Recent studies have clearly revealed that the
monocyte/macrophage system exists in almost all tissues and organs
of the organism and consists of important cell types that mediate
immune response (1–3). Especially, the polarization of
macrophages towards M1 or M2 type has more important pathological
significance and is closely related to the occurrence and
development of diseases, such as tumors or inflammation. Since
abnormal polarization of macrophages has important pathological
value, it is crucial to reveal the molecular mechanism of this
polarization phenomenon, as it is the molecular basis for targeted
intervention or reversal of abnormal polarization of macrophages to
play a therapeutic role in diseases. As for the molecular mechanism
of abnormal polarization of macrophages, most of previous studies
have mainly focused on the research of transcriptional regulatory
factors (4–6). In the recent years, with the progress
of basic research on epigenetics, it has been observed that
microRNAs (miRNAs or miRs) also play an important role in
macrophage polarization, especially in the local pathological
processes of diseases such as tumors and inflammation. miRNA is an
endogenous non-coding RNA (ncRNA), which regulates ~30 to 90% of
genes in life and affects the synthesis of corresponding proteins
by targeting to inhibit or promote the expression of mRNA (7). In addition to transcription factors
(TFs), previous studies have shown that multiple miRNAs were also
involved in regulating macrophage polarization, thereby
participating in the development of inflammation and tumors
(8–11). The present review focuses on the
important role of macrophage polarization in tissue homeostasis and
pathological regulation, as well as the latest research progress in
the relationship between miRNAs and macrophage polarization towards
M1 and M2, providing a theoretical basis for the treatment of
inflammation and tumors.
miRNA is a class of single-strand non-coding small
molecule RNA, with a length of ~18-24 nucleotides. The traditional
miRNA biogenesis supports that the primary miRNA is generated by
type II RNA polymerase in the nucleus, which is split by the
ribonuclease Drosha/DGCR8 complex to generate precursor miRNA, and
then to produce mature miRNAs after being processed by the Dicer
enzyme in the cytoplasm (12).
Usually, one of the main mechanisms by which miRNA acts is by
targeting the 3′ untranslated region of downstream genes to exert a
sponge effect, which in turn inhibits the expression level of
downstream target genes, regulates gene expression at the
post-transcription level, and thus participates in important
cellular processes (7). It is
interesting that changes in the expression levels of certain
important miRNAs can often be achieved through changes in the
expression of target genes to regulate immune response and affect
immune homeostasis. A single miRNA can target hundreds of mRNAs and
affect the expression of multiple genes (13). In addition, miRNA can also be
packaged with proteins or other RNAs (mRNA, circular RNA and long
ncRNA) to form exosomes or microbubbles and get secreted out of
cells, and subsequently taken up by receptor cells through direct
membrane fusion or endocytosis, thus regulating their normal
cellular activity. For example, exosome miR-223 which is derived
from stem cells, can inhibit the expression of some
pro-inflammatory cytokines by targeting Semaphorin 3A and STAT3 in
macrophages of sepsis models (14).
It is well known that miRNA is widely present in
various tissues and involved in an important role in various
biological processes. In addition, numerous miRNAs, for example,
miR-223 and miR-142-3p were also associated to the proliferation,
differentiation and function of numerous kinds of immune cells
(15). Cancer could be classified
as a signaling pathway disease, which was induced by abnormal
signaling pathways (16,17); however, miRNA can regulate the
downstream of numerous target genes, thus affecting the status of
almost all signaling pathways in cancer. On the other hand, miRNAs
can also participate in the occurrence and development of certain
inflammatory diseases, such as atherosclerosis (AS). For example, a
recent study revealed that miR-205-5p can regulate ERBB4/AKT
signaling pathway and has an inhibitory effect on the occurrence of
AS (18).
It is precisely due to the widespread involvement of
miRNA in diseases that it has become one of the targets for tumor
treatment and inflammation control, especially in the treatment of
tumors that it has demonstrated great potential. Usually, miRNAs
exhibit abnormal expression or mutations in most malignant tumors
and can function as oncogenes or tumor suppressor genes. Therefore,
miRNA-targeted therapy has exhibited potential value for cancer
treatment. At present, miRNA-mediated clinical trials have shown a
favorable effect in cancer treatment. miRNAs or their analogues can
be used to treat cancer by regulating and restoring the expression
of cancer suppressor gene-related miRNAs, or inhibiting
proto-oncogene-related miRNAs in cancer cells (19). In addition, the use of nanosomes to
deliver miRNA and small molecule drugs has become increasingly
widespread. For example, in the human pancreatic cancer cell line,
two miR-205 mimics were used to reduce the metastasis and invasion
of cancer cells (20). Therefore, a
more important task is to delve into the key miRNA types which are
closely related to diseases such as tumors, or upstream and
downstream key genes associated with miRNAs, and screen for more
significant targets or signaling pathways through in vitro
and in vivo experiments. Among them, the macrophage
polarization by miRNAs has also been widely concerned by scholars
at home and abroad, and some important progress has been made. As
for the research progress of miRNA in macrophage polarization, and
as the marker for the treatment of tumors and inflammation, the
present review provided a detailed introduction in the relevant
paragraphs below.
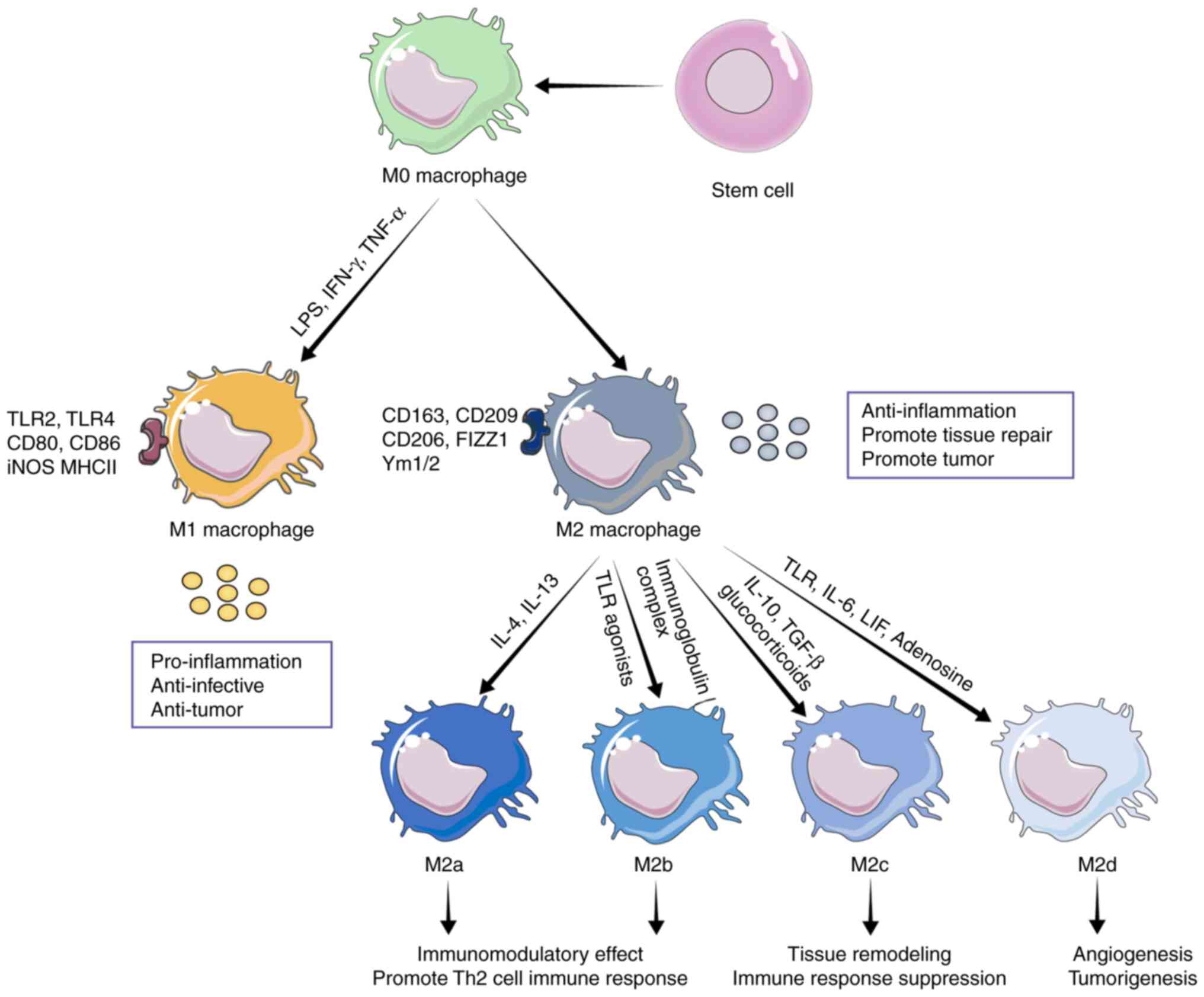
It has been demonstrated that macrophages not only
regulate phagocytosis, exogenous antigen presentation and secretion
of cytokines, but also play roles in system metabolism,
hematopoiesis, angiogenesis, malignant tumors and reproduction
(21). Macrophages have functional
diversity and high heterogeneity. The change of the local
microenvironment or under the action of different stimulators can
obtain different phenotypes and then exert different functions, and
this process is called polarization (Fig. 1) (22). Macrophage polarization has a
significant impact on tissue repair and maintenance of tissue
homeostasis, which were generally divided into two phenotypes
(Fig. 1). For example, one has
classically activated macrophages, namely, M1-type macrophages,
which were usually induced by toll-like receptors (TLR) ligands.
This subset of macrophages expresses TLR2 and TLR4, CD80, CD86,
inducible nitric oxide synthase (iNOS) and major histocompatibility
complex II (MHCII), and can produce a large number of cytokines to
induce further polarization of macrophages in the feedback cycle,
with high antigen presentation and expression of pro-inflammatory
cytokines, such as IL-12, IL-23 and TNF-α, and has antitumor
effects (23–25). Another alternative is to replace
activated macrophages, namely M2 macrophages, which express
specific antigens, such as CD206, CD163, CD209, FIZZ1 and Ym1/2. M2
macrophages can be divided into subgroups M2a, M2b, M2c and M2d.
Certainly, these different subtypes of M2 cells can exert immune
regulatory effects through different mechanisms of action (26–28).
Although recent research clearly indicates the types
of macrophage subtypes, the polarization process of macrophages is
very complex. It has been confirmed that the local microenvironment
state of the tissue can affect the polarization state of
macrophages, and this polarization between M1 and M2 is often
reversible, and rapid type change occurs under the induction of
some factors, or when responding to changes in the microenvironment
(29). Thus, M1 macrophages and M2
macrophages can transform each other under different conditions. If
the M2 macrophage could be induced to switch to M1 macrophage, the
M1 macrophage would play an antitumor immune role to inhibit tumor
growth, which indicates that the reversible way of polarization
from M2 to M1 has potential therapeutic value in clinical treatment
of tumors.
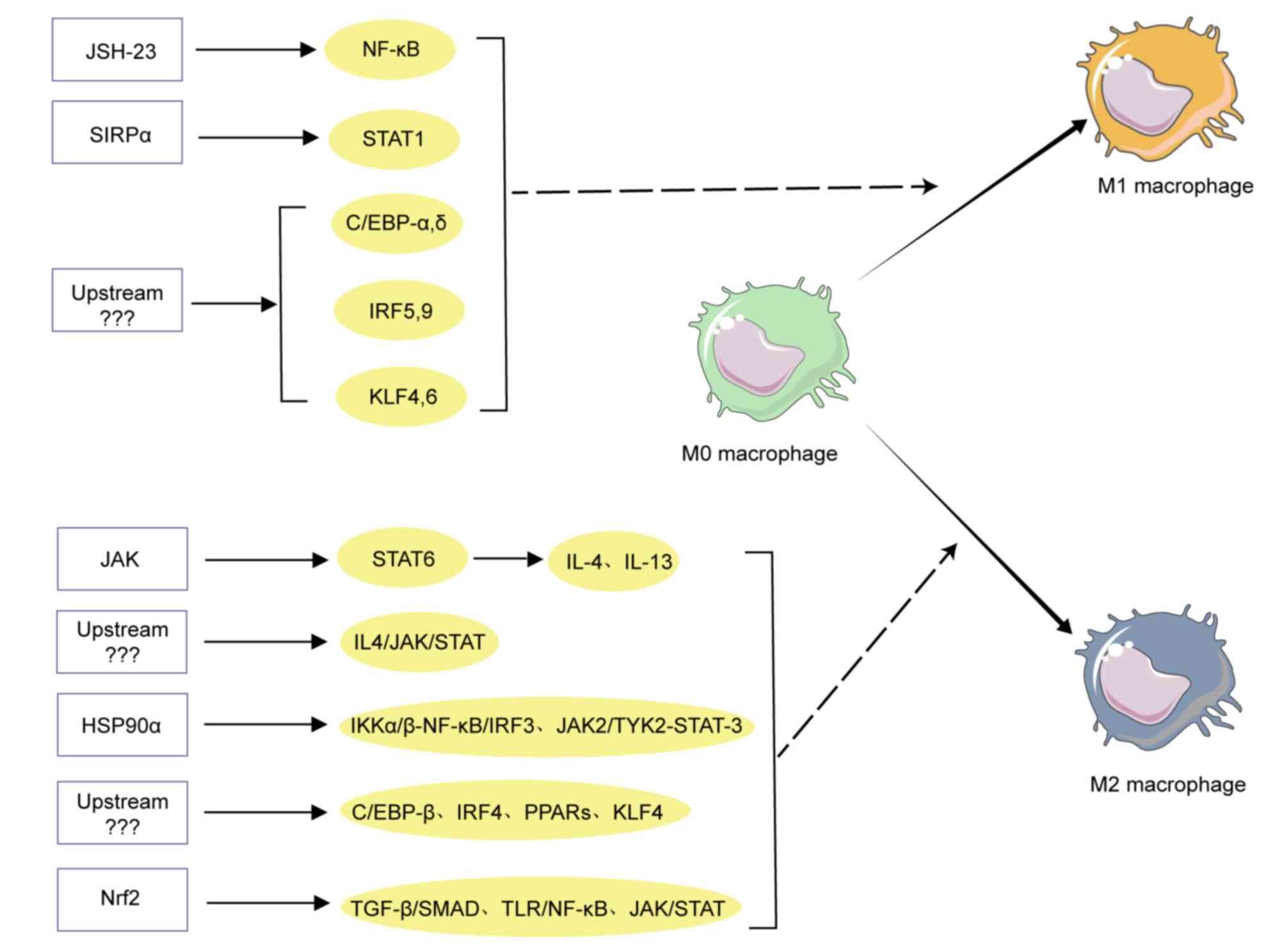
As aforementioned, the polarization of macrophages
is a process of multifactorial interaction, regulated by multiple
activating molecules and signaling pathways (Fig. 2). These activating molecules bind to
relevant receptors on the surface of macrophages (30), activating downstream signaling
pathways and further inducing phenotype-specific gene expression
(31), and participating in
macrophage polarization at the transcriptional level. Multiple
specific signaling molecules and TFs have been demonstrated to
activate macrophages, such as NF-κB, STATs, interferon regulatory
factors (IRFs), CCAAT enhancer binding protein (C/EBP), peroxisome
proliferator activated receptor (PPAR) and Kruppel-like factors
(KLFs) (32). Among them, some are
related to the M1 polarization and the others are related to M2
polarization. For example, the NF-κB signaling pathway was involved
in host immune responses as a pro-inflammatory signaling pathway
(33), which is involved in the
regulation of macrophage polarization (34). Wu et al (35) observed that macrophages in patients
with Behcet's disease (BD), expressed higher CD86 antigen, higher
serum IL-12, TNF-α and lower CD163 antigen, which could enhance
cell phagocytic ability and promote differentiation of Th1 cells,
while the application of NF-κB inhibitors could weaken the M1 like
phenotype stimulated by BD serum, indicating NF-κB has a regulatory
effect on M1 polarization stimulated by BD serum. A recent study
also reported that NF-κB phosphorylation participates in M1
polarization of macrophages in foreign body reaction (FBR). In the
lipopolysaccharides (LPS)-induced inflammatory microenvironment
in vitro, JSH-23, an inhibitor of NF-κB, could precisely
inhibit PLA induced NF-κB phosphorylation and M1 macrophage
polarization, which results in the inhibition of FBR through their
anti-inflammatory and anti-adhesion effects (36). These all indicated that NF-κB was
involved in macrophage polarization.
Janus family of kinases (JAKs) are composed of four
members, such as JAK1, JAK2, JAK3 and Tyk2 (37). The JAK kinase family can
phosphorylate STATs, called the JAK-STAT signaling pathway, to
regulate its downstream genes. In the STAT family, STAT1 and STAT3
are two important family types, but they sometimes exhibit
different functional characteristics. For example, in the presence
of IFN-γ, STAT1 is an important mediator that activates the
polarization of pro-inflammatory macrophages, while STAT3 can
activate the polarization of anti-inflammatory macrophages
(38). A recent related study have
demonstrated that by disrupting the synthesis of hyaluronic acid in
glioblastoma or blocking its binding to the receptor CD44 on
macrophages, signal-regulatory protein alpha (SIRPα), can be
induced to increase or enhance the phosphorylation of STAT1 in
macrophages and inhibits STAT3 phosphorylation, which can induce M1
type macrophage generation and inhibit the growth of glioblastoma
(39). In addition, the TF KLF4 in
rheumatoid arthritis can promote M1 polarization by regulating
STAT1 (40). The JAK-STAT6 pathway
is related to the M2 polarization, which is mainly due to the
modulation of IL-4 and IL-13, thereby activating PPARγ. On the
other hand, the expression of key nuclear TFs such as KLF4 can
induce the specific markers of M2 macrophage (41). Fan et al (42) demonstrated that extracellular heat
shock protein 90α (HSP90α) can induce activation of JAK2/TYK2/STAT3
signaling pathways to promote M2 polarization (42). Liu et al (43) indicated that activating the
IL4/JAK/STAT signaling pathway through mechanical stimulation can
also regulate macrophage polarization towards the M2 subtype,
thereby promoting tendon-bone healing in mouse rotator cuff repair.
A different study also pointed out that activating nuclear factor
erythroid 2-related factor 2 (Nrf2) can inhibit M1 polarization and
promote M2 polarization through three signaling pathways, such as
JAK/STAT, TGFβ/SMAD and TLR/NF-κB and potential signal transduction
pathways, as well as signaling pathways, such as NLRP3, Notch,
PI3K/Akt and MAPK (44). In
addition, the SENP1-Sirt3 signaling pathway can promote M2
macrophage polarization by reducing glutamate dehydrogenase1
(GLUD1) acetylation and promoting GLUD1-mediated aKG production
(45). The aforementioned studies
indicated that M1 or M2 polarization is regulated by specific
signaling molecules and TFs. In summary, based on the
aforementioned literature analysis and the results of literature
analysis not specifically presented, it is preliminarily indicated
that TF C/EBPα, C/EBPδ, STAT1, IRF9, NF-κB or KLF6 were usually
involved in M1 macrophage polarization, while STAT3, STAT6, C/EBPβ,
PPARs, c-myc, KLF4, IRF4 and GATA3 are associated with M2
macrophage polarization (Fig. 2).
As for the specific roles of these listed transcription regulatory
factors, although most literature results have reached consistent
conclusions, their functional characteristics under different
environmental conditions still need further exploration.
To further demonstrate the complexity of the role of
transcriptional regulatory proteins, or their role in macrophage
polarization, only some illustrative examples were provided. For
example, it is interesting that even different members of the same
transcriptional regulatory proteins play different roles in the
polarization process of macrophages, for instance, in the IRF
family, IRF4 promotes M2 polarization by upregulating expression of
IL-10 in colon mucosal cancer (46). However, IRF5 plays a
pro-inflammatory role by activating Akt2 to participate in M1
macrophage polarization (47). As
for lipoxin A4 (LXA4), not only does it participate in the M1
polarization process in LPS-induced M1 macrophage polarization, but
it is also related to the M2 polarization process in IL-4-induced
M2 macrophage polarization, through the FPR2/IRF5 signaling pathway
and FPR2/IRF4 signaling pathway respectively (48). C/EBPα of the C/EBP family can
promote M1-type macrophage polarization, while C/EBPβ promotes M2
polarization (49). Akt1 inhibits
the sensitivity of macrophages to inflammatory stimuli and promotes
M2 macrophage polarization while Akt2 can promote M1 macrophage
polarization, and knocking down Akt2 can enhance the expression of
C/EBPβ and promotes polarization of M2 macrophages (50). Therefore, macrophage polarization is
considered an important regulator of homeostasis and pathology in
the organism's tissues, and a key determinant of disease
occurrence, development and regression. Macrophages involve
multiple TFs and signaling pathways in maintaining M1/M2 phenotype
balance. Therefore, understanding the signaling pathway mechanisms
that regulate macrophage polarization is an extremely important
step in the treatment of diseases.
The reason why the transcriptional regulatory
factors related to macrophage polarization were first introduced or
their related signaling pathways were summarized, is because miRNA
often interacts with these important transcriptional regulatory
proteins, thereby exerting the polarization regulation process of
macrophages (51–53). Macrophage polarization, as a key
regulator of environmental homeostasis in the human organism,
relies on the expression of key TFs, whose expression is modulated
by miRNA (54–56). Recently, with the gradual discovery
of some miRNAs that regulate macrophage polarization, significant
progress has been made in the study of the role of miRNA in
macrophage polarization (7,8,57), and
some research results have achieved positive consensus and
demonstrated favorable application potential (58,59).
Therefore, next, the progress of miRNAs in macrophage polarization
and the impact of miRNA on the treatment of tumor and non-tumor
diseases were mainly summarized.
miRNAs are involved in M1 macrophage polarization.
Among them, the miRNAs in macrophage polarization, have received
widespread attention from domestic and foreign scholars, and some
important progress has been made. First, as a star miRNA, miRNA-155
has been widely studied for its role in macrophage polarization,
and many studies so far revealed that miRNA-155 was involved in
promoting M1 polarization (60–65),
for example, the expression of miRNA-155 was significantly
upregulated when macrophages polarized to the M1 phenotype,
however, its expression was obviously downregulated when
macrophages polarized to M2 phenotype. In addition, silencing
miR-155 significantly promoted the polarization of M2 macrophages,
and overexpression of miRNA-155 could induce a switch from M2 to M1
phenotype (60). It has been
reported that miR-155 participates in inflammation and tumors by
regulating multiple signaling molecules, which can modulate
macrophage polarization in the immune microenvironment, and affect
the host's anti-infection or tumor ability. However, the potential
mechanisms of macrophage polarization during inflammation and tumor
development remain unclear and complicated. In some cases, there
may even be different mechanisms and outcomes. For example, one
study investigated the mechanism by which miR-155 affects
tumor-associated macrophage (TAM) polarization at a molecular level
in hepatocellular carcinoma (HCC) initiated by hepatitis B virus
infection. As compared with HBV− HCC tissues, miR-155
was significantly highly expressed in HBV+ HCC tissues.
In addition, miR-155 overexpression significantly promoted M2-type
macrophage polarization by the miR-155/SHIP1 axis, which
accelerated HCC cell invasion, proliferation and migration. This
finding provides new insights into the development of novel
therapeutic strategies for combatting HBV+ HCC and a new
reference for exploring antitumor immunotherapy (61). Recently, it has been reported that
miR-155-5p can regulate M1-type macrophage polarization by
targeting downstream SOCS1/JAK1/STAT1 axis and participate in liver
fibrosis and hepatic lymphangiogenesis in cirrhosis (62). Besides, miR-155-5p can regulate M1
polarization by other pathways, such as SOCS1/NF-κB pathway
(63), and let-7a-5p can also
target suppressor of cytokine signaling 1 (SOCS1) to modulate
NF-κB, and activate M1 macrophages (64). Shenlian extract was demonstrated to
inhibit the M1 polarization by inhibiting miR-155, upregulating
SOCS3 and blocking the JAK2/STAT3 signaling pathway, thereby
reducing tissue damage and cell apoptosis (65). Another literature study on the role
of miRNA-155 in macrophage polarization suggested that miR-155
could induce M1 polarization by way of directly targeting the
IL13Rα1 (IL-13 receptor α 1, IL13Rα1), which interferes with the
activation of STAT6 and indirectly regulates the expression of
other M2 related genes (66).
In addition to miRNA-155 playing a role in
macrophage polarization, other miRNAs have also been reported to
play a role in macrophage polarization. Histone demethylase jumonji
domain containing 1C (JMJD1C) targets methyltransferase like 3
(METTL3) by upregulating miR-302a, inhibits SOCS2 expression
through m6A modification, and promotes M1 polarization to prevent
the occurrence of glioma (67).
Overexpression of miR-130b-3p in LPS-treated mice inhibited M1
polarization in lung and peritoneal macrophages by inhibiting the
expression of IRF1, thereby reducing inflammation in mouse lung
tissue (68). It was revealed that
the concentration of M2-related miRNAs, such as miR-146a and
miR-223 in the serum of patients with sepsis was significantly
reduced, and overexpression of miR-146a could inhibit expressions
of TLR4-NF-κB pathway protein interleukin 1 receptor associated
kinase 1 (IRAK1) and TNF receptor associated factor 6 (TRAF6),
thereby inducing inhibition of M1 macrophage polarization, and
reducing inflammation caused by sepsis (69). On the contrary, miR-495 can promote
the M1 polarization and inflammatory cytokines by inhibiting the
expression of the obesity-related gene FTO alpha-ketoglutarate
dependent dioxygenase (FTO), which leads to the exacerbation of
inflammatory response in adipose tissue (57). Furthermore, the PI3K/AKT signaling
pathway activated by miR-21 can cause M1-type polarization of
macrophages and lead to fibrosis in pig liver tissue (70), the activation of PPARδ regulated by
miR-9 in monocytes may play an important role in human M1
pro-inflammatory cells, while it does not play a role in M2
anti-inflammatory macrophages (71), and a recent study demonstrated that
miR-9 enriched in HPV+ head and neck squamous cell
carcinoma extracellular vesicles (EVs) can be transported to
macrophages and result in arrest in M1 phenotype by downregulation
of the expression of PPARδ (72).
Another study revealed that efficient gene transfer complex RM125b
carrying miR-125b can promote the polarization of M1 type
macrophages. At the same time, it was also observed that RM125b can
significantly inhibit the growth of tumor through TAM M1
polarization and reduce the proliferation of tumor cells (73). In addition, miR-125b-5p can promote
M1 polarization after mycobacterium tuberculosis infection, which
is related to A20/NF-κB-axis (74).
In summary, some miRNAs have been confirmed to be closely polarized
with M1, and the target and function of their action have been
preliminarily determined (Table
I).
miRNAs are involved in M2 macrophage polarization.
In addition to playing an important role in M1 polarization, some
miRNAs also play important roles in M2 polarization. Among the M2
polarization related miRNAs, one representative miRNA with
consistent research results is miRNA-21, which has attracted the
attention of numerous scholars. Some scholars indicated that
expression of miR-21 was significantly increased in patients with
non-small cell lung cancer (NSCLC) and radiation-induced lung
injury (RILI). The protective effects of miR-21-overexpressing bone
marrow mesenchymal stem cells (BMSCs) against RILI was also
assessed in rat models. Animal-based experiments demonstrated that
treatment with BMSCs had a remarkable effect on alleviating RILI of
rats, and cell-based experiments demonstrated that BMSCs notably
inhibited M1 polarization with a miR-21 dependent manner. These
results indicated that BMSCs with miR-21 overexpression could be a
potential therapeutic strategy for RILI (75). Furthermore, Xue et al
(76) revealed that in the livers
of mice exposed to arsenite, there were elevated levels of miRNA-21
and more extensive liver fibrosis. Arsenite induces the M2
polarization of macrophages via miR-21 regulation of PTEN, which is
involved in the activation of hepatic stellate cells and hepatic
fibrosis, which establish a previously unknown mechanism for
arsenicosis-induced fibrosis. To identify the molecular mechanism
by which miR-21 regulates macrophage polarization in sepsis-induced
intestinal injury, Li et al (77) used a bioinformatics approach to
predict the putative binding site between miR-21 and STAT1, and
their targeting relationship was demonstrated by a luciferase
reporter assay. The results indicated that miR-21 overexpression
significantly inhibited the total and phosphorylated levels of
STAT1, the miR-21 targeted STAT1 signaling and overexpression of
miR-21 significantly promoted M2 polarization, thereby alleviating
intestinal injury caused by sepsis. miR-21 in EVs can promote
macrophage polarization towards the M2-type by targeting programmed
cell death protein 4 PDCD4, reducing sepsis (78). A different study has confirmed that
cigarette smoke extract can induce the M2 polarization by
modulating the expression of miR-21, and the downregulation of
miR-21 could inhibit M2 polarization in chronic obstructive
pulmonary disease (79). However, a
number of previous studies have reported opposite results to the
aforementioned results, as miRNA-21appeared to play a role in
promoting M1 polarization and inhibiting M2 polarization under
certain conditions. For instance, Wang et al (80) identified that prostaglandin E2
(PGE2) induced M2 polarization was contributed to the inhibition of
miR-21 expression by activation of its direct target STAT3, and
silencing the STAT3 gene could abolish this PGE2-mediated
expression of M2 genes in miR-21 deficient macrophages. These data
suggested that the M1 polarization will be induced if miR-21 was
upregulated. In a different study which investigated the
relationship between miRNA-21 regulation of macrophage polarization
and disease occurrence and development, most results indicated that
miRNA-21 not only plays an important role in balancing inflammatory
states, but also participates in the process of tumor occurrence
and development (81). According to
another study, miR-21 can promote chemotherapy resistance in
ovarian cancer by regulating M2 polarization (82). In hypoxic environments, high levels
of miR-21 expression can promote M2 polarization and induce the
progression of lung cancer by targeting IRF1 (83). In addition, miR-21 in the exocrine
body of bladder cancer cells can regulate PI3K/AKT signalling by
inhibiting PTEN activation of macrophages and enhancing STAT3
expression, promoting M2 polarization and leading to an increase of
migration and invasion of cancer cells (84). Ma et al (85) observed that miR-182 expression in
macrophages could directly inhibit the expression of TLR4, leading
to the inactivation of NF-κB to induce M2 polarization of TAMs. In
addition, the therapeutic delivery of miR-182 antagonist with EVs
can lead to inhibition of miR-182, which resulted in tumor
suppression in various models of breast cancer (BC). This is
consistent with a previous study which demonstrated that miR-182
can alleviate inflammation in myocardial infarction by regulating
TLR4 in macrophages (86). Through
literature review, it was observed that another miRNA type related
to M2 macrophage polarization is miRNA-146a. For instance, miR-146a
overexpression can lead to an increase in M2 phenotype markers. On
the contrary, knocking down miR-146a promotes M1 polarization and
reduces M2 polarization (87). In
terms of its molecular mechanism of action, a previous study
revealed that miR-146a, at least partially, targets Notch1, PPARγ
and inhibin beta A subunit (INHBA) to regulate macrophage
polarization. miR-146a can induce M2 polarization by inhibiting
TLR4/NF-κB axis, thus promoting the healing of diabetes ulcers
(88). In addition, miR-125a-5p is
a regulatory factor for the immune regulation of M2b macrophages
(89). However, the secretion of
miR-21a-5p from mesenchymal stem cells (MSCs) could induce M2
polarization and alleviate AS by targeting KLF6 and ERK1/2 pathways
(90). Chen et al (91) demonstrated that lung adenocarcinoma
(LUAD) cells can induce M2 polarization of TAMs in vivo, and
the M2 polarization can promote the invasion, migration and tumor
metastasis of LUAD cells. miR-19b-3p derived from exosomes of LUAD
cells also inhibited STAT3 dephosphorylation by targeting protein
tyrosine phosphatase receptor type D (PTPRD) in TAMs, leading to
activation of STAT3 and polarization of M2 macrophages, thus
positive feedback aggravates the development of cancer. The small
miR-27a-3p released from EVs of glioblastoma can induce macrophage
polarization towards the M2 type through the EZH1/KDM3A/CTGF axis,
promoting the occurrence of glioblastoma (92). The EV miR-34a, secreted by
adipocytes, could inhibit M2 polarization by downregulating KLF4
expression, promoting obesity-induced adipose inflammation
(93). After reviewing the
aforementioned articles, it was observed that some miRNAs have been
confirmed to be closely related to the polarization of M2
macrophages and have clear targets and characteristics of function
(Table I).
Numerous other miRNAs involved in macrophage
polarization need to be further clarified. The results reported in
the aforementioned studies indicated that certain types of miRNAs
may play an important role in the polarization, function and
regulation of macrophage polarization. The main molecular mechanism
by which these miRNAs manipulate the macrophage polarization
process is often through the regulation of downstream key target
genes or signaling pathways, thereby affecting the balance of
pro-inflammatory and anti-inflammatory responses, or playing an
important role in the occurrence and development of tumors.
Therefore, in addition to understanding the positive miRNAs
associated with macrophage polarization as aforementioned, it is
necessary to further detect other new miRNAs and analyze whether
they make sense in clinical validation. In a recent study, Zhang
et al (94) investigated the
differential expression of 109 miRNAs during the polarization of M1
and M2 macrophages in humans and mice. The results showed that in
LPS and IFN-γ stimulated mouse bone marrow-derived macrophages
(BMDM), the expression of miR-127-3p, miR-155-5p, miR-181a,
miR-204-5p and miR-451 were significantly upregulated, while the
expression of miR-125-5p, miR-143-3p, miR-145-5p and miR-146a-3p
were significantly increased in IL-4-induced mouse BMDM cells. In a
different study, whether in polarized BMDM cells or PMA-induced
THP-1 cells, after exposure to IFN-γ/LPS treatment, miR-27a,
miR-29b, miR-125a, miR-146a and miR-155 were significantly
upregulated. However, after IL-4 co-treatment, the expression of
miR-26a and miR-193b was significantly increased (95). Curtale et al (96) confirmed that miR-155 is highly
expressed in M1 polarized macrophages, while miR-146a, miR-125b and
miR-127 are highly expressed under M2 polarized conditions.
Furthermore, the important role of miR-15 in macrophage
polarization and inflammation was elucidated (60,97,98).
In addition, previous studies demonstrated that miR-127 and
miR-125b induce M1 polarization by targeting the expression levels
of Bcl-6 and IRF4 genes, respectively, thereby increasing the
expression and release of pro-inflammatory cytokines (99,100).
It should be particularly emphasized at this point that the
inhibitory effect of miR-127 on Bcl-6 will lead to a decrease in
the expression of dual specificity phosphatase 1 (Dusp1) and an
increase in the phosphorylation level of JNK. Knocking down its
expression leads to a decrease in the expression of M1
characteristic genes and promotes the transcription level of
M2-related genes (99). There are
also results confirming that overexpression of miR-720 can reduce
the expression level of GATA3, ultimately leading to inhibition of
the M2 polarization level (101).
Other miRNAs that are highly expressed in M2 macrophages include
miR-511-3p, miR-223 and let-7c (96,102),
which have been revealed to significantly promote M2 polarization.
Some slightly reported miRNA types are involved in the polarization
process of macrophages to varying degrees. For example, miR-511-3p
is also highly expressed in TAMs (103) and promotes the expression level of
M2-related genes. miR-223 can limit the polarization and
pro-inflammatory activity of M1 macrophages by targeting the TF
Pknox1 (104,105). Similarly, Zhang et al
(106) demonstrated that there was
a loss of let-7c and elevated expression of p21-activated kinase 1
(PAK1) in human and murine macrophages induced by inflammatory
stimuli, and the let-7c dependent upregulation of PAK1 by upstream
EZH2 could promote macrophage M1 polarization. Usually, the
expression of let-7c is higher in M2 macrophage than that in M1
macrophage, the overexpression of let-7c reduces the expression of
M1-related genes and increases the M2 markers, and the opposite
result will occur when knocking down let-7c (107). These results preliminarily
indicated that macrophage polarization is also related to miR-23a,
miR-27a and miR-24-2, their expression is downregulated in M1
polarization and upregulated in M2 polarization. More
interestingly, overexpression of miR-23a or miR-27a can promote the
expression of pro-inflammatory cytokines by acting on different
signaling pathways, while inhibiting the expression level of
M2-type cytokines. For example, miR-23a reduces M2 cytokine
production by targeting TNF inducible protein 3 (TNFAIP3), then
JAK1 and STAT6, while miR-27a can target interferon regulatory
factor 4 (IRF4) and peroxisome proliferators γ (PPAR γ) to regulate
the express of ion-inflammatory factors and activate their
receptors (108).
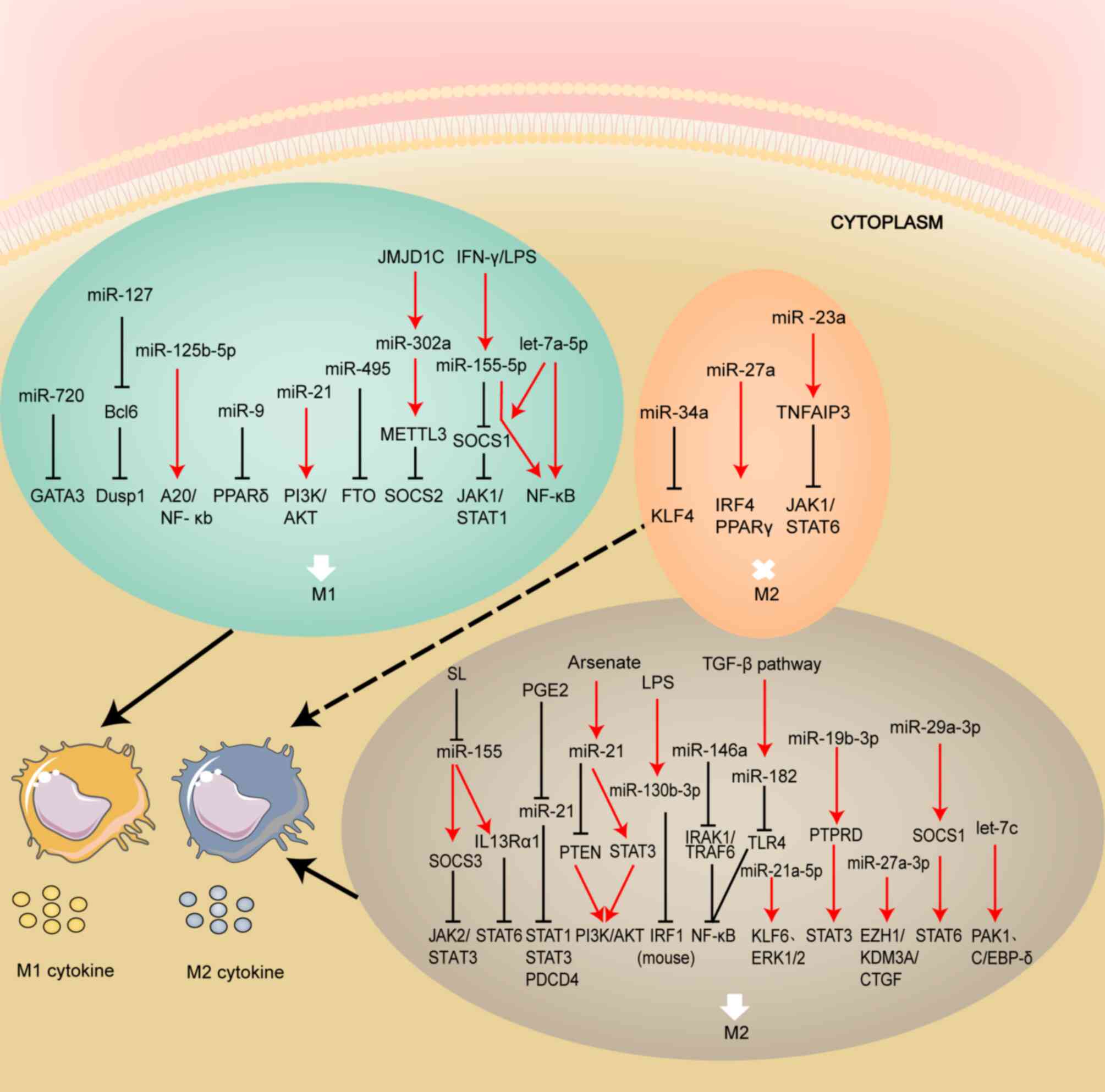
In summary, in this section, a preliminary summary
and analysis of some miRNAs that have been less reported have been
conducted. However, the molecular mechanism of miRNA in macrophage
polarization is relatively complex, involving the interaction
between its upstream regulatory factors and downstream target
genes. In terms of the types of miRNAs polarized by macrophages M1
and M2, both directions of polarization have relatively specific
associations with miRNA types and have different effects on
inhibiting or promoting macrophage polarization (Fig. 3). As for their exact role and
molecular pathways in driving macrophage polarization, further
validation and clarification are needed. Therefore, in addition to
the confirmed miRNAs related to M1 or M2 polarization
aforementioned, other related miRNAs have recently been discovered
and are being further confirmed (Table
II).
Although it has been confirmed that numerous miRNAs
can regulate different macrophage functions, it is known that only
a few miRNAs are closely related to the polarization of
macrophages. Increasing evidence suggests that miRNAs in different
tissues and cell types have their specificity, and numerous studies
have detected miRNA patterns in various macrophage types and their
potential roles in macrophage polarization (109). So far, it has been observed that
specific miRNA mimics or anti-inflammatory drugs can control immune
and inflammatory responses, and it has been preliminarily confirmed
that the use or intervention of these miRNAs can play a role in
treating inflammatory diseases (110–112). However, in addition to the
potential role of miRNA in acute and chronic inflammation, it is
necessary to conduct more research on miRNA expression in diseases,
such as inflammation and tumors (113). Identifying specific targets for
any single miRNA remains a major challenge, and some advanced
technologies are continuously determining the target of miRNA
action (114). Therefore, further
studies are needed to determine the precise function and effects of
miRNAs, especially their regulatory effects on macrophage
polarization before being used as targets for therapies.
Macrophage polarization induced by miRNA for
treatment of tumors. With the continuous deepening of research on
the mechanism of action and biological functions of miRNAs, it has
been identified that certain important miRNAs have certain
physiological and pathological effects. Therefore, some scholars
have gradually transitioned from basic research to applied research
and have achieved favorable therapeutic effects in disease
experiments and clinical treatments. Especially prominent is the
research on the treatment of inflammatory diseases and tumors with
miRNAs. One of the therapeutic effects is based on the role of
miRNA in macrophage polarization. Because whether it is an
inflammatory disease or a malignant tumor, it is to some extent
related to the polarization of macrophages. Thus, whether miRNA is
a new tool for human cancer treatment has attracted the attention
of numerous scholars. For instance, in the research of tumors,
miRNAs are mainly divided into two categories based on their
different effects on tumor cells, such as promoting tumors and
inhibiting tumors. However, it is worth noting that certain miRNAs
occasionally have a dual effect of promoting or inhibiting tumors
under different environmental conditions. In conclusion,
macrophages are important immune cells in the tumor
microenvironment (TME), and miRNAs can regulate the proliferation,
metastasis and therapeutic response of tumor cells mainly by
influencing the polarization of macrophages, which has been
observed in various cancers. Naturally, miRNAs associated with
macrophages are a feasible new treatment method for tumor
immunotherapy. Therefore, after determining the exact role of a
certain miRNA, its targeted therapeutic effect in treatment should
be evaluated and a series of necessary clinical studies should be
conducted. With the deepening understanding of immunotherapy, the
current anticancer treatment research is increasingly focused on
the direction of the TME. It is known that miRNAs play a crucial
role in regulating genetic information and expression and mediate
interactions between tumor cells and numerous components in TME.
Macrophages are abundant in TME, and their different polarization
directions can promote or inhibit tumor growth and progression by
regulating biological behaviors such as macrophage recruitment,
infiltration and polarization. In a review article describing the
relationship between macrophage activation and miRNA, Zhou et
al (115) focused on the
progress and prospects of targeted therapy based on miRNA, novel
clinical biomarkers and drug delivery systems. Through analysis of
research studies, it was observed that crosstalk between
tumor-related macrophages and miRNAs plays a key role in the TME.
It was also pointed out that miRNA-based therapies can be designed
in two different directions. One is to reduce the level of
carcinogenic miRNAs or increase the content of tumor suppressive
miRNAs (116). In terms of the
relationship between cancer miRNAs, tumor suppressor miRNAs and
macrophage polarization aforementioned, various miRNAs play
different roles in the M2 polarization of cancer TAM. Among them,
miRNAs that promote M2 polarization or inhibit M1 polarization are
referred to as ‘oncogenes’, mainly including miR-19a-3p, miR-21,
miR-29a-3p, miR-145, miR-195-5p, miR-224, miR-301a-3p, miR-1246 and
miR-let-7 that promote M2 polarization. Correspondingly, another
type of miRNAs that promotes M1 polarization or inhibits M2
polarization is called a ‘tumor inhibitor’, such as miR-142-3p and
miR-155 that promote M1 polarization. The other two types of ncRNAs
are designated as ‘juggle tumor inhibitors’ or ‘juggle oncogenes’.
Juggle tumor inhibitors refer to ncRNAs that promote M1
polarization, and these Juggle oncogenes inhibit M1 polarization.
Multiple tumor suppressor miRNAs include miR-16, miR-34a and
miR-142-3p, while the main oncogene miRNAs include miR-503, among
others. Therefore, further research on the role of miRNAs in cancer
macrophage polarization may lead to more precise screening of
macrophage polarization modulators for different cancer treatment
methods (117). It has been proved
so far that miRNA plays a significant role in the diagnosis and
treatment of clinical diseases, the first batch of human miRNA
therapy drugs have entered the first phase of clinical trials, and
recently entered the second phase of clinical trials for advanced
tumors. As proof, the miR-155 oligomeric inhibitor Cobomarsen is
used for the treatment of T-cell leukemia/lymphoma. In addition,
the miR-16 analog TargomiR can be used for the treatment of
mesothelioma (118). ncRNA, as a
regulatory factor for macrophage polarization, can also mediate
immune responses to various cancers by reprogramming TME, thereby
regulating immune responses (119). MiRNA mimics and antagonists that
can reprogram TME are currently being tested in human clinical
trials and may become a new promising treatment strategy (120). In particular, miR-138 mimics can
specifically target and bind to PDL-1 and CTLA-4 mRNA, mimic the
effects of anti-PD-1 and anti-CTLA-4 antibodies, and inhibit their
expression (121). Li et al
(122) observed that miR-498 may
inhibit esophageal cancer by inhibiting macrophage autophagy and
M2-like polarization through MDM2/ATF3. There are also a number of
studies indicating that miRNA-related therapies have improved
efficacy and safety than small interfering RNA (siRNA)-based
therapies (123). In subsequent
studies on miRNA therapy, it was revealed that injecting
miRNA-based drugs into tumors can improve their specificity and
efficacy while reducing side effects. For example, injecting
cationic liposomes/pVAXmiR-143 complex into tumors can inhibit
subcutaneous tumor growth in vivo (124). Intra-tumoral injection of
miR-19a-3p can downregulate the expression of fos-related antigen-1
(Fra-1) and effectively reduce the invasive ability of BC (125). The administration of miR-142-3p
microbubbles derived from TAM can significantly inhibit tumor
growth in tumor-bearing mice, indicating the potential anticancer
value of miRNA administration in TME (126). In addition, due to the ability of
miRNAs to interact with specific target genes, they have a
significant role in regulating gene expression, and interference in
their related signaling pathways will also be a targeted
intervention pathway for tumor treatment. In TME, tumors and
mesenchymal cells can cross-communicate through various factors.
One of the strategies for the immune escape of tumor cells is to
release miRNA, which regulates the polarization and activity of
circulating or local monocytes/macrophages to perform
tumor-promoting effects. On the other hand, miRNAs derived from
macrophages can also exert antitumor functions. In a previous
comprehensive article, the author provided a detailed summary and
analysis of the latest developments in miRNA-mediated crosstalk
between tumor cells and macrophages and their uptake patterns in
TME (127). However, there is
relatively little systematic research on the function and mechanism
of miRNA in tumor tissue TAMs. Li et al (128) demonstrated that miR-146a promotes
the expression of a number of M2 macrophage phenotype molecules and
confirmed that overexpression of miR-222 inhibits TAM chemotaxis by
targeting C-X-C motif chemokine ligand 12 (CXCL12) and inhibiting
C-X-C motif chemokine receptor 4 (CXCR4), thereby inhibiting tumor
cell proliferation and tumor growth. The main reason is that miRNA
affects the growth of breast tumors by promoting M2-type
polarization or regulating the recruitment of TAMs. These results
suggested that endogenous miRNAs may play an important role in
controlling the polarization and function of TAMs in BC. Pirlog
et al (129) analyzed the
role of miRNAs in TME macrophage polarization and the role of this
macrophage polarization in NSCLC regions. The results confirmed
that the normal recovery of macrophage polarization in TME can
produce significant antitumor effects. A different study also
revealed that miR-16 can produce significant antitumor activity by
promoting the polarization of M1-like macrophages (130). In terms of research on miR-19a-3p,
it has been detected that it is involved in the induction of the
polarization of M2-like macrophages and is involved in the
progression and invasion of breast tumors (110), or in the promotion of the
occurrence of colitis-related colorectal cancer (CRC). However, so
far, the mechanism by which miR-19a-3p induces M2-like macrophages
has not been elucidated (131).
In addition, a number of studies have also
demonstrated that other miRNAs involved in M2 macrophage
polarization play a role in promoting tumor occurrence and
development. For instance, overexpression of miR-19a-1p induces the
progression and metastasis of BC (125), tumor-derived exosomes miR-21 cause
polarization of M2 macrophages, promoting the growth of head and
neck tumors (132), and miR29a-3p
promotes polarization of M2 macrophages by activating SOCS1/STAT6
signals, leading to invasion of oral squamous cell carcinoma and
tumor cell proliferation (133).
On the contrary, significant progress has been made regarding the
role of M1 polarization in tumors. For instance, Zhang et al
(134) comprehensively summarized
the role of miRNA-34 in tumors and concluded that miRNA-34 exhibits
dysregulation in various human cancers. miR-34a can inhibit M2
polarization and drive M1 polarization. Currently, its main
functional localization is tumor-suppressive miRNAs. With the
development of phase I clinical trials of miR-34a mimetic MRX34,
the importance of miR-34 has become increasingly recognized and
plays a crucial role in inhibiting tumor progression. In addition,
it has potential value as a candidate therapeutic drug for miRNAs
(135,136). A different study also showed that
the expression of miR-34a in triple-negative BC mediated M1
polarization while antagonizing miR-34a could promote M2
polarization. However, there have been opposing studies regarding
miR-34a stimulation of invasion and metastasis in CRC (135). miR-142-3p is a tumor inhibitor
that promotes the polarization of macrophage M1 phenotype and has
been revealed to inhibit the growth of glioma. It has favorable
therapeutic potential in anti-glioma therapy (136). miR-145 was revealed to be a
communication tool between TAM and cancer cells, leading to the
tumor-promoting effect induced by TME. The main reason is that it
has inhibitory effects on M1 and promotes polarization of M2-like
macrophages, indicating that its siRNA has favorable therapeutic
prospects for tumors (137). As
previously described, miR-155 is one of the most widely studied
miRNAs in different cancers and is involved in driving M1
polarization in macrophages and its mimetics are candidate drugs
for treating various tumors (60,138).
Furthermore, a number of miRNAs involved in potential tumor
treatment have attracted the attention of scholars. For example, it
was revealed that miRNA-224 is involved in inhibiting the
progression of prostate cancer (PC) by downregulating TRIB1 to
induce the transformation of M2 macrophages into M1 cells (139). miR-301a-3p promotes the metastatic
phenotype of human pancreatic carcinoma cell line PANC-1 cells by
inducing M2 macrophages to differentiate from stromal macrophages.
In addition, knocking out miR-301a-3p significantly weakens the
polarization of macrophages towards the M2 type, thereby reducing
the invasion, migration and metastasis ability of PANC cells in
vivo and in vitro (140). miR-503 plays a crucial role in
promoting brain metastasis by inducing M1 to M2 macrophage
polarization in BC patients (141). miR-1246, as an EV derived from
hypoxic glioma cells, is assigned to induce M2-like macrophage
polarization (142), while in
ovarian cancer, miR-1245 also enhances chemotherapy resistance
through M2-like cell polarization (143). miRNA let-7b plays an important
role in regulating macrophage polarization, thereby enhancing the
presence of TAM in PC. When treated with let-7b inhibitors, it
leads to reduced migration and angiogenesis of PC cells (144). According to previous studies,
let-7c also has a role in regulating inflammation and related
cytokines through macrophages in the occurrence and development of
lung cancer (145). Additionally,
it has been reported that the lin-28B-let-7-HMGA2 axis is involved
in the induction of BC through M1 macrophage activation (146), and miR-let-7a has also been
exhibited to participate in M2 macrophage polarization (147). Although it is not yet clear how
miRNA-let-7b regulates macrophage phenotype and function, results
have confirmed that TAM treated with let-7b inhibitors reduces
angiogenesis and migration in PC (144). However, further research is needed
to verify the role of miRNA in tumor therapy by regulating
macrophage polarization.
The role of macrophage polarization is induced by
exosomal miRNAs in treatment of tumors. An increasing number of
results demonstrated that cells in some microenvironments can
regulate the process of macrophage polarization in the form of an
exocrine body or microbubble. For example, in the study of the lung
metastasis model of breast adenocarcinoma mice, Xun et al
(148) preliminarily confirmed
that miR-138-5p was transferred from BC cells to tumor-related
macrophages through exosomes, and the polarization of M2
macrophages was achieved by reducing the expression of lysine
demethylase 6B (KDM6B). Therefore, interfering with this
exosome-derived miR-138-5p may become a potential target for cancer
treatment. Xu et al (149)
preliminarily demonstrated that miR-3184-3p is enriched in
cerebrospinal fluid EVs of glioma patients, and promotes tumor
progression by directly promoting glioma cell proliferation and
promoting M2-like macrophage polarization. The results indicate
that interfering with the exosomes miR-3184 may be a possible
pathway for future glioma treatment (149). Ma et al (85) used cationic mannan-modified EVs to
effectively target macrophages in the breast tumor mouse model
experiment, thereby inhibiting M2 cell polarization by inhibiting
the expression of miR-182 and achieving the goal of treating
cancer. Macrophages are abundant in TME and their M2 dominant
polarization is conducive to the malignant proliferation of tumors.
Various forms of miRNAs, including exo-miRNAs, can dually
induce/inhibit macrophage polarization and regulate tumor
progression and treatment response by influencing various molecular
pathways (150). In the gastric
cancer liver metastasis (GC-LM) model, it was revealed that the
expression level of miR-519a-3p in serum EVs of patients with GC-LM
was significantly higher than that of patients without LM. This
exo-miR-519a-3p mainly activates the MAPK/ERK pathway by targeting
DUSP2, leading to M2-like polarization in macrophages. M2 like
polarized macrophages can accelerate the development of GC-LM. The
results indicated that exo-miR-519a-3p plays a crucial role in
mediating the interaction between primary GC cells and hepatic
macrophages, and is a potential therapeutic target for GC-LM
(151). Ma et al (152) investigated whether exosomes
derived from NSCLC affect TAMs and whether TAMs provide feedback
regulation on the progression of NSCLC. The results demonstrated
that miR-181b was upregulated in EVs derived from NSCLC patient
serum and NSCLC cells. This EV derived from NSCLC cells can enhance
the polarization of macrophage M2 by regulating the
miR-181b/JAK2/STAT3 axis. The silencing of miR-181b in NSCLC cells
and the use of JAK2 inhibitors in macrophages blocked this effect.
Therefore, the involvement of extracellular miR-181b in crosstalk
between NSCLC cells and TAMs is also a potential therapeutic target
for NSCLC (152). Although some
studies have confirmed that macrophage-derived exosomes (MDE) are
involved in tumor progression, their role in glioma is not fully
understood. In an experiment on the activation of macrophage, it
was observed that the circRNA BTG (circBTG2) in macrophage exosomes
is upregulated, which contributed to inhibiting tumor progression
through circBTG2/miR-25-3p/PTEN pathway, indicating that miRNA-25
and circBTG2 can be considered as diagnostic biomarkers and
potential targets for the treatment of glioma (153). Chuang et al (154) confirmed that the exosomes
transfected with miR-155 and miR-125b could reverse M2 phenotype
polarization induced by pancreatic cancer and promote M2-like cells
to transform into M1 macrophages. Similarly, macrophages in
glioblastoma TME secrete EVs containing miR-21, and their levels
are related to the M2 polarization state. Reducing the secretion of
miR-21 EVs by macrophages can reduce the polarization state of M2
and achieve the goal of inhibiting tumor growth. In addition,
whether it is miR-195-5p (155),
miR-130a (156), or miR-31-3p
(157,158), they are all related to the
polarization of macrophages and may also mediate the interaction
between tumor cells and macrophages. Interfering with exosomes
containing these miRNAs has the potential for targeted treatment of
tumors. Binenbaum et al (159) observed that MDE significantly
reduced the sensitivity of pancreatic ductal adenocarcinoma (PDAC)
cells to gemcitabine in vitro and in vivo. This
effect is mediated by the transfer of miR-365 in MDE. MiR-365
weakens the effect of gemcitabine by upregulating the adenosine
triphosphate pool and inducing cytidine deaminase in cancer cells.
In mice carrying PDAC, miR-365 translocation in TAM was found to
induce gemcitabine resistance. The use of MDE as antagomir carriers
can improve the efficacy of chemotherapy in cancer and uncover new
treatment options for combating malignant tumors (159). The research results of
Moradi-Chaleshtori et al (160) indicated that exosomes can
effectively deliver miR-130 to macrophages, leading to upregulation
of M1-specific markers and cytokines, as well as downregulation of
M2-specific markers and cytokines. The use of miRNA-containing EVs
to reverse M2 macrophages to the M1 phenotype may be one of the
therapeutic strategies for combating cancer invasion and
metastasis. Chen et al (161) focused on the regulatory effects of
miRNA on macrophage differentiation, functional polarization and
cell crosstalk, and detected that crosstalk between tumor cells and
macrophages is crucial for the formation and progression of TME,
miRNA can act as different forms of communication mediators, such
as microbubbles or EVs. Papillary thyroid cancer (PTC) is an
endocrine malignancy, and the role and molecular mechanism of
miR-655-3p in PTC are currently unclear. A study conducted by Qiao
et al (162) revealed that
overexpression of miR-655-3p with mimics significantly reduced
tumor cell viability, chemotaxis and invasiveness. Exosomes
miR-655-3p inhibits growth, invasion, and macrophage M2
polarization in papillary thyroid carcinoma by targeting CXCR4. The
results indicated that the regulation of exosomes miR-655-3p/CXCR4
may be a potential therapeutic strategy for PTC (162). Some scholars have also conducted
corresponding research on the relationship between extracellular
miRNAs and drug resistance. Previous studies have shown that
cancer-associated fibroblasts (CAFs) regulate gemcitabine
resistance by transferring exosomes miRNA-106b to cancer cells.
Recently, it has been proven that TAM can promote resistance to
gemcitabine. However, the role of CAF in regulating cancer TAM
function remains unclear. Zhao et al (163) extracted primary CAFs from tumor
tissue of PC patients and obtained CAFs-derived exosomes
(CAFs-Exo). The results showed that conditional mediators derived
from CAFs have a higher potential to promote M2 polarization in
macrophages. Furthermore, it was revealed that miRNA-320a can
transfer from CAFs to macrophages through EVs, thereby promoting M2
polarization. Pretreatment of CAFs with miRNA-320a inhibitors
reduced the expression of miRNA-320a in CAFs-Exo and resulted in
reduced polarization of M2 macrophages. Therefore, targeted
intervention of this pathway may be an effective way to combat PC
(163). Some scholars have
observed that in addition to the prevention and treatment of EV
delivery, miRNA can also mediate the transmission of miRNA between
cells through EVs. Loading of miR-124 into 293T-derived EVs formed
miR-124-EVs. The results revealed that miR-124-EVs had an effective
antitumor effect in both glioblastoma cells and microglia cells.
The main mechanism of its effect is that EV-mediated miR-124
delivery can inhibit the growth of human glioblastoma cells and
inhibit the polarization of M2 microglia. These findings provide
substantial evidence for the development of potential therapeutic
strategies using miRNA-loaded EVs (164).
Therapeutic potential of miRNA-induced macrophage
polarization in inflammation-related disease. It has been proven
that the polarization state of macrophages under different
pathological conditions can promote or alleviate various
inflammations (158,165–167). Therefore, miRNAs that regulate
macrophage polarization can also regulate inflammatory responses or
become targets for the treatment of inflammatory diseases such as
sepsis, obesity, cancer and multiple sclerosis. Among them, sepsis
is a severe inflammatory response syndrome and the main cause of
death in hospital intensive care units (168–171). The pathophysiology of sepsis is
very complex, often involving simultaneous activation of
pro-inflammatory and anti-inflammatory responses (172). Given the complex balance between
pro-inflammatory and anti-inflammatory responses, the role of
macrophage polarization in sepsis remains unclear to this day.
However, the study of miRNAs involved in macrophage polarization
will help reveal the pathogenesis of sepsis (173–179). Numerous research results have
preliminarily confirmed that miRNAs that inhibit M1 polarization or
activate M2 polarization may have favorable therapeutic potential
in sepsis. For example, previous studies have exhibited that serum
concentrations of M2 phenotype-related miRNAs, such as miR-146a and
miR-223, in patients with sepsis are significantly reduced
(180). Among them, overexpression
of miR-146a can inhibit expression of TLR4-NF-κB pathway proteins,
and block sepsis-induced inflammatory cell infiltration (69). Wang et al (180) observed the role of circulating
miR-223 in sepsis and observed an association between a decrease in
miR-223 levels and an increase in sepsis severity. However, a
recent study by Benz et al revealed that serum miR-223
levels cannot predict the prognosis or survival rate of sepsis in
critically ill patients (181).
The conflicting results between these two studies can be partially
explained by differences in experimental procedures. The
aforementioned research results appear to confirm that miR-146a and
miR-223, as macrophage polarization modulators, appear to be
related to clinical outcomes in patients with sepsis. However,
despite extensive research on the changes in miRNA expression in
sepsis, the exact role of miRNA-induced macrophage polarization in
sepsis remains unclear and further exploration is needed (173). There are also other studies on the
role of miRNA-induced macrophage polarization in other
inflammatory-related diseases. For example, polarized macrophages
are involved in different disease processes such as obesity
(182–187), cancer (158,165–167), and multiple sclerosis (188,189). Regulating macrophage activation or
replacing activated miRNAs in these disease states may have
therapeutic effects. Overall, overexpression of miRNAs such as
M2-related miR-223 (15,105) and inhibition of miRNAs such as
M1-related miR-33 and miR-155 are beneficial for the control of
inflammation (190,191). Similarly, the increased expression
of pro-inflammatory miR-155 (188)
and the downregulation of anti-inflammatory miR-124 (189) is associated with the deterioration
of multiple sclerosis, and the intervention of these two miRNAs is
also a possible target for inflammation control. In addition, the
increased expression levels of miR-19a-3p (125), miR-16 (130), miR-155 (60,192),
and miR-511-3p (103) all promote
the activation of TAMs to varying degrees, affecting local
inflammation of the tumor. By contrast, as aforementioned,
MSC-derived EVs contain miR-21a-5p, which activates macrophage
polarization and reduces macrophage infiltration by targeting the
KLF6 and ERK1/2 signaling pathways, thereby slowing down the
development of AS (90). The
disruption of miRNA-33-mediated aerobic glycolysis and
mitochondrial oxidative phosphorylation balance is also related to
the M2 polarization level of macrophages and is mainly due to the
targeted regulation of miR-33 on AMP-activated protein kinase
(AMPK). The antagonism of miR-33 can reduce plaque inflammation and
play a protective role in AS (193). According to previous studies,
miR-101 plays a crucial role in macrophage polarization and innate
immune response. Overexpression of miR-101 in macrophages increases
M1-related cytokine expression levels (194). Gao et al (195) demonstrated that the miR-101/MKP-1/
mitogen-activated protein kinase pathway plays a potential
anti-inflammatory target. Liu et al (196) revealed that macrophages treated
with TLR2, TLR3, or TLR4 ligands showed a decrease in the
expression level of miR-147 while silencing miR-147 increased the
expression level of inflammatory cytokines. In addition, miR-203
has been demonstrated to play a negative role in regulating the
immune response to LPS, subsequently reducing inflammatory
mediators such as TNF-α and IL-6 expression (197). Xie et al have confirmed
that overexpression of miR-27a in MDM can increase the level of
pro-inflammatory cytokines while knocking down miR-27a can reduce
the expression of these cytokines. miR-27a mainly prevents
excessive inflammatory response driven by TLR2/4 in macrophages by
reducing the secretion of IL-10 (198). Therefore, the aforementioned
literature research results clearly indicated that in addition to
the antitumor effect of miRNA by regulating M1 and M2 polarization,
an increasing number of research results indicated that this
regulatory mode also plays an important role in inflammation
prevention and control. For instance, M1 and M2 phenotypes play a
unique role in the progression of inflammatory-related diseases
such as sepsis, obesity, cancer and multiple sclerosis. Therefore,
miRNA regulation of macrophage polarization also demonstrates the
potential for targeted therapy in the treatment of
inflammatory-related diseases (9).
Lv et al (199)
investigated the role of extracellular miRNAs derived from tubular
epithelial cells (TECs) in the development of renal
tubulointerstitial inflammation. Research has revealed that
extracellular miR-19b-3p mediates communication between damaged
TECs and macrophages, leading to M1 macrophage activation. The
EV/miR-19b-3p/SOCS1 axis plays a crucial pathological role in renal
tubulointerstitial inflammation and is a new therapeutic target for
renal diseases (199). Macrophage
polarization is also involved in the development and progression of
asthma. Some miRNAs can participate in the development of asthma by
inducing M1/M2 polarization. Therefore, targeting miRNAs to
regulate macrophage polarization may have therapeutic potential in
allergic asthma and other allergic diseases (58). The results of the study conducted by
Arora et al (200)
suggested that miRNAs and other TFs can induce changes in the
macrophage phenotype. Understanding the mechanism of macrophage
polarization and its phenotype regulation will help design new
inflammatory treatment strategies (200). Qiu et al (201) reviewed and analyzed the key role
of epigenetic modifications in the regulation of macrophage
polarization. They support that miRNA epigenetically-mediated
macrophage polarization may be a potential target for the treatment
of ischemic stroke and may provide a promising treatment strategy
for neuronal damage after cerebral ischemia (201). Recent studies have also shown that
EVs derived from mesenchymal stromal cells (MSCs) play an important
role in macrophage immune regulation after myocardial
ischemia/reperfusion (I/R) and in heart injury repair. MSC-derived
exosome (MSC-Exo) alters the polarization state of macrophages by
shuttle miR-182, thereby alleviating myocardial I/R injury in mice.
It was also hypothesized that MSC-Exo can serve as a potential
therapeutic tool for myocardial I/R injury (86). Dang and Leelahavanichkul explored
the polarization phenomenon of miRNA-induced anti-inflammatory
macrophages based on the close relationship between inflammatory
macrophages and sepsis. The research results revealed that
overexpression of miR-223 and miR-146a in RAW264.7 plays an
inducing role in M2 macrophage polarization. Further research
confirmed that the anti-inflammatory state induced by miR-223 is
not only related to HIF-1 α. The downregulation of the interfering
glycolytic pathway is related and can prevent LPS-induced
polarization of M1 macrophages. In the LPS model, pretreatment with
miR-223 overexpressed macrophages and IL-4 reduced the severity of
sepsis. Therefore, the concept of inducing anti-inflammatory
macrophages through cell energy destruction for the treatment of
sepsis was proposed (202).
Neuroinflammation is the main cause of secondary neuronal damage,
but the immune mechanism of brain cell damage in neonatal
hypoxic-ischemic encephalopathy remains unclear. A previous study
demonstrated that miR-210 is a new regulator of microglia
activation in neonatal hypoxic-ischemic brain injury, and also a
potential therapeutic target for neonatal hypoxic-ischemic brain
injury (203). Macrophages also
play a crucial role in the pathogenesis of AS, but their molecular
mechanism remains unclear. For example, Xu et al (204) observed that miR-34a plays a
central role in the regulation of macrophage cholesterol outflow,
inflammation and AS, which indicates that miR-34a is a promising
target for the treatment of heart metabolic diseases. There is
increasing research on the role of MSCs and MSC-Exos in alleviating
myocardial I/R injury. Gao et al (205) attempted to find an ideal microRNA
candidate and determine whether it could replicate the
cardioprotective effects of MSCs and MSC-Exos. The results
indicated that miR-125a-5b is enriched in MSC-Exos, and its
modified oligonucleotide miR-125a-5p atomic can increase the
polarization of M2 macrophages, promote angiogenesis and help
improve myocardial cell apoptosis and inflammation, thereby
achieving effective therapeutic goals (205). The aforementioned studies
indicated that miRNAs play crucial regulatory role in macrophage
polarization, inflammation and tumor development. Given that the
balance between M1/M2 macrophages plays an important role in the
occurrence and development of numerous diseases, and miRNA can
regulate the balance between M1/M2 cells in various ways,
identifying miRNAs related to the dynamic changes in macrophage
polarization and understanding their role in regulating this
process is of great significance for exploring the molecular basis
of disease progression and developing new miRNA-targeted therapy
strategies.
In conclusion, it is known that macrophage
polarization plays an important role in numerous physiological and
pathological conditions, such as infection, inflammation, immunity,
regeneration and tumors. In the past few years, significant
progress has been made in regulating TFs of macrophage
polarization, as well as epigenetic modification mechanisms
including miRNA. It has been revealed that miRNAs can play
inflammatory and immune regulatory roles by participating in the
polarization process of macrophages. However, the molecular
mechanism of miRNA in macrophage polarization is relatively
complex, involving the interaction between its upstream regulatory
factors and downstream target genes. The current research focus
remains on how miRNA regulates the polarization process of
macrophages by regulating downstream target genes or signal axes.
In terms of the types of miRNAs polarized by macrophages M1 and M2,
both directions of polarization have relatively specific
associations with miRNA types and have different effects on
inhibiting or promoting macrophage polarization. The specific
regulatory effect of the same miRNA is related to the specific
pathological microenvironment and induction drugs. Some miRNAs even
exhibit opposite reported results of inhibiting or promoting
macrophage polarization. It is precisely due to the important role
of miRNA in regulating macrophage polarization that it plays an
important role in inflammation, immune response and tumor growth.
At present, there are numerous studies on the regulation of
macrophage polarization by miRNAs involved in inflammation and
tumor occurrence and development, and some important miRNA types
have been preliminarily confirmed. At the same time, in
vitro and in vivo experiments have confirmed that these
miRNA mimics or inhibitors play an important role in inflammation
control and tumor treatment. miRNA is expected to become a target
for treating diseases related to inflammation or tumors. Although
both basic research and early clinical findings indicated
significant potential for drug development based on miRNA
expression interference, there have been no studies of phase III
clinical studies to date. Therefore, in-depth exploration of miRNA
on macrophage polarization will help to improve understanding of
the biological functions of macrophages, and further provide a more
effective theoretical basis and treatment strategies for treating
diseases centered on macrophage polarization.
Not applicable.
The present study was supported by the ‘Twelfth Five-Year’
National Science and Technology Support Program (grant no.
2013BAI07B02), the Natural Science Foundation of China (grant no.
81573467), the Natural Science Foundation of Shandong (grant nos.
ZR2020QH160 and ZR2021MH080), The Foundation for Jinan's Clinical
Science and Technology Innovation (grant no. 202134001) and the
Cultivation Fund of the first affiliated hospital of Shandong First
Medical University (Shandong Qianfoshan Hospital; grant no.
QIPY2020NSFC0819).
Not applicable.
QL and GSJ conceived and designed the article. CZW,
XDW and DFZ surveyed the literature and wrote the manuscript. XLS,
YHW and JW surveyed the literature and provided suggestions. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Haniffa M, Bigley V and Collin M: Human
mononuclear phagocyte system reunited. Semin Cell Dev Biol.
41:59–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Santoni G, Morelli MB, Amantini C, Santoni
M, Nabissi M, Marinelli O and Santoni A: ‘Immuno-Transient Receptor
Potential Ion Channels’: The role in monocyte- and
macrophage-mediated inflammatory responses. Front Immunol.
9:12732018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawakami A, Iwamoto N and Fujio K:
Editorial: The role of monocytes/macrophages in autoimmunity and
autoinflammation. Front Immunol. 13:10934302022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou D, Huang C, Lin Z, Zhan S, Kong L,
Fang C and Li J: Macrophage polarization and function with emphasis
on the evolving roles of coordinated regulation of cellular
signaling pathways. Cell Signal. 26:192–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Juhas U, Ryba-Stanislawowska M, Szargiej P
and Mysliwska J: Different pathways of macrophage activation and
polarization. Postepy Hig Med Dosw (Online). 69:496–502. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lawrence T and Natoli G: Transcriptional
regulation of macrophage polarization: Enabling diversity with
identity. Nat Rev Immunol. 11:750–761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Jiang T, Li MQ, Zheng XL and Zhao
GJ: Transcriptional regulation of macrophages polarization by
MicroRNAs. Front Immunol. 9:11752018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kishore A and Petrek M: Roles of
macrophage polarization and macrophage-derived miRNAs in pulmonary
fibrosis. Front Immunol. 12:6784572021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Essandoh K, Li Y, Huo J and Fan GC:
MiRNA-mediated macrophage polarization and its potential role in
the regulation of inflammatory response. Shock. 46:122–131. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohapatra S, Pioppini C, Ozpolat B and
Calin GA: Non-coding RNAs regulation of macrophage polarization in
cancer. Mol Cancer. 20:242021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okada C, Yamashita E, Lee SJ, Shibata S,
Katahira J, Nakagawa A, Yoneda Y and Tsukihara T: A high-resolution
structure of the pre-microRNA nuclear export machinery. Science.
326:1275–1279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Gu H, Qin D, Yang L, Huang W,
Essandoh K, Wang Y, Caldwell CC, Peng T, Zingarelli B, et al:
Exosomal miR-223 contributes to mesenchymal stem Cell-elicited
cardioprotection in polymicrobial sepsis. Sci Rep. 5:137212015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ying W, Tseng A, Chang RC, Morin A, Brehm
T, Triff K, Nair V, Zhuang G, Song H, Kanameni S, et al:
MicroRNA-223 is a crucial mediator of PPARγ-regulated alternative
macrophage activation. J Clin Invest. 125:4149–4159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Derynck R, Turley SJ and Akhurst RJ: TGFβ
biology in cancer progression and immunotherapy. Nat Rev Clin
Oncol. 18:9–34. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Majumder S, Crabtree JS, Golde TE, Minter
LM, Osborne BA and Miele L: Targeting Notch in oncology: The path
forward. Nat Rev Drug Discov. 20:125–144. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang P, Zhang Y, Wang F, Qin M and Ren L:
MiRNA-205-5p regulates the ERBB4/AKT signaling pathway to inhibit
the proliferation and migration of HAVSMCs induced by ox-LDL.
Pathol Res Pract. 233:1538582022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu S, Cheng X, Wang R, Tan Y, Ge M, Li D,
Xu Q, Sun Y, Zhao C, Chen S and Liu H: Restoration of microRNA
function impairs MYC-dependent maintenance of MLL leukemia.
Leukemia. 34:2484–2488. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mittal A, Chitkara D, Behrman SW and
Mahato RI: Efficacy of gemcitabine conjugated and miRNA-205
complexed micelles for treatment of advanced pancreatic cancer.
Biomaterials. 35:7077–7087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wculek SK, Dunphy G, Heras-Murillo I,
Mastrangelo A and Sancho D: Metabolism of tissue macrophages in
homeostasis and pathology. Cell Mol Immunol. 19:384–408. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang N, Liang H and Zen K: Molecular
mechanisms that influence the macrophage m1-m2 polarization
balance. Front Immunol. 5:6142014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu YC, Zou XB, Chai YF and Yao YM:
Macrophage polarization in inflammatory diseases. Int J Biol Sci.
10:520–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martinez FO and Gordon S: The M1 and M2
paradigm of macrophage activation: Time for reassessment.
F1000Prime Rep. 6:132014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR
and Yang SM: Macrophages in tumor microenvironments and the
progression of tumors. Clin Dev Immunol. 2012:9480982012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liao X, Sharma N, Kapadia F, Zhou G, Lu Y,
Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, et
al: Krüppel-like factor 4 regulates macrophage polarization. J Clin
Invest. 121:2736–2749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Locati M, Mantovani A and Sica A:
Macrophage activation and polarization as an adaptive component of
innate immunity. Adv Immunol. 120:163–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou L, Cao X, Fang J, Li Y and Fan M:
Macrophages polarization is mediated by the combination of PRR
ligands and distinct inflammatory cytokines. Int J Clin Exp Pathol.
8:10964–10974. PubMed/NCBI
|
|
31
|
El KK and Stenmark KR: Contribution of
metabolic reprogramming to macrophage plasticity and function.
Semin Immunol. 27:267–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schultze JL, Schmieder A and Goerdt S:
Macrophage activation in human diseases. Semin Immunol. 27:249–256.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alharbi KS, Fuloria NK, Fuloria S, Rahman
SB, Al-Malki WH, Javed Shaikh MA, Thangavelu L, Singh SK, Rama Raju
Allam VS, Jha NK, et al: Nuclear factor-kappa B and its role in
inflammatory lung disease. Chem Biol Interact. 345:1095682021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ning H, Chen H, Deng J, Xiao C, Xu M, Shan
L, Yang C and Zhang Z: Exosomes secreted by FNDC5-BMMSCs protect
myocardial infarction by anti-inflammation and macrophage
polarization via NF-κB signaling pathway and Nrf2/HO-1 axis. Stem
Cell Res Ther. 12:5192021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu X, Wang Z, Shi J, Yu X, Li C, Liu J,
Zhang F, Chen H and Zheng W: Macrophage polarization toward M1
phenotype through NF-κB signaling in patients with Behçet's
disease. Arthritis Res Ther. 24:2492022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang S, Lu M, Wang W, Yu S, Yu R, Cai C,
Li Y, Shi Z, Zou J, He M, et al: Macrophage polarization modulated
by NF-κB in polylactide membranes-treated peritendinous adhesion.
Small. 18:e21041122022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu P, Shen P, Yu B, Xu X, Ge R, Cheng X,
Chen Q, Bian J, Li Z and Wang J: Janus kinases JAKs): The efficient
therapeutic targets for autoimmune diseases and myeloproliferative
disorders. Eur J Med Chem. 192:1121552020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang Y, Yang N, Pan G, Jin B, Wang S and
Ji W: Elevated IL-33 promotes expression of MMP2 and MMP9 via
activating STAT3 in alveolar macrophages during LPS-induced acute
lung injury. Cell Mol Biol Lett. 23:522018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yan T, Wang K, Li J, Hu H, Yang H, Cai M,
Liu R, Li H, Wang N, Shi Y, et al: Suppression of the hyaluronic
acid pathway induces M1 macrophages polarization via STAT1 in
glioblastoma. Cell Death Discov. 8:1932022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ye Q, Luo F and Yan T: Transcription
factor KLF4 regulated STAT1 to promote M1 polarization of
macrophages in rheumatoid arthritis. Aging (Albany NY).
14:5669–5680. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rinnenthal JL, Goebel HH, Preusse C,
Lebenheim L, Schumann M, Moos V, Schneider T, Heppner FL and
Stenzel W: Inflammatory myopathy with abundant macrophages (IMAM):
The immunology revisited. Neuromuscul Disord. 24:151–155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fan C, Chen C, Chen L, Chua KV, Hung H,
Hsu JT and Huang TS: Extracellular HSP90α Induces MyD88-IRAK
Complex-associated IKKα/β-NF-κB/IRF3 and JAK2/TYK2-STAT-3 signaling
in macrophages for tumor-promoting M2-polarization. Cells.
11:2292022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu Y, Wang L, Li S, Zhang T, Chen C, Hu
J, Sun D and Lu H: Mechanical stimulation improves rotator cuff
tendon-bone healing via activating IL-4/JAK/STAT signaling pathway
mediated macrophage M2 polarization. J Orthop Translat. 37:78–88.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang L and He C: Nrf2-mediated
anti-inflammatory polarization of macrophages as therapeutic
targets for osteoarthritis. Front Immunol. 13:9671932022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou W, Hu G, He J, Wang T, Zuo Y, Cao Y,
Zheng Q, Tu J, Ma J, Cai R, et al: SENP1-Sirt3 signaling promotes
α-ketoglutarate production during M2 macrophage polarization. Cell
Rep. 39:1106602022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu L, Li S, Li H, Lai B and Wen H:
Interferon regulatory factor 4 (IRF4) promotes
lipopolysaccharide-induced colonic mucosal epithelial cell
proliferation by regulating macrophage polarization. Eur Surg Res.
63:257–268. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hedl M, Yan J and Abraham C: IRF5 and IRF5
Disease-risk variants increase glycolysis and human m1 macrophage
polarization by regulating proximal signaling and akt2 activation.
Cell Rep. 16:2442–2455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yuan J, Lin F, Chen L, Chen W, Pan X, Bai
Y, Cai Y and Lu H: Lipoxin A4 regulates M1/M2 macrophage
polarization via FPR2–IRF pathway. Inflammopharmacology.
30:487–498. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yuan Q, Zhao B, Cao YH, Yan JC, Sun LJ,
Liu X, Xu Y, Wang XY and Wang B: BCR-associated Protein 31
regulates macrophages polarization and wound healing function via
early growth response 2/C/EBPβ and IL-4Rα/C/EBPβ pathways. J
Immunol. 209:1059–1070. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Arranz A, Doxaki C, Vergadi E, Martinez De
La Torre Y, Vaporidi K, Lagoudaki ED, Ieronymaki E, Androulidaki A,
Venihaki M, Margioris AN, et al: Akt1 and Akt2 protein kinases
differentially contribute to macrophage polarization. Proc Natl
Acad Sci USA. 109:9517–9522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vaghf A, Khansarinejad B, Ghaznavi-Rad E
and Mondanizadeh M: The role of microRNAs in diseases and related
signaling pathways. Mol Biol Rep. 49:6789–6801. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Panni S, Lovering RC, Porras P and Orchard
S: Non-coding RNA regulatory networks. Biochim Biophys Acta Gene
Regul Mech. 1863:1944172020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu ZP, Wu C, Miao H and Wu H: RegNetwork:
An integrated database of transcriptional and post-transcriptional
regulatory networks in human and mouse. Database (Oxford).
2015:bav0952015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gov E and Arga KY: Interactive cooperation
and hierarchical operation of microRNA and transcription factor
crosstalk in human transcriptional regulatory network. Iet Syst
Biol. 10:219–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Saliminejad K, Khorram KH, Soleymani FS
and Ghaffari SH: An overview of microRNAs: Biology, functions,
therapeutics, and analysis methods. J Cell Physiol. 234:5451–5465.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ghafouri-Fard S, Abak A, Tavakkoli AS,
Shoorei H, Taheri M and Samadian M: The impact of non-coding RNAs
on macrophage polarization. Biomed Pharmacother. 142:1121122021.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Saradna A, Do DC, Kumar S, Fu QL and Gao
P: Macrophage polarization and allergic asthma. Transl Res.
191:1–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Viktoriia K, Polina V, Andrey E, Timur F
and Gennady S: Biochemical and molecular inducers and modulators of
M2 macrophage polarization in clinical perspective. Int
Immunopharmacol. 122:1105832023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cai X, Yin Y, Li N, Zhu D, Zhang J, Zhang
CY and Zen K: Re-polarization of tumor-associated macrophages to
pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol.
4:341–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fei Y, Wang Z, Huang M, Wu X, Hu F, Zhu J,
Yu Y, Shen H, Wu Y, Xie G and Zhou Z: MiR-155 regulates M2
polarization of hepatitis B virus-infected tumor-associated
macrophages which in turn regulates malignant progression of
hepatocellular carcinoma. J Viral Hepat. 30:417–426. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bi J, Liu J, Chen X, Shi N, Wu H, Tang H
and Mao J: MiR-155-5p-SOCS1/JAK1/STAT1 participates in hepatic
lymphangiogenesis in liver fibrosis and cirrhosis by regulating M1
macrophage polarization. Hum Exp Toxicol. 42:96032712211416952023.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang HT, Li LL, Li SN, Wu JT, Chen K, Song
WF, Zhang GB, Ma JF, Fu HX, Cao S, et al: MicroRNA-155 inhibition
attenuates myocardial infarction-induced connexin 43 degradation in
cardiomyocytes by reducing pro-inflammatory macrophage activation.
Cardiovasc Diagn Ther. 12:325–339. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yan C, Zhou Q, Wu J, Xu N, Du Y, Li J, Liu
JX, Koda S, Zhang BB, Yu Q, et al: Csi-let-7a-5p delivered by
extracellular vesicles from a liver fluke activates M1-like
macrophages and exacerbates biliary injuries. Proc Natl Acad Sci
USA. 118:e21022061182021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Song M, Cui X, Zhang J, Li Y, Li J, Zang
Y, Li Q, Yang Q, Chen Y, Cai W, et al: Shenlian extract attenuates
myocardial ischaemia-reperfusion injury via inhibiting M1
macrophage polarization by silencing miR-155. Pharm Biol.
60:2011–2024. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gwiggner M, Martinez-Nunez RT, Whiteoak
SR, Bondanese VP, Claridge A, Collins JE, Cummings JRF and
Sanchez-Elsner T: MicroRNA-31 and MicroRNA-155 are overexpressed in
ulcerative colitis and regulate IL-13 signaling by targeting
interleukin 13 receptor α-1. Genes (Basel). 9:852018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhong C, Tao B, Yang F, Xia K, Yang X,
Chen L, Peng T, Xia X, Li X and Peng L: Histone demethylase JMJD1C
promotes the polarization of M1 macrophages to prevent glioma by
upregulating miR-302a. Clin Transl Med. 11:e4242021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Guo Q, Zhu X, Wei R, Zhao L, Zhang Z, Yin
X, Zhang Y, Chu C, Wang B and Li X: miR-130b-3p regulates M1
macrophage polarization via targeting IRF1. J Cell Physiol.
236:2008–2022. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gao M, Wang X, Zhang X, Ha T, Ma H, Liu L,
Kalbfleisch JH, Gao X, Kao RL, Williams DL and Li C: Attenuation of
cardiac dysfunction in polymicrobial sepsis by MicroRNA-146a is
mediated via targeting of IRAK1 and TRAF6 expression. J Immunol.
195:672–682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cui W, Zhou S, Wang Y, Shi X and Liu H:
Cadmium exposure activates the PI3K/AKT signaling pathway through
miRNA-21, induces an increase in M1 polarization of macrophages,
and leads to fibrosis of pig liver tissue. Ecotoxicol Environ Saf.
228:1130152021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Thulin P, Wei T, Werngren O, Cheung L,
Fisher RM, Grandér D, Corcoran M and Ehrenborg E: MicroRNA-9
regulates the expression of peroxisome proliferator-activated
receptor delta in human monocytes during the inflammatory response.
Int J Mol Med. 31:1003–1010. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tong F, Mao X, Zhang S, Xie H, Yan B, Wang
B, Sun J and Wei L: HPV + HNSCC-derived exosomal miR-9 induces
macrophage M1 polarization and increases tumor radiosensitivity.
Cancer Lett. 478:34–44. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hu A, Chen X, Bi Q, Xiang Y, Jin R, Ai H
and Nie Y: A parallel and cascade control system: Magnetofection of
miR-125b for synergistic tumor-association macrophage polarization
regulation and tumor cell suppression in breast cancer treatment.
Nanoscale. 12:22615–22627. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Luo XB, Li LT, Xi JC, Liu HT, Liu Z, Yu L
and Tang PF: Negative pressure promotes macrophage M1 polarization
after Mycobacterium tuberculosis infection via the lncRNA
XIST/microRNA-125b-5p/A20/NF-κB axis. Ann N Y Acad Sci.
1514:116–131. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bao P, Zhao W, Mou M and Liu X:
MicroRNA-21 mediates bone marrow mesenchymal stem cells protection
of radiation-induced lung injury during the acute phase by
regulating polarization of alveolar macrophages. Transl Cancer Res.
9:231–239. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xue J, Xiao T, Wei S, Sun J, Zou Z, Shi M,
Sun Q, Dai X, Wu L, Li J, et al: miR-21-regulated M2 polarization
of macrophage is involved in arsenicosis-induced hepatic fibrosis
through the activation of hepatic stellate cells. J Cell Physiol.
236:6025–6041. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li Z, Yang B, Gao M, Xiao X, Zhao S and
Liu Z: Naringin improves sepsis-induced intestinal injury by
modulating macrophage polarization via PPARγ/miR-21 axis. Mol Ther
Nucleic Acids. 25:502–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yao M, Cui B, Zhang W, Ma W, Zhao G and
Xing L: Exosomal miR-21 secreted by IL-1beta-primed-mesenchymal
stem cells induces macrophage M2 polarization and ameliorates
sepsis. Life Sci. 264:1186582021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lu J, Xie L and Sun S: The inhibitor
miR-21 regulates macrophage polarization in an experimental model
of chronic obstructive pulmonary disease. Tob Induc Dis. 19:1–10.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang Z, Brandt S, Medeiros A, Wang S, Wu
H, Dent A and Serezani CH: MicroRNA 21 is a homeostatic regulator
of macrophage polarization and prevents prostaglandin E2-mediated
M2 generation. PLoS One. 10:e1158552015.
|
|
81
|
Sheedy FJ: Turning 21: Induction of miR-21
as a key switch in the inflammatory response. Front Immunol.
6:192015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
An Y and Yang Q: MiR-21 modulates the
polarization of macrophages and increases the effects of M2
macrophages on promoting the chemoresistance of ovarian cancer.
Life Sci. 242:1171622020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Jin J and Yu G: Hypoxic lung cancer
cell-derived exosomal miR-21 mediates macrophage M2 polarization
and promotes cancer cell proliferation through targeting IRF1.
World J Surg Oncol. 20:2412022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lin F, Yin HB, Li XY, Zhu GM, He WY and
Gou X: Bladder cancer cell-secreted exosomal miR-21 activates the
PI3K/AKT pathway in macrophages to promote cancer progression. Int
J Oncol. 56:151–164. 2020.PubMed/NCBI
|
|
85
|
Ma C, He D, Tian P, Wang Y, He Y, Wu Q,
Jia Z, Zhang X, Zhang P, Ying H, et al: miR-182 targeting
reprograms tumor-associated macrophages and limits breast cancer
progression. Proc Natl Acad Sci USa. 119:e21140061192022.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X,
Gao L, Xie J and Xu B: Mesenchymal stromal cell-derived exosomes
attenuate myocardial ischaemia-reperfusion injury through
miR-182-regulated macrophage polarization. Cardiovasc Res.
115:1205–1216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Huang C, Liu XJ, Qun Zhou, Xie J, Ma TT,
Meng XM and Li J: MiR-146a modulates macrophage polarization by
inhibiting Notch1 pathway in RAW264.7 macrophages. Int
Immunopharmacol. 32:46–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Peng X, He F, Mao Y, Lin Y, Fang J, Chen
Y, Sun Z, Zhuo Y and Jiang J: miR-146a promotes M2 macrophage
polarization and accelerates diabetic wound healing by inhibiting
the TLR4/NF-κB axis. J Mol Endocrinol. 69:315–327. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Schulert GS, Fall N, Harley JB, Shen N,
Lovell DJ, Thornton S and Grom AA: Monocyte MicroRNA expression in
active systemic juvenile idiopathic arthritis implicates
MicroRNA-125a-5p in polarized monocyte phenotypes. Arthritis
Rheumatol. 68:2300–2313. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ma J, Chen L, Zhu X, Li Q, Hu L and Li H:
Mesenchymal stem cell-derived exosomal miR-21a-5p promotes M2
macrophage polarization and reduces macrophage infiltration to
attenuate atherosclerosis. Acta Biochim Biophys Sin (Shanghai).
53:1227–1236. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chen J, Zhang K, Zhi Y, Wu Y, Chen B, Bai
J and Wang X: Tumor-derived exosomal miR-19b-3p facilitates M2
macrophage polarization and exosomal LINC00273 secretion to promote
lung adenocarcinoma metastasis via Hippo pathway. Clin Transl Med.
11:e4782021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhao G, Yu H, Ding L, Wang W, Wang H, Hu
Y, Qin L, Deng G, Xie B, Li G and Qi L: microRNA-27a-3p delivered
by extracellular vesicles from glioblastoma cells induces M2
macrophage polarization via the EZH1/KDM3A/CTGF axis. Cell Death
Discov. 8:2602022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Pan Y, Hui X, Hoo R, Ye D, Chan C, Feng T,
Wang Y, Lam KSL and Xu A: Adipocyte-secreted exosomal microRNA-34a
inhibits M2 macrophage polarization to promote obesity-induced
adipose inflammation. J Clin Invest. 129:834–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhang Y, Zhang M, Zhong M, Suo Q and Lv K:
Expression profiles of miRNAs in polarized macrophages. Int J Mol
Med. 31:797–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Graff JW, Dickson AM, Clay G, McCaffrey AP
and Wilson ME: Identifying functional microRNAs in macrophages with
polarized phenotypes. J Biol Chem. 287:21816–21825. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Curtale G, Rubino M and Locati M:
MicroRNAs as molecular switches in macrophage activation. Front
Immunol. 10:7992019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Martinez-Nunez RT, Louafi F and
Sanchez-Elsner T: The interleukin 13 (IL-13) pathway in human
macrophages is modulated by microRNA-155 via direct targeting of
interleukin 13 receptor alpha1 (IL13Ralpha1). J Biol Chem.
286:1786–1794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang Y, Zhang M, Li X, Tang Z, Wang X,
Zhong M, Suo Q, Zhang Y and Lv K: Silencing MicroRNA-155 attenuates
cardiac injury and dysfunction in viral myocarditis via promotion
of M2 phenotype polarization of macrophages. Sci Rep. 6:226132016.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ying H, Kang Y, Zhang H, Zhao D, Xia J, Lu
Z, Wang H, Xu F and Shi L: MiR-127 modulates macrophage
polarization and promotes lung inflammation and injury by
activating the JNK pathway. J Immunol. 194:1239–1251. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chaudhuri AA, So AY, Sinha N, Gibson WS,
Taganov KD, O'Connell RM and Baltimore D: MicroRNA-125b potentiates
macrophage activation. J Immunol. 187:5062–5068. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhong Y and Yi C: MicroRNA-720 suppresses
M2 macrophage polarization by targeting GATA3. Biosci Rep.
36:e003632016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Cobos JV, Bradley EJ, Willemsen AM, van
Kampen AH, Baas F and Kootstra NA: Next-generation sequencing of
microRNAs uncovers expression signatures in polarized macrophages.
Physiol Genomics. 46:91–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Squadrito ML, Pucci F, Magri L, Moi D,
Gilfillan GD, Ranghetti A, Casazza A, Mazzone M, Lyle R, Naldini L
and De Palma M: miR-511-3p modulates genetic programs of
tumor-associated macrophages. Cell Rep. 1:141–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Liu Y, Chen Q, Song Y, Lai L, Wang J, Yu
H, Cao X and Wang Q: MicroRNA-98 negatively regulates IL-10
production and endotoxin tolerance in macrophages after LPS
stimulation. Febs Lett. 585:1963–1968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhuang G, Meng C, Guo X, Cheruku PS, Shi
L, Xu H, Li H, Wang G, Evans AR, Safe S, et al: A novel regulator
of macrophage activation: miR-223 in obesity-associated adipose
tissue inflammation. Circulation. 125:2892–2903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhang W, Liu H, Liu W, Liu Y and Xu J:
Polycomb-mediated loss of microRNA let-7c determines inflammatory
macrophage polarization via PAK1-dependent NF-kappaB pathway. Cell
Death Differ. 22:287–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Banerjee S, Xie N, Cui H, Tan Z, Yang S,
Icyuz M, Abraham E and Liu G: MicroRNA let-7c regulates macrophage
polarization. J Immunol. 190:6542–6549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ma S, Liu M, Xu Z, Li Y, Guo H, Ge Y, Liu
Y, Zheng D and Shi J: A double feedback loop mediated by
microRNA-23a/27a/24-2 regulates M1 versus M2 macrophage
polarization and thus regulates cancer progression. Oncotarget.
7:13502–13519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wu XQ, Dai Y, Yang Y, Huang C, Meng XM, Wu
BM and Li J: Emerging role of microRNAs in regulating macrophage
activation and polarization in immune response and inflammation.
Immunology. 148:237–248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Alam MM and O'Neill LA: MicroRNAs and the
resolution phase of inflammation in macrophages. Eur J Immunol.
41:2482–2485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Liu G and Abraham E: MicroRNAs in immune
response and macrophage polarization. Arterioscler Thromb Vasc
Biol. 33:170–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Bi Y, Liu G and Yang R: MicroRNAs: Novel
regulators during the immune response. J Cell Physiol. 218:467–472.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wu XQ, Huang C, Liu XH and Li J: MicroRNA
let-7a: A novel therapeutic candidate in prostate cancer. Asian J
Androl. 16:327–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Thomas M, Lieberman J and Lal A:
Desperately seeking microRNA targets. Nat Struct Mol Biol.
17:1169–1174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhou X, Chen B, Zhang Z, Huang Y, Li J,
Wei Q, Cao D and Ai J: Crosstalk between Tumor-associated
macrophages and MicroRNAs: A key role in tumor microenvironment.
Int J Mol Sci. 23:132582022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang C, Han X, Yang L, Fu J, Sun C, Huang
S, Xiao W, Gao Y, Liang Q, Wang X, et al: Circular RNA circPPM1F
modulates M1 macrophage activation and pancreatic islet
inflammation in type 1 diabetes mellitus. Theranostics.
10:10908–10924. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
van Zandwijk N, Pavlakis N, Kao SC, Linton
A, Boyer MJ, Clarke S, Huynh Y, Chrzanowska A, Fulham MJ, Bailey
DL, et al: Safety and activity of microRNA-loaded minicells in
patients with recurrent malignant pleural mesothelioma: A
first-in-man, phase 1, open-label, dose-escalation study. Lancet
Oncol. 18:1386–1396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Cortez MA, Anfossi S, Ramapriyan R, Menon
H, Atalar SC, Aliru M, Welsh J and Calin GA: Role of miRNAs in
immune responses and immunotherapy in cancer. Genes Chromosomes
Cancer. 58:244–253. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Shah MY, Ferrajoli A, Sood AK,
Lopez-Berestein G and Calin GA: microRNA therapeutics in cancer-an
emerging concept. EBioMedicine. 12:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Smolle MA, Calin HN, Pichler M and Calin
GA: Noncoding RNAs and immune checkpoints-clinical implications as
cancer therapeutics. FEBS J. 284:1952–1966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Li D, Yan M, Sun F, Song J, Hu X, Yu S,
Tang L and Deng S: miR-498 inhibits autophagy and M2-like
polarization of tumor-associated macrophages in esophageal cancer
via MDM2/ATF3. Epigenomics. 13:1013–1030. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Garofalo M and Croce CM: MicroRNAs as
therapeutic targets in chemoresistance. Drug Resist Updat.
16:47–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Jiang Q, Yuan Y, Gong Y, Luo X, Su X, Hu X
and Zhu W: Therapeutic delivery of microRNA-143 by cationic
lipoplexes for non-small cell lung cancer treatment in vivo. J
Cancer Res Clin Oncol. 145:2951–2967. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Yang J, Zhang Z, Chen C, Liu Y, Si Q,
Chuang TH, Li N, Gomez-Cabrero A, Reisfeld RA, Xiang R and Luo Y:
MicroRNA-19a-3p inhibits breast cancer progression and metastasis
by inducing macrophage polarization through downregulated
expression of Fra-1 proto-oncogene. Oncogene. 33:3014–3023. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zhang J, Shan WF, Jin TT, Wu GQ, Xiong XX,
Jin HY and Zhu SM: Propofol exerts anti-hepatocellular carcinoma by
microvesicle-mediated transfer of miR-142-3p from macrophage to
cancer cells. J Transl Med. 12:2792014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Syed SN, Frank AC, Raue R and Brune B:
MicroRNA-A tumor trojan horse for tumor-associated macrophages.
Cells. 8:14822019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Li Y, Zhao L, Shi B, Ma S, Xu Z, Ge Y, Liu
Y, Zheng D and Shi J: Functions of miR-146a and miR-222 in
Tumor-associated macrophages in breast cancer. Sci Rep.
5:186482015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Pirlog R, Cismaru A, Nutu A and
Berindan-Neagoe I: Field Cancerization in NSCLC: A new perspective
on MicroRNAs in macrophage polarization. Int J Mol Sci. 22:7462021.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Jang JY, Lee JK, Jeon YK and Kim CW:
Exosome derived from epigallocatechin gallate treated breast cancer
cells suppresses tumor growth by inhibiting tumor-associated
macrophage infiltration and M2 polarization. BMC Cancer.
13:4212013. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wang T, Xu X, Xu Q, Ren J, Shen S, Fan C
and Hou Y: miR-19a promotes colitis-associated colorectal cancer by
regulating tumor necrosis factor alpha-induced protein 3-NF-κB
feedback loops. Oncogene. 36:3240–3251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Hsieh CH, Tai SK and Yang MH:
Snail-overexpressing cancer cells promote M2-like polarization of
tumor-associated macrophages by delivering MiR-21-abundant
Exosomes. Neoplasia. 20:775–788. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Cai J, Qiao B, Gao N, Lin N and He W: Oral
squamous cell carcinoma-derived exosomes promote M2 subtype
macrophage polarization mediated by exosome-enclosed miR-29a-3p. Am
J Physiol Cell Physiol. 316:C731–C740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhang L, Liao Y and Tang L: MicroRNA-34
family: A potential tumor suppressor and therapeutic candidate in
cancer. J Exp Clin Cancer Res. 38:532019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Rokavec M, Oner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Xu S, Wei J, Wang F, Kong LY, Ling XY,
Nduom E, Gabrusiewicz K, Doucette T, Yang Y, Yaghi NK, et al:
Effect of miR-142-3p on the M2 macrophage and therapeutic efficacy
against murine glioblastoma. J Natl Cancer Inst. 106:dju1622014.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Shinohara H, Kuranaga Y, Kumazaki M,
Sugito N, Yoshikawa Y, Takai T, Taniguchi K, Ito Y and Akao Y:
Regulated polarization of tumor-associated macrophages by miR-145
via colorectal cancer-derived extracellular vesicles. J Immunol.
199:1505–1515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Sethupathy P, Borel C, Gagnebin M, Grant
GR, Deutsch S, Elton TS, Hatzigeorgiou AG and Antonarakis SE: Human
microRNA-155 on chromosome 21 differentially interacts with its
polymorphic target in the AGTR1 3′untranslated region: A mechanism
for functional single-nucleotide polymorphisms related to
phenotypes. Am J Hum Genet. 81:405–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Lin ZY, Huang YQ, Zhang YQ, Han ZD, He HC,
Ling XH, Fu X, Dai QS, Cai C, Chen JH, et al: MicroRNA-224 inhibits
progression of human prostate cancer by downregulating TRIB1. Int J
Cancer. 135:541–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wang X, Luo G, Zhang K, Cao J, Huang C,
Jiang T, Liu B, Su L and Qiu Z: Hypoxic Tumor-derived exosomal
miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to
promote pancreatic cancer metastasis. Cancer Res. 78:4586–4598.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Xing F, Liu Y, Wu SY, Wu K, Sharma S, Mo
YY, Feng J, Sanders S, Jin G, Singh R, et al: Loss of XIST in
breast cancer activates MSN-c-Met and reprograms microglia via
exosomal miRNA to promote brain metastasis. Cancer Res.
78:4316–4330. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Qian M, Wang S, Guo X, Wang J, Zhang Z,
Qiu W, Gao X, Chen Z, Xu J, Zhao R, et al: Hypoxic glioma-derived
exosomes deliver microRNA-1246 to induce M2 macrophage polarization
by targeting TERF2IP via the STAT3 and NF-κB pathways. Oncogene.
39:428–442. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Kanlikilicer P, Bayraktar R, Denizli M,
Rashed MH, Ivan C, Aslan B, Mitra R, Karagoz K, Bayraktar E, Zhang
X, et al: Exosomal miRNA confers chemo resistance via targeting
Cav1/p-gp/M2-type macrophage axis in ovarian cancer. EBioMedicine.
38:100–112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Wang Z, Xu L, Hu Y, Huang Y, Zhang Y,
Zheng X, Wang S, Wang Y, Yu Y, Zhang M, et al: miRNA let-7b
modulates macrophage polarization and enhances tumor-associated
macrophages to promote angiogenesis and mobility in prostate
cancer. Sci Rep. 6:256022016. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Shin JI and Brusselle GG: Mechanistic
links between COPD and lung cancer: A role of microRNA let-7? Nat
Rev Cancer. 14:702014. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Guo L, Cheng X, Chen H, Chen C, Xie S,
Zhao M, Liu D, Deng Q, Liu Y, Wang X, et al: Induction of breast
cancer stem cells by M1 macrophages through Lin-28B-let-7-HMGA2
axis. Cancer Lett. 452:213–225. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Jerome T, Laurie P, Louis B and Pierre C:
Enjoy the silence: The story of let-7 MicroRNA and cancer. Curr
Genomics. 8:229–233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Xun J, Du L, Gao R, Shen L, Wang D, Kang
L, Chen C, Zhang Z, Zhang Y, Yue S, et al: Cancer-derived exosomal
miR-138-5p modulates polarization of tumor-associated macrophages
through inhibition of KDM6B. Theranostics. 11:6847–6859. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Xu H, Li M, Pan Z, Zhang Z, Gao Z, Zhao R,
Li B, Qi Y, Qiu W, Guo Q, et al: miR-3184-3p enriched in
cerebrospinal fluid exosomes contributes to progression of glioma
and promotes M2-like macrophage polarization. Cancer Sci.
113:2668–2680. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Entezari M, Sadrkhanloo M, Rashidi M,
Asnaf SE, Taheriazam A, Hashemi M, Ashrafizadeh M, Zarrabi A,
Rabiee N, Hushmandi K, et al: Non-coding RNAs and macrophage
interaction in tumor progression. Crit Rev Oncol Hematol.
173:1036802022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J,
Xuan Z, Fang L, Yang J, Zhang L, et al: Gastric cancer-derived
exosomal miR-519a-3p promotes liver metastasis by inducing
intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin
Cancer Res. 41:2962022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ma J, Chen S, Liu Y, Han H, Gong M and
Song Y: The role of exosomal miR-181b in the crosstalk between
NSCLC cells and tumor-associated macrophages. Genes Genomics.
44:1243–1258. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Shi L, Cao Y, Yuan W, Guo J and Sun G:
Exosomal circRNA BTG2 derived from RBP-J overexpressed-macrophages
inhibits glioma progression via miR-25-3p/PTEN. Cell Death Dis.
13:5062022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chuang HY, Su YK, Liu HW, Chen CH, Chiu
SC, Cho DY, Lin SZ, Chen YS and Lin CM: Preclinical evidence of
STAT3 inhibitor pacritinib overcoming temozolomide resistance via
downregulating miR-21-enriched exosomes from M2
Glioblastoma-associated macrophages. J Clin Med. 8:9592019.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Wang T, Ren Y, Liu R, Ma J, Shi Y, Zhang L
and Bu R: miR-195-5p suppresses the proliferation, migration, and
invasion of oral squamous cell carcinoma by targeting TRIM14.
Biomed Res Int. 2017:73781482017. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Pakravan G, Foroughmand AM, Peymani M,
Ghaedi K, Hashemi MS, Hajjari M and Nasr-Esfahani MH:
Downregulation of miR-130a, antagonized doxorubicin-induced
cardiotoxicity via increasing the PPARγ expression in mESCs-derived
cardiac cells. Cell Death Dis. 9:7582018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Anandappa G, Lampis A, Cunningham D, Khan
KH, Kouvelakis K, Vlachogiannis G, Hedayat S, Tunariu N, Rao S,
Watkins D, et al: miR-31-3p expression and benefit from Anti-EGFR
inhibitors in metastatic colorectal cancer patients enrolled in the
prospective phase II PROSPECT-C trial. Clin Cancer Res.
25:3830–3838. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Sokilde R, Persson H, Ehinger A, Pirona
AC, Ferno M, Hegardt C, Larsson C, Loman N, Malmberg M, Rydén L, et
al: Refinement of breast cancer molecular classification by miRNA
expression profiles. BMC Genomics. 20:5032019. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Binenbaum Y, Fridman E, Yaari Z, Milman N,
Schroeder A, Ben David G, Shlomi T and Gil Z: Transfer of miRNA in
Macrophage-derived exosomes induces drug resistance in pancreatic
adenocarcinoma. Cancer Res. 78:5287–5299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Moradi-Chaleshtori M, Shojaei S,
Mohammadi-Yeganeh S and Hashemi SM: Transfer of miRNA in
tumor-derived exosomes suppresses breast tumor cell invasion and
migration by inducing M1 polarization in macrophages. Life Sci.
282:1198002021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Chen C, Liu JM and Luo YP: MicroRNAs in
tumor immunity: Functional regulation in tumor-associated
macrophages. J Zhejiang Univ Sci B. 21:12–28. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Qiao L, Dong C, Jia W and Ma B: Exosomal
miR-655-3p inhibits growth, and invasion and macrophage M2
polarization through targeting CXCR4 in papillary thyroid
carcinoma. Acta Biochim Pol. 69:773–779. 2022.PubMed/NCBI
|
|
163
|
Zhao M, Zhuang A and Fang Y:
Cancer-associated fibroblast-derived exosomal miRNA-320a promotes
macrophage M2 polarization in vitro by regulating PTEN/PI3Kγ
signaling in pancreatic cancer. J Oncol. 2022:95146972022.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Hong S, You JY, Paek K, Park J, Kang SJ,
Han EH, Choi N, Chung S, Rhee WJ and Kim JA: Inhibition of tumor
progression and M2 microglial polarization by extracellular
vesicle-mediated microRNA-124 in a 3D microfluidic glioblastoma
microenvironment. Theranostics. 11:9687–9704. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Labonte AC, Tosello-Trampont AC and Hahn
YS: The role of macrophage polarization in infectious and
inflammatory diseases. Mol Cells. 37:275–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Bashir S, Sharma Y, Elahi A and Khan F:
Macrophage polarization: The link between inflammation and related
diseases. Inflamm Res. 65:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Biswas SK, Chittezhath M, Shalova IN and
Lim JY: Macrophage polarization and plasticity in health and
disease. Immunol Res. 53:11–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Hawiger J, Veach RA and Zienkiewicz J: New
paradigms in sepsis: From prevention to protection of failing
microcirculation. J Thromb Haemost. 13:1743–1756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Mayr FB, Yende S and Angus DC:
Epidemiology of severe sepsis. Virulence. 5:4–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Martin GS: Sepsis, severe sepsis and
septic shock: Changes in incidence, pathogens and outcomes. Expert
Rev Anti Infect Ther. 10:701–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Melamed A and Sorvillo FJ: The burden of
sepsis-associated mortality in the United States from 1999 to 2005:
An analysis of multiple-cause-of-death data. Crit Care. 13:R282009.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Gentile LF, Cuenca AG, Efron PA, Ang D,
Bihorac A, McKinley BA, Moldawer LL and Moore FA: Persistent
inflammation and immunosuppression: A common syndrome and new
horizon for surgical intensive care. J Trauma Acute Care Surg.
72:1491–1501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Essandoh K and Fan GC: Role of
extracellular and intracellular microRNAs in sepsis. Biochim
Biophys Acta. 1842:2155–2162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Tsujimoto H, Ono S, Efron PA, Scumpia PO,
Moldawer LL and Mochizuki H: Role of Toll-like receptors in the
development of sepsis. Shock. 29:315–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Cristofaro P and Opal SM: The Toll-like
receptors and their role in septic shock. Expert Opin Ther Targets.
7:603–612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Savva A and Roger T: Targeting Toll-like
receptors: Promising therapeutic strategies for the management of
sepsis-associated pathology and infectious diseases. Front Immunol.
4:3872013. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Weighardt H and Holzmann B: Role of
Toll-like receptor responses for sepsis pathogenesis.
Immunobiology. 212:715–722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Foley NM, Wang J, Redmond HP and Wang JH:
Current knowledge and future directions of TLR and NOD signaling in
sepsis. Mil Med Res. 2:12015.PubMed/NCBI
|
|
179
|
Salomao R, Martins PS, Brunialti MK,
Fernandes ML, Martos LS, Mendes ME, Gomes NE and Rigato O: TLR
signaling pathway in patients with sepsis. Shock. 30 (Suppl
1):S73–S77. 2008. View Article : Google Scholar
|
|
180
|
Wang JF, Yu ML, Yu G, Bian JJ, Deng XM,
Wan XJ and Zhu KM: Serum miR-146a and miR-223 as potential new
biomarkers for sepsis. Biochem Biophys Res Commun. 394:184–188.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Fabian B, Sanchari R, Christian T,
Christoph R and Tom L: Circulating MicroRNAs as Biomarkers for
Sepsis. Int J Mol Sci. 17:782016. View Article : Google Scholar
|
|
182
|
Arner P and Kulyte A: MicroRNA regulatory
networks in human adipose tissue and obesity. Nat Rev Endocrinol.
11:276–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Monteiro R and Azevedo I: Chronic
inflammation in obesity and the metabolic syndrome. Mediators
Inflamm. 2010:2896452010. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Xu H, Barnes GT, Yang Q, Tan G, Yang D,
Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA and Chen H:
Chronic inflammation in fat plays a crucial role in the development
of obesity-related insulin resistance. J Clin Invest.
112:1821–1830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Gregor MF and Hotamisligil GS:
Inflammatory mechanisms in obesity. Annu Rev Immunol. 29:415–445.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Nishimura S, Manabe I and Nagai R: Adipose
tissue inflammation in obesity and metabolic syndrome. Discov Med.
8:55–60. 2009.PubMed/NCBI
|
|
187
|
Bastard JP, Maachi M, Lagathu C, Kim MJ,
Caron M, Vidal H, Capeau J and Feve B: Recent advances in the
relationship between obesity, inflammation, and insulin resistance.
Eur Cytokine Netw. 17:4–12. 2006.PubMed/NCBI
|
|
188
|
Moore CS, Rao VT, Durafourt BA, Bedell BJ,
Ludwin SK, Bar-Or A and Antel JP: miR-155 as a multiple
sclerosis-relevant regulator of myeloid cell polarization. Ann
Neurol. 74:709–720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Ponomarev ED, Veremeyko T, Barteneva N,
Krichevsky AM and Weiner HL: MicroRNA-124 promotes microglia
quiescence and suppresses EAE by deactivating macrophages via the
C/EBP-alpha-PU.1 pathway. Nat Med. 17:64–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Karunakaran D, Richards L, Geoffrion M,
Barrette D, Gotfrit RJ, Harper ME and Rayner KJ: Therapeutic
inhibition of miR-33 promotes fatty acid oxidation but does not
ameliorate metabolic dysfunction in diet-induced obesity.
Arterioscler Thromb Vasc Biol. 35:2536–2543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Yang Y, Yang L, Liang X and Zhu G:
MicroRNA-155 promotes atherosclerosis inflammation via targeting
SOCS1. Cell Physiol Biochem. 36:1371–1381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Yu F, Jia X, Du F, Wang J, Wang Y, Ai W
and Fan D: miR-155-deficient bone marrow promotes tumor metastasis.
Mol Cancer Res. 11:923–936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Ouimet M, Ediriweera HN, Gundra UM, Sheedy
FJ, Ramkhelawon B, Hutchison SB, Rinehold K, van Solingen C,
Fullerton MD, Cecchini K, et al: MicroRNA-33-dependent regulation
of macrophage metabolism directs immune cell polarization in
atherosclerosis. J Clin Invest. 125:4334–4348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Zhu QY, Liu Q, Chen JX, Lan K and Ge BX:
MicroRNA-101 targets MAPK phosphatase-1 to regulate the activation
of MAPKs in macrophages. J Immunol. 185:7435–7442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Gao Y, Liu F, Fang L, Cai R, Zong C and Qi
Y: Genkwanin inhibits proinflammatory mediators mainly through the
regulation of miR-101/MKP-1/MAPK pathway in LPS-activated
macrophages. PLoS One. 9:e967412014. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Liu G, Friggeri A, Yang Y, Park YJ,
Tsuruta Y and Abraham E: miR-147, a microRNA that is induced upon
Toll-like receptor stimulation, regulates murine macrophage
inflammatory responses. Proc Natl Acad Sci USA. 106:15819–15824.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Wei J, Huang X, Zhang Z, Jia W, Zhao Z,
Zhang Y, Liu X and Xu G: MyD88 as a target of microRNA-203 in
regulation of lipopolysaccharide or Bacille Calmette-Guerin induced
inflammatory response of macrophage RAW264.7 cells. Mol Immunol.
55:303–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Xie N, Cui H, Banerjee S, Tan Z, Salomao
R, Fu M, Abraham E, Thannickal VJ and Liu G: miR-27a regulates
inflammatory response of macrophages by targeting IL-10. J Immunol.
193:327–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Lv LL, Feng Y, Wu M, Wang B, Li ZL, Zhong
X, Wu WJ, Chen J, Ni HF, Tang TT, et al: Exosomal miRNA-19b-3p of
tubular epithelial cells promotes M1 macrophage activation in
kidney injury. Cell Death Differ. 27:210–226. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Arora S, Dev K, Agarwal B, Das P and Syed
MA: Macrophages: Their role, activation and polarization in
pulmonary diseases. Immunobiology. 223:383–396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Qiu M, Xu E and Zhan L: Epigenetic
regulations of Microglia/macrophage polarization in ischemic
stroke. Front Mol Neurosci. 14:6974162021. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Dang CP and Leelahavanichkul A:
Over-expression of miR-223 induces M2 macrophage through glycolysis
alteration and attenuates LPS-induced sepsis mouse model, the
cell-based therapy in sepsis. PLoS One. 15:e2360382020. View Article : Google Scholar
|
|
203
|
Li B, Dasgupta C, Huang L, Meng X and
Zhang L: MiRNA-210 induces microglial activation and regulates
microglia-mediated neuroinflammation in neonatal hypoxic-ischemic
encephalopathy. Cell Mol Immunol. 17:976–991. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Xu Y, Xu Y, Zhu Y, Sun H, Juguilon C, Li
F, Fan D, Yin L and Zhang Y: Macrophage miR-34a is a key regulator
of cholesterol efflux and atherosclerosis. Mol Ther. 28:202–216.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Gao L, Qiu F, Cao H, Li H, Dai G, Ma T,
Gong Y, Luo W, Zhu D, Qiu Z, et al: Therapeutic delivery of
microRNA-125a-5p oligonucleotides improves recovery from myocardial
ischemia/reperfusion injury in mice and swine. Theranostics.
13:685–703. 2023. View Article : Google Scholar : PubMed/NCBI
|