Introduction
Ecklonia cava (E. cava) is a perennial
brown alga found widely along the coastal regions of Korea and
Japan that has been used traditionally as a food and food with
medicinal properties (1). E.
cava contains numerous natural, bioactive compounds and their
various derivatives, including the major compound, phlorotannins
(PT), as well as peptides, carotenoids and sulfated polysaccharides
(2,3). Previous studies have reported the
beneficial effects of E. cava extract, such as antioxidant
properties (4,5), anti-human immunodeficiency virus
type-1 activity (6), stimulatory
effects for apoptosis (7),
inhibitory effects for angiotensin 1-converting enzyme (8), anti-inflammatory effects (9) and anti-diabetic effects (10).
The potential for anti-tumor drugs from E.
cava has been previously assessed; however, most studies used
only E. cava-derived compounds, such as dieckol (11), dioxinodehydroeckol (7) and 6,6′-bieckol (12). Among these E. cava-derived
compounds, the anti-tumor activity of dieckol has been widely
reported in several cells and animal models (11–16).
Dieckol inhibited the expression of vascular endothelial growth
factor (VEGF) protein and matrix metallopeptidase (MMP)-9, and
suppressed cell movement as well as stimulated tissue inhibitor of
metalloproteinases (TIMP)-1/2 in MCF-7 cells (13). Moreover, dieckol was reported to
have had anti-tumor effects, including apoptosis activation, tumor
growth suppression, increased reactive oxygen species (ROS)
production and suppression of the Akt/p38 signaling pathway in
ovarian cancer cells and a xenograft model (11). Similar suppressive effects on
invasion and angiogenesis were reported in an
N-nitrosodiethylamine-induced hepatocarcinogenesis model after
dieckol treatment (14).
Furthermore, dioxinodehydroeckol derived from E. cava was
reported to have had inhibitory effects against the proliferation
of MCF-7 cells via regulation of the nuclear factor (NF)-κB
signaling pathway (7). Another
compound, 6,6′-bieckol, was reported to have inhibited the
expression of MMP-2/9, cell migration and the NF-κB signaling
pathway (12). Certain extracts and
complexes derived from E. cava have been reported to have
had similar anti-tumor effects in numerous cancer cells. For
example, the bioactive compounds extracted from E. cava
using carbohydrase hydrolysis suppressed the growth and
proliferation of CT26 colon cancer cells (15). The complex sulfated polysaccharides
derived from E. cava was also reported to have had
anti-tumor effects on cell growth, apoptotic body formation,
caspase (Cas)-9/poly (ADP-ribose) polymerase expression and the
Bax/Bcl-2 signaling pathway (16).
However, to the best of our knowledge, there have been no reports
on the anti-tumor effects and mechanism of the aqueous extract of
E. cava (AEC) against colon cancer.
The present study was intended to be assess the
anti-tumor effects and molecular mechanism of AEC in CT26 cells and
BALB/cKorl syngeneic mice with a CT26 tumor.
Materials and methods
Preparation of AEC and PT
AEC samples were prepared as described previously
(17) with slight modification.
Firstly, dried samples of E. cava were purchased from Para
Jeju Co. Ltd., and were deposited as voucher specimens (accession
no. WPC-19-001) at the Functional Materials Bank of the Wellbeing
RIS Center at Pusan National University (Pusan, Korea). After
extraction using mixture of AEC samples and distilled
dH2O solvent (1:15 ratio), the lyophilized AEC pellets
were harvested in an N-1100 series rotary evaporator (Tokyo
Rikakikai Co., Ltd.).
PT was extracted according to the method previously
described by Lee et al (18)
with slight modification. The dried E. cava powder (30 g)
was mixed with 70% ethanol (300 ml; v/v) and the extract was then
collected by shaking for 12 h at 37°C. After repeating the
extraction process three times, the total extracted solution was
filtered using a membrane with 8 mm pore size and then evaporated
at 40°C. These extracts were dissolved in dH2O, and
sequentially fractionated using n-hexane, chloroform and ethyl
acetate. Finally, ethyl acetate fraction was evaporated at 40°C to
remove the solvent, and used as the PT-rich sample, as reported
previously (18,19).
Determination of four bioactive
compounds in AEC
Total phenolic content (TPC) concentration was
assessed using the Folin-Denis method, as previously described
(20). Briefly, after consistently
mixing the AEC solution, Folin-Ciocalteu reagent and 10%
Na2CO3 solution for 1 h at 37°C, the
absorbance of each well was measured at 725 nm. The concentration
of TPC in AEC was estimated using a standard calibration curve for
caffeic acid (MilliporeSigma) because it is considered as one of
the major phenolic acids. Total flavonoid content (TFC) was
assessed using the Davis method as previously described (21). Briefly, after constantly mixing AEC
solution, 1 N NaNO2 and 90%
C4H10O3 for 1 h at 37°C, the
absorbance of each well was measured at 420 nm. The concentration
of TFC in AEC was estimated using a standard calibration curve for
naringin (MiliporeSigma) because naringin and its aglycone
naringenin belong to this series of flavonoids. Total condensed
tannin content (TTC) was assessed using the Vanillin method as
previously described (22).
Briefly. the concentration of TTC in AEC was estimated based on a
standard calibration curve for a purified (+)-catechin hydrate
standard (MilliporeSigma) because catechin has all the
characteristics of condensed tannin. Finally, the concentration of
total PT content (TPTC) was determined using the Folin-Ciocalteu
method as described previously (23). The concentration of TPTC in AEC was
estimated using a standard calibration curve for phloroglucinol
(MilliporeSigma) because PT is a complex polymer of phloroglucinol,
which is a specific compound found in brown algae. TPC, TFC, TTC
and TPTC in AEC were presented as caffeic acid, naringin, catechin
hydrate and phloroglucinol equivalents (mg) of AEC,
respectively.
Analysis for free radical scavenging
activity
The scavenging activity of
2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals was analyzed as
previously described by Go et al (23). Briefly, after incubation of AEC and
0.1 mM DPPH solution (MilliporeSigma) for 30 min at room
temperature, the absorbance of each well was measured at 517 nm
using a VersaMax plate reader (Molecular Devices, LLC). AEC
scavenging activity for DPPH radicals activity was presented as the
half-maximal inhibitory concentration (IC50) based on
the percentage decrease in absorbance relative to the control
group.
Liquid chromatography-mass
spectrometry (LC-MS) analysis
Bioactive compounds in AEC were assessed using an
Agilent 1290 Infinity high-performance liquid chromatography (HPLC)
system (Agilent Technologies Deutschland GmbH), coupled with a
hybrid quadrupole time-of-flight mass spectrometer (Agilent
Technologies Deutschland GmbH). Detailed conditions and the column
for LC-MS analysis as well as data analysis were performed as
described previously (17).
Briefly, LC-MS signals were detected on a mass spectrometer
operating in the positive ionization mode. A HSS T3 Column (2.1×100
mm, 1.8 µm; Waters Corporation) was used for chromatographic
separation under the following conditions: 0.2 ml/min in flow rate,
10 µl of injection volume, water as mobile phase A, and 100%
acetonitrile as mobile phase B. For MS detection, the operating
parameters were as follows: Gas temperature, 300°C; gas flow, 9
l/min; nebulizer pressure, 45 psi; sheath temperature, 350°C;
sheath gas flow, 11 l/min; VCap, 4,000 V; fragmentor voltage, 175
V. All the acquisition and analysis of data were controlled by
MassHunter software (version B. 0600, Agilent Technologies).
Cell culture and viability assay
Murine colorectal carcinoma CT26 cells from a BALB/c
mouse, were used to evaluate the anti-tumor effects of AEC because
they successfully form solid tumors and metastasize into other
organs in BALB/c or immunocompromised mice. A HCT116 cancer cell
line from the colon tissue of an adult male with colon cancer, was
used to evaluate whether anti-tumor effects of AEC in colon cancer
cells of murine were similar in human colon cancer cells.
Fibroblast Detroit 551 cells from the skin of a normal human
embryo, were used to assess whether AEC was toxic to normal cells.
These three cell lines were purchased from the American Type
Culture Collection. The medium with 10% fetal bovine serum
(Welgene, Inc.) were prepared appropriately for each cell line; a
Roswell Park Memorial Institute 1640 Medium (Welgene, Inc.) for
CT26 cells, McCoy's 5a Medium (Welgene, Inc.) for HCT116 cells and
Minimum Essential Medium Eagle (Welgene, Inc.) for Detroit 551. The
viability of the CT26, HCT116 and Detroit 551 cells was determined
after treatment with AEC using the tetrazolium compound, MTT
(MilliporeSigma) as described previously (21). The optimal concentration of AEC was
determined based on the anti-tumor activity of E. cava
extract in human breast cancer cells (21) and the dose-response curve of AEC in
CT26, HCT116 and Detroit 551 cell lines (Fig. S1A, B and C). Cells at 70–80%
confluence were treated with either the dH2O (Vehicle
treated group, Vehicle) or pretreated with 5 µg/ml Cisplatin (Cis
treated group, Cis), 600 (Low dose AEC, LoAEC treated group), 800
(Medium dose AEC, MiAEC treated group) or 1,000 µg/ml (High dose
AEC, HiAEC treated group) AEC for 24 h at 37°C. In the case of the
PT treatment, the CT26 cells were treated with 100 (Low dose PT,
LoPT treated group), 150 (Medium dose PT, MiPT treated group) or
200 µg/ml (High dose PT, HiPT treated group) PT for 24 h at 37°C.
The optimal dosage of PT was determined based on previous results
which reported IC50 from 56.3 to 219 µg/ml in MKN-28,
Caco-2 and HT-29 cells (24) and
the dose-response curve of PT in CT26 cell lines (Fig. S1D). After incubation for 24 h, 200
µl of fresh medium and 50 µl of MTT solution (2 mg/ml in 1× PBS)
were added to each well, with the supernatants discarded. After
incubation at 37°C for 4 h, the formazan precipitate in each well
was dissolved entirely in dimethyl sulfoxide (DMSO, Duchefa
Biochemie, B.V.), and the absorbance was read at 570 nm using a
Vmax plate reader (Molecular Devices LLC). Before the detection of
the level of the formazan precipitate, the morphological changes of
cells were observed using an optical microscope (Leica Microsystems
GmbH) at a 400× magnification.
Furthermore, the cell viability assessed using the
MTT assay was also evaluated based on the quantification of the ATP
present, because ATP concentration indicates the presence of
metabolically active cells, using CellTiter-Glo assay kit (Promega
Corporation) according to the manufacturer's protocol. After mixing
cell culture medium and CellTiter-Glo® Reagent, the
luminescent signal from each well was measured under an
GloMax® 20/20 Luminometer (Promega Corporation) and ATP
concentration were determined using an ATP standard curve.
Immunofluorescence (IF) staining
analysis of cells
AEC treated CT26 cells were fixed using 4%
formaldehyde for 1 h at room temperature and permeabilized using 1%
Triton X-100, were incubated with 0.5% bovine serum albumin (BSA)
for 1 h at room temperature and then Ki67/MKI67 primary antibodies
(1:20; cat. no. NB500-170s; Novus Biologicals, LLC) for 12 h at
4°C. The cells were subsequently incubated with Alexa Fluor 488
goat anti-rabbit IgG (1:100; cat. no. A11008; Thermo Fisher
Scientific, Inc.) secondary antibodies for 1 h at room temperature.
Ki67 positive cells in CT26 cells were detected based on the green
fluorescence intensity using an EVOS™ M5000 Imaging System (Thermo
Fisher Scientific, Inc.). Their number was counted in the total
area of the field of view (67,500 µm2) for each well of
CT26 cells using the Imaging System.
Analysis of apoptotic cells using
fluorescence-activated cell sorting
The number of apoptotic cells was analyzed using a
Muse™ Annexin V and Dead Cell Kit (MilliporeSigma). Briefly, the
wells containing the CT26 cells were divided into six groups (No,
Vehicle, Cis, LoAEC, MiAEC, and HiAEC) for the AEC experiment.
After being treated with aforementioned concentrations of AEC and
Cis for 24 h at 37°C, the cells were treated with annexin V and
7-aminoactinomycin D (7-AAD) for 20 min at room temperature. The
distribution of the stained cells were analyzed using a Muse™ Cell
Analyzer (MilliporeSigma). After gating analyzed data based on the
cell size, cells were classified into four groups: non-apoptotic
cells, early apoptotic cells, late apoptotic cells and primarily
nuclear debris as described previously (21).
Western blotting analyses
Total protein was collected from the CT26 cells and
CT26 tumors of BALB/cKorl syngeneic mice using the Pro-Prep Protein
Extraction Solution (Intron Biotechnology, Inc.). The tissue
homogenates were prepared from two to three solid tumors per group
based on the results of the measurement of weight and hematoxylin
and eosin (H&E) staining analysis. After homogenizing the
tumor, total proteins were harvested, and their concentration was
determined using a SMART™ Bicinchoninic Acid Protein assay kit
(Thermo Fisher Scientific, Inc.). Total proteins (30 µg/lane) were
separated on 4–20% gradient SDS-PAGE for 2 h at 100 V, and they
were subsequently transferred to nitrocellulose blotting membranes
with a 0.45 µm pore size for 2 h at 40 V. Proteins bounded on
nitrocellulose membranes were incubated with primary antibodies
(Table SI) overnight at 4°C. After
removing the non-specifically bound antibodies using washing buffer
(137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and
0.05% Tween 20), the membranes were incubated with HRP-conjugated
goat anti-rabbit IgG (1:1,000; cat. no. HS101; Transgene Biotech
Co., Ltd.). The intensity of each protein was analyzed using a
Chemiluminescence Reagent (Pfizer, Inc.) using the
FluorChem® FC2 imaging system (ProteinSimple). Finally,
the density of each protein was quantified using AlphaView (Version
3.2.2, Cell Biosciences, Inc.).
Animal experiments
The protocol for experimental animal study was
reviewed and approved by the Pusan National University
Institutional Animal Care and Use Committee (approval no.
PNU-2020-0108). To ensure the reliability of the data from the
animal study, the total number of animals was determined as 35
using G-POWER 3.1.9.7 (Heinrich-Heine-Universität Düsseldorf,
Germany) with the α probability of 0.05, effect size of 0.8 and a
power of 0.80. Seven week old male BALB/cKorl mice (n=35) were
supplied by the National Institute of Food and Drug Safety
Evaluation (Cheongju, Korea) of the Korea Food and Drug
Administration (KFDA). The BALB/cKorl syngeneic mice were bred at
the Pusan National University-Laboratory Animal Resources Center,
which is accredited by the KFDA (accredited unit 000231) and The
Association for Assessment and Accreditation of Laboratory Animal
Care International (accredited unit 001525). Mice were provided,
ad libitum, with filtered tap water and a standard
irradiated chow diet (Samtako Co., Ltd.). All mice were maintained
in a specific pathogen-free state, with a strictly regulated 12 h
light/dark cycle, and constant temperature (22±2°C) and relative
humidity (50±10%).
The anti-tumor activity of AEC was evaluated in the
BALB/cKorl syngeneic mice for a 37 day period using a slight
modification of methods reported previously (25,26).
Briefly, 4×105 CT26 cells were injected subcutaneously
into the back region of BALB/cKorl mice on day 1. CT26
tumor-bearing BALB/cKorl syngeneic mice (n=35) were divided
randomly into one of five groups (n=7/group). The first group
(Vehicle treated group, n=7) was orally administrated the same
volume (200 µl) of 1× PBS every day for five weeks. The second
group (Cis treated group, n=7) was injected intraperitoneally with
Cis (4 mg/kg) every two days from day 26 to day 37, because
long-term administration of Cis causes serious toxicity and can
lead to death (27). The other
three groups were administered a low (250 mg/kg; LoAEC treated
group, n=7), medium (500 mg/kg; MiAEC treated group, n=7) or high
concentration of AEC (1,000 mg/kg; HiAEC treated group, n=7) orally
every day for 37 days. The health and behavior of all mice were
monitored every day before treatment was administrated. No animals
died during the experimental period. Bedding was abundantly
provided as special housing condition to help animals maintain
their body temperature. Furthermore, humane endpoints were set when
the tumor exceeded 4,400 mm3 in volume (28) and when body weight of mice decreased
>20% within 1–2 weeks, to prevent pain or distress of mice. At
24 h after the final treatment, all BALB/cKorl syngeneic mice were
euthanized by a trained researchers using an appropriate chamber
with gas regulator and CO2 gas with a minimum purity of
99.0% based on the AVMA Guidelines for the Euthanasia of Animals. A
cage containing animals was placed in the chamber and
CO2 gas of 99.0% was introduced into chamber without
pre-charging, with a fill rate of ~50% of the chamber volume per
minute. The death of mice were confirmed by ascertaining cardiac
and respiratory arrest, or dilated pupils and fixed body. The solid
tumors were then collected from all euthanized BALB/cKorl syngeneic
mice (Fig. S2).
Determination of tumor volume and
weight
The size of CT26 tumors was measured daily during
oral administration of AEC (from day 1 to 36). The tumor volume was
determined based on the measurement results for length and width of
the tumors using the following formula: Tumor volume
(mm3)=(length × width2)/2. Images of each
tumor formed in the mice were taken after the last administration
using a micro computed tomography (microCT) scanner (Nano Focus Ray
Co., Ltd.). The tumor volume in the microCT image was quantified
using ImageJ 1.52a (National Institutes of Health). The weight of
each tumor was assessed, once excised, using an electrical balance
in duplicate.
Histopathological analysis
The CT26 solid tumor collected from the BALB/cKorl
syngeneic mice model was fixed in a 10% formalin solution for 48 h
at room temperature, and then the middle region of solid tumor was
embedded into paraffin block. Following sectioning the tumor block
into 4 mm thick slices, these section were stained with a H&E
solution (Sigma-Aldrich; Merck KGaA) for 4 min 15 sec at room
temperature. After optical microscopic examination of the tumor
sections at 400× magnification, the tumor type and pathological
features were characterized by Professor Beum Seok Han at Hoseo
University (Asana, Korea). The necrotic areas on the H&E
stained tumor sections were measured and quantified, as described
previously (29).
IF staining analysis of tissue
The aforementioned tissue sections of CT26 solid
tumor (two to three samples per group) were deparaffinized with
xylene, rehydrated using a decreasing EtOH series, and then washed
three times with dH2O for 5 min at room temperature.
These sections were blocked using 10% goat serum (Vector
Laboratories, Inc.; Maravai LifeSciences) in 1× PBS solution for 30
min at room temperature. The tissue slides were incubated with
anti-Ki67/MKI67 (1:100; cat. no. NB500-170s; Novus Biologicals)
primary antibodies for 12 h at 4°C, and then with goat fluorescein
isothiocyanate (FITC)-labeled anti-rabbit IgG secondary antibodies
(1:100; cat. no. 65-6111; Thermo Fisher Scientific, Inc.) for 45
min at room temperature. Finally, Ki67 positive cells in tumor
sections were detected based on the green fluorescence intensity
using an EVOS™ M5000 Imaging System (Thermo Fisher Scientific,
Inc.). Their number was counted in the total area of the field of
view (67,500 mm2) for each CT26 tumor section using the
Imaging System.
Whole blood and serum analysis
After anesthesia with an intraperitoneal injection
of anesthetic mixture (4:1 ratio) containing of 40 mg/kg Alfaxan
(Jurox Pty Limited) and 10 mg/kg Rompun (Bayer-Korea, Ltd.) based
on previous report (30), the
abdominal region of BALB/cKorl syngeneic mice were opened by
sterile scissors. Before opening, adequate anesthesia of mice was
ensured by testing the pedal withdrawal reflex (clasping one's toes
with tweezers) and lacking the eye blink reflex. The whole blood
sample was collected from the abdominal veins using 1 ml syringe
(26 SWG), and subsequentially the mice were terminated by cervical
dislocation. The levels of 12 key factors were analyzed by an
automated cell counter (Beckman-Coulter, Inc.) using the Vetscan
HM5 Reagents Pack (cat. no. 89126-098; Abaxis, Inc.; Zoetis
Services LLC) according to the manufacturer's protocols. The levels
of white blood cells, red blood cells, hemoglobin, hematocrit, mean
corpuscular volume, mean corpuscular hemoglobin, mean corpuscular
hemoglobin concentration, corpuscular hemoglobin concentration
mean, corpuscular hemoglobin content, hemoglobin concentration
distribution width, platelets and mean platelet volume were
measured in duplicate for each sample.
Serum samples were obtained from whole blood sample
after centrifugation at 1,500 × g for 10 min at 4°C. The
concentrations of 5 serum biochemical components were analyzed
using a Hitachi 747 automatic serum analyzer (Hitachi, Ltd.). All
analysis were performed in duplicate using fresh samples.
Statistical analysis
One-way ANOVA (SPSS for Windows, Release 10.10, IBM
Corp.) was used to assess statistical significance between the
treatment groups, and followed by Tukey's post-hoc test for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference, and all values were presented
as the mean ± standard deviation (SD).
Results
Composition and antioxidative activity
of AEC
To evaluate the potential antioxidative activity of
AEC, the main phytochemical classes and DPPH radical scavenging
activity of AEC were analyzed. Among the three phytochemicals, TPC
was detected at the highest concentrations (108.05 mg/g), whereas
the TTC and TFC concentrations were relatively low (29.19 and 6.88
mg/g, respectively). Furthermore, the concentration of TPTC was
2.60 mg/g (Fig. 1A). In addition,
the scavenging activity of AEC against the DPPH radical increased
markedly in a dose-dependent manner, with an IC50 value
of 21.96 µg/ml (Fig. 1B). These
concentrations of phytochemicals and DPPH radical scavenging
activity were similar to those reported elsewhere (31). Furthermore, eleven active
components, including 9,12-octadecadienoic acid (32), aspartic acid (33), eckol (34), phlorofucofuroeckol (35) and dieckol (36), were identified and characterized
using LC-MS analyses (Figs. 1C and
S3). Among them, six compounds,
including phloroglucinol, eckol, 6,6′-bieckol, phlorofucofuroeckol,
fucodiphloroethol G and dieckol, belonged to PT, which contains
numerous separate compounds, and is classified into four
subclasses: the fuhalols and phlorethols group, the fucols group,
the fucophlorethols group and the eckols group (5,35,36).
Therefore, these results indicated that AEC had potential
antioxidant activity.
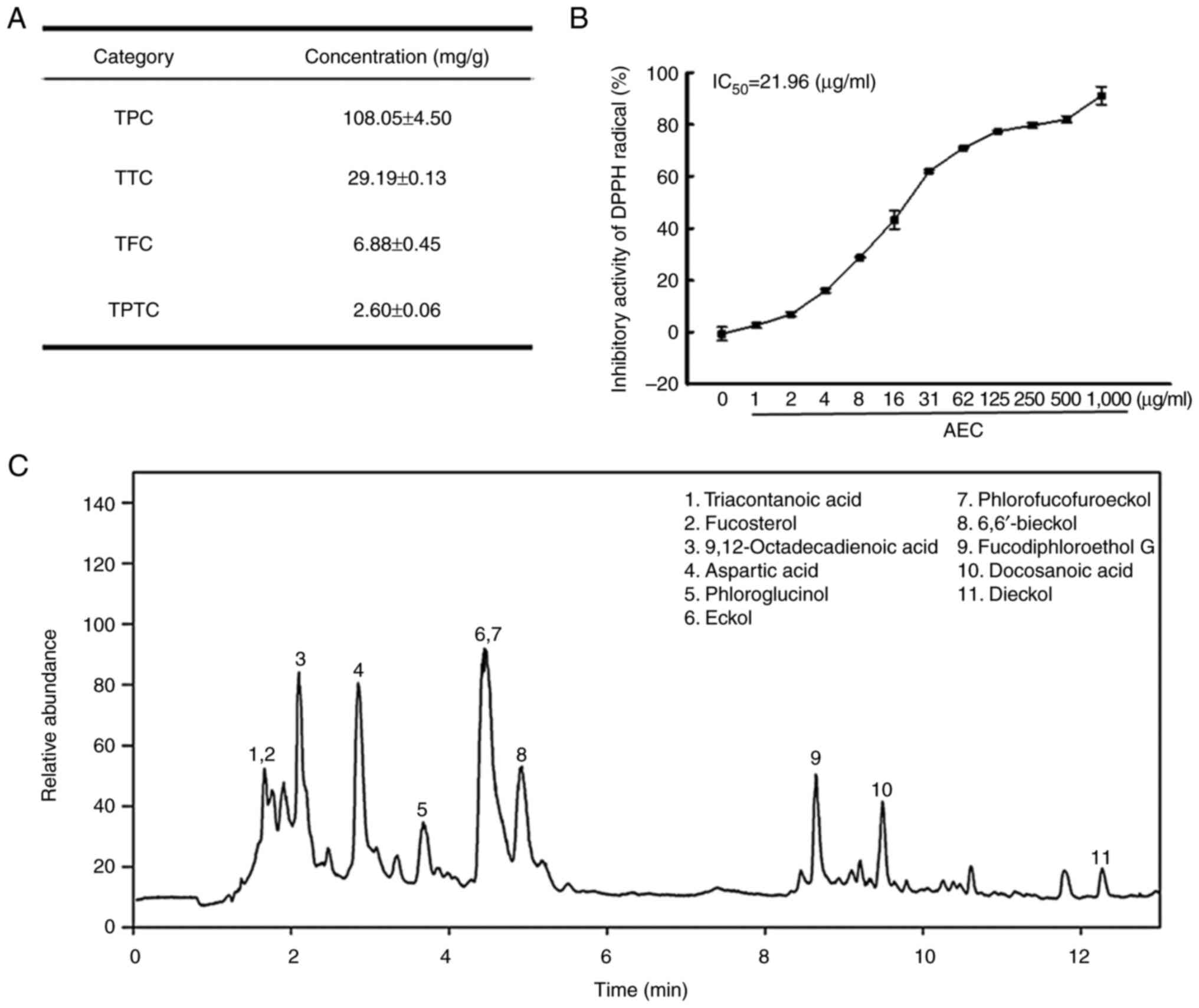 | Figure 1.Major phytochemical composition and
DPPH radical scavenging activity of AEC. (A) TPC, TTC, TFC and TPTC
levels in AEC were analyzed as described in the Materials and
methods. (B) The scavenging activity for DPPH radicals was measured
in solutions containing 0.1 mM DPPH and twelve different
concentrations (0–1,000 µg/ml) of AEC. Two samples for each assay
were analyzed twice by DPPH analysis. The data for above results
are presented as the mean ± SD. (C) LC-MS analysis of AEC. Eleven
bioactive components including triacontanoic acid, fucosterol,
9,12-octadecadienoic acid, aspartic acid, phloroglucinol, eckol,
phlorofucofuroeckol, 6,6′-bieckol, fucodiphloroethanol G,
docosanoic acid and dieckol were detected as different peaks in the
chromatogram. DPPH, 2,2-diphenyl-1-picrylhydrazyl; AEC, aqueous
extract of Ecklonia cava; TPC, total phenolic content; TTC,
total condensed tannin contents; TFC, total flavonoid content;
TPTC, total PT content; IC50, half maximal inhibitory
concentration. |
Cytotoxicity of AEC against CT26
cells
The viability of CT26 cells was determined using an
MTT assay, cell morphological analysis and ATP concentration assay
after exposure to three different AEC concentrations for 24 h to
determine if exposure to AEC induced the cytotoxicity of CT26,
HCT116 and Detroit 551 cells. The viability of CT26 cells in MTT
assay was decreased significantly in a dose-dependent manner, and
the highest cytotoxicity was demonstrated in the HiAEC treated
group (Fig. 2A). The results
detected using the MTT assay were reflected in the morphological
changes seen in CT26 cells (Fig.
2A) and indicated that AEC exerted significant cytotoxicity on
CT26 cells at concentrations <1,000 µg/ml. Furthermore, the
number of cells stained with Ki67 proteins, a marker for cell
proliferation, was significantly decreased after the AEC treatment
compared with the No or Vehicle treatment groups (Fig. 2B). Furthermore, the effects of AEC
on the cell viability assessed using the MTT assay were also
demonstrated in the cell viability assessed based on the
concentration of ATP although there are differences in
dose-dependent responses (Fig. 2C).
Moreover, the cytotoxicity of AEC was similarly demonstrated in
HCT116 cells which are colorectal carcinoma cells derived from an
adult male (Fig. 2D). However, AEC
did not cause any toxicity in Detroit 551 normal fibroblast cell
line derived from a skin tissues of human fetus (Fig. 2E). These results indicated that AEC
exerts significant cytotoxicity in CT26 and HCT116 colon cancer
cells at concentrations <1,000 µg/ml without any significant
toxicity to normal skin fibroblast cells.
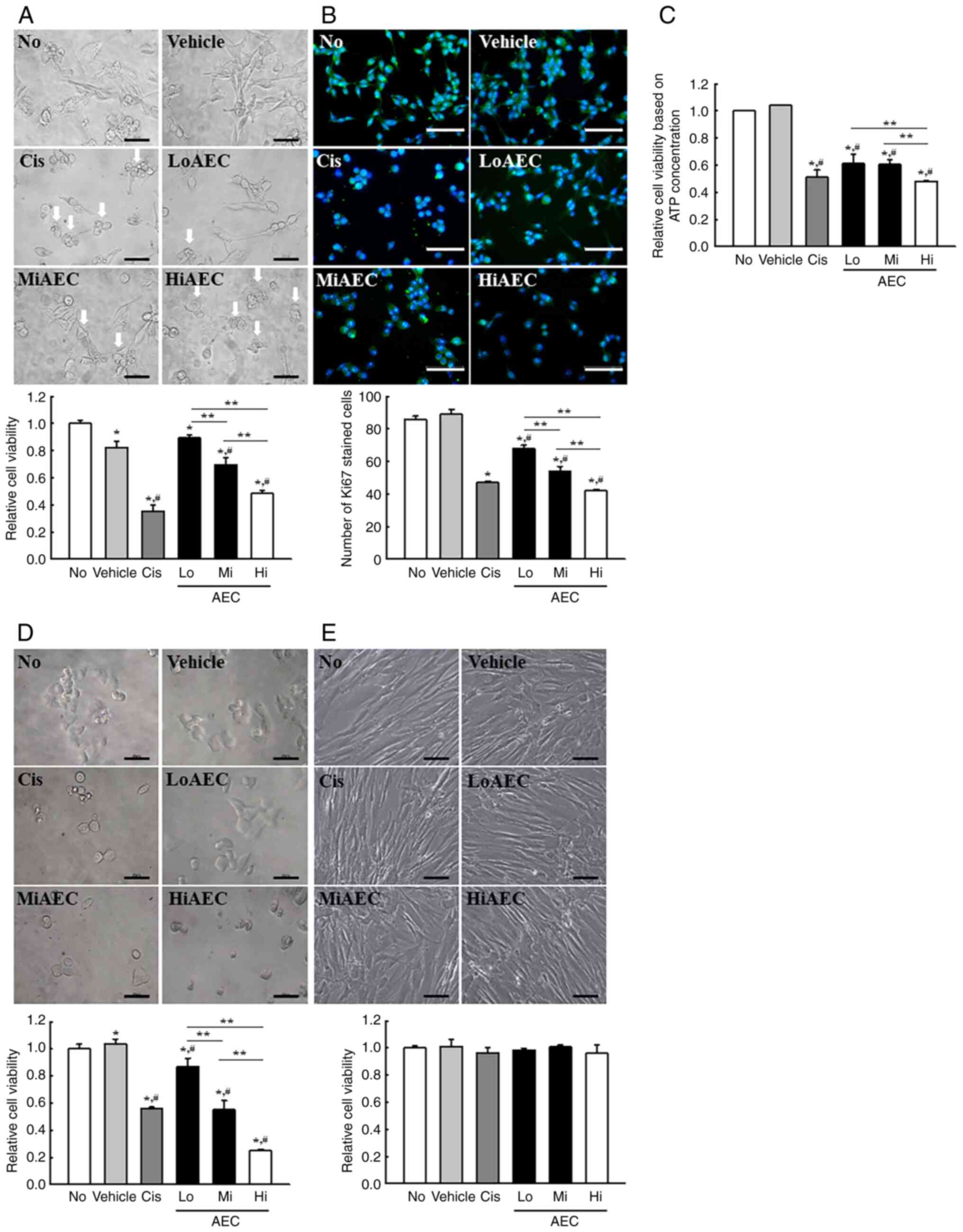 | Figure 2.Cytotoxicity and IF staining of
AEC-treated CT26 cells. (A) Relative cell viability of AEC in CT26
cells assessed using an MTT assay based on the optical density of
the solubilized formazan. After incubating CT26 cells with AEC for
24 h, the morphology of the CT26 cells was observed using an
inverted microscope at 400× magnification. Arrows indicated
abnormal cells. The MTT assay was performed on two to three wells
per group, and the optical density was measured twice for each
well. (B) Protein expression level of Ki67. The preparation of Ki67
stained samples was performed on two to three wells per group, and
Ki67 positive cells was counted in two fields of view (67,500
mm2) in each well. (C) Relative cell viability of AEC in
CT26 cells using CellTiter-Glo luminescent assay. After incubating
CT26 cells with AEC for 24 h, the viability of cells based on the
ATP concentration were assessed using the CellTiter-Glo®
Assay kit. (D) Relative cell viability of AEC in HCT116 cells was
assessed using the MTT assay based on the optical density of the
solubilized formazan. After incubating HCT116 cells with AEC for 24
h, cell viability and morphology were analyzed in the same way as
CT26 cells. (E) Relative cell viability of AEC in Detroit 551 cells
was assessed using the MTT assay based on the optical density of
the solubilized formazan. After incubating Detroit 551 cells with
AEC for 24 h, cell viability and morphology were analyzed in the
same way as CT26 cells. Scale bar=50 µm. The relative level was
calculated as a relative percentage considering the Vehicle group
as 100%. Data was presented as the mean ± SD. *P<0.05 vs. No
group. #P<0.05 vs. Vehicle group. **P<0.05 vs.
between three AEC treated group. IF, immunofluorescence; AEC,
aqueous extract of Ecklonia cava; No, untreated; Cis,
cisplatin; Lo, low concentration; Mi, middle concentration; Hi,
high concentration. |
Effects of AEC on the
apoptosis-associated response of CT26 cells
Experiments in CT26 cells were performed to evaluate
if the cytotoxicity effects of AEC were related to changes in the
apoptosis-associated response. The number of live and apoptotic
CT26 cells were assessed using Annexin V/PI staining analysis, and
the protein expression levels of the mitogen-activated protein
kinases (MAPK) signaling pathway members and apoptosis regulatory
proteins were assessed using specific antibodies. After treatment
with only HiAEC, the number of apoptotic cells was significantly
increased, with a concomitant decrease in the number of live cells,
compared with the Vehicle group (Fig.
3A). The increase in the number of apoptotic cells was
reflected by the protein expression levels of the proteins related
to the Bax/Bcl-2 and MAPK signaling pathways. The AEC-treated group
demonstrated significantly enhanced p-c-Jun N-terminal kinase
(p-JNK), p-extracellular signal-regulated kinase (p-ERK), and p-p38
protein expression levels compared with the Vehicle group, even
though the increase rate of phosphorylation of each protein was
varied (Fig. 3B). A similar
response was demonstrated in the relative protein expression levels
of Cleaved Cas-3/Cas-3 and Bax/Bcl-2, which were increased
significantly in the LoAEC, MiAEC and HiAEC treated groups compared
with the Vehicle group (Fig. 3B).
These results indicated that the cytotoxic effects of AEC may be
tightly linked to the promotion of the apoptosis-related response
and activation of the MAPK and Bax/Bcl-2 signaling pathways.
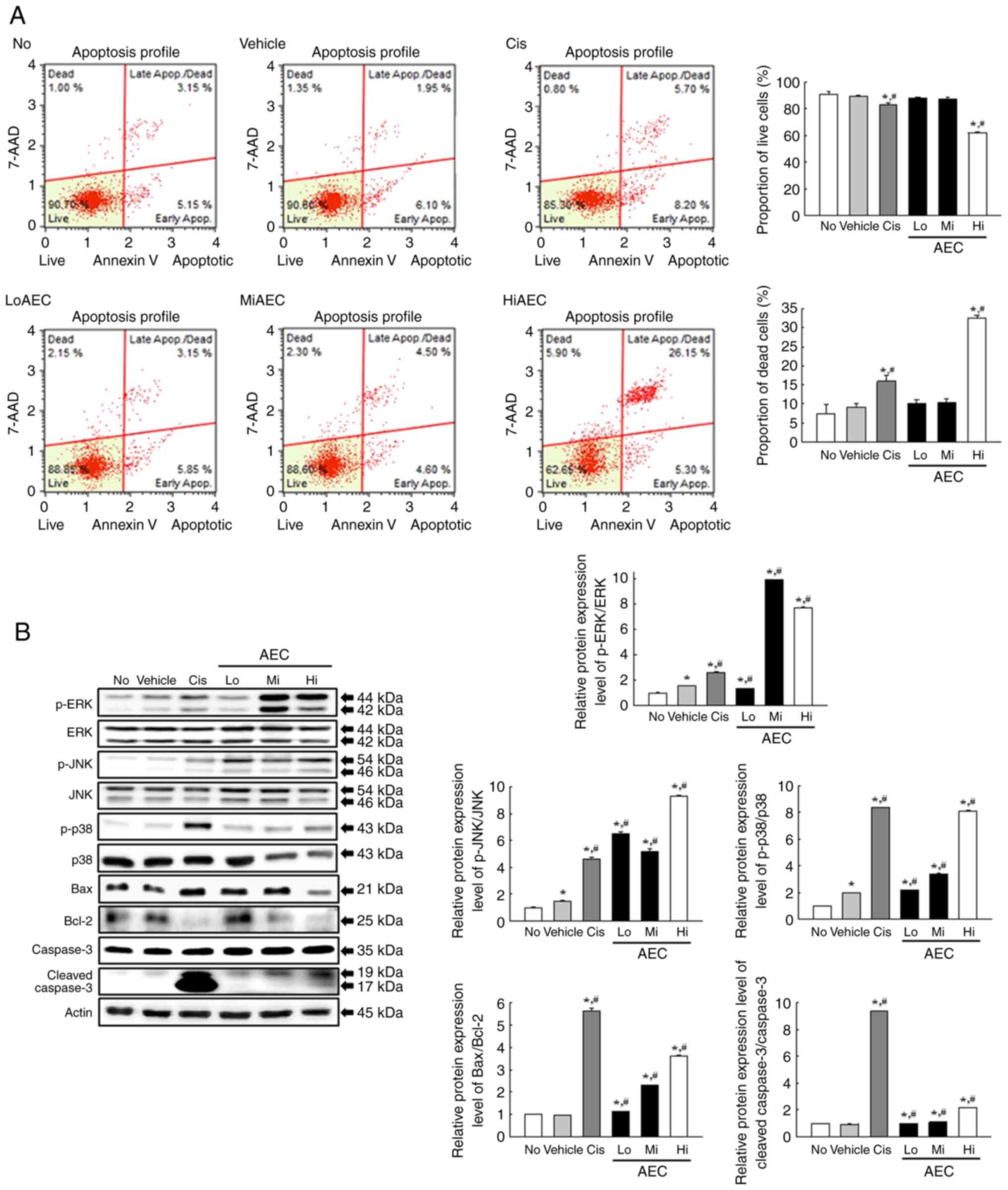 | Figure 3.Apoptotic cells and related protein
analysis of AEC treated CT26 cells. (A) Apoptosis analysis of CT26
cells. After treatment with AEC for 24 h, the number of CT26 cells
was analyzed in each group stained with annexin V and 7-AAD.
Annexin V and 7-AAD staining were performed on two to three wells
per group, and the dead cells and live cells were counted twice for
each well. (B) MAPK and Bax/Bcl-2 protein analysis. After treatment
with AEC for 24 h, the band density for specific protein was
analyzed using densitometry. The preparation of the cell
homogenates was performed on two to three dishes per group, and
western blotting was analyzed twice for each sample. The level of
each protein was normalized to β-actin. The data was presented as
the mean ± SD. *P<0.05 vs. No group. #P<0.05 vs.
Vehicle group. AEC, aqueous extract of Ecklonia cava; No,
untreated; Cis, cisplatin; Lo, low concentration; Mi, middle
concentration; Hi, high concentration; p, phosphorylated. |
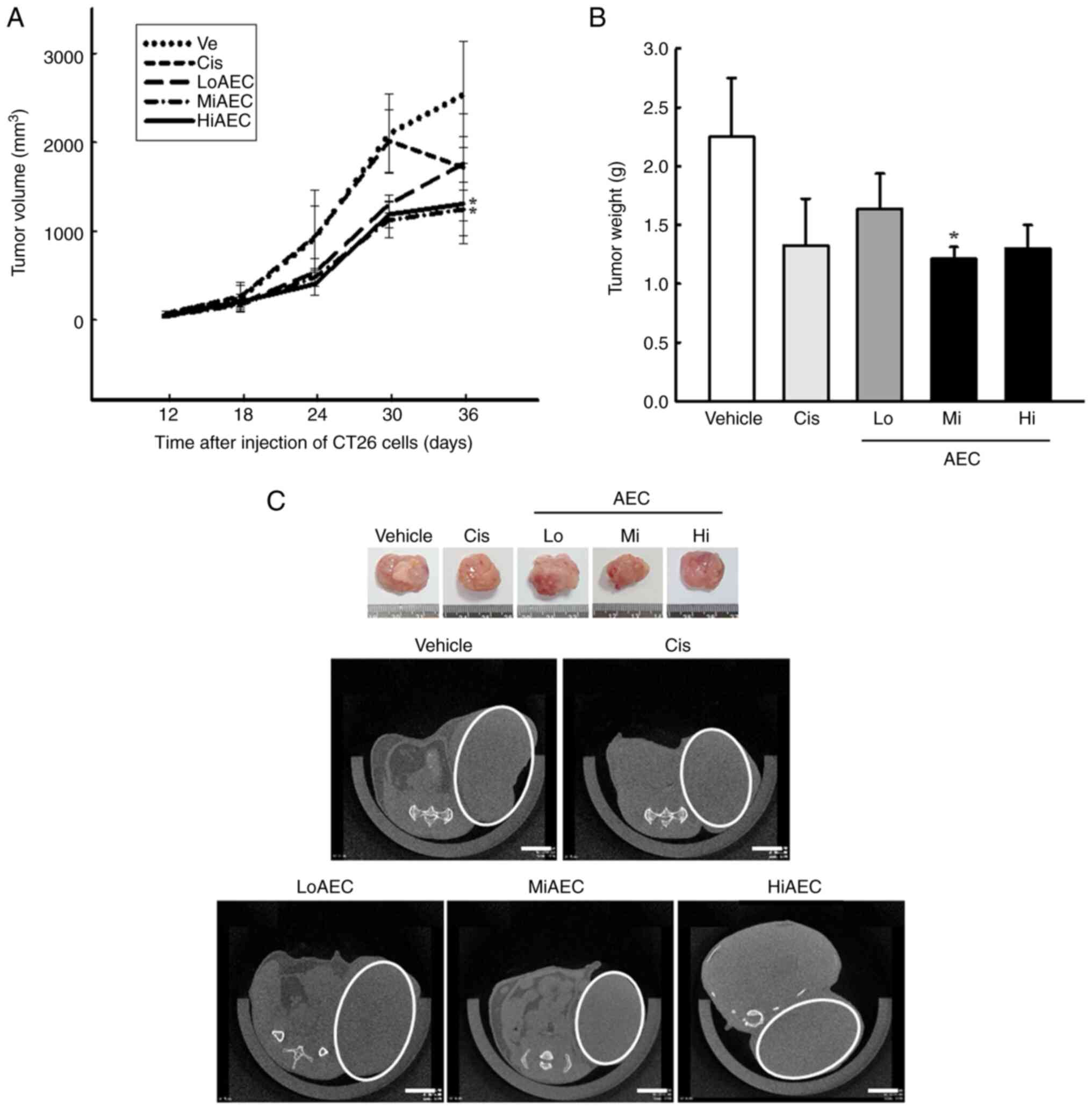
Inhibitory effect of AEC on the growth
of CT26 tumors in BALB/cKorl syngeneic mice
The present study evaluated whether the anti-tumor
activity of AEC in CT26 colon cancer cells could be reproduced
entirely in the BALB/cKorl syngeneic mice with CT26 tumors. The
changes in the volume and histopathological structure of the tumor
were analyzed in CT26 tumors of BALB/cKorl syngeneic mice treated
with three different doses of AEC for five weeks. The volume of the
tumor markedly decreased in the AEC treated groups compared with
the Vehicle group, and a significant reduction in the weight of
them was demonstrated in the MiAEC treated group compared with the
Vehicle group, but not HiAEC treated group (Fig. 4A and B). A similar effect on the
tumor volume was also demonstrated in actual and microCT imaging
(Fig. 4C). Furthermore, few spots
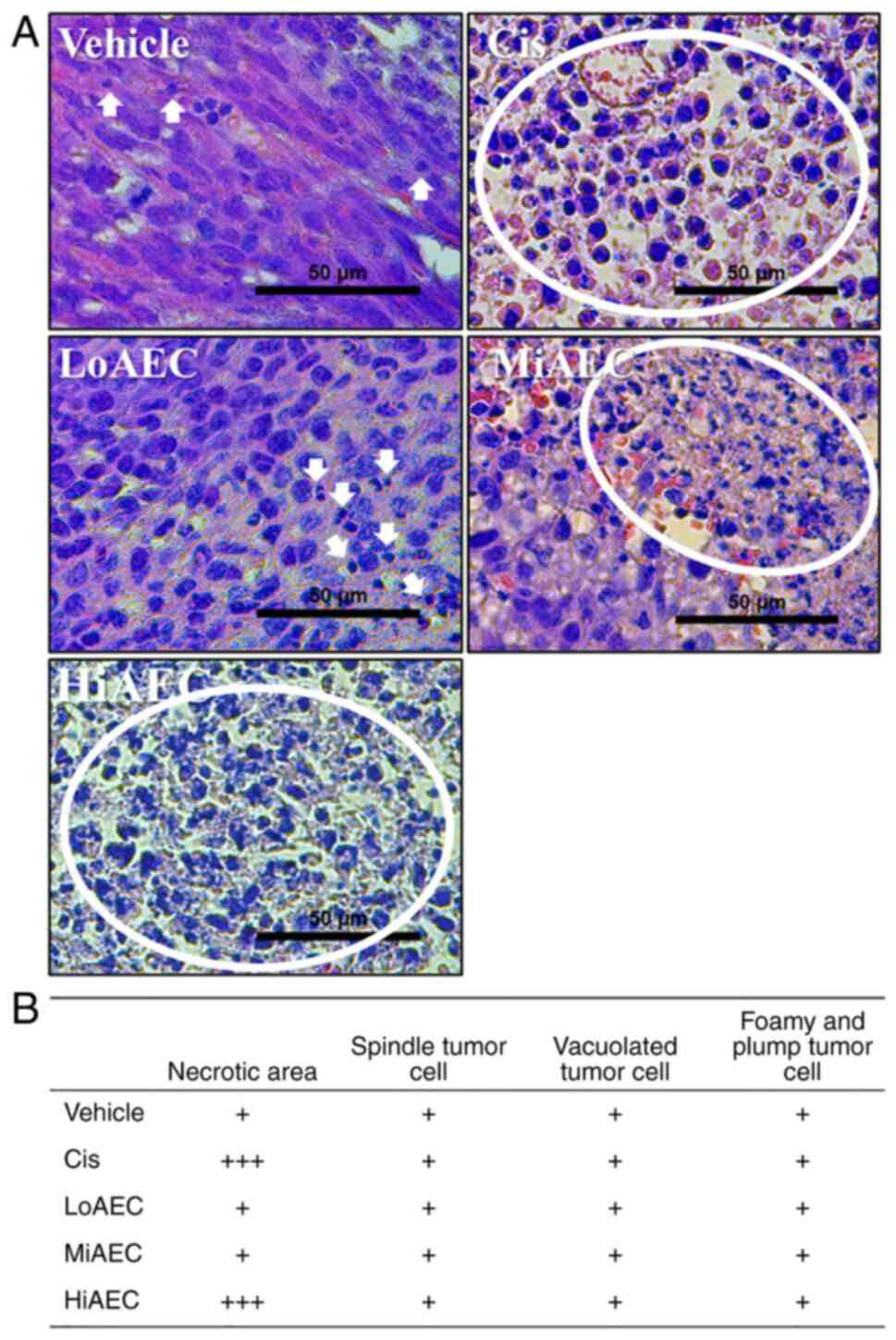
containing cell necrosis were detected in the H&E stained
sections of CT26 tumors in the Vehicle treated group. The necrotic
region of these spots was markedly expanded after the AEC treatment
compared with the Vehicle treated group. Certain pathological
alterations, including hemorrhage, cyst formation and angiogenesis,
were observed in the AEC treated tumors of BALB/cKorl syngeneic
mice (Fig. 5A and B). By contrast,
no significant differences in the toxicity parameters, including
body, kidney and liver weight, cell composition of the blood,
metabolites of the serum, and the histopathological structure of
the liver and kidney were demonstrated between the Vehicle and AEC
treated groups (Fig. S4, Fig. S5, Fig.
S6, Fig. S7). These results
indicated that AEC treatment could inhibit the growth of CT26
tumors in BALB/cKorl syngeneic mice without significant
toxicity.
Effects of AEC on the cell
proliferation in CT26 tumors of BALB/cKorl syngeneic mice
The tissue distribution of Ki67 and proliferating
cell nuclear antigen (PCNA) proteins were evaluated using specific
antibodies in AEC treated tumors of BALB/cKorl syngeneic mice to
evaluate if the inhibitory activity of AEC on CT26 tumor growth was
accompanied by a change in cell proliferation ability. The
fluorescent intensity for the Ki67 proteins significantly decreased
with increasing AEC dose in CT26 tumors of BALB/cKorl syngeneic
mice (Fig. 6A). A similar pattern
was observed with a significant decrease of the protein expression
level of PCNA (Fig. 6B). These
results indicated that the inhibitory activity of AEC on CT26 tumor
growth could be linked to the suppression of tumor cell
proliferation in BALB/cKorl syngeneic mice.
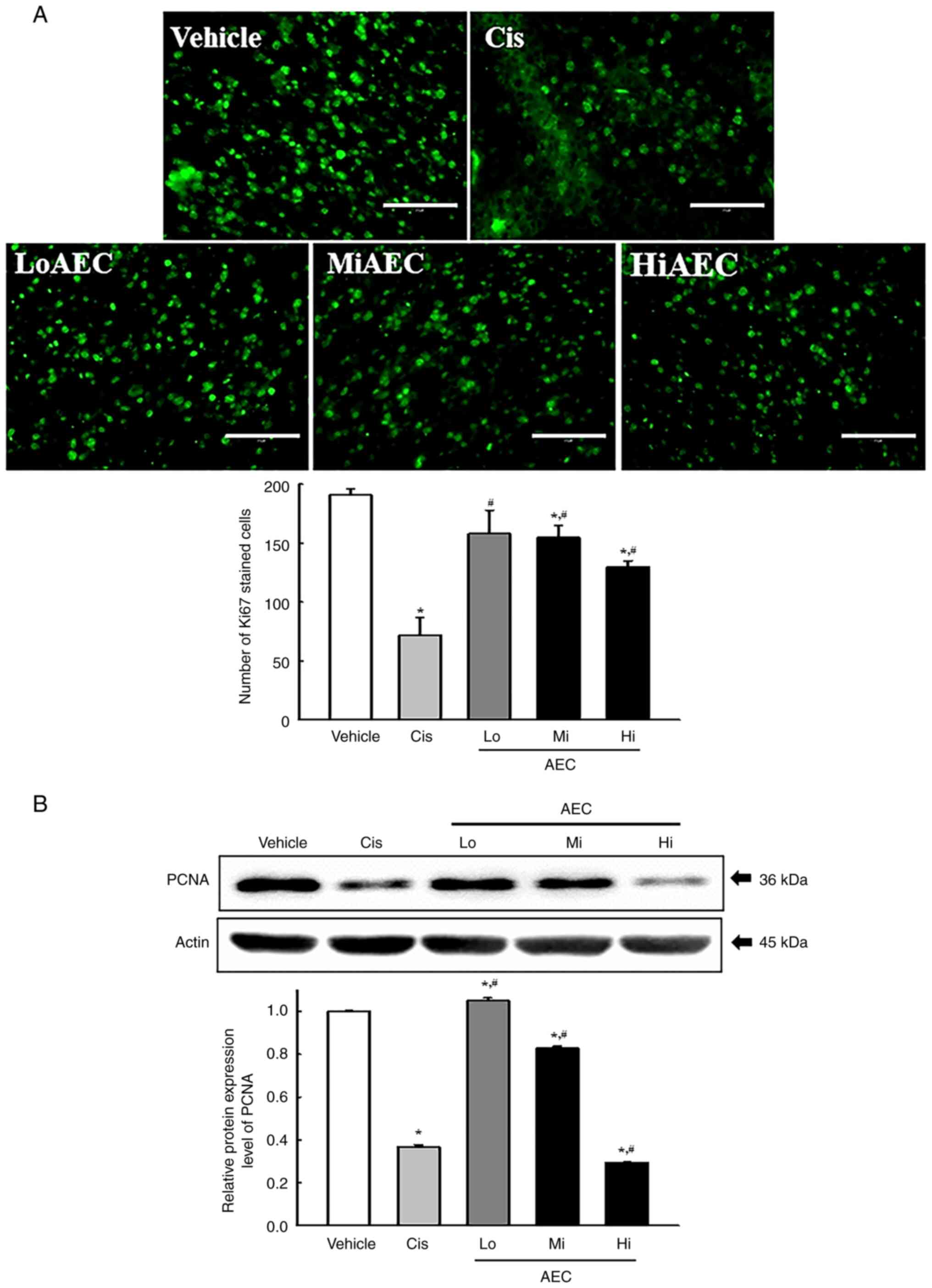 | Figure 6.Protein expression levels of Ki67 and
PCNA in CT26 tumors in BALB/cKorl syngeneic mice. (A) IF assays for
Ki67. After IF staining, Ki67 positive cells were assessed in the
total area of the field of view (67,500 mm2) for each
CT26 tumor section. The Ki67-stained slides were prepared for two
to three tumors per group based on H&E staining results, and
Ki67 positive cells was counted two view fields for each sample.
Scale bar=75 µm. (B) PCNA protein analysis. After collecting the
CT26 tumors, the band density for the specific protein was analyzed
using densitometry. The tissue homogenates were prepared from two
to three tumors per group based on the results of the weight
measurement and H&E staining analysis, and the western blot was
analyzed twice for each sample. The level of each protein was
normalized to β-actin. The data was presented as the mean ± SD.
*P<0.05 vs. Vehicle group. #P<0.05 vs. Cis group.
PCNA, proliferating cell nuclear antigen; IF, immunofluorescence;
AEC, aqueous extract of Ecklonia cava; Cis, cisplatin; Lo,
low concentration; Mi, middle concentration; Hi, high
concentration; H&E, hematoxylin and eosin. |
Effects of AEC on the
apoptosis-associated response in CT26 tumors of BALB/cKorl
syngeneic mice
Alterations to the protein expression levels of the
major proteins within the MAPK and Bax/Bcl-2 signaling pathways
were assessed in the AEC treated tumors of BALB/cKorl syngeneic
mice to evaluate if the inhibitory effects of AEC on CT26 tumor
growth were accompanied by changes in the apoptosis-associated
response. In the MAPK signaling pathway, the phosphorylation of
ERK, JNK and p38 increased significantly in all AEC treated groups
compared with the Vehicle treated group (Fig. 7A). Furthermore, a similar pattern of
increase was demonstrated in the Bax/Bcl-2 signaling pathway, where
the protein expression levels of Bax/Bcl-2 and Cleaved Cas-3
proteins were increased significantly in the AEC treated group
compared with the Vehicle group (Fig.
7B). These results indicated that the inhibitory effects of AEC
on CT26 tumor growth may be associated with the enhanced
phosphorylation of several members of the MAPK signaling pathway
and the critical proteins in the Bax/Bcl-2 signaling pathway.
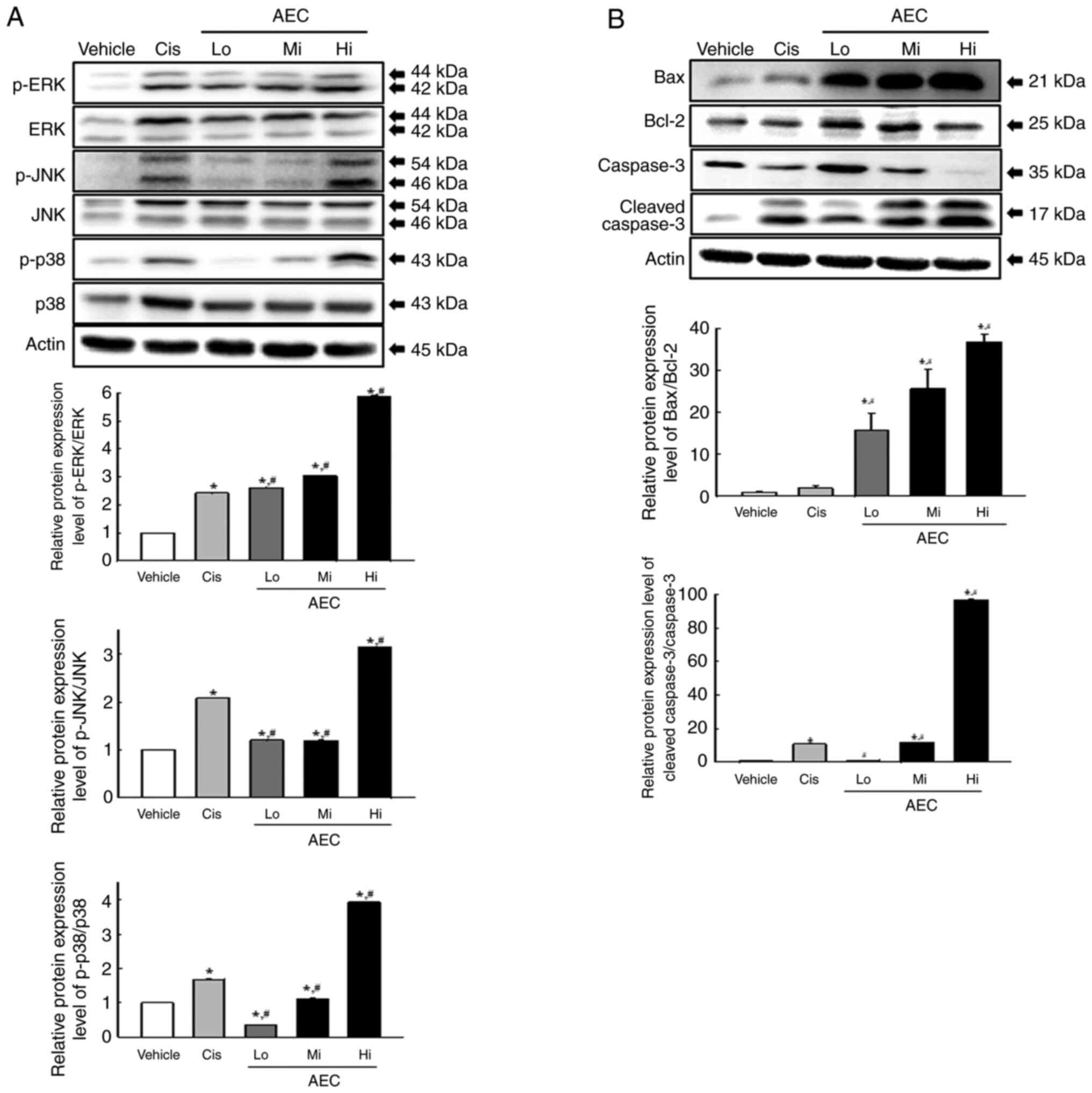 | Figure 7.MAPK and Bax/Bcl-2 protein expression
levels in a CT26 tumor in BALB/cKorl syngeneic mice. (A) MAPK
signaling pathway analysis. After collecting the CT26 tumor, the
protein expression levels of the phosphorylated form and
unphosphorylated forms of the key members proteins were analyzed
using specific antibodies and densitometry. (B) Bax/Bcl-2 pathway
analysis. After collecting the CT26 tumors, the protein expression
levels of Bax, Bcl-2, Cas-3 and Cleaved Cas-3 were assessed using
specific antibodies and densitometry. The tissue homogenates were
prepared from two to three tumors per group and western blot was
analyzed twice for each sample. The level of each protein was
normalized to β-actin. The data was presented as the mean ± SD.
*P<0.05 vs. Vehicle group. #P<0.05 vs. Cis group.
MAPK, mitogen-activated protein kinases; p, phosphorylated; AEC,
aqueous extract of Ecklonia cava; Cis, cisplatin; Lo, low
concentration; Mi, middle concentration; Hi, high
concentration. |
Effects of AEC on the migration
ability in CT26 tumors of BALB/cKorl syngeneic mice
The alteration of the protein expression levels of
key proteins in the myosin light chain (MLC)/focal adhesion kinase
(FAK)/Akt signaling pathway were assessed in the AEC treated tumors
of BALB/cKorl syngeneic mice to evaluate if the inhibitory activity
of AEC on the CT26 tumor growth were accompanied by suppression of
the migration ability. Protein expression levels of the vascular
endothelial growth factor A (VEGFA), matrix metallopeptidase
(MMP)2, and MMP9 proteins were significantly lower in the AEC
treated group compared with the Vehicle treated group, even though
their rate was varied (Fig. 8A).
Furthermore, a similar decreasing pattern was demonstrated in the
regulation of the MLC/FAK/Akt signaling pathway proteins. In the
MLC/FAK signaling pathway, the phosphorylation levels of FAK and
MLC decreased markedly in the AEC treated groups compared with the
Vehicle treated group (Fig. 8B). In
the phosphoinositide 3-kinases (PI3K)/Akt signaling pathway, the
phosphorylation levels of PI3K and Akt proteins were significantly
decreased in the AEC treated group compared with the Vehicle group
(Fig. 8C). Therefore, the
inhibitory effects of AEC on CT26 tumor growth may be associated
with suppression of the migration ability of tumor cells through
alternative control of the MLC/FAK/Akt signaling pathway.
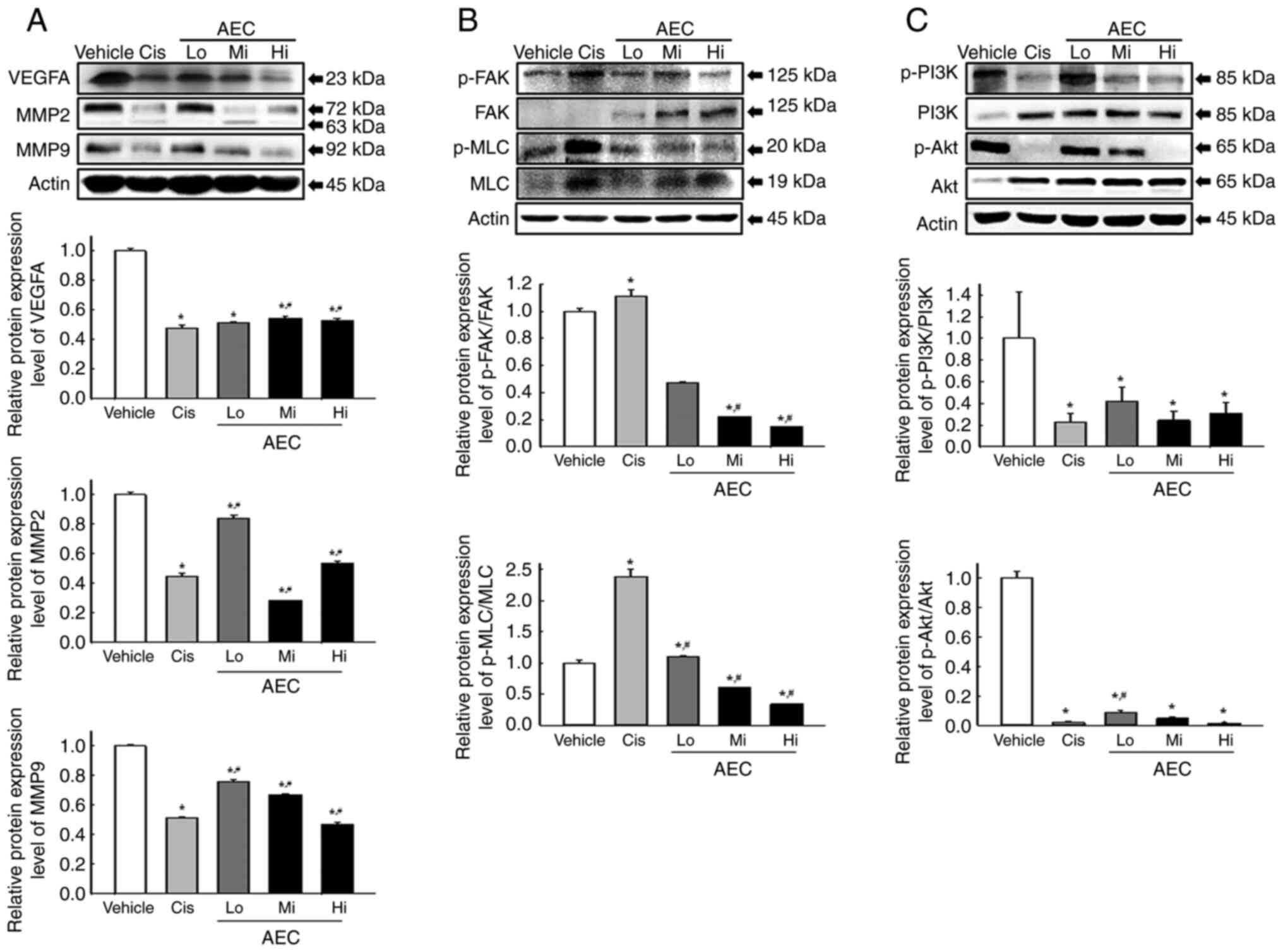 | Figure 8.Analyses of the migration
ability-related proteins in CT26 tumors of BALB/cKorl syngeneic
mice. After collecting the CT26 tumor, the protein expression
levels of (A) VEGFA, MMP2 and MMP9, (B) p-FAK, FAK, p-MLC and MLC,
and (C) p-PI3K, PI3K, p-Akt and Akt in CT26 tumor homogenates were
analyzed using specific antibodies and densitometry. The level of
each protein was normalized to β-actin. The tissue homogenates was
prepared from two to three tumors per group and western blots were
analyzed twice for each sample. The data was presented as the mean
± SD. *P<0.05 vs. Vehicle group. #P<0.05 vs. Cis
group. p, phosphorylated; AEC, aqueous extract of Ecklonia
cava; Cis, cisplatin; Lo, low concentration; Mi, middle
concentration; Hi, high concentration; MLC, myosin light chain;
FAK, focal adhesion kinase; PI3K, phosphoinositide 3-kinases. |
Effects of AEC on the tumor
suppressing activity in CT26 tumors of BALB/cKorl syngeneic
mice
Whether the inhibitory activity of AEC on CT26 tumor
growth was accompanied by changes in the protein expression level
of tumor suppression-related proteins and the NF-κB signaling
pathway was evaluated. Changes in the protein expression levels of
the tumor suppression-related and the NF-κB signaling pathway
proteins were evaluated in the AEC treated tumors of BALB/cKorl
syngeneic mice. The protein expression levels of p53, mouse double
minute 2 (MDM2) and p27 increased markedly in a dose-dependent
manner in the MiAEC and HiAEC treated groups compared with the
Vehicle group (Fig. 9A). However,
protein expression levels in the NF-κB signaling pathway were
significantly inhibited under the same conditions, where the
protein expression levels of p-NF-κB and p-IκB-α were significantly
lower in the AEC treated group compared with the Vehicle group
(Fig. 9B). Therefore, the
inhibitory effects of AEC on CT26 tumor growth could be linked to
the upregulation of tumor suppression-related proteins through the
inhibition of the NF-κB signaling pathway in CT26 tumors in
BALB/cKorl syngeneic mice.
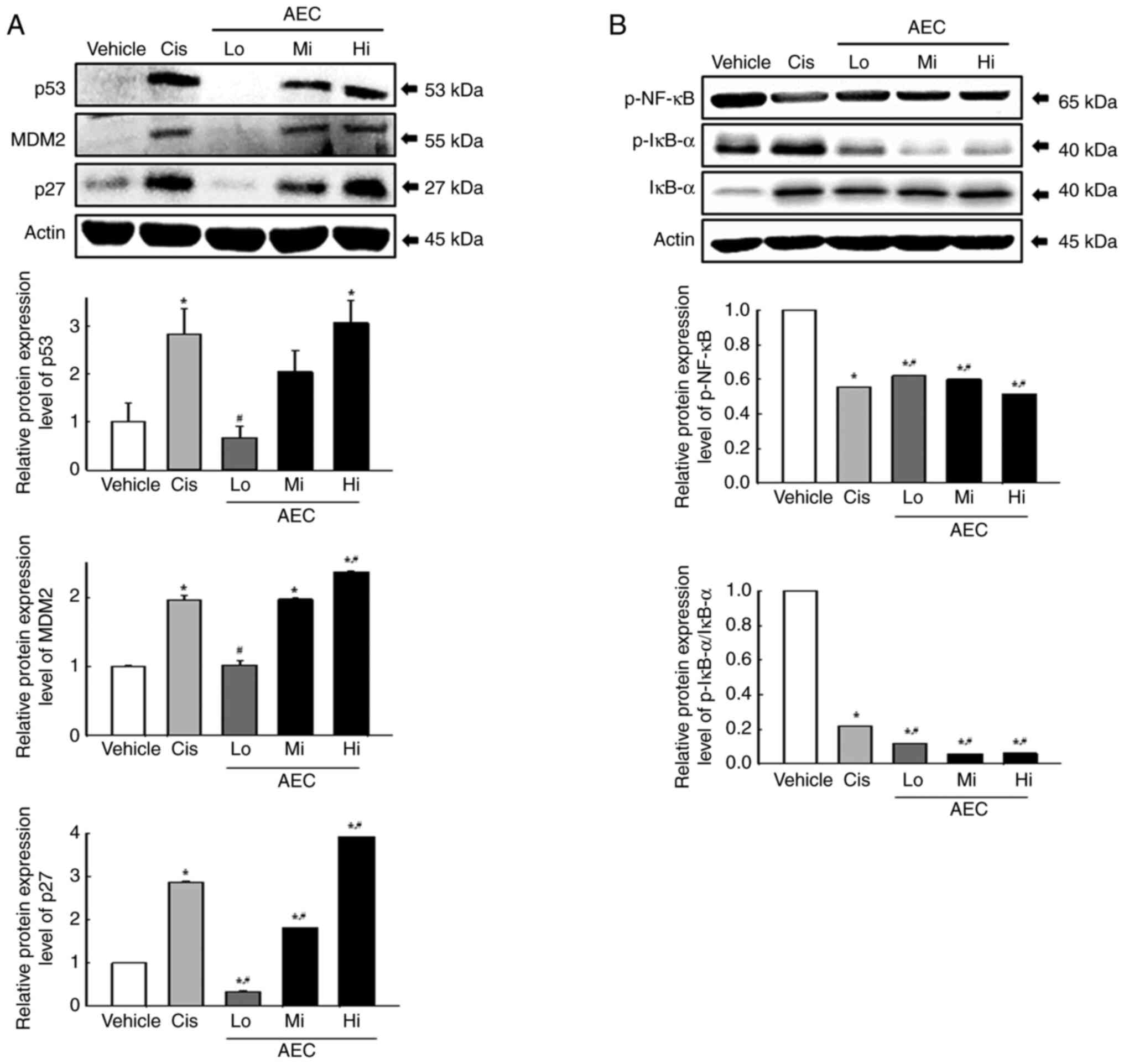 | Figure 9.Analyses of tumor suppression-related
proteins and NF-κB signaling pathway proteins in CT26 tumors in
BALB/cKorl syngeneic mice. Protein expression levels of (A) p53,
MDM2 and p27, and (B) p-NF-κB, p-IκB-α and IκB-α proteins in CT26
tumor homogenates were analyzed using specific antibodies. The
level of each protein was normalized to β-actin. The tissue
homogenates was prepared from two to three tumors per group and
western blots were analyzed twice for each sample. The data was
presented as the mean ± SD. *P<0.05 vs. Vehicle group.
#P<0.05 vs. Cis group. p, phosphorylated; AEC,
aqueous extract of Ecklonia cava; Cis, cisplatin; Lo, low
concentration; Mi, middle concentration; Hi, high concentration;
MDM2, mouse double minute 2. |
Evaluation of the potential for the
role of PT as the primary bioactive substance in AEC in CT26
cells
Finally, the anti-tumor effects of PT as the
bioactive compound candidates in AEC were confirmed in CT26 cells.
Changes in the cytotoxicity and apoptosis activation were assessed
in CT26 cells after PT treatment. The viability of CT26 cells was
significantly decreased in the PT treated groups compared with the
No or Vehicle group (Fig. 10A).
The number of cells stained with Ki67 protein were significantly
decreased in the PT treated groups compared with the No or Vehicle
groups (Fig. 10B). The PT treated
groups in CT26 cells demonstrated an increase in the
phosphorylation levels of ERK, JNK and p38 proteins compared with
Vehicle group although the increased rate of JNK was the lowest
among them (Fig. 10C). Also,
similar enhancements in the protein expression levels of Bax/Bcl-2
and Cleaved Cas-3/Cas-3 were observed in the CT26 cells treated
with PT and HiAEC compared with the No or Vehicle group (Fig. 10D). Therefore, PT in AEC can be
considered one of the main bioactive compounds which exert an
anti-tumor effect of AEC.
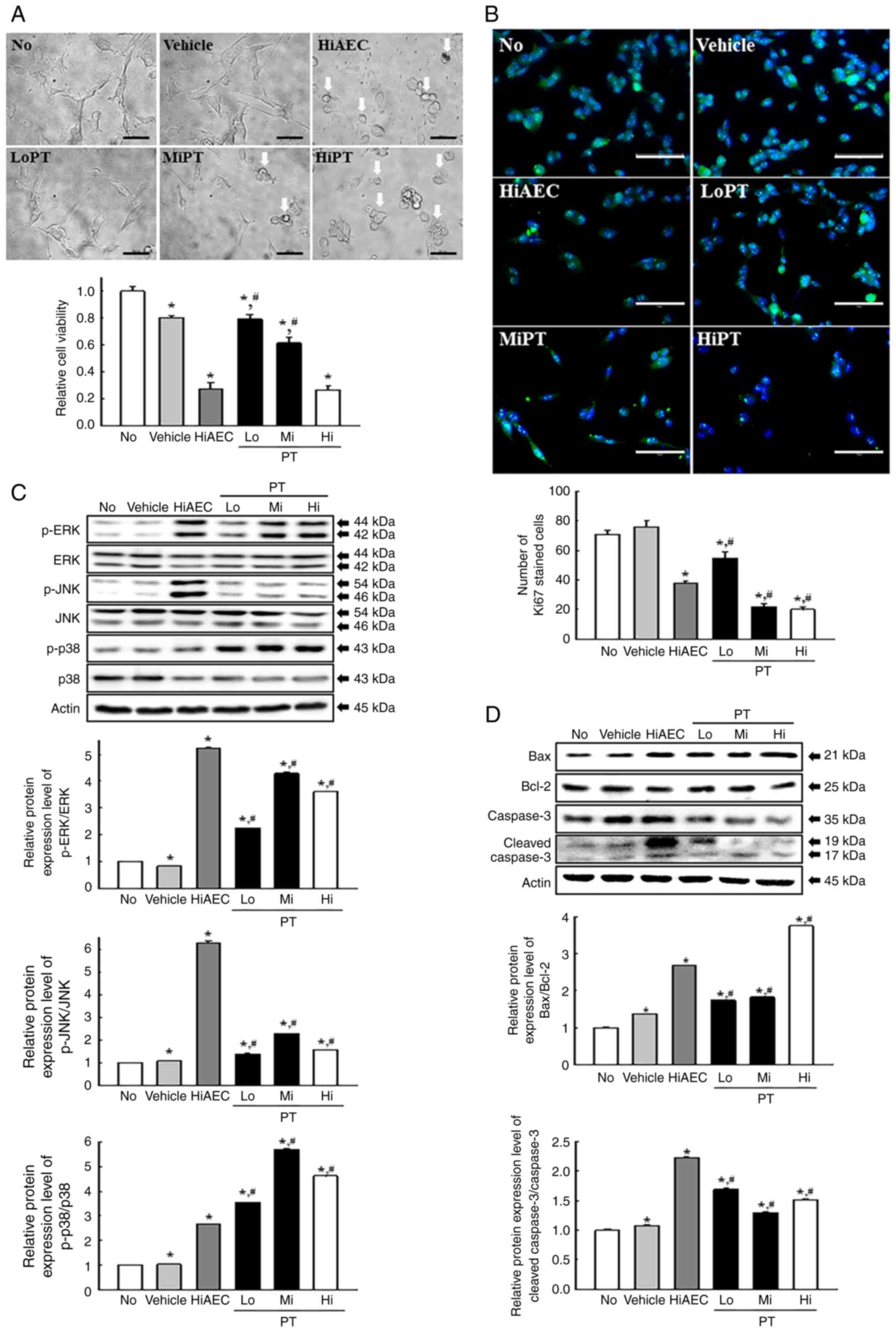 | Figure 10.Anti-tumor effects of PT-treated CT26
cells. (A) Relative cell viability of PT in CT26 cells. After
incubating CT26 cells with PT for 24 h, the morphology of the CT26
cells was observed under an inverted microscope at 400×
magnification (Top). Scale bar=50 µm. An MTT assay based on the
optical density of the solubilized formazan was performed on two to
three wells per group, and the OD was measured twice for each
sample (Bottom). (B) IF assays for Ki67. After IF staining, the
number of Ki67 positive cells were counted in two field of views
with total area of 67,500 mm2 per field of view in each
well. The Ki67-stained samples were prepared on two to three wells
per group and Ki67 positive cells was counted twice for each
sample. Scale bar=75 µm. (C) MAPK and (D) Bax/Bcl-2 signaling
pathway analysis. After treatment with PT for 24 h, the expression
of Bax, Bcl-2, Cas-3 and Cleaved Cas-3 proteins as well as p-ERK,
ERK, p-JNK, JNK, p-p38 and p38 proteins were detected using
specific antibodies. The cell homogenates were prepared on two to
three dishes per group and western blots were analyzed twice for
each sample. The data was presented as the mean ± SD. *P<0.05
vs. No group. #P<0.05 vs. Vehicle group. AEC, aqueous
extract of Ecklonia cava; PT, phlorotannin; IF,
immunofluorescence; MAPK, mitogen-activated protein kinases; p,
phosphorylated; No, untreated; HiAEC, high concentration of AEC;
Lo, low concentration; Mi, middle concentration; Hi, high
concentration; p, phosphorylated. |
Discussion
Tannins are found in numerous parts of the plant,
including the bark of trees, wood, leaves, fruits, seeds and roots,
to protect them from fungi and bacteria (37). Based on these characteristics,
numerous studies on the therapeutic effects of tannins have been
reported. Among these, the anti-carcinogenic and anti-mutagenic
potential of tannins have attracted considerable attention because
their high antioxidative properties protect against cellular
oxidative damage (38). The present
study evaluated the anti-tumor effects and the molecular mechanisms
of AEC in CT26 cells and CT26 tumors in BALB/cKorl syngeneic mice
to identify a novel function of E. cava, which contains
certain tannins, for tumor therapy. The results from the present
study suggested that AEC had anti-tumor effects, including strong
cytotoxicity, activation of apoptosis, suppression of cell
proliferation, inhibition of migration ability and enhanced tumor
suppression ability in CT26 colon cancer cells. Furthermore, the
results from the PT treated CT26 cells indicated that PT was one of
the bioactive component candidates with anti-tumor activity in
AEC.
The AEC used in the present study was extracted from
the same raw materials of E. cava as the tannin-enriched
extract of E. cava used in a previous study (17). However, there was a difference in
the extraction method, and this difference is hypothesized to be
the cause of the difference in the phytochemicals content, radical
scavenging activity and active compound content. In the present
study, eleven bioactive compounds including triacontanoic acid,
fucosterol, 9,12-octadecadienoic acid, aspartic acid,
phloroglucinol, eckol, phlorofucofuroeckol, 6,6′-bieckol,
fucodiphloroethanol G, docosanoic acid and dieckol were identified
in AEC using LC-MS analyses. Identification of most of the
aforementioned compounds were consistent with previous studies
which identified novel bioactive compounds from E. cava
(39,40,41). A
total of forty-one compounds containing PT and other bioactive
compounds were reported to have been detected in the total ion
chromatograms of the sub-fractions of E. cava eluted with
30% MeOH and 70% MeOH (39).
Previously, seven PTs and three sterol derivatives were purified
from E. cava using the comprehensive spectral analysis for
nuclear magnetic resonance and mass spectrometry data (40). Furthermore, the high pressure liquid
chromatography peaks of eckol and dieckol were detected in the
ethyl acetate fraction of E. cava (41). However, active compounds identified
from E. cava differ between studies. In the present study,
few compounds including triacontanoic acid, fucosterol,
9,12-octadecadienoic acid, docosanoic acid with low solubility were
detected in AEC, which was extracted with water solvent (Fig. 1). Among these compounds,
9,12-octadecadienoic acid and docosanoic acid were also found in
water extracts of Cladosporium perangustum and Desmodium
gangeticum (42,43). These data demonstrated that the low
water-soluble compounds could be extracted during an extraction
process including a 100°C boiling step. However, the present study
had certain limitations in that it did not fully reflect the
variation between batches in the separation and isolation of
bioactive components because many bioactive components in marine
plants are affected by numerous environmental conditions.
Therefore, more studies will be required to separate and isolate
bioactive components of AEC and investigate their biological
effects one by one.
Apoptosis is one of the targets for a potential
anti-tumor drug because it is the best-studied model of programmed
cell death of damaged, worthless or outdated cells (44). During these processes, the MEK/ERK
and Bcl-2/Bax signaling pathways are deregulated in many human
tumors, including pediatric leukemia (45). Certain compounds derived from E.
cava exert different effects on the activation of apoptosis.
Phloroglucinol did not induce cytotoxicity of endothelial
progenitor cells at concentrations <100 µM (46). PT and PT enriched extracts induced
significant cytotoxicity of ovarian cancer A2780 and SKOV3 cells
(47). The number of apoptotic
cells was increased after treatment with PT enriched extracts,
while the expression levels of the pro-Cas-3, 8 and 9 proteins, and
Bcl-2 and Bcl-xL decreased under the same conditions (47). Furthermore, similar effects on the
stimulation of apoptosis for SKOV3 cells were reported using
dieckol isolated from E. cava (16). The phloroglucinol derivative from
E. cava induced apoptosis and increased the Cas-3/9 activity
in breast cancer MCF-7 cells (7).
The present study analyzed the changes in certain major factors
related to cytotoxicity, the expression levels of Bax/Bcl-2 and
Cas-3, and the MAPK signaling pathway in CT26 cells and CT26 tumors
in BALB/cKorl syngeneic mice after AEC treatment. The AEC treatment
induced an increase in the number of apoptotic cells, and Bax/Bcl-2
and Cleaved Cas-3/Cas-3 protein expression levels in the cell line
and solid tumor. These results were similar to those previously
reported for PT, PT enriched extracts and dieckol treated ovarian
cancer cells, even though the types of tumor cells were different
in each study. The results from the present study indicated that
AEC could effectively stimulate the apoptosis of colon cancer
cells.
The proliferative activity of the cell population is
typically determined using standard markers, including Ki67, PCNA
and minichromosome maintenance proteins because their expression
manifests during the proliferation of normal and neoplastic cells
(48). In particular, the
expression levels of the aforementioned markers was also reported
in numerous malignant tumors, including multiple myeloma (49), prostate cancer (50) and breast cancer (51). They were significantly lower in
tumors after treatment using various natural products, including
grape seed proanthocyanidins extract, gallatannin and French
maritime pine bark (52–54); however, the efficacy of E.
cava extracts has not been previously reported to the best of
our knowledge. High anti-proliferative activity was reported in
MCF-7 breast cancer cells after treatment with the phloroglucinol
derivative from E. cava (7).
The present study evaluated the efficacy of E. cava extracts
on the suppression of proliferative activity in CT26 tumors in
BALB/cKorl syngeneic mice. The protein expression levels of Ki67
and PCNA decreased markedly in the AEC treated group. These results
were consistent with the aforementioned, previous reports.
Therefore, the results provided novel evidence that AEC could
inhibit the proliferative activity of CT26 tumors in BALB/cKorl
syngeneic mice by regulation of the Ki67 and PCNA proteins.
The present study evaluated the protein expression
level of cell migration proteins and the MLC/FAK/Akt signaling
pathway to investigate the inhibition effects of AEC on the
migration ability of CT26 tumors. The AEC treated group exhibited
markedly lower VEGFA, MMP2 and MMP9 protein expression levels and
inhibition of the MLC/FAK/Akt signaling pathway in CT26 tumors in
BALB/cKorl syngeneic mice compared with the control. These results
on metastasis were similar to those of certain previous reports
(55–61). The metastatic process in the tumor
tissue includes numerous molecular events, including the loss of
cell-cell adhesion ability, alterations in the cell-matrix
interaction, initiation of angiogenesis and degradation of the
basement membrane and extracellular matrix (55,56).
During the migration and invasion process, the PI3K/Akt mediated
signaling pathway and the level of certain related proteins, such
as MMPs and VEGF, can contribute as key regulators (57,58). A
similar effect on the inhibition of metastasis was reported in
previous studies that analyzed cancer cells treated with dieckol
isolated from E. cava. Dieckol treatment induced a decrease
in MMP2, MMP9, NF-κB and FAK expression and inhibited cell
migration in human fibrosarcoma HT1080 cells (59,60).
Similar effects, including the inhibition of gap closure and
decreased MMP9 and VEGF expression, were reported in MCF-7 cells.
In contrast, lung cancer cells treated with dieckol exhibited the
suppression of migration, decreased N-cadherin levels and
activation of key members in the PI3K/Akt signaling pathway
(57,61). However, further research involving
scratch and Transwell cell migration assays will be required to
verify the inhibition effects of AEC on the migration ability of
tumor cells based on the decrease in the expression of proteins
involved in migration.
p53, a key tumor suppressor, and NF-κB, a key
regulator of inflammation, are the major regulators of the
physiological stress response (62). During this process, p53 and NF-κB
proteins translocate from the cytoplasmic region to the nucleus and
control the transcription of certain response genes (63,64).
However, severe dysregulation on p53 and the NF-κB mediated pathway
is commonly reported in cancer development and progression
(64,65). In tumors, the constitutive
activation of NF-κB causes chronic inflammation, which suppresses
the tumor suppressor activity of p53, even though they are in
reciprocal negative regulation (66). To the best of our knowledge there
have been few previous reports of the correlation between these two
regulators and the anti-cancer effects of E. cava.
Down-regulation of the NF-κB family and dependent activated genes
were only reported in MCF-7 cells after exposure to one of the
phloroglucinol derivatives from E. cava (7). The present study evaluated p53 protein
expression levels and the NF-κB signaling pathway in CT26 tumors of
AEC treated BALB/cKorl syngeneic mice to determine the effects of
AEC on the physiological stress response. AEC treatment induced a
marked increase in p53 protein expression levels and inhibited the
NF-κB signaling pathway in CT26 tumors in BALB/cKorl syngeneic
mice. The results of the present study provided the first evidence,
to the best of our knowledge, that AEC may be associated with the
regulation of the major regulators of the physiological stress
response.
PTs are polymers composed of phloroglucinol
(1,3,5-trihydroxybenzene) monomer units linked by different binding
patterns. They have been reported in many marine brown algae
including E. cava, Ecklonia stolonifera and Ecklonia
kurome (67,36). PTs can be categorized into four
subgroups based on the types of linkage; PTs with an ether linkage
group (fuhalols and phlorethols), PTs with a phenyl linkage group
(fucols), PTs with an ether and phenyl linkage group
(fucophloroethols) and PTs with a dibenzodioxin linkage group
(eckols) (36). Twelve compounds
were previously reported as a subclass of PT from marine brown
algae (5,68–70).
PTs in particular is very abundant in E. cava compared with
other brown algae (2). Therefore,
PTs are considered to be one of major causes of the beneficial
functions of E. cava although their content is low (36). The amount of PT (0.26% w/w) from
E. cava detected in this study differed from that detected
in previous studies. In the 95% EtOH extract of E. cava
~5.66% w/w of PT was detected and a slightly higher concentration
(7.52% w/w) of PT was detected in the 70% EtOH extract of
Colonia cava (Laminariales) and Sargassum horneri
(Fucales) (71,72). It was hypothesized that that this
difference was due to the difference in extraction solvents and
extraction processes between studies.
Furthermore, the present study analyzed the role of
PT as one of the bioactive compound candidates for the anti-tumor
effects of AEC. The level of cytotoxicity increased by 11
percentage points to 52% at 600, 800 and 1,000 µg/ml of AEC, while
those levels increased by 21 percentage points to 64% at 100, 150
and 200 µg/ml of PT. The anti-tumor effects of dieckol, one of the
components of PT, was previously reported in numerous cancer cell
lines. In human fibrosarcoma HT1080 cells, dieckol treatment (50
and 100 µg/ml) inhibited cell migration and invasion, intracellular
ROS production, MMP2 and 9 expression, and the NF-kB signaling
pathway (59,60). In human breast cancer MCF-7 cells,
similar effects, including cell movement, MMP expression and
apoptosis, were reported after the dieckol treatment (100, 200 and
400 µg/ml) (13,73). Furthermore, dieckol induced the
inhibition of invasive and migratory properties and stimulated
apoptosis, while it increased the activity of caspases 3, 8 and 9
(61). When comparing the
anti-tumor effects of dieckol with those of AEC and PT, similar
effects of dieckol on cytotoxicity were measured at relatively
lower concentrations (50–400 µg/ml) than AEC and PT. Therefore,
these results and previous studies indicated a possible correlation
between AEC, PT and dieckol during the anti-tumor activity despite
the limitations in comparing different studies directly.
The present study had certain limitations on the
concentration analyses of active compounds in AEC. The six
compounds that belong to PT were not quantified even though the
total amount of PT was determined. In previous studies, however,
the concentration of each compound was well quantified in water
extract of E. cava. Among these compounds, the highest
concentration (1.01 µg/mg) was detected in eckol, followed in order
by dieckol (4.64 µg/mg), 6,6′-bieckol (4.64 µg/mg), 8,8′-bieckol
(1.18 µg/mg), and phloroglucinol (0.18 µg/mg) (74). The differences in the concentration
of bioactive compounds between individual plants should be fully
considered to support the consistency of the concentration of
various components between studies; however, the aforementioned
values are provided to give an indication of non-quantified active
compounds in the present study.
The results of the present study provided
additional scientific evidence that AEC has anti-tumor effects
associated with the cytotoxicity, activation of apoptosis,
suppression of migration ability and enhancement of tumor
suppression ability in CT26 cells or CT26 tumors in BALB/cKorl
syngeneic mice. Moreover, AEC is a potential anti-tumor drug
without significant toxicity and PT may be one of bioactive
component candidates. However, despite the evidence provided based
on scientific analysis strategies, the low anti-tumor activity of
AEC is a weak point in developing a drug for humans. Nevertheless,
further studies will be required to evaluate the effects of the
single bioactive compounds and the molecular mechanisms responsible
for the anti-tumor effects as well as to evaluate the possibility
of using a combined treatment of standard chemotherapy and
extracts.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors wish to thank Miss Jin Hyang Hwang, the
animal technician, for directing animal care at the Laboratory
Animal Resources Center in Pusan National University (Miryang,
Republic of Korea).
Funding
This project was supported by a 2021 and 2022 grant of BIOREIN
(Laboratory Animal Bio Resources Initiative) received from the
Ministry of Food and Drug Safety (Republic of Korea). The present
study was also supported by the BK21 FOUR Program through the
National Research Foundation of Korea funded by the Ministry of
Education, Korea (grant nos. F21YY8109033 and F22YY8109033).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
JEG performed data curation and validation as well
as design of the method. JEG, JEK and SHP performed the formal
analysis. The investigation was performed by JEG, JEK, SJL and YJC.
YWC and HSL provided resources and determined the bioactive
compounds of AEC. SIC and JTH reviewed and edited the manuscript,
analyzed the data and discussed the results. DYH was a major
contributor to the experimental design, funding management and
wrote the original manuscript. All authors have read and agreed to
the published version of the manuscript. JEG, JEK, SJL and YJC
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The protocol for experimental animal study was
reviewed and approved by the Pusan National University
Institutional Animal Care and Use Committee (approval no.
PNU-2020-0108).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maegawa M, Yokohoma Y and Aruga Y:
Critical light conditions for young Ecklonia cava and
Eisenia bicyclis with reference to photosynthesis.
Hydrobiologia. 151:447–455. 1987. View Article : Google Scholar
|
|
2
|
Heo SJ, Park EJ, Lee KW and Jeon YJ:
Antioxidant activities of enzymatic extracts from brown seaweeds.
Bioresour Technol. 96:1613–1623. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang KA, Lee KH, Chae S, Zhang R, Jung MS,
Lee Y, Kim SY, Kim HS, Joo HG, Park JW, et al: Eckol isolated from
Ecklonia cava attenuates oxidative stress induced cell
damage in lung fibroblast cells. FEBS Lett. 579:6295–6304. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahn GN, Kim KN, Cha SH, Song CB, Lee J,
Heo MS, Yeo IK, Lee NH, Jee YH, Kim JS, et al: Antioxidant
activities of phlorotannins purified from Ecklonia cava on
free radical scavenging using ESR and
H2O2-mediated DNA damage. Eur Food Res
Technol. 226:71–79. 2007. View Article : Google Scholar
|
|
5
|
Li Y, Qian ZJ, Ryu B, Lee SH, Kim MM and
Kim SK: Chemical components and its antioxidant properties in
vitro: An edible marine brown alga, Ecklonia cava. Bioorg
Med Chem. 17:1963–1973. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Artan M, Li Y, Karadeniz F, Lee SH, Kim MM
and Kim SK: Anti-HIV-1 activity of phloroglucinol derivative,
6,6′-bieckol, from Ecklonia cava. Bioorg Med Chem.
16:7921–7926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong CS, Kim JA, Yoon NY and Kim SK:
Induction of apoptosis by phloroglucinol derivative from
Ecklonia cava in MCF-7 human breast cancer cells. Food Chem
Toxicol. 47:1653–1658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Athukorala Y and Jeon YJ: Screening for
angiotensin 1-converting enzyme inhibitory activity of Ecklonia
cava. Prev Nutr Food Sci. 10:134–139. 2005. View Article : Google Scholar
|
|
9
|
Jung WK, Ahn YW, Lee SH, Choi YH, Kim SK,
Yea SS, Choi I, Park SG, Seo SK, Lee SW and Choi IW: Ecklonia
cava ethanolic extracts inhibit lipopolysaccharide-induced
cyclooxygenase-2 and inducible nitric oxide synthase expression in
BV2 microglia via the MAP kinase and NF-kappaB pathways. Food Chem
Toxicol. 47:410–417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SH, Li Y, Karadeniz F, Kim MM and Kim
SK: α-Glycosidase and α-amylase inhibitory activities of
phloroglucinal derivatives from edible marine brown alga,
Ecklonia cava. J Sci Food Agric. 89:1552–1558. 2009.
View Article : Google Scholar
|
|
11
|
Ahn JH, Yang YI, Lee KT and Choi JH:
Dieckol, isolated from the edible brown algae Ecklonia cava,
induces apoptosis of ovarian cancer cells and inhibits tumor
xenograft growth. J Cancer Res Clin Oncol. 141:255–268. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang C, Li Y, Shi X and Kim SK:
Inhibition of the expression on MMP-2, 9 and morphological changes
via human fibrosarcoma cell line by 6,6′-bieckol from marine alga
Ecklonia cava. BMB Rep. 43:62–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim EK, Tang Y, Kim YS, Hwang JW, Choi EJ,
Lee JH, Lee SH, Jeon YJ and Park PJ: First evidence that
Ecklonia cava-derived dieckol attenuates MCF-7 human breast
carcinoma cell migration. Mar Drugs. 13:1785–1797. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sadeeshkumar V, Duraikannu A, Ravichandran
S, Kodisundaram P, Fredrick WS and Gobalakrishnan R: Modulatory
efficacy of dieckol on xenobiotic-metabolizing enzymes, cell
proliferation, apoptosis, invasion and angiogenesis during
NDEA-induced rat hepatocarcinogenesis. Mol Cell Biochem.
433:195–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Athukorala Y, Kim KN and Jeon YJ:
Antiproliferative and antioxidant properties of an enzymatic
hydrolysate from brown alga, Ecklonia cava. Food Chem
Toxicol. 44:1065–1074. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahn G, Lee W, Kim KN, Lee JH, Heo SJ, Kang
N, Lee SH, Ahn CB and Jeon YJ: A sulfated polysaccharide of
Ecklonia cava inhibits the growth of colon cancer cells by
inducing apoptosis. EXCLI J. 14:294–306. 2015.PubMed/NCBI
|
|
17
|
Kim JE, Choi YJ, Lee SJ, Gong JE, Lee YJ,
Sung JE, Jung YS, Lee HS, Hong JT and Hwang DY: Antioxidant
activity and laxative effects of tannin-enriched extract of
Ecklonia cava in loperamide-induced constipation of SD rats.
PLoS One. 16:e02463632021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee HS, Kim SW, Oak C, Kang HW, Oh J, Jung
MJ, Kim SB, Won JH and Lee KD: Rabbit model of tracheal stenosis
using cylindrical diffuser. Lasers Surg Med. 49:372–379. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HS, Jeong MS, Ko SC, Heo SY, Kang HW,
Kim SW, Hwang CW, Lee KD, Oak C, Jung MJ, et al: Fabrication and
biological activity of polycaprolactone/phlorotannin endotracheal
tube to prevent tracheal stenosis: An in vitro and in vivo study. J
Biomed Mater Res B Appl Biomater. 108:1046–1056. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gutfinger T: Polyphenols in olive oils. J
Am Oil Chem Soc. 58:966–968. 1981. View Article : Google Scholar
|
|
21
|
Xu ML, Hu JH, Wang L, Kim HS, Jin CW and
Cho DH: Antioxidant and anti-diabetes activity of extracts from
Machilus thunbergii S. et Z. Korean J Medicinal Crop Sci.
18:34–39. 2010.
|
|
22
|
Price ML, Hagerman AE and Butler LG:
Tannin content of cowpeas, chickpeas, pigeon peas, and mung beans.
J Agric Food Chem. 28:459–461. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Go J, Kim JE, Koh EK, Song SH, Sung JE,
Lee HA, Lee YH, Lim Y, Hong JT and Hwang DY: Protective effect of
gallotannin-enriched extract isolated from Galla rhois
against CCl4-induced hepatotoxicity in ICR mice. Nutrients.
8:1072016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Catarino MD, Fernandes I, Oliveira H,
Carrascal M, Ferreira R, Silva AMS, Cruz MT, Mateus N and Cardoso
SM: Antitumor activity of Fucus vesiculosus-derived
phlorotannins through activation of apoptotic signals in gastric
and colorectal tumor cell lines. Int J Mol Sci. 22:76042021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu JW, Bhattacharya S, Yanamandra N,
Kilian D, Shi H, Yadavilli S, Katlinskaya Y, Kaczynski H, Conner M,
Benson W, et al: Tumor-immune profiling of murine syngeneic tumor
models as a framework to guide mechanistic studies and predict
therapy response in distinct tumor microenvironments. PLoS One.
13:e02062232018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsieh YH, Wu CJ, Chow KP, Tsai CL and
Chang YS: Electroporation-mediated and EBV LMP1-regulated gene
therapy in a syngenic mouse tumor model. Cancer Gene Ther.
10:626–636. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park HR, Jo SK, Cho HH and Jung U:
Synergistic Anti-cancer Activity of MH-30 in a murine melanoma
model treated with cisplatin and its alleviated effects against
cisplatin-induced toxicity in mice. In Vivo. 34:1845–1856. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noto FK, Sangodkar J, Adedeji BT, Moody S,
McClain CB, Tong M, Ostertag E, Crawford J, Gao X, Hurst L, et al:
The SRG rat, a Sprague-Dawley Rag2/Il2rg double-knockout validated
for human tumor oncology studies. PLoS One. 15:e02401692020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Na K, Li K, Sang T, Wu K, Wang Y and Wang
X: Anticarcinogenic effects of water extract of sporoderm-broken
spores of Ganoderma lucidum on colorectal cancer in
vitro and in vivo. Int J Oncol. 50:1541–1554. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Erickson RL, Blevins CE, Souza Dyer C and
Marx JO: Alfaxalone-Xylazine anesthesia in laboratory mice (Mus
musculus). J Am Assoc Lab Anim Sci. 58:30–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Best I, Casimiro-Gonzales S, Portugal A,
Olivera-Montenegro L, Aguilar L, Muñoz AM and Ramos-Escudero F:
Phytochemical screening and DPPH radical scavenging activity of
three morphotypes of Mauritia flexuosa L.f. from Peru, and
thermal stability of a milk-based beverage enriched with
carotenoids from these fruits. Heliyon. 6:e052092020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mazumder K, Nabila A, Aktar A and
Farahnaky A: Bioactive variability and in vitro and in vivo
antioxidant activity of unprocessed and processed flour of nine
cultivars of Australian lupin species: A comprehensive
substantiation. Antioxidants (Basel). 9:2822020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu DZ, Lin YS and Hou WC:
Monohydroxamates of aspartic acid and glutamic acid exhibit
antioxidant and angiotensin converting enzyme inhibitory
activities. J Agric Food Chem. 52:2386–2390. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Manandhar B, Paudel P, Seong SH, Jung HA
and Coi JS: Characterizing eckol as a therapeutic aid: A systematic
review. Mar Drugs. 17:3612019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fernando IP, Kim M, Son KT, Jeong Y and
Jeon YJ: Antioxidant activity of marine algal polyphenolic
compounds: A mechanistic approach. J Med Food. 19:615–628. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Wijesekara I, Li Y and Kim S:
Phlorotannins as bioactive agents from brown algae. Process
Biochem. 46:2219–2224. 2011. View Article : Google Scholar
|
|
37
|
Aristri MA, Lubis MAR, Iswanto AH,
Fatriasari W, Sari RK, Antov P, Gajtanska M, Papadopoulos AN and
Pizzi A: Bio-based polyurethane resins derived from tannin: Source,
synthesis, characterization, and application. Forests.
12:1516–1538. 2021. View Article : Google Scholar
|
|
38
|
Chung KT, Wong TY, Wei CI, Huang YW and
Lin Y: Tannins and human health: A review. Crit Rev Food Sci Nutr.
38:421–464. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cho HM, Doan TP, Ha TKQ, Kim HW, Lee BW,
Pham HTT, Cho TO and Oh WK: Dereplication by high-performance
liquid chromatography (HPLC) with quadrupole-time-of-flight mass
spectroscopy (qTOF-MS) and antiviral activities of phlorotannins
from Ecklonia cava. Mar Drugs. 17:1492019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee JH, Ko JY, Oh JY, Kim CY, Lee HJ, Kim
J and Jeon YJ: Preparative isolation and purification of
phlorotannins from Ecklonia cava using centrifugal partition
chromatography by one-step. Food Chem. 158:433–437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee JW, Seok JK and Boo YC: Ecklonia
cava extract and dieckol attenuate cellular lipid peroxidation
in keratinocytes exposed to PM10. Evid Based Complement Alternat
Med. 2018:82483232018.PubMed/NCBI
|
|
42
|
Govindappa M, Lavanya M, Aishwarya P, Pai
K, Lunked P, Hemashekhar B, Arpitha BM, Ramachandra YL and
Raghavendra VB: Synthesis and characterization of endophytic fungi,
Cladosporium perangustum mediated silver nano-particles and
their antioxidant, anticancer and nano-toxicological study.
Bionanoscience. 10:928–941. 2020. View Article : Google Scholar
|
|
43
|
Kurian GA and Paddikkala J: Oral delivery
of insulin with Desmodium gangeticum root aqueous extract
protects rat hearts against ischemia reperfusion injury in
streptozotocin induced diabetic rats. Asian Pac J Trop Med.
3:94–100. 2010. View Article : Google Scholar
|
|
44
|
Baig S, Seevasant I, Mohamad J, Mukheem A,
Huri HZ and Kamarul T: Potential of apoptotic pathway-targeted
cancer therapeutic research: Where do we stand? Cell Death Dis.
7:e20582016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vitagliano O, Addeo R, D'Angelo V, Indolfi
C, Indolfi P and Casale F: The Bcl-2/Bax and Ras/Raf/MEK/ERK
signaling pathways: Implications in pediatric leukemia pathogenesis
and new prospects for therapeutic approaches. Expert Rev Hematol.
6:587–597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kwon YH, Jung SY, Kim JW, Lee SH, Lee JH,
Lee BY and Kwon SM: Phloroglucinol inhibits the bioactivities of
endothelial progenitor cells and suppresses tumor angiogenesis in
LLC-tumor-bearing mice. PLoS One. 7:e336182012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang YI, Ahn JH, Choi YS and Choi JH:
Brown algae phlorotannins enhance the tumoricidal effect of
cisplatin and ameliorate cisplatin nephrotoxicity. Gynecol Oncol.
136:355–364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Juríková M, Danihel Ľ, Polák Š and Varga
I: Ki67, PCNA, and MCM proteins: Markers of proliferation in the
diagnosis of breast cancer. Acta Histochem. 118:544–552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alexandrakis MG, Passam FH, Kyriakou DS,
Dambaki K, Niniraki M and Stathopoulos E: Ki-67 proliferation
index: Correlation with prognostic parameters and outcome in
multiple myeloma. Am J Clin Oncol. 27:8–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li R, Heydon K, Hammond ME, Grignon DJ,
Roach M III, Wolkov HB, Sandler HM, Shipley WU and Pollack A: Ki-67
staining index predicts distant metastasis and survival in locally
advanced prostate cancer treated with radiotherapy: An analysis of
patients in radiation therapy oncology group protocol 86–10. Clin
Cancer Res. 10:4118–4124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Urruticoechea A, Smith IE and Dowsett M:
Proliferation marker Ki-67 in early breast cancer. J Clin Oncol.
23:7212–7220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Abd Eldaim MA, Tousson E, El Sayed IET,
Abd El-Aleim AEH and Elsharkawy HN: Grape seeds proanthocyanidin
extract ameliorates Ehrlich solid tumor induced renal tissue and
DNA damage in mice. Biomed Pharmacother. 115:1089082019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Al-Halabi R, Bou Chedid M, Abou Merhi R,
El-Hajj H, Zahr H, Schneider-Stock R, Bazarbachi A and
Gali-Muhtasib H: Gallotannin inhibits NFĸB signaling and growth of
human colon cancer xenografts. Cancer Biol Ther. 12:59–68. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kimura Y and Sumiyoshi M: French maritime
pine bark (Pinus maritima Lam.) extract (Flavangenol)
prevents chronic UVB radiation-induced skin damage and
carcinogenesis in melanin-possessing hairless mice. Photochem
Photobiol. 86:955–963. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Folkman J and Shing Y: Angiogenesis. J
Biol Chem. 267:10931–10934. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Martin TA and Jiang WG: Loss of tight
junction barrier function and its role in cancer metastasis.
Biochim Biophys Acta. 1788:872–891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim D, Kim S, Koh H, Yoon SO, Chung AS,
Cho KS and Chung J: Akt/PKB promotes cancer cell invasion via
increased motility and metalloproteinase production. FASEB J.
15:1953–1962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang SX, Polley E and Lipkowitz S: New
insights on PI3K/AKT pathway alterations and clinical outcomes in
breast cancer. Cancer Treat Rev. 45:87–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang C, Li Y, Qian ZJ, Lee SH, Li YX and
Kim SK: Dieckol from Ecklonia cava regulates invasion of
human fibrosarcoma cells and modulates MMP-2 and MMP-9 expression
via NF-κB pathway. Evid Based Complement Alternat Med.
2011:1404622011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Park SJ and Jeon YJ: Dieckol from
Ecklonia cava suppresses the migration and invasion of
HT1080 cells by inhibiting the focal adhesion kinase pathway
downstream of Rac1-ROS signaling. Mol Cells. 33:141–149. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang CH, Li XF, Jin LF, Zhao Y, Zhu GJ and
Shen WZ: Dieckol inhibits non-small-cell lung cancer cell
proliferation and migration by regulating the PI3K/AKT signaling
pathway. J Biochem Mol Toxicol. 33:e223462019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gudkov AV, Gurova KV and Komarova EA:
Inflammation and p53: A tale of two stresses. Genes Cancer.
2:503–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lane D and Levine A: p53 Research: The
past thirty years and the next thirty years. Cold Spring Harb
Perspect Biol. 2:a0008932010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Levine AJ and Oren M: The first 30 years
of p53: Growing ever more complex. Nat Rev Cancer. 9:749–758. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kawauchi K, Araki K, Tobiume K and Tanaka
N: Loss of p53 enhances catalytic activity of IKKbeta through
O-linked beta-N-acetyl glucosamine modification. Proc Natl Acad Sci
USA. 106:3431–3436. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Pal Singh I and Bharate SB: Phloroglucinol
compounds of natural origin. Nat Prod Rep. 23:558–591. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yoon NY, Chung HY, Kim H and Choi J:
Acetyl- and butyrylcholinesterase inhibitory activities of sterols
and phlorotannins from Ecklonia stolonifera. Fish Sci.
74:200–207. 2008. View Article : Google Scholar
|
|
69
|
Nagayama K, Shibata T, Fujimoto K, Honjo T
and Nakamura T: Algicidal effect of phlorotannins from the brown
alga Ecklonia kurome on red tide microalgae. Aquaculture.
218:601–611. 2003. View Article : Google Scholar
|
|
70
|
Yoon NY: Cholinestarase and lens aldose
reductase inhibitory activities of phlorotannins from Ecklonia
stolonifera and their protective effects on tacrine induced
hepatotoxicity and hyperlipidemic rat models. Ph.D. Thesis, Pukyong
National University, Korea. 4:1112008.
|
|
71
|
Kim MM, Ta QV, Mendis E, Rajapakse N, Jung
WK, Byun HG, Jeon YJ and Kim SK: Phlorotannins in Ecklonia
cava extract inhibit matrix metalloproteinase activity. Life
Sci. 79:1436–1443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sanjeewa KKA, Fernando IPS, Kim SY, Kim
WS, Ahn G, Jee Y and Jeon YJ: Ecklonia cava (Laminariales)
and Sargassum horneri (Fucales) synergistically inhibit the
lipopolysaccharide-induced inflammation via blocking NF-κB and MAPK
pathways. Algae. 34:45–56. 2019. View Article : Google Scholar
|
|
73
|
You SH, Kim JS and Kim YS: Apoptosis and
cell cycle arrest in two human breast cancer cell lines by dieckol
isolated from Ecklonia cava. J Breast Dis. 6:39–45. 2018.
View Article : Google Scholar
|
|
74
|
Yang H, Lee SY, Lee SR, Pyun BJ, Kim HJ,
Lee YH, Kwon SW, Suh DH, Lee CH, Hong EJ and Lee HW: Therapeutic
effect of Ecklonia cava extract in letrozole-induced
polycystic ovary syndrome rats. Front Pharmacol. 9:13252018.
View Article : Google Scholar : PubMed/NCBI
|