in 11 cases. The international prognostic
index (IPI) score (31) of the
lymphomas was 0–2 in 16 cases and 3–5 in 10 cases. The histological
classification was 14 cases in the non-germinal center B-cell like
lymphoma (non-GCB) subtype and 12 cases in the GCB subtype. A total
of 22 healthy individuals who volunteered in the same period were
selected as the normal control group, which included 10 males and
12 females (median age, 48.5 years; age range, 26–62 years).
Immunohistochemistry (IHC)
Tissue sections used for IHC were assessed by two
pathologists at Hunan Cancer Hospital (Changsha, Hunan, China), and
IHC staining was performed as previously reported (12). Briefly, fresh tissues collected
immediately after surgery were routinely fixed using 10% formalin
solution at room temperature for 24 h, embedded in paraffin,
followed by slicing into 5 µm sections. Then, tissue sections were
dewaxed in xylene at room temperature and rehydrated in graded
alcohols, and tissue antigen retrieval was performed in boiled 1 µM
sodium citrate solution (pH, 6.0) at 100°C for 2 min. After
blocking with normal goat serum (Thermo Fisher Scientific, Inc.) at
room temperature for 1 h, slices were sequentially incubated with
the primary antibodies at 4°C overnight and secondary antibodies
for 2 h at room temperature, stained with DAB solution at room
temperature for 5 min, and counterstained with hematoxylin at room
temperature for 1 min. The antibodies used for IHC staining were as
follows: Rabbit anti-PD-1 (1:200, clone D4W2J, cat. no. 86163S,
Cell Signaling Technologies, Inc.), rabbit anti-PD-L1 (1:500,
17952-1-AP, Proteintech Group, Inc.), mouse anti-CD8 (1:5,000,
clone 1G2B10, cat. no. 66868-1-Ig, Proteintech Group, Inc.), rabbit
anti-CD5 (1:800, clone E8X3S, cat. no. 39300S, Cell Signaling
Technologies, Inc.), rabbit anti-CD10 (1:500, clone E5P7S, cat. no.
65534S, Cell Signaling Technologies, Inc.), rabbit anti-CD20
(1:200, clone E7B7T, cat. no. 48750S, Cell Signaling Technologies,
Inc.), rabbit anti-CD79a (1:250, clone D1X5C, cat. no. 13333S, Cell
Signaling Technologies, Inc.), mouse anti-Ki67 (1:2,000, clone 8D5,
cat. no. 9449S, Cell Signaling Technologies, Inc.), goat anti-mouse
IgG (HRP-linked, 1:2,000, cat. no. AP124P, MilliporeSigma), and
goat anti-rabbit IgG (HRP-linked, 1:2,000, cat. no. AP132P,
MilliporeSigma).
Tissue slices were viewed under an inverted
microscope, and representative images were presented in the
figures. Three fields of view per section were analyzed. Positivity
for CD5, CD10, CD8, PD-L1 and PD-1 was defined as ≥5% positively
stained cells, and positivity for CD20, CD79a and Ki67 was defined
as ≥50% positively stained cells. One-fifth of the cases were
scored by two observers to assess reproducibility. For cases to be
considered suitable for evaluation, ≥25% area of a tissue slice had
to be available for morphologic analysis following staining and at
least one positively stained tumor-infiltrating macrophage was
required as a positive internal control. Analysis of IHC staining
of CD3, CD5, CD8, CD10 and CD20 was performed according to the
manufacturers' protocols and as previously reported (32).
Statistical analysis
Data analysis was performed using SPSS version 22.0
(IBM, Corp.). Statistical graphs were drawn using GraphPad Prism
5.0 (GraphPad Software; Dotmatics). Unpaired, two-sided Student's
t-test was used to assess the statistical significance. The
relationship between the PD-L1 level in plasma exosomes and the
clinicopathological characteristics of GDLBCL patients was
determined using the χ2 or Fisher's exact test. Data
were presented as the mean ± SD from three independent biological
replicates. P<0.05 was considered to indicate a statistically
significant difference.
Results
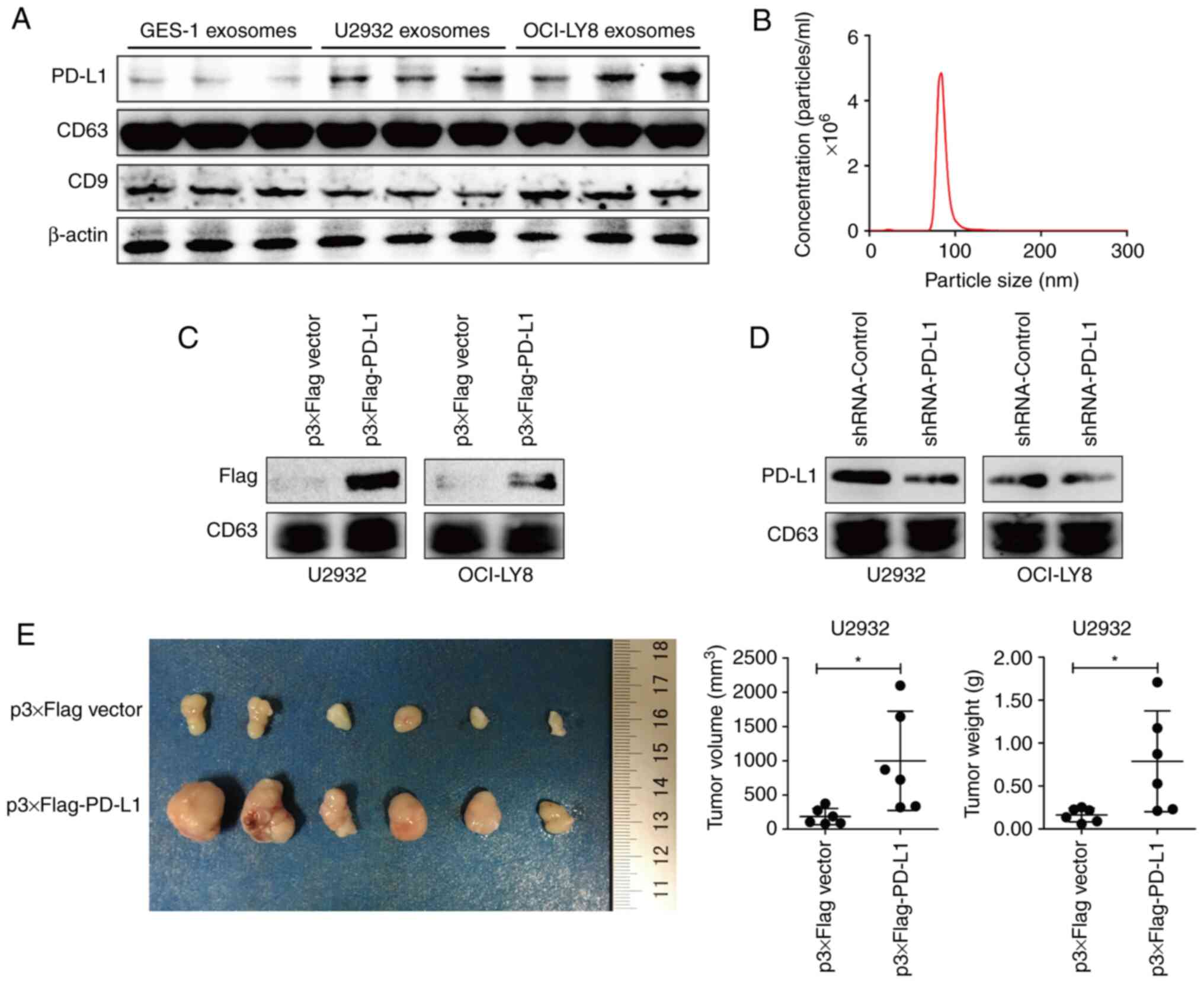
PD-L1 in exosomes derived from DLBCL
cells promotes oncogenesis
To assess the regulatory role of exosomal PD-L1 in
the immune microenvironment of GDLBCL, U2932 and OCI-LY8 DLBCL
cells were chosen as experimental cell models and the supernatants
of the cell culture medium were collected to extract exosomes by
ultracentrifugation. The protein markers CD9 and CD63 detected
using immunoblotting indicated the successful extraction of
exosomes (Fig. 1A). NTA
demonstrated that the particle size of exosomes in the supernatants
of cultured cells presented a normal distribution and was mainly
concentrated between 70 and 120 nm (Fig. 1B). Interestingly, compared with the
protein expression level of exosomal PD-L1 derived from human
epithelial gastric mucosal GES-1 cells, the protein expression
level of PD-L1 in exosomes derived from DLBCL cells U2932 and
OCI-LY8 was much higher (Fig. 1A).
Wit was hypothesized that the high protein expression level of
exosomal PD-L1 might facilitate the initiation and development of
GDLBCL.
Next, Flag-tagged PD-L1 was overexpressed or PD-L1
expression was silenced using shRNA gene-silencing plasmids in
U2932 and OCI-LY8 cells. Assessment of the protein content using
immunoblotting, demonstrated that Flag-tagged PD-L1 appeared in
exosomes derived from the supernatants of U2932 and OCI-LY8 cells
(Fig. 1C). Moreover, the protein
expression level of exosomal PD-L1 was markedly decreased with the
inhibition of cell intrinsic PD-L1 (Fig. 1D). These findings indicated that the
protein expression level of exosomal PD-L1 was consistent with the
protein expression level of cell-intrinsic PD-L1.
To further illustrate the regulatory role of
exosomal PD-L1, tumor xenograft and tail vein injection experiments
were performed in NOD-SCID mice. One week after injecting wild-type
U2932 cells into mice, exosomes derived from the supernatants of
U2932 cells overexpressing PD-L1 and the control vector were
administered into mice through tail vein injection. Four weeks
after cell injection, significantly larger and heavier tumors were
observed in the mice injected with exosomes derived from
PD-L1-overexpressing cells compared with that of the control
(Fig. 1E). These results indicated
that, exosomal PD-L1 contributed to tumor growth in
vivo.
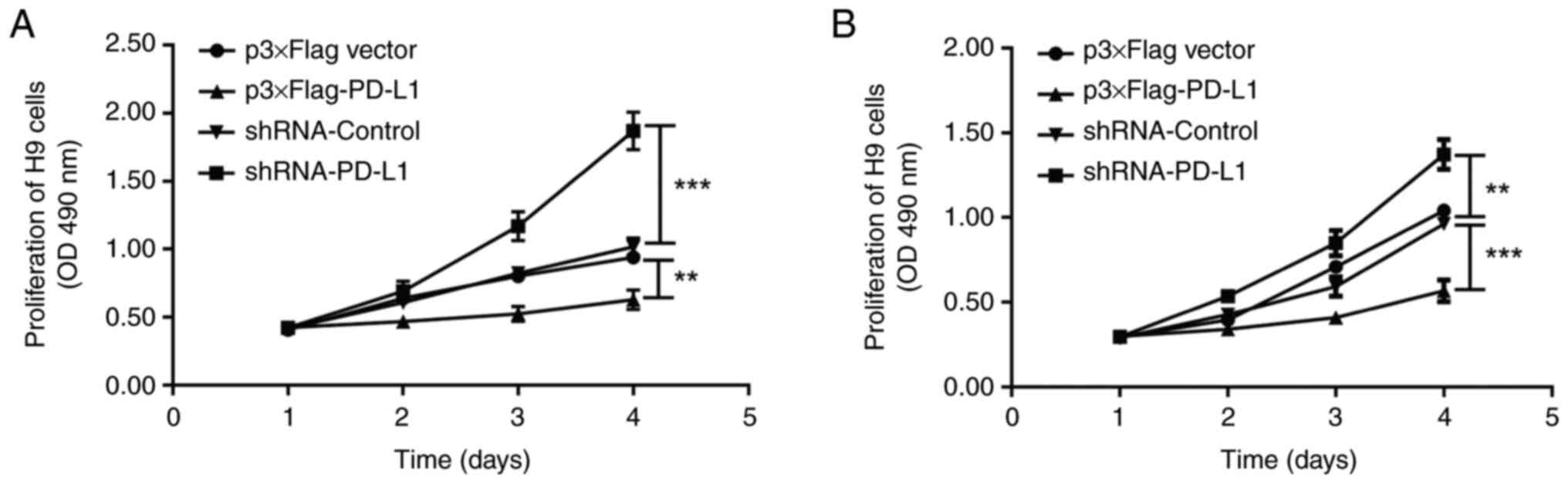
Exosomal PD-L1 inhibits the
proliferation of T lymphocytes
As exosomal PD-L1 contributes to the immune evasion
of cancer cells, it was hypothesized that exosomal PD-L1 might
influence T lymphocytes in the immune microenvironment. Therefore,
the influence of exosomal PD-L1 from the supernatants of cultured
DLBCL cells on the proliferation of H9 human T lymphocytes was
assessed. In MTT experiments, exosomes with PD-L1 overexpression or
PD-L1 silencing as well as those of controls derived from U2932 and
OCI-LY8 cells were added to the culture medium of H9 cells. The
proliferation of H9 cells was significantly inhibited by treatment
with exosomes with PD-L1 overexpression compared with the control
and was significantly promoted by treatment with exosomes with
PD-L1 silencing compared with the control (Fig. 2). These results demonstrated that
PD-L1 in exosomes derived from DLBCL cells inhibited the
proliferation of T lymphocytes. Therefore, exosomal PD-L1
originating from DLBCL cells may interact with the surface PD-1 of
T lymphocytes, resulting in immune suppression.
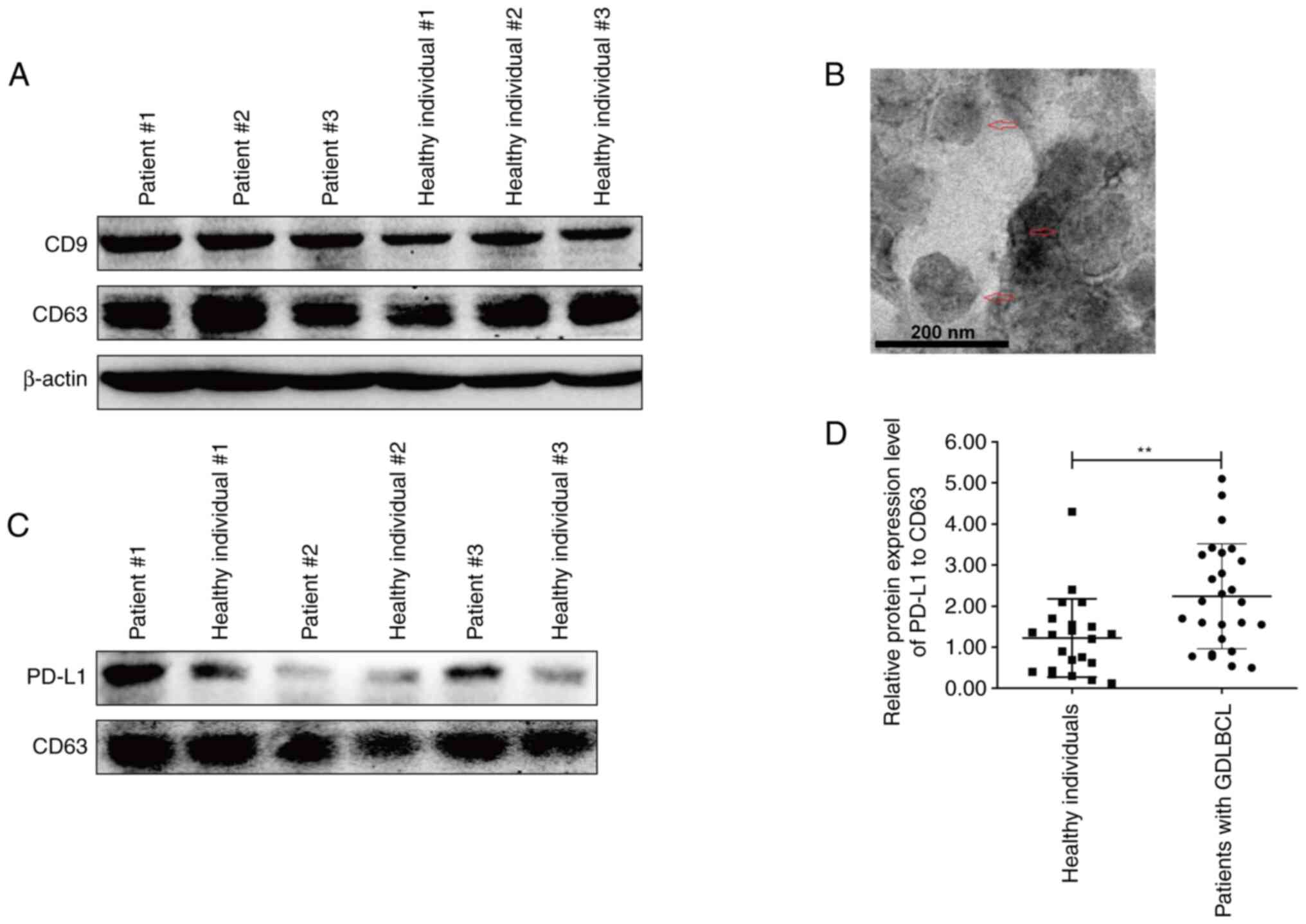
High levels of exosomal PD-L1 are
associated with malignant transformation and poor prognosis in
GDLBCL
Exosomes from the plasma of GDLBCL patients and
healthy individuals were obtained for analysis. The detection of
CD9 and CD63 proteins indicated the successful extraction of plasma
exosomes (Fig. 3A). The exosomes
extracted from the plasma were also assessed using TEM (Fig. 3B). Interestingly, the protein
expression level of exosomal PD-L1 in three representative GDLBCL
patients was markedly higher than that in healthy individuals
(Fig. 3C). The statistical analysis
demonstrated significant upregulation of exosomal PD-L1 in the
plasma of GDLBCL patients (Fig.
3D). These data indicated that exosomes with high levels of
PD-L1 may participate in the occurrence and development of
GDLBCL.
The correlation between the protein expression level
of exosomal PD-L1 and clinicopathological features of GDLBCL was
further analyzed, including gender, age, Lugano stage, IPI score
and pathological subtypes of lymphoma (Table I). Although no significant
relationship was demonstrated between the protein expression level
of exosomal PD-L1 in plasma and gender or age, the protein
expression level of exosomal PD-L1 was demonstrated to be
significantly, positively related with the IPI score, non-GCB
pathological type or Lugano stage of GDLBCL. These results
indicated that a high level of exosomal PD-L1 may lead to malignant
transformation and poor prognosis of GDLBCL.
 | Table I.The relationship between the protein
expression level of programmed death-ligand 1in plasma exosomes and
the clinicopathological characteristics of patients with gastric
diffuse large B-cell lymphoma. |
Table I.
The relationship between the protein
expression level of programmed death-ligand 1in plasma exosomes and
the clinicopathological characteristics of patients with gastric
diffuse large B-cell lymphoma.
|
|
| Exosomal PD-L1
level |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Number of
patients | High | Low | P-value |
|---|
| Gender |
|
|
| 0.6882 |
|
Male | 10 | 4 | 6 |
|
|
Female | 16 | 9 | 7 |
|
| Age, years |
|
|
| 0.4110 |
|
>60 | 9 | 6 | 3 |
|
|
≤60 | 17 | 7 | 10 |
|
| IPI score |
|
|
| 0.0414 |
|
0-2 | 16 | 5 | 11 |
|
|
3-5 | 10 | 8 | 2 |
|
| Pathological
type |
|
|
| 0.0183 |
|
Non-GCB | 14 | 10 | 4 |
|
|
GCB | 12 | 3 | 9 |
|
| Lugano stage |
|
|
| 0.0055 |
| I +
II | 15 | 4 | 11 |
|
| III +
IV | 11 | 9 | 2 |
|
A high level of exosomal PD-L1 is a
potential indicator of the immunosuppressive microenvironment of
GDLBCL
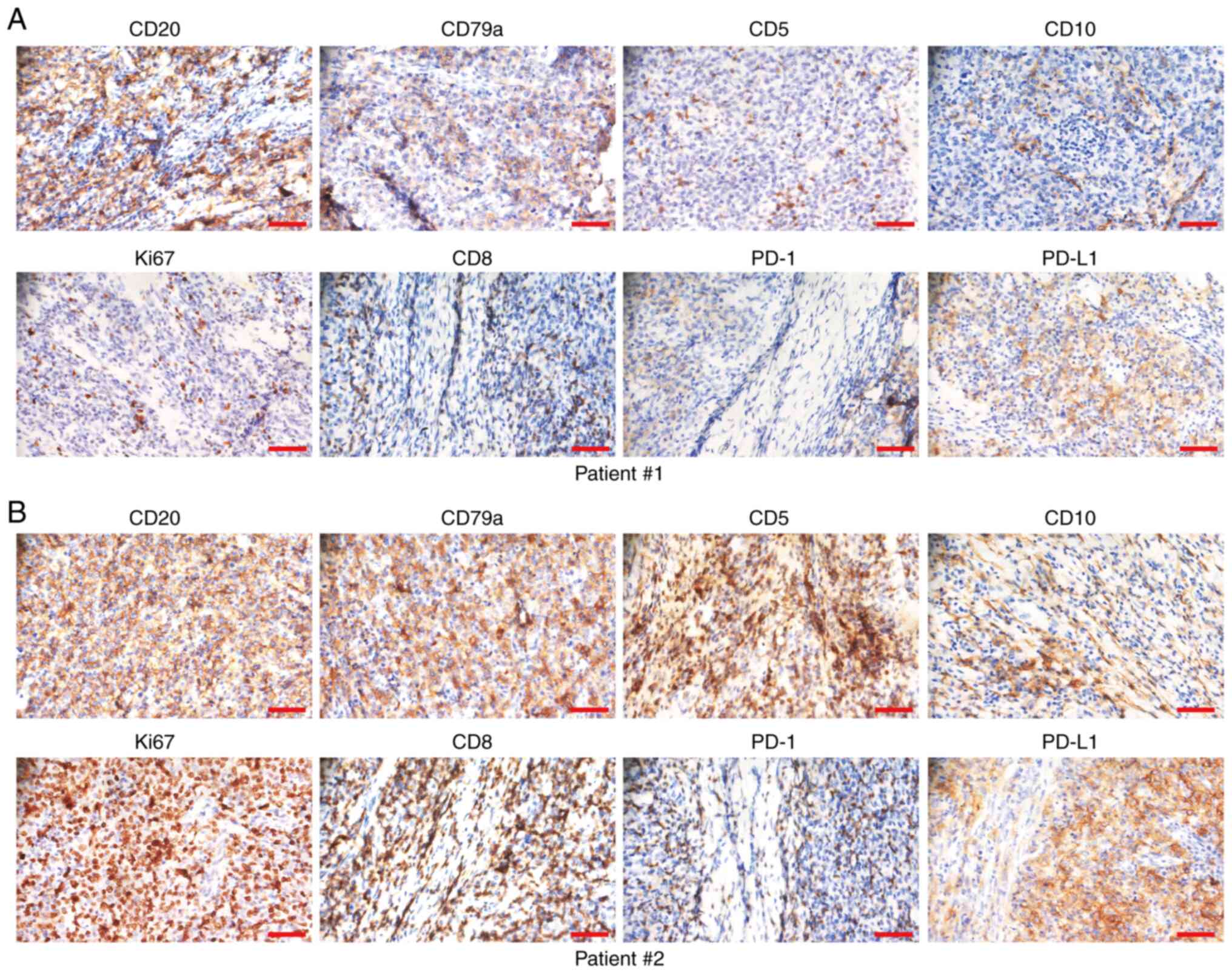
To evaluate the relationship between exosomal PD-L1
in the plasma and the immune microenvironment of GDLBCL, the
protein expression levels of CD20, CD79a, CD5, CD10, Ki67, CD8,
PD-1 and PD-L1 were assessed using IHC staining in a series of
consecutive slices of GDLBCL tissue specimens (Fig. 4A and B). The relationship between
the protein expression level of PD-L1 in plasma exosomes and the
immune microenvironment was evaluated (Table II). The protein expression levels
of PD-L1 and CD8 in tissue specimens were significantly and
positively related to the upregulation of PD-L1 expression in
plasma exosomes (P=0.0004 and P=0.0183, respectively; Table II). Exosomal PD-L1 from gastric
cancer cells interacts with the surface PD-1 on CD8+ T
cells, which leads to immune evasion of cancer cells (21). Therefore, a high expression level of
exosomal PD-L1 in the plasma may reflect the immunosuppressive
microenvironment of GDLBCL. There was no significant relationship
between the protein expression level of PD-L1 in plasma exosomes
and that of CD20, CD79a, CD5, CD10, Ki67 or PD-1 in GDLBCL tissue
specimens (Table II).
 | Table II.The relationship between the protein
level of PD-L1 in plasma exosomes and the immune microenvironment
of gastric diffuse large B-cell lymphoma. |
Table II.
The relationship between the protein
level of PD-L1 in plasma exosomes and the immune microenvironment
of gastric diffuse large B-cell lymphoma.
|
|
| Exosomal PD-L1
level |
|
|---|
|
|
|
|
|
|---|
| Protein level | Number of
patients | High | Low | P-value |
|---|
| CD20 |
|
|
| >0.9999 |
| + | 21 | 11 | 10 |
|
| - | 5 | 2 | 3 |
|
| CD79a |
|
|
| 0.6447 |
| + | 20 | 9 | 11 |
|
| - | 6 | 4 | 2 |
|
| CD5 |
|
|
| >0.9999 |
| + | 3 | 1 | 2 |
|
| - | 23 | 12 | 11 |
|
| CD10 |
|
|
| 0.6914 |
| + | 11 | 6 | 5 |
|
| - | 15 | 7 | 8 |
|
| Ki67 |
|
|
| 0.3783 |
| + | 19 | 8 | 11 |
|
| - | 7 | 5 | 2 |
|
| CD8 |
|
|
| 0.0183 |
| + | 12 | 9 | 3 |
|
| - | 14 | 4 | 10 |
|
| PD-1 |
|
|
| 0.2393 |
| + | 13 | 5 | 8 |
|
| - | 13 | 8 | 5 |
|
| PD-L1 |
|
|
| 0.0004 |
| + | 15 | 12 | 3 |
|
| - | 11 | 1 | 10 |
|
Discussion
Immunotherapies targeting PD-1/PD-L1 have been
reported to be effective strategies for the treatment of
malignances (33,34). However, only 10–30% of patients have
a persistent response to PD-L1/PD-1 antibody therapy in the clinic
(35), which may be partly caused
by the heterogeneity of the tumor microenvironment (36,37).
In the present study, it was demonstrated that the exosomal PD-L1
of DLBCL cells promoted tumor growth in vivo and inhibited
the proliferation of T lymphocytes in vitro. The PD-L1
protein expression level in plasma exosomes was significantly
correlated with the positive rate of PD-L1 and CD8 in GDLBCL
tissue. Moreover, a higher level of plasma exosome PD-L1 was
demonstrated be significantly related to patients with IPI scores
≥2 and advanced Lugano stage, which may be linked with the poorer
prognosis of GDLBCL. The tumor microenvironment induces the
upregulation of PD-L1 in cancer cells, especially on the surface of
aggressive B-cell lymphomas, which inhibits cytotoxic T cells by
binding with the PD-1 receptor on effector T cells (38,39).
Moreover, immunosuppressive lymphocytes such as regulatory T cells
and myeloid-derived suppressor cells hinder the progression of the
cell cycle and the proliferation of cytotoxic T cells as well as
the function of T cells, and induce the depletion of T cells, which
leads to immune tolerance (40,41).
The downregulation of cytokines such as IFN-γ and IL-2 also
aggravates peripheral immune tolerance, which leads to immune
evasion by cancer cells (42).
Exosomes have lipid bilayer membrane structures,
which can provide a protective barrier for vulnerable biomolecules.
Based on biofunctions such as transmitting biological information
through protein or RNA, exosomes have been regarded as promising
drug carriers (43). Regarding the
regulation of the tumor immune microenvironment, exosomes with high
levels of PD-L1 inhibit T-cell activation and become a major
regulator in cancer progression; moreover, inhibition of exosomal
PD-L1 can lead to persistent systemic anticancer immunity (21,44,45).
In a model of anti-PD-L1 antibody resistance, removal of exosomal
PD-L1 inhibited tumor growth (44).
In the present study, the inhibitory effect, of exosomes with high
expression of PD-L1, on the proliferation of T cells was
demonstrated. Moreover, PD-L1 levels in plasma exosomes were
increased with the occurrence and development of GDLBCL, which was
associated with CD8 and PD-L1 staining in tumor tissues. Apart from
the inhibitory effect on CD8+ T cells and exosomal PD-L1
also inhibits the proliferation of CD4+ T cells
(46,47), decreases cytotoxic activity of NK
cells against tumor cells (46,48),
increased the secretion of IL-6, TNF-α and CCL2 by THP1 cells
(49), promotes PD-L1 expression
and phosphorylation of STAT3 in CD14+ monocytes
(50), and predicts the efficacy of
anti-PD-1/PD-L1 therapy (21). All
these findings suggested that an immunosuppressive microenvironment
was constructed by exosomes with high expression of PD-L1. However,
exosomes imaged using TEM were not clear, which may have been due
to the residual salt in the buffer solution of the exosome sample.
Nevertheless, it was still possible to distinguish the typical
saucer shape of exosomes in the TEM images from the present study.
The quality of the TEM images needs to be improved in future
studies.
PD-L1 is widely expressed in numerous types of
lymphoma tissues and lymphoma cell lines (39,51). A
previous retrospective study reported that the positive rate of
PD-L1 in cancer tissues of GDLBCL was 60.6%, which was
significantly correlated with advanced Lugano stage and high IPI
score, as well as poor prognosis (13). Rossille et al (52) reported that the expression level of
PD-L1 was correlated with the prognosis of DLBCL patients. Compared
with patients with high plasma soluble PD-L1 levels at the initial
diagnosis, the 3-year overall survival of patients with low plasma
soluble PD-L1 was 76% vs. 89% (P<0.001) (52). Patients with high levels of soluble
PD-L1 at the time of initial diagnosis were reported to return to
normal levels after achieving complete remission. In the present
study, the high level of PD-L1 protein in plasma exosomes was
significantly correlated with the positive rate for PD-L1 and CD8
in GDLBCL tissues. Moreover, a high level of exosomal PD-L1 in
plasma was significantly associated with the non-GCB subtype and
poor prognosis, which led to an IPI score ≥2 and advanced Lugano
stage (P<0.05). These findings suggested that a high level of
PD-L1 in plasma exosomes may be a biomarker for the poor prognosis
of GDLBCL.
Exosomal PD-L1 is positively correlated with head
and neck squamous cancer progression and administration of
anti-PD-L-1 antibodies inhibits the immunosuppressive function of
PD-L1 (53). PD-L1 is a ligand for
PD-1 on the surface of T cells; however, this review (53) presents no evidence about the
relationship between exosomal PD-L1 and T cell activity. Another
study reported that genetic blockage of exosomal PD-L1 promoted T
cell activity in the draining lymph node to induce systemic
antitumor immunity and memory (44). The aforementioned study (44) directly identified the inhibitory
effect of exosome PD-L1 on T cell activity, but GDLBCL was not
involved. In comparison, the present study demonstrated the
suppression of exosomal PD-L1 on T-cell activation in GDLBCL, which
indicated the significance of exosomal PD-L1 in the formation of an
immunosuppressive microenvironment.
The present study demonstrated the prognostic role
of PD-L1 in plasma exosomes in GDLBCL and analyzed the association
between the protein expression level of exosomal PD-L1 and the
immune microenvironment, which highlighted the importance of
exosomal PD-L1 in the development and immune evasion of GDLBCL. The
significance and innovations of the present study were as follows:
Firstly, high expression of exosomal PD-L1 was demonstrated to be
positively related with the malignant transformation and poor
prognosis of GDLBCL. Secondly, the upregulated expression of PD-L1
in plasma exosomes was identified as a potential indicator for the
immunosuppressive microenvironment of GDLBCL. The present study
further demonstrated the significance of the PD-1/PD-L1 axis in the
development and therapy of GDLBCL and indicated the possibility of
exosomal PD-L1 as a predictor of clinical anti-PD-1
immunotherapy.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the National Natural
Science Foundation of China (grant no. 82170192).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
CZ and YL conceived and designed the experiments.
HZe, JW, BX and HD performed the main experiments and analyzed the
data. MP, RD and SS collected tissue and blood samples. QH
established the cell lines. JLi and JLin conducted the protein
experiments. HZh helped to design the experiments. YL and CZ wrote
the manuscript. RD and HZh performed language correction. HZe, CZ
and YL confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
All tissue specimens used in this study were
obtained from Hunan Cancer Hospital with informed consent from
patients and the present study was approved by the institutional
review boards of Hunan Cancer Hospital (approval no. 2021-012) and
in accordance with the Declaration of Helsinki.
Animal experiments were approved by the Animal Care
and Experiment Committee of Hunan Cancer Hospital (approval no.
SBQLL-2021-050). All procedures performed in the experiments were
in accordance with the Animal Care and Experiment Committee of
Hunan Cancer Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PGL
|
primary gastric lymphoma
|
|
GDLBCL
|
gastric diffuse large B-cell
lymphoma
|
|
DLBCL
|
diffuse large B-cell lymphoma
|
|
NTA
|
nanoparticle tracking analysis
|
|
TEM
|
transmission electron microscope
|
|
IPI
|
international prognostic index
|
|
IHC
|
Immunohistochemistry
|
|
PD-L1
|
programmed death-ligand 1
|
|
NK
|
natural killer
|
|
R-CHOP
|
rituximab, cyclophosphamide,
doxorubicin, vincristine, and prednisolone
|
|
CD9
|
cluster of differentiation 9
|
|
CD63
|
cluster of differentiation 63
|
|
GCB
|
germinal center B-cell like
lymphoma
|
References
|
1
|
Juarez-Salcedo LM, Sokol L, Chavez JC and
Dalia S: Primary gastric lymphoma, epidemiology, clinical
diagnosis, and treatment. Cancer Control. 25:10732748187782562018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujishima F, Katsushima H, Fukuhara N,
Konosu-Fukaya S, Nakamura Y, Sasano H and Ichinohasama R: Incidence
rate, subtype frequency, and occurrence site of malignant lymphoma
in the gastrointestinal tract: Population-based analysis in miyagi,
Japan. Tohoku J Exp Med. 245:159–165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rotaru I, Găman GD, Stănescu C and Găman
AM: Evaluation of parameters with potential prognosis impact in
patients with primary gastric diffuse large B-cell lymphoma
(PG-DLBCL). Rom J Morphol Embryol. 55:15–21. 2014.PubMed/NCBI
|
|
4
|
Deng Y, Su W, Zhu J, Ji H, Zhou X, Geng J,
Zhu J and Zhang Q: Helicobacter pylori infection disturbs the tumor
immune microenvironment and is associated with a discrepant
prognosis in gastric de novo diffuse large B-cell lymphoma. J
Immunother Cancer. 9:e0029472021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zepeda-Gomez S, Camacho J, Oviedo-Cardenas
E and Lome-Maldonado C: Gastric infiltration of diffuse large
B-cell lymphoma: endoscopic diagnosis and improvement of lesions
after chemotherapy. World J Gastroenterol. 14:4407–4409. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or
refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schumacher TN, Kesmir C and van Buuren MM:
Biomarkers in cancer immunotherapy. Cancer Cell. 27:12–14. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk
O, Villacorta-Martin C, Castro de Moura M, Putra J, Camprecios G,
Bassaganyas L, Akers N, et al: Identification of an Immune-specific
Class of Hepatocellular Carcinoma, Based on Molecular Features.
Gastroenterology. 153:812–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li CJ, Lin LT, Hou MF and Chu PY: PDL1/PD1
blockade in breast cancer: The immunotherapy era (Review). Oncol
Rep. 45:5–12. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sohn BS, Kim SM, Yoon DH, Kim S, Lee DH,
Kim JH, Lee SW, Huh J and Suh C: The comparison between CHOP and
R-CHOP in primary gastric diffuse large B cell lymphoma. Ann
Hematol. 91:1731–1739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng R, Zuo C, Li Y, Xue B, Xun Z, Guo Y,
Wang X, Xu Y, Tian R, Chen S, et al: The innate immune effector
ISG12a promotes cancer immunity by suppressing the canonical
Wnt/β-catenin signaling pathway. Cell Mol Immunol. 17:1163–1179.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Shang S, Li J, Deng H, Ouyang L,
Xie H, Zhu H, Li Y and Zuo C: PD-L1 and miR-34a are prognostic
factors for primary gastric diffuse large B-cell lymphoma patients
treated with R-CHOP. Cancer Manag Res. 12:4999–5008. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Niel G, D'Angelo G and Raposo G:
Shedding light on the cell biology of extracellular vesicles. Nat
Rev Mol Cell Biol. 19:213–228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shao H, Im H, Castro CM, Breakefield X,
Weissleder R and Lee H: New technologies for analysis of
extracellular vesicles. Chem Rev. 118:1917–1950. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lobb RJ, Hastie ML, Norris EL, van
Amerongen R, Gorman JJ and Moller A: Oncogenic transformation of
lung cells results in distinct exosome protein profile similar to
the cell of origin. Proteomics. 17:16004322017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meehan K and Vella LJ: The contribution of
tumour-derived exosomes to the hallmarks of cancer. Crit Rev Clin
Lab Sci. 53:121–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moller A and Lobb RJ: The evolving
translational potential of small extracellular vesicles in cancer.
Nat Rev Cancer. 20:697–709. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakaoka A, Nakahana M, Inubushi S, Akasaka
H, Salah M, Fujita Y, Kubota H, Hassan M, Nishikawa R, Mukumoto N,
et al: Exosome-mediated radiosensitizing effect on neighboring
cancer cells via increase in intracellular levels of reactive
oxygen species. Oncol Rep. 45:132021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Theodoraki MN, Yerneni SS, Hoffmann TK,
Gooding WE and Whiteside TL: Clinical significance of
PD-L1+ exosomes in plasma of head and neck cancer
patients. Clin Cancer Res. 24:896–905. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M,
Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1
contributes to immunosuppression and is associated with anti-PD-1
response. Nature. 560:382–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Y, Guo J, Yu L, Guo T, Wang J, Wang X
and Chen Y: PD-L1+ exosomes from bone marrow-derived
cells of tumor-bearing mice inhibit antitumor immunity. Cell Mol
Immunol. 18:2402–2409. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Z, You L, Wang L, Huang X, Liu H, Wei
JY, Zhu L and Qian W: Dual effect of DLBCL-derived EXOs in lymphoma
to improve DC vaccine efficacy in vitro while favor tumorgenesis in
vivo. J Exp Clin Cancer Res. 37:1902018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saibil SD and Ohashi PS: Targeting T cell
activation in immuno-oncology. Curr Oncol. 27 (Suppl 2):S98–S105.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen DS and Mellman I: Elements of cancer
immunity and the cancer-immune set point. Nature. 541:321–330.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Galaly TC, Cheah CY, Kristensen D,
Hutchison A, Hay K, Callreus T and Villa D: Potentials, challenges
and future of chimeric antigen receptor T-cell therapy in
non-Hodgkin lymphomas. Acta Oncol. 59:766–774. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang T, Xu L, Gao L, Tang G, Chen L, Chen
J, Wang Y, Fu W, Yue W, Ye M, et al: Chimeric antigen receptor
T-cell therapy combined with autologous stem cell transplantation
improved progression-free survival of relapsed or refractory
diffuse large B-cell lymphoma patients: A single-center,
retrospective, cohort study. Hematol Oncol. 40:637–644. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thery C, Amigorena S, Raposo G and Clayton
A: Isolation and characterization of exosomes from cell culture
supernatants and biological fluids. Curr Protoc Cell Biol. Chapter
3:Unit 3.22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shang S, Wang J, Chen S, Tian R, Zeng H,
Wang L, Xia M, Zhu H and Zuo C: Exosomal miRNA-1231 derived from
bone marrow mesenchymal stem cells inhibits the activity of
pancreatic cancer. Cancer Med. 8:7728–7740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheson BD, Ansell S, Schwartz L, Gordon
LI, Advani R, Jacene HA, Hoos A, Barrington SF and Armand P:
Refinement of the lugano classification lymphoma response criteria
in the era of immunomodulatory therapy. Blood. 128:2489–2496. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sehn LH, Berry B, Chhanabhai M, Fitzgerald
C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J,
et al: The revised International Prognostic Index (R-IPI) is a
better predictor of outcome than the standard IPI for patients with
diffuse large B-cell lymphoma treated with R-CHOP. Blood.
109:1857–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malipatel R, Patil M, Pritilata Rout P,
Correa M and Devarbhavi H: Primary Gastric Lymphoma:
Clinicopathological Profile. Euroasian J Hepatogastroenterol.
8:6–10. 2018.PubMed/NCBI
|
|
33
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Postow MA, Callahan MK and Wolchok JD:
Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol.
33:1974–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Page DB, Postow MA, Callahan MK, Allison
JP and Wolchok JD: Immune modulation in cancer with antibodies.
Annu Rev Med. 65:185–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei Y, Zhao Q, Gao Z, Lao XM, Lin WM, Chen
DP, Mu M, Huang CX, Liu ZY, Li B, et al: The local immune landscape
determines tumor PD-L1 heterogeneity and sensitivity to therapy. J
Clin Invest. 129:3347–3360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
You W, Shang B, Sun J, Liu X, Su L and
Jiang S: Mechanistic insight of predictive biomarkers for antitumor
PD1/PDL1 blockade: A paradigm shift towards immunome evaluation
(Review). Oncol Rep. 44:424–437. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Juneja VR, McGuire KA, Manguso RT, LaFleur
MW, Collins N, Haining WN, Freeman GJ and Sharpe AH: PD-L1 on tumor
cells is sufficient for immune evasion in immunogenic tumors and
inhibits CD8 T cell cytotoxicity. J Exp Med. 214:895–904. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen BJ, Chapuy B, Ouyang J, Sun HH,
Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA and Rodig
SJ: PD-L1 expression is characteristic of a subset of aggressive
B-cell lymphomas and virus-associated malignancies. Clin Cancer
Res. 19:3462–3473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanaka A and Sakaguchi S: Regulatory T
cells in cancer immunotherapy. Cell Res. 27:109–118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Groth C, Hu X, Weber R, Fleming V,
Altevogt P, Utikal J and Umansky V: Immunosuppression mediated by
myeloid-derived suppressor cells (MDSCs) during tumour progression.
Br J Cancer. 120:16–25. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Boussiotis VA: Molecular and biochemical
aspects of the PD-1 checkpoint pathway. N Engl J Med.
375:1767–1778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bunggulawa EJ, Wang W, Yin T, Wang N,
Durkan C, Wang Y and Wang G: Recent advancements in the use of
exosomes as drug delivery systems. J Nanobiotechnology. 16:812018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Poggio M, Hu T, Pai CC, Chu B, Belair CD,
Chang A, Montabana E, Lang UE, Fu Q, Fong L and Blelloch R:
Suppression of exosomal PD-L1 induces systemic anti-tumor immunity
and memory. Cell. 177:414–427.e13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Daassi D, Mahoney KM and Freeman GJ: The
importance of exosomal PDL1 in tumour immune evasion. Nat Rev
Immunol. 20:209–215. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wen SW, Sceneay J, Lima LG, Wong CS,
Becker M, Krumeich S, Lobb RJ, Castillo V, Wong KN, Ellis S, et al:
The biodistribution and immune suppressive effects of breast
cancer-derived exosomes. Cancer Res. 76:6816–6827. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Morrissey SM and Yan J: Exosomal PD-L1:
Roles in tumor progression and immunotherapy. Trends Cancer.
6:550–558. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharma P, Diergaarde B, Ferrone S,
Kirkwood JM and Whiteside TL: Melanoma cell-derived exosomes in
plasma of melanoma patients suppress functions of immune effector
cells. Sci Rep. 10:922020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu L, Zhang X, Zhang B, Shi H, Yuan X, Sun
Y, Pan Z, Qian H and Xu W: Exosomes derived from gastric cancer
cells activate NF-κB pathway in macrophages to promote cancer
progression. Tumour Biol. 37:12169–12180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gabrusiewicz K, Li X, Wei J, Hashimoto Y,
Marisetty AL, Ott M, Wang F, Hawke D, Yu J, Healy LM, et al:
Glioblastoma stem cell-derived exosomes induce M2 macrophages and
PD-L1 expression on human monocytes. Oncoimmunology.
7:e14129092018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Andorsky DJ, Yamada RE, Said J, Pinkus GS,
Betting DJ and Timmerman JM: Programmed death ligand 1 is expressed
by non-hodgkin lymphomas and inhibits the activity of
tumor-associated T cells. Clin Cancer Res. 17:4232–4244. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rossille D, Gressier M, Damotte D,
Maucort-Boulch D, Pangault C, Semana G, Le Gouill S, Haioun C,
Tarte K, Lamy T, et al: High level of soluble programmed cell death
ligand 1 in blood impacts overall survival in aggressive diffuse
large B-Cell lymphoma: Results from a French multicenter clinical
trial. Leukemia. 28:2367–2375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sinha D, Roy S, Saha P, Chatterjee N and
Bishayee A: Trends in research on exosomes in cancer progression
and anticancer therapy. Cancers (Basel). 13:3262021. View Article : Google Scholar : PubMed/NCBI
|