Introduction
There are numerous microorganisms in the human
intestinal tract, the number of which is >10-fold that of human
cells, which can be divided into beneficial bacteria for human
vitamin synthesis, food digestion and inhibition of the production
of toxic substances; harmful bacteria that affect the function of
the immune system and produce harmful substances; and neutral
bacteria with dual roles, such as Escherichia coli (1). There is a dynamic balance between the
intestinal flora and the host, which can maintain the normal
physiological activities of the host. When the flora is imbalanced,
it can cause inflammation and even diseases. The tumor
microenvironment is the internal environment of tumor cells, which
is not only limited to tumor cells but also includes various
components of the microenvironment and nearby interstitial cells,
blood vessels, cytokines and biomolecules (2). The present study conducted a computer
search for articles related to the intestinal flora and the tumor
microenvironment published in the Wan fang Data Knowledge Service
Platform (https://www.wanfangdata.com.cn), CNKI (https://www.cnki.net), China Biomedical Literature
Service System (http://www.sinomed.ac.cn/zh/), PubMed (https://pubmed.ncbi.nlm.nih.gov/advanced/) and Medline
databases (https://ovidsp.ovid.com/autologin.cgi). The search
time limit was from the establishment of each database until
February 2023. The literature search revealed that recent studies
have shifted their focus from the target of tumor treatment to the
tumor microenvironment, and have revealed that the intestinal flora
plays an important role in the regulation of the tumor
microenvironment (3). The present
review describes in detail the specific mechanisms of intestinal
microflora affecting tumor microenvironment and introduces the
application status and potential biological targets of intestinal
microflora in tumor microenvironment intervention, aiming to
provide a new direction for disease intervention and treatment.
Gut flora
There is a large number of symbiotic bacteria in the
human gut, which dynamically changes under the influence of dietary
habits, drug use and specific physiological conditions. The type
and abundance of Gut microbiota are affected by genetic,
environmental and economic factors as well as living habits, and
cohabitation factors are more influential than genetic factors
(4). With the continuous
development and improvement of modern sequencing technology,
genomic technology and in vitro culture technology of
intestinal flora, the importance and mechanism of intestinal flora
and various diseases have been gradually revealed. Intestinal flora
can not only act as an intestinal barrier to resist the invasion of
pathogens (5), but also play a role
in the occurrence of various diseases such as solid tumors
(colorectal, lung, and pancreatic cancer, etc.) and other diseases
(leukemia, Alzheimer's disease, etc.), and their development and
treatment are inextricably associated (Table I) (6–16).
 | Table I.Types and mechanisms of gut
microbiota in different tumors. |
Table I.
Types and mechanisms of gut
microbiota in different tumors.
| Tumor type | Cancer type | Change in relevant
flora | Mechanism of
action | (Refs.) |
|---|
| Solid tumors | Colorectal
cancer | Bacteroides
fragilis, Escherichia coli, Streptococcs bovis gallolyticus,
Enteroccocus faecalis, Fusobacterium nucleatum increase | Changes in flora
promote the secretion of inflammatory mediators and produce
reactive oxygen species | (6) |
|
| Liver cancer | Microflora
depletion | Produces bile acid
and affects the liver immune function | (7) |
|
| Pancreatic
cancer | Helicobacter
pylori, Pseudomonas aeruginosa, Bifidobacterium | Immune reaction,
inflammation, anti-apoptosis | (8) |
|
| Thyroid
carcinoma | Increased number of
bacteria causing cancer and inflammation | Impaired intestinal
barrier and increased intestinal permeability make it easier for
antigens to pass through and stimulate the immune system;
microflora affects the supply of essential micronutrients | (9) |
|
| Lung cancer |
Firmicutes/Bacteroidetes reduction | Decreased
circulating SCFAs, thus affecting host system immunity and
inflammation | (10) |
| Other | Leukemia | Change in flora
abundance | Biological
antagonism, immune regulation | (11) |
| diseases | Chronic stress | Change in
flora | Brain gut axis and
ileum immune regulation | (12) |
|
| Alzheimer's
disease | Helicobacter
pylori infection | Altered level of
certain neurotransmitters, proteins and receptors involved in
synaptic plasticity | (13) |
|
| Diabetes | Significantly
decreased number of Bifidobacterium, Clostridium and
Firmicutes, and increased number of enterococcal feces | Low level of SCFAs
leading to intestinal inflammation and insulin resistance | (14) |
|
| Parkinson's
disease | Chronic stress
promotes inflammation in the intestinal environment and increases
intestinal permeability | Brain-intestine
axis | (15) |
|
| Bipolar
depression | Abundance of
Akkermania muciniphila and Citrobacter spp. | Brain-intestine
axis | (16) |
Tumor microenvironment
The tumor microenvironment is a special biological
environment formed by changes in the surrounding tissue structure
during tumor growth and development. It was first described as
‘seed and soil’, with tumor cells as seeds, and the appropriate
target organ and growth environment called the tumor
microenvironment (17). In addition
to ‘seed’ tumor cells, the tumor microenvironment also includes
immune cells, adipocytes, stromal cells, extracellular matrix and
acellular components (cytokines, signaling molecules and
chemokines), which together provide nutrition, blood vessels,
collagen and signaling molecules to form a complex and dynamic
network system that provides support for the occurrence,
proliferation, metastasis and immune escape of tumor cells
(18). Since tumor cells have the
characteristic of malignant proliferation, they consume large
quantities of oxygen and nutrients in the soil, which is
accompanied by the production of reducing substances (reactive
oxygen species). Previous studies have revealed that a hypoxic
microenvironment can promote tumor resistance (19), an acidic microenvironment is
conducive to tumor cell metastasis (20) and highly reducing substances affect
tumor treatment (21). The tumor
microenvironment is an important condition to support tumor growth,
and in-depth research and effective regulation of it will provide
effective means for tumor treatment (Table II) (22–27).
 | Table II.Tumor microenvironment
components. |
Table II.
Tumor microenvironment
components.
| Tumor
microenvironment components | Physiological
function | Association with
tumor | (Refs.) |
|---|
| CAFs | Stroma cell | Stimulation of
tumor cell proliferation, invasion and metastasis; chemotherapy
resistance; inhibition of T cells in tumors; regulation of the
inflammatory response and immune system | (22) |
| TANs | Contain multiple
lysosomes and havee strong chemotaxis and phagocytosis
capacities | Recruitment of
macrophages and regulation of T cells | (23) |
| TAMs | First line of
defense against microbial infection | Promotion of cancer
cell proliferation, angiogenesis, metastasis and
immunosuppression | (24) |
| MDSCs | Myeloid suppressor
cells have heterogeneity and differentiation potential, and usually
play an immunosuppressive role in the tumor microenvironment | Immunosuppression
promotes tumor cell proliferation and metastasis | (25) |
| DCs | As the main
antigen-presenting cells, DCs are the bridge between adaptive and
innate immunity |
Immunosuppression | (26) |
| Hyaluronic
acid | Extracellular
matrix | Promotion of tumor
cell proliferation, invasion, immune escape and drug
resistance | (27) |
Gut microbiota regulates the tumor
microenvironment
In recent years, with the continuous development of
technology and the increase in research, the concept of the tumor
biological microenvironment has been proposed, which includes cell
metabolites, the immune system, systemic metabolism, body
circulation, and intestinal flora related to tumorigenesis,
development and metastasis (28).
Among these, the intestinal flora plays the most significant
regulatory role on the tumor microenvironment, mainly through
changes in the flora, brain-gut axis,
hypothalamus-pituitary-adrenal axis, gut-liver axis and bacterial
translocation, which affect the physiological state of target
organs from a long distance. This, in turn, creates a favorable
environment for tumor invasion (29–31).
Regulation of the components of the
tumor microenvironment
Dendritic cells (DCs)
DCs can be divided into conventional DCs and
plasmacytoid DCs (pDCs), and the two phenotypes interact to
maintain the morphology of DCs and the antigen expression capacity
of CD8+ T cells. A previous study has demonstrated that
DC antitumor activity is activated under specific conditions, and,
after maturation, T cells are stimulated to produce the cytokine
IL-2 to convert macrophages in the tumor microenvironment to the M1
phenotype (32). It was revealed
that the antitumor function of DCs was enhanced by injecting
lipopolysaccharide (LPS) into antibiotic-treated mice in
vitro; thus, it was speculated that the microflora may contact
or migrate to the tumor site to form an antitumor microenvironment
through the bacterial components similar to LPS (33) (Fig.
1).
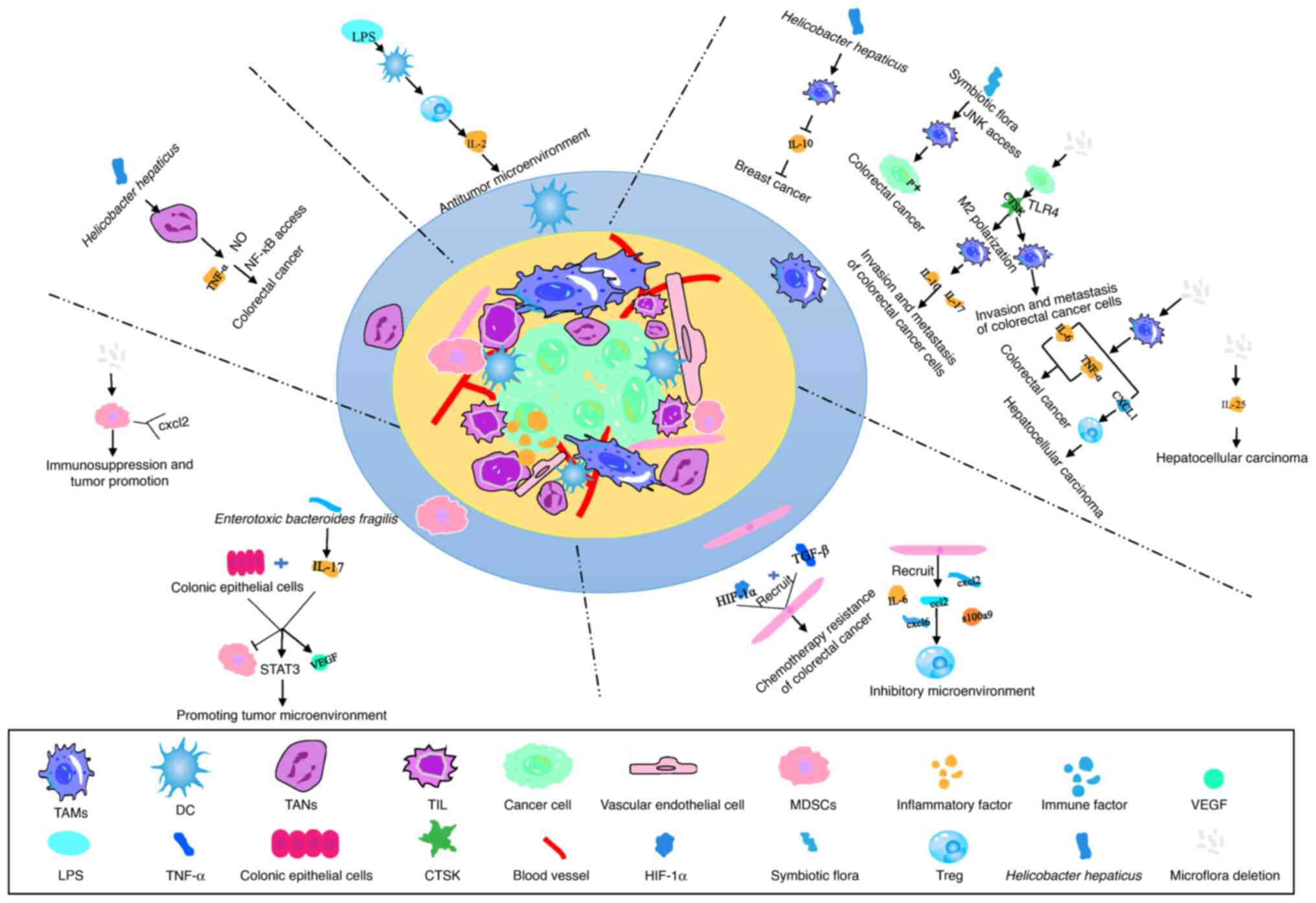 | Figure 1.Intestinal flora regulates tumor
microenvironment components. TAMs: Helicobacter hepaticus
can activate TAMs and inhibit the production of IL-10, thus
inhibiting the development of breast cancer. The imbalance of gut
microbiota directly activates macrophages, macrophages release
IL-6, TNF-α and CXCL10. IL-6 and TNF-α accelerate the development
of CRC by promoting EMT, while CXCL10 induces T-cell infiltration
in the tumor microenvironment and promotes the development of HCC.
Intestinal microbiota bacterial diseases induce tumor cells to
secrete CTSK, thereby activating macrophages through mTOR-dependent
pathways. CTSK stimulates the macrophage secretion of IL-10 and
IL-17, thus promoting the invasion and metastasis of CRC cells.
Some symbiotic microbiota stimulate macrophages to increase c-Jun
phosphorylation in CRC cells through the JNK signaling pathway,
thus accelerating CRC cell proliferation. Intestinal microbiota
bacterial diseases also induce the production of IL-25 from cluster
cells, which promotes EMT and the migration of HCC cells. CAFs:
CAFs influence the tumor immune microenvironment by recruiting
chemokines and immune factors. MDSCs: IL-17 recruits bone MDSCs
into the colon tumor microenvironment of mice colonized with
enterotoxin bacteria, which indirectly induce the ectopic
production of chemokines and growth factors through direct
interaction with IL-17 receptors in CECs, and the expression of
IL-17 in submucosa. IL-17 and transformed CECs jointly promote
tumor development with angiogenic mediators, namely MMP-9 and VEGF,
by inhibiting immune effector cells and activating the STAT3
signaling pathway. Early lack of microbiota in mice enhances the
expression of C-X-C motif receptor 2 ligands. A number of gut
pathogenic microorganisms or gut microbiota and their synergistic
interactions with cytokines activate and proliferate MDSCs in the
tumor microenvironment, thus mediating the immune escape of tumor
cells. TANs: Helicobacter hepaticus can induce the
generation of neutrophils, thereby inducing nitric oxide and TNF-α
increased content, which activates the NF-κB signaling pathway and
promotes tumor generation. DCs: Lipopolysaccharide and TNF-α
stimulate DCs to mature and activate, and subsequently activate T
cells to produce IL-2 to form an antitumor microenvironment. TAMs,
tumor-associated macrophages; CRC, colorectal cancer; EMT,
epithelial-mesenchymal transition; HCC, hepatocellular carcinoma;
CTSK, carnosine K; CAFs, cancer-associated fibroblasts; MDSCs,
myeloid-derived suppressor cells; CECs, colon epithelial cells;
DCs, dendritic cells; TANs, tumor-associated neutrophils; CXCL,
C-X-C motif ligand. |
Tumor-associated neutrophils
(TANs)
TANs can be divided into two phenotypes, tumor
suppressor and tumorigenic, with notably high inhibitory and
polarized properties. TANs are associated with tumorigenesis,
proliferation and immune regulation, and they transform into a
pro-angiogenic subtype under the synergistic effect of chemokines.
TANs release neutrophil extracellular traps (NETs) to kill harmful
microorganisms. NETs activate specific signaling pathways to
stimulate dormant cancer cells, restore their proliferative
activity, and promote tumor recurrence and metastasis (34). A previous study has shown that
Fusobacterium nucleatum can change the composition and
phenotype of tumor-associated macrophages (TAMs), TANs and
myeloid-derived suppressor cells (MDSCs) in the tumor
microenvironment, and can activate the E-cadherin/β-catenin
signaling pathway to promote the malignant transformation of
epithelial cells (35).
Helicobacter hepaticus can stimulate the secretion of nitric
oxide and TNF-α from neutrophils to promote the progression of
colorectal cancer (CRC) (32)
(Fig. 1).
TAMs
TAMs are markedly adaptable, and with subtle changes
in the tumor microenvironment, their phenotype would tranform from
the antitumor M1 to the M2 one, which promotes tumor development
and remodeling (32). TAMs are
affected by the combined effects of various microbiota to regulate
the progression of breast and colon cancer. Zhou et al
(35) compared the fecal microbiota
of patients with hepatocellular carcinoma (HCC) and healthy
controls, and observed that the abundance of specific flora in
patients with HCC was altered, and the authors predicted that tumor
cells could alter the intestinal flora to produce TAMs, and reduce
the level of antitumor immunity (Fig.
1).
MDSCs
Previous studies have revealed that, under the
action of IL-17, MDSCs interact with Bacteroides fragilis
(Bf) to indirectly induce ectopic colonic epithelial cells, and to
induce the expression of IL-17 in intestinal epithelial tissue.
Increased IL-17 expression and activated STAT3 signaling, as well
as vascular growth factors and proangiogenic mediators,
collectively promote colorectal tumor progression (32).
In addition, F. nucleatum promoted the
regeneration of intestinal epithelial tissue by increasing the
number of MDSCs in the tumor microenvironment. In the absence of
microorganisms, the expression of the MDSC ligand C-X-C motif
chemokine receptor 2 was enhanced, exhibiting immunosuppressive and
tumor-promoting effects (32)
(Fig. 1).
Cancer-associated fibroblasts
(CAFs)
CAFs induce chemoresistance in CRC through the
synergistic action of hypoxia-inducible factor (HIF)-1α and TGF-β.
It was revealed that CAFs can assist in tumor immune escape and
resist the action of immunosuppressive drugs. They can induce an
inhibitory T-cell microenvironment by recruiting chemokines and
immune factors (CCL2, CXCL2, CXCL6, S100A9, IL6) (36) (Fig.
1).
Cytokines
The tumor microenvironment includes inflammatory,
immune and hypoxia factors (Table
III) (37–50). Numerous factors connect different
components in the tumor microenvironment through specific signaling
pathways, so that different tumor microenvironments are linked
together to form a dynamic tumor-promoting or tumor-suppressing
microenvironment (Fig. 1).
 | Table III.Cytokines in the tumor
microenvironment. |
Table III.
Cytokines in the tumor
microenvironment.
| Classification | Name | Role | (Refs.) |
|---|
| Inflammatory
factors | IL-1β | Low concentration
of IL-1β induces local inflammatory and protective immune
responses, while high concentrations can lead to
inflammation-related cancer tissue damage | (37) |
|
| TNF-α | TNF-α can
continuously activate NF-κB, which regulates inflammation | (38) |
|
| IL-6 | Participates in the
inititation of the inflammatory pathway | (39) |
|
| IL-10 | Inhibits the
inflammatory response | (40) |
| Immune factors | IL-17 |
Pro-inflammatory | (41) |
|
| IL-22 |
Pro-inflammatory | (42) |
|
| IL-35 | Antitumor
activity | (43) |
|
| TGF-β | Suppresses the
immune response | (44) |
| Chemokines | IL-8 | Cancer-promoting
factors promote tumor immune escape | (45) |
|
| Recombinant
chemokine CXCR6 | Increases immune
cell infiltration in tumor tissue | (46) |
|
| Recombinant human
CCL4 | Achieves antitumor
effects by recruiting regulatory T cells and promoting tumor
macrophages | (47) |
|
| Recombinant human
CCL3 | Participates in
immune monitoring and tolerance | (48) |
|
| Recombinant human
CXCL5/CXCR2 | By blocking the
bridge between tumor and host cells in the tumor microenvironment,
it can improve immune efficacy | (49) |
|
| Recombinant human
CXCL12 | Antitumor
activity | (50) |
Regulatory mechanism
Metabolites
Microbial metabolites
Short-chain fatty acids (SCFAs) are the fermentation
products of dietary fiber. Ohtani Haraand Hara (51) identified that, in addition to
maintaining intestinal homeostasis, intestinal microbiota
metabolites could also transport SCFAs to the liver through the
portal vein, and produce bile acids that induce DNA damage to
remodel the tumor microenvironment and regulate liver function.
Huang et al (52) collected
feces from patients with liver cancer and healthy individuals for
bioinformatics analysis, and observed that bile acids, a metabolite
of the gut flora, can affect tumor treatment and prognosis by
changing the immune microenvironment. In addition, butyrate and
tryptophan metabolites produced by intestinal flora metabolism can
affect the adaptive immunity of the body and promote antitumor
therapeutic effects (53,54). Cholesterol is metabolized in the gut
to produce three metabolites: Bile acids, steroids and vitamin D.
Among them, bile acids can modulate the composition of the gut
microbiota to affect peripheral and autoimmune immunity, while the
metabolic reprogramming of cholesterol in the tumor
microenvironment can cause tumor microbiota to change to an
immunosuppressive type, thus providing an environment conducive to
the proliferation of cancer cells (55) (Fig.
2).
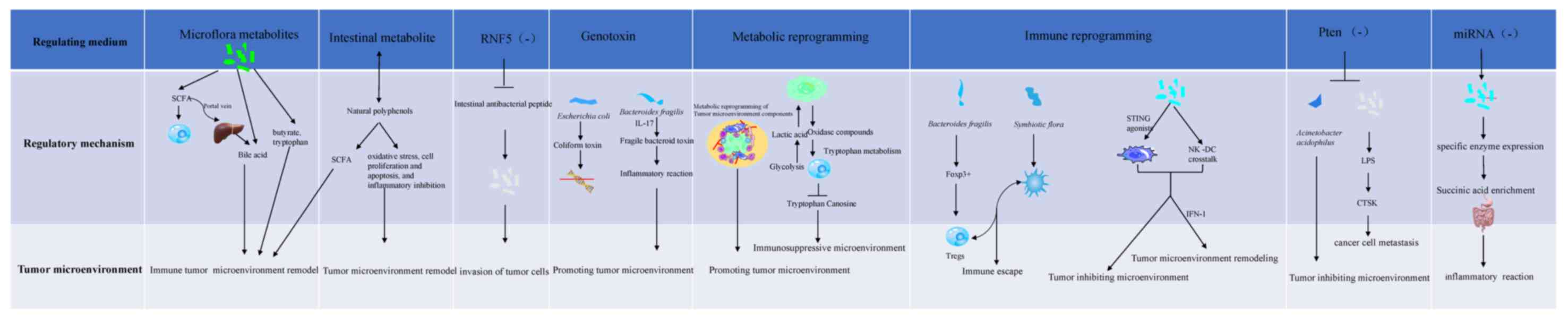 | Figure 2.Mechanisms of intestinal
flora-mediated regulation of the tumor microenvironment.
Metabolites: i) Metabolites of flora: SCFAs can be transmitted to
the liver through the portal vein to produce bile acid and change
the tumor microenvironment. Butyrate and tryptophan affect the
development of tumors by affecting their immune response. ii)
Intestinal metabolites: Intestinal metabolism produces polyphenol
analogues, changes the type of gut microbiota, stimulates the
production of SCFAs and reshape the tumor microenvironment.
Non-hematopoietic components of the intestinal membrane: When
intestinal epithelial cells lack ubiquitin ligase, the secretion of
intestinal cathelicidin decreases, and epithelial cells die, which
causes changes in the gut microbiota and regulates the tumor immune
microenvironment. Genotoxins: PKS gene-positive Escherichia
coli can destroy the single-stranded DNA of intestinal cells,
playing an indirect role in the development of CRC, and colon
epithelial cells can cause DNA damage and activate Wnt and NF under
the action of fragile bacteroid toxins-κB. STAT3 and other colon
epithelial signal transduction pathways affect the self-renewal of
colon mucosal epithelial cells and promote tumor formation.
Metabolic reprogramming: The tumor microenvironment provides
nutrients for malignant proliferation and metastasis of tumor cells
by changing the metabolism of different components of the tumor
microenvironment. Anaerobic glycolysis of tumor cells produces
lactic acid, which can stimulate the production of hyaluronic acid
and the expression of CD44, and is conducive to tumor metastasis.
The hypoxic acidic tumor microenvironment caused by aerobic
glycolysis inhibits the normal metabolism of immune cells and T
cell function. Tumor cells mediate the metabolism of tryptophan in
T cells by overexpressing indoleamine 2,3-dioxygenase and
tryptophan 2,3-dioxygenase in order to transform tryptophan into
caninurenine and its metabolites. Lack of tryptophan and
accumulation of caninurenine metabolites would inhibit the function
of effector T cells. Immune reprogramming: i) Immune escape:
Bacteroides fragilis, as an inducer of forkhead box
P3+ (a powerful inducer that mediates gastrointestinal
immunity and peripheral tolerance), can induce the production of
IL-10-mediated mucosal surface tolerance, which can induce the
generation of Tregs, while symbiotic microorganisms can promote the
escape of pDCs. Both pDCs and Tregs work together to mediate immune
escape; ii) reprogramming of immune cells: STING agonists derived
from microbiota induce the production of IFN-I through mononuclear
phagocytes in tumors, thus forming an antitumor microenvironment.
By regulating the natural killer cell-DC crosstalk, the content of
IFN-I is regulated, reshaping the tumor microenvironment, and
facilitating the response to immune checkpoint blockade. In the
absence of PTEN (gene of phosphate and tension homology deleted on
chromosome ten) expression, miRNA expression and the
proinflammatory bacteria Akkermania muciniphila in the gut
microbiota decrease, forming a microenvironment that inhibits tumor
growth. When the gut microbiota is altered, the lipopolysaccharide
content, the expression of the cathepsin K gene and the risk of
colon cancer cell metastasis increase. Due to the loss of miRNA,
succinate produced by the microbiota accumulates in the colon,
leading to diarrhea in weaned piglets. SCFAs, short-chain fatty
acids; CRC, colorectal cancer; STING, stimulator of intereferon
genes; Tregs, regulatory T cells; pDCs, plasmacytoid dendritic
cells; miRNA, microRNA. |
Intestinal metabolites
Radiotherapy and chemotherapy have serious side
effects during tumor treatment; thus, an increasing number of
experts recommend a diet therapy. Due to their strong antioxidant
function, natural polyphenols are often used as targeted regulators
for colon cancer prevention and treatment (56). In addition, it was demonstrated that
natural polyphenols can not only regulate oxidative stress, cell
proliferation, apoptosis and inflammatory inhibition, but can also
change the type of gut microbiota that stimulates the production of
SCFAs to remodel the tumor microenvironment (56) (Fig.
2).
Non-hematopoietic components of the
intestinal membrane
A previous study has shown that the lack of the
ubiquitin ligase ring finger protein 5 in intestinal epithelial
cells can lead to decreased secretion of intestinal antimicrobial
peptides and cell death, which in turn changes the intestinal
flora, regulates the activity of lymphoid organs and affects tumor
cell invasion (57) (Fig. 2).
Genotoxins
The intestinal flora mediates cancer through
genotoxins, such as colicin produced by Escherichia coli,
which acts as a DNA alkylating agent to damage host DNA (58) and induce cell senescence. Bf toxin
is activated by IL-17 in colonic epithelial cells. NF-kB signal
transduction produces a series of inflammatory responses and
accelerates the transformation of colitis to colon cancer (59). It has been revealed that genotoxin
expression is exacerbated when the gut microbiota is altered
(60) (Fig. 2).
Metabolic reprogramming
The tumor microenvironment supports the malignant
proliferation of tumor cells by providing nutrients and redox
requirements for tumor cell proliferation through aerobic
glycolysis, and metabolic reprogramming of fibroblasts, T cells,
TAMs and adipocytes (61). A
previous study has demonstrated that tumor metabolic reprogramming
can mediate tumor immune escape. Lactic acid produced by glycolysis
can stimulate tumor cell metastasis, and oxidized compounds highly
expressed by tumor cells can accelerate the metabolism of
tryptophan to kynurenine by T cells. The lack of tryptophan and the
increase in kynurenine alter the function of T cells, rendering
them unable to be activated by antigens, thus forming an
immunosuppressive microenvironment (62) (Fig.
2).
Immune reprogramming
Immune escape
Gut microbes influence tumor immunotherapy in
multiple ways. Some bacteria achieve antitumor efficacy by
activating immunity, while some help cancer cells to escape the
immune system by mediating immunosuppression (63). Thus, increasing evidence has shown
that the clinical treatment effect can be improved by regulating or
supplementing microorganisms in vitro (64).
Bf can induce forkhead box P3+ (a potent
inducer of gastrointestinal immunity and peripheral tolerance) to
induce regulatory T-cell (Treg) generation, while commensal
microorganisms can promote the efflux of pDCs. pDCs and Tregs work
together to mediate immune escape (65).
Reprogramming of immune cells
Mononuclear phagocytes are highly plastic, and the
gut flora interferes with the reprogramming of mononuclear
phagocytes in the tumor microenvironment into immunostimulatory
monocytes and DCs, making the tumor microenvironment shift to a
tumor suppressor environment (66,67).
The mechanism is a microbiota-derived IFN-stimulating factor
agonist that modulates macrophage polarization and natural killer
(NK) cell-DC interactions through monocyte-induced IFN-1.
Subsequent in vitro experiments confirmed that IFN-1
increased in mice under a high-fiber diet, and mononuclear
phagocytes in the tumor microenvironment were remodeled, the number
of DCs increased, and the efficacy of immune blocking agents was
improved (68) (Fig. 2).
Signaling pathways and cytokines
The tumor microenvironment mainly includes
inflammatory cytokines, immune cytokines and hypoxia cytokines.
Research has revealed that, in colon cancer and other diseases, the
intestinal flora, and the inflammatory, immune and hypoxic
microenvironments cross-talk, are closely related and interact with
each other. When the intestinal flora is imbalanced, it leads to
the inflammation of epithelial cells, which leads to hypoxia.
Increased HIF levels induce an increase in inflammatory (NF-κB) and
immune (Th17, IL-17) factors, which aggravates inflammation and
leads to cancer. The intestinal flora stimulates the release of
TNF-α and VEGF, promotes angiogenesis in the tumor
microenvironment, aggravates the hypoxia of the tumor
microenvironment, increases the content of HIF, and further
aggravates the hypoxia and inflammation of the microenvironment
(69–72) (Fig.
3).
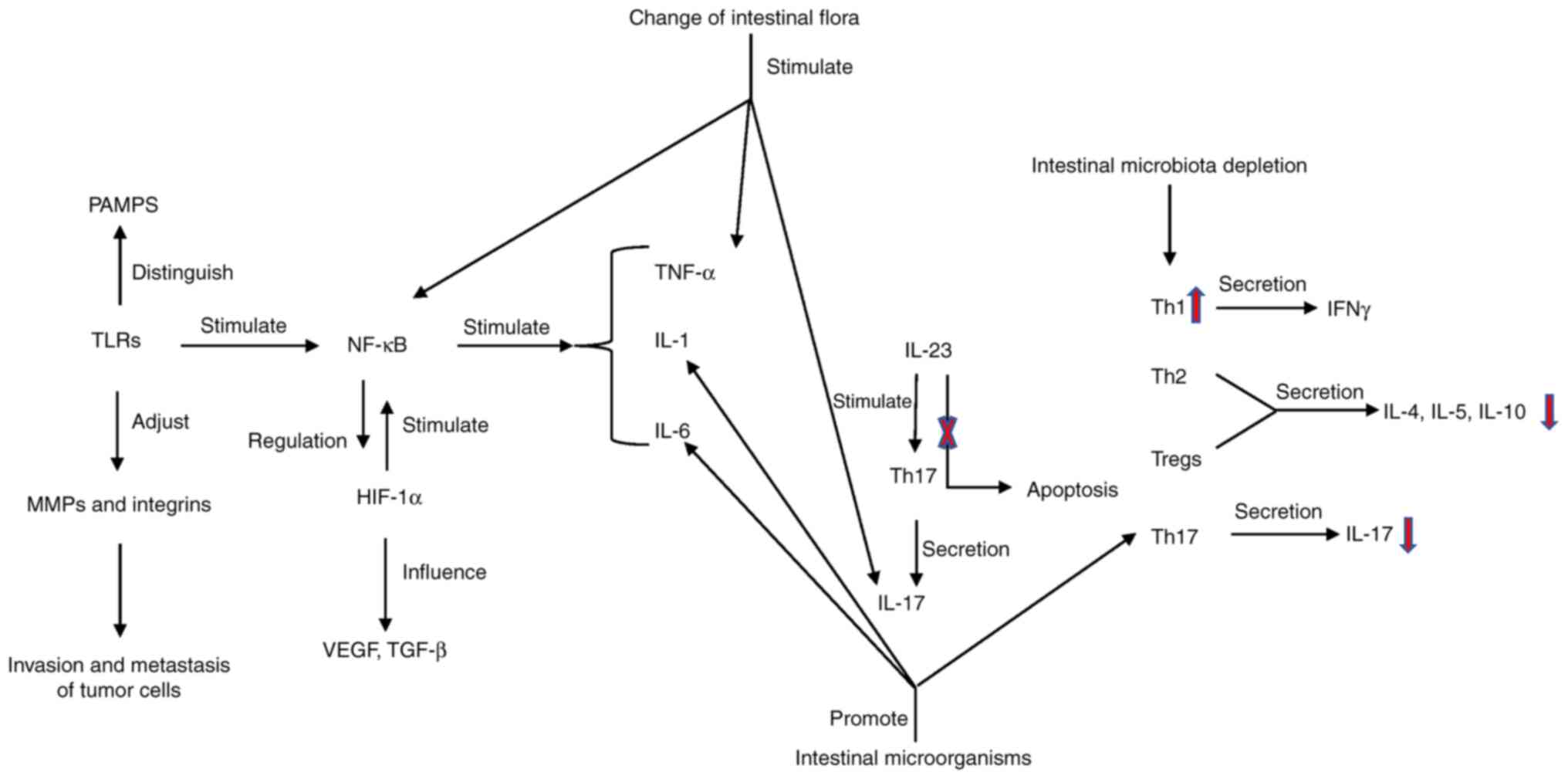 | Figure 3.Signaling pathways and cytokines. The
signal transmission of innate immunity in the intestine is mainly
achieved through the recognition of NOD like receptors and TLRs to
generate perception. Pathogen related molecular patterns of
different microorganisms are recognized by TLRs, TLRs activate
NF-κB and stimulate the production of inflammatory factors and
chemokines. When the gut microbiota changes abnormally, TLRs will
be abnormally activated, producing a series of inflammatory and
tumor-promoting reactions. At the same time, they can regulate the
invasion and metastasis of cancer cells via MMPs and integrins.
Th17 cells can secrete IL-17 and IL-23, which can regulate Th17
cells. Blocking IL-23 secretion can alleviate inflammation. The
content of HIF-1α in a hypoxic tumor environment increases, which
activates NF-κB. NF-κB can increase macrophage HIF-1α expression in
the tumor microenvironment. HIF-1α and TGF-β have synergistic
effects. HIF-1α is positively associated with the angiogenic factor
VEGF. Th cells mature into Th1, Th2, Treg and Th17 cells. Th1 cells
secrete IFN-γ, while Th2 cells and Tregs secrete IL-4, IL-5 and
IL-10 for antitumor effects, and Th17 cells secrete IL-17. After
gut microbiota depletion, the content of IFN-γ increases and the
IL-17 content decreases. PAMPs, pathogen-associated molecular
pattern molecules; MMPs, matrix metalloproteinases; TLRs, toll-like
receptors; Th, helper T cell; HIF, hypoxia-inducible factor; Treg,
regulatory T cell. |
Other mechanisms
Phosphatase and tensin homolog (PTEN), as a tumor
suppressor gene, can antagonize PI3K-Akt signaling to suppress
tumorigenesis (73). Although PTEN
deficiency is not sufficient to induce tumorigenesis, it can
accelerate tumor progression. Howe et al (73) revealed that the pro-inflammatory
Acinetobacter acidophilus was greatly reduced in the
microenvironment of PTEN gene-knockout mice. Therefore, it was
considered that Adenobacter acidophilus could help to
prevent the protumor microenvironment caused by PTEN deficiency and
form a preventive tumor microenvironment. The aforementioned study
linked genetic changes to the gut microbiota and tumor
microenvironment, thus providing new insights for subsequent
studies on the role of gut microbiota in shaping the tumor
microenvironment.
Cathepsin K (CTSK) mainly acts on bone remodeling
and resorption. As the only upregulated metastasis-related signal
in colon cancer cells, it has been revealed that intestinal flora
dysbiosis leads to increased LPS content, which in turn promotes
the expression of the CTSK gene and changes the tumor stromal
microenvironment to promote colon cancer cell migration and
invasion into the bone (74). As
transcriptional regulators, microRNAs (miRNAs) play a significant
role in various physiological activities such as immunity and
metabolism. Through genomic analysis, it was identified that gut
microbiota may reshape the tumor microenvironment by affecting
miRNAs, thereby affecting the metastasis and prognosis of CRC
(75,76).
In the treatment of diarrhea in piglets, it was
identified that diarrhea was caused after weaning. Concurrently,
the deletion of specific miRNAs would change the abundance of
specific bacteria in the intestinal flora, resulting in increased
expression of specific enzymes. Succinic acid is enriched in the
intestine and promotes intestinal epithelial tissue fluid. The
secretion of fluid causes an inflammatory response leading to
diarrhea (77).
In summary, further studies are required to
investigate whether the deletion of a certain miRNA can also cause
changes in the homeostasis of a target organ (such as inflammatory
response, pathway stimulation or immune response) and can help to
regulate tumors through interacting with their microenvironment for
the treatment and diagnosis of tumors (78).
Clinical application
Probiotics
A reasonable use of probiotics (as a common mean of
regulating intestinal flora imbalances) in the treatment of colon
cancer can not only change the composition of the flora but also
regulate the immune response of the intestinal tract, thereby
preventing and treating colon cancer (79). Galunisertib, a TGF-β blocker, was
revealed to relieve immunosuppression by enhancing the infiltration
of specific effector T cells and promoting DC maturation in the
tumor microenvironment, when combined with Bifidobacterium
probiotics (80).
Fecal microbiota transplantation
(FMT)
As a new therapy, FMT mainly transplants healthy
human gut microbiota into patients to remodel and partly restore
intestinal homeostasis. In 2013, it was used in the treatment of
Clostridium difficile infection. Icreasing evidence has
clarified the therapeutic effect of fecal transplantation for other
diseases (18). For example, in
2021, allogeneic fecal bacterial transplantation was applied in
phase I clinical trials in patients with anti-programmed cell death
1 refractory metastatic melanoma, and it was revealed that it could
alter the infiltration and gene expression characteristics of
immune cells in the tumor microenvironment (82). In addition, when using trastuzumab
to treat a HER2-positive breast cancer mouse model, the researchers
observed that allogeneic fecal bacterial transplantation enhanced
the efficacy of trastuzumab in blocking cancer cell proliferation
and improving immune cell infiltration in the tumor
microenvironment (83). In recent
years, the concept of autologous fecal transplantation has emerged,
which is similar in concept to the preservation of neonatal
umbilical cord blood, and implies the rejuvenation of intestinal
flora. Although this concept has certain feasibility, its efficacy
and safety have yet to be verified (84).
Natural extracts
Numerous studies have shown that natural plant
extracts [triterpenoid saponins (85), safflower (86), Astragalus polysaccharides
(87), puerarin (88)] and traditional Chinese medicine
formulas [SWY (89), Wu Mei Wan
(90), and parthenolide (91)] alter the tumor microenvironment by
modulating the gut microbiota in vitro. However, their
research is currently limited to animal experiments, and have yet
to be used in clinical practice (92) (Table
IV).
 | Table IV.Regulation of intestinal flora by
natural extracts. |
Table IV.
Regulation of intestinal flora by
natural extracts.
| Plant | Extract | Flora change | Regulatory
mechanism | Disease
treatment | Subjects | (Refs.) |
|---|
| Carthamus
tinctorius | Safflower
yellow | Barnesiella
and Ersipelotrichaceae incertae sedis return to normal
levels | Improvement of the
immune microenvironment | Hepatocellular
carcinoma | In vivo test
in mice | (86) |
| Astragalus
membranaceus | Astragalus
polysaccharides | Regulation of
changes in Bifidobacterium pseudolongum, Lactobacillus
johnsonii and Lactobacillus flora | Improvement of the
metabolism of glutamate and creatine could control tumor
growth | Melanoma | In vivo test
in mice | (87) |
| Pueraria
lobata | Puerarin | Restoring imbalance
of firmicutes to acteroides | Regulation of the
content of SCFAs and aminoacid metabolites by adjusting the
gut/bone axis could improve the bone microenvironment | Osteoporosis | In vivo test
in mice | (88) |
| Zingiber
officinale Roscoe, Allium tuberosum Rottler ex Spreng.,
Pyrus Bretschneider Rehder, Nelumbo nucifera
Gaertn. | Chinese herbal
compound extract | The expression of
the class Bacilli, the genus Turicibacter, the family
Turicibacteae, and the order Turicibacterales return to normal
levels | Regulation of
metabolism, changes in the tumor microenvironment | Esophageal
precancerous lesion | In vivo test
in mice | (89) |
| Wumei, Huanglian,
Xixin, Guizhi, Dangshen, Fuzi, Huajiao, Ganjiang, Huangbai,
Danggui | Chinese herbal
compound extract | Bacteroidetes and
firmicutes imbalance restoration | Downregulation of
the NF-κB/IL-6/STAT3 signaling pathway to regulate the inflammatory
environment of the intestine | Colorectal
cancer | In vivo test
in mice | (90) |
| Feverfew | Parthenolide |
Alloprevotella displayed high
abundance | Improves the
Treg/Th17 balance in intestinal mucosa mediated through increased
microbiota-derived SCFA production | Inflammatory bowel
disease | In vivo test
in mice | (91) |
Diet
Diet is the most direct and important factor
affecting the intestinal flora. It has been demonstrated that a
high-fat diet can change the intestinal flora to accelerate
intestinal inflammation through direct or extraintestinal effects,
and change the metabolism and tumor immune microenvironment
(93). A previous study has also
revealed that a high-fat diet increases the sensitivity of the gut
to carcinogens (94). Therefore,
scientists suggest that a ketogenic and high-fiber diet can be used
to regulate intestinal flora metabolism and tumor microenvironment
(95). Dietary carrageenan, as a
food additive, alters the gut microbiota resulting in SCFA
reduction, mucosal thinning and changes in intestinal homeostasis
to form a proinflammatory microenvironment. Therefore, it was
speculated that this inflammatory response can be reversed by
supplementation with probiotics (96). In addition to the direct factor of
diet, the intestinal flora of the human body also includes the host
environment. Since the growth of intestinal flora requires the body
to provide ATP to support its growth and form colonies, factors
such as a poor diet, antibiotics and intestinal diseases weaken the
control of the body over the flora, thus resulting in changes in
flora homeostasis. It is thus possible to quantify the conditions
that control the growth of the microbiota, thereby defining the
homeostasis and imbalances of the gut microbiota, and regulating
microbiota imbalances (97).
Biological targets
The tumor microenvironment, as the place of tumor
growth, regulates the occurrence, development and metastasis of
tumors. As an important factor influencing the tumor
microenvironment, intestinal microbiota has been demonstrated to
interact with the tumor microenvironment. Based on extensive
literature review, it has been revealed that the role of
microorganisms in mediating the tumor microenvironment and then
influencing tumor progression is played by a group of bacteria
rather than a specific strain. The following is a summary of
relevant biological targets, providing potential insights for their
clinical applications (Table
V).
 | Table V.Mechanism of action and diseases of
biological targets. |
Table V.
Mechanism of action and diseases of
biological targets.
| Target action | Target name | Mechanism of
action | Disease | (Refs.) |
|---|
| Prevent | Anti-inflammatory
characteristic bacteria with potential for producing butyric
acid | Changes in
microbiota result in changes in the content of butyric acid, a
metabolite of the microbiota | Colon cancer | (98) |
|
| Bile acid | Formation of an
inflammatory microenvironment | Hepatocellular
carcinoma risk prediction and grading | (99) |
| Diagnosis | Number of DNA
fragments from Fusobacterium nucleatum | Microbiota dictates
tolerogenic vs. immunogenic cell death of intestinal epithelial
cells | Staging,
metastasis, chemotherapy resistance, and prognosis of colon
cancer | (100) |
|
| Increased
Leptotrichia and decreased Porphyromonas in
saliva | Gut microbiota can
be connected in series with related flora through the mesentery
lymphatic pathway | Early diagnosis of
pancreatic cancer | (101) |
|
| Abundance of
Akkermansia | Secretion of
interferon by CD8+ T cells causes tumor killing | Diagnostic and
therapeutic targets of ovarian cancer | (102) |
| Treatment | Beneficial
microbiota such as Bifidobacterium, Bacteroides fragilis and
Akkermansia muciniphia | Regulation of the
tumor immune microenvironment | May be used as a
sensitive target for immunotherapy | (103,105) |
| Prognosis | CTSK | CTSK secreted by
tumors can bind to Toll-like receptor 4 and stimulate M2
polarization of tumor-associated macrophages through the
mTOR-dependent pathway, thereby inhibiting the development and
metastasis of colorectal cancer | Invasion and
metastasis of colon cancer | (74) |
|
| Pseudomonas,
Saccharopolysaccharide, Streptomyces and
Clostridium | Effects on tumor
growth and immune infiltration | Prediction of
long-term survival of patients with pancreatic cancer | (109) |
Prevention
It has been identified that the induction of SCFA is
strain-specific, thus, its inductive ability can be inferred from
the abundance of gut flora to predict changes in the tumor
microenvironment (98).
Quantitative analysis of the composition of bile
acid, the metabolite of gut microbiota, and bile salts in feces can
be used to identify the risk index of HCC, and to further combine
the composition and category of gut microbiota to grade the risk of
HCC. This may be related to the inflammatory environment formed by
the gut microbiota and the tumor microenvironment, which can
stimulate the occurrence and development of tumors. Therefore, the
observation and intervention of gut microbiota can be a good means
for the prevention and treatment of HCC (99).
Diagnosis
The quantity of Fusobacterium nucleatum DNA
in the intestinal flora has been revealed to be positively
associated with tumor stage, metastasis and patient survival. In
clinical practice, the level of Fusobacterium nucleatum DNA
can be measured for colon cancer tumor staging, metastasis,
chemotherapy resistance, sex and prognosis (100). There are differences in the types
and abundance of gut microbiota among different diseases, such as
Bacteroides, Lachnospiraceae incertae sedis, and
Clostridium XIV, which can be used to identify small liver
cancer (52).
Pancreatic cancer, as the most lethal malignant
tumor, can not be diagnosed by common detection methods in the
early, or even in the mid or late stages (101). Yang et al (101) revealed that Leptotrichia
increased and Porphyromonas decreased in the saliva of
patients with pancreatic cancer, suggesting that it could be used
as a marker for early diagnosis. Gut microbiota can enter the
pancreas through the mesentery lymphatic pathway to connect
different flora, and affect the occurrence and development of
pancreatic cancer remotely.
Wang et al (102) revealed that Akkermansia in
the intestines of patients with ovarian cancer was significantly
reduced by analyzing the abundance of gut microbiota of patients.
In addition, when the gut microbiota of these patients was
inoculated into mice by fecal bacteria transplantation, the
progression of ovarian cancer in mice was accelerated. The addition
of Akkermania can significantly inhibit the progression of
ovarian cancer in mice. This research has shown that
Akkermania restores the integrity of the intestinal mucosa,
activates T-cell immune response in the tumor microenvironment, and
strengthens immune monitoring. This aforementioned study (102) provided a new direction for the
relationship between gut microbiota and the immune microenvironment
in ovarian cancer, and also suggests that Akkermania can be
used as a new target for diagnosis and treatment of ovarian
cancer.
Treatment
A previous study has demonstrated that the
intestinal flora inhibits apoptosis, changes epigenetic
transplantation, repairs damaged DNA and participates in other
mechanisms to generate therapeutic resistance, but it can also be
used as a target to manipulate and improve the therapeutic effect
of treatments (103).
Traditional radiotherapy is the most effective
method to treat tumors. As an important factor in regulating the
tumor microenvironment, gut microbes are impacted from the effect
of radiotherapy. It has been revealed that there are differences in
the sensitivity of different gut microbiota to radiotherapy, but
the specific mechanism remains unknown (104). Therefore, the sensitivity of
patients to radiotherapy may be evaluated by analyzing the types of
intestinal flora, with the intent that the treatment plan can be
timely adjusted.
Traditional radiotherapy and chemotherapy are aimed
at the tumor cells themselves, using physical rays and chemical
drugs to kill them, but drug resistance is prone to occur. It has
been revealed that the combination of traditional therapy and
immunotherapy can greatly reduce the drug resistance of tumor cells
and improve the therapeutic effect. Research has shown that gut
microbiota can affect the effectiveness of immunotherapy (105). PD-1/PD-L1 has good efficacy in the
treatment of solid tumors, and has been demonstrated that, in in
vitro experiments in mouse models, mice with oral microbiota
have improved anti-PD-1 efficacy than untreated mice (32). Transplanting fecal bacteria from
patients who have responded to anti-PD-1 antibodies into germ-free
mice could significantly improve the control effect of T cells on
tumors, and have a favorable effect on PD-1/PD-L1 immunotherapy.
Immune checkpoint inhibitors (ICIs), as a new treatment method,
exhibit favorable curative effects, but some patients are
insensitive to them or develop resistance to their long-term use
(106). It was shown that patients
who responded well to ICIs had a high number of beneficial bacteria
in the gut (Bifidobacterium, Bf, Akkermansia
muciniphia), which could help to restore and enhance the
therapeutic effect of ICI and immunotherapy in patients (79). As the latest targeted therapy,
chimeric antigen receptor T-cell immunotherapy (CAR-T) is based on
the principle of isolating the T lymphocytes of the patient,
expanding them in vitro to make them carry tumor cell
antigens, and then infusing them back into the body of the patient,
in order to achieve rapid and precise tumor treatment. Through
clinical stool sample observation and genome sequencing analysis,
it was identified that, in patients with B-cell malignancies, there
was a strong association between changes in gut microbiota and
clinical treatment outcomes of CAR-T therapy (107).
In addition, studies have demonstrated that
microorganisms in tumors, oral microbiota, and other factors can
affect the tumor immune microenvironment, thereby affecting tumor
immunotherapy. Therefore, understanding the relationship between
microorganisms, the tumor microenvironment and diseases provides a
new target for better use of microorganisms to treat diseases
accurately (108).
Prognosis
Pancreatic cancer is a malignant tumor of the
digestive tract with extremely high mortality, because its early
diagnosis is difficult. Yang et al (101) revealed that the imbalance of
intestinal microbiota is closely related to the incidence and
prognosis of pancreatic cancer. In addition, Huang et al
(52) revealed that high bile acid
metabolism, low levels of Bacteroides, Lachnospiracea incertae
sedis, and Clostridium XIVa and content of operational
taxonomy unit markers related to bile acid metabolism could be used
to predict the postoperative survival time of patients with liver
cancer.
As a measure of gut microbiota imbalance and CRC
metastasis, CTSK secreted by CRC could accelerate the phenotype
transformation of TAMs to M2 by regulating the TLR4-mTOR signaling
pathway, thereby accelerating the progression of CRC. Concurrently,
it can secrete inflammatory factors to promote cancer cell invasion
and metastasis. Therefore, it has been suggested that CTSK may
serve as a new prognostic and therapeutic target for CRC (74). In other research, four
characteristic microbes in the tumor microbiota (Pseudomonas,
Glycopolysaccharides, Streptomyces and Clostridium),
which could predict the long-term survival of patients with
pancreatic cancer, were also identified. Using donor fecal
microbiota transplantation, it was determined that the tumor
microbiota may be regulated differently and affect tumor growth and
immune infiltration (109).
Conclusions
Overall, the gut microbiota regulates intestinal and
distant tumors through changes in the microbiota, brain-gut axis,
hypothalamic pituitary adrenal axis, intestinal liver axis and
bacterial translocation. It is mainly manifested in the regulation
of each component in the tumor microenvironment, to achieve the
regulation of the tumor microenvironment. Its regulatory mechanisms
include changes in gut and microbiota metabolites, gene toxins,
metabolic reprogramming, immune reprogramming, signaling pathways
and cytokines. The transplantation of probiotics and fecal
microbiota methods in the treatment of tumors in the
microenvironment of gut microbiota regulation have matured.
However, other treatment methods are still at the theoretical stage
and have not been clinically validated, and their adverse effects
on the body remain unclear. In addition, the present review also
summarized and compared the biological targets of gut microbiota
regulating the tumor microenvironment, providing a theoretical
basis for future applications in the prevention, diagnosis,
treatment and prognosis of tumors (Fig.
4).
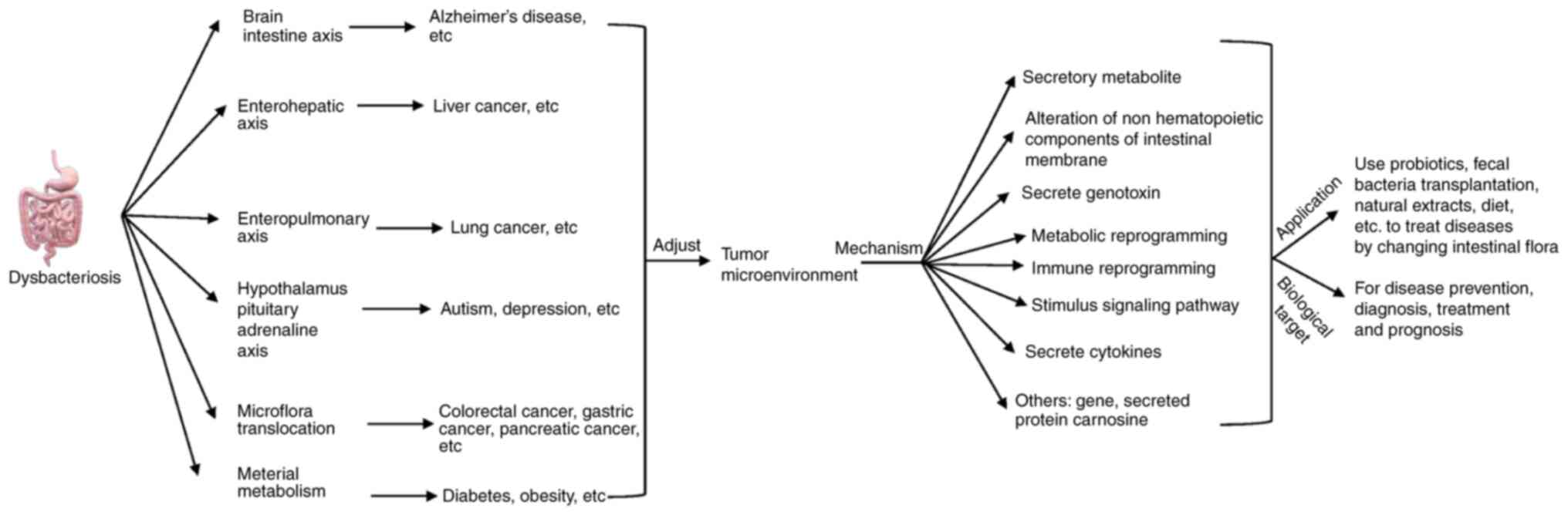 | Figure 4.Intestinal flora, disease, tumor
microenvironment, influence mechanism and application. Dysbiosis of
intestinal flora can affect the tumor microenvironment through
various pathways such as the intestinal-liver and intestinal-brain
axes, which can cause various diseases such as Alzheimer's disease,
liver cancer, lung cancer and Parkinson's disease. The mechanisms
by which the intestinal flora regulates the tumor microenvironment
mainly involve metabolites, non-hematopoietic components of the
intestinal membrane, genotoxins, metabolic reprogramming, immune
reprogramming and regulation of signaling pathways. Study of the
association between intestinal flora, disease and tumor
microenvironment revealed that probiotics, fecal transplants and
natural extracts could be used to modify the intestinal flora to
treat diseases, while the related regulatory mediators could be
applied as biological targets for disease prevention, diagnosis,
treatment and prognosis. |
At present, most of the specific microbiota and
pathway changes in the regulatory mechanism of disease-gut
microbiota-tumor microenvironment lack in vivo and in
vitro experiments. Numerous studies have demonstrated that
substances such as vitamins (81)
and lactic acid produced by glycolysis in the tumor
microenvironment (110) can change
the intestinal flora or tumor microenvironment, however whether
these substances mediate the regulation of gut microbiota in the
tumor microenvironment has not yet been elucidated, which will open
up new directions for future research. As a key cell in tumor
immune regulation, tumor-infiltrating myeloid cells, a component of
the tumor microenvironment, require modification and activation by
m6A methylation (111). Previous
studies have revealed that the methylation of intestinal flora can
interfere with the expression of the oncogenic gene p53 and
activate it. SCFAs promote early onset and metastasis of tumors
(112), and it has been shown that
quantification of m6A methylation may serve as a potential
biological target for pancreatic cancer prognosis (113). Thus, whether the methylation of
gut microbiota also regulates tumors by affecting the function of
tumor-infiltrating myeloid cells, provides a new objective for
future research.
There are complex connections between the gut
microbiota and the tumor microenvironment, and the gut
microbiota-tumor microenvironment directly affects the prevention,
diagnosis, treatment and prognosis of diseases. Therefore, an
in-depth study of the association between the gut microbiota and
the tumor microenvironment will provide new means for the targeted
treatment of clinically common and difficult tumors by regulating
the intestinal flora and tumor microenvironment in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Inner Mongolia Autonomous
Region Health Science and Technology Plan Project Assignment (grant
no. 202201184), the Inner Mongolia Medical University Zhiyuan
Talent Program (Good Learning Talent Program) (grant no.
ZY0202031), the Program for Young Talents of Science and Technology
in Universities of Inner Mongolia Autonomous Region (grant no.
NJYT23050) and the Inner Mongolia Autonomous Region ‘Grassland
Talent’ project youth innovation and entrepreneurship talent
project (grant no. 2022073).
Availability of data and materials
Not applicable.
Authors' contributions
CH and GH conceived and designed this review. TX and
YL wrote the first draft. SJ critically revised the review for
important intellectual content. ZR, SR, ZY, ZJ and TC contributed
in the writing of the manuscript. All authors read and approved the
final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peng-Xu W, Xin-Ru D, Chen-Hong Z and
Hui-Juan Y: Gut microbiota and metabolic syndrome. Chin Med J.
133:808–816. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Freeman JW: Structural biology of the
tumor microenvironment. Adv Exp Med Biol. 1350:91–100. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong-Rolle A, Wei HK, Zhao C and Jin C:
Unexpected guests in the tumor microenvironment: Microbiome in
cancer. Protein Cell. 12:426–435. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gacesa R, Kurilshikov A, Vich Vila A,
Sinha T, Klaassen MAY, Bolte LA, Andreu-Sánchez S, Chen L, Collij
V, Hu S, et al: Environmental factors shaping the gut microbiome in
a Dutch population. Nature. 604:732–739. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sebastián Domingo JJ and Sánchez Sánchez
C: From the intestinal flora to the microbiome. Rev Esp Enferm Dig.
110:51–56. 2018.PubMed/NCBI
|
|
6
|
Lucas C, Barnich N and Nguyen HTT:
Microbiota, inflammation and colorectal cancer. Int J Mol Sci.
18:13102017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma C, Han M, Heinrich B, Fu Q, Zhang Q,
Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, et al: Gut
microbiome-mediated bile acid metabolism regulates liver cancer via
NKT cells. Science. 360:eaan59312018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rabelo-Gonçalves EM, Roesler BM and
Zeitune JM: Extragastric manifestations of Helicobacter pylori
infection: Possible role of bacterium in liver and pancreas
diseases. World J Hepatol. 7:2968–2979. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Knezevic J, Starchl C, Tmava Berisha A and
Amrein K: Thyroid-gut-axis: How does the microbiota influence
thyroid function? Nutrients. 12:17692020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng Y, Fang Z, Xue Y, Zhang J, Zhu J,
Gao R, Yao S, Ye Y, Wang S, Lin C, et al: Specific gut microbiome
signature predicts the early-stage lung cancer. Gut Microbes.
11:1030–1042. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao X, Miao R, Zhu Y, Lin C, Yang X, Jia
R, Linghan K, Wan C and Deng J: A new insight into acute
lymphoblastic leukemia in children: Influences of changed
intestinal microfloras. BMC Pediatr. 20:2902020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Westfall S, Caracci F, Estill M, Frolinger
T, Shen L and Pasinetti GM: Chronic Stress-induced depression and
anxiety priming modulated by Gut-brain-axis immunity. Front
Immunol. 12:6705002021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Angelucci F, Cechova K, Amlerova J and
Hort J: Antibiotics, gut microbiota, and Alzheimer's disease. J
Neuroinflammation. 16:1082019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma Q, Li Y, Li P, Wang M, Wang J, Tang Z,
Wang T, Luo L, Wang C, Wang T and Zhao B: Research progress in the
relationship between type 2 diabetes mellitus and intestinal flora.
Biomed Pharmacother. 117:1091382019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dodiya HB, Forsyth CB, Voigt RM, Engen PA,
Patel J, Shaikh M, Green SJ, Naqib A, Roy A, Kordower JH, et al:
Chronic stress-induced gut dysfunction exacerbates Parkinson's
disease phenotype and pathology in a rotenone-induced mouse model
of Parkinson's disease. Neurobiol Dis. 135:1043522020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Z, Lai J, Zhang P, Ding J, Jiang J, Liu
C, Huang H, Zhen H, Xi C, Sun Y, et al: Multi-omics analyses of
serum metabolome, gut microbiome and brain function reveal
dysregulated microbiota-gut-brain axis in bipolar depression. Mol
Psychiatry. 27:4123–4135. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paget S: The distribution of secondary
growths in cancer of the breast 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
18
|
Arneth B: Tumor microenvironment. Medicina
(Kaunas). 56:152019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Zhao L and Li XF: Hypoxia and the
tumor microenvironment. Technol Cancer Res Treat.
20:153303382110363042021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng X, Xu Y and Ning X: Tumor
microenvironment acidity modulates ROR1 to promote
epithelial-mesenchymal transition and hepatocarcinoma metastasis. J
Cell Sci. 134:jcs2553492021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greenwood E: A perfect mismatch. Nat Rev
Cancer. 2:76–77. 2002. View
Article : Google Scholar
|
|
22
|
Denton AE, Roberts EW and Fearon DT:
Stromal cells in the tumor microenvironment. Adv Exp Med Biol.
1060:99–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Russo M and Nastasi C: Targeting the tumor
microenvironment: A close up of tumor-associated macrophages and
neutrophils. Front Oncol. 12:8715132022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ngambenjawong C, Gustafson HH and Pun SH:
Progress in tumor-associated macrophage (TAM)-targeted
therapeutics. Adv Drug Deliv Rev. 114:206–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin Z, Li C, Wang J and Xue L:
Myeloid-derived suppressor cells: Roles in the tumor
microenvironment and tumor radiotherapy. Int J Cancer. 144:933–946.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Patente TA, Pinho MP, Oliveira AA,
Evangelista GCM, Bergami-Santos PC and Barbuto JAM: Human dendritic
cells: Their heterogeneity and clinical application potential in
cancer immunotherapy. Front Immunol. 9:31762018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spinelli FM, Vitale DL, Sevic I and Alaniz
L: Hyaluronan in the tumor microenvironment. Adv Exp Med Biol.
1245:67–83. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu M, Bai J, Ma C, Wei J and Du X: The
role of gut microbiota in tumor immunotherapy. J Immunol Res.
2021:50615702021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Zhang F, Zhao C, Xu Q, Liang C,
Yang Y, Wang H, Shang Y, Wang Y, Mu X, et al: Dysbiosis of the gut
microbiome is associated with thyroid cancer and thyroid nodules
and correlated with clinical index of thyroid function. Endocrine.
64:564–574. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Quigley EMM: Microbiota-brain-gut axis and
neurodegenerative diseases. Curr Neurol Neurosci Rep. 17:942017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Y, Zhou J and Wang L: Role and
mechanism of gut microbiota in human disease. Front Cell Infect
Microbiol. 11:6259132021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang X, Guo Y, Chen C, Shao B, Zhao L,
Zhou Q, Liu J, Wang G, Yuan W and Sun Z: Interaction between
intestinal microbiota and tumour immunity in the tumour
microenvironment. Immunology. 164:476–493. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maito F, Souza A, Pereira L, Smithey M and
Bonorino C: Intratumoral TLR-4 agonist injection is critical for
modulation of tumor microenvironment and tumor rejection. Isrn
Immunology. 2012:9268172012. View Article : Google Scholar
|
|
34
|
Albrengues J, Shields MA, Ng D, Park CG,
Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A,
Küttner V, et al: Neutrophil extracellular traps produced during
inflammation awaken dormant cancer cells in mice. Science.
361:eaao42272018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou Z, Chen J, Yao H and Hu H:
Fusobacterium and colorectal cancer. Front Oncol. 8:3712018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Monteran L and Erez NL: The dark side of
fibroblasts: Cancer-associated fibroblasts as mediators of
immunosuppression in the tumor microenvironment. Front Immunol.
10:18352019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang W, Borcherding N and Kolb R: IL-1
signaling in tumor microenvironment. Adv Exp Med Biol. 1240:1–23.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Long Q, Huang C, Meng Q, Peng J, Yao F, Du
D, Wang X, Zhu W, Shi D, Xu X, et al: TNF patterns and tumor
microenvironment characterization in head and neck squamous cell
carcinoma. Front Immunol. 12:7548182021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karakasheva TA, Lin EW, Tang Q, Qiao E,
Waldron TJ, Soni M, Klein-Szanto AJ, Sahu V, Basu D, Ohashi S, et
al: IL-6 mediates Cross-talk between tumor cells and activated
fibroblasts in the tumor microenvironment. Cancer Res.
78:4957–4970. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Batchu RB, Gruzdyn OV, Kolli BK,
Dachepalli R, Umar PS, Rai SK, Singh N, Tavva PS, Weaver DW and
Gruber SA: IL-10 signaling in the tumor microenvironment of ovarian
cancer. Adv Exp Med Biol. 1290:51–65. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gorczynski RM: IL-17 signaling in the
tumor microenvironment. Adv Exp Med Biol. 1240:47–58. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang R and Sun B: IL-22 signaling in the
tumor microenvironment. Adv Exp Med Biol. 1290:81–88. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu K, Huang A, Nie J, Tan J, Xing S, Qu Y
and Jiang K: IL-35 regulates the function of immune cells in tumor
microenvironment. Front Immunol. 12:6833322021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lan Y, Moustafa M, Knoll M, Xu C, Furkel
J, Lazorchak A, Yeung TL, Hasheminasab SM, Jenkins MH, Meister S,
et al: Simultaneous targeting of TGF-β/PD-L1 synergizes with
radiotherapy by reprogramming the tumor microenvironment to
overcome immune evasion. Cancer Cell. 39:1388–403.e10. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jobe NP, Rösel D, Dvořánková B, Kodet O,
Lacina L, Mateu R, Smetana K and Brábek J: Simultaneous blocking of
IL-6 and IL-8 is sufficient to fully inhibit CAF-induced human
melanoma cell invasiveness. Histochem Cell Biol. 146:205–217. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Di Pilato M, Kfuri-Rubens R, Pruessmann
JN, Ozga AJ, Messemaker M, Cadilha BL, Sivakumar R, Cianciaruso C,
Warner RD, Marangoni F, et al: CXCR6 positions cytotoxic T cells to
receive critical survival signals in the tumor microenvironment.
Cell. 184:4512–30.e22. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mukaida N, Sasaki SI and Baba T: CCL4
signaling in the tumor microenvironment. Adv Exp Med Biol.
1231:23–32. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ntanasis-Stathopoulos I, Fotiou D and
Terpos E: CCL3 signaling in the tumor microenvironment. Adv Exp Med
Biol. 1231:13–21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang W, Wang H, Sun M, Deng X, Wu X, Ma
Y, Li M, Shuoa SM, You Q and Miao L: CXCL5/CXCR2 axis in tumor
microenvironment as potential diagnostic biomarker and therapeutic
target. Cancer Commun (Lond). 40:69–80. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Portella L, Bello AM and Scala S: CXCL12
signaling in the tumor microenvironment. Adv Exp Med Biol.
1302:51–70. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ohtani N and Hara E: Gut-liver
axis-mediated mechanism of liver cancer: A special focus on the
role of gut microbiota. Cancer Sci. 112:4433–4443. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang H, Ren Z, Gao X, Hu X, Zhou Y, Jiang
J, Lu H, Yin S, Ji J, Zhou L and Zheng S: Integrated analysis of
microbiome and host transcriptome reveals correlations between gut
microbiota and clinical outcomes in HBV-related hepatocellular
carcinoma. Genome Med. 12:1022020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gargaro M, Manni G, Scalisi G, Puccetti P
and Fallarino F: Tryptophan metabolites at the crossroad of
immune-cell interaction via the aryl hydrocarbon receptor:
Implications for tumor immunotherapy. Int J Mol Sci. 22:46442021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
He Y, Fu L, Li Y, Wang W, Gong M, Zhang J,
Dong X, Huang J, Wang Q, Mackay CR, et al: Gut microbial
metabolites facilitate anticancer therapy efficacy by modulating
cytotoxic CD8+ T cell immunity. Cell Metab.
33:988–1000.e7. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xi Y, Yani Z, Jing M, Yinhang W, Xiaohui
H, Jing Z, Quan Q and Shuwen H: Mechanisms of induction of tumors
by cholesterol and potential therapeutic prospects. Biomed
Pharmacother. 144:1122772021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Long J, Guan P, Hu X, Yang L, He L, Lin Q,
Luo F, Li J, He X, Du Z and Li T: Natural polyphenols as targeted
modulators in colon cancer: Molecular mechanisms and applications.
Front Immunol. 12:6354842021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sepich-Poore GD, Zitvogel L, Straussman R,
Hasty J, Wargo JA and Knight R: The microbiome and human cancer.
Science. 371:eabc45522021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Arthur JC, Perez-Chanona E, Mühlbauer M,
Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B,
Rogers AB, et al: Intestinal inflammation targets cancer-inducing
activity of the microbiota. Science. 338:120–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Boleij A, Hechenbleikner EM, Goodwin AC,
Badani R, Stein EM, Lazarev MG, Ellis B, Carroll KC, Albesiano E,
Wick EC, et al: The Bacteroides fragilis toxin gene is prevalent in
the colon mucosa of colorectal cancer patients. Clin Infect Dis.
60:208–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dutilh BE, Backus L, van Hijum SA and
Tjalsma H: Screening metatranscriptomes for toxin genes as
functional drivers of human colorectal cancer. Best Pract Res Clin
Gastroenterol. 27:85–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nenkov M, Ma Y, Gaßler N and Chen Y:
Metabolic reprogramming of colorectal cancer cells and the
microenvironment: implication for therapy. Int J Mol Sci.
22:62622021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cheong JE, Ekkati A and Sun L: A patent
review of IDO1 inhibitors for cancer. Expert Opin Ther Pat.
28:317–330. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He Y, Huang J, Li Q, Xia W, Zhang C, Liu
Z, Xiao J, Yi Z, Deng H, Xiao Z, et al: Gut Microbiota and tumor
immune escape: A new perspective for improving tumor immunotherapy.
Cancers (Basel). 14:53172022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Spencer CN, McQuade JL, Gopalakrishnan V,
McCulloch JA, Vetizou M, Cogdill AP, Khan MAW, Zhang X, White MG,
Peterson CB, et al: Dietary fiber and probiotics influence the gut
microbiome and melanoma immunotherapy response. Science.
374:1632–1640. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ling Z, Shao L and Liu X, Cheng Y, Yan C,
Mei Y, Ji F and Liu X: Regulatory T cells and plasmacytoid
dendritic cells within the tumor microenvironment in gastric cancer
are correlated with gastric microbiota dysbiosis: A preliminary
study. Front Immunol. 10:5332019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Nakamura K and Smyth MJ: Myeloid
immunosuppression and immune checkpoints in the tumor
microenvironment. Cell Mol Immunol. 17:1–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Maier B, Leader AM, Chen ST, Tung N, Chang
C, LeBerichel J, Chudnovskiy A, Maskey S, Walker L, Finnigan JP, et
al: A conserved dendritic-cell regulatory program limits antitumour
immunity. Nature. 580:257–262. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lam KC, Araya RE, Huang A, Chen Q, Di
Modica M, Rodrigues RR, Lopès A, Johnson SB, Schwarz B, Bohrnsen E,
et al: Microbiota triggers STING-type I IFN-dependent monocyte
reprogramming of the tumor microenvironment. Cell.
184:5338–5356.e21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sethi V, Kurtom S, Tarique M, Lavania S,
Malchiodi Z, Hellmund L, Zhang L, Sharma U, Giri B, Garg B, et al:
Gut microbiota promotes tumor growth in mice by modulating immune
response. Gastroenterology. 155:33–37.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gagliani N, Hu B, Huber S, Elinav E and
Flavell RA: The fire within: Microbes inflame tumors. Cell.
157:776–783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tang YA, Chen YF, Bao Y, Mahara S, Yatim
S, Oguz G, Lee PL, Feng M, Cai Y, Tan EY, et al: Hypoxic tumor
microenvironment activates GLI2 via HIF-1α and TGF-β2 to promote
chemoresistance in colorectal cancer. Proc Natl Acad Sci USA.
115:E5990–E5999. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rius J, Guma M, Schachtrup C, Akassoglou
K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG and Karin M:
NF-kappaB links innate immunity to the hypoxic response through
transcriptional regulation of HIF-1alpha. Nature. 453:807–811.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Howe C, Kim SJ, Mitchell J, Im E, Kim YS,
Kim YS and Rhee SH: Differential expression of tumor-associated
genes and altered gut microbiome with decreased Akkermansia
muciniphila confer a tumor-preventive microenvironment in
intestinal epithelial Pten-deficient mice. Biochim Biophys Acta Mol
Basis Dis. 1864:3746–3758. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li R, Zhou R, Wang H, Li W, Pan M, Yao X,
Zhan W, Yang S, Xu L, Ding Y and Zhao L: Gut microbiota-stimulated
cathepsin K secretion mediates TLR4-dependent M2 macrophage
polarization and promotes tumor metastasis in colorectal cancer.
Cell Death Differ. 26:2447–2463. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhou S, Zhu C, Jin S, Cui C, Xiao L, Yang
Z, Wang X and Yu J: The intestinal microbiota influences the
microenvironment of metastatic colon cancer by targeting miRNAs.
FEMS Microbiol Lett. 369:fnac0232022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xing SC, Huang CB, Wu RT, Yang YW, Chen
JY, Mi JD, Wu YB, Wang Y and Liao XD: Breed differences in the
expression levels of gga-miR-222a in laying hens influenced
H2S production by regulating methionine synthase genes
in gut bacteria. Microbiome. 9:1772021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhou X, Liu Y, Xiong X, Chen J, Tang W, He
L, Zhang Z, Yin Y and Li F: Intestinal accumulation of
microbiota-produced succinate caused by loss of microRNAs leads to
diarrhea in weanling piglets. Gut Microbes. 14:20913692022.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li Z, Zhang X, Liu C and Ma J: Non-immune
cell components in the gastrointestinal tumor microenvironment
influencing tumor immunotherapy. Front Cell Dev Biol. 9:7299412021.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ding S, Hu C, Fang J and Liu G: The
protective role of probiotics against colorectal cancer. Oxid Med
Cell Longev. 2020:88845832020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shi L, Sheng J, Wang M, Luo H, Zhu J,
Zhang B, Liu Z and Yang X: Combination therapy of TGF-β blockade
and commensal-derived probiotics provides enhanced antitumor immune
response and tumor suppression. Theranostics. 9:4115–4129. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yue Y, Ye K, Lu J, Wang X, Zhang S, Liu L,
Yang B, Nassar K, Xu X, Pang X and Lv J: Probiotic strain
Lactobacillus plantarum YYC-3 prevents colon cancer in mice by
regulating the tumour microenvironment. Biomed Pharmacother.
127:1101592020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Baruch EN, Youngster I, Ben-Betzalel G,
Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S,
Bloch N, et al: Fecal microbiota transplant promotes response in
immunotherapy-refractory melanoma patients. Science. 371:602–609.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Di Modica M, Gargari G, Regondi V, Bonizzi
A, Arioli S, Belmonte B, De Cecco L, Fasano E, Bianchi F,
Bertolotti A, et al: Gut microbiota condition the therapeutic
efficacy of trastuzumab in HER2-positive breast cancer. Cancer Res.
81:2195–2206. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ke S, Weiss ST and Liu YY: Rejuvenating
the human gut microbiome. Trends Mol Med. 28:619–630. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chen L, Brar MS, Leung FC and Hsiao WL:
Triterpenoid herbal saponins enhance beneficial bacteria, decrease
sulfate-reducing bacteria, modulate inflammatory intestinal
microenvironment and exert cancer preventive effects in ApcMin/+
mice. Oncotarget. 7:31226–31242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fu H, Liu X, Jin L, Lang J, Hu Z, Mao W,
Cheng C and Shou Q: Safflower yellow reduces DEN-induced
hepatocellular carcinoma by enhancing liver immune infiltration
through promotion of collagen degradation and modulation of gut
microbiota. Food Funct. 12:10632–10643. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ding G, Gong Q, Ma J, Liu X, Wang Y and
Cheng X: Immunosuppressive activity is attenuated by Astragalus
polysaccharides through remodeling the gut microenvironment in
melanoma mice. Cancer Sci. 112:4050–4063. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li B, Liu M, Wang Y, Gong S, Yao W, Li W,
Gao H and Wei M: Puerarin improves the bone micro-environment to
inhibit OVX-induced osteoporosis via modulating SCFAs released by
the gut microbiota and repairing intestinal mucosal integrity.
Biomed Pharmacother. 132:1109232020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shi HJ, Chen XY, Chen XR, Wu ZB, Li JY,
Sun YQ, Shi DX and Li J: Chinese medicine formula Siwu-Yin inhibits
esophageal precancerous lesions by improving intestinal flora and
macrophage polarization. Front Pharmacol. 13:8123862022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jiang F, Liu M, Wang H, Shi G, Chen B,
Chen T, Yuan X, Zhu P, Zhou J, Wang Q and Chen Y: Wu Mei wan
attenuates CAC by regulating gut microbiota and the NF-kB/IL6-STAT3
signaling pathway. Biomed Pharmacother. 125:1099822020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Liu YJ, Tang B, Wang FC, Tang L, Lei YY,
Luo Y, Huang SJ, Yang M, Wu LY, Wang W, et al: Parthenolide
ameliorates colon inflammation through regulating Treg/Th17 balance
in a gut microbiota-dependent manner. Theranostics. 10:5225–5241.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li T, Han L, Ma S, Lin W, Ba X, Yan J,
Huang Y, Tu S and Qin K: Interaction of gut microbiota with the
tumor microenvironment: A new strategy for antitumor treatment and
traditional Chinese medicine in colorectal cancer. Front Mol
Biosci. 10:11403252023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tong Y, Gao H, Qi Q, Liu X, Li J, Gao J,
Li P, Wang Y, Du L and Wang C: High fat diet, gut microbiome and
gastrointestinal cancer. Theranostics. 11:5889–5910. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Jin H and Zhang C: High fat high calories
diet (HFD) increase gut susceptibility to carcinogens by altering
the gut microbial community. J Cancer. 11:4091–4098. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
AlHilli MM and Bae-Jump V: Diet and gut
microbiome interactions in gynecologic cancer. Gynecol Oncol.
159:299–308. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wu W, Zhou J, Xuan R, Chen J, Han H, Liu
J, Niu T, Chen H and Wang F: Dietary κ-carrageenan facilitates gut
microbiota-mediated intestinal inflammation. Carbohydr Polym.
277:1188302022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lee JY, Tsolis RM and Bäumler AJ: The
microbiome and gut homeostasis. Science. 377:eabp99602022.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Peterson CT, Perez Santiago J, Iablokov
SN, Chopra D, Rodionov DA and Peterson SN: Short-Chain fatty acids
modulate healthy gut microbiota composition and functional
potential. Curr Microbiol. 79:1282022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yu LX and Schwabe RF: The gut microbiome
and liver cancer: Mechanisms and clinical translation. Nat Rev
Gastroenterol Hepatol. 14:527–539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Roberti MP, Yonekura S, Duong CPM, Picard
M, Ferrere G, Tidjani Alou M, Rauber C, Iebba V, Lehmann CHK, Amon
L, et al: Chemotherapy-induced ileal crypt apoptosis and the ileal
microbiome shape immunosurveillance and prognosis of proximal colon
cancer. Nat Med. 26:919–931. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yang Q, Zhang J and Zhu Y: Potential roles
of the gut microbiota in pancreatic carcinogenesis and
therapeutics. Front Cell Infect Microbiol. 12:8720192022.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang Z, Qin X, Hu D, Huang J, Guo E, Xiao
R, Li W, Sun C and Chen G: Akkermansia supplementation reverses the
tumor-promoting effect of the fecal microbiota transplantation in
ovarian cancer. Cell Rep. 41:1118902022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sevcikova A, Izoldova N, Stevurkova V,
Kasperova B, Chovanec M, Ciernikova S and Mego M: The impact of the
microbiome on resistance to cancer treatment with chemotherapeutic
agents and immunotherapy. Int J Mol Sci. 23:4882022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Liu J, Liu C and Yue J: Radiotherapy and
the gut microbiome: Facts and fiction. Radiat Oncol. 16:92021.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Qiu Q, Lin Y, Ma Y, Li X, Liang J, Chen Z,
Liu K, Huang Y, Luo H, Huang R and Luo L: Exploring the emerging
role of the gut microbiota and tumor microenvironment in cancer
immunotherapy. Front Immunol. 11:6122022020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang D, Hao H, Li X and Wang Z: The effect
of intestinal flora on immune checkpoint inhibitors in tumor
treatment: A narrative review. Ann Transl Med. 8:10972020.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Smith M, Dai A, Ghilardi G, Amelsberg KV,
Devlin SM, Pajarillo R, Slingerland JB, Beghi S, Herrera PS,
Giardina P, et al: Gut microbiome correlates of response and
toxicity following anti-CD19 CAR T cell therapy. Nat Med.
28:713–723. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ma J, Huang L, Hu D, Zeng S, Han Y and
Shen H: The role of the tumor microbe microenvironment in the tumor
immune microenvironment: Bystander, activator, or inhibitor? J Exp
Clin Cancer Res. 40:3272021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sethi V, Vitiello GA, Saxena D, Miller G
and Dudeja V: The role of the microbiome in immunologic development
and its implication for pancreatic cancer immunotherapy.
Gastroenterology. 156:2097–2115.e2. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wei D, Wang L, Zuo X and Bresalier RS:
Vitamin D: Promises on the Horizon and Challenges Ahead for
Fighting Pancreatic Cancer. Cancers (Basel). 13:27162021.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Xiong J, He J, Zhu J, Pan J, Liao W, Ye H,
Wang H, Song Y, Du Y, Cui B, et al: Lactylation-driven
METTL3-mediated RNA m6A modification promotes
immunosuppression of tumor-infiltrating myeloid cells. Mol Cell.
82:1660–1677.e10. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kumar H, Lund R, Laiho A, Lundelin K, Ley
RE, Isolauri E and Salminen S: Gut microbiota as an epigenetic
regulator: Pilot study based on whole-genome methylation analysis.
mBio. 5:e02113–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wang L, Zhang S, Li H and Xu Y, Wu Q, Shen
J, Li T and Xu Y: Quantification of m6A RNA methylation modulators
pattern was a potential biomarker for prognosis and associated with
tumor immune microenvironment of pancreatic adenocarcinoma. BMC
Cancer. 21:8762021. View Article : Google Scholar : PubMed/NCBI
|


















