Introduction
In 2018, head and neck cancers accounted for 8% of
all cancers (~1.45 million individuals) and 5% of all
cancer-related deaths (~500,000 individuals) according to analysis
of 39 cancer types in 185 countries, and the number of cases is
increasing annually (1). The main
type of head and neck cancer is squamous cell carcinoma, and the
standard treatments consist of chemotherapy, radiotherapy and
surgery. For chemotherapy, cisplatin is a key drug, either as a
single agent or in combination with other drugs (2,3).
The standard dose of cisplatin as a single agent is
80–100 mg/m2, but the response rate is only 25–50%
(3,4). In addition, cisplatin should not be
administered at the standard dose to patients with renal
dysfunction (5). Thus, there is
still no standard treatment for head and neck squamous cell
carcinoma (HNSCC) in patients with renal dysfunction, and thus it
is necessary to develop new treatments. Candidate therapies include
antimalarial drugs such as artemisinin, a type of Chinese herbal
medicine extracted from the wormwood plant [Artemisia annua
(A. annua)]. Artemisinin is considered to be less toxic than
other antimalarial drugs, and artesunate, a derivative of
artemisinin with enhanced effects, is currently used for the
treatment of malaria (6).
Previously, artemisinin derivatives have been studied for their
application to various human diseases (7). For example, Sun et al (8) reported that artemisinin and its
derivatives exert antitumor effects on mouse leukemia cell lines
and human hepatoma cell lines. Since then, the antitumor effects of
artemisinin and its derivatives have been examined on various
tumors (9,10). Previously, it has been reported that
artesunate has antitumor effects (11). Although the combined effect of
artesunate with other chemotherapy agents has been demonstrated in
glioma and liver cancer (12,13),
its antitumor effect in head and neck cancer has been reported only
by Roh et al (14). This
antitumor activity is reported to exert anti-proliferative effects
through the following mechanisms: i) By inducing G1-phase arrest
via upregulation of p16 protein expression and downregulation of
CDK4 and cyclin D1 expression, as well as downregulation of ERK1/2
in GBC-SD and NOZ gallbladder cancer cell lines (15); ii) by inducing cell apoptosis and
inhibiting cell proliferation in human acute promyelocyte leukemia
HL-60 cells and acute myeloid leukemia KG1a cells via suppression
of the MEK/ERK and PI3K/Akt pathways (16); iii) by reducing angiogenesis in the
highly angiogenic KS-IMM cell line derived from Kaposi's sarcoma
(17) and iv) by enhancing the
radiosensitivity of U373MG cells through increased ROS generation
(18). It has also been reported
that artesunate affects various signal transduction pathways
(including the Wnt/catenin pathway in RAW 264.7 murine macrophages,
HT-29 colorectal cancer and A431 epidermoid cancer; the AMPK
pathway in SHSY5Y neuroblastoma; metastatic pathways in RAW 264.7
macrophages, in H1395, A549, LXF289, H460, Calu3, and H1299 lung
cancer, and in CaSki and HeLa cervical cancer) and signal
transducers (including NF-κB in HT-1080 fibrosarcoma; MYC/MAX in 39
human cell lines derived from the colon, non-small cell lung
cancer, and ovarian cancer; AP-1 in H1299 cells lung cancer; CREBP
in RAW 264.7 macrophages; mTOR in Rh30 and RD rhabdomyosarcoma and
in SHSY5Y neuroblastoma) (19–21).
However, the details remain unclear (20–22).
In the present study, the antitumor effects of artesunate combined
with cisplatin and iron in HNSCC cell lines were confirmed. Certain
of the effects of this combined treatment on the expression of
molecules related to cell proliferation were also clarified.
Materials and methods
Cell lines
In the present study, the HNSCC-derived UM-SCC-23
and UM-SCC-81B cell lines (a kind gift from Dr Thomas E. Carey,
University of Michigan, Ann Arbor, MI) were used (22). The cells were maintained in
RPMI-1640 medium containing 1% Pen-Strep solution (10,000 U/ml
penicillin and 10,000 µg/ml streptomycin; Thermo Fisher Scientific,
Inc.) and 10% fetal bovine serum (HyClone; Cytiva) at 37°C in an
atmosphere containing 5% CO2 under humidified
conditions.
Cell viability assay
The drugs used were artemisinin (CAS RN®:
63968-64-9; cat. no. A2118; Tokyo Chemical Industry Co., Ltd.),
deoxyartemisinin (CAS RN®: 72826-63-2; cat. no. D232150;
Toronto Research Chemicals), dihydroartemisinin (CAS
RN®: 81496-82-4, cat. no. D3793; Tokyo Chemical Industry
Co., Ltd.), artesunate (CAS RN®: 88495-63-0, cat. no.
A2191; Tokyo Chemical Industry Co., Ltd.), and cisplatin (Randa
Injection; Nippon Kayaku Co., Ltd.). Artemisinin and its
derivatives are poorly soluble in water; thus they were dissolved
in dimethyl sulfoxide (cat. no. D8418; Sigma-Aldrich; Merck
KGaA).
Cell viability was measured with a WST-1 assay (Cell
Counting Kit-8; Dojindo Laboratories, Inc.) after incubating each
cell line with artemisinin and its derivatives (0.2–200 µM),
cisplatin (0.6–4 µg/ml) and iron (0.0008-0.1 mM). UM-SCC-23 and
UM-SCC-81B cells were seeded at 1.7×103 and
1.0×103 cells/well, respectively, in 96-well flat-bottom
plates (cat. no. 353072; Corning, Inc.). The following day, each
drug was added to each well and the cells were cultured further. At
72 h after drug administration, 10 µl of the WST-1 reagent was
added and the cells were incubated for another 3 h. The color that
developed after agitation was measured with a microplate reader
(iMark; Bio-Rad Laboratories, Inc.) at the main wavelength of 450
nm and the secondary wavelength of 650 nm to measure cell viability
(23). The following formula was
used to calculate the percentage of survival: cell viability
(%)=[(As-Ab)/(Ac-Ab)] ×100 [As, absorbance of the sample (wells
containing cells, test substance, and WST-1 solution); Ac,
absorbance of negative control (wells containing cells and WST-1
solution without test substance); Ab, blank absorbance (no cells,
wells with medium, and WST-1 solution)].
Cell cycle and apoptosis analyses by
flow cytometry
For cell cycle analysis, UM-SCC-23 and UM-SCC-81B
cells were seeded at 0.5×105 and 0.3×105
cells/well, respectively, on six-well plates (353046; Corning,
Inc.). The next day, artesunate (3.125 or 12.5 µM), cisplatin (0.25
or 0.5 µg/ml), and/or ferric nitrate (0.02 mM) was added to the
medium, and the cells were collected and analyzed after incubation
for 96 or 72 h. For propidium iodide (PI) staining, the collected
cells were fixed in 70% ethanol overnight at 4°C, stained with a
solution consisting of 0.1% Nonidet P40 (cat. no. 56009; BDH
Laboratory Supplies), 50 µg/ml ribonuclease (cat. no. R5125), and
50 µg/ml PI (cat. no. P4170; both from Sigma-Aldrich; Merck KGaA)
for 30 min at room temperature, and measured by flow cytometry
(LSRFortessaX-20; Becton-Dickinson and Company) (23).
For the analysis of apoptosis, UM-SCC-23 and
UM-SCC-81B cells were seeded at 1.0×105 and
0.5×105 cells/well, respectively, in six-well plates,
and artesunate (37.5 or 46 µM), cisplatin (1.0 or 1.25 µg/ml), and
ferric nitrate (0.01 mM) were added to the medium after 24 h. After
incubation for 48 or 72 h, the cells were collected and stained
with a MEBCYTO-Apoptosis Kit (Annexin V-FITC Kit; 4700; Medical
& Biological Laboratories Co., Ltd.) for 15 min at room
temperature and measured by flow cytometry (23). The cell cycle and apoptosis were
analyzed using a FlowJoV9 (Becton-Dickinson and Company).
Western blot analysis
UM-SCC-23 and UM-SCC-81B cells were seeded at
0.5×105 cells/well on six-well plates and incubated for
24 h. After incubation, artesunate and cisplatin were added to the
medium at a final concentration of 9.375 or 46 µM and 0.75 or 1.0
µg/ml, respectively. At 72 h after the addition of the drugs, the
cells were lysed with Tris-BES sample buffer (Tris-HCl pH 8.4 200
mM, glycerol 12%, SDS 2%, BPB 0.005%) and cell lysates were
collected. The collected samples (UM-SCC-23, 1.7×103
cells/lane; UM-SCC-81B, 1.75×104 cells/lane) were
electrophoresed on a 4–12% Bis-Tris gel (Invitrogen NuPAGE;
NP0322BOX; Thermo Fisher Scientific, Inc.) and the separated
proteins were transferred to a PVDF membrane (Immobilon-P; RPN2232;
EMD Millipore). After blocking with EveryBlot Blocking Buffer (cat.
no. 12010020; Bio-Rad Laboratories, Inc.) for 30 min at room
temperature, the membrane was incubated overnight at 4°C with
primary antibodies against anti-phosphorylated (p-)-retinoblastoma
protein (Rb) (Ser780) (1:500; cat. no. 9307S; Cell Signaling
Technology, Inc.), Rb (1:1,000; cat. no. 9309; Cell Signaling
Technology, Inc.), CDK2 (1:200; cat. no. 2546; Cell Signaling
Technology, Inc.), CDK4 (1:200; cat. no. 2906; Cell Signaling
Technology, Inc.), CDK6 (1:200; cat. no. sc-7961; Santa Cruz
Biotechnology, Inc.), cyclin B1 (1:500; cat. no. 4138; Cell
Signaling Technology, Inc.), cyclin D1 (1:500; cat. no. 26939-1-AP;
Proteintech Group, Inc.), cyclin E (1:200; cat. no. 11554-1-AP;
Proteintech Group, Inc.) and β-actin (1:1,000; cat. no. M177-3;
Medical & Biological Laboratories Co., Ltd.). The membranes
were then incubated with ImmPRESS-HRP reagent anti-rabbit IgG
(1:1,000; cat. no. MP-7401; Vector Laboratories, Inc.) or
ImmPRESS-HRP reagent anti-mouse IgG (1:1,000; cat. no. MP7402;
Vector Laboratories, Inc.) as secondary antibodies for 1 h at room
temperature. Signals were visualized with a chromogenic reagent
(Clarity Western ECL Substrate, cat. no. 1705060 or Clarity MAX
Western ECL Substrate, cat. no. 1705062; Bio-Rad Laboratories,
Inc.), and the chromogenic bands were detected using an Amersham
Imager 600 (Cytiva) (23). The
concentrations of bands detected by western blotting were measured
and compared using ImageJ 1.52a software (National Institutes of
Health). All protein concentration comparisons were normalized
according to the concentration of β-actin.
Transfection of HNSCC cell lines with
small interfering RNAs (siRNAs) targeting Rb into and their effect
on cell proliferation and the cell cycle
To suppress Rb protein expression, validated
Invitrogen Silencer™ Select Pre-Designed siRNAs (s523: 4390824;
sense: ACGGATAGCAAAACTAGA, antisense: TCTAGTTTTGCTATCCGT) were
used, and Invitrogen Silencer™ Select Negative Control No. 1 siRNA
(4390843; the sequences have not been published by Invitrogen) was
used as a control. Lipofectamine™ RNAiMAX Transfection Reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) was utilized for
transfection of siRNA into cells. In practice, UM-SCC-23 and
UM-SCC-81B cells were seeded at 0.5×105 and
0.3×105 cells/well, respectively, in six-well
flat-bottom plates (cat. no. 353046; Corning, Inc.). A total of 3
days later, the cells in each well were transfected at 37°C for 48
h with siRNA (25 pmol/ml for WST-1 assays, 10 pmol/ml for cell
cycle and apoptosis analyses, and western blot analysis) according
to the manufacturer's protocol.
To confirm the suppression of Rb expression by
siRNA, the cells were collected at 24 and 48 h after transfection
and western blotting was performed. To examine the effect of Rb
suppression on cell proliferation, the cells were collected at 24 h
after siRNA transfection and UM-SCC-23 and UM-SCC-81B cells were
seeded at 1.7×103 and 1.0×103 cells/well,
respectively, in 96-well flat-bottom plates (cat. no. 353072;
Corning, Inc.). Cell numbers were measured using a WST-1 assay
after 24, 48 and 72 h, and the proliferative potential of cells in
the presence and absence of Rb repression was compared. To examine
the effect of Rb repression on the cell cycle, the cells were
collected at 24 and 48 h after siRNA transfection and analyzed by
flow cytometry after DNA staining. The distribution of cells in
each phase of the cell cycle was analyzed using the Watson model in
FlowJo v10 (FlowJo LLC).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) analysis
RT-qPCR was performed to confirm the level Rb-1 mRNA
of expression. UM-SCC-23 and UM-SCC-81B cells were seeded
separately in six-well plates (cat. no. 353046; Corning, Inc.) at
1.0×105 cells/well. The next day, artesunate (9.375 or
46 µM) was added to the medium. Total RNA was extracted and
purified using NucleoSpin RNA Kit (Takara Bio, Inc.) following the
manufacturer's instructions after incubation for 48 h. cDNA was
synthesized using High Capacity cDNA Reverse Transcription Kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. qPCR was performed using Taqman Gene
Expression Assay (Rb-1, Hs01078066_m1; cat. no. 4331182; and GAPDH,
Hs04420632; cat. no. 4448489 as control; both from Applied
Biosystems; Thermo Fisher Scientific, Inc.; the sequences were not
available by the company), TaqMan Gene Expression Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.), and ABI 7900
HT Fast Real-Time PCR System (Thermo Fisher Scientific, Inc.).
Thermocycling conditions included DNA polymerase activation at 50°C
for 2 min and at 95°C for 2 min followed by 40 cycles of
denaturation at 95°C for 15 sec, annealing and extension at 60°C
for 1 min. The results were calculated using the 2−ΔΔCq
method (24).
Statistical analysis
The significance of the results for each
experimental group was analyzed by one-way analysis of variance
using JMP Ver. 13.2.1 (SAS Institute, Inc.). In addition, the
Tukey-Kramer honestly significant difference test was used to
compare and confirm the significance of the results for each
experimental group. The results were considered to indicate a
statistically significant difference when P<0.05 was
obtained.
Results
Artesunate inhibits the growth of
HNSCC cell lines
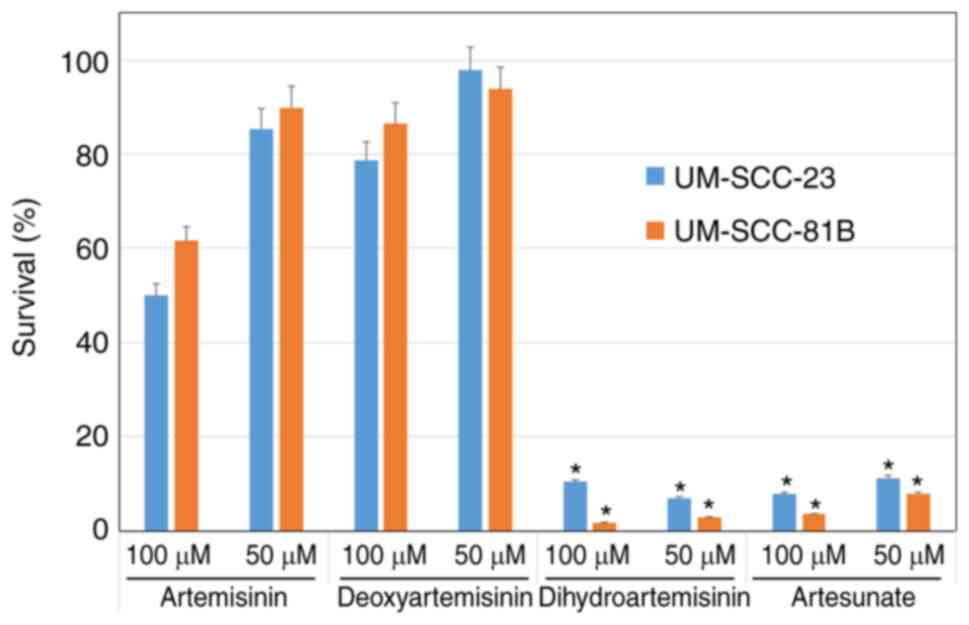
A total of four artemisinin compounds (artemisinin,
deoxyartemisinin, dihydroartemisinin and artesunate) were tested
for their growth inhibitory effects on HNSCC cell lines (UM-SCC-23
and UM-SCC-81B). In both cell lines, artesunate and
dihydroartemisinin inhibited cell proliferation to ~90% of that of
untreated cells at 50 and 100 µM. However, artemisinin and
deoxyartemisinin were less effective at inhibiting cell
proliferation (Fig. 1). Therefore,
it was decided to use artesunate, which is already used clinically
as an antimalarial drug and has inhibitory effects on cell
proliferation, in the following experiments.
Artesunate enhances the growth
inhibitory effect of cisplatin on HNSCC cell lines
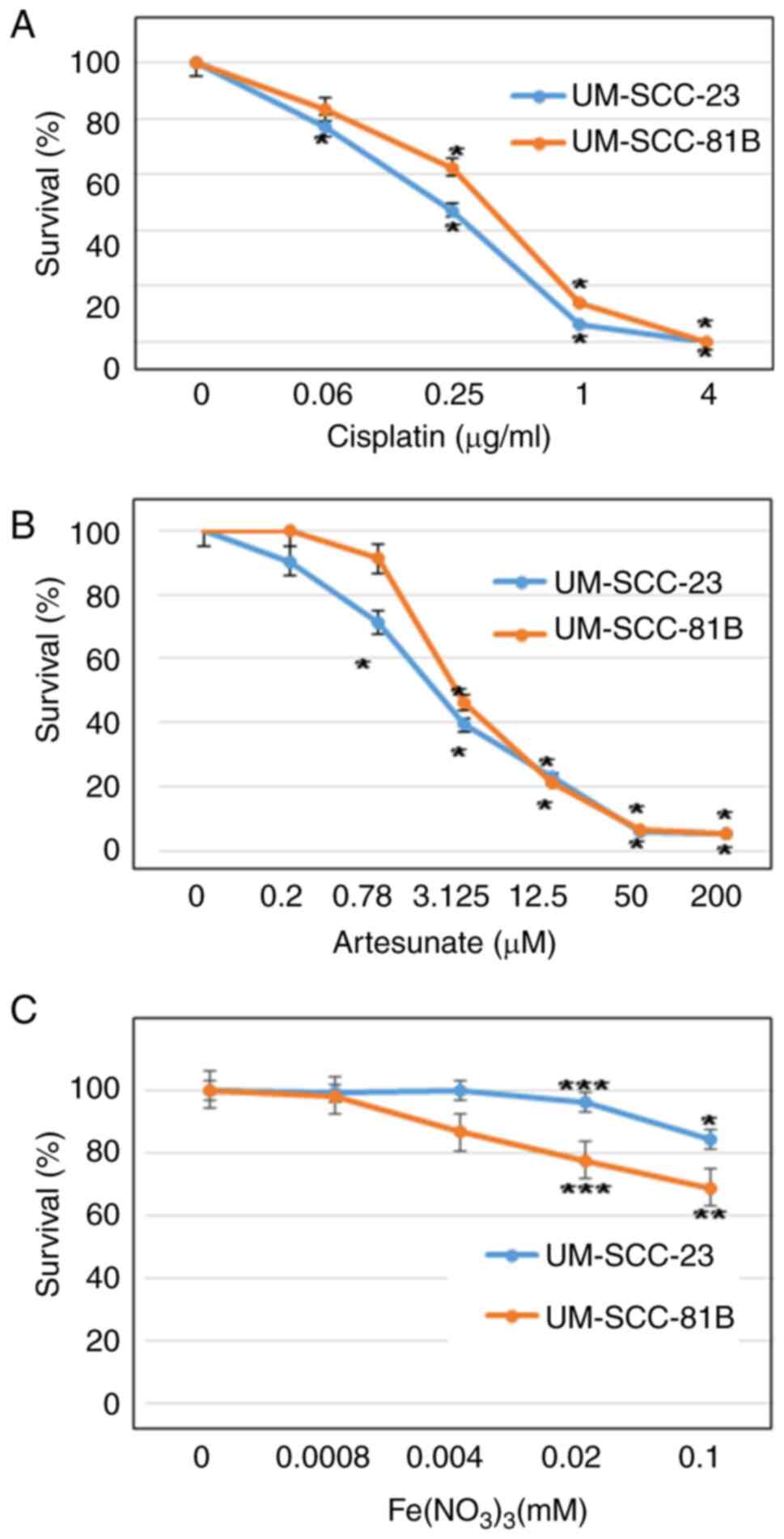
Prior to the examination of the combined effect of
cisplatin and artesunate, the individual effects of cisplatin,
artesunate and iron on HNSCC cell lines were assessed. Cisplatin
exhibited a concentration-dependent inhibition of cell
proliferation in UM-SCC-23 and UM-SCC-81B cells, with half maximal
inhibitory concentration (IC50) values of 0.23 and 0.44
µg/ml, respectively (Fig. 2A). In
the case of artesunate, a concentration-dependent inhibitory effect
on the proliferation of UM-SCC-23 and UM-SCC-81B cells was
revealed, with IC50 values of 2.27 and 2.92 µM,
respectively (Fig. 2B). As for
iron, it had no significant inhibitory effect on growth in either
of the cell lines at the concentrations (0.8 µM-0.1 mM) examined
(Fig. 2C).
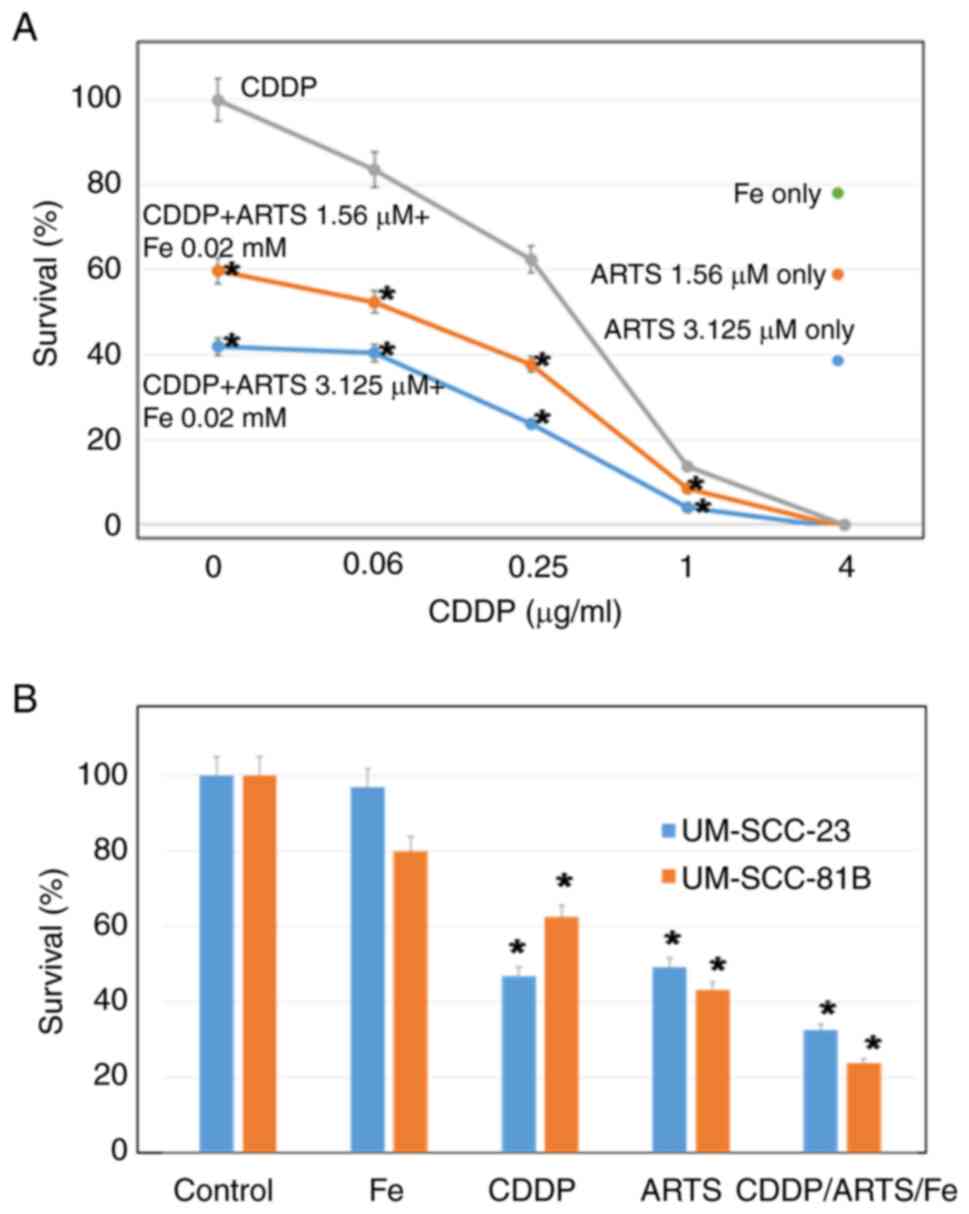
The results of the combined effect of cisplatin,
artesunate and iron in UM-SCC-81B cells were explored (Fig. 3A). The inhibitory effect on growth
of this combination was higher than that of either drug alone. The
lower the concentration of cisplatin, the higher was the combined
effect. This effect was similar for UM-SCC-23 cells (data not
shown). In UM-SCC-23 and UM-SCC-81B cells, the antitumor effects of
cisplatin and artesunate were examined using IC50
approximations of 0.25 µg/ml and 3.1 µM, respectively;
Fe(NO3)3 was examined at 0.02 mM, which had
little effect on its own. In both cell lines, the combination of
the drugs showed a higher antitumor effect (P<0.001) compared
with treatment with each drug alone (Fig. 3B).
Cell cycle analysis
The effects of artesunate, cisplatin and ferric
nitrate alone and in combination on the cell cycle in UM-SCC-23 and
UM-SCC-81B cell lines were investigated. The results for UM-SCC-23
cells are demonstrated in Fig.
4.
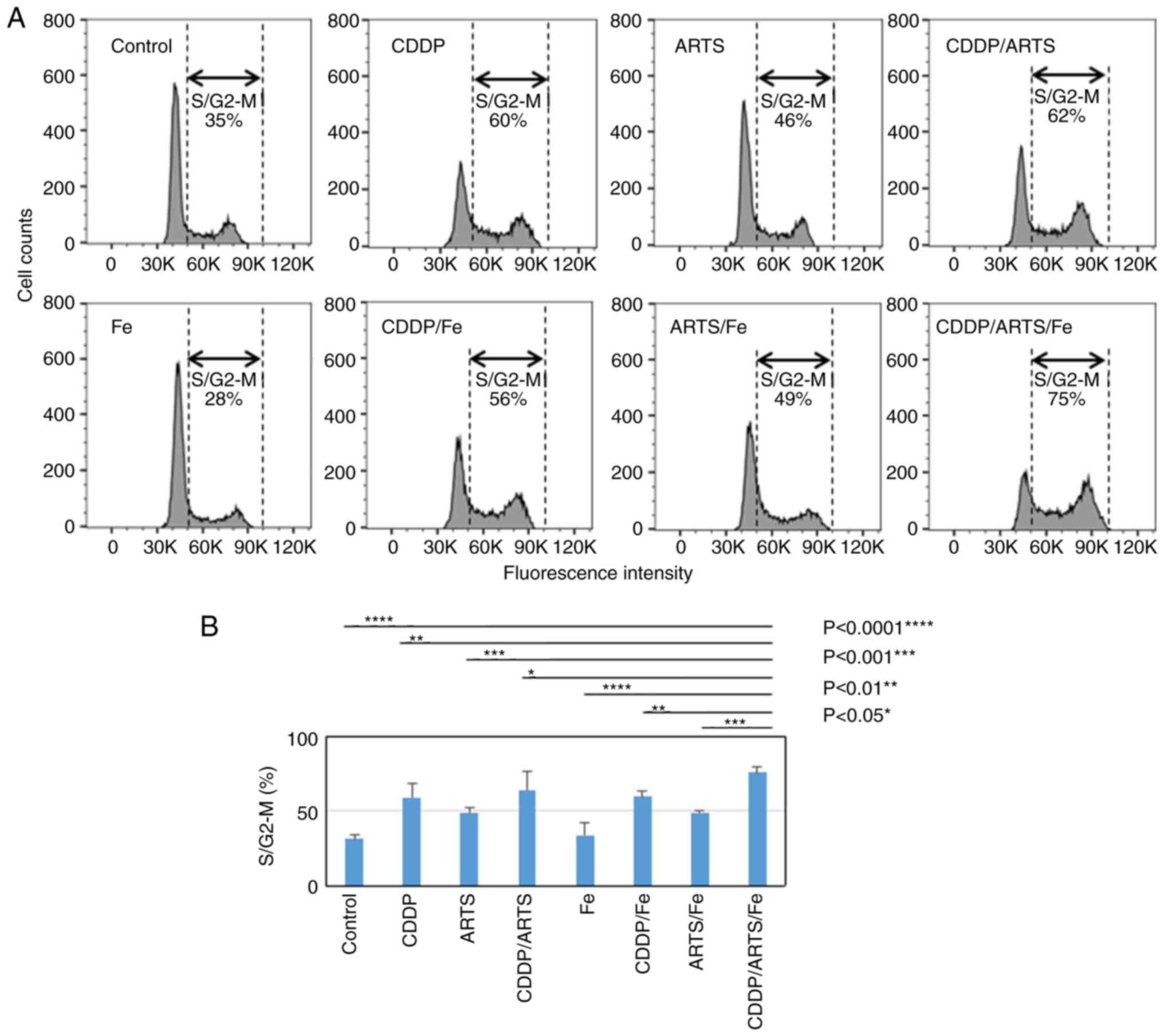 | Figure 4.ARTS induces the accumulation of
HNSCC cells in the S/G2 M phase, which was further enhanced by the
combination of ARTS, Fe and CDDP. (A) HNSCC cells (UM-SCC-23) were
treated with and without a combination of ARTS (3.1 µM), Fe (0.02
mM) and CDDP (0.3 µg/ml) for 72 h, and cell cycle distribution was
examined by flow cytometry. The experiments were performed
independently three times and the figure shows a representative
experiment. (B) Data in the columns are presented as the mean ±
standard deviation of three independent experiments. The results of
statistical analysis are depicted for S/G2-M. *P<0.05,
**P<0.01, ***P<0.001 and ****P<0.0001. ARTS, artesunate;
HNSCC, head and neck squamous cell carcinoma; CDDP, cisplatin; Fe,
Fe(NO3)3. |
Although iron alone had no effect on the cell cycle
at the concentrations used, the population of treated cells
distributed in the S/G2-M phase increased from 35 to 60 and 46%,
respectively, in the groups treated with cisplatin and artesunate
alone compared with the control. In addition, a greater
accumulation of cells in the S/G2-M phase (S/G2-M arrest) was
observed with the combination of cisplatin and artesunate (62%) and
all three drugs (75%) (P<0.05 to P<0.0001) (Fig. 4A and B). A similar trend was
observed for UM-SCC-81B cells (data not shown).
Artesunate enhances the
apoptosis-inducing effect of cisplatin
The ability of artesunate and the combination of
artesunate and cisplatin to induce apoptosis in the UM-SCC-23 and
UM-SCC-81B cell lines was investigated. As demonstrated in Fig. 5, in UM-SCC-23 cells (UM-SCC-81B data
not shown), after 48 h of drug treatment, apoptosis, as detected
with positive annexin V staining and negative PI staining, was
induced in 2, 11, 5, 4 and 9% of the cells in the control,
cisplatin, artesunate, iron alone, and the three-drug combination
treatment group, respectively. When PI staining was included,
apoptosis was detected in 4, 22, 14, 7 and 36% of cells,
respectively, and apoptosis was clearly induced in the cisplatin,
artesunate, and three-drug combination groups compared with iron
treatment alone. This ability to induce apoptosis was observed more
strongly with the combination of the three drugs compared with
treatment with the single drugs. Furthermore, the number of annexin
V-positive and PI-negative cells decreased from 9 to 5% and the
number of annexin V-positive and PI-positive cells, which are
considered dead cells, increased from 28 to 43% from 48 to 72 h in
the three-drug combination group, indicating that cell death by
apoptosis progressed over time.
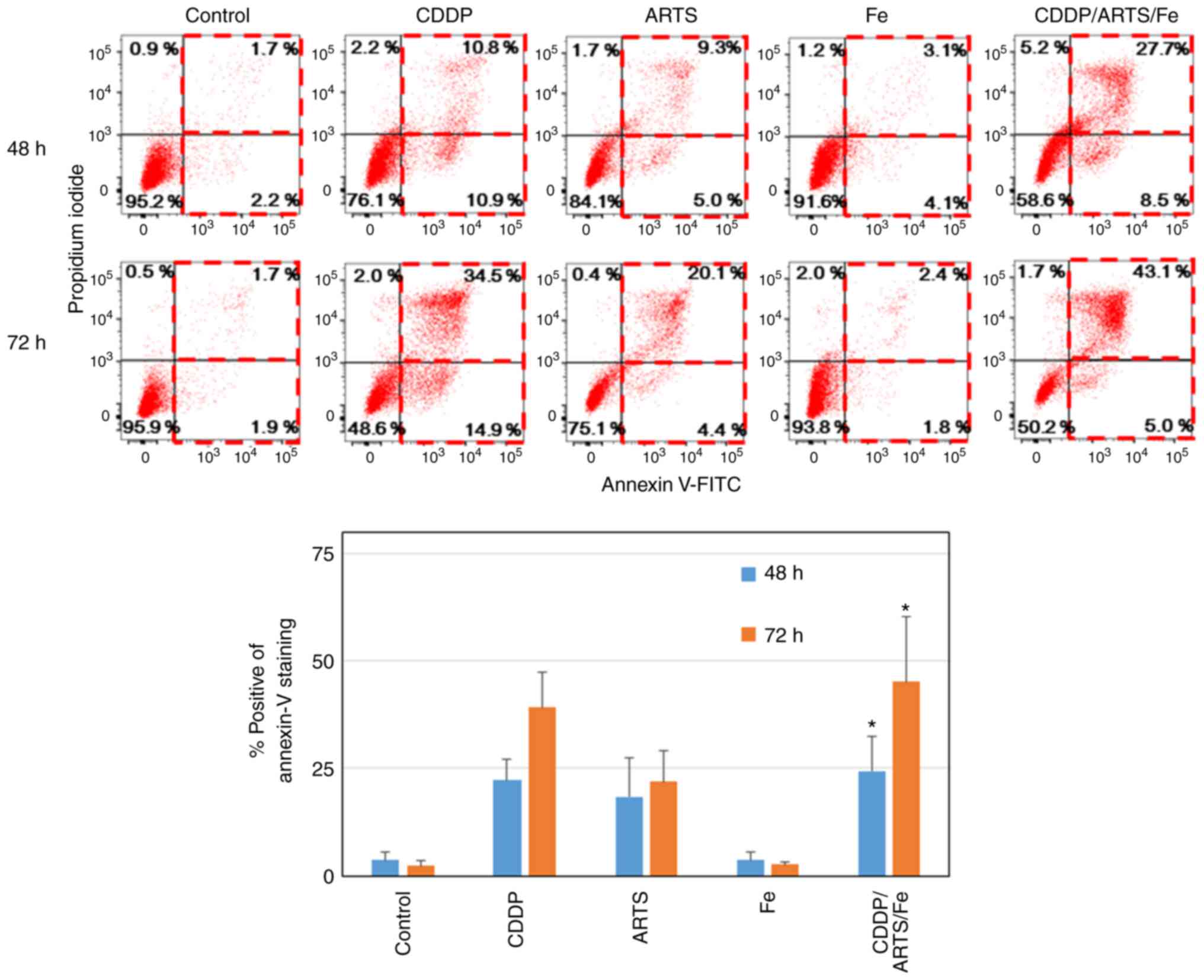 | Figure 5.ARTS induces apoptosis in HNSCC
cells, which was further enhanced by the combination of ARTS, Fe
and CDDP. HNSCC cells (UM-SCC-23) were treated with or without a
combination of ARTS (37.5 µM), CDDP (1.0 µg/ml) and Fe (0.01 mM)
for 48 or 72 h, and apoptosis was examined by flow cytometry using
annexin V and propidium iodide. (A) Flow cytometric profiles of
live, apoptotic and necrotic cells at 48 and 72 h after treatment.
(B) Data in the columns are presented as the mean ± standard
deviation. *P<0.01 vs. control. ARTS, artesunate; HNSCC, head
and neck squamous cell carcinoma; CDDP, cisplatin; Fe,
Fe(NO3)3; FITC, fluorescein
isothiocyanate. |
Artesunate suppresses the expression
of Rb
As cisplatin and artesunate induced cell cycle
arrest, the levels of Rb and p-Rb, which are involved in the cell
cycle and cell proliferation, were investigated. The UM-SCC-23 and
UM-SCC-81B cell lines were treated with cisplatin and artesunate
alone or in combination and examined by western blotting at 72 h
after drug treatment. As shown in Fig.
6A and B, there was a reduction in Rb and p-Rb levels following
cisplatin or artesunate treatment, but this reduction was more
pronounced following treatment with artesunate alone and with
combined treatment of cisplatin and artesunate. To confirm whether
the decreased expression of p-Rb induced by artesunate was due to
decreased phosphorylation or decreased expression of Rb, the ratio
of p-Rb to Rb was compared in the four groups and it was confirmed
that it was due to a decrease in Rb expression (Fig. 6C). Furthermore, it was confirmed
that this decrease also occurred at the mRNA level (Fig. 6D), where artesunate demonstrated a
trend toward a decrease in Rb mRNA levels or a decrease in Rb
expression. It was also considered that the depletion of p-Rb was
due to the depletion of Rb overall.
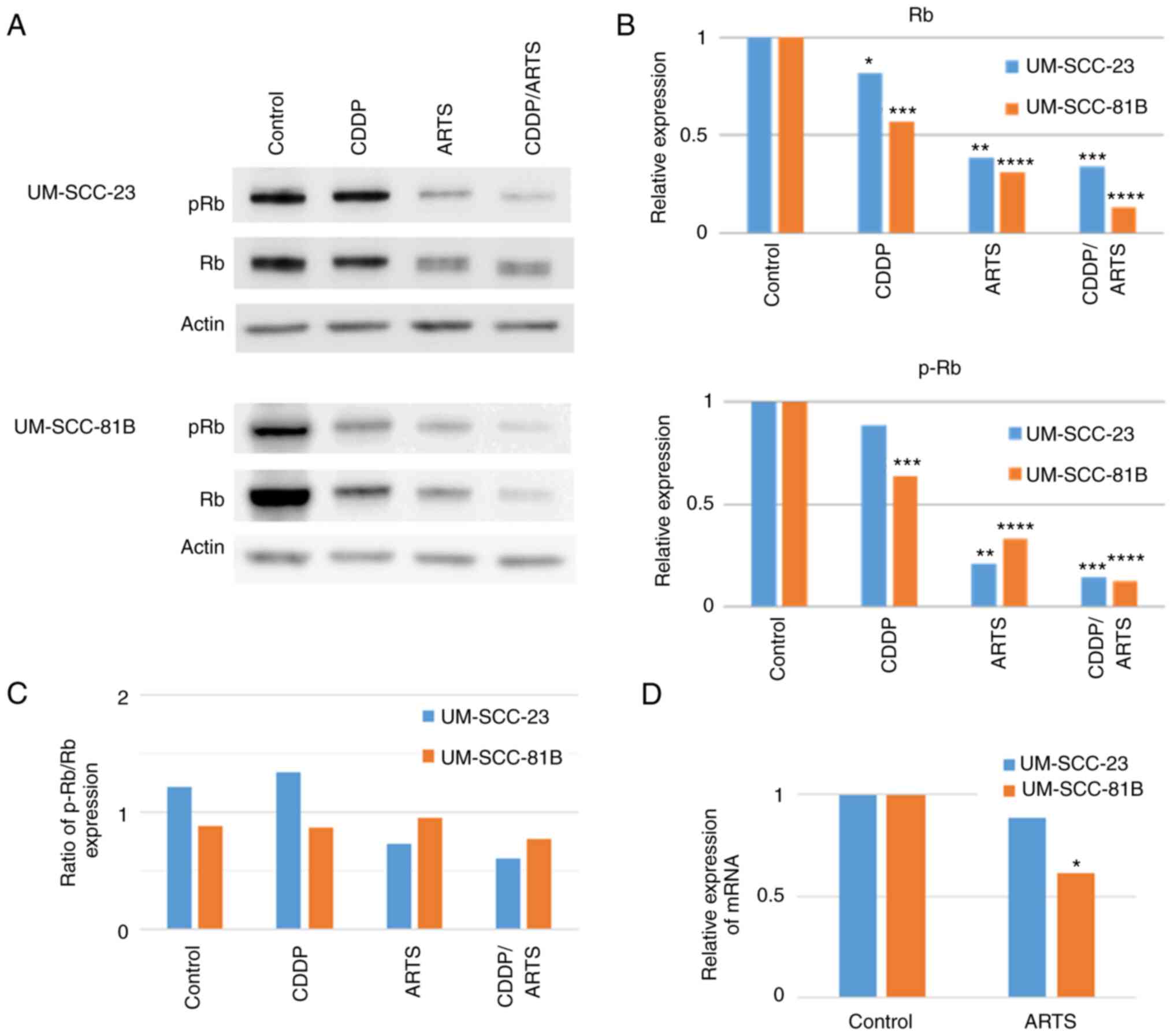 | Figure 6.ARTS reduces Rb and pRb levels in
HNSCC cells. (A) Western blotting was performed for pRb and Rb.
ARTS and CDDP were added to each cell line at final concentrations
of 9.375 µM and 0.75 µg/ml or 46 µM and 1.0 µg/ml, respectively.
The HNSCC cells (UM-SCC-23 and UM-SCC-81) were collected after 72 h
and the levels of Rb and pRb were examined by western blotting.
β-actin was used as a loading control. (B and C) Results are shown
as relative values for the concentration of each band obtained by
western blotting compared with (B) the untreated control and (C)
pRb and Rb. Results are shown comparing the mean from three
experiments. (D) Results are shown as relative values for the
concentration of each band obtained by RT-qPCR compared with the
untreated control. ARTS and CDDP were added to each cell line at
final concentrations of 9.375 and 46 µM, respectively. Results show
a comparison of data from three experiments. *P<0.05,
**P<0.01, ***P<0.001 and ****P<0.0001. ARTS, artesunate;
Rb, retinoblastoma protein; pRb, phosphorylated Rb; HNSCC, head and
neck squamous cell carcinoma; CDDP, cisplatin; RT-qPCR, reverse
transcription-quantitative PCR. |
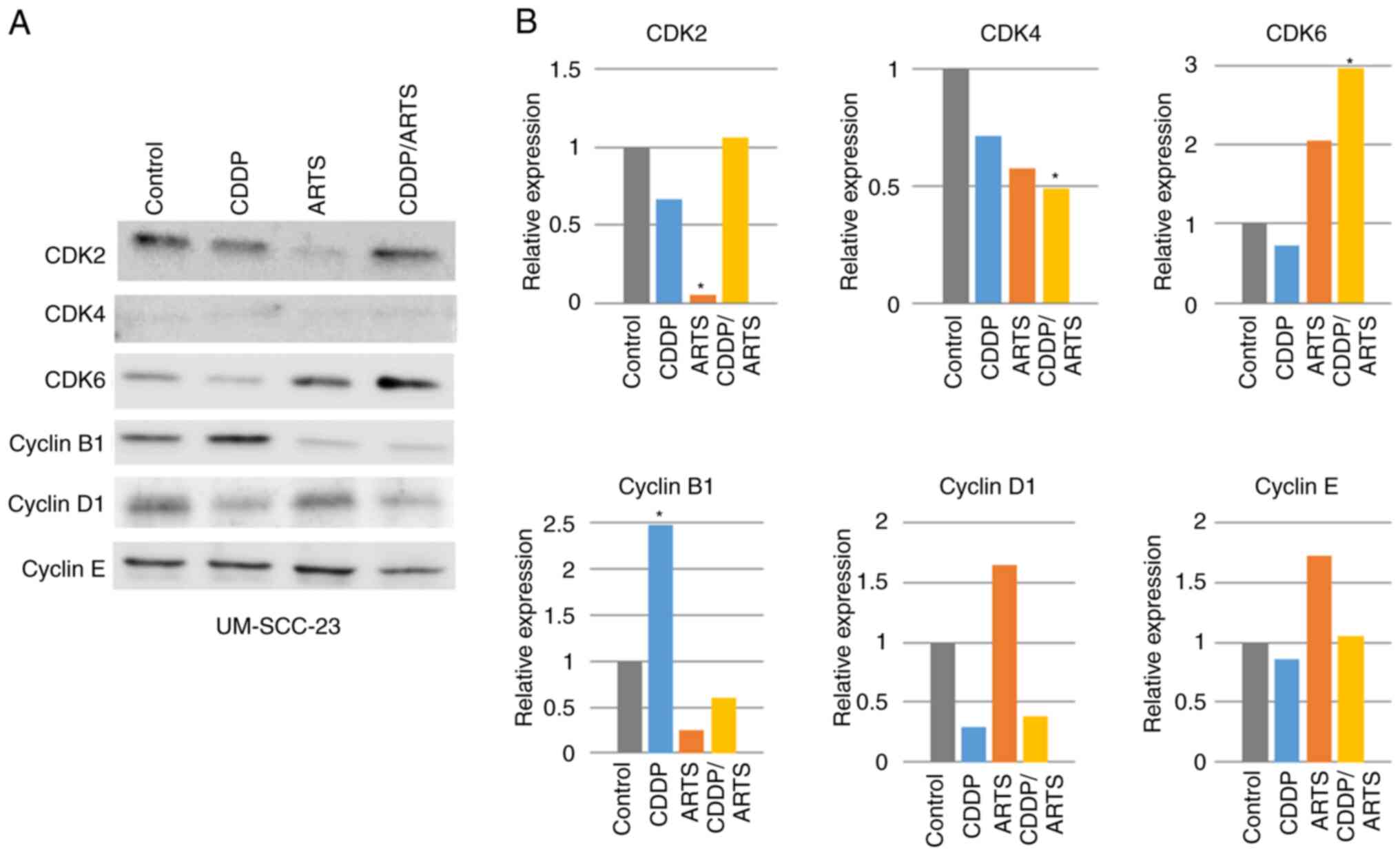
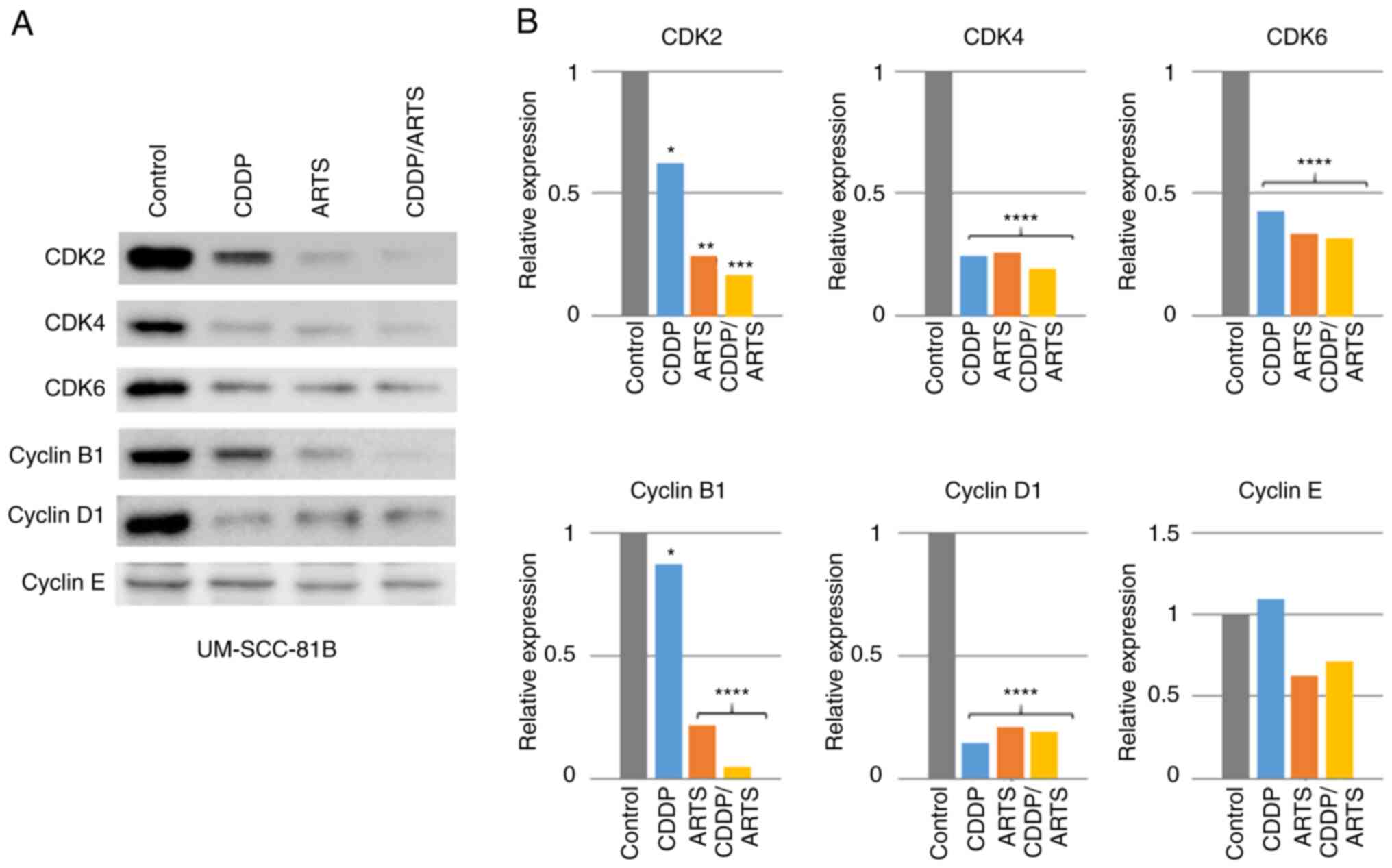
Effects of artesunate on other cell
cycle-related molecules
Since artesunate suppressed the levels of Rb and
p-Rb, its effects on other cell cycle-related molecules were
examined (Figs. 7 and 8). In UM-SCC-23 cells (Fig. 7A and B), all of the observed
molecules were expressed, but CDK4 was expressed at a very low
level. Artesunate significantly suppressed the expression of CDK2
(P<0.05), and a suppressive trend was also observed for CDK4 and
cyclin B1. Conversely, artesunate increased the levels of CDK6,
cyclin D1 and cyclin E, but not significantly. Cisplatin
significantly increased cyclin B1 expression, but a trend toward
suppression was observed for the other molecules. In terms of the
combined effect of artesunate and cisplatin, CDK2 was expressed at
control levels, with a reduction of the suppression observed when
each drug was administered alone. For CDK4, combination treatment
significantly suppressed its expression (P<0.05), while CDK6
expression was significantly enhanced (P<0.05). For cyclin B1,
the cisplatin-induced increase of its expression was suppressed by
combination treatment to a level similar to that observed for
artesunate treatment. For cyclin D1, the artesunate-induced
increase of expression was reversed by combination treatment, and
its expression was suppressed to a level similar to that of
cisplatin treatment alone. For cyclin E, the artesunate-induced
enhancement of expression was suppressed by combination treatment
to a level similar to that of the control. In UM-SCC-81B cells
(Fig. 8A and B), artesunate and
cisplatin significantly suppressed the expression of CDK2
(P<0.01 and P<0.05, respectively), CDK4 (P<0.0001 and
P<0.0001, respectively), CDK6 (P<0.0001 and P<0.0001,
respectively), cyclin B1 (P<0.05 and P<0.0001, respectively)
and cyclin D1 (P<0.0001 and P<0.0001, respectively).
Cisplatin had no effect on cyclin E levels, but a trend toward
suppression was observed with artesunate. In artesunate and
cisplatin combination therapy, significant suppression was also
observed for CDK2 (P<0.001), CDK4 (P<0.0001), CDK6
(P<0.0001), cyclin B1 (P<0.0001) and cyclin D1 (P<0.0001),
while a trend toward suppression was observed for cyclin E.
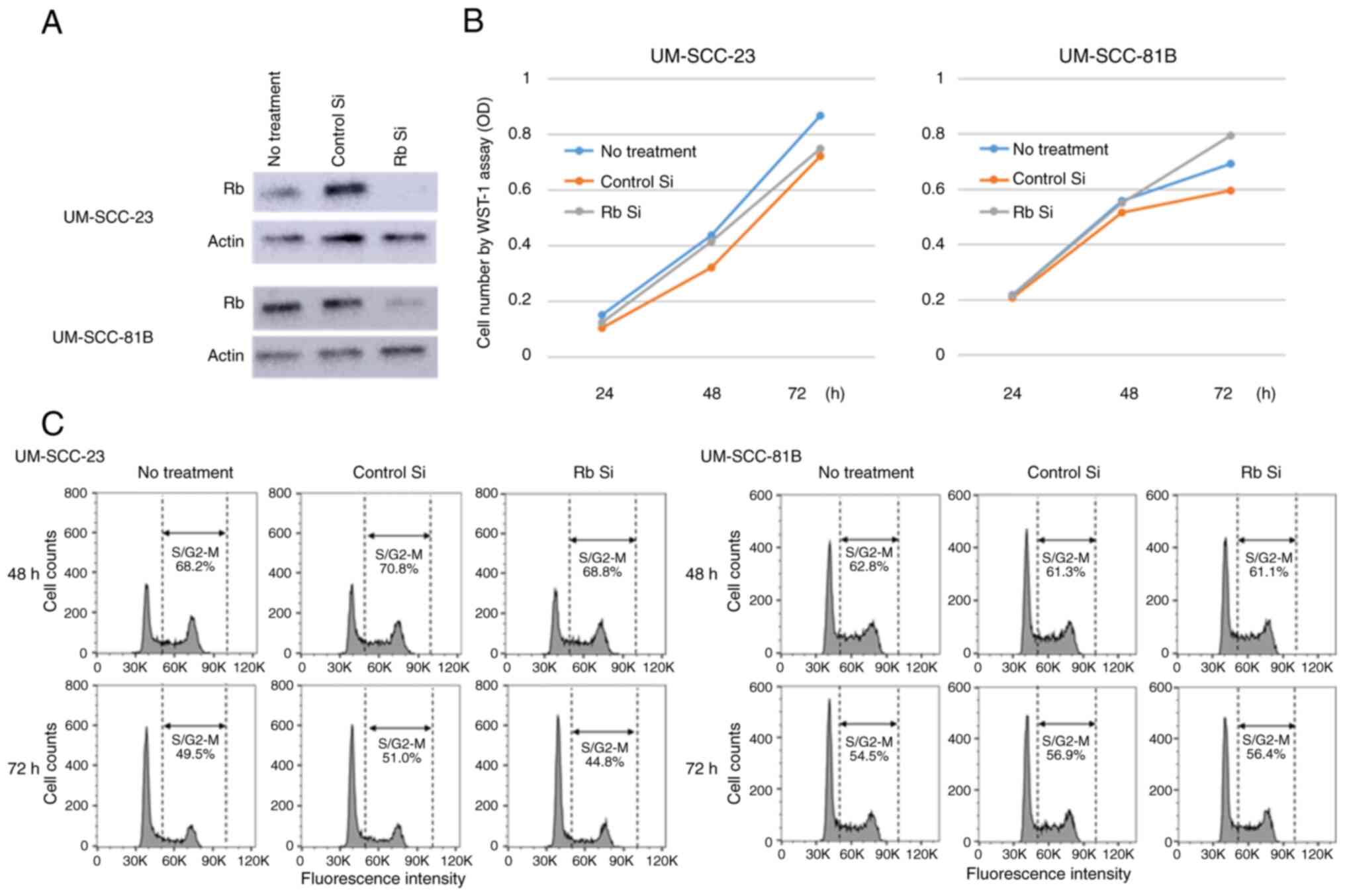
Effects of suppressing Rb expression
in HNSCC cell lines
In the HNSCC cell lines, artesunate treatment
suppressed Rb expression and induced apoptosis along with S/G2-M
arrest. Therefore, it was investigated whether suppressing Rb
expression with siRNA affects the cell cycle and growth inhibition.
As a result (Fig. 9A-C), cell death
was not observed in any of the cell lines in which Rb expression
was suppressed, and these cells had the same proliferative ability
as untreated cells, with no effect on the cell cycle.
Discussion
Currently, there are three standard treatments for
head and neck cancer: Surgery, radiotherapy, and chemotherapy.
Although anticancer drug therapy is expected to reduce tumor size,
numerous cases of head and neck cancer do not respond to anticancer
drugs. In fact, even with standard doses of cisplatin (80–100
mg/m2), the response rate is <50% (3,4). In
addition, although cisplatin is useful, it must be avoided in the
elderly and in patients with renal dysfunction due to its
nephrotoxicity.
Previously, artesunate, an antimalarial drug derived
from the medicinal herb A. annua, has been reported to be
effective against numerous carcinomas (9,10).
Zhang et al (25)
demonstrated that the combination of dihydroartemisinin and
cisplatin was effective in inhibiting cell proliferation and
inducing apoptosis in A549 lung cancer cell lines and A549-derived
cell lines that were not sensitive to cisplatin. However, there is
a paucity of studies on the efficacy of artesunate against head and
neck cancer and its use in combination with cisplatin, a
therapeutic agent for head and neck cancer. In the present study,
the antitumor effect of the artemisinin derivative artesunate and
the combined effect of artesunate and cisplatin were investigated
in head and neck cancer. It was revealed that artesunate alone
showed antitumor effects on HNSCC cell lines. In addition, the
combination of cisplatin and iron enhanced the antitumor effect of
artesunate and cisplatin compared with that of each agent alone.
Furthermore, the combination of artesunate and iron was found to be
effective even at lower cisplatin concentrations. Razavi et
al (26) reported that
artesunate therapy could ameliorate proteinuria and suppress the
progression of glomerular lesions in an experimental model of
nephrotic syndrome, suggesting that artesunate is not only well
tolerated by the kidney but is also effective in improving renal
function in patients with impaired renal function, and is expected
to be used as an adjunct drug when cisplatin cannot be administered
in sufficient doses.
Given this context, cell proliferative capacity,
cell cycle and apoptosis were explored after first knocking down Rb
using siRNA. No difference was observed between artesunate
treatment alone and that after suppressing Rb expression in any of
the experiments conducted. However, even if Rb expression is
knocked down and the suppressive effect of artesunate is no longer
observed, it does not necessarily mean that artesunate is exerting
its effects only through Rb; other signaling pathways may be
involved.
To investigate the inhibition of cell proliferation
by artesunate, its effect on the cell cycle was examined by using
two of the cell lines previously used in studies by the authors on
drug resistance in human oral squamous cell carcinoma (22). S/G2-M arrest was observed in both of
the HNSCC cell lines examined. In a similar study with artemisinin
and its derivatives, artemisinin-induced cell cycle arrest was not
limited to a specific phase but occurred in various phases. Wang
et al (27) reported that
G0/G1 arrest was induced by dihydroartemisinin in pancreatic cancer
cells, and Zhao et al (28)
indicated that G0/G1 arrest was induced by artesunate in bladder
cancer cells. Tran et al (29) revealed the induction of G1 arrest by
artemisinin in human Ishikawa endometrial cancer cells, and Jia
et al (15) reported G1
arrest following artemisinin administration to gall bladder cancer
cells. In addition, Ji et al (30) found that dihydroartemisinin or
artesunate induces G2/M arrest in human osteosarcoma cells, and
Chen et al (31) also
observed the same phenomenon in breast cancer cells. Each study
suggested that the induction of cell cycle arrest by artemisinin
and its derivatives was related to the expression of various
molecules involved in cell proliferation in the target cells. As
demonstrated in the present results, the induction of cell cycle
arrest by cisplatin or artesunate was accompanied by the induction
of apoptosis, which was also induced by their combination.
In the present study, artesunate was found to induce
S/G2-M arrest as well as apoptosis in both HNSCC cell lines used.
To confirm the effect of artesunate on the cell cycle, the
expression and phosphorylation of Rb protein and other cell
cycle-related molecules was examined by western blotting. As a
result, the loss of bands for Rb and p-Rb was observed. The
disappearance of these bands by anticancer agents was reported by
An and Dou (32) in the human
promyelocytic leukemia HL-60 cell line and human monocytic leukemia
U-937 cell line treated with etoposide and cytarabine and by Chen
et al (33) in HL-60 cells
treated with etoposide and cytarabine. In their review, Tan and
Wang (34) described their own
experiments in the human osteoblastic osteosarcoma Saos-2 cell
line, in which they found that cisplatin cleaved Rb. Unfortunately,
Tan and Wang did not publish their results, thus it remains unknown
how this cleavage occurred. There are few stuides on the effect of
artemisinin compounds on Rb expression. Fan et al (35) reported that treatment of gastric
cancer cells with dihydroartemisinin resulted in a dose-dependent
decrease in Rb mRNA levels, and western blotting analysis showed
that there was a decrease in p-Rb with a corresponding decrease in
CDK4 and cyclin D1 levels. In the present study, it was observed
that artesunate reduced the expression of Rb at the mRNA level; and
furthermore, that the reduced expression of p-Rb was due to
decreased expression of Rb, rather than suppression of Rb
phosphorylation. It is not clear whether this artesunate-induced
decrease in the transcriptional level of Rb expression is due to
epigenetic factors. It is also difficult to ascertain whether
artesunate suppresses Rb expression at the translational level from
the results obtained in the current study. Therefore, further
research is needed to clarify these issues.
The induction of Rb expression by artemisinin and
its derivatives was reported by Hou et al (13) in hepatocellular carcinoma, but they
did not observe the suppression of Rb expression in their
experiments. Almasan et al (36) have shown in experiments with mouse
embryonic fibroblasts that the absence of Rb generates a DNA
replication signal that can activate a constitutive p53-related
apoptotic response. Therefore, in the present study, it was also
examined whether the artesunate-induced reduction of Rb levels was
associated with cell proliferation and the induction of apoptosis
by inhibiting Rb expression with siRNA. The siRNA-induced reduction
of Rb expression in the HNSCC cell lines revealed no
artemisinin-like effects on cell proliferation or the cell cycle,
and there was no direct association between the reduction of Rb
levels and apoptosis. This suggested that in the HNSCC cell lines
used in the present study, a different mechanism may be at work in
the induction of apoptosis by artesunate than that described by
Almasan et al (36). In
addition, although the data are not shown, the effects of
artesunate in cells in which Rb expression was knocked down with
siRNA were no different from those in cells in which Rb expression
was intact. This suggested that artesunate may operate via Rb alone
but, given the limited number of cell lines in the current
experiment, such a conclusion cannot be derived at this time. It is
possible that other signaling pathways are involved, and thus
further studies are warranted.
As for the effects of artemisinin on the expression
of other cell cycle-related molecules, the common effect observed
in the cell lines used was the suppression of CDK4 expression,
while the effects on the other molecules differed between the cell
lines, indicating the complex effects of artemisinin on tumor
cells. As an effect of combined artesunate and cisplatin treatment,
cisplatin inhibited cell proliferation by cross-linking DNA, but
this is only effective when the cell cycle is in the S/G2 phase.
Artesunate treatment decreased Rb and p-Rb levels, and thus
advanced the cell cycle to the G1/S phase, which is considered to
allow cisplatin to be more effective. Furthermore, these results
indicated that low concentrations of cisplatin bind to DNA
efficiently and that artesunate is effective when used in
combination with low concentrations of cisplatin. From these
results, it can be inferred that the combination of cisplatin and
artesunate not only enhances the efficacy of cisplatin but may also
have similar effects on other platinum-based drugs.
In the present study, focus was addressed on the
antitumor effect of artemisinin in relation to the cell cycle but
it was found that it is difficult to explain how artemisinin exerts
its antitumor effect only in relation to the cell cycle. The
current results were obtained from experiments using only two cell
lines, and thus it is considered that the results should be
interpreted cautiously. Future analysis of the molecular mechanism
for the antitumor effect of artemisinin, including the phenomena
observed in the present study, is awaited.
In conclusion, it was identified that artesunate
exerted an antitumor effect on HNSCC cells, and this effect was
enhanced when it was combined with cisplatin. This effect was also
observed in combination with low-dose cisplatin, suggesting that
artesunate could enhance the antitumor effect of cisplatin and
could be used widely in the elderly, including those with renal
dysfunction. It is hoped that the molecular mechanism underlying
this combined effect will be elucidated in the future and that it
will be applied in clinical practice.
Acknowledgements
Not applicable.
Funding
The present study was supported by JSPS KAKENHI (grant no.
17K11412).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KY and TO designed the outline of the study. HO, AS,
DI and SY conducted the experiments and contributed to data
interpretation and manuscript preparation. KY, SS and HO confirm
the authenticity of all the raw data. HO and KY wrote the
manuscript. YF, RU, SS, RS, HU, TO and KY supervised the study and
contributed to data interpretation and manuscript
preparation/revision. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morton RP, Rugman F, Dorman EB, Stoney PJ,
Wilson JA, McCormick M, Veevers A and Stell PM: Cisplatinum and
bleomycin for advanced or recurrent squamous cell carcinoma of the
head and neck: A randomised factorial phase III controlled trial.
Cancer Chemother Pharmacol. 15:283–289. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kish JA, Weaver A, Jacobs J, Cummings G
and Al-Sarraf M: Cisplatin and 5-fluorouracil infusion in patients
with recurrent and disseminated epidermoid cancer of the head and
neck. Cancer. 53:1819–1824. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kish J, Drelichman A, Jacobs J, Hoschner
J, Kinzie J, Loh J, Weaver A and Al-Sarraf M: Clinical trial of
cisplatin and 5-FU infusion as initial treatment for advanced
squamous cell carcinoma of the head and neck. Cancer Treat Rep.
66:471–474. 1982.PubMed/NCBI
|
|
5
|
Ogawa T, Niho S, Nagai S, Kojima T,
Nishimura Y, Ohe Y, Kondo N, Yamaguchi T, Endo K, Izumi K and
Minami H: Moderate renal dysfunction may not require a cisplatin
dose reduction: A retrospective study of cancer patients with renal
impairment. Int J Clin Oncol. 18:977–982. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenthal PJ: Artesunate for the treatment
of severe falciparum malaria. N Engl J Med. 358:1829–1836. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ho WE, Peh HY, Chan TK and Wong WS:
Artemisinins: Pharmacological actions beyond anti-malarial.
Pharmacol Ther. 142:126–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun WC, Han JX, Yang WY, Deng DA and Yue
XF: Antitumor activities of 4 derivatives of artemisic acid and
artemisinin B in vitro. Acta Pharmacol Sin. 13:541–543. 1992.
|
|
9
|
Efferth T, Dunstan H, Sauerbrey A, Miyachi
H and Chitambar CR: The anti-malarial artesunate is also active
against cancer. Int J Oncol. 18:767–773. 2001.PubMed/NCBI
|
|
10
|
Efferth T, Sauerbrey A, Olbrich A, Gebhart
E, Rauch P, Weber HO, Hengstler JG, Halatsch ME, Volm M, Tew KD, et
al: Molecular modes of action of artesunate in tumor cell lines.
Mol Pharmacol. 64:382–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Våtsveen TK, Myhre MR, Steen CB, Wälchli
S, Lingjærde OC, Bai B, Dillard P, Theodossiou TA, Holien T, Sundan
A, et al: Artesunate shows potent anti-tumor activity in B-cell
lymphoma. J Hematol Oncol. 11:232018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berte N, Lokan S, Eich M, Kim E and Kaina
B: Artesunate enhances the therapeutic response of glioma cells to
temozolomide by inhibition of homologous recombination and
senescence. Oncotarget. 7:67235–67250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou J, Wang D, Zhang R and Wang H:
Experimental therapy of hepatoma with artemisinin and its
derivatives: In vitro and in vivo activity, chemosensitization, and
mechanisms of action. Clin Cancer Res. 14:5519–5530. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roh JL, Kim EH, Jang H and Shin D: Nrf2
inhibition reverses the resistance of cisplatin-resistant head and
neck cancer cells to artesunate-induced ferroptosis. Redox Biol.
11:254–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia J, Qin Y, Zhang L, Guo C, Wang Y, Yue
X and Qian J: Artemisinin inhibits gallbladder cancer cell lines
through triggering cell cycle arrest and apoptosis. Mol Med Rep.
13:4461–4468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen S, Gan S, Han L, Li X, Xie X, Zou D
and Sun H: Artesunate induces apoptosis and inhibits the
proliferation, stemness, and tumorigenesis of leukemia. Ann Transl
Med. 8:7672020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dell'Eva R, Pfeffer U, Vené R, Anfosso L,
Forlani A, Albini A and Efferth T: Inhibition of angiogenesis in
vivo and growth of Kaposi's sarcoma xenograft tumors by the
anti-malarial artesunate. Biochem Pharmacol. 68:2359–2366. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SJ, Kim MS, Lee JW, Lee CH, Yoo H,
Shin SH, Park MJ and Lee SH: Dihydroartemisinin enhances
radiosensitivity of human glioma cells in vitro. J Cancer Res Clin
Oncol. 132:129–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Efferth T: From ancient herb to modern
drug: Artemisia annua and artemisinin for cancer therapy.
Semin Cancer Biol. 46:65–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong YK, Xu C, Kalesh KA, He Y, Lin Q,
Wong WSF, Shen HM and Wang J: Artemisinin as an anticancer drug:
Recent advances in target profiling and mechanisms of action. Med
Res Rev. 37:1492–1517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu C, Zhang H, Mu L and Yang X:
Artemisinins as anticancer drugs: Novel therapeutic approaches,
molecular mechanisms, and clinical trials. Front Pharmacol.
11:5298812020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishimura K, Tsuchiya Y, Okamoto H, Ijichi
K, Gosho M, Fukayama M, Yoshikawa K, Ueda H, Bradford CR, Carey TE
and Ogawa T: Identification of chemoresistant factors by protein
expression analysis with iTRAQ for head and neck carcinoma. Br J
Cancer. 111:799–806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muramatsu H, Sumitomo M, Morinaga S,
Kajikawa K, Kobayashi I, Nishikawa G, Kato Y, Watanabe M, Zennami
K, Kanao K, et al: Targeting lactate dehydrogenase-A promotes
docetaxel-induced cytotoxicity predominantly in
castration-resistant prostate cancer cells. Oncol Rep. 42:224–230.
2019.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang JL, Wang Z, Hu W, Chen SS, Lou XE
and Zhou HJ: DHA regulates angiogenesis and improves the efficiency
of CDDP for the treatment of lung carcinoma. Microvasc Res.
87:14–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Razavi A, Nouri HR, Mehrabian F and
Mirshafiey A: Treatment of experimental nephrotic syndrome with
artesunate. Int J Toxicol. 26:373–380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang SJ, Gao Y, Chen H, Kong R, Jiang HC,
Pan SH, Xue DB, Bai XW and Sun B: Dihydroartemisinin inactivates
NF-kappaB and potentiates the anti-tumor effect of gemcitabine on
pancreatic cancer both in vitro and in vivo. Cancer Lett.
293:99–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao F, Vakhrusheva O, Markowitsch SD,
Slade KS, Tsaur I, Cinatl J Jr, Michaelis M, Efferth T, Haferkamp A
and Juengel E: Artesunate impairs growth in cisplatin-resistant
bladder cancer cells by cell cycle arrest, apoptosis and autophagy
induction. Cells. 9:26432020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tran KQ, Tin AS and Firestone GL:
Artemisinin triggers a G1 cell cycle arrest of human Ishikawa
endometrial cancer cells and inhibits cyclin-dependent kinase-4
promoter activity and expression by disrupting nuclear factor-κB
transcriptional signaling. Anticancer Drugs. 25:270–281. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ji Y, Zhang YC, Pei LB, Shi LL, Yan JL and
Ma XH: Anti-tumor effects of dihydroartemisinin on human
osteosarcoma. Mol and Cell Biochem. 351:99–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen K, Shou LM, Lin F, Duan WM, Wu MY,
Xie X, Xie YF, Li W and Tao M: Artesunate induces G2/M cell cycle
arrest through autophagy induction in breast cancer cells.
Anticancer Drugs. 25:652–662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
An B and Dou QP: Cleavage of
retinoblastoma protein during apoptosis: An interleukin 1
beta-converting enzyme-like protease as candidate. Cancer Res.
56:438–442. 1996.PubMed/NCBI
|
|
33
|
Chen WD, Otterson GA, Lipkowitz S, Khleif
SN, Coxon AB and Kaye FJ: Apoptosis is associated with cleavage of
a 5 kDa fragment from RB which mimics dephosphorylation and
modulates E2F binding. Oncogene. 14:1243–1248. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan X and Wang JY: The caspase-RB
connection in cell death. Trends Cell Biol. 8:116–120. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fan HN, Zhu MY, Peng SQ, Zhu JS, Zhang J
and Qu GQ: Dihydroartemisinin inhibits the growth and invasion of
gastric cancer cells by regulating cyclin D1-CDK4-Rb signaling.
Pathol Res Pract. 216:1527952020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Almasan A, Yin Y, Kelly RE, Lee EY,
Bradley A, Li W, Bertino JR and Wahl GM: Deficiency of
retinoblastoma protein leads to inappropriate S-phase entry,
activation of E2F-responsive genes, and apoptosis. Proc Natl Acad
Sci USA. 92:5436–5440. 1995. View Article : Google Scholar : PubMed/NCBI
|