Introduction
Lung cancer is the leading cause of death worldwide,
the main type of which is lung adenocarcinoma (LUAD) (1). The robust intratumoral heterogeneity
of LUAD has received wide attention, that is associated with
limited therapeutic, therapeutic resistance and refractory
metastasis (2). To address this
serious question, a histologic subtype classification system has
been proposed in 2011, which refers to lepidic (LEP), acinar (ACI),
papillary (PAP), micropapillary (MIP) and solid (SOL) (3). According to the clinicopathologic
characteristics and patient prognosis of these five main
pathological subtypes, tumors with predominant histologic subtype
were classified into three groups: a low-risk group containing LEP,
an intermediate-risk group containing ACI and PAP and a high-risk
group containing MIP and SOL (4).
LncRNA is a class of transcripts originating from
non-protein coding genome regions with a length of >200
nucleotides (5). Increasing
evidence indicates that lncRNAs are widely expressed in tissues and
play important roles in various physiological and pathological
processes by regulating activation and suppression of
transcription, RNA sponging, degradation, apoptosis, proliferation,
epigenetic modifications and chromatin remodeling (6). Li et al (7) revealed that lncRNA lnc-APUE promotes
G1/S phase transition and tumor growth in hepatocellular carcinoma
(HCC) by acting as a miR-20b sponge. Dysregulation of lncRNAs has
been reported to involve in the malignant progression of numerous
kinds of cancer (8). For instance,
lncRNA Smyca drives multiple tumor progression and therapy
resistance by coactivating the TGF-β/Smad and c-Myc pathways
(9). Moreover, the potential
mechanism of lncRNAs in multiple tumor metabolic regulation
processes is a hot topic of research. It has been demonstrated that
lncRNAs regulate glycolysis in different types of cancer. Ma et
al (10) reported that LncRNA
FGF13-AS1 impairs glycolysis and stemness properties via the
FGF13-AS1/IGF2BPs/Myc feedback loop to inhibit the malignant
progression of breast cancer. To the best of our knowledge,
however, an investigation of lncRNA has not yet been reported to
address the molecular mechanism of histologic patterns.
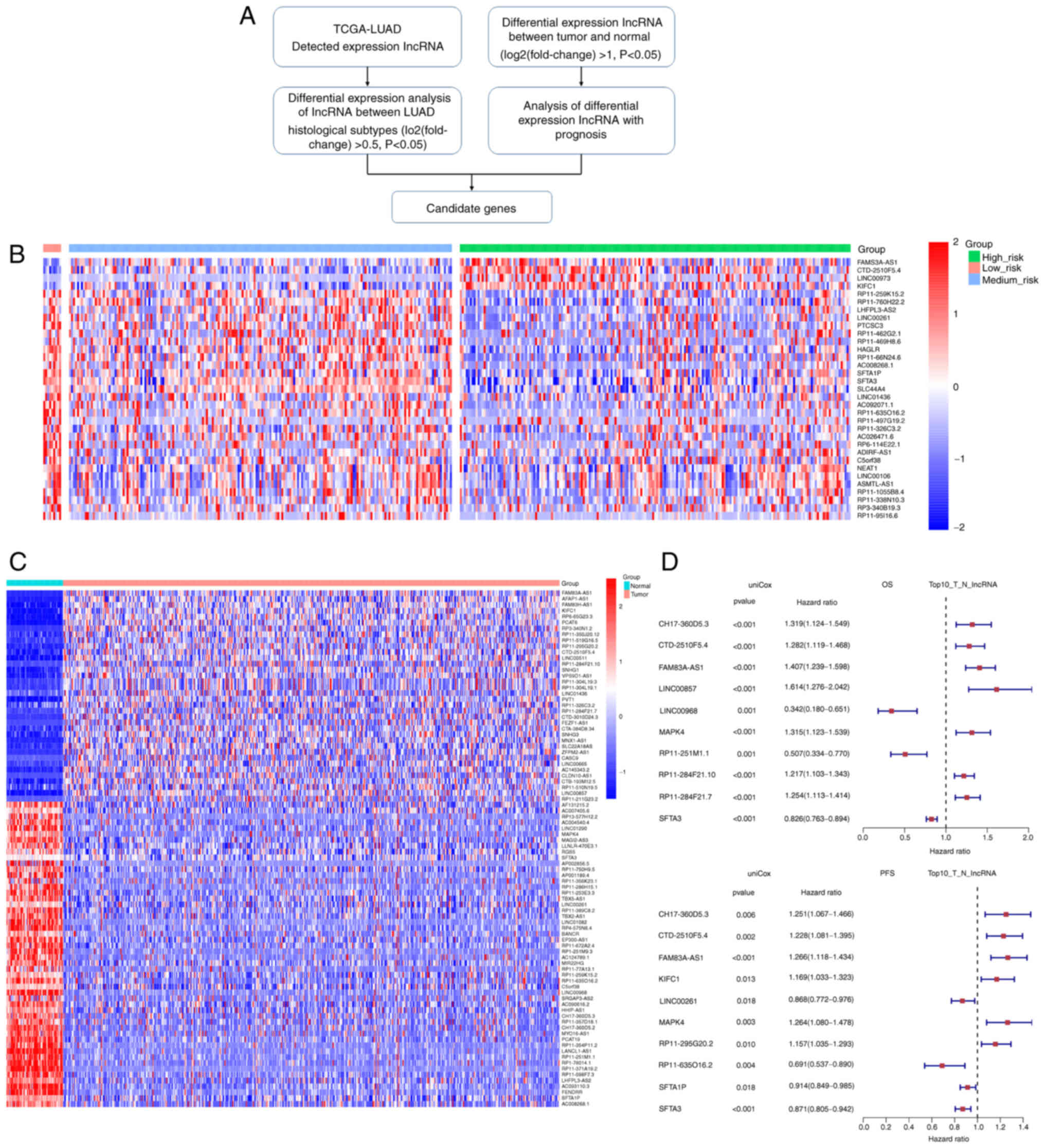
In the present study, our group first downloaded
LUAD pathological H&E from The Cancer Genome Atlas (TCGA) as
well as the transcriptional expression data. According to the
annotation label, lncRNA expression data in the three risk groups
of different pathological subtypes were pairwise compared.
Overlapping the differentially expressed lncRNAs between the three
histological pathology risk groups, and between tumor tissues and
adjacent normal tissues of LUAD (Fig.
1A), focus was addressed on the candidate lncRNA, FAM83A-AS1,
which was associated with poorer prognosis and higher risk of
recurrence. Pan-cancer analysis revealed that FAM83A-AS1 was
positively correlated with high tumor mutational burden (TMB) in
LUAD. Functional assays identified that FAM83A-AS1 promoted cell
migration and invasion and growth in LUAD cancer cell lines.
Mechanistically, FAM83A-AS1 sponged miR-202-3p to regulate the
expression of hexokinase II (HK2) in post-transcription, which
facilitated the malignancy and glycolysis. The present study
identified that FAM83A-AS1 was positively associated with high-risk
pathological subtype and higher clinical stages, and emphasized the
biological roles of FAM83A-AS1/miR-202-3p/HK2 axis in regulating
malignancy and glycolysis of LUAD, which provided a novel avenue to
address the molecular mechanism of histologic patterns.
Materials and methods
Clinical samples
For the present study, 40 tumor and paired normal
tissues were obtained from patients (Age, 40–75; sex, 28 males and
12 females) who underwent surgery in the Department of Thoracic
Surgery of Jiangsu Cancer Hospital (Nanjing, China) from July 2020
to December 2021 (Table I). An area
of 0.5 cm3 in the center of the tumor and a paired
normal tissue 5 cm from the edge of the tumor were selected. None
of the patients had received chemotherapy or immunotherapy before
surgery. After being promptly frozen in liquid nitrogen, all
tissues were kept at −80°C. The present study was approved
(approval no. IACUC-2017088-1) by the Ethics Committee of the The
First Affiliated Hospital of Nanjing Medical University (Nanjing,
China) and complied with the ethical standards of the institution.
Written informed consent was provided by all enrolled
participants.
 | Table I.Clinicopathological characteristics
of 399 patients included in the The Cancer Genome Atlas-lung
adenocarcinoma dataset. |
Table I.
Clinicopathological characteristics
of 399 patients included in the The Cancer Genome Atlas-lung
adenocarcinoma dataset.
| Clinicopathological
characteristics | High risk no.
(%) | Medium risk no.
(%) | Low risk no.
(%) | P-value |
|---|
| Age, years [mean
(SD)] | 65.1 (11.3) | 65.4 (9.1) | 66.8 (12.0) | 0.858 |
| Sex |
|
|
| 0.704 |
|
Male | 92 (46.7) | 82 (42.5) | 4 (44.4) |
|
|
Female | 105 (53.3) | 111 (57.5) | 5 (55.6) |
|
| T stage |
|
|
| 0.456 |
| T1 | 66 (33.5) | 75 (38.9) | 6 (66.7) |
|
| T2 | 110 (55.8) | 94 (48.7) | 3 (33.3) |
|
| T3 | 14 (7.1) | 14 (7.3) | 0 (0.0) |
|
| T4 | 7 (3.6) | 8 (4.1) | 0 (0.0) |
|
|
Unknown | 0 (0.0) | 2 (1.0) | 0 (0.0) |
|
| N stage |
|
|
| 0.038 |
| N0 | 148 (75.1) | 123 (64.1) | 9 (100.0) |
|
| N1 | 25 (12.7) | 40 (20.8) | 0 (0.0) |
|
| N2 | 22 (11.2) | 21 (10.9) | 0 (0.0) |
|
|
Unknown | 2 (1.0) | 8 (4.2) | 0 (0.0) |
|
| M stage |
|
|
| 0.921 |
| M0 | 130 (66.3) | 127 (66.8) | 6 (66.7) |
|
| M1 | 9 (4.6) | 11 (5.8) | 0 (0.0) |
|
|
Unknown | 57 (29.1) | 52 (27.4) | 3 (33.3) |
|
| Stage |
|
|
| 0.089 |
| I | 129 (65.5) | 106 (54.9) | 9 (100.0) |
|
| II | 29 (14.7) | 49 (25.4) | 0 (0.0) |
|
|
III | 26 (13.2) | 24 (12.4) | 0 (0.0) |
|
| IV | 9 (4.6) | 11 (5.7) | 0 (0.0) |
|
|
Unknown | 4 (2.0) | 3 (1.6) | 0 (0.0) |
|
| Smoke |
|
|
| 0.004 |
|
Smoking | 178 (92.2) | 154 (79.8) | 8 (88.9) |
|
|
Non-smoking | 15 (7.6) | 39 (20.2) | 1 (11.1) |
|
Cell culture and treatment
Short tandem repeat sequence analysis was used to
identify all cell lines (HBE, A549, H358, SW1573, H1975 and PC9)
that were purchased from the Shanghai Institute of Cell Biology,
Chinese Academy of Sciences. All cell lines were cultured in a
humidified atmosphere supplemented with 5% CO2 at
37°C.
Dataset
Gene expression (transcripts per million) data and
associated clinical information were downloaded from TCGA
(http://cancergenome.nih.gov/; http://portal.gdc.cancer.gov/projects/TCGA-LUAD).
DNA methylation dataset (Illumina Human Methylation 450K BeadChip
Kit) and LUAD pathological H&E-stained whole-slide images were
also downloaded from TCGA. The data from TCGA used in this paper
are publicly available for reasonable use.
Pan-cancer analysis
Univariate Cox regression (uniCox) and Kaplan-Meier
analyses were conducted to explore the influence of FAM83A-AS1 on
the survival of patients in pan-cancer using the R package
‘survminer. (https://rpkgs.datanovia.com/survminer/index.html)
and ‘survival. (https://cran.r-project.org/web/packages/survival/).
Overall survival (OS) was evaluated (P<0.05 was considered to
indicate a statistically significant difference). Spearman's
correlation analysis was used to evaluate the relationship between
gene expression and TMB.
RNA extraction and reverse
transcription-quantitative (RT-qPCR)
The sequences of the primer sets were listed in
Table SI. Total RNA from cells and
fresh-frozen tissues were extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. cDNA was synthesized using the PrimeScript
RT Reagent Kit (Takara Biotechnology Co., Ltd.). The reaction was
carried out for 15 min at 37°C, 5 min at 85°C, and then 4°C until
further use. To quantitatively express the contents of RNA, RT-qPCR
analysis was carried out using the SYBR Green Premix ExTaqTM kit
(Takara Biotechnology Co., Ltd.) in the biological system 7500
sequence detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Each reaction is performed three times. GAPDH,
18S, or U6 were used as the internal controls for microRNA and
mRNA. The relative level of each target RNA was calculated using
the 2−ΔΔCq method (11).
Cell transfection
The 5-nmol small interfering (si)RNAs (Table SII) for FAM83A-AS1, miR-202
inhibitors, and si-negative control (si-NC) were obtained from
Guangzhou RiboBio Co., Ltd. The sequence of si-FAM83A-AS1 and
miR-202-3p inhibitor are listed in Table SII; however, the sequence of the
corresponding control could not be provided due to confidentiality
of the purchasing company. Lipofectamine iMAX (Thermo Fisher
Scientific, Inc.) was used for transfection (si)RNAs in PC9 cells.
FAM83A-AS1 was created by Invitrogen; Thermo Fisher Scientific,
Inc. and then cloned into the pcDNA3.1 expression vector.and
Lipofectamine 3000 (Thermo Fisher Scientific, Inc.) was used for
transfection in A549 cells. The temperature and duration of
transfection were according to the manufacturer's protocol. After
transfecting for 24 h, PC9 and A549 cells were used for follow-up
experiments.
Cell counting kit-8 (CCK-8)
Using the CCK-8 assay, cell proliferation was
evaluated (cat. no. C0038; Beyotime Institute of Biotechnology).
CCK8 reagent was applied to each well for 1 h at 37°C following 24,
48, or 72 h of siRNA and overexpression. Then, a microplate reader
with an automated setting was used to measure the absorbance at 450
nm (BioTek Instruments, Inc.).
Fluorescence-activated cell sorting
(FACS)
For apoptosis detection, the A549 and PC9 tumor
cells were seeded into a 6-well plate and transfected after 24 h.
Cells (1×106), both floating and adherent, were
trypsinized and rinsed with PBS after the indicated treatments.
Annexin V-FITC Apoptosis Detection kit I (BD Biosciences) was used
to detect apoptotic cells by staining with Annexin V-FITC and PI
according to the manufacturer's instructions at room temperature
for 15 min. A flow cytometer (BD Biosciences) was used to access
the early and late apoptosis of the cells.
5-Ethynyl-2′-deoxyuridine (EdU)
assay
LUAD cells (4×104) were seeded into
96-well templates and harvested at 48 h post-transfection. Cells
were then incubated with 50 mM EdU for 2 h at 37°C, fixed with 4%
paraformaldehyde and incubated with Apollo Dye Solution to label
proliferating cells both 20–40 min at room temperature. Cell nuclei
were counterstained by DAPI (1 µg/ml). Proliferating cells with
green signals were visualized by a Leica DM4000 B LED fluorescent
microscope.
Matrigel and Transwell assay
Cells (4×104) were sown in the upper
Transwell assay chambers of 8-µm pore filters (for the migration
assay). For the invasion assay, 4×104 cells were seeded
in serum-free media into the upper Matrigel assay chambers with a
membrane coated (−20°C) with Matrigel (Corning, Inc.). The medium
in the lower chamber contained 10% FBS as chemokine. Non-migrating
or non-invading cells were gently removed after incubation at 37°C
for 24 h for migration and 48 h for invasion. Cells that migrated
to the bottom of the membrane were then fixed with 4%
paraformaldehyde at room temperature for 30 min, stained with 0.5%
crystal violet solution at room temperature for 30 min, and
observed randomly under a fluorescent microscope at a magnification
of ×100.
Bioinformatics analysis
TargetScan (https://www.targetscan.org/vert_80/) and StarBase
(https://starbase.sysu.edu.cn/starbase2/) database were
used to predict RNA and potential interaction sites of RNA. miRanda
database (ttp://www.microrna.org/microrna/home.do) was used to
predict the interaction sites of miRNA and mRNA. Kyoto Encyclopedia
of Genes and Genomes pathway enrichment analysis was used to
evaluate the degree of gene pathway enrichment.
RNA-binding protein
immunoprecipitation (RIP)
The Magna RIP kit was used to carry out a RIP assay
(cat. no. 17-704; MilliporeSigma). An overnight incubation at 4°C
with 50 µl magnetic beads coated with antibodies against AGO2 (cat.
no. 2897; Cell Signaling Technology, Inc.) or IgG (cat. no. 17-704;
MilliporeSigma) was performed on a total of 1×107 A549
or PC9 cells after they had been collected, lysed and incubated.
The immunoprecipitated RNAs were purified, and quantified by
RT-qPCR, and the beads were then washed with washing buffer.
Pull-down assay
Biotinylated FAM83A-AS1 probes and oligo probes (as
negative controls) for the pull-down assay were created and
manufactured by Shanghai GenePharma Co., Ltd. Streptavidin magnetic
beads (Thermo Fisher Scientific, Inc.) were treated with
biotinylated FAM83A-AS1 probes for 2 h at room temperature to
create probe-coated magnetic beads. The microspheres were then
treated with the cell lysate at 4°C for an overnight period.
Afterwards, the beads were washed. TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to extract the RNA and
RT-qPCR was used to analyze it. The sequence of the FAM83A-AS1
probe was 5′-GGGCCTAAACCGGTCGATTA-3′ and the sequence of the oligo
probe was 5′-ACACTTCTCGGATATACGCCCT-3′.
MiRNA pull down
By transfecting A549 and PC9 cells with 100 nM
3′-biotinylated miRNA mimics, a miRNA pull-down test was carried
out. After the cells had been incubated at 37°C for 24 h, they were
twice rinsed with ice-cold PBS before being lysed with miRNA
pull-down lysis buffer. Biotin-labeled miRNAs were extracted for
the miRNA pull-down test by incubating the beads with 100 µl of
cell lysate and 100 µl of the miRNA pull-down lysis buffer at 4°C
for 4 h while rotating the beads. The TRIzol® reagent
was then used to isolate biotin-labeled miRNAs and the RNAs that
interact with them. By using RT-qPCR, miRNA interacting RNAs were
found.
Fluorescence in situ hybridization
(FISH)
After being fixed in 4% paraformaldehyde at room
temperature for 10 min, A549 cells (1×107) were then
rinsed with PBS. Afterwards, cells were permeabilized for 15 min at
4°C using 0.5% Triton X-100 in precooled PBS. Cells were incubated
for 4 h at 37°C with a mixture of a Cy3-labeled FAM83A-AS1 probe, a
Fam-labeled miR-202-3p targeting probe, and other substances
included in the kit. According to the instructions, a FISH kit
(Shanghai GenePharma Co., Ltd.) was used to find the probe signal.
DAPI (1 µg/ml) was used to stain the nucleus. Images were captured
with a TCS SP5II confocal microscope (Leica Microsystems GmbH).
Dual-luciferase reporter assay
TargetScan (http://www.targetscan.org) anticipated the 3′ UTR
binding sites of miR-202-3p and HK2 mRNA. The miR-202-3p seed
sequence was altered (from TTCCATGTCCCTGTATAATTCTA to
TAGGTACAGGGTGTATAATTCTA) and the wild-type (WT) and mutant (MUT)
HK2 3′-UTR was introduced into the pGL3 basic vector to verify the
binding specificity (Promega Corporation). Sequencing was used to
confirm the validity of each vector, and the dual luciferase assay
kit (cat. no. E1910; Promega Corporation) was used to measure
luciferase activity. The relative luciferase activity was
normalized to Renilla luciferase activity.
Western blot analysis
Western blot analyses were carried out according to
standard protocols. For protein extract preparation, cells were
lysed on ice with RIPA Lysis Buffer (Thermo Fisher Scientific,
Inc.) containing complete protease and phosphatase inhibitor
cocktail (Roche Diagnostics). Soluble protein extracts were
separated by centrifugation at 13,000 g for 20 min. A bicinchoninic
acid (BCA) Protein Assay kit was used for protein determination.
The obtained cell lysates (20 µg protein per lane) were resolved on
4–20% sodium dodecyl-sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred on polyvinylidene difluoride
membraneHybond TM-P (Amersham Bioscience). Membranes were saturated
with 5% bovine serum albumin (Thermo Fisher Scientific, Inc.) at
room temperature for 2 h and incubated with the primary antibodies
anti-HK2 (1:1,000; 2867S; Cell Signaling Technology, Inc.) at 4°C
overnight. Secondary anti-GAPDH (cat. no. 5174S; CST, Cell
Signaling Technology, Inc.) all conjugated to Alexa Fluor 680
(Abcam) were incubated with the membranes for 2 h at room
temperature at 1:10,000 dilution. All bands of western blot were
detected and qualified with gray scale ratio by Odyssey CLx imaging
systems (LI-COR Biosciences).
Extracellular flux assays
The extracellular flux analyzer Seahorse XF96e was
used to quantify the extracellular acidification rate (ECAR) in
cell lines (Agilent Technologies, Inc.). Following the
manufacturer's instructions, glucose, oligomycin, and
2-Deoxy-D-glucose were sequentially added to the Seahorse Analyzer
using the Glycolysis Stress Test kit (cat. no. 103020-100; Agilent
Technologies Inc.) to measure the ECAR, which is used to evaluate
important parameters of glycolytic flux (such as basal glycolysis
and glycolytic capacity). Using a bicinchoninic acid (BCA) Protein
Assay kit (cat. no. P0012; Beyotime, Institute of Biotechnology),
all data were standardized to the protein concentrations.
D-Lactate assay
Intracellular lactate levels were measured using a
D-Lactate Assay kit (cat. no. ab83429; Abcam) according to the
manufacturer's instructions.
Statistical analysis
The statistical analysis was performed using
GraphPad Prism 8.0 software (GraphPad Software, Inc.) and R version
4.1.2 software. R package ‘Limma’ was used to identify
differentially expressed genes (DEGs) between different LUAD
subtype. The false positive results using the default
Benjamini-Hochberg false discovery rate (FDR) method. Survival
analysis was based on the Kaplan-Meier method with a two-sided
log-rank test. The majority of graphs display the mean and standard
deviation as well as graphs for each data point. Paired Student's
t-test was used to determine the significance, and the P-value was
shown by an asterisk. One-way ANOVA test was used for data with
multiple comparisons in data sets of three or more groups (e.g.,
Fig. 2A), and an LSD post-hoc test
was used. For Fig. 2E, a chi-square
test was used to characterize the association of FAM83A-AS1 with
the TN stage.
Results
Identification of high-risk
pathological subtype-associated lncRNA, FAM83A-AS1
According to the annotation of H&E from
TCGA-LUAD dataset, a total of 399 patients were included in the
present study, composed of 9 patients in a low risk group, 193
patients in an intermediate risk group and 197 patients in the
high-risk group. To identify the differentially expressed lncRNAs
across the three histological pathology groups, transcriptional
data of lncRNA were pairwise compared. Compared with the
intermediate or high-risk group, there were 130 lncRNAs
dysregulated in the low-risk group, consisting of 10 downregulated
and 120 upregulated (|log2FC|>0.5, P<0.05, Table SIII). Compared with the low or
high-risk group, there were 13 lncRNAs dysregulated in the
intermediate-risk group, consisting of 3 downregulated and 10
upregulated (|log2FC|>0.5, P<0.05, Table SIV). Compared with the low and
intermediate-risk group, there were 16 lncRNAs dysregulated in the
high-risk group, consisting of 13 downregulated and 3 upregulated
(|log2FC|>0.5, P<0.05, Table
SV). Screening the differentially expressed lncRNAs between
groups according to risk classification, it was found that
FAM83A-AS1, CTD-2510F5.4, LINC00973 and KIFC1 were significantly
upregulated in pathological subtypes ranging from low to high risk
(Fig. 1B). the differentially
expressed lncRNAs between the LUAD tumor tissues and the adjacent
normal tissues in the TCGA-LUAD dataset were also characterized,
and the differential expression of FAM83A-AS1 ranked the top
(Fig. 1C). Univariate regression
analysis was performed, which revealed that FAM83A-AS1 was
associated with a poorer prognosis and a higher risk of recurrence
(Fig. 1D).
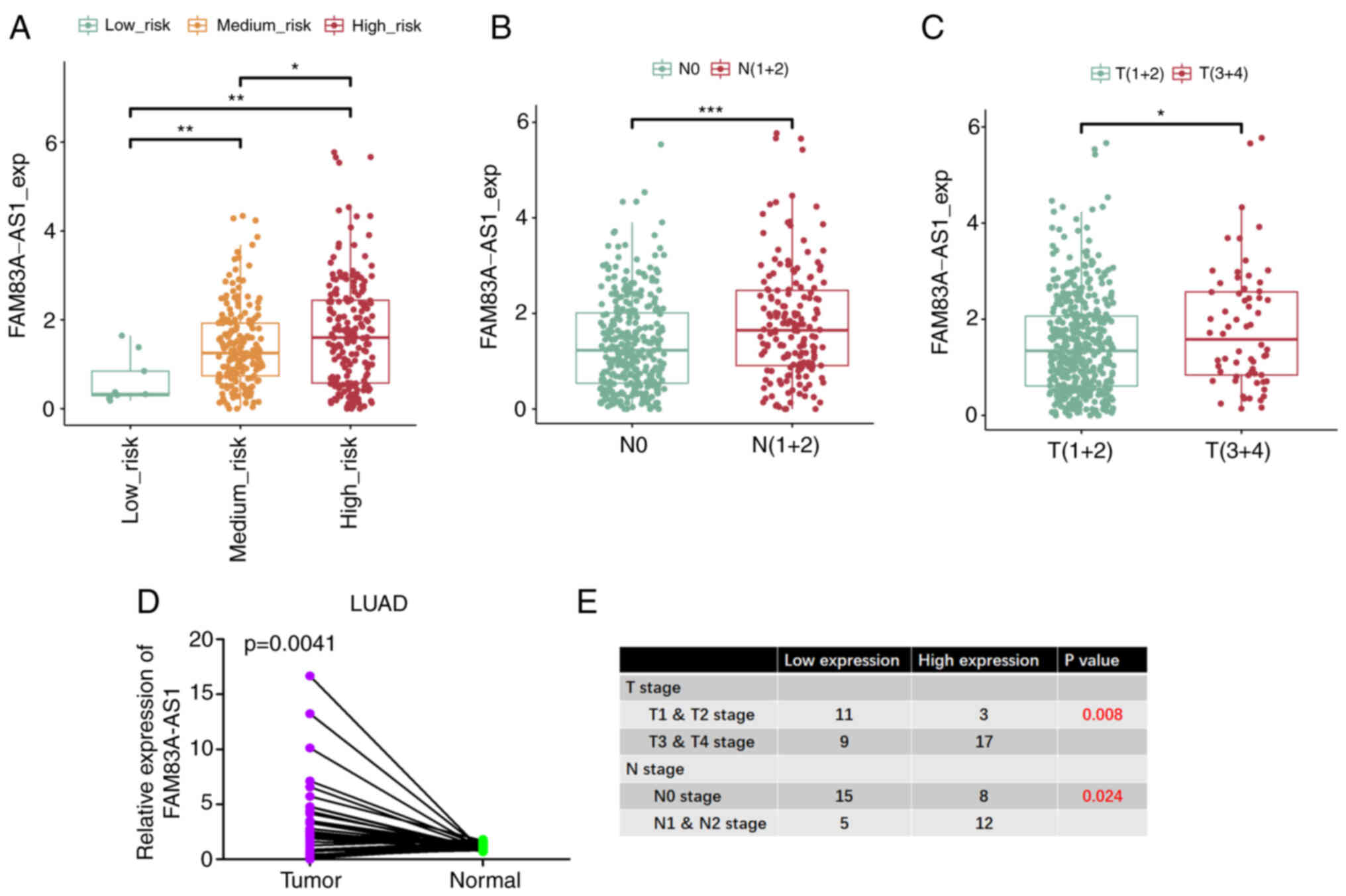
It was also identified that the expression of
FAM83A-AS1 increased gradually from the low-risk group to the
high-risk group (Fig. 2A).
Concurrently, it was also significantly increasingly expressed in
higher N-stage and T-stage (Fig. 2B and
C). The mRNA expression levels were also analyzed using PCR in
our paired adenocarcinoma tissues with clinical stage data and
differentiated low and high based on the median expression level of
FAM83A-AS1, which confirmed that FAM83A-AS1 is highly expressed in
tumors as well as in higher clinical stages (Fig. 2D and E). Collectively, these data
revealed that FAM83A-AS1 was upregulated in tumor tissues compared
with normal tissues, particularly in patients with high-risk
pathological subtypes and higher clinical stages.
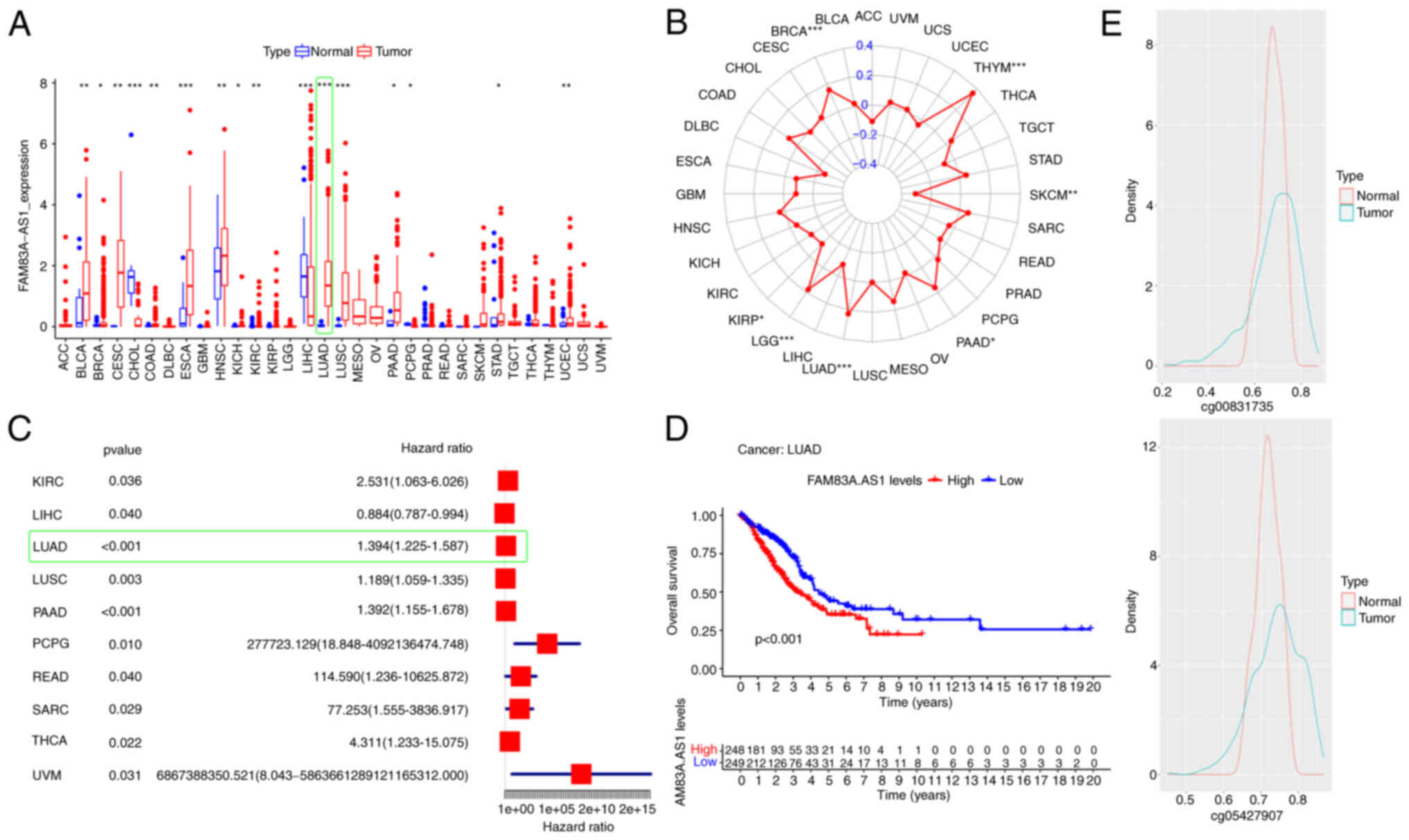
Pan-cancer analysis of FAM83A-AS1
Next, features of FAM83A-AS1 were characterized
through pan-cancer analysis. Pan-cancer analysis showed that
FAM83A-AS1 was upregulated in multiple cancers, particularly in
LUAD (Fig. 3A). Spearman's
correlation analysis revealed that high expression of FAM83A-AS1
was significantly associated with high TMB both in Thymoma (THYM)
and LUAD (Fig. 3B). Concurrently,
FAM83A-AS1 was revealed to be a potential risk factor for tumor
patients in most cancers (Fig. 3C).
In LUAD, Kaplan-Meier survival curves indicated that patients with
high FAM83A-AS1 expression had significantly worse OS than patients
with low expression (Fig. 3D). The
DNA methylation level of the FAM83A-AS1 promoter region was also
explored. The average distribution of the beta values of the
methylation probes cg0831735 and cg054279078 located in the
FAM83A-AS1 promoter region was not significantly different between
tumor and normal tissue, and they were both located in the fully
methylated region (beta >0.6); however, in the partially
methylated region [beta in (0.2, 0.6)], the distribution in tumor
tissue was significantly more than that in normal tissue (Fig. 3E). Methylation levels are negatively
correlated with gene expression in most cases. Therefore, the
methylation differences of FAM83-AS1 promoter region between tumor
and normal tissues in LUAD may be a possible epi-regulatory
mechanism leading to its upregulated expression of FAM83A-AS1 in
tumors. Collectively, the pan-cancer analysis revealed that
increased expression level of FAM83A-AS1 was positively correlated
with the poor prognosis of patients and was also significantly
associated with high TMB in LUAD, which may be caused by the
dysregulated methylation of the promoter region of FAM83A-AS1.
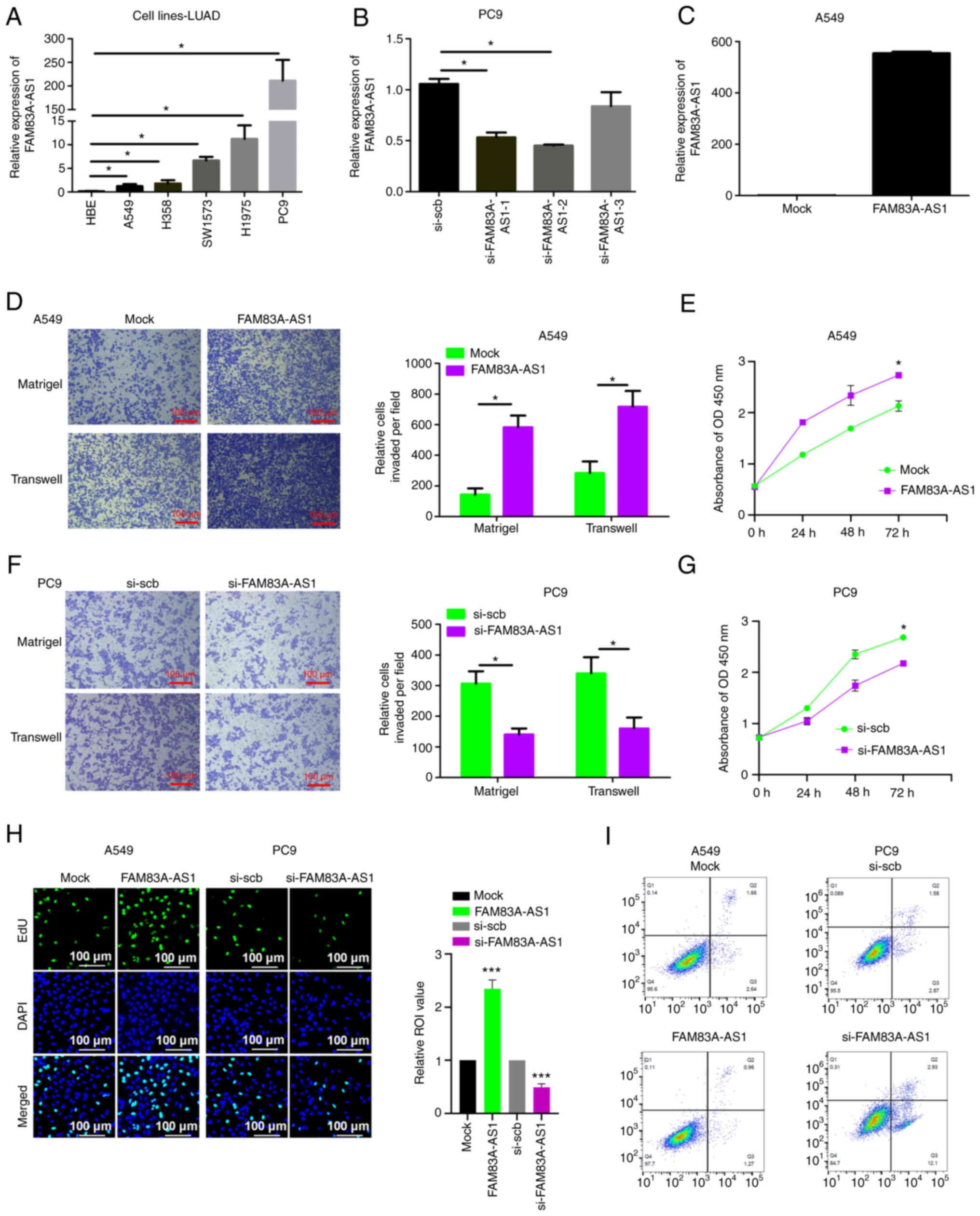
FAM83A-AS1 promotes malignancy of LUAD
in vitro
The role of FAM83A-AS1 in LUAD malignant progression
was further validated through functional assays. Firstly, the
endogenous expression of FAM83A-AS1 in LUAD cell lines was
investigated using RT-qPCR, which revealed that the endogenous
expression of FAM83A-AS1 was higher in all cancer cell lines than
that of normal lung epithelial cell lines HBE, and the expression
of FAM83A-AS1 was relatively higher in PC9 cell lines while it was
relatively lower in A549 cell lines (Fig. 4A), both of which were selected for
the next experimental assays. A total of three pairs of siRNAs were
designed to decrease the expression of FAM83A-AS1 (si-FAM83A-AS1-1,
si-FAM83A-AS1-2 and si-FAM83A-AS1-3), which were transfected into
the PC9 cell line. The RT-qPCR results revealed that
si-FAM83A-AS1-2 was the most efficient siRNA in FAM83A-AS1
knockdown (Fig. 4B), which was
selected to inhibit FAM83A-AS1 expression. In addition, A549 cell
lines were transfected with the plasmid of mock or FAM83A-AS1,
which revealed that overexpression of FAM83A-AS1 significantly
increases FAM83A-AS1 expression (Fig.
4C). Transwell migration and Matrigel invasion assays revealed
that overexpressing FAM83A-AS1 significantly promoted the migration
and invasion of LUAD cell lines (Fig.
4D). While CCK-8 and EdU assays were performed to identify that
overexpression of FAM83A-AS1 efficiently facilitates cell growth in
A549 cells (Fig. 4E and H).
Overexpression of FAM83A-AS1 also reduced cell apoptosis (Fig. 4I). By contrast, the knockdown of
FAM83A-AS1 significantly suppressed migration, invasion, and growth
and induced apoptosis of LUAD cell lines (Fig. 4F and G). Taken together, the
aforementioned data indicated that FAM83-AS1 is functionally
important in promoting the malignancy of LUAD.
FAM83A-AS1 acts as a sponge to
interact with miR-202-3p
Next, the potential biological mechanism of
FAM83A-AS1 was explored. LncRNAs are known to play numerous
important roles, one of which is acting as miRNA sponges (12). To explore the miRNA absorption
capacity of FAM83H-AS1, a RIP experiment of Ago2 was conducted both
in A549 and PC9 cells. The results demonstrated that FAM83H-AS1 was
significantly enriched by an anti-Ago2 antibody compared with IgG
(Fig. 5A), which indicated that the
biological function of FAM83A-AS1 may be through miRNA sponging
(13). The potential miRNAs that
interacted with FAM83A-AS1 were further identified through
bioinformatics analysis of two databases, TargetScan and StarBase
(14). After screening the
potential sponged miRNAs, miR-202-3p was selected, the seed
sequences of which are partially complementary to FAM83A-AS1
(Fig. 5B). To confirm the
interaction between FAM83A-AS1 and miR-202, FAM83A-AS1 and miR-202
mRNA expression was examined in patients with LUAD in TCGA database
and it was observed that miR-202 negatively correlated with
FAM83A-AS1 expression (R=−0.13, P=0.0025) (Fig. 5C). To verify whether this candidate
miRNA could directly bind to LncFAM83H-AS1, the lncRNA pull-down
experiment was conducted using the FAM83H-AS1 probe labeled with
biotin. As expected, the biotin probe bonded specifically to
FAM83H-AS1 (Fig. 5D). In both A549
and PC9 cell lines, miR-202-3p was significantly enriched by the
lncFAM83H-AS1 probe compared with the control probe (Fig. 5E). To further confirm the
interaction between FAM83A-AS1 and miR-202-3p, the MUT biotinylated
probes of FAM83A-AS1 and miR-202-3p were designed, respectively,
according to the complementary sequences. Compared with the
wide-type probe of FAM83A-AS1, the MUT probe of FAM83A-AS1 could
not significantly enrich miR-202-3p both in A549 and PC9 cell lines
(Fig. 5F). A biotinylated
miR-202-3p pull-down experiment was also conducted and the results
revealed that the enrichment of FAM83H-AS1 by the MUT miR-202-3p
probe was significantly reduced compared with the WT miR-202-3p
probe (Fig. 5G). In addition, FISH
analysis revealed co-localization of miR-202-3p and lncFAM83H-AS1
in the cytoplasm (Fig. 5H). A
significant negative correlation was also found between FAM83H-AS1
and miR-202-3p in LUAD tissues (R2=−0.3804, P<0.001) (Fig. 5I). Collectively, these results
suggested that FAM83H-AS1 acts as a sponge to directly bind
miR-202-3p.
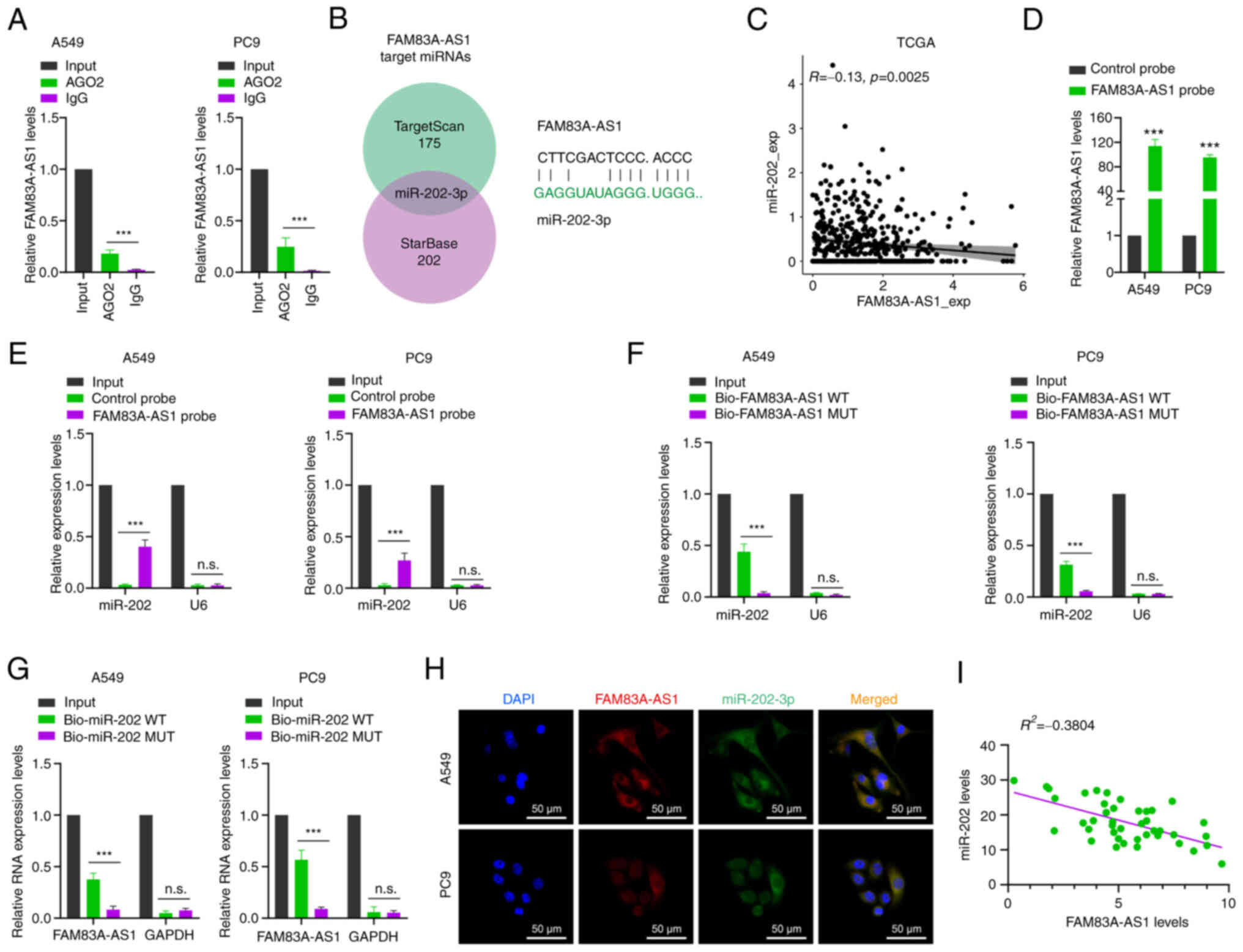 | Figure 5.FAM83A-AS1 acts as a sponge to
interact with miR-202-3p. (A) RNA Binding Protein
Immunoprecipitation assay was performed using an Ago2 antibody, and
IgG served as a negative control. (B) The Venn diagram shows the
intersection of miRNA lists. (C) FAM83A-AS1 mRNA and miR-202-3p
expression was analyzed in patients with LUAD in TCGA. (D) RT-qPCR
results showed that FAM83A-AS1 could be specifically enriched by
FAM83A-AS1 probe. (E) The relative expression levels of FAM83H-AS1
were detected by RT-qPCR in A549 and PC9 cell lysates using the
control probe or FAM83H-AS1 probe. (F) The expression levels of
miR-202-3p and small nuclear RNA U6 were detected by RT-qPCR in
A549 and PC9 cell lysates using WT Bio-FAM83H-AS1 probe or MUT
Bio-FAM83H-AS1 probe. (G) FAM83A-AS1 was enriched by biotinylated
WT or MUT-miR-202-3p, and RT-qPCR was used to determine the
relative FAM83A-AS1 mRNA levels. (H) RNA fluorescence in situ
hybridization images showed the localization of FAM83A-AS1 and
miR-202-3p in A549 cells (scale bar, 50 µm). (I) Expression of
FAM83A-AS1 and miR-202-3p was measured using RT-qPCR in LUAD
tissues. All the results are presented as the mean ± SD (n=3),
which were three separate experiments performed in triplicate.
***P<0.001 (Student's t-test). LUAD, lung adenocarcinoma; miRNA
or miR, microRNA; TCGA, The Cancer Genome Atlas; RT-qPCR, reverse
transcription-quantitative PCR; WT, wild-type; MUT, mutant; n.s.,
no significance. |
FAM83A-AS1 regulates miR-202-3p/HK2
axis to mediate glycolysis
Previous studies have found that lncRNA may play an
important role in tumor progression through the lncRNA-miRNA-mRNA
signaling pathway (15). To explore
the downstream target of FAM83A-AS1, the co-expression pattern of
FAM83A-AS1 and mRNAs was first analyzed according to the expression
data from TCGA. Kyoto Encyclopedia of Genes and Genomes pathway
enrichment analysis showed that FAM83A-AS1 was significantly
enriched in central carbon metabolism, glycolysis and mannose type
O-glycan biosynthesis (Fig. 6A,
upper). The potential target genes of miR-202-3p were also
predicted by the intersection of miRanda (Table SVI). Overlapping the
FAM83A-AS1-associated genes referred to glycolysis (Table SVII) and the potential target genes
of miR-202-3p (Fig. 6A, lower),
focus was addressed on the HK2 mRNA: i) There was a positive
correlation between FAM83A-AS1 and HK2 transcriptional expression
in TCGA database (Fig. 6B). ii) The
3′UTR of HK2 harbored sequences complementary to miR-202-3p
sequences (Fig. 6C). iii) RT-qPCR
and western blot analysis were used to detect the transcription and
protein level of HK2, respectively, when cells were transfected
with knockdown or overexpression of FAM83A-AS1 in A549 and PC9 cell
lines. The results revealed that overexpressing FAM83A-AS1
increased the expression level of HK2 both in transcription and
protein levels, while decreasing FAM83A-AS1 suppressed the
expression level of HK2 (Fig. 6D and
E). Subsequently, the dual-luciferase reporter assay showed
that the luciferase activity was significantly reduced after
co-transfection of miR-202-3p and HK2 3′ UTR WT reporter plasmid,
but not by MUT HK2 plasmid (Fig.
6F). HK2 has been reported to promote aerobic glycolysis of
tumor cells (16). Therefore,
lactate levels were measured, which showed that overexpression of
FAM83A-AS1 significantly increased tumor cell lactate level while
knockdown of FAM83A-AS1 significantly reduced the lactate level
(Fig. 6G and J). Additionally, the
Seahorse XF24e Extracellular Flux Analyzer was utilized to detect
the ECAR of LUAD cells, referred to as glycolysis. It was
demonstrated that FAM83A-AS1 knockdown significantly impaired
glycolysis in A549 (Fig. 6H and I)
and PC9 cell lines (Fig. 6K and L),
which was consistent with the reduced level of lactate production.
Collectively, these data indicated that FAM83A-AS1 sponges
miR-202-3p to regulate the expression of HK2, which medicates
cancer metabolic reprogramming.
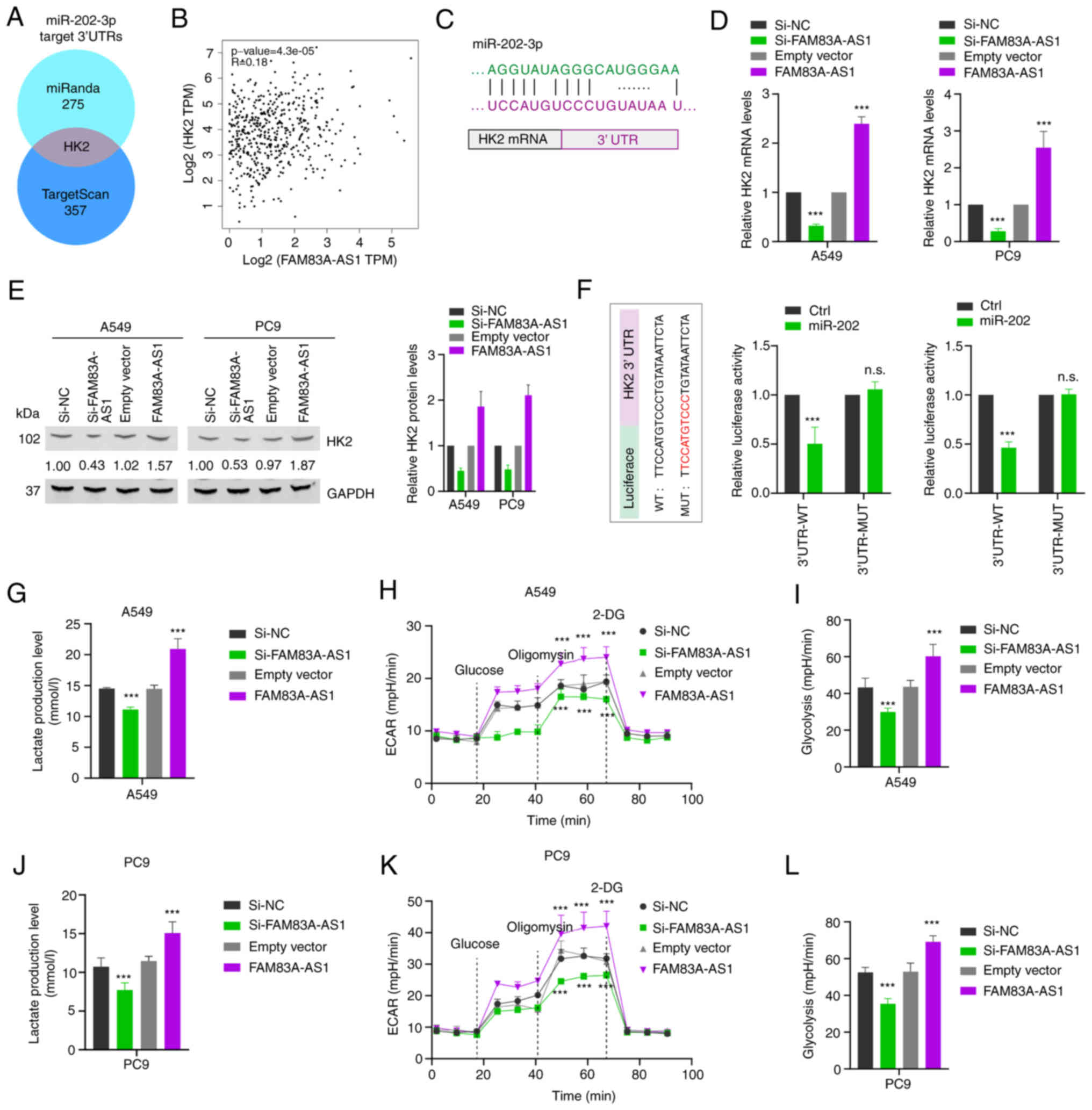 | Figure 6.FAM83A-AS1 regulates miR-202-3p/HK2
axis to mediate glycolysis. (A) Left: Kyoto Encyclopedia of Genes
and Genomes enrichment analysis showed the revealed signaling
pathways associated with FAM83A-AS1. Right: The Venn diagram shows
overlapping the FAM83A-AS1 associated genes referred to glycolysis
and the potential target genes of miR-202-3p by miRanda. (B)
Expression of FAM83A-AS1 and HK2 in The Cancer Genome Atlas
database. (C) MiR-202-3p can bind to the 3′-UTR of HK2 mRNA. (D)
The mRNA level of HK2 in A549 and PC9 cells after knockdown or
overexpression of FAM83A-AS1 was determined by reverse
transcription-quantitative PCR, random oligo as a NC. (E) The
expression levels of HK2 protein in A549 and PC9 cells with
knockdown or overexpression of FAM83A-AS1 were detected by western
blot analysis. (F) Left: Schematic graph illustrated the WT and
mutation MUT of potential binding site between miR-202-3p and the
3′-UTR regions of HK2. Right: The direct binding between HK2 3′-UTR
and miR-202-3p was analyzed by dual-luciferase reporter assay. (G
and J) Lactic acid levels were detected after FAM83A-AS1 knockdown
or overexpression in A549 and PC9 cells. (H and I, K and L) ECAR
and glycolysis levels were detected after FAM83A-AS1 knockdown or
overexpression in (H and I) A549 and (K and L) PC9 cells; 2-DG is a
glucose analogue. All the results are presented as the mean ± SD
(n=3), which were three separate experiments performed in
triplicate. ***P<0.001 (Student's t-test). HK2, hexokinase II;
miR, microRNA; UTR, untranslated region; NC, negative control; WT,
wild-type; MUT, mutant; ECAR, extracellular acidification rate;
2-DG, 2-Deoxy-D-glucose; n.s., no significance. |
FAM83A-AS1 promotes the malignant
progression of LUAD depending on miR-202-3p
To further confirm the biological function of
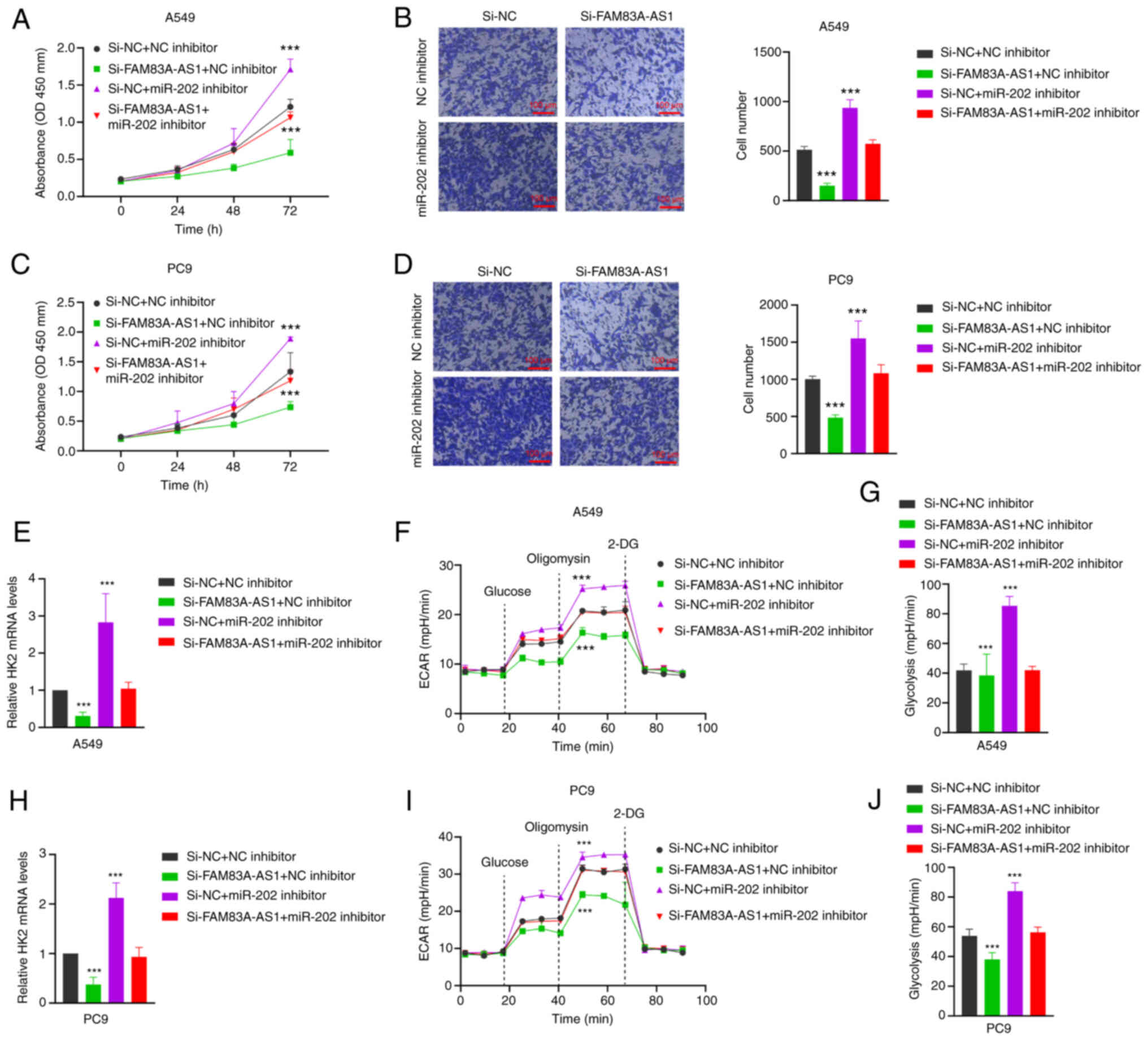
FAM83A-AS1 dependent on miR-202-3p, FAM83A-AS1 siRNA and miR-202-3p
inhibitor were co-transfected into A549 and PC9 cell lines. CCK-8
and Transwell experiments revealed that knockdown of lncFAM83H-AS1
successfully inhibited cell proliferation and migration, while
co-transfection of miR-202-3p inhibitor reversed the inhibition by
FAM83A-AS1 (Fig. 7A and B).
Additionally, it was explored whether the regulatory relationship
between FAM83A-AS1 and HK2 is dependent on miR-202-3p. HK2 mRNA
expression levels were decreased both in A549 and PC9 cell lines
when transfected with the knockdown of FAM83A-AS1, while they were
rescued when miR-202-3p inhibitors were co-transfected (Fig. 7C and D). Finally, a decreased
glycolysis level was observed when the expression level of
FAM83-AS1 was inhibited, which was consistent with the previous
data. While co-transfection of FAM83A-AS1 and miR-202-3p inhibitors
could partly rescue the glycolysis level of A549 and PC9 cell lines
(Fig. 7E-J). These results
suggested that LncFAM83H-AS1 promotes the malignant progression and
glycolysis of LUAD depending on miR-202-3p.
Discussion
In the present study, the differential expressed
lncRNAs across the three different histological pathology risk
groups were explored, and FAM83A-AS1 was selected as the candidate
lncRNA, which was positively associated with high-risk histological
subtypes and higher clinical stages. Pan-cancer analysis revealed
that FAM83A-AS1 was positively correlated with high TMB and poor
prognosis in LUAD. The increased expression of FAM83A-AS1 may
result from the hypomethylation of the promoter region. It was also
revealed that FAM83A-AS1 promoted the progression of LUAD cancer
cell lines in vitro. Additionally, FAM83A-AS1 functioned as
a ceRNA to sponge miR-202-3p to upregulate the expression of HK2,
which facilitated the malignant progression and glycolysis
(16).
The aforementioned study mainly focused on the
genomic diversity in the potential molecular mechanisms that
determine the individual histological subtype of LUAD. Caso et
al (17) employed targeted
next-generation sequencing (NGS), and revealed that tumors in the
high-risk group had higher copy number amplifications, as well as
statistical significance alteration in three oncogenic pathways
(p53, Wnt, Myc), compared with those in the intermediate or
low-risk group. Whole-genome sequencing of tumor cell clusters from
MIP in breast cancer shares high-frequency copy-number loss of
PRDM16 and IGSF9 and the copy-number gain of ALDH2 (18). In addition to the genomic
instability, non-genetic factors also contributed to the functional
and phenotypic heterogeneity of histological pathology. Integrating
with molecular data from >2,000 LUAD patients, Tavernari et
al (19) identified that
epigenetic and transcriptional reprogramming reshaped cancer cell
identity to decipher the transition of histological pathology from
indolent to aggressive patterns. In addition, the tumor
microenvironment also contributed to the determination of the
individual histological subtypes. It has been demonstrated that
paracrine TGF-β signaling activation secreted by cancer-associated
fibroblasts induced a solid-to acinar transition in lung cancer
cells (20). In the current study,
focus was addressed on the important members of transcriptome RNA,
lncRNAs, and the increased expression of FAM83A-AS1 was identified;
it showed a positive correlation with high-risk histological
subtype group and higher clinical stages. It was also revealed that
FAM83A-AS1 promoted migration, invasion and growth of LUAD cancer
cells, which was positively associated with poorer prognosis.
Certain studies have been reported to explore the
potential molecular mechanisms that determine the individual
histological subtype of LUAD. Utilizing NGS, the genomic diversity
of different histologic subtypes has been revealed (17). Compared with the intermediate or
low-risk group, patients in the high-risk group had higher TMB, a
rate of whole genome doubling, and several oncogenic pathways
altered. Tumors with a higher percentage of MIP tended to harbor
more chromosome instability, as well as the alteration in
cyclin-dependent kinase inhibitor 2A, mammalian target of
rapamycin, transcription termination factor 1 and brain-specific
angiogenesis inhibitor 3 (21). In
addition to the genomic mutation spectrum of lung cancer cells,
transcriptomic features have also been reported to be associated
with the histologic phenotype of lung cancer (22,23).
However, as one of the important members of transcriptome RNA,
little is known about the roles of lncRNA in the molecular features
of LUAD histologic patterns.
LncRNAs play an important role in regulating the
human genome due to their specific functions in various
physiological and pathological processes (6). LncRNA FAM83A-AS1, which has been
suggested to promote the development of tumors, is transcribed from
the antisense strand of the FAM83A gene located at 8q24.13
(24). An increasing number of
studies have reported that lncRNA-FAM83A-AS1 has potential
carcinogenic ability in esophageal cell squamous carcinoma
(25) HCC (26), and particularly LUAD. Wang et
al (27) determined that
FAM83A-AS1 increased FAM83A expression by enhancing FAM83A pre-mRNA
stability and promoted the tumorigenesis of LUAD. One of the main
mechanisms that lncRNA modulates gene expression is its interaction
with miRNA as a ceRNA to prevent miRNA from binding to the target
RNA, including FAM83A-AS1 (14).
For example, Xiao et al (28) identified that FAM83A-AS1 promotes
LUAD cell migration and invasion by targeting miR-150-5p and
modifying MMP14. In addition, there are several studies explaining
the ceRNA mechanism between FAM83A-AS1 and miR-141-3p (13) or miR-214 (25). In the present study, a ceRNA model
involving FAM83A-AS1, miR-202-3p and HK2 in LUAD was proposed. The
potential miRNAs that interacted with FAM83A-AS1 were identified
through bioinformatics analysis of two databases, TargetScan and
StarBase, and miR-202-3p was finally selected. At the same time, it
was found that overexpressing FAM83A-AS1 increased the expression
level of HK2 both in transcription and protein levels, while
decreasing FAM83A-AS1 suppressed the expression level of HK2. In
summary, these findings support the authors' hypothesis and reveal
a novel ceRNA regulatory axis (FAM83A-AS1-miR-203-3p-HK2) in LUAD
cells.
The abnormal metabolism of tumor cells is considered
one of the hallmarks of cancer, while glycolysis is regarded as the
main way of energy metabolism in the tumor (29). Glycolysis is closely correlated with
the tumor malignant degree. The rate-limiting enzyme HK2 in the
glycolytic pathway plays an important role in the regulation of
aerobic glycolysis (30). For
example, Tantai et al (31)
reported that TRIM46 activates AKT/HK2 signaling by modifying
PHLPP2 ubiquitylation to promote glycolysis of lung cancer cells.
Except the AMPK, the PI3K/Akt pathway, HIF-1α, and c-Myc,
increasing evidence showed that non-coding RNAs, including lncRNAs,
participate in glycolysis by regulating HK2 gene expression in
different types of cancer (32).
Chen et al (33) stated that
lncRNA PVT1 modulates HK2 expression by competitively binding to
miR-143 to regulate aerobic glucose metabolism in gallbladder
cancer. Yu et al (34)
reported that MIR210HG regulates glycolysis, cell proliferation,
and metastasis of pancreatic cancer cells through
miR-125b-5p/HK2/PKM2 axis. In the present study, it was found that
overexpressing FAM83A-AS1 increased the expression level of HK2
both in transcription and protein levels through sponging
miR-202-3p. Meanwhile, overexpression of FAM83A-AS1 significantly
increased tumor cell lactate levels. Moreover, FAM83A-AS1 knockdown
significantly impaired glycolysis by detecting the ECAR of LUAD
cells, suggesting that FAM83A-AS1 promotes LUAD cell glycolysis. It
was revealed that FAM83A-AS1 sponged miR-202-3p to regulate the
expression of HK2 in post-transcription, which facilitated the
malignancy and glycolysis. The present study, to the best of our
knowledge, is the first to document and interlink the
FAM83A-AS1/miR-202-3p/HK2 axis in regulating malignancy and
glycolysis of LUAD, which provided a novel avenue to addressing the
determination of histologic patterns. In the present study, focus
was addressed on the function and the mechanism of FAM83A-AS1 in
the intrinsic characteristics of the tumor. However, it is unknown
whether FAM83A-AS1 is expressed in other cell types in the tumor
microenvironment such as macrophages, and CD8+ T cells
also, which may have a distinct function and mechanism, all of
which should be explored further both in vivo and in
vitro.
In conclusion, it was identified that FAM83A-AS1 was
positively associated with high-risk pathological subtype and
higher clinical stages, and it functioned as a ceRNA to sponge
miR-202-3p to regulate the HK2 expression at post-transcription,
which promotes the malignancy and glycolysis of LUAD.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The Jiangsu Province ‘Six
Talent Peaks Project’ (grant no. WSN-027).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XX and LZ contributed to sample and data acquisition
and manuscript drafting. XX, LZ, BZ and DX offered technical
support. XX, LZ, CC, GD and WX made substantial contributions to
the conception and design of the study, funding of the study, and
supervision. GD and WX confirm the authenticity of all the raw
data. All authors were involved in writing the paper and read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
IACUC-2017088-1) by the Ethics Committee of the The First
Affiliated Hospital of Nanjing Medical University (Nanjing, China)
and complied with the ethical standards of the institution. Written
informed consent was provided by all enrolled participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He D, Wang D, Lu P, Yang N, Xue Z, Zhu X,
Zhang P and Fan G: Single-cell RNA sequencing reveals heterogeneous
tumor and immune cell populations in early-stage lung
adenocarcinomas harboring EGFR mutations. Oncogene. 40:355–368.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dong ZY, Zhang C, Li YF, Su J, Xie Z, Liu
SY, Yan LX, Chen ZH, Yang XN, Lin JT, et al: Genetic and immune
profiles of solid predominant lung adenocarcinoma reveal potential
immunotherapeutic strategies. J Thorac Oncol. 13:85–96. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schneider F and Dacic S: Histopathologic
and molecular approach to staging of multiple lung nodules. Transl
Lung Cancer Res. 6:540–549. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yousefi H, Maheronnaghsh M, Molaei F,
Mashouri L, Reza Aref A, Momeny M and Alahari SK: Long noncoding
RNAs and exosomal lncRNAs: Classification, and mechanisms in breast
cancer metastasis and drug resistance. Oncogene. 39:953–974. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li SY, Zhu Y, Li RN, Huang JH, You K, Yuan
YF and Zhuang SM: LncRNA Lnc-APUE is repressed by HNF4α and
promotes G1/S phase transition and tumor growth by regulating
MiR-20b/E2F1 axis. Adv Sci (Weinh). 8:20030942021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parasramka MA, Maji S, Matsuda A, Yan IK
and Patel T: Long non-coding RNAs as novel targets for therapy in
hepatocellular carcinoma. Pharmacol Ther. 161:67–78. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen HY, Chan SJ, Liu X, Wei AC, Jian RI,
Huang KW, Lang YD, Shih JH, Liao CC, Luan CL, et al: Long noncoding
RNA Smyca coactivates TGF-β/Smad and Myc pathways to drive tumor
progression. J Hematol Oncol. 15:852022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma F, Liu X, Zhou S, Li W, Liu C, Chadwick
M and Qian C: Long non-coding RNA FGF13-AS1 inhibits glycolysis and
stemness properties of breast cancer cells through
FGF13-AS1/IGF2BPs/Myc feedback loop. Cancer Lett. 450:63–75. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Venkatesh J, Wasson MD, Brown JM, Fernando
W and Marcato P: LncRNA-miRNA axes in breast cancer: Novel points
of interaction for strategic attack. Cancer Lett. 509:81–88. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang H, Yang C, Zhang Q, Zhuo T, Li X, Li
N, Zhu L, Luo C, Gan J and Wu Y: Long non-coding RNA FAM83A
antisense RNA 1 (lncRNA FAM83A-AS1) targets microRNA-141-3p to
regulate lung adenocarcinoma cell proliferation, migration,
invasion, and epithelial-mesenchymal transition progression.
Bioengineered. 13:4964–4977. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang J, Bi Y, Liu XP, Yu D, Yan X, Yao J,
Liu T and Li S: To construct a ceRNA regulatory network as
prognostic biomarkers for bladder cancer. J Cell Mol Med.
24:5375–5386. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su L, Li R, Zhang Z, Liu J, Du J and Wei
H: Identification of altered exosomal microRNAs and mRNAs in
Alzheimer's disease. Ageing Res Rev. 73:1014972022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi T, Ma Y, Cao L, Zhan S, Xu Y, Fu F,
Liu C, Zhang G, Wang Z, Wang R, et al: B7-H3 promotes aerobic
glycolysis and chemoresistance in colorectal cancer cells by
regulating HK2. Cell Death Dis. 10:3082019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caso R, Sanchez-Vega F, Tan KS,
Mastrogiacomo B, Zhou J, Jones GD, Nguyen B, Schultz N, Connolly
JG, Brandt WS, et al: The underlying tumor genomics of predominant
histologic subtypes in lung adenocarcinoma. J Thorac Oncol.
15:1844–1856. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi Q, Shao K, Jia H, Cao B, Li W, Dong S,
Liu J, Wu K, Liu M, Liu F, et al: Genomic alterations and evolution
of cell clusters in metastatic invasive micropapillary carcinoma of
the breast. Nat Commun. 13:1112022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tavernari D, Battistello E, Dheilly E,
Petruzzella AS, Mina M, Sordet-Dessimoz J, Peters S, Krueger T,
Gfeller D, Riggi N, et al: Nongenetic evolution drives lung
adenocarcinoma spatial heterogeneity and progression. Cancer
Discov. 11:1490–1507. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato R, Imamura K, Semba T, Tomita Y,
Saeki S, Ikeda K, Komohara Y, Suzuki M, Sakagami T, Saya H and
Arima Y: TGFβ signaling activated by cancer-associated fibroblasts
determines the histological signature of lung adenocarcinoma.
Cancer Res. 81:4751–4765. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang S, Xu Y, Zhao P, Bao H, Wang X, Liu
R, Xu R, Xiang J, Jiang H, Yan J, et al: Integrated analysis of
genomic and immunological features in lung adenocarcinoma with
micropapillary component. Front Oncol. 11:6521932021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang M, Abbas HA, Negrao MV, Ramineni M,
Hu X, Hubert SM, Fujimoto J, Reuben A, Varghese S, Zhang J, et al:
The histologic phenotype of lung cancers is associated with
transcriptomic features rather than genomic characteristics. Nat
Commun. 12:70812021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nguyen TT, Lee HS, Burt BM, Wu J, Zhang J,
Amos CI and Cheng C: A lepidic gene signature predicts patient
prognosis and sensitivity to immunotherapy in lung adenocarcinoma.
Genome Med. 14:52022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Z, Hu Z, Sui Q, Huang Y, Zhao M, Li
M, Liang J, Lu T, Zhan C, Lin Z, et al: LncRNA FAM83A-AS1
facilitates tumor proliferation and the migration via the
HIF-1α/glycolysis axis in lung adenocarcinoma. Int J Biol Sci.
18:522–535. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jia J, Li H, Chu J, Sheng J, Wang C, Jia
Z, Meng W, Yin H, Wan J and He F: LncRNA FAM83A-AS1 promotes ESCC
progression by regulating miR-214/CDC25B axis. J Cancer.
12:1200–1211. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He J and Yu J: Long noncoding RNA
FAM83A-AS1 facilitates hepatocellular carcinoma progression by
binding with NOP58 to enhance the mRNA stability of FAM83A. Biosci
Rep. 39:BSR201925502019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang W, Zhao Z, Xu C, Li C, Ding C, Chen
J, Chen T and Zhao J: LncRNA FAM83A-AS1 promotes lung
adenocarcinoma progression by enhancing the pre-mRNA stability of
FAM83A. Thorac Cancer. 12:1495–1502. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao G, Wang P, Zheng X, Liu D and Sun X:
FAM83A-AS1 promotes lung adenocarcinoma cell migration and invasion
by targeting miR-150-5p and modifying MMP14. Cell Cycle.
18:2972–2985. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lunt SY and Vander Heiden MG: Aerobic
glycolysis: Meeting the metabolic requirements of cell
proliferation. Annu Rev Cell Dev Biol. 27:441–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou L, Li M, Yu X, Gao F and Li W:
Repression of hexokinases II-mediated glycolysis contributes to
piperlongumine-induced tumor suppression in non-small cell lung
cancer cells. Int J Biol Sci. 15:826–837. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tantai J, Pan X, Chen Y, Shen Y and Ji C:
TRIM46 activates AKT/HK2 signaling by modifying PHLPP2
ubiquitylation to promote glycolysis and chemoresistance of lung
cancer cells. Cell Death Dis. 13:2852022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Li Y, Bai S, Zhang J, Liu Z and Yang
J: NR2F1-AS1/miR-140/HK2 axis regulates hypoxia-induced glycolysis
and migration in hepatocellular carcinoma. Cancer Manag Res.
13:427–437. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, Yu Y, Li H, Hu Q, Chen X, He Y,
Xue C, Ren F, Ren Z, Li J, et al: Long non-coding RNA PVT1 promotes
tumor progression by regulating the miR-143/HK2 axis in gallbladder
cancer. Mol Cancer. 18:332019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu T, Li G, Wang C, Gong G, Wang L, Li C,
Chen Y and Wang X: MIR210HG regulates glycolysis, cell
proliferation, and metastasis of pancreatic cancer cells through
miR-125b-5p/HK2/PKM2 axis. RNA Biol. 18:2513–2530. 2021. View Article : Google Scholar : PubMed/NCBI
|