Introduction
Colorectal cancer (CRC) is the third most common
cause of cancer-related death in the United States (1). In 2020, 147,950 individuals were
predicted to be diagnosed with CRC, and 53,200 succumbed to the
disease (1). The high mortality of
patients with CRC is attributed to various factors, among which the
late discovery leading to the loss of optimal chance of surgery may
be the most important (2). However,
although surgery accompanied with appropriate chemoradiotherapy are
used in clinical practice, once patients with CRC have reached the
advanced stage of the disease, a small part of them benefit from
these therapeutic strategies; this outcome is notably noted for
patients with CRC and distal metastasis (3). Therefore, the identification of novel
biomarkers and therapeutic targets is required.
Long non-coding RNAs (lncRNAs) are a class of
single-stranded RNAs >200 nucleotides (nt) in length, which lack
the ability to encode proteins. In the past decade, increasing
experimental evidence has indicated that lncRNAs play crucial roles
in the development of cancer, including CRC (4,5). For
example, lncRNA CRART16 promoted the resistance of CRC to
5-fluorouracil by sponging microRNA (miR)-193b-5p (6); lncRNA AGAP2-AS1 expression was
upregulated by E2F transcription factor 4, which accelerated the
progression of CRC by regulating the miR-182-5p/cofilin 1 axis
(7); lncRNA small nucleolar RNA
host gene 17 (SNHG17) promoted the tumorigenesis and metastasis of
CRC by regulating the tripartite motif containing 23-pescadillo
ribosomal biogenesis factor 1 axis and the miR-339-5p-FOS like
2-SNHG17 positive feedback loop (8). Currently, lncRNA muscle blind-like 1
antisense RNA 1 (MBNL1-AS1) was identified to be downregulated in
several types of cancer and to be involved in cancer development
(9,10). For example, MBNL1-AS1 suppressed the
progression of retinoblastoma by regulating the
miR-338-5p/Wnt/β-catenin signaling pathway (11). MBNL1-AS1 expression has been shown
to be downregulated and to suppress the progression of colon cancer
by regulating the miR-413-3p/myosin light chain 9 signaling
(12). However, the underlying
mechanisms by which MBNL1-AS1 suppresses the progression of CRC are
unclear.
Blood vessel epicardial substance (BVES) is a tight
junction-associated protein which is downregulated in the majority
of cancer types, including CRC (13). BVES has been identified as a tumor
suppressor in CRC, due to the fact that restoration of its
expression can reduce the proliferation of CRC cells (14). With regard to the associated
mechanism of action, BVES can induce β-catenin redistribution to
inhibit WNT/β-catenin signaling, and the interaction of BVES with
tight junction protein 1 and Rho/Rac guanine nucleotide exchange
factor 2 can result in the decreased levels of activated ras
homolog family member A in CRC (14). Several studies have demonstrated
that the downregulation of BVES expression is involved in promoting
hypermethylation in cancer cells (13,15,16).
However, whether the stability of BVES mRNA is altered in CRC
remains unknown.
In the present study, the expression levels of
MBNL1-AS1 were decreased in CRC tissues compared with those noted
in matched normal tissues. The co-expression analysis based on The
Cancer Genome Atlas (TCGA) database indicated the strongest
correlation between the expression of MBNL1-AS1 and BVES mRNA in
CRC. Furthermore, MBNL1-AS1 enhanced the stability of BVES mRNA in
CRC cells and suppressed their proliferation by acting as a
competing endogenous RNA (ceRNA) to sponge miR-29b-3p, which in
turn directly targeted BVES mRNA 3′ untranslated region (UTR).
Materials and methods
Patients and samples
A total of 20 patients with CRC were enrolled in the
present study. Inclusion criteria were as follows: i) CRC was
diagnosed by pathological examination; ii) patients had no other
severe diseases, including other cancers and high blood pressure
with complications and iii) patients were willing to participate
and signed the informed consents. Exclusion criteria were as
follows: i) Incomplete information and ii) patients received chemo-
or radiotherapy before collecting tissue samples. The fresh CRC
samples were collected after the patients had been completed the
surgery but within 10 min and stored in liquid nitrogen
immediately. All fresh CRC samples were collected from November
2020 to December 2021 in the Affiliated Hospital of Southwest
Medical University. The present study was approved (approval no.
KY2023025) by the Ethics Committee of the Affiliated Hospital of
Southwest Medical University (Luzhou, China), and informed consent
was signed by all patients. Clinicopathological characteristics of
patients are provided in Table
I.
 | Table I.Clinical information of the patients
with colorectal cancer. |
Table I.
Clinical information of the patients
with colorectal cancer.
|
| Expression level of
MBNL1-AS1 |
|
|---|
| Clinicopathological
characteristics |
|
|
|---|
| Low | High | P-value |
|---|
| Sex |
|
| 0.7301 |
|
Male | 15 | 13 |
|
|
Female | 5 | 7 |
|
| Age, years |
|
| 1.0000 |
|
≤63 | 11 | 12 |
|
|
>63 | 9 | 8 |
|
| Tumor size,
cm3 |
|
| 0.0500 |
|
≤28 | 16 | 9 |
|
|
>28 | 4 | 11 |
|
| TNM stage |
|
| 0.7403 |
| I and
II | 6 | 8 |
|
| III and
IV | 14 | 12 |
|
| Lymph node
metastasis |
|
| 0.7150 |
|
Yes | 16 | 14 |
|
| No | 4 | 6 |
|
Animal model
A total of 24 4-week-old female BALB/c nude mice
(weight, 16±3 g) were purchased from Hunan SJA Laboratory Animal
Co., Ltd. and housed in laminar flow cabinets under a specific
pathogen-free condition (37°C; 12/12-h light/dark cycle; free
access to food and water). Mice experiments were approved (approval
no. swmu20230055) by the Ethics Committees of Southwest Medical
University. The nude mice were divided into four groups randomly,
and each group included six nude mice. A total of ~1×107
cells resuspended in 50 µl PBS were injected into the subcutaneous
tissues of each nude mice. A total of seven, 10, 13, 16, 19 and 22
days after the cells were injected, the formed xenografts were
measured, respectively. The nude mice were sacrificed before the
longest diameter of xenografts exceeded 15 mm. They were euthanized
by cervical dislocation after receiving satisfactory anesthesia by
injection of 50 mg/kg pentobarbital sodium. Then, the xenografts
were taken out and weighted. The volumes of xenografts were
calculated based on the following formula: V=π/6 × L × W × H, which
is used to calculate the volume of an ellipse.
Cell culture and transfection
Human colon cell lines (SW480, SW620, HCT116 and
T84) and the normal epithelial cell line, CD 841 CoN were purchased
from the National Collection of Authenticated Cell Cultures and
cultured with RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.), supplemented with 10% fetal bovine serum (FBS; cat. no.
SV30087.03; Hyclone; Cytiva), in 5% CO2 at 37°C. Cell
transfection was performed by using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Routinely, the transfection was
carried out in six-well plates at room temperature. For each well,
2 µg overexpression/shRNA vector or 50 nM siRNA were used to
incubate with cells for 24 h. After that, cells were harvested for
subsequent analysis.
RNA extraction
Total RNAs of transfected SW480 or SW620 cells were
extracted by using an RNA Extraction kit (Chongqing Van der Waals
Biotechnology, Co., Ltd.) according to the manufacturer's protocol.
Briefly, 106 cells were lysed using 100 µl Lysis Buffer
I, and then 300 µl Lysis Buffer II was added. The solution was
added into RNA Binding Columns and centrifuged at 13,000 × g for 30
sec at 4°C. After washing, 50 µl Elution Buffer was added into the
RNA Binding Columns to dissolve the RNAs. The stability of mRNA was
performed by incubating 1 µg total RNA with 2 µg/ml Actinomycin D
(Sigma Aldrich; Merck KGaA) at indicated time points, and analyzed
by PCR.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNAs were reversely transcribed into cDNAs by
using a PrimeScript™ RT reagent kit (Takara Bio, Inc.) and the
cDNAs were amplified by using a SYBR® Premix Ex TaqTM II
reagent (Takara Bio, Inc.). Initial denaturation was performed at
95°C for 10 min, followed by 40 cycles of denaturation (95°C for 10
sec), annealing (60°C for 30 sec), and extension (72°C for 30 sec).
The expression of miR-29c-3p and BVES was calculated by using the
2−ΔΔCq method (17).
ACTB served as the internal reference. The primers of miR-29c-3p
were purchased from (Shanghai, GenePharma Co., Ltd.), and the
primers of MBNL1-AS1, BVES and ACTB were shown in Table SII.
Construction of vectors and small
interfering RNA (siRNA) for BVES
The full-length sequence of MBNL1-AS1 was
synthesized and inserted into pcDNA3.1 vectors (Addgene, Inc.) or
pLV-Puro vectors (Addgene, Inc.,) to construct the
MBNL1-AS1-overexpressing vectors or the cell lines that stably
overexpress MBNL1-AS1. The short hairpin RNA (shRNA) sequence for
MBNL1-AS1 was synthesized and inserted into pLV-Puro vectors
(Addgene, Inc.) to construct the cell lines that stably low express
MBNL1-AS1 expression. The wild-type or mutant sequences of 3′UTR of
BVES mRNA or MBNL1-AS1 containing the binding sites of miR-29c-3p
were inserted into pGL3-Basic vectors (Addgene, Inc.) to construct
the wild-type pGL3-BVES 3′UTR vector, the mutant pGL3-BVES 3′UTR
vector, the wild-type pGL3-MBNL1-AS1 vector, or the mutant
pGL3-MBNL1-AS1 vector. The recombinant vectors were selected by
ampicillin after transforming in receptive bacteria. The shRNA
sequences were shown in Table II.
The siRNAs for BVES were purchased from Thermo Fisher Scientific,
Inc. (cat. no. AM16708) and sequences were unavailable due to
confidentiality issues. The transfection of these vectors or siRNAs
were performed as aforementioned.
 | Table II.The DNA sequences used in the present
study. |
Table II.
The DNA sequences used in the present
study.
| Name | Sequence
(5′→3′) |
|---|
| MBNL1-AS1 | Sense:
CAGGAGAGTGGCAGGAGATGAC |
|
| Antisense:
GTGGTTCGCAGGCATTCTAAGC |
| BVES | Sense:
GGCGTCGTCCTCTTCACAGA |
|
| Antisense:
GCACAGCATCCTACCATTCCAA |
| ACTB | Sense:
TGTCCACCTTCCAGCAGATGT |
|
| Antisense:
TGTCACCTTCACCGTTCCAGTT |
|
MBNL1-AS1-shRNA1 | Sense:
GATCCGAACGAAAGGAGCAGGGTATTTCAAGAG |
|
|
AATACCCTGCTCCTTTCGTTTTTTTA |
|
| Antisense:
AGCTTAAAAAAACGAAAGGAGCAGGGTATT |
|
|
CTCTTGAAATACCCTGCTCCTTTCGTTCG |
|
MBNL1-AS1-shRNA2 | Sense:
GATCCGCCAGAACCTAGTCTCATGTTTCAAGAGA |
|
|
ACATGAGACTAGGTTCTGGTTTTTA |
|
| Antisense:
AGCTTAAAAACCAGAACCTAGTCTCATGTTC |
|
|
TCTTGAAACATGAGACTAGGTTCTGGCG |
| NC-shRNA | Sense:
GATCCCCTTCTCCGAACGTGTCACGTTTCAAGAG |
|
|
AACGTGACACGTTCGGAGAATTTTT |
|
| Antisense:
AGCTAAAAATTCTCCGAACGTGTCACGTTCT |
|
|
CTTGAAACGTGACACGTTCGGAGAAGGG |
Dual-luciferase reporter assay
A total of ~1 µg of the wild-type pGL3-BVES 3′UTR
vectors, the mutant pGL3-BVES 3′UTR vectors, the wild-type
pGL3-MBNL1-AS1, or the mutant pGL3-MBNL1-AS1 vectors, and 0.05 µg
of pRL-TK vectors (Promega Corporation) which served as control,
were transfected into HEK293T cells, respectively, while the cells
were also transfected with NC or miR-29c-3p mimics using
Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. A total of 24 h after
transfection at 37°C, the cells were lysed, and the luciferase
activity was measured using GloMax 20/20 Luminometer (Promega
Corporation). The relative luciferase activity was calculated by
normalizing Firefly luciferase to Renilla luciferase
activity.
Cell proliferation
For Cell Counting Kit-8 (CCK-8) assay, ~2,000
transfected SW480 or SW620 cells were seeded into 96-well plates
and cultured for 1, 2, 3, 4, and 5 days, respectively. For each
well, 10 µl CCK-8 reagent (MedChemExpress) were diluted into 100 µl
medium to incubate the cell and analyzed their proliferation based
on the absorbance at 450 nm. For colony formation assay, ~1,600
transfected SW480 or SW620 cells were seeded into six-well plates
and cultured for 2 weeks. Then the cell plates were fixed by 4%
paraformaldehyde for 10 min at room temperature and stained by 0.1%
crystal violet for 10 min at room temperature. For
5-ethynyl-2′-deoxyuridine (EdU) assay, the transfected cells were
cultured in 15-mm slides and incubated with EdU solution for 2 h at
37°C. The staining was performed by EdU-567 kit (Guangzhou RiboBio
Co., Ltd.) for 30 min at 37°C and the nucleus was stained by 5
mg/ml DAPI.
Cell apoptosis by flow cytometry
A total of ~20,000 transfected SW480 or SW620 cells
were cultured in six-well plate and were harvested by trypsin
without EDTA. Cells were resuspended by binding buffer, containing
2.5 µl AnnexinV-FITC and 2.5 µl PI for 30 min in the dark (cat. no.
BD 556570; BD Biosciences). The percentage of apoptotic cells was
recorded using a Beckman Flow Cytometer (Beckman Coulter, Inc.)
using count model and analyzed using FlowJo software (Version 10.0;
FlowJo LLC).
Western blot analysis
Total proteins of transfected SW480 or SW620 cells
extracted by RIPA lysis buffer (Beyotime Institute of
Biotechnology) and concentration were measured by BCA kit (Beyotime
Institute of Biotechnology). For electrophoresis, 30 µg protein
samples were loaded each lane and were separated using 10% SDS-PAGE
gels. Then, the proteins in the gels were transferred onto PVDF
membranes, which were then incubated in a blocking buffer (3%
bovine serum albumin; Beijing Solarbio Science & Technology
Co., Ltd.) for 1 h at room temperature. Then, the PVDF membranes
were incubated with anti-BVES antibody (1:500; cat. no. 12920-1-AP;
Proteintech Group, Inc.) or anti-GAPDH antibody (1:10,000; cat. no.
60004-1-Ig; Proteintech Group, Inc.) at 4°C overnight. After
washing by 0.1% TBST solution, the PVDF membranes were incubated
with HRP-conjugated goat-anti-rabbit secondary antibody (1:1,000;
cat. no. 7074;) or goat-anti-mouse secondary antibody (1:1,000;
cat. no. 7076; both from Cell Signaling Technology, Inc.) at room
temperature for 1 h. After washing, the PVDF membranes were exposed
using SuperSignal West Dura Extended Duration Substrate Kit (Thermo
Fisher Scientific, Inc.).
Bioinformatic analysis
The enrichment of signaling pathways were analyzed
by Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/). The correlation of the
expression between two genes were acquired from TCGA database
(https://www.cancer.gov/ccg/research/genome-sequencing/tcga)
and analyzed using Spearman's correlation (two-tailed). The binding
sites of miR-29b-3p and miR-29c-3p were predicted by
TargetScanHuman 7.1 (https://www.targetscan.org/vert_71/).
Statistical analysis
The data are presented as the mean ± standard
deviation (SD). The differences between two groups were analyzed
using unpaired Student's t-test or Mann-Whitney U test and among
more than two groups were analyzed using one-way ANOVA followed by
Tukey's HSD test. P<0.05 was considered to indicate
statistically significant difference. Graphs were generated by
GraphPad Prism 8.0.1 (Dotmatics).
Results
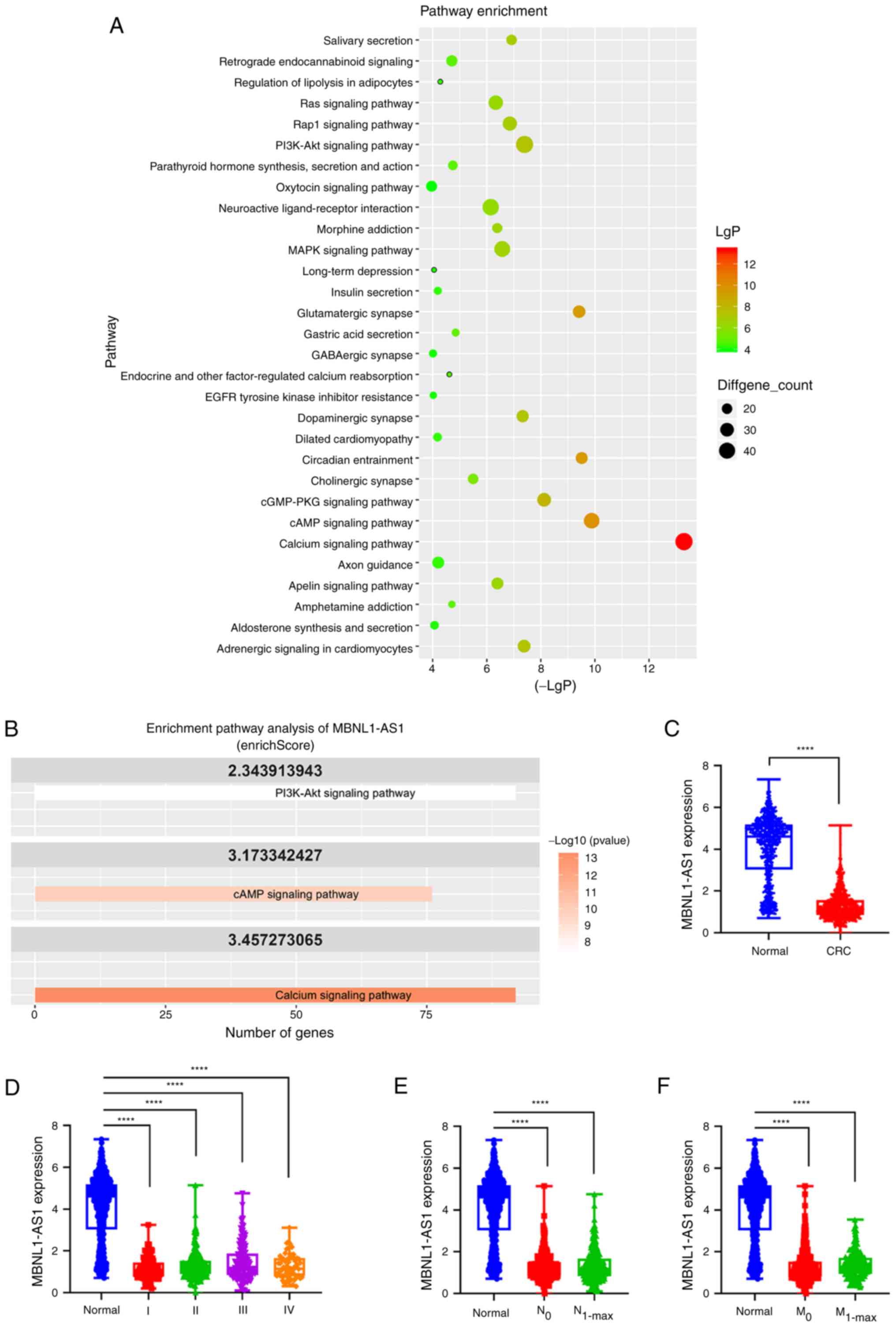
MBNL1-AS1 is involved in the PI3K-AKT
signaling pathway and is downregulated in CRC tissues and cell
lines
To investigate the potential lncRNAs that regulate
the development of CRC, TCGA database was used to explore the
altered signaling pathways. Enrichment analysis of KEGG revealed
that the PI3K-AKT signaling pathway had the highest number of
dysregulated genes among other pathways, with 46 downregulated
genes and 26 upregulated genes. Additionally, the Calcium signaling
pathway showed 46 downregulated genes in CRC tissues compared with
their corresponding expression levels in normal tissues (Table SI, Fig.
1A). Subsequently, the dysregulated lncRNAs implicated in these
pathways were examined. Notably, the present analysis revealed that
the lncRNA MBNL1-AS1 exhibited significantly high enrichScores in
the Calcium, PI3K-AKT and cAMP signaling pathways (Fig. 1B, Table
SII), indicating its potential involvement in CRC
progression.
Subsequently, the expression levels of MBNL1-AS1
were analyzed in CRC using the TCGA database; the analysis included
620 human CRC tissue samples and 830 normal colorectal tissue
samples. The results indicated that MBNL1-AS1 expression was
significantly decreased in CRC tissues compared with that noted in
normal tissues (Fig. 1C). However,
MBNL1-AS1 expression did not correlate with the TNM stage, lymph
node metastasis, or distal metastasis of CRC (Fig. 1D-F). Moreover, MBNL1-AS1 expression
was also significantly decreased in four CRC cell lines, including
SW480, SW620, HCT116, and T84 compared with that noted in the
normal epithelial cell line, CD 841 CoN (Fig. S1). Taken together, these results
suggested that MBNL1-AS1 expression is decreased in CRC and that it
is associated with the PI3K-AKT signaling pathway.
MBNL1-AS1 suppresses CRC cell
proliferation in vitro and in vivo
Given that MBNL1-AS1 expression was not altered in
the metastatic CRC tissues compared with that noted in the
non-metastatic CRC tissues, the effect of MBNL1-AS1 was
investigated on the proliferation of CRC cells. Given that SW620
and SW480 cells exhibited the lowest and highest, respectively,
expression of MBNL1-AS1 than the other CRC cell lines (Fig. S1), forced expression of MBNL1-AS1
was established in SW620 cells (Fig.
2A); the stable knockdown variant of MBNL1-AS1 was also
established in SW480 cells (Fig.
2B). By using CCK-8, colony formation and EdU assays, it was
found that the ectopic expression of MBNL1-AS1 significantly
inhibited the proliferation of SW620 cells, while knockdown of its
expression significantly promoted the ability of SW480 cells to
proliferate (Fig. 2C-L). Moreover,
both overexpression or knockdown of MBNL1-AS1 expression displayed
no apparent impact on cell apoptosis (Fig. 2M-P).
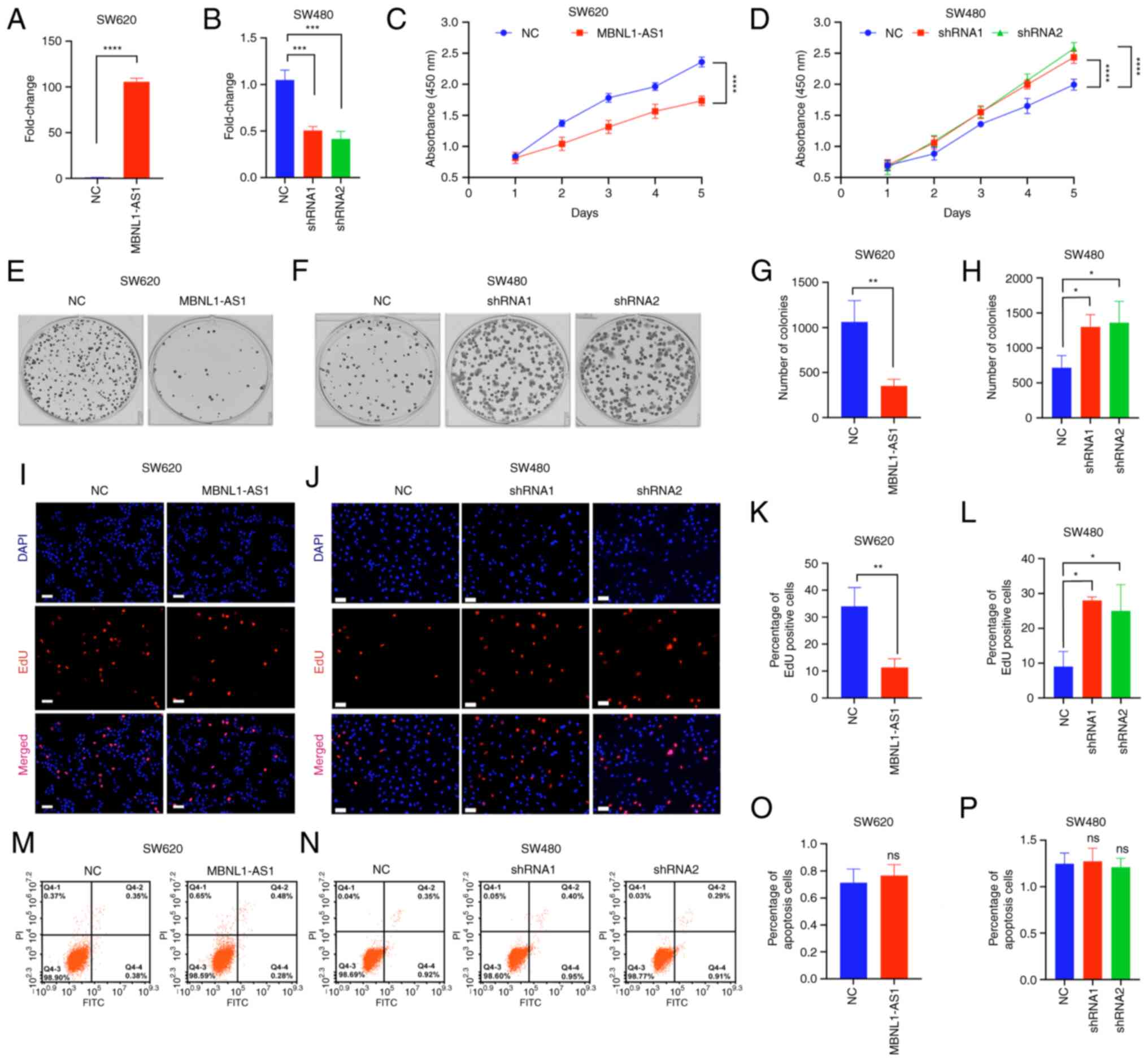 | Figure 2.MBNL1-AS1 suppresses CRC cell
proliferation in vitro. (A and B) MBNL1-AS1 expression in
CRC cells which were transfected with the MBNL1-AS1-overexpressing
vectors or the shRNA 1 or shRNA 2 sequences of MBNL1-AS1. (C and D)
The absorbance of SW620 and SW480 cells as detected by the Cell
Counting Kit-8. (E-H) The number of colonies of SW620 and SW480
cells, as detected by the colony formation assay. (I-L) The
percentage of EdU positive cells of SW620 and SW480 cells, as
detected by the EdU kit. (M-P) The percentage of apoptotic SW620
and SW480 cells, as detected by the Annexin-FITC/PI kit. For A-P,
three individual replicates were performed for each experiment.
*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001.
MBNL1-AS1, muscle blind like splicing regulator 1 antisense RNA 1;
CRC, colorectal cancer; EdU, 5-ethynyl-2′-deoxyuridine; shRNA,
short hairpin RNA; NC, negative control. |
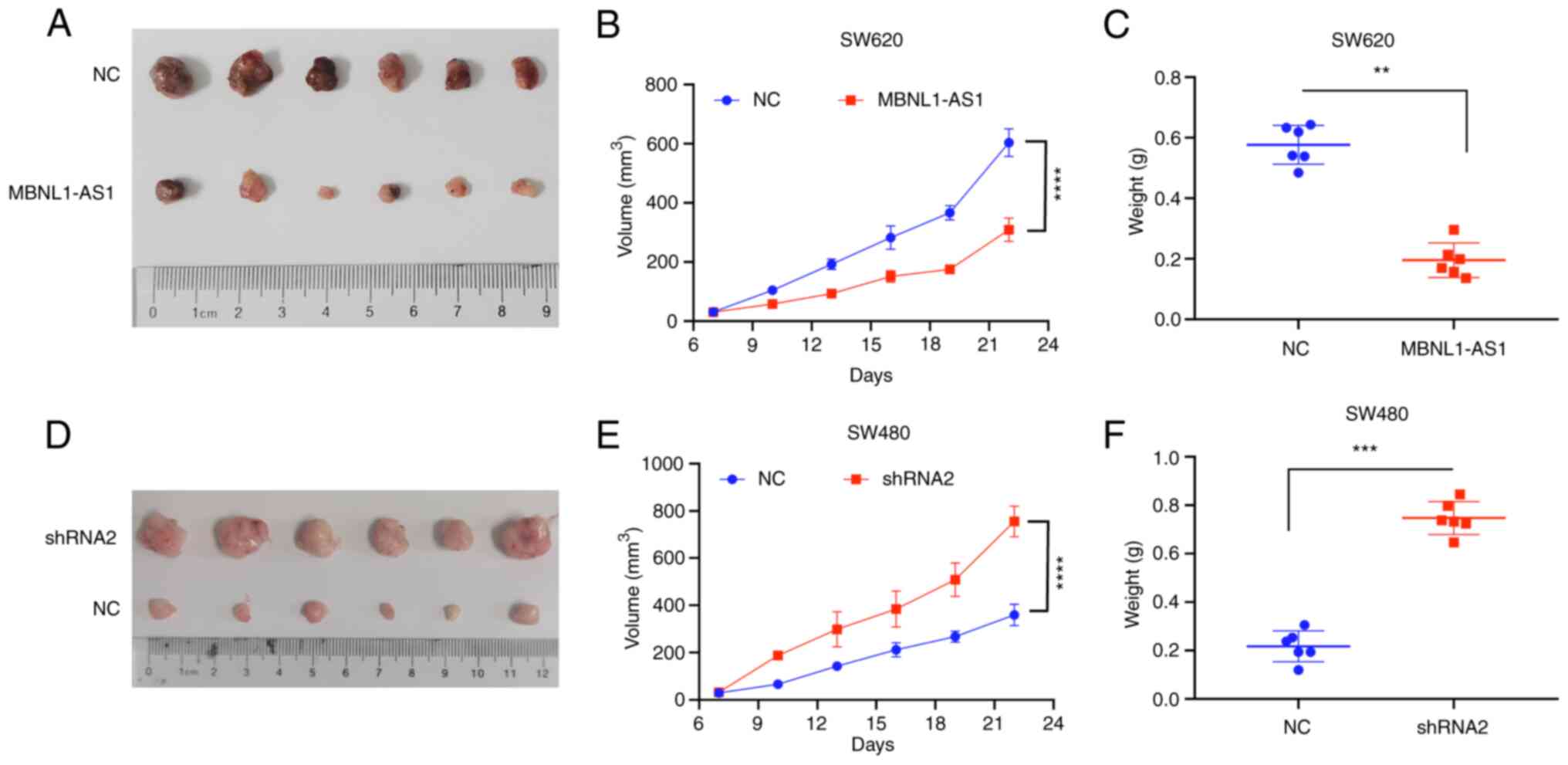
To further evaluate the roles of MBNL1-AS1 on the
proliferation of CRC, CRC cells were subcutaneously injected into
nude mice. One week after the injection, the xenografts were
formed, and their sizes were measured and recorded. A total of four
weeks after the injection, the nude mice were sacrificed, and the
xenografts were removed (Fig. 3A and
D). Ectopic expression of MBNL1-AS1 significantly decreased the
sizes and weights of the xenografts (Fig. 3B and C), while knockdown of
MBNL1-AS1 expression revealed the opposite effects (Fig. 3E and F). Collectively, the results
indicated that MBNL1-AS1 acted as a tumor suppressor that inhibited
CRC cell proliferation.
MBNL1-AS1 expression is positively
correlated with BVES expression in CRC tissues and MBNL1-AS1
promotes the stability of BVES mRNA in CRC cells
To further explore the underlying mechanisms by
which MBNL1-AS1 suppresses CRC cell proliferation, co-expression
analysis of lncRNAs was performed with mRNAs in CRC tissues based
on the TCGA database (Table SIII).
The data indicated that BVES and MBNL1-AS1 exhibited the highest
correlation coefficient followed by cholinergic receptor muscarinic
2 (CHRM2), a crucial member in the PI3K-AKT signaling pathway
(18) (Table SIV). However, the present study
focused on BVES instead of CHRM2, since the former has been
reported to be involved in the development of CRC. Correlation
analysis revealed that BVES mRNA expression positively correlated
with MBNL1-AS1 expression in CRC tissues based on the TCGA database
(Fig. 4A).
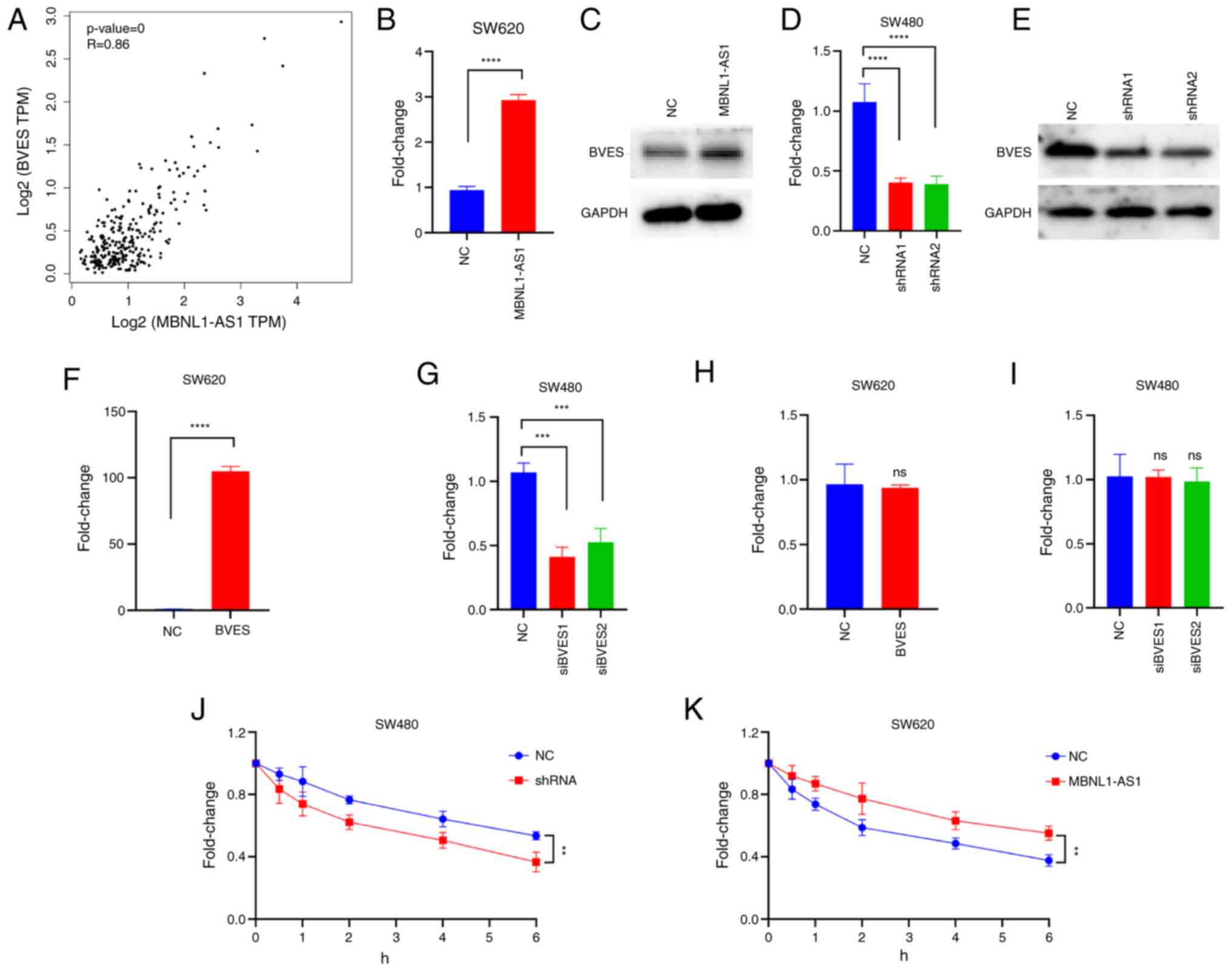 | Figure 4.MBNL1-AS1 expression is positively
correlated with BVES expression in CRC tissues and MBNL1-AS1
promotes the stability of BVES mRNA in CRC cells. (A) Pearson
analysis indicated a significant correlation between the expression
levels of BVES mRNA and MBNL1-AS1 in CRC tissues. (B and C) The
mRNA and protein expression of BVES in SW620 cells, which were
transfected with NC or MBNL1-AS1-overexpressing vectors are
demonstrated. (D and E) The mRNA and protein expression levels of
BVES in SW480 cells, which were transfected with NC or
MBNL1-AS1-inhibiting vectors are indicated. (F and G) The mRNA
expression levels of BVES in SW620 cells transfected with NC or
BVES-overexpressing vectors or in SW480 cells transfected with NC
or the siRNA sequences specific to BVES are revealed. (H and I) The
mRNA expression levels of MBNL1-AS1 in SW620 cells transfected with
NC or BVES-overexpressing vectors or in SW480 cells transfected
with NC or the siRNA sequences specific to BVES are indicated. (J
and K) The mRNA expression levels of BVES in SW480 cells
transfected with NC or MBNL1-AS1-inhibiting vectors or in SW620
cells transfected with NC or MBNL1-AS1-overexpressing vectors in
the presence of actinomycin D are shown. For B-K, three individual
replicates were performed for each experiment. **P<0.01,
***P<0.001 and ****P<0.0001. MBNL1-AS1, muscle blind like
splicing regulator 1 antisense RNA 1; BVES, blood vessel epicardial
substance; CRC, colorectal cancer; NC, negative control; siRNA,
small interfering RNA; ns, non-significant. |
Subsequent studies determined whether MBNL1-AS1
could regulate BVES expression and the data indicated that the
ectopic expression of MBNL1-AS1 significantly increased the
expression levels of BVES mRNA and protein (Fig. 4B and C), while knockdown of
MBNL1-AS1 expression caused a significant decrease in these levels
(Fig. 4D and E). Then, the forced
expression of MBNL1-AS1 was established in SW620 cells (Fig. 4F); the stable knockdown variant of
MBNL1-AS1 was also established in SW480 cells (Fig. 4G). However, BVES did not alter
MBNL1-AS1 expression in CRC cells (Fig.
4H and I). These data suggested that MBNL1-AS1 was an upstream
regulatory factor of BVES. Subsequent studies were performed to
assess the mechanism by which MBNL1-AS1 increased BVES mRNA
expression. Actinomycin D was used to inhibit RNA transcription in
CRC cells and the data indicated that knockdown of MBNL1-AS1
significantly decreased BVES mRNA expression while the ectopic
expression of MBNL1-AS1 increased BVES mRNA expression (Fig. 4J and K, respectively), suggesting
that it regulated BVES mRNA expression at the post-transcriptional
instead of the transcriptional level. Collectively, these results
suggested that MBNL1-AS1 increased the stability of BVES mRNA.
Moreover, their expression levels indicated a positive correlation
in CRC tissues.
MBNL1-AS1 acts as a ceRNA to sponge
miR-29b-3p, which directly targets BVES mRNA
A large number of studies have shown that lncRNAs
serve as ceRNAs to sponge miRs, which target and regulate the mRNA
molecules (19). To explore whether
MBNL1-AS1 serves as a ceRNA to sponge miRs, co-expression analysis
was performed for MBNL1-AS1, miRs and BVES in CRC tissues based on
the TCGA database. The results indicated that miR-29b-3p and
miR-29c-3p were candidate miRs (Table
SV), and their expression levels were both upregulated in CRC
tissues compared with those observed in normal tissues (Fig. 5A and B). TargetScanHuman 7.1 was
used to predict the binding sites of miR-29b-3p and miR-29c-3p with
BVES mRNA and MBNL1-AS1. The data indicated that the seed sequences
of the two miRs were both complementary to the 3,217-3,223 nt of
BVES mRNA and to the 1,214-1,222 nt of MBNL1-AS1 (Fig. 5C). Then, the forced expression of
miR-29b-3p and miR-29c-3p were established in SW620 cells (Fig. 5D and E). Subsequent experiments
demonstrated that miR-29c-3p mimics significantly decreased the
mRNA and protein expression levels of BVES, whereas miR-29b-3p
mimics exhibited a weaker effect compared with that of miR-29c-3p
mimics (Fig. 5F-I). Therefore, the
present study focused on miR-29c-3p. Furthermore, luciferase
reporter vectors were constructed that contained the binding sites
of miR-29c-3p to BVES mRNA and MBNL1-AS1, as well as the
corresponding mutant vectors. The results revealed that miR-29c-3p
mimics significantly decreased luciferase activity levels in 293
cells transfected with the wild-type vectors compared with those of
the mutant vectors (Fig. 5J).
Furthermore, a significantly negative correlation was observed
between the expression levels of MBNL1-AS1 and miR-29c-3p, and
between those of BVES and miR-29c-3p (Fig. 5K and L). Collectively, these results
suggested that MBNL1-AS1 increases BVES expression by serving as a
ceRNA to sponge miR-29c-3p in CRC cells.
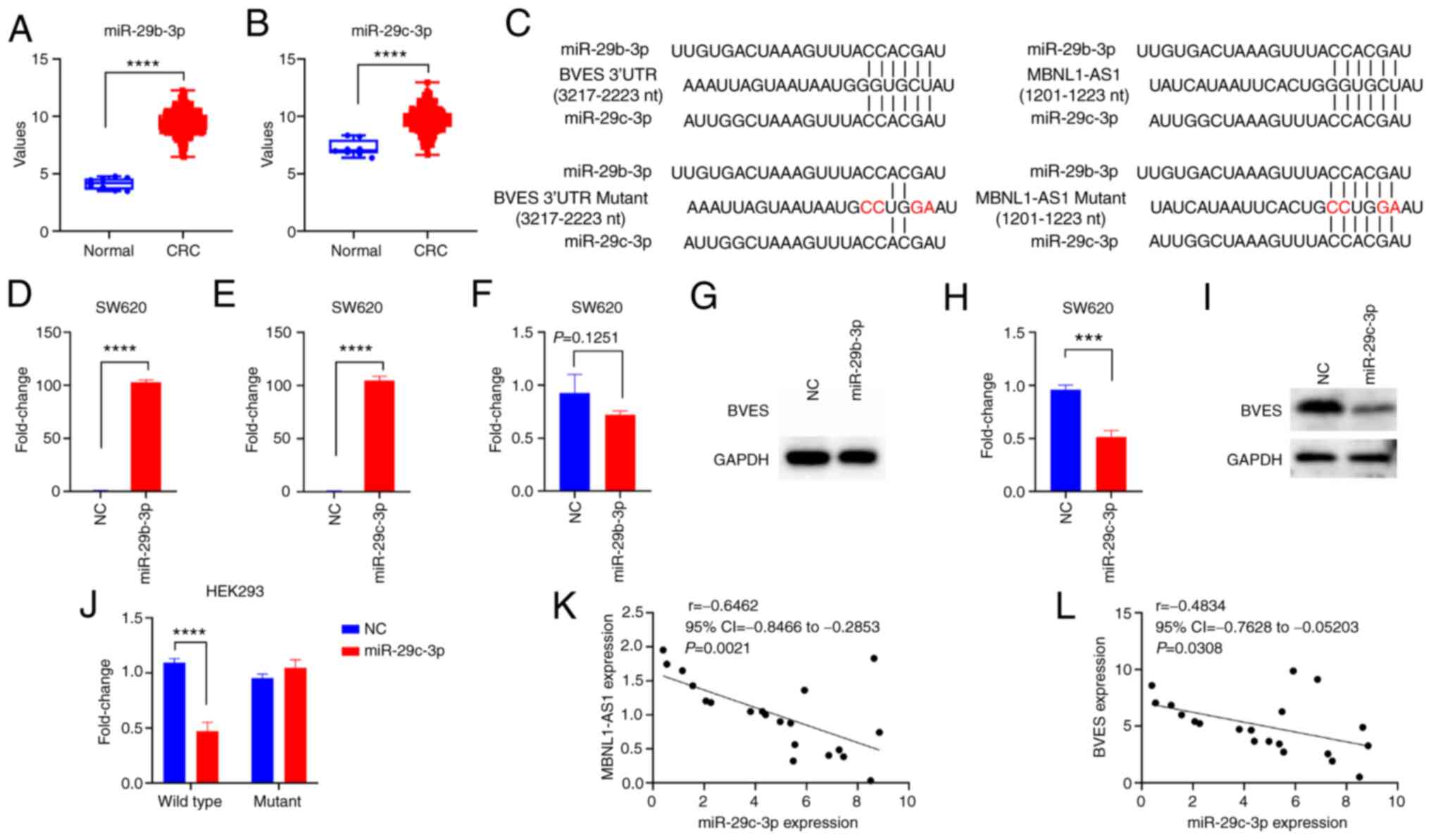 | Figure 5.MBNL1-AS1 acts as a ceRNA to sponge
miR-29b-3p, which directly targets BVES mRNA. (A and B) The
expression levels of miR-29b-3p and miR-29c-3p in normal and CRC
tissues derived from The Cancer Genome Atlas database are
indicated. (C) The binding sites of BVES 3′UTR or MBNL1-AS1 to
miR-29b-3p or miR-29c-3p, and their corresponding mutant binding
sites. (D and E) The expression levels of miR-29b-3p or miR-29c-3p
in SW620 cells transfected with NC, miR-29b-3p mimics, or
miR-29c-3p mimics, are shown. (F-I) The mRNA and protein expression
levels of BVES in SW620 cells transfected with NC, miR-29b-3p
mimics or miR-29c-3p mimics are revealed. (J) The luciferase
activity levels in 293 cells transfected with NC or the luciferase
reporter vector containing the binding site of the wild-type or
mutant BVES with miR-29c-3p are demonstrated. (K and L) Pearson's
correlation analysis indicating negative correlations between the
expression levels of miR-29c-3p and MBNL1-AS1 or BVES mRNA in CRC
tissues (n=20). For D-J, three individual replicates were performed
for each experiment. ***P<0.001 and ****P<0.0001. MBNL1-AS1,
muscle blind like splicing regulator 1 antisense RNA 1; ceRNA,
competing endogenous RNA; miR, microRNA; BVES, blood vessel
epicardial substance; CRC, colorectal cancer; siRNA, small
interfering RNA; UTR, untranslated region; NC, negative
control. |
MBNL1-AS1 inhibits CRC cell
proliferation by regulating miR-29c-3p/BVES signaling
The present study further determined whether
MBNL1-AS1 inhibited CRC cell proliferation by regulating
miR-29c-3p/BVES signaling. BVES siRNAs were used to suppress the
MBNL1-AS1-mediated increase in BVES expression (Fig. 6A and B). Furthermore, miR-29c-3p
mimics also suppressed the MBNL1-AS1-mediated increase in BVES
expression (Fig. 6C and D). CCK-8,
colony formation and EdU assays indicated that significantly
reversed the MBNL1-AS1-mediated decrease in the proliferation of
SW620 cells, while miR-29c-3p mimics significantly suppressed the
MBNL1-AS1-mediated decrease identified in the proliferation of
SW620 cells (Fig. 6E-J) without
affecting cell apoptosis (Fig. 6K and
L). Collectively, these results suggested that MBNL1-AS1
inhibited CRC cell proliferation by regulating miR-29c-3p/BVES
signaling.
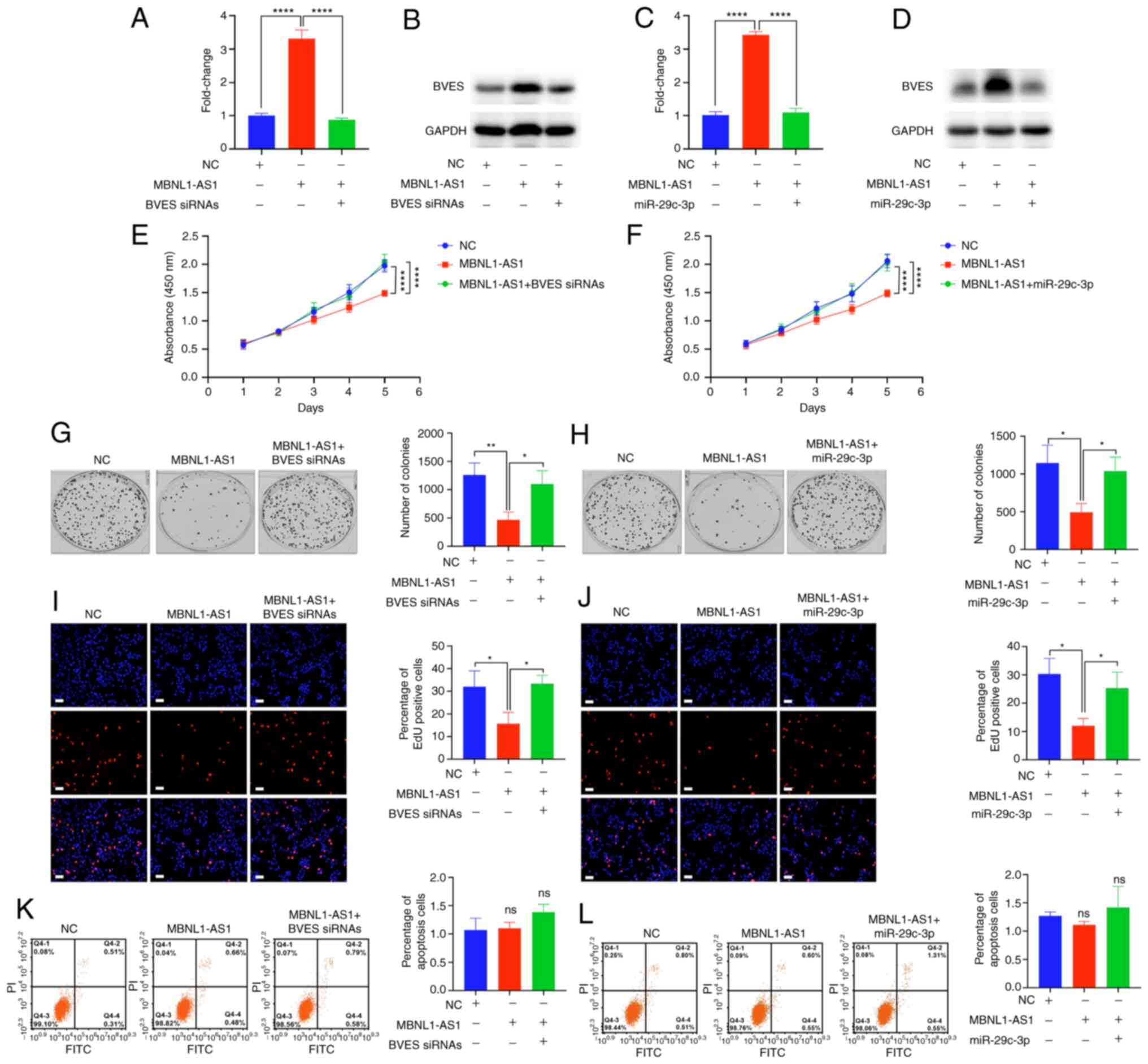 | Figure 6.MBNL1-AS1 inhibits colorectal cancer
cell proliferation via regulating the miR-29c-3p/BVES signal. (A
and B) The mRNA and protein expression levels of BVES in SW620
cells transfected with NC, MBNL1-AS1-overexpressing vectors or
co-transfected with MBNL1-AS1-overexpressing vectors and BVES
siRNAs are indicated. (C and D) The mRNA and protein expression
levels of BVES in SW620 cells transfected with NC,
MBNL1-AS1-overexpressing vectors or co-transfected with
MBNL1-AS1-overexpressing vectors and miR-29c-3p mimics are shown.
(E and F) The absorbance (450 nm) of SW620 cells transfected with
NC, MBNL1-AS1-overexpressing vector or co-transfected with
MBNL1-AS1-overexpressing vectors and BVES siRNAs, or co-transfected
with MBNL1-AS1-overexpressing vectors and miR-29c-3p mimics is
demonstrated. (G and H) The number of colonies of SW620 cells
transfected with NC, MBNL1-AS1-overexpressing vector or
co-transfected with MBNL1-AS1-overexpressing vectors and BVES
siRNAs, or co-transfected with MBNL1-AS1-overexpressing vectors and
miR-29c-3p mimics is indicated. (I and J) The percentage of
5-ethynyl-2′-deoxyuridine positive cells of SW620 cells transfected
with NC, MBNL1-AS1-overexpressing vector or co-transfected with
MBNL1-AS1-overexpressing vectors and BVES siRNAs, or co-transfected
with MBNL1-AS1-overexpressing vectors and miR-29c-3p mimics is
shown. (K and L) The percentage of apoptotic cells of SW620 cells
transfected with NC, MBNL1-AS1-overexpressing vector or
co-transfected with MBNL1-AS1-overexpressing vectors and BVES
siRNAs, or co-transfected with MBNL1-AS1-overexpressing vectors and
miR-29c-3p mimics is indicated. For A-L, three individual
replicates were performed for each experiment. *P<0.05,
**P<0.01 and ****P<0.0001. MBNL1-AS1, muscle blind like
splicing regulator 1 antisense RNA 1; miR, microRNA; BVES, blood
vessel epicardial substance; siRNA, small interfering RNA; NC,
negative control; ns, non-significant. |
Discussion
MBNL1-AS1 is involved in the development of several
types of cancer (11,12,20).
However, its roles and the underlying mechanisms are largely
unknown in CRC. The present study indicated that MBNL1-AS1
expression was significantly decreased in CRC tissues compared with
that observed in normal tissues. Moreover, MBNL1-AS1 suppressed the
ability of CRC cells to proliferate. The expression levels of
MBNL1-AS1 and BVES mRNA exhibited the strongest correlation in CRC
tissues based on the TCGA database analysis and demonstrated that
MBNL1-AS1 was an upstream regulatory factor which increased BVES
expression in CRC cells. With regard to its mechanism of action, it
was observed that MBNL1-AS1 served as a ceRNA to sponge miR-29c-3p,
which directly targeted BVES mRNA 3′ UTR. Finally, it was
demonstrated that MBNL1-AS1 inhibited CRC cell proliferation by
regulating miR-29c-3p/BVES signaling. Therefore, these findings
suggested that the expression levels of MBNL1-AS1, miR-29c-3p and
BVES may be used as potential biomarkers for the diagnosis of CRC.
Moreover, the MBNL1-AS1/miR-29c-3p/BVES axis may be a potential
therapeutic target for CRC. Also, for further studies, the
signaling pathway involved in MBNL1-AS1/miR-29c-3p/BVES axis will
be elucidated.
Currently, the TCGA database has accumulated
numerous data from tens of thousands of clinical samples. The
present study analyzed the aberrant expression of genes associated
with the key signaling pathways in 620 CRC tissues and 830 normal
colorectal tissues based on the TCGA database, which have been
widely used to analyze gene expression in cancer (21,22).
KEGG enrichment analysis indicated that 46 dysregulated genes were
involved in the PI3K-AKT signaling pathway and a large number of
studies have confirmed that this pathway plays crucial roles in the
development of CRC, suggesting that it may be one of the most
important pathways involved in the development of this disease
(23). Subsequently, the
dysregulated lncRNAs involved in this signaling pathway were
analyzed and MBNL1-AS1 was identified as the lncRNA with the
highest association since it had the highest enrichScore in the
PI3K-AKT signaling pathway. MBNL1-AS1 expression has been shown to
be decreased in the majority of cancer types, such as in
retinoblastoma (11), prostate
(9), lung (24), bladder (25), gastric (20), colon (12) and breast cancers (26). With the exception of the clinical
samples in the TCGA database, MBNL1-AS1 expression was detected in
human clinical samples and the results demonstrated that it was
significantly decreased in CRC tissues compared with that
identified in matched normal tissues. Therefore, it was deduced
that MBNL1-AS1 expression was downregulated in CRC.
The present findings revealed the highest
correlation coefficient between the expression levels of MBNL1-AS1
and BVES mRNA in 620 CRC tissues. BVES has been shown to play
important roles in cancer development (13–16,27),
including CRC (14). Whether
MBNL1-AS1 can regulate BVES expression remains unclear. The
findings of the present study demonstrated that MBNL1-AS1 promoted
the stability of BVES mRNA, since MBNL1-AS1 upregulation caused an
increase in BVES mRNA expression in the presence of actinomycin D.
By using the TargetScanHuman 7.1 and the co-expression analysis of
the miRs with MBNL1-AS1 or BVES in CRC tissues, it was found that
miR-29c-3p may be a potential candidate in the connection between
MBNL1-AS1 and BVES. Subsequently, miR-29c-3p was shown to directly
target both MBNL1-AS1 and BVES mRNA 3′UTR, suggesting that it
increased BVES expression by serving as a ceRNA to sponge
miR-29c-3p. Increasing experimental evidence has indicated that
miR-29c-3p is upregulated in CRC tissues compared with the
corresponding expression noted in normal tissues and that it
promotes CRC cell proliferation (28,29).
The findings of the present study indicated that MBNL1-AS1
inhibited CRC cell proliferation by regulating miR-29c-3p/BVES
signaling. MBNL1-AS1 has been shown to suppress cell proliferation
and enhance cell apoptosis by regulating the
miR-135a-5p/PHLPP2/FOXO1 axis (25). It has also been revealed to suppress
breast cancer progression by modulating the miR-423-5p/CREBZF axis
(26). These data suggested that
MBNL1-AS1 acts as a tumor suppressor. Additionally, the five-year
survival rate of breast cancer patients with high expression of
lncRNA MBNL1-AS1 was significantly improved than that of those with
low expression, as observed in a cohort of 80 patients (30). However, whether the expression
levels of MBNL1-AS1, miR-29c and BVES were associated with the
survival rates of patients with CRC requires further
investigation.
In conclusion, the data of the current study
indicated that the expression levels of MBNL1-AS1 and BVES were
downregulated and those of miR-29c-3p were upregulated in CRC
tissues compared with those noted in normal tissues, demonstrating
that MBNL1-AS1 increased BVES expression by serving as a ceRNA to
sponge miR-29c-3p. The latter directly targeted BVES mRNA 3′UTR and
MBNL1-AS1. Collectively, the findings revealed that MBNL1-AS1
inhibited CRC cell proliferation by regulating miR-29c-3p/BVES
signaling and suggest its application as a potential therapeutic
target for CRC.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81901629).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WSC performed all experiments and drafted the
manuscript. XZ and ZFZ participated to perform the cell culture and
RT-qPCR experiments. XMC designed this study and drafted the
manuscript. WSC and XMC confirm the authenticity of all raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
KY2023025) by the Ethics Committee of the Affiliated Hospital of
Southwest Medical University (Luzhou, China), and informed consent
was obtained from all participants for use of their tissue. Mice
experiments were approved (approval no. swmu20230055) by the Ethics
Committee of Southwest Medical University (Luzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamali Zonouzi S, Pezeshki PS, Razi S and
Rezaei N: Cancer-associated fibroblasts in colorectal cancer. Clin
Transl Oncol. 24:757–769. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mammes A, Pasquier J, Mammes O, Conti M,
Douard R and Loric S: Extracellular vesicles: General features and
usefulness in diagnosis and therapeutic management of colorectal
cancer. World J Gastrointest Oncol. 13:1561–1598. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen S, Fang Y, Sun L, He R, He B and
Zhang S: Long Non-coding RNA: A potential strategy for the
diagnosis and treatment of colorectal cancer. Front Oncol.
11:7627522021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang C, Liu J, Hu Q, Zeng S and Yu L:
Metastatic colorectal cancer: Perspectives on long non-coding RNAs
and promising therapeutics. Eur J Pharmacol. 908:1743672021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Zhang X, Zhang J, Chen S, Zhu J
and Wang X: Long noncoding RNA CRART16 confers 5-FU resistance in
colorectal cancer cells by sponging miR-193b-5p. Cancer Cell Int.
21:6382021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo Z, Liu X and Shao H: E2F4-induced
AGAP2-AS1 up-regulation accelerates the progression of colorectal
cancer via miR-182-5p/CFL1 axis. Dig Liver Dis. 54:878–889. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bian Z, Zhou M, Cui K, Yang F, Cao Y, Sun
S, Liu B, Gong L, Li J, Wang X, et al: SNHG17 promotes colorectal
tumorigenesis and metastasis via regulating Trim23-PES1 axis and
miR-339-5p-FOSL2-SNHG17 positive feedback loop. J Exp Clin Cancer
Res. 40:3602021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding X, Xu X, He XF, Yuan Y, Chen C, Shen
XY, Su S, Chen Z, Xu ST and Huang YH: Muscleblind-like 1 antisense
RNA 1 inhibits cell proliferation, invasion, and migration of
prostate cancer by sponging miR-181a-5p and regulating
PTEN/PI3K/AKT/mTOR signaling. Bioengineered. 12:803–814. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Q, Wu Y, Chen J, Tan F, Mou J, Du Z,
Cai Y, Wang B and Yuan C: The regulatory role of both MBNL1 and
MBNL1-AS1 in several common cancers. Curr Pharm Des. 28:581–585.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu L, Zhu S, Tang A and Liu W: LncRNA
MBLN1-AS1 inhibits the progression of retinoblastoma through
targeting miR-338-5p-Wnt/β-catenin signaling pathway. Inflamm Res.
70:217–227. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu K, Wang Y, Liu L, Li S and Yu W: Long
non-coding RNA MBNL1-AS1 regulates proliferation, migration, and
invasion of cancer stem cells in colon cancer by interacting with
MYL9 via sponging microRNA-412-3p. Clin Res Hepatol Gastroenterol.
44:101–114. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Parang B, Kaz AM, Barrett CW, Short SP,
Ning W, Keating CE, Mittal MK, Naik RD, Washington MK, Revetta FL,
et al: BVES regulates c-Myc stability via PP2A and suppresses
colitis-induced tumourigenesis. Gut. 66:852–862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams CS, Zhang B, Smith JJ, Jayagopal
A, Barrett CW, Pino C, Russ P, Presley SH, Peng D, Rosenblatt DO,
et al: BVES regulates EMT in human corneal and colon cancer cells
and is silenced via promoter methylation in human colorectal
carcinoma. J Clin Invest. 121:4056–4069. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng Q, Hawes SE, Stern JE, Wiens L, Lu H,
Dong ZM, Jordan CD, Kiviat NB and Vesselle H: DNA methylation in
tumor and matched normal tissues from non-small cell lung cancer
patients. Cancer Epidemiol Biomarkers Prev. 17:645–654. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim M, Jang HR, Haam K, Kang TW, Kim JH,
Kim SY, Noh SM, Song KS, Cho JS, Jeong HY, et al: Frequent
silencing of popeye domain-containing genes, BVES and POPDC3, is
associated with promoter hypermethylation in gastric cancer.
Carcinogenesis. 31:1685–1693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jagannathan K, Calhoun VD, Gelernter J,
Stevens MC, Liu J, Bolognani F, Windemuth A, Ruaño G, Assaf M and
Pearlson GD: Genetic associations of brain structural networks in
schizophrenia: A preliminary study. Biol Psychiatry. 68:657–666.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jorgensen BG and Ro S: MicroRNAs and
‘sponging’ competitive endogenous RNAs dysregulated in colorectal
cancer: Potential as noninvasive biomarkers and therapeutic
targets. Int J Mol Sci. 23:21662022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su J, Chen D, Ruan Y, Tian Y, Lv K, Zhou
X, Ying D and Lu Y: LncRNA MBNL1-AS1 represses gastric cancer
progression via the TGF-β pathway by modulating miR-424-5p/Smad7
axis. Bioengineered. 13:6978–6995. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang H, Shi Z, Li Y, Zhu G, Chen C, Zhang
Z, Shi R, Su L, Cao P, Pan Z, et al: Pyroptosis-related LncRNA
signatures correlate with lung adenocarcinoma prognosis. Front
Oncol. 12:8509432022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Guo Y, Li S, Xu J, Wang X, Ning W,
Ma L, Qu Y, Zhang M and Zhang H: Identification of
N6-methyladenosine-related lncRNAs as a prognostic signature in
glioma. Front Oncol. 12:7892832022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sanaei MJ, Baghery Saghchy Khorasani A,
Pourbagheri-Sigaroodi A, Shahrokh S, Zali MR and Bashash D: The
PI3K/Akt/mTOR axis in colorectal cancer: Oncogenic alterations,
non-coding RNAs, therapeutic opportunities, and the emerging role
of nanoparticles. J Cell Physiol. 237:1720–1752. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao G, Tan B, Wei S, Shen W, Wang X, Chu
Y, Rong T and Gao C: Down-regulation of MBNL1-AS1 contributes to
tumorigenesis of NSCLC via sponging miR-135a-5p. Biomed
Pharmacother. 125:1098562020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei X, Yang X, Wang B, Yang Y, Fang Z, Yi
C, Shi L and Song D: LncRNA MBNL1-AS1 represses cell proliferation
and enhances cell apoptosis via targeting miR-135a-5p/PHLPP2/FOXO1
axis in bladder cancer. Cancer Med. 9:724–736. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang J, Jiang G, Mao W, Huang L, Huang C,
Wang S, Xue H, Ke J and Ni Q: Up-regulation of long noncoding RNA
MBNL1-AS1 suppresses breast cancer progression by modulating
miR-423-5p/CREBZF axis. Bioengineered. 13:3707–3723. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han P, Fu Y, Liu J, Wang Y, He J, Gong J,
Li M, Tan Q, Li D, Luo Y, et al: Netrin-1 promotes cell migration
and invasion by down-regulation of BVES expression in human
hepatocellular carcinoma. Am J Cancer Res. 5:1396–1409.
2015.PubMed/NCBI
|
|
28
|
Li C, Wang P, Du J, Chen J, Liu W and Ye
K: LncRNA RAD51-AS1/miR-29b/c-3p/NDRG2 crosstalk repressed
proliferation, invasion and glycolysis of colorectal cancer. IUBMB
Life. 73:286–298. 2021. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rapado-González Ó, Majem B, Álvarez-Castro
A, Díaz-Peña R, Abalo A, Suárez-Cabrera L, Gil-Moreno A, Santamaría
A, López-López R, Muinelo-Romay L and Suarez-Cunqueiro MM: A novel
saliva-based miRNA signature for colorectal cancer diagnosis. J
Clin Med. 8:20292019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin Y, Xu L, Zhao B, Bao W, Ye Y, Tong Y,
Sun Q and Liu J: Tumour-suppressing functions of the lncRNA
MBNL1-AS1/miR-889-3p/KLF9 axis in human breast cancer cells. Cell
Cycle. 21:908–920. 2022. View Article : Google Scholar : PubMed/NCBI
|