Introduction
Pancreatic cancer (PaCa) is a type of
gastrointestinal cancer that is relatively resistant to treatment;
accordingly, the prognosis of patients with PaCa is poor. In both
Japan and the United States of America, PaCa is the fourth leading
cause of cancer-related deaths, and the number of deaths is
increasing every year (1).
Multidisciplinary treatments combining surgery, chemotherapy, and
radiotherapy have recently been developed to treat PaCa. In the
past 10 years, with advances in treatment methods, the 5-year
survival rate of patients with PaCa has increased from 6 to 11%,
although it is still far from satisfactory (1). One of the main reasons for this lack
of efficacy is that PaCa readily becomes resistant to
chemotherapy.
Gemcitabine (Gem) has been used to treat PaCa since
1977 (2) and is still a key drug
used as both adjuvant chemotherapy (3) and in Gem plus nab-paclitaxel (GnP)
combination treatment for distant metastases (4). Therefore, Gem resistance in PaCa is a
major clinical problem, and the identification of factors
associated with Gem resistance and the development of therapies
targeting these factors are necessary to improve prognoses. Some
studies have discovered the importance of integrin-linked kinase
(ILK) in chemoresistance (5,6). A
previous study by the authors revealed similar findings and
demonstrated that ILK is important, even after its effect on Gem
becomes inadequate. ILK is an intracellular effector related to
cell-matrix interactions and is associated with multiple signaling
pathways, including AKT, glycogen synthase kinase (GSK)-3β
(7). In cancer tissues, ILK is
often upregulated, suggesting that ILK contributes to
proliferation, invasion, angiogenesis, and metastasis (8,9).
Therefore, downregulation of downstream signaling pathways via
suppression of ILK may play a major role in cancer control, and
ILK-targeted therapies are being developed for numerous cancer
types (9–12). Previous research has described the
efficacy of ILK inhibitors in PaCa (13). However, no functional analyses of
ILK have been reported in established Gem-resistant (Gem-R) cell
lines, and the antitumor effects of ILK inhibitors in Gem-R cell
lines have not been elucidated. ILK has been demonstrated to be
involved in chemotherapy resistance by promoting apoptosis and the
epithelial-mesenchymal transition (EMT) (5,6,14).
However, in these studies, ordinary cancer cells were used.
Therefore, in the present study, the role of ILK was
evaluated using a Gem-R PaCa cell line that was established by the
authors, originally. Preliminary experiments revealed that ILK
expression was upregulated in this Gem-resistant cell line compared
to the Gem-S PaCa cell line. Therefore, it was determined how the
increased expression of ILK affects the behavior of the Gem-R
PaCa-resistant lines. The effect of ILK inhibitors on PaCa cell
lines was also investigated, including the Gem-R PaCa cell
lines.
Materials and methods
Reagents
Compound 22 (Cpd22,
C30H30F3N5O) and
dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (Merck
KGaA). Cpd22 was dissolved in DMSO at 50 mM and used as a stock
solution. The solution was aliquoted into 200-µl tubes and stored
at −20°C. GSK690693 was purchased from Selleck Chemicals.
Establishment of Gem-R PaCa cell
lines
In this experiment, Gem-R PaCa cell lines were
utilized that had been previously established by the authors,
originally (15). The method of
establishment is briefly described below. The half maximal
inhibitory concentration (IC50) of GEM against parental
MIA PaCa-2 or AsPC-1 cells was determined using WST-1 assay. The
IC50 of GEM for each PaCa cell line was established by
constructing a dose-response curve. Each PaCa cell line was
passaged with GEM at the IC50 concentration of the
original cell line for 2 to 3 weeks. Following passage, the
IC50 of the cell line to GEM was determined again. The
PaCa cell lines were then passaged for 2 to 3 weeks with exposure
to the IC50 concentration of the redetermined Gem. This
process was repeated with increasing doses of GEM until the cell
line exhibited an IC50 value to GEM that was at least
50-fold higher than that of the parental line.
Cell culture
Human PaCa cell lines (AsPC-1, BxPC-3, Capan2, MIA
PaCa-2, PANC-1, and SW1990) and the immortalized human endothelial
cell line EA.hy926 were purchased from American Type Culture
Collection (ATCC). The human pancreatic ductal epithelial (HPDE)
cell line H6C7 was purchased from Kerafast, Inc. AsPC-1, BxPC-3,
Capan2, and EA.hy926 cells were maintained in RPMI-1640 medium
(Sigma Aldrich; Merck KGaA). MIA PaCa-2, PANC-1, and SW1990 cells
were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma
Aldrich; Merck KGaA). H6C7 cells were maintained in keratinocyte
serum-free medium (Gibco; Thermo Fisher Scientific, Inc.). Gem-R
PaCa cell lines (MIA PaCa-2, AsPC-1), which were previously
established and had been stored in liquid nitrogen, were used.
Fetal bovine serum (FBS; 10%; Gibco; Thermo Fisher Scientific,
Inc.) was added to both RPMI-1640 and DMEM. All media were
supplemented with 10 mg/ml streptomycin, 10,000 U/ml penicillin,
and 25 µg amphotericin B (Gibco; Thermo Fisher Scientific, Inc.).
All cell lines were cultured at 37°C in a humidified incubator
containing 5% CO2.
RNA interference
ILK small interfering RNA (siRNA; ID s7405: Sense,
5′-CGACCCAAAUUUGACAUGAtt-3′ and antisense,
5′-UCAUGUCAAAUUUGGGUCGct-3′) and nontargeted negative control siRNA
(ncRNA; Silencer Select Negative Control No. 1; cat. no. 4390843;
sequences not provided) were purchased from Thermo Fisher
Scientific, Inc. PaCa cells were seeded in 6-well plates at
2.0×105 cells/well and cultured to 60% confluence before
transfection with siRNA. According to the manufacturer's
instructions, siRNA and Lipofectamine RNAiMAX (Invitrogen; Thermo
Fisher Scientific, Inc.) were mixed in Opti-MEM (Invitrogen; Thermo
Fisher Scientific, Inc.), and the mixture was then incubated at
room temperature for 5 min. The siRNA-lipid complex was diluted in
cell-appropriate medium to a final siRNA concentration of 10 nM. In
the adjusted medium, the cells were cultured in a 5% CO2
incubator at 37°C for 24 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the samples used using
QIAcube and RNeasy Plus Mini Kit (Qiagen GmbH) according to the
manufacturer's protocol. Total RNA was then quantified using a
NanoDrop 1000 (Thermo Fisher Scientific, Inc.). The RNA was then
reverse transcribed using SuperScript III First-Strand Synthesis
SuperMix (Invitrogen; Thermo Fisher Scientific, Inc.) and a T100
Thermal Cycler (Bio-Rad Laboratories, Inc.). The temperature
protocol for reverse transcription was as follows: 25°C for 10 min,
50°C for 30 min, and 85°C for 5 min. RT-qPCR was performed using
TaqMan Gene Expression Assays (cat. no. 4331182; Applied
Biosystems; Thermo Fisher Scientific, Inc.) and predesigned primers
for ILK (Hs01101168_g1) and Cxcl8 (Hs00174103_m1)
from Thermo Fisher Scientific, Inc., on a CFX Connect Real-Time
System (Bio-Rad Laboratories, Inc.). The thermocycling conditions
were as follows: Initial denaturation at 95°C for 20 sec, followed
by 60 cycles at 95°C for 1 sec and 60°C for 20 sec. Glyceraldehyde
3-phosphate dehydrogenase (GAPDH; Hs99999905_m1; Thermo
Fisher Scientific, Inc.) was used as a loading control to normalize
mRNA levels, and samples were quantified using the standard curve
method (16).
Western blotting
Proteins were extracted from cells using
radioimmunoprecipitation lysis buffer containing Protease Inhibitor
Single Use Cocktail and Phosphatase Inhibitor Cocktail (both Thermo
Fisher Scientific, Inc.). Protein concentrations were measured
using a Pierce BCA protein assay kit (Thermo Fisher Scientific,
Inc.). Protein extracts (40 µg) were denatured at 90°C for 5 min
and separated on 10% Mini-PROTEAN TGX Precast gels (Bio-Rad
Laboratories, Inc.). The protein bands were then transferred to
nitrocellulose membranes and blocked in iBind Flex Solution (iBind
Flex Buffer, iBind Flex Additive, and distilled water; Thermo
Fisher Scientific, Inc.) for 20 min at room temperature. The
primary and secondary antibody reactions were performed for 3 h at
room temperature using the iBind Flex Western System (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
following primary antibodies were used: Anti-ILK antibody (1:1,000;
cat. no. GTX101691; GeneTex, Inc.), anti-Akt (pan) (1:1,000; cat.
no. 4691), anti-phoshorylated (p)-Akt (Ser473) (1:1,000; cat. no.
4060) and anti-GAPDH (1:1,000; cat. no. 2118; all from Cell
Signaling Technology, Inc.). Horseradish peroxidase-conjugated goat
anti-rabbit Polychronus (1:2,000; cat. no. P0448; DAKO; Agilent
Technologies, Inc.) was used as the secondary antibody. The
protein-antibody complexes were visualized using SuperSignal West
Femto Chemiluminescent Substrate or SuperSignal West PICO PLUS
Chemiluminescent Substrate or Pierce ECL Western Blotting Substrate
(Thermo Fisher Scientific, Inc.). Immunoreactive protein bands were
detected using an Amersham Imager 600 (Cytiva), and the densities
of the detected bands were calculated using ImageJ software 1.52v
(National Institutes of Health).
Enzyme-linked immunosorbent assay
(ELISA)
Cells in the control group were seeded in 6-well
plates in DMEM or RPMI-1640 medium appropriate for each cell line
containing 5% FBS (1.0×105 cells/well). After overnight
incubation, the medium was replaced (1 ml/well), and after 48 h,
the culture supernatant was collected and centrifuged at 400 × g
and 4°C for 5 min to remove particulates. In the RNA transfection
group, cells were collected 24 h after transfection and seeded into
6-well plates (1.0×105 cells/well). After overnight
incubation, the medium was replaced (1 ml/well), and the culture
supernatant was collected 48 h later. In the Cpd22 group, cells
were pretreated with 1.0 µM Cpd22 for 48 h and seeded into 6-well
plates (1.0×105 cells/well). After overnight incubation,
the medium was replaced (1 ml/well), and the culture supernatant
was collected 48 h later. The assay was performed using a Human
IL-8/CXCL8 Quantikine ELISA Kit (cat. no. D8000C; R&D Systems,
Inc.) with a SpectraMax ABS microplate reader (Molecular Devices,
LLC). The concentration of each protein was determined according to
the manufacturer's protocol.
Invasion assay
In vitro invasion assays were performed using
Corning BioCoat Matrigel Invasion Chambers (Corning, Inc.)
according to the manufacturer's protocol. Four groups of PaCa cell
lines were used when suppressing ILK: Untreated PaCa cell lines,
PaCa cell lines treated with siRNA or ncRNA, and PaCa cell lines
treated with Cpd22. The PaCa cell lines were exposed to 1 µM of
Cpd22 for 48 h. During Akt inhibition, untreated PaCa cell lines
and PaCa cell lines treated with GSK690693 were used to evaluate
invasive capacity. The PaCa cell lines were exposed to GSK690693 at
5 µM for 24 h. Cells were cultured at 5% CO2 at 37°C in
all groups. Next, 1.0×105 PaCa cells were conditioned in
FBS-free medium and seeded in the upper chambers. The inducer used
in the lower chamber was 10% FBS added to the medium. After 24 h of
incubation at 37°C, the top surface of the upper chamber was
swabbed, and the invasive cells were fixed and stained using a
Diff-Quick cell staining kit (Dade Behring), under room
temperature, all samples were fixed for 10 sec and stained with two
different staining solutions, each for 30 sec. The number of cells
in nine randomly defined fields of view (magnification, ×200) was
counted under a light microscope.
Tube formation assay for analysis of
angiogenesis
Tube formation was determined using angiogenesis
assays with EA.hy926 cells and Matrigel (Corning, Inc.). Cells in
the control group were seeded in 6-well plates (1.0×105
cells/well) using DMEM or RPMI-1640 medium containing 5% FBS, as
appropriate for each cell line. After overnight incubation, the
medium was replaced, and cells were incubated in medium containing
2% FBS for 48 h at 37°C. In the RNA transfection group, cells were
collected 48 h after transfection and seeded into 6-well plates
(1.0×105 cells/well). In the Cpd22 group, cells were
pretreated with 1.0 µM Cpd22 for 48 h and seeded into 6-well plates
(1.0×105 cells/well). After overnight incubation, cells
were replaced with medium containing 1% FBS (1 ml/well), and
culture supernatants were collected 48 h later. The collected cell
supernatants were centrifuged at 400 × g and 4°C for 5 min, and
particulates were discarded. Matrigel was added to a 96-well plate
(50 µl/well), and the plates were incubated at 37°C for 30 min to
solidify the Matrigel. Next, EA.hy926 cells (1.2×104
cells/well) were added on top of the Matrigel. The cells were
incubated in mixed medium (50 µl RPMI-1640 medium with 1% FBS and
50 µl/well of the aforementioned supernatant) for 16 h to form
capillary-like structures; cells incubated in RPMI-1640 medium with
1% FBS alone served as controls. The number of endotubes was
counted under a confocal microscope (magnification, ×40), and 6
views per group were analyzed.
Statistical analysis
All statistical analyses were performed using EZR
version 1.54 (Saitama Medical Center, Jichi Medical University,
Saitama, Japan), which is a graphical user interface for R (The R
Foundation for Statistical Computing). Data from experiments
conducted at least in triplicate were expressed as the means ±
standard deviations. Differences between the two samples were
analyzed using unpaired t-tests. Comparisons of multiple groups
were performed by one-way analysis of variance (ANOVA), and
subsequent comparisons of individual groups were performed using
post hoc Bonferroni tests; results with a P-value <0.05 were
considered to indicate a statistically significant difference.
Results
ILK expression is upregulated in PaCa
cells compared with HPDE cells
H6C7 is an immortalized epithelial cell line derived
from normal HPDE cells. The expression of ILK in H6C7 and PaCa cell
lines (AsPC-1, BxPC-3, Capan2, MIA PaCa-2, PANC-1, and SW1990) was
evaluated using RT-qPCR and western blotting. ILK mRNA
expression (Fig. 1A) was
significantly higher in PaCa cells than in HPDE cells (P<0.05).
Protein expression levels (Fig. 1B and
C) were also significantly upregulated in PaCa cells, except
for MIA PaCa-2 cells (P<0.05).
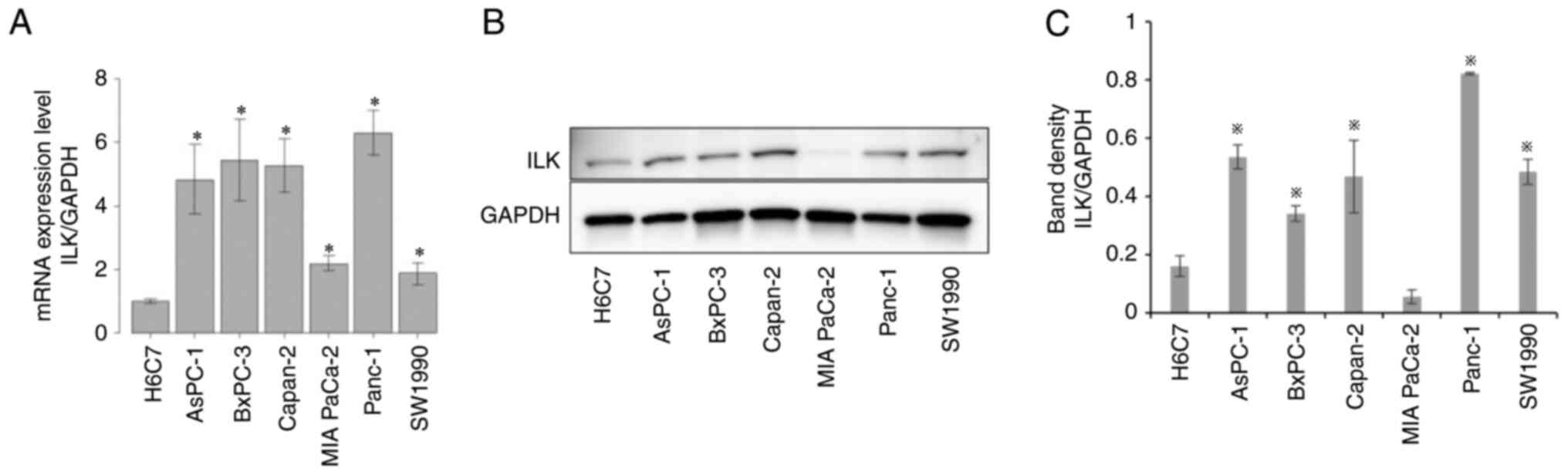 | Figure 1.Comparison of ILK expression in human
pancreatic ductal epithelial cells and PaCa cells. (A) ILK mRNA
expression in HPDE cells (H6C7) and PaCa cells (AsPC-1, BxPC-3,
Capan-2, MIA PaCa-2, PANC-1, SW1990) was assessed using reverse
transcription-quantitative polymerase chain reaction. The mRNA
expression status for each sample was normalized to the expression
of GAPDH. (B) ILK protein expression levels in HPDE cells
(H6C7) and PaCa cells (AsPC-1, BxPC-3, Capan-2, MIA PaCa-2, PANC-1,
SW1990) were evaluated using western blotting. (C) The relative
expression of ILK compared with GAPDH was assessed. Significant
differences in ILK expression between HPDE (H6C7) and PaCa cells
were assessed using one-way analysis of variance. *P<0.05. PaCa,
pancreatic cancer; ILK, integrin-linked kinase. |
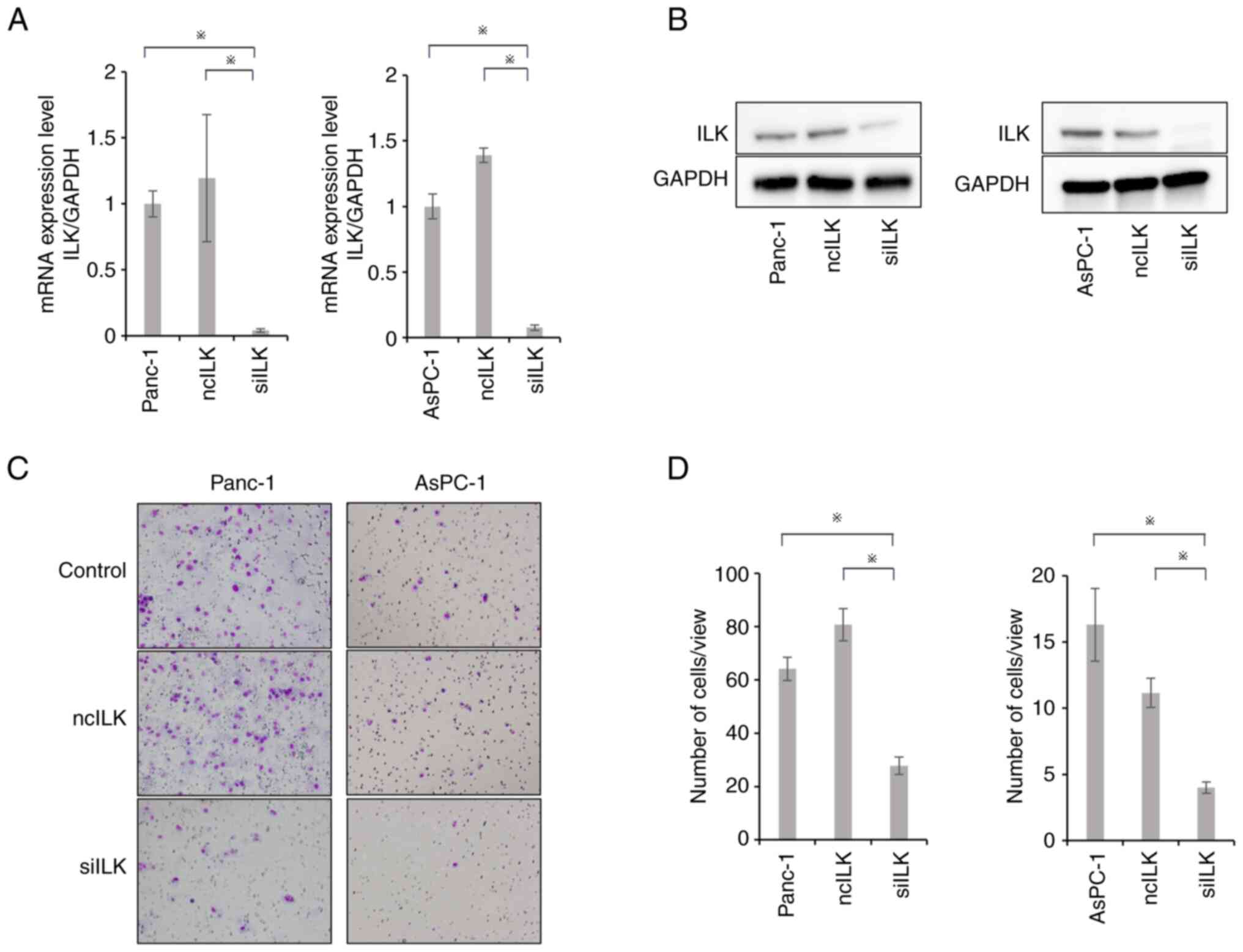
Changes in ILK expression levels after
ILK knockdown in PaCa cell lines
RT-qPCR and western blotting were performed to
evaluate changes in ILK mRNA expression in PaCa cell lines
transfected with ILK siRNA. AsPC-1 and PANC-1 cells with
high ILK expression were selected for ILK siRNA
transfection. PaCa cell lines transfected with ILK siRNA
exhibited significant downregulation of ILK compared with control
cells or cells transfected with negative control siRNA (P<0.05;
Fig. 2A and B).
Effects of ILK knockdown on PaCa cell
invasion
Matrigel invasion assays were performed to evaluate
the invasive potential of PaCa cell lines. Cell invasion ability
was significantly suppressed in the ILK-knockdown group compared
with that in the control and negative control groups (P<0.05;
Fig. 2C and D).
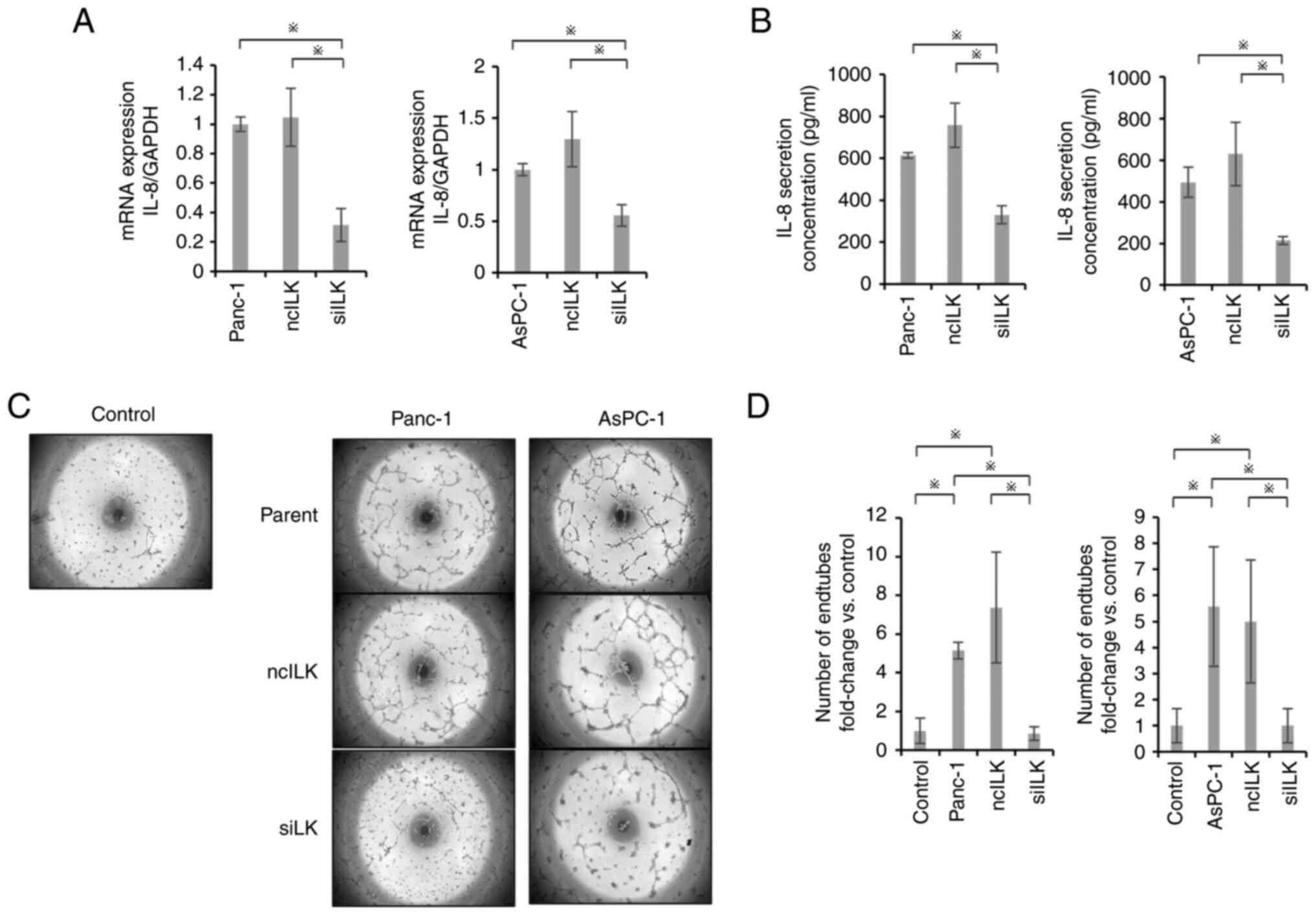
Effects of ILK knockdown on the
angiogenic potential of PaCa cell lines
The secretion of IL-8 as an angiogenic factor was
evaluated by RT-qPCR and ELISA. IL-8 mRNA expression and
IL-8 secretion were significantly suppressed in the ILK-knockdown
group compared with those in the control and negative control
groups (P<0.05; Fig. 3A and B).
Tube formation assays also showed significant suppression in the
ILK-knockdown group compared with the control and negative control
groups (P<0.05; Fig. 3C and
D).
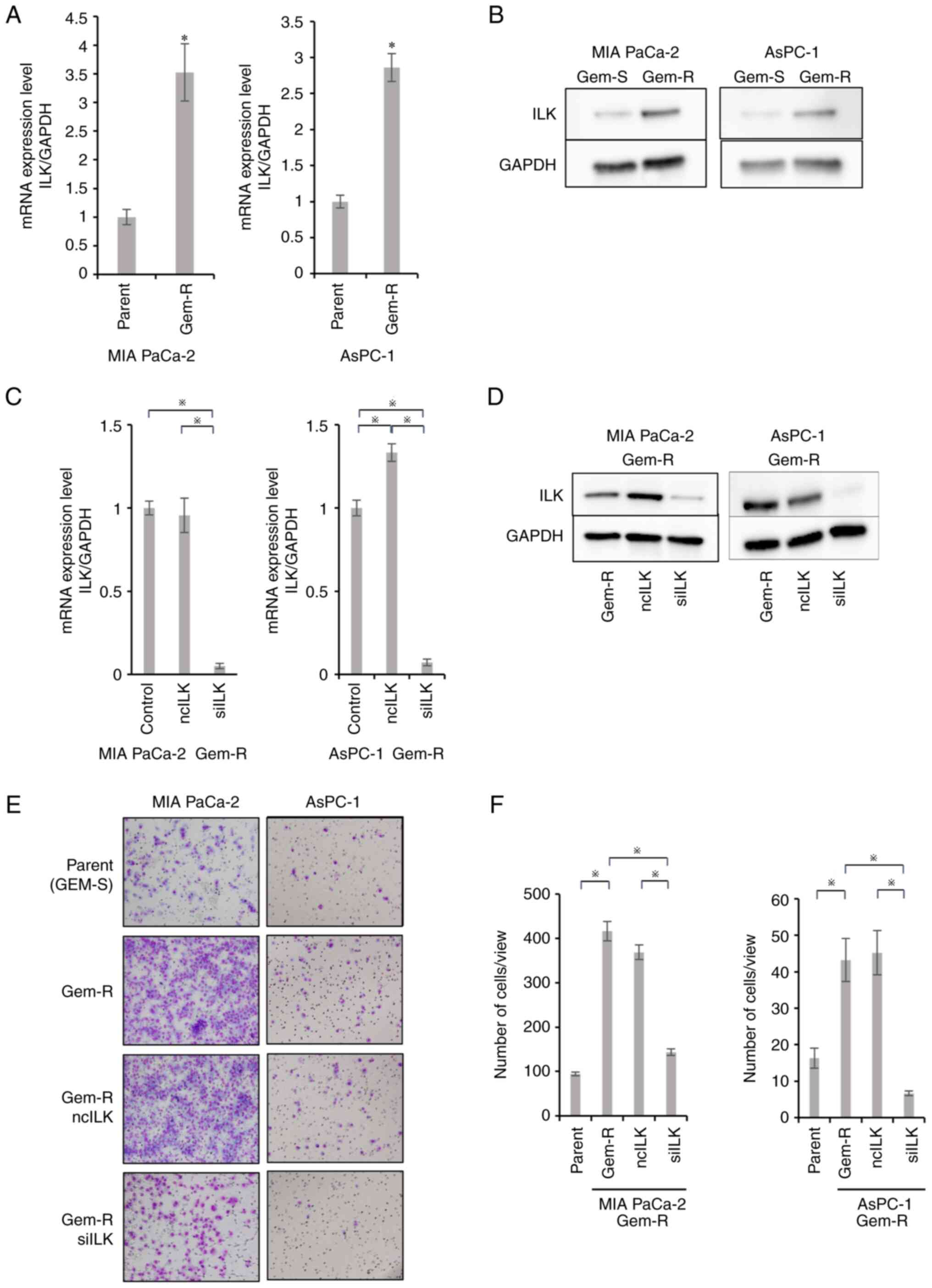
Enhanced expression of ILK in Gem-R
PaCa cells
RT-qPCR showed that ILK mRNA expression was
significantly enhanced in Gem-R PaCa cells compared with
Gem-sensitive (Gem-S) PaCa cells (P<0.05; Fig. 4A). Western blotting also confirmed
that ILK protein expression was enhanced, consistent with the
results of PCR (Fig. 4B).
Changes in ILK expression levels after
ILK knockdown in Gem-R PaCa cell lines
RT-qPCR and western blotting were performed to
assess changes in ILK expression in Gem-R PaCa cells transfected
with ILK siRNA. ILK expression was downregulated in
Gem-R PaCa cells transfected with ILK siRNA compared with
that in control cells or cells transfected with negative control
siRNA (P<0.05; Fig. 4C and
D).
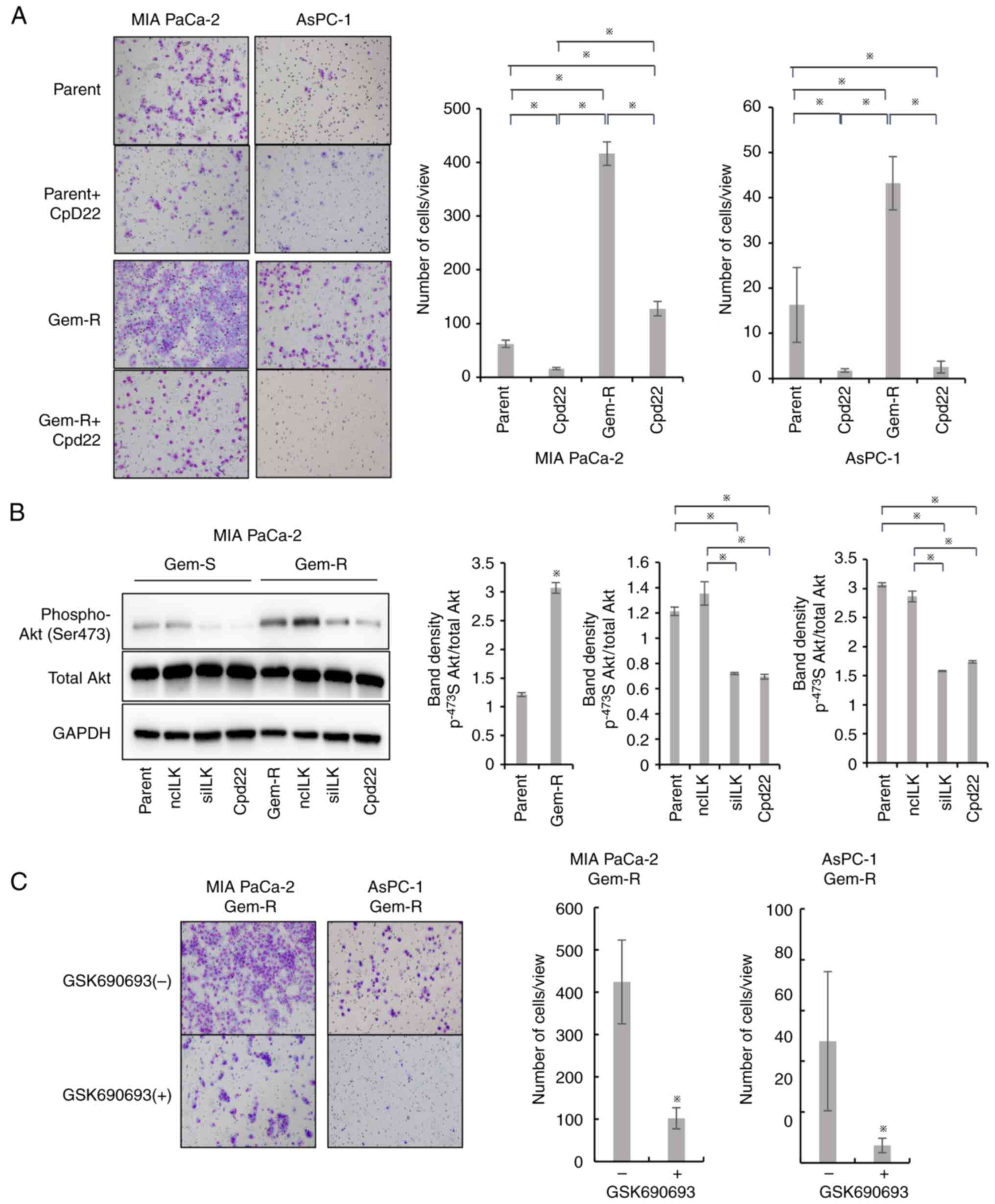
Enhanced invasive potential of Gem-R
PaCa cells and effects of ILK knockdown on cell invasive
potential
Matrigel invasion assays were performed to evaluate
the invasive potential of Gem-R PaCa cells. Gem-R PaCa cells showed
significantly higher invasive ability than Gem-S cells.
Furthermore, ILK siRNA transfection inhibited the invasive
ability, similar to that observed in Gem-S cells (P<0.05;
Fig. 4E and F).
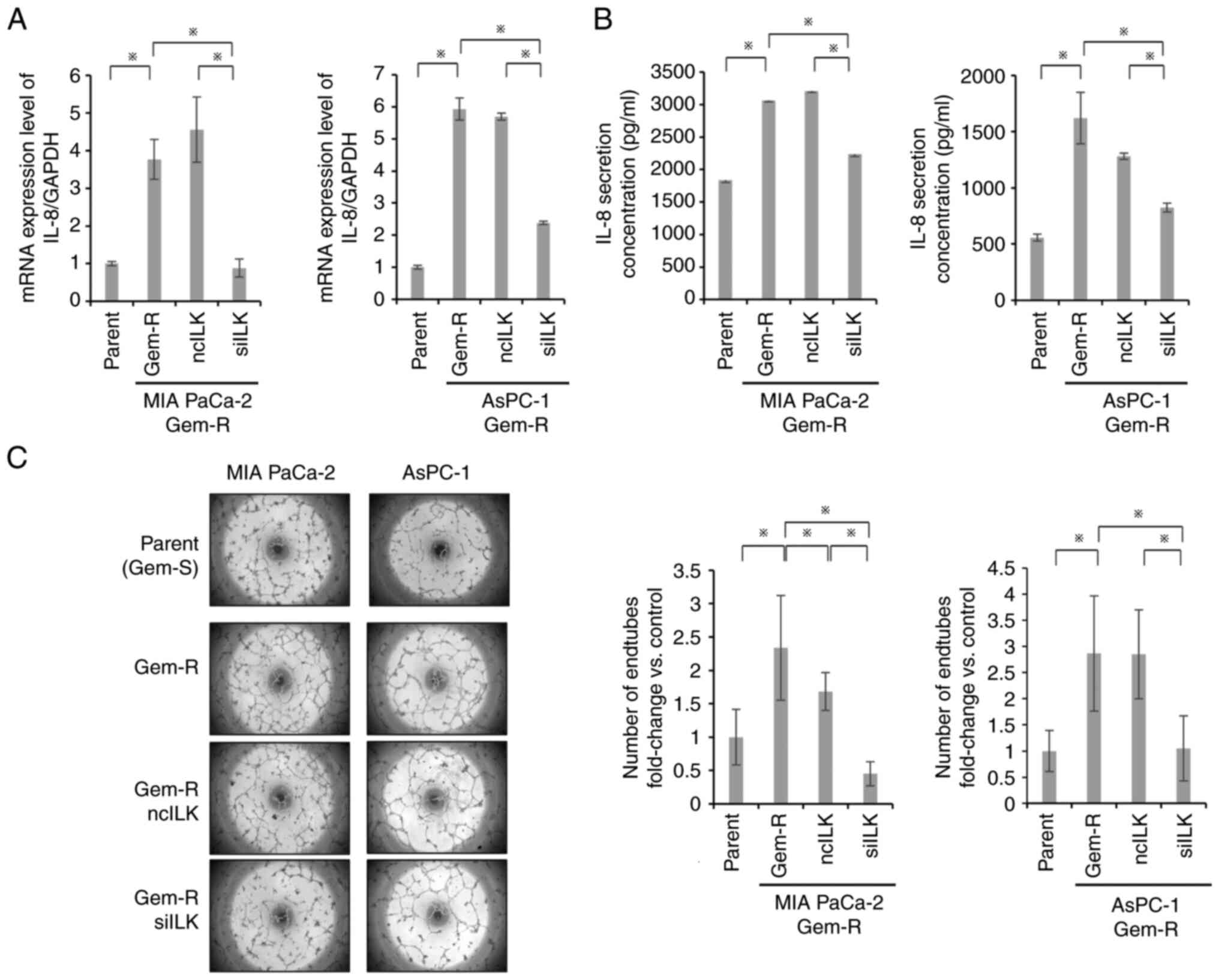
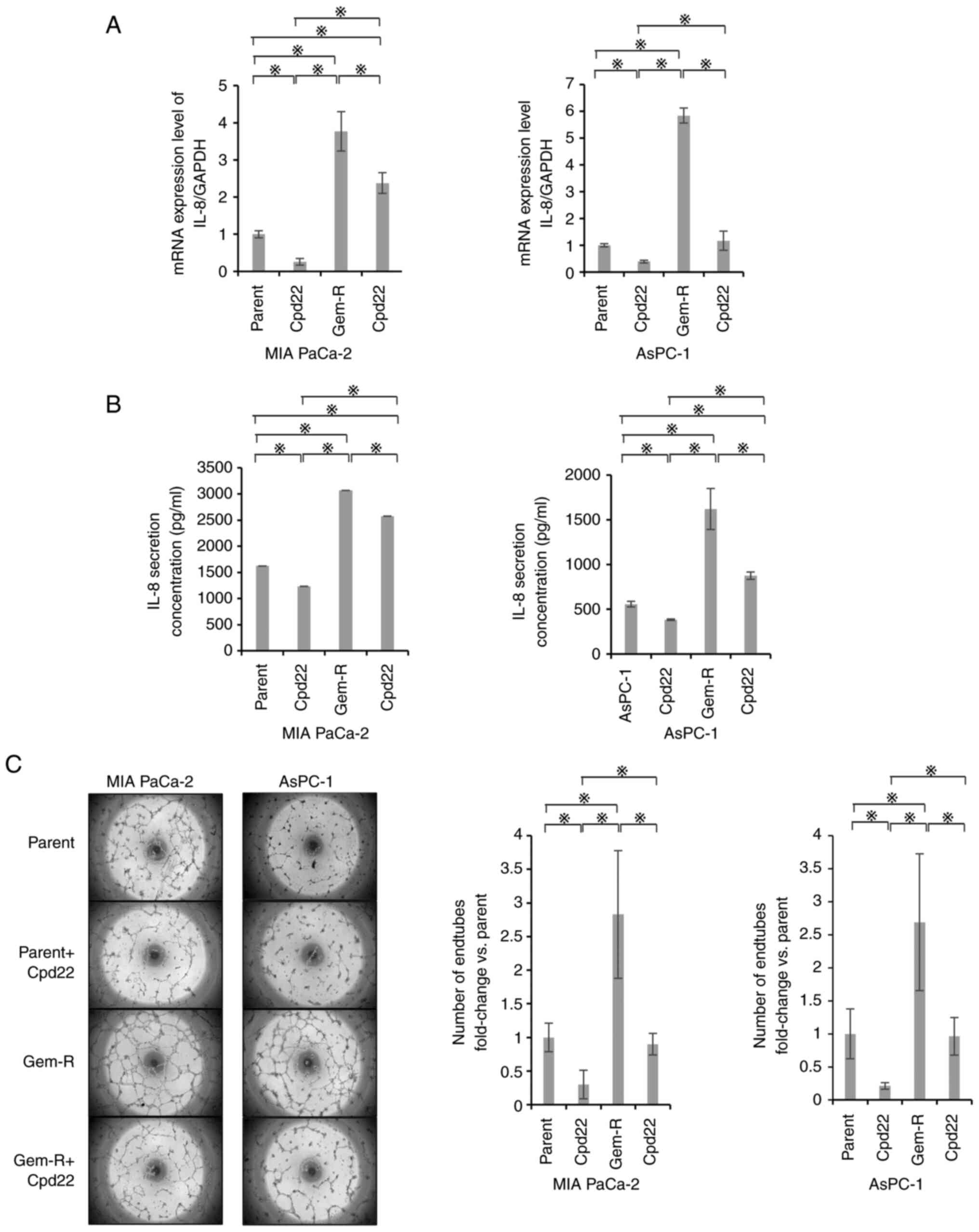
Angiogenic potential of Gem-R PaCa
cells and effects of ILK knockdown
RT-qPCR and ELISA were used to evaluate IL-8 in
Gem-R PaCa cells. Notably, Gem-R PaCa cells showed significantly
higher IL-8 mRNA expression and IL-8 protein secretion than
Gem-S PaCa cells (P<0.05; Fig. 5A
and B). Tube formation assays showed that tube formation was
enhanced in Gem-R PaCa cells compared with that in Gem-S PaCa cells
and was significantly suppressed in the ILK-knockdown group
(P<0.05; Fig. 5C).
Effects of Cpd22 on proliferation in
Gem-S and Gem-R PaCa cells
Gem-S and Gem-R PaCa cells were incubated with
various concentrations of Cpd22 for 72 h, and the effects of Cpd22
on cell proliferation were then examined using WST-1 assays. Cpd22
inhibited proliferation in both Gem-R and Gem-S PaCa cell lines in
a concentration-dependent manner. The IC50 values, as
calculated from the WST-1 assay results, were as follows: 1.96 µM
for PANC-1, 2.54 µM for SW1990, 2.05 µM for AsPC-1, and 2.16 µM for
MIA PaCa-2 (Fig. 6A and B). By
contrast, the IC50 values for Gem-R AsPC-1 and Gem-R MIA
PaCa-2 cells were 2.48 and 2.83 µM, respectively. To avoid the
cytotoxic effects of Cpd22, the concentration of Cpd22 was set
below the IC50 value in subsequent experiments.
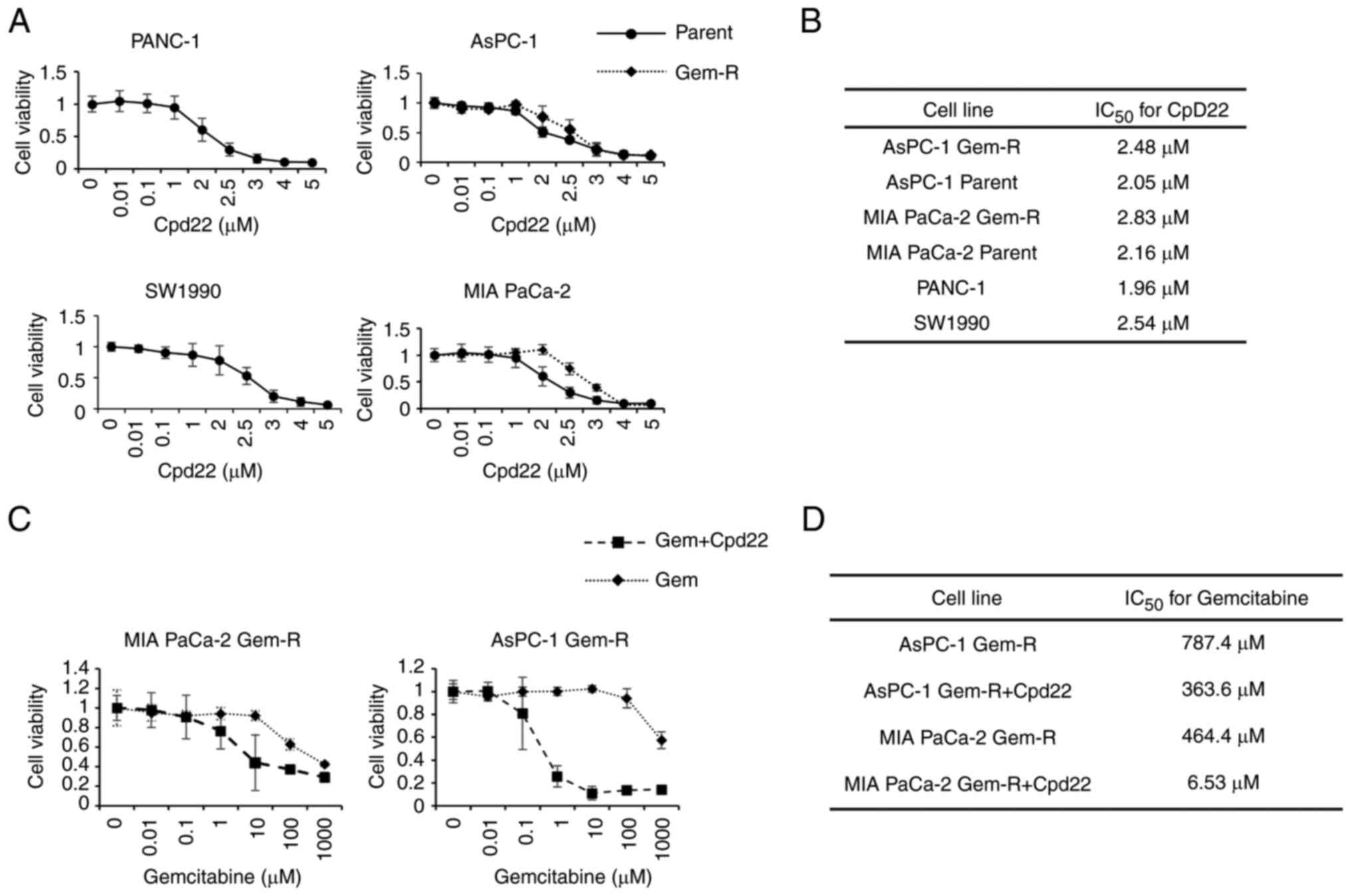 | Figure 6.Inhibitory effects of Cpd22 on the
proliferation of Gem-S (PANC-1, SW1990, AsPC-1, MIA PaCa-2) and
Gem-R (AsPC-1, MIA PaCa-2) PaCa cell lines. (A) Cells were exposed
to Cpd22 at the indicated concentrations for 48 h, and the degree
of cell proliferation was evaluated using WST-1 assays. (B)
IC50 values for Cpd22 were measured in PANC-1, SW1990,
AsPC-1 Gem-S and Gem-R, and MIA PaCa-2 Gem-S and Gem-R cell lines.
(C) Additive effects of Cpd22 on Gem sensitivity in Gem-R PaCa
cells. Cells were exposed to Gem and 1 µM Cpd22 for 48 h, and the
proliferation of each cell line was then evaluated using WST-1
assays. (D) The IC50 for Gem was measured in MIA-PaCa2
and AsPC-1 cells. Cpd22, compound 22; Gem, gemcitabine, Gem-S,
gemcitabine-sensitive; Gem-R, gemcitabine-resistant,
IC50, half-maximal inhibitory concentration. |
Effects of Cpd22 on the drug
sensitivity of Gem-R cells
Cpd22 was added to cells at 1 µM (a concentration
that did not affect cell proliferation ability) in combination with
Gem, and cells were cultured for 72 h. The sensitivity of AsPC-1
Gem-R cells to Gem increased from 787.4 to 363.6 nM, whereas that
of MIA PaCA-2 Gem-R cells increased from 464.4 to 6.53 µM when
Cpd22 was added (Fig. 6C and
D).
Effects of Cpd22 on the invasive
potential of PaCa cells
Matrigel invasion assays were performed to evaluate
the invasive potential of PaCa cell lines via ILK suppression by
Cpd22. In Gem-S and Gem-R PaCa cells (MIA PaCa-2, AsPC-1), cell
invasion ability was significantly suppressed in the Cpd22 group
compared with that in the untreated group (P<0.05; Fig. 7A).
Differences in the ILK/Akt pathway
between Gem-S and Gem-R cells
Inhibition of ILK suppressed the phosphorylation of
Akt at Ser473 in both Gem-S and Gem-R cell lines. The
phosphorylation of Akt at Ser473 was significantly enhanced in
Gem-R cells compared with that in Gem-S cells (P<0.05; Fig. 7Β). The phosphorylation of Akt at
Ser473, which was enhanced in Gem-R PaCa cells, was suppressed to
the same extent as in the parental lines by inhibition of ILK.
Effect of GSK on the invasive
potential of Gem-R PaCa cell lines
Matrigel invasion assay was performed to evaluate
the invasive potential of PaCa cell lines via suppression of Akt by
GSK. In Gem-R PaCa cells (MIA PaCa-2, AsPC-1), cell invasive
potential was significantly suppressed in the GSK group compared to
the untreated group (P<0.05; Fig.
7C).
Effects of Cpd22 on the angiogenic
potential of PaCa cells
RT-qPCR and ELISA were used to evaluate the effects
of Cpd22-dependent ILK suppression on the secretory capacity of
IL-8. In Gem-S cells, mRNA expression was significantly suppressed
by Cpd22. IL-8 secretion was significantly suppressed in the Cpd22
group compared with that in the untreated group in MIA PaCa-2 and
AsPC-1 cells (P<0.05; Fig. 8A and
B). In Gem-R cells, both mRNA expression and protein secretion
were suppressed. Tube formation assays also showed that
angiogenesis was significantly suppressed in the Cpd22 group for
both Gem-S and Gem-R cells (P<0.05; Fig. 8C).
Discussion
The aim of the present study was to clarify the
roles of ILK in the resistance of PaCa cells to Gem and to
determine whether Cpd22, an ILK inhibitor, exerted antitumor
effects on Gem-S and Gem-R PaCa cells. The results of the present
study confirmed that ILK was upregulated in Gem-R cells compared
with that in Gem-S cells. Furthermore, invasion and angiogenesis
assays demonstrated that invasive and angiogenic potentials were
enhanced with ILK expression and that the malignant potential of
the cells was increased. By contrast, inhibition of ILK expression
suppressed invasiveness and angiogenesis in both Gem-S and Gem-R
cells, and Cpd22, an ILK inhibitor, suppressed tumor growth,
invasiveness, and angiogenesis in both Gem-S and Gem-R cells. The
use of Cpd22 in addition to Gem improved Gem sensitivity in Gem-R
cells. Overall, the findings of the present study also suggested
that the degree of ILK expression contributed to malignancy in PaCa
cells.
ILK has been demonstrated to interfere with the Akt,
GSK-3β, and HIPPO pathways (17)
and is involved in various mechanisms related to tumor progression,
such as cell proliferation, apoptosis, invasion, and angiogenesis
(9). Increased ILK expression has
been reported in numerous malignant tumors, including lung
(18), ovarian (19), colon (20,21),
stomach (22), and liver cancers
(23). Increased ILK expression may
be associated with prognosis (8).
The authors of the present study previously reported that ILK is
significantly and strongly expressed in patients with PaCa and that
high ILK expression and retroperitoneal invasion are poor
prognostic factors (24). In the
present study, it was determined that ILK expression tended to be
higher in Gem-S PaCa cells than in HPDE cells. Moreover, ILK
expression was even higher in more aggressive Gem-R PaCa cells than
in Gem-S cells, providing support for the association between ILK
and tumorgenicity.
The relationship between ILK and invasive potential
has been discussed in previous studies. The invasive potential of
PaCa is markedly high, resulting in low resectability rates.
Additionally, ILK has been suggested to be involved in invasion
through promotion of the EMT and lobular pseudopodia formation
(7,9,25). ILK
has also been demonstrated to be involved in invasion in PaCa cells
(24). In the present study, ILK
suppression in Gem-S PaCa cells blocked invasive potential,
consistent with previous findings (24). Notably, invasive ability was
enhanced in Gem-R PaCa cells in which ILK was upregulated compared
with that in Gem-S PaCa cells. However, when ILK was suppressed,
invasive ability was also inhibited to the same extent as that in
Gem-S PaCa cells. It was also demonstrated that ILK is likely to
affect Akt to regulate invasive potential. The Akt pathway
functions downstream of ILK (26,27).
Akt is a signal transducer that is frequently activated in PaCa and
is associated with tumor aggressiveness (28), and the relationship between the Akt
pathway and tumor invasiveness is widely known. In the present
study, it was determined that phosphorylation of Akt was enhanced
in Gem-R PaCa cells in which ILK expression was upregulated
compared with that in Gem-S PaCa cells. Furthermore, Akt
phosphorylation was found to be suppressed to the same level as in
Gem-S PaCa cells when ILK expression was suppressed. GSK690393 is
an Akt inhibitor with strong selectivity for Akt kinase (29). To investigate whether inhibition of
Akt can suppress the invasive potential of Gem-R PaCa cells with
increased expression of ILK, the changes in invasive potential
after exposure to GSK690393 were evaluated. Inhibition of Akt
activity in the Gem-R PaCa cells suppressed invasive potential.
While several pathways, including CXCL12/CXCR4 signaling (30) and NF-κB (31), as well as Akt, are reported to be
involved in the invasive potential of cancer, the enhancement of
the Akt pathway by upregulation of ILK may be involved in the
enhancement of the invasive potential of Gem-R PaCa cells.
Subsequently, the angiogenic potential of the cells
was evaluated. Several studies have shown that ILK promotes
angiogenesis via vascular endothelial growth factor in cancer
(32,33). PaCa, which is generally considered
an ischemic tumor, has a high microvascular density, and the
association between microvascular density and prognosis has been
noted (34,35). It has been previously reported by
the authors that IL-8 is involved in angiogenesis via the
IL-8/CXCR2 axis in PaCa and that IL-8 expression is upregulated in
Gem-R PaCa cell lines (15,36). In addition, Akt has been shown to be
involved in the regulation of IL-8 (37,38).
Therefore, the association between ILK and IL-8 were assessed and
it was revealed that there was a relationship between the intensity
of ILK/Akt pathway activity and the transcription and secretion of
IL-8. Although further validation of the signaling pathway is
required, the results of the present study suggest that ILK may be
involved in the expression of IL-8 and may act to promote
angiogenesis.
Cpd22, an ILK inhibitor, is a cell-permeable
drug-like compound that has been reported to inhibit cell
proliferation (IC50, 1–2.5 µM) in vitro and to
exert antiproliferative effects by inducing apoptosis. Cpd22 was
revealed to block the Akt Ser473 phosphorylation, a downstream
signal of ILK (9). In the present
study, Cpd22 was used at the same concentration as previously
reported and it was demonstrated that this concentration inhibited
the proliferation of both Gem-R and Gem-S PaCa cells. Furthermore,
Cpd22 inhibited the invasive and angiogenic potentials of these
cell lines. Similar to the suppression of ILK expression using
ILK siRNA, the suppression of ILK activity by Cpd22 was able
to block the phosphorylation of Akt at Ser473.
ILK may be involved in chemotherapy resistance. In
fact, ILK has been shown to be associated with the mechanism of
chemotherapy resistance via the EMT, cancer stem cell markers, and
the MRP1 pathway (6,39,40).
In PaCa cells, ILK overexpression increases resistance to Gem
chemotherapy by enhancing the phosphorylation of Akt and GSK-3β,
whereas inhibition of ILK expression has been reported to induce
Gem-induced apoptosis (5). Because
activation of the Akt pathway resists apoptosis and phosphorylation
of Akt is inhibited by Cpd22, the improved sensitivity may be the
result of increased induction of apoptosis by Gem when used in
combination with Cpd22. To the best of our knowledge, this is the
first study to confirm the roles of ILK and the effects of ILK
inhibitors using established Gem-R PaCa cells. The findings of the
present study strongly indicated that Cpd22 exerted antitumor
effects against PaCa cells and could be effective even after the
cells acquired Gem resistance, supporting the potential application
of Cpd22 as a novel therapeutic agent.
The present study had several limitations. Firstly,
the behaviors of PaCa cell lines were not evaluated when ILK was
overexpressed. In addition, animal studies are required to confirm
the observed effects in vivo.
In conclusion, the results of the present study
suggested that Gem-R PaCa cells exerted higher invasive and
angiogenic potential and increased aggressiveness mediated by
enhanced ILK/Akt signaling. Cpd22, an ILK inhibitor, suppressed the
invasive and angiogenic potential of the cells and blocked cell
proliferation in Gem-S and Gem-R PaCa cell lines. Cpd22 also
enhanced Gem sensitivity in Gem-R cells. Therefore, Cpd22 may be an
effective alternative therapy in patients with PaCa after
acquisition of Gem resistance.
Acknowledgements
The authors would like to thank Ms Seiko Inumaru
(Department of Gastroenterology, Nagoya City University Graduate
School of Medicine, Nagoya, Japan), a laboratory assistant, for
preparing the experimental reagents.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated or analyzed in this study are
included in the published article.
Authors' contributions
HM and YM contributed to the conception and design
of this study, analyzed and interpreted the data, and wrote and
reviewed the manuscript. HM, TK, YM, YH, HI, KS, MM, HT and ST were
responsible for the design of this study. HM, TK, YA, YM, KN and YD
acquired the data. TK, MK, AM and YM confirm the authenticity of
all raw data. HM, YD, KN, YH, MM and RO wrote the Materials and
methods section of the manuscript. YM, HT, RO and ST assisted in
the technical, administrative, and material aspects of performing
RT-qPCR, western blotting, and invasion assays. YM and ST provided
technical, administrative, and material support. YM supervised the
study. All authors have read the final manuscript and are equally
responsible for all aspects of the study and guarantee its
completeness and accuracy.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oettle H, Post S, Neuhaus P, Gellert K,
Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C,
et al: Adjuvant chemotherapy with gemcitabine vs observation in
patients undergoing curative-intent resection of pancreatic cancer:
A randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duxbury MS, Ito H, Benoit E, Waseem T,
Ashley SW and Whang EE: RNA interference demonstrates a novel role
for integrin-linked kinase as a determinant of pancreatic
adenocarcinoma cell gemcitabine chemoresistance. Clin Cancer Res.
11:3433–3438. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia Z: Role of integrin-linked kinase in
drug resistance of lung cancer. Onco Targets Ther. 8:1561–1565.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McDonald PC and Dedhar S: New perspectives
on the role of integrin-linked kinase (ILK) signaling in cancer
metastasis. Cancers (Basel). 14:32092022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McDonald PC, Fielding AB and Dedhar S:
Integrin-linked kinase-essential roles in physiology and cancer
biology. J Cell Sci. 121:3121–3132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ning Z, Zhu X, Jiang Y, Gao A, Zou S, Gu
C, He C, Chen Y, Ding WQ and Zhou J: Integrin-linked kinase is
involved in the proliferation and invasion of esophageal squamous
cell carcinoma. J Cancer. 11:324–333. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ji C, Zhang M, Hu J, Cao C, Gu Q, Liu Y,
Li X, Xu D, Ying L, Yang Y, et al: The kinase activity of
integrin-linked kinase regulates cellular senescence in gastric
cancer. Cell Death Dis. 13:5772022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li B, Wang X, Wang R, Rutz B, Ciotkowska
A, Gratzke C, Herlemann A, Spek A, Tamalunas A, Waidelich R, et al:
Inhibition of neurogenic and thromboxane A2-induced
human prostate smooth muscle contraction by the integrin α2β1
inhibitor BTT-3033 and the integrin-linked kinase inhibitor Cpd22.
Prostate. 80:831–849. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sarı Kılıçaslan SM and İncesu Z: Effects
of integrin-linked kinase on protein kinase b, glycogen synthase
kinase-3β, and β-catenin molecules in ovarian cancer cells. Iran J
Basic Med Sci. 24:1500–1508. 2021.PubMed/NCBI
|
|
13
|
Yau CY, Wheeler JJ, Sutton KL and Hedley
DW: Inhibition of integrin-linked kinase by a selective small
molecule inhibitor, QLT0254, inhibits the PI3K/PKB/mTOR, Stat3, and
FKHR pathways and tumor growth, and enhances gemcitabine-induced
apoptosis in human orthotopic primary pancreatic cancer xenografts.
Cancer Res. 65:1497–1504. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng S, Pöttler M, Lan B, Grützmann R,
Pilarsky C and Yang H: Chemoresistance in Pancreatic Cancer. Int J
Mol Sci. 20:45042019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Imafuji H, Matsuo Y, Ueda G, Omi K,
Hayashi Y, Saito K, Tsuboi K, Morimoto M, Koide S, Ogawa R, et al:
Acquisition of gemcitabine resistance enhances angiogenesis via
upregulation of IL8 production in pancreatic cancer. Oncol Rep.
41:3508–3516. 2019.PubMed/NCBI
|
|
16
|
Bustin SA: Quantification of mRNA using
real-time reverse transcription PCR (RT-PCR): trends and problems.
J Mol Endocrinol. 29:23–39. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Serrano I, McDonald PC, Lock F, Muller WJ
and Dedhar S: Inactivation of the Hippo tumour suppressor pathway
by integrin-linked kinase. Nat Commun. 4:29762013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takanami I: Increased expression of
integrin-linked kinase is associated with shorter survival in
non-small cell lung cancer. BMC Cancer. 5:12005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahmed N, Riley C, Oliva K, Stutt E, Rice
GE and Quinn MA: Integrin-linked kinase expression increases with
ovarian tumour grade and is sustained by peritoneal tumour fluid. J
Pathol. 201:229–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marotta A, Parhar K, Owen D, Dedhar S and
Salh B: Characterisation of integrin-linked kinase signalling in
sporadic human colon cancer. Br J Cancer. 88:1755–1762. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bravou V, Klironomos G, Papadaki E,
Stefanou D and Varakis J: Integrin-linked kinase (ILK) expression
in human colon cancer. Br J Cancer. 89:2340–2341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ito R, Oue N, Zhu X, Yoshida K, Nakayama
H, Yokozaki H and Yasui W: Expression of integrin-linked kinase is
closely correlated with invasion and metastasis of gastric
carcinoma. Virchows Arch. 442:118–123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peroukides S, Bravou V, Varakis J,
Alexopoulos A, Kalofonos H and Papadaki H: ILK overexpression in
human hepatocellular carcinoma and liver cirrhosis correlates with
activation of Akt. Oncol Rep. 20:1337–1344. 2008.PubMed/NCBI
|
|
24
|
Sawai H, Okada Y, Funahashi H, Matsuo Y,
Takahashi H, Takeyama H and Manabe T: Integrin-linked kinase
activity is associated with interleukin-1 alpha-induced progressive
behavior of pancreatic cancer and poor patient survival. Oncogene.
25:3237–3246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. J Cell Sci. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Troussard AA, Mawji NM, Ong C, Mui A,
St–Arnaud R and Dedhar S: Conditional knock-out of integrin-linked
kinase demonstrates an essential role in protein kinase B/Akt
activation. J Biol Chem. 278:22374–22378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qu Y, Hao C, Xu J, Cheng Z, Wang W and Liu
H: ILK promotes cell proliferation in breast cancer cells by
activating the PI3K/Akt pathway. Mol Med Rep. 16:5036–5042. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schlieman MG, Fahy BN, Ramsamooj R,
Beckett L and Bold RJ: Incidence, mechanism and prognostic value of
activated AKT in pancreas cancer. Br J Cancer. 89:2110–2115. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heerding DA, Rhodes N, Leber JD, Clark TJ,
Keenan RM, Lafrance LV, Li M, Safonov IG, Takata DT, Venslavsky JW,
et al: Identification of 4- (2- (4- amino- 1,2,5- oxadiazol- 3-
yl)- 1- ethyl- 7- {[(3S)- 3-piperidinylmethyl]oxy}-
1H-imidazo[4,5-c]pyridin- 4-yl)- 2- methyl- 3- butyn- 2- ol
(GSK690693), a novel inhibitor of AKT kinase. J Med Chem.
51:5663–5679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kato T, Matsuo Y, Ueda G, Murase H, Aoyama
Y, Omi K, Hayashi Y, Imafuji H, Saito K, Morimoto M, et al:
Enhanced CXCL12/CXCR4 signaling increases tumor progression in
radiation-resistant pancreatic cancer. Oncol Rep. 47:682022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maier HJ, Schmidt-Strassburger U, Huber
MA, Wiedemann EM, Beug H and Wirth T: NF-kappaB promotes
epithelial-mesenchymal transition, migration and invasion of
pancreatic carcinoma cells. Cancer Lett. 295:214–228. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng CC, Hu HF, Hong P, Zhang QH, Xu WW,
He QY and Li B: Significance of integrin-linked kinase (ILK) in
tumorigenesis and its potential implication as a biomarker and
therapeutic target for human cancer. Am J Cancer Res. 9:186–197.
2019.PubMed/NCBI
|
|
33
|
Tan C, Cruet-Hennequart S, Troussard A,
Fazli L, Costello P, Sutton K, Wheeler J, Gleave M, Sanghera J and
Dedhar S: Regulation of tumor angiogenesis by integrin-linked
kinase (ILK). Cancer Cell. 5:79–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stipa F, Lucandri G, Limiti MR, Bartolucci
P, Cavallini M, Di Carlo V, D'Amato A, Ribotta G and Stipa S:
Angiogenesis as a prognostic indicator in pancreatic ductal
adenocarcinoma. Anticancer Res. 22:445–449. 2002.PubMed/NCBI
|
|
35
|
Benckert C, Thelen A, Cramer T, Weichert
W, Gaebelein G, Gessner R and Jonas S: Impact of microvessel
density on lymph node metastasis and survival after curative
resection of pancreatic cancer. Surg Today. 42:169–176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsuo Y, Ochi N, Sawai H, Yasuda A,
Takahashi H, Funahashi H, Takeyama H, Tong Z and Guha S: CXCL8/IL-8
and CXCL12/SDF-1alpha co-operatively promote invasiveness and
angiogenesis in pancreatic cancer. Int J Cancer. 124:853–861. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grzesiak JJ, Smith KC, Burton DW, Deftos
LJ and Bouvet M: GSK3 and PKB/Akt are associated with
integrin-mediated regulation of PTHrP, IL-6 and IL-8 expression in
FG pancreatic cancer cells. Int J Cancer. 114:522–530. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Tang C, Cao H, Li K, Pang X, Zhong
L, Dang W, Tang H, Huang Y, Wei L, et al: Activation of IL-8 via
PI3K/Akt-dependent pathway is involved in leptin-mediated
epithelial-mesenchymal transition in human breast cancer cells.
Cancer Biol Ther. 16:1220–1230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tsoumas D, Nikou S, Giannopoulou E,
Champeris Tsaniras S, Sirinian C, Maroulis I, Taraviras S, Zolota
V, Kalofonos HP and Bravou V: ILK Expression in colorectal cancer
is associated with EMT, Cancer stem cell markers and
chemoresistance. Cancer Genomics Proteomics. 15:127–141.
2018.PubMed/NCBI
|
|
40
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|