Introduction
Hepatocellular carcinoma (HCC) is a chronic disease
and one of the most significant causes of cancer-associated death;
furthermore, the clinical prognosis is usually poor (1). The etiology of liver cancer is
diverse, including viral infection, alcoholic and non-alcoholic
fatty liver disease, aflatoxins, genetic and metabolic factors and
infection, among which viral infection [via hepatitis B virus (HBV)
and hepatitis C virus (HCV)] is the most important factor (2,3). In
recent years, with the prevalence of obesity, diabetes and various
metabolic syndromes increasing, the incidence of HCC has also been
shown to be on a clear upward trajectory (1,4,5). Liver
cancer is divided into primary and secondary HCC, and the most
important type of HCC is primary HCC (cancer originating from
mutations in hepatocytes or other cell types in the liver), which
accounts for ~80% of all cases of HCC; other types of HCC include
intrahepatic cholangiocarcinoma and mixed hepatocellular
cholangiocarcinoma (3,6). Although various types of liver cancer
are significant, the present review will focus mainly on primary
HCC. Clinically, the majority of cases of HCC are identified at a
nearly advanced stage, in part due to the fact that HCC is not
generally associated with obvious physical signs or symptoms in the
early stages. By contrast, early HCC can usually only be detected
either by ultrasound imaging or by measuring the blood
α-fetoprotein concentration, although its specificity is not high
(2,6). As a result, HCC is not easily detected
in the early stages, resulting in only a small number of, and poor,
options of therapy being available. It is worth noting that the
treatment of HCC is complex and several years of study have
demonstrated that treatment of patients with HCC depends on the
clinical manifestation, liver function and tumor staging, although
multidisciplinary treatments exist, which mainly include surgical
resection and liver transplantation, radiofrequency ablation,
chemical drug targeting inhibition and immune suppression (3,7,8).
However, due to its high invasiveness and high metastasis rate, the
prognosis for patients with HCC continues to be poor (9,10).
Therefore, there is an urgent need to identify novel early markers
and therapeutic targets to prevent, diagnose and treat the
disease.
Ion channels are specialized proteins found in cell
membranes, which facilitate the movement of specific ions across
the plasma membrane. These are involved in fundamental cellular
processes, such as nerve impulse transmission, cell proliferation,
apoptosis, hormone secretion and sensory transduction (11,12).
The aberrant expression and function of ion channels leads to
impairment of these processes, allowing normal cells to transform
into malignant derivatives that exhibit uncontrolled proliferation
and spread, which are hallmarks of cancer (13). Previous studies have confirmed that
ion channels fulfill an important role in the development and
progression of cancer, including the infinite proliferation of
cells and their invasion and metastasis (14–16),
and ion channels have been approved as effective drug targets for
cancer therapy (17,18). As the most widely distributed type,
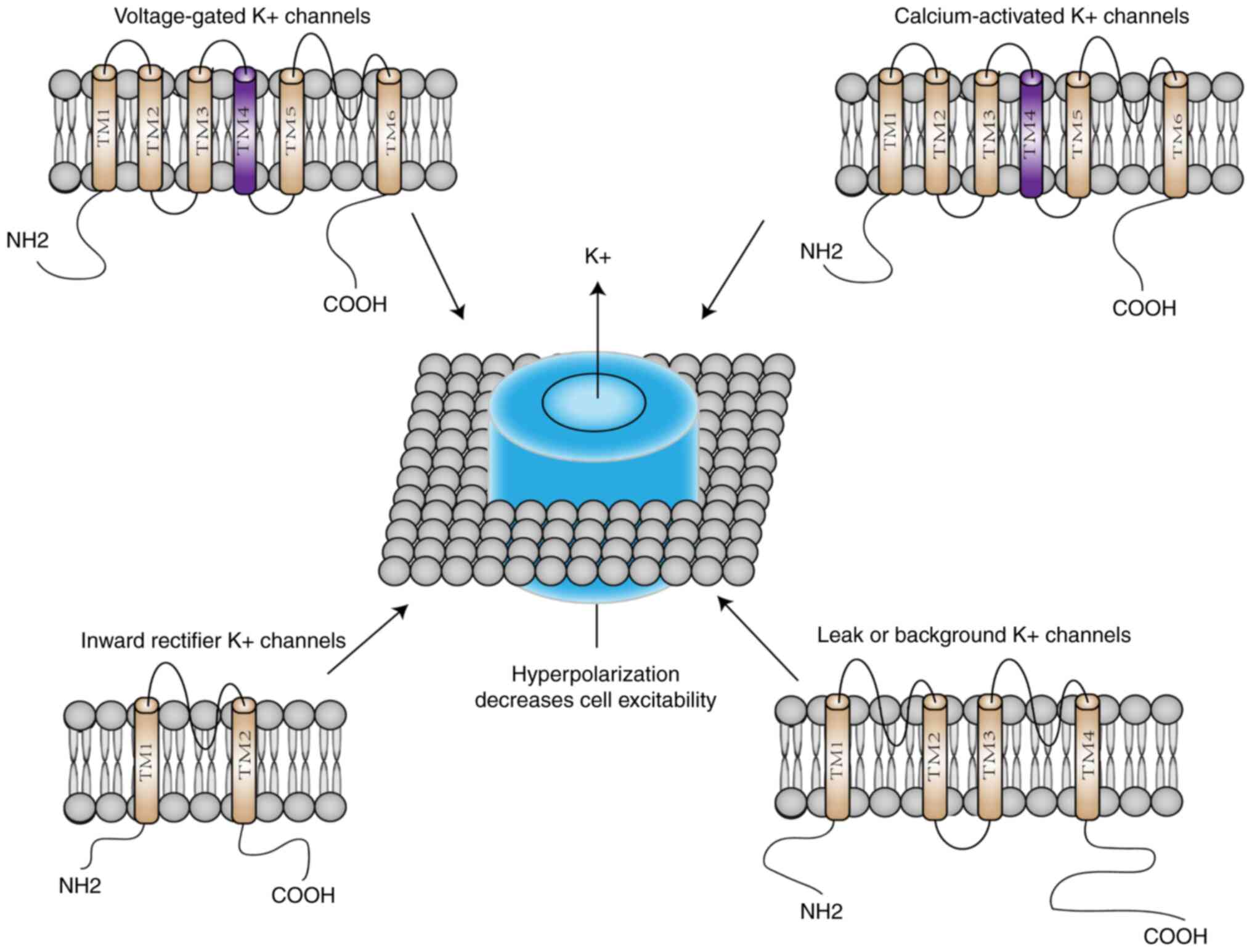
K+ channels regulate a variety of biological processes
by controlling the flow of K+ across cell membranes. The
K+ channel family has a total of 78 members, which can
be divided into four major groups based on their domains and
activation mechanisms: i) Voltage-gated K+ (Kv)
channels, ii) calcium-activated K+ (KCa) channels, which
themselves are divided into large conductance (BK), medium
conductance (IK) and small conductance (SK) channels, iii) inward
recirculated K+ channels and iv) two-pore domain
K+ channels (15,19)
(Fig. 1). As important contributors
to the resting membrane potential, K+ channels affect a
variety of physiological processes (including regulating the heart
rate, muscle contraction, neurotransmitter release, neuronal
excitability, cell volume regulation, cell proliferation and
differentiation, as well as cell cycle progression, apoptosis and
metabolism) through regulating the intracellular K+
concentration (20). There is an
increasing body of evidence to suggest that a wide variety of
K+ channels are expressed on tumor cells, and
dysregulation of their expression has been identified at the
genomic, transcriptional, post-translational and epigenetic levels
(15). In general, K+
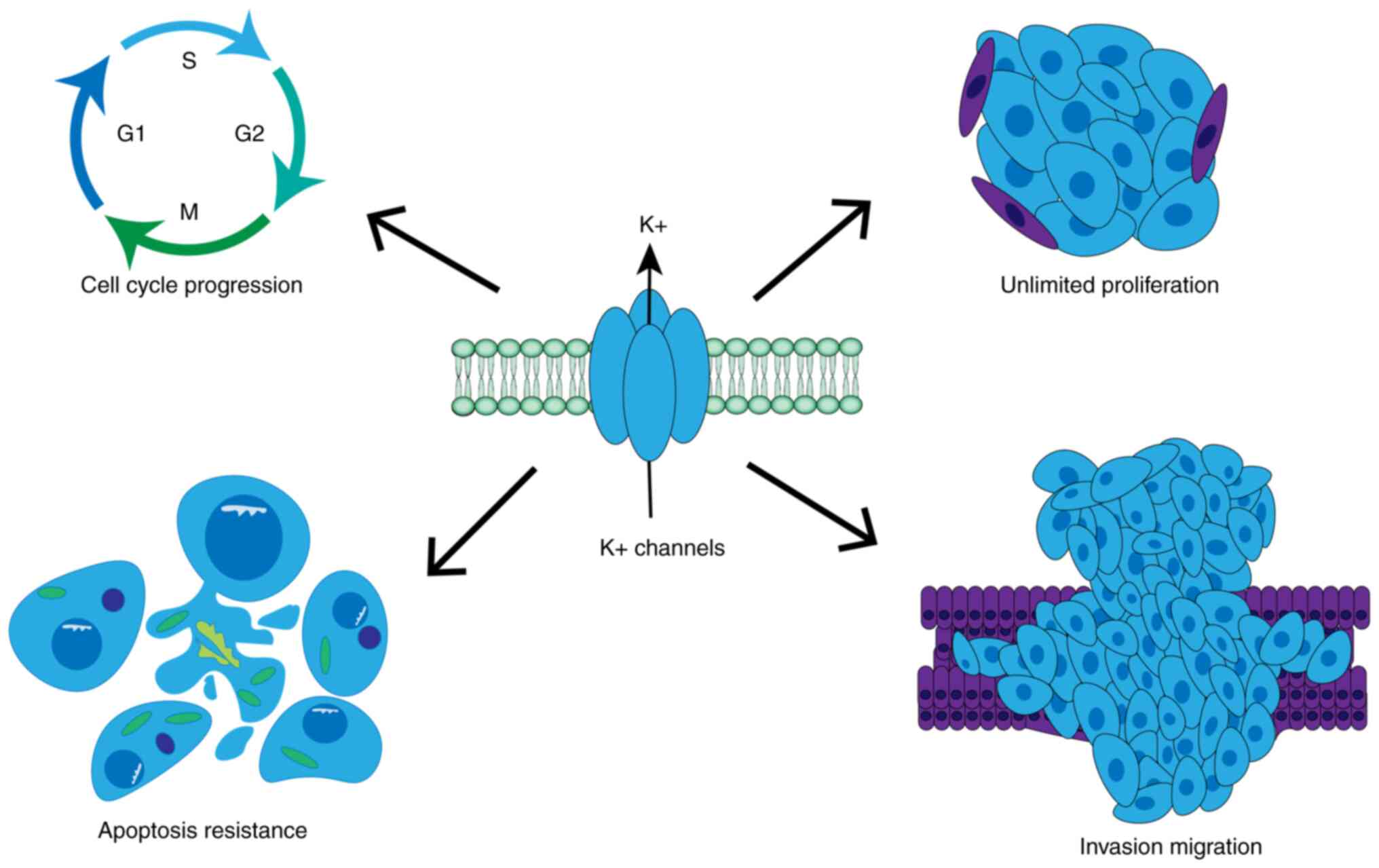
channels have been shown to regulate cell proliferation through
four mechanisms: i) Establishment of oscillating membrane
potentials; ii) control of cell volume dynamics; iii) regulation of
calcium signaling; and iv) promotion of malignant growth through
atypical non-ionic osmotic functions (15). Therefore, these channels have been
shown to fulfill important roles in regulating tumor cell
proliferation, cell cycle progression and apoptosis (21,22)
(Fig. 2). For instance,
upregulation of Kv1.1 has been demonstrated to be a marker for a
subgroup of medulloblastomas (23);
elevated levels of Kv1.3 expression are detected in numerous human
malignancies, including breast, colon and prostate cancer (24); a high expression level of Kv11.1 [or
human ether-a-go-go-related gene (hERG)] was shown to serve as a
marker for solid and blood cancers (25); and the overexpression of Kv10.1 [or
Ether-a-go-go-1 (EAG1)] has been identified in cancers of various
human organs (26). In addition,
K+ channel modulators exert antitumor effects primarily
as regulators of various types of cancer cell behaviors, including
proliferation and migration (27).
Furthermore, the abnormal expression and dysfunction of specific
K+ channels may lead to the development of various
diseases. In addition, the expression and activity of K+
channels have been indicated to be significantly correlated with
the grade of malignancy of tumors. Targeting K+ channels
may therefore have great therapeutic potential in terms of the
treatment of a wide range of human diseases (27,28).
Certain studies have indicated that targeting the inhibition of
K+ channels may either directly inhibit tumor growth or
improve the efficacy of chemotherapy or cytotoxic drugs, and this
may be used as a combined treatment strategy for exerting
anti-tumor effects (29,30). However, to date, to the best of our
knowledge, the role of K+ channels in HCC has rarely
been studied.
Therefore, the aim of the present review was to
provide an overview of the dysregulation of K+ channels
in HCC, also discussing their role in the proliferation, invasion
and migration of HCC. The latest progress that has been made in
terms of research on drugs targeting K+ channels for the
treatment of HCC is also discussed, as well as the existing
limitations and future research directions in this field. These
research efforts are geared towards providing novel opportunities
for the early detection and treatment of HCC.
Role of K+ channels in regulating
metabolism, proliferation and injury of liver cells
The liver is a complex organ composed of a variety
of different types of cells, which fulfills crucial roles in the
body's metabolism. The roles of liver cells mainly comprise
carbohydrate, lipid, protein and amino acid metabolism, bile acid
intake, synthesis and output, as well as the metabolism of drugs
and other foreign substances and the excretion of biological
molecules (31). Therefore, damage
sustained to the liver cells has an adverse effect on human health.
K+ channels fulfill an important role in acute and
chronic hepatocyte injury. For instance, margatoxin (MgTX), a
Kv1.3-specific blocker, has been shown to reduce the serum levels
of TNF-α, IL-6, alanine transaminase and aspartate transaminase to
reduce the expression ratio of C-C motif chemokine receptor
2/glutathione reductase 1 double-positive cells and ionized calcium
binding adaptor molecule 1/c-type lectin domain family 4 member F
positive cells in peripheral mononuclear macrophages, as well as
reducing the infiltration rate of peripheral mononuclear
macrophages into the liver (32).
MgTX has also been shown to markedly protect the liver from acute
liver injury. In addition, it is a novel target for the prevention
and treatment of alcoholic fatty liver disease, precisely since it
is able to regulate the function of macrophages. Further studies
have indicated that the Kv1.3 pathway may alleviate the development
of liver fibrosis by reducing the expression of TGF-β (32). It has been reported that KCa3.1
expression is increased in hepatocytes with liver fibrosis and that
these increases coincide with the progression of liver injury. In
addition, the inhibition of KCa3.1 has been shown to lead to cell
apoptosis and increase the level of DNA damage, and also to
stimulate the proliferation of hepatic stellate cells and aggravate
liver fibrosis, which demonstrates that KCa3.1 channels exert a
protective role in liver injury (33). The activity of two-pore domain
K+ (KCNK2) channels determines resting membrane
potential and Ca2+ levels, thereby fulfilling a role in
extracellular matrix production and the cell proliferation of
hepatic stellate cells, providing a potential therapeutic target
for hepatic fibrosis (34).
In recent years, the role of K+ channels
in the pathogenesis and treatment of HCC has been gradually
uncovered. Several studies have shown that K+ ions are a
key regulator of hepatocyte function, which is manifested in
inhibiting hepatocyte proliferation and inducing apoptosis, thereby
preventing the metastasis of hepatocytes during the process of
carcinogenesis. First, K+ has been shown to inhibit the
proliferation of hepatocytes, particularly HepG2 cells. In
addition, the results of cell-cycle analysis experiments have
indicated that K+ is able to block the S-phase of the
cell cycle and inhibit the growth of L02 and HepG2 cells via
preventing normal DNA replication (35). Furthermore, it is well-established
that the pro-apoptotic protein Bax and the anti-apoptotic protein
Bcl-2 fulfill a key role in the regulation of cell apoptosis, and
that a decrease in the Bcl-2/Bax ratio can promote cell apoptosis.
K+ ions promote the expression of the channel protein
hERG in a dose-dependent manner, possibly by upregulating the
expression of voltage-dependent anion-selective channel protein 1
or through disrupting the balance of the Bcl-2/Bax ratio, which
prevents cells from being transferred to the precancerous pathway,
leading to the imbalance of the mitochondrial membrane potential,
thereby inducing mitochondria to release cytochrome c and to
activate caspase proteins (35).
These steps consequently result in an imbalance of the caspase-3/7
ratio, eventually leading to apoptosis. In conclusion, targeted
activation or inhibition of K+ channels can be a
potential therapeutic approach to inhibit the development and
progression of HCC.
K+ channels in the development,
migration, proliferation and invasion of HCC
Although a large number of ion channels associated
with the proliferation, invasion and metastasis of HCC have been
reported, the number of specific biomarkers and therapeutic targets
available remains limited (36). As
one of the most extensive ion channels, various studies have
confirmed that K+ channels have an important role in the
development, migration, proliferation and invasion of HCC and may
be recruitable as novel tumor markers and targeted therapeutic
targets (Table I). Although the
underlying mechanisms involved have yet to be fully elucidated,
this may help to provide a foundation upon which further research
can be based.
 | Table I.Role of K+ channels in the
development of HCC. |
Table I.
Role of K+ channels in the
development of HCC.
| Name | Role | Therapeutic
strategy | (Refs.) |
|---|
| BK | Exhibits high
expression in HCC | Inhibition | (37) |
|
| Promotes the
migration of HCC cells |
|
|
| KCa3.1 | Exhibits high
expression in HCC | Inhibition | (38–40) |
|
| Promotes the
proliferation, migration and transfer of HCC and ICC cells |
|
|
| EAG1 | Exhibits high
expression in HCC | Inhibition | (41) |
|
| Promotes the
proliferation, migration and invasion of HCC |
|
|
| KCCN2, KCNK15,
KCNK17 | Low expression in
HCC Utility as diagnostic markers and predictors of prognosis | Requires further
research | (42) |
| KCNK9 | Exhibits high
expression in HCC | Requires
further | (42) |
|
| Utility as a
diagnostic marker | research |
|
| KCNQ1 | Shows low
expression in HCC | Activation | (43) |
|
| Promotes the
invasion of cells |
|
|
| KCNQ1OT1 | Exhibits high
expression in HCC | Inhibition | (44–46) |
|
| Promotes the
proliferation and transfer of HCC cells, and inhibits
apoptosis |
|
|
| ATP1A1FXYD6 | Exhibits high
expression in HCC | Inhibition | (47–49) |
|
| Promotes the
occurrence, proliferation and migration of HCC cells |
|
|
| Kv1.3 | Exhibits high
expression in PBC Promotes cell proliferation and apoptosis | Inhibition | (50) |
BK channels
Large-conductance calcium-activated potassium
channels, i.e. BK channels, belong to the Ca2+-activated
K+ channel family, which also contains two other
members, namely IK and SK Ca2+-activated K+
channels (37). BK channels were
first identified in chromaffin cells in 1981 (38), and were later found to be expressed
in neurons of the vibratory nervous system, where they can be
activated by membrane depolarization and increased cytosolic
Ca2+ levels (39).
Previous studies have reported a variety of functions of BK
channels; for instance, BK channels are involved in the regulation
of the cell cycle and proliferation, and they have also been shown
to be involved in the migration of cancer cells (40). Previous studies have reported on
disorders of BK channels in various types of cancer cells,
including triple-negative breast cancer cells, neuroblastoma cells,
glioblastoma and human astrocytoma cells (40). The patch-clamp technique has also
been used to identify BK channels in SMMC-7721 and Huh7 cells. The
results obtained indicated that BK channels were functionally
expressed in both HCC cells and normal stem cells (40). At the same time, the expression of
BK channels in patients with HCC was detected, and it was found
that the expression of BK channels in tumor tissues was higher
compared with that in non-tumor tissues. More interestingly, the
prognosis of patients with a high expression of BK channels was
found to be significantly lower compared with that of patients with
low BK channel expression (40). A
significant role has been identified for BK channels in terms of
promoting cancer cell migration and two possible explanations have
been proposed: i) Through reducing the expression of
epithelial-to-mesenchymal transition (EMT)-associated proteins,
such as E-cadherin, vimentin and N-cadherin, and cell-cell contact
is thereby reduced, which promotes cancer cell migration; and ii)
through reducing cell volume to promote tumor cell migration
(40).
KCa3.1 channels
The IK Ca2+-activated K+
channel (KCa3.1) belongs to a medium-conductance calcium-activated
K+ channel family and is a potential tumor-targeting
molecule, which regulates intracellular ion homeostasis and cell
volume under physiological conditions (41). It has been confirmed that this
channel is overexpressed in a variety of different types of cancer
and regulates the migration, invasion, proliferation and treatment
resistance of cancer cells (42,43).
It is well-known that HCC stem cells fulfill an important role in
tumorigenesis, tumor recurrence and metastasis. A previous study
found that KCa3.1 is highly expressed in liver cancer stem cells.
KCa3.1 has also been shown to promote the proportions of
CD133+ and CD44+ cell subsets, the expression
of stem cell transcription factors and the ability of pellet
formation in vitro, and to increase the incidence of tumor
and tumor growth in vivo (44). Several studies have used
immunohistochemical analysis to observe that the expression of
KCa3.1 in HCC tissues is significantly upregulated, and this
upregulation of gene expression is not only associated with the
serum α-fetoprotein level, but it is also associated with an
increased risk of recurrence in patients with early HCC (45,46). A
number of studies have investigated the potential underlying
mechanism(s) governing how KCa3.1 exerts a role in HCC. On the one
hand, silencing the expression of KCa3.1 has been shown to lead to
a reduction in the levels of extracellular signal-regulated kinases
1 and 2 (ERK1/2) and matrix metalloproteinase 9 (MMP-9) (47). Both ERK1/2 and MMP-9 are considered
important biomarkers in the MAPK/ERK signaling pathway. MMP-9 is
able to induce the degradation of the extracellular matrix, thereby
reducing the stability of cancer cells and making them more prone
to metastasis. Activation of ERK1/2 may lead to the transcriptional
activation of downstream target genes that are associated with cell
proliferation, migration and metastasis (47). In addition, it has been conclusively
shown that KCa3.1 promotes the migration of HCC cells through the
MAPK/ERK signaling pathway, thereby promoting tumor migration and
invasion. Knockdown or inhibition of KCa3.1 expression has also
been shown to reduce the migratory and invasive capabilities of HCC
cells (46). Alternatively, other
studies have found that KCa3.1 can promote the proliferation,
invasion and migration of HCC cells via regulating the S-phase
kinase-associated protein 2 (SKP2)/P27/P21 signaling pathway. The
possible underlying mechanism is that upregulation of KCa3.1
expression changes the half-life of the SKP2 protein, which
subsequently mediates the degradation of P21/P27 to promote HCC
cell cycle progression. By contrast, knockdown of KCa3.1 was found
to significantly reduce the migratory or invasive potential of LM3
and Huh7 cells (45). These results
corroborated that KCa3.1 may be a potential molecular target for
the treatment of HCC. In HCC, KCa3.1 channels have also been
implicated in the development of intrahepatic cholangiocarcinoma
(ICC). The expression of the KCa3.1 channel is significant in ICC
and this was found to be correlated with the age, lymph node
metastasis and TNM stage of the patients. Finally, pharmacological
inhibition or knockdown of KCa3.1 was also shown to reduce the
proliferative and invasive capabilities of ICC cells (43).
Eag1 channel
The Eag1 channel, also known as Kv10.1, is a
voltage-gated K+ channel that is mainly distributed in
the cell membrane and participates in various physiological
processes of the body, including cell action-potential
repolarization, Ca2+ signal transduction and cell volume
regulation, thereby promoting cell proliferation and migration
(27). It is worth noting that this
channel has been found to have carcinogenic properties and that it
has therefore garnered great interest among cancer researchers
(48). A previous study reported
that Eag1 is expressed at a low level in normal tissues, with the
exception of the central nervous system and abnormal expression in
tumor cells of different origins (26). A previous study also reported that,
the mRNA and protein expression of Eag1 in cirrhotic tissues and
pretumor lesions was significantly higher compared with that in
normal liver (49). To investigate
the role of Eag1 in HCC, the authors found that the
colony-formation capabilities and proliferation rates of the LM3
and Huh7 cell lines were significantly decreased following
downregulation of Eag1. On the other hand, Eag1 overexpression
promoted the colony-formation capability and proliferation of the
cells. In addition, macroscopic observations of the tumor volume
showed that the tumors with Eag1 overexpression were larger
compared with those in the control group with normal expression of
Eag1 (50). In the same study, the
authors identified the putative mechanism to account for the above
effects: On the one hand, Eag1 is able to regulate SKP2 through
ubiquitination to improve cell proliferation, and the expression of
Eag1 was shown to be positively correlated with SKP2.
Overexpression of Eag1 led to a prolongation of the half-life of
SKP2, which subsequently stimulated the degradation of P21/P27,
thereby accelerating the cell cycle progression of HCC. On the
other hand, Eag1 has also been shown to promote the migration and
invasion of tumor cells via promoting the formation of pseudopodia
(50). In conclusion,
downregulation of Eag1 expression may be used as a therapeutic
strategy for HCC.
KCNK channels
KCNK channels are K+-selective channels.
The majority of KCNKs act as outward rectifying channels, or are
almost voltage-independent at physiological K+
concentrations to maintain the resting membrane potential, thereby
regulating biological metabolism and apoptosis (51–53).
It is worth noting that KCNKs are able to contribute to oncogenes
in various cancers. For instance, in ovarian cancer, certain
regulators of KCNK2 have been shown to inhibit apoptosis and to
promote cell proliferation (54).
The expression level of the long non-coding RNA (lncRNA)
KCNK15-antisense 1 was found to be downregulated in pancreatic
cancer, which consequently inhibited the invasiveness of tumor
cells (55). By contrast, KCNK9 is
upregulated in breast and colorectal cancer, and increases tumor
tolerance via its anti-apoptotic activity on the cancer cells
(56,57). In HCC, different KCNK channels have
been demonstrated to have different expression levels. Through
analyzing the UALCAN database, researchers observed that the
expression levels of KCNK2, KCNK15 and KCNK17 were decreased in
HCC, whereas the expression level of KCNK9 was increased, and these
trends were associated with poor prognosis for patients with HCC.
Additionally, the receiver operating characteristic curve analysis
suggested that the levels of KCNK2, KCNK9, KCNK15 and KCNK17 levels
may be used as biomarkers for the diagnosis and prognosis of HCC
(52). This study provided
important information for the early diagnosis of HCC.
Potassium voltage-gated channel,
KQT-like subfamily, member 1 (KCNQ1) channels
KCNQ1 channels, as a class of voltage-gated
K+ channels, are usually expressed in a variety of
tissues, including the heart, stomach, intestine and pancreas,
which mediate the outflow of K+ ions from cells and
regulate ion homeostasis in tissues (58). Previous studies have confirmed that
the expression of KCNQ1 is markedly downregulated in human HCC cell
lines compared with non-malignant cells or normal human liver
tissues (59,60). On the other hand, overexpression of
KCNQ1 inhibited the invasion of HCC, and the same study revealed
that pharmacological activation of KCNQ1 channels inhibited tumor
metastasis of HCC in nude mice. The putative underlying mechanism
may be that KCNQ1 affects the cellular distribution of β-catenin,
thereby inhibiting the activity of the Wnt/β-catenin signaling
pathway, which acts as one of the main pathways involved in both
the initiation and progression of HCC (59) and in the mRNA expression of its
downstream targets, ultimately fulfilling a tumor-suppressor role
(60). In conclusion, it has been
demonstrated that the KCNQ1 channel may be used as both a biomarker
and potential therapeutic target for HCC.
Long non-coding RNA (lncRNA)
K+ voltage-gated channel subfamily Q member 1
overlapping transcript 1 (KCNQ1OT1) channels
The lncRNA KCNQ1OT1 is a chromatin-regulatory lncRNA
that has been shown to participate in the regulation of various
types of cancer as an oncogene, including rectal cancer and lung
cancer (61). However, at present,
little is known concerning the mechanism via which KCNQ1OT1
promotes carcinogenesis. However, certain studies have found that
the short-strand repeat polymorphism in KCNQ1OT1 contributes to the
initiation of HCC, which may affect the expression of KCNQ1OT1 and
cyclin-dependent kinase inhibitor 1C (CDKN1C) through a
structure-dependent mechanism (62). Previous studies have also reported
that the expression of KCNQ1OT1 is associated with the development
of HCC. Several studies have reported that KCNQ1OT1 is able to
affect the growth of HCC; for example, by inhibiting the expression
of microRNA (miR-504) (61),
through targeting miR-338-3p (63),
by regulating the miR-506-3p/forkhead box (Fox)Q1 axis (64) and through regulating the
miR-146A-5p/alkaline ceramidase 3 signaling axis (65). Of note, the latter study (65) reported that the expression of
KCNQ1OT1 is significantly upregulated in HCC; furthermore, it was
found that silencing KCNQ1OT1 inhibited cell proliferation,
improved the sensitivity to radiotherapy and promoted cell
apoptosis, as well as hindering the metastasis of HCC cells. In
terms of treatment strategies, a previous study (66) reported that KcNQ1OT1 knockdown
enhanced the sensitivity of sorafenib through targeting miR-506,
induce cell apoptosis and inhibit the metastasis of
sorafenib-resistant HCC cells. In conclusion, KCNQ1OT1 may also
provide novel therapeutic strategies and opportunities for HCC.
Na+/K+-ATPase
(NKA) channel
NKA channel, as a member of the P-type ATPase
family, is composed of three peptides: The α and β subunits and
FXYD protein (a type of small molecule single transmembrane
protein, which regulators of Na+/K+-ATPase). It pumps three
Na+ ions out and two K+ ions in for each
ATPase hydrolyzed, acting as a multifunctional protein, which
fulfills roles in cell attachment, adhesion, motility and signal
transduction (67,68). In recent years, it was shown that
NKA is abnormally expressed and has abnormal activity in various
types of cancer, which signified that it may be anticipated to
become a novel target for tumor therapy. Of note, the dysregulation
of NKA subunits has been shown to vary among different types of
cancer. Previous studies have found that the expression of ATPase
Na+/K+ transporting subunit α1 (ATP1A1) in
HCC is significantly higher compared with that in adjacent
non-tumor tissues (69,70). ATP1A1 has been shown to have the
following roles: i) Knockdown of ATP1A1 in HepG2 and MHCC97H cells
significantly reduced their proliferation in vitro and
inhibited the tumorigenicity of MHCC97H cells in vivo; ii)
downregulation of ATP1A1 expression in of HepG2 cells led to
cell-cycle arrest in G2/M phase and apoptosis, and in
Hep3B, it increased cell migration; and iii) downregulation of
ATP1A1 expression led to the production of excessive reactive
oxygen species (ROS), which led to DNA damage and cell-cycle
arrest, and prevented the replication of damaged and defective DNA
(69). In addition, in
non-alcoholic steatohepatitis (NASH)-associated malignancies,
α1-NKA signaling was shown to activate the PI3K/Akt signaling
pathway, which simultaneously inhibited the FoxO3 circuit, leading
to the downregulation of the anti-apoptotic survival protein and
pro-apoptotic protein, second mitochondria-derived activator of
caspase/direct inhibitor of apoptosis-binding protein with low pI,
a process that is conducive to cell division and the development of
HCC (71). Therefore, ATP1A1 not
only serves as a potential biomarker for the diagnosis and
prognosis of HCC (72), but it may
also offer a therapeutic path for HCC. FXYD proteins are regulators
of Na+/K+-ATPase that are located in the cell
membrane, which regulate the kinetic properties of
Na+/K+-ATPase by changing the rate and
affinity of Na+ and K+ transport (73). FXYD6 was shown to be upregulated in
HCC, promoting the migration and proliferation of HCC cells
(74). The upregulation of FXYD6 is
also positively correlated with an increase of
Na+/K+-ATPase activity and it mainly exerts
its antitumor activity through activating the downstream SrC-ERK
signaling pathway. In addition, the blocking of FXYD6 by
self-produced anti-FXYD6 functional antibody was shown to
significantly inhibit the growth of mouse xenograft tumors,
indicating that FXYD6 can act as a novel therapeutic target for HCC
(74). Taken together, these
results demonstrated that FXYD6 fulfills a key role in the
progression of HCC, suggesting that FXYD6-targeting therapy may be
of benefit for the clinical treatment of patients with HCC.
Therefore, each subunit of NKA may be used as a novel potential
therapeutic target for HCC, offering further options for the
treatment of HCC.
Kv1.3
Kv1.3, an important regulatory protein of the immune
response, has been found in lymphocytes and is mainly involved in
immune regulation of the body's immune system (75). Kv1.3 protein on cell membranes is
involved in cell proliferation, whereas Kv1.3 channels located on
mitochondria are involved in cell apoptosis, and therefore, Kv1.3
located on mitochondria is considered to be a novel tumor biomarker
(32,76). It has been reported that the Kv1.3
channel is involved in the proliferation and apoptosis of a variety
of different types of tumor. It is noteworthy that the expression
of Kv1.3 has been shown to vary with the tumor stage and
downregulation of Kv1.3 may significantly inhibit cell
proliferation and increase cell apoptosis (77). However, to date, only a small number
of studies have been published on the role of this channel in HCC,
although a previous study reported that Kv1.3 blocker can regulate
the hyperreactivity of B lymphocytes and inhibit the abnormal
hypersecretion of antimitochondrial antibodies in primary biliary
cirrhosis to achieve the purpose of treating HCC (78). However, the precise role of Kv1.3 in
HCC requires further study.
Targeted therapeutic agents for HCC
At present, the treatment of HCC mainly includes
surgery, radiotherapy and chemotherapy, although HCC is not
sensitive to radiotherapy and conventional chemotherapy drugs, such
as doxorubicin, fluorouracil and cisplatin, have serious side
effects (7,79). Sorafenib is the first-line drug in
the treatment of HCC supported by the currently available data,
although the efficacy varies among different patients and the
sensitivity to the drug is typically reduced following long-term
treatment (80); therefore, it is
urgent to identify novel therapeutic strategies. With this as the
aim, molecular targeted therapy has been attracting increasing
attention. Research confirming that the abnormal expression of
certain K+ channels serves an important role in the
development and progression of HCC, has also found several drugs
able to target and inhibit the ion channels, thereby exerting
antitumor effects (Table II). For
instance, pharmacological inhibition of voltage-gated K+
channels has long been reported to reduce the adhesion and
proliferation of HCC cells, thereby exerting their antitumor
effects (81).
 | Table II.Drugs targeting K+ channels in
HCC. |
Table II.
Drugs targeting K+ channels in
HCC.
| Drugs | Mechanism | (Refs.) |
|---|
| IbTX | Blocks the BK
channel | (37) |
|
| Inhibits migration
and invasion |
|
| Astemizole | Blocks the Eag1
channel | (82) |
|
| Inhibits
proliferation and promotes apoptosis |
|
| Procyanidin B1 | Blocks the Eag1
channel | (79) |
|
| Inhibits
proliferation and promotes apoptosis |
|
| TRAM-34 | Blocks the KCa3.1
channel | (38,83) |
|
| Inhibits
proliferation and promotes apoptosis |
|
| Berberine and
Ouabain | Combined with
Na+/K+-ATPase enhances the anti-liver cancer effect of
sorafenib | (47,84) |
| Sodium
orthovanadate | Inhibition of
ATPase reverses sorafenib resistance of HCC cells | (85) |
Iberiotoxin (IbTX)
IbTX, a BK channel antagonist, selectively binds to
the pore-forming α-subunit of the BK channel (40). It has been reported that blockade of
BK channels inhibits both hypoxia-induced migration and
chemotherapy resistance to cisplatin in human glioblastoma cells
(82). In HCC, the authors of a
previously published study (40)
experimentally found that blocking BK channels with IbTX led to a
marked inhibition of the migration and invasion of HCC cells under
hypoxic conditions (40).
Subsequently, the same authors identified that the mechanism of its
action was to induce G2 phase arrest of HCC cells,
thereby inhibiting their migration and invasion, and regulating the
growth of the tumor (40). This
finding provided a novel strategy for the treatment of HCC.
Astemizole
Astemizole is an antihistamine that penetrates lipid
bilayers and binds to channels inside cell membranes (83). Owing to its inhibitory effects on
several cancer-associated proteins, including K+
channels, histamine receptors and P-glycoproteins, research has
focused on its potential therapeutic role in cancer. A previous
study identified that astemizole was able to inhibit the
proliferation and increase the apoptosis of HepG2 and Huh-7 cells
(84). Although astemizole has
diverse targets, exploration of the mRNA and protein expression
levels of Eag1 in these cells has revealed that the inhibition of
Eag1 channels mediated by astemizole in HCC may potentially be the
mechanism that accounts for its anti-proliferative and
pro-apoptotic effects (49,85). Treatment with astemizole was also
shown to reduce the mRNA and protein expression levels of Eag1 in
diethylnitrosamine-treated mice, resulting in both improved
histological features and appearance of the liver (85). It was thereby determined that
astemizole may have clinical utility in terms of the prevention and
treatment of HCC. Another study reported that serious adverse
reactions to the use of astemizole as a therapeutic agent only
occur in excessive use of the drug with HCC (84), and thus, it holds promise as a
selective agent both to prevent the risk of HCC and as a promising
therapeutic agent for patients with HCC.
Procyanidin B1
Procyanidin B1, a natural compound derived from
grape seed, not only induces the apoptosis of cells, but also
inhibits tumor growth (86,87). A previous study reported that
procyanidin B1 directly binds to the Eag1 channel, thereby
inhibiting its current, whereas its effect on other K+
channels was negligible, and this provided the putative underlying
mechanism to account for its inhibition of the migration and
proliferation of HCC cells (79).
Of note, procyanidin B1 was found to not exert any adverse effects
on normal metabolism in mice compared with conventional antitumor
drugs. In conclusion, procyanidin B1 was demonstrated to be a
significant potential antitumor drug for HCC.
1-[(2-Chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34)
A previous study reported that inhibition of KCa3.1
via genetic and pharmacological means led to a significant
reduction in the proliferation of tumor cells and the
susceptibility of tumors to certain therapeutic interventions was
also changed (88). TRAM-34, a
specific KCa3.1 blocker, has been reported to be effective in
inhibiting cell proliferation and motility in a variety of
different types of cancer, including glioblastoma and lung cancer
(89,90). Of note, long-term treatment with
TRAM-34 for atherosclerosis therapy at therapeutic concentrations
did not lead to any significant side effects (91). TRAM-34 has also been shown to
inhibit the proliferation and induce the apoptosis of HepG2 cells.
On the one hand, TRAM-34 can inhibit cell proliferation by
mediating decreases in both the mRNA expression of estrogen
receptor-α and nuclear factor-κB activation (92); on the other hand, the apoptosis of
HCC cells was found to be promoted by regulating the intracellular
level of ROS and through promoting p53 activation. In addition,
treatment with TRAM-34 also led to a marked inhibition of the
migration of HepG2, thereby reducing the development of HCC
(93). In addition to fulfilling a
role in HCC, inhibition of KCa3.1 channels by TRAM-34 was also
shown to inhibit hepatocyte fibrosis (94) and ICC tumor growth (43). Taken together, these results
indicated that TRAM-34 may potentially be a drug for the treatment
of HCC.
Berberine and ouabain
Berberine, a natural dibenzyl isoquinoline alkaloid
isolated from Berberis (common name: Barberry), has been studied as
a drug against a variety of different types of cancer, including
HCC. It was demonstrated that berberine could induce the
phosphorylation of Src in a
Na+/K+-ATPase-dependent manner, leading to
activation of p38-MAPK and the epidermal growth factor receptor
(EGFR)/ERK signaling pathway (95).
In addition, the Na+/K+-ATPase ligand ouabain
has also been shown to induce the phosphorylation of Src, EGFR,
insulin-like growth factor 1 receptor, ERK1/2 and p38-MAPK in HCC
cells, leading to the inhibition of cell growth and migration
through inhibiting EMT in HCC cells both in vivo and in
vitro (69). It was found that
treatment of HCC with sorafenib led to a significant induction of
cell death and inhibition of the growth of HCC xenografts in
vivo (95). Therefore,
targeting the Na+/K+-ATPase with berberine
and ouabain is a novel strategy to enhance the effects of
sorafenib. In addition to the drugs that have already been studied,
it would be useful to identify other drugs that target this channel
in future studies.
Sodium orthovanadate (SOV)
It has been reported that
Na+/K+-ATPase activity is increased in
drug-resistant tumors and its enhanced activity contributes towards
the biological behaviors observed in, and drug resistance of,
cancer, such as prostate, breast and lung cancer or leukemia
(96). Blocking
Na+/K+-ATPase through the use of specific
inhibitors has been shown to re-sensitize cancer to chemotherapy
drugs (97,98). It was found that the sorafenib
resistance of HCC cells was associated with higher levels of
Na+/K+-ATPase activity. SOV, a phosphate
analogue, is a recognized ATPase inhibitor and treatment of
sorafenib-resistant HCC with SOV has been shown to re-sensitize the
cancer to sorafenib, enhancing its antitumor effects (99). Although the exact mechanism
underlying its inhibition of ATPase requires further study, what
has been discovered to date is at least sufficient to demonstrate
that SOV may be an effective candidate drug to overcome sorafenib
resistance in the treatment of HCC.
Conclusions and prospects
A large number of studies have demonstrated that the
regulation of ion channels is associated with the development and
progression of HCC (77). In the
present review, the abnormal expression of K+ channels
in HCC was discussed. These K+ channels are known to be
involved in the development, proliferation and invasion of HCC, and
so they may serve as novel tumor markers and potential therapeutic
targets for HCC. Although an increasing body of evidence has
indicated the abnormal expression and function of K+
channels in HCC, research on ion-channel-targeted therapy in cancer
remains in its infancy and the mechanisms of action have yet to be
fully elucidated. Therefore, further systematic exploration of the
mechanisms involved is required to help improve the quality of life
of patients with HCC. Although certain K+ channels have
been shown to be abnormally expressed in HCC, whether they can be
selectively targeted in tumor cells remains to be determined.
Although the expression levels of K+ channels in tumor
and non-tumor tissues have been compared, the differences in
K+ channel expression at the different stages of HCC
remain poorly understood. The majority of channels studied so far
have been confined to the plasma membrane, but these ion channels
may also fulfill important roles in other organelles, such as the
mitochondria, and these may be useful as novel targets for tumor
therapy in the future. The majority of studies performed to date
have been laboratory-based and further studies are required. In the
future, it is expected that these targeted drugs will be tested in
clinical trials and their efficacy either alone or in combination
with other antitumor drugs in patients with HCC or at risk of
developing HCC may be further tested. To date, differences between
K+ channels in HCC and other tumors have not been
reported, and whether K+ channels may be used to
distinguish different cancers by comparing the expression levels or
expression sites requires further research.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science
Foundation of China (grant no. 82073087), and the Collaborative
Innovation Center of Chinese Ministry of Education (grant no.
2020-39).
Availability of data and materials
Not applicable.
Authors' contributions
XC made substantial contributions to the conception
and design of the study, as well as writing the manuscript and
performing the literature search. LZha, LHe, LZhe and BT were
involved in revising the manuscript critically for important
intellectual content. Data authentication is not applicable. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McGlynn KA, Petrick JL and El-Serag HB:
Epidemiology of hepatocellular carcinoma. Hepatology. 73 (Suppl
1):S4–S13. 2021. View Article : Google Scholar
|
|
2
|
Fujiwara N, Friedman S, Goossens N and
Hoshida Y: Risk factors and prevention of hepatocellular carcinoma
in the era of precision medicine. J Hepatol. 68:526–549. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Calderaro J, Ziol M, Paradis V and
Zucman-Rossi J: Molecular and histological correlations in liver
cancer. J Hepatol. 71:616–630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Craig A, von Felden J, Garcia-Lezana T,
Sarcognato S and Villanueva A: Tumour evolution in hepatocellular
carcinoma. Nat Rev Gastroenterol Hepatol. 17:139–152. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sia D, Villanueva A, Friedman S and Llovet
J: Liver cancer cell of origin, molecular class, and effects on
patient prognosis. Gastroenterology. 152:745–761. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang JD, Hainaut P, Gores GJ, Amadou A,
Plymoth A and Roberts LR: A global view of hepatocellular
carcinoma: Trends, risk, prevention and management. Nat Rev
Gastroenterol Hepatol. 16:589–604. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Llovet J, Montal R, Sia D and Finn R:
Molecular therapies and precision medicine for hepatocellular
carcinoma. Nat Rev Clin Oncol. 15:599–616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan LK and Ng IO: Joining the dots for
better liver cancer treatment. Nat Rev Gastroenterol Hepatol.
17:74–75. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kunzelmann K: Ion channels and cancer. J
Membr Biol. 205:159–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lang F, Föller M, Lang KS, Lang PA, Ritter
M, Gulbins E, Vereninov A and Huber SM: Ion channels in cell
proliferation and apoptotic cell death. J Membr Biol. 205:147–157.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prevarskaya N, Skryma R and Shuba Y: Ion
channels in cancer: Are cancer hallmarks oncochannelopathies?
Physiol Rev. 98:559–621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prevarskaya N, Skryma R and Shuba Y: Ion
channels and the hallmarks of cancer. Trends Mol Med. 16:107–121.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang X and Jan LY: Targeting potassium
channels in cancer. J Cell Biol. 206:151–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Conti M: Targeting K+ channels for cancer
therapy. J Exp Ther Oncol. 4:161–166. 2004.PubMed/NCBI
|
|
17
|
Teisseyre A, Gąsiorowska J and Michalak K:
Voltage-gated potassium channels Kv1.3-potentially new molecular
target in cancer diagnostics and therapy. Adv Clin Exp Med.
24:517–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kale VP, Amin SG and Pandey MK: Targeting
ion channels for cancer therapy by repurposing the approved drugs.
Biochim Biophys Acta. 1848:2747–2755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuang Q, Purhonen P and Hebert H:
Structure of potassium channels. Cell Mol Life Sci. 72:3677–3693.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bates E: Ion channels in development and
cancer. Annu Rev Cell Dev Biol. 31:231–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Comes N, Serrano-Albarrás A, Capera J,
Serrano-Novillo C, Condom E, Ramón Y Cajal S, Ferreres JC and
Felipe A: Involvement of potassium channels in the progression of
cancer to a more malignant phenotype. Biochim Biophys Acta.
1848:2477–2492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zúñiga L, Cayo A, Gonzalez W, Vilos C and
Zúñiga R: Potassium channels as a target for cancer therapy:
Current perspectives. Onco Targets Ther. 15:783–797. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taylor MD, Northcott PA, Korshunov A,
Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S,
Gajjar A, et al: Molecular subgroups of medulloblastoma: The
current consensus. Acta Neuropathol. 123:465–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Comes N, Bielanska J, Vallejo-Gracia A,
Serrano-Albarrás A, Marruecos L, Gómez D, Soler C, Condom E, Ramón
Y Cajal S, Hernández-Losa J, et al: The voltage-dependent K(+)
channels Kv1.3 and Kv1.5 in human cancer. Front Physiol. 4:2832013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pillozzi S, Masselli M, De Lorenzo E,
Accordi B, Cilia E, Crociani O, Amedei A, Veltroni M, D'Amico M,
Basso G, et al: Chemotherapy resistance in acute lymphoblastic
leukemia requires hERG1 channels and is overcome by hERG1 blockers.
Blood. 117:902–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hemmerlein B, Weseloh RM, Mello de Queiroz
F, Knötgen H, Sánchez A, Rubio ME, Martin S, Schliephacke T, Jenke
M, Heinz-Joachim-Radzun, et al: Overexpression of Eag1 potassium
channels in clinical tumours. Mol Cancer. 5:412006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pardo LA and Stühmer W: The roles of K(+)
channels in cancer. Nat Rev Cancer. 14:39–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bachmann M, Li W, Edwards MJ, Ahmad SA,
Patel S, Szabo I and Gulbins E: Voltage-gated potassium channels as
regulators of cell death. Front Cell Dev Biol. 8:6118532020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen Z, Yang Q and You Q: Researches
toward potassium channels on tumor progressions. Curr Top Med Chem.
9:322–329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z: Roles of K+ channels in regulating
tumour cell proliferation and apoptosis. Pflugers Arch.
448:274–286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ben-Moshe S and Itzkovitz S: Spatial
heterogeneity in the mammalian liver. Nat Rev Gastroenterol
Hepatol. 16:395–410. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Lv XW, Zhang L, Wang H, Li J and Wu
B: Review on biological characteristics of Kv1.3 and its role in
liver diseases. Front Pharmacol. 12:6525082021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sevelsted Møller L, Fialla AD, Schierwagen
R, Biagini M, Liedtke C, Laleman W, Klein S, Reul W, Koch Hansen L,
Rabjerg M, et al: The calcium-activated potassium channel KCa3.1 is
an important modulator of hepatic injury. Sci Rep. 6:287702016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kondo R, Deguchi A, Kawata N, Suzuki Y and
Yamamura H: Involvement of TREK1 channels in the proliferation of
human hepatic stellate LX-2 cells. J Pharmacol Sci. 148:286–294.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia Z, Huang X, Chen K, Wang H, Xiao J, He
K, Huang R, Duan X, Liu H, Zhang J and Xiang G: Proapoptotic role
of potassium ions in liver cells. Biomed Res Int. 2016:17291352016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Craig A and Villanueva A: Liver capsule:
Molecular-based signatures in hepatocellular carcinoma. Hepatology.
63:20182016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ghatta S, Nimmagadda D, Xu X and O'Rourke
ST: Large-conductance, calcium-activated potassium channels:
Structural and functional implications. Pharmacol Ther.
110:103–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marty A: Ca-dependent K channels with
large unitary conductance in chromaffin cell membranes. Nature.
291:497–500. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Knaus HG, Schwarzer C, Koch RO, Eberhart
A, Kaczorowski GJ, Glossmann H, Wunder F, Pongs O, Garcia ML and
Sperk G: Distribution of high-conductance Ca(2+)-activated K+
channels in rat brain: Targeting to axons and nerve terminals. J
Neurosci. 16:955–963. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He Y, Lin Y and He F, Shao L, Ma W and He
F: Role for calcium-activated potassium channels (BK) in migration
control of human hepatocellular carcinoma cells. J Cell Mol Med.
25:9685–9696. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wulff H and Castle N: Therapeutic
potential of KCa3.1 blockers: Recent advances and promising trends.
Expert Rev Clin Pharmacol. 3:385–396. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Todesca LM, Maskri S, Brömmel K, Thale I,
Wünsch B, Koch O and Schwab A: Targeting Kca3.1 channels
in cancer. Cell Physiol Biochem. 55:131–144. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song P, Du Y, Song W, Chen H, Xuan Z, Zhao
L, Chen J, Chen J, Guo D, Jin C, et al: KCa3.1 as an effective
target for inhibition of growth and progression of intrahepatic
cholangiocarcinoma. J Cancer. 8:1568–1578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fan J, Tian R, Yang X, Wang H, Shi Y, Fan
X, Zhang J, Chen Y, Zhang K, Chen Z and Li L: KCNN4 promotes the
stemness potentials of liver cancer stem cells by enhancing glucose
metabolism. Int J Mol Sci. 23:69582022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Du Y, Song W, Chen J, Chen H, Xuan Z, Zhao
L, Chen J, Jin C, Zhou M, Tuo B, et al: The potassium channel
KCa3.1 promotes cell proliferation by activating SKP2 and
metastasis through the EMT pathway in hepatocellular carcinoma. Int
J Cancer. 145:503–516. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li QT, Feng YM, Ke ZH, Qiu MJ, He XX, Wang
MM, Li YN, Xu J, Shi LL and Xiong ZF: KCNN4 promotes invasion and
metastasis through the MAPK/ERK pathway in hepatocellular
carcinoma. J Investig Med. 68:68–74. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ranjan A, Iyer SV, Ward C, Link T, Diaz
FJ, Dhar A, Tawfik OW, Weinman SA, Azuma Y, Izumi T and Iwakuma T:
MTBP inhibits the Erk1/2-Elk-1 signaling in hepatocellular
carcinoma. Oncotarget. 9:21429–21443. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rodríguez-Rasgado J, Acuña-Macías I and
Camacho J: Eag1 channels as potential cancer biomarkers. Sensors
(Basel). 12:5986–5995. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chávez-López MG, Zúñiga-García V,
Pérez-Carreón JI, Avalos-Fuentes A, Escobar Y and Camacho J: Eag1
channels as potential early-stage biomarkers of hepatocellular
carcinoma. Biologics. 10:139–148. 2016.PubMed/NCBI
|
|
50
|
Chen J, Xuan Z, Song W, Han W, Chen H, Du
Y, Xie H, Zhao Y, Zheng S and Song P: EAG1 enhances hepatocellular
carcinoma proliferation by modulating SKP2 and metastasis through
pseudopod formation. Oncogene. 40:163–176. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lotshaw D: Biophysical, pharmacological,
and functional characteristics of cloned and native mammalian
two-pore domain K+ channels. Cell Biochem Biophys. 47:209–256.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kheradpezhouh E, Ma L, Morphett A, Barritt
GJ and Rychkov GY: TRPM2 channels mediate acetaminophen-induced
liver damage. Proc Natl Acad Sci USA. 111:3176–3181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li WC, Xiong ZY, Huang PZ, Liao YJ, Li QX,
Yao ZC, Liao YD, Xu SL, Zhou H, Wang QL, et al: KCNK levels are
prognostic and diagnostic markers for hepatocellular carcinoma.
Aging (Albany NY). 11:8169–8182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Innamaa A, Jackson L, Asher V, van
Schalkwyk G, Warren A, Keightley A, Hay D, Bali A, Sowter H and
Khan R: Expression and effects of modulation of the K2P potassium
channels TREK-1 (KCNK2) and TREK-2 (KCNK10) in the normal human
ovary and epithelial ovarian cancer. Clin Transl Oncol. 15:910–918.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
He Y, Hu H, Wang Y, Yuan H, Lu Z, Wu P,
Liu D, Tian L, Yin J, Jiang K and Miao Y: ALKBH5 inhibits
pancreatic cancer motility by decreasing long non-coding RNA
KCNK15-AS1 methylation. Cell Physiol Biochem. 48:838–846. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Alvarez-Baron C, Jonsson P, Thomas C,
Dryer S and Williams C: The two-pore domain potassium channel
KCNK5: Induction by estrogen receptor alpha and role in
proliferation of breast cancer cells. Mol Endocrinol. 25:1326–1336.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim CJ, Cho YG, Jeong SW, Kim YS, Kim SY,
Nam SW, Lee SH, Yoo NJ, Lee JY and Park WS: Altered expression of
KCNK9 in colorectal cancers. APMIS. 112:588–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Peroz D, Rodriguez N, Choveau F, Baró I,
Mérot J and Loussouarn G: Kv7.1 (KCNQ1) properties and
channelopathies. J Physiol. 586:1785–1789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
White BD, Chien AJ and Dawson DW:
Dysregulation of Wnt/β-catenin signaling in gastrointestinal
cancers. Gastroenterology. 142:219–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fan H, Zhang M and Liu W: Hypermethylated
KCNQ1 acts as a tumor suppressor in hepatocellular carcinoma.
Biochem Biophys Res Commun. 503:3100–3107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li C, Miao R, Zhang J, Qu K and Liu C:
Long non-coding RNA KCNQ1OT1 mediates the growth of hepatocellular
carcinoma by functioning as a competing endogenous RNA of miR-504.
Int J Oncol. 52:1603–1612. 2018.PubMed/NCBI
|
|
62
|
Wan J, Huang M, Zhao H, Wang C, Zhao X,
Jiang X, Bian S, He Y and Gao Y: A novel tetranucleotide repeat
polymorphism within KCNQ1OT1 confers risk for hepatocellular
carcinoma. DNA Cell Biol. 32:628–634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhong W, Dai Q and Huang Q: Effect of
lncRNA KCNQ1OT1 on autophagy and drug resistance of hepatocellular
carcinoma cells by targeting miR-338-3p. Cell Mol Biol
(Noisy-le-grand). 66:191–196. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jiang M, Cui BW, Wu YL, Zhang Y, Shang Y,
Liu J, Yang HX, Qiao CY, Zhan ZY, Ye H, et al: P2X7R orchestrates
the progression of murine hepatic fibrosis by making a feedback
loop from macrophage to hepatic stellate cells. Toxicol Lett.
333:22–32. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang G, Zhou L, Xu Q, Meng F, Wan Y, Meng
X, Wang L and Zhang L: LncRNA KCNQ1OT1 inhibits the
radiosensitivity and promotes the tumorigenesis of hepatocellular
carcinoma via the miR-146a-5p/ACER3 axis. Cell Cycle. 19:2519–2529.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang J, Zhao X, Ma X, Yuan Z and Hu M:
KCNQ1OT1 contributes to sorafenib resistance and programmed
death-ligand-1-mediated immune escape via sponging miR-506 in
hepatocellular carcinoma cells. Int J Mol Med. 46:1794–1804.
2020.PubMed/NCBI
|
|
67
|
Xie Z and Askari A: Na(+)/K(+)-ATPase as a
signal transducer. Eur J Biochem. 269:2434–2439. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rajasekaran SA, Palmer LG, Quan K, Harper
JF, Ball WJ Jr, Bander NH, Peralta Soler A and Rajasekaran AK:
Na,K-ATPase beta-subunit is required for epithelial polarization,
suppression of invasion, and cell motility. Mol Biol Cell.
12:279–295. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhuang L, Xu L, Wang P, Jiang Y, Yong P,
Zhang C, Zhang H, Meng Z and Yang P: Na+/K+-ATPase α1 subunit, a
novel therapeutic target for hepatocellular carcinoma. Oncotarget.
6:28183–28193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Xu ZW, Wang FM, Gao MJ, Chen XY, Hu WL and
Xu RC: Targeting the Na(+)/K(+)-ATPase alpha1 subunit of hepatoma
HepG2 cell line to induce apoptosis and cell cycle arresting. Biol
Pharm Bull. 33:743–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Udoh US, Banerjee M, Rajan PK, Sanabria
JD, Smith G, Schade M, Sanabria JA, Nakafuku Y, Sodhi K, Pierre SV,
et al: Tumor-suppressor role of the α1-Na/K-ATPase signalosome in
NASH related hepatocellular carcinoma. Int J Mol Sci. 23:73592022.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tang S, Yang X, Zhou C, Mei Y, Ye J, Zhang
X, Feng G, Zhang W, Zhang X and Fan W: Sodium pump Na + /K + ATPase
subunit α1-targeted positron emission tomography imaging of
hepatocellular carcinoma in mouse models. Mol Imaging Biol.
24:384–393. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Garty H and Karlish SJD: Role of FXYD
proteins in ion transport. Annu Rev Physiol. 68:431–459. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gao Q, Chen X, Duan H, Wang Z, Feng J,
Yang D, Song L, Zhou N and Yan X: FXYD6: A novel therapeutic target
toward hepatocellular carcinoma. Protein Cell. 5:532–543. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Feske S, Wulff H and Skolnik EY: Ion
channels in innate and adaptive immunity. Annu Rev Immunol.
33:291–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Teisseyre A, Palko-Labuz A, Sroda-Pomianek
K and Michalak K: Voltage-gated potassium channel Kv1.3 as a target
in therapy of cancer. Front Oncol. 9:9332019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zúñiga-García V, Chávez-López Mde G,
Quintanar-Jurado V, Gabiño-López NB, Hernández-Gallegos E,
Soriano-Rosas J, Pérez-Carreón JI and Camacho J: Differential
expression of ion channels and transporters during hepatocellular
carcinoma development. Dig Dis Sci. 60:2373–2383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Prosdocimi E, Checchetto V and Leanza L:
Targeting the mitochondrial potassium channel Kv1.3 to kill cancer
cells: Drugs, strategies, and new perspectives. SLAS Discov.
24:882–892. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Na W, Ma B, Shi S, Chen Y, Zhang H, Zhan Y
and An H: Procyanidin B1, a novel and specific inhibitor of Kv10.1
channel, suppresses the evolution of hepatoma. Biochem Pharmacol.
178:1140892020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhao W, Bai B, Hong Z, Zhang X and Zhou B:
Berbamine (BBM), a natural STAT3 inhibitor, synergistically
enhances the antigrowth and proapoptotic effects of sorafenib on
hepatocellular carcinoma cells. ACS Omega. 5:24838–24847. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhou Q, Kwan HY, Chan HC, Jiang JL, Tam SC
and Yao X: Blockage of voltage-gated K+ channels inhibits adhesion
and proliferation of hepatocarcinoma cells. Int J Mol Med.
11:261–266. 2003.PubMed/NCBI
|
|
82
|
Rosa P, Catacuzzeno L, Sforna L, Mangino
G, Carlomagno S, Mincione G, Petrozza V, Ragona G, Franciolini F
and Calogero A: BK channels blockage inhibits hypoxia-induced
migration and chemoresistance to cisplatin in human glioblastoma
cells. J Cell Physiol. 233:6866–6877. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang X, Chen Y, Zhang Y, Guo S, Mo L, An H
and Zhan Y: Eag1 voltage-dependent potassium channels: Structure,
electrophysiological characteristics, and function in cancer. J
Membr Biol. 250:123–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
García-Quiroz J and Camacho J: Astemizole:
An old anti-histamine as a new promising anti-cancer drug.
Anticancer Agents Med Chem. 11:307–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
de Guadalupe Chávez-López M, Pérez-Carreón
JI, Zuñiga-García V, Díaz-Chávez J, Herrera LA, Caro-Sánchez CH,
Acuña-Macías I, Gariglio P, Hernández-Gallegos E, Chiliquinga AJ
and Camacho J: Astemizole-based anticancer therapy for
hepatocellular carcinoma (HCC), and Eag1 channels as potential
early-stage markers of HCC. Tumour Biol. 36:6149–6158. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Roy AM, Baliga MS, Elmets CA and Katiyar
SK: Grape seed proanthocyanidins induce apoptosis through p53, Bax,
and caspase 3 pathways. Neoplasia. 7:24–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Mantena SK and Katiyar SK: Grape seed
proanthocyanidins inhibit UV-radiation-induced oxidative stress and
activation of MAPK and NF-kappaB signaling in human epidermal
keratinocytes. Free Radic Biol Med. 40:1603–1614. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Mohr CJ, Steudel FA, Gross D, Ruth P, Lo
WY, Hoppe R, Schroth W, Brauch H, Huber SM and Lukowski R:
Cancer-associated intermediate conductance
Ca2+-Activated K+ Channel KCa3.1. Cancers
(Basel). 11:1092019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Catacuzzeno L, Fioretti B and Franciolini
F: Expression and role of the intermediate-conductance
calcium-activated potassium channel KCa3.1 in glioblastoma. J
Signal Transduct. 2012:4215642012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Bulk E, Ay AS, Hammadi M, Ouadid-Ahidouch
H, Schelhaas S, Hascher A, Rohde C, Thoennissen NH, Wiewrodt R,
Schmidt E, et al: Epigenetic dysregulation of KCa 3.1 channels
induces poor prognosis in lung cancer. Int J Cancer. 137:1306–1317.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Toyama K, Wulff H, Chandy KG, Azam P,
Raman G, Saito T, Fujiwara Y, Mattson DL, Das S, Melvin JE, et al:
The intermediate-conductance calcium-activated potassium channel
KCa3.1 contributes to atherogenesis in mice and humans. J Clin
Invest. 118:3025–3037. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Freise C, Ruehl M, Seehofer D, Hoyer J and
Somasundaram R: The inhibitor of Ca(2+)-dependent K+ channels
TRAM-34 blocks growth of hepatocellular carcinoma cells via
downregulation of estrogen receptor alpha mRNA and nuclear
factor-kappaB. Invest New Drugs. 31:452–457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu Y, Zhao L, Ma W, Cao X, Chen H, Feng
D, Liang J, Yin K and Jiang X: The blockage of KCa3.1 channel
inhibited proliferation, migration and promoted apoptosis of human
hepatocellular carcinoma cells. J Cancer. 6:643–651. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Freise C, Heldwein S, Erben U, Hoyer J,
Köhler R, Jöhrens K, Patsenker E, Ruehl M, Seehofer D, Stickel F
and Somasundaram R: K+-channel inhibition reduces portal perfusion
pressure in fibrotic rats and fibrosis associated characteristics
of hepatic stellate cells. Liver Int. 35:1244–1252. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yang S, Yang S, Zhang H, Hua H, Kong Q,
Wang J and Jiang Y: Targeting Na+/K+-ATPase
by berbamine and ouabain synergizes with sorafenib to inhibit
hepatocellular carcinoma. Br J Pharmacol. 178:4389–4407. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Alevizopoulos K, Calogeropoulou T, Lang F
and Stournaras C: Na+/K+ ATPase inhibitors in cancer. Curr Drug
Targets. 15:988–1000. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Simpson CD, Mawji IA, Anyiwe K, Williams
MA, Wang X, Venugopal AL, Gronda M, Hurren R, Cheng S, Serra S, et
al: Inhibition of the sodium potassium adenosine triphosphatase
pump sensitizes cancer cells to anoikis and prevents distant tumor
formation. Cancer Res. 69:2739–2747. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Durlacher CT, Chow K, Chen XW, He ZX,
Zhang X, Yang T and Zhou SF: Targeting Na+/K+-translocating
adenosine triphosphatase in cancer treatment. Clin Exp Pharmacol
Physiol. 42:427–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Jiang W, Li G, Li W, Wang P, Xiu P, Jiang
X, Liu B, Sun X and Jiang H: Sodium orthovanadate overcomes
sorafenib resistance of hepatocellular carcinoma cells by
inhibiting Na+/K+-ATPase activity and
hypoxia-inducible pathways. Sci Rep. 8:97062018. View Article : Google Scholar : PubMed/NCBI
|