Introduction
Breast cancer is one of the major causes of
cancer-related mortality among women worldwide. In 2020, over 2.3
million new cases of breast cancer were diagnosed (1). Recent advances in the early detection
and treatment have led to improvements in the outcomes of breast
cancer patients; however, these patients continue to have a high
risk of developing disease recurrence, progression and mortality.
The presence of distant metastases markedly worsens the prognosis
and quality of life of women with breast cancer. Currently, the
5-year survival rate of patients with localized disease is 99%;
however, in patients with distant metastases, this is reduced to
27% (2). Breast cancer has an
affinity towards bone tissue. It has been estimated that in >70%
of patients with advanced-stage breast cancer, disease progression
will lead to bone metastases (3).
Bone metastases are the major cause of excessive bone resorption,
which is known to lead to severe skeletal complications, such as
hypercalcemia, cancer-induced bone pain, spinal cord compression
and pathological fractures (4). The
multidirectional treatment for breast cancer comprises surgery,
radiotherapy, chemotherapy and hormonotherapy. In addition,
bisphosphonates (BPs) are used as an adjuvant therapy to inhibit
skeletal-related events (5).
Nitrogen-containing BPs (N-BPs) are a standard treatment for
patients with breast cancer with bone metastases, as well as in
patients who are at risk of developing treatment-induced
osteoporosis (6). However, there is
increasing evidence to suggest that BPs also exert direct and
indirect antitumor effects. The direct mechanisms underlying the
antitumor effects of BPs comprises the inhibition of tumor cell
proliferation, pro-apoptotic effects and synergism with cytostatics
(7–9). In turn, the indirect anticancer
effects include the inhibition of cell adhesion and motility, the
stimulation of immune surveillance and anti-angiogenic activity
(10,11). Nevertheless, the translation of
preclinical findings to clinical settings is not straightforward.
Several trials have demonstrated that adjuvant therapy with BPs may
have some beneficial effects on breast cancer patients by improving
their overall survival rate, disease-free survival, and by
prolonging recurrence-free survival. On the other hand, others have
demonstrated that BPs exhibit limited efficacy and even induce
side-effects [reviewed in (3)].
Thus far, BPs have only been administered to patients with breast
cancer with osteolytic bone metastatic disease. Recently, the
American Society of Clinical Oncology has recommended that
zoledronate or clodronate can be administered as an adjuvant
therapy to post- and pre-menopausal patients prior to treatment who
have ovarian suppression-induced menopause (12). The ESMO Clinical Practice Guidelines
also recommend that BPs can be administered to patients with
early-stage breast cancer with low estrogen levels, particularly
those who are at a high risk of relapse and those with
treatment-related bone loss (13).
However, it remains unclear whether BPs may exert beneficial
effects on non-skeletal metastases. Another clinical question is
whether the effectiveness and adverse effects depend on the BPs
used.
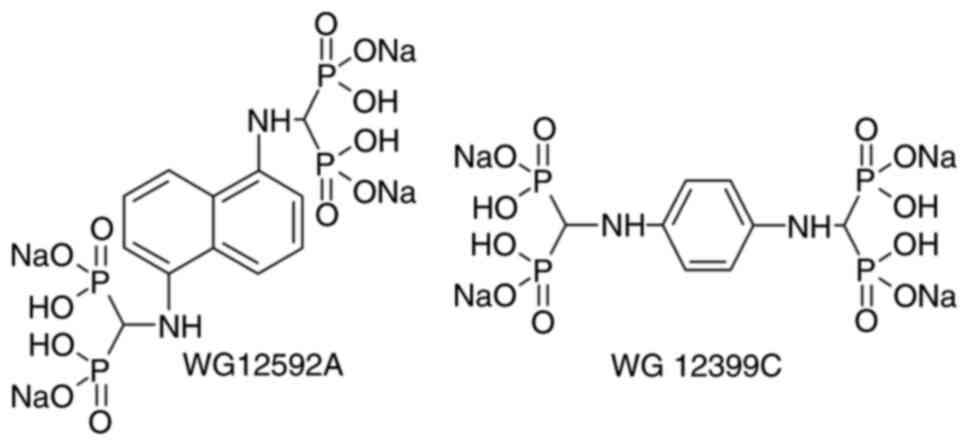
In previous studies, the authors synthesized two
novel aminomethylidene-BPs, namely
benzene-1,4-bis[aminomethylidene(bisphosphonic)] acid (WG12399C)
and naphthalene-1,5-bis[aminomethylidene(bisphosphonic)] acid
(WG12592A) (14). Both BPs
exhibited notable anti-proliferative and pro-apoptotic activity
against osteoclast precursors and tumor cells (15,16).
Moreover, the intravenous (i.v.) administration of WG12399C and
WG12592A significantly prevented ovariectomy-induced bone loss
through the reduction of osteoclastogenesis and the inhibition of
the resorptive activity of mature osteoclasts (17). These findings suggest the potential
beneficial effects of WG12399C and WG12592A in combined anticancer
treatment, as bone-protecting agents and as drugs modulating the
tumor-microenvironment interactions.
Due to the results of these previous studies, the
authors were encouraged to perform an extended analysis of the
biological activity of WG12592A and WG12592A in in vivo
experiments. Therefore, the specific aim of the present study was
to evaluate the in vivo anticancer activity of these BPs (as
a tetrasodium salt; Fig. 1).
The present study used 4T1 breast adenocarcinoma
cells inoculated into BALB/c mice as models of lung and bone
metastasis. The effects of WG12399C and WG12592A on tumor growth
and metastasis were examined in these animals. In addition, the
anti-proliferative activity of these amino BPs in combination with
cytostatic drugs was assessed in 4T1 cells. In addition, the
effects of BPs on cell motility and the cellular mechanisms of
action were investigated.
Materials and methods
Compounds
Tetrasodium salts of
benzene-1,4-bis[aminomethylidene(bisphosphonic)] acid (WG12399C)
and naphthalene-1,5-bis[aminomethylidene(bisphosphonic)] acid
(WG12592A) were synthesized from appropriate acids as described in
a previous study by the authors (17). The starting free bisphosphonic acids
were obtained by dealkylating their octaethyl esters as previously
described (15). Reference
zoledronate was prepared using a previously described procedure
(18).
In vivo experiments
Animals
A total of 155 female BALB/c mice (8–10 weeks old,
weighing 16–23 g) were purchased from the Center of Experimental
Medicine of the Medical University of Bialystok (Bialystok, Poland)
and maintained in specific pathogen-free facility under controlled
environmental conditions (temperature, 22±2°C; humidity, 55±10%;
exposure to a 12-h day/night cycle), with water and food
(S8435-S023, ssniff-Spezialdiäten GmbH) ad libitum. All the
experiments were performed in accordance with the EU Directive
2010/63/EU on the protection of animals used for scientific
purposes. The study procedures were approved by the first Local
Committee for Experiments with the Use of Laboratory Animals,
Wroclaw, Poland (Permission nos. 4/2015 and 80/2015).
Toxicity analyses
The subacute toxicity of WG12399C and WG12592A was
determined according to the OECD Test Guideline no. 407 (https://www.oecd-ilibrary.org/environment/test-no-407-repeated-dose-28-day-oral-toxicity-study-in-rodents_9789264070684-en)
with minor modifications. In total, 35 female mice were randomly
divided into groups, each consisting of 5 BALB/c females (weighing
16–19 g). The mice were maintained in specific pathogen-free
facility under controlled environmental conditions, with water and
food ad libitum. The mice received WG12399C and WG12592A by
i.v. administration. The total doses of the compounds were divided
into four weekly injections: WG12399C: 18, 35 and 50 mg/kg body
weight (b.w.); WG12592A: 1, 2 and 4 mg/kg b.w. The doses were
established based on the 50% lethal dose (LD50) value.
The animals in the control group received the vehicle (saline). The
mice were observed every day for 28 days, and weighed twice a week.
The following were observed during the 28-day period: Food and
water intake; the condition of skin, fur and eyes; signs of
diarrhea; and changes in behavior. On day 28 of the experiment,
blood samples for biochemical analyses were obtained under
anesthesia with 2–3% isoflurane from the eye venous sinus. The
animals were then euthanized by cervical dislocation, and the major
visceral organs and tumors were excised for gross examination and
histopathological analysis.
Cell transplantation
i) Orthotopic model
The 4T1 cells (cat. no. CRL 2539ATTC, ATCC) were
suspended in Hanks' medium [Laboratory of Analytical Chemistry
(IIET)] and inoculated orthotopically into the second mammary fat
pad (2×104/mouse). A total of 45 female mice were
subjected to the procedure of orthotopic tumor cell
inoculation.
ii) Intracardiac model
At day ‘0’, 4T1-luc2-tdTomato cells (CVCL_5J46,
Caliper Life Sciences, USA) were suspended in 100 µl
phosphate-buffered saline (PBS) and injected into the left
ventricle of the heart (2.5×104/mouse) under general
anesthesia with isoflurane (5% for induction and 2–3% for
maintenance). The anterior chest wall was washed with iodine and
70% alcohol. Subsequently, a 30-gauge needle on a tuberculin
syringe was aimed straight and inserted through the intercostal
space into the left ventricle of the heart. The proper positioning
of the needle was confirmed by the spontaneous influx of blood into
the syringe. A total of 75 female mice were subjected to the
procedure of tumor cell inoculation. As this procedure poses a high
risk of post-operative complications (19), 17 deaths were recorded. Thus, the
final number of mice in each group differed at the end of the
experiment.
Drug administration
i) Orthotopic model
Mice (weighing 18–22 g) were divided into five
groups so that the mean tumor volume was similar in each treatment
group. The mice were maintained in a specific pathogen-free
facility under controlled environmental conditions, with water and
food ad libitum. The following groups were included in the
study: An untreated group and groups treated with WG12399C,
WG12592A, zoledronate, or cyclophosphamide. The animals were placed
in groups of 4–5 mice per cage. In the orthotopic model (n=9 per
group), when the tumors reached a mean volume of ~50
mm3, the mice began receiving i.v. injections of the
vehicle or WG12399C at 50 mg/kg, WG12592A at 5 mg/kg, or
zoledronate at 100 µg/kg, with the total dose divided into four and
administered every 6th day (Table
I) (20,21). As a reference, cyclophosphamide (25
mg/kg b.w.) was injected intraperitoneally (i.p.) three times a
week. The doses of the BPs applied in the experiment were selected
based on subacute toxicity experiment and previous research on the
antiresorptive activity of WG12399C and WG12952A, in which they did
not induce toxicity (17). The
doses of cyclophosphamide and zoledronate were selected based on
the literature data (20) and
previous studies by the authors (21). The starting number of mice was 9 in
each group. Of note, one animal in the WG12399C-treated group died
during the course of experiment; however, this death was not
related to treatment, tumor growth, or other experimental
conditions.
 | Table I.Treatment strategy for mice bearing
4T1 or 4T1-luc2-tdTomato cells in orthotopic or intracardiac model,
respectively. |
Table I.
Treatment strategy for mice bearing
4T1 or 4T1-luc2-tdTomato cells in orthotopic or intracardiac model,
respectively.
|
|
|
| Commencement of
treatment | Scheme of
treatment |
|---|
|
|
|
|
|
|
|---|
| Compound | Total dose | Route | Orthotopic
model | Intracardiac
model | Orthotopic
model | Intracardiac
model |
|---|
| WG12399C | 50 mg/kg | i.v. | 50 mm3
tumor volume | 6 Days prior tumor
cells injection | Every 6 days | Day-6, −1, 5 and
9a |
| WG12592A | 5 mg/kg |
|
|
|
|
|
| Zoledronate | 100 µg/kg |
|
|
|
|
|
| CY | 200 mg/kg | i.p. |
|
| Three times a
week | Three times a week
starting from day-6 |
ii) Intracardiac model
Mice (weighing 18–23 g) were randomly divided into
five groups as follows: An untreated group and groups of mice
treated with WG12399C, WG12592A, zoledronate or CY. The mice were
maintained in a specific pathogen-free facility under controlled
environmental conditions, with water and food ad libitum.
The animals received i.v. injections of the vehicle or WG12399C at
50 mg/kg, WG12592A at 5 mg/kg, or zoledronate at 100 µg/kg, with
the total dose divided into four (Table
I). The first two doses were administered prior to tumor cell
inoculation on days 6 and 1, and the following two doses were
administered on days 5 and 9. Cyclophosphamide at the dose of 25
mg/kg b.w. was injected i.p. three times a week commencing on day
6.
Estimation of antitumor activity
i) Orthotopic model
All mice were observed daily and weighed three times
a week throughout the experiment. When tumors became palpable,
their volume was calculated three times a week according to the
formula (a2 × b)/2 (where a is the width and b is the
length). Tumor growth inhibition was calculated by using the value
of the control, untreated mice as 100%. The experiment was
completed after 29 days of tumor cell inoculation. At the end of
the experiment, the animals were anesthetized with 2–3% isoflurane
and blood samples were collected. The animals were then euthanized
by cervical dislocation under anesthesia with 2–3% isoflurane.
Tumors and other organs (lungs, livers, ovaries and lymph nodes)
were excised post-mortem, weighed and stored for further analyses.
The lungs were fixed in formalin (Avantor Performance Materials
Poland S.A.) and the metastatic foci were visually counted.
ii) Intracardiac model
All mice were observed daily and weighed three times
a week throughout the experiment. At 11 days after tumor cell
injection, the animals were anesthetized with 2–3% isoflurane and
blood samples were collected. Tumors and organs (lungs, livers,
kidneys, spleens, ovaries and bones) were excised post-mortem,
weighed and analyzed. The post-mortem visualization of
4T1-luc2-tdTomato tumor metastases was performed using an in
vivo MS FX PRO system (Carestream Health). Briefly, on day 11
after tumor cell inoculation, each animal received an an injection
of 150 mg/kg D-luciferin potassium salt (Synchem UG & Co. KG)
i.p. After 10 min, the mice were anesthetized with a 3–5% (v/v)
isoflurane (Forane, Abbott Laboratories) in synthetic air (200
ml/min), euthanized by cervical dislocation, and the internal
organs were excised, weighed and analyzed for the presence of
neoplastic foci. X-ray images were captured with the following
settings: t=2 min, f-stop=5.57 and FOV=198.6. The visualization of
metastases was performed under the following conditions: t=3 min,
binning 2×2, FOV=198.6, f-stop=5.57. Images were analyzed using
Carestream MI SE version 5.0 software. The intensity of the
luminescence was determined as the sum intensity of the region of
interest and expressed in arbitrary units.
Histopathological examination
To detect micrometastases, axillary and inguinal
lymph nodes and lungs were excised and fixed in 6% buffered
formalin at room temperature for 1 week. To quantify the density of
tumor blood vessel, samples of tumor tissue were excised and fixed
in buffered formalin. Paraffin-embedded samples of tissues were cut
into 4-µm-thick sections, then dewaxed with xylene and rehydrated
in decreasing concentrations of ethanol. Finally, the slides were
washed in distilled water. The cytoplasm was stained at room
temperature with 1% eosin (Aqua Med Sp. z o.o.) for 3 sec and the
nuclei were counterstained with 1% hematoxylin (Merck KGaA) for 5
min. at room temperature. The stained sections were dehydrated in
an alcohol gradient and mounted with a coverslip. The number of
metastases in the lungs and lymph nodes was counted at a
magnification of ×40 or ×400. Microvessel density was quantified at
a magnification of ×200 in five intratumoral areas of the lesion
and the final score was expressed as a mean of the five analyzed
areas.
Blood analysis
Blood was collected into heparinized tubes, and then
analyzed using the Mythic 18 hematology analyzer (C2 Diagnostics).
For biochemical analyses, blood plasma was obtained by the
centrifugation of blood at 2,000 × g for 15 min at 4°C. Alkaline
phosphatase, alanine and aspartate aminotransferases, albumin,
cholesterol and calcium were measured in the plasma samples using
the Cobas c 111 z ISE device (Roche Diagnostics).
Measurement of plasma levels of
cytokines
The expression of selected proteins was determined
in tumor homogenates and plasma. Thus, blood samples were collected
from the animals prior to euthanasia and immediately centrifuged at
2,000 × g for 15 min at 4°C. The samples of plasma were stored at
−80°C for further analysis. Frozen tumor tissue was homogenized in
RIPA buffer (MilliporeSigma) containing a cocktail of protease and
phosphatase inhibitors (MilliporeSigma) using the Fast
Prep®−24 MP Bio homogenizer (MP Biomedicals LLC).
Following 20 min of incubation on ice, the samples were centrifuged
(4°C, 15 min, 12,000 × g), and the supernatants were transferred to
new tubes, centrifuged again, and then kept at −80°C for further
analysis. Prior to the enzyme-linked immunosorbent assays (ELISAs),
the protein concentration was quantified using the Bio-Rad Protein
Assay kit (Bio-Rad Laboratories, Inc.). ELISAs were performed
according to the manufacturer's protocols. The absorbance (at 450
nm) or chemiluminescence were read using the BioTek Hybrid H4
reader (BioTek Instruments, Inc.). Based on the standard curves,
the concentrations of the following proteins in the test samples
were calculated: Vascular endothelial cell growth factor A (VEGF-A;
KMG0112, Thermo Fisher Scientific, Inc.), matrix metalloproteinase
(MMP)-2 (E-EL-M0780), MMP-9 (E-EL-M0627) and transforming growth
factor (TGF)-β1 (E-EL-M0051) (all from Elabscience Biotechnology
Co., Ltd.).
In vitro experiments
Cells and cell culture
Mouse mammary adenocarcinoma 4T1 cells were
purchased from ATCC (cat. no. CRL 2539). The cells were cultured in
RPMI-1640 medium (Laboratory of Analytical Chemistry, IIET) with
Opti-MEM® (Thermo Fisher Scientific, Inc.) (1:1, v/v).
The medium was supplemented with 5% FBS (HyClone, Thermo Fisher
Scientific, Inc.), 2 mM glutamine, 4.5 g/l glucose, 1.0 mM sodium
pyruvate (all from Merck KGaA), and antibiotics (penicillin and
streptomycin; Polfa Tarchomin). The 4T1-luc2-tdTomato-, luciferase-
and red fluorescent protein (tdTomato)-expressing variant of the
4T1 cell line was obtained from Caliper Life Sciences, Inc. The
cells were cultured in RPMI-1640 + Gluta-MAX™ medium
(Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Merck
KGaA) and antibiotics. Both cell lines were cultured under standard
conditions (37°C, humidified atmosphere, 5% CO2).
Combination with cytostatics
The cells were seeded in 96-well plates (Sarstedt,
Inc.) at a density of 1×104 cells/well in 100 µl culture
medium without FBS and antibiotics, and immediately pre-treated
with WG12399C (5 µg/ml) or WG12592A (10 µg/ml), or one of the
cytostatics: doxorubicin (1,000, 100, 10 and 1 ng/ml,
MilliporeSigma), 5-fluorouracil (5-FU) (10, 1, 0.1 and 0.01 µg/ml,
EBEWE Pharma Ges.m.b.H), or paclitaxel (10, 1, 0.1 and 0.01 µg/ml,
Fluorochem Ltd.). BPs at serial concentrations of 100, 50, 25, 10,
5, 2.5, 1, and 0.1 µg/ml were added to control cells for the
calibration curve. The plates were incubated under standard
conditions for 24 h. Subsequently, doxorubicin, 5-FU, paclitaxel or
BPs (12.5 µg/ml of WG12399C or 10 µg/ml of WG12592A) were added to
obtain the following combinations: i) Pre-treatment with BPs
followed by cytostatics; ii) pre-treatment with cytostatics
followed by BPs; and iii) BPs and cytostatics applied
simultaneously. The concentrations of the BPs applied in this assay
were based on the calibration curves and did not exceed an
inhibitory concentration (IC)20. Untreated cells, as
well as cells treated with BPs or cytostatics alone were used as
controls. Following an additional 72 h of incubation under standard
conditions, sulforhodamine B (SRB) assay was performed as
previously described by Skehan et al (22). The absorbance of the samples was
read using a Synergy H4 Hybrid Reader (BioTek Instruments, Inc.) at
a wavelength of 540 nm. In total, two reference agents were used:
Incadronic acid, which exhibits a chemical similarity to that of
the test compounds, and zoledronic acid, with the highest
antiproliferative activity among N-BPs.
To define the interactions between the BPs and
cytostatics, the results of SRB assay were analyzed using the
CalcuSyn program (Biosoft). Based on the method described by Chou
and Talalay (23,24), the combination index (CI) was
calculated. Values of CI <1, =1 and >1 indicate synergism, an
additive effect and antagonism, respectively.
GGOH-FOH assay
The cells were seeded in 96-well plates at a density
of 1×104 cells/well in 100 µl culture medium. After 24
h, 50 µl of either 10 or 20 µM geranylgeraniol (GGOH; Enzo Life
Sciences, Inc.) or farnesol (FOH; Merck KGaA) at a concentration of
5 or 10 µM was added to the cells for 1 h in 37°C. The cells were
then incubated with WG12399C or WG12592A (100, 10, 1 and 0.1 µg/ml)
for an additional 48 h. Untreated cell, as well as cells treated
with WG12399C, WG12592A or zoledronate alone served as controls.
The anti-proliferative activity of BPs was determined using SRB
assay as described above. Prolab-3 software version 4.0
Professional (INFORM-TECH) was used for the calculation of the
IC50 values of the compounds.
Cell cycle analysis
At 24 h prior to treatment, the 4T1 and
4T1-luc2-tdTomato cells were seeded in 35-mm Petri dishes at a
density of 1.5×104 cells/ml in 4 ml of the culture
medium. The cells were then treated with WG12399C at a
concentration of 22.1 µM and WG12592A at a concentration of 16.8 µM
for 24, 48 and 72 h. Untreated cells and cells treated with
zoledronic acid (8.6 µM) were used as controls. The concentrations
of BPs applied in this assay were based on the calibration curves
from antiproliferative tests and did not exceed IC20.
Following incubation with compounds under standard conditions, the
cells were trypsinized, washed twice with cold PBS and fixed for at
least 24 h in 70% (v/v) ethanol at −20°C. The cells were then
washed again and 4′6-diamidino-2-phenylindole (DAPI) staining
solution [1 µg/ml, Triton X-100 0.1% (v/v); Merck KGaA] was added
to each sample. After 30 min of incubation on ice, the samples were
analyzed using the BD LSRFortessa cytometer (BD Biosciences) and
the ModFit LT 3.0 program (Verity Software House).
Measurement of caspase-3 activity
The 4T1 and 4T1-luc2-tdTomato cells were seeded in
24-well plates at a density of 3×103 cells/ml in the
culture medium. The cells were cultured overnight, and treated with
WG12399C at a concentration of 23.9 µM or WG12592A at a
concentration of 23.6 µM for 24, 48 and 72 h. Untreated cells and
cells treated with zoledronic acid (24.1 µM) were used as controls.
The concentrations of BPs applied in this assay were based on the
calibration curves from antiproliferative tests and did not exceed
IC30-40. Following incubation under standard conditions,
the cells were lysed for 30 min with ice-cold lysis buffer [50 mM
HEPES, 150 mM NaCl, 10% saccharose, 5 mM EDTA, 0.1% Triton-X 100,
10 mM dithiothreitol (DTT), pH 7.5] (IIET). Subsequently, 40 µl of
each sample was transferred to an opaque, 96-well plate (Corning,
Inc.) containing 160 µl of the reaction buffer (10 µM Ac-DEVD-ACC
substrate, 20 mM HEPES, 100 mM NaCl, 10% saccharose, 1 mM EDTA, 10
mM DTT, pH 7.5) (IIET). The fluorescence of each sample was
continuously recorded for 2 h at 37°C using the BioTek Synergy H4
Hybrid Reader (λex=360 nm, λem=460 nm). In
parallel, the SRB assay was performed in order to normalize to the
protein content. The results obtained are expressed as mean
relative caspase-3/7 activity in comparison to the untreated cells
± standard deviation (SD).
Annexin-V assay
The 4T1 cells were seeded in 24-well plates at a
density of 3×103 cells/ml in the culture medium. The
cells were cultured overnight and treated with WG12399C at a
concentration of 23.9 µM and WG12592A at a concentration of 23.6 µM
for 24, 48 and 72 h. Untreated cells and cells treated with 24.1 µM
zoledronic acid were used as controls. The concentrations of BPs
applied in this assay were based on the calibration curves from
antiproliferative tests and did not exceed IC30-40.
Following incubation under standard conditions, the cells were
harvested, washed with PBS and suspended in 200 µl binding buffer
(10 mM HEPES/NaOH, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4)
containing 5 µl of APC-Annexin V (Enzo Lifesciences Ltd.). The
cells were stained for 15 min in the dark at room temperature, and
were then washed again in PBS, and suspended in 190 µl binding
buffer. Prior to the FACS analysis, 5 µl DAPI solution (2 mg/ml)
were added for 15 min at room temperature. The cells were analyzed
using the BD LSRFortessa cytometer (BD Biosciences) and the Diva
Software version 6.2 program. Based on the obtained two-color dot
plots, cells were categorized as follows: DAPI+/Annexin
V+ were apoptotic cells, DAPI−/Annexin
V+ were early apoptotic cells, DAPI+/Annexin
V+/− were necrotic cells, and double-negative cells were
live cells.
Migration and invasion assay
The 4T1 and 4T1-luc2-tdTomato cells were seeded in
24-well plates at a density of 5×103 cells/ml in 500 µl
culture medium. The cells were cultured for 24 h and then treated
for an additional 48 h with WG12399C (at a concentration of 23 µM
for 4T1 cells and 20.1 µM for 4T1-luc2-tdTomato cells) and WG12592A
(at a concentration of 20.2 µM for 4T1 cells and 16.8 µM for
4T1-luc2-tdTomato cells). The concentrations of BPs applied in this
assay were based on the calibration curves from antiproliferative
tests and did not exceed IC20-30. Untreated cells and
cells treated with zoledronic acid were used as controls. For the
migration and invasion assay, the cells in the number of
2×104 cells/insert were applied on the upper side of
Matrigel-coated inserts (BioCoat Matrigel Invasion Chamber, BD
Biosciences) placed in the bottom chambers with the culture medium
containing 20% FBS as a chemoattractant. The migration and invasion
assay was performed under standard culture conditions for 24 h. The
cells on the top of the membrane were then carefully removed using
a cotton swab moistened with PBS, and the cells that migrated to
the bottom side of the porous membrane were fixed in Diff-Quick
Fix, stained with Diff-Quick I and II (for 2 min in each solution
at room temperature) (Medion Diagnostics, Düdingen, Switzerland),
and counted under a light microscope (Olympus Corporation). The
assay was repeated at least three times.
Statistical analysis
The results were analyzed using GraphPad Prism 7.03
(GraphPad Software Inc.). The Shapiro-Wilk's normality test and
Bartlett's test were used to confirm the assumptions for the
analysis of variance (ANOVA). Following ANOVA, Dunnett's or Tukey's
multiple comparisons test were used. For non-parametric data, the
Kruskal-Wallis test with Dunn's multiple comparison test was
applied. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Subacute toxicity of WG12399C and
WG12592A
In previous research by the authors, the
LD50 values of the studied BPs were determined and these
were as follows: 73.8 mg/kg for WG12399C and 3.3 mg/kg for WG12592A
(17). Thus, the total doses of the
compounds selected for subacute toxicity tests were as follows: 18,
35 and 50 mg/kg for WG12399C, and 1, 2 and 4 mg/kg for WG12592A.
These total doses were divided into four weekly i.v. injections. It
was observed that neither of the compounds affected the overall
well-being of the mice, as manifested by an increase in body weight
during the course of the experiment (17). No treatment-related deaths were
recorded. Moreover, no significant differences in the weights of
internal organs excised following euthanasia were observed between
the control and treated animals (Fig.
S1). In addition, the studied BPs did not induce any
significant changes in blood morphology that may be attributed to
the toxicity of these compounds. The only significant BP-induced
change in morphological parameters was an increase in the number of
erythrocytes in the animals treated with the highest dose of
WG12399C as compared with the control mice (Fig. S2).
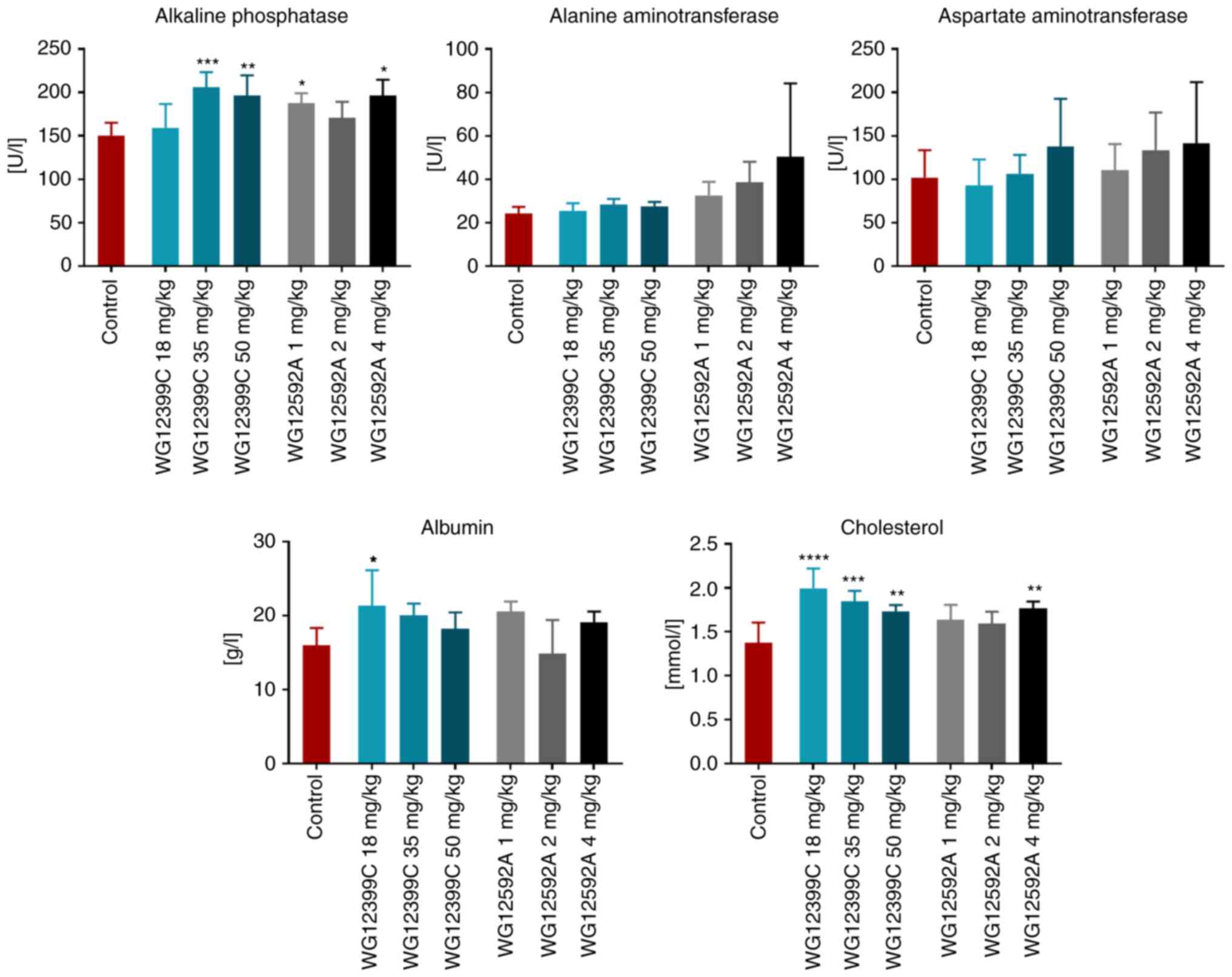
The analysis of blood biochemical parameters at the
end of the experiment revealed an increase in the activity of
alkaline phosphatase in the groups of mice administered WG12399C
and WG12592A. The concentration of albumin was also increased in
the mice treated with a low dose of WG12399C (Fig. 2). The activity of alanine and
aspartate aminotransferases was also found to be elevated in the
groups administered higher doses of BPs (although the difference
was not statistically significant). The administration of WG12399C
and WG12592A (at higher doses) also led to an increase in
cholesterol concentrations in the blood. Nevertheless, the weekly
doses of WG12399C (e.g., 12.5 mg/kg) and WG12592A (e.g., 1 mg/kg)
were well-tolerated by the animals and the BPs did not evoke any
clinical signs of toxicity. Based on these observations and the
data from previous research (17),
the following total doses, which were divided into four and
administered every 6th day, were selected for treatment of the
mice: WG12399C at 50 mg/kg and WG12592A at 5 mg/kg.
Antitumor activity of WG12399C and
WG12592A
The 4T1 cells were injected orthotopically into the
mammary fad pads of 8- to 10-week-old syngeneic BALB/c mice. The
animals were observed and the kinetics of tumor growth were
recorded. As presented in Fig. 3A,
the treatment applied in the experiment did not significantly
affect the mean weight of the animals in any of the groups. In
contrast to the reference agent, cyclophosphamide, which inhibited
primary tumor growth by 46%, none of the tested BPs affected the
kinetics of tumor growth in a significant manner (Fig. 3B). However, despite the lack of
antitumor activity, WG12399C inhibited lung metastases. The number
of macroscopically visible metastatic foci in the lungs of mice
receiving WG12399C was reduced by ~66% in comparison to the control
(Fig. 3C).
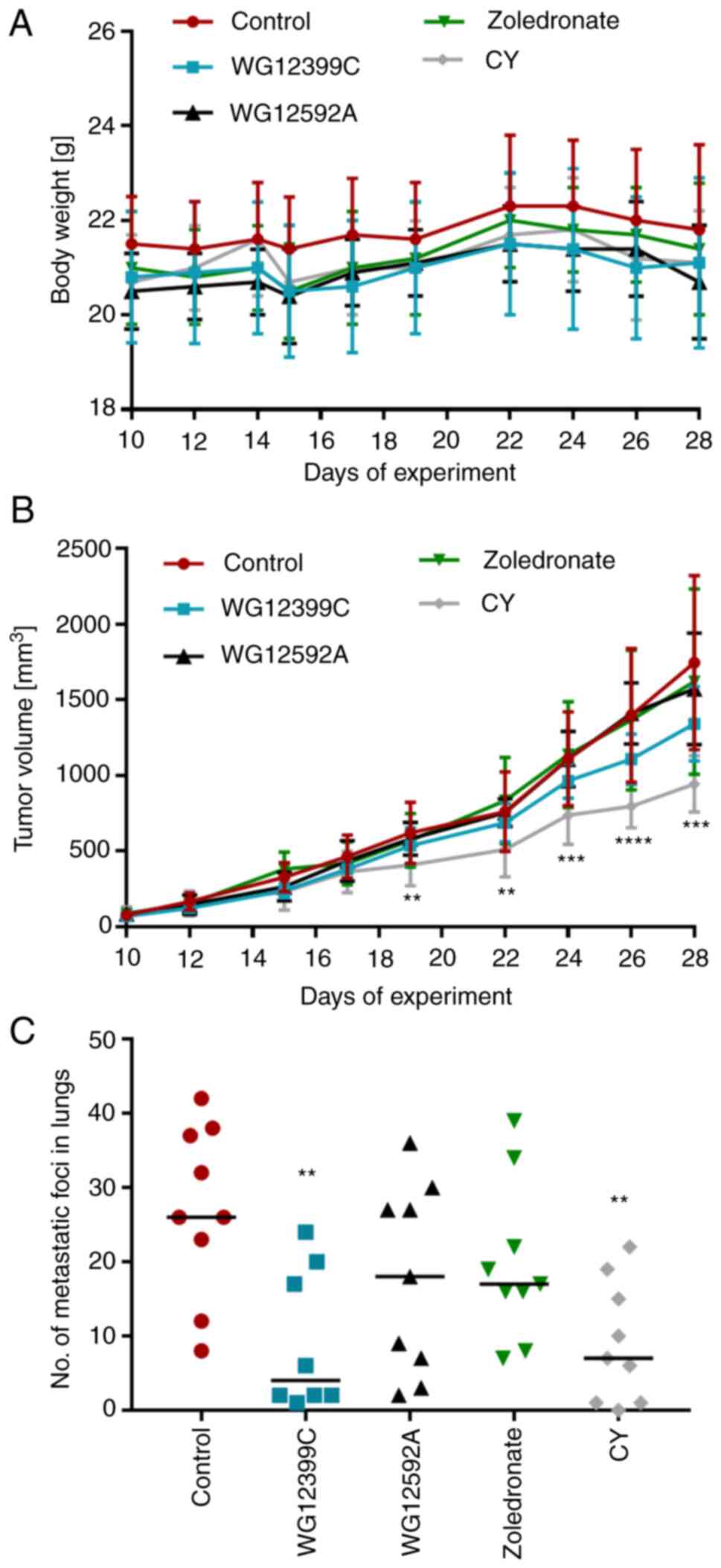 | Figure 3.Effect of bisphosphonates on 4T1
tumor progression. (A) Body weight changes. (B) Kinetics of primary
tumor growth. (C) Number of macroscopically visible metastatic foci
in the lungs on day 28. Mice were inoculated orthotopically with
4T1 cells (day 0). From day 8, the mice began receiving treatment
in the following regimens: WG12399C, total dose of 50 mg/kg,
intravenous administration, every 6th day; WG12529A, total dose of
5 mg/kg, intravenous administration, every 6th day; zoledronate,
total dose of 100 µg/kg, intravenous administration, every 6th day;
and CY, total dose of 225 mg/kg, intraperitoneally, three times a
week. n=8-9 mice per group. Data are presented as (A and C) the
mean ± SD, or as (B) data for individual animals with median
values. **P<0.01, ***P<0.001 and ****P<0.0001 vs. the
control, assessed using one-way ANOVA with Dunnett's multiple
comparisons tests. CY, cyclophosphamide. |
On day 28 of the experiment, the animals were
euthanized, and the internal organs were excised, weighed and
histologically analyzed. The post-mortem analysis of the internal
organs revealed that the weights of the lungs, heart and ovaries
were reduced in the mice receiving WG12399C as compared to the
control animals (Fig. 4A). However,
although these changes were evident, the differences were not
statistically significant. The histopathological analysis of the
lungs revealed the presence of 4T1 cell-derived metastases in 88%
of the WG12399C-treated animals and in 100% of the animals in the
remaining groups (Fig. 4B and C).
The differences in the size and number of metastatic lesions were
recorded. In the control group, as well as in the WG12592A- and
zoledronate-treated mice, the metastatic foci were numerous, large
subpleural and intrapleural. In the cyclophosphamide group,
numerous large and small lesions were observed in the lungs,
whereas in the lungs of the WG12399C-treated mice, single large or
numerous, yet small foci prevailed. Moreover, WG12399C and WG12592A
impaired the formation of metastases in the sentinel lymph nodes.
Metastatic foci were observed only in 2 out of 8 mice, and in 5 out
of 9 mice in the WG12399C- and WG12592A-treated groups,
respectively, whereas in the untreated group, tumor metastases were
noted in 8 out of 9 animals. Zoledronic acid did not prevent the
metastasis of 4T1 tumors to the lungs or lymph nodes (Fig. 4B).
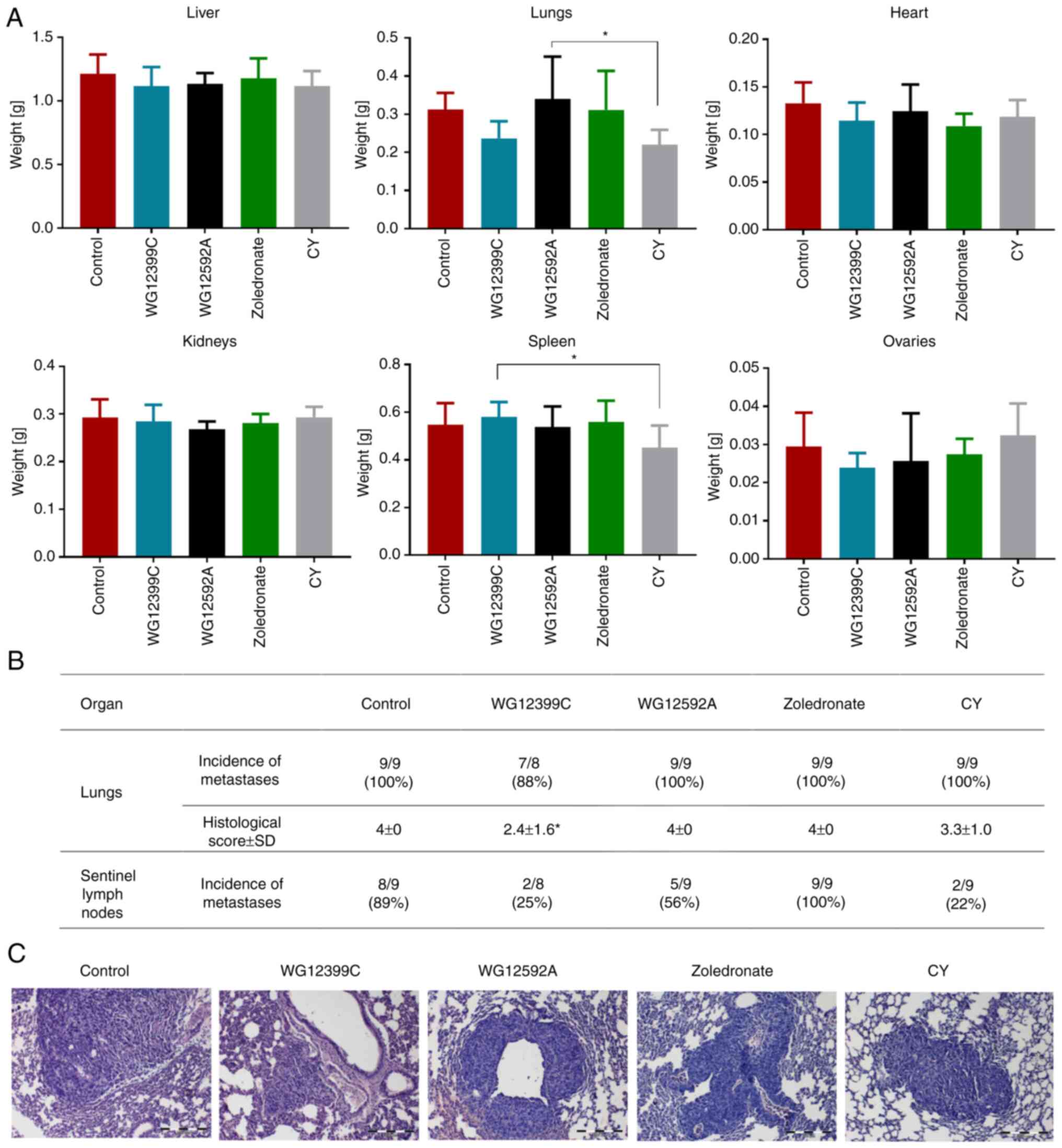 | Figure 4.Effect of bisphosphonates on 4T1
metastasis. (A) Weights of the internal organs. (B) Incidence of
histologically confirmed metastatic foci in the lungs and lymph
nodes on day 28. (C) Representative images of metastases in the
lungs; n=8-9 mice per group. Data are presented as (A and B) the
mean ± SD. Histological scoring: 0, no metastatic foci; 1, single
small foci; 2, single large foci; 3, numerous small foci; 4,
numerous large foci. *P<0.05, assessed using one-way ANOVA with
Tukey's multiple comparisons tests (weight organs, vs. control) or
with the Kruskal-Wallis test with Dunn's multiple comparisons tests
(histological scoring, vs. control, WG12592A and zoledronate). CY,
cyclophosphamide. |
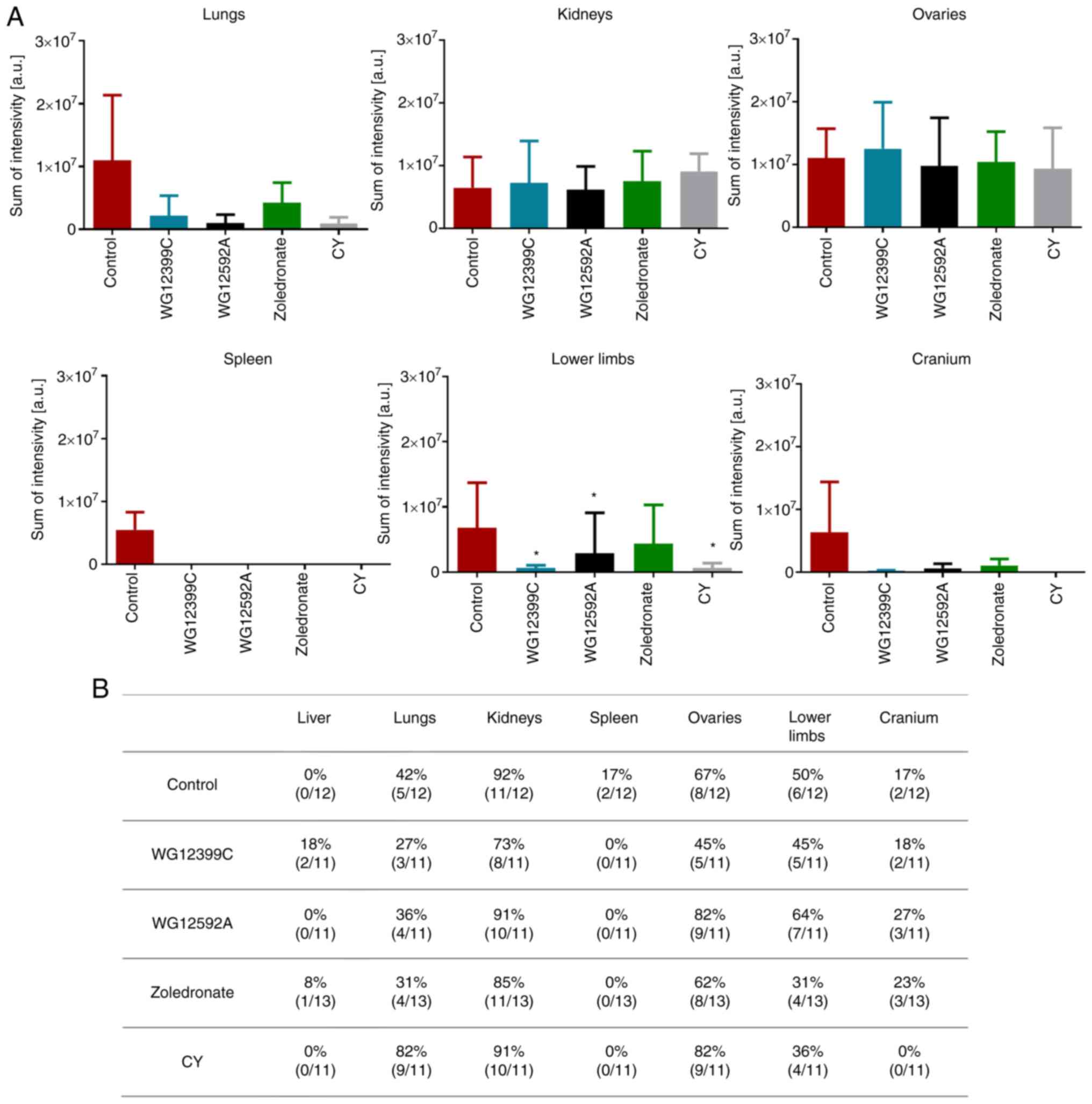
The anti-metastatic effects of the BPs were further
evaluated in the experimental metastasis model of 4T1-luc2-tdTomato
cells that were intracardially inoculated into a tail vein of
female BALB/c mice. The 4T1-luc2-tdTomato cells are derived from a
parental 4T1 cell line, labeled with fluorochrome, and they express
luciferase (25). Thus, these cells
may be visualized in the internal organs, and allow the monitoring
of the metastasis process. The intracardiac injection of cancer
cells successfully leads to their colonization of internal organs,
such as the lungs, kidneys, spleen, ovaries and bones (26–29).
In the present study, mice were pre-treated with BPs at 1 and 6
days prior to transplantation, and the treatment was repeated on
days 4 and 9. The post-mortem visualization (day 11) of the
metastatic lesions in the internal organs revealed that the
administered BPs affected the metastasis of 4T1-luc-tdTomato cells
in BALB/c mice at a similar rate as observed for cyclophosphamide
(Figs. 5 and S3). The luminescence analysis of the
lungs revealed a reduction in the size of metastatic lesions in the
mice treated with either WG12399C or WG12592A (Fig. 5A). Furthermore, treatment with
WG12399C reduced the incidence of tumor metastases in the lungs
from 42% in the control mice to 27% in the mice in the WG12399C
group (Fig. 5B). The effects of the
BPs on other internal organs varied. In total, 2 out of the 12
control mice were diagnosed with spleen metastases, whereas no such
metastases were recorded in the treated groups. However, no marked
differences were observed for metastasis in the livers, kidneys, or
ovaries. In turn, treatment with BPs had a beneficial effect on
tumor metastasis to the bones, although the incidence of bone
metastases was not significantly affected (Fig. 5B); however, both WG12399C and
WG12595A markedly reduced the size and/or the number of metastatic
foci in the crania and bones of lower limbs (Fig. 5A). The reference agent, zoledronate,
reduced the number of mice diagnosed with metastases in long bones;
however, its effects on the size of metastases were less
pronounced. These results suggest that WG12399C and WG12592A BPs
significantly prevent the metastasis of 4T1 cells to secondary
sites, not only including bones, but also internal organs.
Expression of proteins involved in
tumor progression in plasma and tumor tissue
On day 28 following the inoculation of 4T1 cells
into BALB/c mice, samples of plasma and tumor tissue were collected
and the concentrations of several proteins involved in tumor growth
and progression were measured. The plasma levels of VEGF and MMP-9
were significantly elevated in the untreated control mice
inoculated with 4T1 cells compared to the healthy animals (Fig. 6A). In the tumor-bearing untreated
control mice, the concentration of TGF-β1 was increased by 31%
compared to the healthy animals; however, the difference was not
statistically significant. Treatment with WG12399C and WG12592A did
not affect the plasma levels of VEGF or MMPs in a significant
manner. An increase in the plasma concentration of TGF-β1 was
observed in the mice treated with the BPs compared to the untreated
control animals, with the differences ranging from 55% (for
WG12399C and WG12595A) to 151% (for zoledronate, P=0.0019). No
significant changes in the levels of MMP-2 and TGF-β1 in tumor
tissue were observed in the mice receiving treatment in comparison
to the untreated control group (Fig.
6B). The administration of zoledronate led to a significant
decrease in the tumor concentration of VEGF and MMP-9. By contrast,
such effects were not observed in the animals treated with WG12399C
and WG12592A. This may suggest that the antitumor activity of
WG12399C and WG12592A is related to mechanisms other than the
modulation of the tumor microenvironment.
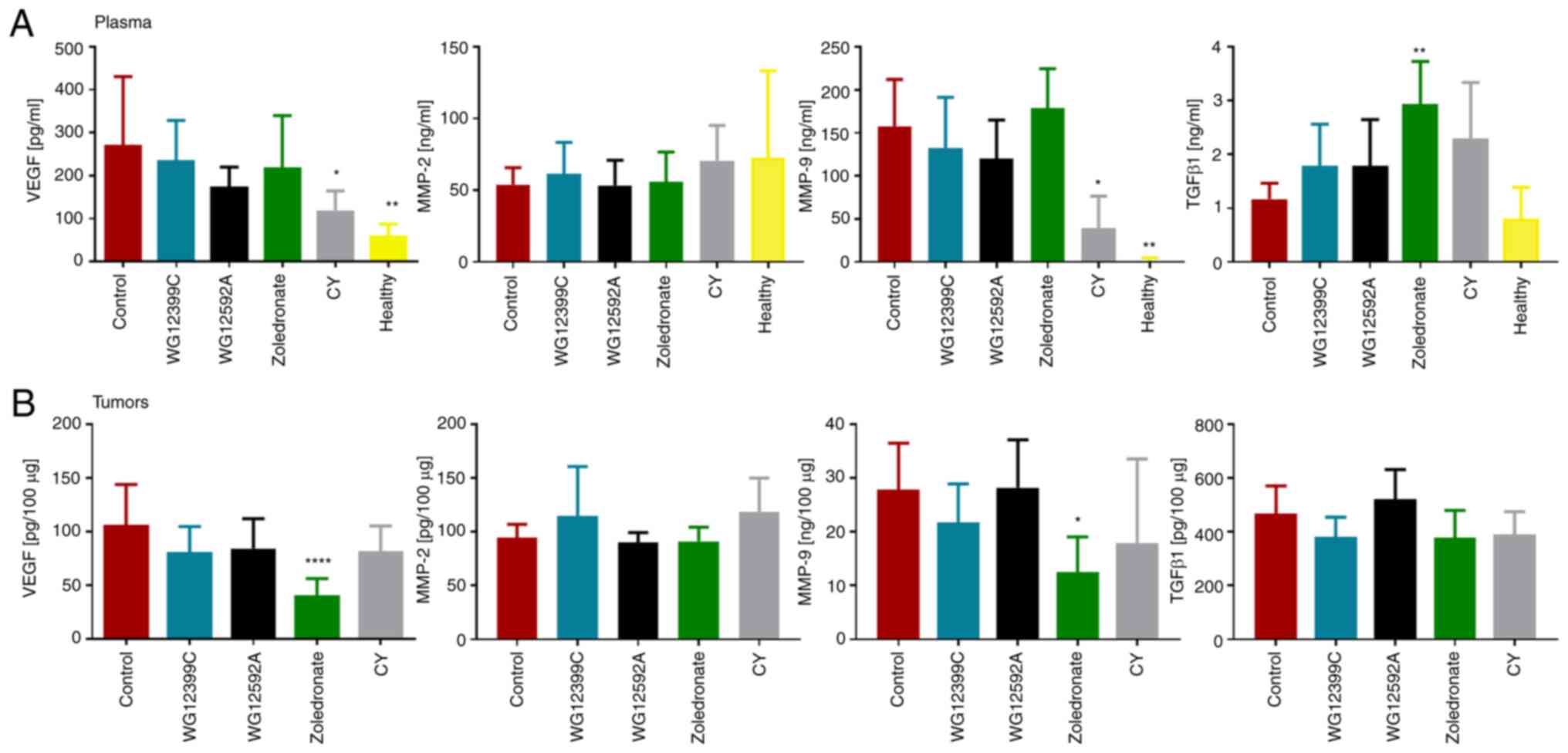 | Figure 6.Expression of proteins involved in
tumor progression in (A) plasma and (B) tumors of mice inoculated
with 4T1 cells. Samples of plasma and tumor tissue were obtained on
day 28 of the experiment. The concentrations of VEGF, MMP-2, MMP-9
and TGF-β1 proteins were assessed using ELISA; n=5-8 samples per
group. Data are presented as mean ± SD. *P<0.05, **P<0.01,
****P<0.0001 vs. control, assessed using one-way ANOVA with
Dunnett's multiple comparison tests (VEGF, TGF-β1, MMP-9 in tumors,
and MMP-2 in plasma) or with Kruskal-Wallis test with Dunn's
multiple comparisons tests (MMP-2 in tumors and MMP-9 in plasma).
CY, cyclophosphamide; VEGF, vascular endothelial growth factor;
MMP, matrix metalloproteinase; TGF, transforming growth factor. |
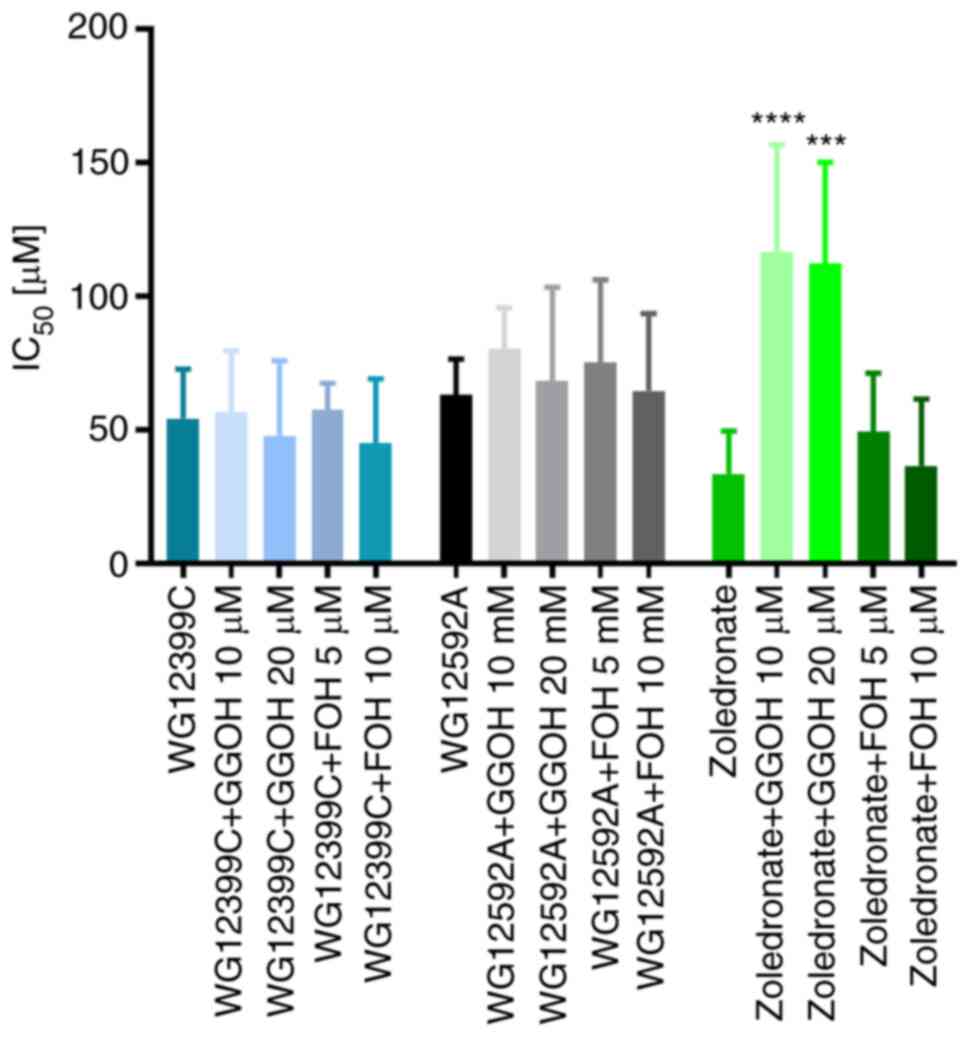
Anti-proliferative activity of
WG12399C and WG12592A alone and in combination with
cytostatics
As previously reported, WG12399C and WG12592A
exhibit antiproliferative activity against HL-60 promyelocytic
leukemia, MCF-7 breast cancer cells and J774E macrophages, which
are the osteoclast surrogates for in vitro studies (15). In the present study, it was observed
that WG12399C and WG12592A potently inhibited the proliferation of
4T1 cells at IC50 values of 54.3 and 63.2 µM,
respectively, in comparison to the IC50 value of 33.5 µM
calculated for zoledronate (Fig.
7). Furthermore, the anti-proliferative effects of WG12399C and
WG12592A against 4T1 cells were not abrogated by GGOH and FOH,
which are intermediate metabolites in the mevalonate pathway with a
proven ability to reverse the effects of amino BPs (30,31).
Contrary to the positive control, zoledronate, the IC50
values of the WG12399C and WG12592A BPs applied alone or following
pre-treatment with GGOH or FOH did not vary significantly. These
results indicate the different mechanisms of action of zoledronate
and tested the BPs.
Further analyses revealed that WG12399C and WG12592A
augmented the anti-proliferative activity of doxorubicin, 5-FU and
paclitaxel (Table II). The cells
were simultaneously treated with the BPs and cytostatics or
pre-treated with a BP or cytostatic 24 h prior to the addition of
the second compound. The most pronounced effects were observed with
the combination of BPs and paclitaxel, irrespective of the
treatment schedule. The CI values for the BP + paclitaxel
combinations ranged from 0.042 to 0.782. A strong synergism was
also recorded for the combination of WG12592A with 5-FU or low
doses of doxorubicin, regardless of the order of treatment of the
cells with these compounds. The effects of WG12399C on the
anti-proliferative activity of 5-FU and doxorubicin was complex,
and was found to be dependent on the concentration of cytostatics
and the treatment schedule. Both synergistic and antagonistic
effects were observed.
 | Table II.CI for combined treatment of WG12399C
and WG12592A and cytostatics against 4T1 cells. |
Table II.
CI for combined treatment of WG12399C
and WG12592A and cytostatics against 4T1 cells.
|
WG12399Ca |
|---|
|
|---|
|
| Doxorubicin
(µg/ml) | 5-Fluorouracil
(µg/ml) | Paclitaxel
(µg/ml) |
|---|
|
|
|
|
|
|---|
|
| 0.001 | 0.01 | 0.1 | 1 | 0.001 | 0.01 | 0.1 | 1 | 0.001 | 0.01 | 0.1 | 1 |
|---|
| WG12399C +
cytostatic simultaneously | 0.724 | 0.702 | 1.489 | 1.170 | 1.129 | 0.655 | 0.681 | 1.955 | 0.782 | 0.486 | 0.268 | 0.532 |
| Pre-treatment with
WG12399C + cytostatic | 1.727 | 0.934 | 1.078 | 1.424 | 2.317 | 0.836 | 0.787 | 1.902 | 1.412 | 0.681 | 0.413 | 0.593 |
| Pre-treatment with
cytostatic + WG12399C | 1.790 | 0.922 | 0.684 | 0.752 | 1.523 | 1.347 | 0.487 | 0.954 | 1.376 | 0.449 | 0.304 | 0.730 |
|
|
WG12592A |
|
|
| Doxorubicin
(µg/ml) | 5-Fluorouracil
(µg/ml) | Paclitaxel
(µg/ml) |
|
|
|
|
|
|
| 0.001 | 0.01 | 0.1 | 1 | 0.001 | 0.01 | 0.1 | 1 | 0.001 | 0.01 | 0.1 | 1 |
|
| WG12592A +
cytostatic simultaneously | 0.275 | 0.675 | 1.287 | 1.604 | 0.627 | 0.345 | 0.375 | 2.301 | 0.220 | 0.092 | 0.126 | 1.130 |
| Pre-treatment with
WG12592A + cytostatic | 0.564 | 0.447 | 0.952 | 1.037 | 0.522 | 0.611 | 0.348 | 1.833 | 0.537 | 0.158 | 0.072 | 0.635 |
| Pre-treatment with
cytostatic + WG12592A | 0.610 | 0.447 | 1.220 | 1.156 | 0.429 | 0.394 | 0.421 | 1.692 | 0.074 | 0.042 | 0.176 | 1.037 |
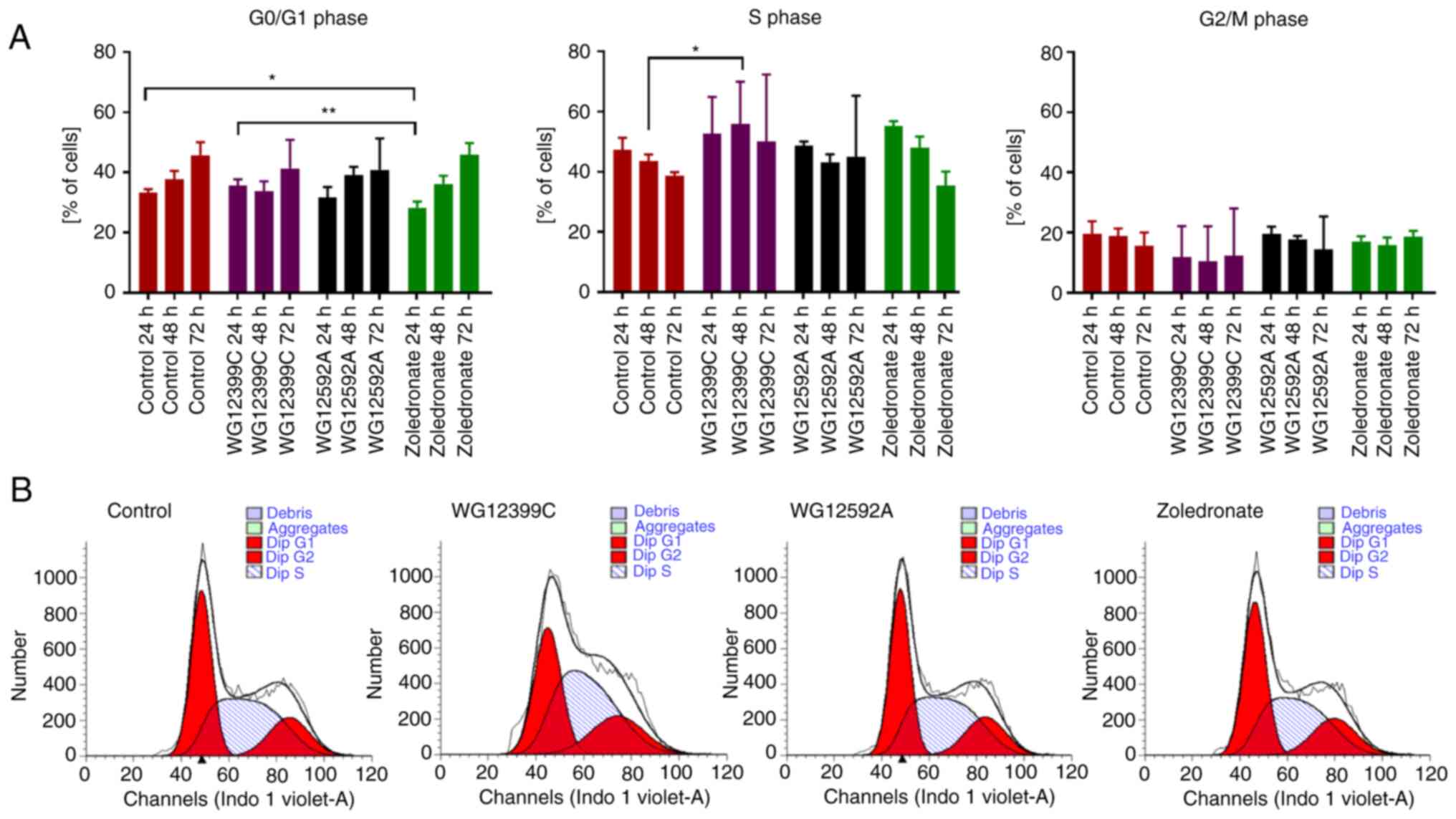
Effect of BPs on cell cycle
progression
To investigate the mechanisms underlying the
anti-proliferative activity of WG12399C and WG12592A against 4T1
cells, the effects of these BPs on the cell cycle progression were
examined. The 4T1 cells were incubated with the compounds at their
IC20 concentrations for 24, 48 and 72 h. WG12592A did
not significantly affect the progression of the cell cycle
(Fig. 8). On the other hand,
zoledronate led to the transitory arrest of cell cycle progression
in the S phase, leading to an increase in the number of cells in
this phase from 47% (control) to 55% after 24 h (although the
differences were not statistically significant), as previously
described (32,33). The most profound effect was observed
in the 4T1 cells treated with WG12399C for 48 h, where the number
of cells in the S phase increased from 44% (control) to 56%
(P=0.0492).
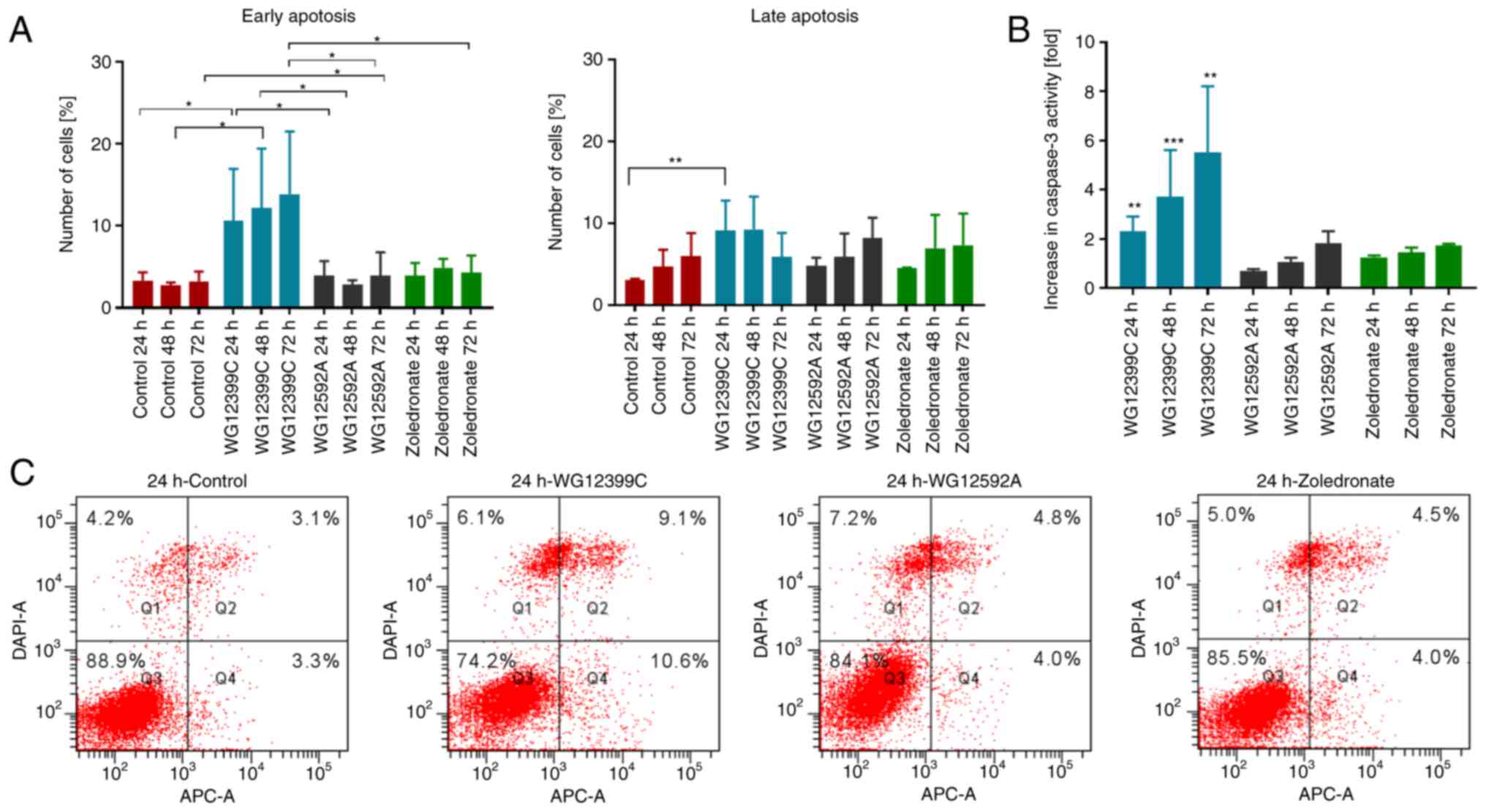
Pro-apoptotic activity of BPs
Since the cytostatic activity may result not only
from the inhibition of the cell cycle, but also from the direct
pro-apoptotic effects of the compounds, the effects of BPs on
apoptosis were investigated. The most substantial pro-apoptotic
activity in the 4T1 cells was observed for WG12399C compared to the
untreated control and the reference agent, zoledronate (Fig. 9). The pro-apoptotic activity of the
WG12399C BP increased in a time-dependent manner. Following
incubation for 24 h, an almost 2-fold increase in the activity of
caspase-3 was noted, while following an additional 48 h, the enzyme
activity increased almost 6-fold compared to the control cells
(Fig. 9B). In the WG12592A-treated
cells, the activity of caspase-3 increased with the time of
incubation; however, the highest increase was approximately 2-fold.
The enhanced activity of caspase-3 was accompanied by an increase
in the percentage of early and late apoptotic BP-treated cells
(Fig. 9A and C). Following 24 and
48 h of incubation with WG12399C, the percentage of Annexin
V-positive cells (early + late apoptosis) increased 3-fold in
comparison to the control. The observed results suggest that the
pro-apoptotic effects may be one of the mechanisms of the direct
antitumor activity of the WG12399C and WG12592A BPs.
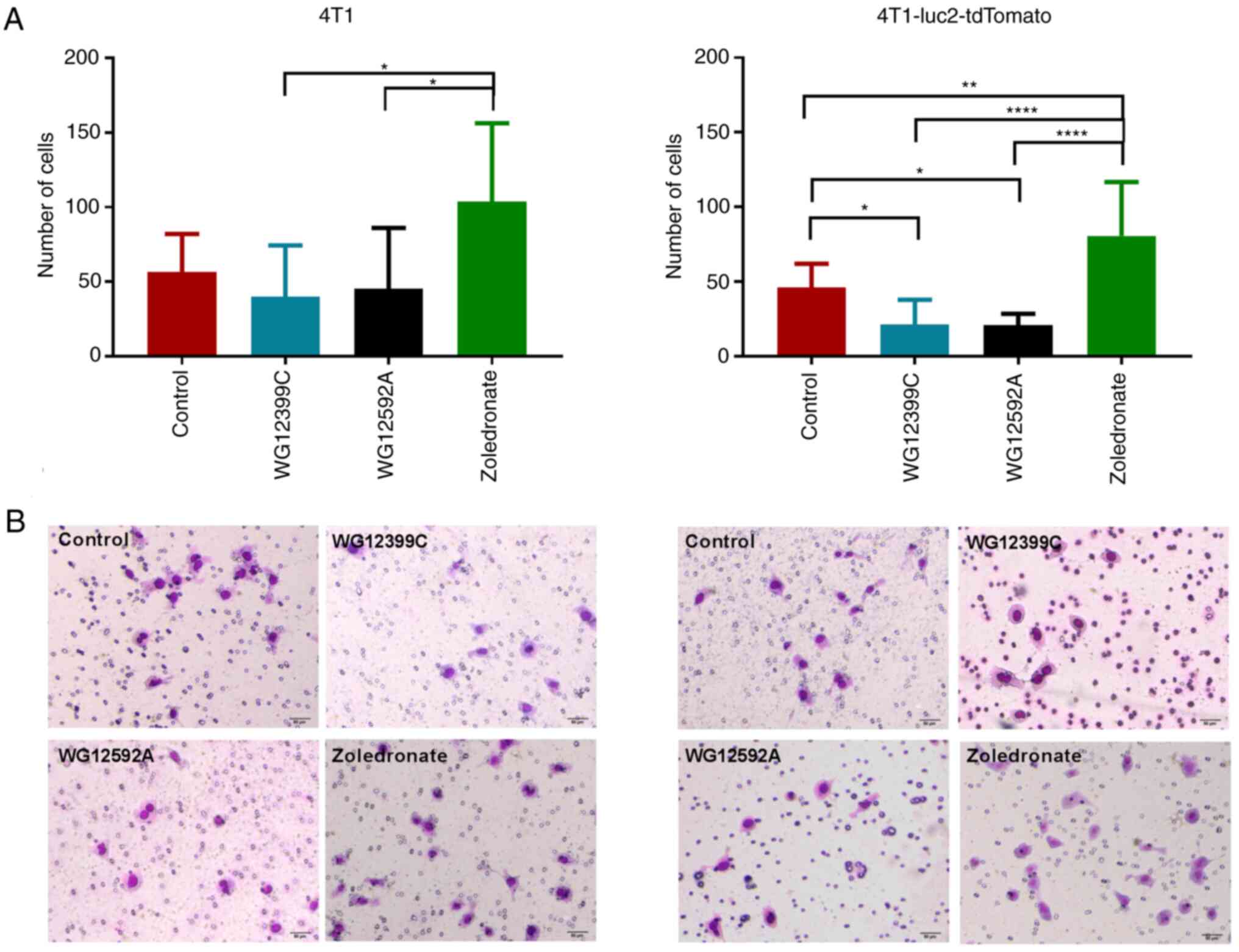
Effects of BPs on the invasiveness of
4T1 cells
The motility of tumor cells is a critical biological
characteristic, as it is directly associated with tumor progression
and metastasis. The migratory properties of cancer cells are
closely related to their invasiveness and enable the cells to leave
the primary tumor and colonize distant organs. Therefore, the
present study examined the effects of WG912399C and WG12592A on the
migratory properties of 4T1 cells and their counterpart
4T1-luc2-tdTomato cells using Matrigel-coated wells. Notably,
zoledronate stimulated the invasion of both 4T1 cell lines through
Matrigel by ~90% (Fig. 10). By
contrast, both WG12399C and WG12592A inhibited the migration of 4T1
cells through porous membranes; however, the effect varied
depending on the cell type and compound. In the case of
4T1-luc-tdTomato cells, both WG12592A and WG12399C inhibited
migration through Matrigel by >50% (P=0.0192 and P=0.0319,
respectively). For the 4T1 cells, the effects were less evident;
however, the effects of WG12399C and WG12592A were still beneficial
compared to those of the reference agent, zoledronate. The
impairment of the invasiveness of 4T1 cells may, at least in part,
explain anti-metastatic effects of the WG12399C and WG12592A
BPs.
Discussion
Previously, the authors developed a novel efficient
method of the synthesis of N-substituted aminomethylidene-BPs
(14). Two compounds,
benzene-1,4-bis[aminomethylidene(bisph osphonic)] acid (WG12399C)
and naphthalene-1,5-bis[aminomethylidene(bisphosphonic)] acid
(WG12592A), exhibited promising antiresorptive and
antiproliferative properties. The present study evaluated the
anticancer activity of WG12399C and WG12592A in murine models of
breast adenocarcinoma. The administered weekly doses (e.g., 12.5
mg/kg for WG12399C and 1 mg/kg for WG12592A) were well-tolerated by
the animals and did not induce any clinical signs of toxicity. The
only exception was an increase in the activity of alkaline
phosphatase and aspartate aminotransferase observed in the groups
receiving higher doses of BPs, which may indicate liver damage.
However, the histopathological analysis revealed only single
lymphocytic infiltrates in some BP-treated mice (Fig. S1).
Much of the clinical and experimental data
concerning the anti-metastatic activity of BPs refers to their
influence on the metastasis to bone tissue. Local high
concentrations of these compounds are achieved in the bone
microenvironment and therefore, bone metastases are most likely to
be positively affected by BPs (36–38).
Clinical studies on the effects of BPs on non-osseous metastases
have yielded conflicting results (34,35).
The overall effect of BPs on tumor growth may be dependent on
several conditions, including the hormone status or the drug doses
and regimen (39,41,42).
To analyze the effects of WG12399C and WG12592A on primary tumor
growth, as well as bone and visceral metastasis, two models of
metastasizing 4T1 mammary breast cancer were applied, an orthotopic
and intracardiac model. Each of these models has unique advantages.
Orthotopically transplanted 4T1 cells form solid primary tumors and
metastasize spontaneously to the lungs and lymph nodes, while
intracardially injected 4T1 cells efficiently colonize the kidneys,
ovaries, brains and bones (40). In
the present study, BPs were administered intravenously at the
following total doses divided into four weekly injections: WG12399C
at 50 mg/kg, WG12592A at 5 mg/kg and zoledronate at 100 µg/kg. A
main limitation of the present study was that single doses of the
BPs were applied. However, the aim was to evaluate the biological
activity of WG12399C and WG12592A in comparison to zoledronate,
which is the most active BP used in clinical practice. Thus, the
maximum well-tolerated doses of WG12399C and WG12592A were applied
in the present study. It was found that neither zoledronate nor the
new aminomethylidene-BPs significantly affected the growth of
4T1-derived tumor cells in BALB/c mice. These findings are
consistent with the results reported by other authors, in that
zoledronate at doses similar to the one applied herein, did not
inhibit the growth of murine and human transplantable breast
cancers. At higher concentrations, zoledronate has been shown to
stimulate the growth of MDA-MB-436 cells (42). Although no inhibitory effects on
primary tumors were observed, WG12399C significantly inhibited the
formation of spontaneous metastatic foci of 4T1 tumors in the lymph
nodes and lungs in comparison to the untreated animals. The number
of macroscopically visible foci in the lungs was significantly
reduced, which was also reflected by decreased lung weights in
comparison to the untreated control animals. Histopathological
analyses reveled a decrease in the number of metastases-positive
animals and a reduction in the metastatic foci size. Furthermore,
pre-treatment and subsequent treatment with WG12399C or WG12592A
efficiently prevented the colonization of lungs, crania and bones
of lower limbs by 4T1 cells. Of note, the effects observed for
these BPs were comparable to those observed for cyclophosphamide
and were much more profound compared to that in zoledronate-treated
animals.
Several mechanisms have been postulated for the
anticancer activity of BPs. One of these is the changes in the
tumor microenvironment, including reduced vascularization. It has
been demonstrated that in zoledronate-treated patients, the plasma
levels of VEGF are significantly decreased and this effect is
long-lasting (43,44). In the present study, elevated
concentrations of plasma VEGF were observed in tumor-bearing
animals in comparison to healthy animals, irrespective of the drug
administered. In contrast to zoledronate, WG12399C and WG12592A did
not reduce the levels of VEGF in tumor tissue. Histopathological
analyses did not reveal any notable decrease in the density of
tumor vasculature as a result of treatment with the BPs (Table SI).
Metastasis is a multistep process that leads to the
formation of secondary tumors in organs distant to the primary
site. One of its crucial steps is the invasion of secondary tissue
by circulating cells. Thus, to identify the possible mechanism
responsible for the anti-metastatic activity of aminomethylidene
BPs, the present study evaluated their effects on the invasive
potential of 4T1 cells. In the present study, 4T1-luc2-tdTomato
cells treated with WG12399C or WG12595A exhibited a markedly
reduced invasiveness through Matrigel by 54%. The effect observed
in parental 4T1 cells was less pronounced. WG12399C inhibited the
invasiveness of 4T1 cells by 29% and WG12592A inhibited this by 20%
in comparison to the untreated cells. Notably, zoledronate
significantly increased the metastatic potential of both cell lines
by 75–83%; however, the mechanism responsible for this effect
cannot be postulated based on the findings of the present
study.
The exact mechanisms underlying the anti-metastatic
activity of WG12399C and WG12592A remain unclear; however, the
pro-apoptotic and anti-proliferative activity of these compounds
may, at least in part, explain the observed effects. It was noted
that WG12399C or WG12595A efficiently inhibited the proliferation
of 4T1 cells, with IC50 values approximately 2-fold
higher than those calculated for zoledronate. It is widely accepted
that N-BPs exert biological effects by inhibiting the mevalonate
pathway (45). The inhibition of
protein prenylation induces the apoptosis of normal and tumor cells
(8,46). The present study confirmed that the
addition of 10 µM GGOH to the culture medium induced a significant
decrease in the anti-proliferative activity of zoledronate in 4T1
cells. By contrast, the anti-proliferative activity of WG12399C and
WG12592A was not affected by the intermediate metabolites of the
mevalonate pathway. This may suggest that the mechanism of action
of these two amino BPs differs from that of classic N-BPs. It was
hypothesized that this difference may be due to the variations in
the structure of the studied BPs and zoledronate. Zoledronic acid,
similar to other clinically applied N-BPs, belongs to the class of
hydroxy BPs, in which the carbon atom of the bisphosphonic group is
connected to the hydroxyl group, while the nitrogen atom is present
in the side chain (47). WG12399C
and WG12592A are aminomethylidene-BPs, in which the carbon atom of
the bisphosphonic group is connected to the nitrogen atom of the
amino group. Furthermore, these BPs contain two bisphosphonic
groups in their structures. The presence of phenyl and naphthyl
rings bound directly to the amino group decreases the basicity and
increases the hydrophobicity of these compounds.
It has been widely documented that N-BPs exert
pro-apoptotic effects on cancer cells by inhibiting the mevalonate
pathway, as mentioned above (9,48,49).
However, the exact mechanisms responsible for the pro-apoptotic
activity remain to be elucidated. Herein, it was observed that
despite the lack of inhibition of the mevalonate pathway, WG12399C
exerted a significant pro-apoptotic effect on 4T1 cells. It
increased the percentage of Annexin V-positive cells by almost
3-fold and the activity of caspase-3 by almost 6-fold in comparison
to the untreated cells.
The results of several in vitro experiments
have revealed that N-BPs can influence the anti-proliferative
activity of various cytostatics, such as cisplatin, taxanes or
etoposide (50–53). For example, Van Beek et al
(54) reported that the combination
of docetaxel, at a concentration minimally affecting tumor growth,
with risedronate led to an almost complete inhibition of tumor
growth and exerted a protective effect on bone integrity. Previous
studies have demonstrated that both the simultaneous and subsequent
treatment of precursors of osteoclasts with cytostatics and
aminomethylidene-BPs results in a synergistic anti-proliferative
effect, which is most pronounced in cells pretreated with BP
(16). In the present study, it was
found that both WG12399C and WG12592A, at low concentrations,
enhanced the effectiveness of cytostatics commonly used as
anticancer agents in breast cancer patients. The most beneficial
synergistic effects were observed for 5-FU and paclitaxel,
irrespective of the treatment regimen. The effects of WG12399C and
WG12592A on the anti-proliferative activity of doxorubicin were
found to be dependent on the concentration of the cytostatic and
the treatment regimen. These results indicate the potential
usefulness of the studied aminomethylidene-BPs in the adjuvant or
combined treatment of cancer malignancies. However, further studies
are required to evaluate the effects of these BPs on the antitumor
activity of drugs in animal models.
The mechanisms regulating cell cycle progression
play a role in controlling the growth and proliferation of cells.
Previous studies have shown that N-BPs inhibit the progression of
the cell cycle in the S phase (33,55);
however, the overall effect of zoledronate on cell cycle
distribution may be dependent on the cell type, proliferation rate,
or the activation of particular signaling pathways. For example,
Wang et al (56) reported
that zoledronic acid significantly induced cell cycle arrest in the
G1 phase of cervical cancer cells-derived CSCs (cancer
stem cells) in a concentration-dependent manner, although this
compound did not affect cell cycle progression in parental cells.
In the present study, it was observed that zoledronate induced the
arrest of the cell cycle of 4T1 cells in the S phase, with a
corresponding decrease in the number of cells in other phases;
however, the changes were not statistically significant. WG12592A
did not disrupt the cell cycle distribution at the concentration
equaling its IC20-30 value. In turn, treatment with
WG12399C resulted in cell accumulation in the
G0G1 phase and a decrease in the percentage
of cells in the G2M phase.
In conclusion, the findings of the present study
provide some support for the adjuvant role of aminomethylidene-BPs
in breast cancer therapy. WG12399C and WG12592A exhibit
anti-metastatic activity in the murine breast cancer model, both in
bone and in the extraosseous environment, and potentiate the
anti-proliferative activity of common anticancer drugs. Further
studies are required however, to elucidate the exact mechanisms
underlying the anti-metastatic effects of WG12399C and WG12592A;
however, it can be postulated that these compounds exert direct
effects on tumor cells through their anti-proliferative and
pro-apoptotic activity, as well as indirect effects by inhibiting
cell motility.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Science Centre
(grant no. 2014/13/B/NZ4/01105).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ANG was involved in the conceptualization of the
study, as well as in data investigation and analysis, funding
acquisition, and in the writing of the original draft. WG was
involved in the conceptualization of the study, as well as in data
investigation, and in the writing, reviewing and editing of the
manuscript. DP was involved in data investigation and analysis. MN
was involved in data investigation and analysis, as well as in the
writing, reviewing and editing of the manuscript. EM was involved
in data investigation, and in the writing, reviewing and editing of
the manuscript. JW was involved in the conceptualization and
supervision of the study, and in the writing, reviewing and editing
of the manuscript. All authors have read and approved the final
manuscript. ANG and WG confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The in vivo procedures were approved by the
first Local Committee for Experiments with the Use of Laboratory
Animals, Wroclaw, Poland (Permission nos. 4/2015 and 80/2015).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BP
|
bisphosphonate
|
|
N-BPs
|
nitrogen-containing
bisphosphonates
|
|
GGOH
|
geranylgeraniol
|
|
FOH
|
farnesol
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
References
|
1
|
Breast cancer. https://www.who.int/news-room/fact-sheets/detail/breast-cancer
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gainford MC, Dranitsaris G and Clemons M:
Recent developments in bisphosphonates for patients with metastatic
breast cancer. BMJ. 330:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Oronzo S, Wood S and Brown JE: The use
of bisphosphonates to treat skeletal complications in solid
tumours. Bone. 147:1159072021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldvaser H and Amir E: Role of
bisphosphonates in breast cancer therapy. Curr Treat Options Oncol.
20:262019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boissier S, Ferreras M, Peyruchaud O,
Magnetto S, Ebetino FH, Colombel M, Delmas P, Delaissé JM and
Clézardin P: Bisphosphonates inhibit breast and prostate carcinoma
cell invasion, an early event in the formation of bone metastases.
Cancer Res. 60:2949–2954. 2000.PubMed/NCBI
|
|
8
|
Senaratne SG, Pirianov G, Mansi JL, Arnett
TR and Colston KW: Bisphosphonates induce apoptosis in human breast
cancer cell lines. Br J Cancer. 82:1459–1468. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buranrat B and Bootha S: Antiproliferative
and antimigratory activities of bisphosphonates in human breast
cancer cell line MCF-7. Oncol Lett. 18:1246–1258. 2019.PubMed/NCBI
|
|
10
|
Misso G, Porru M, Stoppacciaro A,
Castellano M, De Cicco F, Leonetti C, Santini D and Caraglia M:
Evaluation of the in vitro and in vivo antiangiogenic effects of
denosumab and zoledronic acid. Cancer Biol Ther. 13:1491–1500.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka Y, Iwasaki M, Murata-Hirai K,
Matsumoto K, Hayashi K, Okamura H, Sugie T, Minato N, Morita CT and
Toi M: Anti-tumor activity and immunotherapeutic potential of a
bisphosphonate prodrug. Sci Rep. 7:59872017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dhesy-Thind S, Fletcher GG, Blanchette PS,
Clemons MJ, Dillmon MS, Frank ES, Gandhi S, Gupta R, Mates M, Moy
B, et al: Use of adjuvant bisphosphonates and other bone-modifying
agents in breast cancer: A cancer care Ontario and American society
of clinical oncology clinical practice guideline. J Clin Oncol.
35:2062–2081. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cardoso F, Kyriakides S, Ohno S,
Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S and Senkus E;
ESMO Guidelines Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Early breast cancer: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up†. Ann Oncol. 30:1194–1220. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldeman W, Kluczyński A and Soroka M: The
preparation of N-substituted aminomethylidenebisphosphonates and
their tetraalkyl esters via reaction of isonitriles with trialkyl
phosphites and hydrogen chloride. Part 1. Tetrahedron Lett.
53:5290–5292. 2012. View Article : Google Scholar
|
|
15
|
Goldeman W and Nasulewicz-Goldeman A:
Synthesis and antiproliferative activity of aromatic and aliphatic
bis[aminomethylidene(bisphosphonic)] acids. Bioorg Med Chem Lett.
24:3475–3479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nasulewicz-Goldeman A, Goldeman W,
Mrówczyńska E and Wietrzyk J: Biological effects of aromatic
bis[aminomethylidenebis(phosphonic)] acids in osteoclast precursors
in vitro. Chem Biol Drug Des. 94:1835–1848. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nasulewicz-Goldeman A, Goldeman W, Nikodem
A, Nowak M, Papiernik D, Goszczyński TM and Wietrzyk J: Aromatic
bis[aminomethylidenebis(phosphonic)] acids prevent
ovariectomy-induced bone loss and suppress osteoclastogenesis in
mice. Int J Mol Sci. 22:95902021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh SK, Manne N, Ray PC and Pal M:
Synthesis of imidazol-1-yl-acetic acid hydrochloride: A key
intermediate for zoledronic acid. Beilstein J Org Chem. 4:422008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Balathasan L, Beech JS and Muschel RJ:
Ultrasonography-guided intracardiac injection: An improvement for
quantitative brain colonization assays. Am J Pathol. 183:26–34.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hiraga T, Williams PJ, Ueda A, Tamura D
and Yoneda T: Zoledronic acid inhibits visceral metastases in the
4T1/luc mouse breast cancer model. Clin Cancer Res. 10:4559–4567.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blazejczyk A, Switalska M, Chlopicki S,
Marcinek A, Gebicki J, Nowak M, Nasulewicz-Goldeman A and Wietrzyk
J: 1-methylnicotinamide and its structural analog
1,4-dimethylpyridine for the prevention of cancer metastasis. J Exp
Clin Cancer Res. 35:1102016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Skehan P, Storeng R, Scudiero D, Monks A,
McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S and Boyd MR:
New colorimetric cytotoxicity assay for anticancer-drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chou TC and Talalay P: Generalized
equations for the analysis of inhibitions of Michaelis-Menten and
higher-order kinetic systems with two or more mutually exclusive
and nonexclusive inhibitors. Eur J Biochem. 115:207–216. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Features T and Information G: Bioware
ultra cell line NCI-H460-luc2. 2–3. 2010.http://www.caliperls.com/assets/027/8876.pdf
|
|
26
|
Zhang Z, Hu Z, Gupta J, Krimmel JD,
Gerseny HM, Berg AF, Robbins JS, Du H, Prabhakar B and Seth P:
Intravenous administration of adenoviruses targeting transforming
growth factor beta signaling inhibits established bone metastases
in 4T1 mouse mammary tumor model in an immunocompetent syngeneic
host. Cancer Gene Ther. 19:630–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Campbell JP, Merkel AR, Masood-Campbell
SK, Elefteriou F and Sterling JA: Models of bone metastasis. J Vis
Exp. e42602012.PubMed/NCBI
|
|
28
|
Werbeck JL, Thudi NK, Martin CK,
Premanandan C, Yu L, Ostrowksi MC and Rosol TJ: Tumor
microenvironment regulates metastasis and metastasis genes of mouse
MMTV-PymT mammary cancer cells in vivo. Vet Pathol. 51:868–881.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Z, Qutaish M, Han Z, Schur RM, Liu Y,
Wilson DL and Lu ZR: MRI detection of breast cancer micrometastases
with a fibronectin-targeting contrast agent. Nat Commun.
6:79842015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Denoyelle C, Hong L, Vannier JP, Soria J
and Soria C: New insights into the actions of bisphosphonate
zoledronic acid in breast cancer cells by dual RhoA-dependent and
-independent effects. Br J Cancer. 88:1631–1640. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goffinet M, Thoulouzan M, Pradines A,
Lajoie-Mazenc I, Weinbaum C, Faye JC and Séronie-Vivien S:
Zoledronic acid treatment impairs protein geranyl-geranylation for
biological effects in prostatic cells. BMC Cancer. 6:602006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iguchi T, Miyakawa Y, Yamamoto K, Kizaki M
and Ikeda Y: Nitrogen-containing bisphosphonates induce S-phase
cell cycle arrest and apoptosis of myeloma cells by activating MAPK
pathway and inhibiting mevalonate pathway. Cell Signal. 15:719–727.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okamoto S, Jiang Y, Kawamura K, Shingyoji
M, Tada Y, Sekine I, Takiguchi Y, Tatsumi K, Kobayashi H, Shimada
H, et al: Zoledronic acid induces apoptosis and S-phase arrest in
mesothelioma through inhibiting Rab family proteins and
topoisomerase II actions. Cell Death Dis. 5:e15172014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Diel IJ, Solomayer EF, Costa SD, Gollan C,
Goerner R, Wallwiener D, Kaufmann M and Bastert G: Reduction in new
metastases in breast cancer with adjuvant clodronate treatment. N
Engl J Med. 339:357–363. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saarto T, Blomqvist C, Virkkunen P and
Elomaa I: Adjuvant clodronate treatment does not reduce the
frequency of skeletal metastases in node-positive breast cancer
patients: 5-Year results of a randomized controlled trial. J Clin
Oncol. 19:10–17. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kohno N, Aogi K, Minami H, Nakamura S,
Asaga T, Iino Y, Watanabe T, Goessl C, Ohashi Y and Takashima S:
Zoledronic acid significantly reduces skeletal complications
compared with placebo in Japanese women with bone metastases from
breast cancer: A randomized, placebo-controlled trial. J Clin
Oncol. 23:3314–3321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lawson MA, Xia Z, Barnett BL, Triffitt JT,
Phipps RJ, Dunford JE, Locklin RM, Ebetino FH and Russell RG:
Differences between bisphosphonates in binding affinities for
hydroxyapatite. J Biomed Mater Res B Appl Biomater. 92:149–155.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hortobagyi GN, Theriault RL, Porter L,
Blayney D, Lipton A, Sinoff C, Wheeler H, Simeone JF, Seaman J and
Knight RD: Efficacy of pamidronate in reducing skeletal
complications in patients with breast cancer and lytic bone
metastases. Protocol 19 aredia breast cancer study group. N Engl J
Med. 335:1785–1792. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Steinman RA, Brufsky AM and Oesterreich S:
Zoledronic acid effectiveness against breast cancer metastases-a
role for estrogen in the microenvironment? Breast Cancer Res.
14:2132012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Farhoodi HP, Segaliny AI, Wagoner ZW,
Cheng JL, Liu L and Zhao W: Optimization of a syngeneic murine
model of bone metastasis. J Bone Oncol. 23:1002982020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Michigami T, Hiraga T, Williams PJ,
Niewolna M, Nishimura R, Mundy GR and Yoneda T: The effect of the
bisphosphonate ibandronate on breast cancer metastasis to visceral
organs. Breast Cancer Res Treat. 75:249–258. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ottewell PD, Mönkkönen H, Jones M, Lefley
DV, Coleman RE and Holen I: Antitumor effects of doxorubicin
followed by zoledronic acid in a mouse model of breast cancer. J
Natl Cancer Inst. 100:1167–1178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Santini D, Vincenzi B, Dicuonzo G,
Avvisati G, Massacesi C, Battistoni F, Gavasci M, Rocci L,
Tirindelli MC, Altomare V, et al: Zoledronic acid induces
significant and long-lasting modifications of circulating
angiogenic factors in cancer patients. Clin Cancer Res.
9:2893–2897. 2003.PubMed/NCBI
|
|
44
|
Bellone F, Catalano A, Sottile AR, Gaudio
A, Loddo S, Corica F and Morabito N: Early Changes of VEGF levels
after zoledronic acid in women with postmenopausal osteoporosis: A
potential role of vitamin D. Front Med (Lausanne). 8:7484382021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Luckman SP, Hughes DE, Coxon FP, Graham R,
Russell G and Rogers MJ: Nitrogen-containing bisphosphonates
inhibit the mevalonate pathway and prevent post-translational
prenylation of GTP-binding proteins, including ras. J Bone Miner
Res. 13:581–589. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Benford HL, Frith JC, Auriola S, Mönkkönen
J and Rogers MJ: Farnesol and geranylgeraniol prevent activation of
caspases by aminobisphosphonates: Biochemical evidence for two
distinct pharmacological classes of bisphosphonate drugs. Mol
Pharmacol. 56:131–140. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Russell RGG, Watts NB, Ebetino FH and
Rogers MJ: Mechanisms of action of bisphosphonates: Similarities
and differences and their potential influence on clinical efficacy.
Osteoporos Int. 19:733–759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Suyama K, Noguchi Y, Tanaka T, Yoshida T,
Shibata T, Saito Y and Tatsuno I: Isoprenoid-independent pathway is
involved in apoptosis induced by risedronate, a bisphosphonate, in
which Bim plays a critical role in breast cancer cell line MCF-7.
Oncol Rep. 18:1291–1298. 2007.PubMed/NCBI
|
|
49
|
Miwa A, Takezako N, Hayakawa H, Hayakawa
M, Tominaga S and Yanagisawa K: YM-175 induces apoptosis of human
native monocyte-lineage cells via inhibition of prenylation. Am J
Hematol. 87:1084–1088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Benassi MS, Chiechi A, Ponticelli F,
Pazzaglia L, Gamberi G, Zanella L, Manara MC, Perego P, Ferrari S
and Picci P: Growth inhibition and sensitization to cisplatin by
zoledronic acid in osteosarcoma cells. Cancer Lett. 250:194–205.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Matsumoto S, Kimura S, Segawa H, Kuroda J,
Yuasa T, Sato K, Nogawa M, Tanaka F, Maekawa T and Wada H: Efficacy
of the third-generation bisphosphonate, zoledronic acid alone and
combined with anti-cancer agents against small cell lung cancer
cell lines. Lung Cancer. 47:31–39. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Koto K, Murata H, Kimura S, Horie N,
Matsui T, Nishigaki Y, Ryu K, Sakabe T, Itoi M, Ashihara E, et al:
Zoledronic acid inhibits proliferation of human fibrosarcoma cells
with induction of apoptosis, and shows combined effects with other
anticancer agents. Oncol Rep. 24:233–239. 2010.PubMed/NCBI
|
|
53
|
Ottewell PD, Deux B, Mönkkb̈nen H, Cross
S, Coleman RE, Clezardin P and Holen I: Differential effect of
doxorubicin and zoledronic acid on intraosseous versus extraosseous
breast tumor growth in vivo. Clin Cancer Res. 14:4658–4666. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Van Beek ER, Lowik CWGM, Van Wijngaarden
J, Ebetino FH and Papapoulos SE: Synergistic effect of
bisphosphonate and docetaxel on the growth of bone metastasis in an
animal model of established metastatic bone disease. Breast Cancer
Res Treat. 118:307–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhao Z, Shen W, Zhu H, Lin L, Jiang G, Zhu
Y, Song H and Wu L: Zoledronate inhibits fibroblasts' proliferation
and activation via targeting TGF-β signaling pathway. Drug Des
Devel Ther. 12:3021–3031. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang L, Liu Y, Zhou Y, Wang J, Tu L, Sun
Z, Wang X and Luo F: Zoledronic acid inhibits the growth of cancer
stem cell derived from cervical cancer cell by attenuating their
stemness phenotype and inducing apoptosis and cell cycle arrest
through the Erk1/2 and Akt pathways. J Exp Clin Cancer Res.
38:932019. View Article : Google Scholar : PubMed/NCBI
|