Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumours in humans and is the third most common type of
cancer and the second leading cause of cancer-related mortality
worldwide (1). Chemotherapy is the
main treatment modality for CRC, particularly for patients with
stage IV CRC and patients with tumour progression following
surgery, who have a high mortality rate. 5-Fluorouracil (5-FU) and
oxaliplatin are the first-line chemotherapy agents (2). 5-FU-based therapies, such as FOLFOX
(5-FU, folinic acid and oxaliplatin) have been used as standard
therapies for advanced-stage CRC (3).
However, chemoresistance significantly affects the
effectiveness of CRC therapy, resulting in tumour recurrence
accompanied by metastasis. Cancer stem cells (CSCs) are responsible
for tumour initiation, tumour growth, metastasis and resistance to
chemotherapy (4,5). Therefore, the elucidation of the
mechanisms that maintain colorectal CSCs is essential for the
development of novel therapeutic treatment strategies for CRC.
Heterogeneous ribonucleoprotein AB (hnRNPAB) is a
main member of the widely expressed RNA-binding protein hnRNP
family, which is mainly involved in the process of mRNA selective
splicing, mRNA stabilization and gene transcription regulation
(6). Research has indicated that
the inhibition of hnRNPAB interaction with the p53 family member,
p63α, may be responsible for craniofacial diseases, such as
Hay-Wells syndrome (7).
In addition to craniofacial and neurodegenerative
diseases (7,8), hnRNPAB is closely related to tumours
and functions as a transcriptional repressor or activator, as it
binds DNA and leads to the enhancement or inhibition of the
expression of downstream genes, which are involved in the
progression and metastasis of a series of tumours, including lung
(9), prostate (10) and liver cancers (11). Hua et al (10) found that hnRNPAB interacted with
lncRNA PCAT19 to activate a series of cell cycle-related genes, and
promoted the growth and metastasis of prostate cancer. Zhou et
al (12) found that the
overexpression of hnRNPAB induced the epithelial-mesenchymal
transition process and the metastasis of liver cancer. Another
study confirmed that hnRNPAB promoted the progression of liver
cancer by inhibiting the expression of lnc-ELF209 (11).
A previous study by the authors confirmed that
hnRNPAB was highly expressed in CRC tissues and was closely
associated with the poor prognosis of patients (13). However, the role of hnRNPAB in the
stemness characteristics and drug resistance of colorectal CSCs is
not yet not fully understood. In the present study, the properties
and drug resistance of hnRNPAB in colorectal CSCs were
investigated. It was found that hnRNPAB expression was increased in
colorectal CSCs compared with their parental cells, and stem cell
properties and drug resistance were increased. The knockdown of the
hnRNPAB gene in colorectal CSCs reduced the characteristics
of human colorectal CSCs and enhanced their sensitivity to drugs by
changing the cell cycle and increasing apoptosis. These results
suggest that hnRNPAB may be involved in the regulation of
colorectal CSCs and may function as a regulator of the drug
resistance process in CRC.
Materials and methods
Cell lines and colorectal CSC
culture
The human CRC cell lines, SW480 (cat. no.
TCHu172)and HT29 (cat. no. TCHu103), were purchased from The Cell
Bank of Type Culture Collection of the Chinese Academy of Sciences.
The SW480 and HT29 cells were cultured in Leibovitz's L-15 or
McCoy's 5A medium (HyClone; Cytiva) supplemented with 10% foetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C
with 5% CO2. The SW480 and HT29 cells with good cell
growth and 80% confluency were washed with PBS and grown in
serum-free Leibovitz's L-15 or McCoy's 5A medium supplemented with
stem cell culture medium [20 ng/ml epidermal growth factor (EGF);
Invitrogen; Thermo Fisher Scientific, Inc.] B27 (1:50; Gibco;
Thermo Fisher Scientific, Inc.) and 10 ng/ml basic fibroblast
growth factor (bFGF; Invitrogen; Thermo Fisher Scientific, Inc.).
At 72 h after the replacement of the stem cell culture medium,
SW480CSCs and HT29CSCs were obtained by culturing the cells in stem
cell medium for 14 consecutive days, which has been confirmed in
previous experiments (14), and
harvested for subsequent experiments as needed.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated using RNAiso Plus (cat. no.
9108; Takara Biotechnology Co., Ltd.), and cDNA was synthesized
using a PrimeScriptTM RT Reagent kit (cat. no. RR037A;
Takara Biotechnology Co., Ltd.) according to the manufacturer's
instructions. mRNA expression levels were analysed using qPCR with
SYBR Premix Ex TaqTM (cat. no. RR820; Takara
Biotechnology Co., Ltd.). The forward and reverse primers were
designed and synthesized by Dalian Bao Biotech Co., Ltd. The
thermocycling conditions were as follows: Denaturation at 95°C for
5 min, followed by 35 cycles of denaturation at 95°C for 15 sec and
annealing/elongation at 60°C for 30 sec. GAPDH was used as an
internal control. The sequences were as follows: hnRNPAB forward,
5′-AAGAAGTCTATCAGCAGCAGCAGTATG-3′ and reverse,
5′-CTCCACCTCCACCACCACCTC-3′; GAPDH forward,
5′-ATGACATCAAGAAGGTGGTGAAGCAGG-3′ and reverse,
5′-GCGTCAAAGGTGGAGGAGTGGG-3′. The samples were normalized to GAPDH.
Relative expression levels were calculated using the
2−ΔΔCq method (15).
Western blot analysis
Total protein was extracted from the cells using the
Protein Extraction kit (cat. no. R0010; Beijing Solarbio Science
& Technology Co., Ltd), and the protein concentration was
quantified using a BCA Protein Assay kit (cat. no. PC0020; Beijing
Solarbio Science & Technology Co., Ltd.). Equal amounts (40 µg
per lane) of protein were separated using 10% SDS-polyacrylamide
gels. The protein was transferred onto a nitrocellulose membrane at
4°C under a constant flow of 200 mA for 1 h using a dry transfer
system. The membrane was blocked in 5% skimmed milk for 1 h at room
temperature and incubated overnight at 4°C with anti-hnRNPAB
(1:1,000; cat. no. ab199724; Abcam), anti-octamer-binding
transcription factor 4 (OCT4; 1:1,000; cat. no. HRP-60242;
Proteintech Group, Inc.), anti-SOX2 (1:1,000; cat. no. 11064-1-AP;
Proteintech Group, Inc.), anti-Bcl-2 (1:1,000; cat. no. ab32124;
Abcam), anti-Bax (1:1,000; cat. no. ab32503; Abcam), anti-caspase-3
(1:1,000; cat. no. ab32351; Abcam) and anti-GAPDH (1:2,000; cat.
no. ab8245; Abcam) primary antibodies. Subsequently, the membrane
was washed and incubated in an HRP-conjugated antibody solution
(1:5,000; cat. no. 7074; Cell Signaling Technology, Inc.) for 1 h
at room temperature. The membrane was washed and visualized using
Super ECL plus supersensitive luminescent solution (Bio-Rad
Laboratories, Inc.) and exposed using X-ray film. Quantity One
software v4.6.6 (Bio-Rad Laboratories, Inc.) was used to quantify
the band intensities.
Cell transfection
Lentiviral constructs expressing hnRNPAB shRNA
(HBLV-sh-hnRNPAB-PURO) and negative control (HBLV-PURO) were
purchased from Hanbio Biotechnology Co, Ltd. The sequences for
hnRNPAB shRNA-1, hnRNPAB shRNA-2 and negative control shRNA were as
follows: 5′-GGUAGUACAAACUACGGCATT-3′,
5′-GGAGAGGTCGTTGACTGTACAATAA-3′ and 5′-UUCUCCGAACGUGUCACGUTT-3′,
respectively. A total of 1×105 cells from each of the
HT29CSCs and SW480CSCs were plated in a low-adherence six-well
plate and one well was taken as an example. Subsequently, 120 µl
HBLV-PURO (NC control virus), HBLV-sh-hnRNPAB-1-PURO and
HBLV-sh-hnRNPAB-2-PURO virus (both viral titers were
1×108 TU/ml; multiplicity of infection, 100) was added,
and 5 µl Polybrene (2 mg/ml) were added to bring the medium to 2
ml. After an additional 72 h, the cells were collected, and the
effects of gene silencing were verified by RT-qPCR and western blot
analysis after 48–72 h.
Cell Counting Kit-8 (CCK-8) assay
The cells were infected with the lentivirus
according to the aforementioned steps. The transfected cells were
counted, and 1×104 cells were spread in a 96-well plate.
5-FU (0.025, 1, 10 and 100 µmol/l; cat. no. 51-21-8;
MilliporeSigma) and oxaliplatin (0, 0.02, 0.1, 1, 1 and 10 µmol/l;
cat. no. 61825-94-3; MilliporeSigma) were added followed by
incubation at 37°C in a cell incubator for 48 h. Subsequently, 10
µl CCK-8 detection solution (cat. no. CK04; Dojindo Laboratories,
Inc.) were added to each well, incubated at 37°C in a cell
incubator for 2 h, and the absorbance was measured at 450 nm using
a microplate reader at 37°C. The cell growth inhibition rate was
calculated as follows: Inhibition rate (IR)=(absorbance of the
control group NC-absorbance of the experimental group)/absorbance
of the control group. The concentration at which each drug produced
50% inhibition of growth (IC50) was estimated using the
IR.
Sphere formation assay
For the sphere formation assay, a total of 800 cells
were suspended in serum-free medium and plated into an attachment
plate. The cells were then cultured in Leibovitz's L-15 or McCoy's
5A medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with 20 ng/ml EGF (Invitrogen; Thermo Fisher Scientific, Inc.), B27
(1:50; Gibco; Thermo Fisher Scientific, Inc.) and 10 ng/ml bFGF
(Invitrogen; Thermo Fisher Scientific, Inc.). For serial passaging,
the primary spheres were collected and resuspended in Leibovitz's
L-15 or McCoy's 5A medium with the aforementioned supplements
following trypsin dissociation. Finally, the number of spheres was
counted under a microscope (IX53; Olympus Corporation), and the
size of spheres was estimated using ImageJ v6.0 software (National
Institutes of Health), as previously described (16).
Flow cytometry
For the flow cytometric analysis of CSC markers, the
cells were digested into single-cell suspensions and washed with
PBS. A total of 1×106 cells were then resuspended in 100
µl PBS containing 0.5% BSA and 10 µl fluorophore-conjugated primary
antibodies against CD133 (1:100; cat. no. ab305371; Abcam) and CD44
(1:100; cat. no. ab284640; Abcam) for 10 min in the dark at 4°C.
Subsequently, the tubes were removed by centrifugation (500 × g, 5
min at 37°C) and washed twice with 500 µl PBS buffer. The cells
were then suspended in 200 µl PBS each and analysed using a FACS
Vantage SE (BD Biosciences). For the detection of apoptosis, the
HT29CSCs and SW480CSCs were infected with the lentivirus and
treated with 5-FU (10 µmol/l) or oxaliplatin (1 µmol/l) for 48 h.
The cells were resuspended in 100 µl 1X conjugated buffer, mixed
with 5 µl of Annexin V/FITC, and incubated at room temperature in
the dark for 5 min. Subsequently, 10 µl of 20 µg/ml propidium
iodide solution (PI) were added. The cells were then suspended in
400 µl PBS and analysed using a fluorescence-activated cell sorting
(FACS) Vantage SE (BD Biosciences).
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). Comparisons between two groups were performed using a
Student's unpaired t-test. For testing among multiple groups,
one-way analysis of variance (ANOVA) with the SNK-q test or Tukey's
post hoc test was conducted. Statistical analyses were performed
using SPSS software (version 19.0; IBM Corp.). Each experiment was
performed three times, and P<0.05 was considered to indicate a
statistically significant difference.
Results
hnRNPAB is highly expressed in
colorectal CSCs and may be related to the stemness of CSCs
The SW480CSCs and HT29CSCs were obtained from a
suspension culture of the human CRC cell lines, SW480 and HT29,
respectively, grown in suspension (Fig.
1A), which was confirmed by a previous study by the authors
(14). The present study detected
the mRNA and protein expression of hnRNPAB in the SW480CSCs,
HT29CSCs and their parental SW480 cells and HT29 cells using
RT-qPCR and western blot analysis, respectively. The relative mRNA
expression of hnRNPAB in the SW480, SW480CSC, HT29 and HT29CSC
groups was 1.06±0.03, 3.15±0.03, 0.73±0.05 and 2.59±0.07,
respectively, as detected using RT-qPCR. Compared with those in the
SW480 and HT29 groups, the mRNA expression levels of hnRNPAB in the
SW480CSC and HT29CSC groups were increased (P<0.01; Fig. 1B). Furthermore, the same trend was
observed for the hnRNPAB protein levels detected using western blot
analysis (P<0.01; Fig. 1C and
D).
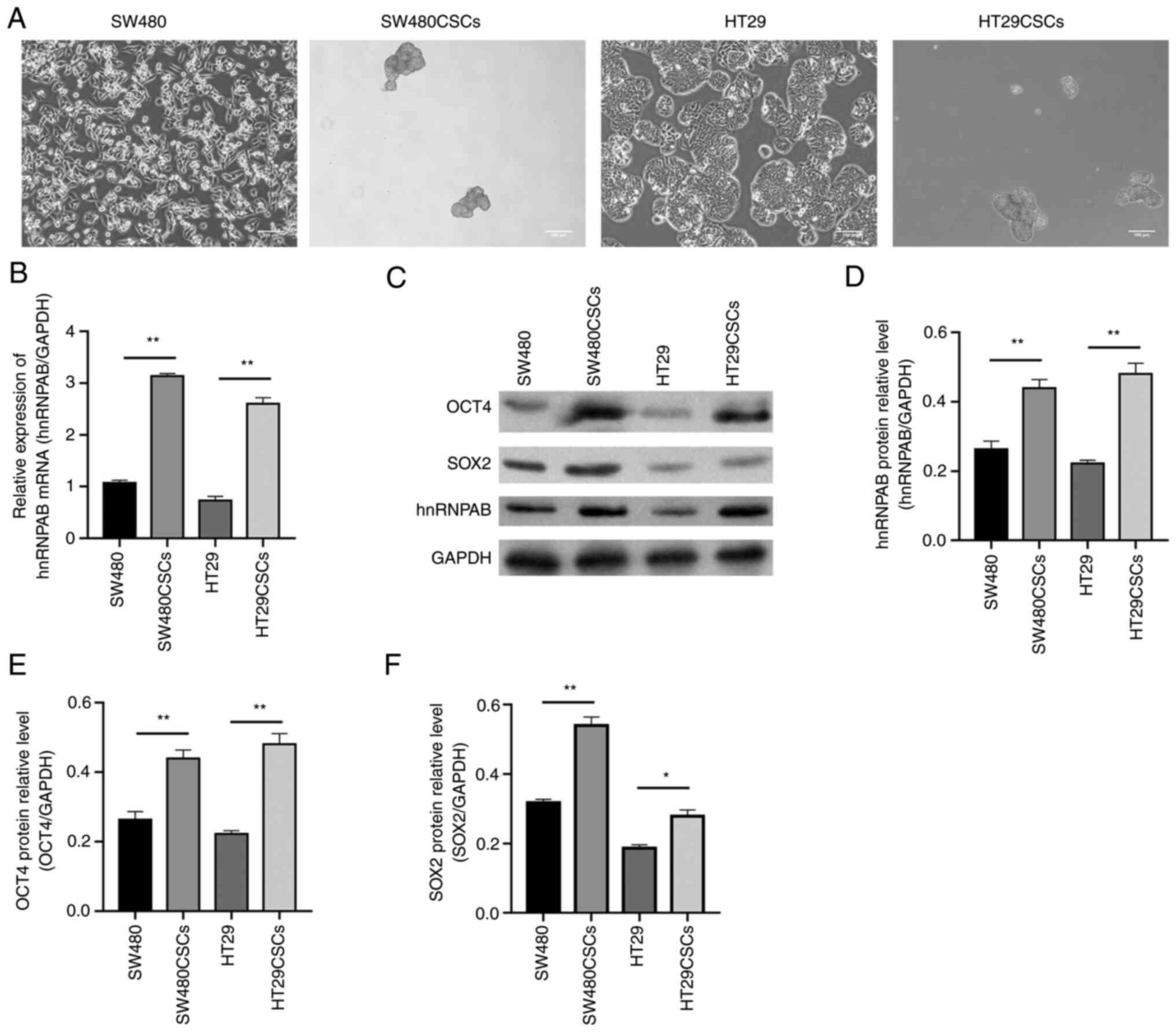 | Figure 1.hnRNPAB is highly expressed in
SW480CSCs and HT29CSCs and may be related to the stemness of CSCs.
(A) Morphology of SW480, SW480CSCs, HT29, HT29CSCs, SW480CSCs and
HT29CSCs aggregated to form three-dimensional spheres. (B) The mRNA
expression levels of hnRNPAB in SW480, SW480CSCs, HT29 and HT29CSCs
were measured using reverse transcription-quantitative PCR. (C-F)
The protein expression levels of OCT4, SOX2 and hnRNPAB in SW480,
SW480CSCs, HT29 and HT29CSCs were examined using (C) western blot
analysis and (D-F) the protein levels were quantified. The
expression levels of (D) hnRNPAB, (E) OCT4, and (F) SOX2 increased
in colorectal CSCs. Each bar represents the mean ± SD of three
independent experiments. *P<0.05 and **P<0.01. hnRNPAB,
heterogeneous ribonucleoprotein AB; CSCs, cancer stem cells; OCT4,
octamer-binding transcription factor 4. |
The present study also detected the protein
expression of OCT4 and SOX2, which are characteristic markers of
embryonic stem cells. The results of western blot analysis revealed
that the protein expression levels of OCT4 in the SW480, SW480CSC,
HT29 and HT29CSC groups were 0.26±0.02, 0.44±0.02, 0.22±0.01 and
0.48±0.02, respectively; the protein expression levels of SOX2 in
the SW480, SW480CSC, HT29, and HT29CSC groups were 0.32±0.01,
0.54±0.02, 0.19±0.01 and 0.28±0.01, respectively. The protein
expression levels of OCT4 and SOX2 in the SW480CSCs and HT29CSCs
were significantly higher than those in their parental SW480 cells
and HT29 cells, consistent with the same trend observed for hnRNPAB
expression (P<0.05; Fig. 1C-F).
These results suggested that hnRNPAB may be related to the stemness
of CSCs.
OCT4 and SOX2 expression levels
decrease in colorectal CSCs following the knockdown of the hnRNPAB
gene
To investigate the effects of hnRNPAB on the
stemness of colorectal CSCs, stable hnRNPAB-silenced colorectal
CSCs were established using two independent shRNAs (sh-hnRNPAB-1
and sh-hnRNPAB-2), with shRNA-NC (sh-NC) as a negative control.
Compared with that in the sh-NC group, the mRNA level of hnRNPAB in
the experimental groups (sh-hnRNPAB-1 and sh-hnRNPAB-2) of
SW480CSCs and HT29CSCs was significantly decreased, as measured
using RT-qPCR (P<0.01; Fig. 2A).
Furthermore, the same trend was observed for the hnRNPAB protein
levels detected using western blot analysis (P<0.01; Fig. 2B-E). These results suggested that
the hnRNPAB gene in SW480CSCs and SW620CSCs was successfully
silenced.
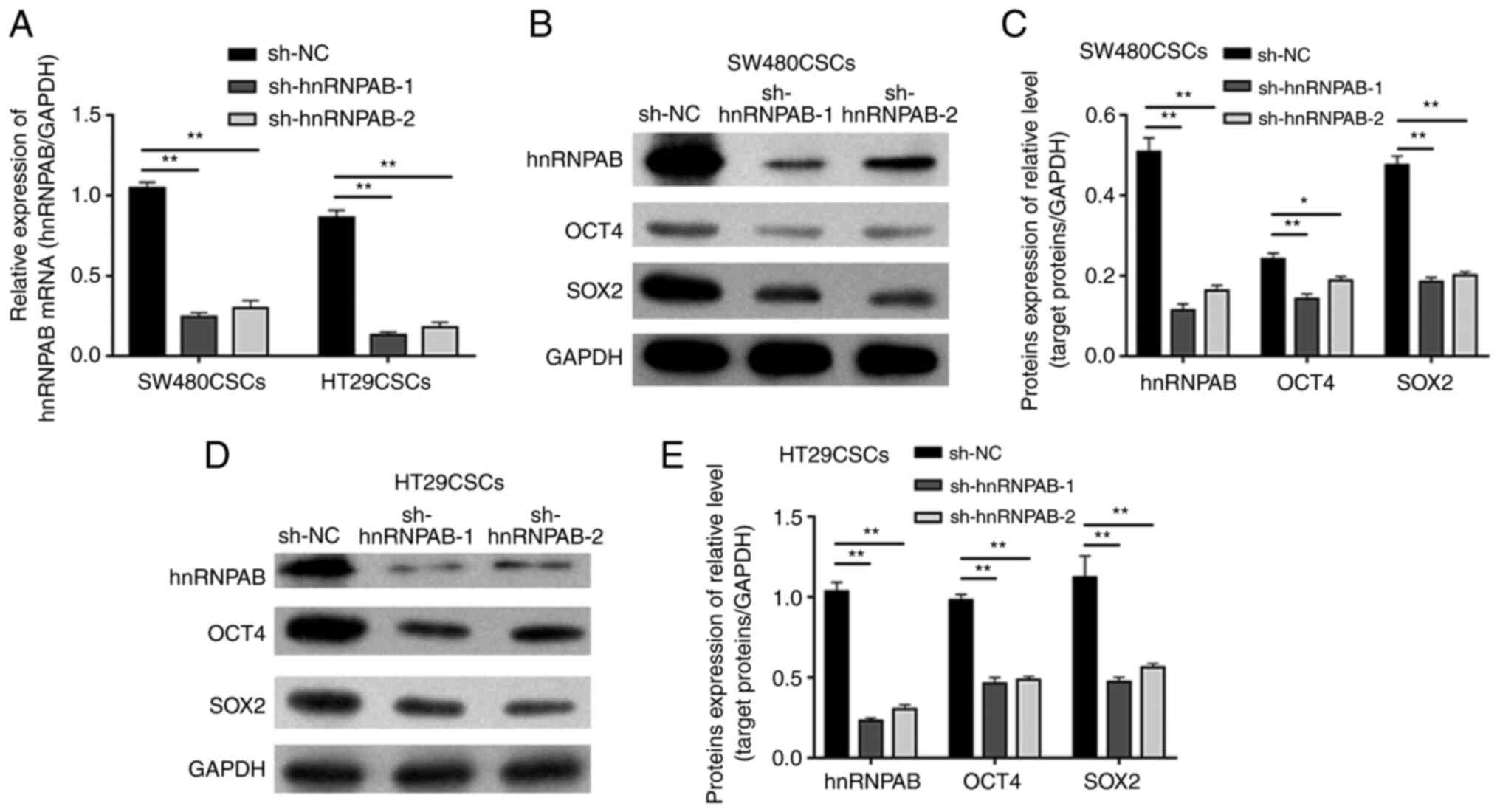 | Figure 2.Expression of hnRNPAB, OCT4 and SOX2
in colorectal CSCs in which the hnRNPAB gene was silenced.
(A) The mRNA expression levels of hnRNPAB in SW480CSCs and HT29CSCs
transfected with sh-hnRNPAB-1, sh-hnRNPAB-2 and sh-NC were analysed
using reverse transcription-quantitative PCR. (B and C) The protein
expression levels of hnRNPAB, OCT4, and SOX2 in SW480CSCs
transfected with sh-hnRNPAB-1, sh-hnRNPAB-2 and sh-NC were examined
using (B) western blot analysis and (C) the protein levels were
quantified. (D and E) The protein expression levels of hnRNPAB,
OCT4, and SOX2 in HT29CSCs transfected with sh-hnRNPAB-1,
sh-hnRNPAB-2 and sh-NC were examined using (D) western blot
analysis and (E) the protein levels were quantified. Each bar
represents the mean ± SD of three independent experiments.
*P<0.05 and **P<0.01. hnRNPAB, heterogeneous
ribonucleoprotein AB; CSCs, cancer stem cells; OCT4,
octamer-binding transcription factor 4. |
To examine the effects of EC cell properties, the
levels of two EC cell biomarkers, OCT4 and SOX2, were detected
using western blot analysis. The protein expression of OCT4 in the
SW480CSC sh-NC, sh-hnRNPAB-1 and sh-hnRNPAB-2 groups was 0.24±0.01,
0.14±0.01 and 0.19±0.01, respectively, and the protein expression
of SOX2 in the SW480CSC sh-NC, sh-hnRNPAB-1 and sh-hnRNPAB-2 groups
was 0.48±0.02, 0.19±0.01 and 0.20±0.01, respectively. The results
revealed that the protein expression of OCT4 and SOX2 in the
sh-hnRNPAB-1 and sh-hnRNPAB-2 groups of SW480CSCs decreased
significantly compared with that in the sh-NC group of SW480CSCs
(P<0.05; Fig. 2B and C). In
addition, the protein expression of OCT4 and SOX2 in the
sh-hnRNPAB-1 and sh-hnRNPAB-2 groups of HT29CSCs decreased
significantly compared with that in the sh-NC group of HT29CSCs
(P<0.01; Fig. 2D and E).
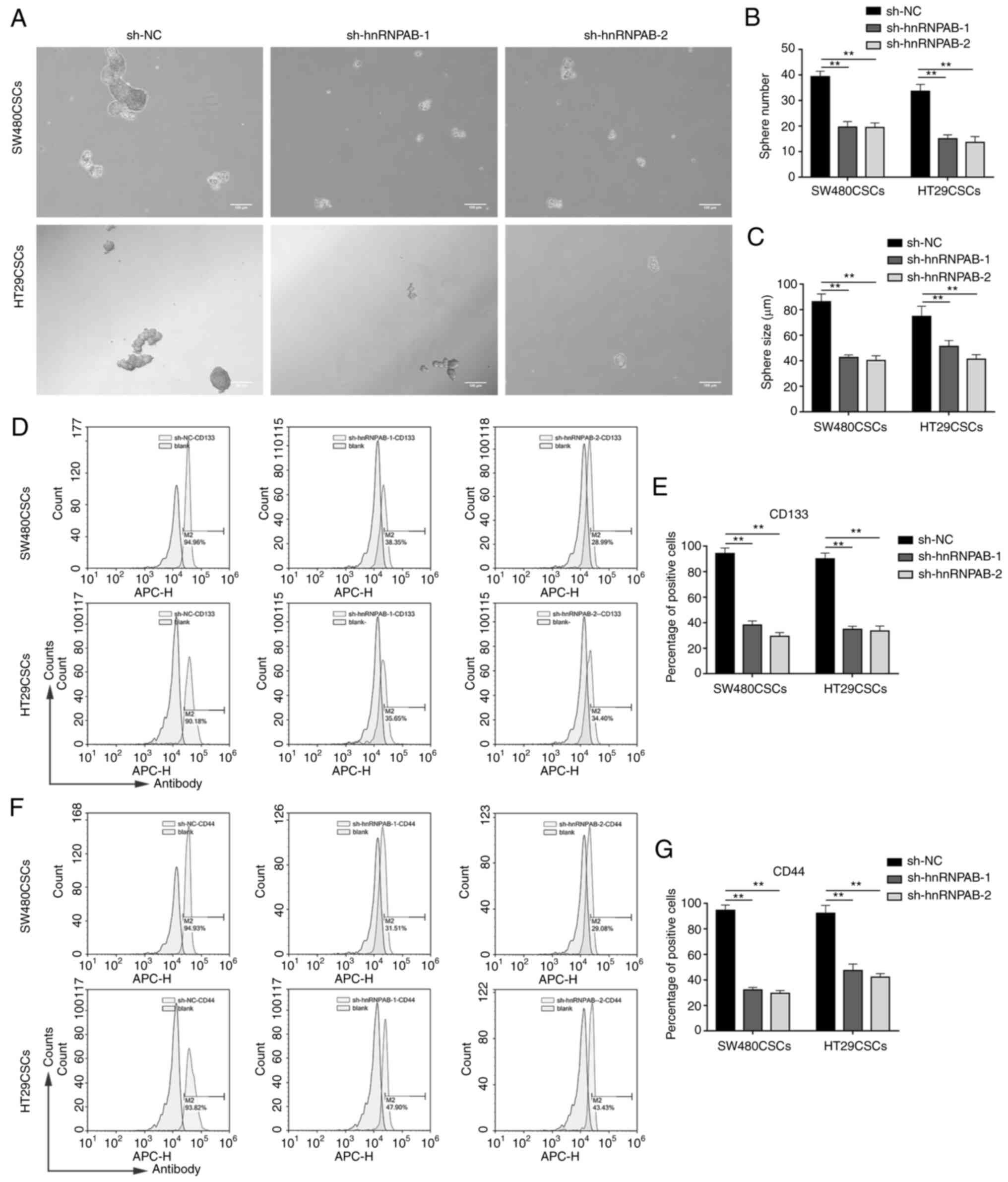
Stemness is reduced in colorectal CSCs
after the silencing of the hnRNPAB gene
To verify the effects of hnRNPAB on the stemness of
CSCs, spheroid formation assays were employed. The number and
average diameter of the spheres derived from the colorectal CSCs in
which hnRNPAB was knocked down were less than those derived from
the sh-NC group (P<0.01; Fig.
3A-C), confirming that the stemness of the colorectal CSCs
decreased after the silencing of the hnRNPAB gene.
Subsequently, the levels of two CSC markers, CD133
and CD44, were detected. Flow cytometric analysis revealed that
CD133 was expressed in 94.23±4.31% of sh-NC cells, 38.13±3.27% of
sh-hnRNPAB-1 cells and in 29.34±2.91% of sh-hnRNPAB-2 cells in the
SW480CSCs; it was also expressed in 90.03±4.56% of sh-NC cells,
34.85±2.39% of sh-hnRNPAB-1 cells and in 33.47±3.93% of
sh-hnRNPAB-2 cells in the HT29CSCs (P<0.01; Fig. 3D and E). In addition, flow
cytometric analysis revealed that CD44 was expressed in 94.56±4.16%
of sh-NC cells, 32.13±1.98% of sh-hnRNPAB-1 cells and in
29.53±2.15% of sh-hnRNPAB-2 cells in the SW480CSCs; it was also
expressed in 93.23±6.25% of sh-NC cells, 47.37±5.10% of
sh-hnRNPAB-1 cells and in 42.23±2.72% of sh-hnRNPAB-2 cells in the
HT29CSCs (P<0.01; Fig. 3F and
G). These results demonstrated that the expression levels of
CD133 and CD44 were significantly decreased in the sh-hnRNPAB-1 and
sh-hnRNPAB-2 groups compared with their sh-NC group in colorectal
CSCs.
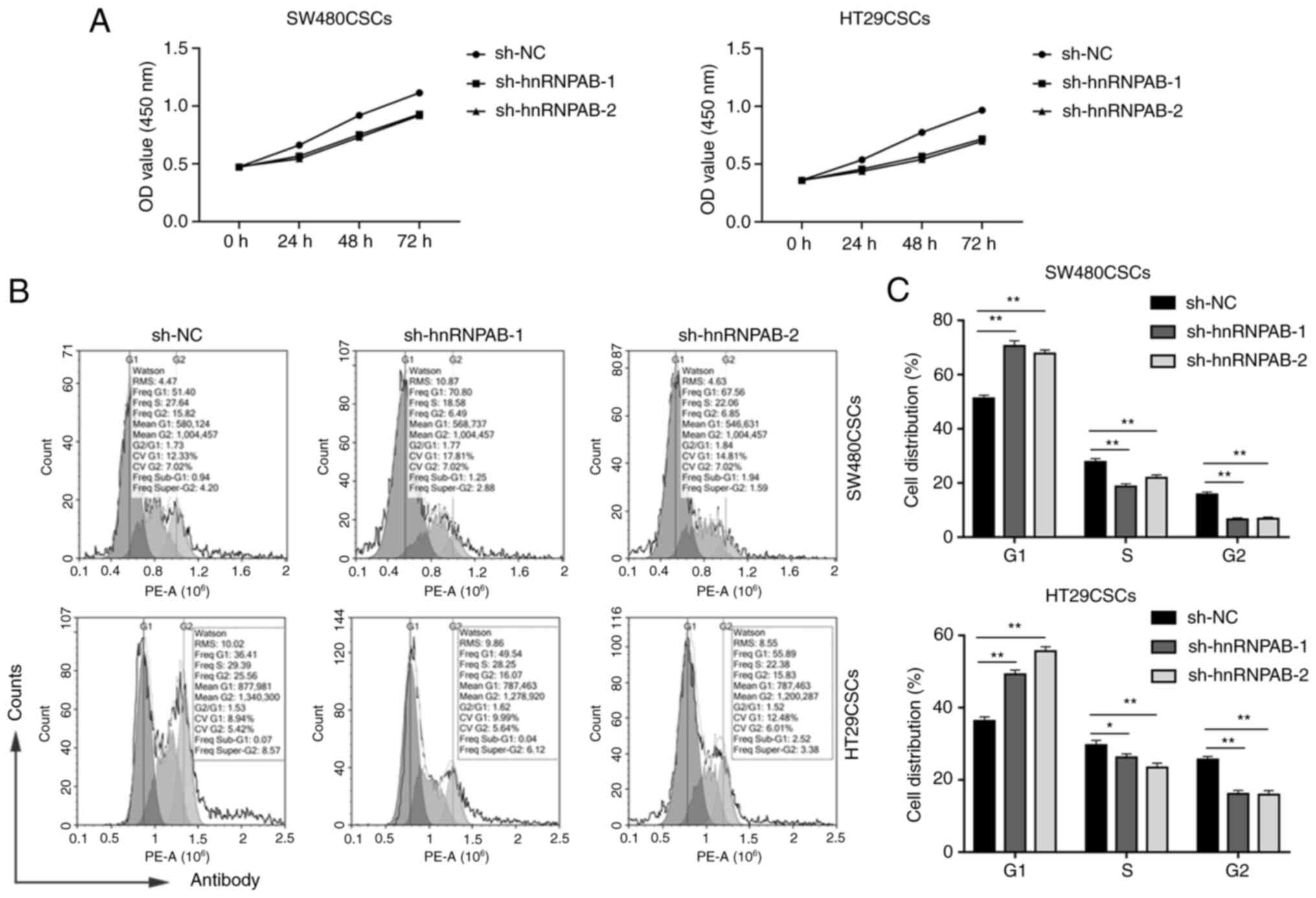
Silencing of hnRNPAB inhibits
colorectal CSC proliferation and prevents cell G1/S transition in
vitro
A CCK-8 assay was then performed to examine the
effects of hnRNPAB on the growth of colorectal CSCs. The results
revealed that hnRNPAB silencing markedly attenuated the viability
of the SW480CSCs and HT29CSCs in the sh-hnRNPAB-1 and sh-hnRNPAB-2
groups compared with the sh-NC group (Fig. 4A).
To explore the mechanisms through which hnRNPAB
accelerated cell proliferation, the effects of hnRNPAB on cell
cycle progression were detected using flow cytometry. As presented
in Fig. 4B and C, compared with the
sh-NC group, the sh-hnRNPAB-1 and sh-hnRNPAB-2 groups of SW480CSCs
and HT29CSCs exhibited a significantly increased percentage of
cells in the G1 phase (51.32±1.09% vs. 70.56%±2.01 and 67.79±1.37%;
and 36.32±1.13% vs. 49.24±1.21 and 55.67±1.26%, respectively), a
decreased percentage of cells in the S phase (27.82±1.21% vs.
18.76±0.92 and 21.87±1.15%; and 29.67±1.29% vs. 26.21±1.03 and
23.48±1.21%, respectively), as well as a corresponding decrease in
the proportion of cells in the G2 phase (15.86±0.87% vs. 6.61±0.54
and 6.83±0.63%; and 25.62±0.92% vs. 16.12±0.98 and 15.92±1.17%,
respectively) (P<0.05; Fig. 4B and
C). Compared with the sh-NC group, the percentage of G1 phase
cells in the sh-hnRNPAB group increased significantly, indicating
that hnRNPAB silencing induced G1 phase arrest. These results
suggested that hnRNPAB accelerated colorectal CSC cell cycle
progression by facilitating the G1/S transition to promote cell
proliferation.
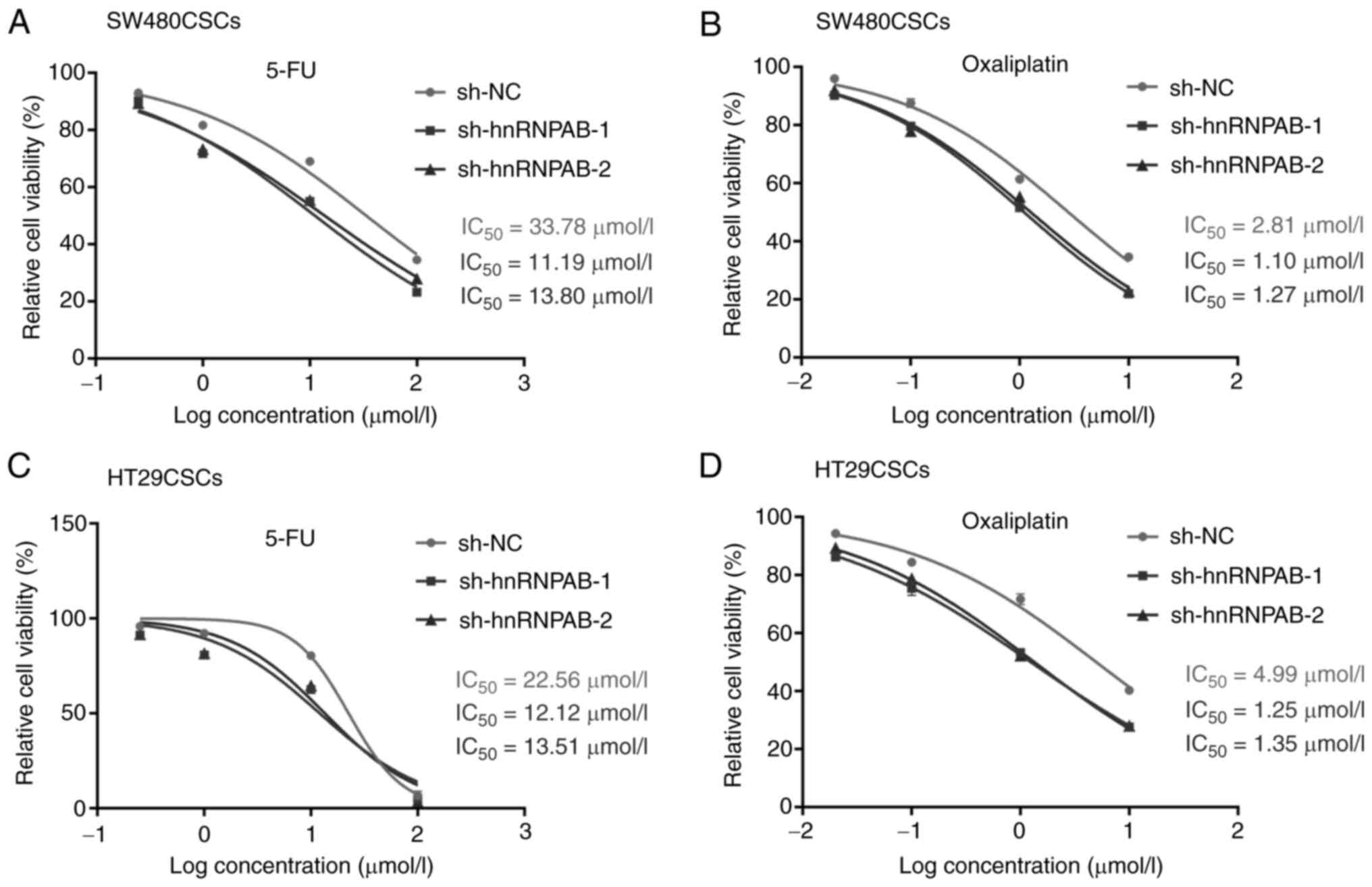
Silencing of hnRNPAB enhances the
sensitivity of colorectal CSCs to the chemotherapeutic drugs, 5-FU
and oxaliplatin
To investigate the role of hnRNPAB in sensitivity to
the chemotherapeutic drugs, 5-FU and oxaliplatin, a cytotoxicity
assay was performed using the hnRNPAB knockdown groups
(sh-hnRNPAB-1 and sh-hnRNPAB-2 groups) in the SW480CSCs and
HT29CSCs. As shown in Fig. 5A,
compared with the sh-NC group, the viability of the cells in the
sh-hnRNPAB-1 and sh-hnRNPAB-2 groups decreased markedly following
treatment with 5-FU in the SW480CSCs. The amount of 5-FU required
to achieve the same level of cell death as the sh-NC group in the
SW480CSCs was much lower in the sh-hnRNPAB-1 and sh-hnRNPAB-2
groups. The respective IC50 values for 5-FU were 11.19
µmol/l (sh-hnRNPAB-1), 13.80 µmol/l (sh-hnRNPAB-2) and 33.78 µmol/l
(sh-NC) in the SW480CSCs (Fig. 5A).
As shown in Fig. 5B, compared with
the sh-NC group, the viability of the cells in the sh-hnRNPAB-1 and
sh-hnRNPAB-2 groups decreased significantly following treatment
with oxaliplatin in the SW480CSCs. Similar results were obtained in
the sh-hnRNPAB-1 and sh-hnRNPAB-2 groups of HT29CSCs (Fig. 5C and D).
The results revealed that the viability of the cells
in the sh-hnRNPAB group and the sh-NC group decreased significantly
with the increasing drug concentrations, regardless of whether 5-FU
or oxaliplatin were added. However, the sh-hnRNPAB groups exhibited
are more significant decrease than the sh-NC group.
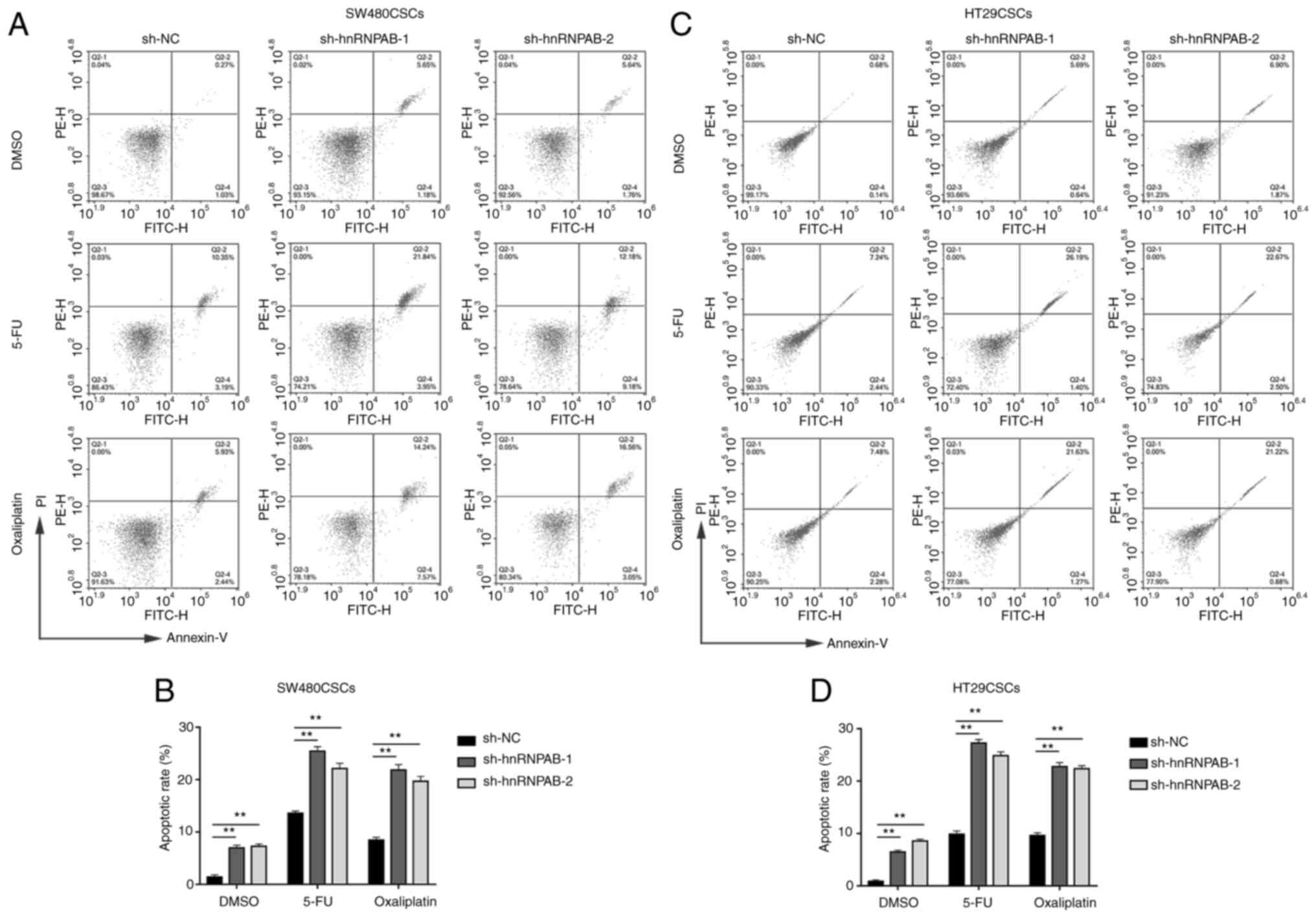
Knockdown of hnRNPAB increases the
apoptotic rate of colorectal CSCs treated with the chemotherapeutic
drugs, 5-FU and oxaliplatin
The present study then examined whether hnRNPAB
exerts any effect on colorectal CSC apoptosis following treatment
with the chemotherapeutic drugs, 5-FU and oxaliplatin. The
sh-hnRNPAB-1 and sh-hnRNPAB-2 groups, compared with the sh-NC
group, exhibited a higher percentage of apoptotic SW480CSCs treated
with DMSO (6.95±0.53 and 7.27±0.44% vs. 1.42±0.39%, respectively),
5-FU (25.43±0.67 and 22.12±0.56% vs. 13.58±0.42%, respectively) and
oxaliplatin (21.83±1.03 and 19.68±0.92% vs. 8.46±0.52%,
respectively), as determined using flow cytometry (P<0.01;
Fig. 6A and B). In addition, the
sh-hnRNPAB-1 and sh-hnRNPAB-2 groups, compared with the sh-NC
group, exhibited a higher percentage of apoptotic cells in the
HT29CSCs treated with DMSO (6.45±0.34 and 8.53±0.38% vs.
0.87±0.26%, respectively), 5-FU (27.28±0.69 and 24.87±0.73% vs.
9.83±0.65%, respectively) and oxaliplatin (22.75±0.82 and
22.40±0.56% vs. 9.62±0.51%, respectively), as determined using flow
cytometry (P<0.01; Fig. 6C and
D). In the two types of colorectal CSCs, the apoptotic rate of
the sh-hnRNPAB groups and sh-NC group was increased following
treatment with 5-FU and oxaliplatin. Compared with the sh-NC group,
the knockdown of hnRNPAB in the SW480CSCs and HT29CSCs resulted in
a higher apoptotic rate in response to the chemotherapeutic drugs,
5-FU and oxaliplatin.
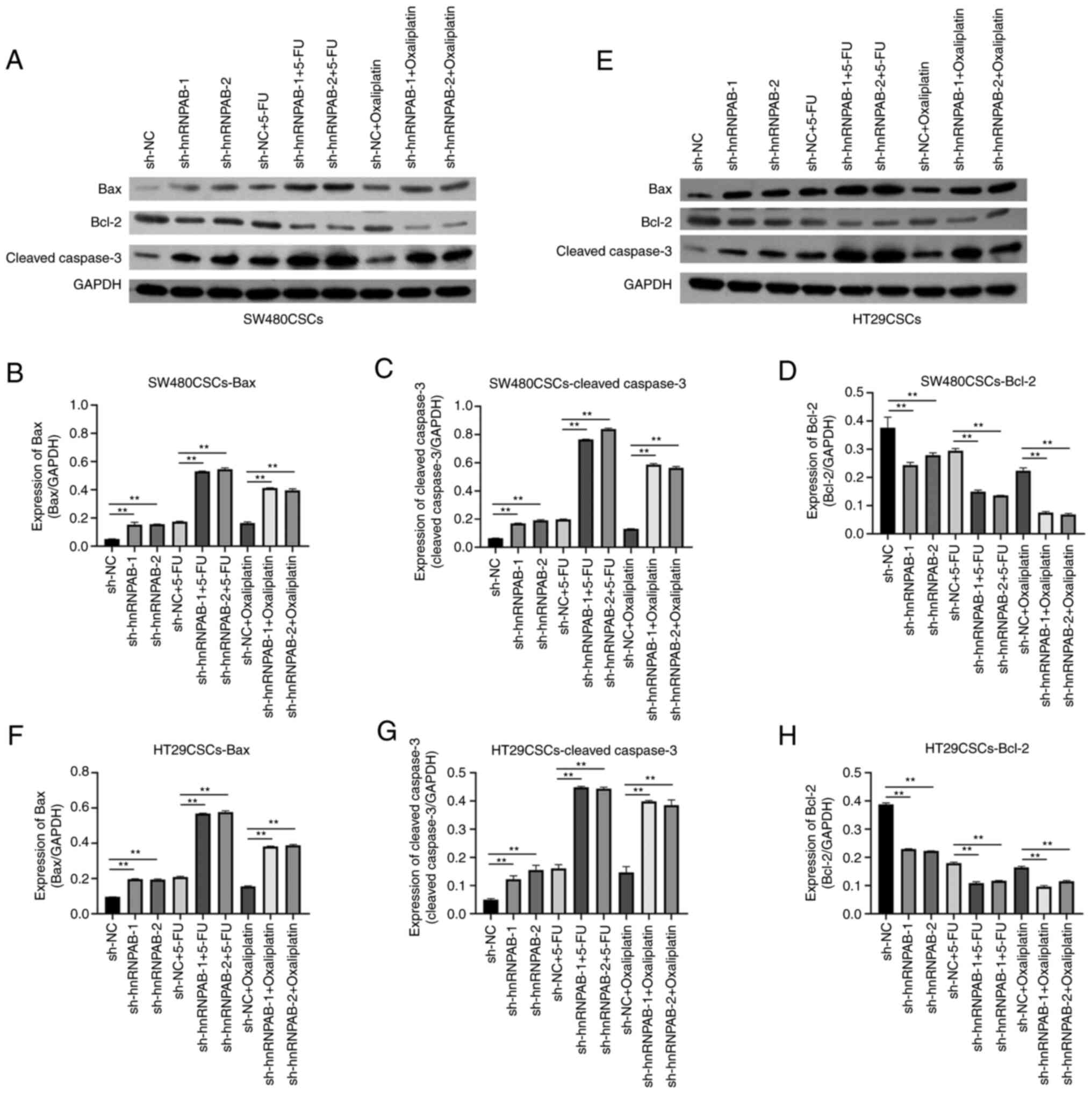
In addition, the levels of apoptosis-related
indicators (Bax, Bcl-2 and cleaved caspase-3) were examined using
western blot analysis. The results revealed that compared with the
sh-NC group, in the sh-hnRNPAB-1 and sh-hnRNPAB-2 groups of
SW480CSCs treated with 5-FU or oxaliplatin, the expression of the
pro-apoptotic proteins, Bax and cleaved caspase-3, was increased,
while the expression of the anti-apoptotic protein, Bcl-2, was
decreased; the expression of apoptosis-related proteins in the
sh-hnRNPAB groups increased or decreased more significantly
(P<0.01; Fig. 7A-D). In
addition, the same trend was observed for the expression of Bax,
Bcl-2 and cleaved caspase-3 in the HT29CSCs with hnRNPAB silencing
treated with 5-FU or oxaliplatin (P<0.01; Fig. 7E-H). The above results suggested
that silencing hnRNPAB increased the sensitivity of SW480CSCs and
HT29CSCs to chemotherapeutic drugs by promoting cell apoptosis.
Discussion
hnRNPs are a large class of RNA-binding proteins
with important roles in multiple aspects of nucleic acid
metabolism, including the packaging of nascent transcripts, mRNA
alternative splicing and gene translational regulation. Studies
have found that the abnormal expression of hnRNPs plays a crucial
role in the occurrence and development of lung, liver, colorectal,
breast, pancreatic cancer and other malignant tumours (17). As an early classification of the
hnRNP family, hnRNPAB was first purified from 40S granules of human
HeLa cells, and it can be divided into four subtypes: hnRNPA1,
hnRNPA2/B1, hnRNPA3 and hnRNPA0 (18). Previous studies have found that
hnRNPAB is highly expressed in CRC and is closely related to
various prognoses of CRC. In the present study, it was found that
the stemness characteristics of colorectal CSCs were increased
compared with their parent cells, the resistance to
chemotherapeutic drugs was increased, and the expression of hnRNPAB
was increased. The knockdown of hnRNPAB expression in colorectal
CSCs decreased the stemness of CSCs, which were sensitive to
chemotherapeutic drugs and cell apoptosis increased.
CSCs are considered subsets of tumour cells with
high tumorigenicity, multilineage differentiation potential, and
self-renewal capacity (19).
Several preclinical studies have demonstrated the presence of CSCs
in human CRC and have demonstrated significant contributions of CRC
CSCs to clinical tumour progression, chemoresistance, and treatment
failure (20). CSCs can express
specific surface markers. CRCs that recur following chemotherapy
failure are enriched in cells expressing tumour stem cell markers,
i.e., enriched in CSCs (21). For
CRC, the CSC markers are OCT4, SOX2, CD44, CD133, CD29, Nanog and
Lgr5 (22–24). It has been have found that the
increase in identifiable stemness-related biomarkers in tumour
cells is associated with treatment resistance and cancer recurrence
(25).
Determining the function of hnRNPAB in CSCs is
critical for understanding the molecular mechanisms of tumour
occurrence and development. According to previous research methods
(14), the authors cultured
colorectal cancer CSCs, termed SW480CSCs and HT29CSCs, and found
that the expression of hnRNPAB increased in colorectal CSCs, as
shown by RT-qPCR and western blot analysis. To further elucidate
the role of hnRNPAB in CSCs, shRNPAB in SW480CSCs and HT29CSCs was
silenced using two independent shRNAs. Spheroid formation
experiments have been used to verify the proliferation,
self-renewal and differentiation abilities of different cell
populations of CSCs (26). In the
present study, the spheroid colony formation ability of colorectal
CSCs with hnRNPAB silencing was lower than that of the normal
control group (the number and size of cells were decreased).
Accumulating evidence has indicated that hnRNPAB can
regulate stem cell proliferation, the cell cycle and apoptosis
(27,28). Choi et al (27) found that hnRNPA2/B1 knockout reduced
the expression of the embryonic stem cell markers, OCT4, NANAG and
SOX2, inhibited the proliferation of human embryonic stem cells and
induced cell arrest in the G0/G1 phase, thus regulating the
self-renewal and multipotential of human embryonic stem cells. Chen
et al (28) reported that
miRNA-8064 targeted hnRNPAB to inhibit the self-renewal of
colorectal CSCs through the Wnt/β-catenin pathway. The stemness
capability of colorectal CSCs was previously assessed using flow
cytometry to examine the percentage of cells expressing tumour stem
cell markers, such as OCT4, SOX2, CD44 and CD133 in vitro
(29). In the present study, the
levels of the colorectal CSC markers, CD44, CD133, OCT4 and SOX2,
were reduced in colorectal CSCs after hnRNPAB silencing, suggesting
a reduction in stemness.
Konishi et al (30) found that hnRNPA0 was highly
expressed in CRC cells. The knockdown of hnRNPA0 in the HCT116
cells reduced the number of cells in the G1 phase, and increased
that in the S and G2/M phase; it also increased the expression of
cleaved caspase-3 and promoted apoptosis. These results indicate
that hnRNPA0 inhibits the apoptosis of CRC cells by maintaining the
promotion of the G2/M phase. Liu et al (31) found that the silencing hnRNPA2/B1
reduced the total clone number of CRC cells with cetuximab
treatment and significantly prevented cell migration and invasion,
and the MIR100HG/hnRNPA2B1/TCF7L2 regulatory loop, regulated
cetuximab resistance and tumour metastasis. In the present study,
it was found that the knockdown of hnRNPAB reduced cell
proliferation by blocking the cell transition from the G1/S to the
S/G2 phase. It was hypothesized that hnRNPAB may be one of the
kinases that promotes the transformation of cells from the G1 to
the S phase. Thus, hnRNPAB promoted the growth of cancer cells by
regulating CSC properties, as well as alterations in the cell
cycle.
In addition, the present study examined the effects
of hnRNPAB in colorectal CSCs treated with the chemotherapeutic
drugs, oxaliplatin and 5-FU. The results revealed that colorectal
CSCs with a high hnRNPAB expression were more resistant to the
chemotherapeutic drugs, oxaliplatin and 5-FU. The knockdown of
hnRNPAB enhanced the sensitivity of colorectal CSCs to the
chemotherapeutic drugs and promoted cell apoptosis.
In conclusion, the present study demonstrated that
the knockdown of hnRNPAB reduced the growth of colorectal CSCs,
enhanced the sensitivity to the chemotherapeutic drugs, 5-FU and
oxaliplatin, and promoted cell apoptosis. Therefore, the inhibition
of hnRNPAB may be considered a novel molecular therapeutic strategy
for CRC and drug-resistant patients. However, the underlying
mechanisms require further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Health Commission of
Hubei Province scientific research project (WJ2021M093) and
Xianning Central Hospital, Hubei University of Science and
Technology First Affiliated Hospital Key Project (2020XYA003,
2020XYA008 and 2020XYA010).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, SC, JD and JLi conceived and designed the
experiments. JZ, JLiu and SC performed the experiments and the
statistical analysis. JZ, JD and JLi participated in the discussion
and interpretation of the data. JZ wrote the manuscript. JD and JLi
supervised all the experimental work. JD and JLi confirmed the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Colin. 71:209–249. 2021.
View Article : Google Scholar
|
|
2
|
Meyerhardt JA and Mayer RJ: Systemic
therapy for colorectal cancer. N Engl J Med. 352:476–487. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sinicrope FA, Foster NR, Thibodeau SN,
Marsoni S, Monges G, Labianca R, Kim GP, Yothers G, Allegra C,
Moore MJ, et al: DNA mismatch repair status and colon cancer
recurrence and survival in clinical trials of 5-fluorouracil-based
adjuvant therapy. J Natl Cancer Inst. 103:863–875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Visvader JE and Lindeman GJ: Cancer stem
cells: Current status and evolving complexities. Cell Stem Cell.
10:717–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeuner A, Todaro M, Stassi G and De Maria
R: Colorectal cancer stem cells: from the crypt to the clinic. Cell
Stem Cell. 15:692–705. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aranburu A, Liberg D, Honoré B and
Leanderson T: CArG box-binding factor-A interacts with multiple
motifs in immunoglobulin promoters and has a regulated subcellular
distribution. Eur J Immunol. 36:2192–2202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fomenkov A, Huang YP, Topaloglu O,
Brechman A, Osada M, Fomenkova T, Yuriditsky E, Trink B, Sidransky
D and Ratovitski E: P63 alpha mutations lead to aberrant splicing
of keratinocyte growth factor receptor in the Hay-Wells syndrome. J
Biol Chem. 278:23906–23914. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mao Y, Tamura T, Yuki Y, Abe D, Tamada Y,
Imoto S, Tanaka H, Homma H, Tagawa K, Miyano S and Okazawa H: The
hnRNP-Htt axis regulates necrotic cell death induced by
transcriptional repression through impaired RNA splicing. Cell
Death Dis. 7:e22072016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tauler J, Zudaire E, Liu H, Shih J and
Mulshine JL: hnRNP A2/B1 modulates epithelial-mesenchymal
transition in lung cancer cell lines. Cancer Res. 70:7137–7147.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hua JT, Ahmed M, Guo H, Zhang Y, Chen S,
Soares F, Lu J, Zhou S, Wang M, Li H, et al: Risk SNP-mediated
promoter-enhancer switching drives prostate cancer through lncRNA
PCAT19. Cell. 174:564–575.e18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Chen Q, Piao HY, Wang B, Zhu GQ,
Chen EB, Xiao K, Zhou ZJ, Shi GM, Shi YH, et al: HNRNPAB-regulated
lncRNA-ELF209 inhibits the malignancy of hepatocellular carcinoma.
Int J Cancer. 146:169–180. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou ZJ, Dai Z, Zhou SL, Hu ZQ, Chen Q,
Zhao YM, Shi YH, Gao Q, Wu WZ, Qiu SJ, et al: HNRNPAB induces
epithelial-mesenchymal transition and promotes metastasis of
hepatocellular carcinoma by transcriptionally activating SNAIL.
Cancer Res. 74:2750–2762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou JM, Jiang H, Yuan T, Zhou GX, Li XB
and Wen KM: High hnRNP AB expression is associated with poor
prognosis in patients with colorectal cancer. Oncol Lett.
18:6459–6468. 2019.PubMed/NCBI
|
|
14
|
Zhou JM, Hu SQ, Jiang H, Chen YL, Feng JH,
Chen ZQ and Wen KM: OCT4B1 promoted EMT and regulated the
self-renewal of CSCs in CRC: Effects associated with the balance of
miR-8064/PLK1. Mol Ther Oncolytics. 15:7–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Su K, Sun X, Jiang Y, Wang L, Hu C,
Zhang C, Lu M, Du X and Xing B: Sec62 promotes stemness and
chemoresistance of human colorectal cancer through activating
Wnt/β-catenin pathway. J Exp Clin Cancer Res. 40:1322021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu Y, Wang X, Gu Q, Wang J, Sui Y, Wu J
and Feng J: Heterogeneous nuclear ribonucleoprotein A/B: An
emerging group of cancer biomarkers and therapeutic targets. Cell
Death Discov. 8:3372022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han SP, Tang YH and Smith R: Functional
diversity of the hnRNPs: Past, present and perspectives. Biochem J.
430:379–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lobo NA, Shimono Y, Qian D and Clarke MF:
The biology of cancer stem cells. Annu Rev Cell Dev Biol.
23:675–699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilson BJ, Schatton T, Zhan Q, Gasser M,
Ma J, Saab KR, Schanche R, Waaga-Gasser AM, Gold JS, Huang Q, et
al: ABCB5 identifies a therapy-refractory tumor cell population in
colorectal cancer patients. Cancer Res. 71:5307–5316. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang TY, Lan KC, Chiu CY, Sheu ML and Liu
SH: ANGPTL1 attenuates cancer migration, invasion, and stemness
through regulating FOXO3a-mediated SOX2 expression in colorectal
cancer. Clin Sci (Lond). 136:657–673. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wen K, Fu Z, Wu X, Feng J, Chen W and Qian
J: Oct-4 is required for an antiapoptotic behavior of
chemoresistant colorectal cancer cells enriched for cancer stem
cells: Effects associated with STAT3/survivin. Cancer Lett.
333:56–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kantara C, O'Connell M, Sarkar S, Moya S,
Ullrich R and Singh P: Curcumin promotes autophagic survival of a
subset of colon cancer stem cells, which are ablated by
DCLK1-siRNA. Cancer Res. 74:2487–2498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park SY, Kim JY, Jang GB, Choi JH, Kim JH,
Lee CJ, Lee S, Baek JH, Park KK, Kim JM, et al: Aberrant activation
of the CD45-Wnt signaling axis promotes stemness and therapy
resistance in colorectal cancer cells. Theranostics. 11:8755–8770.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beck B and Blanpain C: Unravelling cancer
stem cell potential. Nature reviews. Cancer. 13:727–738.
2013.PubMed/NCBI
|
|
27
|
Choi HS, Lee HM, Jang YJ, Kim CH and Ryu
CJ: Heterogeneous nuclear ribonucleoprotein A2/B1 regulates the
self-renewal and pluripotency of human embryonic stem cells via the
control of the G1/S transition. Stem Cells. 31:2647–2658. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen ZQ, Yuan T, Jiang H, Yang YY, Wang L,
Fu RM, Luo SQ, Zhang T, Wu ZY and Wen KM: MicroRNA-8063 targets
heterogeneous nuclear ribonucleoprotein AB to inhibit the
self-renewal of colorectal cancer stem cells via the Wnt/β-catenin
pathway. Oncol Rep. 46:2192021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Wang C, Jiang Y, Wang Q, Tao Y,
Zhang H, Zhao Y, Hu Y, Li C, Ye D, et al: Bcl-3 promotes Wnt
signaling by maintaining the acetylation of β-catenin at lysine 49
in colorectal cancer. Signal Transduct Target Ther. 5:522020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Konishi H, Fujiya M, Kashima S, Sakatani
A, Dokoshi T, Ando K, Ueno N, Iwama T, Moriichi K, Tanaka H and
Okumura T: A tumor-specific modulation of heterogeneous
ribonucleoprotein A0 promotes excessive mitosis and growth in
colorectal cancer cells. Cell Death Dis. 11:2452020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu H, Li D, Sun L, Qin H, Fan A, Meng L,
Graves-Deal R, Glass SE, Franklin JL, Liu Q, et al: Interaction of
lncRNA MIR100HG with hnRNPA2B1 facilitates m6A-dependent
stabilization of TCF7L2 mRNA and colorectal cancer progression. Mol
Cancer. 21:742022. View Article : Google Scholar : PubMed/NCBI
|